Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
High-precision deposition and controlled release of high molecular weight hyaluronic acid from contact lens surfaces using nanoelectrospray†
Rahul Tiwaria,
Matthew Alexanderb,
Julie Sandersona,
Robert A. Broadc,
Cheng-Chun Pengd,
Dharmendra Janid and
Sheng Qi *ae
*ae
aSchool of Chemistry, Pharmacy and Pharmacology, University of East Anglia, Norwich, Norfolk, NR4 7TJ, UK. E-mail: sheng.qi@liverpool.ac.uk
bSchool of Engineering, Mathematics, and Physics, University of East Anglia, Norwich, Norfolk, NR4 7TJ, UK
cCooperVision International Ltd (CVi), 36 School Lane, Chandlers Ford, Eastleigh, SO53 4LY, UK
dCooperVision Inc., 6101 Bollinger Canyon Rd, Suite 500, San Ramon, CA 94583, USA
eSchool of Pharmacy and Pharmaceutical Sciences, Institute of Systems Molecular & Integrative Biology, Faculty of Health & Life Sciences, University of Liverpool, Liverpool, L69 3GE, UK
First published on 29th December 2025
Abstract
Using additive manufacturing processes to selectively modify soft and wet polymer surfaces, such as soft contact lenses, with micrometer-level precision for applications, including controlled delivery of active ingredients, can be challenging. This study demonstrates the use of a novel nanoelectrospray (nES) process as a technical solution to deposit precise amounts of high molecular weight hyaluronic acid (HA), a highly water-soluble anionic glycosaminoglycan, onto predefined locations on the surface of soft contact lenses, and subsequently release it in a sustained manner. nES allows precise deposition of nano- to micrometer-thick layers outside the central optical zone. To achieve the sustained release of HA from the lens, a chemical modification of the polymer surface was developed to allow the lens surface to be covalently linked with a semi-interpenetrating network (IPN) layer containing entrapped HA after deposition by nES. Additional zein barrier layers applied by nES over the HA layer led to further reduction in the release rate of HA from the lenses. The results confirmed that the selective nES deposition allowed modification of the lens surface without affecting optical properties in the central vision zone of the soft contact lenses. The results suggested that the HA release kinetics can be strongly affected by multiple factors, including the degree of crosslinking, the molecular size of the crosslinker, the addition of a photoinitiator and the polymeric barrier layer. This study demonstrated the potential of nES as an alternative approach for surface modification and drug loading to commercially available contact lenses for treating ocular conditions.
Introduction
Hyaluronic acid (HA) is a naturally occurring linear polymer, a component of the vertebrate extracellular matrix, and has been found to have an array of physiological/pharmacological functions.1 It is a glycosaminoglycan with repeating units of D-glucuronic acid and N-acetylglucosamine disaccharides.2 HA is found throughout the tissues of the eye, including the tear film,2 where it acts as a natural lubricant for the eye and contributes to the stabilization of the tear film.3HA is a component of many commercially available eye drops that can be used in conjunction with contact lenses to provide soothing effects and improve comfort. Despite its mucoadhesive properties, the residence time of HA at the ocular surface is limited, with effects reported for up to 30 minutes after instillation4–6 via eye drops. Enhanced comfort therefore has a limited time span, and frequent application of HA-containing eye drops is needed. A contact lens that releases HA has therefore been proposed,7 which would be anticipated to improve the contact lens-wearing experience by enhancing surface water retention,8 reducing protein adsorption,9 and improving wearer comfort. Furthermore, HA has been shown to be effective in improving the signs and symptoms of dry eye disease.10 Therefore, contact lenses capable of providing sustained release of HA would offer significant benefits to wearers who experience dry eye symptoms. This study aims to achieve this by developing a novel type of contact lens with an HA coating.
Controlled drug release by soft contact lenses, also known as drug-eluting contact lenses (DECLs), is seen as a highly promising drug delivery method to the eye11,12 due to, for example, improved bioavailability caused by the extended residence time in the post-lens tear film and low dosing needed to achieve therapeutic effects. Various approaches to load contact lenses that deliver HA to the eye have been reported,13–15 including methods such as soaking,7 molecular imprinting,16 implant-laden contact lenses,17 physical entrapment of HA in the contact lens7,18,19 and semi-circular ring-implanted contact lenses.20,21 However, achieving sustained release kinetics of HA from contact lenses is highly challenging.8,18,22–26 This is mainly due to the extremely high aqueous solubility of HA, which leads to the rapid diffusion and release of the loaded HA from the bulk of the lens material to the external aqueous environment.
A new additive manufacturing method, nanoelectrospray (nES) additive coating, for precision deposition of drugs onto commercially available contact lenses was reported recently and was used in this study to develop controlled release of HA from contact lenses.27–29 Using a custom-built nES printing system, mask-free deposition on the lens surface can be carried out, enabling patterned coating of the peripheral region of the contact lens to ensure that the central optical area of the lens remains unaffected. The contact lenses with formulations deposited on their inner surface via nES exhibited optical transmittance above the acceptable threshold (>95%) at 600 nm. This confirms that nES deposition provides high spatial precision, enabling targeted coating while preserving the clarity of the optical zone.27–29
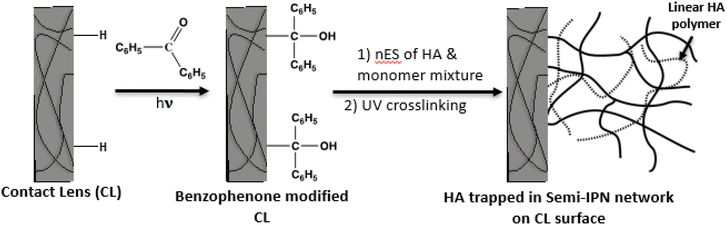
The present study explored a chemical modification approach, which enables permanent linking via chemical bonding between the surface and the grafted polymer to form stable systems.30–35 Chemically linking the lens surface to the nES-printed HA-loaded polymer aimed to avoid delamination and allow extended release of HA. Among chemical modifications, photo-induced grafting is known to be a useful technique for the modification of polymeric materials.1,36–38 Poly(2-hydroxyethyl methacrylate) (pHEMA)-based soft contact lenses were surface-modified with benzophenone before HA-acrylic monomer-based coatings were deposited on the lens. Photopolymerisation by UV light enabled the formation of a cross-linked semi-interpenetrating (semi-IPN) polymer network on the lens surface that traps the HA and provides sustained release of HA. A similar approach has been used, in which a modest 2% reduction in optical acuity was observed for HA-grafted pHEMA, yet transparency remained above >92%, indicating that HA grafting does not significantly impact the optical properties of pHEMA.39
Burst release is a common issue of drug-eluting contact lenses and is particularly challenging for water-soluble actives such as HA.15 While simple soaking methods result in a rapid initial release within hours, advanced techniques such as embedding HA-modified nanoparticles, micelles, or polymeric implants within the lens structure are necessary for true sustained release, with studies demonstrating controlled delivery for over 12 days to several weeks.16,40–43 In this study, we evaluated the application of nES for rapidly depositing a double-layer coating to slow down the diffusion and release of HA from the lens. UV-induced grafting was used due to its low cost, easy operation and mild reaction conditions.44–46 The biological effect of HA is molecular weight (Mw)-dependent.47–50 HA with a high Mw (average 2M Da) is reported to have greater anti-inflammatory activity as well as higher water binding capacity to provide better lubricating and soothing effects.51–53 It was therefore used in this study. In the double-layer coating, the base coat contained HA and the top coats chosen in this study, serving as the barrier layer, were zein and poly(lactic-co-glycolic acid) (PLGA). Both polymers have excellent biocompatibility and biodegradability, as well as effective film-forming properties, which make them commonly used in tablet and therapeutic product coatings.27,54,55 Zein is considered amphiphilic, but more specifically it is a hydrophobic protein with a high concentration of non-polar amino acids.51 It has been used previously for controlled drug delivery.52,53 PLGA is a hydrophobic biodegradable polymer that has been widely used for controlled and sustained drug delivery in long-acting injectables and implants, mostly in the form of microparticles.54,55 We hypothesize that, as a top coat over the HA formulations on contact lenses, its hydrophobic properties may prevent rapid water diffusion into the PLGA coat, thereby restricting the diffusion of HA polymers and resulting in sustained HA release.
Experimental
Materials
Sodium hyaluronate (average Mw of 2M Da) (HA) and Biomedics 38 soft contact lenses (poly(2-hydroxyethyl methacrylate)-based), with a center thickness range from approximately 0.03 to 1.00 mm, were supplied by CooperVision Inc. (Polymacon, San Ramon, USA; product no longer commercially available). The chemicals used to modify the lens surface were: 2-hydroxyethyl methacrylate (HEMA) (≥99%), 2-(diethylamino)ethyl methacrylate (DEAEMA) (99%), poly(ethylene glycol) diacrylate (PEGDA) (average Mw 575 and 700 Da), benzophenone (BP), 2,2-dimethoxy-2-phenylacetophenone (DMPA), and PLGA Resomer® RG 756 S (Mw 76–115 kDa, lactide![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glycolide 75
glycolide 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25). These chemicals and phosphate-buffered saline (PBS) tablets (pH 7.3) were purchased from Merck Life Science UK (Haverhill, UK). Purified zein was obtained from Thermo Scientific (Loughborough, UK). Ethanol, acetone, and sodium nitrate were purchased from Fisher Scientific (Loughborough, UK). Milli-Q (Merck Millipore, Watford, UK) ultrapure water was used for all aqueous solutions. The chemical structures of the monomers, crosslinker and photoinitiators used are shown in Fig. 1.
25). These chemicals and phosphate-buffered saline (PBS) tablets (pH 7.3) were purchased from Merck Life Science UK (Haverhill, UK). Purified zein was obtained from Thermo Scientific (Loughborough, UK). Ethanol, acetone, and sodium nitrate were purchased from Fisher Scientific (Loughborough, UK). Milli-Q (Merck Millipore, Watford, UK) ultrapure water was used for all aqueous solutions. The chemical structures of the monomers, crosslinker and photoinitiators used are shown in Fig. 1.
Chemical surface modification of contact lenses with HA trapped in a semi-IPN layer
The process involved two steps, as depicted in Fig. 2. In the first step, the contact lens was surface-modified with a Norrish-type photoinitiator, benzophenone (BP). The lens was rinsed with Milli-Q water before being transferred into a glass vial containing BP (10 mg mL−1) in acetone, and the solution with the lens was degassed with nitrogen for 10 minutes to remove oxygen. After degassing, the solution with the lens was placed under UV light (New Iradifire 48 W UV LED floodlight with a true 365 nm wide beam (100-degree beam angle), UV Gear, Surrey, UK) for 45–60 minutes. Although the light intensity was not measured, the height of the UV light source was fixed for all experiments to ensure no fluctuation of light intensity caused by height. The lens was then removed and washed with acetone and ethanol several times to remove excess unreacted BP and then washed with PBS media three times to remove the solvent from the lens. The BP-modified lenses were stored in a hydrated condition in the dark until further use.Nanoelectrospray (nES) coating of contact lenses
Fig. 3a shows a schematic of the custom-made experimental setup for the nES used in this study (PCE Automations, Beccles, UK). The system features a 2.5 mL Luer lock syringe, equipped with a metal nozzle with a 100 μm internal diameter (Nordson EFD, Dunstable, UK). This syringe is mounted on a motorized z-translation stage (EGSC-BS-KF-32-100-8P, Festo SE & Co. KG, Ostfildern, Germany) that allows for vertical movement. Pneumatic pressure, adjustable via an in-line pressure gauge, facilitates the nES printing of highly viscous liquids. A high-magnification digital camera (MVL6X12Z, Thorlabs Ltd, Ely, UK) monitors the spraying process. A custom-made contact lens holder, as described in previous studies,27–29 is mounted on a 2D motorized x–y translation stage (5155-1000A, Festo SE & Co. KG, Ostfildern, Germany), enabling simultaneous circular movements. All x, y, and z movements, as well as spraying parameters, are controlled via the machine's built-in digital control panel. | ||
| Fig. 3 nES setup was used for depositions on contact lenses. Schematic illustration (a) of the nES setup; (b) polymer layer printed at the periphery of contact lens via nES. | ||
Externally connected components include high-voltage power supplies (HCP 146500 model, FuG Elektronik GmbH, Schechen, Germany) linked to a high-voltage switch (PVX-4140 model, Direct Energy, Houston, TX, USA), which switches the voltage to pulsation mode. A function generator (TG 1000 model, Aim-TTi, Huntingdon, UK) controls the frequency and amplitude of the generated square waves. A current amplifier (DLPCA-200, Laser Components, Chelmsford, UK) amplifies the voltage and connects to a digital storage oscilloscope (TBS1104, Tektronix UK, Bracknell, UK) to monitor the waveform. The amplifier's measurement resistance is set to 106 Ω. A power supply capable of up to 6 kV provides a voltage range of 1.5–3.5 kV to maintain a stable cone-jet. The contact lens is positioned on a motorized xy-translational stage, 3 mm beneath the cone-jet, to enable circular movement. The number of rotations was 90, with a rotation speed of 10 mm s−1, for all the coating formulations, including the top and base coats of the double-layer-coated lenses. A 100 µm nozzle was used for spraying, with a nozzle-to-substrate distance (NSD) of 3 mm. A voltage range of 1.5–3.5 kV was applied to produce a stable cone-jet for all formulations. Fig. 3b shows a schematic illustration of the printed polymer layer (grey colour) at the periphery of the contact lens, with no coating in the vision zone of the lens for both single-layer and double-layer deposition. For lenses coated with UV-curing treatment, the lenses were washed with Milli-Q water after nES to remove excess unreacted material.
In the second step of the chemical surface modification of contact lenses, monomers HEMA (for hydrogen bonding with HA) and DEAEMA (cationically charged for electrostatic interaction with HA), crosslinkers (PEGDA700 and PEGDA575), an additional surface-curing photoinitiator DMPA (to induce the polymerisation process and reduce the polymerisation time), and HA (Mw, 2M Da, 2 mg ml−1, dissolved in water) were mixed in different ratios and deposited on the BP-modified contact lens via nES, which was kept hydrated after step 1 described earlier. The HA loadings of the lenses were assessed prior to UV light exposure. After nES, the coated contact lens was exposed to UV light for 15–45 minutes to create the semi-IPN with trapped HA.
For the double-layer-coated lenses, the lenses were coated with a base layer as described above. The single-layer-coated lenses were returned to a Petri dish containing water to allow the lenses to relax for 2 hours to their original shape prior to top-layer coating. The PLGA (20 mg ml−1 in acetone) and zein (25 mg ml−1 in ethanol/H2O (70/30) mixture) solutions used for coating were prepared for double-layer-coated lens, as described in previous studies.27,28
Attenuated total reflection-Fourier transform infrared (ATR-FTIR) spectroscopy
An FTIR spectrophotometer (VERTEX 70, Bruker Optics, Ettlingen, Germany), equipped with a Golden Gate, ATR accessory (Specac Ltd, Orpington, UK) and a diamond internal reflection element, was used to examine the contact lens before and after modification. The spectra were collected over a wavenumber range of 500–4000 cm−1, with a resolution of 4 cm−1 and 64 scans at room temperature.In vitro HA release from HA-loaded contact lenses
HA was assayed using a high-performance liquid chromatography (HPLC) 200 series system (PerkinElmer, MA, USA) with an RI detector set at 35 °C and a Waters HSPgel AQ MB-H, 6.0 × 150 mm, Part# 186001790 column (Waters, MA, USA). 0.2 M sodium nitrate dissolved in Milli-Q water was used as the mobile phase. The column temperature was 65 °C, and all methods operated at a flow rate of 0.5 mL min−1. The sample volume was 100 μL, and the measurement time was 10 minutes. The HA calibration assay was carried out using HA-2M in the range of 3.05–54.13 µg mL−1 in PBS (pH 7.3). The retention time for HA (Mw 2M Da) was 3.8 min, and the area under the peak was used to plot the concentration calibration of HA.The total amount of HA deposited on nES-coated lenses was quantified using HPLC. Each HA-loaded lens was subjected to ultrasonication for 2 minutes in a 6 mL glass vial containing 2 mL of phosphate-buffered saline (PBS, pH 7.3). The vials were sonicated for 2 minutes and then incubated in a shaking incubator for 24 hours to ensure complete delamination and dispersion of the HA polymer from the lens surface into the PBS medium. Triplicate lenses were used to determine the average HA loading on the lens.
The in vitro HA release tests from HA-loaded lenses were performed in 6 mL glass vials containing 2 mL of PBS and placed in a shaking incubator (125 rpm at 35 °C; IKA, Staufen, Germany). At each sampling time point, 500 μL of the releasing medium sample was extracted and replaced with fresh PBS pH 7.3 to maintain the perfect sink conditions throughout the release experiment. All samples were filtered through a 0.45 µm filter (to remove any large fragments delaminated from the lens) prior to HPLC analysis, and the HA release profile was plotted as the cumulative HA release (%) over time. The HA release experiments were carried out in triplicate for each sample. Statistical analysis of the drug release data at each time point was performed using one-way ANOVA followed by Tukey's post-hoc test, with p ≤ 0.05 considered statistically significant.
Cryogenic-scanning electron microscopy (Cryo-SEM)
Cryo-SEM studies were used to examine the surface of nES-printed polymer coats on different substrates using a Gemini 300 series microscope (Zeiss, Ostfildern, Germany), operating at 2–10 kV acceleration voltage. To perform cryo-SEM on contact lenses, the coated hydrated lens was cut into quarters using a scalpel. All the cryo-SEM samples were sublimated for 2 minutes and sputtered with platinum for 60 s at 10 mA.Viscosity and electrical conductivity measurements
As the viscosity and electrical conductivity of the formulation used for top and base coats can significantly affect nES performance, both properties were measured for all formulations used in this study. The viscosity of each formulation used for nES was measured on a Discovery Hybrid Rheometer (TA Instruments, Delaware, USA) using a 40 mm steel cone plate across an increasing range of shear (0.01–200 s−1) at 25 °C. Data were analysed using TRIOS software (TA Instruments, Delaware, USA). The Carreau model was used to calculate the zero-shear viscosity values of the formulations. Electrical conductivity measurements of all nES formulations were performed using a Jenway 4510 conductivity meter (Jenway Ltd, Stone, UK) with a microvolume conductivity probe. All measurements were performed at room temperature of 22 ± 2 °C.Results and discussion
Loading HA on benzophenone (BP)-modified contact lens using nES
The soft contact lenses were modified with the photoinitiator BP under UV light to create an active site for photopolymerisation. Fig. 4a illustrates the appearance of a contact lens after modification with BP, which remained fully transparent. In this study, it is assumed that the chemical modification and nES coating did not introduce significant changes to the thickness, mechanical properties, or wearability of the contact lenses. These parameters will be systematically evaluated in follow-up experiments beyond the scope of the present study. ATR-FTIR spectra of the modified lens shows a new carbonyl peak at 1650 cm−1 and two benzene peaks (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretches) at 1595 and 1577 cm−1 (Fig. 4b), indicating the successful modification of photoinitiator on the lens surface.38,56,57
C stretches) at 1595 and 1577 cm−1 (Fig. 4b), indicating the successful modification of photoinitiator on the lens surface.38,56,57
The HA-acrylic monomer-based mixture containing monomers HEMA, cationically charged DEAEMA, crosslinkers PEGDA700 and/or PEGDA575 with HA was successfully deposited on the BP-modified lens via nES at the periphery of the contact lens surface. Through UV exposure, the goal was to create a semi-IPN with HA entrapped in the network, thus slowing down the diffusion and release of HA from the lens.
A range of HA-acrylic monomer-based mixture formulations for nES coating were tested. The detailed the amounts of monomers (HEMA and DEAEMA or a mixture of both) and crosslinker (PEGDA700) of each formulation are shown in Table 1. The zero-shear viscosity and conductivity of all formulations were measured and were in the range of 50–80 cP and 30–55 µS cm−1, respectively. All coating formulations contained HA, with HA-2M lenses without monomer or crosslinkers serving as a control (no UV curing was performed on the control lenses). The control lenses and the lenses with a code containing ‘UV’ were all BP-modified lenses. The HA loadings of the lenses were assessed prior to UV curing. The loading values ranged from 11.55 to 14.84 µg per lens, as shown in Table 1. No significant difference in HA loading was observed between the control lenses and those containing the monomer/crosslinker (UV-01 to UV-05).
| Lens code | HAa (mL) | PEGDA700 crosslinker (mL) | PEGDA575 crosslinker (mL) | HEMA monomer (mL) | DEAEMA monomer (mL) | Amount of HA deposited on CL (µg) |
|---|---|---|---|---|---|---|
| a HA (Mw of 2M Da, 1 mL, 2 mg mL−1 concentration) further diluted with 1 mL water to make the final concentration of 1 mg mL−1 for spraying; HA quantities deposited are presented as mean ± SD; n = 3. | ||||||
| HA-2Ma | 1.0 | — | — | — | — | 14.84 ± 1.17 |
| UV-01 | 1.0 | 0.7 | — | 0.3 | — | 12.01 ± 2.60 |
| UV-02 | 1.0 | 0.9 | — | 0.1 | — | 12.85 ± 3.37 |
| UV-03 | 1.0 | 0.9 | — | 0.05 | 0.05 | 11.55 ± 2.16 |
| UV-04 | 1.0 | 0.9 | — | — | 0.1 | 14.73 ± 2.35 |
| UV-05 | 1.0 | — | 0.9 | 0.1 | — | 12.08 ± 1.40 |
In vitro HA release from HA-loaded contact lenses
Release profiles of HA from lenses coated with formulations UV-01 to UV-04 (Table 1) are shown in Fig. 5a. The control lenses coated with HA without any chemical modification showed that over 95% of the HA was released within the first 60 minutes (Fig. 5a). UV-01 showed a higher level of initial burst release compared to the other three formulations on the chemically modified lenses (UV-02, UV-03 and UV-04).The diffusion of macromolecules such as HA through a crosslinked polymer network is mainly affected by the size and configuration of the molecule and the crosslinked network, respectively. The surrounding crosslinked polymer network acts as a steric barrier for HA to diffuse. A larger mesh size of the polymer network results in faster diffusion. In addition, HA, being a long-chain molecule, diffuses differently compared to simple particles. It behaves like a sequence of interconnected particles, with each segment of the chain needing to follow the one in front of it. This chain-like structure restricts movement, leading to what is known as the reptation model of diffusion.16,58,59 These factors may have contributed to the reduced release rate observed for HA in the chemically modified lenses.
Two different monomers, two types of crosslinkers and two crosslinker-to-monomer ratios were used in these formulations. As seen in Fig. 5a, the order of the HA release rate is UV-01 > UV-04 > UV-02 ≈ UV-03 during the first 4 hours of the tests. UV-01, as expected, had the fastest HA release among the tested formulations since it has the lowest crosslinker to monomer ratio (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) among all other formulations in Table 1, leading to a less crosslinked polymer network with, therefore, a larger mesh size. UV-02 and UV-04 have the same crosslinker-to-monomer ratio (9
3) among all other formulations in Table 1, leading to a less crosslinked polymer network with, therefore, a larger mesh size. UV-02 and UV-04 have the same crosslinker-to-monomer ratio (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) but different monomers. With a tertiary amine group, DEAEMA (UV-04) is positively charged, which introduces ionic character to the polymer network and provides electrostatic interaction with the negatively charged carboxyl groups of HA. Although electrostatic interaction is stronger than the hydrogen bonding occurring between HEMA (UV-02) and HA, the difference seen in the HA release rate is not significant. UV-03 used a high crosslinker-to-monomer ratio and HEMA-DEAEMA mixed monomers. It showed a similar HA release rate to UV-04 and UV-02. The overall HA release for these formulations is still relatively fast, reaching 80% release within 14 hours for UV-02, UV-03 and UV-04, with a notable burst release in the first 4 hours.
1) but different monomers. With a tertiary amine group, DEAEMA (UV-04) is positively charged, which introduces ionic character to the polymer network and provides electrostatic interaction with the negatively charged carboxyl groups of HA. Although electrostatic interaction is stronger than the hydrogen bonding occurring between HEMA (UV-02) and HA, the difference seen in the HA release rate is not significant. UV-03 used a high crosslinker-to-monomer ratio and HEMA-DEAEMA mixed monomers. It showed a similar HA release rate to UV-04 and UV-02. The overall HA release for these formulations is still relatively fast, reaching 80% release within 14 hours for UV-02, UV-03 and UV-04, with a notable burst release in the first 4 hours.
To further reduce the HA release rate from the lenses, a lower molecular weight crosslinker, PEGDA575, was used (UV-05). This was expected to produce a less porous polymer IPN structure on the lens surface.60,61 The PEGDA575 crosslinker resulted in slower HA release compared to PEGDA700 (Fig. 5b). UV-05 exhibited an initial burst release of 55% HA within the first 30 minutes, which is likely dominated by HA on the surface of the polymer matrix that is not fully entrapped within the bulk of the IPN. However, after the first 30 minutes, the release rate of HA from UV-05-treated lenses was notably slower than that from lenses treated with the PEGDA700-based crosslinker, indicating that the shorter-chain PEGDA575 crosslinker created a less porous IPN, which slowed down the diffusion and release of HA from the polymer matrix.
To accelerate the polymerisation process for nES HA coating, the photoinitiator DMPA (50 mg mL−1) was incorporated with the monomers (HEMA and DEAEMA) and the crosslinker (PEGDA575) (Table 2). DMPA was selected due to its fast reaction rate and efficiency, even at low concentrations.62,63 The addition of the DMPA initiator further shortened the needed polymerisation time under UV light from 45 minutes to 10 minutes for UV-06 and UV-08. In contrast, UV-07, which did not include DMPA, required 45 minutes to achieve a crosslinked polymer network on the lens surface.
| Lens code | HAa (mL) | PEGDA575 (mL) | HEMA (mL) | DEAEMA (mL) | DMPAa (µL) | UV time (mins) | Amount of HA deposited on CL (µg) |
|---|---|---|---|---|---|---|---|
| a HA (Mw of 2M Da) has a concentration of 2 mg mL−1, and DMPA has a concentration of 50 mg mL−1; HA quantities deposited are presented as mean ± SD; n = 3. | |||||||
| UV-06 | 1.5 | 0.9 | 0.05 | 0.05 | 20 | 10 | 22.98 ± 3.88 |
| UV-07 | 1.5 | 0.9 | 0.1 | — | — | 45 | 18.80 ± 2.25 |
| UV-08 | 1.5 | 0.9 | 0.1 | — | 50 | 10 | 19.13 ± 1.55 |
The HA release profile showed a significantly slower initial release in UV-06 and UV-08 than in UV-07 (Fig. 6). In UV-07, BP on the lens surface, through the modification process prior to nES, is the only available initiator for the reaction at the contact lens/HA coat interface, leading to a longer polymerisation time and a crosslinked grafted polymer network.64,65 In contrast, DMPA, through its radical formation and rapid curing process, allowed faster photopolymerisation of the sprayed HA ink from the surface to the bottom of the coating in UV-06 and UV-08. Taking into consideration the effects on sustaining the HA release and reducing the curing time, including DMPA in the formulation was carried forward for further formulation optimisation.
 | ||
| Fig. 6 Cumulative HA release profiles of the lenses with printed semi-IPN polymer rings with the additional initiator DMPA in the ink formulations (data are presented as mean ± SD; n = 3). | ||
To further reduce the HA release rate, a barrier coating of either PLGA or zein was added to the semi-IPN HA layer, creating a bilayer coating (Table 3). These formulations have an HA-2M semi-IPN base coat (UV-09 with no topcoat), and either zein (UV-10) or PLGA (UV-11) as the top coat.
| Top coat (for double-layer coated lenses) | ||||
|---|---|---|---|---|
| Lens code | Polymer used | H2O (%) | Ethanol (%) | Acetone (%) |
| a HA (Mw 2M Da) has a concentration of 2 mg mL−1, and DMPA has a concentration of 50 mg mL−1; HA quantity deposited is presented as mean ± SD; n = 3. | ||||
| UV-10 | Zein (25 mg mL−1) | 30 | 70 | 0 |
| UV-11 | PLGA (20 mg mL−1) | 0 | 0 | 100 |
Both HEMA and DEAEMA monomers were included with the cross-linker PEGDA575 in the base coat of UV-10 and UV-11. The photoinitiator (DMPA) was included, and a 10-minute exposure to UV light was used to facilitate photopolymerisation. For UV-09, the HA-monomer formulation was applied to the lens and cured under UV light for 10 minutes. For UV-10 and UV-11, after the 10-minute curing of the base coats, a top coat of zein or PLGA was deposited on top to act as a secondary barrier to HA release. Cryo-SEM images revealed the morphology of different locations on the contact lens surface with a bilayer nES coating. Fig. 7a shows the top coat at the peripheral region of the contact lens, as illustrated in Fig. 7b, while the central region (optical zone diameter: ∼8 mm) remained uncoated (Fig. 7c).
The release of HA was evaluated from the double-layer coated lenses (UV-10 and UV-11) and compared to the results of single-layer coated lenses (UV-09) (Fig. 8). The single-layer coated lenses (UV-09) exhibited a rapid initial burst of HA, with over 50% released within the first hour and over 80% within 5 hours. The double-layer coated UV-11 lenses with a PLGA top coat showed no statistically significant difference in the cumulative release and release rate of HA compared to UV-09. This may be attributed to the higher hydrophobicity of PLGA, which led to some level of delamination of the coating during the HA release experiment.
The double-layer coated lenses with zein as the top coat (UV-10) showed a significant reduction of the total HA release to 35% within 24 hours (Fig. 8a) in comparison with UV-09 and UV-11. HA in the base coat can have dual-directional diffusion, leading to release from both the front of the lens and across the zein top coat at the back of the lens, as illustrated in Fig. 8b. There was almost no release within the first 30 minutes, which could be attributed to the lag time for the HA to diffuse across the zein layer as well as the full thickness of the lens. This was followed by an 18% release in 1 hour and an additional 17% release between 1 and 6 hours. Between 6 and 24 hours, the HA release rate significantly reduced, resulting in 35% cumulative release by 24 hours. The reason for the incomplete HA release within 24 hours could be attributed to the interactions between HA and zein within the hydrated zein network. It has been reported that, in the hydrated or film form of zein, it can form a cross-linked or semi-interconnected network.66–69 While HA is diffusing through the hydrated zein network, it could interact with zein through electrostatic attraction, hydrogen bonding between NH2 of HA and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of glutamine in zein, and hydrophobic interactions.66 These interactions could retain HA within the zein layer, leading to the slow and incomplete release observed.
O of glutamine in zein, and hydrophobic interactions.66 These interactions could retain HA within the zein layer, leading to the slow and incomplete release observed.
There are two main limitations of nES as a surface deposition method. In the context of contact lens coating, while nES can successfully deliver active ingredients to the surface of both high-water content hydrogel lenses and silicone hydrogel lenses, these compounds are prone to rapid bidirectional diffusion through the lens material, as illustrated in Fig. 8b. This presents a particular challenge for highly hydrophilic molecules such as HA, which may require chemical modification or advanced formulation strategies to achieve sustained release. More broadly, nES delivers relatively small volumes of formulation per application. Although this is generally sufficient for ocular therapeutics, where drug doses are typically low, it may limit the applicability of nES for other medical devices with small surface areas that require high drug loading.
Conclusions
This study used high molecular weight HA (2M), which is an ocular surface comfort agent used in eye drops and has been clinically used for treating dry eye syndrome, to demonstrate the capability of nES for material deposition on contact lens surfaces with high precision. An IPN approach was developed to ‘dock’ HA at the peripheral section of the contact lens surface. The selection of the monomer type (for hydrogen bonding and electrostatic interaction) with different lengths of the crosslinker (Mw of 700 and 575) and the additional rapidly cured photoinitiator impacted on the density of the IPN formed and the speed of the curing process. The different mesh-sized IPNs slowed down HA release from the lens surface after an initial burst release. The deposition of an additional zein or PLGA coat to act as a release barrier on top of HA trapped in the IPN base layer led to further sustained HA release beyond 24 hours from the zein top-coated lenses. nES can be employed to selectively coat the peripheral regions of soft contact lenses, enabling sustained release of HA from the lens. The results of this study demonstrate the potential of nES as a versatile technique for precise surface deposition of active ingredients, with broader applicability across a range of medical devices.Author contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Data availability
Data are available upon request.Acknowledgements
The authors thank CooperVision Inc. for funding the project and for providing hyaluronic acid and soft contact lenses for this study.References
- K. T. Dicker, L. A. Gurski, S. Pradhan-Bhatt, R. L. Witt, M. C. Farach-Carson and X. Jia, Hyaluronan: a simple polysaccharide with diverse biological functions, Acta Biomater., 2014, 10, 1558–1570 CrossRef CAS PubMed.
- C. A. Scheuer, M. J. Rah and W. T. Reindel, Increased concentration of hyaluronan in tears after soaking contact lenses in Biotrue multipurpose solution, Clin Ophthalmol., 2016, 10, 1945–1952 Search PubMed.
- L. S. Mengher, K. S. Pandher, K. S. Pandher, A. J. Bron, A. J. Bron, C. C. Davey and C. C. Davey, Effect of sodium hyaluronate (0.1%) on break-up time (NIBUT) in patients with dry eyes, Br. J. Ophthalmol., 1986, 70, 442–447 Search PubMed.
- R. Gurny, J. E. Ryser, C. Tabatabay, M. Martenet, P. Edman and O. Camber, Precorneal residence time in humans of sodium hyaluronate as measured by gamma scintigraphy, Graefe's Arch. Clin. Exp. Ophthalmol., 1990, 228, 510–512 CrossRef CAS.
- S. Garcia-Lázaro, D. Madrid-Costa, T. Ferrer-Blasco, R. Montés-Micó and A. Cerviño, OCT for assessing artificial tears effectiveness in contact lens wearers, Optom Vis Sci, 2012, 89, E62–E69 CrossRef.
- S. Kaya, D. Schmidl, L. Schmetterer, K. J. Witkowska, A. Unterhuber, V. Aranha Dos Santos, C. Baar, G. Garhöfer and R. M. Werkmeister, Effect of hyaluronic acid on tear film thickness as assessed with ultra-high resolution optical coherence tomography, Acta Ophthalmol., 2015, 93, 439–443 CrossRef CAS.
- F. A. Maulvi, T. G. Soni and D. O. Shah, Extended release of hyaluronic acid from hydrogel contact lenses for dry eye syndrome, J. Biomater. Sci., Polym. Ed., 2015, 26, 1034–1050 CrossRef PubMed.
- A. Singh, P. Li, V. Beachley, P. McDonnell and J. H. Elisseeff, A hyaluronic acid-binding contact lens with enhanced water retention, Cont Lens Anterior Eye., 2015, 38, 79–84 CrossRef.
- A. Weeks, A. Boone, D. Luensmann, L. Jones and H. Sheardown, The effects of hyaluronic acid incorporated as a wetting agent on lysozyme denaturation in model contact lens materials, J. Biomater. Appl., 2012, 28, 323–333 CrossRef.
- L. A.-O. X. Hynnekleiv, M. A.-O. Magno, R. R. Vernhardsdottir, E. Moschowits, K. A. Tønseth, D. A. Dartt, J. A.-O. Vehof and T. P. Utheim, Hyaluronic acid in the treatment of dry eye disease, Acta Ophthalmol., 2022, 100, 844–860 CrossRef CAS.
- C. C. Li and A. Chauhan, Ocular transport model for ophthalmic delivery of timolol through p-HEMA contact lenses, J. Drug Delivery Sci. Technol., 2007, 17, 69–79 CrossRef CAS.
- A. Soluri, A. Hui and L. Jones, Delivery of Ketotifen Fumarate by Commercial Contact Lens Materials, Optom. Vis. Sci., 2012, 89, 1140–1149 CrossRef.
- T. Y. Kim, G.-H. Lee, J. Mun, S. Cheong, I. Choi, H. Kim and S. K. Hahn, Smart contact lens systems for ocular drug delivery and therapy, Adv. Drug Delivery Rev., 2023, 196, 114817 CrossRef CAS PubMed.
- J. Xu, Y. Xue, G. Hu, T. Lin, J. Gou, T. Yin, H. He, Y. Zhang and X. Tang, A comprehensive review on contact lens for ophthalmic drug delivery, J. Controlled Release, 2018, 281, 97–118 Search PubMed.
- O. L. Lanier, K. G. Christopher, R. M. Macoon, Y. Yifan, S. Poorvajan and A. Chauhan, Commercialization challenges for drug eluting contact lenses, Expert Opin. Drug Delivery, 2020, 17, 1133–1149 CrossRef PubMed.
- M. Ali and M. E. Byrne, Controlled release of high molecular weight hyaluronic Acid from molecularly imprinted hydrogel contact lenses, Pharm Res., 2009, 26, 714–726 CrossRef CAS.
- A. R. Desai, F. A. Maulvi, D. M. Desai, M. R. Shukla, K. M. Ranch, B. A. Vyas, S. A. Shah, S. Sandeman and D. O. Shah, Multiple drug delivery from the drug-implants-laden silicone contact lens: Addressing the issue of burst drug release, Mater. Sci. Eng., C, 2020, 112, 110885 CrossRef CAS PubMed.
- A. Weeks, L. N. Subbaraman, L. Jones and H. Sheardown, Physical entrapment of hyaluronic acid during synthesis results in extended release from model hydrogel and silicone hydrogel contact lens materials, Eye Contact Lens., 2013, 39, 179–185 CrossRef.
- C. Huang, X. A.-O. Zhang, Y. Li and X. Yang, Hyaluronic acid and graphene oxide loaded silicon contact lens for corneal epithelial healing, J. Biomater. Sci., Polym. Ed., 2021, 32, 372–384 CrossRef CAS.
- A. R. Desai, F. A. Maulvi, M. M. Pandya, K. M. Ranch, B. A. Vyas, S. A. Shah and D. O. Shah, Co-delivery of timolol and hyaluronic acid from semi-circular ring-implanted contact lenses for the treatment of glaucoma: in vitro and in vivo evaluation, Biomater. Sci., 2018, 6, 1580–1591 RSC.
- A. R. Desai, F. A. Maulvi, D. M. Desai, M. R. Shukla, K. M. Ranch, B. A. Vyas, S. A. Shah, S. Sandeman and D. O. Shah, Multiple drug delivery from the drug-implants-laden silicone contact lens: Addressing the issue of burst drug release, Mater. Sci. Eng., C, 2020, 112, 110885 CrossRef CAS PubMed.
- F. A. Maulvi, T. G. Soni and D. O. Shah, Extended release of hyaluronic acid from hydrogel contact lenses for dry eye syndrome, J. Biomater. Sci., Polym. Ed., 2015, 26, 1035–1050 CrossRef CAS.
- C. Huang, X. Zhang, Y. Li and X. Yang, Hyaluronic acid and graphene oxide loaded silicon contact lens for corneal epithelial healing, J. Biomater. Sci., Polym. Ed., 2021, 32, 372–384 CrossRef CAS PubMed.
- J. G. Alauzun, S. Young, R. D’Souza, L. Liu, M. A. Brook and H. D. Sheardown, Biocompatible, hyaluronic acid modified silicone elastomers, Biomaterials, 2010, 31, 3471–3478 CrossRef CAS PubMed.
- M. George, K. Khong and I. Maltseva, Hyaluronic Acid (HA) Release of HA-Containing Lens Care Solutions with Silicone Hydrogel Lenses, Cont. Lens Anterior Eye, 2019, 42, e17–e18 CrossRef.
- F. A. Maulvi, S. S. Singhania, A. R. Desai, M. R. Shukla, A. S. Tannk, K. M. Ranch, B. A. Vyas and D. O. Shah, Contact lenses with dual drug delivery for the treatment of bacterial conjunctivitis, Int. J. Pharm., 2018, 548, 139–150 CrossRef CAS.
- C. H. Tam, M. S. Alexander, J. Sanderson and S. Qi, Selectively coated contact lenses by nanoelectrospray (nES) to fabricate drug-eluting contact lenses for treating ocular diseases, Med. Eng. Phys., 2024, 124, 104110 CrossRef.
- C. H. Tam, M. S. Alexander and S. Qi, Precision coating of ocular devices/contact lenses by nanoelectrospray additive printing, Mater. Des., 2022, 219, 110782 Search PubMed.
- G. Bebawy, J. Sanderson and S. Qi, Nanoelectrospray fabrication of pH-responsive double-layered drug-eluting contact lenses for ocular drug delivery, Int. J. Pharm., 2025, 686, 126323 CrossRef CAS.
- W.-L. Chen, R. Cordero, H. Tran and C. K. Ober, 50th Anniversary Perspective: Polymer Brushes: Novel Surfaces for Future Materials, Macromolecules, 2017, 50, 4089–4113 CrossRef CAS.
- K. L. Mittal and K.-W. Lee, Polymer surfaces and interfaces: characterization, modification and application, Vsp, 1997 Search PubMed.
- E. Ruckenstein and Z. Li, Surface modification and functionalization through the self-assembled monolayer and graft polymerization, Adv. Colloid Interface Sci., 2005, 113, 43–63 CrossRef CAS PubMed.
- K. J. Kim, A. G. Fane and C. J. D. Fell, The performance of ultrafiltration membranes pretreated by polymers, Desalination, 1988, 70, 229–249 CrossRef CAS.
- A.-S. Jönsson and B. Jönsson, The influence of nonionic and ionic surfactants on hydrophobic and hydrophilic ultrafiltration membranes, J. Membr. Sci., 1991, 56, 49–76 CrossRef.
- C. M. Chan, T. M. Ko and H. Hiraoka, Polymer surface modification by plasmas and photons, Surf. Sci. Rep., 1996, 24, 1–54 CrossRef CAS.
- M. Sangermano and N. Razza, Light induced grafting-from strategies as powerful tool for surface modification, eXPRESS Polym. Lett., 2019, 13, 135–145 CrossRef CAS.
- S. Luan, J. Zhao, H. Yang, H. Shi, J. Jin, X. Li, J. Liu, J. Wang, J. Yin and P. Stagnaro, Surface modification of poly(styrene-b-(ethylene-co-butylene)-b-styrene) elastomer via UV-induced graft polymerization of N-vinyl pyrrolidone, Colloids Surf., B, 2012, 93, 127–134 CrossRef CAS PubMed.
- N. De Smet, M. Rymarczyk-Machal and E. Schacht, Modification of polydimethylsiloxane surfaces using benzophenone, J. Biomater. Sci., Polym. Ed., 2009, 20, 2039–2053 CrossRef CAS PubMed.
- M. Korogiannaki, J. Zhang and H. Sheardown, Surface modification of model hydrogel contact lenses with hyaluronic acid via thiol-ene “click” chemistry for enhancing surface characteristics, J. Biomater. Appl., 2017, 32, 446–462 CrossRef CAS PubMed.
- M. Van Beek, L. Jones and H. Sheardown, Hyaluronic Acid Containing Hydrogels for the Reduction of Protein Adsorption, Biomaterials, 2008, 29, 780 CrossRef CAS PubMed.
- M. Van Beek, A. Weeks, L. Jones and H. Sheardown, Immobilized Hyaluronic Acid Containing Model Silicone Hydrogels Reduce Protein Adsorption, J. Biomater. Sci., Polym. Ed., 2008, 19, 1425 CrossRef CAS.
- L. Jones, A. Mann, K. Evans, V. Franklin and B. Tighe, An in Vivo Comparison of the Kinetics of Protein and Lipid Deposition on Group II and Group IV Frequent-Replacement Contact Lenses, Optom. Vis. Sci., 2000, 77, 503–510 CrossRef CAS.
- J. Mun, J. w. Mok, S. Jeong, S. Cho, C.-K. Joo and S. K. Hahn, Drug-eluting contact lens containing cyclosporine-loaded cholesterol-hyaluronate micelles for dry eye syndrome, RSC Adv., 2019, 9, 16578–16585 RSC.
- S. Hans, C. Andre, C. Jean-Francois, G. Hui, L. Reto, S. Michael and S. Gajendran, Surface immobilization of biomolecules by light, Opt. Eng., 1995, 34, 2339–2348 Search PubMed.
- A. Collioud, J. F. Clemence, M. Saenger and H. Sigrist, Oriented and covalent immobilization of target molecules to solid supports: Synthesis and application of a light-activatable and thiol-reactive cross-linking reagent, Bioconjugate Chem., 1993, 4, 528–536 Search PubMed.
- A. Ulman, Formation and Structure of Self-Assembled Monolayers, Chem. Rev., 1996, 96, 1533–1554 Search PubMed.
- A. G. Tavianatou, I. Caon, M. Franchi, Z. Piperigkou, D. Galesso and N. K. Karamanos, Hyaluronan: molecular size-dependent signaling and biological functions in inflammation and cancer, FEBS J., 2019, 286, 2883–2908 Search PubMed.
- L. Hu, S. Nomura, Y. Sato, K. Takagi, T. Ishii, Y. Honma, K. Watanabe, Y. Mizukami and J. Muto, Anti-inflammatory effects of differential molecular weight Hyaluronic acids on UVB-induced calprotectin-mediated keratinocyte inflammation, J. Dermatol. Sci., 2022, 107, 24–31 CrossRef CAS.
- J. B. Lee, S. Chu, H. B. Ben Amara, H. Y. Song, M. J. Son, J. Lee, H. Y. Kim, K. A.-O. Koo and I. A.-O. Rhyu, Effects of hyaluronic acid and deproteinized bovine bone mineral with 10% collagen for ridge preservation in compromised extraction sockets, J. Periodontol., 2021, 92, 1564–1575 Search PubMed.
- M. Litwiniuk, A. Krejner, M. S. Speyrer, A. R. Gauto and T. Grzela, Hyaluronic Acid in Inflammation and Tissue Regeneration, Wounds., 2016, 28, 78–88 Search PubMed.
- W. A.-O. X. Müller-Lierheim, Why Chain Length of Hyaluronan in Eye Drops Matters, Diagnostics (Basel)., 2020, 10, 511 CrossRef.
- T. C. Laurent, U. B. Laurent and J. R. Fraser, The structure and function of hyaluronan: An overview, Immunol. Cell Biol., 1996, 74, A1–A7 CrossRef CAS.
- M. Frescura, M. Berry, A. Corfield, S. Carrington and D. L. Easty, Evidence of hyaluronan in human tears and secretions of conjunctival cultures, Biochem. Soc. Trans., 1994, 22, 228S CrossRef CAS.
- M. Mastromatteo, G. Barbuzzi, A. Conte and M. A. Del Nobile, Controlled, release of thymol from zein based film, Innovative Food Sci. Emerging Technol., 2009, 10, 222–227 CrossRef CAS.
- T. Lin, C. Lu, L. Zhu and T. Lu, The biodegradation of zein in vitro and in vivo and its application in implants, AAPS PharmSciTech., 2011, 12, 172–176 CrossRef CAS.
- L. Yuan, B. Qu, J. Chen, H. Lv and X. Yang, Engineering modifiers bearing benzophenone with enhanced reactivity to construct surface microstructures, Polym. Chem., 2019, 10, 4859–4865 RSC.
- İ. Türkcan, A. D. Nalbant, E. Bat and G. Akca, Examination of 2-methacryloyloxyethyl phosphorylcholine polymer coated acrylic resin denture base material: surface characteristics and Candida albicans adhesion, J. Mater. Sci.: Mater. Med., 2018, 29, 107 CrossRef.
- P. G. de Gennes, Reptation of a Polymer Chain in the Presence of Fixed Obstacles, J. Chem. Phys., 1971, 55, 572–579 CrossRef.
- N. A. Peppas, Hydrogels in medicine and pharmacy, CRC Press, Boca Raton, FL, 1986 Search PubMed.
- M. Kędzierska, M. Bańkosz, K. Sala, J. Dudzik, P. Potemski and B. A.-O. Tyliszczak, Investigating the Effect of the Crosslinking Factor on the Properties of Hydrogel Materials Containing Tilia platyphyllos Hydrolate, Molecules., 2023, 28, 7035 CrossRef.
- C. M. González-Henríquez, G. d. C. Pizarro, M. A. Sarabia-Vallejos, C. A. Terraza and Z. E. López-Cabaña, In situ-preparation and characterization of silver-HEMA/PEGDA hydrogel matrix nanocomposites: Silver inclusion studies into hydrogel matrix, Arabian J. Chem., 2019, 12, 1413–1423 Search PubMed.
- V. Mucci and C. Vallo, Efficiency of 2,2-dimethoxy-2-phenylacetophenone for the photopolymerization of methacrylate monomers in thick sections, J. Appl. Polym. Sci., 2012, 123, 418–425 Search PubMed.
- L. M. Barcelos, M. G. Borges, C. J. Soares, M. S. Menezes, V. Huynh, M. G. Logan, A. P. P. Fugolin and C. S. Pfeifer, Effect of the photoinitiator system on the polymerization of secondary methacrylamides of systematically varied structure for dental adhesive applications, Dent. Mater., 2020, 36, 468–477 Search PubMed.
- J.-S. Chen, T.-Y. Liu, H.-M. Tsou, Y.-S. Ting, Y.-Q. Tseng and C.-H. Wang, Biopolymer brushes grown on PDMS contact lenses by in situ atmospheric plasma-induced polymerization, J. Polym. Res., 2017, 24, 69 CrossRef.
- D. Keskin, T. Mokabbar, Y. Pei and P. Van Rijn, The Relationship between Bulk Silicone and Benzophenone-Initiated Hydrogel Coating Properties, Polymers, 2018, 10, 534 CrossRef.
- S. Kim, D. J. Sessa and J. W. Lawton, Characterization of zein modified with a mild cross-linking agent, Ind. Crops Prod., 2004, 20, 291–300 CrossRef CAS.
- J. R. N. Taylor, S. K. Johnson, J. Taylor, S. Njila and C. Jackaman, Oxidation of commercial (α-type) zein with hydrogen peroxide improves its hydration and dramatically increases dough extensibility even below its glass transition temperature, J. Cereal Sci., 2016, 70, 108–115 Search PubMed.
- T. J. Schober, S. R. Bean, M. Tilley, B. M. Smith and B. P. Ioerger, Impact of different isolation procedures on the functionality of zein and kafirin, J. Cereal Sci., 2011, 54, 241–249 CrossRef CAS.
- B. M. Smith, S. R. Bean, G. Selling, D. Sessa and F. M. Aramouni, Role of non-covalent interactions in the production of visco-elastic material from zein, Food Chem., 2014, 147, 230–238 CrossRef CAS PubMed.
Footnote |
| † The authors Rahul Tiwari, Matthew Alexander, Robert A Broad and Cheng-Chun Peng are no longer at these addresses. Robert A Broad and Cheng-Chun Peng are no longer employees of CooperVision. |
| This journal is © The Royal Society of Chemistry 2026 |