Open Access Article
Open Access ArticleAn evaluation of the pharmaceutical properties of the Nigerian baobab polysaccharide for sustained release oral tablets
Munirah A.
Ajiboye
a,
Yogesh
Rawat
b,
Nihad
Mawla
a,
Rand
Abdulhussain
a,
Haja
Muhamad
a,
Adeola O.
Adebisi
a,
Barbara R.
Conway
 a,
Alan M.
Smith
a,
Alan M.
Smith
 a,
Gordon A.
Morris
a,
Gordon A.
Morris
 b,
Katie
Addinall
b,
Maria
Dimopoulou
c,
Athanasios
Angelis-Dimakis
b,
Katie
Addinall
b,
Maria
Dimopoulou
c,
Athanasios
Angelis-Dimakis
 b and
Kofi
Asare-Addo
b and
Kofi
Asare-Addo
 *a
*a
aDepartment of Pharmacy, University of Huddersfield, Huddersfield, HD1 3DH, UK. E-mail: k.asare-addo@hud.ac.uk
bDepartment of Physical and Life Sciences, University of Huddersfield, Huddersfield HD1 3DH, UK
cSchool of Health and Life Sciences, Teesside University, TS1 3BX, UK
First published on 9th October 2025
Abstract
Natural polysaccharides have various applications, and their use is on the rise due to properties including biodegradability, biocompatibility, cytocompatibility and the absence of negative immune responses. Natural polysaccharides have also been reported to be efficient biopolymers that can be used in oral dosage forms. To this end, this study investigated the potential of using the Nigerian baobab polysaccharide as a renewable pharmaceutical excipient and its impact on the release of theophylline as a model drug. The results indicate that the extraction process yielded an amorphous polysaccharide from the baobab oblong fruit. The thermogravimetric analysis showed weight loss to occur in three phases typical of polysaccharide decomposition. Differential scanning calorimetry (DSC) revealed that the polysaccharide was stable until around 175 °C, after which thermal degradation takes place. Tablet formulations containing different concentrations of baobab were evaluated for mechanical properties, flowability, and dissolution characteristics. An increase in baobab content improved the mechanical strength of the tablets. The increase in the baobab concentration simultaneously brings about a decrease in the porosity of the compacts from 11% to 9%, demonstrating its suitability for use in tablet formulations. In vitro dissolution studies in acidic media (pH 1.2) showed that formulations with higher baobab content (30%–57.5%) demonstrated sustained release characteristics, with no burst release observed. At pH 6.8, however, an increase in the polysaccharide content seemed to promote a “burst release”. These distinctive behaviours at different pH values suggest significant potential for exploiting and understanding the functional properties of the polysaccharide to aid formulators in manipulating drug release. These pH-dependent behaviours mean that a formulator can tune release by adjusting the baobab![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MCC ratio. Higher baobab content (30–57.5%) enables sustained, burst-free release at pH 1.2, while at pH 6.8, increasing baobab (to B4) enhances the 10 min burst, and the baobab-only formulation (B5) achieves the fastest overall release through rapid erosion.
MCC ratio. Higher baobab content (30–57.5%) enables sustained, burst-free release at pH 1.2, while at pH 6.8, increasing baobab (to B4) enhances the 10 min burst, and the baobab-only formulation (B5) achieves the fastest overall release through rapid erosion.
1. Introduction
Excipients are essential in drug formulations, acting as vehicles that provide the necessary weight, consistency, and volume for the accurate delivery of active components. Excipients frequently perform additional functions that affect active component storage, drug release, and bioavailability.1,2 Many synthetic excipients have been incorporated into pharmaceutical formulations, but there is growing interest in the use of natural and sustainable products.3–5Polysaccharides are natural polymers that are derived directly from plant sources6 and are often included in pharmaceutical applications due to their hydrophilic nature, ability to swell upon contact with water and thickening properties,7–9 making them excellent candidate materials for use as excipients.
A class of polysaccharides that have recently attracted research attention comprises those extracted from the Malvaceae family including baobab (Adansonia digitata).10 Baobab trees are found in the northern part of Nigeria where the fruits and leaves are used as food. The leaf, for instance, is ground into a powder and can be prepared as a soup which is referred to as “Kuka”. Due to the various traditional uses of the baobab tree in medicine and nutrition, it is commonly referred to as the “chemist tree”.11,12 Polysaccharides extracted from the baobab tree generally contain high molecular weight random coiled chains with particularly high intrinsic viscosity depending on the extraction process.13 Depending on the technology or solvent employed for the extraction process, different polysaccharides with distinct physicochemical compositions, macromolecular and structural characteristics, and functional and biological characteristics can be achieved.12,14 Polysaccharides extracted from the baobab fruit pulp have been shown to contain linear xylogalacturonans8,13 with small amounts of co-extracted glucans15 depending on the pH used for extraction.8 Polysaccharides extracted from baobab, as with polysaccharides extracted from other novel sources, are referred to as pectin-like polysaccharides as they usually consist of lower amounts of galacturonic content (<65%) and larger amounts of neutral sugars (glucose, arabinose, rhamnose and galactose) and therefore cannot be classified as pectin for pharmaceutical or food applications.16 Pectin is a complex polysaccharide present in the wall of plant cells. Pectin has been investigated as an excipient in a variety of dosage forms, including film coatings for colon-specific drug delivery systems with ethyl cellulose, microparticulate delivery systems for ophthalmic preparations, and matrix-type transdermal patches. Although it has great potential as a hydrophilic polymeric material for controlled release matrix drug delivery systems, its water solubility adds to the drug's premature and rapid release from these matrices.1,17 Pectin can form gels in a variety of ways depending on its structure. Gelation can be induced by acidification, cross-linking with calcium ions, or the alginate reaction.2 Research shows that it has a molecular composition (phenolic compounds, galacturonic acid, acetyl groups, proteins and neutral sugars) that makes it suitable for use in pharmaceutical applications.10,13,18,19
This study first aimed at extracting baobab polysaccharide from the oblong fruit and characterising its solid-state properties. An investigation into the mechanical properties of the Nigerian baobab polysaccharide relevant to its use as an excipient was conducted. The study then sought to investigate the polysaccharide as a potential matrix former to control the release of a model drug (theophylline) in a bid to encourage the use of abundant renewable sources of raw materials suitable for use in the pharmaceutical industry in the developing world.
2. Materials and methods
2.1. Materials
The oblong-shaped baobab (Adansonia digitata) fruit was dried at 45 °C and ground to a fine powder before being subjected to extraction. For the extraction process, all buffer salts, sodium azide, disodium phosphate heptahydrate, monosodium phosphate monohydrate, ethanol and dialysis membranes (molecular weight cut-off 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da) were purchased from Sigma Aldrich (Gillingham, UK) and were of analytical grade. Microcrystalline cellulose (MCC) (Avicel PH101) was purchased from FMC (Pentre, UK), magnesium stearate was purchased from Merck (Darmstadt, Germany) and theophylline, the model drug used, was purchased from TCI Chemicals (Oxford, UK). pH 6.8 buffer solutions used for dissolution analysis consisting of sodium hydroxide and potassium phosphate monobasic were purchased from Fisher Scientific (Loughborough, UK) and Honeywell Fluka (Loughborough, UK), respectively. The pH 1.2 medium was prepared using hydrochloric acid purchased from Fisher Scientific (UK) and potassium chloride was purchased from Honeywell Fluka (Loughborough, UK).
000 Da) were purchased from Sigma Aldrich (Gillingham, UK) and were of analytical grade. Microcrystalline cellulose (MCC) (Avicel PH101) was purchased from FMC (Pentre, UK), magnesium stearate was purchased from Merck (Darmstadt, Germany) and theophylline, the model drug used, was purchased from TCI Chemicals (Oxford, UK). pH 6.8 buffer solutions used for dissolution analysis consisting of sodium hydroxide and potassium phosphate monobasic were purchased from Fisher Scientific (Loughborough, UK) and Honeywell Fluka (Loughborough, UK), respectively. The pH 1.2 medium was prepared using hydrochloric acid purchased from Fisher Scientific (UK) and potassium chloride was purchased from Honeywell Fluka (Loughborough, UK).
2.2. Extraction of the baobab polysaccharide
718.6 g of the dried Nigerian baobab fruit sample was used in the extraction process. The polysaccharide was extracted on a bench scale according to the experimental design that was reported in ref. 20 and 21 with minor modifications. The first step was aqueous extraction (pH 6.0) at 80 °C for 1 h at a mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25. Subsequently, the extracted material was cooled and neutralised (pH 7) with sodium hydroxide at 25 °C, followed by centrifugation, rotary evaporation at 80 °C, ethanol precipitation and overnight storage. The precipitate was centrifuged, oven-dried at 60 °C for 1 h and finally extensively dialysed against distilled water (molecular weight cut-off: 12
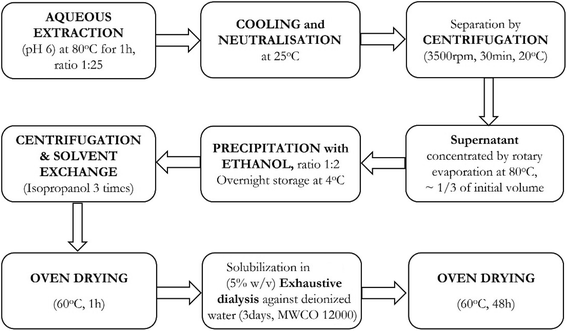
25. Subsequently, the extracted material was cooled and neutralised (pH 7) with sodium hydroxide at 25 °C, followed by centrifugation, rotary evaporation at 80 °C, ethanol precipitation and overnight storage. The precipitate was centrifuged, oven-dried at 60 °C for 1 h and finally extensively dialysed against distilled water (molecular weight cut-off: 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da) and oven dried at 60 °C for 48 h. This whole process is depicted in Fig. 1.
000 Da) and oven dried at 60 °C for 48 h. This whole process is depicted in Fig. 1.
The obtained polysaccharide from the extraction process was milled with a Retsch ball mill (Retsch MM400). A frequency of 30 Hz for 2 min was used to reduce the particle size of the produced extract and the final powder was passed through a 250 µm sieve, which was sealed and stored till required.
2.3. Solid-state characterisation of the baobab polysaccharide
2.4. Preparation of tablets and mechanical strength
A Tubular™ mixer (Willy A. Bachofen, Switzerland) was used to mix the Nigerian baobab polysaccharide powder, microcrystalline cellulose, theophylline and magnesium stearate as per the ratios in Table 1. Each batch was prepared using 5 g of powder mixture, which included all excipients and the active pharmaceutical ingredient. All the components shown in Table 1 without the magnesium stearate were mixed for 8 min to ensure uniformity, after which magnesium stearate was then added and then the final blend was mixed for a further 2 min.| Formulation code | Baobab polysaccharide (%) | Theophylline (%) | MCC (%) | Magnesium stearate (%) |
|---|---|---|---|---|
| B1 | 10 | 41.5 | 47.5 | 1 |
| B2 | 20 | 41.5 | 37.5 | 1 |
| B3 | 30 | 41.5 | 27.5 | 1 |
| B4 | 50 | 41.5 | 7.5 | 1 |
| B5 | 57.5 | 41.5 | 0.0 | 1 |
 | (1) |
2.5. Tablet swelling
The swelling behaviour of the baobab polysaccharide tablets was investigated using a digital camera (Leica ICC50HD) linked to Keyence image analysis software. A tablet compact was placed vertically in a plastic Petri dish, and 70 mL of deionized water was added at ambient temperature. The Petri dish was placed on a plane with a light source (tungsten lamp), and the camera, equipped with a macro lens, was placed above. The data were obtained at 20 min intervals for up to 120 min. The swelling index study was performed in triplicate.2.6. In vitro dissolution testing
The in vitro theophylline release was carried out using an automated USP Type II (paddle method) dissolution apparatus, Pharma Test DT 70 (PharmaTest, Germany). Dissolution vessels were filled with 900 mL of dissolution media (acid solution (pH 1.2) or buffer solution (pH 6.8)) equilibrated to a temperature of 37 ± 0.5 °C with a paddle speed of 50 rpm. Dissolution was studied from 10 min to 240 min, with the theophylline content in the samples primed automatically at a set time point by a pump into an attached UV spectrophotometer, where the theophylline content was measured with the UV spectrophotometer at 286 nm. All experiments were conducted in triplicate.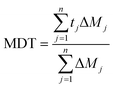 | (2) |
 | (3) |
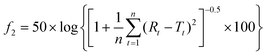 | (4) |
This is a mathematical treatment of the dissolution data, where n = number of pull points for tested samples; Rt = reference assay at time point t; and Tt = test assay at time point t.
The similarity factor was calculated using the drug release profile obtained from the formulation containing 57.5% baobab polysaccharide content (B5) as the reference standard. An f2 value ranging from 50 to 100 suggests a similarity in the test and the reference drug release profiles.7 The closer the f2 value is to 100, the more similar or identical the release profiles are. Additionally, dissimilarity occurs with a decrease in the f2 value.25
The zero-order model represents a constant rate of drug release, independent of the drug concentration,26,27 as shown in the following equation:
| Q = Q0 + k0t | (5) |
In contrast, in the first-order kinetics, the drug release is directly proportional to the remaining drug concentration,28 expressed as
| In(Qt) = In(Q0) + k1 | (6) |
The Higuchi model describes drug release in systems where the concentration of the drug exceeds its solubility, leading to release rates that follow the square root of time.29 This relationship is represented by
| Mt = kH√t | (7) |
The Korsmeyer–Peppas (power–law model) is used for cases where drug release mechanisms may involve diffusion, swelling, or matrix dissolution. In this model:
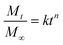 | (8) |
3. Results and discussion
3.1. Extraction and solid-state properties of the baobab polysaccharide
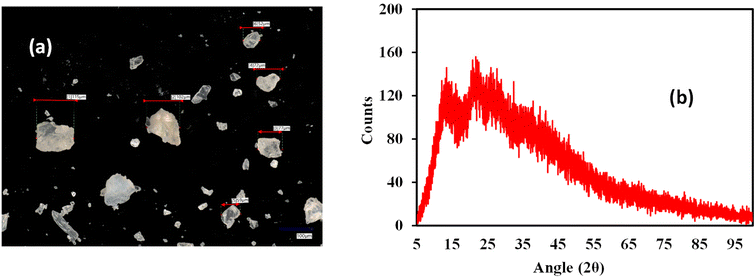
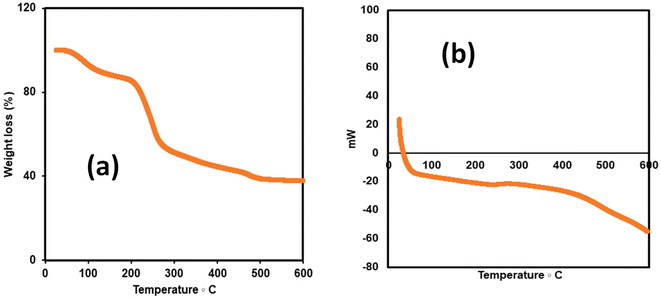
The extraction of polysaccharides from baobab fruit involved several steps, as shown in Fig. 1. The process resulted in 144 g (20% yield) of polysaccharide extract from the dried sample. Our bench-scale yield for Nigerian oblong baobab (20%) is lower than literature values for similar protocols (∼29–30% from oblong/oval fruits), but techno-economic analysis indicates viability when ethanol is recycled and utilities are optimised. Published models report minimum selling prices of ∼£23–£35 per 100 g in the UK at ∼30% yield and ∼£27 per 100 g in Nigeria, with steam cost being the dominant sensitivity.31 Using focus stacking digital microscopy, the maximum grain width of the extracted, milled and sieved polysaccharide was found to be 115 µm (Fig. 2a). The XRPD data for the extracted baobab polysaccharide suggest that it is completely amorphous in nature (Fig. 2b) with broad halos,32,33 which have also been observed in Grewia polysaccharides.30Thermal stability is an essential characteristic that determines the processing conditions and potential uses of polysaccharides. TGA and DSC analyses were carried out over a range of temperatures between 25 °C and 600 °C as shown in Fig. 3a and b. During the TGA analysis, weight loss occurred in three phases, which is a typical behaviour for polysaccharide decomposition.34 The first weight loss of approximately 13.1% is seen to occur due to water loss.30,34 When the temperature was increased to 175 °C, the TGA showed a major mass change of approximately 49%, which may be due to the deterioration of the polysaccharide structure at 250 °C.35,36 The final stage of degradation occurred between 300 °C and 500 °C with approximately 11% weight loss. The last two phases coincided with the broad endothermic enthalpy change in DSC, as seen in Fig. 3b. The char yield of the sample was 37%. The thermal analysis studies on the sample indicate that, after accounting for the initial water loss, the material is stable until around 175 °C, after which thermal degradation takes place.
3.2. Physical properties of the baobab polysaccharide and formulation blends and tablets
The true density of the pure baobab (milled baobab polysaccharide from a <250 µm sieve) was calculated to be 1.63 g cm−3 with the bulk and tap densities recorded as 0.73 ± 0.01 and 0.97 ± 0.01 g cm−3, respectively, giving the baobab polysaccharide a Carr's consolidation index of 29%, which is indicative of poor flow properties.The compaction profiles of the polysaccharide (milled baobab polysaccharide from a <250 µm sieve) from 5 kN to 15 kN are depicted in Fig. 4. This shows the tablet porosity and mechanical strength of the compacted baobab polysaccharide properties. Fig. 4 shows that an increase in the compression and compaction of the polysaccharide led to an increase in the mechanical strength of the compacts, which was accompanied by a simultaneous decrease in the porosity of the compacts. This increase in mechanical strength with an increase in compression has been observed and reported for other polysaccharides.22 This showed that the extracted polysaccharide is a compressible polysaccharide and therefore has the potential for use in tabletted formulations.
Formulations B1–B5 (Table 1) had true densities similar to that of the pure baobab polysaccharide (1.55–1.59 g cm−3). This slight decrease upon blending with other formulation components has also been observed and reported.30 It is important to note that all formulation blends were compacted at 10 kN only. It was observed that there was a general increase in the tablet's mechanical strength with an increase in the MCC content in the formulation (68–148 kN) (Fig. 5). MCC has been reported to be used widely for direct compression to reduce tablet density and has good mechanical strength.37,38 The porosities of the formulated blends ranged from 9–11% demonstrating a real impact of the formulation changes on the porosity of the compacts. This suggests that porosity may not necessarily be a contributing factor to potential dissolution performance.
 | ||
| Fig. 5 Formulated tablet properties of hardness and porosity using the baobab polysaccharide. Note: formulations B1–B5 have constituents as identified in Table 1. All experiments were conducted in triplicate. | ||
3.3. Tablet swelling
The swelling capacity of tablets compacted with the pure baobab polysaccharide powder was evaluated in deionized water. The process was set for the compacts to be evaluated for up to 120 min; however, the swelling of the compacts lasted for 45 min only. The compacts at this time point were observed to have lost their structure and began to gradually dissolve in the Petri dish (Fig. 6). A similar behaviour for Grewia polysaccharides has been reported in deionised media. The authors however reported different behaviours for the Grewia mollis polysaccharide at pH 12 and pH 6.8.22 Such observed behaviour can impact drug release. It has been reported in the literature that the composition of ions in a medium can impact drug release.7,22,25,30 | ||
| Fig. 6 Photograph of pure baobab polysaccharide tablets swelling in deionized water over a 45 min time period. All experiments were conducted in triplicate. | ||
3.4. Theophylline release from baobab polysaccharide tablets
Drug release in acidic media is shown in Fig. 7a. Fig. 7a illustrates how the acid solution used as the release medium influences theophylline release from the tablet matrices with five differing baobab concentrations. The solubility of theophylline is largely unchanged over the pH range studied. Theophylline's solubility at pH 1.2 has been reported to be 13.1 ± 0.1 mg ml−1 and 10.5 ± 0.1 mg ml−1 at pH 6.8.39 There was a gradual release of theophylline from all the tablet matrices (B2–B5) except for those containing 10% baobab (B1), which showed a relatively faster release of theophylline. It is important to note that all tablets contain the same amounts of theophylline content (41.5%) and magnesium stearate (1%). After about 10 min in acidic media, the profiles of B2–B5 showed that 12.6% of theophylline was released from the tablets containing 20% baobab with 37.5% MCC (B2), 11.8% of theophylline was released from tablets containing 30% baobab with 27.5% MCC, 9.43% of theophylline was released from tablets containing 50% baobab and 9.9% from tablets containing 57.5% baobab with no MCC, indicating no burst release. This shows a gradual decrease in the initial release of the model drug with an increase in baobab content.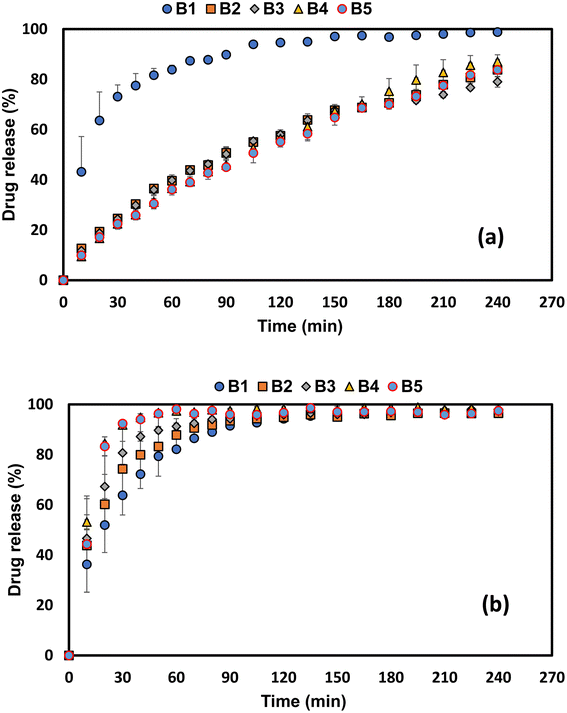 | ||
| Fig. 7 Effect of theophylline release from baobab polysaccharide matrices in acidic media (a) and basic media (b). All experiments were conducted in triplicate. Note: formulation codes B1–B5 are explained in Table 1. | ||
The drug release after 240 min for formulations B2–B5 are relatively similar (Fig. 7a). This is also confirmed in Table 2, where the dissolution parameters of DE (52–54%), MDT (78–92 min) and MDR (0.37–0.40% min−1) are similar. Using drug release from B5 (baobab-only matrices) as the reference standard, f2 values indicated similarity (71–77%). It was not possible to determine MDT and MDR for the B1 dissolution profile due to its quick release. Its profile was dissimilar to the reference standard (B5) used (f2 = 18%). The faster release also made it impossible to determine its kinetics of drug release. When baobab was used alone with no further excipients, the matrices had an n value of 0.68, which suggests that anomalous transport occurred. This was also the case for formulations B2–B4. It was however interesting to note that there was an increase in the contribution of erosion with an increase in baobab content associated with a decrease in the MCC content. The incorporation of MCC therefore seems to be really advantageous for improving the tablet hardness of the manufactured matrices as well, indicating to a formulator that there may well be a concentration where it might be inadequate or not be beneficial as in the case of formulation B1.
| Formulation code | Powder formulation | MDT (min) | DE (%) | MDR (% min−1) | Similarity factor (f2) | Zero order (RSQ) | First order (RSQ) | Higuchi (RSQ) | Peppas (RSQ) | Diffusion exponent (n) |
|---|---|---|---|---|---|---|---|---|---|---|
| All experiments were conducted in triplicate. | ||||||||||
| B1 | 10% BBP + 47.5% MCC + drug | — | 15.88 | — | 17.94 | — | — | — | — | — |
| B2 | 20% BPP + 37.5% MCC + drug | 84.53 | 54.28 | 0.38 | 73.18 | 0.970 | 0.993 | 0.996 | 0.997 | 0.625 |
| B3 | 30% BPP + 27.5% MCC + drug | 77.78 | 53.41 | 0.37 | 71.26 | 0.972 | 0.995 | 0.998 | 0.997 | 0.653 |
| B4 | 50% BPP + 7.5% MCC + drug | 89.05 | 51.76 | 0.40 | 77.26 | 0.985 | 0.998 | 0.997 | 0.997 | 0.710 |
| B5 | 57.5% BPP + drug | 91.62 | 51.79 | 0.37 | — | 0.981 | 0.998 | 0.997 | 0.997 | 0.676 |
In contrast to drug release in acidic media, in buffer, the tablets containing 10% baobab with 47.5% MCC (B1) released approximately 36.3% of theophylline in the first 10 min, 43.8% of theophylline was released from the 20% baobab with 37.5% MCC (B2), and 46.6% of theophylline was released from the 30% baobab with 27.5% MCC (B3). The tablets containing 50% baobab powder with 7.5% MCC (B4) released approximately 53.2% of theophylline. This showed that an increase in the baobab content contributed to the burst release observed in the phosphate buffer media. Of interest, however, was the behaviour of the baobab-only matrices (B5), i.e., the tablets containing 57.5% baobab with no MCC content. These matrices released approximately 44% of theophylline after 10 min but released their total drug content relatively faster than all the matrices with MCC content. This suggests that MCC may have a beneficial effect in reducing burst release in phosphate buffer media. The faster release of theophylline (after 240 min, the tablet matrices were completely dissolved for all five formulations) meant that it was not possible to calculate dissolution parameters and kinetics for drug release in the buffer. The viscosity of baobab fruit polysaccharide has been studied in both acidic and basic media using a Kinexus rheometer (Malvern Instruments, UK) fitted with a 20 mm plate-plate geometry and a 1 mm gap.21 The authors reported that an increase in temperature by applying the time–temperature superposition principle results in a monotonic reduction of both viscoelastic functions (G′, G″). The authors also found that although the shape of the curves produced remained unchanged (meaning no measurable structural changes), the construction of the master curves of viscoelasticity revealed significant differences in the relaxation behaviour of samples at different pH values. The samples in acidic media were found to be typical of entangled viscoelastic chains, indicating a strong frequency dependency.21 This behaviour is thus attributed to the observed performance of the polysaccharides in different media. It is therefore anticipated that the baobab polysaccharide in combination with the MCC therefore forms a swelling gel matrix which hinders drug diffusion, slows down the drug release, and thus eliminates significant burst release. This behaviour has been observed in the literature for hydrophilic matrices as acidic media enhance hydrogen bonding and gel formation.40,41 This suggests that the baobab polysaccharide might not be suitable for targeting drug release in the lower small intestine or colon. These dissolution behaviours also explain why porosity is not a likely dominant release-controlling factor.
4. Conclusion
The extraction process for the baobab polysaccharide was efficient and therefore feasible for large-scale production. The extracted polysaccharide however had poor flow properties, as indicated by its true density and Carr's index. When incorporated into tablet formulations as a potential matrix former, it is compressible and forms tablets or compacts with adequate mechanical strength. In acidic media, tablets with higher baobab content (formulations B2–B5) demonstrated a slower, more controlled release of theophylline. The absence of burst release in formulations with higher baobab content (B4 and B5) further supports its potential for use in controlled release systems. In basic media, tablets with higher baobab content (B4 and B5) depicted a faster release of theophylline, with the drug releasing relatively quickly. This suggests that while the baobab polysaccharide can be effective for controlled release in acidic environments, it might not be suitable for targeting drug release in the lower small intestine or colon. These findings support the feasibility of a baobab-derived, renewable polysaccharide as an excipient aligned with our initial motivation to use green materials in pharmaceutical applications. Bench-scale extraction delivered approximately 20% yield, and the material formed mechanically robust, compressible tablets and provided sustained release in acidic medium with faster release in basic medium. This demonstrates the suitability of the formulations across conditions relevant to oral delivery. Moreover, these regionally sourced biopolymers could help diversify excipient supply chains where access to conventional materials is limited. Future work should address process standardisation, batch-to-batch variability, and safety/regulatory evaluation to translate this potential into practice.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Data availability
The authors confirm that the data supporting the findings of this study are available within the article.Acknowledgements
The authors are grateful to the University of Huddersfield for support.References
- C. E. Beneke, A. M. Viljoen and J. H. Hamman, Molecules, 2009, 14, 2602–2620 CrossRef PubMed.
- D. A. Bhaskar, K. J. Uttam, A. Mahendrasingh, C. M. Jayram and S. R. Bhanudas, J. Adv. Pharm. Educ. Res., 2013, 3, 387–402 Search PubMed.
- M. Naghshineh, K. Olsen and C. A. Georgiou, Food Chem., 2013, 136, 472–478 CrossRef PubMed.
- M. Wongkaew, B. Tinpovong, K. Sringarm, N. Leksawasdi, K. Jantanasakulwong, P. Rachtanapun and S. R. Sommano, Foods, 2021, 10, 627 CrossRef PubMed.
- P. M. Nadar, M. A. Merrill, K. Austin, S. M. Strakowski and J. M. Halpern, Transl. Psychiatry, 2022, 12, 372 CrossRef PubMed.
- M. J. Abdelhak, J. Adv. Bio-Pharm. Pharmacovigil., 2019, 1, 15–25 Search PubMed.
- E. I. Nep, N. Kaur, S. Shaboun, A. O. Adebisi, A. M. Smith, B. R. Conway and K. Asare-Addo, Colloids Surf., B, 2020, 188, 110809 CrossRef CAS PubMed.
- K. Alba, M. Dimopoulou and V. Kontogiorgos, LWT–Food Sci. Technol., 2021, 144, 111235 CrossRef CAS.
- L. I. Atanase, Polymers, 2021, 13, 477 CrossRef CAS PubMed.
- K. Alba, P. T. Nguyen and V. Kontogiorgos, Food Hydrocolloids, 2021, 118, 106749 CrossRef CAS.
- X. N. Li, J. Sun, H. Shi, L. L. Yu, C. D. Ridge, E. P. Mazzola and P. Chen, Food Res. Int., 2017, 99, 755–761 CrossRef CAS.
- H. Bougatef, F. Tandia, A. Sila, A. Haddar and A. Bougatef, S. Afr. J. Bot., 2023, 157, 387–397 CrossRef CAS.
- K. Alba, V. Offiah, A. P. Laws, K. O. Falade and V. Kontogiorgos, Food Hydrocolloids, 2020, 106, 105874 CrossRef CAS.
- F. M. Kpodo, J. K. Agbenorhevi, K. Alba, A. M. Smith, G. A. Morris and V. Kontogiorgos, Int. J. Biol. Macromol., 2019, 122, 866–872 CrossRef CAS PubMed.
- O. A. Patova, A. Luanda, N. M. Paderin, S. V. Popov, J. J. Makangara, S. P. Kuznetsov and E. N. Kalmykova, Carbohydr. Polym., 2021, 262, 117946 CrossRef CAS.
- C. D. May, Carbohydr. Polym., 1990, 12, 79–99 CrossRef.
- J. Fan, K. Wang, M. Liu and Z. He, Carbohydr. Polym., 2008, 73, 241–247 CrossRef.
- J. Liu, S. Willför and C. Xu, Bioact. Carbohydr. Diet. Fibre, 2015, 5, 31–61 CrossRef.
- R. Rojas, J. C. Contreras-Esquivel, M. T. Orozco-Esquivel, C. Muñoz, J. A. Aguirre-Joya and C. N. Aguilar, Waste Biomass Valorization, 2015, 6, 1095–1102 CrossRef.
- M. Dimopoulou, K. Alba, G. Campbell and V. Kontogiorgos, J. Sci. Food Agric., 2019, 99, 6191–6198 CrossRef PubMed.
- M. Dimopoulou, K. Alba, I. M. Sims and V. Kontogiorgos, Carbohydr. Polym., 2021, 273, 118540 CrossRef PubMed.
- E. I. Nep, K. Asare-Addo, M. U. Ghori, B. R. Conway and A. M. Smith, Int. J. Pharm., 2015, 496, 689–698 CrossRef.
- H. Al-Hamidi, A. A. Edwards, D. Douroumis, K. Asare-Addo, A. M. Nayebi, S. Reyhani-Rad and A. Nokhodchi, Colloids Surf., B, 2013, 103, 189–199 CrossRef PubMed.
- M. M. Talukdar and R. Kinget, Int. J. Pharm., 1995, 120, 63–72 CrossRef.
- K. Asare-Addo, M. Levina, A. R. Rajabi-Siahboomi and A. Nokhodchi, Colloids Surf., B, 2010, 81, 452–460 CrossRef PubMed.
- S. Dash, P. N. Murthy, L. Nath and P. Chowdhury, Acta Pol. Pharm., 2010, 67, 217–223 Search PubMed.
- P. Paarakh, P. Jose, C. Setty and P. Christoper, Release Kinetics - Concepts and Applications, 2018 Search PubMed.
- J. Siepmann and N. A. Peppas, Adv. Drug Delivery Rev., 2001, 48, 139–157 CrossRef.
- J. Siepmann and F. Siepmann, Int. J. Pharm., 2013, 453, 12–24 CrossRef PubMed.
- E. I. Nep, M. H. Mahdi, A. O. Adebisi, C. Dawson, K. Walton, P. J. Bills and K. Asare-Addo, Int. J. Pharm., 2017, 532, 352–364 CrossRef PubMed.
- M. Dimopoulou, V. Offiah, K. Falade, A. M. Smith, V. Kontogiorgos and A. Angelis-Dimakis, Sustainability, 2021, 13, 9915 CrossRef CAS.
- A. Charlesby, J. Polym. Sci., 1953, 11, 521–529 CrossRef CAS.
- N. S. Murthy and F. Reidinger, X-ray Analysis. A Guide to Materials, 1996 Search PubMed.
- B. A. Stone, B. Svensson, M. E. Collins and R. A. Rastall, Glycoscience, 2008, 2325 Search PubMed.
- Y. Peng and S. Wu, J. Anal. Appl. Pyrolysis, 2010, 88, 134–139 CrossRef CAS.
- M. S. Iqbal, S. Massey, J. Akbar, C. M. Ashraf and R. Masih, Food Chem., 2013, 140, 178–182 CrossRef CAS.
- M. El-Sakhawy and M. L. Hassan, Carbohydr. Polym., 2007, 67, 1–10 CrossRef CAS.
- N. Saigal, S. Baboota, A. Ahuja and J. Ali, J. Young Pharm., 2009, 1, 6 CrossRef CAS.
- K. Asare-Addo, B. R. Conway, M. J. Hajamohaideen, W. Kaialy, A. Nokhodchi and H. Larhrib, Colloids Surf., B, 2013, 111, 24–29 CrossRef CAS PubMed.
- K. M. Keogh, F. J. O'Brien and N. J. Dunne, Front. Mater., 2021, 8, 752813 CrossRef.
- Y. Qiu and K. Park, Polymers, 2017, 9, 137 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2026 |