Open Access Article
Open Access Article‘Clip-Cycle’ approaches to functionalised pyrrolidines, pyrrolizidines and indolizidines
Lee C.
Duff†
,
Selin
Yilmaz†
,
Athanasia
Agora
,
Christopher J.
Maddocks
,
Ian J. S.
Fairlamb
 *,
William P.
Unsworth
*,
William P.
Unsworth
 * and
Paul A.
Clarke‡
* and
Paul A.
Clarke‡

Department of Chemistry, University of York, YO10 5DD, Heslington, York, UK. E-mail: william.unsworth@york.ac.uk; ian.fairlamb@york.ac.uk
First published on 15th December 2025
Abstract
The ‘Clip-Cycle’ approach is a versatile and modular synthetic method for the synthesis of aza-heterocycles via sequential cross metathesis and aza-Michael reactions. In this manuscript, a series of innovations to the ‘Clip-Cycle’ approach are reported. First, the asymmetric ‘Clip-Cycle’ syntheses of 2,5- and 3,5-pyrrolidines from chiral starting materials are reported for the first time, using a kinetic resolution method. A two-directional ‘Clip-Cycle’ approach for the synthesis of pyrrolizidines and indolizidines is then introduced.
Introduction
Aza-heterocycles are highly important in synthetic and medicinal chemistry and modular methods to make them from simple building blocks are valuable.1,2 The ‘Clip-Cycle’ approach, developed within the laboratory of Prof Paul A. Clarke§ at the University of York, is one such method (Scheme 1a). ‘Clip-Cycle’ operates via a two-step sequence.3 The ‘Clip’ phase of the method uses a cross metathesis reaction catalysed by Hoveyda–Grubbs second generation catalyst (HG-II), to couple an amine- or alcohol-tethered alkene 1 with a thioacrylate 2. This affords a functionalised Michael acceptor 3, that can undergo cyclisation via an acid-catalysed intramolecular conjugate addition reaction in the ‘Cycle’ phase, to form the heterocyclic product 4. The ‘Cycle’ phase can be done racemically (e.g. using racemic camphorsulfonic acid, rac-CSA) or enantioselectively using a chiral phosphoric acid (CPA).4 The thioester moiety is key to the ‘Clip-Cycle’ approach; the Michael acceptor must be sufficiently reactive to enable the intramolecular conjugate addition in the ‘Cycle’ step, but not too reactive so that conjugate addition takes place spontaneously following cross metathesis in the ‘Clip’ phase, as this would preclude asymmetric CPA catalysis.3a Prior to this manuscript, the successful application of the ‘Clip-Cycle’ approach for the enantioselective synthesis of pyrrolidines 4a,3a,btetrahydropyrans 4b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3c and piperidines 4c
3c and piperidines 4c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3d was described.
3d was described.
 | ||
| Scheme 1 Extensions to the ‘Clip-Cycle’ method to synthesize functionalised pyrrolidine, pyrrolizidines and indolizidines. | ||
In this manuscript, a series of innovations to the ‘Clip-Cycle’ method are reported. Firstly, the synthesis of 2,5- and 3,5-disubstituted pyrrolidines is reported using a kinetic resolution approach.5 The ‘Clip-Cycle’ method results in the generation of a new stereogenic centre. However, with the exception of a single scaffold (2 examples, vide infra),2c all previously published examples have started from achiral starting materials; this simplifies the reaction significantly, as diastereoselectivity and the resolution of a chiral starting material are not considerations. The requirement to use achiral starting materials places a significant limitation on the scope of the method however. To address this, the synthesis of 2,5- and 3,5-disubstituted pyrrolidines from chiral amine derivatives is described herein for the first time (Scheme 1b). Furthermore, a novel two-directional ‘Clip-Cycle’ approach is introduced and used in the synthesis of pyrrolizidines and indolizidines from amino diene precursors (Scheme 1c).
‘Clip-Cycle’ synthesis of 2,5- and 3,5-pyrrolidines via kinetic resolution
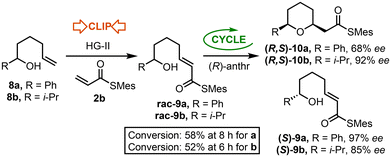
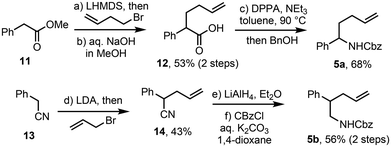
Previously reported ‘Clip-Cycle’ reactions made use of achiral starting materials, with the exception of the synthesis of tetrahydropyrans (R,S)-10a and (R,S)-10b (Scheme 2).3c In this series, cross metathesis of alcohols 8a and 8b with thioacrylate 2b, mediated by HG-II, afforded racemic Michael acceptors rac-9a and rac-9b respectively. Cyclisation with a chiral CPA catalyst was then performed aiming for ≈50% conversion (58% and 52% respectively), which resulted in effective kinetic resolution of both racemic starting materials, with enantioenriched tetrahydropyran products (R,S)-10a (68% ee) and (R,S)-10b (92% ee) isolated, along with recovered starting materials (S)-9a (97% ee) and (S)-9b (85% ee), concomitantly enriched as the R-enantiomer.To extend the kinetic resolution hypothesis to 2,5- and 3,5-pyrrolidines, the syntheses of racemic amines 5a and 5b were performed. To synthesise the 2-phenyl amine 5a, methyl phenylacetate 11 was first alkylated with 4-bromo-1-butene, mediated by LHMDS, and the resultant ester hydrolysed to afford carboxylic acid 12. Subsequent Curtius rearrangement, followed by trapping with benzyl alcohol, afforded amine 5a in good overall yield. Synthesis of the 3-substituted amine 5b proceeded via alkylation of nitrile 13 with allyl bromide to give alkene 14, reduction using LiAlH4 and Cbz-protection to afford 5b (Scheme 3).
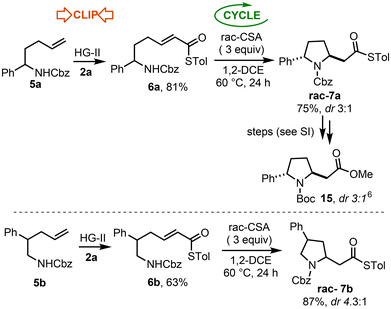
The ‘Clip-Cycle’ of secondary amines 5a and 5b was then examined, initially to generate racemic pyrrolidine products. In both cases, the cross metathesis with thioacrylate 2a proceeded smoothly, to afford Michael acceptors 6a and 6b in good yields, both as single E-isomers. Cyclisation was then promoted by excess rac-CSA, as a Brønsted acid catalyst, to afford the expected 2,5- and 3,5-pyrrolidine products rac-7a and rac-7b respectively (Scheme 4). These experiments confirm the approach is synthetically viable for these substrate classes, and also provided racemic product standards, which were needed for the asymmetric studies to follow. In the case of 2,5-pyrrolidine rac-7a, the product was obtained as a ≈ 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of diastereoisomers, with the trans isomer shown assigned as the major diastereoisomer. To make this assignment, rac-7a was converted into its N-Boc protected amino ester derivative and its NMR spectroscopic data compared to those of the known trans diastereoisomer 15.6 3,5-Pyrrolidine rac-7b was isolated as a 4.3
1 mixture of diastereoisomers, with the trans isomer shown assigned as the major diastereoisomer. To make this assignment, rac-7a was converted into its N-Boc protected amino ester derivative and its NMR spectroscopic data compared to those of the known trans diastereoisomer 15.6 3,5-Pyrrolidine rac-7b was isolated as a 4.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of diastereoisomers; the relative stereochemistry of the major and minor diastereoisomers could not be unequivocally determined in this case.
1 mixture of diastereoisomers; the relative stereochemistry of the major and minor diastereoisomers could not be unequivocally determined in this case.
Attention was then placed on assessing the feasibility of achieving the kinetic resolution of both racemic substrates rac-6a and rac-6b. Based on our previous pyrrolidine ‘Clip-Cycle’ work,3a,b the CPA catalyst (R)-TRIP (20 mol%, see Scheme 5 box) was selected as the cyclisation catalyst, with cyclohexane as the solvent at 50 °C. In both cases, the reactions were analysed at regular intervals by chiral HPLC and stopped when around 50% of the starting material was converted. Details of the reaction conversion and ee at all measured time points is included in the SI; a summary of the end point of the reactions is summarised in Scheme 5 and described below.
After reacting rac-6a for 9 hours, 52% conversion into 2,5-pyrrolidine (S,S)-7a was observed by chiral HPLC analysis, hence the reaction was stopped at this time point. The ee of (S,S)-7a was measured to be 69% ee, while the remaining 48% of the reaction mixture was accounted for by unreacted starting material, enriched to 79% ee as the (R)-enantiomer (R)-6a. The assignment of the absolute stereochemistry of (S,S)-7a and (R)-6a was made assuming the same sense of selectivity as that observed during our previous work on the asymmetric ‘Clip-Cycle’ synthesis of 2,5-pyrrolidines.3a,b The diastereomeric ratio of (S,S)-7a was not measured in this case, as the trans- and cis-diastereoisomers of 7a were not resolved by the HPLC analytical method used.
For the resolution of rac-6b, 52% conversion into 3,5-pyrrolidine (S)-7b was observed by chiral HPLC after 7 hours at 50 °C and the reaction halted at this time point. In this case, the chiral HPLC method used was able to resolve both the cis- and trans-diastereoisomers of 7b and each of their enantiomeric pairs, meaning that both the dr, and the ee for both isomers could be monitored simultaneously. A high ee was observed for both diastereoisomers (77% and 93% for the major and minor isomers respectively), with enantioenriched unreacted starting material 6b′ (48% of the reaction mixture). The newly formed stereogenic centre at the 5-position is again likely to be the (S)-enantiomer shown based on our previous work.3a,b For rac-7b, the relative stereochemistry of the major diastereomer of (S)-7b was not determined, and hence the absolute configuration of the major enantiomer of 6b′ could not be deduced also.
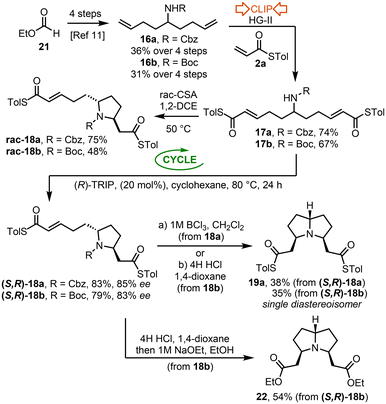
3,5-Disubstituted pyrrolizidines and Indolizidines
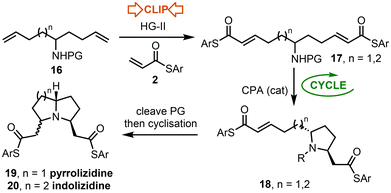
The second half of this study focuses on the synthesis of 3,5-disubstituted pyrrolizidines and indolizidines using the ‘Clip-Cycle’ approach. Pyrrolizidines and indolizidines are common motifs in biologically active alkaloids, isolated from a variety of natural sources, ranging from poison dart frogs and ants to plants and trees.7 In view of their importance, various synthetic approaches to make them have been established,8 with two-directional approaches based on the elaboration of symmetrical (or pseudo-symmetrical) precursors amongst the most effective.9 We recognised an opportunity to utilise the ‘Clip-Cycle’ approach, summarised in Scheme 6. Thus, a double cross metathesis reaction (16 → 17, ‘Clip’) followed by an aza-Michael reaction10 (17 → 18, ‘Cycle’) would deliver a desymmetrised pyrrolidine intermediate 18 asymmetrically using a suitable CPA catalyst. Cleavage of the N-protecting group (PG below) would then be expected to facilitate a second aza-Michael reaction spontaneously, to afford either a pyrrolizidine 19 or indolizidine 20 depending on the chain length (n = 1 or 2).The ‘Clip-Cycle’ pyrrolizidine synthesis started with the 4-step conversion of ethyl formate 21 into dienes 16a and 16b, using a modified literature procedure, based on a report by Nicolai and Waser.11 Both dienes 16a and 16b were then reacted with thioacrylate 2a and the Hoveyda–Grubbs second generation catalyst, which promoted a double cross metathesis reaction to form dienes 17a and 17b in the ‘Clip’ phase of the process. Each was formed as a single E,E-geometrical isomer. Next, the ‘Cycle’ step was performed, first using rac-CSA to form pyrrolidines rac-18a and rac-18b, with both pyrrolidines isolated as a single trans-diastereoisomer.12 The ‘Cycle’ step was then repeated using our standard conditions for asymmetric pyrrolidine formation,3a,b using CPA catalyst (R)-TRIP (20 mol%) in cyclohexane as the solvent. For both diene substrates the cyclisation worked well; pyrrolidines (S,R)-18a and (S,R)-18b were isolated in 83% and 79% yields, and in 85% ee and 83% ee respectively. As for the racemic reaction, both products were isolated as a single trans-diastereoisomer.12
Attention then turned to N-protecting group cleavage and the second aza-Michael reaction. In the case of N-Cbz derivative 18a, the alkene groups precluded a standard hydrogenolysis approach, therefore a Lewis acidic method was chosen, using BCl3;13 these conditions promoted Cbz-cleavage and spontaneous aza-Michael reaction to afford pyrrolizidine 19a in 38% yield. The same pyrrolizidine 19a was also obtained from 18b, following reaction with 4 M HCl in 1,4-dioxane. Both methods furnished 19a as a single diastereoisomer. The optical rotation of 19a was measured to be zero; as an enantioenriched starting material was used, this indicated that meso-isomer has likely be formed. This assignment was corroborated by the formation of pyrrolizidine 22, the diethyl ester analogue of 19a. Three of the possible diastereoisomers of pyrrolizidine 22 have been reported previously by two groups, Stockman and coworkers9c and Spring and coworkers.9e Comparison of our NMR spectroscopic data with those published, confirmed the assignment of 19a as the meso-isomer shown (Scheme 7).
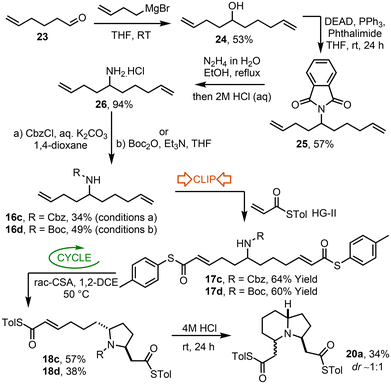
The ‘Clip-Cycle’ indolizidine synthesis started with the conversion of aldehyde 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 into secondary alcohol 24 using a Grignard reagent (Scheme 8). Subsequent Mitsunobu reaction, hydrazinolysis and carbamate formation then delivered unsymmetrical amino dienes 16c and 16d. The ‘Clip’ step worked as expected, with HG-II catalyst promoting a double cross metathesis reaction to form dienes 17c and 17d, both as single E,E-geometrical isomers. The ‘Cycle’ step was then performed using rac-CSA, which furnished pyrrolidines 18c and 18d as the trans diastereoisomers shown; notably this diastereoselectivity matches that observed during the synthesis of 2,5-pyrrolidine 7a described in the first half of this manuscript. Finally, the conversion of one of these 2,5-pyrrolidines (Boc-protected derivative 18d) into indolizidine 20a was completed by reaction with 4 M HCl at RT, which promoted concomitant Boc-cleavage and aza-Michael reaction to form indolizidine 20a with a 1
14 into secondary alcohol 24 using a Grignard reagent (Scheme 8). Subsequent Mitsunobu reaction, hydrazinolysis and carbamate formation then delivered unsymmetrical amino dienes 16c and 16d. The ‘Clip’ step worked as expected, with HG-II catalyst promoting a double cross metathesis reaction to form dienes 17c and 17d, both as single E,E-geometrical isomers. The ‘Cycle’ step was then performed using rac-CSA, which furnished pyrrolidines 18c and 18d as the trans diastereoisomers shown; notably this diastereoselectivity matches that observed during the synthesis of 2,5-pyrrolidine 7a described in the first half of this manuscript. Finally, the conversion of one of these 2,5-pyrrolidines (Boc-protected derivative 18d) into indolizidine 20a was completed by reaction with 4 M HCl at RT, which promoted concomitant Boc-cleavage and aza-Michael reaction to form indolizidine 20a with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of separable diastereomers, in 34% unoptimised yield.
1 mixture of separable diastereomers, in 34% unoptimised yield.
Conclusion
In conclusion the synthetic potential of the ‘Clip-Cycle’ approach for the synthesis of aza-heterocycles is expanded significantly by the innovations reported in this study. First, the asymmetric ‘Clip-Cycle’ synthesis of 2,5- and 3,5-pyrrolidines from chiral starting materials is reported for the first time, using an effective kinetic resolution method. Furthermore, a two-directional ‘Clip-Cycle’ approach for the synthesis of pyrrolizidines and indolizidines has also been disclosed, from appropriate amino diene precursors.Future work on the 2,5- and 3,5-pyrrolidine series should focus on expanding the preliminary kinetic resolution results described, further optimising for improved ee, and unequivocally establishing the relative and absolute stereoselectivity for the 3,5-pyrrolidine system. Expanding the substrate scope of the asymmetric kinetic resolution method and demonstrating its efficacy in preparative reactions is also important. Here, automated methods (HTE) and data science tools, including machine learning approaches, may be particularly useful.14,15
For the pyrrolizidine synthesis, it is unfortunate that the second aza-Michael reaction delivers a meso-product 19a, as this means that the enantioselectivity imparted in the preceding cyclisation step is lost. Future work can therefore focus on developing strategies to maintain chirality in the indolizidine product following cyclisation. This could be done by using an unsymmetrical diene precursor; for example, a diene analogous to 17a/b with one of the thioesters replaced by a simple ethyl ester would likely react similarly to afford a chiral indolizidine product via the same route. In the indolizidine series, future work can focus on combining the indolizidine synthesis described with the 2,5-pyrrolidine kinetic resolution method, to enable asymmetric indolizidine synthesis. For both the pyrrolizidine and indolizidine series, future applications in target synthesis are of interest.7,9 We encourage other researchers interested in continuing to study ‘Clip-Cycle’ reactivity based on the results described herein and elsewhere3 to do so.§
Author contributions
Synthetic studies on 2,5- and 3,5-pyrrolidines were done by S. Y. and A. A. Synthetic studies on pyrrolizidines and indolizidines were done by L. C. D. and C. J. M. Both projects were conceived, designed and led by P. A. C. The paper was written by W. P. U. and I. J. S. F., with contributions from L. C. D. and S. Y.Conflicts of interest
There are no conflicts to declare.Data availability
The data that support the findings of this study are available in the published article and its supplementary information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5ob01746g.Acknowledgements
The authors thank the University of York for funding that supported this research and the TR Ministry of National Education, Turkey, for funding the PhD studentship of S. Y. I. J. S. F. thanks the Royal Society for an Industry Fellowship (2021–25) and the EPSRC IAA scheme for funding.References
- (a) C.-V. T. Vo, G. Mikutis and J. W. Bode, Angew. Chem., Int. Ed., 2013, 52, 1705–1708 Search PubMed; (b) W.-Y. Siau and J. W. Bode, J. Am. Chem. Soc., 2014, 136, 17726–17729 CrossRef CAS PubMed; (c) J. A. Rossi-Ashton, A. K. Clarke, R. J. K. Taylor and W. P. Unsworth, Org. Lett., 2020, 22, 1175–1181 CrossRef CAS PubMed; (d) I. Zalessky, J. M. Wootton, J. K. F. Tam, D. E. Spurling, W. C. Glover-Humphreys, J. R. Donald, W. E. Orukotan, L. C. Duff, B. J. Knapper, A. C. Whitwood, T. F. N. Tanner, A. H. Miah, J. M. Lynam and W. P. Unsworth, J. Am. Chem. Soc., 2024, 146, 5702–5711 Search PubMed.
- (a) E. Vitaku, D. T. Smith and J. T. Njardarson, J. Med. Chem., 2014, 57, 10257–10274 CrossRef CAS PubMed; (b) N. M. Nasir, K. Ermanis and P. A. Clarke, Org. Biomol. Chem., 2014, 12, 3323–3335 RSC; (c) R. D. Taylor, M. MacCross and A. D. G. Lawson, J. Med. Chem., 2014, 57, 5845–5859 CrossRef CAS PubMed.
- (a) C. J. Maddocks, K. Ermanis and P. A. Clarke, Org. Lett., 2020, 22, 8116–8121 Search PubMed; (b) C. J. Maddocks and P. A. Clarke, Tetrahedron, 2021, 78, 131789 CrossRef CAS; (c) K. Alomari, N. S. P. Chakravarthy, B. Duchadeau, K. Ermanis and P. A. Clarke, Org. Biomol. Chem., 2022, 20, 1181–1185 RSC; (d) S. Ravi, C. Maddocks, I. J. S. Fairlamb, W. P. Unsworth and P. A. Clarke, Org. Biomol. Chem., 2025, 23, 649–653 RSC.
- Z. Sun, G. A. Winschel, P. M. Zimmerman and P. Nagorny, Angew. Chem., Int. Ed., 2014, 53, 11194–11198 CrossRef CAS PubMed.
- For selected asymmetric methods for the synthesis of pyrrolidines, see: (a) X. Fang and C.-J. Wang, Org. Biomol. Chem., 2018, 16, 2591–2601 RSC; (b) X. Ma, I. R. Hazelden, T. Langer, R. H. Munday and J. F. Bower, J. Am. Chem. Soc., 2019, 141, 3356–3360 Search PubMed.
- A. Farwick and G. Helmchen, Adv. Synth. Catal., 2010, 352, 1023–1032 Search PubMed.
- (a) J. Robertson and K. Stevens, Nat. Prod. Rep., 2014, 31, 1721–1788 RSC; (b) J. P. Michael, Nat. Prod. Rep., 2001, 18, 520–542 RSC; (c) J. Robertson and K. Stevens, Nat. Prod. Rep., 2017, 34, 62–89 RSC; (d) J. Zhang, S. L. Morris-Natschke, D. Ma, X.-F. Shang, C.-J.- Yang, Y.-Q. Liu and K.-H. Lee, Med. Res. Rev., 2021, 41, 928–960 CrossRef CAS PubMed; (e) W. T. Bradner, Cancer Treat. Rev., 2001, 27, 35–50 CrossRef CAS PubMed; (f) M. Tomasz, Chem. Biol., 1995, 2, 575–579 CrossRef CAS PubMed; (g) K. Whitby, T. C. Pierson, B. Geiss, K. Lane, M. Engle, Y. Zhou, R. W. Doms and M. S. Diamond, J. Virol., 2005, 79, 8698–8706 CrossRef CAS PubMed.
- (a) N. K. Ratmanova, I. A. Andreev, A. V. Leontiev, D. Momotova, A. M. Novoselov, O. A. Ivanova and I. V. Trushkov, Tetrahedron, 2020, 76, 131031 CrossRef CAS; (b) C. Bhat and S. G. Tilve, RSC Adv., 2014, 4, 5405–5452 Search PubMed.
- For relevant 2-directional approaches to related bicyclic scaffolds, see: (a) S. R. Magnuson, Tetrahedron, 1995, 51, 2167–2213 CrossRef CAS; (b) A. F. Newton, S. J. Roe, J. C. Legeay, P. Aggarwal, C. Gignoux, N. J. Birch, R. Nixon, M.-L. Alcarazc and R. A. Stockman, Org. Biomol. Chem., 2009, 7, 2274–2277 RSC; (c) J. C. Legeay, W. Lewis and R. A. Stockman, Chem. Commun., 2009, 45, 2207–2209 RSC; (d) A. Barthelme, D. Richards, I. R. Mellorb and R. A. Stockman, Chem. Commun., 2013, 49, 10507–10509 RSC; (e) M. D. Galvilan, W. R. J. D. Galloway, K. M. G. O'Connell, J. T. Hodkingson and D. R. Spring, Chem. Commun., 2010, 46, 776–778 Search PubMed; (f) M. Guerola, M. Sánchez-Roselló, C. Mulet, C. del Pozo and S. Fustero, Org. Lett., 2015, 17, 960–963 CrossRef CAS PubMed.
- For aza-Michael reactions leading to azacycles, see: (a) G. J. Noordzij and C. H. R. M. Wilsens, Front. Chem., 2019, 729 CrossRef CAS PubMed; (b) P. Sharma, R. Gupta and R. K. Bansal, Beil. J. Org. Chem., 2021, 17, 2585–2610 CrossRef CAS PubMed; (c) K. Y. Palate, Z. Yang, A. C. Whitwood and W. P. Unsworth, RSC Chem. Biol., 2022, 3, 334–340 Search PubMed; (d) A. Y. Rulev, Eur. J. Org. Chem., 2023, e202300451 CrossRef CAS; (e) Z. Yang, I. Zalessky, R. G. Epton, A. C. Whitwood, J. M. Lynam and W. P. Unsworth, Angew. Chem., Int. Ed., 2023, 62, e202217178 CrossRef CAS PubMed.
- S. Nicolai and J. Waser, Org. Lett., 2011, 13, 6324–6327 CrossRef CAS PubMed.
- The assigned trans-relative stereochemistry agrees with the diastereoselectivity observed in the 2,5-pyrrolidine syntheses described earlier in this manuscript and was confirmed retrospectively based on the assignment pyrrolizidine 22. It is likely that a smaller amount of the cis-pyrrolidine was also formed during the cyclisation step but was removed during chromatographic purification.
- D. R. Williams, D. L. Brown and J. W. Benbow, J. Am. Chem. Soc., 1989, 111, 1923–1925 CrossRef CAS.
- D. Yepes, F. Neese, B. List and G. Bistoni, J. Am. Chem. Soc., 2020, 142, 3613–3625 CrossRef CAS PubMed.
- A. F. Zahrt, J. J. Henle, B. T. Rose, Y. Wang, W. T. Darrow and S. E. Denmark, Science, 2019, 363, 6424 CrossRef PubMed.
Footnotes |
| † These authors contributed equally to this work. |
| ‡ Deceased. |
| § The development of the ‘Clip-Cycle’ approach will not continue at the University of York as Prof Paul A. Clarke passed away in November 2023. |
| This journal is © The Royal Society of Chemistry 2026 |