Pore-wall functionalization of covalent organic framework palladium catalysts boosts the multicomponent reaction of CO2
Shiyuan
Wei†
,
Benling
Yu†
,
Jiawei
Li†
 *,
Jianhui
Zhu
,
Yaqi
Li
and
Jianhan
Huang
*,
Jianhui
Zhu
,
Yaqi
Li
and
Jianhan
Huang
 *
*
College of Chemistry and Chemical Engineering, Hunan Provincial Key Laboratory of Micro and Nano Material Interface Science, Central South University, Changsha 410083, Hunan, P. R. China. E-mail: lijiawei@csu.edu.cn; jianhanhuang@csu.edu.cn
First published on 18th November 2025
Abstract
Although supported metal nanoparticles (NPs) have demonstrated great potential in heterogeneous catalysis, the regulation of their interaction with the support framework remains a significant challenge. Herein, we employed a pore-wall functionalization strategy to construct four covalent organic frameworks (COFs) with distinct chemical microenvironments. Palladium nanoparticles were incorporated into the frameworks (Pd@TA, Pd@TA-4F, Pd@TA-OCH3, and Pd@TA-OHex), and the materials were applied in the multicomponent reaction of carbon dioxide. Impressively, modifications in the pore-wall microenvironment exhibited a regular modulating effect on the catalytic performance of the Pd NPs. Among them, Pd@TA-OCH3, which combines electron-donating effects and low steric hindrance, demonstrated the best catalytic performance. Furthermore, due to the altered surface microenvironment, Pd@TA-OCH3 exhibited optimal enrichment and adsorption (k = 0.32 h−1) behavior toward the substrates. In-depth catalytic and adsorption experiments, along with DFT calculations, confirmed the structure–activity relationship between the microenvironments of the COFs and their catalytic performance.
Introduction
The increasing atmospheric concentration of CO2 is contributing to increased global warming and climate change. In this context, it is in line with green and sustainable development to convert cheap, easily available and non-toxic CO2 as a renewable C1 resource into high-value chemicals through chemical fixation. To date, various CO2 fixation reactions involving C–C, C–N, C–O or C–H bond formation have been developed for the preparation of diverse fine chemicals.1,2 In particular, the multicomponent reactions (MCRs) between CO2 and simple raw materials in a one-pot manner are extremely fascinating by minimizing the number of synthesis operations while maximizing the structural and functional complexity of products with prominent economic and ecological advantages.3,4 However, the rational construction of functionalized catalysts that can drive smooth MCRs remains challenging.Palladium has received tremendous attention in organometallic catalysis, owing to its excellent selectivity, adjustable electronic structure and variable oxidation state, which have led to its widespread applications ranging from Nobel Prize-winning cross-coupling reactions to industrially significant oxidation reactions such as the Wacker process.5,6 The Pd-catalyzed one-pot MCR between CO2, isonitrile and 2-iodoaniline is particularly promising through the simultaneous construction of C–C, C–N, and C–O bonds for the facile preparation of quinazolinone and its derivatives,7 significantly important N-containing heterocyclic compounds widely utilized in various pharmaceuticals and bioactive molecules.8 Nevertheless, the gas–liquid–solid three phase nature of this MCR poses an additional requirement for the fabrication of a highly efficient catalytic system. Meanwhile, the thermodynamically favored Pd0 multimolecular agglomeration pathway to form Pd black in the reaction process further compromised the catalytic efficiency and selectivity.9 In addition, Pd-catalyzed coupling reactions usually involve a stepwise approach including oxidation addition, migration insertion, transmetallation, and reductive elimination, which are primarily dominated by the electronic properties and geometrical configuration of the Pd center.10,11 Therefore, in this case, constructing a platform that can not only regulate the electronic states and steric hindrance of a Pd catalyst, as well as maintain its long-term stability, but also facilitate the enrichment of the three-phase components is highly desirable while being extremely challenging.
Covalent organic frameworks (COFs) are an emerging class of porous materials with unique structures and chemical diversities, making them promising candidates for heterogeneous catalysis.12,13 Owing to their high versatility and tunability, the electronic structures and chemical microenvironment of the catalytic centers can be precisely adjusted for specific transformations. For instance, Jiang et al. first proposed a microenvironmental regulation strategy by integrating PdCu2 nanoparticles (NPs) onto the pore walls of MOFs/COFs with different functional groups. This modification changed the surface electronic state of the Pd NPs and significantly regulated the activity and selectivity in the semi-hydrogenation of alkynes.14–16 On the other hand, the robust and confined pore structure of COFs inhibits the active Pd0 from deactivation to Pd black and facilitates the confined growth of ultrafine Pd NPs within the frameworks. We reported the first vinyl-linked PPh3-based COFs for the confined growth of well-dispersed Pd NPs (<5 nm), and the corresponding Pd@TMBen-PPh3 could serve as outstanding heterogeneous catalysts for the Pd-catalyzed carbonylation coupling reaction.17 Additionally, the highly porous and hydrophobic nature of most COF skeletons is beneficial for the favorable enrichment and adsorption of gas and organic substrates, which promotes their interactions and enhances the reaction kinetics.18 All these features indicate COFs to be an ideal platform to drive the highly efficient Pd-catalyzed MCR between CO2, isonitrile and 2-iodoaniline, and to the best of our knowledge, it has never been reported before.
Here in this work, we reported the construction of a series of imine-based COF Pd catalysts (Pd@TA, Pd@TA-4F, Pd@TA-OCH3, and Pd@TA-OHex) through pre-functionalization and post-synthetic modification strategies. By decorating the pore wall with different functional groups (–H, –4F, –OMe, and –OHex), the electronic properties and steric hindrance effects of the Pd catalysts can be facilely regulated, which showed significant impacts on the catalytic performance of the one-pot MCR between CO2, isonitrile and 2-iodoaniline. Besides, due to the distinct surface microenvironments of these COFs, their enrichment and adsorption behavior towards substrates differed, with Pd@TA-OCH3 representing the most remarkable substrate enrichment effects. The results showed that Pd@TA-OCH3 gave apparently superior catalytic performance in the MCR than its homogeneous counterpart, a physical mixture of a homogeneous Pd catalyst and a COF, and other functionalized COFs, highlighting the overall regulation of electronic states, steric hindrance and the substrate binding effect of the catalyst on the activity in the Pd-catalyzed MCR.
Results and discussion
The four imine-based COF Pd catalysts were prepared via solvothermal synthesis followed by post-modification. COF-TA was synthesized from TAPB and terephthalaldehyde (TA) in o-dichlorobenzene/n-butanol at 120 °C for 72 h with 6 M acetic acid. COF-TA-OCH3 was obtained by reacting TAPB with 2,5-dimethoxybenzaldehyde (TA-OCH3) in mesitylene/dioxane under the same conditions. Similarly, replacing TA-OCH3 with tetrafluoro-terephthaldehyde (TA-4F) or 2,5-bis(hexyloxy)terephthalaldehyde (TA-OHex) yielded COF-TA-4F and COF-TA-OHex. Pd@TA, Pd@TA-OCH3, Pd@TA-4F, and Pd@TA-OHex were then prepared by stirring the COFs in Pd(OAc)2/acetonitrile at 50 °C for 12 h, followed by NaBH4 reduction. The synthetic scheme is shown in Scheme 1. | ||
| Scheme 1 The synthetic route, chemical structure of Pd@COFs and corresponding Pawley refinements of the PXRD patterns. | ||
The successful synthesis of the pristine COFs has been confirmed through various characterization techniques. First, powder X-ray diffraction (PXRD) demonstrated high crystallinity (>85%) for all four COFs, with strong diffraction peaks observed near 2.9°, attributed to the (100) plane, along with corresponding (110) and (200) planes (Fig. S2). Further XRD simulations indicated an AA stacking mode (Scheme 1). The framework structures of these COFs were also verified using 13C CP-MAS NMR, confirming their successful syntheses (Fig. 1a and Fig. S5).19,20 N2 adsorption–desorption experiments at 77 K revealed type IV isotherms for all COFs, with BET surface areas calculated as 1043, 607, 1994, and 1535 m2 g−1 for COF-TA, TA-4F, TA-OCH3, and TA-OHex, respectively (Fig. 1b and Fig. S6–S8). Pore size distribution analysis confirmed their mesoporous structures, with a trend of decreasing pore size as the functional group volume increased (Fig. S9). Fourier-transform infrared (FT-IR) spectroscopy clearly showed characteristic peaks corresponding to imine bonds and side-chain functional groups, along with the disappearance of aldehyde and amine peaks from the corresponding monomers, confirming the successful formation of these COFs (Fig. 1c and Fig. S10–S12). Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) images revealed that COF-TA, TA-OCH3, and TA-OHex exhibited similar agglomerated particle morphologies, while COF-TA-4F displayed a dendritic structure (Fig. S22–S26).
After the successful synthesis of pristine COFs, post-modification metallization was then carried out. PXRD analysis indicates that the crystallinity of the metallized COFs is slightly reduced, but is basically similar to that of the pristine COFs (Scheme 1 and Fig. S3). PXRD only detected weak peaks corresponding to the (111), (200), and (220) crystal planes of Pd NPs, which can be attributed to their high dispersion and small particle size (Fig. S4). Meanwhile, the specific BET surface areas and pore sizes of these COFs all decreased, which might be due to the occupation of the pores by Pd NPs (Fig. 1b and Fig. S6–S9). The FT-IR spectra show that the imine bonds of COFs remain intact, indicating that their structures have not been damaged (Fig. 1c and Fig. S10–S12). The X-ray photoelectron spectroscopy (XPS) analysis confirms the successful loading of Pd (Fig. 1d and Fig. S13–S16). The results indicated that Pd is basically in its zero-valent form, and the presence of some divalent Pd may be due to the partial oxidation (Fig. 1e).21,22 The XPS spectrum of COF-TA-OCH3 in the N 1s region shows a binding energy (BE) peak of imine bond (C–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) at 398.3eV.23 Notably, the BE of C
C) at 398.3eV.23 Notably, the BE of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–C in Pd@TA-OCH3 moved to a higher value by 0.3 eV owing to the Pd coordination to C
N–C in Pd@TA-OCH3 moved to a higher value by 0.3 eV owing to the Pd coordination to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N (Fig. 1f). The same shift was observed after loading Pd catalysts on other COFs (Fig. S17–S19). Meanwhile, it can be found that by modifying electron-withdrawing or -donating substituents, the BE of Pd0 3d5/2 is shifted from 336.2 eV for Pd@TA to 336.6 eV for Pd@TA-4F and to 335.8 eV for Pd@TA-OCH3 (Fig. 1e). In addition, the SEM images of these COF Pd catalysts did not undergo significant changes, indicating good retention of their morphology (Fig. S27–S30). Moreover, the TEM images show the uniform distribution of Pd NPs on the surface and in the interlayers of the COFs (Fig. S31b, e, h and k). Taking Pd@TA-OCH3 for example, the Pd NPs are uniformly distributed with a size of about 5 nm, and the size of the Pd NPs is inversely proportional to the size of the functional groups (Fig. S32). By analyzing the lattice stripes of individual Pd NPs, it is concluded that their spacing is 0.23 nm, which corresponds to the (111) crystalline surface (Fig. S31h).24 The energy dispersive X-ray spectroscopy (EDS)-mapping images show the uniform distribution of C, N, O, and Pd over the framework (Fig. S31). Inductively coupled plasma mass spectrometry (ICP-MS) showed that the Pd content in these COFs is approximately 13 wt% (Table S4). All these results verified the successful syntheses of COFs.
N (Fig. 1f). The same shift was observed after loading Pd catalysts on other COFs (Fig. S17–S19). Meanwhile, it can be found that by modifying electron-withdrawing or -donating substituents, the BE of Pd0 3d5/2 is shifted from 336.2 eV for Pd@TA to 336.6 eV for Pd@TA-4F and to 335.8 eV for Pd@TA-OCH3 (Fig. 1e). In addition, the SEM images of these COF Pd catalysts did not undergo significant changes, indicating good retention of their morphology (Fig. S27–S30). Moreover, the TEM images show the uniform distribution of Pd NPs on the surface and in the interlayers of the COFs (Fig. S31b, e, h and k). Taking Pd@TA-OCH3 for example, the Pd NPs are uniformly distributed with a size of about 5 nm, and the size of the Pd NPs is inversely proportional to the size of the functional groups (Fig. S32). By analyzing the lattice stripes of individual Pd NPs, it is concluded that their spacing is 0.23 nm, which corresponds to the (111) crystalline surface (Fig. S31h).24 The energy dispersive X-ray spectroscopy (EDS)-mapping images show the uniform distribution of C, N, O, and Pd over the framework (Fig. S31). Inductively coupled plasma mass spectrometry (ICP-MS) showed that the Pd content in these COFs is approximately 13 wt% (Table S4). All these results verified the successful syntheses of COFs.
After successfully constructing these COF Pd catalysts, we investigated their catalytic performance in the one-pot Pd-catalyzed MCR involving 2-iodoaniline (1a), tert-butyl isonitrile (1b), and CO2. Impressively, the MCR in the presence of Pd@TA-OCH3 (2 mol% Pd) at 80 °C for 12 h showed excellent activity with a yield of 98% and a turnover number (TON) of 49 (Fig. 2a and Fig. S33, S34). Under the optimal reaction conditions, we further compared the catalytic performance of different catalytic systems (Fig. 2b and Table S2). When the homogeneous catalyst Pd(OAc)2 was used, the catalyst loading had to be increased to 10 mol% to drive the MCR, resulting in a significantly reduced TON of only 8.2. This phenomenon may be attributed to the Pd0 multimolecular agglomeration pathway to form Pd black in the homogeneous system, which leads to a prominent decrease in catalytic activity. For comparison, the excellent stability of Pd@TA-OCH3 guarantees its long-term activity in the MCR, and it could run five consecutive catalytic cycles without apparent loss of activity (Fig. S35). To verify its high stability, the recovered Pd@TA-OCH3 was characterized using different characterization methods. Firstly, SEM and TEM images of the recovered Pd@TA-OCH3 showed that the distribution state of Pd NPs did not change after catalysis (Fig. S37 and S38). Besides, negligible Pd leaching was confirmed by ICP-MS (Table S4). Moreover, the N2 adsorption isotherm and PXRD pattern of the recovered Pd@TA-OCH3 indicated that the porosity and crystallinity were well maintained after catalysis (Fig. S39 and S40). The above results unambiguously demonstrated the remarkable effect of Pd@TA-OCH3 to stabilize the Pd0 species. Furthermore, the physical mixture of Pd(OAc)2 or Pd NPs with TA-OCH3 was also evaluated in this MCR, but it showed an obviously reduced catalytic activity compared to Pd@TA-OCH3 (Table S2, entries 11–13). These results highlighted the significant synergistic effect of the active Pd site and COF skeleton of Pd@TA-OCH3. We proposed that the hydrophobic nature of Pd@TA-OCH3 facilitates the enrichment of the substrates around the active Pd site, thus achieving higher catalytic efficiency. Indeed, the adsorption results showed that Pd@TA-OCH3 exhibited favorable adsorption towards CO2 and 1b (Fig. 2c, d and Fig. S43, S44). For comparison, Pd(OAc)2/Pd NPs and TA-OCH3 are separated in the physical mixture system, and their interactions are compromised in the solution, which is unfavorable for adsorption and activation of the substrates, thereby leading to a dramatic decrease in the activity.
Subsequently, we investigated the catalytic performances of other Pd@COFs bearing different substituents, since the electronic states and spatial resistance of Pd exert prominent influence on the key steps, such as oxidative addition and reductive elimination of the reaction process (Fig. 2b). The catalytic results showed that Pd@TA-OCH3 and Pd@TA-OHex bearing electron-donating groups exhibited enhanced activity with yields as high as 98% and 70%, respectively, which were significantly better than those of Pd@TA (64%) and Pd@TA-4F (55%). This activity enhancement may be attributed to the more negative electronic states of Pd in Pd@TA-OCH3 and Pd@TA-OHex, which promote the activation of carbon–halogen bonds and further accelerate the reaction rate of the oxidative addition step, as witnessed in previous reports. Besides, Pd@TA-OCH3 exhibited the best catalytic performance with much higher yields than Pd@TA-OHex, which may be ascribed to the steric hindrance brought about by the long-chain-OHex substituent, resulting in restricted reaction rates. In order to verify the above hypothesis, we carried out a reaction kinetics study. The results showed that the yield of Pd@TA-OCH3 was significantly higher than that of the other three catalysts since the beginning of the reaction (Fig. 2e). Based on the first-order kinetic equation −ln(1−xA) = kt, we fitted the curves for these four Pd@COF catalysts, and the results indicated that the catalytic rate of Pd@TA-OCH3 (0.32 h−1) was 3.9, 4.7, and 3.4 times higher than those of Pd@TA, Pd@TA-4F and Pd@TA-OHex, respectively (Fig. 2f). Attempts to fit second-order kinetics yielded less satisfactory correlation, further supporting the dominance of first-order behavior in this system (Fig. S36). DFT calculations using COF fragments and Pd cluster models revealed that Pd@TA-OCH3 possesses the highest electron density due to the methoxy group's electron-donating effect, followed by Pd@TA-OHex, Pd@TA and Pd@TA-4F. These results align with the catalytic performance, emphasizing the role of pore-wall functionalization (Fig. 3 and Fig. S45, S46).25 Compared to existing reports, this system achieves more efficient catalysis under milder conditions (Table S5).
 | ||
| Fig. 3 (a) The calculated charge and (b) the calculated ELF diagrams of the Pd cluster in Pd@TA-OCH3. | ||
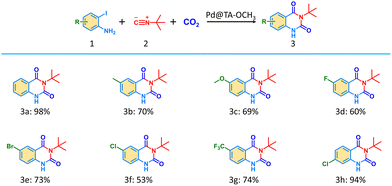
After obtaining the optimal reaction conditions, the substrate scope was investigated (Table 1). When 2-iodoanilines bearing electron-donating groups, such as methyl and methoxy substituents, were employed, separation yields of 70% and 69% were obtained. This may be attributed to the increased electron density on the aromatic ring caused by these electron-donating groups, which could hinder the oxidative addition of Pd0. 2-Iodoanilines substituted with various electron-withdrawing groups, such as –CF3, –Cl, –Br, or –F groups, gave moderate yields (53–74%). Although electron-withdrawing substituents are more favorable for facilitating Pd0 insertion, they inevitably reduce the electron density on the amino group, thus lowering its nucleophilicity. Notably, when a chlorine atom was positioned para to the iodine atom on 2-iodoaniline, the yield of product 3h (94%) was higher than that of 2-iodo-4-chloroaniline (3f), in which the –Cl group is at the para position to the –NH2 group. This suggests that the presence of an electron-withdrawing group in the para-position relative to the iodine atom results in better catalytic performance. These findings provide useful guidance for the rational design of aryl iodide substitution patterns.
| a Isolated yields. |
|---|
|
Based on the above results and relevant literature,26,27 a feasible mechanism for the MCR is proposed (Fig. 4). Initially, 1a engages in oxidative addition with Pd0 in the presence of Pd@TA-OCH3 to generate intermediate A. This intermediate then participates in a migratory insertion with 2a to furnish intermediate B. After the iodine atom departs, CO2 coordinates with the Pd catalyst to form a carbonyl complex. Simultaneously, DBU promotes deprotonation of the ortho-amino group, enhancing the nucleophilicity of the nitrogen atom and enabling it to attack the carbonyl group, thereby closing the ring. Intermediate C is subsequently transformed via reductive elimination to deliver intermediate D, thus completing the Pd catalytic cycle. DBU-mediated deprotonation induces electronic rearrangement of D to produce the open-chain intermediate E. Finally, E undergoes a second intramolecular nucleophilic cyclization followed by protonation, affording the desired product 3a.
Conclusions
In summary, we synthesized a series of COF-based Pd catalysts featuring different pore-wall substituents with tailored electron-donating and -withdrawing properties. Pd@TA-OCH3 exhibited the optimal catalytic efficiency in the one-pot MCR of CO2, isonitrile, and 2-iodoaniline owing to its balanced electronic effects and steric environment, as well as the enhanced reactant enrichment and catalyst stability. This work establishes concrete design principles for COF-based Pd catalysts, unequivocally positioning pore-wall engineering as a critical strategy for modulating catalytic behavior. Notably, this pore-wall engineering strategy is expected to be extended to other heterogeneous catalytic systems for a wider range of chemical transformations, potentially opening up new avenues for the rational design of high-performance, tailor-made catalysts.Conflicts of interest
There are no conflicts to declare.Data availability
The data associated with this article are available in the main text and its supplementary information (SI). Supplementary information: synthetic procedures and characterization spectra. See DOI: https://doi.org/10.1039/d5qm00708a.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 22308389 and 22478442).Notes and references
- X. Ding, W. Liu, J. Zhao, L. Wang and Z. Zou, Photothermal CO2 Catalysis toward the Synthesis of Solar Fuel: From Material and Reactor Engineering to Techno-Economic Analysis, Adv. Mater., 2025, 37, 2312093 CrossRef CAS.
- G. Wang, J. Chen, Y. Ding, P. Cai, L. Yi, Y. Li, C. Tu, Y. Hou, Z. Wen and L. Dai, Electrocatalysis for CO2 conversion: from fundamentals to value-added products, Chem. Soc. Rev., 2021, 50, 4993–5061 RSC.
- F. Yang, J. Wang, Y. Wang, B. Yu, Y. Cao, J. Li, L. Wu, J. Huang and Y.-N. Liu, Perfluoroalkyl-Decorated Noble-Metal-Free MOFs for the Highly Efficient One-Pot Four-Component Coupling between Aldehydes, Amines, Alkynes, and Flue Gas CO2, Angew. Chem., Int. Ed., 2024, 63, e202318115 CrossRef CAS PubMed.
- D. Tang, B. Ma, R. Liu, J. Tian and C. Zhao, One-Pot Solvent-Free Conversion of Furfural and CO2 into 2,5-Furandicarboxylic Acid, ACS Sustainable Chem. Eng., 2024, 12, 15752–15761 CrossRef CAS.
- J. He, M. Wasa, K. S. L. Chan, Q. Shao and J.-Q. Yu, Palladium-Catalyzed Transformations of Alkyl C–H Bonds, Chem. Rev., 2017, 117, 8754–8786 CrossRef CAS PubMed.
- P. Ruiz-Castillo and S. L. Buchwald, Applications of Palladium-Catalyzed C–N Cross-Coupling Reactions, Chem. Rev., 2016, 116, 12564–12649 CrossRef CAS PubMed.
- P. Xu, F. Wang, T.-Q. Wei, L. Yin, S.-Y. Wang and S.-J. Ji, Palladium-Catalyzed Incorporation of Two C1 Building Blocks: The Reaction of Atmospheric CO2 and Isocyanides with 2-Iodoanilines Leading to the Synthesis of Quinazoline-2,4(1H,3H)-diones, Org. Lett., 2017, 19, 4484–4487 CrossRef CAS PubMed.
- C. Liu, Y. Cao, Y. Zuo, C. Zhang, S. Ren, X. Zhang, C. Wang, Y. Zeng, J. Ling, Y. Liu, Z. Chen, X. Cao, Z. Wu, C. Zhang and J. Lu, Hybridization-based discovery of novel quinazoline-2-indolinone derivatives as potent and selective PI3Kα inhibitors, J. Adv. Res., 2025, 68, 459–475 CrossRef CAS PubMed.
- M.-B. Li and J.-E. Bäckvall, Efficient Heterogeneous Palladium Catalysts in Oxidative Cascade Reactions, Acc. Chem. Res., 2021, 54, 2275–2286 CrossRef CAS.
- S.-T. Kim, B. Pudasaini and M.-H. Baik, Mechanism of Palladium-Catalyzed C–N Coupling with 1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU) as a Base, ACS Catal., 2019, 9, 6851–6856 CrossRef CAS.
- R. G. Rao, R. Blume, T. W. Hansen, E. Fuentes, K. Dreyer, S. Moldovan, O. Ersen, D. D. Hibbitts, Y. J. Chabal, R. Schlögl and J.-P. Tessonnier, Interfacial charge distributions in carbon-supported palladium catalysts, Nat. Commun., 2017, 8, 340 CrossRef PubMed.
- S. S. A. Shah, M. S. Javed, T. Najam, M. A. Nazir, A. ur Rehman, A. Rauf, M. Sohail, F. Verpoort and S.-J. Bao, Covalent Organic Frameworks (COFs) for heterogeneous catalysis: Recent trends in design and synthesis with structure-activity relationship, Mater. Today, 2023, 67, 229–255 CrossRef CAS.
- S. Daliran, A. R. Oveisi, Y. Peng, A. López-Magano, M. Khajeh, R. Mas-Ballesté, J. Alemán, R. Luque and H. Garcia, Metal–organic framework (MOF)-, covalent-organic framework (COF)-, and porous-organic polymers (POP)-catalyzed selective C–H bond activation and functionalization reactions, Chem. Soc. Rev., 2022, 51, 7810–7882 RSC.
- M. Guo, Q. Meng, W. Chen, Z. Meng, M.-L. Gao, Q. Li, X. Duan and H.-L. Jiang, Dual Microenvironment Modulation of Pd Nanoparticles in Covalent Organic Frameworks for Semihydrogenation of Alkynes, Angew. Chem., Int. Ed., 2023, 62, e202305212 CrossRef CAS.
- L. Jiao, J. Wang and H.-L. Jiang, Microenvironment Modulation in Metal–Organic Framework-Based Catalysis, Acc. Mater. Res., 2021, 2, 327–339 CrossRef CAS.
- L. Jiao, Y. Wang, H.-L. Jiang and Q. Xu, Metal–Organic Frameworks as Platforms for Catalytic Applications, Adv. Mater., 2018, 30, 1703663 CrossRef.
- B. Yu, L. He, S. Wei, J. Li, Y. Wang, J. Zhong, J. Huang and Y.-N. Liu, Robust phosphine-based covalent-organic framework palladium catalysts for the highly efficient carbonylation coupling reaction, Chem. Commun., 2025, 61, 508–511 RSC.
- Y. Qian and H.-L. Jiang, Structural Regulation of Covalent Organic Frameworks for Catalysis, Acc. Chem. Res., 2024, 57, 1214–1226 CrossRef CAS PubMed.
- P. Shao, J. Li, F. Chen, L. Ma, Q. Li, M. Zhang, J. Zhou, A. Yin, X. Feng and B. Wang, Flexible Films of Covalent Organic Frameworks with Ultralow Dielectric Constants under High Humidity, Angew. Chem., Int. Ed., 2018, 57, 16501–16505 CrossRef CAS.
- H. Yu, F. Zhang, Q. Chen, P.-K. Zhou, W. Xing, S. Wang, G. Zhang, Y. Jiang and X. Chen, Vinyl-Group-Anchored Covalent Organic Framework for Promoting the Photocatalytic Generation of Hydrogen Peroxide, Angew. Chem., Int. Ed., 2024, 63, e202402297 CrossRef CAS.
- Z. Ouyang, G. Sheng, Y. Zhong, J. Wang, J. Cai, S. Deng and Q. Deng, Palladium Single Atom-supported Covalent Organic Frameworks for Aqueous-phase Hydrogenative Hydrogenolysis of Aromatic Aldehydes via Hydrogen Heterolysis, Angew. Chem., Int. Ed., 2025, 64, e202418790 CrossRef CAS PubMed.
- M.-Y. Sun, S. C. Cheung, X.-Z. Wang, J.-K. Jin, J. Guo, D. Li and J. He, Structural Reassignment of Covalent Organic Framework-Supported Palladium Species: Heterogenized Palladacycles as Efficient Catalysts for Sustainable C–H Activation, ACS Cent. Sci., 2024, 10, 1848–1860 CrossRef CAS PubMed.
- H. Pang, G. Liu, D. Huang, Y. Zhu, X. Zhao, W. Wang and Y. Xiang, Embedding Hydrogen Atom Transfer Moieties in Covalent Organic Frameworks for Efficient Photocatalytic C−H Functionalization, Angew. Chem., Int. Ed., 2023, 62, e202313520 CrossRef CAS.
- Y. Qiu, D. Jiao, H. Huang, J. Wu, M. Wang, T. Gao, X. Zhao, X. Ge, W. Zhang, W. Zheng, D. J. Singh, J. Fan and X. Cui, Locking interstitial hydrogen atoms in Pd metallenes for efficient oxygen reduction reaction, Nat. Commun., 2025, 16, 6103 CrossRef CAS PubMed.
- X. Li, J. Liu, J. Wu, L. Zhang, D. Cao and D. Cheng, Constructing a Highly Active Pd Atomically Dispersed Catalyst for Cinnamaldehyde Hydrogenation: Synergistic Catalysis between Pd–N3 Single Atoms and Fully Exposed Pd Clusters, ACS Catal., 2024, 14, 2369–2379 CrossRef CAS.
- D. Liu, B. Song, J. Wang, B. Li, B. Wang, M. Li, A. Qin and B. Z. Tang, CO2-Involved and Isocyanide-Based Three-Component Polymerization toward Functional Heterocyclic Polymers with Self-Assembly and Sensing Properties, Macromolecules, 2021, 54, 4112–4119 CrossRef CAS.
- S. Ren, S. Lin, Z. Ren, M. Wang, Y. Yang, H.-T. Tang, P. Chen, L. Zhou and Y.-M. Pan, Single-Site Palladium “Microreactor” for Carbon Dioxide Coupling/Cyclization Reactions, ACS Sustainable Chem. Eng., 2025, 13, 2001–2010 CrossRef CAS.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © the Partner Organisations 2026 |



