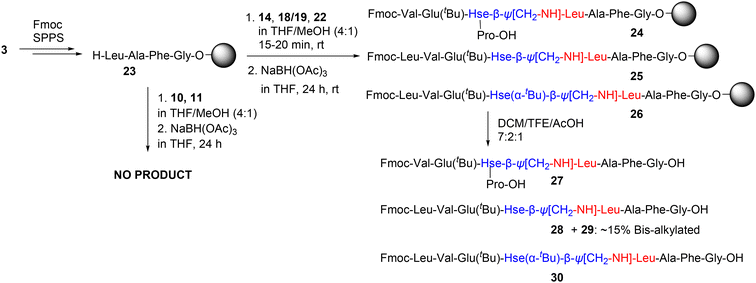Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Use of homoserinyl γ-aldehyde–containing peptides in solid-phase reductive amination
Dimitrios
Tolis
,
Dimitra
Magkafa
and
Spyridon
Mourtas
 *
*
Department of Chemistry, University of Patras, 26510 Rio Patras, Greece. E-mail: s.mourtas@upatras.gr
First published on 5th January 2026
Abstract
Reduced peptide bonds of the methyleneamino Ψ[CH2–NH] type are widely recognized as peptide bond isosteres with significant value in drug discovery and development. Solid-Phase Synthesis (SPS) enables Solid-Phase Fragment Condensation (SPFC), a key strategy for the construction of complex peptides. In this study, we report the SPS of peptides containing selectively side-chain deprotected homoserine (Hse) residues, followed by solution-phase oxidation of the liberated Hse side-chain hydroxyl group to the corresponding γ-aldehydes. The intrinsic instability of these intermediates, primarily due to intramolecular cyclization to γ-hydroxy lactam or lactone products, is systematically examined, and stabilization strategies to overcome these limitations are developed. The resulting stabilized homoserinyl γ-aldehyde peptides were subsequently employed, as proof of concept, in solid-phase reductive amination with the N-terminus of resin-bound peptides. Overall, this approach enables the efficient formation of Hse-β-Ψ[CH2–NH] reduced peptide bonds and establishes a versatile platform for broader peptide ligation and modification strategies.
1. Introduction
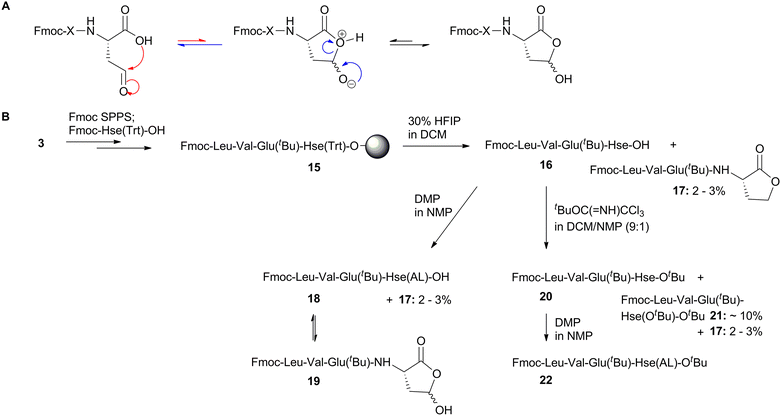
Peptides are well known for their high biological specificity and favorable safety profile, which are major factors contributing to their extensive use as therapeutic agents. However, the clinical application of native peptides has been limited primarily due to their poor metabolic stability and short half-life.1 Various strategies have been proposed to overcome these limitations, including chemical modifications and novel drug delivery approaches, or combinations thereof, to enable intact peptides to reach their targets.1 In the development of chemically modified peptides and peptide conjugates, several synthetic strategies have been employed, such as lipidation, incorporation of non-natural amino acids into the peptide backbone, cyclization, replacement of amide bonds with pseudopeptide bonds, and prodrug synthesis, among others.2–8Pseudopeptides are peptidomimetics in which one or more peptide bonds are replaced by an isostere (also known as isosteric surrogate). The most straightforward examples of isostere replacements are summarized in Fig. 1. As shown, the replacement of amide bond [CO–NH] 1 by the general formula bond [CH2–Y], where Y can be CH2, NH, O, S, leads to the formation of the corresponding Ψ[CH2–CH2] (2a), Ψ[CH2–NH] (2b), Ψ[CH2–O] (2c), and Ψ[CH2–S] (2d) isosteres. This replacement represents a viable strategy for the rational design of peptidomimetics, not only because it increases the metabolic stability of the resulting compounds, but also due to enhanced potency and biological activity they often exhibit.9,10
Solid-Phase Synthesis (SPS) and Solid-Phase Fragment Condensation (SPFC) strategies have been extensively employed in the synthesis of peptides and their analogues, including chemically modified peptides. These methodologies have enabled the production of numerous commercially available peptides and continue to shape the future of peptide-based therapeutics.3,7,11–14 Among the various types of resins developed over the years, the acid labile 2-chlorotrityl chloride (CLTR) resin is widely used in Solid-Phase Peptide Synthesis (SPPS) due to its unique advantages. For example, it prevents racemization during the loading of the first amino acid, inhibits diketopiperazine formation, and allows cleavage under relatively mild acidic conditions. Additionally, the use of the Fmoc/tBu strategy combined with different side-chain protecting groups enables an orthogonal protection approach, facilitating selective deprotection during synthesis.13–16
As part of our ongoing efforts to develop SPS methods and strategies for drug design and development, we have established procedures for replacing peptide bonds with the [CH2–S] isostere,17 and for synthesizing thiol-containing peptoids by incorporating N-mercaptoalkylglycine residues into peptide chains, effectively substituting amino acids with their N-mercaptoalkylglycine counterparts.18
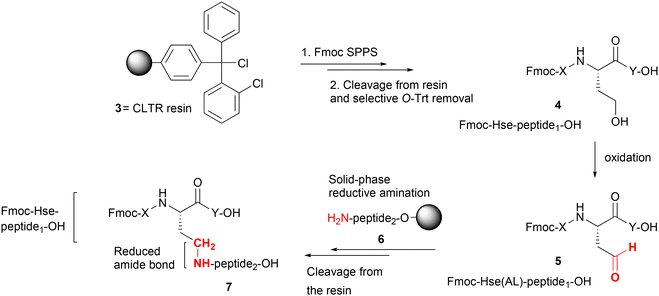
In this work, we rationalized the SPS of homoserine (Hse)-containing peptides synthesized on CLTR resin 3 using the Fmoc/tBu methodology, with the acid-labile Trt group serving as the side-chain protecting group for Hse. This orthogonal protection strategy enables the synthesis of Hse(Trt)-containing peptides, which upon cleavage from the resin and subsequent selective detritylation, could yield homoserine-containing peptides 4. Controlled oxidation of the liberated γ-hydroxyl side-chain of Hse could then allow formation of the corresponding homoserinyl γ-aldehyde peptides 5. These aldehyde peptides could be further employed in solid-phase reductive amination with the N-terminus of a second solid-phase synthesized peptide 6, to afford a side-chain-elongated peptide 7, wherein a linkage between the two peptides (peptide1–peptide2) would be based on a newly formed pseudopeptide bond of Hse-β-Ψ[CH2–NH] type (Scheme 1).
While the formation of reduced amide bonds of Ψ[CH2–NH] type in peptide-based drug design has been proposed as a strategy to enhance metabolic stability, potency and biological activity,9,19 the solid-phase reductive amination between homoserinyl peptide aldehydes and the N-terminus of resin-bound peptides has not been reported. This paper presents the challenges encountered in the synthesis of homoserinyl γ-aldehyde peptides and describes problem-solving approaches aimed at their efficient synthesis and subsequent application in solid-phase fragment condensation under reductive amination conditions.
2. Results and discussion
2.1. Methods to prepare homoserinyl γ-aldehyde peptides – challenges
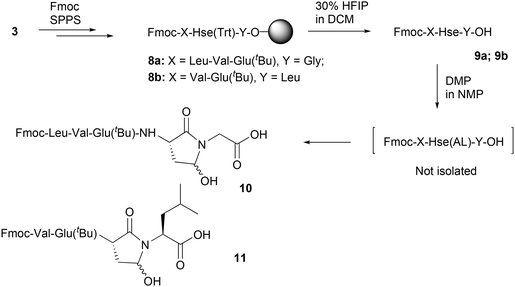
In order to investigate the synthesis of homoserinyl γ-aldehyde peptides and their further use in SPS, we initially considered the solid-phase synthesis (SPS) of selectively side-chain deprotected homoserine peptides. For this, we applied standard solid-phase peptide synthesis (SPPS) methods, by using 2-chlorotrityl chloride resin (CLTR) 3 and Fmoc/tBu methodology. The orthogonal protection of Hse was achieved by using the trityl (Trt) group as the side-chain protecting group, which allows selective removal of O-Trt group without affecting the tBu-type protecting groups.20–22 Thus, we synthesized small peptides containing both Hse(Trt) and Glu(tBu) (as a representative tBu-protected amino acid). The coupling of the Fmoc-amino acids during the peptide assembly was performed using N-hydroxybenzotriazole (HOBt) and N,N′-diisopropylcarbodiimide (DIC) as the condensing agents, while removal of the Fmoc group was achieved by using 25% piperidine in NMP. By this method, the Hse(Trt)-containing peptidyl-resin 8a/b (Y = Gly; Leu respectively) was obtained (Scheme 2).Treatment of resin 8a with 30% 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP) in dichloromethane (DCM), simultaneously cleaved the peptide from the resin and removed the Trt-type protecting group, to afford the selectively Hse deprotected peptide, 9a (Fig. S1) which was further subjected to standard Dess–Martin oxidation.23–25 The reaction process was followed by HPLC analysis, where a clear consumption of the starting material (9a) and the formation of a new product was evidenced, which was isolated. However, the isolated product was not identified as the expected Hse aldehyde peptide. In fact, although the isolated product had the expected mass, the 1H-NMR and 13C-NMR analysis revealed the absence of the expected aldehyde-H/C. In addition, when this product was subjected to standard reductive amination process (as it is analytically described in section 2.3), this was not able to react. Thus, we concluded that the isolated product corresponds to γ-hydroxy lactam peptide 10 (Fig. S2). In order to further investigate this finding, we replaced Gly with Leu, by synthesizing the Hse-containing resin-bound peptide 8b and the corresponding 9b (Fig. S3), which was also oxidized with DMP reagent. The oxidation reaction was followed by HPLC analysis, showing the successful oxidation of 9b. However, also in this case, we did not obtain the expected Hse aldehyde peptide, but instead, we obtained a product with the expected mass, but no aldehyde-H/C in 1H and 13C-NMR analysis. In addition, this product was unable to further react under standard reductive animation conditions (as analytically described in section 2.3). Thus, we concluded that the isolated product corresponds to the γ-hydroxy lactam peptide 11 (Fig. S4).
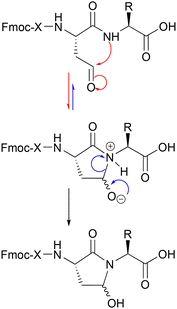
As an explanation for this finding, we propose the mechanism of Scheme 3. According to this, the initially formed aldehyde (after DMP oxidation) is transformed to the corresponding γ-hydroxy lactam through an intramolecular nucleophilic attack of the nitrogen of the neighboring amide bond (of the n + 1 residue relative to Hse) to an initially formed intermediate, which upon proton migration affords the finally formed γ-hydroxy lactam. The results indicate that the final step of this transformation is not an equilibrium, as no aldehyde-H/C were evidenced in 1H/13C-NMR of the isolated product, nor was the isolated product able to react under reductive amination conditions (as it is analytically discussed in section 2.3).
A similar behavior has been proposed for homoserinyl peptides during the conversion of Hse to Asp by oxidizing the side-chain γ-hydroxyl group to the corresponding carboxylic acid group. In that case, the rapid oxidation of homoserine to Asp reduced the formation of γ-hydroxy lactam.26 In our case, where the aldehyde was the desired product, γ-hydroxy lactam was finally formed. A clear difficulty was therefore presented in our attempt to propose a protocol for the solid-phase synthesis of homoserinyl γ-aldehyde peptides and their use in solid-phase reductive amination. Strategies to overcome these difficulties/limitations were adopted, as described in the following section.
2.2. Methods to prepare homoserinyl γ-aldehyde peptides – effective approaches
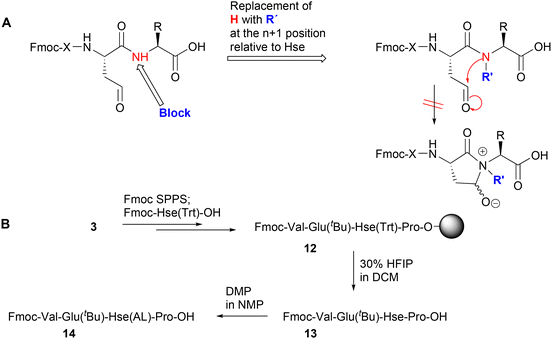
In order to overcome the aforementioned difficulties, due to the formation of homoserinyl γ-hydroxy lactams instead of γ-aldehydes, we investigated the possibility to stabilize the synthesized homoserinyl γ-aldehyde peptides. For this, two strategies were investigated: (a) the replacement of the n + 1 residue of the peptide chain (relative to the Hse residue) with an N-alkyl-α-amino acid, and (b) the use of C-terminal homoserinyl γ-aldehyde peptides. Both approaches are analytically presented and discussed in sections 2.2.1 and 2.2.2, respectively.The experimental part for this approach confirmed our hypothesis, as the solid-phase synthesis of the resin-bound peptide 12, where the n + 1 residue was replaced by proline (Pro), a naturally occurring N-alkyl-α-amino acid, afforded, after selective detritylation with 30% HFIP, the homoserinyl γ-aldehyde peptide 13 (Hse selectively deprotected), which, after DMP oxidation afforded 14 (Scheme 4B). The reaction progress was followed by HPLC analysis, where a clear oxidation of 13 to form 14 was evidenced (Fig. S5 and S6). In contrary to the non-alkylated Hse-containing peptide aldehydes, 1H and 13C-NMR analysis of the isolated 14 revealed the presence of the expected aldehyde-H/C, which provides evidence of our hypothesis (Fig. S11–S13). In addition, peptide 14 was effectively used in solid-phase reductive amination processes (as it is analytically described in section 2.3).
Thus, we initially synthesized the resin-bound C-terminal Hse(Trt) peptide 15 and the corresponding C-terminal Hse peptide 16, using standard SPPS and 30% HFIP, which enables cleavage from the resin and selective removal of the Hse(Trt) protecting group (Scheme 5B). This treatment also produced a small amount (2–3%) of lactone 17 (Fig. S7). Given the low percentage of 17 and the fact that this byproduct would not participate in the subsequent chemical reactions, we proceeded to the next step with this mixture, which was subjected to DMP oxidation, allowing conversion of 16 to the C-terminal homoserinyl γ-aldehyde peptide 18 (17 was not affected during this treatment) (Fig. S8).
In addition to this approach, we tert-butylated 16, by using tert-butyl 2,2,2-trichloroacetimidate (TBTA).27–29 The preferable tert-butylation of carboxylic acids over hydroxyl groups has also been reported,29–31 and therefore, we used this reagent to selectively tert-butylate the C-terminal α-carboxylic acid group over Hse hydroxyl group. This reaction, although relatively slow, finally afforded the C-terminal homoserinyl a-tert-butylated γ-hydroxy peptide 20 (Scheme 5B). Besides 20, the bis-tert-butylated 21 was also formed at approximately 10% (integrated by HPLC analysis), while lactone 17 (approx. 2–3%) was also identified in the final mixture (as remnant of the previous step) (Fig. S9). Nevertheless, this mixture was effectively subjected to DMP oxidation, to afford the C-terminal homoserinyl a-tert-butylated γ-aldehyde 22 (no participation of both 17 or 21 was evidenced in this reaction) (Fig. S10). Since the presence of both 17 and 21 in the final product was not considered as a problem for the reductive amination reaction, we used this mixture directly to the next step.
It should be noted that the progress and purity of the reactions and products were monitored by HPLC analysis, and the products and byproducts were identified by ESI-MS analysis. To further support our findings, we also performed 1H and 13C-NMR analysis (Fig. S14 and S15). No aldehyde-H/C was observed in the case of 18/19, whereas 22 showed clear signals for both aldehyde-H/C. These results provide evidence for the cyclization of peptide aldehyde 18 to the corresponding γ-hydroxy lactone 19, while no such cyclization occurred in the case of 22.
It is worth noting that the C-terminal homoserine γ-hydroxy 16 was found to be prone to lactonization (forming the corresponding peptide lactone 17) during storage. In fact, 16 cyclized to 17 at a percentage of 13–15% within 15 days at 4 °C, as determined by HPLC analysis. However, 16 remained stable even after 30 days at −20 °C.
2.3. Solid-phase reductive amination of Hse(AL) containing peptides
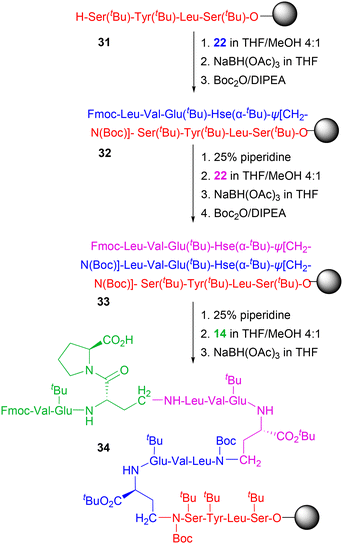
In this part of the work, products 10, 11, 14, 18/19 and 22 were subjected to the solid-phase reductive amination reaction. As a model substrate, a tetrapeptide was initially synthesized on CLTR resin 3 by using standard SPPS (Scheme 6). Additionally, Phe was incorporated into the peptide sequence to facilitate monitoring of reaction completion by HPLC analysis. In the final step of the peptide synthesis, the N-terminal Fmoc group was removed to yield the corresponding peptidyl-resin 23.For the solid-phase reductive amination, our protocol was based on a two-step procedure: first, the formation of the corresponding imine between the N-terminus of the resin-bound peptide 23 and the aldehyde group of the Hse-containing peptides followed by washing of the unreacted peptide; second, reduction of the imine using the mild reducing agent sodium triacetoxyborohydride [NaBH(OAc)3].32–34 This procedure enabled the formation of the resin-bound pseudopeptides 24–26, which were subsequently treated with a mixture of DCM/2,2,2-trifluoroethanol (TFE)/AcOH (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to finally yield the corresponding Hse-β-Ψ[CH2–NH] containing 27, 28 and 30 (Scheme 6).
1) to finally yield the corresponding Hse-β-Ψ[CH2–NH] containing 27, 28 and 30 (Scheme 6).
It should be noted that, although in case of 10 and 11, no reaction was noticed (Fig. S16), the use of the Hse(AL)-Pro containing peptide 14 was efficiently subjected to solid-phase reductive amination to form the peptidyl-resin 24 and the corresponding 27 (Fig. S17). In case of 18/19, where the Hse residue was introduced at the C-terminus of the peptide chain, the γ-aldehyde group of Hse, in equilibrium with the corresponding γ-hydroxy lactone ring (as discussed in section 2.2.2), allowed the solid-phase reductive amination, obviously by the open form of the peptide, finally resulting to the peptidyl-resin 25 and the corresponding pseudopeptide 28. In this case, a small percentage (approx. 15%) of the bis-alkylated peptide 29 was also formed (a well-known by-product of reductive amination processes) (Fig. S18).35 In the case of peptide 22, where the C-terminal α-carboxylic acid group of the peptide was tert-butylated, this was also effectively reacted with resin 23, allowing the formation of the corresponding resin-bound 26 and finally the formation of 30 (Fig. S19). For this set of experiments (solid-phase peptide synthesis, solid-phase reductive amination processes) the reaction progress and completion, as well as the purity of the reaction products, were monitored by HPLC analysis and the products/byproducts were identified by ESI-MS analysis where the expected masses were identified.
The incapability of the Hse-containing peptides 10 and 11 to react with resin 23 (under the same reductive conditions that were applied for peptides 14, 18/19 and 22), is clearly due to the chemical stability of the γ-hydroxy lactam ring and its inability to equilibrate with its open form. This indicates that, while the synthesis of the C-terminal Hse-containing peptide aldehydes could be an effective approach for preparing γ-hydroxy lactam peptides, these could not serve as alkylating agents in reductive amination processes, at least under the described protocol.
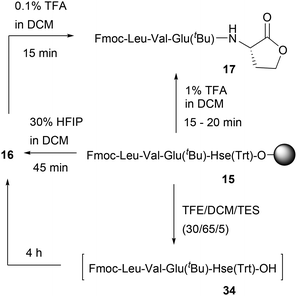
Among the many opportunities for peptide modification and conjugation strategies enabled by the stabilized Hse peptide aldehydes developed in this work, as well as by the proposed solid-phase reductive amination protocol, we provide as an illustrative example the Solid-Phase Fragment Condensation (SPFC) of peptide 34 (Scheme 7), which contains three sequentially formed Hse-β-Ψ[CH2–NH] bonds. This peptide was assembled on resin 31 by initial reductive amination with fragment aldehyde 22 (using the previously reported protocol). The resulting Hse-β-Ψ[CH2–NH] bond was then protected with the Boc group to afford the resin-bound fragment 32. Subsequent Fmoc removal with 25% piperidine enabled a second imine formation and reduction step with fragment 22 allowing the formation of the second Hse-β-Ψ[CH2–NH] bond, which was also protected with Boc group to afford 33. After another Fmoc removal, fragment aldehyde 14 was reacted using the same imine-amine protocol to introduce the third Hse-β-Ψ[CH2–NH] bond. The reaction progress was monitored by HPLC analysis after treatment of resin samples with DCM/TFE/AcOH (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), which afforded the corresponding fully protected (C-terminal deprotected) resin-cleaved fragments of 32–34 (as well as their non-Boc-protected precursors) (Fig. S20). This example demonstrates that the methodology enables the efficient formation of Hse-β-Ψ[CH2–NH] bonds andmation offers significant advantages for fragment preparation suitable for further use/modification in SPFC strategies.
1), which afforded the corresponding fully protected (C-terminal deprotected) resin-cleaved fragments of 32–34 (as well as their non-Boc-protected precursors) (Fig. S20). This example demonstrates that the methodology enables the efficient formation of Hse-β-Ψ[CH2–NH] bonds andmation offers significant advantages for fragment preparation suitable for further use/modification in SPFC strategies.
2.4. Investigating O-Trt cleavage from Hse(Trt)-containing peptides – lactone formation
As already presented in sections 2.2.2 and 2.3, the use of C-terminal Hse peptides in solid-phase reductive amination processes is a viable method. In one of the methods applied herein, treatment of 15 with 30% HFIP afforded the Hse peptide 16 and a small percentage (2–3%) of lactone 17 (as previously described in section 2.2.2).To further investigate this issue, we treated resin 15 with 1% TFA in DCM at rt, where the formation of lactone 17 was confirmed within 15–20 min (Scheme 8). The same product was obtained by the treatment of resin 15 with 30% HFIP in DCM for 45 min to initially afford the liberated O-Trt deprotected peptide 16 followed by treatment with only 0.1% TFA in DCM (or 1% TFA in DCM), which afforded lactone 17 within 15 min (Fig. S21–S23).
 | ||
| Scheme 8 Protocols tested for the SPS of C-terminal Hse-containing peptides and C-terminal peptide lactones. | ||
No racemization was measured during lactone formation (as evidenced by 1H-NMR analysis of H-Leu-Val-Glu(tBu)-L-Hse-lactone and H-Leu-Val-Glu(tBu)-D-Hse-lactone) (Fig. S24). Thus, we concluded that treatment of 15 with 30% HFIP followed by treatment with only 0.1% TFA or treatment of 15 with 1% TFA, could be used for the synthesis of the corresponding lactone 17, but these cleavage protocols cannot be used for the formation of the C-terminal Hse(OL) peptides (as they would rapidly cyclize to lactones).
Further studies on the preparation of C-terminal Hse O-Trt deprotected peptides from the resin, by replacing HFIP with TFE, showed that treatment of resin 15 with 30% TFE in DCM did not afford any of the desired O-Trt deprotected peptide, in agreement with previous studies.21 However, treatment of the resin 15 with TFE/DCM/TES (30/65/5) afforded the O-Trt deprotected peptide alcohol 16 within 4 h, through an initial fast release of the Hse O-Trt protected peptide 34 followed by O-Trt deprotection, with limited lactone formation (2–3%) within 4 h, as determined by HPLC analysis (Fig. S25 and 26).
Summarizing this section, treatment of CLTR resin-bound C-terminal Hse(Trt) peptides with TFE/DCM/TES 30/65/5 represents an alternative method for peptide cleavage from the resin and removal of the O-Trt protecting group(s), allowing the synthesis of C-terminal Hse-containing peptides, which worked well for the Glu(tBu)/Hse(Trt)-containing substrate tested.
In addition, the use of TFA for the cleavage of Hse(Trt)-containing peptides from CLTR resin, besides the well-known limitation of trifluoroacetylation of the liberated hydroxy groups,36,37 has a second limitation in the case of the C-terminal Hse peptides: the rapid formation of lactones. Nevertheless, this method, i.e. treatment of the resin-bound C-terminal Hse(Trt) peptides, or the initially cleaved C-terminal Hse peptides with TFA, could be used for the easy and efficient cyclization to the corresponding C-terminal peptide lactones.
3. Conclusions
In this work, we propose two complementary approaches for solid-phase reductive amination using homoserinyl (Hse) γ-aldehyde containing peptides. Both strategies rely on the initial synthesis of peptides bearing side-chain selectively deprotected Hse residues, followed by oxidation of the Hse side-chain hydroxyl group to the corresponding aldehyde.In the first approach, replacement of the amide hydrogen at the n + 1 position relative to the Hse residue with an N-alkyl group, effectively prevents intramolecular cyclization to the corresponding γ-hydroxy lactam, thereby stabilizing the peptide aldehyde, enabling its use in subsequent solid-phase reductive amination. In the second approach, a C-terminal Hse γ-aldehyde peptide is generated. In this case, although cyclization to the corresponding γ-hydroxy lactone may occur, the lactone remains reactive under reductive amination condition, presumably via its open-chain form. Additional stabilization strategies for C-terminal Hse γ-aldehyde peptides, through tert-butylation of the α-COOH group of the C-terminal Hse residue, are also demonstrated.
As proof of concept, all three types of Hse-derived peptide aldehydes, i.e. N-alkylated at the n + 1 position, or present as the C-terminal aldehyde (in equilibrium with the γ-hydroxy lactone) or as its stabilized precursor, were successfully employed in solid-phase reductive amination, leading to ligated peptides via formation of a methyleneamino Hse-β-Ψ[CH2–NH] isosteric bond.
Beyond this specific demonstration, the methodology offers broad applicability to a wide range of peptide ligation and modification strategies that exploit Hse γ-aldehyde peptide intermediates and the incorporation of the Hse-β-Ψ[CH2–NH] isostere, thus providing a versatile platform for modern peptide chemistry and conjugate development.
4. Experimental
4.1. Materials
2-Chlorotrityl chloride resin (CLTR) 100–200 mesh, 1% divinylbenzene (DVB) (loading capacity 1.2–2.0 mmol g−1) and Fmoc-protected amino acids were kindly provided by CBL Patras S.A. (Industrial area of Patras, Building block 1, GR-25018, Patras, Greece). Plastic reactors for Peptide Synthesis (polypropylene syringes equipped with porous polyethylene frits at the bottom) with a pore size of 25 μm were obtained from Carl Roth Gmbh + Co. KG, Karlsruhe, Germany. Tetrahydrofuran (THF) (ACS reagent, Reag. Ph. Eur., ≥99.9%, containing 250 ppm BHT as inhibitor) was purchased from Honeywell Riedel de Haen (Germany). THF was dried over molecular sieves type 4A (beads, diameter 1.7–2.4 mm) 48 h prior to use (Sigma-Aldrich O.M. Ltd, Athens, Greece). Methanol (MeOH) (HPLC grade, ≥99.8%) was purchased from Fischer Chemicals (Thermo Fisher Scientific, Geel, Belgium). All other chemicals were purchased from Sigma-Aldrich O.M. Ltd (Athens, Greece). All chemicals were used without further purification.4.2. Analytical methods
High Performance Liquid Chromatography (HPLC) analysis was performed on a Waters 2695 multisolvent delivery system (Milford, MA, USA), combined with Waters 996 photodiode array detector. The following columns were used: (A) Column: Lichrospher RP-18, 5 μm, 125–4 mm; (B) Column: Purospher RP-8e, 5 μm, 125–4 mm; (C) Column: Purospher RP-8e, 5 μm, 250–4 mm. ESI-MS spectra were recorded on a Waters Micromass ZQ 4000 mass detector (positive mode), controlled by MassLynx 4.1 software, by direct infusion, using a syringe pump at a flow rate of 5 mL min−1. Cone voltage was set at 30 V and scan time at 1 s, with the interscan delay at 0.1 s. NMR spectra were recorded on a Brucker DPX 600 MHz instrument (Peoria, IL, USA). The sample spectra were recorded at 25 °C. Chemical shifts (δ) were referenced to the corresponding solvent peaks and are reported in parts per million (ppm).5. Experimental procedures
5.1. Solid-phase synthesis – general protocol
Solid-phase peptide synthesis was carried out manually, either in plastic peptide synthesis reactors attached to a Visiprep Solid Phase Extraction Vacuum Manifold, or in Eppendorf tubes, where the reaction mixture was transferred to either microfilters or polypropylene syringes for washing.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 (3 × 20 mL g−1 resin for 5, 10, 15 min, respectively), N-methyl-2-pyrrolidone (NMP) (×5), isopropyl alcohol (iPrOH) (×3), diethyl ether (DEE) (×2) and dried in vacuo.
5 (3 × 20 mL g−1 resin for 5, 10, 15 min, respectively), N-methyl-2-pyrrolidone (NMP) (×5), isopropyl alcohol (iPrOH) (×3), diethyl ether (DEE) (×2) and dried in vacuo.
Removal of the Fmoc group for the first amino acid attachment was performed directly after attachment of the Fmoc-amino acid, as described in protocol: “Fmoc removal during solid-phase peptide assembly”.
Resin loading was estimated on the N-deprotected amino acid resin by measuring the resin weight gain, using the following formula: S(wt) = [Wt(g) × 1000]/[Wt(add) × Wt(t)], where S(wt): weight gain substitution (mmol g−1); Wt(g): weight gained by resin (g); Wt(add): molecular weight added to the resin = MW of amino acid minus MW of leaving group (g mol−1); Wt(t): total weight gain of the resin after loading.
Completion of the coupling reaction was monitored using the Kaiser test. Briefly, a small sample of the resin was taken and washed sequentially with NMP, iPrOH, DEE several times. The sample was then transferred to a small glass tube, and two drops of each Kaiser reagent were added. The tube was heated at 110–120 °C for 4–5 min. A positive test (blue resin beads) indicated incomplete coupling, while a negative test (colorless or yellowish beads) indicated complete coupling. In the case of incomplete coupling, recoupling was carried out using a freshly prepared solution of the activated Fmoc-amino acid.
Finally, the resin was filtered and washed with NMP (×5), iPrOH (×3), DEE (×2) and dried in vacuo.
5.2. General procedures for acidic cleavage of resin-bound peptides – preparation of peptide alcohols 9a, 9b, 13, 16
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) for 15 min at rt (10–15 mL g−1 resin), to cleave the fully protected peptides from the resin. The cleavage solution was filtered, and the resin was washed with an additional portion of the cleavage mixture, followed by DCM (×2). The combined filtrates were concentrated under reduced pressure on a rotary evaporator until an oily residue was obtained. DEE was then added to precipitate the peptide as a white solid, which was collected by filtration and washed with DEE (×3). The crude peptide was finally dried in vacuo.
1) for 15 min at rt (10–15 mL g−1 resin), to cleave the fully protected peptides from the resin. The cleavage solution was filtered, and the resin was washed with an additional portion of the cleavage mixture, followed by DCM (×2). The combined filtrates were concentrated under reduced pressure on a rotary evaporator until an oily residue was obtained. DEE was then added to precipitate the peptide as a white solid, which was collected by filtration and washed with DEE (×3). The crude peptide was finally dried in vacuo.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 65
65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) for 4–5 h at rt (10 mL g−1 resin). The cleavage solution was filtered, and the resin was washed with an additional portion of the cleavage mixture, followed by DCM (×2). The combined filtrates were concentrated under reduced pressure on a rotary evaporator until an oily residue was obtained. DEE was added to precipitate the peptide as a white solid, which was collected by filtration and washed with DEE (×3). The resulting side-chain deprotected peptide alcohols were obtained as white solids and further dried in vacuo.
5) for 4–5 h at rt (10 mL g−1 resin). The cleavage solution was filtered, and the resin was washed with an additional portion of the cleavage mixture, followed by DCM (×2). The combined filtrates were concentrated under reduced pressure on a rotary evaporator until an oily residue was obtained. DEE was added to precipitate the peptide as a white solid, which was collected by filtration and washed with DEE (×3). The resulting side-chain deprotected peptide alcohols were obtained as white solids and further dried in vacuo.
Yields from 1.0 g resin (resin 3) were as follows: Fmoc-Leu-Val-Glu(tBu)-Hse-Gly-OH (9a): 0.70 g (yield: 88.0%); Fmoc-Val-Glu(tBu)-Hse-Leu-OH (9b): 0.68 g (yield: 92.0%); Fmoc-Val-Glu(tBu)-Hse-Pro-OH (13): 0.75 g (yield: 89.7%); Fmoc-Leu-Val-Glu(tBu)-Hse-OH (16): 0.65 g (yield: 88.0%) [both cleavage methods gave comparable results].
5.3. tert-Butylation
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (2.2 mL) (both solvents were dried over molecular sieves 4A for at least 48 h prior to use). To this solution, tert-butyl 2,2,2-trichloroacetimidate (TBTA) (0.44 mmol; 96.53 mg) was added, and the reaction mixture was stirred at rt. Reaction progress was monitored by HPLC analysis. After 24 h and 48 h, fresh portions of TBTA (0.44 mmol; 96.53 mg) were added, and the reaction was terminated at 72 h. The reaction mixture was diluted with DCM, and the organic layer was sequentially extracted with 10% aqueous sodium hydrogen carbonate (NaHCO3) (×2) and water (×3). The organic layer was then dried over magnesium sulfate (MgSO4), filtered, and concentrated under reduced pressure to yield an oily residue. This residue was treated with DEE to precipitate a white solid, which was collected by filtration and washed with DEE.
1) (2.2 mL) (both solvents were dried over molecular sieves 4A for at least 48 h prior to use). To this solution, tert-butyl 2,2,2-trichloroacetimidate (TBTA) (0.44 mmol; 96.53 mg) was added, and the reaction mixture was stirred at rt. Reaction progress was monitored by HPLC analysis. After 24 h and 48 h, fresh portions of TBTA (0.44 mmol; 96.53 mg) were added, and the reaction was terminated at 72 h. The reaction mixture was diluted with DCM, and the organic layer was sequentially extracted with 10% aqueous sodium hydrogen carbonate (NaHCO3) (×2) and water (×3). The organic layer was then dried over magnesium sulfate (MgSO4), filtered, and concentrated under reduced pressure to yield an oily residue. This residue was treated with DEE to precipitate a white solid, which was collected by filtration and washed with DEE.
This procedure afforded the desired Fmoc-Leu-Val-Glu(tBu)-Hse-OtBu (20) (115.0 mg; yield: 65.5%). The final product contained 2–3% lactone 17 and 11.0% of the di-tert butylated derivative 21.
5.4. DMP oxidation
Upon completion, DCM (twice the volume of NMP) was added, and the mixture was cooled in an ice-bath for 10 min. Water (twice the volume of DCM) was then added, and the mixture was stirred for 10 min. The aqueous and organic layers were separated, and the aqueous phase was washed once more with DCM. The combined organic layers were treated with an aqueous 10% sodium thiosulfate (Na2S2O3) (approx. of equal volume to the combined DCM and NMP fractions), under vigorous stirring in an ice bath for 15 min. After phase separation, the organic layer was washed with a 10% aqueous NaHCO3 (×2) and water (×1), then acidified to pH 3–4 using 10% aqueous citric acid and finally washed with water (×3). The organic phase was dried over MgSO4, filtered, and concentrated under reduced pressure to yield an oily residue. This was treated with DEE to precipitate the desired γ-hydroxy lactam peptides as white solids.
Yields were as follows: Fmoc-Leu-Val-Glu(tBu)-Hse-Gly-OH (9a) (0.251 mmol; 200 mg) afforded γ-hydroxy lactam of Fmoc-Leu-Val-Glu(tBu)-Hse(AL)-Gly-OH (10) (169.57 mg; yield: 85%); Fmoc-Val-Glu(tBu)-Hse-Leu-OH (9b) (0.406 mmol; 300 mg) afforded γ-hydroxy lactam of Fmoc-Val-Glu(tBu)-Hse-Leu-OH (11) (263.45 mg; yield: 88%); Fmoc-Val-Glu(tBu)-Hse-Pro-OH (13) (0.277 mmol; 200 mg) afforded Fmoc-Val-Glu(tBu)-Hse(AL)-Pro-OH (14) (163.54 mg; yield: 82%); Fmoc-Leu-Val-Glu(tBu)-Hse-O-tBu (20) (0.12 mmol; 95 mg) afforded Fmoc-Leu-Val-Glu(tBu)-Hse(AL)-O-tBu (22) (84.33 mg; yield: 89%).
Upon completion, DCM (twice the volume of NMP) was added, followed by an aqueous solution of 10% Na2S2O3 (approx. equal in volume to the combined DCM and NMP). The mixture was stirred vigorously for 15 min. The organic and aqueous layers were then separated, and the aqueous phase was washed once more with DCM. The combined organic layers were subsequently treated with water, resulting in the formation of a white solid at the interface. The phases were separated, and this washing procedure with water was repeated multiple times. After four washings, interfacial precipitation consistently yielded a white solid (formed by combining all collected solids formed at the interface during extractions). The combined solids were further washed with a mixture of DCM and water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (×2), then dried over MgSO4 to afford 18.
1) (×2), then dried over MgSO4 to afford 18.
Fmoc-Leu-Val-Glu(tBu)-Hse-OH (16) (0.135 mmol; 100 mg) afforded Fmoc-Leu-Val-Glu(tBu)-Hse(AL)-OH (18) as a white solid (84.77 mg; yield: 85%).
5.5. Solid-phase reductive amination
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (5 mL g−1 resin) was added, followed by the addition of 10, 11 (1.2 eq.; 0.12 mmol) or the peptide aldehydes (14, 18/19, 22) (1.2 eq.; 0.12 mmol). The suspension was agitated for 15–20 min at rt. The resin was then filtered and washed with anhydrous THF (×3) to remove any excess unreacted peptide aldehyde.
1) (5 mL g−1 resin) was added, followed by the addition of 10, 11 (1.2 eq.; 0.12 mmol) or the peptide aldehydes (14, 18/19, 22) (1.2 eq.; 0.12 mmol). The suspension was agitated for 15–20 min at rt. The resin was then filtered and washed with anhydrous THF (×3) to remove any excess unreacted peptide aldehyde.
Next, the resin was transferred to a new Eppendorf tube, and anhydrous THF (5 mL g−1 resin) was added, followed by solid sodium triacetoxyborohydride NaBH(OAc)3 (2.2 eq.; 0.22 mmol). The reaction mixture was stirred at rt for 24 h. Reaction completion was monitored by HPLC analysis of the crude cleavage mixture of a resin sample. In general, the reaction was completed within 24 h. If incomplete conversion was observed by HPLC analysis, the resin was washed as described below and the imine–amine procedure was repeated to ensure full transformation.
After the reduction step, the reaction mixture was removed from the resin and discarded. The resin was then washed by addition and decantation of THF (3 × 4–5 volumes), and the resin was subsequently transferred to a filter and washed with THF, THF/MeOH (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (4 × 15 min), THF (×5), NMP (×5), iPrOH (×3), and DEE (×2).
1) (4 × 15 min), THF (×5), NMP (×5), iPrOH (×3), and DEE (×2).
The final cleavage from the resin was performed by treating the resin with DCM/TFE/AcOH (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (15 min), followed by DCM (×2). The combined filtrates were concentrated under reduced pressure to afford an oily residue, which was treated with DEE to precipitate the product. The resulting white solid was filtered and washed with DEE to yield the desired Hse-β-Ψ[CH2–NH] peptides.
1) (15 min), followed by DCM (×2). The combined filtrates were concentrated under reduced pressure to afford an oily residue, which was treated with DEE to precipitate the product. The resulting white solid was filtered and washed with DEE to yield the desired Hse-β-Ψ[CH2–NH] peptides.
Products and yields for a typical resin loading (0.9–1.0 mmol g−1) were as follows: Fmoc-Val-Glu(tBu)-Hse(Pro-OH)-β-Ψ[CH2NH]-Leu-Ala-Phe-Gly-OH (27), obtained by reductive amination between peptide aldehyde 14 and resin 23 (78.6 mg; yield: 78.6%); Fmoc-Leu-Val-Glu(tBu)-Hse-β-Ψ[CH2NH]-Leu-Ala-Phe-Gly-OH (28) and bis-alkylated (∼15%) (29), obtained by reductive amination between peptide aldehyde 18/19 and resin 23 (70.5 mg; yield: 69.5%); Fmoc-Leu-Val-Glu(tBu)-Hse(α-tBu)-β-Ψ[CH2NH]-Leu-Ala-Phe-Gly-OH (30), obtained by reductive amination between peptide aldehyde 22 and resin 23 (75.2 mg; yield: 70.6%); no products were identified for the reductive amination between 10, 11 and resin 23.
Boc protection of each Hse-β-Ψ[CH2–NH] bond was performed by first washing the resin with NMP to remove residual solvents. The resin was then suspended in a mixture of DCM/NMP (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (5 mL g−1 resin). DIPEA (10 eq.) and di-tert-butyl dicarbonate Boc2O (10 eq.) were added sequentially, and the resin was agitated for 2 × 24 h at rt. HPLC analysis indicated that approximately 15–20% of the Hse-β-Ψ[CH2–NH] bonds remained unreacted after 24 h; therefore, a second protection cycle was required to achieve complete Boc protection. The resin was then washed with NMP (×5), iPrOH (×3), and DEE (×2) to afford the resin-bound Hse-β-Ψ[CH2–N(Boc)]-containing peptides.
1) (5 mL g−1 resin). DIPEA (10 eq.) and di-tert-butyl dicarbonate Boc2O (10 eq.) were added sequentially, and the resin was agitated for 2 × 24 h at rt. HPLC analysis indicated that approximately 15–20% of the Hse-β-Ψ[CH2–NH] bonds remained unreacted after 24 h; therefore, a second protection cycle was required to achieve complete Boc protection. The resin was then washed with NMP (×5), iPrOH (×3), and DEE (×2) to afford the resin-bound Hse-β-Ψ[CH2–N(Boc)]-containing peptides.
5.6. C-terminal peptide lactone formation (17)
This residue was directly treated with 0.1% TFA in DCM (1.5 mL), and the cyclization to the corresponding C-terminal peptide lactone 17 was completed within 15–20 min (as monitored by HPLC analysis). The reaction mixture was concentrated under reduced pressure, and the oily residue was treated with DEE to induce precipitation. The resulting solid was filtered, washed with DEE (×2), and dried in vacuo to afford lactone 17 (34.8 mg; yield: 96.6%).
The reaction mixture was concentrated under reduced pressure, and the oily residue was treated with DEE to precipitate the product. The resulting white solid was filtered, washed with DEE (×2), and dried in vacuo to yield lactone 17 (34.1 mg; yield: 94.6%).
Author contributions
Conceptualization, S. M.; methodology, S. M.; validation, S. M.; formal analysis, S. M., D. T., D. M.; investigation, D. T., S. M., D. M.; resources, S. M.; data curation, S. M.; writing – original draft preparation, S. M.; writing – review and editing, S. M.; supervision, S. M.; project administration, S. M.; funding acquisition, S. M. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5ob01718a.Acknowledgements
This paper has been partly financed by the funding programme “MEDICUS” of the University of Patras—Research Committee, Code 81762; The publication of the article in OA mode was financially supported by HEAL-Link; The authors acknowledge CBL Patras S.A., Patras, Greece and Emeritus Prof. K. Barlos for kindly providing 2-chlorotrityl chloride resin and Fmoc-amino acids; The authors acknowledge Prof. Dimitrios Gatos for access to HPLC equipment.References
- O. Al Musaimi, L. Lombardi, D. R. Williams and F. Albericio, Pharmaceuticals, 2022, 15, 1283 CrossRef CAS.
- L. Gentilucci, R. De Marco and L. Cerisoli, Curr. Pharm. Des., 2010, 16, 3185 CrossRef CAS PubMed.
- C. Lamers, Future Drug Discovery, 2022, 4(2), FDD75 Search PubMed.
- I Avan, C. D. Hall and A. R. Katritzky, Chem. Soc. Rev., 2014, 43, 3575 Search PubMed.
- N. Qvit, S. J. S. Rubin, T. J. Urban, D. Mochly-Rosen and E. R. Gross, Drug Discovery Today, 2017, 22, 454 Search PubMed.
- J. M. Ahn, N. A. Boyle, M. T. MacDonald and K. D. Janda, Mini-Rev. Med. Chem., 2002, 2, 463 Search PubMed.
- L. Wang, N. Wang, W. Zhang, X. Cheng, Z. Yan, G. Shao, X. Wang, R. Wang and C. Fu, Signal Transduction Targeted Ther., 2022, 7, 48 CrossRef CAS.
- X. Zhang, X. Li, Q. You and X. Zhang, Eur. J. Med. Chem., 2017, 139, 542 CrossRef CAS PubMed.
- P. L. Scognamiglio, G. Morelli and D. Marasco, Methods Mol. Biol., 2015, 1268, 159 Search PubMed.
- A. F. Spatola, Methods Neurosci., 1993, 13, 19 CAS.
- P. Barman, S. Joshi, S. Sharma, S. Preet, S. Sharma and A. Saini, Int. J. Pept. Res. Ther., 2023, 29, 61 CrossRef CAS.
- L. Otvos Jr and J. D. Wade, Front. Chem., 2014, 2, 62 Search PubMed.
- K. Barlos and D. Gatos, in Fmoc Solid Phase Peptide Synthesis - A Practical Approach, ed. W. C. Chan and P. D. White, Oxford University Press, 2000, pp. 215–228 Search PubMed.
- J. M. Palomo, RSC Adv., 2014, 4, 32658 RSC.
- F. Albericio, Biopolymers, 2000, 55, 123 CrossRef CAS.
- M. Amblard, J. A. Fehrentz, J. Martinez and G. Subra, Mol. Biotechnol., 2006, 33, 239 CrossRef CAS.
- S. Mourtas, C. Katakalou, D. Gatos and K. Barlos, Molecules, 2020, 25, 218 CrossRef CAS.
- S. Mourtas, D. Gatos and K. Barlos, Molecules, 2019, 24, 4261 Search PubMed.
- J.-M. Ahn, K. Kassees, T.-K. Lee, B. Manandhar and A. M. Yousif, in Comprehensive Medicinal Chemistry III, Elsevier, 2017, vol. 6, DOI:10.1016/B978-0-12-409547-2.12413-8.
- K. Barlos, D. Gatos and S. Koutsogianni, J. Pept. Res., 1998, 51, 194 CrossRef CAS.
- A Wester, A. M. Hansen, P. R. Hansen and H. Franzyk, Amino Acids, 2021, 53, 1455 Search PubMed.
- A Isidro-Llobet, M. Álvarez and F. Albericio, Chem. Rev., 2009, 109, 2455 CrossRef CAS PubMed.
- AG. Myers, B. Zhong, M. Movassaghi, D. W. Kung, B. A. Lanman and S. Kwon, Tetrahedron Lett., 2000, 41, 1359 Search PubMed.
- S. I. Al-Gharabli, S. T. A. Shah, S. Weik, M. F. Schmidt, J. R. Mesters, D. Kuhn, G. Klebe, R. Hilgenfeld and J. Rademann, ChemBioChem, 2006, 7, 1048 CrossRef CAS.
- R. Colombo, Z. Wang, J. Han, R. Balachandran, H. N. Daghestani, D. P. Camarco, A. Vogt, B. W. Day, D. Mendel and P. Wipf, J. Org. Chem., 2016, 81, 10302 Search PubMed.
- W. Yang, S. Ramadan, B. Yang, K. Yoshida and X. Huang, J. Org. Chem., 2016, 81, 12052 CrossRef CAS.
- A Armstrong, I. Brackenridge, R. F. W. Jackson and J. M. Kirk, Tetrahedron Lett., 1988, 29, 2483 Search PubMed.
- N. S. Mahajani, R. I. L. Meador, T. J. Smith, S. E. Canarelli, A. A. Adhikari, J. P. Shah, C. M. Russo, D. R. Wallach, K. T. Howard, A. M. Millimaci and J. D. Chisholm, J. Org. Chem., 2019, 84, 7871 CrossRef CAS.
- Y. Tian, D. Wang, J. Li, C. Shi, H. Zhao, X. Niu and Z. Li, Chem. Commun., 2016, 52, 9275 RSC.
- H.-K. Cui, Y. Guo, Y. He, F.-L. Wang, H.-N. Chang, Y.-J. Wang, F.-M. Wu, C.-L. Tian and L. Liu, Angew. Chem., Int. Ed., 2013, 52, 9558 CrossRef CAS.
- L. J. Dowman, S. M. Agten, J. Ripoll-Rozada, B. M. Calisto, P. J. B. Pereira and R. J. Payne, Chem. Commun., 2021, 57, 10923 RSC.
- AF. Abdel-Magid, C. A. Mayano and K. G. Carson, Tetrahedron Lett., 1990, 31, 5595 CrossRef CAS.
- AF. Abdel-Magid, K. G. Carson, B. D. Harris, C. A. Maryanoff and R. D. Shah, J. Org. Chem., 1996, 61, 3849 CrossRef CAS.
- AF. Abdel-Magid and S. J. Mehrman, Org. Process Res. Dev., 2006, 10, 971 CrossRef CAS.
- AF. Abdel-Magid, B. D. Harris and C. A. Maryanoff, Synlett, 1994, 81 Search PubMed.
- L. Kocsis, F. Ruff and G. Orosz, J. Pept. Sci., 2006, 12, 428 CrossRef CAS PubMed.
- S. B. H. Kent, A. R. Mitchell, M. Engelhard and R. B. Merrifield, Proc. Natl. Acad. Sci. U. S. A., 1979, 76, 2180 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2026 |