 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
N-to-C peptide elongation by ammonia-Ugi reaction: synthesis of potent self-assembling elastin-like short peptides
Keisuke Tomohara *ab,
Naoki Tanakac,
Yukino Kotakea,
Miwa Ushiroa,
Misato Adachia,
Hikaru Kimuraa,
Hisanori Nambu
*ab,
Naoki Tanakac,
Yukino Kotakea,
Miwa Ushiroa,
Misato Adachia,
Hikaru Kimuraa,
Hisanori Nambu *a and
Takeru Nose
*a and
Takeru Nose *bc
*bc
aFaculty of Pharmaceutical Sciences, Kyoto Pharmaceutical University, 1 Misasagishichono-cho, Yamashina-ku, Kyoto 607-8412, Japan. E-mail: tomohara47@mb.kyoto-phu.ac.jp
bFaculty of Arts and Science, Kyushu University, 744 Motooka, Nishi-ku, Fukuoka 819-0395, Japan
cDepartment of Chemistry, Graduate School of Science, Kyushu University, 744 Motooka, Nishi-ku, Fukuoka 819-0395, Japan
First published on 15th December 2025
Abstract
The ammonia-Ugi reaction employing ammonium carboxylates of N-protected amino acids (or peptides), ketones, and α-isocyano esters enabled N-to-C peptide elongation, together with in situ construction of α,α-disubstituted amino acid residues. This method offered an effective synthetic method of novel elastin-like short peptides, which exhibited highly potent self-assembling properties.
Peptides possess considerable potential both as drug delivery vehicles and therapeutic agents against pharmacologically diverse and otherwise intractable biological targets.1 Incorporation of unnatural α,α-disubstituted amino acid residues into peptides can significantly impact their metabolic resistance, hydrophobicity, membrane permeability, biological activity and selectivity, and immunogenicity, ultimately enhancing the druggability of peptide-based therapeutics.2
Peptide synthesis is typically performed via stepwise coupling of N-protected amino acids to a growing peptide chain anchored on a solid support using condensation agents.3 Although such a solid-phase peptide synthesis (SPPS) works well with proteinogenic and other α-monosubstituted amino acids, it often results in poor yields with sterically hindered α,α-disubstituted amino acids, requiring excessive amounts of coupling agents under harsh conditions such as heating or microwave irradiation.4 Moreover, preparation of N-protected α,α-disubstituted amino acids generally requires multi-step and tedious processes. Alternatively, highly reactive condensation agents5 and catalytic reaction systems6 have offered efficient methods for unnatural peptide synthesis; however, these still use expensive unnatural α,α-disubstituted amino acids as substrates. These synthetic challenges have long posed significant barriers to the discovery and development of bioactive peptides.
The ammonia-Ugi reaction, a variant of the Ugi reaction,7,8 is a four-component coupling reaction that involves ammonia, an aldehyde or a ketone, a carboxylic acid, and an isocyanide, enabling a straightforward synthesis of peptides.9 Although the ammonia-Ugi reaction had long been considered impractical and unsuccessful,10 we recently reported an efficient synthetic protocol of unnatural dipeptides using the ammonia-Ugi reaction: by stirring ammonium carboxylates derived from N-protected amino acids 1, ketones, and isocyanides in trifluoroethanol (TFE) at ambient temperature, a variety of dipeptides 2 were obtained in good yields (Scheme 1a).11 Of note, these dipeptides 2 contained unnatural α,α-disubstituted amino acids, which were constructed in situ during the ammonia-Ugi reaction from readily available ketones as building blocks. Mechanistically, ammonium (NH4+) dissociates into NH3 and H+, which in turn facilitates the thermodynamically unfavourable N-unsubstituted imine formation (Scheme 1a).12 With its success, however, the products 2 were obtained as amides at their C-termini, rendering them unsuitable for further N-to-C peptide elongation. To the best of our knowledge, neither the ammonia-Ugi reaction nor the Ugi reaction has ever been applied for N-to-C peptide chain elongation.13,14 Here, this study proposes a novel strategy for N-to-C elongation of unnatural peptides by the ammonia-Ugi reaction employing α-isocyano ester 3 as an elongation unit (Scheme 1b). The expected ammonia-Ugi adducts 4 possess ester moieties at their C-termini, providing a versatile platform for subsequent peptide chain elongation.
 | ||
| Scheme 1 (a) Dipeptide synthesis by ammonia-Ugi reaction, (b) N-to-C peptide elongation by sequential ammonia-Ugi reaction (this study). | ||
Using a commercially available ethyl isocyanoacetate (3, CN-Gly-OEt), a series of unnatural tripeptides 4 were synthesized (Scheme 2). The key ammonium carboxylates of N-protected amino acids 1 were prepared by stirring N-protected amino acids with aqueous ammonia in acetonitrile or THF at 0 °C (Table S1).11 Then, the ammonia-Ugi reaction using Boc-Phe-ONH4, cyclopentanone, and CN-Gly-OEt (3) afforded the tripeptide Boc-Phe-Ac5c-Gly-OEt (4a) in 93% yield, with no detectable racemization of the chiral α-carbon of phenylalanine (>99.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 er). Herein, the unnatural 1-aminocyclopentane-1-carboxylic acid (Ac5c) residue in 4a was constructed in situ from cyclopentanone as a substrate. In general, diethylglycine (Deg) is a challenging substrate to be incorporated into peptides under conventional SPPS conditions because of the steric hindrance,15 whereas the present ammonia-Ugi reaction successfully delivered Boc-Phe-Deg-Gly-OEt (4b) in 87% yield. Again, no racemization was observed during this process (>99.5
0.5 er). Herein, the unnatural 1-aminocyclopentane-1-carboxylic acid (Ac5c) residue in 4a was constructed in situ from cyclopentanone as a substrate. In general, diethylglycine (Deg) is a challenging substrate to be incorporated into peptides under conventional SPPS conditions because of the steric hindrance,15 whereas the present ammonia-Ugi reaction successfully delivered Boc-Phe-Deg-Gly-OEt (4b) in 87% yield. Again, no racemization was observed during this process (>99.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 er). The chiral α-carbon of serine is known to be susceptible to racemization during peptide synthesis;16 however, the present reaction conditions afforded 4c in an excellent yield (97%) with a perfect stereochemical integrity (>99.5
0.5 er). The chiral α-carbon of serine is known to be susceptible to racemization during peptide synthesis;16 however, the present reaction conditions afforded 4c in an excellent yield (97%) with a perfect stereochemical integrity (>99.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 er). Even the sterically demanding Boc-Aib was compatible with the present reaction conditions, affording the sterically congested tripeptide 4d, composed of contiguous α,α-disubstituted amino acids {α-aminoisobutyric acid (Aib) and α-methylhomophenylalanine [(αMe)Hph]}, in 78% yield. In addition to Boc, various N-protecting groups, including Cbz, Bz, and formyl (For), were well tolerated, giving the tripeptides 4e, 4f, and 4g in 83%–99% yields. Unfortunately, the stereochemical integrity of Bz-Phe was slightly lost (98
0.5 er). Even the sterically demanding Boc-Aib was compatible with the present reaction conditions, affording the sterically congested tripeptide 4d, composed of contiguous α,α-disubstituted amino acids {α-aminoisobutyric acid (Aib) and α-methylhomophenylalanine [(αMe)Hph]}, in 78% yield. In addition to Boc, various N-protecting groups, including Cbz, Bz, and formyl (For), were well tolerated, giving the tripeptides 4e, 4f, and 4g in 83%–99% yields. Unfortunately, the stereochemical integrity of Bz-Phe was slightly lost (98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 er), whereas that of Ac-Phe remained intact under the same conditions.11 It is worth noting that the N-formyl group in 4g can serve as a precursor of an isocyano group, potentially enabling inverse C-to-N peptide elongation.14 Scale-up experiments (up to 5.4 mmol) proceeded smoothly without any detrimental effects on the reaction system, providing the corresponding products 4a, 4b, and 4g in excellent yields (83%–93%). Overall, the ammonia-Ugi reaction using α-isocyano ester and N-protected amino acids enabled the efficient synthesis of unnatural tripeptides containing α,α-disubstituted amino acids. These tripeptides possess ester groups at their C-termini, offering a platform for subsequent N-to-C peptide chain elongation.
2 er), whereas that of Ac-Phe remained intact under the same conditions.11 It is worth noting that the N-formyl group in 4g can serve as a precursor of an isocyano group, potentially enabling inverse C-to-N peptide elongation.14 Scale-up experiments (up to 5.4 mmol) proceeded smoothly without any detrimental effects on the reaction system, providing the corresponding products 4a, 4b, and 4g in excellent yields (83%–93%). Overall, the ammonia-Ugi reaction using α-isocyano ester and N-protected amino acids enabled the efficient synthesis of unnatural tripeptides containing α,α-disubstituted amino acids. These tripeptides possess ester groups at their C-termini, offering a platform for subsequent N-to-C peptide chain elongation.
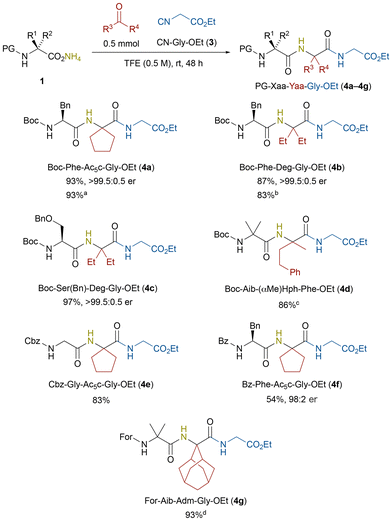 | ||
Scheme 2 Synthesis of tripeptides 4a–4g by ammonia-Ugi reaction using ammonium carboxylates of N-protected amino acids. a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.4 mmol, b 5.4 mmol, b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.5 mmol, c 3.5 mmol, c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mmol, d 1 mmol, d![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.5 mmol. 4.5 mmol. | ||
Peptides containing glycine are frequently found in biomaterials17 such as elastin,18 collagen,19 and silk fibroin.20 Owing to glycine's small size and conformational flexibility, it plays a crucial role in modulating peptide structure and function. The development of efficient synthetic strategies for unnatural analogues of glycine-rich peptides is therefore of significant interest in both synthetic and biomedical research. In this study, we developed an efficient N-to-C peptide elongation method for synthesizing glycine-containing unnatural peptides by the ammonia-Ugi reaction employing CN-Gly-OEt (3) (Scheme 3). The C-terminal ester moieties of tripeptides 4a and 4b were hydrolyzed under basic conditions and subsequently treated with aqueous ammonia to give the corresponding ammonium carboxylates 5a and 5b in excellent yields. Under the present ammonia-Ugi reaction conditions, both tripeptides were successfully elongated into the pentapeptides Boc-Phe-Xaa-Gly-Aib-Gly-OEt 6a and 6b in good yields, together with in situ construction of Aib residue from acetone. The resulting peptides again possess ester moieties at C-termini, potentially providing a versatile platform for further N-to-C elongation or other chemical modifications. Totally, starting from N-protected amino acids 1, the first ammonia-Ugi reaction furnished tripeptides 4 (Scheme 2), and the second ammonia-Ugi reaction extended them to pentapeptides 6 (Scheme 3). Each step elongated peptides by two amino acid residues at a time while constructing α,α-disubstituted amino acids in situ. This streamlined approach requires no condensation agents, thus providing an environmentally friendly synthetic method of glycine-containing unnatural peptides.
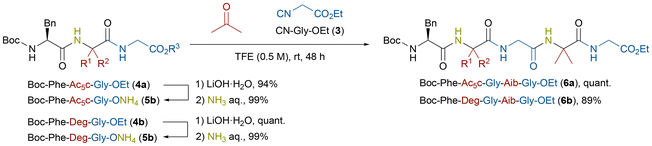 | ||
| Scheme 3 Synthesis of pentapeptides 6a and 6b by ammonia-Ugi reaction starting from ammonium carboxylates of N-protected tripeptides 4a and 4b. | ||
The obtained pentapeptides 6a and 6b share the repeating amino acid sequence of a short elastin-like peptide (sELP) H-(Phe-Pro-Gly-Val-Gly)n-NH2 (7, Scheme 4a).21 sELP exhibits reversible lower critical solution temperature (LCST)-type behaviour, being soluble at low temperatures and insoluble at high temperatures.22 Such reversible temperature-responsive and self-assembling properties make sELP a promising candidate for drug delivery applications. The development of unnatural analogs with enhanced self-assembling properties is therefore of considerable interest. Herein, we designed a novel unnatural analogue, H-(Phe-Ac5c-Gly-Aib-Gly)2-NH2 (8a), by modifying the original sELP sequence (Scheme 4b). Specifically, the Pro-2 in 7 was replaced with Ac5c, a noncanonical amino acid structurally related to Pro, and the Val-4, located at a guest position in the original sequence,23 was substituted with Aib, a noncanonical amino acid analogous to Val. The decapeptide 8a was synthesized in a good yield via segment coupling between Boc-Phe-Ac5c-Gly-Aib-Gly-OH (9a) and H-Phe-Ac5c-Gly-Aib-Gly-NH2 (10a) under standard condensation conditions, followed by Boc deprotection under acidic conditions. We also designed and synthesized a structurally related analogue, H-(Phe-Deg-Gly-Val-Gly)2-NH2 (8b), in which Ac5c residue in 8a was replaced with Deg, a noncyclic analogue of Ac5c. Both 8a and 8b were obtained as TFA salts in pure form after purification by reversed-phase (RP)-HPLC (Fig. S1). Additionally, we designed and synthesized H-(Phe-Pro-Gly-Aib-Gly)2-NH2 (11) as an Aib analogue of the original sELP 7 via a standard SPPS protocol (see, SI).
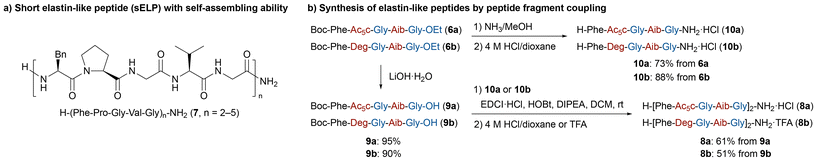 | ||
| Scheme 4 (a) Chemical structure of short elastin-like peptide (sELP) 7, (b) synthesis of unnatural sELP analogues 8a and 8b by conventional peptide segment coupling. | ||
The self-assembling properties of peptides 8a, 8b, and 11 were then investigated under buffered aqueous conditions. Each peptide was dissolved in phosphate buffer containing NaCl (pH 7.4; 27.4 mM Na2HPO4, 17.8 mM NaH2PO4, and 3M NaCl), and their turbidities at 400 nm were recorded upon increasing and decreasing temperatures (Fig. 1a, b and Table S2). The results revealed that these synthetic peptides 8a and 8b exhibited reversible LCST behaviour at concentrations of approximately 1–3.5 mM. The control peptide 11 showed the similar behaviour; however, it required significantly higher concentrations to initiate aggregation compared to the peptides 8a and 8b (Fig. 1c). Herein, the transition temperature (Tt) was defined as the temperature at which turbidity reached half of its maximum value during heating. The relationship between Tt and peptide concentration of 8a and 8b fitted well to a power function (Tt = aCb), while that of 11 followed a conventional logarithmic function (Tt = a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log(C) + b) as previously demonstrated for ELPs, where C is peptide concentration and a and b are constants (Fig. 1d).24 A clear correlation between peptide concentration and Tt demonstrated that the self-assembling capabilities of unnatural analogues 8a and 8b were approximately 100-fold stronger than that of reference peptide 11 (Fig. 1d). The reversibility of structural changes in 8a and 8b was roughly investigated by circular dichroism measurements (Fig. S2 and S3). Furthermore, the self-assembling behaviours were confirmed by bright-field microscopy (Fig. S4) and dynamic light scattering measurements (Fig. S5). Taken together, both peptides 8a and 8b were found to form aggregates above their respective Tt values. Ultra performance liquid chromatography (UPLC)-MS analysis showed peptides 8a and 8b were more hydrophobic than peptide 11 (Fig. S1), suggesting that the increased hydrophobicity of 8a and 8b may contribute to their enhanced self-assembling properties.
log(C) + b) as previously demonstrated for ELPs, where C is peptide concentration and a and b are constants (Fig. 1d).24 A clear correlation between peptide concentration and Tt demonstrated that the self-assembling capabilities of unnatural analogues 8a and 8b were approximately 100-fold stronger than that of reference peptide 11 (Fig. 1d). The reversibility of structural changes in 8a and 8b was roughly investigated by circular dichroism measurements (Fig. S2 and S3). Furthermore, the self-assembling behaviours were confirmed by bright-field microscopy (Fig. S4) and dynamic light scattering measurements (Fig. S5). Taken together, both peptides 8a and 8b were found to form aggregates above their respective Tt values. Ultra performance liquid chromatography (UPLC)-MS analysis showed peptides 8a and 8b were more hydrophobic than peptide 11 (Fig. S1), suggesting that the increased hydrophobicity of 8a and 8b may contribute to their enhanced self-assembling properties.
Conclusions
This study, although preliminary, developed a streamlined N-to-C peptide elongation method for synthesizing unnatural peptides using the ammonia-Ugi reaction. Starting from readily available N-protected amino acids, ketones, and α-isocyano ester as building blocks, a series of tripeptides 4a–4g were synthesized in high yields and with excellent stereochemical integrity. Notably, the sterically demanding residues such as Aib, Ac5c, (αMe)Hph, and Deg were constructed in situ from simple ketones, and successfully incorporated into peptides, overcoming limitations of conventional peptide synthesis. The tripeptides 4 were further elongated into pentapeptides 6 via the second ammonia-Ugi reaction, demonstrating the method's versatility. Interestingly, both 8a and 8b exhibited reversible LCST behaviour at approximately 100-fold lower concentrations than 11, due probably to the increased hydrophobicity. Future work will focus on synthesis of longer peptides and more sterically congested peptides using α-isocyano α,α-disubstituted amino acids. Extensions in these directions will be reported from our laboratory in due course.Author contributions
KT, conceptualization, investigation, methodology, and writing – original draft; KT, NT, YK, MU, MA, HK, investigation; HN and TN, supervision and writing – review & editing.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information: experimental procedures, supplementary figures and tables, NMR spectra of all new compounds, and HPLC charts. See DOI: https://doi.org/10.1039/d5ob01834j.Acknowledgements
This study was financially supported by JSPS KAKENHI Grant No. JP25K09905 (KT) and JP24K03120 (TN), and the UBE foundation (KT), Kyoto Health and Science Research Center (KT), and JST Adaptable and Seamless Technology transfer Program through Target-driven R&D (A-STEP) Grant Number JPMJTM22EA (KT). We thank Dr Yasunao Hattori (Kyoto Pharmaceutical University) for HRMS measurements and Dr Kazuya Kobayashi (Kyoto Pharmaceutical University) for insightful advice on peptide synthesis.References
- For selected reviews, see: S. Li, J. C. M. van Hest, R. Xing and X. Yan, Chem. Commun., 2025, 61, 13841 Search PubMed; K. Sharma, K. K. Sharma, A. Sharma and R. Jain, Drug Discovery Today, 2023, 28, 103464 Search PubMed; A. D. Cunningham, N. Qvit and D. Mochly-Rosen, Curr. Opin. Chem. Biol., 2017, 44, 59 Search PubMed; L. Nevola and E. Giralt, Chem. Commun., 2015, 51, 3302 Search PubMed.
- For selected reviews, see: T. Misawa, N. Ohoka, M. Oba, H. Yamashita, M. Tanaka, M. Naito and Y. Demizu, Chem. Commun., 2019, 54, 7792 Search PubMed; B. C. Doak, J. Zheng, D. Dobritzsch and J. Kihlberg, J. Med. Chem., 2016, 59, 2312 Search PubMed.
- R. B. Merrifield, J. Am. Chem. Soc., 1963, 85, 2149 Search PubMed.
- F. Zieleniewski, D. N. Woolfson and J. Clayden, Chem. Commun., 2020, 56, 12049 Search PubMed.
- For recent examples, see: T. Liu, Z. Peng, M. Lai, L. Hu and J. Zhao, J. Am. Chem. Soc., 2024, 146, 4270 Search PubMed; D. Uehara, S. Adachi, A. Tsubouchi, Y. Okada, V. V. Zhdankin, A. Yoshimura and A. Saito, Chem. Commun., 2024, 60, 956 Search PubMed; J. Yang, D. Zhang, Y. Chang, B. Zhang, P. Shen, C. Han and J. Zhao, Org. Chem. Front., 2024, 11, 5422 Search PubMed; Z. Wang, X. Wang, P. Wang and J. Zhao, J. Am. Chem. Soc., 2021, 143, 10374 Search PubMed.
- M. Todorovic and D. M. Perrin, Pept. Sci., 2020, 112, e24210 Search PubMed.
- I. Ugi, Angew. Chem., Int. Ed. Engl., 1962, 1, 8 Search PubMed; A. Dömling and I. Ugi, Angew. Chem., Int. Ed., 2000, 39, 3168 Search PubMed.
- For recent examples, see: J. Zhang, P. Yu, S.-Y. Li, H. Sun, S.-H. Xiang, J. Wang, K. N. Houk and B. Tan, Science, 2018, 361, eaas8707 Search PubMed; K. Tomohara, N. Ohashi, T. Uchida and T. Nose, Sci. Rep., 2022, 12, 15568 Search PubMed.
- T. M. Vishwanatha, K. Kurpiewska, J. Kalinowska-Tłuścik and A. Dömling, J. Org. Chem., 2017, 82, 9585 Search PubMed; A. L. Brown, Q. I. Churches and C. A. Hutton, J. Org. Chem., 2015, 80, 9831 Search PubMed; S. de Castro, M.-J. Camarasa, J. Balzarini and S. Velázquez, Eur. J. Med. Chem., 2014, 83, 174 Search PubMed; M. Abbas and L. A. Wessjohann, Org. Biomol. Chem., 2012, 10, 9330 Search PubMed; S. Ackermann, H.-G. Lerchen, D. Häbich, A. Ullrich and U. Kazmaier, Beilstein J. Org. Chem., 2012, 8, 1652 Search PubMed; R. Pick, M. Bauer, U. Kazmaier and C. Hebach, Synlett, 2005, 757 Search PubMed; U. Kazmaier and C. Hebach, Synlett, 2003, 1591 Search PubMed; M. Waki and J. Meienhofer, J. Am. Chem. Soc., 1977, 99, 6075 Search PubMed.
- I. P. Bhela, M. Serafini, E. D. Grosso, G. C. Tron and T. Pirali, Org. Lett., 2021, 23, 3610 Search PubMed.
- K. Tomohara, S. Kusaba, M. Masui, T. Uchida, H. Nambu and T. Nose, Org. Biomol. Chem., 2024, 22, 6999 Search PubMed; K. Tomohara, T. Nose and H. Nambu, Synlett, 2025, 2671 Search PubMed.
- Y. Kondo, H. Morimoto and T. Ohshima, Synlett, 2024, 379 Search PubMed.
- K. Ghosh and W. D. Lubell, J. Pept. Sci., 2025, 31, e70019 Search PubMed.
- Lubell and co-workers reported inverse C-to-N peptide elongation by the ammonia-Ugi reaction. F. M. Mir, M. Crisma, C. Toniolo and W. D. Lubell, Chem. Sci., 2019, 10, 6908 Search PubMed.
- M. D. Zotti and J. Clayden, Org. Lett., 2019, 21, 2209 Search PubMed.
- Y. E. Jad, S. N. Khattab, B. G. de la Torre, T. Govender, H. G. Kruger, A. El-Faham and F. Albericio, Org. Biomol. Chem., 2014, 12, 8379 Search PubMed; W. Van Den Nest, S. Yuval and F. Albericio, J. Pept. Sci., 2001, 7, 115 Search PubMed; A. D. Fenza, M. Tancredi, C. Galoppini and P. Rovero, Tetrahedron Lett., 1998, 39, 8529 Search PubMed.
- M. V. Katti, R. Sami-Subbu, P. K. Ranjekar and V. S. Gupta, Protein Sci., 2000, 9, 1203 Search PubMed.
- R. M. Senior, G. L. Griffin, R. P. Mecham, D. S. Wrenn, K. U. Prasad and D. W. Urry, J. Cell Biol., 1984, 99, 870 Search PubMed; D. W. Urry, J. Protein Chem., 1984, 3, 403 Search PubMed.
- J. A. M. Ramshaw, N. K. Shah and B. Brodsky, J. Struct. Biol., 1998, 122, 86 Search PubMed.
- C.-Z. Zhou, F. Confalonieri, M. Jacquet, R. Perasso, Z.-G. Li and J. Janin, Proteins, 2001, 44, 119 Search PubMed.
- S. Taniguchi, N. Watanabe, T. Nose and I. Maeda, J. Pept. Sci., 2016, 22, 36 Search PubMed; I. Maeda, S. Taniguchi, N. Watanabe, A. Inoue, Y. Yamasaki and T. Nose, Protein Pept. Lett., 2015, 22, 934 Search PubMed.
- K. Suyama, E. N. Mai, I. Maeda and T. Nose, ACS Synth. Biol., 2025, 14, 2999 Search PubMed; N. Tanaka, K. Suyama, K. Tomohara and T. Nose, Biomacromolecules, 2024, 25, 7156 Search PubMed; N. Tanaka, K. Suyama, K. Tomohara, I. Maeda and T. Nose, J. Pept. Sci., 2023, 29, e3449 Search PubMed; D. Tatsubo, K. Suyama, N. Sakamoto, K. Tomohara, S. Taniguchi, I. Maeda and T. Nose, Biochemistry, 2023, 62, 2559 Search PubMed; S. Sumiyoshi, K. Suyama, N. Tanaka, T. Andoh, A. Nagata, K. Tomohara, S. Taniguchi, I. Maeda and T. Nose, Sci. Rep., 2022, 12, 19414 Search PubMed; K. Suyama, M. Shimizu, I. Maeda and T. Nose, Biopolymers, 2022, 113, e23521 Search PubMed; K. Suyama, M. Mawatari, D. Tatsubo, I. Maeda and T. Nose, ACS Omega, 2021, 6, 5705 Search PubMed; K. Suyama, D. Tatsubo, W. Iwasaki, M. Miyazaki, Y. Kiyota, I. Takahashi, I. Maeda and T. Nose, Biomacromolecules, 2018, 19, 3201 Search PubMed; D. Tatsubo, K. Suyama, M. Miyazaki, I. Maeda and T. Nose, Biochemistry, 2018, 57, 1582 Search PubMed; K. Suyama, S. Taniguchi, D. Tatsubo, I. Maeda and T. Nose, J. Pept. Sci., 2016, 22, 236 Search PubMed.
- D. L. Barreiro, I. J. Minten, J. C. Thies and C. M. J. Sagt, ACS Biomater. Sci. Eng., 2023, 9, 3796 Search PubMed.
- D. E. Meyer and A. Chilkoti, Biomacromolecules, 2004, 5, 846 Search PubMed.
| This journal is © The Royal Society of Chemistry 2026 |

