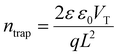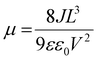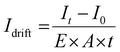Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Dual-site hydrogen bonding in 3D hybrid halide perovskitoids towards stable and sensitive ultraviolet light detection
Yao Li a,
Hang Lia,
Qianwen Guan
a,
Hang Lia,
Qianwen Guan a,
Huang Yea,
Chengshu Zhangac,
Lijun Xua,
Haiqing Zhongab,
Xinwei Zhoua and
Junhua Luo
a,
Huang Yea,
Chengshu Zhangac,
Lijun Xua,
Haiqing Zhongab,
Xinwei Zhoua and
Junhua Luo *ad
*ad
aState Key Laboratory of Functional Crystals and Devices, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou, Fujian 350002, China. E-mail: jhluo@fjirsm.ac.cn
bCollege of Chemistry, Fuzhou University, Fuzhou, Fujian 350108, China
cSchool of Physical Science and Technology, ShanghaiTech University, Shanghai 201210, China
dFujian College, University of Chinese Academy of Sciences, Fuzhou 350000, China
First published on 23rd December 2025
Abstract
Three-dimensional (3D) organic–inorganic hybrid perovskites (OIHPs) are known for excellent charge transport and large light absorption coefficients, making them promising materials for ultraviolet (UV) photodetection. However, the traditional 3D ABX3 perovskite structure is constrained by the tolerance factor, limiting its capacity to accommodate larger A-site cations. Therefore, it is necessary to explore novel 3D perovskitoids that can incorporate larger organic cations for UV photodetection. Herein, we introduced the diamine 3-methylaminopropylamine (3-MAPA) cation into the lead-iodide framework and successfully synthesized a 3D perovskitoid compound (3-MAPA)Pb2I6 (MPI). By forming an extensive network of hydrogen bonds between the 3-MAPA2+ cations and the inorganic framework, MPI effectively suppresses ion migration, thereby achieving an ultralow dark-current drift of 8.184 × 10−8 nA cm−1 s−1 V−1. Owing to its 3D inorganic lattice, MPI exhibits a high carrier mobility–lifetime product (μτ) of 2.613 × 10−3 cm2 V−1. These synergistic effects of extensive hydrogen bonding and the 3D lead-halide framework collectively enable stable UV photodetection under periodic 377 nm illumination with high responsivity (R ≈ 464.42 mA W−1) and detectivity (D* ≈ 4.05 × 1012 Jones). This work establishes stable UV photodetection via 3D perovskitoid compounds, expanding the candidate materials.
Introduction
Ultraviolet (UV) photodetection technology plays an indispensable role in national defense,1 environmental monitoring,2 and biomedical applications.3 However, mainstream UV photodetector materials (e.g., GaN,4 AlGaN,5 diamond,6 and AlN7) are hindered by high-temperature fabrication processes and escalating production costs.8,9 These shortcomings severely limit their practical deployment in UV photodetection.10–14 In recent years, organic–inorganic hybrid perovskites (OIHPs) are considered highly promising for UV photodetection due to their tunable bandgaps, strong light absorption, high charge-carrier mobility, and low-cost solution processability.15–19 Among them, 3D perovskites exhibit exceptional performance in UV detection, featuring high sensitivity, ultrafast response, and low detection limits,20–22 which are attributed to their 3D charge transport networks, superior light absorption efficiency and low exciton binding energy.23 However, conventional 3D perovskites usually suffer from inherent phase instability, undergoing rapid decomposition under moisture, heat, and UV irradiation, which significantly compromises device operational lifetime.24–27 Therefore, it is crucial to develop ultraviolet photodetectors that exhibit excellent stability and outstanding semiconductor properties.Enhancement of the environmental stability of OIHP materials is commonly achieved through modifications at the molecular level, including compositional engineering28 and dimensional control.29 For instance, researchers have synthesized a series of two-dimensional hybrid perovskites by incorporating chain-like or aromatic amines into the metal–halide framework (e.g. (BA)2PbBr4,30 (i-BA)2(MA)Pb2Cl7,31 (C8H11FN)2PbBr4,32 etc.), which effectively suppresses ion migration and enhances ultraviolet photochemical stability. However, restricted by the Goldschmidt tolerance factor,33–37 the introduction of bulky cations leads to the separation of inorganic and organic layers. These low-dimensional perovskites exhibit significant quantum confinement effects, which impede carrier transport and consequently hinder the development of high-performance UV photodetectors.38–42 In recent years, 3D hybrid perovskitoid materials with extended lattices have attracted widespread attention due to their capability to accommodate larger organic cations,43 offering a promising avenue for designing a stable 3D structure. These structures feature both corner-sharing and edge-sharing octahedral connectivity, enabling the accommodation of larger A-site cations by breaking the traditional constraints of the tolerance factor. Inspired by this concept, researchers have synthesized novel 3D perovskitoids (e.g. (3-MAPA)Pb2Br6,44 (DMPZ)Pb2Br6,45 etc.) and integrated them into X-ray detectors, yielding devices that are exceptionally stable and have high carrier mobility. The performance enhancement originates from the extensive network of hydrogen bonds between the diamine cations and the inorganic framework, which effectively suppresses ion migration, while the preserved 3D inorganic scaffold facilitates efficient charge carrier transport.46,47 Extending this strategy to UV photodetection, the exploration of diamine-derived 3D perovskitoid compounds holds significant promise.
Here, by incorporating the diammonium cation 3-methylaminopropylammonium (3-MAPA) into a metal–halide framework, we have successfully synthesized a novel 3D perovskitoid (3-MAPA)Pb2I6 (MPI)—featuring conformationally diverse organic cations. Abundant hydrogen bond interactions between the diamine cations and the inorganic lattice overcome the conventional tolerance-factor limitations associated with 3D perovskites. The 3D inorganic framework endows MPI with a high carrier mobility–lifetime product (μτ = 2.613 × 10−3 cm2 V−1). Devices fabricated from MPI single crystals demonstrate high responsivity (R ≈ 464.42 mA W−1) and specific detectivity (D* ≈ 4.05 × 1012 Jones), and exhibit excellent operational light stability under periodic 377 nm illumination. Additionally, under a 10 V bias and sustained ON/OFF switching over 103 cycles, the photocurrent remains stable without degradation. Crucially, the dark current drift at 10 V over 1000 seconds is extremely low, at 8.184 × 10−8 nA cm−1 s−1 V−1. MPI also exhibits significantly superior environmental stability relative to conventional 3D hybrid perovskites. This work provides a new perspective on the application of perovskitoids in UV photodetection by elucidating the critical role of hydrogen bonding in enhancing material performance and stability.
Results and discussion
Micromorphology and optoelectronic properties
Large red single crystals of (3-MAPA)Pb2I6 (MPI) with dimensions of 8 × 2 × 16 mm3 were successfully synthesized via a slow-cooling solution method (Fig. S1 and S2). Scanning electron microscopy (SEM) and atomic force microscopy (AFM) analyses revealed a smooth and well-faceted morphology (Fig. S3 and S4), indicating low density of surface defects. Such high crystal quality is beneficial for minimizing surface trap states and enhancing the transport of photogenerated charge carriers. Phase purity was confirmed by powder X-ray diffraction (PXRD), where the experimental pattern exhibited sharp and intense reflections in excellent agreement with the simulated pattern (Fig. S5), indicating a high-purity, single-phase material with excellent quality. High-resolution rocking curve measurements of the (080) plane yielded a narrow full width at half maximum (FWHM) of 0.018° (Fig. S6), indicative of minimal mosaicity and exceptional lattice coherence throughout the crystal. This exceptional material quality facilitates comprehensive investigation of the intrinsic stability and ultraviolet photoresponse under low-intensity illumination.Single-crystal X-ray diffraction analysis revealed that MPI crystallizes in the orthorhombic Pbam space group (Table S1). The structure comprises a 3D framework constructed from edge-sharing [Pb2I10]6− dimers, which are further interconnected via corner-sharing to form an extended network along the [001] direction (Fig. 1a–c and S7). The coexistence of edge-sharing and corner-sharing motifs along different crystallographic axes induces pronounced structural anisotropy, leading to direction-dependent charge transport and photoresponse behaviors. This unique topology creates well-defined cavities capable of accommodating the bulky divalent organic cation 3-MAPA2+. The MPI crystal accommodates two distinct molecular conformations within its framework cavities. While the N–H⋯I hydrogen bonds are a hallmark of conventional 3D perovskites, the emergence of C–H⋯I interactions is particularly noteworthy: the conformation of the 3-MAPA2+ cation brings C–H moieties into closer proximity to I sites (Fig. S8). Additionally, the local electrostatic influence of the NH3+ group enhances the delocalization of hydrogen electron density, thereby activating the C–H group as a hydrogen-bond donor and enabling the formation of the C–H⋯I hydrogen bond.48 To better understand the interactions within the molecule, we performed Hirshfeld surface analysis using Crystal Explorer software, where the red regions indicate stronger hydrogen bond intermolecular forces between N–H⋯I and C–H⋯I (Fig. 1d). Compared with single-amine cations, in the A′Pb2I6 perovskite built from diammonium cations we observe a relatively rich hydrogen-bond network between the organic cations and the inorganic framework (Fig. 1e and Table S2).49 Such an extensive hydrogen-bonding network contributes to enhanced structural stability. Moreover, the existence of multiple conformations of the organic cation enables multidirectional hydrogen-bond formation with the inorganic lattice, enhancing crystallographic symmetry and suppressing lattice distortion. This is evidenced by the notably lower calculated values (distortion index D = 0.00516 and bond angle variance σ2 = 1.92167) of MPI relative to other diammonium A′Pb2I6 perovskites (Fig. 1f and S8, Tables S3, S4). Different conformational states of organic cations can reinforce crystal rigidity and moisture resistance by modulating hydrogen-bonding networks and electrostatic interactions, thereby improving phase stability.50,51
In general, the application of 3D perovskites in optoelectronic devices is primarily governed by their intrinsic semiconductor and optical properties. Therefore, we employed a synergistic approach combining first-principles calculations with experimental characterization. The UV-vis absorption spectrum exhibits a sharp absorption edge around 606 nm, corresponding to an optical bandgap (Eg) of 2.13 eV, as determined by Tauc plot analysis (Fig. 2a). Density functional theory (DFT) calculations reveal that MPI possesses a direct bandgap with both the valence band maximum (VBM) and conduction band minimum (CBM) located at the same Brillouin zone. The calculated bandgap of 2.12 eV (Fig. 2b) aligns well with the experimental value, indicating the reliability of the employed computational approach. Under 450 nm excitation, MPI exhibits a pronounced photoluminescence (PL) peak at 584 nm (Fig. S9), corresponding to an optical bandgap of approximately 2.12 eV, as determined using the photon energy equation. The intense PL emission indicates efficient radiative recombination, highlighting MPI's potential for optoelectronic applications. Partial density of states (PDOS) analysis indicates that the VBM is primarily composed of iodine 5p orbitals, while the CBM is dominated by lead 6p orbitals, confirming that the electronic transitions are largely governed by the inorganic sublattice (Fig. 2c). Partial charge density mappings (Fig. S10) further illustrate that electronic states near the VBM are predominantly localized around equatorial I atoms, whereas CBM states are concentrated around Pb centers, indicative of strong electronic anisotropy. This electronic anisotropy is structurally rooted in the unique coexistence of edge-sharing and corner-sharing [PbI6] octahedra along distinct crystallographic axes (Fig. 1a), which induces pronounced structural anisotropy. The Pb–I–Pb bond angles along the a and b axes (151.43° and 92.20°, respectively) deviate more from linearity than the c-axis (179.36°) (Fig. S11), leading to differences in charge distribution and optical transition probabilities.
The crystallographic anisotropy manifests as distinct direction-dependent charge transport characteristics. To further elucidate the optoelectronic properties of MPI, we fabricated devices along different crystallographic axes and systematically characterized their carrier mobility (Fig. S12) and photoresponse (Fig. S13). The mobility–lifetime product (μτ), a critical parameter reflecting charge transport efficiency in ultraviolet photodetectors, was quantitatively extracted using a modified Hecht equation, which describes the average carrier drift distance under an applied electric field.52,53
To comprehensively evaluate the semiconductor properties of the material, we systematically investigated the charge transport characteristics and photoresponse behavior of photoconductive devices based on MPI. The defect density of MPI was evaluated using the space-charge-limited current (SCLC) method.54 Fig. 2e reveals three characteristic regimes: (1) an ohmic region (n = 1) at a low bias, (2) a trap-filled limit (TFL) region (n = 2) at an intermediate bias, and (3) the Child's region (n > 3). This behavior suggests trap-dominated charge injection. The trap density (ntrap) was derived from the following equation:
The μ value of (3-MAPA)Pb2I6 along the c-axis direction was calculated to be 104.25 cm2 V−1 s−1. Furthermore, based on the slope of the I–V curve, the resistivity (ρ) of MPI was determined to be 1.14 × 1010 Ω cm, indicating high intrinsic resistivity (Fig. S14). This suggests low carrier concentration under dark conditions, which is advantageous for improving detection sensitivity. Notably, this resistivity is significantly higher than that of conventional 3D perovskites such as MAPbBr3, CsPbBr3, and MAPbI3 (typically in the range of 106–109 Ω cm),55–57 enabling effective suppression of the dark current and noise, thereby promoting more stable and sensitive photodetection. The temporal response of the photodetector, defined by the rise (τrise) and fall (τfall) times corresponding to the increase from 10% to 90% and decay from 90% to 10% of the photocurrent, is a key indicator of device performance. MPI demonstrates rapid switching behavior, with τrise = 206 μs and τfall = 230 μs, underscoring its suitability for high-speed optoelectronic applications (Fig. 2f).
Performance of UV detection
Superior semiconductor properties are fundamental to achieving high-performance photodetectors. To evaluate the semiconductor characteristics and photodetection potential of MPI, a planar electrode photodetector was fabricated along the c-axis, which favors efficient carrier transport, based on high-quality MPI single crystals (Fig. 3a). Photocurrent measurements across the 266–637 nm wavelength range revealed a photoresponse consistent with the absorption spectrum, with a peak observed at 377 nm under an illumination intensity of 4.965 mW cm−2 (Fig. 3b). Consequently, the irradiation of a 377 nm laser was selected for further photoelectrical performance. Under 377 nm ultraviolet illumination, the device exhibited a pronounced photoresponse, as the photocurrent density increased with increasing light intensity (Fig. 3c). Notably, the device demonstrated an ultralow dark current of only 9.9 × 10−11 A, and under an illumination intensity of 4.965 mW cm−2 with a 10 V bias, it generated a photocurrent of 1.57 × 10−7 A, yielding a high on/off current ratio of 1586, indicative of its excellent light responsiveness. The photocurrent remained stable and discernible through repeated light on/off switching cycles, demonstrating both sensitivity and operational stability under low light power (Fig. 3d). This capability is attributed to the low defect density and superior charge transport in the MPI single crystal, which enabled a low detection limit down to the nW cm−2 level, showing significant potential for UV signal detection. Furthermore, under a 10 V bias voltage, the device showed a linear increase in the photocurrent with increasing light intensity from 1.215 to 4.523 mW cm−2 (Fig. 3e), further confirming its excellent linear response. To elucidate the detector's enhanced performance under low light, the relationship between the photocurrent (Iph = Ilight − Idark) and incident light power density (P) was analyzed using the empirical power law Iph ∼ Pβ (Fig. 3f). This model describes the photoresponse behavior across different illumination intensities, where the exponent β reflects the sensitivity of the device to light intensity and is closely linked to the photogeneration, trapping, and recombination of carriers. Fitting results revealed that under light, the β value reached 1.590, which is markedly higher than 0.727 under strong illumination. This elevated β indicates more efficient photoresponses at low intensities, likely due to suppressed carrier recombination and enhanced charge extraction, thereby improving detection sensitivity. In conclusion, the MPI-based UV photodetector exhibits excellent photoresponse characteristics under illumination, suggesting great potential for applications in low-intensity ultraviolet detection, such as environmental monitoring, biosensing, and early warning systems.The MPI based photodetector exhibits a pronounced and stable photoresponse along the c-axis under ultraviolet (UV) illumination, characterized by high responsivity (R) and specific detectivity (D*) values. Both R and D* decrease with increasing incident light power density, following power-law decay, which is typically associated with carrier recombination mechanisms (Fig. 4a). At low power densities, the device achieves a responsivity of 464.42 mA W−1 and a detectivity of 4.051 × 1012 Jones, indicating its superior sensitivity to UV light. Notably, the MPI based photodetector demonstrates exceptional R and D*, surpassing most perovskite materials employed in photoelectric detection, including (BPA)2PbBr4,53 (PA)2PbBr4,49 (i-PA)2CsAgBiBr7,58 and MAPbI3 nanoribbon arrays59 (Fig. 4b and Table S6). The MPI photodetector demonstrates exceptional photostability under periodic ultraviolet (UV) illumination at 377 nm, with the photocurrent maintaining 98.5% of its initial value after 1000 s of multiple cycle testing (Fig. 4c). Further investigation into the photoresponse dynamics reveals pronounced and rapid on/off switching behavior under periodic UV excitation, indicating swift response times suitable for high-frequency communication and fast imaging applications. After 103 consecutive light on/off cycles under a 10 V bias, the photocurrent remains stable without significant degradation, highlighting the device's reliability and fatigue resistance (Fig. 4d). Environmental stability assessments show remarkable resistance to degradation, with the device retaining 96.5% of its initial photoresponse after 60 days of storage under ambient conditions (25 °C, 40–60% RH) (Fig. 4e). To more intuitively evaluate the device performance and stability of the materials, a comparative study was conducted between MPI and the conventional 3D perovskite MAPbI3 in terms of the dark current drift, thermal robustness, and structural stability. The dark current drift (Idrift) was employed as a quantitative indicator of the dark-state stability of the photodetectors, calculated using the following equation:
In terms of thermal stability, thermogravimetric analysis (TGA) revealed that the decomposition temperature of MPI reaches 612 K, significantly higher than that of MAPbI3 (570 K), and surpassing many reported OIHPs (Fig. 4g). Furthermore, X-ray diffraction (XRD) patterns confirmed that MPI retained its crystalline structure after storage under 50% relative humidity for four months, with no detectable formation of secondary phases or notable peak attenuation (Fig. 4h). In contrast, MAPbI3 showed pronounced degradation within just one week under identical conditions. The exceptional structural and environmental stability of MPI is ascribed to the synergistic effect between the multi-conformational cationic arrangement and the diamine-anchored topological network structure. This work represents the first demonstration of utilizing a multi-conformational 3D perovskitoid material to construct high-performance ultraviolet photodetectors with outstanding long-term stability, thereby advancing the development of reliable UV detection technologies.
Conclusions
In summary, we synthesized a novel 3D perovskitoid material designated as (3-MAPA)Pb2I6 (MPI) and systematically investigated its potential for ultraviolet light photodetection. By forming an extensive network of hydrogen bonds between the 3-MAPA2+ cations and the inorganic framework, MPI effectively suppresses ion migration, thereby achieving an ultralow dark-current drift of 8.184 × 10−8 nA cm−1 s−1 V−1. Benefiting from the structural advantages of its 3D inorganic framework, the MPI detector exhibits an exceptional carrier mobility–lifetime product (μτ = 2.613 × 10−3 cm2 V−1). These synergistic effects of extensive hydrogen bonding and the 3D lead-halide framework collectively enable stable UV photodetection under periodic 377 nm illumination with high responsivity (R ≈ 464.42 mA W−1) and detectivity (D* ≈ 4.05 × 1012 Jones). This work presents novel design strategy for 3D hybrid perovskitoids, based on their high responsivity, excellent stability, and ultralow detection limit down to the nW cm−2 level, which provides a new pathway for constructing high-performance UV photodetectors.Author contributions
Yao Li wrote the manuscript; Hang Li and Qianwen Guan performed data analysis; Huang Ye and Chengshu Zhang performed validation; Lijun Xu, Haiqing Zhong and Xinwei Zhou performed data curation; Junhua Luo performed the methodology and reviewed this article.Conflicts of interest
The authors declare no conflict of interest.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information: additional computational details and experimental details, materials, and methods, including crystal morphology, crystal structure data, PXRD patterns, the TG curve, and basic photoelectric properties. See DOI: https://doi.org/10.1039/d5qi02302e.CCDC 2486626 contains the supplementary crystallographic data for this paper.60
Acknowledgements
This work was supported by the National Natural Science Foundation of China (22435005, 22193042), the Key Research Program of Frontier Sciences of the Chinese Academy of Sciences (ZDBS-LY-SLH024), the Natural Science Foundation of Fujian Province (2023J05076, 2021J01523, and 2020J01112), the Youth Innovation Promotion of CAS (2019301, Y202069, and 2020307), the Young Talent Supporting Project of Fujian Association of Science and Technology (2021000008) and the National Key Research and Development Program of China (2019YFA0210402).References
- Y. Zhang, Y. Cai, J. Zhou, Y. Xie, Q. Xu, Y. Zou, S. Guo, H. Xu, C. Sun and S. Liu, Surface acoustic wave-based ultraviolet photodetectors: a review, Sci. Bull., 2020, 65, 587–600 CrossRef CAS.
- L. Jia, W. Zheng and F. Huang, Vacuum-ultraviolet photodetectors, PhotoniX, 2020, 1, 1–25 CrossRef.
- H. Chen, K. Liu, L. Hu, A. A. Al-Ghamdi and X. Fang, New concept ultraviolet photodetectors, Mater. Today, 2015, 18, 493–502 CrossRef CAS.
- D. Y. Um, B. Chandran, J. Y. Kim, J. K. Oh, S. U. Kim, J. U. An, C. R. Lee and Y. H. Ra, New Charge Carrier Transport-Assisting Paths in Ultra-Long GaN Microwire UV Photodetector, Adv. Funct. Mater., 2023, 33, 2306143 CrossRef CAS.
- Q. Cai, H. You, H. Guo, J. Wang, B. Liu, Z. Xie, D. Chen, H. Lu, Y. Zheng and R. Zhang, Progress on AlGaN-based solar-blind ultraviolet photodetectors and focal plane arrays, Light: Sci. Appl., 2021, 10, 1–31 Search PubMed.
- E. Pace, R. Di Benedetto and S. Scuderi, Fast stable visible-blind and highly sensitive CVD diamond UV photodetectors for laboratory and space applications, Diamond Relat. Mater., 2000, 9, 987–993 CrossRef CAS.
- H. Wu, C. Wu, X. Cheng, C. Guo, J. Hu, D. Guo and S. He, Highly Wavelength-Selective Self-Powered Solar-Blind Ultraviolet Photodetector Based on Colloidal Aluminum Nitride Quantum Dots, Small, 2025, 21, 2312127 Search PubMed.
- W. Guo, H. Xu, W. Weng, L. Tang, Y. Ma, Y. Liu, L. Hua, B. Wang, J. Luo and Z. Sun, Broadband Photoresponses from Ultraviolet to Near-Infrared (II) Region through Light-induced Pyroelectric Effects in a Hybrid Perovskite, Angew. Chem., Int. Ed., 2022, 61, e202213477 Search PubMed.
- Z. Huo, Y. Zhang, X. Han, W. Wu, W. Yang, X. Wang, M. Zhou and C. Pan, Piezo-phototronic effect enhanced performance of a p-ZnO NW based UV-Vis-NIR photodetector, Nano Energy, 2021, 86, 106090 Search PubMed.
- Z. Jin, Q. Zhou, Y. Chen, P. Mao, H. Li, H. Liu, J. Wang and Y. Li, Graphdiyne : ZnO Nanocomposites for High-Performance UV Photodetectors, Adv. Mater., 2016, 28, 3697–3702 CrossRef CAS.
- Y. Ping, H. Long, H. Liu, C. Chen, N. Zhang, H. Jing, J. Lu, Y. Zhao, Z. Yang, W. Li, F. Ma, X. Fang, Z. Wei and H. Xu, Polarization Sensitive Solar-Blind Ultraviolet Photodetectors Based on Ultrawide Bandgap KNb3O8 Nanobelt with Fringe-Like Atomic Lattice, Adv. Funct. Mater., 2022, 32, 2111673 CrossRef CAS.
- H. Y. Lee, C. W. Lin and C. T. Lee, Aluminum-nanosphere-stacked MgNiO metal-semiconductor-metal ultraviolet photodetectors, J. Alloys Compd., 2019, 773, 210–216 Search PubMed.
- H. Chen, P. Yu, Z. Zhang, F. Teng, L. Zheng, K. Hu and X. Fang, Ultrasensitive Self-Powered Solar-Blind Deep-Ultraviolet Photodetector Based on All-Solid-State Polyaniline/MgZnO Bilayer, Small, 2016, 12, 5809–5816 CrossRef CAS.
- J. Kazmi, A. Abbas, D. J. Young, J. H. Shah, W. Ahmad, S. S. A. Shah, S. R. A. Raza, M. A. Mohamed, A. O. Govorov and Z. Wang, ZnO nanowire UV photodetectors: At the intersection of flexibility, biocompatibility, and visible blindness, Mater. Today, 2025, 82, 139–180 CrossRef CAS.
- S. Wang, L. Li, W. Weng, C. Ji, X. Liu, Z. Sun, W. Lin, M. Hong and J. Luo, Trilayered Lead Chloride Perovskite Ferroelectric Affording Self-Powered Visible-Blind Ultraviolet Photodetection with Large Zero-Bias Photocurrent, J. Am. Chem. Soc., 2020, 142, 55–59 Search PubMed.
- Q. Guan, P. Xu, B. Xu, H. Ye, Z. Zhu, S. Wang, C. Zhang, H. Li, C. Ji, Z. Lin and J. Luo, Unprecedented Ultraviolet Circularly Polarized Light-Dependent Anomalous Photovoltaics in Chiral Hybrid Perovskites, Adv. Sci., 2025, 12, 2412506 CrossRef CAS PubMed.
- Z. Xu, W. Weng, Y. Li, X. Liu, T. Yang, M. Li, X. Huang, J. Luo and Z. Sun, 3D-to-2D Dimensional Reduction for Exploiting a Multilayered Perovskite Ferroelectric toward Polarized-Light Detection in the Solar-Blind Ultraviolet Region, Angew. Chem., Int. Ed., 2020, 59, 21693–21697 CrossRef CAS.
- D. Fu, W. Jia, S. Wu, J. Chang, Z. Chen and J. Luo, Bilayered Dion-Jacobson Hybrid Perovskite Bulk Single Crystals Constructed with Aromatic Diammonium for Ultraviolet-Visible-Near-Infrared Photodetection, Chem. Mater., 2023, 35, 2541–2548 CrossRef CAS.
- C. Ji, D. Dey, Y. Peng, X. Liu, L. Li and J. Luo, Angew. Chem., Int. Ed., 2020, 59(43), 18933–18937 CrossRef CAS.
- J. J. Yoo, G. Seo, M. R. Chua, T. G. Park, Y. Lu, F. Rotermund, Y. K. Kim, C. S. Moon, N. J. Jeon, J. P. Correa-Baena, V. Bulović, S. S. Shin, M. G. Bawendi and J. Seo, Nature, 2021, 590(7847), 587–593 CrossRef CAS PubMed.
- G. A. Elbaz, D. B. Straus, O. E. Semonin, T. D. Hull, D. W. Paley, P. Kim, J. S. Owen, C. R. Kagan and X. Roy, Nano Lett., 2017, 17(3), 1727–1732 CrossRef CAS.
- M. Jaysankar, B. A. L. Raul, J. Bastos, C. Burgess, C. Weijtens, M. Creatore, T. Aernouts, Y. Kuang, R. Gehlhaar, A. Hadipour and J. Poortmans, ACS Energy Lett., 2019, 4(1), 259–264 CrossRef CAS.
- D. Sudarsan, R. Ganguly, A. L. Koner and S. K. Batabyal, Adv. Mater. Technol., 2025, 10(11), 2401573 CrossRef CAS.
- G. Maculan, A. D. Sheikh, A. L. Abdelhady, M. I. Saidaminov, M. A. Haque, B. Murali, E. Alarousu, O. F. Mohammed, T. Wu and O. M. Bakr, Phys. Chem. Lett., 2015, 6(19), 3781–3786 Search PubMed.
- J. He, S. Jiang, L. Lu, W. Li, J. Zhang, W. Wei, Z. Guo, B. Hu, Z. Wan, Y. Yun, Y. Tian, K. Huang, M. Chen and C. Li, Nanotechnology, 2023, 34(31), 315202 Search PubMed.
- L. Gao, K. Zeng, J. Guo, C. Ge, J. Du, Y. Zhao, C. Chen, H. Deng, Y. He, H. Song, G. Niu and J. Tang, Nano Lett., 2016, 16(12), 7446–7454 CrossRef CAS PubMed.
- J. Zhang, J. Zhao, Y. Zhou, Y. Wang, K. S. Blankenagel, X. Wang, M. Tabassum and L. Su, Adv. Opt. Mater., 2021, 9(17), 2100524 CrossRef CAS.
- L. Tang, W. Weng, H. Chen, L. Hua, W. Guo, Y. Liu, Y. Ma, Y. Chen, J. Luo and Z. Sun, Adv. Funct. Mater., 2023, 33(22), 2214858 Search PubMed.
- X. Zhou, Z. Lu, L. Zhang and Q. Ke, Nano Energy, 2023, 117, 108908 Search PubMed.
- L. Liang, X. Niu, X. Zhang, Z. Wang, J. Wu and J. Luo, Adv. Opt. Mater., 2022, 10(24), 2201342 Search PubMed.
- L. Lu, W. Weng, Y. Ma, Y. Liu, S. Han, X. Liu, H. Xu, W. Lin, Z. Sun and J. Luo, Angew. Chem., Int. Ed., 2022, 61(26), e2205030 Search PubMed.
- Y. Wang, B. Su, G. Lin, H. Lou, S. Wang, C. Y. Yue and X. Lei, CrystEngComm, 2021, 24(12), 2258–2263 Search PubMed.
- C. C. Stoumpos and M. G. Kanatzidis, Adv. Mater., 2016, 28(28), 5778–5793 Search PubMed.
- V. M. Goldschmidt, Naturwissensenschaffen, 1926, 14(21), 477–485 CrossRef CAS.
- S. Meloni, T. Moehl, W. Tress, M. Franckeviius, M. Saliba, Y. H. Lee, P. Gao, M. K. Nazeeruddin, S. M. Zakeeruddin, U. Rothlisberger and M. Graetzel, Nat. Commun., 2016, 7, 10334 Search PubMed.
- Q. Guan, S. You, Z. K. Zhu, R. Li, H. Ye, C. Zhang, H. Li, C. Ji, X. Liu and J. Luo, Angew. Chem., Int. Ed., 2024, 63(11), e202320180 Search PubMed.
- D. Umeyama, L. Leppert, B. A. Connor, M. A. Manumpil, J. B. Neaton and H. I. Karunadasa, Angew. Chem., Int. Ed., 2020, 59(43), 19087–19094 CrossRef CAS PubMed.
- J. Lv, X. Lu, X. Li, M. Xu, J. Zhong, X. Zheng, Y. Shi, X. Zhang and Q. Zhang, Small, 2022, 18(27), 2201715 Search PubMed.
- Y. Zhang, S. Li, Z. Li, H. Liu, X. Liu, J. Chen and X. Fang, Nano Lett., 2021, 21(1), 382–388 Search PubMed.
- Y. Wang, C. Ji, X. Liu, S. Han, J. Zhang, Z. Sun, A. Khan and J. Luo, Inorg. Chem. Front., 2018, 5(10), 2450–2455 RSC.
- Q. Wang, H. Wang, M. Sun, R. Xue, M. Ning, S. Li, P. Chen and Z. Li, Opt. Mater., 2023, 145, 114408 CrossRef CAS.
- T. Guo, C. Tian, S. Zhao, Z. Chu, J. Ma, Y. Li, Z. Shi and G. Ran, J. Phys. Chem. C., 2021, 125(25), 13909–13916 CrossRef CAS.
- X. Li, M. Kepenekian, L. Li, H. Dong, C. C. Stoumpos, R. Seshadri, C. Katan, P. Guo, J. Even and M. G. Kanatzidis, J. Am. Chem. Soc., 2022, 144(9), 3902–3912 Search PubMed.
- A. Wang, C. Zhang, Q. Guan, H. Ye, R. Li, H. Li, Y. Geng, C. Qu, Z. Wang, C. Ji and J. Luo, Small, 2024, 21, 2407843 CrossRef PubMed.
- L. Xu, D. Wang, H. Ye, H. Li, C. Zhang, Q. Guan, H. Zhong, Z. Han and J. Luo, Small, 2025, 21(9), 2410517 CrossRef CAS PubMed.
- S. Guan, Y. Li, C. Xu, N. Yin, C. Xu, C. Wang, M. Wang, Y. Xu, Q. Chen, D. Wang, L. Zuo and H. Chen, Adv. Mater., 2024, 36(25), 2400342 Search PubMed.
- X. Meng, J. Lin, X. Liu, X. He, Y. Wang, T. Noda, T. Wu, X. Yang and L. Han, Adv. Mater., 2019, 31(42), 1903721 CrossRef CAS PubMed.
- K. Dong, X. Yang, F. Yao, H. Cong, H. Zhou, S. Zhou, H. Cui, S. Wang, C. Tao, C. Sun, H. Fu, W. Ke and G. Fang, Adv. Mater., 2024, 36(24), 2313889 CrossRef CAS.
- C. Zhang, H. Xiao, Q. Guan, T. Zhu, L. Liang, R. Li, H. Ye, X. Niu and J. Luo, J. Mater. Chem. C, 2023, 11(15), 5116–5122 Search PubMed.
- A. Glushkova, A. Arakcheeva, P. Pattison, M. Kollár, P. Andričević, B. Náfrádi, L. Forró and E. Horváth, CrystEngComm, 2018, 20(25), 3543–3549 RSC.
- Y. Tian, X. Zhang, K. Zhao, X. Miao, T. Deng, W. Fan, D. Jin, X. Jiang, S. Zhong, X. Wang, S. Wang, P. Shi, L. Tian, L. Yao, S. Gong, X. Yu, X. Gao, Z. Chen, X. Chen, Y. Lu, V. Shrote, Y. Yang, D. Yang, R. Wang and J. Xue, Nat. Photonics, 2024, 18(9), 960–966 CrossRef CAS.
- L. Zhao, Y. Zhou, Z. Shi, Z. Ni, M. Wang, Y. Liu and J. Huang, Nat. Photonics, 2023, 17(4), 315–323 Search PubMed.
- C. Ji, S. Wang, Y. Wang, H. Chen, L. Li, Z. Sun, Y. Sui, S. Wang and J. Luo, Adv. Funct. Mater., 2020, 30(5), 1905529 Search PubMed.
- J. Sworakowski and K. Pigon, Solid State Commun., 1969, 30, 491–496 Search PubMed.
- A. Jaffe, Y. Lin, C. M. Beavers, J. Voss, W. L. Mao and H. I. Karunadasa, ACS Cent. Sci., 2016, 2(4), 201–209 CrossRef CAS PubMed.
- G. Cen, Y. Liu, C. Zhao, G. Wang, Y. Fu, G. Yan, Y. Yuan, C. Su, Z. Zhao and W. Mai, Small, 2019, 15(36), 1902135 Search PubMed.
- Y. Liu, Y. Zhang, K. Zhao, Z. Yang, J. Feng, X. Zhang, K. Wang, L. Meng, H. Ye, M. Liu and S. (Frank) Liu, Adv. Mater., 2018, 30(29), 1707314 Search PubMed.
- Y. Li, T. Yang, Z. Xu, X. Liu, X. Huang, S. Han, Y. Liu, M. Li, J. Luo and Z. Sun, Angew. Chem., 2020, 132(9), 3457–3461 Search PubMed.
- S. Lim, M. Ha, Y. Lee and H. Ko, Adv. Opt. Mater., 2018, 6(21), 1800615 Search PubMed.
- CCDC 2486626: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2pgjrg.
| This journal is © the Partner Organisations 2026 |