Synergistic activation of persulfate by Cu(II) and Fe(III) enhanced with catechin for chloramphenicol removal from soil
Yanlin
Wu
 *a,
Ruixin
Ji
b,
Tian
Qiu
b,
Olivier
Monfort
*a,
Ruixin
Ji
b,
Tian
Qiu
b,
Olivier
Monfort
 *c,
Wenbo
Dong
b and
Jie
Guan
a
*c,
Wenbo
Dong
b and
Jie
Guan
a
aSchool of Resources and Environmental Engineering, Shanghai Polytechnic University, Shanghai 201209, China. E-mail: wuyanlin@fudan.edu.cn
bShanghai Key Laboratory of Atmospheric Particle Pollution and Prevention, Department of Environmental Science and Engineering, Fudan University, Shanghai 200433, China
cDepartment of Inorganic Chemistry, Faculty of Natural Sciences, Comenius University Bratislava, Ilkovicova 6, Mlynska dolina, 842 15 Bratislava, Slovakia. E-mail: olivier.monfort@uniba.sk
First published on 2nd December 2025
Abstract
The combined pollution of heavy metals and antibiotics has become a serious environmental problem in recent years. In this study, an efficient and innovative technique to remove chloramphenicol (CAP), a pharmaceutical compound, from the Cu-contaminated soil was investigated. Fe(III) was added to form a bimetallic system to activate persulfate (PS) to produce reactive oxidative species (ROS). Moreover, polyphenol catechin (CAT) was used to enhance the Fe(II)/Fe(III) and Cu(I)/Cu(II) cycles, thus producing more ROS. The optimal ratio between Fe(III)/Cu(II) and the CAT concentration was determined. The bimetallic system with Fe(III)/Cu(II)/PS/CAT was more stable and efficient than monometallic ones and its efficiency in CAP degradation (100% after 2 h) was slightly affected by carbonate ions. In addition, 9 intermediate degradation products were identified, and the mineralization degree, determined by TOC analysis, reached 28.2% after 12 h. Among ROS, SO4˙−, ˙OH, and Cu(III) contributed to CAP degradation by 82.5%, 12.4%, and 5.1%, respectively. Cu(I) was found to play an important role in the PS activation mechanism, especially in Fe(III) reduction. The toxicity of the treated soil significantly decreased. This study highlighted the application potential of the combination of copper and iron in the activation of PS to treat pharmaceutical pollution.
1. Introduction
With industrial and agricultural development worldwide, the issue of mixed pollutant exposure has become an increasingly serious concern. Combination of heavy metals and antibiotics is a dangerous “cocktail”, which is particularly common in polluted cropland, orchard and farm environments. As a heavy metal, copper (Cu) plays essential roles in the growth and development of animals and plants, but it also causes serious pollution problems when it enters the soil at high concentrations. Cu pollution usually occurs due to wastewater irrigation and extensive use of chemical fertilizers and fungicides.1,5 Normally, the total concentrations of Cu in the soil should range from 7.0 to 263.0 mg kg−1 (ref. 2–4), and the limit of Cu concentration for agricultural land is 150 mg kg−1 in China. However, the highest Cu concentration in a mine water irrigation area in Brazil reached 1526.2 mg kg−1 and the bioavailable/dissolved copper concentration reached 860.3 mg kg−1,5 which is a very serious environmental issue. Simultaneously, antibiotics are widely used in agriculture, especially in livestock and poultry breeding industries, and their excessive use also caused significant environmental issues. Chloramphenicol (CAP) is a broad-spectrum antibiotic that has been widely used in agriculture. However, since the year 2000, it has been listed as a prohibited drug for food-producing animals in various countries, such as the United States, Canada, the European Union and China, due to its carcinogenic activity.6,7 Although the use of CAP has been banned in these regions, this highly recalcitrant product continues to be detected in food,6,7 rivers and lakes,8,9 sediments and soils.10,11 The concentration levels of CAP in sediments and soils has reached the mg L−1 to mg kg−1 range.10,11 In addition, soils contaminated with heavy metals could enhance both the occurrence and spread of antibiotic resistance.12 Therefore, removal of antibiotics from heavy metal-contaminated soils is of particular importance.Sulfate radical-based advanced oxidation processes (SR-AOPs) are frequently used for the degradation of antibiotics, including CAP, in water treatment13–15 and soil remediation.16 SR-AOPs are a widely used approach to treat organic pollution due to their benefits including good stability, strong oxidation activity, low operational cost and high selectivity.16,17 Among the existing methods to activate persulfate (PS) or peroxymonosulfate (PMS) into highly reactive radicals, activation using transition metal ions (such as iron, copper and cobalt) has received the most attention, due to their high efficiency and low energy consumption. Iron was the most widely studied metal activator,18,19 and has been efficiently used for the removal of antibiotics from soils.16,20 Meanwhile, copper-based catalysts, such as Cu@Fe3O421 and natural bornite,22 have also been applied as PS activators for the degradation of organic pollutants. Apart from the confirmed high efficiency of Fe–Cu bimetallic catalysts in the activation of PS, the mechanism of metal valence state conversion has not been elucidated yet, and few studies have applied Fe–Cu bimetallic activators for soil remediation purposes.
On the other hand, plant polyphenols have been introduced in many AOPs as modifying agents, due to their capacity for the complexation and reduction of metal ions.23,24 Gallic acid (GA), obtained from Rheum palmatum L. and Cornus officinalis, has been used to modify Fe(III)/PS19,20 and Fe3O4/PMS25 systems, where Fe(III) could be efficiently reduced to Fe(II), with Fe(II) being the key species required for the activation of PS/PMS. Catechin (CAT), obtained from Camellia sinensis (L.) Kuntze, is a natural and environmentally friendly antioxidant, which reacts more rapidly with Fe(III) than GA through both complexation and reduction. For water pollution remediation, CAT has already been proven to enhance Fenton-like, Fe(III)/PS and Fe(III)/PMS systems.16,26,27
In this study, Fe(III) was added to the simulated Cu-contaminated soil, forming a Fe–Cu bimetallic system for PS activation in CAP degradation. CAT was applied to enhance Cu(II)/Cu(I) and Fe(III)/Fe(II) cycles, thus improving the efficiency of ROS production. The effect of different Fe(III)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu(II) ratios on CAP degradation was investigated. Furthermore, the effect of CAT on the metal valence state was investigated, and the ROS involved in CAP removal were identified. The toxicity of degradation byproducts and their degree of mineralization were also assessed. Finally, mechanistic insights into PS activation and CAP degradation were provided. In summary, the main advantage of this system is that wastes can be used to treat wastes with stable and efficient decontamination rates. Indeed, Cu is usually present in contaminated soil while CAT can be extracted from tea leaves.
Cu(II) ratios on CAP degradation was investigated. Furthermore, the effect of CAT on the metal valence state was investigated, and the ROS involved in CAP removal were identified. The toxicity of degradation byproducts and their degree of mineralization were also assessed. Finally, mechanistic insights into PS activation and CAP degradation were provided. In summary, the main advantage of this system is that wastes can be used to treat wastes with stable and efficient decontamination rates. Indeed, Cu is usually present in contaminated soil while CAT can be extracted from tea leaves.
2. Materials and methods
2.1. Chemicals and reagents
The chemicals and reagents used in this research are detailed in Text S1 in the supplementary information (SI).2.2. Experimental procedure
The soil was collected from the banks of the Suzhou River (Shanghai, China), pretreated according to the procedure described in Text S2 (SI) to reduce the organic matter content and desorb other organic contaminants. The physical and chemical parameters of the soil after pretreatment are provided in Table S1 (SI).Prior to experiments, the CAP solution was mixed with uncontaminated soil to a concentration of 32.3 mg kg−1. The degradation reactions were performed in a 20 mL brown glass vial. The CAP contaminated soil samples were mixed with deionized water, CAT, PS, Fe(III) and/or Cu(II) solutions. The detailed mixing process was the same as that of our previous research.16 The initial CAP concentration in the contaminated soil mixture mud was 40 µM and the pH was 6.5, without adjustment. The degradation reactions were terminated at fixed time intervals with the addition of an excess of Na2S2O3. After extraction, centrifugation and filtration, the CAP concentrations were analyzed by high-performance liquid chromatography (HPLC, Ultimate 3000, Dionex, US). The CAP extraction procedure is described in Text S3 (SI), and the CAP recovery rate after extraction was stable between 90 and 92%. All experiments were performed at least twice.
2.3. Analytical methods
HPLC analysis of CAP in soil was performed using a C18 reverse phase column (100-5C18, Kromasil, Sweden), with the detection wavelength set at 278 nm, and the CAP retention time was 5.15 min. The mobile phase for CAP analysis consisted of methanol and water at a 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 v/v ratio in the isocratic mode. The intermediate products obtained from the CAP degradation were analyzed by ESI-QTOF-MS.
40 v/v ratio in the isocratic mode. The intermediate products obtained from the CAP degradation were analyzed by ESI-QTOF-MS.
To identify the contribution of ROS, 2-propanol (2-Pr), tert-butanol (TBA) and sodium bromide (NaBr) were added as selective quenching agents of SO4˙−, ˙OH and Cu(III) in the bimetallic system. Electron spin resonance (ESR, EMX-plus, Bruker, Germany) was also performed to confirm the scavenging studies.
Total iron and Fe(II) concentrations were determined by the addition of Ferrozine and the resulting Fe(II)–ferrozine complex could be detected using a UV-vis spectrophotometer at 562 nm.27 The Cu(I) concentration was determined by the addition of neocuproine, forming a yellow complex which could be detected at 455 nm.28,29
The degree of mineralization of CAP was measured using a total organic carbon analyzer with a solid sampler (TOC-L CPH, Shimadzu, Japan). The biotoxicity of the samples was evaluated based on the survival rate of fluorescent bacteria, using a fluorescence tester (DACY-3, Hengmei, China) set at 600 nm (OD600t) after mixing with the test solution.
3. Results and discussion
3.1. Enhanced CAP removal in the bimetallic activation system
CAP degradation in the Fe(III)/Cu(II)/PS/CAT system was investigated and the control experiments were carried out (Fig. 1). Regarding the control experiments, CAP could not be degraded by only Fe(III) or CAT, while a negligible extent of degradation was observed after 120 min of reaction with the Fe(III)/PS system (9.8%) and direct PS oxidation (6.5%).The CAP degradation efficiency was significantly improved by the addition of the plant polyphenol CAT. The CAP removal rate significantly increased to 68.4% in the Cu(II)/PS/CAT system, while reaching 94.3% in the Fe(III)/PS/CAT system under the same reaction conditions, indicating that CAP removal was more efficient by the iron system than the copper system, because copper is less active than iron for PS activation. The bimetallic system, obtained by replacing half of the Fe(III) content with Cu(II), led to an enhanced degradation rate of 96.8%. It indicated that there was a synergistic effect between Fe(III) and Cu(II) in the PS activation, and thus in the production of ROS. In the case where there were no additional effects of the cations in the Fe(III)/Cu(II)/PS/CAT system, the CAP degradation rate would be expected to be approx. 81%. So, the synergistic effect between Fe(III) and Cu(II) increased the CAP degradation rate by approx. 15%.
Previous studies24,25 reported that CAT was able to efficiently reduce Fe(III) and Cu(II). Therefore, it could be reasonably assumed that the newly formed Cu(I) could also reduce Fe(III) to Fe(II), thus improving PS activation, as described in eqn (1)–(5). In other words, Cu(I) could enhance the redox Fe(III)/Fe(II) cycle to increase the regeneration of Fe(II), which is very important in PS activation. Consequently, the CAP degradation efficiency significantly increased.
| CAT + Cu(II) → Cu(I) + CATproducts | (1) |
| CAT + Fe(III) → Fe(II) + CATproducts | (2) |
| Cu(I) + Fe(III) → Cu(II) + Fe(II) | (3) |
| Fe(II) + S2O82− → Fe(III) + SO4˙− | (4) |
| OH− + SO4˙− → SO42− + HO˙ | (5) |
3.2. Effect of the Fe(III)/Cu(II) ratio
As discussed above, Cu(II) played an important role in enhancing the Fe(III)/PS/CAT system. Therefore, real Cu-contaminated soil might improve PS activation, supporting the effective degradation of organic pollutants (such as CAP), while Fe was often naturally present in most of the soils, and CAT can be extracted from green tea leaves. In the present study, 100 µM of dissolved Cu(II) was added to the reaction soil mixture. Usually, only about 1–3% of the copper in soil was transformed to dissolved Cu(II) because the majority of the copper tends to immediately form Cu(OH)2, a CuCO3 precipitate or a copper complex in the soil.30 This corresponds to a potential original soil content of 632 mg kg−1, which is a simulated Cu-contaminated relevant concentration.To investigate the effect of the Fe(III)/Cu(II) ratio, different concentrations of Fe(III) ranging from 20 to 1000 µM were added to the simulated Cu-contaminated soil to form various Fe(III)/Cu(II)/PS/CAT systems and the degradation of CAP was monitored (Fig. 2). After 120 min of the reaction, the CAP degradation rate was 53.6% without the addition of Fe(III) and it increased to 82.2% with the addition of 20 µM Fe(III). This significant enhancement tends to validate the synergistic effect described in the previous section. The addition of a low concentration of Fe(III) achieved a significant improvement in reaction efficiency. The CAP degradation rate was the highest when 100 µM Fe(III) was added, i.e. 96.8% after 120 min in Fe(III)/Cu(II) = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. When the ratio of Fe(III)/Cu(II) exceeded 1
1 ratio. When the ratio of Fe(III)/Cu(II) exceeded 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the CAP degradation efficiency was adversely affected.
1, the CAP degradation efficiency was adversely affected.
From these results, it could be deduced that Cu(II) was reduced to Cu(I) by the reaction with CAT, driving the reduction of Fe(III) to Fe(II). With increasing Fe(III) concentrations, Fe(II) was continuously produced, the rate of ROS production was improved and the CAP degradation rate was increased. However, when the molar ratio of Fe(III)/Cu(II) was excessively high, a high amount of Fe(II) was generated, resulting in radical depletion, and this inhibited the rate of CAP degradation. Therefore, the ratio of Fe(III) to Cu(II) should be controlled in order to achieve an optimal CAP degradation efficiency.
3.3. The role of CAT
According to our previous studies,26,27 CAT could react with Fe(III), generating Fe(II) by complexation and reduction, and the optimal molar ratio of Fe(III) to CAT was ∼2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in the Fe(III)/PS/CAT system.16 In the present study, the optimal molar ratio of Cu(II) to CAT in the monometallic Cu(II)/PS/CAT system was also established to be ∼2.5
1 in the Fe(III)/PS/CAT system.16 In the present study, the optimal molar ratio of Cu(II) to CAT in the monometallic Cu(II)/PS/CAT system was also established to be ∼2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. S1). This indicated that the reaction mechanisms of the two metal ions with CAT were similar, i.e. Cu(I) produced through Cu(II) reduction by CAT.
1 (Fig. S1). This indicated that the reaction mechanisms of the two metal ions with CAT were similar, i.e. Cu(I) produced through Cu(II) reduction by CAT.
The optimal CAT concentration was investigated in the bimetallic system, with Fe(III) and Cu(II) both at 100 µM, by varying the dosage of CAT from 0 to 100 µM and monitoring the resulting CAP degradation (Fig. 3). The reaction rate increased rapidly upon increasing the CAT concentration from 0 to 80 µM, and then reached a plateau at a CAT concentration of 100 µM. At Fe(III)/Cu(II) = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the total concentration of metal ions was 200 µM. Therefore, the optimal CAT dosage was 80 µM, corresponding to a molar ratio of ∼2.5
1, the total concentration of metal ions was 200 µM. Therefore, the optimal CAT dosage was 80 µM, corresponding to a molar ratio of ∼2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. An excess of CAT would competitively consume ROS and lead to a decreased efficiency in CAP degradation. Indeed, CAT was an antioxidant and its dosage should be controlled appropriately to reach the maximum efficiency of the Fe(III)/Cu(II)/PS/CAT system in the oxidative degradation of CAP. Therefore, in practical applications, the optimal dosage of CAT should be determined according to the content of metal ions within the soil.
1. An excess of CAT would competitively consume ROS and lead to a decreased efficiency in CAP degradation. Indeed, CAT was an antioxidant and its dosage should be controlled appropriately to reach the maximum efficiency of the Fe(III)/Cu(II)/PS/CAT system in the oxidative degradation of CAP. Therefore, in practical applications, the optimal dosage of CAT should be determined according to the content of metal ions within the soil.
3.4. Identification of the ROS
Firstly, 2.0 mM 2-Pr was added as a selective quenching agent for SO4˙− and ˙OH within the bimetallic system (Fig. 4a). 2-Pr could quench both SO4˙− and HO˙ due to its high reactivity (k2-Pr,HO˙ = 1.9 × 109 M−1 s−1,31k2-Pr,SO4˙− = 8.5 × 107 M−1 s−1 (ref. 32)). Under such conditions, the CAP degradation rate was almost completely inhibited and decreased to 4.9%, indicating that almost total CAP degradation occurred via the combined effects of SO4˙− and HO˙. The existence of these radicals was further confirmed by ESR using 5,5-dimethyl-1-oxopyrroline (DMPO) to form the spin adduct compounds DMPO–HO˙ and DMPO–SO4˙− (Fig. 4b). | ||
| Fig. 4 (a) Effects of different quenchers on CAP degradation; (b) ESR spectra ([Fe(III)]0 = [Cu(II)]0 = 100 µM, [PS]0 = 1.0 mM, [CAT]0 = 80 µM, pH = 6.5). | ||
The remaining 4.9% of CAP degradation might occur due to the oxidation by Cu(III), which was formed from the reaction between PS and Cu(II),33 as shown in eqn (6). Br− was added to determine the effect of Cu(III) according to eqn (7)–(10). Br− could react with high second order rate constant with radicals and Cu(III) (kHO˙/Br− = 1.1 × 1010 M−1 s−1; kSO4˙−/Br− = 3.5 × 109 M−1 s−1 (ref. 34); kCu(III)/Br− = (1.9–3.4) × 108 M−1 s−1 (ref. 35)), thus making it difficult to prove the presence of Cu(III) due to interference from large amounts of radicals in the Fe–Cu bimetallic system (Fig. 4a). As shown in Fig. 4, CAP degradation was significantly inhibited by the addition of Br−, making it difficult to prove the presence of Cu(III) due to interference from large amounts of radicals in the Fe–Cu bimetallic system. However, the oxidation capability of both Br˙ (2.2 V) and Br2˙− (1.66 V) was stronger than that of Cu(III) (1.3–1.5 V), but weaker than that of SO4˙− (2.5–3.1 V) and ˙OH (2.8 V). By adding Br− to the monometallic Cu(II)/PS/CAT system, a slight increase in CAP degradation was observed (Fig. S2), because the generated Br˙ and Br2˙− have higher oxidation capabilities than Cu(III). The slight promotion of CAP degradation confirmed the existence of Cu(III), meaning that the main ROS were not radicals. Thus, it could be concluded that the contribution of Cu(III) to CAP degradation in the Fe(III)/Cu(III)/PS/CAT system was small.
| S2O82− + Cu(II) → Cu(III) + SO4˙− + SO42− | (6) |
| Br− + SO4˙− → Br˙ + SO42− | (7) |
| Br− + HO˙ → Br˙ + OH− | (8) |
| Br− + Cu(III) → Cu(II) + Br˙ | (9) |
| Br− + Br˙ → Br2˙− | (10) |
TBA is a known quencher of ˙OH (kTBA,HO˙ = 6.0 × 108 M−1 s−1 (ref. 31)) and it could also reduce Cu(III).36 Therefore, the addition of TBA could effectively quench both Cu(III) and ˙OH, while preserving the oxidation of SO4˙−. Following the addition of TBA to the bimetallic system, the rate of CAP degradation decreased from 96.8% to 79.9%, indicating that SO4˙− had a larger contribution than ˙OH. Therefore, the relative contributions of SO4˙−, ˙OH and Cu(III) to CAP degradation were 82.5%, 12.4% and 5.1%, respectively.
3.5. Effect of carbonate ions
In the environment, many coexisting anions such as NO3−, SO42−, Cl−, and CO32−/HCO3− affect chemical reactions, including the degradation of organic pollutants. Among them, carbonates have the most significant inhibitory effect on pollutant degradation.37–39 So the effect of CO32− on CAP degradation was investigated in bimetallic and monometallic systems by varying the concentration of CO32− from 0.1 mM to 10 mM (Fig. 5).The most significant inhibitory effect was observed in the monometallic Fe(III)/PS/CAT system (Fig. 5a). CAP degradation decreased from 94.3% to 51.4% after CO32− addition. In this system, SO4˙− and HO˙ were the predominant ROS, and they react with CO32− to produce CO3˙−,16,18,40 which had low oxidation ability (eqn (11) and (12)). Meanwhile, the introduction of CO32− would increase the pH which might detrimentally affect the efficiency of Fe(III) in the generation of ROS. In the Cu(II)/PS/CAT system (Fig. 5b), a slight effect was observed on CAP degradation with CO32− addition. The CAP degradation extent decreased from 68.3% to 58.4%, i.e. a 10% reduction. In this system, Cu(III) was the predominant oxidative species besides radicals, and Cu(III) was not affected by CO32−. In the bimetallic system (Fig. 5c), there was about 20% inhibition of CAP degradation, and a decrease of the extent of degradation from 96.8% to 76.7%. Compared to the monometallic system, the bimetallic one had the same metal cation concentrations; however, the CAP degradation rate was higher than that of the copper system and the inhibitory effect of carbonates was lower than that of the iron system. Generally, the bimetallic system has great application potential. It simultaneously has high oxidation efficiency and anti-interference ability.
| SO4˙− + CO32− → SO42− + CO3˙− k = 1.6 × 106 M−1 s−1 | (11) |
| HO˙ + CO32− → OH− + CO3˙− k = 8.5 × 106 M−1 s−1 | (12) |
3.6. Proposed activation mechanism in the Fe(III)/Cu(II)/PS/CAT system
In the present work, the identification and role of ROS are discussed in Section 3.4, where SO4˙− and ˙OH were the dominant species in the bimetallic system. In addition, it was concluded that Cu(III) and Cu(I) were present within the Fe(III)/Cu(II)/PS/CAT system. Although Cu(III) could not be directly detected, its role was confirmed by the addition of Br− (Section 3.4). To highlight the role of Cu(I) and Fe(II) in the mechanism of PS activation, their concentrations were measured in the Cu(II)/CAT, Fe(III)/CAT and Fe(III)/Cu(II)/CAT systems (Fig. 6). The Cu(I) concentration in the Cu(II)/CAT system was about 60 µM due to its reduction by CAT (eqn (1)), and it decreased to 40 µM in the Fe(III)/Cu(II)/CAT system, as Cu(I) reacted with Fe(III) to form Cu(II) and Fe(II) (eqn (3)). Meanwhile, the Fe(II) concentration in the Fe(III)/CAT system was about 80 µM due to its reduction by CAT (eqn (2)) and increased to about 90 µM in the Fe(III)/Cu(II)/CAT system, due to further reduction of Fe(III) by Cu(I) (eqn (3)). | ||
| Fig. 6 Cu(I) and Fe(II) concentrations in different systems ([Fe(III)]0 = [Cu(II)]0 = 100 µM, [PS]0 = 1.0 mM, and [CAT]0 = 80 µM). | ||
Therefore, in the Fe(III)/Cu(II)/PS/CAT system, both iron and copper underwent continuous redox cycling between Fe(II)/Fe(III) and Cu(I)/Cu(II), with PS being more efficiently activated than in the monometallic systems. The higher Fe(II) concentration in the bimetallic system highlights that Fe(II) played a key role in PS activation into ROS, thus accelerating the degradation of CAP. The activation mechanism is summarized in Fig. 7.
3.7. The degradation byproducts of pollutant CAP
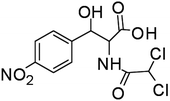
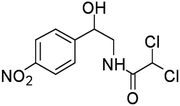
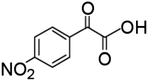
The degradation of CAP was further analyzed by ESI-QTOF-MS, and 9 degradation byproducts were identified (Table 1). The CAP degradation pathway was similar to that in our previous research study,16 especially the products P1–P5 and P9.The carbon atoms on the benzene ring of CAP were attacked by HO˙, and P1 was generated.16 Product 2 (P2) and product 3 (P3) were produced from the oxidation of alcohol to produce carboxylic acid and ketone products.16 Product 4 (P4) and Product 5 (P5) were formed through the fracture of the C–C bond.16 P9 was obtained from the further fragmentation of P5.
The products 6–8 (P6–P8) were unique byproducts resulting from the present bimetallic system. They were produced by the breaking of the amide bond, and the benzene ring part was retained. The carbon atom in the para-position of the nitro group was oxidized and three different carboxylic acid groups were formed in P6–P8. This might be due to the oxidation by Cu(III).
3.8. Mineralization degree and toxicity assessment
The TOC was analyzed to assess the mineralization degree (Fig. 8a). The TOC was monitored in three different systems after 12 hours of reaction. The TOC removal rate was about 14.8% in the copper monometallic system and 25.1% in the iron monometallic system, while 28.2% was obtained in the bimetallic system. The mineralization degrees were consistent with the degradation efficiency in three systems. The Fe(III)/Cu(II)/PS/CAT system had not only the highest degradation efficiency, but also performed best in the mineralization rate. | ||
| Fig. 8 (a) Degradation of TOC ([PS]0 = 1.0 mM, [CAT]0 = 80 µM, pH = 6.5) and (b) luminescent bacteria inhibition rate of different solutions. | ||
After the degradation of CAP, many degradation byproducts remained in the solution, so the toxicity of the treated samples was assessed. The inhibition rate of the luminescent bacteria was applied to evaluate the toxicity of the treated samples.41,42 The luminescence intensity is closely related to their cellular activity. With the exposure of luminescent bacteria to toxic substances, their luminescence ability will be inhibited and the inhibition rate can be calculated to reflect the toxicity level of the analyzed samples. The toxicities of CAP, CAT and PS were also measured along with the monometallic and bimetallic systems (Fig. 8(b)). CAP had the highest inhibition rate of the luminescent bacteria (85.4%), indicating that CAP was toxic to organisms, while CAT had the lowest inhibition rate (25.2%), which implied it was environmentally friendly. However, the 52.1% inhibition rate of PS might not be due to its toxicity, because the strong oxidizing properties of PS (2.01 V) might affect the growth of bacteria. The inhibition rate of luminescent bacteria was about 49.0% in the iron monometallic system, 70.5% in the copper monometallic system, and 49.1% in the bimetallic system. The results showed that toxicity effectively decreased after treatment. The highest toxicity in the copper system was not only mainly due to the incomplete degradation of CAP (only 68.3% degradation of CAP) but also the remaining copper ions. The Fe(III)/Cu(II)/PS/CAT system had both high degradation efficiency and low toxicity. Furthermore, the bimetallic system needed a smaller amount of Fe(III) than the iron monometallic system and could potentially utilize the copper from a contaminated soil. In other words, the bimetallic systems have great advantages and potential in terms of sustainability since they can use waste to treat waste.
4. Conclusions
In this study, collaborative persulfate activation by Cu(II) and Fe(III) ions was enhanced using polyphenol CAT. Pollutant CAP was effectively degraded (96.8% after 120 min) using the Fe(III)/Cu(II)/PS/CAT system with a mineralization rate of 28.2% after 12 hours of reaction. The CAT played an important role in the formation of Fe(II) and Cu(I), and Cu(I) was confirmed to further enhance the Fe(III) reduction. The optimal ratio of Fe(III) to Cu(II) was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, with the CAT concentration being about a quarter of the total concentration of metal ions. Furthermore, the active oxidative species were identified, showing that SO4˙−, ˙OH and Cu(III) contributed to CAP degradation, with relative contributions of 82.5%, 12.4% and 5.1%, respectively. The degradation efficiency of the Fe(III)/Cu(II)/PS/CAT system was slightly inhibited by the presence of carbonate ions. During the CAP degradation, 9 degradation byproducts were identified, of which three were new compared with other published studies. The toxicity of the treated samples exhibited a significant reduction, indicating that such a bimetallic system has promising application potential. Indeed, this bimetallic system provided a sustainable approach since it could potentially use wastes, i.e., highly Cu-contaminated soils and CAT extracted from green tea leaves, along with naturally available iron to treat waste like pharmaceutical pollutants.
1, with the CAT concentration being about a quarter of the total concentration of metal ions. Furthermore, the active oxidative species were identified, showing that SO4˙−, ˙OH and Cu(III) contributed to CAP degradation, with relative contributions of 82.5%, 12.4% and 5.1%, respectively. The degradation efficiency of the Fe(III)/Cu(II)/PS/CAT system was slightly inhibited by the presence of carbonate ions. During the CAP degradation, 9 degradation byproducts were identified, of which three were new compared with other published studies. The toxicity of the treated samples exhibited a significant reduction, indicating that such a bimetallic system has promising application potential. Indeed, this bimetallic system provided a sustainable approach since it could potentially use wastes, i.e., highly Cu-contaminated soils and CAT extracted from green tea leaves, along with naturally available iron to treat waste like pharmaceutical pollutants.
Author contributions
Yanlin Wu: writing – original draft, investigation, funding acquisition, and validation; Ruixin Ji: formal analysis and software; Tian Qiu: data curation and visualization; Olivier Monfort: writing – review and editing, funding acquisition, and resources; Wenbo Dong: conceptualization, methodology, and supervision; Jie Guan: funding acquisition and project administration.Conflicts of interest
There are no conflicts to declare.Data availability
All relevant data supporting this article have been included in the manuscript and supplementary information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5nj03893f.Acknowledgements
This work was supported by the Science and Technology Development Fund of Pudong New Area (PKJ2024-N03), Project of Ministry of Science and Technology of China for China-Slovakia International Cooperation (No. 9-7), Project of China-Central Eastern European Countries Higher Education Institutions consortium (No. 2023264), and the National Natural Science Foundation of China (NSFC 52070127 and NSFC 52370142). This work was also supported by the INOPHER project financed by the Slovak Research and Development Agency under contract no. SK-CN-23-0002.References
- C. Ballabio, P. Panagos, E. Lugato, J. H. Huang, A. Orgiazzi, A. Jones, O. Fernández-Ugalde, P. Borrelli and L. Montanarella, Copper distribution in European topsoils: An assessment based on LUCAS soil survey, Sci. Total Environ., 2018, 636, 282–298 CrossRef CAS PubMed.
- W. Li, M. Zhang and H. Shu, Distribution and Fractionation of Copper in Soils of Apple Orchards, Environ. Sci. Pollut. Res., 2005, 12, 168–172 CrossRef CAS PubMed.
- D. Jeon, B. Robinson and N. Dickinson, Organic matter mitigates biotic impact of copper in fruit orchard soil, Environ. Pollut., 2024, 363, 125145 CrossRef CAS.
- K. A. Mackie, T. Müller, S. Zikeli and E. Kandeler, Long-term copper application in an organic vineyard modifies spatial distribution of soil micro-organisms, Soil Biol. Biochem., 2013, 65, 245–253 CrossRef CAS.
- D. P. Oliveira, G. N. Nobrega, F. Ruiz, F. Perlatti, A. A. Soares, X. L. Otero and T. O. Ferreira, Risk assessment and copper geochemistry of an orchard irrigated with mine water: a case study in the semiarid region of Brazil, Environ. Geochem. Health, 2019, 41, 603–615 CrossRef CAS.
- I. D. Morariu, L. Avasilcai, M. Vieriu, O. Cioanca and M. Hancianu, Immunochemical assay of chloramphenicol in honey, Farmacia, 2019, 67(2), 235–239 CrossRef CAS.
- V. Dumont, A. C. Huet, I. Traynor, C. Elliott and P. Delahaut, A surface plasmon resonance biosensor assay for the simultaneous determination of thiamphenicol, florefenicol, florefenicol amine and chloramphenicol residues in shrimps, Anal. Chim. Acta, 2006, 567(2), 179–183 CrossRef CAS.
- H. Liu, G. Zhang, C. Q. Liu, L. Li and M. Xiang, The occurrence of chloramphenicol and tetracyclines in municipal sewage and the Nanming River, Guiyang City, China, J. Environ. Monit., 2009, 11, 1199–1205 RSC.
- L. M. Nguyen, N. T. T. Nguye, T. T. T. Nguyen, T. T. Nguyen, D. T. C. Nguyen and T. V. Tran, Occurrence, toxicity and adsorptive removal of the chloramphenicol antibiotic in water: a review, Environ. Chem. Lett., 2022, 20, 1929–1963 CrossRef CAS PubMed.
- X. Ji, Q. Shen, F. Liu, J. Ma, G. Xu, Y. Wang and M. Wu, Antibiotic resistance gene abundances associated with antibiotics and heavy metals in animal manures and agricultural soils adjacent to feedlots in Shanghai; China, J. Hazard. Mater., 2012, 235–236, 178–185 CrossRef CAS.
- N. Karikalan, A. Yamuna and T. Y. Lee, Ultrasensitive detection of ineradicable and harmful antibiotic chloramphenicol residue in soil, water, and food samples, Anal. Chim. Acta, 2023, 1243, 340841 CrossRef CAS.
- I. Timková, M. Lachká, L. Nosálová, L. Malinicová, P. Pristas and J. Sedláková-Kaduková, What antibiotic threat do the heavy metals contaminated sites of mine hide?, Inzy. Miner., 2020, 2(1), 205–210 Search PubMed.
- F. Cheng, P. Zhou, Y. Liu, X. Huo, J. Zhang, Y. Yuan, H. Zhang, B. Lai and Y. Zhang, Graphene oxide mediated Fe(III) reduction for enhancing Fe(III)/H2O2 Fenton and photo-Fenton oxidation toward chloramphenicol degradation, Sci. Total Environ., 2021, 797, 149097 CrossRef CAS PubMed.
- C. Q. Tan, Y. J. Dong, D. F. Fu, N. Y. Gao, J. X. Ma and X. Y. Liu, Chloramphenicol removal by zero valent iron activated peroxymonosulfate system: Kinetics and mechanism of radical generation, Chem. Eng. J., 2018, 334, 1006–1015 CrossRef CAS.
- Y. Q. Gao, J. Q. Zhou, H. Ning, Y. Y. Rao and N. Y. Gao, Electrochemically activated peroxymonosulfate for the abatement of chloramphenicol in water: performance and mechanism, Environ. Sci. Pollut. Res., 2022, 29(12), 17866–17877 CrossRef CAS.
- T. Qiu, L. Y. Li, W. B. Dong, G. Mailhot, Y. L. Wu and X. Zeng, Chloramphenicol degradation in soil with catechin-enhanced ferric iron/persulfate system, J. Cleaner Prod., 2022, 372, 133742 CrossRef CAS.
- Y. M. Zhang, S. H. Nie, M. H. Nie, C. X. Yan, L. H. Qiu, L. L. Wu and M. J. Ding, Remediation of sulfathiazole contaminated soil by peroxymonosulfate: Performance, mechanism and phytotoxicity, Sci. Total Environ., 2022, 830, 154839 CrossRef CAS PubMed.
- Y. Li, Y. L. Wu and W. B. Dong, Trace catechin enhanced degradation of organic pollutants with activated peroxymonosulfate: Comprehensive identification of working oxidizing species, Chem. Eng. J., 2022, 429, 132408 CrossRef CAS.
- L. Y. Li, D. Q. Zheng, X. Y. Gu, C. J. Sun, Y. K. Liu, W. B. Dong and Y. L. Wu, Ibuprofen degradation by gallic acid improved Fe(III)/persulfate reaction: Mechanism, Environ. Pollut., 2022, 314, 120318 CrossRef CAS PubMed.
- Y. K. Liu, T. Qiu, Y. L. Wu, S. Y. Wang, M. Liu and W. B. Dong, Remediation of soil contaminated with ibuprofen by persulfate activated with gallic acid and ferric iron, Chem. Eng. J., 2021, 426, 127653 CrossRef CAS.
- V. L. Pham, D. G. Kim and S. O. Ko, Oxidative degradation of the antibiotic oxytetracycline by Cu@Fe3O4 core-shell nanoparticles, Sci. Total Environ., 2018, 631–632, 608–618 CrossRef CAS PubMed.
- X. Zhang, H. H. Deng, G. Q. Zhang, F. L. Yang and G. E. Yuan, Tetracycline degradation by natural bornite as an efficient and cost-effective persulfate activator: Performance and mechanism, Chem. Eng. J., 2020, 381, 122717 CrossRef CAS.
- Y. Wang, S. Y. Chen, X. Yang and J. Chen, Enhanced removal of Cr(VI) in the Fe(II)/peroxymonosulfate system with the addition of tea polyphenols: Performance and mechanism, J. Hazard. Mater., 2020, 398, 122–929 Search PubMed.
- Z. Shi, Z. Li, J. Gao, L. Tian and X. C. Ma, Enhanced oxidation of bisphenol A by permanganate in the presence of epigallo catechin gallate: Kinetics and mechanism, Sep. Purif. Technol., 2020, 247, 117125 CrossRef.
- Y. Li, L. R. Xiang, L. Y. Li, X. Y. Gu, W. B. Dong and Y. L. Wu, Chloramphenicol degradation via heterogeneous activation of peroxymonosulfate by Fe3O4 and gallic acid, Chemosphere, 2023, 344, 140376 CrossRef CAS PubMed.
- Z. Y. Fang, J. Zhao, Y. Li, Y. Wang, T. Qiu, Y. L. Wu, W. B. Dong and G. Mailhot, Catechin-improved Fenton-like system: Effects and mechanism of an environmental-friendly polyphenol, Chem. Eng. J., 2021, 426, 127946 CrossRef CAS.
- Y. L. Wu, O. Monfort, W. B. Dong, M. Brigante and G. Mailhot, Catechin-enhanced iron-mediated activation of persulfate: From generation of reactive species to atenolol degradation in water, Sci. Total Environ., 2019, 697, 134188 CrossRef CAS PubMed.
- A. Afkhami, M. Bahram and H. Madrakian, Spectrophotometric Determination of Acetylcysteine by Cu(I)-Neocuproine, Anal. Lett., 2005, 38(11), 1823–1834 Search PubMed.
- M. K. Kalinowska, M. Jezowska-Bojczuk, M. Wozniak and A. Kozlowski, Neocuproine Copper(II) Complexes with Bioactive Ligands in Aqueous Solution, J. Inorg. Biochem., 2004, 98(6), 1030–1038 Search PubMed.
- T. C. Hoang, L. J. Schuler, E. C. Rogevich, P. M. Bachman, G. M. Rand and R. A. Frakes, Copper Release, Speciation, and Toxicity Following Multiple Floodings of Copper Enriched Agriculture Soils: Implications in Everglades Restoration, Water Air Soil Poll., 2009, 199(1–4), 79–93 CrossRef CAS.
- G. V. Buxton, C. L. Greenstock, W. P. Helman and A. B. Ross, Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (˙OH/˙O-) in aqueous solution, J. Phys. Chem. Ref. Data, 1988, 17(2), 513–886 CrossRef CAS.
- L. Dogliotti and E. Hayon, Flash photolysis of persulfate ions in aqueous solutions. Study of the sulfate and ozonide radical anions, J. Phys. Chem., 1967, 71(8), 2511–2516 CrossRef CAS.
- C. Liu, K. Shih, C. Sun and F. Wang, Oxidative degradation of propachl or by ferrous and copper ion activated persulfate, Sci. Total Environ., 2012, 416, 507–512 CrossRef CAS PubMed.
- A. D. Luca, X. He, D. D. Dionysiou, R. F. Dantas and S. Esplugas, Effects of bromide on the degradation of organic contaminants with UV and Fe(II) activated persulfate, Chem. Eng. J., 2017, 318, 206–213 CrossRef.
- D. Meyerstein, Trivalent copper. I. Pulse radiolytic study of the chemical properties of the aquo complex, Inorg. Chem., 1971, 10, 638–641 CrossRef CAS.
- A. N. Pham, G. Xing, C. J. Miller and T. D. Waite, Fenton-like copper redox chemistry revisited: hydrogen peroxide and superoxide mediation of copper-catalyzed oxidant production, J. Catal., 2013, 301, 54–64 CrossRef CAS.
- S. Wang and J. Wang, Radiation-induced degradation of sulfamethoxazole in the presence of various inorganic anions, Chem. Eng. J., 2018, 351, 688–696 CrossRef CAS.
- Y. Wang, Q. Ning, L. Fu, S. Wu, Q. Chen, Z. Liu and Y. Wang, Influence and mechanism of water matrices on H2O2-based Fenton-like oxidation processes: A review, Chem. Eng. J., 2021, 423, 130157 CrossRef.
- X. R. Yang, X. Cao, L. Zhang, Y. L. Wu, L. Zhou, G. L. Xiu, C. Ferronato and J. M. Chovelon, Sulfate radical-based oxidation of the aminopyralid and picloram herbicides: The role of amino group on pyridine ring, J. Hazard. Mater., 2021, 405, 124181 CrossRef CAS PubMed.
- H. Liu, J. Zhao, Y. Wang, Y. Wu, W. Dong, M. Nie and X. Wang, Enhancement of peroxymonosulfate activation by sinapic acid accelerating Fe(III)/Fe(II) cycle, Chem. Eng. J., 2022, 446, 137177 CrossRef CAS.
- Y. Liang, J. Liu, Y. Zhang, H. Liang and J. Liu, Effects of 4-chlorophenol wastewater treatment on sludge acute toxicity, microbial diversity and functional genes expression in an activated sludge process, Sci. Total Environ., 2020, 743, 140595 Search PubMed.
- Y. Wang, Z. Wang, H. Liang and J. Liu, Toxicity of surface water from Huangpu River to luminous bacteria (Vibrio qinghaiensis SP Q67) and zebrafish (Danio rerio) embryos, Ecotoxicol. Environ. Saf., 2019, 169, 726–732 Search PubMed.
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2026 |