Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
From spheres to worms to vesicles: kinetic control of nanostructure formation from the same block copolymer
Juliane
Eberhardt
ab,
Stephanie
Hoeppener
 cd,
Ulrich
Mansfeld
b and
Johannes C.
Brendel
cd,
Ulrich
Mansfeld
b and
Johannes C.
Brendel
 *ab
*ab
aMacromolecular Chemistry I, University of Bayreuth, Universitätsstr. 30, 95447 Bayreuth, Germany. E-mail: johannes.brendel@uni-bayreuth.de
bInstitute of Macromolecular Research (BIMF) and Bavarian Polymer Institute (BPI), University of Bayreuth, Universitätsstr. 30, 95447 Bayreuth, Germany
cLaboratory of Organic and Macromolecular Chemistry (IOMC), Friedrich Schiller University Jena, Humboldtstr. 10, 07743 Jena, Germany
dJena Center for Soft Matter (JCSM), Friedrich Schiller University Jena, Philosophenweg 7, 07743 Jena, Germany
First published on 16th December 2025
Abstract
Block copolymer self-assembly in solution offers a versatile platform for designing nanostructures with tailored morphologies in aqueous environments. While morphology is commonly controlled by adjusting block ratios, kinetic trapping of nanostructures represents a powerful yet underexplored strategy to direct shape and structure formation, for example in biomedical applications. In this study, we investigate the self-assembly behavior of the amphiphilic block copolymer poly[(butyl acrylate)50-co-(pyridyl disulfide ethyl acrylate)5]-block-(poly ethylene oxide)125-N3 (P(BA50-PDSA5)-b-PEO125-N3) using a solvent switch method. The polymer features a neutral, biocompatible hydrophilic block and a hydrophobic block with a low glass transition temperature (Tg). By systematically varying the initial solvent composition (DMSO/acetone), polymer concentration (1, 4, 7 mg mL−1), and water addition rate (1, 2, 4, 20 mL h−1), we demonstrate precise control over nanoparticle morphology. DMSO content above 80% favored vesicle formation, while balanced DMSO/acetone mixtures stabilized worm-like micelles. Lower polymer concentration of 1 mg mL−1 resulted in a decrease in the formation of non-spherical morphologies, and faster water addition rate of 4 mL h−1 broadened the worm phase, indicating a strong influence of kinetics on the final morphology. Characterization via asymmetric flow field-flow fractionation (AF4), dynamic light scattering (DLS), and cryogenic transmission electron microscopy (cryo-TEM) revealed sharp transitions and mixed phases, highlighting the sensitivity of the system to subtle assembly conditions. These findings provide mechanistic insights into morphology control and underscore the potential of kinetically guided self-assembly for designing shape-specific nanostructures, which is particularly relevant for biomedical applications where nanoparticle shape influences biodistribution, cellular uptake, and therapeutic efficacy.
Introduction
The self-assembly of block copolymers has been a subject of intense research for several decades, and continues to be of great interest due to the broad applicability of the resulting nanostructures. Early studies were enabled by the development of living polymerization techniques, which allowed the synthesis of well-defined block copolymers.1 The field expanded significantly with the advent of reversible deactivation radical polymerizations, which broadened the scope of accessible monomers.2–7 Initial efforts focused on understanding the mechanisms of self-assembly and identifying the parameters that govern morphology formation.8–10 More recently, research has shifted towards designing functional block copolymers for tailor-made applications, thereby expanding the applicability of these nanomaterials in areas like biomedicine, lithography, energy conversion and storage, as well as emulsifiers.11–18Block copolymer self-assembly is driven either by the immiscibility between chemically distinct blocks or insolubility of one block in selective solvents, leading to microphase separation in bulk or formation of nanostructures in solution. The latter structures typically range from 5 to 200 nm in size, showing distinct morphologies, including spheres, worm-like micelles, and vesicles.19 Despite decades of research, challenges in this field remain, e.g., the targeting of specific morphologies, in particular non-spherical structures,20–22 and achieving reproducible control over particle morphology.23 Traditionally, controlled variations in morphology have been accomplished by adjusting the block ratio of the copolymer, thereby tuning the balance between solvophobic and solvophilic interactions.24,25 Another widely used method is polymerization-induced self-assembly (PISA), where the self-assembly occurs concurrently with the polymerization process, enabling access to a range of morphologies.26 Nevertheless, kinetic barriers can limit the versatility of structures, restricting the formation of the thermodynamic equilibrium.27 As a result, only a small number of monomers, which are soluble in water, allow access to the broad variety of morphologies.28
The concept of the packing parameter provides a useful, though limited, framework for predicting morphologies. This dimensionless parameter depends on the volume of the hydrophobic block, the effective interfacial area, and the length of the hydrophobic chain.18,29 While being developed and effective for surfactants, the packing parameter is often insufficient for the prediction of block copolymer assemblies since the chain conformation plays a significant role which limits the precise determination of the specific parameters.30 As a result, extensive experimental screening is typically necessary to gain a more comprehensive understanding of the resulting morphologies.31,32
Moreover, the concept of the packing parameter relies on structures in a thermodynamically favored equilibrium state. The self-assembly of block copolymers in selective solvents, however, can lead to kinetically trapped states, in particular if aqueous solutions are utilized.33 With increasing amounts of the non-solvent (water), the mobility of the insoluble core blocks decreases. Initially, micelles grow and compact to reduce interfacial energy, but once the core reaches a glassy or highly viscous state, further morphological transitions are hindered or impossible.24 Rapid kinetic trapping of polymer nanostructures in metastable states, e.g., by quenching the assembly by water, can be a beneficial strategy, particularly when compounds are to be encapsulated in the polymer nanostructure. Possible applications of this approach include e.g. the formulation of polymer nanoparticle based drug delivery systems by nanoprecipitation.34,35 However, this method is limited mainly to the formation of spherical particles with a limited size range.36 To allow for more morphological variety and better control over the nanostructures, it is more common to dissolve the block copolymer in a non-selective solvent and the selective solvent is added slowly. If the concentration of the solution is above the critical micelle concentration, this causes the solvophobic blocks to aggregate, leading to the formation of micelles. The selective non-solvent is afterwards removed by evaporation or dialysis.12,37,38 This technique is referred to as the solvent switch method.
In particular, the group of Eisenberg pioneered investigations on the impact of kinetics on the self-assembly behavior.24,37,39–43 Focusing on highly asymmetric block copolymers with a large polystyrene block and short hydrophilic poly(acrylic acid) blocks, they were able to create spheres, worms or vesicles depending on the addition rate of the water as selective solvent. Several parameters like the concentration of the polymer in solution or the solubility of both blocks in the good solvent and the selective solvent were scrutinized to gain detailed insights into the process. Inspired by these results, subsequent investigations expanded the scope of this methodology towards non-ionic or cationic block polymers and more complex architectures.44–49 For example, Vena et al. utilized PS250-b-PEO45 to perform a series of self-assembly experiments under varying conditions, resulting in spherical particles with distinct surface and internal structure.46 A common aspect of most of the studied systems is the asymmetric composition of the block copolymer, comprising polystyrene blocks of a rather high degree of polymerization (>100). Furthermore, this hydrophobic block features a high glass transition temperature Tg, which has often been considered crucial to trap polymers and decrease their mobility. Although the glass transition has an impact on the chain equilibrium in micelle formation,50 there have been reports that also low Tg block copolymers can form kinetically trapped or frozen nanostructures,33,51 which is commonly related to very low critical micelle concentrations and a high surface tension induced by dissolution of the respective hydrophobic blocks.52,53 Our recent work further demonstrated that it is possible to trap such low Tg block copolymers in different morphologies starting from the same block copolymer.54 It was demonstrated that spheres, worm-like micelles or vesicles can be created from P(BA50-PDSA5)-b-PEO125-N3, a block copolymer with similar molar masses of both blocks, depending on the organic non-selective solvent used for the solvent switch.
In this study, we investigate the assembly behavior of the selected polymer to evaluate the sensitivity and reproducibility of kinetically controlled nanoparticle formulations. Our objective is to identify the parameters that determine the final nanoparticle shape and to highlight key factors in self-assembly that enable selective and reproducible targeting of specific pure morphologies. We further examine whether similar governing factors can be identified for this symmetric, non-ionic, low-Tg block copolymer, as has previously been reported for highly asymmetric polystyrene block copolymers. Therefore, the ratio of acetone and DMSO in the organic co-solvent was systematically varied, several concentrations of the polymer were tested, and, most importantly, the water addition rate during the solvent switch was altered to determine their impact on the morphology. Understanding the critical parameters that control block copolymer self-assembly is essential for advancing these complex systems toward practical applications, particularly as carriers in biomedical contexts where particle shape and morphology play a decisive role.21,54
Results and discussion
We focused on the amphiphilic block copolymer poly[(butyl acrylate)50-co-(pyridyl disulfide ethyl acrylate)5]-block-(poly ethylene oxide)125-N3 (P(BA50-PDSA5)-b-PEO125-N3), which consists of a hydrophobic copolymer composed of butyl acrylate (BA) and pyridyldisulfide ethyl acrylate (PDSA), and a hydrophilic block of polyethyleneoxide (PEO) terminated with an azide group, which can be seen in Fig. 1, for the self-assembly study, which was prepared according to the previously published procedure.54 NMR and GPC data of the polymer are shown in Fig. S1 and S2. The PDSA (pyridyldisulfide ethyl acrylate) in the hydrophobic block enables crosslinking of the nanoparticles after their formulation, if required to enhance stability during analysis or for further application.55,56 The hydrophilic block is made out of poly(ethylene oxide) facilitated with an azide-endgroup allowing further modification with labels or targeting units, which can be useful for further applications. The self-assembly process was induced by the well-established solvent switch process.57 Therefore, the polymer was first dissolved in acetone, DMSO or a mixture of both before water was continuously added via a syringe pump until a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was reached. This ratio is generally sufficient to induce the self-assembly process of the copolymer. Then, the organic solvent was removed by dialysis before the obtained assembled structures were crosslinked by the addition of 1,6-hexane dithiol, which reacts with the PDSA units. Finally, particles were again dialyzed before characterization.
1 was reached. This ratio is generally sufficient to induce the self-assembly process of the copolymer. Then, the organic solvent was removed by dialysis before the obtained assembled structures were crosslinked by the addition of 1,6-hexane dithiol, which reacts with the PDSA units. Finally, particles were again dialyzed before characterization.
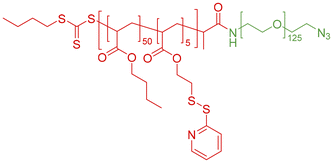 | ||
| Fig. 1 Chemical structure of the amphiphilic block copolymer P(BA50-PDSA5)-b-PEO125-N3 used for nanoparticle formation. | ||
Our previous studies already revealed that in general three different morphologies (spherical micelles, worm-like micelles, and vesicles) can be formed by this amphiphilic block copolymer.54 In this study we aimed to elucidate the critical parameters for creating the distinct structures and gain a better understanding and control of the assembly process. Therefore, the effect of (i) the initial concentration of the polymer, (ii) the rate of water addition, and (iii) gradual changes in the initial solvent composition (DMSO/acetone) were examined to determine their respective influence on the morphology (Fig. 2). The concentration of the block copolymer was varied between 1, 4 and 7 mg mL−1, while 1, 2 and 4 mL h−1 were initially tested as rates of water addition. In all cases, a set of solvent mixtures were applied in the starting solution, which gradually changed from pure acetone to DMSO (in steps of 10%). After crosslinking and purification, all obtained morphologies were analyzed by DLS and AF4 measurements. Selected samples were examined using cryo-TEM investigations.
Impact of starting solvent composition
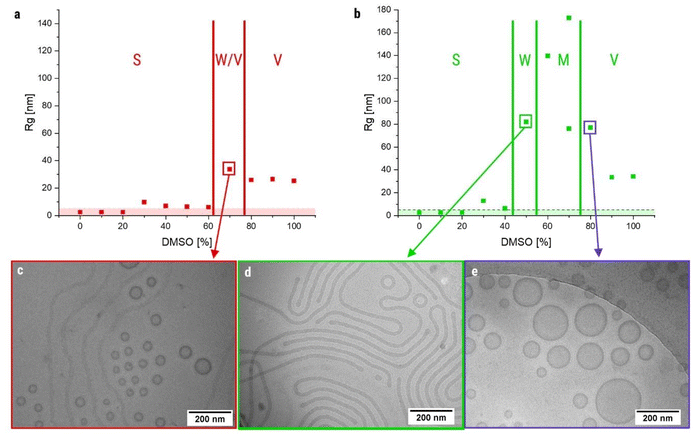
As a starting point, we first evaluated the previously established procedure in more detail,54 focusing on systematic changes of the solvent composition (acetone/DMSO). The solvents were selected based on their miscibility with water and their ability to dissolve both polymer blocks. The initial concentration of the polymer was set to 4 mg mL−1 and the water addition rate was kept at 2 mL h−1. AF4 measurements revealed significant differences in elution times and the corresponding radii of gyration (Rg) at the peak elution time of each nanostructure vary significantly when the solvent composition is changed (summary depicted in Fig. 3a, exemplarily chosen AF4 elugrams are presented in Fig. 3f–i). At high acetone content (DMSO <30%), mostly small particles (<20 nm) are detected, which correspond well to spherical micelles in terms of their size. The AF4 results are further confirmed by DLS measurements (Fig. S3), as the hydrodynamic radii Rh remain below 30 nm. Since all other morphologies are significantly larger in size, following samples with Rg values below 10 nm were considered to be small spherical micelles. At 40% DMSO, a second peak or shoulder appears in the AF4 elugrams indicating the formation of larger structures. In accordance, the corresponding Rg values at this second peak are significantly larger (∼100 nm). Additional TEM measurements indeed revealed the presence of both spherical and worm-like micelles in the samples confirming the onset of a morphology transition (Fig. 3b). Interestingly, the next step to 50% DMSO yielded already a pure worm-phase (Fig. 3c) and no more spherical micelles could be observed. The complete transition is also confirmed by the AF4 measurements (Fig. S4), since no signal appears at low elution times (<20 min), which would be representative for spherical micelles. A further increase of the DMSO content to 60% does not appear to induce a major change in the AF4 results, since elution times and the corresponding Rg do not change significantly (Fig. 3h). However, TEM images revealed already the presence of several vesicles besides the observed worm-like micelles (Fig. 3d). Increasing the DMSO content in the initial solvent mixture further, the ratio of vesicles in the mixture increases (Fig. 3e) and concomitantly the elution times shift towards higher values (Fig. 3i), although the shift is not as clear as for the transition between spherical and worm-like micelles. The corresponding Rg values also decrease, which is related to the smaller radii of gyration for round vesicles compared to the highly elongated worm-like micelles. Consequently, the presence of a pure vesicle phase can be assigned at contents of 80% DMSO and higher (Fig. S5).These results clearly illustrate that small changes in the starting solvent composition have already a major impact on resulting morphologies. Nevertheless, all major morphologies can be obtained as pure phases, although a broader transition range is present between worm-like micelles and vesicles. In this case, it is difficult to find out which morphology is thermodynamically favored. One possible hint would be the transition of thermodynamically non-favored morphologies over long periods of time.58 Therefore, a sample of non-crosslinked worm-like micelles, which are assumed as thermodynamically less stable morphologies due to its rare occurrence, was measured in AF4 again after one year at room temperature. The resulting elugram showed only a slight shift but no significant change of the peak shape, that is common for worm-like micelles, or the Rg value, which was 75.2 nm directly after assembly and 76.7 nm after 1 year of storage (Fig. S6). To have a closer look on the thermodynamic stability of all morphologies, non-crosslinked samples were kept at 80 °C for one week, which should enhance dynamics in the system.59 No significant changes in their size were observed and the morphologies seem preserved (Fig. S7). Hence, it is difficult to judge on the thermodynamically favored morphology of the system.
Influence of the initial concentration of the polymer
After revealing the impact of the solvent composition, we further expanded the parameter space by varying the initial concentration of the block copolymer in the organic solvent mixture. A lower (1 mg mL−1) and a higher (7 mg mL−1) concentration were tested and compared to the initially applied concentration of 4 mg mL−1. The initial concentration of 4 mg mL−1 was chosen to provide sufficiently concentrated samples for application in the Ussing chamber experiments reported previously.54 The now tested concentrations were selected to ensure that the polymer remains fully dissolved without aggregation for 7 mg mL−1, while the final samples prepared from 1 mg mL−1 still provide a reliable signal in AF4 measurements without need for further treatment. In both cases, the same stepwise change in the solvent composition was applied in order to detect changes in the phase transitions (Fig. 4a and b). TEM-images were made for the batches with 50% and 80% DMSO to confirm the predicted morphologies (Fig. 4d and e).At a lower concentration (1 mg mL−1), the composition range has expanded up to 60% of DMSO, where pure spherical micelles are formed. At 70% DMSO a slight increase of particle size was observed indicating a morphological transition. Interestingly, cryo-TEM analysis of this sample revealed that mainly small vesicles were formed and only few worm-like micelles can be observed (Fig. 4c). When increasing the DMSO content further, the peaks in the AF4 measurement become more narrow and lower Rg values are obtained, which indicates the formation of only vesicles (Fig. S4, S9 and S11). Those vesicles have an Rg of approximately 25 nm, which are smaller in comparison to vesicles which are formed at higher concentrations (4 mg mL−1). This trend was found as well when comparing the particles in DLS measurements (Fig. S3, S8 and S10). The decreased concentration in general has a significant impact on the formation of different morphologies. The formation of worm-like micelles is nearly completely impeded at low polymer concentrations. We assume that the worm-like micelles are formed by fusion of first aggregates,60 which only can take place if their concentration is high enough. At this low concentration the formation of spherical micelles prevails, while at high DMSO levels the process switches directly to the vesicle formation. This straight transition indicates that the vesicles are emerging from individual nucleates rather than form by fusion of particles. This fundamentally important aspect deserves attention in subsequent studies. These require more sophisticated kinetic experiments to identify the exact mechanism, which was beyond the scope of this study.24,39
Based on these results, we assumed that an increase of the polymer concentration might broaden the range where worm-like micelles are formed. However, the results of the AF4 measurements reveal a very similar picture for 7 mg mL−1 (Fig. 4b) as observed for the experiments with 4 mg mL−1 (Fig. 3a). Again, a broad transition between worm-like micelles and vesicles is observed, but no significant shift of the phase. Only the size of the obtained nanostructures appears increased when comparing the Rg values and the results of the DLS measurements for nanoparticles at different concentrations. Vesicles formed in pure DMSO at different concentrations were compared, revealing that the vesicles with the lowest concentration (1 mg mL−1) had an Rg of 25.2 nm. At a concentration of 4 mg mL−1, the Rg increased to 32.6 nm. The largest vesicles, with an Rg of 34.2 nm, were observed at a concentration of 7 mg mL−1. This observation was also reported by Eisenberg et al.,24 who found that an increase in concentration leads to a corresponding increase in the aggregation number. This leads to larger micelle cores and to a higher degree of stretching of the hydrophobic block, which is in our case butyl acrylate. However, the general assembly process seems not to be influenced by the increased concentration and other factors appear to be more critical in this regard.
Varying the rate of water addition
Besides the polymer concentration, we also considered the time for the polymers to assemble a critical factor. In this regard, we varied the rate at which the water is added to the polymer solution in the different solvent mixtures. Using an initial addition rate of 2 mL h−1, we now tested a lower rate (1 mL h−1) and a higher rate (4 mL h−1).In all cases, the transition from small particles (most likely small spherical micelles) to worm-like micelles occurred at 40% to 50% DMSO content (Fig. 5a–c). This transition seems therefore determined by the solvent composition and cannot be affected by the other tested parameters. However, the second transition between worm-like micelles and vesicles appears more sensitive to changes in the water addition rate. While at slower addition rates of 1 mL h−1 and 2 mL h−1, the pure worm-like micelle phase is only observed at 50% DMSO, at 4 mL h−1, this phase is extended up to 70% DMSO in the initial solvent mixture compared to the slower addition rates. Additionally, at 2 mL h−1, a broader range of mixed morphologies, including worm-like micelles and vesicles, is observed compared to 1 mL h−1. The shift of the transition point is best illustrated in the cryo-TEM images recorded from batches prepared at a solvent mixture of 70% DMSO and 30% acetone and different water addition rates (Fig. 5d–f). While at 1 mL h−1 only vesicles are formed, an increase to 2 mL h−1 already causes a mixed phase of worm-like micelles and vesicles and speeding the addition further up provides a pure phase of worm-like micelles. Interestingly, at this rate the change to 80% DMSO in the starting solvent appears to induce a straight transition towards vesicles considering the narrow distribution observed in AF4 measurements and the decrease of Rg values. AF4 measurements also confirmed a broader worm phase at 4 mL h−1, as evidenced by the irregular peak shape observed when non-spherical particles are measured (Fig. S4, S13 and S15). DLS measurements of samples with different water addition rates can be seen in Fig. S3, S12 and S14. The increase of the addition rate therefore narrows down the transition range, where mixed structures are obtained, and thus this parameter represents a critical factor to ensure pure morphologies, which are often desired. Overall, the assembly process appears to be kinetically driven, although the influence of the water addition rate is not as significant as for the previously reported asymmetric polystyrene block copolymers.39 At lower addition rates, competing processes forming either worm-like micelles or vesicles can still occur in parallel. However, increasing the rate discriminates one process over the other and thus causes selection.
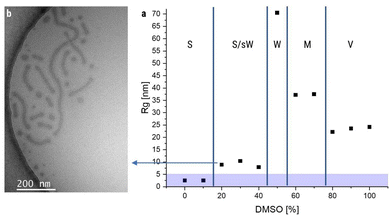
Intrigued by this observation, we expanded the study and tested a much higher water addition rate of 20 mL h−1. To our surprise, the AF4 measurements (Fig. S17) indicated a change in the structures at much lower DMSO contents of around 20%, which before led in every case to the formation of pure spherical micelles. At this high addition rate, the Rg increased between 10% and 20% DMSO (Fig. 6a). Cryo-TEM measurements (Fig. 6b) indeed revealed that for 20 mL h−1 and 20% DMSO already short worm-like micelles (∼150 nm) were formed besides the expected spherical micelles. Surprisingly, those short worms did not show an irregular peak shape in AF4, this seems to arise only for longer worm-like micelles. However, only at 50% and more DMSO content again worm-like micelles of length >1 µm were obtained. DLS measurements for those samples can be seen in Fig. S16. The mechanism inducing the formation of short worm-like micelles at very low DMSO content remains unclear, but it has to be kept in mind that the high addition rate (1 mL is added in only 3 min) turbulences in the vial might cause additional aggregation and inhomogeneities may enhance the aggregation of first particles. A better control of the mixing process might provide a clearer picture of the impact of such high addition rates and experiments using microfluidics are currently being developed.
Conclusion
In this study, we systematically investigated the self-assembly behavior of the amphiphilic block copolymer P(BA50-PDSA5)-b-PEO125-N3 using a solvent switch method. By varying the solvent composition, polymer concentration, and water addition rate, we demonstrated precise control over nanoparticle morphology, enabling the formation of spherical micelles, worm-like micelles, and vesicles from a single polymer system. The results showed that even slight changes in these parameters significantly influence the final morphology of the nanoparticles.The type of non-selective solvent emerged as the most critical parameter affecting particle shape. In this context, it is important to consider that the solvent's affinity for the hydrophobic core strongly influences both the core size and the degree of stretching of the hydrophobic block during the assembly process.24 In this work, higher concentrations of DMSO favored the formation of vesicles, while balanced DMSO/acetone mixtures stabilized worm-like micelles. A higher acetone content led to the formation of spherical particles. An increase in polymer concentration generally resulted in larger particles, particularly vesicles. In contrast, lower concentrations led to the disappearance of the worm-like phase. The water addition rate had a pronounced effect on morphology evolution: faster addition (4 mL h−1) broadened the worm-like phase. Interestingly, a very high water addition rate (20 mL h−1) resulted in the formation of short worm-like micelles at DMSO contents below 50%, suggesting that turbulence and local inhomogeneities may play a role in early-stage aggregation. These effects may also contribute to the formation of mixed phases, which were frequently observed between pure morphologies. The free energy of two morphologies can be nearly equivalent, making subtle local differences sufficient to dictate the resulting structure.42 Additionally, polymer dispersity may further contribute to the formation of different morphologies, as chains of varying length may transition to the next morphology at different times.25
These findings highlight the complex interplay of thermodynamic and kinetic factors in block copolymer self-assembly and underscore the potential of solvent switch techniques for designing shape-specific nanostructures. Notably, the obtained nanostructures remained stable for over a year, and even annealing at elevated temperatures did not induce morphological transitions, indicating that kinetic effects may play a decisive role in the assembly process. The work highlights that despite decades of research, several uncertainties remain regarding polymeric self-assembly in aqueous environments. However, the ability to target all major morphologies using a single block copolymer opens up exciting possibilities, particularly in applications such as nanomedicine, where nanoparticle morphology influences biodistribution, cellular uptake, and therapeutic efficacy.21,54 Further experiments could show the influence of particle shape excluding all effects related to particle composition.
Experimental part
Materials and methods
All chemicals and solvents were purchased from Sigma-Aldrich, Acros Organics, Carl Roth, Merck, Jena Bioscience and were used without further purification unless mentioned otherwise. Pyridyldisulfide ethyl acrylate (PDSA) and 2-(butylthiocarbonothioylthio)propanoic hydroxysuccinimide (PABTC-NHS) were prepared via previously reported procedures.61,62 1,4-Dioxane and butyl acrylate (BA) were treated 24 h with inhibitor remover resin prior to use. N3-PEO125-NH2 was purchased from Rapp Polymere.DLS was performed on a ZetaSizer Nano ZS (Malvern, Herrenberg, Germany) equipped with a He–Ne laser operating at a wavelength of λ = 633 nm. Counts were detected at an angle of 173°. The particle size was approximated as the effective diameter (Z-average) obtained by the cumulants method assuming a spherical shape. All measurements were conducted at 25 °C in semi-micro cuvettes after equilibration times of 30 s in triplicate. Every measurement included 10 runs, in which every run took 30 seconds. Apparent hydrodynamic radii were calculated using the Stokes–Einstein equation (1):
| Rh = kT·6πηD | (1) |
AF4 measurements were performed on an AF2000 MT System from Postnova Analytics GmbH (Landsberg, Germany), equipped with a tip and focus pump (PN1130), an autosampler (PN5300), and a channel oven unit (PN4020) set to 25 °C. The channel was coupled to a multiangle laser light scattering (MALLS) detector (PN3621) equipped with a 532 nm laser and measuring 21 angles, a refractive index (RI) detector (PN3150), and a UV-detector (PN3212) set to 280 nm. The channel had a trapezoidal geometry with a nominal height of 350 μm. Regenerated cellulose (RC) membrane from Postnova Analytics GmbH (10 kDa RC membrane) with a molar mass cutoff of 10 kDa was used as accumulation wall. As the mobile phase aqueous solution with 0.02 wt% of NaN3 was used. 50 μL of the sample at a concentration of 1 mg mL−1 was injected with an injection flow rate of 0.2 mL min−1, a focus flow rate of 0.8 mL min−1, and a cross-flow rate of 0.7 mL min−1, resulting in a detector flow rate of 0.3 mL min−1. The focusing time was 4 min before switching to elution at an exponentially decaying crossflow from 0.7 mL min−1 to 0.2 mL min−1 in 76.2 min. Thereafter the crossflow profile was set to decay in a linear way from 0.05 mL min−1 to 0.04 mL min−1 in 71 min. Before the start of the next measurement, a rinsing step was performed at 1.5 mL min−1 flow of the tip pump for 20 min. After each sample measurement, a blank measurement was run which was subtracted from the data of the sample measurement for analysis. The MALLS data of the scattering angles from 20°–148° was analysed via ZIMM plot to obtain the radius of gyration (Rg) at the specified elution times.
All Cryo-TEM measurements, exept Fig. 6b, were performed on a FEI Tecnai G2 20 equipped with a LaB6 filament with an acceleration voltage of 200 kV. Samples were prepared on Quantifoil grids (R2/2) which were treated with Ar plasma prior to use for hydrophilization and cleaning. 8.5 μL of the solution were vitrified on Quantifoil grids using a Vitrobot Mark IV system. Liquid ethane was used as a cryogen. Samples were transferred to a Gatan 626 cryo holder and were maintained at a temperature <−175 °C during the entire process. All images were acquired with a Mega View (OSIS, Olympus Soft Imaging Systems) or an Eagle 4k CCD camera, respectively.
Cryo-TEM of Fig. 6b was performed on a JEOL JEM-2200FS operating at 200 kV and equipped with a field emission gun and an in-column energy omega filter. The zero-loss energy-filtered, bright field micrographs were recorded at cryo conditions (Holder T = −174 °C) with a bottom-mounted CMOS 4K camera (OneView, Gatan). The images were processed with digital processing software (Digital Micrograph 3.6, Gatan). For cryo preparation, 4 μL of the aqueous sample solution were deposited on a Cu grid with a holey carbon film (Quantifoil, Germany) that was glow-discharged before. The grid was then blotted at 15 °C and 90% humidity with filter paper and plunged into liquid ethane using a Leica EM GP plunge freezer (Leica, Germany). After preparation, the grids were stored and transferred to the TEM under liquid nitrogen.
Non-cryo-TEM investigations were conducted by blotting 15 µL of the solution (1 mg mL−1) onto freshly cleaned carbon support films (Quantifoil, Jena).
Author contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.Conflicts of interest
There are no conflicts of interest to declare.Data availability
The data supporting this article is presented in the main manuscript or have been included as part of the supplementary information (SI). Supplementary information is available and includes SEC and NMR data of the polymer, DLS data and AF4 elugrams of the assembled structures, as well as further TEM images. See DOI: https://doi.org/10.1039/d5py01038a.Acknowledgements
This work was financially supported by the DFG-funded Collaborative Research Centre PolyTarget (project-ID: 316213987 – SFB 1278; projects A05, C04, Z01). J. C. Brendel further thanks the DFG for funding within the Emmy-Noether Programme (project-ID: 358263073) and the Heisenberg-Programme (project-ID: 517761335). Cryo-TEM investigations were conducted at (i) the Jena Center for Soft Matter (JCSM) electron microscopy facilities established with funds by the DFG and the European Funds for Regional development (EFRE) and (ii) at the Keylab Electron and Optical Microscopy within the Bavarian Polymer Institute. We further gratefully acknowledge the support of Ivo Nischang and his team in performing the AF4 measurements.References
- K. Matyjaszewski and A. H. Müller, Controlled and living polymerizations: from mechanisms to applications, John Wiley & Sons, 2009 Search PubMed.
- F. H. Schacher, P. A. Rupar and I. Manners, Angew. Chem., Int. Ed., 2012, 51, 7898–7921 CrossRef CAS.
- A.-L. Buckinx, L. J. Weerarathna, A. Sokolova and T. Junkers, Polym. Chem., 2024, 15, 4615–4621 RSC.
- A.-L. Buckinx, K. Verstraete, E. Baeten, R. F. Tabor, A. Sokolova, N. Zaquen and T. Junkers, Angew. Chem., Int. Ed., 2019, 58, 13799–13802 CrossRef CAS.
- J. Jennings, G. He, S. M. Howdle and P. B. Zetterlund, Chem. Soc. Rev., 2016, 45, 5055–5084 RSC.
- H. Dau, G. R. Jones, E. Tsogtgerel, D. Nguyen, A. Keyes, Y.-S. Liu, H. Rauf, E. Ordonez, V. Puchelle, H. Basbug Alhan, C. Zhao and E. Harth, Chem. Rev., 2022, 122, 14471–14553 CrossRef CAS.
- G. Theodosopoulos and M. Pitsikalis, Block Copolymers by Anionic Polymerization: Recent Synthetic Routes and Developments, Springer Japan, Tokyo, 2015 Search PubMed.
- S. E. Webber, J. Phys. Chem. B, 1998, 102, 2618–2626 CrossRef CAS.
- K. Mori, H. Hasegawa and T. Hashimoto, Polymer, 1990, 31, 2368–2376 CrossRef CAS.
- H. W. Deckman, J. H. Dunsmuir, S. Garoff, J. A. McHenry and D. G. Peiffer, J. Vac. Sci. Technol., B: Microelectron. Process. Phenom., 1988, 6, 333–336 CrossRef CAS.
- E. Blasco, M. B. Sims, A. S. Goldmann, B. S. Sumerlin and C. Barner-Kowollik, Macromolecules, 2017, 50, 5215–5252 Search PubMed.
- M. Karayianni and S. Pispas, J. Polym. Sci., 2021, 59, 1874–1898 Search PubMed.
- P. Maji and K. Naskar, J. Appl. Polym. Sci., 2022, 139, e52942 CrossRef CAS.
- M. Hasannia, A. Aliabadi, K. Abnous, S. M. Taghdisi, M. Ramezani and M. Alibolandi, J. Controlled Release, 2022, 341, 95–117 CrossRef CAS PubMed.
- C. Barner-Kowollik, A. S. Goldmann and F. H. Schacher, Macromolecules, 2016, 49, 5001–5016 CrossRef CAS.
- C. M. Bates and F. S. Bates, Macromolecules, 2017, 50, 3–22 Search PubMed.
- F. S. Bates and G. H. Fredrickson, Phys. Today, 1999, 52, 32–38 CrossRef CAS.
- U. Tritschler, S. Pearce, J. Gwyther, G. R. Whittell and I. Manners, Macromolecules, 2017, 50, 3439–3463 CrossRef CAS.
- J. C. Brendel and F. H. Schacher, Chem. – Asian J., 2018, 13, 230–239 Search PubMed.
- J. Chen, N. E. Clay, P. No-hyung and H. Kong, Chem. Eng. Sci., 2015, 125, 20–24 CrossRef CAS.
- E. Gardey, J. Eberhardt, S. Hoeppener, F. H. Sobotta, J. C. Brendel and A. Stallmach, Macromol. Biosci., 2024, 24, 2400179 CrossRef CAS PubMed.
- Y. Zhou, D. Gao, Y. Wang, L. Liang, Q. Zhang, W. Han, J. Wang, C. Zhu, X. Zhang and Y. Gan, Chin. Chem. Lett., 2024, 35, 108967 Search PubMed.
- M. Elsabahy and K. L. Wooley, Chem. Soc. Rev., 2012, 41, 2545–2561 RSC.
- Y. Mai and A. Eisenberg, Chem. Soc. Rev., 2012, 41, 5969–5985 Search PubMed.
- O. Terreau, L. Luo and A. Eisenberg, Langmuir, 2003, 19, 5601–5607 CrossRef CAS.
- N. J. W. Penfold, J. Yeow, C. Boyer and S. P. Armes, ACS Macro Lett., 2019, 8, 1029–1054 CrossRef CAS.
- A. Blanazs, A. J. Ryan and S. P. Armes, Macromolecules, 2012, 45, 5099–5107 CrossRef CAS.
- F. H. Sobotta, M. T. Kuchenbrod, C. Grune, D. Fischer, S. Hoeppener and J. C. Brendel, Polym. Chem., 2021, 12, 1668–1680 RSC.
- J. N. Israelachvili, Intermolecular and Surface Forces, Academic Press, San Diego, 3rd edn, 2011 Search PubMed.
- R. Nagarajan, Adv. Colloid Interface Sci., 2017, 244, 113–123 CrossRef CAS.
- S. L. Canning, G. N. Smith and S. P. Armes, Macromolecules, 2016, 49, 1985–2001 CrossRef CAS PubMed.
- M. J. Derry, L. A. Fielding and S. P. Armes, Prog. Polym. Sci., 2016, 52, 1–18 CrossRef CAS.
- T. Nicolai, O. Colombani and C. Chassenieux, Soft Matter, 2010, 6, 3111–3118 RSC.
- C. J. Martínez Rivas, M. Tarhini, W. Badri, K. Miladi, H. Greige-Gerges, Q. A. Nazari, S. A. Galindo Rodríguez, R. Á. Román, H. Fessi and A. Elaissari, Int. J. Pharm., 2017, 532, 66–81 CrossRef.
- H. Fessi, F. Puisieux, J. P. Devissaguet, N. Ammoury and S. Benita, Int. J. Pharm., 1989, 55, R1–R4 CrossRef CAS.
- G. Bovone, L. Cousin, F. Steiner and M. W. Tibbitt, Macromolecules, 2022, 55, 8040–8048 CrossRef CAS PubMed.
- Y. Yu and A. Eisenberg, J. Am. Chem. Soc., 1997, 119, 8383–8384 CrossRef CAS.
- A. Sorrenti, J. Leira-Iglesias, A. J. Markvoort, T. F. A. de Greef and T. M. Hermans, Chem. Soc. Rev., 2017, 46, 5476–5490 RSC.
- L. Zhang and A. Eisenberg, Macromolecules, 1999, 32, 2239–2249 CrossRef CAS.
- L. Zhang and A. Eisenberg, Science, 1995, 268, 1728–1731 Search PubMed.
- Y. Yu, L. Zhang and A. Eisenberg, Macromolecules, 1998, 31, 1144–1154 Search PubMed.
- H. Shen and A. Eisenberg, J. Phys. Chem. B, 1999, 103, 9473–9487 CrossRef CAS.
- A. Choucair and A. Eisenberg, Eur. Phys. J. E, 2003, 10, 37–44 CrossRef CAS PubMed.
- K. Yu and A. Eisenberg, Macromolecules, 1998, 31, 3509–3518 Search PubMed.
- X. Liu and I. Gitsov, Macromolecules, 2019, 52, 5563–5573 Search PubMed.
- M. P. Vena, D. de Moor, A. Ianiro, R. Tuinier and J. P. Patterson, Soft Matter, 2021, 17, 1084–1090 RSC.
- N. P. Truong, J. F. Quinn, M. V. Dussert, N. B. T. Sousa, M. R. Whittaker and T. P. Davis, ACS Macro Lett., 2015, 4, 381–386 CrossRef CAS.
- H. Cui, Z. Chen, S. Zhong, K. L. Wooley and D. J. Pochan, Science, 2007, 317, 647–650 CrossRef CAS PubMed.
- A. H. Gröschel, A. Walther, T. I. Löbling, F. H. Schacher, H. Schmalz and A. H. E. Müller, Nature, 2013, 503, 247–251 Search PubMed.
- R. J. Carrazzone, X. Li, J. C. Foster, V. V. S. Uppala, C. E. Wall, A. R. Esker, L. A. Madsen and J. B. Matson, Macromolecules, 2021, 54, 6975–6981 CrossRef CAS PubMed.
- S. Jain and F. S. Bates, Macromolecules, 2004, 37, 1511–1523 CrossRef CAS.
- Y.-Y. Won, H. T. Davis and F. S. Bates, Macromolecules, 2003, 36, 953–955 CrossRef CAS.
- M. Jacquin, P. Muller, R. Talingting-Pabalan, H. Cottet, J. F. Berret, T. Futterer and O. Théodoly, J. Colloid Interface Sci., 2007, 316, 897–911 CrossRef CAS PubMed.
- E. Gardey, Z. Cseresnyes, F. H. Sobotta, J. Eberhardt, D. Haziri, P. C. Grunert, M. T. Kuchenbrod, F. V. Gruschwitz, S. Hoeppener, M. Schumann, N. Gaßler, M. T. Figge, A. Stallmach and J. C. Brendel, Small, 2024, 20, 2306482 CrossRef CAS.
- E. Gardey, F. H. Sobotta, S. Hoeppener, T. Bruns, A. Stallmach and J. C. Brendel, Biomacromolecules, 2020, 21, 1393–1406 CrossRef CAS PubMed.
- M. Elsabahy, S. Samarajeewa, J. E. Raymond, C. Clark and K. L. Wooley, J. Mater. Chem. B, 2013, 1, 5241 RSC.
- F. V. Gruschwitz, T. Klein, M. T. Kuchenbrod, N. Moriyama, S. Fujii, I. Nischang, S. Hoeppener, K. Sakurai, U. S. Schubert and J. C. Brendel, ACS Macro Lett., 2021, 10, 837–843 CrossRef CAS.
- E. G. Kelley, R. P. Murphy, J. E. Seppala, T. P. Smart, S. D. Hann, M. O. Sullivan and T. H. Epps, Nat. Commun., 2014, 5, 3599 CrossRef PubMed.
- J. van Stam, S. Creutz, F. C. De Schryver and R. Jérôme, Macromolecules, 2000, 33, 6388–6395 CrossRef CAS.
- M. Fauquignon, L. Porcar, A. Brûlet, J.-F. Le Meins, O. Sandre, J.-P. Chapel, M. Schmutz and C. Schatz, ACS Macro Lett., 2023, 12, 1272–1279 CrossRef CAS PubMed.
- B. Remant, B. Thapa and P. Xu, Mol. Pharm., 2012, 9, 2719–2729 CrossRef.
- S. Larnaudie, J. Brendel, K. Jolliffe and S. Perrier, J. Polym. Sci., Part A: Polym. Chem., 2016, 54, 1003–1011 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2026 |