Synthesis of tris-indolylmethanealkaloids by harnessing the nucleophilic reactivity of Indole-BX and studying their interactions with hemoglobin
Sukanya
Das
 a,
Mangal Deep
Burman
a,
Mangal Deep
Burman
 b,
Sagar
Bag
b,
Sagar
Bag
 b,
Ranjit
Soren
a,
Brindaban
Roy
c,
Sudipta
Bhowmik
b,
Ranjit
Soren
a,
Brindaban
Roy
c,
Sudipta
Bhowmik
 *bd and
Raj K.
Nandi
*bd and
Raj K.
Nandi
 *a
*a
aDepartment of Chemistry, Jadavpur University, Kolkata 700032, India. E-mail: rkn.chemistry@jadavpuruniversity.in
bDepartment of Biophysics, Molecular Biology and Bioinformatics, University of Calcutta, 92, A. P. C. Road, Kolkata 700009, India. E-mail: su_sudipta@yahoo.co.in
cDepartment of Chemistry, Kalyani University, Nadia, West Bengal 741235, India
dMahatma Gandhi Medical Advanced Research Institute (MGMARI), Sri Balaji Vidyapeeth (Deemed to be University), Pondy-Cuddalore Main Road, Pillaiyarkuppam, Pondicherry 607402, India
First published on 18th November 2025
Abstract
In the current investigation, we have synthesized tris-indolylmethanealkaloid (TIM) derivatives by exploration of the nucleophilicity of cyclic hypervalent iodine reagent Indole-BX and also described the nature of the interaction between hemoglobin (Hb) with tris-indolylmethanealkaloids (TIM) 10 and 5 using different multi-spectroscopic studies. Fluorescence spectroscopy investigations have revealed a stronger interaction of TIM 10 with Hb than that of TIM 5, which is almost structurally similar. Circular dichroism (CD) analysis indicates that the native Hb conformation unfolds in the presence of both compounds. According to the thermal melting investigation, both TIM 10 and 5 have a small effect on Hb stability. This study demonstrates that subtle structural variations in TIM 10 and 5 can drastically alter interaction properties, potentially aiding drug discovery.
Introduction
Indole is an essential heterocyclic molecule used in many fields of science and industry.1–11 Its flexibility in chemical synthesis and its presence in natural products continue to motivate research and development across a variety of sectors, including materials science and medicine.12–15 Bis- and tris-indole derivatives are important compounds and have been found to have many potential bioactivities.16–20 Isolation as well as synthesis of bis-indolylmethanes (BIMs) (1, 2) (arundine, vibrindole),21,22 bis-indolylarsinolmethane BIAMs (3, 4) (arsinol line A),23–30 tris-indolylmethane (TIM) (5) have received considerable attention from the scientific community (Fig. 1).Hemoglobin (Hb) is one of the most important proteins in our body. It is a tetramer protein, consisting of a pair of α chains and a pair of β chains. Each α chain consists of 141 amino acids, and each β chain consists of 146 amino acids.31 Heme, an iron-containing porphyrin ring, is present in every chain.32 Hb is generally contained within erythrocytes, with only a small amount in plasma. Hb is mainly associated with the gaseous transport in our body. It generally collects oxygen from the lungs, releases it in tissues, collects carbon dioxide from tissues, and delivers it to the lungs.33 Additionally, it also interacts with different endogenous and exogenous compounds reversibly, such as 2,3-bisphosphoglycerate,34 alkaloids,35 flavonoids,36 and food colorants, etc.37 Among different compounds, alkaloids are an important group due to their various medicinal properties, such as, antioxidant,38 antitumor,39 neuroprotective,40 anti-inflammatory,41 and analgesic42 activities. Hence, the interaction between Hb and different alkaloids has been well studied.43 Among the various interactions of this class of molecules, those involving tris-indole derivatives with Hb remain unexplored, aside from a few reports on their antitumor44 and cytotoxic activities.45
As a continuation of our work46–48 in the field of hypervalent iodine chemistry, we wish to explore Waser's Indole-BX49 for the synthesis of different indole derivatives. In general so far this Indole-BX were used as an electrophilic indole source from hypervalent iodine surrogates by suppressing the innate nucleophilicity of Indole nucleus. However, recently, Yoshikai reported50 that many hypervalent iodine reagents also act as nucleophilic group-transfer agents. Motivated by this finding, we plan to investigate the possibility that Indole-BX may function as a nucleophile.
In this study, we first synthesized bis- and tris-indole derivatives from Indole-BX. Then, we examined how structural changes in the ligands affect their interactions with hemoglobin (Hb) using various analytical methodologies. This understanding will be especially important when dealing with novel agents, as it will help predict interactions and their consequences. Besides that, the study also provides useful information to understand how interactions between Hb and ligands affect the normal structure and function of Hb.
Experimental sections
Materials
Lyophilized human Hb and methanol were obtained from Sigma-Aldrich. The Hb concentration was calculated by spectrophotometric absorbance considering the molar extinction coefficient of Hb at 276 nm (120![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 808 M−1 cm−1).35 All experiments were executed using a buffer containing 50 mM KCl, 10 mM KH2PO4, and 1 mM K2EDTA (pH 7.4) at room temperature (25 °C). In each experiment, a minimal methanol concentration (less than 1% by volume) was used. TIMs were dissolved in methanol.
808 M−1 cm−1).35 All experiments were executed using a buffer containing 50 mM KCl, 10 mM KH2PO4, and 1 mM K2EDTA (pH 7.4) at room temperature (25 °C). In each experiment, a minimal methanol concentration (less than 1% by volume) was used. TIMs were dissolved in methanol.
Absorption spectroscopy
The UV-Vis absorption spectra of Hb alone and in the presence of TIMs were recorded on a Hitachi UH5300 spectrophotometer. In this experiment, the concentration of Hb (2.5 µM) was kept constant, and the concentration of TIMs was gradually increased from 0 to 9.5 µM. The experiment was executed using a cuvette with a 1 cm path length. The spectra were collected with a data interval of 1 nm and a scanning speed of 400 nm min−1.The binding constant (Kb) between Hb and TIMs was calculated considering the absorbance of Hb at 405 nm using the modified Benesi–Hildebrand equation.51
Fluorescence spectroscopy
The fluorescence properties of Hb were investigated using fluorescence spectroscopy. Fluorescence spectroscopy was performed on a Fluoromax-4C spectrofluorometer (Horiba Scientific) using a cuvette with a 1 cm path length at 298, 303, and 310 K. In this experiment, the concentration of Hb (2.5 µM) was kept constant, and the concentration of TIMs was gradually increased from 0 to 9.5 µM. In this study, the Hb was excited at 280 nm.Stoichiometry
The stoichiometry between Hb and TIMs was performed using a UV-Vis spectrophotometer. In this experiment, the overall concentration (CHb + CLigand) was held constant, while the molar fractions of Hb and TIMs were continuously changed. The difference in absorbance was calculated (ΔA = AHb − AHb+Ligand) and plotted against the molar fraction of the ligand. The Job plot was carried out, considering the variation in absorbance at the Soret band.Circular dichroism spectroscopy
Circular dichroism (CD) spectroscopy of Hb in the absence and presence of TIMs was taken using a Jasco J-1500 CD spectrometer. In this study, the concentration of Hb was kept at 3 µM, and the concentration of TIMs was increased to obtain Hb: TIMs ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4. The study was performed in a 1 mm path-length cuvette. The scanning speed of the CD spectrometer was 100 nm min−1. Each spectrum was the average of three individual scans. CD results were converted to mean residue ellipticity (MRE) in deg cm2 dmol−1 using the following formula:
4. The study was performed in a 1 mm path-length cuvette. The scanning speed of the CD spectrometer was 100 nm min−1. Each spectrum was the average of three individual scans. CD results were converted to mean residue ellipticity (MRE) in deg cm2 dmol−1 using the following formula:where C is the molar protein concentration, n is the number of amino acid residues, and l is the path length of the cuvette in centimeters.
The quantity of α-helix in different Hb samples can be estimated from the MRE value at 222 nm using the equation of Chen et al.52
UV-Melting study
Thermal denaturation studies of Hb in the absence and presence of TIMs were performed on a Hitachi UH5300 absorption spectrophotometer equipped with a Peltier temperature controller. In this study, the concentration of Hb was kept at 5 µM, and TIMs were used throughout the course at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. The experiment was performed in a cuvette with a 1 cm path length. The samples were heated at the rate of 1 °C min−1, and the change in absorbance was monitored at 280 nm.
1 ratio. The experiment was performed in a cuvette with a 1 cm path length. The samples were heated at the rate of 1 °C min−1, and the change in absorbance was monitored at 280 nm.
Results and discussion
Synthesis of TIMs from Indole-BX
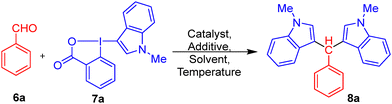
To establish the optimal reaction conditions for the desired transformation, a series of experiments was conducted using benzaldehyde (6a) and N-methylated Indole-BX (7a) as the model substrates (Table 1). Inspired by earlier reports on bis-indole formation from aldehydes and simple indoles,53–56 we initially screened those reported conditions using Indole-BX in place of a regular indole. However, attempts with various additives in the absence of a transition-metal catalyst were unsuccessful. When NBS (10 mol%) or NH4Cl (1 equiv.) was used either neat or in MeCN, no product formation was observed, even after prolonged heating at 120 °C (entries 1–3).| S. no. | Indole-BX (equiv.) | Catalyst (4 mol%) | Additive | Solvent | T (°C) | Time (h) | Yield (%) |
|---|---|---|---|---|---|---|---|
| Optimized reaction conditions: 6a (1 equiv.), 7a (2 equiv.), [RhCp*Cl2]2 (4 mol%), AgSbF6 (20 mol%), gl. AcOH (5 mol%), HFIP (1.5 mL), 110 °C for 6 h; ND = not detected. | |||||||
| 1. | 2 | — | NBS (10 mol%) | — | rt-120 | 24 | ND |
| 2. | 2 | — | NH4Cl (1 equiv.) | — | 120 | 24 | ND |
| 3. | 2 | — | NH4Cl (1 equiv.) | MeCN | 120 | 24 | ND |
| 4. | 2 | — | I2 (20 mol%) | MeCN | rt-120 | 24 | ND |
| 5. | 2 | — | I2 (20 mol%) | HFIP | rt-120 | 24 | ND |
| 6. | 2 | — | gl. AcOH (10 mol%) | — | rt-120 | 24 | ND |
| 7. | 2 | — | gl. AcOH (10 mol%) | HFIP | rt-120 | 24 | ND |
| 8. | 2 | — | gl. AcOH (10 mol%) | DCE | rt-120 | 24 | ND |
| 9. | 2 | [RhCp*Cl2]2 | gl. AcOH (5 mol%) | DCE | rt-120 | 8 | 46 |
| AgSbF6 (20 mol%) | |||||||
| 10. | 2 | [RhCp*Cl2]2 | gl. AcOH (5 mol%) | HFIP | 110 | 6 | 62 |
| AgSbF 6 (20 mol%) | |||||||
| 11. | 2 | — | gl. AcOH (5 mol%) | HFIP | 110 | 6 | ND |
| AgSbF6 (20 mol%) | |||||||
| 12. | 2 | [RhCp*Cl2]2 | AgSbF6 (20 mol%) | HFIP | 110 | 8 | 7 |
| 13. | 2 | [RhCp*Cl2]2 | gl. AcOH (5 mol%) | HFIP | 110 | 12 | 11 |
| 14. | 2 | FeCl3 | gl. AcOH (5 mol%) | HFIP | rt-120 | 24 | ND |
| AgSbF6 (20 mol%) | |||||||
| 15. | 2 | Ru(PPh3)2Cl2 | gl. AcOH (5 mol%) | HFIP | rt-120 | 24 | ND |
| AgSbF6 (20 mol%) | |||||||
Similarly, the use of I2 (20 mol%) in MeCN or HFIP, as well as glacial AcOH (10 mol%) in different solvents, such as HFIP or DCE, failed to yield the desired product 8a (entries 4–8). Encouraged by previous reports on C–H activation using Rh(III) complexes, we turned our attention to [RhCp*Cl2]2 in combination with AgSbF6 as a halide abstractor. Gratifyingly, when [RhCp*Cl2]2 (4 mol%) was used along with gl. AcOH (5 mol%) and AgSbF6 (20 mol%) in DCE, the desired product 8a was obtained in 46% yield after 8 h (entry 9). A further enhancement in yield was observed when HFIP was employed as the solvent at a slightly elevated temperature (110 °C), affording the product in 62% yield within 6 h (entry 10). Control experiments confirmed the essential roles of both the Rh(III) catalyst and additives. In the absence of the rhodium catalyst (entry 11), or when either gl. AcOH (entry 12) or AgSbF6 (entry 13) was omitted; a significant drop in yield was noted, underscoring the synergistic effect of all three components. Attempts to replace Rh(III) with other metal catalysts such as FeCl3 and Ru-based complexes proved ineffective under similar conditions (entries 14 and 15), with no detectable product formation. These results clearly indicate that the combination of [RhCp*Cl2]2 (4 mol%), glacial AcOH (5 mol%), and AgSbF6 (20 mol%) in HFIP at 110 °C provides the most efficient and reproducible conditions for the desired transformation.
With this result, we can say that our hypothesis regarding the innate nucleophilicity of indole also holds for Indole-BX reagents. Encouraged by the optimized conditions, we next investigated the substrate scope to evaluate the versatility of our methodology (Scheme 1). Although numerous protocols for synthesizing bis- and tris-indolyl frameworks were reported in the literature, both metal-free and metal-catalyzed,53–78 often providing high yields under simple and economic conditions, our focus here is on showcasing the unique reactivity of Indole-BX reagents. While our isolated yields are modest in comparison, the method's adaptability and potential for biological application, particularly in haemoglobin interaction studies, prompted us to pursue both the developed protocol and literature-reported approaches for scale-up. Upon introducing electron-donating substituents such as methyl groups at the ortho- and para-positions of the aromatic aldehyde (6) (Scheme 1(A)), under the optimized conditions with N-methylated Indole-BX (7a), we observed a slight increase in yields for the corresponding bis-indole adducts (8b and 8c: 65% and 70%, respectively). Halogen-substituted substrates (para-F) also performed well, affording product 8d in shorter reaction times (2 h) with a moderate 60% yield, underscoring the robustness of the reaction conditions. The electron-donating group (para-NO2) also gives comparable yields (8e: 58%). For the tris-indole series (Scheme 1(B)), employing N-methylated derivatives of both indole-3-aldehyde and Indole-BX reagent, afforded the desired product (10) in 56% yield. In contrast, use of NH-indole-3-aldehyde led to a reduced yield (5, 44%), possibly due to subtle electronic differences or variations in solubility and hydrogen-bonding behaviour under the reaction conditions. Moreover, the reaction with other heteroaromatic aldehydes, such as pyrrole-2-carboxaldehyde, resulted in the formation of the expected product in very low yield (11:18%), whereas thiophene-2-carboxaldehyde failed to yield the corresponding product (12); instead, a complex reaction mixture was observed. We tested aromatic and heteroaromatic aldehydes as well as an aliphatic aldehyde (such as pentanal) under the optimized conditions, but no product formation was observed. Inspired by the well-documented biological relevance of TIMs in medicinal chemistry,16–20 we extended our methodology toward their synthesis with an eye on probing their interactions with haemoglobin in future studies.
A plausible mechanism for this transformation is illustrated in Scheme 2. In the presence of AgSbF6, a partially naked cationic Rh(III) species (A) is generated in situ, which undergoes oxidative addition with Indole-BX (7) to form intermediate B. A coordination followed by nucleophilic addition of the aldehyde (6/9) to B affords intermediate C through attack of the indole moiety at the formyl carbon, followed by coordination of the resulting alkoxide oxygen to the rhodium centre.79,80 A subsequent hydride migration to rhodium furnishes intermediate D along with the formation of ketone 13. The rhodium catalyst is then regenerated through the elimination of 2-iodobenzoic acid (14), which can re-enter the catalytic cycle via a new oxidative addition with another molecule of Indole-BX (7) to regenerate B. Resembles to the earlier step [B-C], the newly formed ketone 13 undergoes nucleophilic addition by indole and coordinate to the Rh(III) centre, generating intermediate E. Reductive elimination from E regenerates the active catalyst. It may produce a peroxy-type intermediate F. Subsequent nucleophilic attack of water at the carbonyl carbon, followed by cleavage through intermediate G, delivers the desired products (8/5/10/11) along with molecular oxygen and 2-iodobenzoic acid (14).
UV-Vis absorption spectroscopy
UV-Vis absorption spectra of Hb possess two major absorption peaks: one at 269 nm corresponding to the phenyl groups of tryptophan and tyrosine residues in the globin portion, and another at 405 nm, called the Soret band, which arises from the heme group. The first peak arises from the π → π* transition of the phenyl amino acids, and the second peak appears due to the π → π* transition in the porphyrin ring of the heme group. Gradual addition of these two TIMs induces Hb spectral changes. These two compounds have no absorption, so the spectral change in Hb is solely due to their interaction with Hb. Following these two TIMs, the intensity of the Soret band decreased, while the maximum absorption wavelengths remained constant. These results indicate that the heme remains unexposed from the crevices at the exterior of the subunit, and these compounds are readily bound to the hydrophobic pocket of Hb.35 The percentage of hypo-chromicity is high in the case of TIM 10 (26.47%) compared to TIM 5 (22.87%) (Fig. 2). Besides that, the binding constant between Hb and TIM 10 was 1.51 × 105 M−1, and Hb and TIM 5 was 5.67 × 104 M−1 (Fig. S1). This result indicates that the interaction between Hb and TIM 10 is stronger than that between Hb and TIM 5. | ||
| Fig. 2 UV-Vis spectroscopic study of the interaction between (A) Hb and TIM 10, and (B) Hb and TIM 5. | ||
Fluorescence spectroscopy
The tryptophan residues of Hb are mainly responsible for its fluorescence. In fluorescence spectrophotometry, efficient energy transfer from tryptophan residues to heme significantly quenches the protein's inherent fluorescence. Hence, in buffer, Hb exhibits very low fluorescence. In this study, TIM 10 induced quenching of the fluorescence property of Hb, indicating interaction between Hb and TIM10. However, TIM 5 entered the hydrophobic cavity of Hb and induced the exposure of heme. As a result, the TIM 5 induced a small increase in the fluorescence intensity of Hb, and the fluorescence quenching of tryptophan by heme was suppressed at different temperatures (Fig. 3 and Fig. S5).81 To gain a comprehensive understanding of the quenching mechanism of the interaction between Hb and TIM 10, the fluorescence spectroscopy results were evaluated using the Stern–Volmer equation.82| F0/F = 1 + Kq × τ0[L] = 1 + Ksv |
| F0/(F0 − F) = 1/fa + 1/Kafa[L] |
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ka = −ΔH°/RT + ΔS°/R Ka = −ΔH°/RT + ΔS°/R |
ΔG° = ΔH° − TΔS° = −RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ka. Ka. |
It was found that both calculated ΔH° and ΔG° of the interaction between Hb and TIM 10 were negative in nature, which implied that the acting bonds were mainly hydrogen bonds and van der Waals forces. The negative value of ΔG° indicated that the interaction was spontaneous (Table S1).86,87
Stoichiometry
The stoichiometry of Hb and TIMs was determined by the continuous variation method. This method allows the protein–ligand complex's characteristics to be recognized. In this experiment, the total concentration of (CHb + CLigand) was kept constant, while the molar fraction of Hb and TIMs was varied. Then, changes in the absorbance of the Soret band were recorded and plotted against the molar fraction of the ligand. It has been found that Hb interacts with TIMs in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio (Fig. 4).
1 ratio (Fig. 4).
Circular dichroism (CD) spectroscopy
To look into the potential impact of these two TIMs binding on the secondary structure of Hb, CD spectroscopy was performed. In buffer media, two negative ellipticity peaks from Hb generally appear, one at 208 nm and another at 222 nm. The peak at 208 appears due to the π → π* transition of the α-helix, while the peak at 222 nm corresponds to π → π* transition of both the α-helix and β-sheets. The percentage of α-helix in native Hb was 49.72%, whereas upon interaction with TIM 10 and TIM 5, the percentage of α-helix decreased to 39.22% and 39.53%, respectively (Fig. 5). Besides that, no alterations in the peaks of maxima were found. Therefore, it can be concluded that the interaction of these two compounds with Hb induced the unfolding of the native conformation of Hb and the π–π stacking interaction force between Hb and TIMs.UV melting study
The effects of these two TIMs on Hb stability were examined using UV melting. The melting temperature of control Hb was 62.5 °C. In the presence of these two compounds, the melting temperature of Hb was altered to 62.39 °C by TIM 10 and 62.66 °C by TIM 5 (Fig. 6). Hence, this study indicates that these two compounds have the least effect on Hb stability. | ||
Fig. 6 Melting study of Hb in the absence and presence of different TIMs in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio; black line = Hb, red line = Hb + TIM 10, and blue line = Hb + TIM 5. 1 ratio; black line = Hb, red line = Hb + TIM 10, and blue line = Hb + TIM 5. | ||
Conclusion
In conclusion, we synthesised a series of bis-indole derivatives using cyclic Indole-BX, in which indole is transferred to the carbonyl carbon as an unusual nucleophilic source in hypervalent iodine chemistry. We also synthesised tris-indole derivatives, TIM5 and TIM10, using our protocol. Several recent studies have shown molecular interactions between proteins and ligands using multi-spectroscopic techniques. The interaction between these two almost similar structures with Hb will depict a picture for drug designing. The spectrophotometric titration study of the interaction between these two ligands and Hb exhibited hypochromic changes in the UV-Vis spectra of Hb, denoting the formation of complexes. TIM 10 induced much greater hypochromicity in Hb compared to TIM 5, indicating a stronger interaction between Hb and TIM 10 than between Hb and TIM 5. The strong binding affinity between TIM 10 and Hb is also supported by the calculated binding constants. TIM 10 induced quenching of the fluorescence property of Hb and exhibited a large binding constant between Hb and TIM 10. These results indicate stronger complex formation between Hb and TIM 10 than between Hb and TIM 5.88 Stoichiometry studies revealed that TIM 10 and 5 interact with Hb in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio.
1 ratio.
Furthermore, CD findings suggested that the presence of TIM 10 and TIM 5 caused unfolding of the native Hb configuration. Melting experiments revealed that both compounds slightly affect Hb stability. This study helps understand how a small structural alteration (the presence of a Met group) can drastically alter the interaction process, which may be important for pharmaceutical manufacturing. This research may be crucial for pharmaceutical and medicinal chemists to establish and comprehend how these alkaloids interact with Hb in blood plasma. The finding may shed light on the varied behaviors of these alkaloids in biological systems and aid understanding of drug transport across membranes. This approach can also be readily applied to drugs of a similar nature and helps in understanding the pharmacokinetics and biological activities of these alkaloids. As a result, this work will aid in understanding the structural basis for screening and the design of appropriate synthetic compounds, which will be critical to advancing clinical and pharmaceutical research.
Author contributions
S. D.: writing – original draft, visualization, methodology, investigation, formal analysis, data curation. M. D. B.: writing – original draft, visualization, methodology, investigation, formal analysis, data curation. S. B.: writing – review & editing, investigation, visualization, formal analysis. R. S.: investigation, formal analysis, data curation. B. R.: investigation, formal analysis, data curation. S. B.: writing – review & editing, supervision, project administration, formal analysis, funding acquisition, conceptualization. R. K. N.: writing – review & editing, supervision, project administration, formal analysis, funding acquisition, conceptualization.Conflicts of interest
There are no conflicts to declareData availability
All data generated or analyzed during this study are included in this published article and its supplementary information (SI). Supplementary information is available with experimental procedure, charecterization data and biological studies results. See DOI: https://doi.org/10.1039/d5nj03073k.Acknowledgements
SD is thankful to DST-INSPIRE, Govt. of India, for her PhD fellowship (SRF) (IF 190973). MDB thanks the University of Calcutta for providing a fellowship (URF, Fellow ID F-2495). Sagar Bag thanks UGC, Government of India, for providing a fellowship and research grant (UGC-Senior Research Fellowship, NTA reference number: 201610001623). SB thanks “Intramural Seed Money Research Committee, SBV” for “SBV-Seed money” (SBV/IRC/SEED MONEY/167/2023). RKN & BR are thankful to SERB (ANRF)-Govt. of India for financial assistance (project No. EEQ/2022/000930 and EEQ/2021/000635, respectively). RKN is also thankful to JU for financial assistance with seed money (Ref. No. S-3/178/23). We also thank DST for the instrumental facility at Jadavpur University under the FIST scheme SR/FST/CS-II-032/2014(G).References
- W. J. Houlihan, Chemistry of Heterocyclic Compounds: Indoles, Part Two, Wiley Interscience, New York, 1972 Search PubMed.
- R. J. Sundberg, Indoles, Elsevier, 1996 Search PubMed.
- J. A. Joule and K. Mills, Heterocyclic Chemistry, Blackwell Science, Oxford, UK, 4th edn, 2000 Search PubMed.
- A. Takeda, S. Kamijo and Y. Yamamoto, Indole synthesis via palladium-catalyzed intramolecular cyclization of alkynes and imines, J. Am. Chem. Soc., 2000, 122, 5662–5663 CrossRef CAS.
- K. R. Roesch and R. C. Larock, Synthesis of isoindolo [2,1-a] indoles by the palladium-catalyzed annulation of internal acetylenes, J. Org. Chem., 2001, 66, 412–420 CrossRef CAS PubMed.
- A. Lerchner and E. M. Carreira, First total synthesis of (±)-strychnofoline via a highly selective ring-expansion reaction, J. Am. Chem. Soc., 2002, 124, 14826–14827 CrossRef CAS PubMed.
- H. Kusama, J. Takaya and N. Iwasawa, A facile method for the synthesis of polycyclic indole derivatives: The generation and reaction of tungsten-containing azomethine ylides, J. Am. Chem. Soc., 2002, 124, 11592–11593 CrossRef CAS PubMed.
- L. Ackermann, General and efficient indole syntheses based on catalytic amination reactions, Org. Lett., 2005, 7, 439–442 CrossRef CAS PubMed.
- N. L. Segraves and P. Crews, Investigation of brominated tryptophan alkaloids from two thorectidae sponges: Thorectandra and Smenospongia, J. Nat. Prod., 2005, 68, 1484–1488 CrossRef CAS PubMed.
- S. A. Patil, R. Patil and D. D. Miller, Indole molecules as inhibitors of tubulin polymerization: potential new anticancer agents, Future Med. Chem., 2012, 4, 2085–2115 CrossRef CAS PubMed.
- T. V. Sravanthi and S. L. Manju, Indoles—A promising scaffold for drug development, Eur. J. Pharm. Sci., 2016, 91, 1–10 CrossRef CAS PubMed.
- B. Biersack and R. Schobert, Indole compounds against breast cancer: recent developments, Curr. Drug Targets, 2012, 13, 1705–1719 CrossRef CAS PubMed.
- A. Ahmad, B. Biersack, Y. Li, D. Kong, B. Bao, R. Schobert, S. B. Padhye and F. H. Sarkar, Targeted regulation of PI3K/Akt/mTOR/NF-κB signaling by indole compounds and their derivatives: mechanistic details and biological implications for cancer therapy, Anti-Cancer Agents Med. Chem., 2013, 13, 1002–1013 CrossRef CAS PubMed.
- S. Olgen, Recent development of new substituted indole and azaindole derivatives as anti-HIV agents, Mini-Rev. Med. Chem., 2013, 13, 1700–1708 CrossRef CAS PubMed.
- N. K. Kaushik, N. Kaushik, P. Attari, N. Kumar, C. H. Kim, A. K. Verma and E. H. Choi, Biomedical importance of indoles, Molecules, 2013, 18, 6620–6662 CrossRef CAS PubMed.
- F. A. Wati, M. Santoso, Z. Moussa, S. Fatmawati, A. Fadlan and Z. M. A. Judeh, Chemistry of trisindolines: natural occurrence, synthesis and bioactivity, RSC Adv., 2021, 11, 25381–25421 RSC.
- C. Bonnesen, I. M. Eggleston and J. D. Hayes, Dietary indoles and isothiocyanates that are generated from cruciferous vegetables can both stimulate apoptosis and confer protection against DNA damage in human colon cell lines, Cancer Res., 2001, 61, 6120–6130 CAS.
- C. Hong, G. L. Firestone and L. F. Bjeldanes, Bcl-2 family-mediated apoptotic effects of 3,3′-diindolylmethane (DIM) in human breast cancer cells, Biochem. Pharmacol., 2002, 63, 1085–1097 CrossRef CAS PubMed.
- S. Safe, S. Papineni and S. Chintharlapalli, Cancer chemotherapy with indole-3-carbinol, bis (3′-indolyl) methane and synthetic analogs, Cancer Lett., 2008, 269, 326–338 CrossRef CAS PubMed.
- M. Rahimi, K.-L. Huang and C. K. Tang, 3,3′-Diindolylmethane (DIM) inhibits the growth and invasion of drug-resistant human cancer cells expressing EGFR mutants, Cancer Lett., 2010, 295, 59–68 CrossRef CAS PubMed.
- R. Bell, S. Carmeli and N. Sar, Vibrindole A, a metabolite of the marine bacterium, Vibrio parahaemolyticus, isolated from the toxic mucus of the boxfish Ostracion cubicus, J. Nat. Prod., 1994, 57, 1587–1590 CrossRef CAS PubMed.
- M. Shiri, M. A. Zolfigol, H. G. Kruger and Z. Tanbakouchian, Bis-and trisindolylmethanes (BIMs and TIMs), Chem. Rev., 2010, 110, 2250–2293 CrossRef CAS PubMed.
- G. de la Herran, A. Segura and A. G. Csaky, Benzylic substitution of gramines with boronic acids and rhodium or iridium catalysts, Org. Lett., 2007, 9, 961–964 CrossRef CAS PubMed.
- X. Guo, S. Pan, J. Liu and Z. Li, One-pot synthesis of symmetric and unsymmetric 1,1-Bis-indolylmethanes via tandem iron-catalyzed C–H bond oxidation and C–O bond cleavage, J. Org. Chem., 2009, 74, 8848–8851 CrossRef CAS PubMed.
- C. Praveen, Y. W. Sagayaraj and P. T. Perumal, Gold(I)-catalyzed sequential cycloisomerization/bis-addition of o-ethynylanilines: An efficient access to bis (indolyl) methanes and di (indolyl) indolin-2-ones, Tetrahedron Lett., 2009, 50, 644–647 CrossRef CAS.
- D. Xia, Y. Wang, Z. Du, Q.-Y. Zheng and C. Wang, Rhenium-catalyzed regiodivergent addition of indoles to terminal alkynes, Org. Lett., 2012, 14, 588–591 CrossRef CAS PubMed.
- L. Zhang, C. Peng, D. Zhao, Y. Wang, H.-J. Fu, Q. Shen and J.-X. Li, Cu(II)-catalyzed C–H (SP3) oxidation and C–N cleavage: base-switched methylenation and formylation using tetramethylethylenediamine as a carbon source, Chem. Commun., 2012, 48, 5928–5930 RSC.
- M. P. Muñoz, M. C. de la Torre and M. A. Sierra, Platinum-catalysed bisindolylation of allenes: a complementary alternative to gold catalysis, Chem. – Eur. J., 2012, 18, 4499–4504 CrossRef.
- J. Beltrá, M. C. Gimeno and R. P. Herrera, A new approach for the synthesis of bisindoles through AgOTf as catalyst, Beilstein J. Org. Chem., 2014, 10, 2206–2214 CrossRef PubMed.
- S. Karmakar, P. Das, D. Ray, S. Ghosh and S. K. Chattopadhyay, Ag(I)-Catalyzed domino cyclization–addition sequence with simultaneous carbonyl and alkyne activation as a route to 2,2′-disubstituted bisindolylarylmethanes, Org. Lett., 2016, 18, 5200–5203 CrossRef CAS PubMed.
- M. Yuan, Q. Nong, H. Guo, Y. Li, H. Tian, J. Zhang, L. Liu, B. He, L. Hu and G. Jiang, pH-dependent cadmium binding to hemoglobin: Implications for human excretion, Sci. Total Environ., 2025, 958, 177700 CrossRef CAS PubMed.
- Y. Yuan, V. Simplaceanu, J. A. Lukin and C. Ho, NMR investigation of the dynamics of tryptophan side-chains in hemoglobins, J. Mol. Biol., 2002, 321, 863–878 CrossRef CAS PubMed.
- S. Singh, P. Gopi, P. Sharma, M. S. S. Rani, P. Pandya and M. S. Ali, Hemoglobin targeting potential of aminocarb pesticide: Investigation into dynamics, conformational stability, and energetics in solvent environment, Biochem. Biophys. Res. Commun., 2024, 736, 150896 CrossRef CAS PubMed.
- G. M. Levitzky, Pulmonary Physiology, McGraw Hill, 2007 Search PubMed.
- M. D. Burman, S. Bag, S. Ghosal, S. Karmakar, G. Pramanik, R. K. Chinnadurai and S. Bhowmik, Exploring the structural importance of the C3
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C4 double bond in plant alkaloids harmine and harmaline on their binding interactions with hemoglobin, ACS Omega, 2023, 8, 37054 CrossRef CAS PubMed.
C4 double bond in plant alkaloids harmine and harmaline on their binding interactions with hemoglobin, ACS Omega, 2023, 8, 37054 CrossRef CAS PubMed. - J.-L. Yuan, H. Liu, X. Kang, Z. Lv and G.-L. Zou, Characteristics of the isomeric flavonoids apigenin and genistein binding to hemoglobin by spectroscopic methods, J. Mol. Struct., 2008, 891, 333 CrossRef CAS.
- A. Basu and G. S. Kumar, Interaction of the dietary pigment curcumin with hemoglobin: energetics of the complexation, Food Funct., 2014, 5, 1949 RSC.
- C.-Y. Chen and Y. Zhang, Berberine: An isoquinoline alkaloid targeting the oxidative stress and gut-brain axis in the models of depression, Eur. J. Med. Chem., 2025, 290, 117475 CrossRef CAS PubMed.
- Q. Cao, Q. Wang, X. Wu, Q. Zhang, J. Huang, Y. Chen, Y. You, Y. Qiang, X. Huang, R. Qin and G. Cao, A literature review: mechanisms of antitumor pharmacological action of leonurine alkaloid, Front. Pharmacol., 2023, 14, 1272546 CrossRef CAS PubMed.
- K. Bhardwaj, R. Bhargav, J. Patocka, R. Sharma, Z. Navratilova, P. Oleksak and K. Kuca, Dendrobine: a neuroprotective sesquiterpenic alkaloid for the prevention and treatment of diseases: a review, Mini Rev. Med. Chem., 2024, 24, 1395–1408 CrossRef CAS.
- R. G. Geetha and S. Ramachandran, Recent advances in the anti-inflammatory activity of plant-derived alkaloid rhynchophylline in neurological and cardiovascular diseases, Pharmaceutics, 2021, 13, 1170 CrossRef CAS PubMed.
- K. Ya, W. Tangamornsuksan, C. N. Scholfield, J. Methaneethorn and M. Lohitnavy, Pharmacokinetics of mitragynine, a major analgesic alkaloid in kratom (Mitragyna speciosa): A systematic review, Asian J. Psychiatr., 2019, 43, 73–82 CrossRef PubMed.
- M. Gaurav, A. Natesh, A. Arundhati and D. Mariam, Biochemical aspects of hemoglobin-xenobiotic interactions and their implications in drug discovery, Biochimie, 2021, 191, 154–163 CrossRef CAS PubMed.
- E. V. Stepanova, A. A. Shtil’, S. N. Lavrenov, V. M. Bukhman, A. N. Inshakov, E. P. Mirchink, A. S. Trenin, O. A. Galatenko, E. B. Isakova, V. A. Glazunova, L. G. Dezhenkova, E. S. Solomko, E. E. Bykov and M. N. Preobrazhenskaya, Tris (1-alkylindol-3-yl) methylium salts as a novel class of antitumor agents, Russ. Chem. Bull., 2010, 59, 2259–2267 CrossRef CAS.
- S. N. Lavrenov, Y. N. Luzikov, E. E. Bykov, M. I. Reznikova, E. V. Stepanova, V. A. Glazunova, Y. L. Volodina, V. V. Tatarsky Jr, A. A. Shtil and M. N. Preobrazhenskaya, Synthesis and cytotoxic potency of novel tris (1-alkylindol-3-yl) methylium salts: Role of N-alkyl substituents, Bioorg. Med. Chem., 2010, 18, 6905–6913 CrossRef CAS PubMed.
- S. Das, T. Datta, M. A. Sk, B. Roy and R. K. Nandi, Isoxazole group directed Rh(III)-catalyzed alkynylation using TIPS-EBX, Org. Biomol. Chem., 2024, 22, 6922 RSC.
- P. Pal, J. Waser and R. K. Nandi, Umpolung of Electron-Rich Heteroarenes with Hypervalent Iodine Reagents, Heterocycles, 2021, 103, 555 CrossRef CAS PubMed.
- (a) S. Das, S. Bag, M. A. Sk, P. Majumdar, U. K. Das, S. Bhowmik and R. K. Nandi, Uracil-BX: A New Class of Cyclic Hypervalent Iodine Reagents for Unraveling Approach Towards Umpolung Functionalization and VEGF G-Quadruplex DNA Identification. ChemRxiv, 2025, preprint DOI:10.26434/chemrxiv-2025-tn425; (b) S. Das, M. A. Sk, P. Majumdar, U. K. Das and R. K. Nandi, Uracil-BX: Bench-Stable Cyclic Hypervalent Iodine Reagents for Umpolung Functionalization, Org. Lett., 2025, 27, 8158–8163 CrossRef CAS PubMed.
- P. Caramenti, S. Nicolai and J. Waser, Indole-and Pyrrole-BX: Bench-Stable Hypervalent Iodine Reagents for Heterocycle Umpolung, Chem. – Eur. J., 2017, 23, 14702 CrossRef CAS PubMed.
- C. Arakawa, K. Kanemoto, K. Nakai, C. Wang, S. Morohashi, E. Kwon, S. Ito and N. Yoshikai, Carboiodanation of arynes: Organoiodine(III) compounds as nucleophilic organometalloids, J. Am. Chem. Soc., 2024, 146, 3910 CrossRef CAS PubMed.
- S.-Q. Liu, J.-J. Xu and H.-Y. Chen, A reversible adsorption–desorption interface of DNA based on nano-sized zirconia and its application, Colloids Surf., B, 2004, 36, 155 CrossRef CAS PubMed.
- Y.-H. Chen and J. T. Yang, A new approach to the calculation of secondary structures of globular proteins by optical rotatory dispersion and circular dichroism, Biochem. Biophys. Res. Commun., 1971, 44, 1285 CrossRef CAS PubMed.
- H. Koshima and W. Matsusaka, N-Bromosuccinimide catalyzed condensations of indoles with carbonyl compounds under solvent-free conditions, J. Heterocycl. Chem., 2002, 39, 1089 CrossRef CAS.
- S. Naskar, A. Hazra, P. Paira, K. B. Sahu, S. Banerjee and N. B. Mondal, NH4Cl-promoted synthesis of symmetrical and unsymmetrical triindolylmethanes under solvent-free conditions, J. Chem. Res., 2008, 2008, 568 CrossRef.
- A. Hazra, P. Paira, K. B. Sahu, S. Banerjee and N. B. Mondal, Molecular iodine: an efficient catalyst for the synthesis of both symmetrical and unsymmetrical triindolylmethanes (TRIM), Catal. Commun., 2008, 9, 1681 CrossRef CAS.
- M. T. El Sayed, K. M. Ahmed, K. Mahmoud and A. Hilgeroth, Synthesis, cytostatic evaluation and structure activity relationships of novel bis-indolylmethanes and their corresponding tetrahydroindolocarbazoles, Eur. J. Med. Chem., 2015, 90, 845 CrossRef CAS PubMed.
- X. Qi, H.-J. Ai, N. Zhang, J.-B. Peng, J. Ying and X.-F. Wu, Palladium-catalyzed carbonylative bis (indolyl) methanes synthesis with TFBen as the CO source, J. Catal., 2018, 362, 74 CrossRef CAS.
- W. Xiu, W. Zhenhua, G. Zhang, W. Zhang, Y. Wu and Z. Gao, Tunable Titanocene Lewis Acid Catalysts for Selective Friedel–Crafts Reaction of Indoles and N-Sulfonylaldimines, Eur. J. Org. Chem., 2016, 502 CrossRef CAS.
- J. Yang, Y. Zhang, H.-C. F. Wong, J. Huang, Y.-L. S. Tse and Y.-Y. Yeung, Design and Applications of Cyclopropenium Chalcogen Dihalides in Catalysis via C (sp3)–H··· X Interactions, ACS Catal., 2024, 14, 3018 CrossRef CAS.
- S. Khaksar, S. M. Vahdat, M. Gholizadeh and S. M. Talesh, An expeditious and efficient synthesis of symmetrical tris (indolyl) methanes under catalyst-free conditions in fluorinated alcohols, J. Fluorine Chem., 2012, 136, 8 CrossRef CAS.
- K. Singh, S. Sharma and A. Sharma, Unique versatility of Amberlyst 15. An acid and solvent-free paradigm towards synthesis of bis (heterocyclyl) methane derivatives, J. Mol. Catal. A: Chem., 2011, 347, 34 CrossRef CAS.
- S. N. Lavrenov, Y. N. Luzikov, E. E. Bykov, M. I. Reznikova, E. V. Stepanova, V. A. Glazunova, Y. L. Volodina, V. V. Tatarsky Jr, A. A. Shtil and M. N. Preobrazhenskaya, Synthesis and cytotoxic potency of novel tris (1-alkylindol-3-yl) methylium salts: Role of N-alkyl substituents, Bioorg. Med. Chem., 2010, 18, 6905 CrossRef CAS PubMed.
- Z. H. Zhang and J. Lin, Efficient and convenient method for the synthesis of symmetrical triindolylmethanes catalyzed by iodine, Synth. Commun., 2007, 37, 209 CrossRef CAS.
- M. Chakrabarty, S. Sarkar, A. Linden and B. K. Stein, First general solvent-free synthesis of symmetrical triindolylmethanes using acid-washed montmorillonite clay, Synth. Commun., 2004, 34, 1801 CrossRef CAS.
- X. L. Luo, W. J. Ji, Z. L. Yu, M. M. Wang, C. B. Pang, X. Q. Chu and D. Ge, Heteroarylation of Organophosphonium Salts with Indoles in Water: Synthesis of Symmetrical and Unsymmetrical 3,3′-Bisindolylmethane Derivatives, Eur. J. Org. Chem., 2024, e202400964 CrossRef CAS.
- P. Nath, M. L. Deb and P. K. Baruah, Synthesis of tris (indolyl) methanes by using dimethyl carbonate as a single carbon source via CH functionalization approach, Chem. Pap., 2024, 78, 1117 CrossRef CAS.
- M. L. Deb, P. J. Borpatra, C. D. Pegu, R. Thakuria, P. J. Saikia and P. K. Baruah, Iodine/tert-Butyl Hydroperoxide-Mediated Reaction of Indoles with Dimethylformamide/Dimethylacetamide to Synthesize Bis-and Tris (indolyl) methanes, ChemistrySelect, 2017, 2, 140 CrossRef CAS.
- M. Barbero, S. Cadamuro, S. Dughera, C. Magistris and P. Venturello, A new practical synthesis of triaryl and trisindolylmethanes under solvent-free reaction conditions, Org. Biomol. Chem., 2011, 9, 8393 RSC.
- M. Kargar, R. Hekmatshoar and A. Mostashari, Synthesis of novel methylene bridge functionalized bis (indolyl) methanes thorough a double Michael addition, Heterocycles, 2011, 83, 2535 CrossRef CAS.
- D. G. Gu and S. J. Ji, Various tri (indolyl) methanes were synthesized by condensation of indole and its derivatives with indole-3-carboxaldehyde using acidic ionic liquids [hmim] HSO4/EtOH as an efficient and green catalyst system, Chin. J. Chem., 2008, 26, 578 CrossRef CAS.
- X. F. Zeng, S. J. Ji and X. M. Su, Facile Synthesis of Symmetrical Triindolylmethanes Catalyzed by Iodine under Solvent-free Condition, Chin. J. Chem., 2008, 26, 413 CrossRef CAS.
- N. G. Singh, C. Kathing, J. W. S. Rani and R. L. Nongkhlaw, Synthesis of pharmacologically active bis (indolyl) and tris (indolyl) derivatives using chlorotrimethylsilane, J. Chin. Chem. Soc., 2014, 61, 442 CrossRef CAS.
- E. Akgün, M. Tunali and U. Pindur, Proton acid-catalysed acylation of indoles by 2-alkoxy-1, 3-dioxolanes, Arch. Pharm., 1987, 320, 397 CrossRef.
- S. Tuengpanya, C. Chantana, U. Sirion, W. Siritanyong, K. Srisook and J. Jaratjaroonphong, One-pot solvent-free synthesis of triaryl-and triheteroarylmethanes by Bi (OTf) 3-catalyzed Friedel-Crafts reaction of arenes/heteroarenes with trialkyl orthoformates, Tetrahedron, 2018, 74, 4373 CrossRef CAS.
- M. Chakrabarty, N. Ghosh, R. Basak and Y. Harigaya, Dry reaction of indoles with carbonyl compounds on montmorillonite K10 clay: a mild, expedient synthesis of diindolylalkanes and vibrindole A, Tetrahedron Lett., 2002, 43, 4075 CrossRef CAS.
- H. Veisi, M. Ataee, P. Darabi-Tabar, E. Amiri and A. R. Faraji, One-pot conversion of aromatic compounds to the corresponding bis (indolyl) methanes by the Vilsmeier–Haack reaction, C. R. Chim, 2014, 17, 305 CrossRef CAS.
- V. Nair, K. G. Abhilash and N. Vidya, Practical synthesis of triaryl-and triheteroarylmethanes by reaction of aldehydes and activated arenes promoted by gold(III) chloride, Org. Lett., 2005, 7, 5857 CrossRef CAS.
- V. D. Kadu, A. A. Patil and P. R. Shendage, Oxone-promoted synthesis of bis (indolyl) methanes from arylmethylamines and indoles, J. Mol. Struct., 2022, 1267, 133502 CrossRef CAS.
- Y. Lian, R. G. Bergman, L. D. Lavis and J. A. Ellman, Rhodium (III)-catalyzed indazole synthesis by C–H bond functionalization and cyclative capture, J. Am. Chem. Soc., 2013, 135, 7122–7125 CrossRef CAS.
- B. Jia, Y. Yang, X. Jin, G. Mao and C. Wang, Rhenium-Catalyzed Phthalide Synthesis from Benzamides and Aldehydes via C–H Bond Activation, Org. Lett., 2019, 21, 6259–6263 CrossRef CAS.
- W. Liu, X. Guo and R. Guo, The interaction between hemoglobin and two surfactants with different charges, Int. J. Biol. Macromol., 2007, 41, 548 CrossRef CAS PubMed.
- J. R. Lakowicz, Principles of fluorescence spectroscopy, Springer, 3rd edn, 2006 Search PubMed.
- W. He, H. Dou, Z. Li, X. Wang, L. Wang, R. Wang and J. Chang, Investigation of the interaction between five alkaloids and human hemoglobin by fluorescence spectroscopy and molecular modeling, Spectrochim. Acta, Part A, 2014, 123, 176–186 CrossRef CAS PubMed.
- S. Lehrer, Solute perturbation of protein fluorescence. Quenching of the tryptophyl fluorescence of model compounds and of lysozyme by iodide ion, Biochemistry, 1971, 10, 3254–3263 CrossRef CAS PubMed.
- W. R. Ware, Oxygen quenching of fluorescence in solution: an experimental study of the diffusion process, J. Phys. Chem., 1962, 66, 455–458 CrossRef CAS.
- A. Sharifi-Rad, J. Mehrzad, M. Darroudi, M. R. Saberi and J. Chamani, Oil-in-water nanoemulsions comprising Berberine in olive oil: biological activities, binding mechanisms to human serum albumin or holo-transferrin and QMMD simulations, J. Biomol. Struct. Dyn., 2021, 39, 1029–1043 CrossRef CAS.
- M. Kaffash, S. Tolou-Shikhzadeh-Yazdi, S. Soleimani, S. Hoseinpoor, M. R. Saberi and J. Chamani, Spectroscopy and molecular simulation on the interaction of Nano-Kaempferol prepared by oil-in-water with two carrier proteins: an investigation of protein–protein interaction, Spectrochim. Acta, Part A, 2024, 309, 123815 CrossRef CAS PubMed.
- S. Rezaei, H.-S. Meftah, Y. Ebtehajpour, H. R. Rahimi and J. Chamani, Investigation on the effect of fluorescence quenching of calf thymus DNA by piperine: caspase activation in the human breast cancer cell line studies, DNA Cell Biol., 2024, 43, 26–38 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2026 |