Open Access Article
Open Access ArticleSustained drug delivery of the β-blocker acebutolol hydrochloride via chitosan–bilimbi leaf extract films
Jennifer P.
Pinto
 a,
Manjunath B.
Megalamani
a,
Manjunath B.
Megalamani
 b,
Ajitkumar Appayya
Hunashyal
c,
Oshin Jacintha
D′souza
a,
Sharanappa T.
Nandibewoor
b,
Ajitkumar Appayya
Hunashyal
c,
Oshin Jacintha
D′souza
a,
Sharanappa T.
Nandibewoor
 d,
Saraswati P.
Masti
d,
Saraswati P.
Masti
 c and
Ravindra B.
Chougale
c and
Ravindra B.
Chougale
 *a
*a
aP. G. Department of Studies in Chemistry, Karnatak University, Dharwad-580 003, India. E-mail: chougalerkud19@gmail.com
bSustainable Energy and Nanomaterials Technology Research Applications Laboratory, Atria Institute of Technology, Bengaluru – 560024, India
cDepartment of Chemistry, Karnatak Science College, Dharwad-580 003, India
dDepartment of Chemistry, School of Advanced Sciences, KLE Technological University, Hubballi-580031, India
First published on 31st October 2025
Abstract
This study presents the development of biocompatible polymeric films designed for the sustained release of the β-blocker acebutolol hydrochloride (AH). The films were fabricated using chitosan (CH), a biodegradable biopolymer, in combination with varying concentrations of Averrhoa bilimbi leaf extract (ABE), a natural additive with potential antimicrobial properties. The composite films were characterized using crystallinity, spectroscopic, mechanical, thermal, and morphological analyses. Functional testing included swelling studies, cumulative drug release profiling, cytotoxicity assessments, and microbial resistance assessments. The results indicated that the incorporation of ABE positively influenced the structural, mechanical, thermal and morphological properties, including swelling behaviour, thickness, and drug release kinetics. The film with the lowest ABE concentration showed optimal swelling (∼200%) and the highest drug release (∼25%), along with notable drug loading (∼78%) and encapsulation efficiency (∼19%). The release kinetics followed the Higuchi and Korsmeyer–Peppas models, indicating a non-Fickian diffusion mechanism. Importantly, the AH integrity was maintained throughout the fabrication process, and all the films exhibited excellent biocompatibility (90% cell viability rate). These findings support the feasibility of using CH/ABE films as promising candidates for sustained drug delivery systems. The use of plant-based and biodegradable components underscores the potential of this green strategy in pharmaceutical formulations, contributing to sustainable chemistry and reducing the environmental impact in drug delivery applications.
1. Introduction
Engineering novel drug delivery systems (DDS) has become a focus in the field of pharmaceutical research. The therapeutic process of drug administration requires a competent drug delivery mechanism. The mechanism involves three major steps: administration of the drug, release of the active pharmaceutical ingredient, and transportation of the drug to its targeted site.1 Drug delivery systems mainly emphasize liberating a particular drug at the appropriate time and concentration towards a specific target site.2 It has been observed over the years that the conventional systems for drug delivery such as tablets, pills, syrups, injectables, etc., have been facing issues of intermittent drug release profiles in addition to the scarcity of bioavailability of the drugs.3 The major loophole of these traditional DDS is the lack of controlled release of the drug accompanied by irregular biodistribution at indefinite sites, resulting in detrimental effects on the health of individuals. To combat this issue, advanced systems of drug delivery that mitigate the controlled release of drugs have been developed. The foremost challenge of these advanced systems lies in limiting the dosage and sustaining the concentration of the drug at a particular location.4The use of natural polymers in tailoring films apt for DDS has gained substantial attention from researchers over the past few decades. The prime rationale behind this could be their biocompatibility and defensive properties.5 In addition, their drug-loading efficiency and ability to confirm controlled release of the drug over a constant time interval at definite proportions add to their advantages. For a polymer to act as a resourceful drug delivery vehicle, the main prerequisites are bioerodibility, bioresorbability and biodegradability.6 Biopolymers such as chitosan, gelatin, pullulan, cellulose, carrageenan, etc., meet these requisites and hence their properties have been effectively harnessed in the area of pharmaceutical research, particularly in DDS.7–11
Chitosan (CH) is one of the natural cationic polymers that has gained much recognition owing to the presence of functionally active groups in its structure which impart massive physicochemical properties. The inherent properties of chitosan such as reduced immunogenicity and toxicity, biodegradability, biocompatibility, and bacterial resistivity allow it to be highly utilised in the field of medicine as drug carriers, wound dressing agents and antibacterials.12 Also the property of CH as a biocompatible and biodegradable polymer ensures its applicability in biomedical applications.13 The abundance of free hydroxyl and amino groups in the structure of CH endows it with mucoadhesive properties since these groups favorably interact with mucosal tissues.14 Barring all these advantages of CH, the property of high-water absorption15 by CH along with its sparse mechanical properties hinders its application in controlled drug release technologies.16 Hence, to tame these drawbacks, blending CH with suitable dopants, extracts or nanofillers is gaining prominence.
Averrhoa bilimbi (AB), commonly known as bilimbi, is a prominent plant found in the tropical and subtropical regions, known for the presence of high concentrations of several phytopharmaceuticals in it.17 In a study reported by Valsan and Raphael describing the pharmacognostic profile of AB leaves, the presence of both primary and secondary metabolites such as carbohydrates, sugars, cardiac glycosides, flavonoids, phenols, saponins, etc., was clearly observed. These potential compounds of pharmacological importance transmit antimicrobial, anticancer and antioxidant properties and are also known for their capacity to control blood cholesterol levels.18 Thus, the medicinal importance of Averrhoa bilimbi leaves can be effectively utilized in DDS by blending its extract with CH.
Acebutolol hydrochloride (AH) is a cardioselective beta-blocker drug primarily used in treating cardiovascular ailments, particularly hypertension, angina and also thyrotoxicosis.19,20 The pharmacokinetic parameter ‘elimination half-life (T1/2)’ of a drug is the time taken for a particular drug to reduce to 50% of its concentration in the body.21 It is found that the T1/2 of AH is nearly 3–4 h in plasma and 8–13 h in the case of its metabolite, diacetolol.22 Notably, it can be inferred that AH can be released into the body at a controlled rate via drug-delivering films, rather than by intravenous administration.
A wide range of research has been carried out on CH films employed as DDS. Affes et al. developed CH films loaded with ciprofloxacin and studied the release behavior of the drug, which proved that CH possessed the ability to release the drug sustainably.7 Barman et al. reported that CH films reinforced natural halloysite nanotubes containing norfloxacin as potential drug delivery vehicles providing enhanced therapeutic benefits.23 Divya Radha, Jisha S. Lal and K. S. Devaky studied the release profile of the anticancer drug 5-fluorouracil from chitosan and banana peel extract.6 However, the use of a cardioselective β-blocker in combination with CH and a leaf extract, which in this case is ABE, has not been previously reported.
The major aim of controlled release drug delivery is to avoid the frequent administration of dosages. Drugs such as AH have relatively short half-lives, which necessitate the requirement of a sustained release system. Therefore, an effective and controlled release drug-delivery system evades the requirement of frequent dosage. Hence, the fabrication of a controlled-release system in the form of a flexible film with CH as the host matrix was chosen. Since CH films have limitations of brittleness and water absorption, they are inhibited by the addition of bioactive constituents such as plant extract, which in this case is ABE. ABE possesses useful phytochemical components that enhance the therapeutic efficiency of the films. The present work aims to fill the research gap of incorporating a β-blocker drug into a plant extract mediated polysaccharide film.
The hypothesis of this study was to examine the effect of AB extract on CH films in enhancing their physicochemical, morphological, and biological properties to develop a biocompatible and sustainable channel for the controlled release of AH. Accordingly, this work focused on creating films using CH and the drug AH with increasing concentrations of AB extract. The release of AH by the incorporation of AB extract and its subsequent addition in increasing concentrations to the CH matrix was studied for understanding swelling, drug loading efficiency and encapsulation, and release kinetics along with the films’ biocompatibility and microbial resistance.
2. Materials and methods
2.1 Chemicals used
Chitosan (viscosity: 5–20 mPa s, molecular weight: 1526.464 g mol−1 and deacetylation degree: 70%) was obtained from Tokyo Chemical Industry (TCI), Japan. Acebutolol hydrochloride (C18H28N2O4·HCl, molecular weight: 372.89 g mol−1) was procured from Sigma Aldrich. Phosphate buffered saline (PBS) (pH: 7.4 ± 0.1) was purchased from Sisco Research Laboratories (SRL) Pvt. Ltd India. Acetic acid (glacial) (C2H4O2, molecular weight: 60.05 g mol−1) was obtained from SD Fine Chemicals, India. Averrhoa bilimbi leaves were obtained from the local areas of Dharwad, Karnataka, India. The plasticizer glycerol (C3H8O3, molecular weight: 92.09 g mol−1) was obtained from Loba Chemie Pvt. Ltd. All chemicals used in this study were of purely analytical grade and millipore water was used throughout this work.2.2 Preparation of Averrhoa bilimbi leaf extract (ABE)
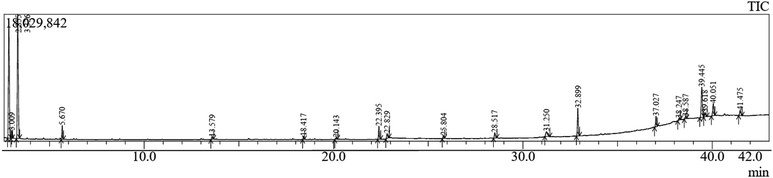
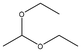
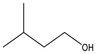
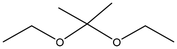
Averrhoa bilimbi leaves were washed thoroughly with water and dried under shade for about a week. The dried leaves were ground using a blender and the powder was collected. 10 g of this dried leaf powder were accurately weighed and soaked in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 solution of ethanol and water. The solution containing the soaked leaves was macerated for three days. The solution was filtered using Whatman filter paper no. 41 and the filtrate was collected and stored at 4 °C until further use. This extract was analyzed for its phytochemicals by measuring the total phenolic content (TPC) and analyzing it with gas chromatography–mass spectrometry (GC-MS) for a detailed overview.
1 solution of ethanol and water. The solution containing the soaked leaves was macerated for three days. The solution was filtered using Whatman filter paper no. 41 and the filtrate was collected and stored at 4 °C until further use. This extract was analyzed for its phytochemicals by measuring the total phenolic content (TPC) and analyzing it with gas chromatography–mass spectrometry (GC-MS) for a detailed overview.
2.3 Preparation of acebutolol hydrochloride (AH) drug-loaded chitosan (CH)/Averrhoa bilimbi leaf extract (ABE) films
The films designed for drug release were obtained by applying the solvent casting technique. The compositions of the films are listed in Table 1. CH (0.5 g) was weighed and dissolved in 50 mL of 1% glacial acetic acid solution. This solution was magnetically stirred. The ABE was added to the CH solution at varying concentrations slowly to ensure a homogeneous solution. The AH drug (20 mg) was added to this composite solution by dissolving it in 5 mL of water. 1% glycerol (1 mL) was added to the solution. The composite solution was stirred for about three hours and then finally cast onto glass petri plates. The solvents were allowed to evaporate, and the dried films so obtained were peeled from the petri plates and stored in a desiccator until further investigation. Fig. 1 shows images of the fabricated films.| Sample code | Chitosan (CH) (g) | Averrhoa bilimbi leaf extract (ABE) (mL) | Acebutolol hydrochloride (AH) (g) |
|---|---|---|---|
| CD | 0.5 | — | 0.02 |
| CAD-1 | 0.5 | 1.0 | 0.02 |
| CAD-2 | 0.5 | 2.0 | 0.02 |
| CAD-3 | 0.5 | 3.0 | 0.02 |
3. Characterisation techniques
3.1 Phytochemical characterisation of the Averrhoa bilimbi leaf extract (ABE)
3.2 Characterisation of the CH/ABE/AH films
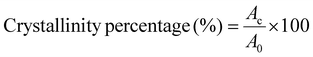 | (1) |
The percentage of crystallinity was also calculated using the data from the thermograms such as ΔHf (the enthalpy of fusion of the film at the melting temperature) and ΔHf100 (the enthalpy of fusion of the 100% crystalline chitosan, i.e. 62.05 J g−1). These were subsequently used in the following eqn (2):26
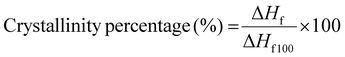 | (2) |
 | (3) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28
28 | (4) |
 | (5) |
Zero order model:
| Mt = Kt | (6) |
First order model:
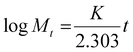 | (7) |
Higuchi model:
| Mt = K√t | (8) |
Korsmeyer–Peppas model:
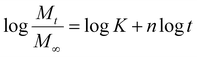 | (9) |
Here, Mt represents the amount of drug (AH) released at time t. K and n correspond to the release rate constant and the release exponent, respectively, while M∞ is the amount of drug present in the sample.
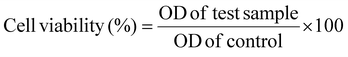 | (10) |
4. Results and discussion
4.1 Phytochemical analysis of the Averrhoa bilimbi leaf extract (ABE)
4.2 Analysis of the properties of the CH/ABE/AH films
| Sample code | Thickness (mm) | Crystallinity percentage (%) as calculated from XRD | Crystallinity percentage (%) as calculated from DSC | Tensile strength (MPa) | Extensibility (%) |
|---|---|---|---|---|---|
| The different superscripts in the columns indicate varied values at p ≤ 0.05 while Tukey's test was used. | |||||
| CD | 0.071 ± 0.012a | 33.214 ± 0.153d | 30.178 ± 0.546a | 28.279 ± 0.398c | 11.607 ± 0.307d |
| CAD-1 | 0.072 ± 0.006bc | 38.669 ± 0.841a | 37.164 ± 0.299c | 26.283 ± 0.648b | 16.128 ± 0.591b |
| CAD-2 | 0.084 ± 0.014a | 42.147 ± 0.624b | 41.879 ± 0.212cd | 11.848 ± 0.941a | 19.740 ± 0.821a |
| CAD-3 | 0.096 ± 0.011b | 52.607 ± 0.517bc | 51.246 ± 0.351b | 13.923 ± 0.164d | 26.731 ± 0.568bc |
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching in amide-I and –OH bending are present at 1586 cm−1 and 1379 cm−1.44 The bands in the range 1011 to 1019 cm−1 were ascribed to the C–O functional groups present in the polymer matrix. There were significant changes in the wavenumbers of the functional groups, indicating potential interactions between the components of the polymer matrix.
O stretching in amide-I and –OH bending are present at 1586 cm−1 and 1379 cm−1.44 The bands in the range 1011 to 1019 cm−1 were ascribed to the C–O functional groups present in the polymer matrix. There were significant changes in the wavenumbers of the functional groups, indicating potential interactions between the components of the polymer matrix.
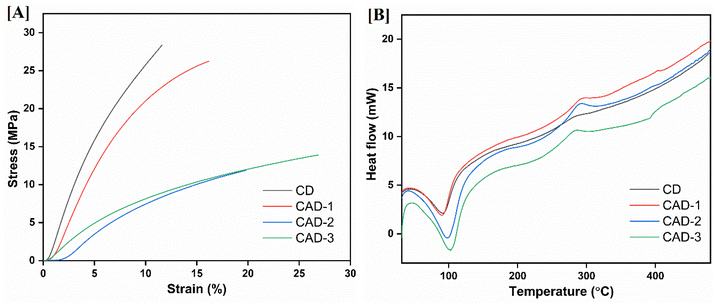
The mechanical properties of the films are displayed as stress–strain curves in Fig. 4[A]. The values of the parameters of mechanical properties such as tensile strength and extensibility are tabulated in Table 3. The films employed for drug delivery may be subjected to human body environments involving stress, which may lead to the disintegration of the films. Hence, an examination of the mechanical parameters of the films is necessarily done to discern this factor.47 The control chitosan film (CD) demonstrated the highest tensile strength of 28.279 MPa, indicative of a rigid, hydrogen-bonded network. While the ABE incorporated films showed a decline in tensile strength, the extensibility of the films increased with increasing ABE concentration. The decrease in tensile strength could be ascribed to the discontinuity in the chains of CH induced by the ABE. This apparently causes the matrix to resist any fracture.48 Hence, the tensile strength of the films reduces on moving from the CAD-1 to CAD-3 films. While the increment in extensibility can be linked to the plasticising ability of the ABE, the polyphenols present in the ABE may have contributed to this extensibility49 The CAD-1 film exhibited the most balanced performance, maintaining relatively higher stiffness while accommodating deformability, whereas excessive plasticization effects dominated CAD-2 and CAD-3.
| Sample code | Drug loading capacity (%) | Encapsulation efficiency (EE) (%) |
|---|---|---|
| The different superscripts present in the columns indicate that the values are significantly (p ≤ 0.05, analysis was done in triplicate) different from each other. | ||
| CD | 82.165 ± 0.084a | 15.318 ± 0.042a |
| CAD-1 | 78.662 ± 0.031c | 19.012 ± 0.027c |
| CAD-2 | 76.751 ± 0.068b | 11.082 ± 0.008d |
| CAD-3 | 75.159 ± 0.017d | 9.777 ± 0.039ab |
| Sample code | Zero-order model | First-order model | Higuchi model | Korsmeyer–Peppas model | |||||
|---|---|---|---|---|---|---|---|---|---|
| R 2 | K | R 2 | K | R 2 | K | R 2 | K | n | |
| CD | 0.817 | 5.225 | 0.888 | 0.138 | 0.942 | 2.509 | 0.918 | 0.439 | 0.627 |
| CAD-1 | 0.893 | 5.453 | 0.515 | 0.115 | 0.946 | 2.610 | 0.946 | 1.044 | 0.504 |
| CAD-2 | 0.776 | 7.089 | 0.609 | 0.117 | 0.916 | 3.484 | 0.931 | 1.225 | 0.527 |
| CAD-3 | 0.722 | 7.874 | 0.778 | 0.131 | 0.867 | 3.937 | 0.906 | 0.909 | 0.584 |
Zero-order kinetics mainly implies the dissolution of the drug at a slower pace, avoiding the consequences of disaggregation. Drug-delivery systems based on this model are known for their prolonged pharmacological activity.69 A straight line was obtained for the zero-order release equation, with correlation coefficients (R2) ranging from 0.72 to 0.82. The CAD-1 sample showed a higher R2 value in this regard, apparently indicating its use for sustained release. The minimal variations obtained in the values of the zero-order rate constant K could be ascribed to the seeping out of the drug AH with a reduced velocity induced by the CS/ABE matrix.70
The first-order kinetics model depicts the absorption or elimination of a water-soluble drug from the matrix based on the porosity and density of the drug-delivery system.69 From Table 5, it is evident that the values of R2 vary widely, indicating that the concentration of AH is independent of the matrix involved. Since AH is highly water soluble and the CS/ABE blend matrix is porous, it causes the rapid dissolution of AH as soon as the solvent medium penetrates the blend matrix.71 On the lines of the Higuchi model, the R2 values of the CAD-1 film confirm the fact that the release of drug AH is driven by the process of diffusion from the CH/ABE matrix. The minimal concentration of ABE in the CAD-1 film allows for the uptake of aqueous media (in this case, PBS), allowing the drug AH to leach out efficiently from the film matrix.
The Korsmeyer–Peppas model is applicable when more than one release mechanism occurs in the drug-delivery system. The release exponent ‘n’ has shown values greater than 0.5, indicating anomalous non-Fickian diffusion.72 This is indicative of the fact that matrix swelling and diffusion of the drug occur simultaneously.73 The constant values of R2 signify the controlled release of AH from the polymer matrix, with CAD-1 showing steady dissolution. From the analysis of these mathematical models, it can be inferred that a lower concentration of ABE allows for smooth dissolution of the drug AH from the CS/ABE matrix at a sustained rate. Also, the minimal increment in the value of n from CAD-1 to CAD-3 could be due to the good anchorage of the bonds between various components of the matrix, as sufficed by FTIR and XRD results, which imply stronger intermolecular CH/ABE/AH interactions with increasing ABE concentration. The observations are consistent with previous reports on CH-based films whose release kinetics followed the exhibited Korsmeyer–Peppas model depicting anomalous non-Fickian diffusion.74–76
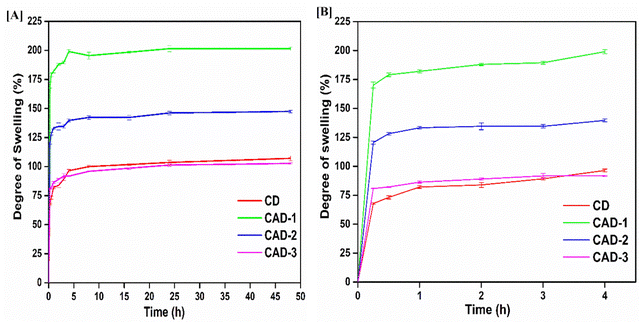
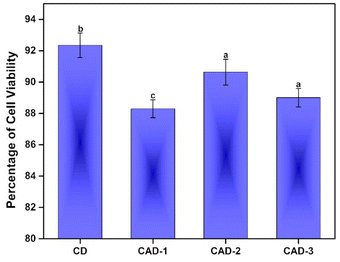
The percentage of cell viability was investigated for the films without ABE, i.e. the control CD film, and for those including ABE (CAD-1, CAD-2, CAD-3). The control CD film showed the highest percentage of cell viability. Furthermore, the percentage of cell viability appeared to increase with the increasing concentration of ABE to a value of 91% for the CAD-2 film and then there was a decrease in the cell viability for the CAD-3 film. The phytochemicals may have been responsible for this progression, which may have aided in keeping the cells active, rather than hindering their activity. The slight decrease in cell viability percentage at the higher concentration of ABE could be ascribed to the presence of certain constituents in the ABE, which may have hindered cell activities.78 These results confirm the non-toxic nature of the CH/ABE matrix, while also indicating that the incorporation of ABE did not induce any detrimental effects, supporting their applicability as drug-delivery films.
| Sample code | E. coli | S. aureus |
|---|---|---|
| Different superscripts within the columns specify that the values are significantly different (p ≤ 0.05, n = 3). | ||
| CD | 5 ± 0.01d | 2 ± 0.06ab |
| CAD-1 | 7 ± 0.04b | 3 ± 0.08a |
| CAD-2 | 9 ± 0.02c | 4 ± 0.01c |
| CAD-3 | 12 ± 0.07a | — |
| Drug used | Film composition | Key findings | Limitations | Ref. |
|---|---|---|---|---|
| Norfloxacin (antibacterial) | CH + halloysite nanotubes | • Controlled release achieved | • Early burst release rate: (>30% in 1 h) | 23 |
| • Release rate: >45% in 6 h | • Use of halloysite nanotubes as drug carriers and then introduction in films | |||
| Ciprofloxacin (antibacterial) | CH + ciprofloxacin + glutaraldehyde | • Controlled release achieved | • Early burst release rate: (>60% in 4 h) | 7 |
| • Release rate: >60% in 24 h | • Use of a synthetic crosslinking agent | |||
| Doxorubicin hydrochloride (anticancer) | CH | • Controlled release achieved | • Early burst release rate: (>20% in 1 h) | 81 |
| • Release rate: >60% in 6 h | • Use of a synthetic crosslinking agent | |||
| 5-Fluorouracil (anticancer) | CH + banana peel extract | • Controlled release achieved | • Early burst release rate: (>25% in 5 h) | 6 |
| • Release rate: >55% in 24 h | ||||
| • Use of natural extract in developing films | ||||
| Acebutolol hydrochloride (cardioselective β-blocker) | CH + Averrhoa bilimbi leaf extract | • Controlled release achieved | • Partial drug release | Present work |
| • Release rate: >25% in 24 h | • Secondary burst at high concentrations of ABE | |||
| • Use of natural extract in developing films |
4. Conclusions
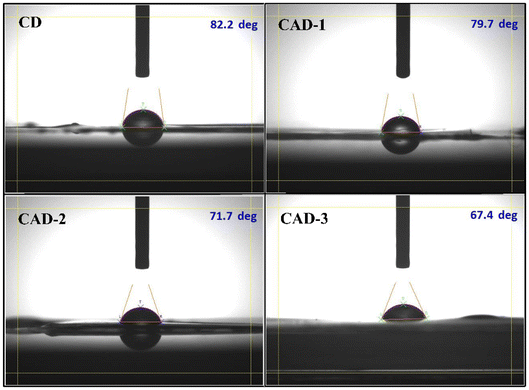
Acebutolol hydrochloride (AH)-loaded chitosan (CH)/Averrhoa bilimbi leaf extract (ABE) films demonstrated exceptional potential as controlled delivery systems for the β-blocker AH. The films were fabricated via a simple and effective solvent evaporation method using biodegradable and biocompatible materials such as CH and ABE. Notably, the films exhibited increased thickness and enhanced crystallinity, indicating a high level of compatibility between the matrix constituents. Spectroscopic analysis, coupled with microscopic imaging, further corroborated these findings. The FTIR spectra revealed stronger hydrogen bonding, while the SEM images displayed a homogeneous surface morphology, underscoring the uniform integration of the components. The films exhibited increasing flexible nature along with pronounced thermal stability. Evaluation of the films’ swelling indices, sustained drug release rates, and drug loading efficiencies suggests that these films are highly effective in modulating the release profile of the β-blocker AH, thereby reducing the frequency of drug administration and mitigating potential adverse health effects. The inclination of the release rates of the developed films towards the Higuchi and Korsmeyer–Peppas models suggests that AH is released via non-Fickian diffusion, highlighting that the release phenomenon is a simultaneous occurrence of both the swelling of the CH/ABE matrix and diffusion of AH from the matrix. Thus, the biocompatibility and bacterial resistance of the films further confirm their safety for human use, positioning them as promising options for controlled drug delivery applications.Author contributions
Jennifer P. Pinto: conceptualization, data interpretation, and writing – original manuscript. Manjunath B. Megalamani: design of the study and methodology. Ajitkumar Appayya Hunashyal: investigation and formal analysis. Oshin Jacintha D'souza: visualisation and data curation. Sharanappa T. Nandibewoor: supervision and validation. Saraswati P. Masti: revising manuscript. Ravindra B. Chougale: supervision, correspondence, review, and formal correction.Conflicts of interest
All authors declare no conflicts of interest.Data availability
The data supporting the findings of this study will be made available from the corresponding author upon reasonable request.Acknowledgements
One of the authors, Jennifer P. Pinto, acknowledges financial support from the University Grants Commission, Government of India, through the Savitribai Jyotirao Phule Single Girl Child Fellowship (SJSGC) – 2023 (Ref. No. UGCES-22-OB-KAR-F-SJSGC-2368). The authors gratefully acknowledge the Sophisticated Analytical Instrument Facility (SAIF), and University Scientific Instrumentation Centre (USIC), Karnatak University, Dharwad, for providing necessary instrumentation amenities.References
-
K. K. Jain, An Overview of Drug Delivery Systems, vol. 2059. 2020. DOI:10.1007/978-1-4939-9798-5_1
.
- V. S. Bheemidi, M. Tiruckovela and P. Varanasi, An imperative note on novel drug delivery systems, J. Nanomed. Nanotechnol., 2011, 2, 100125 CrossRef
.
- S. Adepu and S. Ramakrishna, Controlled drug delivery systems: Current status and future directions, Molecules, 2021, 26(19) DOI:10.3390/molecules26195905
.
- D. Liu, F. Yang, F. Xiong and N. Gu, The smart drug delivery system and its clinical potential, Theranostics, 2016, 6(9), 1306–1323, DOI:10.7150/thno.14858
.
- T. Gazori, M. R. Khoshayand, E. Azizi, P. Yazdizade, A. Nomani and I. Haririan, Evaluation of Alginate/Chitosan nanoparticles as antisense delivery vector: Formulation, optimization and in vitro characterization, Carbohydr. Polym., 2009, 77(3), 599–606, DOI:10.1016/j.carbpol.2009.02.019
.
- D. Radha, J. S. Lal and K. S. Devaky, Release studies of the anticancer drug 5-fluorouracil from chitosan-banana peel extract films, Int. J. Biol. Macromol., 2024, 256, 128460 CrossRef PubMed
.
- S. Affes, I. Aranaz, N. Acosta, Á. Heras, M. Nasri and H. Maalej, Chitosan derivatives-based films as pH-sensitive drug delivery systems with enhanced antioxidant and antibacterial properties, Int. J. Biol. Macromol., 2021, 182, 730–742, DOI:10.1016/j.ijbiomac.2021.04.014
.
- N. Samiraninezhad, K. Asadi, H. Rezazadeh and A. Gholami, Using chitosan, hyaluronic acid, alginate, and gelatin-based smart biological hydrogels for drug delivery in oral mucosal lesions: A review, Int. J. Biol. Macromol., 2023, 126573 CrossRef PubMed
.
- E. Pezik, T. Gulsun, S. Sahin and İ. Vural, Development and characterization of pullulan-based orally disintegrating films containing amlodipine besylate, Eur. J. Pharm. Sci., 2021, 156 DOI:10.1016/j.ejps.2020.105597
.
- T. Maver,
et al., Cellulose based thin films as a platform for drug release studies to mimick wound dressing materials, Cellulose, 2015, 22(1), 749–761, DOI:10.1007/s10570-014-0515-9
.
- J. S. Boateng, H. V. Pawar and J. Tetteh, Polyox and carrageenan based composite film dressing containing anti-microbial and anti-inflammatory drugs for effective wound healing, Int. J. Pharm., 2013, 441(1–2), 181–191, DOI:10.1016/j.ijpharm.2012.11.045
.
- F. Tao,
et al., Chitosan-based drug delivery systems: From synthesis strategy to osteomyelitis treatment – A review, Carbohydr. Polym., 2021, 251, 117063, DOI:10.1016/j.carbpol.2020.117063
.
- R. Jayakumar, M. Prabaharan, P. T. Sudheesh Kumar, S. V. Nair and H. Tamura, Biomaterials based on chitin and chitosan in wound dressing applications, Biotechnol. Adv., 2011, 29(3), 322–337, DOI:10.1016/j.biotechadv.2011.01.005
.
- J. Potaś, E. Szymańska and K. Winnicka, Challenges in developing of chitosan – Based polyelectrolyte complexes as a platform for mucosal and skin drug delivery, Eur. Polym. J., 2020, 140, 110020, DOI:10.1016/j.eurpolymj.2020.110020
.
- B. Kaczmarek, A. Sionkowska, F. J. Monteiro, A. Carvalho, K. Łukowicz and A. M. Osyczka, Characterization of gelatin and chitosan scaffolds cross-linked by addition of dialdehyde starch, Biomed. Mater., 2018, 13(1) DOI:10.1088/1748-605X/aa8910
.
- J. You,
et al., Quaternized Chitosan/Poly(acrylic acid) Polyelectrolyte Complex Hydrogels with Tough, Self-Recovery, and Tunable Mechanical Properties, Macromolecules, 2016, 49(3), 1049–1059, DOI:10.1021/acs.macromol.5b02231
.
- H. Y. Setyawan, S. Sukardi and B. F. Nareswari, The phytochemical potential of Averrhoa bilimbi - A review, IOP Conf. Ser.: Earth Environ. Sci., 2021, 733(1) DOI:10.1088/1755-1315/733/1/012091
.
- A. C. Iwansyah,
et al., Evaluation on the physicochemical properties and mineral contents of Averrhoa bilimbi L. leaves dried extract and its antioxidant and antibacterial capacities, Food Sci. Technol., 2021, 41, 987–992 CrossRef
.
- A. Radha, A. Singh, L. Sharma and K. K. Thakur, Molecular interactions of acebutolol hydrochloride to human serum albumin:
A combined calorimetric, spectroscopic and molecular modelling approach, Mater. Today: Proc., 2021, 44, 1700–1706, DOI:10.1016/j.matpr.2020.11.872
.
- Y. N. Patil, M. B. Megalamani and S. T. Nandibewoor, PVA Capped Mn-Doped ZnS Encapsulated Nontoxic MoS 2 Nano-Sheet Probe for the Sensitive Estimation of Cardiovascular β-Blocking Agent Acebutolol in Biomedical and Environmental Samples, J. Electrochem. Soc., 2023, 170(3), 037505, DOI:10.1149/1945-7111/acbe6d
.
-
K. Boussery, F. M. Belpaire and J. Van de Voorde, Physiological Aspects Determining the Pharmacokinetic Properties of Drugs, Elsevier Ltd, 2008. DOI:10.1016/B978-0-12-417205-0.00023-7
.
- A. Z. Ranjitha and N. G. N. Swamy, Formulation and in vitro evaluation of acebutolol hydrochloride microballoons for sustained drug delivery, Indian J. Novel Drug Deliv., 2014, 6(2), 124–131 Search PubMed
. Available: https://www.embase.com/search/results?subaction=viewrecord&from=export&id=L604474738.
- M. Barman, S. Mahmood, R. Augustine, A. Hasan, S. Thomas and K. Ghosal, Natural halloysite nanotubes /chitosan based bio-nanocomposite for delivering norfloxacin, an anti-microbial agent in sustained release manner, Int. J. Biol. Macromol., 2020, 162, 1849–1861, DOI:10.1016/j.ijbiomac.2020.08.060
.
- L. Valero, M. Gainche, C. Esparcieux, F. Delor-Jestin and H. Askanian, Vegetal Polyphenol Extracts as Antioxidants for the Stabilization of PLA: Toward Fully Biobased Polymer Formulation, ACS Omega, 2023, 9(7) DOI:10.1021/acsomega.3c07236
.
- J. P. Pinto,
et al., Development of Chitosan-Copovidone nanocomposite films with antioxidant and antibacterial properties for food packaging applications, Food Hum., 2023, 1, 378–390, DOI:10.1016/j.foohum.2023.06.003
.
- S. S. Narasagoudr, Y. Shanbhag, R. B. Chougale, B. M. Baraker, S. P. Masti and B. Lobo, Thermal degradation kinetics of ethyl vanillin crosslinked chitosan/poly(vinyl alcohol) blend films for food packaging applications, Chem. Data Collect., 2021, 34 DOI:10.1016/j.cdc.2021.100739
.
- J. Chalitangkoon, M. Wongkittisin and P. Monvisade, Silver loaded hydroxyethylacryl chitosan/sodium alginate hydrogel films for controlled drug release wound dressings, Int. J. Biol. Macromol., 2020, 159, 194–203, DOI:10.1016/j.ijbiomac.2020.05.061
.
- A. B. Reddy, B. Manjula, T. Jayaramudu, E. R. Sadiku, P. Anand Babu and S. Periyar Selvam, 5-Fluorouracil Loaded Chitosan–PVA/Na + MMT Nanocomposite Films for Drug Release and Antimicrobial Activity, Nano-Micro Lett., 2016, 8(3), 260–269, DOI:10.1007/s40820-016-0086-4
.
- A. M. Craciun, L. Mititelu Tartau, M. Pinteala and L. Marin, Nitrosalicyl-imine-chitosan hydrogels based drug delivery systems for long term sustained release in local therapy, J. Colloid Interface Sci., 2019, 536, 196–207, DOI:10.1016/j.jcis.2018.10.048
.
- O. Jacintha, T. Gasti, V. Hiremani, J. Pinto, S. Contractor, A. Shettar, D. Olivia, S. Arakera, S. Masti and R. Chougale,
et al., International Journal of Biological Macromolecules Basella alba stem extract integrated poly (vinyl alcohol)/chitosan composite films: A promising bio-material for wound healing, Int. J. Biol. Macromol., 2023, 225, 673–686, DOI:10.1016/j.ijbiomac.2022.11.130
.
- L. Monjazeb Marvdashti, A. Koocheki and M. Yavarmanesh, Characterization, Release Profile and Antimicrobial Properties of Bioactive Polyvinyl Alcohol-Alyssum homolocarpum Seed Gum- Nisin Composite Film, Food Biophys., 2019, 14(2), 120–131, DOI:10.1007/s11483-018-09562-y
.
- M. R. R. Rahardhian, B. T. Murti, D. Wigati, R. Suharsanti and C. N. Putri, Solvent concentration effect on total flavonoid and total phenolic contents of Averrhoa bilimbi leaf extract, Pharmaciana, 2019, 9(1), 137–144, DOI:10.12928/pharmaciana.v%vi%i.10809
.
- M. T. Islam,
et al., Phytol: A review of biomedical activities, Food Chem. Toxicol., 2018, 121, 82–94, DOI:10.1016/j.fct.2018.08.032
.
- M. L. Gonzalez-Rivera, J. C. Barragan-Galvez, D. Gasca-Martínez, S. Hidalgo-Figueroa, M. Isiordia-Espinoza and A. J. Alonso-Castro, In Vivo Neuropharmacological Effects of Neophytadiene, Molecules, 2023, 28(8) DOI:10.3390/molecules28083457
.
-
S.-K. Kim and F. Karadeniz, Chapter 14 - Biological Importance and Applications of Squalene and Squalane, in Advances in Food and Nutrition Research, ed. S.-K. Kim, Academic Press, 2012, vol. 65, pp. 223–233. DOI:10.1016/B978-0-12-416003-3.00014-7
.
- A. O. Nogueira, Y. I. S. Oliveira, B. L. Adjafre, M. E. A. de Moraes and G. F. Aragão, Pharmacological effects of the isomeric mixture of alpha and beta amyrin from Protium heptaphyllum : a literature review, Fundam. Clin. Pharmacol., 2019, 33(1), 4–12, DOI:10.1111/fcp.12402
.
-
S. D. Ambavade, A. V. Misar and P. D. Ambavade, Pharmacological, nutritional, and analytical aspects of β-sitosterol: A review, 2014, Kluwer Academic Publishers. DOI:10.1007/s13596-014-0151-9
.
-
R. Ashraf and H. N. Bhatti, Chapter 10 – Stigmasterol, in A Centum of Valuable Plant Bioactives, ed. M. Mushtaq and F. Anwar, Academic Press, 2021, pp. 213–232. DOI:10.1016/B978-0-12-822923-1.00019-4
.
- B. Al-Hindi, N. A. Yusoff, I. J. Atangwho, M. Ahmad, M. Z. Asmawi and M. F. Yam, A Soxhlet extract of Gongronema latifolium retains moderate blood glucose lowering effect and produces structural recovery in the pancreas of STZ-induced diabetic rats, Med. Sci., 2016, 4(2), 9 Search PubMed
.
- B. Yerriswamy, C. L. Reddy, C. V. Prasad, M. C. S. Subha and K. C. Rao, Synthesis and characterization of sodium alginate-g-2-hydroxyethyl methacrylate interpenetrating beads for controlled release of acebutolol hydrochloride, Des. Monomers Polym., 2011, 14(1), 25–37, DOI:10.1163/138577210X541178
.
- P. Ramakrishna, B. Mallikarjuna, A. C. Babu, P. Sudhakar, K. C. Rao and M. C. S. Subha, Interpenetrating polymer network of crosslinked blend microspheres for controlled release of acebutolol HCl, J. Appl. Pharm. Sci., 2011, 1(6), 212–219 Search PubMed
.
- S. B. Aziz, Modifying Poly(Vinyl Alcohol) (PVA) from Insulator to Small-Bandgap Polymer: A Novel Approach for Organic Solar Cells and Optoelectronic Devices, J. Electron. Mater., 2016, 45(1), 736–745, DOI:10.1007/s11664-015-4191-9
.
- S. S. Narasagoudr, V. G. Hegde, V. N. Vanjeri, R. B. Chougale and S. P. Masti, Ethyl vanillin incorporated chitosan/poly(vinyl alcohol) active films for food packaging applications, Carbohydr. Polym., 2020, 236, 116049, DOI:10.1016/j.carbpol.2020.116049
.
- D. I. Chan-Matú, V. M. Toledo-López, M. d. L. V. y. Vargas, S. Rincón-Arriaga, A. Rodríguez-Félix and T. J. Madera-Santana, Preparation and characterization of chitosan-based bioactive films incorporating Moringa oleifera leaves extract, J. Food Meas. Charact., 2021, 15(5), 4813–4824, DOI:10.1007/s11694-021-01055-w
.
- K. Rambabu, G. Bharath, F. Banat, P. L. Show and H. H. Cocoletzi, Mango leaf extract incorporated chitosan antioxidant film for active food packaging, Int. J. Biol. Macromol., 2019, 126, 1234–1243, DOI:10.1016/j.ijbiomac.2018.12.196
.
- S. A. Mir, B. N. Dar, A. A. Wani and M. A. Shah, Effect of plant extracts on the techno-functional properties of biodegradable packaging films, Trends Food Sci. Technol., 2018, 80, 141–154, DOI:10.1016/j.tifs.2018.08.004
.
-
P. Ma, et al., Recent advances in mechanical force-responsive drug delivery systems, 2022, Royal Society of Chemistry. 10.1039/d2na00420h
.
- R. B. Bodini, P. J. A. Sobral, C. S. Favaro-Trindade and R. A. Carvalho, Properties of gelatin-based films with added ethanol-propolis extract, LWT-Food Sci. Technol., 2013, 51(1), 104–110, DOI:10.1016/j.lwt.2012.10.013
.
-
V. Kola and I. S. Carvalho, Plant extracts as additives in biodegradable films and coatings in active food packaging, Elsevier Ltd, 2023. DOI:10.1016/j.fbio.2023.102860
.
- J. P. Pinto, O. D’souza, V. Hiremani, N. Dalbanjan, S. Kumar, S. Narasgoudr, S. Masti and R. Chougale,
et al., Functional properties of taro starch reinforced polysaccharide based films for active packaging, Food Biosci., 2023, 56, 103340, DOI:10.1016/j.fbio.2023.103340
.
- Thermal Transitions in Polymers, 1997.
- P. Ramakrishna, B. Mallikarjuna, A. C. Babu, P. Sudhakar, M. C. S. Subha and K. C. Rao, Interpenetrating polymer network of crosslinked blend microspheres for controlled release of Acebutolol HCl, J. Appl. Pharm. Sci., 2011, 1(6), 212–219 Search PubMed
.
- C. Venkata Prasad, B. Yerri Swamy, B. Mallikarjuna, K. Sreekanth, M. Subha, K. Chowdoji Rao and J. Yu,
et al., Preparation and characterization of interpenetrating polymer network beads for controlled release of acebutolol hydrochloride, Adv. Polym. Technol., 2012, 31(2), 87–99, DOI:10.1002/adv.20238
.
- M. O. Tuhin,
et al., Modification of mechanical and thermal property of chitosan-starch blend films, Radiat. Phys. Chem., 2012, 81(10), 1659–1668, DOI:10.1016/j.radphyschem.2012.04.015
.
- S. Yadav, G. K. Mehrotra, P. Bhartiya, A. Singh and P. K. Dutta, Preparation, physicochemical and biological evaluation of quercetin based chitosan-gelatin film for food packaging, Carbohydr. Polym., 2020, 227, 115348, DOI:10.1016/j.carbpol.2019.115348
.
- X.-Y. Wang, J. Wang, C. Zhao, L. Ma, D. Rousseau and C.-H. Tang, Facile fabrication of chitosan colloidal films with pH-tunable surface hydrophobicity and mechanical properties, Food Hydrocolloids, 2023, 137, 108429, DOI:10.1016/j.foodhyd.2022.108429
.
- K. A. Nxumalo, O. A. Fawole and A. O. Aremu, Development of Chitosan-Based Active Films with Medicinal Plant Extracts for Potential Food Packaging Applications, Processes, 2023, 12(1), 23, DOI:10.3390/pr12010023
.
- S. Farris, L. Introzzi, P. Biagioni, T. Holz, A. Schiraldi and L. Piergiovanni, Wetting of Biopolymer Coatings: Contact Angle Kinetics and Image Analysis Investigation, Langmuir, 2011, 27(12), 7563–7574, DOI:10.1021/la2017006
.
- A. A. Shamsuri and S. N. A. M. Jamil, Functional Properties of Biopolymer-Based Films Modified with Surfactants: A Brief Review, Processes, 2020, 8(9), 1039, DOI:10.3390/pr8091039
.
- B. N. Singh, W. R. Thoden and J. Wahl, Acebutolol: A Review of its Pharmacology, Pharmacokinetics, Clinical Uses, and Adverse Effects, Pharmacotherapy, 1986, 6(2), 45–61, DOI:10.1002/j.1875-9114.1986.tb03451.x
.
- B. A. Khan, A. Khan, M. K. Khan and V. A. Braga, Preparation and properties of High sheared Poly(Vinyl Alcohol)/Chitosan blended Hydrogels films with Lawsonia inermis extract as wound dressing, J. Drug Delivery Sci. Technol., 2021, 61, 102227, DOI:10.1016/j.jddst.2020.102227
.
- P. B. Franco, L. A. De Almeida, R. F. C. Marques, M. A. Da Silva and M. G. N. Campos, Chitosan Associated with the Extract of Unripe Banana Peel for Potential Wound Dressing Application, Int. J. Polym. Sci., 2017, 2017(1), 9761047, DOI:10.1155/2017/9761047
.
- C. Y. Chin, J. Jalil, P. Y. Ng and S. F. Ng, Development and formulation of Moringa oleifera standardised leaf extract film dressing for wound healing application, J. Ethnopharmacol., 2018, 212, 188–199, DOI:10.1016/j.jep.2017.10.016
.
- Q. Yuan, J. Shah, S. Hein and R. D. K. Misra, Controlled and extended drug release behavior of chitosan-based nanoparticle carrier, Acta Biomater., 2010, 6(3), 1140–1148, DOI:10.1016/j.actbio.2009.08.027
.
- F. Ordikhani and A. Simchi, Long-term antibiotic delivery by chitosan-based composite coatings with bone regenerative potential, Appl. Surf. Sci., 2014, 317, 56–66, DOI:10.1016/j.apsusc.2014.07.197
.
- W. Huang, C. P. Tsui, C. Y. Tang and L. Gu, Effects of Compositional Tailoring on Drug Delivery Behaviours of Silica Xerogel/Polymer Core-shell Composite Nanoparticles, Sci. Rep., 2018, 8(1), 1–13, DOI:10.1038/s41598-018-31070-9
.
- F. Cui,
et al., Nanoparticles incorporated in bilaminated films: A smart drug delivery system for oral formulations, Biomacromolecules, 2007, 8(9), 2845–2850, DOI:10.1021/bm070339e
.
- M. V. S. Varma, A. M. Kaushal, A. Garg and S. Garg, Factors affecting mechanism and kinetics of drug release from matrix-based oral controlled drug delivery systems, Am. J. Drug Delivery, 2004, 2(1), 43–57, DOI:10.2165/00137696-200402010-00003
.
- D. Samaha, R. Shehayeb and S. Kyriacos, Modeling and comparison of dissolution profiles of diltiazem modified-release formulations, Dissolution Technol., 2009, 16(2), 41–46, DOI:10.14227/DT160209P41
.
- A. Craciun, L. Mititelu Tartau, M. Pinteala and L. Marin, Nitrosalicyl-imine-chitosan hydrogels based drug delivery systems for long term sustained release in local therapy, J. Colloid Interface Sci., 2019, 536, 196–207, DOI:10.1016/j.jcis.2018.10.048
.
- N. V. Mulye and S. J. Turco, An Examination of Assumptions Underlying the First-Order Kinetic Model for Release of Water-Soluble Drugs from Dicalcium Phosphate Dihydrate Matrices, Drug Dev. Ind. Pharm., 1996, 22(7), 673–679 CrossRef
.
- R. W. Korsmeyer, R. Gurny, E. Doelker, P. Buri and N. A. Peppas, Mechanisms of solute release from porous hydrophilic polymers, Int. J. Pharm., 1983, 15(1), 25–35, DOI:10.1016/0378-5173(83)90064-9
.
- J. Siepmann and N. A. Peppas, Higuchi equation: Derivation, applications, use and misuse, Int. J. Pharm., 2011, 418(1), 6–12, DOI:10.1016/j.ijpharm.2011.03.051
.
- J. Chalitangkoon, A. Ronte, T. Sintoppun, N. Manapradit and P. Monvisade, Dual Cross-Linked Chitosan-Based Films with pH-Sensitive Coloration and Drug Release Kinetics for Smart Wound Dressings, ACS Omega, 2025, 10(8), 7770–7782, DOI:10.1021/acsomega.4c08089
.
- T. N. da Silva, F. Reynaud, P. H. d. S. Picciani, K. G. de Holanda e Silva and T. N. Barradas, Chitosan-based films containing nanoemulsions of methyl salicylate: Formulation development, physical-chemical and in vitro drug release characterization, Int. J. Biol. Macromol., 2020, 164, 2558–2568, DOI:10.1016/j.ijbiomac.2020.08.117
.
- A. Craciun, L. Mititelu Tartau, M. Pinteala and L. Marin, Nitrosalicyl-imine-chitosan hydrogels based drug delivery systems for long term sustained release in local therapy, J. Colloid Interface Sci., 2019, 536, 196–207, DOI:10.1016/j.jcis.2018.10.048
.
- M. Sakthivel, D. S. Franklin, S. Sudarsan, G. Chitra and S. Guhanathan, Investigation on pH-switchable (itaconic acid/ethylene glycol/acrylic acid) based polymeric biocompatible hydrogel, RSC Adv., 2016, 6(108), 106821–106831, 10.1039/c6ra21043k
.
- O. J. D′souza, J. P. Pinto, A. K. Shettar, S. S. Narasagoudr, S. P. Masti and R. B. Chougale, Biofunctionalization of chitosan/gelatin composite films reinforced Phyllanthus niruri extract for wound healing application, Surf. Interfaces, 2023, 43, 103567 CrossRef
.
- T. J. Silhavy, D. Kahne and S. Walker, The bacterial cell envelope, Cold Spring Harbor Perspect. Biol., 2010, 2(5), a000414, DOI:10.1101/cshperspect.a000414
.
- S. G. G. Aziz and H. Almasi, Physical characteristics, release properties, and antioxidant and antimicrobial activities of whey protein isolate films incorporated with thyme (Thymus vulgaris L.) extract-loaded Nanoliposomes, Food Bioprocess Technol., 2018, 11(8), 1552–1565, DOI:10.1007/s11947-018-2121-6
.
- J. H. Lee,
et al., Drug-Loaded Biocompatible Chitosan Polymeric Films with Both Stretchability and Controlled Release for Drug Delivery, ACS Omega, 2023, 8(1), 1282–1290, DOI:10.1021/acsomega.2c06719
.
| This journal is © The Royal Society of Chemistry 2026 |