Molecular insights into the role of selenoenzymes in the toxicity of methylmercury
Mikel
Bernabeu de Maria†
a,
Tshering
Zangmo†
a,
Andrzej
Gawor
 bc,
Luigi
Messori
bc,
Luigi
Messori
 d,
Ewa
Bulska
d,
Ewa
Bulska
 bc,
Joanna
Szpunar
bc,
Joanna
Szpunar
 a,
Ryszard
Lobinski
ae,
Karinne
Miqueu
a,
Ryszard
Lobinski
ae,
Karinne
Miqueu
 *a and
Luisa
Ronga
*a and
Luisa
Ronga
 *a
*a
aUniversité de Pau et des Pays de l'Adour, E2S UPPA, CNRS, IPREM UMR 5254, 2 Av. du Président Pierre Angot, 64053 Pau cedex 09, France. E-mail: luisa.ronga@univ-pau.fr; karinne.miqueu@univ-pau.fr
bUniversity of Warsaw, Faculty of Chemistry, Pasteura 1, 02-093, Warsaw, Poland
cUniversity of Warsaw, Biological and Chemical Research Centre, Żwirki i Wigury 101, 02-089, Warsaw, Poland
dLaboratory of Metals in Medicine, Department of Chemistry, University of Florence, via della Lastruccia 3, 50019, Sesto Fiorentino, Florence
eChair of Analytical Chemistry, Warsaw University of Technology, ul. Noakowskiego 3, 00-664 Warszawa, Poland
First published on 25th November 2025
Abstract
Mercury (Hg) is a toxic metal that poses a serious threat to global health. Methylmercury (MeHg), an organic compound of Hg, is among the most toxic forms of the metal. The molecular mechanisms by which methylmercury produces its toxic effects are not fully understood. However, previous studies have shown that certain selenoenzymes, which play a vital role in maintaining cellular and tissue homeostasis (e.g. thioredoxin reductase (TrxR) and glutathione peroxidase (GPx)), are strongly inhibited by methylmercury in both in vitro and in vivo studies, and are therefore probable targets of its toxicity. This study aims to gain a comprehensive mechanistic understanding of the role of selenoproteins in methylmercury toxicity by investigating their reactivity towards MeHg+ and analysing their metal-binding mode using a joint experimental and computational approach. In particular, liquid chromatography (LC) coupled to tandem electrospray mass spectrometry (ESI-MS) was employed to characterise the reactivity of methylmercury with the C-terminal dodecapeptide of TrxR1 and the full-length Gpx1. Remarkably, clear evidence of Se–Hg bond formation in GPx1 has been achieved for the first time in this study. Conversely, DFT calculations provided a rational explanation and detailed description of the underlying reaction mechanisms involving the preferential reactivity of MeHg+ towards SeCys, followed by the participation of neighboring Cys residues. These reactions lead to the formation of robust S–Hg–S(e) bridges within the investigated selenoproteins. We propose that these molecular mechanisms also operate in vivo, determining the potent inhibition of selenoenzymes by MeHg and the associated severe toxicity.
Introduction
Owing to its extreme toxicity, the World Health Organization (WHO) recently included mercury (Hg) in the top ten chemicals of major public health concern.1 Methylmercury (MeHg), an organic form of Hg, is among the most toxic forms of the mercury compounds;2 it bioaccumulates in fish and rice,3,4 resulting in human exposure to MeHg and subsequent neurotoxicity.Mercury ions (MeHg+ and Hg2+), being soft acids, readily react with biomolecules, thereby inhibiting their activities and disrupting normal cellular functions. The thiol (–SH) and selenol (–SeH) groups of cysteine (Cys, C) and selenocysteine (SeCys, U), respectively, in proteins are primary targets of Hg2+ and MeHg+.5,6 However, given the greater nucleophilicity of the –SeH group compared to that of the –SH group, selenoproteins, which contain at least one SeCys and are responsible for the main physiological functions of Se, appear to be favoured targets for MeHg+ toxicity.5,6 In particular, selenoenzymes, which play an essential role in cellular and tissue homeostasis, are strongly inhibited by MeHg+ and Hg2+in vivo.7–11 For example, MeHg+ reduced the activity of glutathione peroxidase 1 (GPx1) in the mouse brains and in cultured neuroblastoma cells, causing significant alterations in oxidative stress and apoptotic markers.12 Furthermore, in zebra-seabreams exposed to MeHg+ in water a decreased thioredoxin reductase (TrxR) activity was found in the liver (40%) and the brain (75%).13
The strong link existing between MeHg+ and SeCys is fascinating and not fully understood, with the potential for valuable therapeutic applications after human exposure to MeHg+. Several studies have examined so far the impact of co-exposure to Hg2+ or MeHg+ and selenite (Na2SeO3) on selenoenzymes. For example, selenite has been shown to reactivate HgCl2-inhibited TrxR activity in HEK 293 cells,14 and selenite supplementation has been found to prevent TrxR10 and GPx15 inhibition induced by Hg2+ in fishes and mice (kidneys), respectively. Ganther et al.16 first observed the antagonistic role of Na2SeO3 against MeHg+ toxicity, but the molecular mechanism of detoxification remains elusive and controversial as additive or even synergistic effects have also been reported in the literature.17 It has been suggested that Se's protective effect depends on its availability for selenoprotein synthesis,18 and it is generally accepted that MeHg+ is more toxic when the Hg level approaches or exceeds equimolar stoichiometry with Se.18 However, our understanding of selenoprotein involvement in MeHg+ toxicity is limited by the lack of formal evidence for Se–Hg bonds and chemical details behind these interactions. We believe that clarifying the way by which MeHg+ binds to selenoproteins in vivo and in vitro could provide further insight into the chemical mechanisms of MeHg+ toxicity at an atomic and molecular level. This could offer clues as to how mercury could be detoxified.
First, Carvalho et al.14 used mass spectrometry to demonstrate that a reduced form of TrxR (potentially presenting 17 free Cys and 1 SeCys) reacts with an excess of MeHg+. This reaction leads to the formation of MeHg–TrxR adducts containing several MeHg moieties (up to eight), which eventually results in enzyme inactivation. However, this study only provided indirect evidence of SeCys involvement in MeHg binding. In 2020 Pickering et al.19 used extended X-ray absorption fine structure spectroscopy to clearly demonstrate, for the first time, the formation of a Se–Hg bond within the catalytic pocket of TrxR upon reaction with MeHg+. Conversely, despite several reports in the literature suggesting that the activity of GPx1 is negatively affected by exposure to MeHg+,12,20 there is a substantial lack of direct evidence of Se–Hg bond formation in GPx1. More broadly, the mechanisms underlying the interaction of MeHg+ with selenoproteins and thiol-containing proteins, and consequent toxicity, remain poorly understood to date. Nevertheless, recent in silico studies investigating the influence of MeHg+ on the oxidation of the selenolate centre to a selenoxide by hydrogen peroxide in model methylmercury (seleno)cysteinate complexes have paved the way for a deeper molecular interpretation of MeHg toxicity.21
Building on the above arguments, this study aims to gain a comprehensive mechanistic understanding of the role of selenoproteins in MeHg toxicity. This will involve investigating their reactivity toward MeHg+ and analysing the binding mode of Hg using a combination of experimental and computational methods.
Firsly, we selected the 12-mer peptide derived from the C-terminus of human TrxR1 (Trx(488–499)) as our model. This peptide contains the reactive Cys–SeCys motif that mimics the active site of the enzyme (Fig. 1A). We investigated its reactivity with MeHgCl using liquid chromatography (LC) coupled to tandem electrospray mass spectrometry (MS/MS), integrating the related experimental evidence with a theoretical study. In particular, we performed density functional theory (DFT) calculations to explore the reaction between MeHgCl and the tripeptide TrxR(497–499) (Fig. 1A). This represents a simplified model focusing on the Cys and SeCys residues.
We then extended the LC-MS/MS approach to investigate the reactivity of MeHgCl with full-length GPx1 selenoprotein, which contains one SeCys residue and five Cys residues (Fig. 1B). The evidence obtained by MS could be better interpreted with the aid of DFT calculations performed on the model motif. This enabled us to elucidate the key role of SeCys in the MeHg+ protein binding mode.
Results and discussion
The reaction of TrxR(488–499) with MeHgCl: LC-MS/MS studies
First, the reactivity of MeHg+ with the dodecapeptide Trx(488–499) was investigated. TrxR(488–499) is derived from the C-terminus of human TrxR1 and contains the Cys–SeCys reactive motif, which mimics the active site of the enzyme. As illustrated in Fig. 2, to facilitate the reaction of TrxR(488–499) and MeHgCl, the peptide was initially treated with 10 equiv. of the reducing agent dithiothreitol (DTT) for 30 min at 37 °C. This protocol resulted in the S–Se bond being extensively reduced, forming free thiol/selenol groups (see Fig. S1). After the above described reduction step, the peptide was reacted with MeHgCl, in the presence of DTT (10 equiv. initially added), under physiological conditions (pH 7 and 37 °C) at different molar ratios of peptide to MeHg+ (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 and 1
0.5 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) and for different incubation times (5 min and 18 h) resulting in the formation of several metal–peptide adducts (see Table S1). The reaction products were monitored and identified by LC-MS/MS (see Fig. S2–S5), and this identification was also corroborated by comparing the experimental and theoretical isotopic patterns (see Fig. S6–S10).
3) and for different incubation times (5 min and 18 h) resulting in the formation of several metal–peptide adducts (see Table S1). The reaction products were monitored and identified by LC-MS/MS (see Fig. S2–S5), and this identification was also corroborated by comparing the experimental and theoretical isotopic patterns (see Fig. S6–S10).
 | ||
| Fig. 2 Scheme of the experiments: TrxR(488–499) incubation with MeHgCl in the presence of DTT, promoting a Hg-bridged adduct, and mono- and bis-metalated complexes. | ||
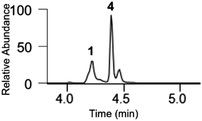
The typical set of results consisted of a total ion current chromatogram (TIC), mass spectra taken at the peak apexes (MS), the relative intensity of each species at their characteristic m/z values (extracted ion chromatogram, XIC) and their MS/MS spectra. These were used to elucidate the metal binding sites (see Fig. S11–12). Fig. 3 shows an example of this set of results for TrxR(488–499) incubated with MeHgCl (3 equiv., 18 h). Briefly, the TIC peaks in Fig. 3A were identified in Fig. 3C–E as the unreacted, the Hg-bridged and the bis-metalated TrxR(488–499) peptides in the mass spectra. MS/MS analysis of the bis-metalated TrxR(488–499) peptide (Fig. 3F) revealed the direct involvement of S and Se in the binding to two MeHg moieties. Furthermore, the relative abundance of these peptides in the reaction medium could be assessed on the basis of the intensity of the XIC of each species (z = 1) (Fig. 3B).
The results of the above-described experiments are summarized in Table 1, which shows the XIC of unreacted and metalated TrxR(488–499) according to the number of MeHgCl equivalents at various times. Reacting TrxR(488–499) with 3 equiv. of MeHgCl yielded a significant amount of bis-metalated adduct and trace amounts of Hg-bridged peptide within 5 minutes of incubation (see Table 1 entry 1 and Fig. S2). The LC-MS profile of this reaction mixture did not change significantly after 18 h of incubation (Table 1 entry 2 and Fig. 3).
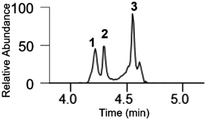
In a second set of experiments, a small quantity of MeHgCl (0.5 equiv.) was reacted with Trx(488–499). After just 5 min of incubation (see Table 1 entry 1 and Fig. S3), TrxR(488–499) was largely converted into the mono-metalated adduct. MS/MS analysis of the mono-metalated Trx(488–499) adduct (see Fig. S12) formed under these conditions showed selective binding of MeHg+ to Se over S.
The LC-MS profile of this reaction mixture underwent a drastic change after 18 h, as can be seen in Table 1 (entry 2) and Fig. S4: the mono-metalated adduct was completely consumed, and the Hg-bridged and bis-metalated adducts formed.
These results demonstrate that a brief reaction involving a substoichiometric quantity of MeHgCl with TrxR(488–499) promotes the selective binding of SeCys498 with MeHg+ in the mono-metalated adduct. Following a prolonged incubation period, the mono-metalated adduct transforms into the Hg-bridged and bis-metalated adducts through Cys499 reaction with either the MeHg moiety linked to SeCys498 (intramolecularly) or a second MeHgCl molecule, respectively.
The reaction of TrxR(497–499) with MeHgCl: DFT calculations
Few theoretical studies have investigated the formation of chalcogen–mercury bonds and the mechanism of action of MeHg+ remains not fully understood. Among the most recent studies, G. Schreckenbach and F. Wang studied the structural, electronic, spectroscopic and thermodynamic properties of five methylmercury–amino acid complexes and their selenium analogs, using the B3LYP and PBE0 functionals, and demonstrated that MeHg-amino acid complexes are slightly different from their selenium analogues.22 More recently, L. Orian et al. explored the Rabestein reaction, a key process in methyl mercury toxicity, involving a ligand (methylchalcogenolate) and a substrate (methylchalcogenolatemethyl mercury). Using (COSMO)-ZORA-B3LYP-D3(BJ)/TZ2P calculations with comparison to a Minnesota functional, they provided a molecular explanation for the inactivation of thiol-dependent enzymes, due to mercury's strong binding to thiol and selenol groups.23To obtain a clearer picture of the role of TrxR(488–499) in MeHg toxicity and gain better insight into the mechanism involved, the reactivity of MeHgCl and MeHgOH with the shorter TrxR(497–499) peptide was investigated by DFT (see the SI for computational details). This tripeptide was designed to focus exclusively on the Cys–SeCys motif directly involved in the reactivity of the dodecapeptide with MeHgCl, as demonstrated by LC-MS/MS. This computational study aimed to clarify the above-described reactivities, which lead to the formation of the three Hg-TrxR(488–499) adducts (2, 3 and 4 in Fig. 2) and improve our understanding of the Hg-demethylation process, generating the bridged S–Hg–Se complex (2). DFT calculations were performed at the SMD(H2O)-B3LYP-D3(BJ)/SDD(Hg), 6-31G** (other atoms)//B3LYP-D3(BJ)/SDD(Hg), 6-31G** (other atoms) level of theory to describe the formation of the mono-, bis-metalated and Hg-bridged TrxR(497–499) adducts (4′, 3′ and 2′ in Scheme 1), as well as to rationalize the selective binding of the Hg center with the Se center in the mono-metalated species. This level of theory (B3LYP) was selected based on prior investigations22–24 on chalcogen–mercury bonds, where it has been shown to provide accurate results. To further support our results, single-point calculations were also performed using the M06 functional, for comparison. Both theoretical levels produced comparable results and led to similar conclusions. Only the results obtained with B3LYP-D3(BJ) are discussed in the main text. The results from the M06 functional are indicated in parentheses in Fig. 4 and discussed in the SI.
First, we performed an in-depth investigation of the formation of the Se- and S-bound mono-adducts (Scheme 1, adducts 4′, 4″ and 4‴). To account for all possible interacting species, two different reduced forms of TrxR(497–499) were considered as the starting point for the process: (i) a free thiol–selenolate form, consistent with the pKa values of Cys and SeCys, ∼8.3 and ∼5.2,25 respectively, in aqueous solution at pH = 7.0 (Scheme 1a) and (ii) a free thiol/selenol form (Scheme 1b), in accordance with experimental evidence (MS) proving the reduction of TrxR(488–499) upon treatment with DTT before reacting with MeHgCl (see Fig. S1 and Table S1) as well as previous literature reports describing the reduction of disulfide derivatives by DTT.26 Moreover, this thiol/selenol form allows a direct comparison between the two chalcogens within an identical chemical environment, enabling the direct evaluation of mercury's relative affinity toward each chalcogen. In addition, regarding the mercury substrates, we considered both MeHgCl (the reactant used for experimental investigations) and MeHgOH, which can be formed in an aqueous environment. Previous studies by C. Meuleman et al.,27 investigating the behavior of methylmercury compounds in aqueous solution, demonstrated that, depending on the pH, a competition occurred between hydroxide ions (OH−) and halide ions (Cl−, Br−, etc.), indicating that the predominant methylmercury species in solution is pH-dependent. At pH 7.0, both MeHgCl and MeHgOH species are expected to coexist.
Considering first the thiol/selenolate form, the formation of the Se–Hg adduct 4′-X− (X: Cl, OH) upon reaction with MeHgCl or MeHgOH (Scheme 1a) was found to be thermodynamically favorable (Table S2). The reaction is strongly exergonic with MeHgCl (ΔG < −25 kcal mol−1), whereas it is only slightly exergonic with MeHgOH (ΔG ∼ 0 to −5.5 kcal mol−1). These findings are consistent with experimental observations, which show the rapid formation of the Se-adduct 4′ within 5 minutes at 37 °C, following the reduction of TrxR(497–499) by DTT and subsequent reaction with MeHgCl in water.
Considering next the thiol/selenol form, and in light of the various Hg-peptide adducts experimentally produced by reacting MeHgCl with TrxR(488–499) in water solution, we have proposed the reaction mechanism illustrated in Scheme 1b and c for the reaction involving TrxR(497–499). Two successive additions of MeHgX (X: Cl, OH) lead to the mono- and bis-metalated complexes, 4′ (or 4″/4‴) and 3′, respectively. Hg-demethylation can then occur through two different pathways. The Hg-bridged complex S–Hg–Se (2′) can be formed either from the mono-metalated complex (4′) by the loss of methane or from the bis-metalated complex (3′) by the loss of dimethylmercury (Me2Hg) through a nucleophilic attack of the chalcogen (S or Se) on the metal center.
To begin with, we studied the first step of the process, i.e., the addition of one equivalent of MeHgX (X: Cl, OH) to the thiol/selenol reduced form of TrxR(497–499), in order to assess the relative affinity of selenium and sulfur for mercury, within the same chemical environment (–SH, thiol and –SeH, selenol). This addition could potentially lead to the formation of two different mono-metalated complexes, with either Se (4′) or S (4″/4‴) involved in the binding with MeHg+. DFT calculations provided insight into the origin of the selectivity of this initial step. In line with the experimental observations, the reaction profile (see Fig. S13 and S14) showed that the formation of the mono-metalated complex involving a Se–Hg bond (4′) is favored, both kinetically and thermodynamically, compared to its analogue involving a S–Hg bond (4‴). Initially, the selenol moiety coordinates with MeHgX to form a stabilized adduct (ΔG: −5.5 kcal mol−1 for X: Cl and −0.5 kcal mol−1 for X: OH) with a Se⋯Hg bond length of 3.083 and 3.180 Å for X: Cl and X: OH, respectively (<∑rvdW radii: 3.95 Å),28 in line with mercury's marked preference for selenium over sulfur.29 Then, the release of HCl or H2O through a four-membered transition state is highly favorable, with low activation barriers of ΔG‡: 1.3 kcal mol−1 (TS1_Se) and 1.6 kcal mol−1 (TS1_Se_OH) for the reactions with MeHgCl and MeHgOH, respectively. Both reactions are exergonic (ΔG = −3.5 kcal mol−1 for X: Cl and −32.1 kcal mol−1 for X: OH), with the first addition of MeHgX being more thermodynamically favorable for MeHgOH, producing water as a by-product. In contrast, the transition state corresponding to the nucleophilic attack of sulfur on the mercury center (TS1_S without stabilizing Se → Hg interaction) lies significantly higher in energy, by 17.7 kcal mol−1 (MeHgCl) and 7.5 kcal mol−1 (MeHgOH). The pronounced difference between the two activation barriers explains the selectivity for Se over S and supports the preferential involvement of Se in the binding to MeHg in the mono-metalated adduct 4. Moreover, a comparison of the relative stabilities of the Se-bound and S-bound adducts (Fig. S13) indicates that adduct 4′ is the most stable one, while the S-bound isomers 4″ (with Se → Hg interaction) and 4‴ (without Se → Hg interaction) were found to be higher in energy by 7.8 and 17.7 kcal mol−1, respectively. These results highlight the higher affinity of selenium compared to that of sulfur for mercury and are consistent with the experimental observation after incubating reduced TrxR(488–499) with 0.5 equiv. of MeHgCl for 5 min in aqueous solution (Table 1 and Fig. S13 and S14). This is the consequence of the greater nucleophilicity of selenium compared to that of sulfur. This is demonstrated by the selenium lone pair being in a more accessible energetic position (HOMO: −6.35/–6.59 for the MeHgCl adduct and −6.23 eV for the MeHgOH adduct) than the sulfur lone pair (HOMO: −6.69/–6.84 eV for the MeHgCl adduct and −7.18 eV for the MeHgOH adduct) in the reduced TrxR(497–499) reactant and its adduct with MeHgCl or MeHgOH (see Fig. S15).
Finally, to further substantiate the higher affinity of selenium for mercury, we analysed the role of the Se → Hg interaction in the mono-metalation of the sulfur atom by computing and comparing the transition states associated with the nucleophilic attack of sulfur on mercury, both in the presence and absence of an intramolecular Se → Hg interaction. Our calculations demonstrated that this stabilising interaction promotes the incorporation of the initial MeHgCl or MeHgOH unit, underscoring the pivotal role of the selenol binding preference for MeHg+. For both MeHgCl and MeHgOH substrates, a significant decrease in the activation barrier of the nucleophilic attack of sulfur is also observed in the presence of this interaction (ΔΔG‡: 7.6 kcal mol−1 for MeHgCl and 5.8 kcal mol−1 for MeHgOH) (see Fig. S13 and S14, TS1_S transition states). Additionally, this Se → Hg interaction was found to strongly stabilise the mono-metalated derivative by 9.9 kcal mol−1 (4″vs.4‴, Fig. S13) through the formation of the S–Hg bond, thereby supporting the stabilising role of this interaction.
Once the mono-metalated complex 4′ is formed, there are two possible competing pathways (Scheme 1c). The sulfur can either attack the metal center to form the bridged Se–Hg–S complex (2′) directly, resulting in the loss of methane, or a second addition of MeHgCl or MeHgOH can lead to the bis-metalated complex (3′). This affords 2′ after the loss of dimethylmercury (MeHgMe). With the MeHgCl substrate, DFT calculations (Fig. 4) revealed that the bis-metalation from 4′ proceeds via an accessible barrier of 14.5 kcal mol−1 but is endergonic (ΔG = +8.4 kcal mol−1), indicating a reversible process. However, the bis-metalated complex 3′ can be stabilised by another molecule of MeHgCl, making the reaction slightly thermodynamically favorable (ΔG = −1.5 kcal mol−1). The activation barrier and the thermodynamics of the reaction are consistent with LC-MS detection of the bis-metalated adduct 3 following a short reaction (5 min) of TrxR(488–499) with an excess of MeHgCl (see Fig. S2). With MeHgOH as the substrate (Fig. 4), the activation barrier of the bis-metalation step was calculated to be higher (ΔG‡: 21.2 kcal mol−1) than that observed with MeHgCl, but it remains readily accessible under the experimental conditions. The formation of 3′ is thermodynamically favored, with ΔG: −15.2 kcal mol−1. The barrier of 21.2 kcal mol−1 is consistent with the longer reaction time of 18 h observed experimentally using 0.5 eq. of MeHgCl in aqueous solution.
The demethylation from the bis-metalated species 3′ was then considered. The release of dimethylmercury is endergonic (ΔG ∼ 9 kcal mol−1) and involves a large barrier (ΔG‡ = 37–40 kcal mol−1, see Fig. S16). This makes the formation of 2′ unfeasible via this pathway. In contrast, the release of methane from 4′ proceeds with a more accessible energy barrier (Fig. 4). These results demonstrate that the Hg-demethylation occurs through a pathway involving a nucleophilic attack by sulfur on mercury bound to selenium in the mono-metalated species 4′. The loss of methane is the rate-determining step of the process (RDS). The analysis of the associated transition states (TS3: C⋯H: 1.397 Å and S⋯H: 1.723 Å) indicates that Hg-demethylation occurs in a synchronous, concerted manner. The S–H bond elongates by 27.8% from the mono-metalated adduct, while the C–H bond forms partially (27.9% elongated from CH4).
To gain a complete understanding of the process, we compared the results obtained from DFT calculations with LC-MS data of TrxR(488–499) incubated with MeHgCl over longer reaction times (18 hours). This revealed the formation of bis-metalated 3 and Hg-bridged 2 peptide-adducts (see Table 1). The above calculations revealed that the Hg-bridged complex 2′ is formed exclusively through the demethylation of Hg (rate determining step) from the mono-metalated-complex 4′via a strongly exergonic process with a relatively high activation barrier (ΔG‡ ∼ 27 kcal mol−1, optimization in solvent, see Fig. S17). This is consistent with the longer reaction time of 18 h. Additionally, in line with the LC-MS results, the Hg-demethylation process appeared to easily compete with the bis-metalation. The formation of 3′ from 4′ occurs with an activation barrier lower than that of Hg-demethylation (ΔΔG‡: 16.8 with MeHgCl and 10.1 with MeHgOH) and is less exergonic. These data suggest that the bis-metalated derivative (3′) is the kinetic product of this reaction, whereas the bridged Se–Hg–S derivative (2′) is the thermodynamic product. Both are formed from the mono-metalated species (4′).
In light of these results and according to the experimental data obtained for the dodecapeptide, it can be hypothesised that, a deficiency of MeHgCl and short reaction times favour the spontaneous conversion of TrxR peptides into the mono-metalated complex with a Se–Hg bond, given that mercury has a stronger affinity for selenium than sulfur. The mono-metalated adduct can then easily lead to the bis-metalated adduct in the presence of an excess of MeHgCl; this bis-metalation process is potentially reversible. Over long timescales, the bis-metalation can compete with Hg-demethylation, which occurs via sulfur attacking the metal centre and releasing methane. This can lead to the formation of both the Hg-bridged and bis-metalated TrxR peptide adducts from the key mono-metalated intermediate.
The reaction of GPx1 protein with MeHgCl: LC-MS/MS studies
Finally, we broadened the scope of our investigation to include a full-length selenoprotein, by examining the reactivity of GPx1 with MeHg+ in an experimental setting. Building on the mechanistic knowledge gained through the integrated experimental and theoretical results outlined above for TrxR peptide reactivity, we reacted GPx1 with a small amount of MeHgCl (0.5 equiv.) for 5 min, followed by an excess of the same reactant (3 equiv.) for 3 h. These incubations were performed under physiological conditions (37 °C and pH 7.0), immediately after a 30 min reduction step of the protein with 10 equiv. of DTT, and the corresponding reaction media were analysed by LC-MS.LC-MS/MS analysis of GPx1 incubated with 0.5 equiv. of MeHgCl for 5 min (see the top panel of Fig. 5 and S18) revealed that the predominant product was the mono-metalated adduct (+MeHg) for both the full-length (FL) and truncated (T) forms of GPx 1, which are characteristic of the unreacted protein (Fig. S19).
To identify the exact MeHg-binding site within the mono-metalated adduct of GPx1, two parallel approaches were undertaken. Firstly, a bottom-up analysis was performed (see the middle panel of Fig. 5 and Fig. S20): the protein was incubated with MeHgCl under the aforementioned conditions and then proteolytically digested by trypsin. A total of 21 peptides were observed, covering 97% of the GPx1 sequence. Of these peptides, those containing Cys80, Cys97, Cys117, Cys158 and SeCys51 were found to be metalated (+MeHg+). The extracted ion chromatogram (XIC) shows the highest intensity of the SeCys51 metalated peptide bearing the MeHg+ moiety on SeCys51 (as demonstrated by MS/MS).
Then, a top-down analysis was also conducted by fragmenting the mono-metalated GPx1 directly in the gas-phase using collision-induced dissociation (CID) (Fig. 5, bottom panel). This investigation was carried out on the FL GPx1 + MeHg adduct (22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 744.4 Da). A charge state of 23+ and an m/z of 990.4590 were selected for CID-MS/MS. The detection of characteristic b and y fragments bound to the MeHg moiety (e.g. MeHg+y154 and MeHg+b76), with a mass increment of +217.0 Da, enabled us to propose SeCys51 as the most probable binding site. Furthermore, the presence of multiple b and y fragments indicates the selective binding of MeHg+ to SeCys51 ruling out the involvement of the 5 Cys residues within the GPx1 sequence.
744.4 Da). A charge state of 23+ and an m/z of 990.4590 were selected for CID-MS/MS. The detection of characteristic b and y fragments bound to the MeHg moiety (e.g. MeHg+y154 and MeHg+b76), with a mass increment of +217.0 Da, enabled us to propose SeCys51 as the most probable binding site. Furthermore, the presence of multiple b and y fragments indicates the selective binding of MeHg+ to SeCys51 ruling out the involvement of the 5 Cys residues within the GPx1 sequence.
A second set of experiments was conducted by reacting GPx1 with 3 equiv. of MeHgCl for 3 h, followed by LC-MS analysis (see Fig. 6 and S21). This revealed extensive conversion of both FL and T forms of GPx1 into several metalated adducts.
The addition of one bare Hg(II) ion appeared to be the most prevalent type of species, with adducts detected at 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 652.4 Da and 22
652.4 Da and 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 728.3 Da for T GPx1 + Hg and FL GPx1 + Hg, respectively. However, the addition of one MeHg moiety was also evident, with adducts at 22
728.3 Da for T GPx1 + Hg and FL GPx1 + Hg, respectively. However, the addition of one MeHg moiety was also evident, with adducts at 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 668.4 Da and 22
668.4 Da and 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 744.3 Da, for T GPx1 + MeHg and FL GPx1 + MeHg, respectively. The addition of two bare Hg(II) ions to the protein were also observed, with adducts at 22
744.3 Da, for T GPx1 + MeHg and FL GPx1 + MeHg, respectively. The addition of two bare Hg(II) ions to the protein were also observed, with adducts at 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 850.4 Da and 22
850.4 Da and 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 927.3 Da, for T GPx1 + 2Hg and FL GPx1 + 2Hg, respectively. In the case of T Gpx1, double addition of one bare Hg(II) and one MeHg+ ion was also observed (T GPx1 + Hg + MeHg at 22
927.3 Da, for T GPx1 + 2Hg and FL GPx1 + 2Hg, respectively. In the case of T Gpx1, double addition of one bare Hg(II) and one MeHg+ ion was also observed (T GPx1 + Hg + MeHg at 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 865.3 Da).
865.3 Da).
In agreement with the observations previously made on TrxR peptides and their interactions with MeHgCl, we can state that a short treatment of GPx1 with a limited quantity of MeHgCl primarily leads to the formation of the mono-metalated protein, whereby the SeCys residue is directly involved in the binding to the MeHg moiety. The subsequent involvement of Cys residues (five of which are found in the GPx1 sequence) is likely to explain the addition of one or two bare Hg(II) ion(s) to Gpx1 following an extended incubation period involving excess MeHgCl. To explain the formation of the GPx1 + Hg adduct, we can hypothesise that Cys80 attacks the Hg centre of the MeHg moiety bound to SeCys51 due to its spatial proximity30 (resulting in demethylation), thereby forming a Se–Hg–S bridge within the protein (see Fig. 7). The formation of the GPx1 + 2Hg adduct can be explained by the binding of a second MeHgCl molecule to two additional Cys (among Cys97, Cys117 and Cys158), which results in the formation of a second S–Hg–S bridge.
Conclusion
In this study we have elucidated the binding mode of MeHg+ to the active sites of TrxR1 and GPx1 by an integrated experimental and theoretical approach. Indeed, by studying two C-terminal peptide models of TrxR1 containing the Cys–SeCys catalytic dyad and the full-length GPx1, containing one SeCys and five Cys residues, we have gained solid mechanistic insights into the MeHg+ reactivity.Notably, in the case of the TrxR-derived dodecapeptide, DFT calculations offered us a detailed and rational description of the reaction mechanism with MeHgCl. This was strongly supported by LC-MS/MS observations. The SeCys residue was found to initiate the reaction by attacking MeHg+ ions, leading to the formation of a mono-metalated Se complex (MeHg–Se–). The higher affinity of selenium for mercury was further supported by comparing the mono-metalation step in a model reaction involving a reduced form of TrxR(488–499) containing a similar chemical environment (thiol/selenol). These results indicate that mono-metalation of the Se atom (MeHg–Se–) is favoured both kinetically and thermodynamically over the monometalation of the S atom (MeHg–S–). This result can be explained by the greater nucleophilicity (and softer character) of selenolate compared to that of thiolate.31,32 This product can then evolve into either an S–Hg–Se bridge, via an intramolecular nucleophilic attack of S on Hg, resulting in the demethylation of Hg, or a bis-metalated adduct, via an intermolecular reaction of S with a second MeHg+ molecule. These two products were observed experimentally when TrxR(488–499) was treated with an excess of MeHgCl over an extended reaction time in aqueous solution. The exclusive detection of the mono-metalated MeHg–Se– peptide following a brief exposure to a small quantity of MeHgCl confirms that the process begins with SeCys metalation and proceeds via intra- and inter-molecular reactions involving S, ultimately forming bis-metalated and Hg-bridged adducts as the final products.
A similar conclusion can be drawn for GPx1, where the protein underwent mono-metalation following brief incubation with a substoichiometric amount of MeHgCl. LC-MS/MS analysis of the mono-metalated GPx1 adduct showed that the MeHg moiety binds directly to SeCys51. This experimental finding provides the first formal evidence of Se–Hg bond formation in GPx1. Conversely, prolonged reaction involving excess MeHgCl resulted in mono- and bis-adducts containing one or two bare Hg(II) ions.
Overall, these data offer a clear molecular explanation for the inhibition of the selenoenzymes TrxR1 and GPx1 by MeHg+, as well as for the associated toxic effects. These processes involve MeHg+ preferentially reacting with SeCys, which is followed by the involvement of neighboring Cys residues. This leads to the formation of S–Hg–Se and S–Hg–S bridges within selenoproteins. The presence of SeCys appears to serve as a favoured entry point for MeHg+, into selenoproteins. This initial event is then followed by a cascade of Hg coordination steps involving Cys residue, which can lead to the inactivation of redox enzymes.
Our study also provides additional mechanistic details and evidence supporting the ability of peptide sequences containing vicinal SeCys and Cys residues to form Se–Hg–S bridges and displace the methyl ligand.29,33 Notably, these findings open new avenues for the use of selenopeptides for MeHg+ detoxification strategies.
Author contributions
Conceptualization: KM and LR; formal analysis: MBdM, TZ, and AG; funding acquisition: RL and LR; investigation: MBdM, TZ, AG, and KM; methodology: MBdM, TZ, KM, and LR; resources: LM, JS, RL, KM, and LR; supervision: KM and LR; validation: LM, EB, RL, JS, KM, and LR; visualization: MBdM, TZ, KM, and LR; writing – original draft: KM and LR; writing – review & editing: MBdM, TZ, AG, LM, EB, JS, RL, KM, and LR.Conflicts of interest
There are no conflicts to declare.Data availability
All data supporting the findings of this study are available within the article and its supplementary information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5qi01958c.Acknowledgements
The authors acknowledge the ANR – FRANCE (French National Research Agency) for its financial support of the HgNeuroDetox project. This research has received partial funding from the Nouvelle Aquitaine Region and STEE-UPPA through the PhD fellowship of T. Z. and E2S-UPPA through the PhD fellowship of M. B. M. The authors thank Dr Simon Godin for training T. Z., M. B. M. and A. G. in ESI-MS.The Fellowship – Scientific Visitor Programme undertaken by A. G. at the Université de Pau et des Pays de l'Adour was co-financed by the ‘Excellence Initiative – Research University (2020–2026)’, implemented under the auspices of the Ministry of Science and Higher Education of the Republic of Poland.
The “Direction du Numérique” of the Université de Pau et des Pays de l'Adour and the Mésocentre de Calcul Intensif Aquitain (MCIA) are acknowledged for their support in providing computational facilities. This work was also granted access to the HPC resources of IDRIS under the allocation 2024-[AD010800045R3] made by GENCI.
References
- World Health Organization, 10 chemicals of public health concern. https://www.who.int/news-room/photo-story/detail/10-chemicals-of-public-health-concern (accessed June 2024).
- Y.-S. Hong, Y.-M. Kim and K.-E. Lee, Methylmercury Exposure and Health Effects, J. Prev. Med. Public Health, 2012, 45, 353–363 CrossRef PubMed.
- L. Feng, P. Li and X. Feng, Methylmercury bioaccumulation in rice and health effects: A systematic review, Curr. Opin. Environ. Sci. Health, 2021, 23, 100285 CrossRef.
- P. Li, X. Feng and G. Qiu, Methylmercury Exposure and Health Effects from Rice and Fish Consumption: A Review, Int. J. Environ. Res. Public Health, 2010, 7, 2666–2691 CrossRef CAS PubMed.
- M. Bernabeu De Maria, J. Lamarche, L. Ronga, L. Messori, J. Szpunar and R. Lobinski, Selenol (-SeH) as a target for mercury and gold in biological systems: Contributions of mass spectrometry and atomic spectroscopy, Coord. Chem. Rev., 2023, 474, 214836 CrossRef CAS.
- Y. Sugiura, Y. Tamai and H. Tanaka, Selenium protection against mercury toxicity: high binding affinity of methylmercury by selenium-containing ligands in comparison with sulfur-containing ligands, Bioinorg. Chem., 1978, 9, 167–180 CrossRef CAS PubMed.
- M. Farina, J. L. Franco, C. M. Ribas, F. C. Meotti, A. L. Dafré, A. R. S. Santos, F. Missau and M. G. Pizzolatti, Protective effects of Polygala paniculata extract against methylmercury-induced neurotoxicity in mice, J. Pharm. Pharmacol., 2005, 57, 1503–1508 CrossRef CAS PubMed.
- J. L. Franco, A. Teixeira, F. C. Meotti, C. M. Ribas, J. Stringari, S. C. Garcia Pomblum, Â. M. Moro, D. Bohrer, A. V. Bairros, A. L. Dafre, A. R. S. Santos and M. Farina, Cerebellar thiol status and motor deficit after lactational exposure to methylmercury, Environ. Res., 2006, 102, 22–28 CrossRef CAS PubMed.
- M. C. Carvalho, J. L. Franco, H. Ghizoni, K. Kobus, E. M. Nazari, J. B. T. Rocha, C. W. Nogueira, A. L. Dafre, Y. M. R. Müller and M. Farina, Effects of 2,3-dimercapto-1-propanesulfonic acid (DMPS) on methylmercury-induced locomotor deficits and cerebellar toxicity in mice, Toxicology, 2007, 239, 195–203 CrossRef CAS PubMed.
- V. Branco, J. Canário, J. Lu, A. Holmgren and C. Carvalho, Mercury and selenium interaction in vivo: Effects on thioredoxin reductase and glutathione peroxidase, Free Radicals Biol. Med., 2012, 52, 781–793 CrossRef CAS PubMed.
- V. Branco and C. Carvalho, The thioredoxin system as a target for mercury compounds, Biochim. Biophys. Acta, Gen. Subj., 2019, 1863, 129255 CrossRef CAS PubMed.
- J. L. Franco, T. Posser, P. R. Dunkley, P. W. Dickson, J. J. Mattos, R. Martins, A. C. D. Bainy, M. R. Marques, A. L. Dafre and M. Farina, Methylmercury neurotoxicity is associated with inhibition of the antioxidant enzyme glutathione peroxidase, Free Radicals Biol. Med., 2009, 47, 449–457 CrossRef CAS PubMed.
- V. Branco, J. Canário, A. Holmgren and C. Carvalho, Inhibition of the thioredoxin system in the brain and liver of zebra-seabreams exposed to waterborne methylmercury, Toxicol. Appl. Pharmacol., 2011, 251, 95–103 CrossRef CAS PubMed.
- C. M. L. Carvalho, J. Lu, X. Zhang, E. S. J. Arnér and A. Holmgren, Effects of selenite and chelating agents on mammalian thioredoxin reductase inhibited by mercury: implications for treatment of mercury poisoning, FASEB J., 2011, 25, 370–381 CrossRef CAS PubMed.
- O. Wada, N. Yamaguchi, T. Ono, M. Nagahashi and T. Morimura, Inhibitory effect of mercury on kidney glutathione peroxidase and its prevention by selenium, Environ. Res., 1976, 12, 75–80 CrossRef CAS.
- H. E. Ganther, C. Goudie, M. L. Sunde, M. J. Kopecky, P. Wagner, S.-H. Oh and W. G. Hoekstra, Selenium: Relation to Decreased Toxicity of Methylmercury Added to Diets Containing Tuna, Science, 1972, 175, 1122–1124 CrossRef CAS PubMed.
- M. A. K. Khan and F. Wang, Mercury-selenium compounds and their toxicological significance: Toward a molecular understanding of the mercury-selenium antagonism, Environ. Toxicol. Chem., 2009, 28, 1567–1577 CrossRef CAS PubMed.
- A. Manceau, A. C. Gaillot, P. Glatzel, Y. Cherel and P. Bustamante, In Vivo Formation of HgSe Nanoparticles and Hg–Tetraselenolate Complex from Methylmercury in Seabirds—Implications for the Hg–Se Antagonism, Environ. Sci. Technol., 2021, 55, 1515–1526 CrossRef CAS PubMed.
- I. J. Pickering, Q. Cheng, E. M. Rengifo, S. Nehzati, N. V. Dolgova, T. Kroll, D. Sokaras, G. N. George and E. S. J. Arnér, Direct Observation of Methylmercury and Auranofin Binding to Selenocysteine in Thioredoxin Reductase, Inorg. Chem., 2020, 59, 2711–2718 CrossRef CAS PubMed.
- V. Glaser, E. M. Nazari, Y. M. R. Müller, L. Feksa, C. M. D. Wannmacher, J. B. T. Rocha, A. F. De Bem, M. Farina and A. Latini, Effects of inorganic selenium administration in methylmercury–induced neurotoxicity in mouse cerebral cortex, Int. J. Dev. Neurosci., 2010, 28, 631–637 CrossRef CAS PubMed.
- A. Madabeni, P. A. Nogara, M. Bortoli, J. B. T. Rocha and L. Orian, Effect of Methylmercury Binding on the Peroxide-Reducing Potential of Cysteine and Selenocysteine, Inorg. Chem., 2021, 60, 4646–4656 CrossRef CAS PubMed.
- A. M. Asaduzzaman, M. A. K. Khan, G. Schreckenbach and F. Wang, Computational Studies of Structural, Electronic, Spectroscopic, and Thermodynamic Properties of Methylmercury-Amino Acid Complexes and Their Se Analogues, Inorg. Chem., 2010, 49, 870–878 CrossRef CAS PubMed.
- A. Madabeni, M. Dalla Tiezza, F. B. Omage, P. A. Nogara, M. Bortoli, J. B. T. Rocha and L. Orian, Chalcogen–mercury bond formation and disruption in model Rabenstein's reactions: A computational analysis, J. Comput. Chem., 2020, 41, 2045–2054 CrossRef CAS PubMed.
- P. Delre, A. Chahine, G. F. Mangiatordi, D. Alberga, M. Saviano, G. Valverdu, L. Ronga, K. Miqueu and P. Karamanis, Shedding light on the HSAB-guided sulfur–selenium antagonism in mercury coordination and reactivity toward biologically relevant systems: a DFT and MD study, Phys. Chem. Chem. Phys., 2025, 27, 16644–16663 RSC.
- C. Z. Chung and N. Krahn, The selenocysteine toolbox: A guide to studying the 21st amino acid, Arch. Biochem. Biophys., 2022, 730, 109421 CrossRef CAS PubMed.
- N. Bhasin, P. Carl, S. Harper, G. Feng, H. Lu, D. W. Speicher and D. E. Discher, Chemistry on a Single Protein, Vascular Cell Adhesion Molecule-1, during Forced Unfolding, J. Biol. Chem., 2004, 279, 45865–45874 CrossRef CAS PubMed.
- C. Meuleman, C. Casais Laiño, P. Lansens and W. Baeyens, A study of the behaviour of methylmercury compounds in aqueous solutions, and of gas/liquid distribution coefficients, using head space analysis, Water Res., 1993, 27, 1431–1446 CrossRef CAS.
- S. S. Batsanov, van der Waals Radii of Elements, Inorg. Mater., 2001, 37, 871–885 CrossRef CAS.
- M. Bernabeu De Maria, D. Tesauro, F. Prencipe, M. Saviano, L. Messori, C. Enjalbal, R. Lobinski and L. Ronga, Disclosing the Preferential Mercury Chelation by SeCys Containing Peptides over Their Cys Analogues, Inorg. Chem., 2023, 62, 14980–14990 CrossRef CAS PubMed.
- E. F. Pettersen, T. D. Goddard, C. C. Huang, E. C. Meng, G. S. Couch, T. I. Croll, J. H. Morris and T. E. Ferrin, in UCSF ChimeraX: Structure visualization for researchers, educators, and developers (version 1.7), Resource for Biocomputing, Visualization, and Informatics, University of California, San Francisco, 2023 Search PubMed.
- R. Mousa, R. Notis Dardashti and N. Metanis, Selenium and Selenocysteine in Protein Chemistry, Angew. Chem., Int. Ed., 2017, 56, 15818–15827 CrossRef CAS PubMed.
- R. E. Huber and R. S. Criddle, Comparison of the chemical properties of selenocysteine and selenocystine with their sulfur analogs, Arch. Biochem. Biophys., 1967, 122, 164–173 CrossRef CAS PubMed.
- A. Geri, S. Zineddu, L. Massai, L. Ronga, R. Lobinski, J. Gailer and L. Messori, Mercury binding to proteins disclosed by ESI MS experiments: The case of three organomercurials, J. Inorg. Biochem., 2024, 252, 112479 CrossRef CAS PubMed.
Footnote |
| † These authors contributed equally. |
| This journal is © the Partner Organisations 2026 |