 Open Access Article
Open Access ArticleFabrication of manganese-loaded lignin-derived carbon via an in situ anchoring strategy for water electrolysis
Yang
Huamei
 ab,
Wang
Beilei
a,
Yu
Mengjun
a,
He
Haiyan
bc and
Li
Changzhi
ab,
Wang
Beilei
a,
Yu
Mengjun
a,
He
Haiyan
bc and
Li
Changzhi
 *bc
*bc
aSchool of Materials and Chemical Engineering, Xuzhou University of Technology, Xuzhou, Jiangsu 221018, China
bCAS Key Laboratory of Science and Technology on Applied Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, 116023, China. E-mail: licz@dicp.ac.cn
cUniversity of Chinese Academy of Sciences, Beijing 100049, China
First published on 19th November 2025
Abstract
Low-cost, high-performance catalysts are pivotal for advancing water electrolysis toward industrial applications. As an abundant, renewable, and readily available carbon feedstock, lignin offers a sustainable platform for the development of a carbon-supported catalyst. In this study, manganese-loaded lignin-derived carbon (Mnx@C) was synthesized via an in situ chelation–anchoring strategy using lignin as the carbon source. The in situ chelation–anchoring carbonization process enabled effective incorporation and uniform dispersion of Mn species. Mn species in Mnx@C were mainly Mn2O3, MnO2, and MnO. Among these Mn species, MnO was dispersed in the lignin-based carbon support as aggregation nanoparticles, while Mn2O3 and MnO2 were primarily dispersed in an amorphous state. The electrocatalytic performances of Mnx@C were systematically evaluated for the oxygen evolution reaction (OER) and hydrogen evolution reaction (HER). The Mn content and NH4Cl modification significantly influenced the OER and HER activities. The Mn1@C prepared at 1 mmol Mn/g lignin, with the final Mn content of 8.7%, exhibited optimal performances of 517 mV (OER) and 448 mV (HER) at 50 mA cm−2. Further improvement was achieved by modulating the pore structure using NH4Cl as a pore-forming agent, resulting in a Mn-loaded and nitrogen-doped carbon (Mn1@NC). NH4Cl modification induced a morphological transition from a bulk structure to a layered microporous structure with a high surface area. The modified Mn1@NC demonstrated excellent OER and HER performances, requiring low overpotentials of 299 mV (OER) and 409 mV (HER) at 50 mA cm−2. In addition, the overall water-splitting demonstrated a low potential of 1.65 V to reach 10 mA cm−2. This in situ anchoring and direct carbonization strategy provides a simple, cost-effective, and scalable route to high-performance Mn-based catalysts from low-cost lignin, offering potential for industrial water electrolysis and high-efficient utilization of lignin.
Introduction
Water electrolysis is a promising sustainable technology for hydrogen production with significant application potential.1,2 Catalysts play a pivotal role in reducing the kinetic barriers associated with the oxygen evolution reaction (OER) and hydrogen evolution reaction (HER) during water electrolysis. State-of-the-art catalysts rely heavily on scarce precious metals (such as Pt, Ru and Ir compounds),3–7 leading to prohibitively high costs that impede large-scale applications. Consequently, developing non-precious metal catalysts with high activity for water electrolysis is imperative as alternatives to precious metal-based catalysts.8,9Transition metals and their oxides, nitrides, phosphides, hydroxides and sulfides have been widely investigated.7,8,10–13 Manganese (Mn), a typical 3d transition metal, is derived primarily from parent materials in soil, and exhibits multiple oxidation states. Mn-based materials have been explored as electrocatalysts for the OER and HER owing to their inherent catalytic activity, environmental compatibility, low cost, and natural abundance.14–19 Particularly, Mn-based oxides (MnOx) exhibit good chemical stability in alkaline electrolytes20,21 and facilitate O–O bond formation steps in the OER owing to its variable oxidation states (commonly +2 in MnO, +3 in Mn2O3, +4 in MnO2, and mixed states in Mn3O4).20 Therefore, focused development of Mn-based catalysts remains necessary, as they possess irreplaceable potential in large-scale commercial applications. However, Mn-based materials always exhibit poor OER and HER performances compared to other transition metal-based materials (such as iron, nickel, and cobalt-based materials), limiting their practical applications.7,12,21,22 The lower electrocatalytic activity of Mn-based catalysts, leading to low intrinsic catalytic activity,3,8,12,23 is primarily caused by the poor electrical conductivity and the electronic configuration that results in weak electronic coupling with intermediates.20,21
The performance of Mn-based catalysts can be improved through the following strategies: constructing heterostructures (e.g., composites with carbon materials or oxides) to increase the number of active sites and enhance the electron transfer rate and charge transfer efficiency. Regulating the morphology (e.g., preparing nanosheets or hollow spheres) to increase specific surface area exposes more active sites for the OER and the HER. Carbon-based materials are employed as electrically active materials or conductive substrates to create oxygen vacancies and heterogeneous interfaces. The OER and HER performance of MnOx-based electrocatalysts can be improved by tuning the morphology of carbon materials and synergistic effects between Mn species and carbon.18,19,24–26 Carbon supports such as activated carbon, carbon nanotubes, graphene, and carbon nanofibers are always employed to prepare Mn-loading carbon catalysts via an impregnation method. However, these carbon supports need to be prepared in advance, making the process labor- and time-intensive. Meanwhile, these carbon supports often possess inert surfaces that limit metal dispersion during impregnation.27 Recently, well-synthetic carbon materials such as carbon dots18,28 and metal–organic frameworks29,30 were employed to incorporate with Mn species. This strategy can provide Mn-based carbon catalysts with high surface areas, enhanced conductivity, greatly promoted electrocatalytic activity and high stability for the OER. However, the reliance on pure chemicals as carbon precursors incurs high costs, hindering large-scale application.
Lignin, a renewable biomass resource, possesses a highly aromatic, three-dimensional conjugated network with abundant oxygen-containing functional groups (e.g., hydroxyl, carbonyl, and methoxy) and high carbon content.31 These structural characteristics endow lignin with compelling potential for synthesizing multifunctional carbon materials, particularly in applications such as energy storage, adsorption, and catalysis.31–37 Notably, the oxygen functional groups can effectively anchor metal species through chelation or coordination, promoting uniform metal dispersion and enhancing catalytic performance.36 Owing to these advantages, lignin is regarded as an ideal precursor for designing high-performance functional carbon supports in catalytic applications. At the same time, lignin is a large-volume by-product of the pulping and papermaking as well as biorefinery industries. Lignin is a large-volume byproduct of the pulping and papermaking as well as biorefinery industries. It exhibits a substantial annual production volume, yet the majority is currently underutilized through low-value applications such as disposal or inefficient combustion, which pose environmental and economic challenges. Using it as a carbon source can achieve “waste resource utilization” and reduce the preparation cost of carbon materials.
In this work, a Mn-loaded lignin-derived carbon catalyst (Mnx@C) was prepared for water electrolysis via an in situ anchoring and one-step carbonization strategy. The effect of Mn proportion was investigated, and Mn1@C obtained at the Mn proportion of 1 mmol g−1 lignin exhibited the best OER and HER performances. Furthermore, morphological modulation using ammonium chloride (NH4Cl) as a pore-forming agent yielded Mn1@NC with a porous lamellar structure, characterized by high surface area and abundant micropores. This optimized structure significantly enhanced bifunctional OER and HER performances. The Mn1@NC catalyst exhibited a remarkably low overpotential of 299 mV for the OER and 409 mV for the HER at a current density of 50 mA cm−2. In addition, the overall water-splitting device demonstrated a low potential of 1.65 V to reach 10 mA cm−2. This performance conclusively demonstrates the viability of fabricating high-performance bifunctional electrocatalysts for water electrolysis through the in situ chelation and carbonization methodology utilizing lignin as a carbon precursor.
Experimental
Experimental instruments and reagents
Lignin, purchased from Shandong Longli Biotechnology Co., Ltd, 200 mesh, vacuum-dried at 80 °C for 12 hours. Polyvinylpyrrolidone (PVP), Shanghai Titan Technology Co., Ltd, RG; carbon black, Shanghai Titan Technology Co., Ltd, RG; anhydrous ethanol, Shanghai Titan Technology Co., Ltd, ≥99.7%; ammonium chloride (NH4Cl), Shanghai Titan Technology Co., Ltd, ≥99.5%; manganese(II) tetrahydrate chloride (MnCl2·4H2O), Shanghai Titan Technology Co., Ltd, ≥99%.Preparation of Mnx@C and Mnx@NC
Mnx@C was prepared via an in situ anchoring and one-step carbonization strategy. The specific process was as follows: lignin molecules first combined with Mn ions to form a stable manganese–lignin complex (Mn–lignin) via coordinate bonds, and then the complex underwent high-temperature carbonization under an inert atmosphere to obtain Mnx@C. Typically, 1 g of lignin was added to 10 mL of ethanol and ultrasonicated for 15 minutes to achieve complete dispersion, forming a suspension. 0.198 g (1 mmol) of MnCl2·4H2O was dissolved in 10 mL of ethanol/water (V![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V 2
V 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Under ultrasonication, the MnCl2 solution was added dropwise using a pipette to the lignin–ethanol suspension, forming a mixture. This mixture was then magnetically stirred for 24 hours. Subsequently, 50 mL of deionized water was added to the ethanol suspension of Mn–lignin, and the suspension was left to stand for 30 minutes. Mn–lignin complex precipitated out and settled. Vacuum filtration was performed to obtain the Mn–lignin complex. The Mn–lignin complex was dried in a vacuum oven at 80 °C for 10 hours. The dried Mn–lignin sample was placed in a porcelain boat and transferred to a tubular furnace for high-temperature carbonization. The heating program, under a nitrogen atmosphere, was as follows: ramp the temperature at 15 °C min−1 to 800 °C and then hold at 800 °C for 2 hours. After carbonization, the furnace was cooled naturally to room temperature under nitrogen, and Mn-loaded lignin carbon-based materials were obtained, which were labelled as Mnx@C (x was the millimole ratio of MnCl2·4H2O to lignin). For example, samples prepared with 0.198 g (1 mmol) of MnCl2·4H2O added to 1 g lignin were labelled as Mn1@C. The effect of Mn loading was investigated with the Mn proportion x being 0 (0 g), 0.05 (0.010 g of MnCl2·4H2O, 0.05 mmol), 0.25 (0.049 g of MnCl2·4H2O, 0.25 mmol), 0.5 (0.099 g of MnCl2·4H2O, 0.5 mmol), 1 (0.198 g of MnCl2·4H2O, 1 mmol), and 2 (0.396 g of MnCl2·4H2O, 2 mmol).
1). Under ultrasonication, the MnCl2 solution was added dropwise using a pipette to the lignin–ethanol suspension, forming a mixture. This mixture was then magnetically stirred for 24 hours. Subsequently, 50 mL of deionized water was added to the ethanol suspension of Mn–lignin, and the suspension was left to stand for 30 minutes. Mn–lignin complex precipitated out and settled. Vacuum filtration was performed to obtain the Mn–lignin complex. The Mn–lignin complex was dried in a vacuum oven at 80 °C for 10 hours. The dried Mn–lignin sample was placed in a porcelain boat and transferred to a tubular furnace for high-temperature carbonization. The heating program, under a nitrogen atmosphere, was as follows: ramp the temperature at 15 °C min−1 to 800 °C and then hold at 800 °C for 2 hours. After carbonization, the furnace was cooled naturally to room temperature under nitrogen, and Mn-loaded lignin carbon-based materials were obtained, which were labelled as Mnx@C (x was the millimole ratio of MnCl2·4H2O to lignin). For example, samples prepared with 0.198 g (1 mmol) of MnCl2·4H2O added to 1 g lignin were labelled as Mn1@C. The effect of Mn loading was investigated with the Mn proportion x being 0 (0 g), 0.05 (0.010 g of MnCl2·4H2O, 0.05 mmol), 0.25 (0.049 g of MnCl2·4H2O, 0.25 mmol), 0.5 (0.099 g of MnCl2·4H2O, 0.5 mmol), 1 (0.198 g of MnCl2·4H2O, 1 mmol), and 2 (0.396 g of MnCl2·4H2O, 2 mmol).
Additionally, NH4Cl was employed to modify the catalytic performance of Mn1@C, which served as a pore-forming agent and a nitrogen source. The preparation of the Mn–lignin complex followed the same procedure as Mn1@C. Wetting grinding was employed to achieve thorough mixing of Mn–lignin and NH4Cl with the ratio of NH4Cl to lignin as 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. 0.5 g NH4Cl was added to the complex of Mn–lignin, and the mixture was subjected to wetting grinding for complete homogenization. The mixture of Mn–lignin and NH4Cl was dried in a vacuum oven at 80 °C for 12 hours. Finally, the mixture of Mn–lignin and NH4Cl was carbonized via the same procedure of Mn1@C. The modified N-doped carbon was labelled as Mn1@NC. An N-doped lignin-based carbon without Mn-loading (Mn0@NC) was also prepared using the same procedure as Mn1@NC.
1. 0.5 g NH4Cl was added to the complex of Mn–lignin, and the mixture was subjected to wetting grinding for complete homogenization. The mixture of Mn–lignin and NH4Cl was dried in a vacuum oven at 80 °C for 12 hours. Finally, the mixture of Mn–lignin and NH4Cl was carbonized via the same procedure of Mn1@C. The modified N-doped carbon was labelled as Mn1@NC. An N-doped lignin-based carbon without Mn-loading (Mn0@NC) was also prepared using the same procedure as Mn1@NC.
For control experiments, activated carbon (AC) was employed to prepare a Mn-loaded activated carbon (Mn1@AC) with 0.198 g of MnCl2·4H2O, using the same method as Mn1@C.
Electrochemical testing
Nickel foam was cut into 1.2 × 1 cm2 squares. The nickel foam squares were cleaned by sequential immersion in 3 mol L−1 hydrochloric acid, acetone, deionized water, and ethanol, vacuum-dried at 60 °C for 12 hours. The high-temperature carbonized samples were weighed according to the mass ratio (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) of carbon black, PVP and samples. Typically, 0.08 g of carbon black, 0.01 g of PVP and 0.01 g of samples were weighed and mixed. The mixture was ground with 2 ml of ethanol to form a homogeneous slurry. The nickel foam was immersed in the above slurry for 1 minute and rapidly dried using a heat lamp. The nickel foam was weighed before and after loading catalytic materials by a microbalance. The average loaded mass was 0.8 ± 0.05 mg cm−2. Then, the loaded foam nickel was placed in a vacuum oven at 60 °C for 12 hours.
1) of carbon black, PVP and samples. Typically, 0.08 g of carbon black, 0.01 g of PVP and 0.01 g of samples were weighed and mixed. The mixture was ground with 2 ml of ethanol to form a homogeneous slurry. The nickel foam was immersed in the above slurry for 1 minute and rapidly dried using a heat lamp. The nickel foam was weighed before and after loading catalytic materials by a microbalance. The average loaded mass was 0.8 ± 0.05 mg cm−2. Then, the loaded foam nickel was placed in a vacuum oven at 60 °C for 12 hours.
All electrochemical OER and HER tests were performed on a CHI660E electrochemical workstation using a standard three-electrode system in 1 M KOH electrolyte (pH = 14) at room temperature. The fabricated nickel foam electrode (clamped with an electrode holder) was used as the working electrode, and the area of the working electrode immersed in the KOH solution was kept as 1 × 1 cm2. The platinum sheet was used as the counter electrode. The mercury/mercury oxide (Hg/HgO) electrode (1 M KOH, 25 °C, standard potential +0.098 V vs. SHE) was used as the reference electrode.
Linear sweep voltammetry (LSV) for the OER was performed in the potential range of 0 to 1 V vs. Hg/HgO. All measured potentials (vs. Hg/HgO) were converted to the reversible hydrogen electrode (RHE) scale using the formula under alkaline conditions: V (vs. RHE) = V (vs. Hg/HgO) +0.098 + 0.059 × pH. Tafel slopes (b) were calculated by fitting the linear region of the Tafel plot using the equation: η = a + b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) j, where η is the overpotential (η = E − Erev, Erev = 1.23 V), a is a constant, and j is the current density. Cyclic voltammetry (CV) was performed within the non-faradaic potential range (typically 0.1 to 0.2 V vs. RHE is recommended; confirm suitability for your system) at various scan rates (e.g., 10, 40, 80, and 100 mV s−1). The double-layer capacitance (Cdl) was estimated from the slope of the plot of the charging current density difference (Δj = |ja − jc|/2) at a fixed potential within this range versus the scan rate. The long-term electrochemical stability of the catalyst was evaluated by chronopotentiometry or chronoamperometry at a constant current density or potential in 1 M KOH (pH = 14) for 20 hours. Electrochemical impedance spectroscopy (EIS) was conducted from a potential of 238 to 458 mV vs. RHE for the OER over a frequency range from 100 kHz to 0.1 Hz with an AC amplitude of 10 mV. LSV measurements for the HER were performed in the potential range of −0.9 to 0.0 V vs. Hg/HgO. LSV measurements for the overall water-splitting were conducted using a two-electrode system in 1.0 M KOH solution, with Mn1@NC serving as both the cathode and the anode (Mn1@NC‖Mn1@NC).
j, where η is the overpotential (η = E − Erev, Erev = 1.23 V), a is a constant, and j is the current density. Cyclic voltammetry (CV) was performed within the non-faradaic potential range (typically 0.1 to 0.2 V vs. RHE is recommended; confirm suitability for your system) at various scan rates (e.g., 10, 40, 80, and 100 mV s−1). The double-layer capacitance (Cdl) was estimated from the slope of the plot of the charging current density difference (Δj = |ja − jc|/2) at a fixed potential within this range versus the scan rate. The long-term electrochemical stability of the catalyst was evaluated by chronopotentiometry or chronoamperometry at a constant current density or potential in 1 M KOH (pH = 14) for 20 hours. Electrochemical impedance spectroscopy (EIS) was conducted from a potential of 238 to 458 mV vs. RHE for the OER over a frequency range from 100 kHz to 0.1 Hz with an AC amplitude of 10 mV. LSV measurements for the HER were performed in the potential range of −0.9 to 0.0 V vs. Hg/HgO. LSV measurements for the overall water-splitting were conducted using a two-electrode system in 1.0 M KOH solution, with Mn1@NC serving as both the cathode and the anode (Mn1@NC‖Mn1@NC).
Characterization
The Mn contents in Mnx@C, Mn1@AC and Mn1@NC were determined by ICP-OES. The contents of carbon (C), hydrogen (H) and nitrogen (N) in lignin, Mnx@C, Mn1@AC and Mn1@NC were determined by using an elemental analyzer. Powder X-ray diffraction patterns were collected using a multi-purpose X-ray diffractometer with Cu Kα radiation (λ = 1.5418 Å). Samples were prepared by pressing the catalyst powder onto a sample holder, cleaning the surface with anhydrous ethanol, and drying. Measurements were performed with a tube voltage of 40 kV, a tube current of 40 mA, a scan rate of 2° min−1, and a scan range of 5° to 90° (2θ). The textural properties (specific surface area, pore volume, and pore size distribution) of the samples were determined by N2 adsorption–desorption isotherms measured at −196 °C. Specific surface area was calculated using the Brunauer–Emmett–Teller (BET) method. Pore size distribution was analyzed using density functional theory (DFT) or non-local density functional theory (NLDFT) methods for micropores (<2 nm) and mesopores. The morphology of the samples was characterized at different scales using scanning electron microscopy (SEM) and transmission electron microscopy (TEM) with energy-dispersive X-ray spectroscopy (EDS).Results and discussion
Structural characterization
Table 1 shows that Mn content in Mnx@C increased to 9.74 wt% with the Mn proportion increasing to 2 mmol g−1. Lignin employed in this work was mainly composed of 63.3 wt% carbon (C), 5.9 wt% hydrogen (H), 28.9 wt% oxygen (O), and 1.9 wt% nitrogen (N), as listed in Table 1. Without the addition of Mn and NH4Cl, the carbon yield from lignin, labelled as Mn0@C, was 35.6%. The content of C, H, O and N was 83.82%, 2.67%, 13.06% and 0.43%, respectively. Mnx@C, prepared through an in situ anchoring and one-step carbonization strategy, had a higher content of C than lignin, and a lower content of H, N and O. With the increase in Mn content, the C content decreased while the contents of N and O increased, suggesting that the introduced Mn immobilized O and N. Activated carbon (AC) was employed to prepare a Mn-loaded catalyst (Mn1@AC) with the Mn proportion of 1 mmol g−1 AC. Table 1 shows that Mn1@AC had a higher C content and a much lower O content than Mn1@C. The Mn content in Mn1@AC was 6.63 wt%, slightly lower than Mn1@C.| Samples | Mna/% | Cb/% | Hc/% | Nd/% | Oe/% | I 40.5/I22.4f |
|---|---|---|---|---|---|---|
| a Determined by ICP-OES. b Determined by using an elemental analyzer. c Determined by using an elemental analyzer. d Determined by using an elemental analyzer. e Determined by the difference of 100 and the sum of a–d. f I 40.5/IC calculated based on XRD, as shown in Fig. 1. | ||||||
| Lignin | — | 63.32 | 5.91 | 1.87 | 28.90 | — |
| Mn0@C | 0.02 | 83.82 | 2.67 | 0.43 | 13.06 | — |
| Mn0.05@C | 0.55 | 83.71 | 2.21 | 0.42 | 13.11 | 0.98 |
| Mn0.25@C | 2.87 | 80.54 | 2.73 | 0.51 | 13.35 | 1.42 |
| Mn0.5@C | 5.84 | 78.63 | 1.32 | 0.74 | 13.47 | 4.65 |
| Mn1@C | 8.65 | 75.48 | 1.45 | 0.82 | 13.60 | 5.28 |
| Mn2@C | 9.74 | 72.22 | 0.94 | 0.85 | 16.25 | 3.74 |
| Mn1@AC | 6.63 | 87.37 | 1.42 | 0.38 | 4.20 | — |
| Mn1@NC | 8.92 | 72.23 | 2.13 | 4.36 | 12.36 | 5.22 |
The addition of NH4Cl during the carbonization process achieved N-doped carbon (Mn1@NC) with the Mn proportion of 1 mmol g−1 lignin, which had a much higher N content than Mn1@C and Mn1@AC as listed in Table 1. As mentioned above, lignin has abundant active functional groups in its structure, including hydroxyl, carbonyl, and carboxyl groups. Meanwhile, lignin was decomposed to form active carbon intermediates during carbonization.38 The co-carbonization of NH4Cl and the Mn–lignin complex achieved nitrogen doping and morphology regulation through a synergistic effect due to the abundant active structures in lignin.39 At around 280 °C, NH4Cl decomposed to generate gases (NH3 and HCl). NH3 reacted with these carbon intermediates or active groups, and N was embedded into the carbon skeleton through substitution, cyclization, and other reactions, realizing in situ N-doping.38–40 As the carbon skeleton further graphitized, nitrogen stably existed in the carbon structure (mainly in three forms: pyrrole nitrogen, pyridine nitrogen, and graphitic nitrogen). The EDS and XPS analysis results in the following section will confirm the N-doping and its existing forms.
Fig. 1(a) shows the XRD patterns of Mnx@C, Mn1@AC and Mn1@NC. Mn0@C, derived from lignin carbonization without Mn-loading, was composed of an amorphous carbon structure with a diffraction peak at 2θ = 22.4°. With the increasing Mn proportion, the diffraction peaks at 2θ = 35.6°, 40.5°, 59.1°, 70.1°, and 73.6° intensified. These peaks corresponded to the diffraction peaks of the MnO standard card (PDF#89-2804), indicating that Mn was successfully loaded onto the carbon support and existed in the form of MnO. Taking the intensity of the carbon peak (2θ = 22.4°, I22.4) as a reference, the intensity ratio of the MnO characteristic peak (I40.5) to I22.4 (I40.5/I22.4) was calculated to semi-quantitatively analyze the loading amount of MnO as listed in Table 1. The loading amount of MnO increased with the increasing Mn proportion, which was consistent with the results of ICP-OES. The loading amount of MnO in Mn1@C reached the maximum with the peak value of I40.5/I22.4 as listed in Table 1. When NH4Cl was added for pore generation, the XRD pattern of Mn1@NC had no significant changes, indicating that the addition of NH4Cl had no effects on Mn species. However, Mn1@AC had no distinct diffraction peaks of MnO as shown in Fig. 1(a). This demonstrated a clear difference of Mn species between the Mnx@C prepared in situ from lignin and Mn1@AC prepared via the conventional method (i.e., impregnation method).
 | ||
| Fig. 1 XRD spectra (a), SEM images (b), TEM images (c), N2 adsorption–desorption curves (d) and elemental mapping (e) of Mnx@C and Mn1@NC. | ||
Fig. 1(b) shows the SEM images of Mn1@C and Mn1@NC. In the absence of NH4Cl, Mn1@C exhibited a bulk morphology with obvious agglomeration of Mn species. The addition of NH4Cl significantly altered the morphology of Mn1@NC, transforming from a bulk structure of Mn1@C to a porous lamellar structure of Mn1@NC. Fig. 1(b) also shows a substantial population of pores in Mn1@NC, partially labelled with red circles. Fig. 1(c) shows the TEM images of Mn1@C and Mn1@NC. Obviously, a large number of pores were observed in Mn1@NC with 7–10 nm of nanoparticles, while Mn1@C possessed large nanoparticles without pores observed. Fig. 1(c) also illustrates the MnO nanocrystals that are surrounded by amorphous carbon or graphene. The lattice fringe with a spacing of 0.25 nm corresponded to the (111) plane of the cubic MnO phase, and a spacing of 0.34 nm corresponded to the highly organized crystal carbon structure, which was thought to be the graphene layer. These results were consistent with the XRD results, as shown in Fig. 1(a). Fig. 1(d) shows the N2 adsorption–desorption isotherm of Mn1@NC. N2 adsorption–desorption test did not provide the isotherm of Mn1@C, which indicated that Mn1@C was a non-porous material. Mn1@NC exhibited a typical type I isotherm, indicating that Mn1@NC was microporous with abundant micropores. The surface area of Mn1@NC was 567.0 m2 g−1 and the micropore area was 549.8 m2 g−1, while the pore diameter in Mn1@NC as shown in Fig. 1(d) was 1.26 nm. As mentioned above, volatiles from lignin decomposition, NH3 and HCl from NH4Cl were formed during the co-carbonization of NH4Cl and the Mn–lignin complex. The evolution of these gases (volatiles, NH3 and HCl) promoted the pore formation in carbon materials. At the same time, NH3 and HCl reacted with carbon-based intermediates or active groups to achieve morphology regulation through a complex synergistic effect.39,40 For example, the released HCl acted as an acid catalyst to break the C–C or C–O bonds and to promote the formation of condensed ring structures during lignin carbonization.41 With the increase in temperature, lignin decomposed extensively to produce small-molecule gases (e.g., CO2, H2O). The continuous escape of these gases generated pores and constructed an interconnected porous network.41,42Fig. 1(e) shows the elemental mapping of carbon (C), nitrogen (N), oxygen (O) and Mn in Mn1@NC. As shown in Fig. 1(e), there was a certain correlation between the distributions of O and Mn, which corresponds to the formation of MnO. N was doped in the prepared Mn1@NC, exhibiting a distribution similar to that of O and Mn.
OER performance
Fig. 2(a) shows the LSV curves for the OER of Mnx@C prepared at different Mn proportions. The overpotentials (E50) of Mnx@C at a current density of 50 mA cm−2 for the OER are illustrated in Fig. 2(b). Fig. 2(a) and (b) indicate that the OER performance of Mnx@C was affected by the Mn proportion. The LSV curves of Mnx@C shifted to a low potential zone with the Mn proportion increasing to 1 mmol g−1. The E50 decreased from 618 mV to 517 mV with the Mn proportion increasing to 1 mmol g−1. When the proportion of Mn was 1 mmol g−1, Mn1@C exhibited the best OER catalytic performance with the lowest E50 of 517 mV. Further increase with the Mn proportion led to the decrease of OER performance with a higher E50 of 565 mV at 2 mmol g−1 than that at 1 mmol g−1. Fig. 2(c) shows the Tafel slopes of Mnx@C corresponding to the LSV curves for the OER. Except Mn1@C, the Tafel slopes of Mnx@C were above 60 mV dec−1, indicating that the rate-determining step (RDS) of the OER was dominated by a single-electron transfer step for the formation of the oxygen intermediate (*O) and the peroxide intermediate (*OOH). Mn1@C exhibited the lowest Tafel slope (51.4 mV dec−1), indicating that Mn1@C exhibited the fastest OER kinetic process among all the in situ prepared catalysts. The Tafel slope of Mn1@C was slightly lower than the theoretical value (60 mV dec−1), indicating that it was still dominated by a single-electron transfer as RDS, accompanied by a slight synergistic effect of multi-step reactions. Fig. 2(d) illustrates that the double-layer capacitance (Cdl) of Mn1@C was higher than that of other Mnx@C, indicating that the electrochemical active area of Mn1@C was larger than other Mnx@C. Above all, Mn1@C exhibited the best OER catalytic performance. | ||
| Fig. 2 The effects of Mn proportion on the OER performance of electrode material Mnx@C. (a) LSV; (b) overpotential at 50 mA cm−2; (c) Tafel value; (d) Cdl. | ||
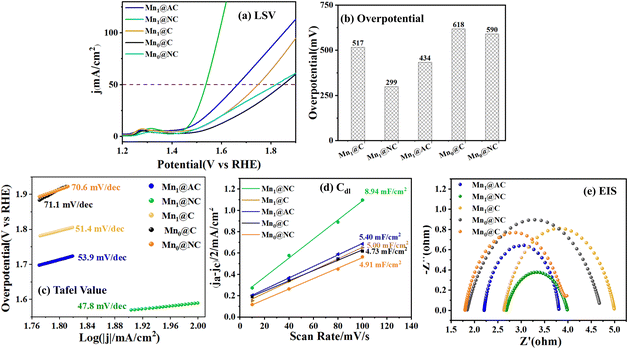
Comparing the OER performance of Mn1@C and Mn1@AC in Fig. 3, the OER performance of Mn1@C was significantly poorer than that of Mn1@AC prepared with AC as the support. As shown in Fig. 3(b), the E50 of Mn1@AC was 434 mV, lower than that of Mn1@C (517 mV). Fig. 3(c) shows that the Tafel slope of Mn1@AC was 53.9 mV dec−1, slightly higher than that of Mn1@C, indicating that Mn1@AC had a slower OER kinetic process than Mn1@C. As illustrated in Fig. 3(d), the Cdl of Mn1@AC (5.40 mF cm−2) was higher than that of Mn1@C (5.00 mF cm−2), indicating that the electrochemical active area of Mn1@AC was higher than that of Mn1@C. The higher electrochemical active area of Mn1@AC was possibly due to the fact that AC has abundant pores, which facilitated substrate adsorption and electron transmission, thereby improving the OER performance.
 | ||
| Fig. 3 NH4Cl effect on the OER performance of electrode materials Mn1@C and Mn1@NC. (a) LSV; (b) overpotential at 50 mA cm−2; (c) Tafel value; (d) Cdl; (e) EIS. | ||
To optimize the pore structure of Mn1@C prepared by in situ carbonization of lignin, NH4Cl was added as a pore-forming agent into the Mn-chelated lignin samples. As discussed in Section 2.1, with the addition of NH4Cl, Mn1@NC had more developed pore structures and higher specific surface area than Mn1@C. Fig. 3(a) and (b) show that the E50 decreased from 517 mV for Mn1@C to 299 mV for Mn1@NC, which was much lower than that of Mn1@AC (434 mV). Fig. 3(c) shows the Tafel slope of Mn1@NC (47.8 mV dec−1) was much lower than that of Mn1@C (51.4 mV dec−1) and Mn1@AC (53.9 mV dec−1), indicating that Mn1@NC had a faster OER kinetic process than Mn1@C and Mn1@AC. As shown in Fig. 3(d), the Cdl of Mn1@NC reached 8.94 mF cm−2, which was much higher than that of Mn1@C (5.38 mF cm−2) and Mn1@AC (5.40 mF cm−2), indicating that the addition of NH4Cl effectively increased the electrochemical active area of the Mn-doped lignin carbon material. Fig. 3(e) illustrates that the charge transfer resistances (Rct) increased in the order of Mn1@NC < Mn1@AC< Mn1@C, indicating that Mn1@NC had the fastest charge transfer rate during the catalytic OER process. Above all, the addition of NH4Cl significantly improved the OER performance of Mn1@NC, due to the adjusted pore structures and high specific surface area.
As shown in Fig. 3, the NH4Cl-added carbon material (Mn0@NC) without Mn-loading was also studied to further illustrate the effects of Mn-loading and NH4Cl-addition on the OER performance. The OER performance of Mn0@NC was slightly enhanced with a lower E50 (590 mV), a smaller Tafel slope (70.6 mV dec−1), and a higher Cdl (4.91 mF cm−2) than Mn0@C, indicating that NH4Cl-addition was beneficial for the improvement of OER performance, possibly due to the regulated morphology and N-doping. Comparing the NH4Cl-free materials (Mn1@C) and Mn-free materials (Mn0@NC) with Mn and N co-doped materials (Mn1@NC), the much better OER performances of Mn1@NC indicated that Mn-loading and NH4Cl-addition played a crucial synergistic role in enhancing the OER performance.
Fig. 4 summarizes the OER overpotential (E10) at 10 mA cm−2 of Mn1@NC and the reported Mn-based catalysts.18,19,28,30,43 As illustrated in Fig. 4, Mn1@NC, prepared via an in situ anchoring and direct carbonization strategy in this work, has a much lower E10 (235 mV) than the reported Mn-based catalysts. In other words, Mn1@NC exhibited more excellent OER catalytic activity than the reported Mn-based catalysts. The in situ anchoring and direct carbonization strategy using lignin as the carbon source provided a simple, cost-effective, and scalable route to high-performance Mn-based catalysts from lignin, offering significant potential for industrial water electrolysis.
HER performance
Fig. 5(a) shows the LSV curves for the HER of Mnx@C prepared with different Mn proportions. The Mn proportion obviously affected the HER performance of Mnx@C. The LSV curves for the HER shifted to a lower potential zone with the Mn proportion increasing to 1 mmol g−1 as shown in Fig. 5(a). Fig. 5(b) illustrates the E50 of Mnx@C at a current density of 50 mA cm−2 for the HER. The E50 decreased from 581 mV to 448 mV with the Mn proportion increasing to 1 mmol g−1. When the Mn proportion was 1 mmol g−1, Mn1@C had the lowest E50 of 448 mV, showing the best HER performance. However, the LSV curve for the HER of Mn2@C shifted to a higher potential zone at the Mn proportion of 2 mmol g−1, as shown in Fig. 5(a), and the E50 of Mn2@C increased to 525 mV, as shown in Fig. 5(b), which indicated that a high Mn proportion of 2 mmol g−1 led to a decreased HER performance of Mn2@C. Fig. 5(c) illustrates the Tafel slope of Mnx@C. All the Tafel slopes were between 40 mV dec−1 and 120 mV dec−1, indicating that the control step was the Heyrovsky reaction in the Volmer–Heyrovsky mechanism (electrochemical desorption mechanism).44–46 As shown in Fig. 5(c), Mn1@C had the smallest Tafel slope (52.4 mV dec−1) among all the prepared catalysts, indicating that Mn1@C exhibited the fastest kinetic process of the HER, and the most excellent catalytic activity for the HER. | ||
| Fig. 5 The effects of Mn proportion (a)–(c) on the HER performance of electrode material Mnx@C, and NH4Cl effect (d)–(f) on the HER performance of electrode materials Mn1@C and Mn1@NC. | ||
Fig. 5(d–f) shows the HER performance of Mn1@C, Mn1@AC and Mn1@NC. Compared with the Mn1@AC prepared via the conventional method, Mn1@C exhibited a lower E50 (448 mV) than Mn1@AC (525 mV) as illustrated in Fig. 5(e), indicating that lignin-derived in situ prepared Mn1@C achieved higher HER performance than Mn1@AC synthesized by the conventional method (i.e., the impregnation method). Similarly with the OER performance, NH4Cl-added and Mn-free carbon materials (Mn0@NC) also exhibited a better HER performance than Mn0@C, as evidenced by a lower E50 (494 mV). Additionally, Mn0@NC outperformed Mn0@C, and Mn1@NC outperformed Mn1@C in HER performance, indicating that the NH4Cl-addition played important roles in improving HER activity. The synergistic effect of the Mn-loading and NH4Cl-addition endowed Mn1@NC with the optimal HER performance. As shown in Fig. 5(d), the LSV curve of Mn1@NC shifted to a lower potential zone than that of Mn1@C. Fig. 5(e) further reveals that Mn1@NC had a lower E50 (409 mV) than Mn1@C (448 mV), demonstrating that NH4Cl-addition obviously lowered the overpotential and improved the HER performance of Mn-loaded carbon materials. Furthermore, Fig. 5(f) shows that the Tafel slope of Mn1@NC (40.2 mV dec−1) was remarkably lower than that of Mn1@C (52.4 mV dec−1), indicating that NH4Cl-addition promoted the Heyrovsky reaction during the HER and accelerated the reaction kinetics. As previously discussed, the decomposition of NH4Cl during the preparation of Mn1@NC generated abundant pores and a high specific surface area, which facilitated proton transfer and thereby improved the HER performance.
Stability test and the overall water splitting
Fig. 6(a) shows the stability of Mn1@C and Mn1@NC at a constant voltage. Both Mn1@C and Mn1@NC exhibited a trend of current decline during the initial testing stage, mainly due to the change in the chemical valence of Mn in Mn1@C and Mn1@NC during the initial power-on process. As the test progressed, the current of Mn1@C and Mn1@NC stabilized at a current retention rate of 94% and 96% following a 24 h stability assessment, respectively, indicating that both Mn1@C and Mn1@NC had good stability. As mentioned earlier, the polar functional groups in the lignin structure can form coordination bonds with Mn and stabilize Mn anchored in the carbon-based support, thereby avoiding the loss of Mn during application. Additionally, the doping of N enhanced the anchoring effect of the carbon-based support on the metal. Hence, Mn1@NC exhibited greater stability than Mn1@C. The LSV curves of Mn1@C and Mn1@NC after the stability test were also tested and are shown in Fig. 6(a). The LSV curve of Mn1@C-tested slightly shifted to a higher potential zone than Mn1@C as prepared, while the LSV curve of Mn1@NC C-tested was almost the same as for Mn1@NC as prepared. This result indicated the good stability of Mn1@NC and the N-doping enhanced the stability. | ||
| Fig. 6 (a) Stability test of Mn1@C and Mn1@NC; (b) LSV curves for overall water splitting using Mn1@NC‖Mn1@NC. | ||
Encouraged by the extraordinary activity of Mn1@NC for the HER and the OER in alkaline media, a two-electrode system was assembled in a 1.0 M KOH solution with Mn1@NC serving as both the cathode and anode (Mn1@NC‖Mn1@NC). The LSV curves for overall water splitting using Mn1@NC‖Mn1@NC are shown in Fig. 6(b). The device afforded the current densities of 10 mA cm−2 at cell voltages of 1.65 V, which outperforms NF‖NF (1.946 V).
The formation process of Mn1@NC and the real active site
Fig. 7 shows the XPS spectra of C 1s, O 1s, Mn 2p, and N 1s of Mn1@NC before test (a–d) and after test (e–h). The high-resolution XPS spectrum of C 1s was deconvoluted into three peaks at 284.8 eV, 286.1 eV, and 288.9 eV, corresponding to C–C (sp2 carbon), C–O–C (ether/alkoxy groups), and O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (carboxyl/ester groups), respectively. More O–C
O (carboxyl/ester groups), respectively. More O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O was detected on the surface of Mn1@NC after the test. Another two peaks observed in Fig. 7(e) were caused by the K adsorbed on the surface of Mn1@NC during the OER test. Three deconvoluted peaks at 531 eV, 532 eV and 533 eV, as shown in Fig. 7(b and f), were assigned to Mn–O, Mn–O–C, and C
O was detected on the surface of Mn1@NC after the test. Another two peaks observed in Fig. 7(e) were caused by the K adsorbed on the surface of Mn1@NC during the OER test. Three deconvoluted peaks at 531 eV, 532 eV and 533 eV, as shown in Fig. 7(b and f), were assigned to Mn–O, Mn–O–C, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O. There was a new deconvoluted peak assigned to defect O at 529.8 eV, as shown in Fig. 7(f), which was beneficial for the OER.47,48 The high-resolution XPS spectrum of Mn 2p1/2 in Fig. 7(c and g) was at 653.6 eV. The high-resolution XPS spectra of Mn 2p3/2 in Fig. 7(c) were well deconvoluted into five peaks at 638.1 eV, 641.1 eV, 642.8 eV, 644.6 eV and 646.9 eV, which were assigned to Mn0, MnO, Mn2O3, MnO2, and MnO.49,50 Mn exhibited multiple chemical oxidation states on the surface of Mn1@NC before the test. Obviously, Mn 2p3/2 of Mn1@NC after the stability test, as shown in Fig. 7(g), was mainly MnOOH, which worked as the real active site for the OER.20 Mn0 and MnO were oxidized to MnOOH. The N content on the surface of Mn1@NC reduced from 3.47 wt% (before test) to 1.52 wt% after the test, resulting from the N leaching out during the OER process. The N on the surface of Mn1@NC existed as graphitic N (401.1 eV), pyridinic N (399.8 eV) and pyrrolic N (398.8 eV) as assigned in Fig. 7(d and h).51 Among these N species, graphitic N was the main N component on the surface of Mn1@NC due to its higher area proportion. The graphitic N was reported to promote the electron transport with high electron-transfer capacity.51,52 The pyridinic N and pyrrolic N brought heteroatomic doping defects on the surface of Mn1@NC.53 The relative area proportion of pyrrolic N decreased, while the relative area proportion of pyridinic N increased, indicating that pyridinic N was more stable than pyrrolic N during the OER process.
O. There was a new deconvoluted peak assigned to defect O at 529.8 eV, as shown in Fig. 7(f), which was beneficial for the OER.47,48 The high-resolution XPS spectrum of Mn 2p1/2 in Fig. 7(c and g) was at 653.6 eV. The high-resolution XPS spectra of Mn 2p3/2 in Fig. 7(c) were well deconvoluted into five peaks at 638.1 eV, 641.1 eV, 642.8 eV, 644.6 eV and 646.9 eV, which were assigned to Mn0, MnO, Mn2O3, MnO2, and MnO.49,50 Mn exhibited multiple chemical oxidation states on the surface of Mn1@NC before the test. Obviously, Mn 2p3/2 of Mn1@NC after the stability test, as shown in Fig. 7(g), was mainly MnOOH, which worked as the real active site for the OER.20 Mn0 and MnO were oxidized to MnOOH. The N content on the surface of Mn1@NC reduced from 3.47 wt% (before test) to 1.52 wt% after the test, resulting from the N leaching out during the OER process. The N on the surface of Mn1@NC existed as graphitic N (401.1 eV), pyridinic N (399.8 eV) and pyrrolic N (398.8 eV) as assigned in Fig. 7(d and h).51 Among these N species, graphitic N was the main N component on the surface of Mn1@NC due to its higher area proportion. The graphitic N was reported to promote the electron transport with high electron-transfer capacity.51,52 The pyridinic N and pyrrolic N brought heteroatomic doping defects on the surface of Mn1@NC.53 The relative area proportion of pyrrolic N decreased, while the relative area proportion of pyridinic N increased, indicating that pyridinic N was more stable than pyrrolic N during the OER process.
 | ||
| Fig. 7 XPS spectra of (a) and (e) C 1s, (b) and (f) O 1s, (c) and (g) Mn 2p, and (d) and (h) N 1s of Mn1@NC before the stability test (a)–(d) and after the stability test (e)–(h). | ||
Above all, the formation process of Mn1@NC and the real active site for the OER process can be summarized as follows. First, Mn2+ underwent self-assembly with lignin due to the chelation–anchoring interaction between the polar oxygen-containing functional groups of lignin and Mn2+. The chelation–anchoring interaction permitted Mn2+ to be well dispersed in lignin carbon. Secondly, carbothermal reduction occurred, generating Mn0 during the in situ carbonization process. Simultaneously, due to the complex and highly reactive nature of lignin depolymerization products, redox reactions took place with Mn0 or Mn2+, forming Mn2O3, MnO2, or MnO. Among these MnOx species, MnO was the main MnOx species, and its aggregation nanoparticles dispersed in the lignin-based carbon support, while Mn2O3, MnO2, and Mn0 were primarily dispersed in an amorphous state on the Mn1@NC surface. NH4Cl was decomposed into HCl and NH3 during the co-carbonization of NH4Cl with Mn–lignin.24 The outgassing of HCl and NH3 acted as a pore-forming agent, regulating the carbonization process of the lignin-derived carbon, which resulted in the formation of a sheet-like porous carbon structure while concurrently achieving N-doped carbon. During the initial stage of the OER, the low-valent manganese species (Mn0 and MnO) in Mn1@NC underwent oxidation to Mn species with +3 valence. MnO2, as a common precursor of Mn-based OER catalysts, underwent reduction–reconstruction reactions to form Mn species with +3 valence in the OER test. The formed Mn species with +3 valence then combined with the hydroxide anion (OH−) in KOH solution to form a stable structure of MnOOH through proton transfer and coordination structure adjustment during the OER test. MnOOH was the real active species. OH− in KOH was first adsorbed on the Mn site of MnOOH forming adsorbed Mn3+–OH intermediates. Then, Mn3+–OH intermediates were oxidized to form Mn4+–O substances with the loss of one electron (e−) and one proton (H+). Two adjacent Mn4+–O intermediates underwent O–O bond coupling to form the Mn–O–O–Mn peroxide intermediate, which continued to undergo an oxidation reaction, losing electrons and adjusting its coordination structure. Finally, the Mn–O bond broke to release O2 molecules. Meanwhile, the Mn active sites were reduced to the initial +3 valence state, recombining with OH− in the electrolyte to enter the next catalytic cycle.20
Conclusions
This study successfully fabricated Mn-loaded lignin-derived carbon catalysts via an in situ anchoring and one-step carbonization strategy. Characterization studies confirmed that the in situ process enabled effective incorporation and uniform dispersion of Mn species (mainly MnO, Mn2O3, and MnO2), where MnO existed as aggregated nanoparticles and Mn2O3/MnO2 as amorphous phases. The Mn proportion had a significant impact on catalytic performance: the optimal Mn proportion was 1 mmol g−1 lignin, and Mn1@C exhibited the best OER and HER activity among Mnx@C samples, and had the lowest overpotential of 517 mV (OER) and 448 mV (HER) at 50 mA cm−2. Notably, NH4Cl modification played a dual role—serving as a pore-forming agent to construct a layered microporous structure (enhancing specific surface area and active site exposure) and a nitrogen source to realize N-doping (improving charge transfer efficiency). This modification enhanced the OER and HER activity of Mn1@NC, 299 mV (OER) and 409 mV (HER) at 50 mA cm−2, which outperformed both Mn1@C and Mn1@AC prepared by the conventional method. The comparison among OER performances of Mn1@NC, Mn1@C, Mn0@NC and Mn0@C indicated that the Mn-loading and NH4Cl-addition played a crucial synergistic role in enhancing the OER and HER performances for water electrolysis. Furthermore, Mn1@NC showed excellent long-term stability and enabled overall water-splitting to reach 10 mA cm−2 at 1.65 V using Mn1@NC‖Mn1@NC. This study simplified the preparation process of metal-loaded carbon-based electrode materials and provided a reference for the preparation and application of biomass carbon-based composite materials.Author contributions
Conceptualization: H. Y. and C. L.; methodology: H. Y. and C. L.; validation: M. Y., H. Y. and B. W.; formal analysis: B. W., H. Y. and M. Y.; data curation: M. Y., H. Y. and B. W.; writing – original draft preparation: H. Y.; writing – review and editing: H. Y. and C. L.; visualization: H. Y.; supervision: H. Y. and C. L.; funding acquisition: C. L. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
There are no conflicts of interest to declare.Data availability
The experiments, observations and data that support the findings of this study are included in this manuscript.Acknowledgements
This work was supported by the National Key R&D Program of China (2023YFA1507902), and the National Natural Science Foundation of China (U25A20332).References
- M. Liu, Z. Yao, J. Gu, C. Li, X. Huang, L. Zhang, Z. Huang and M. Fan, Chem. Eng. J., 2023, 461, 141918 CrossRef CAS.
- H. Yang, Z. Chen, K. Luo, M. Yu and Y. Zhang, New J. Chem., 2024, 48, 8690–8701 RSC.
- K. Qin, H. Yu, W. Zhu, Y. Zhou, Z. Guo, Q. Shao, Y. Wu, X. Wang, Y. Li, Y. Ji, F. Liao, Y. Liu, Z. Kang and M. Shao, Adv. Funct. Mater., 2025, 35, 2402226 CrossRef CAS.
- P. Salimi and M. M. Najafpour, Chem. – Eur. J., 2020, 26, 17063–17068 CrossRef CAS PubMed.
- S. Madadkhani, S. I. Allakhverdiev and M. M. Najafpour, New J. Chem., 2020, 44, 15636–15645 RSC.
- M. S. A. Akbari, S. Nandy, K. H. Chae, P. Aleshkevych and M. M. Najafpour, ACS Appl. Energy Mater., 2024, 7, 3299–3308 CrossRef CAS.
- W. Zhang, M. Liu, X. Gu, Y. Shi, Z. Deng and N. Cai, Chem. Rev., 2023, 123, 7119–7192 CrossRef CAS PubMed.
- Y. Feng, H. Yang, X. Wang, C. Hu, H. Jing and J. Cheng, Int. J. Hydrogen Energy, 2022, 47, 17946–17970 CrossRef CAS.
- H. Wang, K. H. L. Zhang, J. P. Hofmann, V. A. Pena OShea and F. E. Oropeza, J. Mater. Chem. A, 2021, 9, 19465–19488 RSC.
- X. Qiao, X. Yin, L. Wen, X. Chen, J. Li, H. Ye, X. Huang, W. Zhao and T. Wang, Chem, 2022, 8, 3241–3251 CAS.
- M. Farajzadeh and F. R. Rahsepar, ChemElectroChem, 2024, 11, e202300516 CrossRef CAS.
- X. Huo, H. Yu, B. Xing, X. Zuo and N. Zhang, Chem. Rec., 2022, 22, e202200175 CrossRef CAS PubMed.
- X. Song, C. Fan, Y. Tang, Y. Ren, Z. Zang, L. Li, X. Yu, Z. Lu, X. Yang and X. Zhang, Fuel, 2025, 381, 133558 CrossRef CAS.
- M. Gharedaghloo and M. M. Najafpour, ACS Appl. Energy Mater., 2024, 7, 10081–10091 CrossRef CAS.
- M. Huynh, C. Shi, S. J. L. Billinge and D. G. Nocera, J. Am. Chem. Soc., 2015, 137, 14887–14904 CrossRef CAS PubMed.
- H. Tian, L. Zeng, Y. Huang, Z. Ma, G. Meng, L. Peng, C. Chen, X. Cui and J. Shi, Nano-Micro Lett., 2020, 12, 161 CrossRef CAS PubMed.
- Y. Wang, Y. Zhang, G. Gao, Y. Fan, R. Wang, J. Feng, L. Yang, A. Meng, J. Zhao and Z. Li, Nano-Micro Lett., 2023, 15, 219 CrossRef CAS PubMed.
- X. Zhai, X. Wang, X. Pang, J. Zhang, Q. Wu, W. Na, Y. Zhou and L. Tian, J. Taiwan Inst. Chem. Eng., 2021, 126, 383–391 CrossRef CAS.
- Y. Zhang, Z. Liu, F. Guo, M. Li and X. Bo, J. Colloid Interface Sci., 2022, 618, 149–160 CrossRef CAS PubMed.
- P. Wang, S. Zhang, Z. Wang, Y. Mo, X. Luo, F. Yang, M. Lv, Z. Li and X. Liu, J. Mater. Chem. A, 2023, 11, 5476–5494 RSC.
- L. Tian, X. Zhai, X. Wang, J. Li and Z. Li, J. Mater. Chem. A, 2020, 8, 14400–14414 RSC.
- Y. Hu, B. Shen, W. Liu, Y. Pan, J. Huang, X. Zhu, Z. Guo, Y. Xiao, J. Cheng and J. Qu, J. Alloys Compd., 2025, 1010, 178251 CrossRef CAS.
- G. Zhang, B. Hu, Y. Luo, Y. Xie, Y. Chen, Y. Zhang, Y. Ling, J. Zou and Y. Shao, Int. J. Hydrogen Energy, 2024, 53, 1226–1232 CrossRef CAS.
- A. Muzammil, R. Haider, W. Wei, L. Li, L. Wu, Y. Fan and X. Yuan, ACS Appl. Mater. Interfaces, 2025, 17, 22812–22821 CrossRef CAS PubMed.
- X. F. Lu, Y. Chen, S. Wang, S. Gao and X. W. Lou, Adv. Mater., 2019, 31, 1902339 CrossRef PubMed.
- S. Chandrasekaran, E. Arumugam, S. Ramasamy, C. Karuppiah, S. Bhaskaran, C. C. Yang, D. Nallapandi and K. Palanisamy, Int. J. Hydrogen Energy, 2023, 48, 10423–10437 CrossRef CAS.
- J. Liu, J. Zhang, H. Zhou, B. Liu, H. Dong, X. Lin and Y. Qin, J. Colloid Interface Sci., 2023, 629, 822–831 CrossRef CAS PubMed.
- L. Tian, J. Wang, K. Wang, H. Wo, X. Wang, W. Zhuang, T. Li and X. Du, Carbon, 2019, 143, 457–466 CrossRef CAS.
- S. Sangeetha, G. Krishnamurthy, S. Foro and K. Raj, J. Inorg. Organomet. Polym, 2020, 30, 4792–4802 CrossRef CAS.
- N. Zhang, P. Cui, J. Zhang and Y. Qiao, Catalysts, 2025, 15, 579 CrossRef CAS.
- W. Li, G. Wang, W. Zhang, J. Li, B. Zhang and C. Si, Ind. Crops Prod., 2023, 204, 117342 CrossRef CAS.
- K. L. A. Cao, Y. Kitamoto, F. Iskandar and T. Ogi, Adv. Powder Technol., 2021, 32, 2064–2073 CrossRef CAS.
- F. Chen, W. Zhou, H. Yao, P. Fan, J. Yang, Z. Fei and M. Zhong, Green Chem., 2013, 15, 3057–3063 RSC.
- Z. Chen, H. Lai, H. Zhuo, Y. L. Zhong, L. Zhong and X. Peng, Collagen Leather, 2023, 5, 15 CrossRef.
- Z. Cheng, Z. Li, S. Huang, J. Pan, J. Mei, S. Zhang, X. Peng, W. Lu and L. Yan, Catalysts, 2025, 15, 503 CrossRef CAS.
- T. Li, B. Chen, M. Cao, X. Ouyang, X. Qiu and C. Li, AIChE J., 2023, 69, e17877 CrossRef CAS.
- W. Zhang, J. Yin, C. Wang, L. Zhao, W. Jian, K. Lu, H. Lin, X. Qiu and H. N. Alshareef, Small Methods, 2021, 5, 2100896 CrossRef CAS PubMed.
- H. M. Yang, S. Apparia, S. Kudo, J.-I. Hayash and K. Norinaga, Ind. Eng. Chem. Res., 2015, 54(27), 6855–6864 CrossRef CAS.
- W. Chen, Y. Chen, H. Yang, K. Li, X. Chen and H. Chen, Bioresour. Technol., 2018, 249, 247–253 CrossRef CAS PubMed.
- W. Chen, K. Li, M. Xia, Y. Chen, H. Yang, C. Zhiquan, X. Chen and H. Chen, Bioresour. Technol., 2018, 263, 350–357 CrossRef CAS PubMed.
- Z. Wu, L. Hu, Y. Jiang, X. Wang, J. Xu, Q. Wang and S. Jiang, Biomass Convers. Biorefin., 2023, 13, 519–539 CrossRef.
- Y. Chen, Y. Jiang, D. Tian, J. Hu, J. He, G. Yang, L. Luo, Y. Xiao, S. Deng, O. Deng, W. Zhou and F. Shen, Int. J. Biol. Macromol., 2020, 164, 3038–3047 CrossRef CAS PubMed.
- Y. Gorlin and T. F. Jaramillo, J. Am. Chem. Soc., 2010, 132, 13612–13614 CrossRef CAS PubMed.
- J. Wang, W. Zang, X. Liu, J. Sun, S. Xi, W. Liu, Z. Kou, L. Shen and J. Wang, Small, 2024, 20, 2309427 CrossRef CAS PubMed.
- F. Bao, E. Kemppainen, I. Dorbandt, R. Bors, F. Xi, R. Schlatmann, R. van de Krol and S. Calnan, ChemElectroChem, 2021, 8, 195–208 CrossRef CAS.
- C. Wan, Y. Ling, S. Wang, H. Pu, Y. Huang and X. Duan, ACS Cent. Sci., 2024, 10, 658–665 CrossRef CAS PubMed.
- Y. Wu, C. Guo, R. Yao, K. Zhang, J. Li and G. Liu, Adv. Funct. Mater., 2024, 34, 2410193 CrossRef CAS.
- X. Chen, R. Ma, W. Jia, X. Cao, J. Zhang, F. Cheng and L. Jiao, Chem Catal., 2025, 5, 101335 CAS.
- M. C. Biesinger, B. P. Payne, A. P. Grosvenor, L. W. M. Lau, A. R. Gerson and R. S. C. Smart, Appl. Surf. Sci., 2011, 257, 2717–2730 CrossRef CAS.
- E. S. Ilton, J. E. Post, P. J. Heaney, F. T. Ling and S. N. Kerisit, Appl. Surf. Sci., 2016, 366, 475–485 CrossRef CAS.
- G. Qu, P. Jia, S. Tang, M. N. Pervez, Y. Pang, B. Li, C. Cao and Y. Zhao, J. Hazard. Mater., 2024, 461, 132626 CrossRef CAS PubMed.
- T. Marshall-Roth, N. J. Libretto, A. T. Wrobel, K. J. Anderton, M. L. Pegis, N. D. Ricke, T. V. Voorhis, J. T. Miller and Y. Surendranath, Nat. Commun., 2020, 11, 5283 CrossRef CAS PubMed.
- M. Liu, M. Liu, W. Chen, F. Li, S. Cai, S. J. Lin, X. Chen and Z. Cai, Angew. Chem., Int. Ed., 2025, 64, e202421755 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2026 |

