Synthesis of cyclooctyne-sulfamates by the Nicholas cyclisation
Iaroslav A.
Kutuzov
 a,
Ekaterina A.
Khmelevskaya
a,
Alexander F.
Khlebnikov
a,
Ekaterina A.
Khmelevskaya
a,
Alexander F.
Khlebnikov
 a,
Mia D.
Kim
a,
Alexander Yu.
Ivanov
b,
Ivan A.
Rodionov
a,
Mia D.
Kim
a,
Alexander Yu.
Ivanov
b,
Ivan A.
Rodionov
 a,
Irina A.
Balova
a,
Irina A.
Balova
 a and
Natalia A.
Danilkina
a and
Natalia A.
Danilkina
 *a
*a
aInstitute of Chemistry, Saint Petersburg State University (SPbU), Saint Petersburg 199034, Russia. E-mail: n.danilkina@spbu.ru
bCenter for Magnetic Resonance, Research Park, Saint Petersburg State University (SPbU), Saint Petersburg, 199034, Russia
First published on 1st December 2025
Abstract
Here, we present the Nicholas cyclisation as an alternative, simple synthetic method for producing cyclooctyne-sulfamates as promising SPAAC reagents. Co2(CO)6-complexes of both monocyclic and benzene-fused cyclooctyne-sulfamates were prepared using the Nicholas cyclisation. After cobalt deprotection, the non-fused cyclooctyne-sulfamate SNO-Me2 remained stable and underwent SPAAC with benzyl azide (k = 0.0163 M−1 s−1) readily. In contrast, the benzene-fused cyclooctyne-sulfamates B-SNO and B-SNO-Me2 were found to be kinetically unstable. The reactivity and stability of monocyclic and benzene-fused cyclooctyne-sulfamates were predicted and compared using DFT calculations. The crucial influence of benzene-fusion and propargylic methyl groups on geometry, electronic structure, stability and reactivity was demonstrated.
Introduction
Following Carolyn Bertozzi's discovery of SPAAC (Strain-Promoted Azide–Alkyne Cycloaddition)1 and its role in bioorthogonal chemistry,2 cycloalkyne-based reagents became in high demand for a variety of applications.3–7 To meet this demand, organic chemists have been searching for cycloalkyne structures that are synthetically accessible and functionalisable, and that are stable and have fast bioorthogonal reactivity. To date, around twenty types of cycloalkynes have been synthesised and studied as suitable reagents for bioorthogonal transformations.8–11The synthesis of cycloalkynes is not a routine task because of ring strain attributed to these structures. Nevertheless, there are a number of synthetic methods that can be used to introduce a triple bond within a medium-sized cycle (Fig. 1A).12–14
The oldest known method is the elimination reaction (Fig. 1A), which converts vic-dibromides, vinyl bromides, vinyl triflates and (trialkylsilyl)vinyl triflates into cyclic alkynes. This reaction has been used to synthesise cyclooctyne itself,15OCT,2MOFO,16DIFO,17DIBO,18DBCO,19BCN,20BARAC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21 and other alkynes.22–26 Another approach involves decomposition reactions (Fig. 1A), i.e. oxidative decomposition of bishydrazones (synthesis of cyclooctyne,27TMTH derivatives28,29 and ACN
21 and other alkynes.22–26 Another approach involves decomposition reactions (Fig. 1A), i.e. oxidative decomposition of bishydrazones (synthesis of cyclooctyne,27TMTH derivatives28,29 and ACN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30), thermal decomposition of selenadiazoles (DIMAC)31 and photochemical destruction of cyclopropenones (ODIBO,32DPAD
30), thermal decomposition of selenadiazoles (DIMAC)31 and photochemical destruction of cyclopropenones (ODIBO,32DPAD![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33 and others34,35). Other less common methods, like [1,2]-rearrangement of alkylidene carbene for the synthesis of ABC-derivatives36,37 should also be mentioned (Fig. 1A).
33 and others34,35). Other less common methods, like [1,2]-rearrangement of alkylidene carbene for the synthesis of ABC-derivatives36,37 should also be mentioned (Fig. 1A).
Intra- and intermolecular alkylation is very useful and important approach for the synthesis of heterocycloalkynes (Fig. 1B). Cyclodecynes can be obtained using conventional propargylation techniques,38 and cyclononynes containing three heteroatoms can be prepared via Mannich-type ethynylation.39 It was recently found that rather harsh conditions were surprisingly effective for synthesising tricyclic benzoazacyclooctynes (Fig. 1B).40 However, other heterocyclononynes and -octynes cannot be synthesised using alkylation without employing specific techniques to bend the alkyne. In this case, Nicholas reaction41,42—the propargylation of various nucleophiles with Co2(CO)6-stabilised propargylic carbocations—is the method of choice (Fig. 1C). This approach towards cycloalkynes has been originally used for the oxathiacyclooctyne43 and then expanded for the important DACN family.44–46 The Nicholas cyclisation has been also employed in the synthesis of benzene-fused cyclononyne-sulfonamides (ABSACN)47 and for the heterocycle-fused heterocyclononynes BT9N, IC9N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 48 and IC9O.49
48 and IC9O.49
The Nicholas cyclisation enables the formation of a carbon–heteroatom bond easily due to the high stability of the carbocation and the proximity of the reaction centres, which is the result of significant alkyne bending (∼140°). Furthermore, Nicholas reaction products are obtained as stable cobalt carbonyl complexes with reduced RSE (ring strain energy) compared to free of cobalt cyclic alkynes.50 Co2(CO)6-cycloalkyne complexes can be stored and chemically modified in this form.51 They can then be deprotected from cobalt if necessary using mild techniques.48,52
Heterocycloalkynes, which have a heteroatom attached to the propargylic position, are unique bioorthogonal reagents because the heteroatom provides extra bending without significantly increasing the RSE due to stereoelectronic triple-bond stabilisation.53 Furthermore, these effects play a crucial role in the activation of cycloaddition via transition state stabilisation.53,54 In addition to its role in stabilisation/click activation, a heteroatom can serve as a site for cycloalkyne functionalisation, which helps to attach linking groups, fluorescent tags and other reporters to construct heterocycloalkyne-based bioorthogonal reagents.55–57
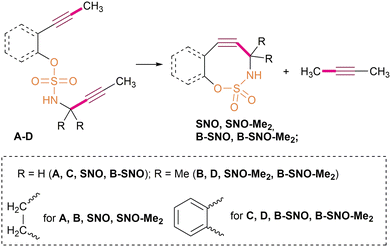
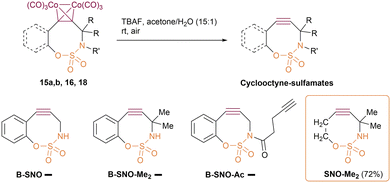
Cyclooctyne-sulfamates, which were recently introduced by Schomaker's group,58 are unique heterocyclooctynes with a sulfamate unit within the strained ring that is linked to the propargylic centre via the nitrogen atom of the sulfamate moiety (Fig. 1D). Several cyclooctyne-sulfamate derivatives are known,59 including difluorocyclooctyne-sulfamates for orthogonal bioorthogonal click reactions,60 and various functionalised cyclooctyne-sulfamates.61 These structures have all been obtained through a sophisticated synthetic route using silylated allenes as the starting material for Rh-catalysed intramolecular aziridination, followed by TBAF-promoted desilylation and the subsequent aziridine ring opening (Fig. 1D). In order to follow this synthetic route, it is first necessary to synthesise the starting silylated allenes from the corresponding functionalised alkynes. Therefore, despite the straightforward and high-yielding nature of the last two stages, the whole synthetic sequence for the cyclooctyne-sulfamates is complicated.
Given the nucleophilic nature of the sulfamate group and its propargylic attachment, Nicholas cyclisation could be considered a useful alternative synthetic approach for the cyclooctyne-sulfamate family (Fig. 1D).
To the best of our knowledge, the Nicholas cyclisation using a sulfamate as a nucleophile has not been described in the literature. Here, we report on the suitability of sulfamate functionality for the Nicholas cyclisation, considering the applicability of this reaction to the synthesis of monocyclic and benzene-fused cyclooctyne-sulfamates. We also provide a comprehensive theoretical study of the effects of benzene fusion and methyl substitution at the propargylic position on the RSE and SPAAC reactivity of cyclooctyne-sulfamates.
Results and discussion
Computational studies
Despite the thorough description of monocyclic sulfamate-cycloalkynes in the literature (see above), their benzene-fused analogues have never been synthesised using the reported approach. On the other hand, the Nicholas reaction could be used as a cyclisation tool, which would provide synthetic availability of both monocyclic and benzene-fused cyclooctyne-sulfamates in a similar way. At the same time, annulating a benzene ring should increase the SPAAC reactivity of sulfamate cyclooctynes due to the proposed stronger alkyne bending and increased RSE,8 as well as the possible additional stabilisation of the SPAAC transition state (TS) by the annulated ring.48In light of the interest in benzene fusion as a SPAAC activation technique8,19 and the fact that the impact of two alkyl substituents at the propargylic position in the cyclooctyne-sulfamate series has never been studied, the current study focused on four cyclooctyne-sulfamates: the non-substituted and dimethyl-substituted benzene-fused systems (cyclooctynes B-SNO and B-SNO-Me2), as well as the methylated and non-methylated monocyclic cyclooctynes SNO and SNO-Me2 (see Fig. 2).
DFT calculations were carried out to analyse the influence of benzene fusion and dimethyl substitution on alkyne bending and RSE values. Geometry optimisations of all compounds were performed using BP86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 62-D3
62-D3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 63 density functional method and def2-TZVP64 basis set using SMD65 solvent model (acetonitrile is the solvent of choice). CREST66 is a submodule of Grimme's XTB67 program, the gfn2-XTB method with default settings, was used with GBSA solvation correction for conformer elucidation. Electronic energies of optimised geometries (top 90% occupation of CREST conformational ensemble) were calculated at M062X-D3
63 density functional method and def2-TZVP64 basis set using SMD65 solvent model (acetonitrile is the solvent of choice). CREST66 is a submodule of Grimme's XTB67 program, the gfn2-XTB method with default settings, was used with GBSA solvation correction for conformer elucidation. Electronic energies of optimised geometries (top 90% occupation of CREST conformational ensemble) were calculated at M062X-D3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 68 density functional coupled to the def2-TZVPP basis set. For the further details see the SI, Section S3.
68 density functional coupled to the def2-TZVPP basis set. For the further details see the SI, Section S3.
Ring strain energies (RSE) were calculated as the enthalpies of fictitious isodesmic reactions where acyclic diynes A–D transform into the corresponding cycloalkynes and but-2-yne (Scheme 1).38,48
Calculations on cycloalkyne-sulfamates revealed that fusing the benzene ring to a cycloalkyne core significantly increases the RSE value (by 3.1 kcal mol−1 for SNO/B-SNO and by 2.4 kcal mol−1 for SNO-Me2/B-SNO-Me2). This correlates well with the increase in alkyne bending of ∼1° for both alkyne angles 1 and 2 (Fig. 3) in the case of both methylated and non-methylated derivatives. Such geometric changes upon benzene fusion would increase SPAAC reactivity and decrease the stability of cyclooctynes.
 | ||
| Fig. 3 Comparison of bending and RSE values for non-substituted and dimethyl-substituted SNO and B-SNO. | ||
We also demonstrated that alkyne bending and RSE values can be regulated by replacing the two hydrogen atoms at the propargylic position, adjacent to the sulfamate group, with methyl groups (Fig. 3).
In the case of SNO, introducing two methyl groups provides a decrease in C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–C(Me)2 alkyne bending by 4.1° and a reduction in the RSE value by 1.4 kcal mol−1. Regarding benzene-fused structures, the same structural change results in a decrease in the C
C–C(Me)2 alkyne bending by 4.1° and a reduction in the RSE value by 1.4 kcal mol−1. Regarding benzene-fused structures, the same structural change results in a decrease in the C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–C(Me)2 alkyne bending angle of 3.7° and a decrease in the RSE of 2.1 kcal mol−1. Therefore, the addition of two substituents at the propargylic position could be assumed as a way to regulate the stability and reactivity of cyclooctyne-sulfamates (Fig. 3).
C–C(Me)2 alkyne bending angle of 3.7° and a decrease in the RSE of 2.1 kcal mol−1. Therefore, the addition of two substituents at the propargylic position could be assumed as a way to regulate the stability and reactivity of cyclooctyne-sulfamates (Fig. 3).
We then turned to the optimisation of SPAAC TSs for the B-SNO and SNO pair and MeN3 as a model azide, as well as the analysis of the molecular orbitals of these two alkynes. The method, basis set and solvation model used for TS optimisation were the same as those mentioned above. Careful verification of the unique imaginary frequency for the transition state was carried out to check whether the imaginary frequency path leads to the desired product (for further details see SI, Section S3). Although these calculations provided higher free activation barriers than RB3LYP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 62,69,70/6-31++G(d,p)/GD3
62,69,70/6-31++G(d,p)/GD3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 63 SMD65 calculations (see SI, Section S3), i.e. the conditions used previously for heterocycle-fused cycloalkynes,48 the calculation algorithm employed here allows using of time- and resource-reasonable calculations with CREST conformational search that provides the same trend in free activation barrier values as calculations with RB3LYP method. The energies of the FMOs of cyclooctynes were obtained using single-point energy calculations with the B3PW91
63 SMD65 calculations (see SI, Section S3), i.e. the conditions used previously for heterocycle-fused cycloalkynes,48 the calculation algorithm employed here allows using of time- and resource-reasonable calculations with CREST conformational search that provides the same trend in free activation barrier values as calculations with RB3LYP method. The energies of the FMOs of cyclooctynes were obtained using single-point energy calculations with the B3PW91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 71 density functional and the 6-31++G(d,p) basis set.
71 density functional and the 6-31++G(d,p) basis set.
We found that annulation of a benzene ring diminishes the FMO gap due to π-extension between the out-of-plane π orbitals of the triple bond and the benzene π system. In the case of B-SNO, the out-of-plane π/π* orbitals of the triple bond impact both the HOMO and the LUMO. For SNO, however, the in-plane π/π* orbitals of the triple bond provide electron density to the FMOs (Fig. 4).
Due to the decrease in the FMO gap value, B-SNO may be less stable than SNO because of possible dimerisation or trimerisation, as well as other side processes.48,72
In terms of reactivity, the LUMO+2 energy of B-SNO with triple-bond π* in-plane impact is lower (−0.79 eV) than that of LUMO of SNO (−0.51 eV) with the same π* in-plane impact. This could be used to predict the expected increased reactivity of B-SNO compared to SNO when alkynes play the role of acceptor in SPAAC, as is known for heterocycloalkynes.53
Calculations on the SPAAC TS (the reaction of SNO and B-SNO with the model methyl azide) revealed that, as predicted, the free activation energies of B-SNO are lower than those of the SNO alkyne. Furthermore, the difference in the free activation barriers of syn- and anti-triazoles varies for SNO and B-SNO series.
Thus, the increased SPAAC reactivity upon benzene fusion was predicted correctly in terms of both geometry and electronics.
Synthesis and properties of cyclooctyne-sulfamates
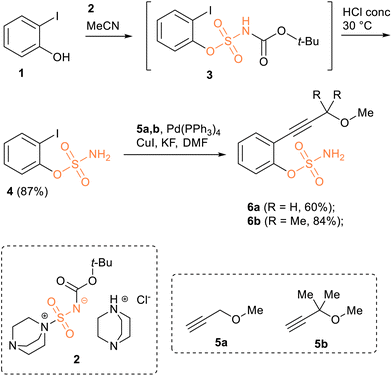
We then turned to synthesising benzene-fused and non-fused cyclooctyne-sulfamates using the Nicholas reaction as a ring-closure technique. Out of the four calculated structures, three were chosen as synthetic targets: B-SNO, B-SNO-Me2 and SNO-Me2.Firstly, we need to synthesise the starting alkyne sulfamates. Several techniques for the sulfamoylation of alcohols and phenols have been reported. These include sulfamoylation using unstable sulfamoyl chloride, which is typically generated in situ from CSI (chlorosulfonyl isocyanate) and formic acid,73 and sulfamoylation using stable sulfamoylated reagents.74 For the synthesis of o-iodophenol sulfamate 4, we opted for a sulfamoylation procedure involving a DABCO-containing stable salt 2,75 which has been successfully employed in the sulfamoylation of phenols (Scheme 2).
It was observed that the originally reported procedure for the sulfamoylation of alcohols and phenols by the reagent 2 in the presence of an additional amount of HCl-dioxane solution in acetonitrile was not effective in the case of o-iodophenol 1. Modifying this known procedure by simply excluding HCl as a reagent to increase the nucleophilicity of the iodophenol and using absolute acetonitrile as a solvent to prevent the hydrolysis of reagent 2 resulted in the formation of intermediate 3. Treating this intermediate with concentrated aqueous HCl in situ afforded the desired 2-iodophenyl sulfamate 4 in 87% yield (Scheme 2).
The Sonogashira coupling of o-iodophenyl sulfamate 4 required the use of a procedure involving KF as a base in absolute DMF, which was developed for cases where more common conditions (using Et3N as a base) were ineffective.50,76 Thus, using Pd(PPh3)4 and CuI as a catalytic system, and KF as a base, for the reaction of o-iodophenyl sulfamate 4 with alkynes 5a and 5b in absolute DMF gave the desired 2-ethynylated aryl sulfamates 6a and 6b in yields of 55–60% (Scheme 2). Using Et3N as a base, on the other hand, resulted only in a complex mixture of by-products.
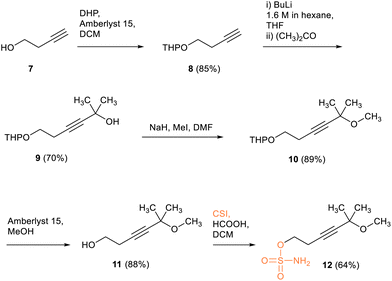
The synthesis of the starting sulfamate 12 for the non-fused cyclooctyne-sulfamate SNO-Me2 was carried out in accordance with Scheme 3. The synthesis started with but-3-yn-1-ol (7), which was THP-protected to form 8 and then converted into dimethylpropargylic alcohol 9 by reacting the lithium acetylide of the protected alkyne 8 with acetone. The alcohol 9 was then methylated using a MeI/NaH system, forming the ether 10, which was subsequently deprotected. The resulting alcohol 11 was then sulfamoylated using the CSI procedure, yielding the desired alkynyl sulfamate 12 in a 64% yield.
The key Nicholas cyclisation step via the sulfamate moiety was successfully accomplished for both phenyl sulfamates 6a,b and the alkyl sulfamate 12, as follows: firstly, the acyclic cobalt complexes were formed from alkyne-sulfamates and octacarbonyl dicobalt in DCM at a concentration of 0.01 M; then, the reaction mixture was diluted with DCM to a concentration of 0.001 M and treated with BF3 diethyl etherate (Scheme 4).
This one-pot, two-step protocol enabled the synthesis of cobalt complexes of both benzene-fused cyclooctyne sulfamates 15a,b and monocyclic cyclooctyne sulfamate 16 in good yields.
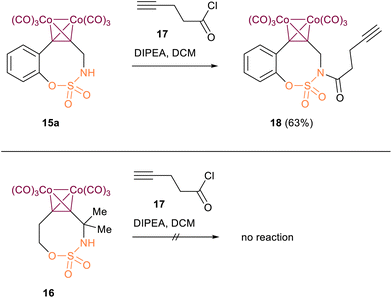
We also demonstrated that the Co-complex 15a fused to benzene ring can undergo additional functionalisation via the NH atom through acylation with pent-4-ynoyl chloride 17, resulting in the formation of the corresponding ethynylated derivative of Co-B-SNO18. However, the non-fused analogue with two additional methyl groups at the propargylic position did not undergo acylation under the same conditions, which can be explained by steric hindrance of two methyl groups adjacent to the closest to the nitrogen carbon atom (Scheme 5).
Finally, we turned to Co-deprotection for all the cobalt complexes of cyclooctyne-sulfamates that were obtained. We have recently shown that the mildest method for Co-deprotection of Co-complexes of heterocycle-fused heterocycloalkynes is the use of TBAF in aqueous acetone under air conditions.48 Of all the cyclooctyne-sulfamates, only the non-fused monocyclic alkyne SNO-Me2 can be easily obtained by TBAF-promoted Co-decomplexation in a high yield (Scheme 6).
This cyclooctyne is a stable compound that can be isolated and stored for months at −18 °C and for at least a week at room temperature. However, all three benzene-fused cyclooctyne-sulfamates (B-SNO, B-SNO-Me2 and B-SNO-Ac) were found to be kinetically unstable. Thus, upon treating their cobalt complexes with TBAF in aqueous acetone, only a complex mixture of non-alkyne products was detected. The instability of the benzene-fused cyclooctyne-sulfamates found is in good agreement with preliminary computational results.
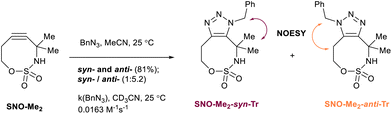
Stable cyclooctyne-sulfamate SNO-Me2 reacted with benzyl azide at room temperature, producing a mixture of syn- and anti-triazoles in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.2 ratio and achieving an overall yield of 81% (Scheme 7, for NOESY spectra see SI, Section S5). The second-order rate constant for SPAAC was found to be 0.0163 M−1 s−1 (see SI, Section S2.3.2 for details). Thus, replacing both hydrogen atoms adjacent to the sulfamate moiety on a propargylic carbon atom leads to a slight decrease in reactivity for SNO-Me2 compared to monoalkylated C5H11-SNO-OCT (0.025 M−1 s−1).58
5.2 ratio and achieving an overall yield of 81% (Scheme 7, for NOESY spectra see SI, Section S5). The second-order rate constant for SPAAC was found to be 0.0163 M−1 s−1 (see SI, Section S2.3.2 for details). Thus, replacing both hydrogen atoms adjacent to the sulfamate moiety on a propargylic carbon atom leads to a slight decrease in reactivity for SNO-Me2 compared to monoalkylated C5H11-SNO-OCT (0.025 M−1 s−1).58
Conclusion
The Nicholas reaction was found to be an efficient and straightforward alternative method for synthesising cyclooctyne-sulfamates. The starting materials required for Nicholas cyclisation—alkyl- and aryl sulfamates containing a methoxypropargyl unit—can be easily synthesised using standard sulfamoylation techniques directly from alkyne alcohols. Formation of Co-complexes of acyclic alkyne-sulfamates followed by the Nicholas cyclisation can be performed as a convenient one-pot process yielding Co-complexes of cyclic non-fused and benzene-fused cyclooctyne-sulfamates.The monocyclic Co-complex can then be converted into cyclooctyne-sulfamate SNO-Me2 using TBAF in aqueous acetone. SNO-Me2 is stable during isolation and storage and exhibits fast SPAAC reactivity (k = 0.0163 M−1 s−1). However, we have demonstrated that benzene fusion decreases the stability of cyclooctyne-sulfamates, meaning that they cannot be obtained in cobalt-free form. The instability of benzene-fused cyclooctyne-sulfamates compared to monocyclic ones can be explained by increased alkyne bending and RSE, as well as a reduction in the FMO gap value. We also demonstrate that introducing methyl groups at the propargylic position reduces RSE and alkyne bending, which can be used to regulate the stability of cycloalkynes.
Author contributions
IAK – investigation, methodology, writing – review & editing; EAK – investigation, writing – review & editing; AFK – investigation, visualization; MDK – investigation; AYI – investigation, formal analysis, visualization; IAR – methodology; IAB – project administration, writing – review & editing; NAD – conceptualization, investigation, data curation, methodology, project administration, supervision, visualization, writing – original draft, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information: all relevant experimental data, characterisation details, copies of all NMR spectra, computational details and kinetic measurement details. See DOI: https://doi.org/10.1039/d5ob01784j.Acknowledgements
This work was funded by the Russian Science Foundation 24-23-00377. The research was carried out using the SPbU Resource Centres: Magnetic Resonance Research Centre, Chemical Analysis and Materials Research Centre, Computer Center. Oussama Abdelhamid Mammeri (SPbU) is thanked for ESI HR MS measurements; Sergey N. Smirnov (SPbU) is thanked for the NMR measurements.References
- N. J. Agard, J. A. Prescher and C. R. Bertozzi, J. Am. Chem. Soc., 2004, 126, 15046–15047 CrossRef CAS PubMed.
- E. M. Sletten and C. R. Bertozzi, Angew. Chem., Int. Ed., 2009, 48, 6974–6998 CrossRef CAS PubMed.
- K. R. Venrooij, L. de Bondt and K. M. Bonger, Top. Curr. Chem., 2024, 382, 24 CrossRef CAS PubMed.
- N. K. Devaraj, ACS Cent. Sci., 2018, 4, 952–959 CrossRef CAS PubMed.
- V. Rigolot, C. Biot and C. Lion, Angew. Chem., Int. Ed., 2021, 60, 23084–23105 CrossRef CAS PubMed.
- T. Luu, K. Gristwood, J. C. Knight and M. Jörg, Bioconjugate Chem., 2024, 35, 715–731 CrossRef CAS PubMed.
- K. Li, D. Fong, E. Meichsner and A. Adronov, Chem. – Eur. J., 2021, 27, 5057–5073 CrossRef CAS PubMed.
- T. Harris and I. V. Alabugin, Mendeleev Commun., 2019, 29, 237–248 CrossRef CAS.
- D. Svatunek, Top. Curr. Chem., 2024, 382, 17 CrossRef CAS PubMed.
- S. E. Beutick, P. Vermeeren and T. A. Hamlin, Chem. – Asian J., 2022, 17, e202200553 CrossRef CAS PubMed.
- J. Dommerholt, F. P. J. T. Rutjes and F. L. van Delft, Top. Curr. Chem., 2016, 374, 16 CrossRef PubMed.
- E. G. Chupakhin and M. Y. Krasavin, Chem. Heterocycl. Compd., 2018, 54, 483–501 CrossRef CAS.
- A. Diana, G. I. Truglio, E. Petricci and D. Lanari, Org. Biomol. Chem., 2025, 23, 5441–5456 RSC.
- Y. Fang, A. S. Hillman and J. M. Fox, Top. Curr. Chem., 2024, 382, 15 CrossRef CAS PubMed.
- L. Brandsma and H. D. Verkruijsse, Synthesis, 1978, 290–290 CrossRef CAS.
- N. J. Agard, J. M. Baskin, J. A. Prescher, A. Lo and C. R. Bertozzi, ACS Chem. Biol., 2006, 1, 644–648 CrossRef CAS PubMed.
- J. M. Baskin, J. A. Prescher, S. T. Laughlin, N. J. Agard, P. V. Chang, I. A. Miller, A. Lo, J. A. Codelli and C. R. Bertozzi, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 16793–16797 CrossRef CAS PubMed.
- X. Ning, J. Guo, M. A. Wolfert and G.-J. Boons, Angew. Chem., Int. Ed., 2008, 47, 2253–2255 CrossRef CAS PubMed.
- M. F. Debets, S. S. van Berkel, S. Schoffelen, F. P. J. T. Rutjes, J. C. M. van Hest and F. L. van Delft, Chem. Commun., 2010, 46, 97–99 RSC.
- J. Dommerholt, S. Schmidt, R. Temming, L. J. A. Hendriks, F. P. J. T. Rutjes, J. C. M. van Hest, D. J. Lefeber, P. Friedl and F. L. van Delft, Angew. Chem., Int. Ed., 2010, 49, 9422–9425 CrossRef CAS PubMed.
- J. C. Jewett, E. M. Sletten and C. R. Bertozzi, J. Am. Chem. Soc., 2010, 132, 3688–3690 CrossRef CAS PubMed.
- B. R. Varga, M. Kállay, K. Hegyi, S. Béni and P. Kele, Chem. – Eur. J., 2012, 18, 822–828 CrossRef CAS PubMed.
- C. Gröst and T. Berg, Org. Biomol. Chem., 2015, 13, 3866–3870 RSC.
- C. Lis and T. Berg, Synlett, 2019, 939–942 CAS.
- C. Lis, S. Rubner, C. Gröst, R. Hoffmann, D. Knappe and T. Berg, Chem. – Eur. J., 2018, 24, 13762–13766 CrossRef CAS PubMed.
- J.-J. Shie, Y.-C. Liu, J.-C. Hsiao, J.-M. Fang and C.-H. Wong, Chem. Commun., 2017, 53, 1490–1493 RSC.
- A. T. Blomquist and L. H. Liu, J. Am. Chem. Soc., 1953, 75, 2153–2154 CrossRef CAS.
- G. de Almeida, E. M. Sletten, H. Nakamura, K. K. Palaniappan and C. R. Bertozzi, Angew. Chem., Int. Ed., 2012, 51, 2443–2447 CrossRef CAS PubMed.
- J. Weterings, C. J. F. Rijcken, H. Veldhuis, T. Meulemans, D. Hadavi, M. Timmers, M. Honing, H. Ippel and R. M. J. Liskamp, Chem. Sci., 2020, 11, 9011–9016 RSC.
- J. Brauer, M. Mötzing, C. Gröst, R. Hoffmann and T. Berg, Chem. – Eur. J., 2022, 28, e202202259 CrossRef CAS PubMed.
- E. M. Sletten and C. R. Bertozzi, Org. Lett., 2008, 10, 3097–3099 CrossRef CAS PubMed.
- C. D. McNitt and V. V. Popik, Org. Biomol. Chem., 2012, 10, 8200 RSC.
- M. J. Holzmann, N. Khanal, P. Yamanushkin and B. Gold, Org. Lett., 2023, 25, 309–313 CrossRef CAS PubMed.
- A. A. Poloukhtine, N. E. Mbua, M. A. Wolfert, G.-J. Boons and V. V. Popik, J. Am. Chem. Soc., 2009, 131, 15769–15776 CrossRef CAS PubMed.
- L. Denninger, H. Brunst, L. J. G. W. van Wilderen, M. Horz, H. M. A. Masood, C. D. McNitt, I. Burghardt, V. V. Popik and J. Bredenbeck, J. Chem. Phys., 2024, 160, 174201 CrossRef CAS PubMed.
- J. M. Dones, N. S. Abularrage, N. Khanal, B. Gold and R. T. Raines, J. Am. Chem. Soc., 2021, 143, 9489–9497 CrossRef CAS PubMed.
- E. Das, M. A. M. Feliciano, P. Yamanushkin, X. Lin and B. Gold, Org. Biomol. Chem., 2023, 21, 8857–8862 RSC.
- T. Harris, G. d. P. Gomes, S. Ayad, R. J. Clark, V. V. Lobodin, M. Tuscan, K. Hanson and I. V. Alabugin, Chem, 2017, 3, 629–640 CAS.
- H. Liu, H. Xing, P. Yu, Y. Wang, J.-L. Yan, J. Liu and M. Wang, J. Org. Chem., 2025, 90, 984–993 CrossRef CAS PubMed.
- T. Miyasaka, K. Hishiki, M. Yamaguchi, Y. Ono and T. Nishikawa, J. Org. Chem., 2025, 90, 14312–14315 CrossRef CAS PubMed.
- K. M. Nicholas and R. Pettit, Tetrahedron Lett., 1971, 12, 3475–3478 CrossRef.
- R. F. Lockwood and K. M. Nicholas, Tetrahedron Lett., 1977, 18, 4163–4165 CrossRef.
- T. Hagendorn and S. Bräse, RSC Adv., 2014, 4, 15493–15495 RSC.
- R. Ni, N. Mitsuda, T. Kashiwagi, K. Igawa and K. Tomooka, Angew. Chem., Int. Ed., 2015, 54, 1190–1194 CrossRef CAS PubMed.
- K. Igawa, S. Aoyama, Y. Kawasaki, T. Kashiwagi, Y. Seto, R. Ni, N. Mitsuda and K. Tomooka, Synlett, 2017, 2110–2114 CrossRef CAS.
- Y. Kawasaki, Y. Yamanaka, Y. Seto, K. Igawa and K. Tomooka, Chem. Lett., 2019, 48, 495–497 CrossRef CAS.
- K. Kaneda, R. Naruse, S. Yamamoto and T. Satoh, Asian J. Org. Chem., 2018, 7, 793–801 CrossRef CAS.
- N. A. Danilkina, A. I. Govdi, A. F. Khlebnikov, A. O. Tikhomirov, V. V. Sharoyko, A. A. Shtyrov, M. N. Ryazantsev, S. Bräse and I. A. Balova, J. Am. Chem. Soc., 2021, 143, 16519–16537 CrossRef CAS PubMed.
- A. A. Vidyakina, A. A. Shtyrov, M. N. Ryazantsev, A. F. Khlebnikov, I. E. Kolesnikov, V. V. Sharoyko, D. V. Spiridonova, I. A. Balova, S. Bräse and N. A. Danilkina, Chem. – Eur. J., 2023, 29, e202300540 CrossRef CAS PubMed.
- N. A. Danilkina, A. G. Lyapunova, A. F. Khlebnikov, G. L. Starova, S. Bräse and I. A. Balova, J. Org. Chem., 2015, 80, 5546–5555 CrossRef CAS PubMed.
- P. Gobbo, T. Romagnoli, S. M. Barbon, J. T. Price, J. Keir, J. B. Gilroy and M. S. Workentin, Chem. Commun., 2015, 51, 6647–6650 RSC.
- Y. Sakata, R. Nabekura, Y. Hazama, M. Hanya, T. Nishiyama, I. Kii and T. Hosoya, Org. Lett., 2023, 25, 1051–1055 CrossRef CAS PubMed.
- B. Gold, G. B. Dudley and I. V. Alabugin, J. Am. Chem. Soc., 2013, 135, 1558–1569 CrossRef CAS PubMed.
- B. Gold, N. E. Shevchenko, N. Bonus, G. B. Dudley and I. V. Alabugin, J. Org. Chem., 2012, 77, 75–89 CrossRef CAS PubMed.
- A. A. Vidyakina, S. A. Silonov, A. I. Govdi, A. Y. Ivanov, E. P. Podolskaya, I. A. Balova, S. Bräse and N. A. Danilkina, Org. Biomol. Chem., 2024, 22, 7637–7642 RSC.
- A. A. Vidyakina, S. A. Silonov, A. Y. Ivanov, E. A. Shpakova, M. D. Kim, E. P. Podolskaya, A. S. Gladchuk, I. A. Balova, S. Bräse and N. A. Danilkina, J. Mater. Chem. B, 2025, 13, 10525–10535 RSC.
- R. Masuda, Y. Kawasaki, K. Igawa, Y. Manabe, H. Fujii, N. Kato, K. Tomooka and J. Ohkanda, Chem. – Asian J., 2020, 15, 742–747 CrossRef CAS PubMed.
- E. G. Burke, B. Gold, T. T. Hoang, R. T. Raines and J. M. Schomaker, J. Am. Chem. Soc., 2017, 139, 8029–8037 CrossRef CAS PubMed.
- E. G. Burke and J. M. Schomaker, J. Org. Chem., 2017, 82, 9038–9046 CrossRef CAS PubMed.
- Y. Hu, J. M. Roberts, H. R. Kilgore, A. S. Mat Lani, R. T. Raines and J. M. Schomaker, J. Am. Chem. Soc., 2020, 142, 18826–18835 CrossRef CAS PubMed.
- Y. Hu, R. Spiegelhoff, K. S. Lee, K. M. Sanders and J. M. Schomaker, J. Org. Chem., 2024, 89, 4512–4522 CrossRef CAS PubMed.
- A. D. Becke, Phys. Rev. A, 1988, 38, 3098–3100 CrossRef CAS PubMed.
- S. Grimme, J. Antony, S. Ehrlich and H. Krieg, J. Chem. Phys., 2010, 132, 154104 CrossRef PubMed.
- F. Weigend and R. Ahlrichs, Phys. Chem. Chem. Phys., 2005, 7, 3297 RSC.
- A. V. Marenich, C. J. Cramer and D. G. Truhlar, J. Phys. Chem. B, 2009, 113, 6378–6396 CrossRef CAS PubMed.
- P. Pracht, F. Bohle and S. Grimme, Phys. Chem. Chem. Phys., 2020, 22, 7169–7192 RSC.
- C. Bannwarth, S. Ehlert and S. Grimme, J. Chem. Theory Comput., 2019, 15, 1652–1671 CrossRef CAS PubMed.
- Y. Zhao and D. G. Truhlar, Theor. Chem. Acc., 2008, 120, 215–241 Search PubMed.
- C. Lee, W. Yang and R. G. Parr, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 785–789 CrossRef CAS PubMed.
- A. D. Becke, J. Chem. Phys., 1993, 98, 5648–5652 CrossRef CAS.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS PubMed.
- E. M. Sletten, H. Nakamura, J. C. Jewett and C. R. Bertozzi, J. Am. Chem. Soc., 2010, 132, 11799–11805 CrossRef CAS PubMed.
- C. G. Espino, P. M. Wehn, J. Chow and J. Du Bois, J. Am. Chem. Soc., 2001, 123, 6935–6936 CrossRef CAS.
- M. A. Sguazzin, J. W. Johnson and J. Magolan, Org. Lett., 2021, 23, 3373–3378 CrossRef CAS PubMed.
- I. Armitage, A. M. Berne, E. L. Elliott, M. Fu, F. Hicks, Q. McCubbin and L. Zhu, Org. Lett., 2012, 14, 2626–2629 CrossRef CAS PubMed.
- A. G. Lyapunova, A. S. D′yachenko and N. A. Danilkina, Russ. J. Org. Chem., 2017, 53, 800–804 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2026 |