 Open Access Article
Open Access ArticleNaringenin-encapsulated nano-cochleate hydrogel for topical delivery: cellular anti-inflammatory activity and dermatokinetic profiling
Anuja Shashikant Kamble†
a,
Ashish Dilip Sutar†a,
Gagandeep Kaura,
Kamalinder K. Singh *bc and
Rahul Shukla*a
*bc and
Rahul Shukla*a
aDepartment of Pharmaceutics, National Institute of Pharmaceutical Education and Research (NIPER-Raebareli), Bijnor-Sisendi Road, Sarojini Nagar, Near CRPF Base Camp, Lucknow, UP 226002, India. E-mail: rahulshuklapharm@gmail.com; rahul.shukla@niperraebareli.edu.in
bSchool of Pharmacy and Biomedical Sciences, University of Lancashire, Preston PR1 2HE, UK. E-mail: KSingh1@lancashire.ac.uk
cBiomedical Evidence based Transdisciplinary (BEST) Health Research Institute, University of Lancashire, Preston PR1 2HE, UK
First published on 14th January 2026
Abstract
Naringenin (NR) is a plant-based flavonoid with poor aqueous solubility, which is indicated for the treatment of psoriasis due to its strong antioxidant and anti-inflammatory properties. The aim of this study was to evaluate the non-steroidal NR-loaded nanocochleate hydrogel (NR-NC-G) for its skin permeation, safety, and efficacy in the treatment of psoriasis. First, nanoliposomes (NR-LIPO) were prepared and further chelated using calcium chloride to transform them into nanocochleates (NR-NCs). NR-NCs exhibited rolled sheet-like nanosized particles with a hydrodynamic diameter of approximately 160–170 nm, an encapsulation efficiency of 81–82%, and good colloidal stability, as indicated by a ζ-potential of −27 mV. To achieve a local reservoir-like action, nanocochleates were loaded into a hydrogel comprising of combination of Carbopol 934P and sodium alginate. NR-NC-G was characterised for its physical and rheological characteristics, and it exhibited uniform drug loading, long-term stability, and the ability to scavenge reactive oxygen species (ROS), as validated by various antioxidant assays. Furthermore, NR-NC-G reduced cellular ROS levels, nitrate accumulation, and mitochondrial healing ability in a lipopolysaccharide-stimulated RAW264.7 inflammation model, thereby proving its enhanced antioxidant and anti-inflammatory effects. The ex vivo skin permeation and dermatokinetic studies showed that NR-NC-G exhibited high permeation across the excised skin of BALB/C mice. The dermatokinetic studies showed that topical application of NR-NC-G provided 3.43 and 3.34-fold greater Cmax and AUC0−t in the epidermal layer, respectively, compared to the bulk NR solution. Overall, this novel nanoformulation enhances ROS scavenging capacity, improves cellular uptake, enhances skin permeation and retention, and suggests potential applications for treating psoriasis.
1. Introduction
Psoriasis is a chronic autoimmune skin disease marked by fast keratinocyte proliferation, epidermal hyperplasia, and inflammatory infiltration.1 Its pathogenesis involves overexpression of inflammatory mediators like TNF-α, IL-6, and IL-17, along with increased oxidative stress (Fig. 1a).2–4 Traditional topical treatments, such as corticosteroids and calcineurin inhibitors, generally lead to local irritation, and drugs like methotrexate carry risks of immunosuppression and hepatotoxicity.5,6Naringenin (NR), a plant-based citrus flavonoid, has dual antioxidant and anti-inflammatory properties by modulating NF-κB and Nrf2 (Fig. 1e) pathways according to various reported studies.7 Because of its anti-inflammatory activity and inhibitory activity on chemokine production, reports have demonstrated its potential use in psoriasis.8,9 But its therapeutic efficacy is compromised by poor aqueous solubility, chemical instability, and limited bioavailability (∼5–9%) because of high first-pass metabolism and low permeability.7,10,11
To overcome these limitations, nanocarrier systems have been investigated.11 While traditional nanoparticulate systems enhance solubility, their colloidal instability and low dermal retention limit their clinical effectiveness.12 Nanocochleates (NCs) are sustained-release depot systems composed of phospholipid precipitates that self-assemble from anionic lipid vesicles binding to divalent cations, such as Ca2+. They consist of a uniform, solid-lipid bilayer sheet that is rolled in a supramolecular pattern (Fig. 1c).12 Nanosize, industrial feasibility, cytocompatibility, fewer side effects, and higher effectiveness are a few of the advantages of NCs that allow them to be explored in topical drug delivery.
Recent advances in hydrogel engineering highlight their capacity to provide sustained, localised drug delivery while resisting mechanical stress in challenging biological environments.13–15 Dual-network and stimuli-responsive hydrogels have demonstrated improved structural stability, controlled release, and enhanced therapeutic efficacy across various applications.16–19 These systems underscore the broader potential of hydrogel platforms to optimise bioavailability, modulate local microenvironments, and support tissue repair.20,21 Such progress provides a strong rationale for developing novel hydrogel-based carriers for topical anti-inflammatory therapeutics such as naringenin.
Embedding NCs into a Carbopol-based hydrogel further enhances topical application by improving spreadability, bioadhesion, and drug residence time at the site of application. In the present study, a naringenin nanocochleate-loaded hydrogel (NR-NC-G) was developed using a Quality by Design (QbD) approach and evaluated for its physicochemical, dermatokinetic, and biological performance to check its potential for topical treatment of psoriasis.
2. Materials and methods
2.1. Materials
Naringenin was purchased from Tokyo Chemical Industry Co., Ltd. Lipoid-S-100 was a gift from Lipoid GmbH, Germany, and cholesterol was procured from Hi-Media. Various excipients, including calcium chloride, Carbopol 934, triethanolamine, bovine serum albumin (BSA), and sodium alginate, were obtained from Sisco Research Laboratories. Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), dichlorodihydrofluorescein diacetate (DCFDA), 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolocarbocyanine iodide (JC-1), lipopolysaccharide (LPS), etc., were procured from Hi-Media. The organic solvents used are of HPLC grade, and the entire formulation procedure was carried out with triple-distilled Milli-Q water.3. Methods
3.1. Drug quantification
NR was solubilised in methanol to make a stock solution and serially diluted to prepare working standards of 2, 4, 6, 8, and 10 μg mL−1 for λmax determination and calibration curve construction using a UV-visible spectrophotometer (Cary 60, UV-Visible spectrophotometer, Agilent). Furthermore, an HPLC analytical method was developed and validated for the quantification of NR in skin samples. Chromatographic detection was performed using a C18 column (250 mm, 4.6 µm) under isocratic conditions with a mobile phase of 80% water and 20% methanol (v/v) at a flow rate of 1.2 mL min−1. The detector was set at 288 nm. The injection volume was 10 µL, and the column temperature was maintained at 25 °C.22,233.2. Nanocochleate (NR-NC) formulation
A QbD approach was used to systematically develop NR-loaded liposomes (NR-LIPOs). This approach was followed in accordance with the ICH Q8 and Q9 guidelines, starting with the definition of the Quality Target Product Profile (QTPP) and the identification of Critical Quality Attributes (CQAs), Critical Material Attributes (CMAs), and Critical Process Parameters (CPPs).24 Particle size (PS) and percent encapsulation efficiency (EE%) were defined as the main CQAs. An Ishikawa (fishbone) diagram (Fig. 1d) was constructed to identify factors affecting the quality and reproducibility of the NR-NC-G system. Key parameters included the properties of NR, lipid and gel material attributes, nanocochleate-formation parameters, and equipment conditions. Each sub-factor, such as the lipid/cholesterol ratio, calcium concentration, stabiliser level, rheology of the gel base, and mixing parameters, was mapped based on early experimental observations. This served as a structured framework to guide risk assessment, parameter control, and optimisation throughout formulation development.In the first step, NR-LIPOs were formulated using the thin film hydration technique. Lipoid-S-100, cholesterol, and NR were dissolved in a sufficient quantity of chloroform, and the solvent was evaporated using a rotary evaporator to form a thin film, which was further hydrated with Milli-Q water to form liposomes. A custom experimental design was applied to optimise Lipoid-S-100 and cholesterol concentrations as independent variables, with the hydrodynamic diameter (HD) and percent encapsulation efficiency (%EE) as response variables.
In the second phase (Fig. 1b), NR-loaded nanocochleates (NR-NCs) were fabricated via the trapping method. Calcium chloride was added in varying volumes to the optimised liposomal suspension and vortexed for 2 minutes to induce structural reorganisation.12,25 Dynamic light scattering (DLS) was employed to assess the changes, and the optimal volume was selected based on the resulting data. Bovine serum albumin (BSA) was incorporated as an aggregation inhibitor. The secondary structure of the developed NCs was evaluated using circular dichroism (CD) spectroscopy to detect any conformational changes in comparison with the BSA solution.
3.3. Characterisation of NR-NCs
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm and 4 °C for 45 minutes to investigate the %EE. The supernatant was collected and analysed using UV-Visible spectrophotometry at an appropriate wavelength. The percentage EE was calculated using the formula given in eqn (1).27
000 rpm and 4 °C for 45 minutes to investigate the %EE. The supernatant was collected and analysed using UV-Visible spectrophotometry at an appropriate wavelength. The percentage EE was calculated using the formula given in eqn (1).27| % EE = [conc. total − conc. free drug] ÷ conc. total × 100 | (1) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–14
000–14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da, HiMedia, India) were pre-activated in distilled water for 12 hours at room temperature. NR dispersion and NR-NCs (equivalent to 5 mg of NR) were sealed in the dialysis bags and immersed in 100 ml of phosphate-buffered saline (pH 7.4 and pH 5.2 for comparative analysis) at 37 ± 0.5 °C with stirring (50 rpm). 1 ml of the sample was withdrawn at intervals up to 36 hours and replaced with fresh medium. All samples were stored at −20 °C and analysed using UV spectroscopy at 288 nm.34–36 Release kinetics were assessed using the DD solver add-in for Microsoft Excel, and regression coefficients (R2) were compared to determine the best fit.37 In addition, a recovery study was performed by adding NR-NC samples at three concentration levels (2, 4 and 6 mg) to PBS (pH 5.2 and 7.4) and placing them in dialysis bags under the same conditions as the release experiment (37 °C, 24 h). Samples collected at 0 h and 36 h were analysed by UV spectrophotometry to determine the recoverable NR fraction.
000 Da, HiMedia, India) were pre-activated in distilled water for 12 hours at room temperature. NR dispersion and NR-NCs (equivalent to 5 mg of NR) were sealed in the dialysis bags and immersed in 100 ml of phosphate-buffered saline (pH 7.4 and pH 5.2 for comparative analysis) at 37 ± 0.5 °C with stirring (50 rpm). 1 ml of the sample was withdrawn at intervals up to 36 hours and replaced with fresh medium. All samples were stored at −20 °C and analysed using UV spectroscopy at 288 nm.34–36 Release kinetics were assessed using the DD solver add-in for Microsoft Excel, and regression coefficients (R2) were compared to determine the best fit.37 In addition, a recovery study was performed by adding NR-NC samples at three concentration levels (2, 4 and 6 mg) to PBS (pH 5.2 and 7.4) and placing them in dialysis bags under the same conditions as the release experiment (37 °C, 24 h). Samples collected at 0 h and 36 h were analysed by UV spectrophotometry to determine the recoverable NR fraction.3.4. Loading of NR-NCs in secondary vehicle (hydrogel)
Blank gel bases were fabricated by dispersing Carbopol-934P at varying concentrations of 0.5, 0.75, and 1% w/v, respectively, along with 0.25% w/v sodium alginate to utilise its adhesive property, in purified water under continuous stirring at 500 rpm for 5–6 hours at 25 °C. Triethanolamine was added to adjust the pH. The hydrogel base was then gradually combined with NR-NCs under constant agitation to incorporate the nano-carriers into the gel matrix.38,393.5. Characterisation of NR-NC-G
To determine the drug content, 1 mL of gel was diluted with 10 mL of water in a measuring cylinder. The gel was then sampled from three distinct areas of the cylinder (top, middle, and bottom), and the drug content was estimated using a UV spectrophotometer.1,41
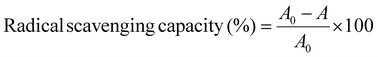 | (2) |
| J = ∂m ÷ (A ∂t) | (3) |
| Kp (cm h−1) = Jss/C0 | (4) |
3.6. In vitro cell-line studies
The RAW 264.7 macrophage cell line was obtained from the National Centre for Cell Sciences (NCCS), Pune, India. Cells were cultured and maintained in DMEM supplemented with 10% fetal bovine serum, 1% antibiotic penicillin and streptomycin, cultured in a T-25 flask, and maintained at 37 °C in an incubator with 5% atmospheric CO2 and observed daily. Bulk NR was dissolved in dimethyl sulfoxide (DMSO) and filtered through a 0.25 μm filter membrane. Samples were diluted in serum-free medium (SFM) to concentrations varying from 1000 to 31.25 μg mL−1.51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per well) and incubated for 24 h, then treated with varying concentrations of NR and NR-NC-G (as per test requirement, volume 100 μL in each well) and again incubated for 24 h and 48 h (as per test requirement). After completion of the incubation period, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) solution (100 μL at a concentration of 5 mg mL−1) was added to each well and further incubated for three hours. After the incubation period, the supernatant was discarded, and 100 μL DMSO was added to solubilise the formazan crystals. Finally, OD570 was measured on a microplate reader (BioTek Synergy H1, Agilent, U.S.) to evaluate the cell viability.52
000 cells per well) and incubated for 24 h, then treated with varying concentrations of NR and NR-NC-G (as per test requirement, volume 100 μL in each well) and again incubated for 24 h and 48 h (as per test requirement). After completion of the incubation period, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) solution (100 μL at a concentration of 5 mg mL−1) was added to each well and further incubated for three hours. After the incubation period, the supernatant was discarded, and 100 μL DMSO was added to solubilise the formazan crystals. Finally, OD570 was measured on a microplate reader (BioTek Synergy H1, Agilent, U.S.) to evaluate the cell viability.52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per well in 24-well plates for 24 h. Then, the cells were treated with bulk NR and NR-NC-G (125 μg mL−1) for comparison. 1 μg mL−1 LPS was incorporated into the cells for two hours, and the cells were further incubated for 6, 12, and 24 hours. Untreated cells with 1 μg mL−1 LPS were kept as a positive control. The cells were washed twice with 1× PBS, and 20 μM DCF-DA was added. The cells were then incubated for 15 min at 37 °C in a CO2 incubator. Finally, the DCF-DA solution was replaced with cold PBS, and fluorescence was examined using a fluorescence microscope (Leica DM3000, Germany) and a camera (DMC 5400, Leica, Germany). Digital images of each well were taken with a 20× objective using an image acquisition system (Leica Application Suite X (LAS X)).54
000 cells per well in 24-well plates for 24 h. Then, the cells were treated with bulk NR and NR-NC-G (125 μg mL−1) for comparison. 1 μg mL−1 LPS was incorporated into the cells for two hours, and the cells were further incubated for 6, 12, and 24 hours. Untreated cells with 1 μg mL−1 LPS were kept as a positive control. The cells were washed twice with 1× PBS, and 20 μM DCF-DA was added. The cells were then incubated for 15 min at 37 °C in a CO2 incubator. Finally, the DCF-DA solution was replaced with cold PBS, and fluorescence was examined using a fluorescence microscope (Leica DM3000, Germany) and a camera (DMC 5400, Leica, Germany). Digital images of each well were taken with a 20× objective using an image acquisition system (Leica Application Suite X (LAS X)).54For quantification, the same experiment was run in a 96-well plate. ROS production was observed as an increase in the fluorescence intensity of the DCF dye, as measured at an excitation of 485 nm and an emission of 535 nm using a fluorescence plate reader.
3.7. Statistical analysis
Statistical analysis was performed using GraphPad Prism (GraphPad Software, San Diego, CA, USA). Following a significant effect examined by one-way or two-way analysis of variance (ANOVA), desirable multiple comparison tests were used to assess differences between group results. Tukey's Honest Significant Difference (HSD) test was used for all pairwise comparisons among group means to control the family-wise error rate. Sidak's multiple comparison test was employed for selected comparisons, offering improved statistical power compared to the Bonferroni method while maintaining control over type I error. Dunnett's test was particularly applied when comparing each treatment group to a single control, providing greater sensitivity by limiting the number of comparisons. All tests were conducted at a significance level of α = 0.05, with corrections for multiple testing based on the chosen procedure.4. Results
4.1. Analytical method development
A UV-Visible spectrophotometric analysis was done, and the λmax of NR was observed at approximately 288 nm (Fig. S1a). It was observed that the absorbance increased linearly with concentrations ranging from 2 to 10 µg mL−1, indicating good linearity.For the HPLC analysis, the retention time for naringenin was found to be around 4.012 minutes (Fig. S1b), with a total run time of 7 minutes. The method was validated (SI data) and a calibration curve was established at concentrations ranging from 2 to 10 µg m−1 (Fig. S1c).
4.2. Optimisation of NR-LIPOs
At first, NR liposomes were optimised using a JMP custom design, and later, using the trapping method, NR-NCs were manufactured.12 The trapping method employed was straightforward, economical, and rapid, proving ideal for preparing NCs as reported in the literature.NR-LIPO optimisation was systematically approached using QbD principles as per ICH guidelines. Based on preliminary trials and risk analysis using an Ishikawa fishbone diagram (Fig. 1d), the amount of Lipoid-S-100 and cholesterol was a key CMA influencing the CQAs, specifically PS and %EE. A RSM design was employed to evaluate the effects and interactions of these CMAs, and second-order polynomial models were developed for both the responses. NR-LIPO is optimised through a systematic and statistical experimental approach integrating data from custom design, regression modelling (Table 2), graphical diagnostics (Fig. 2), and desirability-based optimisation. Ten experimental runs (Table 1) showed a wide distribution in PS, ranging from 167.9 to 298.2 nm, and EE, from 49.77% to 81.22%. The graph and linear equation trend suggested that an increase in Lipoid-S-100 and cholesterol concentrations is directly proportional to a rise in particle size and a concurrent decline in %EE, which may be due to limitations in the formation and entrapment of multilamellar vesicles.
| Components of NR-LIPO | ||||
|---|---|---|---|---|
| Independent variables and their levels | ||||
| Component | Unit | Low (−1) | Mid (0) | High (+1) |
| Lipoid S100 (X1) | mg | 40 | 50 | 60 |
| Cholesterol (X2) | mg | 10 | 15 | 20 |
| Dependent variables and their constraints | ||||
| Particle size (Y1) | nm | Minimized | ||
| Encapsulation efficiency (Y2) | % | Maximized | ||
| Design space of NR-LIPO using custom design, limiting trials to 10 runs | ||||
|---|---|---|---|---|
| Run no. | X1 | X2 | Y1 | Y2 |
| All runs were conducted in triplicate (n = 3). | ||||
| NR-LIPO1 | 40 | 10 | 167.9 ± 2.5 | 81.22 ± 1.25 |
| NR-LIPO2 | 50 | 15 | 203.5 ± 1.25 | 77.23 ± 2 |
| NR-LIPO3 | 50 | 15 | 189.2 ± 3.45 | 77.5 ± 3.65 |
| NR-LIPO4 | 60 | 10 | 253.1 ± 10.25 | 67.07 ± 2.11 |
| NR-LIPO5 | 60 | 20 | 298.2 ± 12.35 | 49.77 ± 2.5 |
| NR-LIPO6 | 50 | 10 | 186 ± 5.36 | 78.96 ± 3.5 |
| NR-LIPO7 | 40 | 20 | 183.6 ± 5.23 | 78.04 ± 3.15 |
| NR-LIPO8 | 50 | 20 | 219.2 ± 3.65 | 68.99 ± 1.25 |
| NR-LIPO9 | 40 | 15 | 168.2 ± 4.23 | 79.57 ± 3.5 |
| NR-LIPO10 | 60 | 15 | 256.4 ± 3.25 | 64 ± 2.5 |
| Summary of fit | ||||
|---|---|---|---|---|
| Response | R2 | Adjusted R2 | RMSE | MR |
| Y1 | 0.9894 | 0.9763 | 6.7026 | 212.53 |
| Y2 | 0.9875 | 0.9720 | 1.6500 | 72.235 |
| ANOVA | ||||||
|---|---|---|---|---|---|---|
| Parameters | DF | SS | MS | F-Ratio | p-Value | Model significance |
| Y1 | ||||||
| Model | 5 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 898.240 898.240 |
3379.65 | 75.2282 | 0.0005 | Significant |
| Error | 4 | 179.701 | 44.93 | — | — | — |
| C. total | 9 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 077.941 077.941 |
— | — | — | — |
| Y2 | ||||||
| Model | 5 | 864.934 | 172.987 | 63.5353 | 0.0007 | Significant |
| Error | 4 | 10.890 | 2.723 | — | — | — |
| C. total | 9 | 875.825 | — | — | — | — |
| Optimal values, desirability search approach | |||||
|---|---|---|---|---|---|
| Sample | X1 | X2 | Y1 | Y2 | Desirability |
| NR-LIPO11 | 40.15 | 13.36 | 164.81 | 81.83 | 0.9565 |
Table 2 summarises the model's statistical performance, showing excellent fit, with R2 values of 0.9894 for PS and 0.9875 for %EE, and adjusted R2 values of 0.9763 and 0.9720, respectively. The lower RMSE values, i.e., 6.70 for PS and 1.65 for EE, again supported the precision of the model. The statistical significance of both models (p < 0.001) was confirmed by ANOVA, and high F-ratios (75.23 for PS and 63.54 for %EE) were found, which indicates that the chosen variables are responsible for most of the variability in the responses. The regression equations found for each response quantify the influence of linear, quadratic, and interactive effects of independent variables:
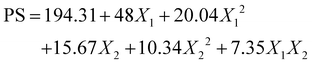 | (5) |
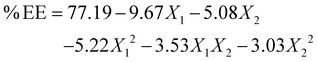 | (6) |
Graphical evaluation of the statistical models through diagnostic and response surface figures validated the significance of using models to understand formulation behaviour. The actual versus predicted plots (Fig. 2a and d) for both PS and %EE showed strong linear correlation, with data points closely aligned along the diagonal, proving that the regression models correctly predicted experimental outcomes. These findings are further supported by the residual versus predicted plots (Fig. 2b and e), which show a random scatter of residuals around zero, confirming that the models met the assumption of homoscedasticity and were free from systematic bias. The 3D response surface (Fig. 2h and i) visually represented the interaction between Lipoid-S-100 and cholesterol. For PS, both variables exhibited a synergistic effect, with increases in either leading to larger vesicles, which may be due to multilamellar vesicle formation and thickness in lipidic layers. In contrast, for %EE, an antagonistic effect was seen; higher Lipoid-S-100 and cholesterol levels led to reduced drug entrapment, potentially due to saturation of the lipid matrix in liposomal vesicles. These opposing trends highlighted the need for a balanced formulation strategy. The desirability plot (Fig. 2g) further integrated these findings by identifying an optimal region in the design space that satisfies both response criteria.
The contour overlay plot (Fig. 2j) represents the design space optimisation of NR-LIPO, based on two CQAs: PS and %EE, and plotted against the concentrations of Lipoid-S-100 on the x-axis and cholesterol on the y-axis. The red contour represents the response surface for PS, where the red dotted region indicates areas that meet the condition of minimised particle size. The blue contour corresponds to %EE, showing areas where %EE is maximised or within the desirable range. The overlapping region between the red and blue contour lines signifies the “sweet spot or design space”. This area is an optimal space where a combination of Lipoid-S-100 and cholesterol concentrations simultaneously satisfies both response criteria: minimising the PS and optimum or high %EE. The desirable design space is relatively confined, emphasising that only specific ranges of Lipoid-S-100 (around 40–50 mg) and cholesterol (around 10–15 mg) allow both criteria to be met. It is found that outside the design space, you either get too large particles or inadequate EE.
The desirability function analysis (Fig. 2g) enabled simultaneous optimisation of both responses. An optimal formulation (NR-LIPO11) was identified, yielding a predicted particle size of 164.81 nm, a %EE of 81.83%, and a desirability score of 0.9565. This solution falls within the robust design space, as confirmed by the overlaid contour plots, indicating a favourable balance between particle size and drug loading efficiency.
4.3. Transformation and stabilisation of NR-LIPO into NR-NCs
The amount of CaCl2 and BSA, used as aggregation inhibitors, was optimised using DLS and CD spectroscopy. DLS was employed to identify the transformation of liposomes into nanocochleates by assessing changes in particle size distribution. The intensity-weighted size distribution curves revealed a distinct flattening, attributed to calcium-induced structural reorganisation. The NR-LIPO11 formulation exhibited a sharp, narrow peak at around 164 nm, indicating a relatively uniform liposome population. Conversely, the NR-NCs exhibited a broader size distribution (Fig. 3a). This flattening of the curve was interpreted as confirmation of the successful transition from liposomes to nanocochleates, mediated by the addition of calcium chloride.CD spectra were utilised to provide critical insights into the structural alterations of BSA upon interaction with NCs. The spectrum of pure BSA (Fig. 3b, blue) exhibited a prominent negative peak at around 210–220 nm, characteristic of a predominant α-helical structure, consistent with the secondary composition of 18.7% α-helix and 58.7% β-sheet. In contradiction, NCs alone (red line) showed no significant CD signal, confirming the absence of intrinsic secondary structures. On addition of BSA in NCs (pink), a decrease in ellipticity was observed, which indicates structural modifications. This change correlated with an increase in α-helical content from 18.7% to 31.6%, an increase in β-sheets from 58.7% to 66.4%, and a marked reduction in disordered structures from 19.9% to 2.0%. These conformational changes suggested that BSA underwent structural tightening, likely driven by hydrophobic and electrostatic interactions with NCs. Based on these observations, the final optimised NR-NC formulation was achieved by adding 0.1 mM (500 µL) calcium chloride and 60 µg mL−1 (1.2 mL) BSA to the NR-LIPO11 system.
4.4. Characterisation of NR-NCs
The HD of the NR-NCs was within the nanoscale range, specifically 168 ± 1.8 nm. Additionally, the PDI was found to be 0.25, indicating a highly uniform PSD. The ζ-potential of 26.6 ± 1.6 mV indicates a moderately charged surface capable of providing electrostatic repulsion; additionally, the BSA coating contributes steric stabilisation, collectively ensuring adequate colloidal stability and minimising aggregation of the nanocochleates. The %EE of the optimised NR-NCs was evaluated using a centrifugation method, yielding a value of 85.23 ± 4.33%. This high EE indicates effective encapsulation of NR within the nanocochleate structures.Morphological SEM analysis revealed that liposomes exhibited smooth, spherical vesicular structures, as shown in Fig. 3c. In contrast, NR-NCs displayed elongated, cylindrical morphologies (Fig. 3f), indicating a successful structural transformation induced by calcium interaction. Zoomed-in micrographs emphasised the uniformity and structural integrity of these rod-like particles. AFM images (Fig. 3d and g) further confirmed the rod-shaped morphology, with topographic height profiles ranging between 135 and 150 nm, consistent with nanoscale dimensions.
TEM images provided a detailed visualisation of the internal lamellar organisation of the NR-NCs. Fig. 3e shows densely packed elongated rods, whereas Fig. 3h depicts an individual, well-defined nanocochleate, clearly revealing the characteristic rolled lipid bilayer architecture.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching near 1650 cm−1, and aromatic C
O stretching near 1650 cm−1, and aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C vibrations between 1600 and 1400 cm−1. These confirmations indicate the presence of phenolic and flavonoid structures in NR. In PM (Fig. 3j, red), notable shifts and intensity loss are observed; these bands suggest intermolecular interactions, such as hydrogen bonding and hydrophobic interactions, especially involving the carbonyl and hydroxyl groups of NR with Lipoid-S-100 and BSA. The appearance of additional bands or shifts near 2900 cm−1 (aliphatic C–H stretching) and 1500–1000 cm−1 (fingerprint region) is due to Lipoid-S-100 and BSA. In the case of NR-NCs (Fig. 3j, blue), suppression or displacement of NR original peaks and the appearance of new band patterns, especially in the 1500–1000 cm−1 range, strongly suggest successful encapsulation. An effective encapsulation of NR within the nanocochleate matrix, without any chemical degradation, is confirmed by the results obtained.
C vibrations between 1600 and 1400 cm−1. These confirmations indicate the presence of phenolic and flavonoid structures in NR. In PM (Fig. 3j, red), notable shifts and intensity loss are observed; these bands suggest intermolecular interactions, such as hydrogen bonding and hydrophobic interactions, especially involving the carbonyl and hydroxyl groups of NR with Lipoid-S-100 and BSA. The appearance of additional bands or shifts near 2900 cm−1 (aliphatic C–H stretching) and 1500–1000 cm−1 (fingerprint region) is due to Lipoid-S-100 and BSA. In the case of NR-NCs (Fig. 3j, blue), suppression or displacement of NR original peaks and the appearance of new band patterns, especially in the 1500–1000 cm−1 range, strongly suggest successful encapsulation. An effective encapsulation of NR within the nanocochleate matrix, without any chemical degradation, is confirmed by the results obtained.4.5. Characterisation of NR-NC-G
Based on rheological evaluation and matching with the marketed formulation (MF), the optimum concentration of the gel formulation was selected to ensure desirable properties of the gel formulation. The results obtained from various gel formulations, including blank gels and NR-NC-G, showed that 1% NR-NC-G in Carbopol 934P gel exhibited superior physicochemical behaviour, making it the optimal choice for further development.In Fig. 5b-1, blank gel, NR-NC-G and MF exhibited a wide linear viscoelastic region (LVER) (marked in the blue background) in the plot, suggesting good internal network stability under low applied stress. However, NR-NC-G demonstrated a higher G′, indicating a more dominant elastic character. This signifies improved structural rigidity, a critical factor for maintaining the formulation's shape and mechanical integrity during application and storage.
Furthermore, the viscoelastic nature was examined through frequency sweep measurements, as illustrated in Fig. 5b-2. Both G′ and G″ remained consistent across the tested angular frequency range, verifying frequency-independent behaviour—an indication of a robust gel network. NR-NC-G consistently shows higher G′ values than MF, reinforcing its superior elastic nature. The inclusion of NR-NCs into the gel did not alter this profile, certifying that NR-NCs were well integrated within the gel matrix. This demonstrates excellent mechanical stability of the hydrogel, even after incorporating the nanocarrier.
Shear stress versus shear strain was examined to evaluate deformation behaviour, as shown in Fig. 5c-1. A nonlinear increase in shear strain with increasing shear stress was observed, indicating pseudo-plastic behaviour. This property is particularly favourable for topical applications, as it suggests that the formulation remains strong under static conditions but becomes more fluid under mechanical stress, ensuring easy application without compromising retention.
Lastly, the complex viscosity versus angular frequency was analysed (see Fig. 5c-2). The viscosity of NR-NC-G was higher than that of MF and showed a decreasing trend with increasing frequency, confirming its shear-thinning nature. High viscosity is often associated with enhanced mucoadhesion. Together, these characteristics indicate that the formulation combines mechanical robustness.
From texture analysis (Fig. 5d), it was found that NR-NC-G has a firmness value of 1866.894 g and a work of shear of 760.670 g s. This indicates that the gel has a moderate level of hardness and a moderate energy requirement for spreading the gel, respectively. According to the results, the gel has sufficient structural integrity while remaining easy to apply.
The pH of the gel was found to be in the range of 5.0 to 5.5, which is compatible with the natural pH of the skin. Drug content analysis based on samples taken from the top, middle, and bottom of the gel showed values of 99.21%, 98.78%, and 97.32%, respectively. These results indicate good uniformity in the drug content of NR-NC-G.
The same trend was observed in the DPPH neutralisation assay (Fig. 4c). The IC50 of NR was 183.71 µg mL−1, while NR-NC-G again exhibited stronger activity with an IC50 value of 92.12 µg mL−1. Ascorbic acid (AA), as expected, showed the highest efficacy. Tukey's post hoc test indicated that NR remained significantly different from AA at all concentrations (****p < 0.0001), whereas NR-NC-G showed non-significant differences at higher concentrations (ns = 0.3394 at 1000 µg mL−1; **p = 0.0014 at 500 µg mL−1), further supporting its improved functional performance. These results confirm that the entrapment of NR into the nano-complex formulation substantially enhances its antioxidant potential, highlighting its promise for applications in targeting oxidative stress.
This enhanced performance is further validated by quantitative permeation metrics. The flux of NR-NC-G was significantly higher at 5.960 µg cm−2 h−1, as compared to 0.410 µg cm−2 h−1 for the NR solution—showing nearly a 15-fold increase in drug transport efficiency. In addition to this, the permeability coefficient of NR-NC-G (2.980 cm h−1) is markedly higher than that of the NR solution (0.205 cm h−1), confirming the formulation's superior skin permeation capacity. Altogether, these results highlight the potential of NR-NC-G as a highly effective topical drug delivery system for enhancing the bioavailability of poorly soluble compounds, such as naringenin, offering a highly permeable dosage formulation for dermal administration.
| NR-Sol (dermis) | NR-NC-G (dermis) | NR-Sol (epidermis) | NR-NC-G (epidermis) | |
|---|---|---|---|---|
| Tskinmax (h) | 4 | 2 | 6 | 2 |
| Cskinmax (µg cm−2) | 29.11 ± 3.42 | 32.94 ± 3.32 | 26.00 ± 2.90 | 89.07 ± 1.76 |
| AUC0−t | 149.99 ± 29.77 | 198.78 ± 26.12 | 147.01 ± 22.74 | 490.59 ± 20.73 |
| Ke | 0.33 ± 0.15 | 0.17 ± 0.03 | 0.30 ± 0.08 | 0.15 ± 0.01 |
In the dermis, NR-NC-G exhibited faster drug absorption, with a Tskinmax of 2 hours, compared to 4 hours for NR-Sol. The maximum dermal concentration (Cskinmax) achieved with NR-NC-G was 32.94 ± 3.32 µg cm−2, higher than that of NR-Sol (29.11 ± 3.42 µg cm−2). Another important factor, AUC0−t, which reflects cumulative drug exposure, was significantly greater for NR-NC-G (198.78 ± 26.12 µg h cm−2) than for NR-Sol (149.99 ± 29.77 µg h cm−2), suggesting more efficient drug deposition. The lower elimination rate constant (Ke) for NR-NC-G (0.17 ± 0.03 h−1) compared to NR-Sol (0.33 ± 0.15 h−1) further supports the slower clearance of NR-NC-G from the dermal compartment.
The differences were even more significant in the epidermis, the primary target site for therapeutic action in psoriasis. Both NR sol. and NR-NC-G reached high epidermal concentration at 2 hours, but NR-NC-G achieved a markedly higher Cskinmax of 89.07 ± 1.76 µg cm−2, compared to NR-Sol. The AUC0−t for NR-NC-G in the epidermis was 490.59 ± 20.73 µg h cm−2, over threefold higher than that of NR-Sol. Similarly, the Ke for NR-NC-G was significantly lower (0.15 ± 0.01 h−1) than for NR-Sol, indicating that NR-NC-G has the ability to retain the drug longer in the skin.
As the concentration decreased, the protective effect of NR-NC-G remained statistically significant but was less pronounced. At 125 and 62.5 μg mL−1, NR-NC-G-treated cells maintained high cell viability percentages, outperforming the bulk NR. Collectively, these findings clearly demonstrate that NR maintained over 50% cell viability up to a concentration of 213.33 μg mL−1, while NR-NC-G exhibited similar tolerance up to 432.58 μg mL−1 and below these concentrations, the reduction in cell viability was not statistically significant compared to control, suggesting relatively high biocompatibility at minimum doses. But at high concentrations above IC50, a visible decline in cell viability was observed.
Furthermore, to examine the cytotoxic effects of LPS on RAW264.7 macrophages, cells were treated with 1 μg mL−1 LPS and assessed for viability at 6, 12, 24, and 48 hours (Fig. 6b). Sidak's multiple comparisons test showed no statistically significant difference in cell viability between the untreated and LPS-treated groups at 6, 12, and 24 hours (p > 0.05), indicating that short-term exposure to LPS does not markedly compromise macrophage viability. In contrast, by 48 hours, LPS treatment led to a pronounced decline in cell viability, compared to the control (p = 0.0053).
Based on these findings, concentrations of NR and NR-NC-G below 200 μg mL−1 were selected for subsequent experiments to ensure minimal cytotoxicity. Additionally, LPS treatment at 1 μg mL−1 for 6 to 24 hours was chosen as the standard condition for further assays examining inflammatory responses to obtain early immune activation.
4.5.8.1. LPS induced inflammation in cells
Morphological alterations in RAW264.7 cells upon exposure to LPS were evaluated using an inverted microscope. Cells in the untreated healthy control group showed a typical resting macrophage morphology, characterised by a round shape and smooth cellular outlines, with no visible pseudopodia (Fig. 6c – control). In contrast, cells stimulated with LPS (1 μg mL−1) for 24 hours displayed morphological features indicative of macrophage activation. These included an enlargement of cell size and the extension of elongated pseudopodia, as highlighted by red arrows (Fig. 6c – LPS treated). These observations support the activation of RAW264.7 cells in response to pro-inflammatory stimulation with LPS.4.5.8.2. Anti-inflammatory activity and NO level estimation
To evaluate the therapeutic efficacy of NR-NC-G in modulating LPS-induced cytotoxicity in RAW264.7 cells, cell viability was examined following 24-hour exposure to NR-NC-G at varying concentrations (62.5, 125, and 250 μg mL−1) and compared to cells treated with LPS alone and untreated control cells. The results showed a significant restoration of cell viability in the NR-NC-G treated groups compared to the LPS-only group at all tested concentrations (Fig. 6d). At 250 μg mL−1, cells treated with NR-NC-G exhibited higher cell viability compared to the LPS-only group (p < 0.0001). Importantly, this value was approximately similar to the untreated control group, indicating that NR-NC-G restored cellular health (p = 0.2192). At 125 and 62.5 μg mL−1, NR-NC-G treatment also resulted in improvements in viability relative to the LPS-only group.The nitrite levels estimation analysis revealed statistically significant differences (p < 0.0001) across all comparisons (Fig. 6e). At 250 μg mL−1, NR-NC-G co-treated with LPS resulted in significantly lower nitrite levels compared to both the bulk NR + LPS and LPS-only groups. Similar trends were observed at 125 μg mL−1, where NR-NC-G + LPS showed a greater reduction in nitrite levels relative to the bulk NR + LPS and LPS-only groups. At 62.5 μg mL−1, the nanoformulation continued to exhibit its superior effect. These results suggest that NR-NC-G exerts a significantly greater anti-inflammatory response than the bulk NR, validating the enhanced therapeutic efficacy.
4.5.8.3. Cellular ROS assessment
To evaluate intracellular ROS levels and antioxidant efficacy of the NR-NC-G formulation in LPS-stimulated RAW264.7 macrophages, DCFDA-based fluorescence imaging and quantitative fluorescence intensity measurements were performed and photographed under a fluorescent microscope. The +ve control group showed consistently high green fluorescence, confirming strong ROS generation upon inflammatory stimulation. Conversely, cells treated with the bulk NR showed time-dependent fluorescence attenuation. This trend suggests partial ROS scavenging by the bulk drug, although fluorescence remained significantly higher than that of NR-NC-G (Fig. 6f).Cells treated with NR-NC-G showed reduced green fluorescence at all time points. At 6 hours, a faint signal was observed (RFU = 121.7), which is significantly lower than those of the bulk NR and the positive control. The fluorescence further diminished at 12 and 24 hours (RFU = 165.3 and 133.3, respectively), suggesting a sustained antioxidant effect of NR-NC-G. Tukey's multiple comparison test confirmed a significant reduction in fluorescence between the 12-hour and 24-hour timepoints in the NR-NC-G group (p = 0.0380), indicating that oxidative stress continued to decline over time with NR-NC-G treatment.
Microscopy images (Fig. 6g) visually validated these quantitative findings: positive control cells showed intense green fluorescence, indicative of high ROS accumulation, whereas untreated cells exhibited no fluorescence. Cells treated with bulk NR showed a modest reduction in green intensity over time. Still, cells treated with NR-NC-G showed minimal green fluorescence, specifically evident at 12 and 24 hours, highlighting the strong ROS-neutralising capacity.
4.5.8.4. Mitochondrial depolarisation
To assess mitochondrial membrane potential (Δψm) in LPS-stimulated RAW264.7 macrophages, JC-1 staining was used, where healthy mitochondria are indicated by JC-1 aggregates (red fluorescence) and depolarised mitochondria by JC-1 monomers (green fluorescence). In untreated cells, high red and minimal green signals were observed, reflected by a significantly elevated red-to-green fluorescence ratio, confirming perfect mitochondrial function (Fig. 7a). On the other hand, the LPS-stimulated cells showed a drastic reduction in red fluorescence and an increased green signal (ratio ∼0.66), indicating mitochondrial dysfunction.Bulk NR-treated cells showed partial preservation of Δψm, with a moderate red-to-green ratio (∼3.17), compared to LPS-only cells. Importantly, this effect was significantly enhanced with NR-NC-G treatment, which restored the red-to-green fluorescence ratio to ∼12.60—comparable to control levels.
Furthermore, microscopy images (Fig. 7b) supported these findings. Control cells exhibited high red fluorescence, accompanied by a low green signal, indicating intact mitochondrial function. In contrast, LPS-stimulated cells displayed strong green fluorescence and a markedly reduced red signal, indicating mitochondrial damage. Bulk NR-treated cells exhibited a transfer toward red aggregates, while still retaining residual green fluorescence. Notably, NR-NC-G-treated cells exhibited intense red fluorescence with a low green signal, and merged images indicated a restoration of mitochondrial function.
Dunnett's multiple comparisons test confirmed that the difference between LPS and the control and LPS and NR-NC-G (p < 0.0001) Δψm was highly significant. However, the difference between LPS and bulk NR Δψm was not statistically significant (p = 0.0577), suggesting that NR-NC-G gave more effective repolarisation of mitochondrial function than unentrapped NR.
5. Discussion
Plant-derived flavonoid, NR with a polyphenolic structure, has well-documented antioxidant and anti-inflammatory effects.57 However, its therapeutic efficacy is hindered by low aqueous solubility, limited chemical stability, and permeability issues. This research aimed to overcome these problems by utilising nanocochleates (NR-NCs) embedded within a hydrogel matrix (NR-NC-G), which synergistically enhanced their bioactivity and targeted skin delivery. The data presented herein unravel not only the macroscopic and dermatokinetic advantages of NR-NC-G but also provide mechanistic insights at the molecular level.The formulation of NR-LIPOs and their conversion into NR-NCs is done via a rational design under the QbD framework. The regression models, supported by high R2 values and minimal residual error, identified Lipoid S-100 and cholesterol content as CMAs. An increase in both caused a larger PS, which may be due to the formation of multilamellar vesicles and enhanced bilayer rigidity, as cholesterol adjusts itself between phospholipid tails, decreasing fluidity. At the molecular level, cholesterol's planar steroid ring system enhances van der Waals interactions with lipid acyl chains, increasing bilayer thickness and reducing membrane permeability, thereby decreasing the %EE. The transformation into nanocochleates, confirmed via DLS and microscopy, is attributed to calcium-mediated inter-bilayer bridging. Calcium ions neutralise the negatively charged phospholipid, facilitating membrane stacking into cochleate cylinders. The divalent Ca2+ ions selectively bind to negatively charged phospholipid headgroups, leading to progressive charge neutralisation and a marked reduction in inter-bilayer repulsive forces. As this electrostatic screening increases, adjacent lamellae collapse, fuse, and reorganise into a compacted multilamellar structure, as seen in Fig. 3f-h. Beyond a threshold Ca2+ concentration, this collapse is energetically sufficient to drive the characteristic folding and rolling of the stacked bilayers into the cylindrical nanocochleate architecture. Thus, the calcium level directly influences the extent and efficiency of cochleation, dictating the rigidity, completeness, and structural integrity of the resulting nanocochleates. CD spectroscopy revealed a conformational tightening of BSA, characterised by an increased β-sheet content. This structural change proves strong electrostatic and hydrophobic interactions between BSA and the NR-NC interface, helping nanocochleate stabilisation via surface adsorption.
DSC and XRD results showed a loss of NR crystallinity upon encapsulation, supporting molecular-level dispersion within the lipid matrix. ATR-IR peak shifts, specifically in O–H and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching regions, confirmed hydrogen bonding and hydrophobic interactions between NR and Lipoid-S-100. These forces are important in preserving the drug within the core of the bilayer. In the release study, we found that the acidic milieu of psoriatic lesions aligns closely with the accelerated NR-NC release observed at pH 5.2, enabling possible drug liberation at inflamed sites. In contrast, the markedly slower release at physiological pH 7.4 will help limit unnecessary diffusion into healthy surrounding skin. This pH-responsive behaviour supports targeted, lesion-specific delivery that can enhance therapeutic precision and overall treatment efficiency. NR-NC-G provided additional rheological stability. The gel showed pseudoplastic, shear-thinning behaviour, which is highly desirable for topical gels.
O stretching regions, confirmed hydrogen bonding and hydrophobic interactions between NR and Lipoid-S-100. These forces are important in preserving the drug within the core of the bilayer. In the release study, we found that the acidic milieu of psoriatic lesions aligns closely with the accelerated NR-NC release observed at pH 5.2, enabling possible drug liberation at inflamed sites. In contrast, the markedly slower release at physiological pH 7.4 will help limit unnecessary diffusion into healthy surrounding skin. This pH-responsive behaviour supports targeted, lesion-specific delivery that can enhance therapeutic precision and overall treatment efficiency. NR-NC-G provided additional rheological stability. The gel showed pseudoplastic, shear-thinning behaviour, which is highly desirable for topical gels.
Ex vivo permeation and dermatokinetic results confirmed that NR-NC-G enhances NR deposition in skin layers. The NR-NC structure, with its strong, rolled bilayer, may facilitate transcellular penetration by disrupting tight junctions and promoting lipid fusion. The hydrogel matrix works as a holding unit or depot, maintaining a local concentration gradient that drives passive diffusion. The low Ke and higher AUC values suggest high NR accumulation in the skin, critical for managing chronic conditions like psoriasis.
NR-NC-G significantly enhanced free radical scavenging activity compared to bulk NR, with low IC50 values in both DPPH and H2O2 assays. This improvement may be due to the restricted mobility and high surface area of nanocochleates, which likely concentrate NR molecules at the interface, facilitating rapid electron donation or hydrogen atom transfer reactions. At the intracellular level in RAW 264.7, the NR-NC-G cleared ROS accumulation, as evidenced by reduced DCFDA fluorescence. This proves that NR-NC-G modulates oxidative stress by preserving the glutathione (GSH) pool and inhibiting NADPH oxidase activity. Moreover, it is possible that NR-NC-G may activate the Nrf2-Keap1 pathway, causing upregulation of antioxidant enzymes (e.g., SOD, catalase), a hypothesis proved by ROS suppression over delayed time.58
NR-NC-G demonstrated a strong reduction of LPS-stimulated nitrite production and restored macrophage viability by inhibition of inducible nitric oxide synthase (iNOS). These effects are believed to happen through inhibition of the Toll-like receptor 4 (TLR4)/MyD88 signalling axis (Fig. 1e),59 which is triggered by LPS and travels via the nuclear factor-kappa B (NF-κB) and mitogen-activated protein kinase (MAPK) pathways. At the molecular level, NR is known to inhibit IκB kinase (IKK), by preventing the degradation of IκB-α and nuclear translocation of the NF-κB p65 subunit. Additionally, NR downregulates the phosphorylation of ERK1/2, JNK, and p38 MAPK, thereby disrupting inflammatory mediators. The nano-encapsulated NR likely enhances this cycle inhibition due to sustained intracellular availability. JC-1 assays demonstrated that NR-NC-G restored Δψm, a crucial factor in maintaining ATP synthesis.
This work primarily highlights the formulation perspective of the naringenin-encapsulated nano-cochleate hydrogel, emphasising its promising cellular anti-inflammatory activity and dermatokinetic behaviour, and establishes a strong foundation for topical delivery applications. While in vivo studies, extended safety profiling, and broader mechanistic analyses are planned as future steps, the current findings clearly demonstrate the system's potential and provide a solid platform for advancing toward more comprehensive evaluations in subsequent research.
6. Conclusion
In this study, we successfully developed a nanocochleate-based gel formulation (NR-NC-G) as an advanced and targeted topical therapy for psoriasis. A custom experimental design limited to ten trials enabled efficient optimisation by evaluating the influence of key excipients. The resulting NR-NCs demonstrated nanometric particle size, high entrapment efficiency, and sustained drug release over 36 hours when incorporated into a Carbopol gel base. Ex vivo dermatokinetic analysis showed enhanced NR accumulation in skin layers, supporting the formulation's skin retention capacity and potential for localised treatment. Furthermore, in vitro studies in LPS-stimulated RAW264.7 macrophages confirmed the anti-inflammatory and antioxidant effects of NR-NC-G, demonstrating its ability to reduce oxidative stress and highlighting its therapeutic relevance in psoriasis and paving the way for future preclinical studies.Author contributions
ASK, ADS: conceptualisation, data curation, and writing – original draft. GK: data curation-cell line studies. KKS: writing – review & editing. RS: conceptualisation, writing – review & editing, and supervision.Conflicts of interest
The authors declare no competing interests.Animal ethics statement
All animal experiments were conducted in accordance with the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Government of India. The experimental protocol was reviewed and approved by the Institutional Animal Ethics Committee (IAEC) of the National Institute of Pharmaceutical Education and Research (NIPER), Raebareli, under approval number NIPER/RBL/IAEC/228/SEP.2024.Abbreviations
| NR | Naringenin |
| NR-LIPO | Naringenin liposomes |
| NR-NCs | Naringenin nanocochleates |
| NR-NC-G | Naringenin nanochochleates hydrogel |
| BSA | Bovine serum albumin |
| ROS | Reactive oxygen species |
| BALB/C | Bagg albino/C |
| AUC | Area under curve |
| TNF-α | Tumour necrosis factor-α |
| IL-6 | Interleukin-6 |
| IL-17 | Interleukin-17 |
| NF-kB | Nuclear factor-kappa B pathway |
| Nrf2 | Nuclear factor erythroid-derived 2 |
| MAPK | Mitogen-activated protein kinase |
| HO-1 | Heme oxygenase-1 |
| PS | Hydrodynamic diameter |
| PDI | Polydispersity index |
| QBD | Quality by design |
| DMEM | Dulbecco's modified Eagle's medium |
| FBS | Fetal bovine serum |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| DCFDA | Dichlorodihydrofluorescein diacetate |
| JC-1 | 5,5′,6,6′-Tetrachloro-1,1′,3,3′-tetraethylbenzimidazolocarbocyanine iodide |
| LPS | Lipopolysaccharide |
| HPLC | High-performance liquid chromatography |
| ICH | International Conference of Harmonization |
| QTPP | Quality target product profile |
| CQAs | Critical quality attributes |
| CMAs | Critical material attributes |
| CPPs | Critical process parameters |
| PS | Particle size |
| EE% | Encapsulation efficiency |
| DLS | Dynamic light scattering |
| CD | Circular dichroism |
| SEM | Scanning electron microscopy |
| TEM | Transmission electron microscopy |
| AFM | Atomic force microscopy |
| DSC | Differential scanning calorimetry |
| ATR-IR | Attenuated total reflectance – infrared spectroscopy |
| XRD | X-ray diffraction |
| RH | Relative humidity |
| PBS | Phosphate buffer saline |
| H&E | Haematoxylin and eosin |
| DMSO | Dimethyl sulfoxide |
| SFM | Serum-free medium |
| Δψm | Mitochondrial membrane potential |
| ANOVA | Analysis of variance |
| HSD | Honest significant difference |
| PM | Physical mixture |
| AIC | Akaike information criteria |
| MSC | Model selection criterion |
| MF | Marketed formulation |
| LVER | Linear viscoelastic region |
| RFU | Relative fluorescence unit |
Data availability
Data will be made available on request.Supplementary information (SI) is available. See DOI: https://doi.org/10.1039/d5pm00268k.
Acknowledgements
The authors acknowledge the Department of Pharmaceuticals, Ministry of Chemicals and Fertilizers, Government of India, for financial support. The authors also gratefully acknowledge the CoE-NDDS and NIPER-R. The NIPER-R communication number for the research article is NIPER-R/Communication/828. The corresponding author RS acknowledges the DST-SERB for providing the SIRE fellowship with award No. SIR/2022/00678. The support provided by University of Lancashire to host the SIRE fellowship is gratefully acknowledged.References
- R. Keshari, A. Tharmatt, M. M. Pillai, D. Chitkara, P. Tayalia and R. Banerjee, et al., Eugenol-Loaded Lipid Nanoparticles-Derived Hydrogels Ameliorate Psoriasis-like Skin Lesions by Lowering Oxidative Stress and Modulating Inflammation, ACS Pharmacol. Transl. Sci., 2024, 7, 3592–3606, DOI:10.1021/acsptsci.4c00493.
- O. FitzGerald, A. Ogdie, V. Chandran, L. C. Coates, A. Kavanaugh and W. Tillett, et al., Psoriatic arthritis, Nat. Rev. Dis. Primers, 2021, 7, 59, DOI:10.1038/s41572-021-00293-y.
- Q. Li, V. Chandran, L. Tsoi, D. O'Rielly, R. P. Nair and D. Gladman, et al., Quantifying Differences in Heritability among Psoriatic Arthritis (PsA), Cutaneous Psoriasis (PsC) and Psoriasis vulgaris (PsV), Sci. Rep., 2020, 10, 4925, DOI:10.1038/s41598-020-61981-5.
- A. D. Sutar and R. Shukla, Emerging Smart Microneedle Technologies in Psoriasis: Convergence of Nanocarriers, Machine learning, and Personalized Delivery, RSC Pharm., 2025, 2(6), 1268–1291, 10.1039/D5PM00173K.
- W. B. Kim, D. Jerome and J. Yeung, Diagnosis and management of psoriasis, Can. Fam. Physician, 2017, 63, 278–285 Search PubMed.
- S. Makuch, M. Dróżdż, A. Makarec, P. Ziółkowski and M. Woźniak, An Update on Photodynamic Therapy of Psoriasis—Current Strategies and Nanotechnology as a Future Perspective, Int. J. Mol. Sci., 2022, 23, 9845, DOI:10.3390/ijms23179845.
- G. Zhang, G. Sun, H. Guan, M. Li, Y. Liu and B. Tian, et al., Naringenin nanocrystals for improving anti-rheumatoid arthritis activity, Asian J. Pharm. Sci., 2021, 16, 816–825, DOI:10.1016/j.ajps.2021.09.001.
- R. Deenonpoe, P. Prayong, N. Thippamom, J. Meephansan and K. Na-Bangchang, Anti-inflammatory effect of naringin and sericin combination on human peripheral blood mononuclear cells (hPBMCs) from patient with psoriasis, BMC Complementary Altern. Med., 2019, 19, 168, DOI:10.1186/s12906-019-2535-3.
- M. Nowak-Perlak, K. Szpadel, I. Jabłońska, M. Pizon and M. Woźniak, Promising Strategies in Plant-Derived Treatments of Psoriasis-Update of In Vitro, In Vivo, and Clinical Trials Studies, Molecules, 2022, 27, 591, DOI:10.3390/molecules27030591.
- J. A. Adetunji, K. D. Fasae, A. I. Awe, O. K. Paimo, A. M. Adegoke and J. K. Akintunde, et al., The protective roles of citrus flavonoids, naringenin, and naringin on endothelial cell dysfunction in diseases, Heliyon, 2023, 9, e17166, DOI:10.1016/j.heliyon.2023.e17166.
- D. Şahin, E.Ş Çağlar, T. Boran, A. E. Karadağ, G. Özhan and N. Üstündağ Okur, Development, characterization of naringenin-loaded promising microemulsion formulations, and demonstration of anti-aging efficacy by in vitro enzyme activity and gene expression, J. Drug Delivery Sci. Technol., 2023, 84, 104422, DOI:10.1016/j.jddst.2023.104422.
- D. B. Bari, S. R. Helaskar, M. B. Gagarani and C. V. Pardeshi, Nose-to-brain delivery of self-assembled curcumin-nanocochleates for glioblastoma treatment, J. Drug Delivery Sci. Technol., 2025, 104, 106557, DOI:10.1016/j.jddst.2024.106557.
- C. Song, R. Liu, B. Kong, Z. Gu and G. Chen, Functional hydrogels for treatment of dental caries, Biomed. Technol., 2024, 5, 73–81, DOI:10.1016/j.bmt.2023.05.002.
- S. Mehnath, M. Sathish Kumar, K. Chitra and M. Jeyaraj, Bone-Adhesive Hydrogel for Effective Inhibition of M. tuberculosis and Osteoblast Regeneration, ACS Infect. Dis., 2023, 9, 2269–2281, DOI:10.1021/acsinfecdis.3c00328.
- S. Mehnath, K. Karthikeyan and M. Jeyaraj, Mechanical Force on Hydrogel Implication on Enhanced Drug Release, Antibacterial, and M2 Macrophage Polarization: New Insights Alleviate Diabetic Wound Healing, ACS Appl. Mater. Interfaces, 2024, 16(41) DOI:10.1021/acsami.4c13633.
- Z. Han, L. Bao, Y. Yu, Y. Zhao, M. Wang and Y. Sun, et al., Injectable short-fiber hydrogel with fatigue resistance and antibacterial properties for synergistic periodontitis therapy, Chem. Eng. J., 2025, 520, 166298, DOI:10.1016/j.cej.2025.166298.
- K.-H. Yi, Thermal degradation of hyaluronic acid dermal fillers, Plast. Aesthetic Res., 2024, 11(56) DOI:10.20517/2347-9264.2024.119.
- Z. Tang, L. Deng, J. Zhang, T. Jiang, H. Xiang and Y. Chen, et al., Intelligent Hydrogel-Assisted Hepatocellular Carcinoma Therapy, Research, 2024, 7, 0477, DOI:10.34133/research.0477.
- Z. Zhang, H. Li, M. Qian, Y. Zheng, L. Bao and W. Cui, et al., Up IGF-I via high-toughness adaptive hydrogels for remodeling growth plate of children, Regener. Biomater., 2025, 12 DOI:10.1093/rb/rbaf004.
- S. Mehnath and M. Jeyaraj, Antibiofilm and enhanced antibiotic delivery by halloysite nanotubes architected dental implant against periodontitis, Mater. Chem. Phys., 2023, 295, 127061, DOI:10.1016/j.matchemphys.2022.127061.
- M. Arjama, S. Mehnath, M. Rajan and M. Jeyaraj, Engineered Hyaluronic Acid-Based Smart Nanoconjugates for Enhanced Intracellular Drug Delivery, J. Pharm. Sci., 2023, 112, 1603–1614, DOI:10.1016/j.xphs.2021.10.005.
- D. K. Jha, D. S. Shah, S. R. Talele and P. D. Amin, Correlation of two validated methods for the quantification of naringenin in its solid dispersion: HPLC and UV spectrophotometric methods, SN Appl. Sci., 2020, 2, 698, DOI:10.1007/s42452-020-2536-3.
- R. Bhandari, A. Kuhad, J. K. Paliwal and A. Kuhad, Development of a new, sensitive, and robust analytical and bio-analytical RP-HPLC method for in vitro and in vivo quantification of naringenin in polymeric nanocarriers, J. Anal. Sci. Technol., 2019, 10, 11, DOI:10.1186/s40543-019-0169-1.
- A. D. Sutar, R. K. Verma and R. Shukla, Quality by Design in Pulmonary Drug Delivery: A Review on Dry Powder Inhaler Development, Nanotherapy Approaches, and Regulatory Considerations, AAPS PharmSciTech, 2024, 25, 178, DOI:10.1208/s12249-024-02900-z.
- S. J. Nadaf and S. G. Killedar, Curcumin nanocochleates: Use of design of experiments, solid state characterization, in vitro apoptosis and cytotoxicity against breast cancer MCF-7 cells, J. Drug Delivery Sci. Technol., 2018, 47, 337–350, DOI:10.1016/j.jddst.2018.06.026.
- S. Hatem and M. El-Kayal, Novel anti-psoriatic nanostructured lipid carriers for the cutaneous delivery of luteolin: A comprehensive in vitro and in vivo evaluation, Eur. J. Pharm. Sci., 2023, 191, 106612, DOI:10.1016/j.ejps.2023.106612.
- M. Tilawat and S. Bonde, Curcumin and quercetin loaded nanocochleates gel formulation for localized application in breast cancer therapy, Heliyon, 2023, 9, e22892, DOI:10.1016/j.heliyon.2023.e22892.
- T. Shanmugam, N. Joshi, N. Ahamad, A. Deshmukh and R. Banerjee, Enhanced absorption, and efficacy of oral self-assembled paclitaxel nanocochleates in multi-drug resistant colon cancer, Int. J. Pharm., 2020, 586, 119482, DOI:10.1016/j.ijpharm.2020.119482.
- D. (Chau Thuy) Nguyen, J. Dowling, R. Ryan, P. McLoughlin and L. Fitzhenry, Controlled release of naringenin from soft hydrogel contact lens: An investigation into lens critical properties and in vitro release, Int. J. Pharm., 2022, 621, 121793, DOI:10.1016/j.ijpharm.2022.121793.
- M. G. El-Melegy, H. M. Eltaher, A. Gaballah and A. H. El-Kamel, Enhanced oral permeability of Trans-Resveratrol using nanocochleates for boosting anticancer efficacy; in vitro and ex vivo appraisal, Eur. J. Pharm. Biopharm., 2021, 168, 166–183, DOI:10.1016/j.ejpb.2021.08.020.
- Y. Tomar, S. Maheshwari, S. Gorantla and G. Singhvi, Curcumin loaded liquid crystalline nanoparticles for enhanced topical application: Design, characterization, ex vivo and dermatokinetic evaluation, J. Drug Delivery Sci. Technol., 2024, 92, 105391, DOI:10.1016/j.jddst.2024.105391.
- X. Yang, Y. Tang, M. Wang, Y. Wang, W. Wang and M. Pang, et al., Co-delivery of methotrexate and nicotinamide by cerosomes for topical psoriasis treatment with enhanced efficacy, Int. J. Pharm., 2021, 605, 120826, DOI:10.1016/j.ijpharm.2021.120826.
- A. G. Yurtsever, A. Ekmekcioglu, M. Muftuoglu, S. Güngör and M. S. Erdal, Formulation development and evaluation of fluvastatin loaded transethosomes: Characterization, stability, in vitro dermal penetration, cytotoxicity and antipsoriatic activity studies, J. Drug Delivery Sci. Technol., 2024, 91, 105234, DOI:10.1016/j.jddst.2023.105234.
- L. L. David, A. Daniels, S. Habib and M. Singh, Gold nanoparticles in transferrin-targeted dual-drug delivery in vitro, J. Drug Delivery Sci. Technol., 2023, 90, 105168, DOI:10.1016/j.jddst.2023.105168.
- F. R. Wibowo, O. A. Saputra, W. W. Lestari, M. Koketsu, R. R. Mukti and R. Martien, pH-Triggered Drug Release Controlled by Poly(Styrene Sulfonate) Growth Hollow Mesoporous Silica Nanoparticles, ACS Omega, 2020, 5, 4261–4269, DOI:10.1021/acsomega.9b04167.
- A. Bergal, M. Andac and F. Trotta, Cyclodextrin-based chemically modified pH-Responsive new kind of aldehyde-functionalized nanosponge nanoparticles for doxorubicin hydrochloride delivery, J. Drug Delivery Sci. Technol., 2025, 107, 106853, DOI:10.1016/j.jddst.2025.106853.
- Y. Zhang, M. Huo, J. Zhou, A. Zou, W. Li and C. Yao, et al., DDSolver: an add-in program for modeling and comparison of drug dissolution profiles, AAPS J., 2010, 12, 263–271, DOI:10.1208/s12248-010-9185-1.
- A. Kumar, B. Valamla, P. Thakor, P. S. Chary, N. Rajana and N. K. Mehra, Development and evaluation of nanocrystals loaded hydrogel for topical application, J. Drug Delivery Sci. Technol., 2022, 74, 103503, DOI:10.1016/j.jddst.2022.103503.
- M. Behera, P. Mahale, A. Gowtham, A. D. Sutar, R. K. Kaundal and R. Shukla, Quality by design based hydrogel formulation of 4-Octyl itaconate-loaded nanostructured lipid carriers for epidermal restoration in atopic dermatitis, J. Pharm. Invest., 2025, 1–22, DOI:10.1007/s40005-025-00748-4.
- V. Kokol, Y. B. Pottathara, M. Mihelčič and L. S. Perše, Rheological properties of gelatine hydrogels affected by flow- and horizontally-induced cooling rates during 3D cryo-printing, Colloids Surf., A, 2021, 616, 126356, DOI:10.1016/j.colsurfa.2021.126356.
- D. Mathure, A. D. Sutar, H. Ranpise, A. Pawar and R. Awasthi, Preparation and Optimization of Liposome Containing Thermosensitive In Situ Nasal Hydrogel System for Brain Delivery of Sumatriptan Succinate, Assay Drug Dev. Technol., 2023, 21, 3–16, DOI:10.1089/adt.2022.088.
- L. Angeli, K. Morozova and M. Scampicchio, A kinetic-based stopped-flow DPPH· method, Sci. Rep., 2023, 13, 7621, DOI:10.1038/s41598-023-34382-7.
- R. T. Polez, M. A. Ajiboye, M. Österberg and M. M. Horn, Chitosan hydrogels enriched with bioactive phloroglucinol for controlled drug diffusion and potential wound healing, Int. J. Biol. Macromol., 2024, 265, 130808, DOI:10.1016/j.ijbiomac.2024.130808.
- F. S. Alfehaid, A. B. Nair, H. Shah, B. Aldhubiab, J. Shah and V. Mewada, et al., Enhanced transdermal delivery of apremilast loaded ethosomes: Optimization, characterization and in vivo evaluation, J. Drug Delivery Sci. Technol., 2024, 91, 105211, DOI:10.1016/j.jddst.2023.105211.
- K. Van Bocxlaer, E. Gaukel, D. Hauser, S. H. Park, S. Schock and V. Yardley, et al., Topical Treatment for Cutaneous Leishmaniasis: Dermato-Pharmacokinetic Lead Optimization of Benzoxaboroles, Antimicrob. Agents Chemother., 2018, 62 DOI:10.1128/AAC.02419-17.
- A. Sharma, D. K. Upadhyay, G. S. Sarma, N. Kaur, G. D. Gupta and R. K. Narang, et al., Squalene integrated NLC based gel of tamoxifen citrate for efficient treatment of psoriasis: A preclinical investigation, J. Drug Delivery Sci. Technol., 2020, 56, 101568, DOI:10.1016/j.jddst.2020.101568.
- K. Saini, N. Modgill, K. Singh and V. Kakkar, Tetrahydrocurcumin Lipid Nanoparticle Based Gel Promotes Penetration into Deeper Skin Layers and Alleviates Atopic Dermatitis in 2,4-Dinitrochlorobenzene (DNCB) Mouse Model, Nanomaterials, 2022, 12, 636, DOI:10.3390/nano12040636.
- A. Qadir, M. Aqil, A. Ali, M. H. Warsi, M. Mujeeb and F. J. Ahmad, et al., Nanostructured lipidic carriers for dual drug delivery in the management of psoriasis: Systematic optimization, dermatokinetic and preclinical evaluation, J. Drug Delivery Sci. Technol., 2020, 57, 101775, DOI:10.1016/j.jddst.2020.101775.
- S. S. Alqarni, M. Afzal, F. A. Al-Abbasi, E. Moglad, A. S. Bawadood and N. A. R. Almalki, et al., Exploring acemannan-loaded nanogel formulation for the treatment of IMQ-induced psoriasis-like inflammation: In vitro characterization and in vivo efficacy assessment, Int. Immunopharmacol., 2025, 148, 114064, DOI:10.1016/j.intimp.2025.114064.
- J. Wu, S. Liu, H. Zhang, X. Zhang, J. Xue and Z. Li, et al., Amlexanox ameliorates imiquimod-induced psoriasis-like dermatitis by inhibiting Th17 cells and the NF-κB signal pathway, Biomed. Pharmacother., 2025, 184, 117922, DOI:10.1016/j.biopha.2025.117922.
- V. K. Singh, D. Sahoo, K. Agrahari, A. Khan, P. Mukhopadhyay and D. Chanda, et al., Anti-inflammatory, anti-proliferative and anti-psoriatic potential of apigenin in RAW 264.7 cells, HaCaT cells and psoriasis like dermatitis in BALB/c mice, Life Sci., 2023, 328, 121909, DOI:10.1016/j.lfs.2023.121909.
- S. Thakur, M. M. Anjum, S. Jaiswal, A. K. Gautam and P. S. Rajinikanth, Tazarotene-calcipotriol loaded Nanostructured lipid carrier enriched hydrogel: A novel dual drug synergistic approach towards Psoriasis management, J. Drug Delivery Sci. Technol., 2023, 88, 104944, DOI:10.1016/j.jddst.2023.104944.
- Y. Tian, S. Zhou, R. Takeda, K. Okazaki, M. Sekita and K. Sakamoto, Anti-inflammatory activities of amber extract in lipopolysaccharide-induced RAW 264.7 macrophages, Biomed. Pharmacother., 2021, 141, 111854, DOI:10.1016/j.biopha.2021.111854.
- A. Shariev, S. Menounos, A. J. Laos, P. Laxman, D. Lai and S. Hua, et al., Skin protective and regenerative effects of RM191A, a novel superoxide dismutase mimetic, Redox Biol., 2021, 38, 101790, DOI:10.1016/j.redox.2020.101790.
- C. Jiang, J. Zhang, H. Xie, H. Guan, R. Li and C. Chen, et al., Baicalein suppresses lipopolysaccharide-induced acute lung injury by regulating Drp1-dependent mitochondrial fission of macrophages, Biomed. Pharmacother., 2022, 145, 112408, DOI:10.1016/j.biopha.2021.112408.
- D. Ji, J. Yin, D. Li, C. Zhu, J. Ye and Y. Pan, Effects of inflammatory and anti-inflammatory environments on the macrophage mitochondrial function, Sci. Rep., 2020, 10, 20324, DOI:10.1038/s41598-020-77370-x.
- R. Joshi, Y. A. Kulkarni and S. Wairkar, Pharmacokinetic, pharmacodynamic and formulations aspects of Naringenin: An update, Life Sci., 2018, 215, 43–56, DOI:10.1016/j.lfs.2018.10.066.
- J. Xu, H. Chen, H. Qian, F. Wang and Y. Xu, Advances in the modulation of ROS and transdermal administration for anti-psoriatic nanotherapies, J. Nanobiotechnol., 2022, 20, 448, DOI:10.1186/s12951-022-01651-y.
- S. Han, H. Gao, S. Chen, Q. Wang, X. Li and L.-J. Du, et al., Procyanidin A1 Alleviates Inflammatory Response induced by LPS through NF-κB, MAPK, and Nrf2/HO-1 Pathways in RAW264.7 cells, Sci. Rep., 2019, 9, 15087, DOI:10.1038/s41598-019-51614-x.
Footnote |
| † Authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2026 |







