Open Access Article
Open Access ArticleHigh-throughput DoE synthesis of chalcones and pyrazolines for fluorescent sensing
Alexander
Ciupa

Materials Innovation Factory, University of Liverpool, 51 Oxford Street, Liverpool L7 3NY, UK. E-mail: ciupa@liverpool.ac.uk
First published on 23rd December 2025
Abstract
Pyrazolines are attractive scaffolds for fluorescent sensors due to their unique photophysical properties and the ease of their synthesis from chalcone precursors. While both chalcones and pyrazolines have been widely explored, a systematic optimization of their synthesis using design of experiments (DoE) has yet to be reported. Here, we apply a data-driven DoE strategy, evaluating multiple variables across more than 105 datapoints, to establish highly optimized conditions for the Claisen–Schmidt condensation of chalcones and their subsequent conversion to pyrazolines. High-throughput screening of the resulting library revealed pyrazoline P10 with unusual “turn on” fluorescence at 590 nm in the presence of Zn2+ only. This integrated DoE–HTS workflow demonstrates a powerful approach for accelerating sensor discovery.
Introduction
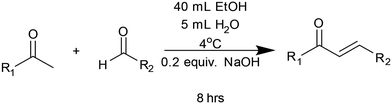
The integration of modern data-driven methodology into synthetic organic chemistry is streamlining synthetic method development.1 Among these, design of experiments (DoE) enables the systematic identification of optimal parameters with a minimal number of experiments, offering a powerful alternative to the traditional one-factor-at-a-time (OFAT) strategy.2 For instance, recent examples include the use of DoE in Heck–Suzuki reactions,3 B(OCH2CF3)3-mediated direct amidation,4 and solvent selection for multicomponent reactions.5 In parallel, high-throughput screening (HTS) platforms are accelerating the discovery process, with improvements reported in drug development,6 enhanced catalyst screening,7 and solar cell optimisation.8 Both DoE and HTS approaches benefit from modular, easily accessible chemical scaffolds. Chalcones are privileged structures9 with a diverse range of pharmacological properties, including anti-cancer,10 anti-inflammatory,11 and anti-infective properties.12 Typically, chalcones are prepared via a Claisen–Schmidt condensation13 from commercially available aromatic ketone and aldehyde starting materials (Fig. 1A). The diverse range of inexpensive, commercially available starting materials enables large modular libraries of chalcones to be synthesized and screened. Interest in Claisen–Schmidt condensations has grown considerably over the past sixty years (Fig. 1B). Importantly, while useful in themselves, chalcones can also serve as valuable precursors for pyrazolines (blue in Fig. 1A), another privileged structure with multiple medicinal properties.14 Pyrazolines with nanomolar activities for cancer cell lines15 and COX inhibitory activity have been reported.16 Pyrazolines can be aromatised to pyrazoles17 (red in Fig. 1A), providing access to another highly attractive privileged structure.18 Despite the wealth of applications chalcones and pyrazolines provide, no DoE study has optimised access to these scaffolds. This study addresses this research gap, providing efficient access to chalcone and pyrazolines via DoE while exploring HTS platforms in pursuit of novel fluorescent sensors. | ||
| Fig. 1 Access to pyrazoline and pyrazoles via the chalcone scaffold (panel A). Claisen–Schmidt condensations reported between 1960 and 2025 via the Reaxys database (panel B). | ||
Pyrazolines display unique fluorescence properties for the design of fluorescent sensors for toxic metals such as Zn2+, Cu2+, and Hg2+.19 The modular nature of the pyrazolines scaffold enables fine-tuning of photophysical properties, a useful feature for sensor development. The use of pyrazoline20 and pyrazole21 sensors for detecting toxic metals in biological environments represents a growing area of interest. Group 12 sensors, which selectively detect and distinguish Zn2+, Cd2+, and Hg2+, are particularly challenging. Zinc, the second most abundant transition metal in the human body, is vital for life,22 cadmium is highly toxic and linked to numerous cancers,23 and mercury displays potent neurotoxicity.24 Few fluorescent sensors can differentiate between these metals at different fluorescence wavelengths (λem).25 Simple pyrazoline A is a highly selective “turn on” sensor for Zn2+ and Cd2+ in MeCN (Fig. 2).26 Further studies revealed pyrazole B with improved selectivity for Zn2+/Cd2+ (Fig. 2).27 Pyrazoline C is a “turn on” sensor for Fe3+/Fe2+ with yellow λem.28 Substitution of the pyrazoline N1 methyl for phenyl reversed the fluorescent response from “turn on” to “turn off” (D in Fig. 2).28 The hydrazone functional group is commonly incorporated into multi-analyte fluorescent sensors.29 A recent breakthrough reported first-in-class hydrazone-pyrazolines sensors distinguishing Zn2+ at 560 nm, Cd2+ at 510 nm, and Hg2+ at 460 nm λem in aqueous environments (E in Fig. 2).30 Typical approaches to chalcones involve the use of polar protic solvents, such as ethanol and methanol, accounting for 50% and 20% of all Claisen–Schmidt condensations reported between 1960–2025 (see SI1 for literature survey). Researchers often use previous synthetic methodologies with little to no experimental optimisation reported. This study addresses this research need using a data-driven design of experiments (DoE) approach. A large-scale chalcone library (>1 g scale) enabled a second DoE study focusing on pyrazoline synthesis. A pyrazoline library was generated, screened across multiple analytes using a HTS platform. This workflow significantly accelerated the discovery process, providing a valuable case study on incorporating data-driven methodology in synthetic organic chemistry.
 | ||
| Fig. 2 Fluorescent sensors for toxic metals. This study incorporated design of experiments (DoE) and high throughput screening (HTS) for pyrazoline sensor development (inset). | ||
Results and discussion
An LC-MS method to monitor the Claisen–Schmidt condensation reaction was established (Fig. 3). An internal standard (acetaminophen) was added to all reaction aliquots ensuring injection consistency, and external calibration curves were generated (see SI3 for full details). A typical chromatogram is displayed in Fig. 3 demonstrating the conversion of 2-acetylpyridine and benzaldehyde to C1. A screening study to confirm the suitability of the LC-MS method and determine the main DoE parameters was performed (SI4). Solvent, consisting of the bulk of the reaction mixture, had a significant impact on chalcone yield. The equivalent of NaOH, temperature, and time were also identified as significant factors and selected for DoE analysis. While this one factor at a time (OFAT) approach identified suitable conditions, it did not identify interconnected variables or provide a system-wide analysis. A DoE workflow was established (Fig. 4) to screen multiple variables; each performed in triplicate. This experimental design provided 45 datapoints to determine the optimum conditions for the Claisen–Schmidt condensation reaction13 for chalcone synthesis using a system-wide approach. | ||
| Fig. 3 Claisen–Schmidt condensation for C1 monitored via LC-MS, starting materials 2-acetylpyridine and benzaldehyde to chalcone C1 with acetaminophen used as an internal standard. | ||
A least squares fit DoE model was generated with an R2 value of 0.97 and a P-value of <0.001, indicating good statistical modelling (Fig. 5C). This model was then used to predict various reaction conditions (Fig. 5A). Solvent was confirmed as influencing the reaction yield, with the polar protic solvents MeOH, EtOH, and IPA providing the best yields. Polar aprotic solvent MeCN and chlorinated solvent dichloromethane should be avoided. Interestingly, the use of 0.2 equivalent NaOH was the most efficient alongside 4 °C reaction temperature. Further increases in catalyst and temperature were detrimental to reaction yield. Time had a minor influence on reaction yield, with 4 h and 8 h the optimal. Using the above model and the 12 principles of green chemistry,31 particularly atom economy and energy efficiency, the following conditions were selected: EtOH, 0.2 equiv. NaOH, 4 °C and 8 h. These reaction conditions were then used to synthesise a structurally diverse library of 27 chalcones in excellent yield. Performing these reactions on a 10 mmol scale enabled gram-scale purification of chalcones directly via recrystallization preventing the requirement for time-consuming column chromatography (Scheme 1).
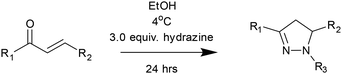
The high yield for chalcones C1–C27 provides further confirmation of the predictive properties of the DoE model and validates the chosen reaction conditions (Table 1). These reaction conditions are likely to be suitable for future chalcone research beyond sensing, for example medicinal chemistry.9–12 These chalcones then served as precursors for a pyrazoline synthesis DoE and, in turn, a pyrazoline library. A suitable DoE approach was developed to determine the optimal reaction conditions for the conversion of chalcone C1 to pyrazoline P1. The same LC-MS method was utilized to monitor the conversion of C1 to P1 with a DoE workflow established to screen solvent, phenylhydrazine equivalent, temperature, and time (Fig. 6).
A least squares fit DoE model was generated with EtOH identified as the optimal medium for the reaction (Fig. 7). Interestingly, temperature exerted only a minor influence on pyrazoline formation, with 4 °C providing the best outcome when used in combination with 3.0 equivalents of hydrazine.
 | ||
| Fig. 7 DoE summary, optimal parameters are displayed in red (panel A), residuals in panel B. A good predictive model (R2 = 0.93) was generated with confidence interval displayed in red (panel C). | ||
Reaction time showed a linear correlation with product yield, and 24 hours was determined to be optimal. Guided by this optimization model and the 12 Principles of Green Chemistry,31 the final reaction conditions were selected as EtOH, 3.0 equiv. hydrazine, 4 °C, and 24 h (Scheme 2). Under these conditions, a structurally diverse pyrazoline library comprising 11 potential sensors was synthesized in good to excellent yields (Fig. 8). Reactions performed on a 2 mmol scale generated sufficient material for purification via recrystallization, eliminating the need for time-consuming column chromatography. This approach was essential for achieving rapid synthesis and enabling high-throughput screening for desirable sensing properties. A preliminary fluorescence screen was performed to identify promising sensors in the presence of Zn2+ and Cd2+ (Fig. 8). Pyrazoline P1–P3, all containing a phenyl-substituted pyrazoline, displayed negligible changes in fluorescence emission with Zn2+ and Cd2+. Interestingly, the pyridine-substituted analogues P7–P9 did display a slight preference for Zn2+ over Cd2+ with green fluorescence emission. This observation suggested that the addition of nitrogen may be beneficial to group 12 metal selectivity. Pyrazoline P11 displayed a “turn off” response, indicating the naphthyl unit is impacting the fluorescence response. The lead sensor was identified as P10, displaying both a nitro-substituted ring and pyridyl units. A highly unusual yellow fluorescence emission was observed in the presence of Zn2+, but not with Cd2+. The substitution of an electronegative halogen (as observed in P8 and P9) for a highly electronegative nitro group profoundly modified the response to Zn2+ and Cd2+ and warrants further investigation. With a library of 11 pyrazolines in hand, a high-throughput screening (HTS) assay was developed using a standard laboratory fluorescence plate reader using a 96-well format. The responses to eleven different metal ions (250 μM) across each pyrazoline (50 μM) were measured in duplicate in acetonitrile.
This approach was designed to rapidly screen for fluorescence sensing properties in the 380–650 nm range using 360 nm excitation (see SI for full analysis, see Fig. 9 for heatmap summary). Pyrazoline P1, P3–5, P7–P8, and P10 all displayed a “turn on” response to a range of group 12 metals. The paramagnetic metals Fe3+, Ru3+, and Cu2+ displayed a “turn off” response across all pyrazolines; this is commonly observed in the literature.32 The biological metals Na+, Li+, and Mg2+ did not alter the fluorescence response, and therefore these sensors are unsuitable for monitoring these analytes. The metal screen for P10 is of particular interest as there is a “turn on” response only for Zn2+, not Cd2+ or Hg2+. The Stokes shift for P10 is also unusually large, >240 nm and this is advantageous for sensing applications. This suggests P10 could be a highly useful Zn2+ specific sensor and will be explored in future work. In summary, a combined DoE and HTS workflow has been established to rapidly synthesise a pyrazoline library from chalcone precursors in high yield. These sensors were rapidly screened for desirable sensing properties, resulting in the unexpected discovery of sensor P10 with future potential.
Conclusion
The optimised synthetic conditions for chalcone and pyrazoline formation has been determined using a data-driven design of experiments (DoE) approach (Fig. 10). These conditions (EtOH, 0.2 equiv. NaOH, 4 °C and 8 h) provided a diverse chalcone library of 27 compounds in good to excellent yield (60–93%, Table 1). The optimal conditions for pyrazoline formation (EtOH, 3.0 equiv. hydrazine, 4 °C, and 24 h) were determined and used to generate a pyrazoline library also in good to excellent yield (11–94%, Fig. 8).The establishment of an HTS assay enabled the rapid identification of fluorescent properties to a range of metals, resulting in the highly expected discovery of P10 with highly unusual yellow fluorescent emission in the presence of Zn2+ only. This workflow demonstrates the significant advantages of incorporating modern data-driven methodology into synthetic organic chemistry. This approach is ideally suited to machine learning (ML) applications in which a large, structurally diverse, and modular chemical library is synthesized and screened, resulting in large datasets of consistent data.33 This dataset can then be used to train ML models to predict and design novel compounds with specific properties (Fig. 11A). These, in turn, are generated and screened, and the resulting data used to improve the model further. A key requirement for this strategy is easily accessible scaffolds that are highly modular, using inexpensive reagents. As highlighted within, pyrazolines are ideally suited to this approach. Potential applications of such workflows include the generation of ML-derived sensors for specific metals and the development of molecular logic gates (Fig. 10B).34 Numerous recent pyrazolines have been reported as logic gates,35 and the application of ML will accelerate development further. This is future work and will be reported in due course.
Author contributions
Alexander Ciupa designed, synthesized, characterised all compounds, and authored the manuscript.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5ob01684c.Acknowledgements
Krzysztof Pawlak is acknowledged for assistance with development of the high throughput screening assay and Steven Robinson for assistance with time-of-flight high resolution mass spectrometry. This work made use of shared equipment located at the Materials Innovation Factory, created as part of the UK Research Partnership Innovation Fund (Research England) and co-funded by the Sir Henry Royce Institute.References
- Selected examples: (a) A. F. de Almeida, R. Moreira and T. Rodrigues, Nat. Rev. Chem., 2019, 3, 589 Search PubMed; (b) L. Himanen, A. Geurts, A. S. Foster and P. Rinke, Adv. Sci., 2019, 6, 1900808 Search PubMed; (c) J. Y. Peng, D. Schwalbe-Koda, K. Akkiraju, T. Xie, L. Giordano, Y. Yu, C. J. Eom, J. R. Lunger, D. J. Zheng, R. R. Rao, S. Muy, J. C. Grossman, K. Reuter, R. Gómez-Bombarelli and Y. Shao-Horn, Nat. Rev. Mater., 2022, 7, 991 CrossRef.
- K. Telford, Johns Hopkins APL Tech. Dig., 2007, 27, 224 Search PubMed.
- A. Ekebergh, C. Lingblom, P. Sandin, C. Wennerås and J. Mårtensson, Org. Biomol. Chem., 2015, 13, 3382 RSC.
- V. Karaluka, R. M. Lanigan, P. M. Murray, M. Badland and T. D. Sheppard, Org. Biomol. Chem., 2015, 13, 10888 RSC.
- P. M. Murray, F. Bellany, L. Benhamou, D.-K. Bučar, A. B. Tabor and T. D. Sheppard, Org. Biomol. Chem., 2016, 14, 2373 Search PubMed.
- J. R. Broach and J. Thorner, Nature, 1996, 384, 14 CrossRef PubMed.
- S. M. Senkan, Nature, 1998, 394, 350 CrossRef CAS.
- X. Rodríguez-Martínez, E. Pascual-San-José and M. Campoy-Quiles, Energy Environ. Sci., 2021, 14, 3301 RSC.
- P. Singh, A. Anand and V. Kumar, Eur. J. Med. Chem., 2014, 85, 758 CrossRef CAS.
- Selected examples: (a) C. Karthikeyan, N. S. H. Narayana Moorthy, S. Ramasamy, U. Vanam, E. Manivannan, D. Karunagaran and P. Trivedi, Recent Pat. Anti-Cancer Drug Discovery, 2015, 10, 97 CrossRef CAS PubMed; (b) S. Ducki, R. Forrest, J. A. Hadfield, A. Kendall, N. J. Lawrence, A. T. McGown and D. Rennison, Bioorg. Med. Chem. Lett., 1998, 8, 1051 CrossRef CAS PubMed; (c) A. Ciupa, N. J. Griffiths, S. K. Light, P. J. Wood and L. Caggiano, Med. Chem. Commun., 2011, 2, 1011 RSC.
- Selected examples: (a) Z. Nowakowska, Eur. J. Med. Chem., 2007, 42, 125 Search PubMed; (b) A. Yadav, V. Sharma and G. Singh, ChemistrySelect, 2024, 9, e202401321 CrossRef CAS.
- D. K. Mahapatra, S. K. Bharti and V. Asati, Eur. J. Med. Chem., 2015, 101, 496 CrossRef CAS PubMed.
- Selected examples: (a) L. Claisen and A. Claparède, Ber. Dtsch. Chem. Ges., 1881, 14, 2460 CrossRef; (b) J. G. Schmidt, Ber. Dtsch. Chem. Ges., 1881, 14, 1459 Search PubMed; (c) D. Coutinho, H. G. Machado, V. H. Carvalho-Silva and W. A. da Silva, Phys. Chem. Chem. Phys., 2021, 23, 6738 RSC; (d) C. L. Perrin and J. Woo, Phys. Chem. Chem. Phys., 2022, 24, 18978 RSC.
- B. Varghese, S. N. Al-Busafi, F. O. Suliman and S. M. Z. Al-Kindy, RSC Adv., 2017, 7, 46999 Search PubMed.
- D. Matiadis and M. Sagnou, Int. J. Mol. Sci., 2020, 21, 5507 CrossRef CAS.
- M. Mantzanidou, E. Pontiki and D. Hadjipavlou-Litina, Molecules, 2021, 26, 3439 Search PubMed.
- Selected examples: (a) S. Hofmann, M. Linden, J. Neuner, F. N. Weber and S. R. Waldvogel, Org. Biomol. Chem., 2023, 21, 4694 RSC; (b) S. V. Gamapwar, N. P. Tale and N. N. Karade, Synth. Commun., 2012, 42, 2617 Search PubMed; (c) A. Ciupa, New J. Chem., 2024, 48, 13900 RSC.
- Selected examples: (a) M. A. Alam, Future Med. Chem., 2023, 15, 2011 CrossRef CAS PubMed; (b) T. Sarwar, G. Mustafa, W. Zafar, A. U. Hassan, S. H. Sumrra and A. Asif, J. Mol. Struct., 2025, 1348, 143523 CrossRef CAS.
- Selected examples: (a) M. Z. Alam, S. Ahmad and S. A. Khan, J. Fluoresc., 2025, 35, 1241 CrossRef CAS; (b) M. Mohasin and S. A. Khan, J. Fluoresc., 2025, 35, 2553 CrossRef CAS PubMed; (c) M. Z. Alam, M. Mohasin, S. Ahmad, U. Salma and S. A. Khan, J. Coord. Chem., 2025, 78, 367 CrossRef CAS.
- Selected examples: (a) Z. Zhang, F. W. Wang, S. Q. Wang, F. Ge, B. X. Zhao and J. Y. Miao, Org. Biomol. Chem., 2012, 10, 8640 RSC; (b) R. Manjunath and P. Kannan, New J. Chem., 2018, 42, 10891 RSC; (c) Y. S. Yang, F. N. Wang, Y. P. Zhang, F. Yang and J. J. Xue, J. Fluoresc., 2024, 34, 159 CrossRef CAS PubMed; (d) Y. P. Zhang, Y. Y. Dong, Y. S. Yang, H. C. Guo, B. X. Cao and S. Q. Sun, Spectrochim. Acta, Part A, 2017, 177, 147 CrossRef CAS; (e) T. T. Zhang, F. W. Wang, M. M. Li, J. T. Liu, J. Y. Miao and B. X. Zhao, Sens. Actuators, B, 2013, 186, 755 CrossRef CAS.
- Selected examples: (a) S. Gond, P. Yadav, A. Singh, S. Garai, A. Shekher, S. C. Gupta and V. P. Singh, Org. Biomol. Chem., 2023, 21, 4482 RSC; (b) A. Tigreros and J. Portilla, Curr. Chin. Sci., 2021, 1, 197 CrossRef CAS; (c) S. Saha, A. De, A. Ghosh, A. Ghosh, K. Bera, K. S. Das, S. Akhtar, N. C. Maiti, A. K. Das, B. B. Das and R. Mondal, RSC Adv., 2021, 11, 10094 RSC; (d) A. Dhara, N. Guchhait, I. Mukherjee, A. Mukherjee and S. Chandra Bhattacharya, RSC Adv., 2016, 6, 105930 RSC.
- C. T. Chasapis, C. A. Spiliopoulou, A. C. Loutsidou and M. E. Stefanidou, Arch. Toxicol., 2012, 86, 521 CrossRef CAS PubMed.
- G. Genchi, S. M. Sinicropi, G. Lauria, A. Carocci and A. Catalano, Int. J. Environ. Res. Public Health, 2020, 17, 3782 CrossRef CAS.
- Y. S. Wu, A. I. Osman, M. Hosny, A. M. Elgarahy, A. S. Eltaweil, D. W. Rooney, Z. Chen, N. S. Rahim, M. Sekar, S. C. B. Gopinath, N. N. Mat Rani, K. Batumalaie and P. S. Yap, ACS Omega, 2024, 9, 5100 CrossRef CAS PubMed.
- Selected examples: (a) Z. Xu, K.-H. Baek, H. N. Kim, J. Cui, X. Qian, D. R. Spring, I. Shin and J. Yoon, J. Am. Chem. Soc., 2010, 132, 601 CrossRef CAS PubMed; (b) Y. Tan, J. Gao, J. Yu, Z. Wang, Y. Cui, Y. Yang and G. Qian, Dalton Trans., 2013, 42, 11465 RSC; (c) G. Tian, Y.-Z. Han and Q. Yang, J. Mol. Struct., 2023, 1273, 134341 CrossRef CAS.
- A. Ciupa, M. F. Mahon, P. A. De Bank and L. Caggiano, Org. Biomol. Chem., 2012, 10, 8753 RSC.
- A. Ciupa, RSC Adv., 2024, 14, 3519 RSC.
- A. Ciupa, RSC Adv., 2024, 14, 34918 RSC.
- A. Ciupa, RSC Adv., 2025, 15, 3465 RSC.
- A. Ciupa, Anal. Methods, 2025, 17, 5782 RSC.
- Selected examples: (a) P. Anastas and N. Eghbali, Chem. Soc. Rev., 2010, 39, 301 RSC; (b) R. A. Sheldon, Chem. Soc. Rev., 2012, 41, 1437 Search PubMed; (c) R. A. Sheldon, Chem. Commun., 2008, 3352 Search PubMed.
- Selected examples: (a) Z. Zhang, F.-W. Wang, S.-Q. Wang, F. Ge, B.-X. Zhao and J.-Y. Miao, Org. Biomol. Chem., 2012, 10, 8640 RSC; (b) Z. L. Gong, F. Ge and B.-X. Zhao, Sens. Actuators, B, 2011, 159, 148 Search PubMed; (c) M. M. Li, F. Wu, X. Y. Wang, T. T. Zhang, Y. Wu, Y. Xiao, J. Y. Miao and B.-X. Zhao, Anal. Chim. Acta, 2014, 826, 77 CrossRef CAS.
- J. Vamathevan, D. Clark, P. Czodrowski, I. Dunham, E. Ferran, G. Lee, B. Li, A. Madabhushi, P. Shah, M. Spitzer and S. R. Zhao, Nat. Rev. Drug Discovery, 2019, 18, 463 CrossRef CAS.
- A. Ciupa, RSC Adv., 2025, 15, 10565 RSC.
- Selected examples: (a) D. C. Magri and A. A. Camilleri, Chem. Commun., 2023, 59, 4459 RSC; (b) D. Sammut, N. Bugeja, K. Szaciłowski and D. C. Magri, New J. Chem., 2022, 46, 15042 RSC; (c) N. Zerafa, M. Cini and D. C. Magri, Mol. Syst. Des. Eng., 2021, 6, 93 Search PubMed.
| This journal is © The Royal Society of Chemistry 2026 |