Interfacial charge transfer-mediated Fermi level pinning in MBE-grown 2D 2H-MoSe2/2H-MoTe2 heterostructures
Kamlesh
Bhatt
 ,
Santanu
Kandar
,
Lipika
,
Ashok
Kapoor
and
Rajendra
Singh
,
Santanu
Kandar
,
Lipika
,
Ashok
Kapoor
and
Rajendra
Singh
 *
*
Department of Physics, Indian Institute of Technology Delhi, Hauz Khas, New Delhi 110016, India. E-mail: rsingh@physics.iitd.ac.in
First published on 11th November 2025
Abstract
MoSe2 and MoTe2 based heterostructures, owing to their remarkable photoresponsivity and tunable electrical characteristics, have emerged as promising candidates for field-effect transistors (FETs) and near-infrared (NIR) optoelectronic applications. However, the contributions of different interfacial processes impose limitations on the band tunability and carrier dynamics of the heterostructure, posing challenges in their device engineering. In this work, we present the scalable, layer-by-layer growth of a trilayer MoSe2/MoTe2 heterostructure over a SiO2 substrate via molecular beam epitaxy (MBE). By leveraging the tunable probing depth of AR-XPS, we successfully resolve the interfacial bonding modifications, such as Te migration across the interface and localized Mo–Se–Te bonding. Our investigations show that these site-specific processes at the interface induce asymmetric energy level shifts, Fermi level pinning, and modulation of the valence band edge. Consequently, deviations from predicted band alignment are observed, with the Fermi level pinned around 0.58 eV above the valence band edge on the MoTe2 side and the anomalous upshift of the valence band maximum of MoSe2 in the heterostructure. These interfacial effects also result in a reduced barrier for hole injection, which can improve bidirectional carrier transport and gate-tunable hole conduction in such heterostructure-based devices. The findings highlight the critical role of interfacial interactions in governing band alignment of the ultrathin transition metal dichalcogenide (TMDC) heterostructures, providing key insights for advancing nanoelectronic and optoelectronic devices through heterostructure band engineering.
New conceptsThis work introduces the concept that localized interfacial charge transfer, beyond conventional interlayer charge transfer, plays a dominant role in redefining band alignment and electronic structure in TMDC van der Waals heterostructures. Unlike previous studies that predominantly focus on band offsets based on interlayer charge redistribution, our findings reveal that site-specific processes at the interface, such as Te migration and localized Mo–Se–Te bonding, can induce asymmetric energy level shifts, Fermi level pinning, and modulation of valence band edge. These effects challenge simplified band alignment models, highlighting the importance of interfacial stoichiometry and site-specific bonding in determining device properties. In addition to the material-level insights, this work also presents a novel, non-destructive approach for probing buried interface physics using angle-resolved X-ray photoelectron spectroscopy (AR-XPS). By leveraging the tunable probing depth of AR-XPS, we successfully deconvolute interfacial contributions from bulk signals. This is traditionally achievable only via cross-sectional TEM or XPS depth-profiling; however, the former is highly localized and involves complex, potentially damaging sample preparation, while the latter results in selective etching of chalcogen atoms in TMDCs. This combination of scalable heterostructure growth with interface-specific probing offers a platform for studying buried interfaces and opens new possibilities in nanoscale device engineering. |
1. Introduction
The exploration of advanced heterostructures based on two-dimensional (2D) transition metal dichalcogenides (TMDCs) has emerged as an important area of research to leverage the distinct characteristics of individual TMDC materials for achieving novel functionalities.1–4 These exciting heterostructure properties extend beyond conventional optical and electrical characteristics, including novel quantum phenomena such as emergent quantum states and excitonic dynamics, which are essential for innovative applications in quantum computing and the development of high-efficiency optoelectronic devices.5–7 MoSe2/MoTe2 heterostructures show great promise in flexible field-effect transistor (FET) devices and near-infrared (NIR) applications owing to their remarkable photoresponsivity and tunable electrical properties, while also laying the groundwork for advancements in quantum technologies.8–16 Both MoSe2 and MoTe2 exhibit band absorption in the NIR-to-visible range.17,18 While MoSe2 exhibits strong excitonic effects with a higher bandgap and better environmental stability, MoTe2 is characterized by strong spin–orbit coupling and higher electron mobility.19–23 In addition, MoTe2 possesses the advantage of an electrically ambipolar nature, enabling broad tunability of its Fermi level across the bandgap for optimizing the heterostructure's properties.24,25 Together, these materials can enable a heterostructure with type-II band alignment, which facilitates efficient spatial separation and transport of carriers, essential for high-performance optoelectronic devices.The interface in such layered heterostructures plays an important role in determining the overall heterostructure properties, as it facilitates crucial interfacial charge transfer processes that can drastically affect the device performance.26 Despite the promise of TMDC heterostructures for high-performance FETs and NIR optoelectronics, a major challenge lies in understanding and controlling the role of these interfacial interactions in determining their electronic band alignment. As a result, experimentally observed band alignments and carrier dynamics often deviate from conventional interlayer charge transfer (ILCT) models. These discrepancies arise from atomically localized interfacial processes, such as interfacial charge redistribution, electronic hybridization from localized bonding modifications, vacancy defect formation and twist-angle-dependent interlayer coupling, which critically alter the local electronic structure.27–31 Such interfacial interactions introduce complexities in heterostructure band tunability, affecting carrier dynamics and recombination kinetics.32 Previous studies have demonstrated electric-field-induced band alignment transitions in MoSe2/WS2 using a photoluminescence (PL) study33 and ultrafast charge transfer in WS2/MoSe2 heterostructures through pump–probe spectroscopy.34 However, the techniques are limited in resolving atomic-scale interfacial bonding changes and lack sufficient depth sensitivity to probe buried interfaces. The lack of understanding of interfacial processes leads to ambiguities in controlling heterostructure characteristics through band engineering, thereby limiting their potential for advanced device applications.
The present study aims to address this gap by providing insights into the interfacial interactions and band modifications in TMDC heterostructures by providing quantitative band alignment determination coupled with depth-resolved bonding analysis. Our investigation on MoSe2/MoTe2 heterostructures reveals how atomic-scale interfacial interactions and bonding modifications, coupled with interlayer charge transfer, govern band alignment and carrier injection barriers in these 2D heterostructures.
To achieve this, we employ scalable, layer-by-layer growth of the MoSe2/MoTe2 heterostructure with controlled trilayer thicknesses on SiO2 substrates using MBE, combined with scanning Kelvin probe force microscopy, ultraviolet photoelectron spectroscopy, and X-ray photoelectron spectroscopy. By leveraging the tunable probing depth of AR-XPS, we successfully resolve the interfacial bonding modifications and observe that Te migration and localized Mo–Se–Te bonding near the interface induce asymmetric energy level shifts, Fermi level pinning, and valence band edge modulation. This results in an experimental heterostructure band alignment that deviates substantially from predictions based on interlayer charge transfer (ILCT) models, with a reduced valence band offset (VBO) in the heterostructure, which can be favorable for enhanced hole injection.
2. Experimental section
2.1. Thin film growth
The two-dimensional MoSe2 and MoTe2 thin films were grown on SiO2/Si substrates using the molecular beam epitaxy technique with a 2D MBE system from Vinci Technology, France, equipped with an in situ reflection high-energy electron diffraction (RHEED) characterization facility. The detailed description of the system and pre-growth calibration have been reported elsewhere previously.35 Before loading the substrate into the MBE chambers, it was ultrasonicated in acetone, IPA and DI water. The substrate was pre-annealed before growth for one hour at 700 °C in a separate UHV chamber to desorb surface impurities. To avoid the cross-contamination of Se and Te, the sample puck was transferred from the growth chamber to the storage chamber after the first material of the heterostructure had been grown and the growth chamber was degassed thoroughly. A small piece of sapphire substrate was clipped on top of the first deposited material as a mask to create a lateral heterojunction for measuring film thickness. The growth temperatures were chosen as 450 °C and 350 °C for MoTe2 and MoSe2 film growth, respectively. The beam equivalent pressures (BEP), a measure of the evaporating elemental flux, for Te and Se were 4.2 × 10−7 mbar and 2.3 × 10−7 mbar, respectively. Since the growth rate is solely determined by the metal flux in TMDCs, the Mo flux was varied to achieve the desired growth rates. The growth rates of ∼40 min/layer and ∼12 min/layer were obtained at the e-beam emission current of 68 mA and 75 mA for MoTe2 and MoSe2, respectively. These lower growth rates are essential to achieve low roughnesses of surface and interfaces, as the slower rates provide sufficient time for Mo adatoms to coalesce properly in the lateral directions. The growth temperature at these particular flux rates was optimized to achieve the layer-by-layer growth modes, which is critical to realize sharp interface heterostructures by surpassing island formation during the growth. For MoSe2, the layer-by-layer growth was achieved comparatively easily over a wider range of growth temperatures, while for MoTe2, it was achieved for the growth temperature ranging from 380 °C to 480 °C only. Outside this temperature regime, the RHEED oscillations could not be obtained for MoTe2. The thickness of each material was specifically chosen to be three unit layers because (1) only bilayer or thicker heterostructures demonstrate band quantization phenomena in 2D materials which are absent in monolayers, (2) the unique 2D characteristics of TMDCs are distinctive only below 4–5 layers and tend towards bulk behavior exceeding this thickness, and (3) the inversion symmetry is broken in the odd number of layers of TMDCs in their 2H phase which is crucial for spin-valley coupled effects36 and non-linear optical effects such as single harmonic generation.372.2. Material characterization
The grown films were characterized during the growth using RHEED (Staib Instruments) to investigate the crystalline quality and layer numbers by observing RHEED diffraction patterns and intensity oscillations. The large area homogeneity was confirmed by taking images of the grown films and heterostructure using the LEICA M125 C microscope at different magnifications. The high-resolution height-sensor images were taken using Atomic Force Microscopy (Bruker Dimension Icon XR) in tapping mode using a TESPA-V2 probe (tip radius ∼7 nm). Raman spectroscopy was performed using a Renishaw inVia Raman spectrometer in backscattering geometry with a 532 nm laser operated at 10 mW power. Raman mapping was performed over a 20 × 20 μm2 area across the lateral edge of the MoSe2/MoTe2 heterostructure, but with a lower power of 5 mW to avoid film damage due to longer laser exposures.2.3. Charge-transfer-study experiments
The scanning Kelvin probe force microscopy (SKPFM) measurements were performed with the same AFM instrument in the amplitude modulation mode using a conductive platinum–iridium coated probe (tip radius ∼5 nm). The work function calibration of the tip was done using a freshly exfoliated HOPG substrate. X-ray photoelectron spectroscopy was performed using an Axis-Supra from Kratos Analytical, equipped with a monochromatic Al Kα (1486.6 eV) source, using a step size of 0.05 eV and a pass energy of 20 eV for acquiring the core level spectra. A conducting path was established from the film surface to the sample stage using conductive carbon tape to avoid a charging effect at the film surface. To investigate the interfacial interactions, angle-resolved XPS (AR-XPS) measurements were performed, in which the angle between the source and the analyzer remained fixed while the stage was rotated in the source–sample–analyzer plane to change the emission angle. The acquired data were fitted and analyzed using the CasaXPS software.38 The binding energy scale was calibrated with the Fermi edge position of gold. To ensure the physical significance of the fitting and avoid the false assignment of components, the peak-area ratio of the two spin–orbit components was kept nearly fixed (e.g., 3d5/2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3d3/2 = 3
3d3/2 = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). The film stoichiometry was determined using the relative sensitivity factor (RSF)-corrected peak areas of different components. The RSF values were imported from the Kratos library as 3.32, 9.51 and 0.85 for Mo 3d, Te 3d and Se 3d, respectively. Ultraviolet photoelectron spectroscopy (UPS) measurements were performed with the same XPS system for acquiring valence band spectra using a He I source (with an energy of 21.22 eV), as the technique provides a greater photoionization cross-section for valence band states with its low photon energy and a much better energy resolution.39 The measurements have been performed with a collimation setting of 27 μm aperture size and a pass energy of 5 eV, with a step size of 0.05 eV, while the sweep times were chosen to achieve the count rates of <15 kcps around the valence band edge. The precise value of the valence band edge was calculated by fitting the VB edge using the Edge Down function in the CasaXPS software.
2). The film stoichiometry was determined using the relative sensitivity factor (RSF)-corrected peak areas of different components. The RSF values were imported from the Kratos library as 3.32, 9.51 and 0.85 for Mo 3d, Te 3d and Se 3d, respectively. Ultraviolet photoelectron spectroscopy (UPS) measurements were performed with the same XPS system for acquiring valence band spectra using a He I source (with an energy of 21.22 eV), as the technique provides a greater photoionization cross-section for valence band states with its low photon energy and a much better energy resolution.39 The measurements have been performed with a collimation setting of 27 μm aperture size and a pass energy of 5 eV, with a step size of 0.05 eV, while the sweep times were chosen to achieve the count rates of <15 kcps around the valence band edge. The precise value of the valence band edge was calculated by fitting the VB edge using the Edge Down function in the CasaXPS software.
2.4. Theoretical calculations
First-principles calculations based on density functional theory (DFT) were performed using the codes of the Vienna ab initio simulation package (VASP). The projector augmented wave (PAW) approach was used to describe the interaction of core valence electrons.40 The exchange and correlation energies were considered in the generalized gradient approximation following the Perdew–Burke–Ernzerhof (PBE) scheme.41 The energy cut-off was set to 500 eV with the Γ-centered k-point mesh of 8 × 8 × 1. Spin–orbit coupling (SOC) effects were considered to capture the electronic structure accurately. All atomic positions were relaxed until forces were below 0.01 eV Å−1, with the electronic self-consistency criterion set to 10−6 eV. The density of states (DOS) calculations were computed using the tetrahedron method to analyze the electronic structure modifications induced by vacancy defects. A 3 × 3 × 3 supercell was employed to model the atomic environment and to avoid interactions between periodic images in the out-of-plane (z) direction, and a vacuum layer of 20 Å was introduced in the simulation cell. The crystal structures of MoSe2 and MoTe2 were obtained from the Crystallography Open Database (COD).42,433. Results and discussion
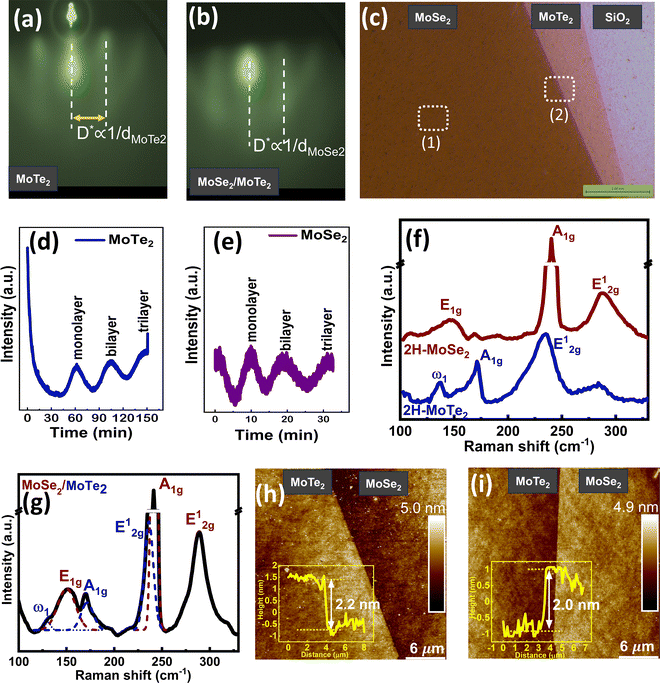
3.1. Layer-by-layer growth of vertical heterostructures (trilayer MoSe2/MoTe2)
The growth of thin films was carried out using the molecular beam epitaxy technique to achieve a high-quality film with precise control over the layer thickness and film stoichiometry. Since controlling the growth mode is crucial for fabricating sharply interfaced vertical heterostructures, the individual growth temperatures and metal fluxes were optimized for both materials (given in the Experimental section) to achieve a layer-by-layer growth mode, as evident from the observation of oscillations in the RHEED intensity (Fig. 1d and e). Each maximum in the intensity oscillations represents the completion of a single-layer growth, thus confirming a precise thickness-controlled growth.44 We have observed that achieving the layer-by-layer growth mode for MoTe2 is much more challenging than for MoSe2. The Mo flux can be adjusted over a wide range to achieve the required growth rate (from 3 minutes per layer to 45 minutes per layer); however, there lies only a narrow window of the growth temperature within which layered growth of the 2H phase of MoTe2 is achieved (refer to the Experimental section).The streaky RHEED pattern in Fig. 1a and b indicates a crystalline growth with a flat surface for both MoTe2 and MoSe2. Since the in-plane lattice constants for the 2H phase of MoSe2 and MoTe2 have nearly the same values (aMoTe2 = 0.352 nm and aMoSe2 = 0.332 nm)45,46 within the FWHM limits of the observed RHEED streaks (∼0.092 nm), we obtain similar streak spacing (D*) for the new set of streaks after the heterostructure formation. Also, the diffraction pattern remains the same for all azimuthal angles, corresponding to the random in-plane orientations of the grown crystalline domains. Fig. 1c shows the optical image taken at the hetero-edge of the uniformly grown MoSe2/MoTe2 heterostructure on SiO2. It is also challenging to achieve a single phase for MoTe2 due to a small energy difference between its two phases (∼40 meV); consequently, it often results in a mixed 2H-1T′ phase.47,48 The Raman spectra of the individual materials and heterostructure further confirm the crystallographic phase purity of the two materials (Fig. 1f and g). The phonon modes around 136 cm−1 (ω1), 171 cm−1 (A1g) and 234 cm−1 (E12g) correspond to the 2H phase of MoTe2, while those around 151 cm−1 (E1g), 241 cm−1 (A1g) and 288 cm−1 (E12g) correspond to the 2H phase of MoSe2.49,50 Furthermore, Raman mapping is performed across the lateral hetero-edge of the MoSe2/MoTe2 heterostructure, and multipoint Raman spectra are acquired from multiple locations across the heterostructure, which confirm the large-area phase uniformity of the grown heterostructure (SI S1). The film thicknesses in the heterostructures are reconfirmed ex situ using AFM by drawing height profiles at the sharp hetero step-edge (Fig. 1h and i). The AFM images, as shown in SI Fig. S2, further confirm the smooth surfaces of the grown films in the heterostructure with the rms roughness of 0.28 nm and 0.33 nm for MoTe2 and MoSe2, respectively. The obtained results from our MBE-grown heterostructures, characterized by large-area uniformity, layer-by-layer growth with precise trilayer thickness control, low surface roughness and consistent phase purity in the 2H phase, address the common challenges reported for CVD-grown TMDC films and heterostructures, including higher surface roughnesses (0.6–1.7 nm),51,52 the coexistence of multiple phases in MoTe2,53 partial film coverage and spatial non uniformity.54–56 Also, our MBE growth at significantly lower growth temperatures (350–450 °C) under UHV conditions, compared to high-temperature CVD processes (>650 °C),51 ensures reduced thermal stress and provides better control over phase formation.
3.2. Inter-layer charge transfer study: SKPFM and valence band spectra
The scanning Kelvin probe force microscopy (SKPFM) measurements were performed to calculate the work functions of the individual materials (Fig. 2a and b) and heterostructures (Fig. 2c and d). SKPFM maps the surface potential by measuring the contact potential difference (VCPD) between the tip and the sample locally, which is then used to calculate the work function of the material with the following equation:| ϕsample = ϕtip − eVCPD, |
| VBO = ϕ(MoSe2) + VBM(MoSe2) − ϕ(MoTe2) − VBM(MoTe2) |
| CBO = ϕ(MoSe2) + VBM(MoSe2) − Eg(MoSe2) − [ϕ(MoTe2) + VBM(MoTe2) − Eg(MoTe2)] |
The heterostructure band alignment is proposed based on the conventional interlayer charge transfer (ILCT) theory, as shown in Fig. 3b, in which the electronic charge is transferred in the heterostructure from MoTe2 (with a lower work function) to MoSe2 (with a higher work function). The heterostructure possesses a type-II band alignment with a valence band offset (VBO) of 0.64 eV and a conduction band offset (CBO) of 0.24 eV. Due to this interlayer charge transfer across the interface, the MoTe2 side of the interface becomes partially positive while the MoSe2 side becomes negative, which leads to interface dipole formation. The interface dipole in such heterostructures creates a local potential gradient across the interface (Δϕ) from the MoTe2 to the MoSe2 side, lowering the vacuum level of MoTe2 relative to MoSe2. The work function of the heterostructure that we obtain using SKPFM will be a combined effect of the intrinsic material and the interface dipole. These ILCT-driven changes in different energy levels are indicated qualitatively in Fig. 3b, where Δϕ1 and Δϕ2 are the interfacial dipole-induced shifts in different energy levels of MoSe2 and MoTe2, respectively.
The SKPFM measurements were carried out around two positions in the heterostructures: (1) away from the hetero step-edge and (2) on and near the hetero step-edge (refer to Fig. 1c). The SKPFM measurements on the vertical heterostructure (Fig. 2c and d), which were performed far from the hetero-edge, show that the interface dipole effectively changes the work function of MoSe2 to 4.84 ± 0.01 eV from 4.99 ± 0.01 eV for pristine MoSe2 (Fig. 2e). To investigate the ILCT effect on MoTe2, we performed SKPFM measurement on the reverse order-stacked MoTe2/MoSe2 heterostructure (Fig. 2d). The work function of MoTe2 after the heterostructure formation remains nearly unchanged (4.72 ± 0.01 eV), which contradicts ILCT predictions according to which the transferred electronic charge from MoTe2 should lead to an increase in its work function (as shown in Fig. 3b). This indicates that the Fermi level is pinned on the MoTe2 side in the heterostructure.
Furthermore, the measurements were performed on the hetero step-edge of the MoSe2/MoTe2 heterostructure over a 30 × 30 μm2 area to observe the lateral charge transfer effects in the heterostructure (Fig. 2f). Interestingly, the work function of MoTe2 near the lateral hetero-edge increases to 4.76 ± 0.01 eV with a decrease in MoSe2's work function to 4.80 ± 0.01 eV. The same is observed in the reverse-stacked MoTe2/MoSe2 heterostructure with work functions of MoSe2 and MoTe2 being 4.91 ± 0.01 eV and 4.77 ± 0.01 eV, respectively (Fig. 2g). This significant change in the work function of MoTe2 demonstrates that the previously observed Fermi level pinning is not an intrinsic property of the grown MoTe2 film itself, but rather results from some other charge distribution processes in the vertical heterostructure, which makes it insensitive to the direct ILCT effects. Furthermore, as we move away from the step-edge on both sides for the measurements, these surface potential and work function values gradually approach those obtained in Fig. 2a–d for pristine materials and vertical heterostructures.
To further investigate the charge transfer process in the MoSe2/MoTe2 heterostructure, the valence band spectra were recorded using UPS. Fig. 4 shows the high-resolution UPS spectra acquired around the valence band edge for the individual materials (Fig. 4a) and for the heterostructure materials (Fig. 4b). The VBM values were measured precisely by fitting the valence band edge using the Edge Down function (SI S3). The valence band edge lies at 0.58 ± 0.05 eV for MoTe2 and at 0.96 ± 0.05 eV for MoSe2 with respect to the Fermi level. In the heterostructure, the valence band edge of MoTe2 does not show any significant shift and lies at 0.56 ± 0.05 eV, which follows its work function change, as previously measured using SKPFM and further confirms that the Fermi level of MoTe2 remains pinned. In the case of MoSe2, the VBM value decreases to 0.88 ± 0.05 eV in the heterostructure. It may be mentioned here that the ILCT process decreases the work function of MoSe2 in the heterostructure (Fig. 2e), which means the Fermi level moves away from the valence band and other core levels. However, this decrement in the energy difference between the VBM and the Fermi level is anomalous, as it contradicts the KPFM results. This reduction in the energy difference may be due to the upshift of the VBM itself.
In the following sections, we investigate the underlying charge redistribution mechanisms for (a) the pinning of the Fermi level of MoTe2 and (b) the abnormal upward shift of the VBM of MoSe2 in the MoSe2/MoTe2 vertical heterostructure.
3.3. Origin of different charge transfer processes: XPS core level spectra and energy shifts
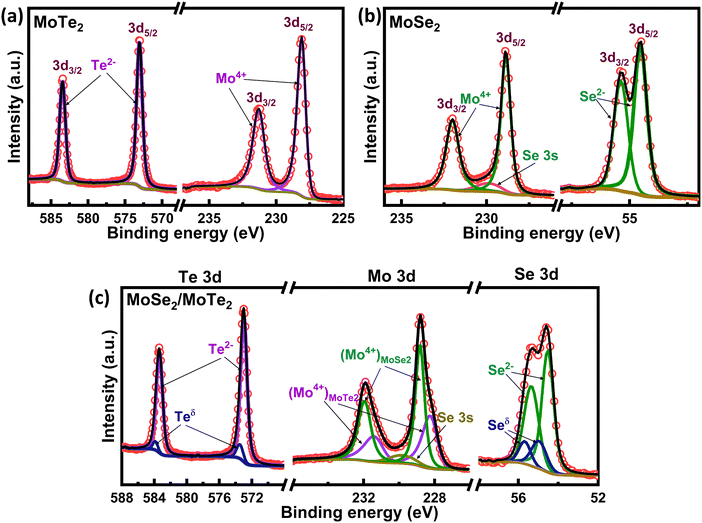
To understand these charge transfer processes in the heterostructure, which lead to the anomaly in the different energy levels, we studied XPS core level spectra of the constituent elements in the pristine materials and the heterostructure under surface normal emission mode (Fig. 5). It is emphasized that it is the energy of a core level from the valence band edge, not the one measured from the Fermi level, that remains constant in the absence of any change in the chemical environment of the element. However, in XPS, the binding energy of a core-level electron is measured from the Fermi level. So, any change in the Fermi level position, as represented by its work function, should also be reflected in the core level binding energy shift. The Mo 3d, Te 3d and Se 3d core levels are taken as the representative core levels in MoTe2 and MoSe2. The spectra are deconvoluted to obtain the contribution of different bonding states of an element in the material (Fig. 5). SI Table S2 includes the binding energy peak position and various fitting parameters corresponding to a particular bonding state.In MoTe2, Mo gets bonded in its 4+ oxidation state while Te is in its 2− oxidation state.57 The Mo 3d core level (Fig. 5a) shows two spin–orbit-splitted peaks corresponding to the Mo–Te bond at 228.32 ± 0.05 eV (Mo 3d5/2) and 231.51 ± 0.05 eV (Mo 3d3/2). Similarly, the Te 3d spectrum contains two peaks at 573.15 ± 0.05 eV (Te 3d5/2) and 583.43 ± 0.05 eV (Te 3d3/2) corresponding to the Mo–Te bond in MoTe2. In MoSe2 (Fig. 5b), the Mo 3d core level overlaps with the Se 3s core level. The spectrum shows the peaks corresponding to the Mo–Se bond at 228.87 ± 0.05 eV and 232.07 ± 0.05 eV, which are at slightly higher binding energies than those for Mo–Te. This reflects a stronger interatomic bonding in MoSe2 than in MoTe2. The additional component around 229.85 ± 0.05 eV is contributed from Se 3s core level electrons. The Se 3d spectrum shows 3d5/2 and 3d3/2 components corresponding to the Mo–Se bond at 54.52 ± 0.05 eV and 55.34 ± 0.05 eV, respectively.
The changes in the binding energies and relative contribution of these different bonding states in the heterostructure strongly depend on the competition between the different charge transfer processes between the two materials (Fig. 5c). The peak corresponding to the Mo–Te bonding state in the Mo 3d spectrum of the heterostructure shows a shift of 0.05 eV towards the lower binding energy (refer to SI Table S2). Although the shift can be considered insignificant within the measurement uncertainty limits, the small reduction in the oxidation state of Mo may also arise from the change in Te interactions around Mo atoms, which is discussed in a later section. For Te 3d, no significant shift is observed in the Mo–Te peak, which aligns well with its VBM changes; however, an additional pair of bonding states (Teδ) is observed at a higher binding energy (573.46 eV), leading to an asymmetric peak shape. Since the additional contribution appears at much lower energy than the 4+ oxidation state of Te in TeO2 (which usually occurs at ∼576.60 eV58), this cannot be assigned to the oxide contribution of tellurium. Therefore, this contribution suggests the presence of another type of bonding of Te with an oxidation state (4+) > δ > (2−).
The peak corresponding to the Mo–Se bond in both Mo 3d and Se 3d spectra shows a shift (∼0.12 eV) towards higher binding energy, which aligns well with its decreased work function. Additionally, the Se 3d spectrum shows a slightly different convoluted peak shape in the heterostructure, which can be fitted if the additional pair of components is introduced at a higher binding energy. These energy shifts in the core level and the work function both confirm that the ILCT process is dominant in the heterostructure, making MoSe2 more n-type. However, the energy difference between the Mo 3d core level and the VBM increases from 228.10 eV in MoSe2 (Fig. 3a) to 228.30 eV in the heterostructure (Fig. 6d). This further confirms the upward shift in the VBM of MoSe2 towards the Fermi level. This shift can possibly arise due to the redistribution of the states near the valence band edge of MoSe2. Since the additional bonding state contributions are observed in both Se and Te core levels, there is a possibility of bonding between Se and Te due to a site-to-site charge transfer leading to this modification in the valence band edge states, which is further investigated in the following section.
3.4. The mechanism behind the unconventional energy shifts: angle-resolved XPS
To investigate the underlying mechanism behind the charge redistribution in the MoSe2/MoTe2 heterostructure, we performed angle-resolved XPS at photoemission angles other than surface normal (90°) (Fig. 6a). The effective photoemission depth is related to the photoemission angle (θ), the angle between the analyzer and the film surface, as follows:deff ∝ λEAL × sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ, θ, | (1) |
In normal emission mode, the intensity of the photoelectrons reaching the surface, which were produced at a depth greater than d, is given by
I = I0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−d/λEAL) exp(−d/λEAL) | (2) |
According to eqn (2), the majority of the detected signal originates from the top few nanometers of the film. About 80% of the total measured signal is contributed from a depth deff ≤ 1.6(λEAL) while about 90% of the total signal is from deff ≤ 2.4(λEAL). If we consider the values of λEAL for MoSe2 (∼2.0 nm) and MoTe2 (∼2.2 nm) from the NIST database,62 the effective photoemission depths at normal emission (sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ = 1) can be approximated as (deff)80% ≈ 3.2 nm and (deff)90% ≈ 4.8 nm.
θ = 1) can be approximated as (deff)80% ≈ 3.2 nm and (deff)90% ≈ 4.8 nm.
At 60° emission, the relative contribution of different binding states remains nearly unchanged (see SI Fig. S4a), as the effective photoemission depth only decreases by a factor of 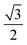 (from eqn (1)), resulting in a combined contribution from the entire MoSe2/MoTe2 stacked structure. However, at 30° emission, the effective photoelectron depth is halved compared to normal emission, and we observe significant changes in the spectra (Fig. 6b). As summarized in Fig. 6c, the relative contribution of the higher binding energy peak (Teδ) in the Te 3d spectrum increases from 8.5% to 16.9%. This indicates that this feature is not uniformly present in the MoTe2 film but is predominantly localized towards the upper layer, i.e., near the MoSe2/MoTe2 interface. Similarly, in the Se 3d spectrum, the higher binding energy component (Seδ) decreases from 26.9% to 10.4% at 30° emission, suggesting that this contribution is not prominent in the upper side of MoSe2, which again shows its confined presence near the heterostructure interface. For further lower-angle emission at 20° (SI Fig. S4b), the signal-to-noise ratio is insufficient to ensure a reliable spectral fitting. Collectively, these observations confirm that the higher binding energy components in both Se 3d and Te 3d spectra originate from a region near the heterostructure interface.
(from eqn (1)), resulting in a combined contribution from the entire MoSe2/MoTe2 stacked structure. However, at 30° emission, the effective photoelectron depth is halved compared to normal emission, and we observe significant changes in the spectra (Fig. 6b). As summarized in Fig. 6c, the relative contribution of the higher binding energy peak (Teδ) in the Te 3d spectrum increases from 8.5% to 16.9%. This indicates that this feature is not uniformly present in the MoTe2 film but is predominantly localized towards the upper layer, i.e., near the MoSe2/MoTe2 interface. Similarly, in the Se 3d spectrum, the higher binding energy component (Seδ) decreases from 26.9% to 10.4% at 30° emission, suggesting that this contribution is not prominent in the upper side of MoSe2, which again shows its confined presence near the heterostructure interface. For further lower-angle emission at 20° (SI Fig. S4b), the signal-to-noise ratio is insufficient to ensure a reliable spectral fitting. Collectively, these observations confirm that the higher binding energy components in both Se 3d and Te 3d spectra originate from a region near the heterostructure interface.
Furthermore, the variation in the film stoichiometry was investigated using the RSF-corrected areas of the individual peaks (SI S8). The Mo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Te ratio changes from 1
Te ratio changes from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.90 at normal emission to 1
1.90 at normal emission to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.60 at 30° emission (Fig. 6c), indicating that some of the Te atoms near the interface migrate from the MoTe2 lattice, leaving Te vacancies there. This Te vacancy creation in MoTe2 is relatively easier owing to the weaker Mo–Te bonding compared to the Mo–Se bonding. The DFT calculations (SI Table S1) reveal that the formation energy of the Te vacancy (2.64 eV) is significantly lower than that of the Mo (2.89 eV) and Se (3.10 eV) vacancies in MoTe2 and MoSe2, respectively. This shows that Te vacancies are the favorable intrinsic defect in the heterostructure. Moreover, there is a high electronegativity difference (∼0.45) between Se (2.55) and Te (2.10), which is even more than that (∼0.06) between Mo (2.16) and Te according to the Pauling scale.63 Therefore, it can preferably lead to the migration of Te near the heterostructure interface, leaving vacancies on the MoTe2 side and forming the Mo–Se–Te/Se–Te bonding on the MoSe2 side. This is experimentally observed in both the Se 3d and Te 3d core levels, as indicated by the Seδ and Teδ contributions in Fig. 5c and Fig. 6b. Due to the higher electronegativity of Se, the electronic charge transfer takes place from Te to Se, giving a slightly positive oxidation state to Te (δ+) and a negative oxidation state to Se (δ−). Since the above charge transfer is localized, affecting the Se and Te atoms near the interface more dominantly, the binding energy of the Mo–Se component remains nearly unaffected by this charge transfer.
1.60 at 30° emission (Fig. 6c), indicating that some of the Te atoms near the interface migrate from the MoTe2 lattice, leaving Te vacancies there. This Te vacancy creation in MoTe2 is relatively easier owing to the weaker Mo–Te bonding compared to the Mo–Se bonding. The DFT calculations (SI Table S1) reveal that the formation energy of the Te vacancy (2.64 eV) is significantly lower than that of the Mo (2.89 eV) and Se (3.10 eV) vacancies in MoTe2 and MoSe2, respectively. This shows that Te vacancies are the favorable intrinsic defect in the heterostructure. Moreover, there is a high electronegativity difference (∼0.45) between Se (2.55) and Te (2.10), which is even more than that (∼0.06) between Mo (2.16) and Te according to the Pauling scale.63 Therefore, it can preferably lead to the migration of Te near the heterostructure interface, leaving vacancies on the MoTe2 side and forming the Mo–Se–Te/Se–Te bonding on the MoSe2 side. This is experimentally observed in both the Se 3d and Te 3d core levels, as indicated by the Seδ and Teδ contributions in Fig. 5c and Fig. 6b. Due to the higher electronegativity of Se, the electronic charge transfer takes place from Te to Se, giving a slightly positive oxidation state to Te (δ+) and a negative oxidation state to Se (δ−). Since the above charge transfer is localized, affecting the Se and Te atoms near the interface more dominantly, the binding energy of the Mo–Se component remains nearly unaffected by this charge transfer.
The experimentally observed Fermi level pinning in MoTe2 (Fig. 2e) can be explained by the interplay of interlayer charge transfer and intrinsic doping induced by these Te-vacancies. In principle, interlayer charge transfer across the heterostructure interface should shift the Fermi level of MoTe2 downward, as electrons redistribute from MoTe2 toward MoSe2 (as indicated in ILCT predicted band-alignment in Fig. 3b). However, our DFT density of states calculations for MoTe2 with Te vacancies reveal that the presence of Te vacancies in MoTe2 modifies its electronic band structure by contributing to localized states just below the conduction band (SI Fig. S5). Since these donor-like states intrinsically drive MoTe2 toward n-type doping, they counteract the ILCT-driven downward shift by pulling the Fermi level upward. The combined effect of these two competing mechanisms results in the apparent pinning of the Fermi level near its experimentally observed position (0.58 ± 0.05 eV above the VBM).
Based on these obtained values of work function and different energy levels, we present the experimentally observed band alignment for the MoSe2/MoTe2 heterostructure (Fig. 6d), in which the energy level shift differs from that in the ILCT-driven band alignment with modified interfacial band bending. The effective VBO decreases to 0.45 eV, which indicates a reduced barrier for hole injection. The enhanced hole injection in such heterostructure-based devices can improve bidirectional carrier transport and gate-tunable hole conduction. A comparative summary is included in Table 1, which compares our findings with reported interfacial band alignments and charge redistribution mechanisms for closely related TMDC heterostructures. It is also important to mention that our study highlights the role of interfacial charge redistribution and bonding modifications, while the twist angle between TMDC layers can also influence interfacial electronic properties. Since the grown films are limited by the multiple in-plane orientations of the grains, further investigations on twist-controlled single-crystalline heterostructures will be essential to understand the contributions of twist angle to the band alignment and carrier dynamics of such heterostructures.
| Heterostructure | Methods | Band alignment | Reported interfacial mechanisms | Key findings | Ref. |
|---|---|---|---|---|---|
| MoSe2/MoTe2 trilayer | AR-XPS, UPS, SKPFM | Modified, Type II, reduced VBO ≈ 0.43 eV | ILCT, Te migration and localized Mo–Se–Te bonding | MoTe2 Fermi level pinning and upshift in the MoSe2 VBM | This work |
| Individual bulk MoSe2 and MoTe2 | SXPS, HAXPES | Type II, VBO ≈ 0.40 eV | Conventional ILCT (calculated based on their ionization potentials) | IP: 5.40 eV (MoSe2), 5.00 eV (MoTe2) | 67 |
| MoTe2(1L)/MoS2 | STS, UPS | Type II, VBO ≈ 0.53 eV | ILCT, interfacial screening | Fermi level pinning due to gap states | 64 |
| MoTe2/MoS2 | DFT | Type I and Type II | ILCT | Biaxial strain and electric field-induced Type II–Type I conversion | 65 |
| MoSe2/WS2 bilayers | Electrical gating and PL | Type I and Type II | ILCT, trapping of interfacial carriers by the interlayer exciton | Field-tuned band re-ordering | 66 |
3.5. Modification of band-edge states in MoSe2: DFT calculations
The valence band edge in most of the TMDC materials is predominantly defined by metal-derived electronic states, so any modifications in the chalcogen bonding do not have a significant impact on the valence band edge; however, in some TMDCs, the chalcogen atoms can also contribute to these band-edge states.67 To investigate this for MoSe2, density functional theory (DFT) calculations were performed, mapping the individual contributions of Mo and Se to the total density of states (DOS) in MoSe2 (Fig. 7). The DOS spectra reveal that the states around the VBM in MoSe2 have significant contributions from 4p orbitals of Se in conjunction with Mo 4d orbitals. Consequently, any change in the chemical bonding of Se atoms can significantly influence the position of the band edge. This makes the valence band edge of MoSe2 sensitive to the chemical environment of Se, particularly at surfaces or interfaces where it is Se-terminated. In the grown MoSe2/MoTe2 heterostructure, the additional features observed in the Se and Te core-level spectra (Fig. 5c and 6b) confirm that the bonding of interfacial Se atoms is modified, with the formation of Mo–Se–Te/Se–Te near the interface. Therefore, these bonding modifications can be responsible for the experimentally obtained upshift of the valence band edge of MoSe2.4. Conclusion
In conclusion, we have successfully achieved the scalable layer-by-layer growth of a trilayer MoSe2/MoTe2 heterostructure uniformly over SiO2 using MBE and observed abnormal shifts in the energy levels of the heterostructure. Our investigation based on SKPFM, XPS and AR-XPS reveals that this anomaly in the energy levels arises from the complex interfacial charge transfer processes occurring around the heterostructure interface. The migration of Te atoms induces n-type doping in MoTe2, which compensates for the effect of inter-layer charge transfer, thus pinning its Fermi level. The modified bonding in MoSe2 near the interface affects the electronic states near its valence band edge, resulting in the upshift of its VBM. Such interfacial effects not only lead to deviations from ILCT-driven band alignment but also introduce an asymmetric band profile with reduced valence band offset.The experimentally established band alignment of the MoSe2/MoTe2 heterostructure in the present study overcomes the ILCT-based ambiguity, which is critical for precise heterostructure band-engineering of future devices. The reduced VBO corresponds to a lower energy barrier for hole transport across the interface, which is favorable for efficient hole injection and extraction in both directions. In photodetectors, such improved hole extraction can result in enhanced carrier collection and responsivity, while the enhanced bidirectional transport can be crucial for ambipolar FETs, where a small VBO supports balanced and symmetric switching between electron and hole conduction. Combined with the demonstrated large area thickness-controlled growth of the ultrathin heterostructure, the findings provide key insights into the intricate charge transfer in TMDC heterostructures and the impact on the band alignment, which is crucial for advancing their nanoelectronic and optoelectronic device applications.
Author contributions
Kamlesh Bhatt: conceptualization, methodology, writing – original draft, formal analysis, writing – review & editing, data curation, and visualization. Santanu Kandar: methodology and investigation. Lipika: formal analysis and investigation. Ashok Kapoor: validation, formal analysis, and writing – review & editing. Rajendra Singh: supervision, conceptualization, writing – review & editing, resources, and funding acquisition.Conflicts of interest
The authors declare no conflict of interest.Data availability
The data that support the findings of this study can be made available from the corresponding author upon reasonable request.Supplementary information (SI) is available. See DOI: https://doi.org/10.1039/d5nh00663e.
Acknowledgements
K. Bhatt would like to acknowledge the University Grant Commission (UGC), India, for providing a fellowship. The authors acknowledge the “Grand Challenge Project on MBE Growth of 2D Materials” sponsored by the Ministry of Human Resource Development (MHRD), India and the Indian Institute of Technology Delhi for providing financial support for this work. The authors also acknowledge the partial funding support from the Department of Science and Technology (DST-Nano Mission) under the project scheme “Consortium for Collective and Engineered Phenomena in Topology (CONCEPT).” The authors also thank Dr B.G. Ganga and Dr Atul Kumar Singh for their kind support with detailed AR-XPS and UPS measurements. The authors also want to acknowledge the Central Research Facility (CRF) and Department of Physics, Indian Institute of Technology Delhi, New Delhi, for the characterization facilities.References
- J. M. Shainline, Appl. Phys. Lett., 2021, 118, 160501 CrossRef CAS.
- L. H. Fowler-Gerace, Z. Zhou, E. A. Szwed, D. J. Choksy and L. V. Butov, Nat. Photonics, 2024, 18, 823–828 CrossRef CAS.
- P. V. Pham, S. C. Bodepudi, K. Shehzad, Y. Liu, Y. Xu, B. Yu and X. Duan, Chem. Rev., 2022, 122, 6514–6613 CrossRef CAS PubMed.
- L. Yin, F. Wang, R. Cheng, Z. Wang, J. Chu, Y. Wen and J. He, Adv. Funct. Mater., 2019, 29, 1804897 CrossRef.
- M. Rahaman, G. Kim, K. Y. Ma, S. Song, H. S. Shin and D. Jariwala, npj 2D Mater. Appl., 2023, 7, 66 CrossRef CAS.
- Y. Wang, L. Yin, W. Huang, Y. Li, S. Huang, Y. Zhu, D. Yang and X. Pi, Adv. Intell. Syst., 2021, 3, 2000099 CrossRef.
- B. Zhao, Z. Wan, Y. Liu, J. Xu, X. Yang, D. Shen, Z. Zhang, C. Guo, Q. Qian, J. Li, R. Wu, Z. Lin, X. Yan, B. Li, Z. Zhang, H. Ma, B. Li, X. Chen, Y. Qiao, I. Shakir, Z. Almutairi, F. Wei, Y. Zhang, X. Pan, Y. Huang, Y. Ping, X. Duan and X. Duan, Nature, 2021, 591, 385–390 CrossRef CAS PubMed.
- Y.-C. Lin, R. K. Ghosh, R. Addou, N. Lu, S. M. Eichfeld, H. Zhu, M.-Y. Li, X. Peng, M. J. Kim, L.-J. Li, R. M. Wallace, S. Datta and J. A. Robinson, Nat. Commun., 2015, 6, 7311 CrossRef CAS.
- X. Zhang, Z. Kang, L. Gao, B. Liu, H. Yu, Q. Liao, Z. Zhang and Y. Zhang, Adv. Mater., 2021, 33, 2104935 CrossRef CAS.
- H. Son, H. Choi, J. Jeon, Y. J. Kim, S. Choi, J. H. Cho and S. Lee, ACS Appl. Mater. Interfaces, 2021, 13, 8692–8699 CrossRef CAS.
- W. Choi, J. Ahn, K. Kim, H. Jin, S. Hong, D. K. Hwang and S. Im, Adv. Mater., 2021, 33, 2103079 Search PubMed.
- Y.-C. Lin, R. K. Ghosh, R. Addou, N. Lu, S. M. Eichfeld, H. Zhu, M.-Y. Li, X. Peng, M. J. Kim, L.-J. Li, R. M. Wallace, S. Datta and J. A. Robinson, Nat. Commun., 2015, 6, 7311 CrossRef CAS PubMed.
- X. Zhou, Z. Tian, H. J. Kim, Y. Wang, B. Xu, R. Pan, Y. J. Chang, Z. Di, P. Zhou and Y. Mei, Small, 2019, 15, 1902528 CrossRef CAS.
- B. Zhao, Z. Wan, Y. Liu, J. Xu, X. Yang, D. Shen, Z. Zhang, C. Guo, Q. Qian, J. Li, R. Wu, Z. Lin, X. Yan, B. Li, Z. Zhang, H. Ma, B. Li, X. Chen, Y. Qiao, I. Shakir, Z. Almutairi, F. Wei, Y. Zhang, X. Pan, Y. Huang, Y. Ping, X. Duan and X. Duan, Nature, 2021, 591, 385–390 CrossRef CAS.
- S. Kandar, K. Bhatt, N. Kumar, A. K. Kapoor and R. Singh, ACS Appl. Nano Mater., 2024, 7, 8212–8220 CrossRef CAS.
- X. Li, M.-W. Lin, J. Lin, B. Huang, A. A. Puretzky, C. Ma, K. Wang, W. Zhou, S. T. Pantelides, M. Chi, I. Kravchenko, J. Fowlkes, C. M. Rouleau, D. B. Geohegan and K. Xiao, Sci. Adv., 2016, 2, 1501882 CrossRef.
- C. Ruppert, B. Aslan and T. F. Heinz, Nano Lett., 2014, 14, 6231–6236 CrossRef CAS PubMed.
- H. Lee, J. Ahn, S. Im, J. Kim and W. Choi, Sci. Rep., 2018, 8, 11545 CrossRef.
- K. L. Seyler, P. Rivera, H. Yu, N. P. Wilson, E. L. Ray, D. G. Mandrus, J. Yan, W. Yao and X. Xu, Nature, 2019, 567, 66–70 CrossRef CAS.
- B. Han, C. Robert, E. Courtade, M. Manca, S. Shree, T. Amand, P. Renucci, T. Taniguchi, K. Watanabe, X. Marie, L. E. Golub, M. M. Glazov and B. Urbaszek, Phys. Rev. X, 2018, 8, 031073 CAS.
- A. Arora, K. Nogajewski, M. Molas, M. Koperski and M. Potemski, Nanoscale, 2015, 7, 20769–20775 RSC.
- C. Jiang, F. Liu, J. Cuadra, Z. Huang, K. Li, A. Rasmita, A. Srivastava, Z. Liu and W.-B. Gao, Nat. Commun., 2017, 8, 802 CrossRef PubMed.
- A. M. Shafi, M. G. Uddin, X. Cui, F. Ali, F. Ahmed, M. Radwan, S. Das, N. Mehmood, Z. Sun and H. Lipsanen, Adv. Sci., 2023, 10, 2303437 CrossRef CAS PubMed.
- Y. Lin, Y. Xu, S. Wang, S. Li, M. Yamamoto, A. Aparecido-Ferreira, W. Li, H. Sun, S. Nakaharai, W. Jian, K. Ueno and K. Tsukagoshi, Adv. Mater., 2014, 26, 3263–3269 CrossRef CAS PubMed.
- M. A. Khan, M. F. Khan, S. Rehman, H. Patil, G. Dastgeer, B. M. Ko and J. Eom, Sci. Rep., 2022, 12, 12085 CrossRef CAS PubMed.
- X. Jiang, T. Yilmaz, E. Vescovo and D. Lu, Phys. Rev. B, 2024, 109, 115112 CrossRef CAS.
- N. Gasparini, F. V. A. Camargo, S. Frühwald, T. Nagahara, A. Classen, S. Roland, A. Wadsworth, V. G. Gregoriou, C. L. Chochos, D. Neher, M. Salvador, D. Baran, I. McCulloch, A. Görling, L. Lüer, G. Cerullo and C. J. Brabec, Nat. Commun., 2021, 12, 1772 CrossRef CAS PubMed.
- G. Wen, S. Fu, M. Bonn and H. I. Wang, J. Chem. Phys., 2024, 161, 054714 CrossRef CAS PubMed.
- F. H. Davies, C. J. Price, N. T. Taylor, S. G. Davies and S. P. Hepplestone, Phys. Rev. B, 2021, 103, 045417 CrossRef CAS.
- Y. Jiang, S. Chen, W. Zheng, B. Zheng and A. Pan, Light: Sci. Appl., 2021, 10, 72 CrossRef CAS.
- T. Naimer, K. Zollner, M. Gmitra and J. Fabian, Phys. Rev. B, 2021, 104, 195156 CrossRef CAS.
- R. Rosati, I. Paradisanos, L. Huang, Z. Gan, A. George, K. Watanabe, T. Taniguchi, L. Lombez, P. Renucci, A. Turchanin, B. Urbaszek and E. Malic, Nat. Commun., 2023, 14, 2438 CrossRef CAS.
- J. Kistner-Morris, A. Shi, E. Liu, T. Arp, F. Farahmand, T. Taniguchi, K. Watanabe, V. Aji, C. H. Lui and N. Gabor, Nat. Commun., 2024, 15, 4075 CrossRef CAS PubMed.
- A. Bian, S. Fu, P. Wang, K. Zhao, J. He, X. Zhang, D. He, Y. Wang and H. Zhao, J. Mater. Chem. C, 2022, 10, 5328–5335 RSC.
- K. Bhatt, S. Kandar, N. Kumar, A. Kapoor and R. Singh, Nanoscale, 2024, 16, 15381–15395 RSC.
- J. Xia, J. Yan and Z. X. Shen, FlatChem, 2017, 4, 1–19 CrossRef CAS.
- H. Jiang, L. Zheng, J. Wang, M. Xu, X. Gan, X. Wang and W. Huang, Nanoscale, 2021, 13, 18103–18111 RSC.
- N. Fairley, V. Fernandez, M. Richard-Plouet, C. Guillot-Deudon, J. Walton, E. Smith, D. Flahaut, M. Greiner, M. Biesinger, S. Tougaard, D. Morgan and J. Baltrusaitis, Appl. Surf. Sci. Adv., 2021, 5, 100112 CrossRef.
- J. J. Yeh and I. Lindau, At. Data Nucl. Data Tables, 1985, 32, 1–155 CrossRef CAS.
- G. Kresse and J. Furthmüller, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 11169–11186 CrossRef CAS.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS.
- P. B. James and M. T. Lavik, Acta Crystallogr., 1963, 16, 1183 CrossRef CAS.
- D. Puotinen and R. E. Newnham, Acta Crystallogr., 1961, 14, 691–692 CrossRef CAS.
- S. Hasegawa, in Characterization of Materials, ed. E. N. Kaufmann, Wiley, 2012, pp. 1–14 Search PubMed.
- D. Puotinen and R. E. Newnham, Acta Crystallogr., 1961, 14, 691–692 CrossRef CAS.
- P. B. James and M. T. Lavik, Acta Crystallogr., 1963, 16, 1183 CrossRef CAS.
- F. Zhang, H. Zhang, S. Krylyuk, C. A. Milligan, Y. Zhu, D. Y. Zemlyanov, L. A. Bendersky, B. P. Burton, A. V. Davydov and J. Appenzeller, Nat. Mater., 2019, 18, 55–61 CrossRef CAS PubMed.
- K.-A. N. Duerloo, Y. Li and E. J. Reed, Nat. Commun., 2014, 5, 4214 CrossRef CAS PubMed.
- S. Kandar, K. Bhatt, N. Kumar, A. Kapoor and R. Singh, Nanoscale, 2024, 16, 18609–18619 RSC.
- N. Kumar, K. Bhatt, S. Kandar, G. Rana, C. Bera, A. K. Kapoor and R. Singh, ACS Appl. Nano Mater., 2024, 7, 23834–23841 CrossRef CAS.
- J. P. Fraser, L. Masaityte, J. Zhang, S. Laing, J. C. Moreno-López, A. F. McKenzie, J. C. McGlynn, V. Panchal, D. Graham, O. Kazakova, T. Pichler, D. A. MacLaren, D. A. J. Moran and A. Y. Ganin, Commun. Mater., 2020, 1, 48 CrossRef.
- M. G. Choi, A. Belianinov, A. Pawlicki, S. Park, H. Lee, O. S. Ovchinnikova and S. Kim, Surf. Interfaces, 2021, 26, 101437 CrossRef CAS.
- V. O. Khaustov, D. Convertino, J. Köster, A. A. Zakharov, M. J. Mohn, Z. M. Gebeyehu, L. Martini, S. Pace, G. Marini, M. Calandra, U. Kaiser, S. Forti and C. Coletti, ACS Appl. Nano Mater., 2024, 7, 18094–18105 CrossRef CAS.
- L. Tang, J. Tan, H. Nong, B. Liu and H.-M. Cheng, Acc. Mater. Res., 2021, 2, 36–47 CrossRef CAS.
- Y. Gong, S. Lei, G. Ye, B. Li, Y. He, K. Keyshar, X. Zhang, Q. Wang, J. Lou, Z. Liu, R. Vajtai, W. Zhou and P. M. Ajayan, Nano Lett., 2015, 15, 6135–6141 CrossRef CAS.
- Y. Ding, N. Zhou, L. Gan, X. Yan, R. Wu, I. H. Abidi, A. Waleed, J. Pan, X. Ou, Q. Zhang, M. Zhuang, P. Wang, X. Pan, Z. Fan, T. Zhai and Z. Luo, Nano Energy, 2018, 49, 200–208 CrossRef CAS.
- Y. Yoo, Z. P. DeGregorio, Y. Su, S. J. Koester and J. E. Johns, Adv. Mater., 2017, 29, 1605461 CrossRef.
- K. Bhatt, S. Kandar, N. Kumar, A. Kapoor and R. Singh, Appl. Surf. Sci., 2024, 659, 159832 CrossRef CAS.
- A. Jablonski and C. J. Powell, J. Phys. Chem. Ref. Data, 2020, 49, 033102 CrossRef CAS.
- F. A. Stevie and C. L. Donley, J. Vac. Sci. Technol., A, 2020, 38, 063204 CrossRef CAS.
- A. Jablonski and C. J. Powell, J. Electron Spectrosc. Relat. Phenom., 2017, 218, 1–12 CrossRef CAS.
- C. J. Powell and A. Jablonski, NIST Electron Effective-Attenuation-Length Database, National Institute of Standards and Technology, Gaithersburg, MD, 2011 Search PubMed.
- J. F. Ogilvie, in Conceptual Trends in Quantum Chemistry, Springer, Netherlands, Dordrecht, 1994, pp. 171–198 Search PubMed.
- H. C. Diaz, Y. Ma, R. Chaghi and M. Batzill, Appl. Phys. Lett., 2016, 108, 191606 CrossRef.
- S. T. Nguyen, N. V. Hieu, H. Le-Quoc, K. Nguyen-Ba, C. V. Nguyen, H. V. Phuc and C. Q. Nguyen, Nanoscale Adv., 2024, 6, 3624–3631 RSC.
- J. Kistner-Morris, A. Shi, E. Liu, T. Arp, F. Farahmand, T. Taniguchi, K. Watanabe, V. Aji, C. H. Lui and N. Gabor, Nat. Commun., 2024, 15, 4075 CrossRef CAS.
- L. A. H. Jones, Z. Xing, J. E. N. Swallow, H. Shiel, T. J. Featherstone, M. J. Smiles, N. Fleck, P. K. Thakur, T.-L. Lee, L. J. Hardwick, D. O. Scanlon, A. Regoutz, T. D. Veal and V. R. Dhanak, J. Phys. Chem. C, 2022, 126, 21022–21033 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2026 |