 Open Access Article
Open Access ArticleMagnetically driven, plant-extract-modified Fe3O4 nanoparticles for sustainable and eco-friendly wastewater detoxification: recent developments
Chanchal
Das
 ab and
Goutam
Biswas
ab and
Goutam
Biswas
 *b
*b
aDepartment of Chemistry, Saheed Kshudiram College, Uttar Kamakhyaguri, Alipurduar, West Bengal-736202, India
bDepartment of Chemistry, Cooch Behar Panchanan Barma University, Vivekananda Street, Cooch Behar, West Bengal-736101, India. E-mail: goutam@cbpbu.ac.in
First published on 4th December 2025
Abstract
The increasing burden of toxic heavy metals, dyes, pharmaceuticals, and pathogenic microorganisms in aquatic environments necessitates the development of sustainable purification strategies. This review comprehensively elucidates recent progress in the synthesis, characterization, and application of phytogen-based synthesis of functionalized magnetic nanoparticles (phytogen@MNPs) for eco-friendly wastewater treatment. Plant-derived bioactive compounds serve as green capping agents, facilitating the synthesis of multifunctional, biocompatible, and surface-reactive MNPs. This review details diverse phytogenic sources, synthesis methodologies, and advanced characterization techniques, highlighting the influence of surface modification on stability, adsorption efficiency, and superparamagnetic behavior. Applications in the adsorption and catalytic degradation of inorganic, organic, and microbial contaminants are critically discussed, along with the kinetics, isotherms, and thermodynamics of pollutant removal. The antibacterial properties, reusability, and impact of real water matrices are covered, highlighting the superior performance and cost-effectiveness of phytogen@MNPs. Mechanistic insights into pollutant–nanoparticle interactions reveal the decisive roles of surface functionalization and particle size. This review also encompasses the advantages of phytogen@MNPs over conventional materials, while also identifying the need for standardized protocols, evaluation of long-term stability, and strategies for scalable production to fully realize their potential in environmental remediation in future work.
1. Introduction
Every year, large amounts of toxic inorganic and organic pollutants, such as harmful heavy metals (cations, oxycations, and oxyanions), organic dyes, and pharmaceutical wastes, are discharged because of overproduction, overuse in various applications, and overprescription.1 These detrimental contaminants are directly or indirectly discharged into rivers and oceans, where they adversely affect the flora and fauna. In addition to these pollutants, pathogenic microorganisms persist in surface water, which is the primary reason why water remains non-potable even after undergoing purification processes.2 The removal of toxic metal ions, organic pollutants, and harmful microbes from wastewater can provide clean and pure drinking water.Among the different types of materials and composites, nanoparticles (NPs) have been extensively developed for wastewater purification applications. NPs can be of various types, such as metals, metal oxides, metal sulfides, and spinel materials. In this study, we focused on magnetite nanoparticles (MNPs). Iron oxide nanoparticles are generally of three types: hematite (α-Fe2O3), maghemite (γ-Fe2O3), and magnetite (Fe3O4).3 The latter two interact strongly with the magnetic field, whereas hematite is weakly magnetic. MNPs are superparamagnetic mixed metal oxides with Fe(II) and Fe(III) ions in octahedral and tetrahedral sites (inverse spinel cubic lattice), respectively. They can also be easily prepared using straightforward methods and recycled for numerous cycles, making them cost-effective materials.4 Surface-modified MNPs can be classified into two categories based on the selection of surface modifiers: chemically modified and biogenic or biomodified. Biomodified magnetic nanoparticles (MNPs) are distinguished by their unique properties, as they can be synthesized in a cost-effective and environmentally sustainable manner under open-air conditions, utilizing extracts from leaves, crude latex, bark, and seeds of various plants. There is growing research interest in the fabrication of MNPs by biomodification, that is, surface modification with various bioactive compounds, including phytogens, enzymes, peptides, and fungi, and their application in water and wastewater treatment. Here, we discuss only phytogen@MNPs, which are found in various plant parts and extracted using specific solvents. Polyphenols, flavonoids, terpenoids, peptides, nucleic acids, enzymes, cellulose, and starch are phytogens. The features of nanoparticles, such as biocompatibility, stability, magnetic properties, functionality, and band gap, can be modified by phytogen coating or biomodification. These phytogen@MNPs were more advantageous than other chemically modified MNPs.
These nanoparticles were characterized with respect to their structural, morphological, magnetic, and thermal stability properties using advanced techniques such as dynamic light scattering (DLS), ultraviolet-visible (UV-vis) spectroscopy, Fourier-transform infrared (FTIR) spectroscopy, powder X-ray diffraction (pXRD), field emission scanning electron microscopy (FE-SEM), high-resolution transmission electron microscopy (HRTEM), superconducting quantum interference device (SQUID)/vibrating-sample magnetometry (VSM)/alternating gradient magnetometry (AGM), and thermogravimetric analysis (TGA).
The properties of these phytogen@MNPs include a high surface area-to-volume ratio, availability, cost-effectiveness, ease of dispersion in solutions, ability to generate reactive oxygen species (ROS), rapid adsorption kinetics, and superparamagnetic properties. Owing to their high saturation magnetization values, facile separation from the reaction mixtures can be achieved using a simple magnet. MNPs have been suggested as crucial agents or carriers in cancer treatment because of their distinct dynamic magnetization in response to alternating magnetic fields, such as in magnetic hyperthermia therapy.5 Magnetic materials with nanoscale to microscale particle sizes are appealing for use in biological and medicinal applications, as well as in wastewater treatment and magnetic recording.6 Most of these applications require nanoparticles that are evenly sized, shaped, and distributed in a solvent.7 For example, nanoparticles at the nanometer scale can bind with DNA or proteins via a capillary tube, whereas microparticles are limited to interactions with cells.6,8 Furthermore, the attainment of single-domain particles and superparamagnetic behavior is contingent upon the critical size being within the range of 30–50 nm.9 Functionalized MNPs have many such applications, including drug carriers,10 drug release,11 cancer therapy,12 magnetic resonance imaging (MRI),13 proton exchange membranes,14 catalysis15–18 and sensors.19 Their biocompatibility and lack of toxicity make them suitable for use in biotechnology.20 A comparison table for phytogen@MNPs with other NPs is given in Table 1.
| Parameter | Phytogen@MNPs | Phytogen coated other metal-oxide (TiO2, ZnO, CuO) NPs |
|---|---|---|
| Separation by an external magnet | Phytogen@MNPs are recovered using an external magnet after preparation and application, which makes wastewater purification easier and effective | These NPs lack inherent magnetism and require complex separation like centrifugation, therefore making recovery difficult and complicated after wastewater purification |
| Mechanism of removal | Adsorption, photocatalytic degradation, Fenton-like oxidation | Adsorption, photocatalytic degradation, but not Fenton-like oxidation |
| Catalytic performance | Low photocatalysis efficiency is observed | Higher photocatalysis efficiency is observed for TiO2 and ZnO under UV/visible irradiation |
| Recyclability | Due to the simple and effective magnetic recovery process, reusability frequency or recyclability are higher | As these are non-magnetic, hence the recovery process is complex and difficult, making it less recyclable |
| Cost analysis | Fe salts are relatively cheaper than other metal salts (Cu, Zn and Ti). Also, convenient magnetic separation makes the cost of production much less | Other metal salts are relatively expensive, and also in some cases use of non-aqueous solvents and an inert atmosphere makes overall production slightly expensive |
| Environmental toxicity | MNPs themselves have been proven to be less/non-toxic towards aquatic as well as normal cell lines at higher concentrations | Toxicity varies depending on concentration, e.g., ZnO and CuO NPs have a considerable toxicity profile while TiO2 NPs are relatively safer at higher concentrations |
Owing to their small size, large surface area, and reactive surface sites, nanomaterials have shown excellent effectiveness in decreasing pollutant concentrations in adsorption and catalytic degradation studies. Batch adsorption methods and kinetic modeling demonstrated that phytogen@MNPs are significantly more cost-effective than traditional adsorbents and photocatalysts. Moreover, they exhibit high efficacy in removing heavy metals, dyes, pharmaceutical residues, and bacteria. Many review articles related to the synthesis and application of biogenic or plant extract-mediated MNPs have been published, discussing either the synthesis or a few specific applications, such as anticancer and drug delivery.21,22 Some review articles of a similar kind discuss the removal of microorganisms,23 and few address synthesis and provide a brief discussion on environmental and biomedical applications.24 Among these articles, either the synthesis methodology, application, or both are missing, along with a brief discussion and mechanism. Therefore, our brief mechanistic findings highlight the potential of tailored MNPs for environmental cleanup and offer insights into the design of more efficient and sustainable materials. This study underscores the importance of functional group modification and the formation of phytogen@MNPs to enhance the performance of MNPs in real-world applications. Future work will focus on scaling the synthetic process and testing the long-term stability and reusability of these materials under various environmental conditions.
2. Various phytogens and phytogen-coated nanoparticles
Plants and their derivatives have garnered significant interest in the synthesis and fabrication of nanoparticles. This interest is due to the presence of phytogenic compounds, such as flavonoids, polyphenols, ascorbic acid, sugars, proteins, gums, and pectic molecules, which contain various coordinating groups, including –OH, –NH2, –CONH2, >C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and –COOH. These compounds act as stabilizing agents, provide multifunctionality, and impart biocompatibility to NPs on the surface. Some common plants containing bioactive molecules are discussed below.
O, and –COOH. These compounds act as stabilizing agents, provide multifunctionality, and impart biocompatibility to NPs on the surface. Some common plants containing bioactive molecules are discussed below.
Tinospora cordifolia is a well-known medicinal plant that is used in traditional medicine to treat various illnesses. Amrita, Guduchi, and Gulancha are common names of the Menispermaceae family. It has been used to treat a wide range of illnesses, including fever, diarrhea, leprosy, skin conditions, and diabetes. It is regarded as an essential plant in the Indian medicinal system. Alkaloids, terpenoids, lignans, steroids, and other chemical compounds have been identified in Tinospora cordifolia, supporting its phytochemical and pharmacological activities. In particular, it has anti-inflammatory, antimicrobial, antibacterial, antifungal, antidiabetic, antistress, hypolipidemic, hepatic, anticancer, anti-HIV, antiosteoporotic, antitoxic, wound-healing, anticomplementary, immunomodulatory, systemic infection, and anti-Parkinson's disease effects.25–27 Using various parts of T. cordifolia, several types of NPs, such as silver nanoparticles (AgNPs),28 titanium dioxide (TiO2) NPs,29 magnesium oxide (MgO) NPs,30 copper nanoparticles (CuNPs),31 and zinc oxide (ZnO) NPs,32 have been synthesized. These nanoparticles have been employed as antibiofilm agents, photocatalysts, antibacterial agents, diabetes regulators, antioxidants, anti-inflammatory agents, and adsorbents for lead, iron, phosphate, and arsenic ions.
Various parts of the Azadirachta indica (A. indica) plant are used in traditional medicines. Some important polyphenolic phytochemicals found in the ethanolic extract of A. indica leaves include ellagic acid, quercetin, quercetin-3-O-glucoside, gallic acid, 2,3-(S)-hexahydroxydiphenoyl-(α/β)-D-glucopyranose, avicularin, and castalagin. Abdulhady et al. reported that these substances exhibit strong cytotoxic and antioxidant properties.33 The presence of hydroxyl groups in the chemical structures of phenolic compounds may account for the substantial free radical scavenging capacity observed in neem leaves.33 Various nanoparticles, including TiO2, ZnO, CuO, α-Fe2O3, Fe3O4@ZnO, and MoO3, have been synthesized using different components of the A. indica plant.34 These nanoparticles have been employed for the adsorption of different adsorbates, degradation of various molecules, sensing, thermal catalysis, cytotoxicity, dye degradation, and antimicrobial activity. Jatropha curcas (J. curcas) is of significant commercial interest because of the industrial-scale extraction of biodiesel from its seeds.35,36 Although Jatropha latex has some ethnomedical uses, such as wound healing and coagulant activities in the blood,37 it is acrid and irritating to the skin.38 Extensive studies on J. curcas have revealed that the major constituents of its latex are curcain (an enzyme), curcacycline A (a cyclic octapeptide), and curcacycline B (a cyclic nonapeptide).39J. curcas plant parts can be used to synthesize NPs, such as lanthanum cobalt oxide (La2CoO4),40 AgNPs,39 and ZnO,41 which have been studied for catalysis, single-molecule magnets, and bacterial removal.
The Cinnamomum tamala (CT) leaf is a well-known Indian spice, called “Indian bay leaf” or “tejpatta”, which is used as a traditional medicine in the treatment of scabies, anal diseases, rectal disorders, heart troubles, bad taste, ozena, diarrhea, etc.42 Because CT leaves have antioxidant and antibacterial properties, they may also be used as antifouling agents for ships and boats in marine and freshwater systems in the future.43,44 The aqueous part of CT leaf extract consists of eugenol and kaempferol (as the main ingredients) and many flavonoids,45,46 which possess various coordinating groups such as hydroxyl (–OH) and carbonyl (>C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) groups. Hence, this extract was used as a surface modifier for nanoparticle synthesis. CT extract has also been used to reduce metal ions to produce metal nanoparticles.47 Using this, AgNPs48 and ZnO49 have been synthesized and successfully applied in catalysis and photocatalysis.
O) groups. Hence, this extract was used as a surface modifier for nanoparticle synthesis. CT extract has also been used to reduce metal ions to produce metal nanoparticles.47 Using this, AgNPs48 and ZnO49 have been synthesized and successfully applied in catalysis and photocatalysis.
Terminalia arjuna (TA) is another traditional medicine used for the treatment of rheumatic heart disease, endothelial dysfunction, hypertension, oxidative stress, and a few other health conditions and has a good antibacterial effect.50,51 In traditional medicine, a combination of elixir from TA bark with milk or ghrita is used to treat ulcers, whereas bark ash is used to treat snakebites and scorpion stings.52 TA bark contains flavonoids, β-sitosterol, triterpenoids, polyphenols, steroids, and glycosides, which have no negative side effects.53,54 Various parts of TA were used to prepare various types of NPs, such as MNPs,54 Cu, and AgNPs embedded in multiwalled carbon nanotubes (MWCNTs),55 which were used for the adsorption of contaminants from water and were successfully applied to study antimicrobial and anticancer activities.
Some parts of Asia and several southern European countries are home to the annual blooming plant Nigella sativa L. (NS). Seeds of this plant, known as black cumin, are well-known spices that are sometimes referred to as black seeds or black caraway seeds. People from the Middle East and Far East Asia have been using this plant to cure ailments such as headaches, asthma, infections, dysentery, back pain, obesity, gastrointestinal disorders, and hypertension, as it is identified to have healing properties in traditional medicine.56 It is generally called ‘kalonji’ or ‘kala jeera’ and is extensively used as traditional medicine and in cooking within India. NS seeds primarily consist of 2-isopropyl-5-methyl-1,4-benzoquinone (thymoquinone, TQ), which has good anticancer, antineoplastic, anti-inflammatory, and antimicrobial properties.57 Furthermore, NS has been documented to possess antioxidant properties in various animal models of neurological disorders, and several human studies corroborate these findings. NS seeds normalize the level of glutathione and thus activate enzymes with antioxidant properties, such as superoxide dismutase, catalase, and glutathione peroxidase.58 NS seed extract and TQ are also effective against a few infectious and non-infectious allergies, as well as other skin disorders,59 and have antidiabetic effects.60 Ali et al.61 studied the toxicological and pharmacological properties of NS and found that it is safer to use. Various NPs functionalized with NS seed phytogens, including AgNPs,62 CuNPs,63 ZnO NPs,64 and TiO2 NPs,65 have been reported to exhibit antibacterial, anti-obesity, and anticancer properties.
Overall, these plant parts and their extracts can be used for efficient fabrication of NPs. A few other common plants and extracts of different plant parts containing these compounds are listed in Table 2.
| Plant | Plant extract containing major compounds | Nanoparticles synthesized | Reference |
|---|---|---|---|

|

|
Fe2O3NPs, Fe3O4NPs, AgNPs, ZnONPs, ZnO-CuONPs | 66–71 |

|
|
BiZnFe NCs, α-Fe2O3NPs, Fe3O4NPs, AgNPs | 72–76 |

|
|
CoNPs, Pd@CuONPs, CuNPs, AgNPs | 77–81 |

|
|
Co3O4NPs, AuNPs, AgNPs, CuNPs, ZnO/NiONPs | 82–85 |

|

|
CuAl2O4NPs, AgNPs, ZnONPs, TiO2NPs | 86–90 |

|

|
MnO2NPs, AgNPs, FeNPs, Ag@α-Fe2O3NPs, CuONPs | 91–97 |

|

|
ZnNPs, MnxCo1-xAl2O4, FeNPs, MnSNPs | 98–102 |

|
|
CuSNPs, AgNPs, CuNPs | 103–107 |
This review explores innovative green synthesis methods for MNPs, emphasizing the role of plant-derived bioactive compounds in the fabrication of nanoparticles.
3. Synthesis of magnetite nanoparticles
Magnetite nanoparticles have been synthesized using various physical, chemical, and biological approaches. Chemical co-precipitation is the simplest and most commonly used method. Other categories of synthetic procedures for MNPs include bottom-up and top-down methods.108 Here, we only discuss the bottom-up approach. Examples of synthetic approaches for all categories are listed in Table 3.| Synthetic procedure | Typical methodology | References |
|---|---|---|
| Thermal decomposition | Fe(III) solution + surfactant solution + heating under an inert environment | 109 and 44 |
| Sonochemical | Fe(III) + Fe(II) solution + HCl solution + ammoniacal hydrazine + ultrasonication in a plastic tube with heating | 110 and 111 |
| Electrochemical | Electrodes: Hg|Hg2SO4, a platinum wire, and an iron rod. Electrolyte: saturated K2SO4 solution | 112–114 |
| Hydrothermal | Fe(III) solution + surfactant + hydrazine + stirring + heating in an autoclave | 115,116 |
| Microemulsion | Lipophilic solvent + aqueous solution of metal ions and surfactant + stirring + evaporation | 117–119 |
| Biological | Biological culture + metal ion containing solution | 120–122 |
| Co-precipitation | Fe(III) + Fe(II) salt solution + coating agent + OH− (pH∼11) | 9 and 123 |
3.1 General synthetic procedure for phytogen@MNPs
Massart et al.124 first synthesized MNPs via co-precipitation in 1981, after which they were widely investigated. The first biosynthesized MNPs were reported by Blakemore et al. (1975)125 in magnetotactic bacteria, and the first images of phytogen@MNPs in magnetotactic bacteria were captured by Williams et al. in the 1990s.126 In subsequent studies, various phytogen@MNPs were synthesized in the laboratory using diverse methodologies. However, this discussion focuses on the synthesis of phytogen@MNPs derived from different plant extracts. Phytogens contribute to the stabilization of nanoparticles and enhance their bioactivity, as well as their adsorption and degradation capabilities. Phytogen@MNPs can also be prepared using the co-precipitation method, which is convenient, eco-friendly, and cost-effective. In a conventional procedure, ferrous salts (e.g., FeCl2, FeSO4) and ferric salts (e.g., FeCl3, Fe2(SO4)3) are combined in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio to form a solution. Subsequently, an extract from a specific plant part was introduced, followed by the addition of NaOH solution to maintain an alkaline pH (∼11). Following additional stirring, magnetic separation, and repeated washing four to five times, the final separation and drying processes yielded the desired phytogen@MNPs (Fig. 1).
2 ratio to form a solution. Subsequently, an extract from a specific plant part was introduced, followed by the addition of NaOH solution to maintain an alkaline pH (∼11). Following additional stirring, magnetic separation, and repeated washing four to five times, the final separation and drying processes yielded the desired phytogen@MNPs (Fig. 1).
Washing several times with deionized water and other solvents is an important step in the preparation of MNPs. This is because some ferrous/ferric hydroxides, along with an excess concentration of the base (e.g., sodium hydroxide, ammonium hydroxide, etc.) and the coating agent, were removed during this process. Drying is crucial for the removal of adsorbed water molecules, as their presence in the FTIR spectra results in a broad and high-intensity peak, which is undesirable.
A comparison of various plant extract-mediated magnetite nanoparticles is shown in Table 4. The analysis of the table indicates that an open-air environment and aqueous solutions are adequate for the synthesis of MNPs when a concentrated plant extract solution is introduced. In most cases, NaOH was used as the base, and no other chemicals were used; hence, the procedure for the preparation of plant extract-mediated MNPs was greener and more cost-effective. Kalu et al.127 synthesized MNPs using an aq. extract of Calotropis procera. They qualitatively analyzed the plant extract and found it to contain polyphenols, flavonoids, alkaloids, tannins, and saponins with total contents of 43.54 (mg g−1), 33.83 (mg g−1), 28.38 (mg g−1), 25.95 (mg g−1), and 24.42 (mg g−1), respectively. Each phytochemical contains several coordinating groups that effectively coordinate with MNPs.
| Phytogen@MNPs | Fe-salts | Plant extract | Base | Synthetic procedure | Reaction environment | pH, temperature, time |
|---|---|---|---|---|---|---|
| TOL@MNPs16 | FeCl3 (1 g), FeSO4 (1 g) | Taraxacum officinale leaf (aq.) | NH3 (30%) | Refluxometry | Open-air | pH 10, rt and then boiling, 60 min and then 190 min |
| TC@MNPs15 | FeCl3 (2 g), FeSO4 (1 g) | Tinospora cordifolia leaf (aq.) | NH4OH (25%) | Refluxometry | Open-air | pH 10, rt and then 100 °C, 60 min then 180 min |
| MC@MNPs128 | FeCl3 (1 M) | Matricaria chamomilla flower (aq.) | NaOH (8 M) | Microwave | Open-air | 260 °C, 5 min |
| NS@MNPs129 | FeCl3 (1.62 g), FeCl2 (0.65 g) | Nigella sativa seed (aq. alc.) | NaOH (3.19 M) | Coprecipitation | Open-air | pH 11, rt, 60 min |
| Pom@MNPs130 | FeCl3 (2 mM), FeCl2 (1 mM) | Pomegranate peel (aq.) | NaOH | Coprecipitation | N2-gas | pH 10, rt (if NaOH is used) or 60 °C (if NH3 is used as base), 65 min |
| ACV@MNPs18 | FeCl3 (1 mM), FeSO4 (1 mM) | Adiantum capillus-veneris (aq.) | NaOH (10%) | Refluxometry | Open-air | pH 10, rt, 180 min |
| TA@MNPs54 | FeCl3 (0.65 g), FeCl2 (0.26 g) | Terminalia arjuna bark (aq.) | NaOH (6.4 M) | Coprecipitation | Open-air | pH 11, rt, 60 min |
| TS@MNPs131 | FeCl3 (13.6 g), FeCl2 (5 g) | Thymus schimperi leaf (aq.) | NaOH (2 M) | Coprecipitation | Open-air | 60 °C, 60 min |
| CP@MNPs127 | FeCl3 (6.25 g), FeCl2 (3.12 g) | Calotropis procera leaf (aq.) | NaOH (1.0 M) | Coprecipitation | Open-air | pH 11, 80 °C, 80 min |
| CS@MNPs132 | FeCl3 (0.2 M), FeSO4 (0.1 M) | Citrus Sinensis peels (aq) | NaOH (0.1 M) | Coprecipitation | Open-air | pH 11–12, rt, 30 min |
3.2 Mechanism of synthesis of magnetite nanoparticles and dependency on various factors
The preparation of MNPs can be described by eqn (1)–(3) applying a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of Fe(III) and Fe(II) at pH ∼11.54
1 molar ratio of Fe(III) and Fe(II) at pH ∼11.54| Fe(III) (aq) + Fe(II) (aq) + 5OH− (aq) = Fe(OH)3 (aq) + Fe(OH)2 (s) | (1) |
| Fe(OH)3 (s) = FeO2H (s) + H2O (l) | (2) |
| 2FeO2H (s) + Fe(OH)2 (s) = Fe3O4 (s) + 2H2O (l) | (3) |
The growth, size, magnetic susceptibility, and nucleation of the produced nanoparticles were regulated by an alkaline medium or [OH−]. The concentrations of Fe(III) and Fe(II) also affected the size and concentration of the Fe3O4 nanoparticles.
Akbar et al.133 altered the Fe3+/Fe2+ ratio to 1, 1.25, 1.5, 1.75, and 2. The application of Fe3+ cations caused iron oxide to form in both the hematite and maghemite phases; however, the use of Fe2+ cations caused the formation of the maghemite phase. Based on their report, it can be said that the maghemite phase was produced by varying the Fe3+/Fe2+ ratio to 1, 1.25, 1.5, and 1.75, whereas the magnetite phase was formed when the ratio was 2.
The introduction of coating materials is another important aspect that determines the stability of MNPs through van der Waals interactions, and electrostatic, covalent, and hydrogen bonding. For instance, Etemadifar et al.134 reported that the stability of zucchini and pomegranate peel extract-containing phytogen@MNPs is due to electrostatic forces, which also helps to prevent agglomeration (Fig. 2).
 | ||
| Fig. 2 Interaction of zucchini and pomegranate peel extract containing phytogen with magnetite nanoparticles (source: this image is taken from an article written by Etemadifar et al.134). | ||
The ratio of the plant extract to the corresponding Fe salt is also an important factor that determines the formation and stability of the phytogen@MNPs. Geneti et al.131 synthesized MNPs using the leaf extract of Thymus schimperi and applied them to remove chromium (Cr) and mercury (Hg). They synthesized MNPs using three different ratios of plant extract to iron salts: 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 1
1, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. They concluded that the formation of crystal planes in the MNPs was indistinct when the plant extract contained twice the amount of salt precursors. Conversely, when the plant extract was half the amount of salt precursors, the crystal planes were well defined (Fig. 3), and the crystal sizes increased. The corresponding powder XRD patterns are shown in Fig. 3a–c.
2. They concluded that the formation of crystal planes in the MNPs was indistinct when the plant extract contained twice the amount of salt precursors. Conversely, when the plant extract was half the amount of salt precursors, the crystal planes were well defined (Fig. 3), and the crystal sizes increased. The corresponding powder XRD patterns are shown in Fig. 3a–c.
 | ||
Fig. 3 pXRD patterns of MNPs synthesized using the leaf extract of Thymus schimperi in different ratios of plant extract to Fe salts: (a) 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, (b) 1 1, (b) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and (c) 1 1, and (c) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (source: this image is taken from an article written by Geneti et al.131). 2 (source: this image is taken from an article written by Geneti et al.131). | ||
4. Characterization techniques
Following the synthesis of MNPs, their characterization is crucial because it offers a definitive understanding of their stability, magnetic properties, and potential applications across various fields.4.1 DLS and zeta potential study
DLS is frequently used to determine the hydrodynamic diameter of MNPs by generating dilute suspensions. The hydrodynamic diameter is defined as the diameter of hydrated nanoparticles, which is generally influenced by the presence of electronegative atom-containing groups, such as –N<, –OH, –CONH–, and –COOH. An increase in the number of these groups typically results in greater hydration and a larger DLS diameter owing to the extensive hydrogen bonding interactions between water molecules and the surface of the coated MNPs. Another important factor in size measurement is the polydispersity index (PDI). The lower the PDI value (generally less than 0.3 or 30%), the narrower is the size range and uniformity of the particles. The measurement of the surface charge, expressed as zeta potential (ζ), is a critical parameter in DLS analyses. This measurement provides insights into the potential of MNPs to attract ions, thereby elucidating the mechanism of pollutant removal in wastewater. The zeta potential (ZP) is a measure of the electrical double layer or charge of nanoparticles. While performing the ZP study for nanoparticles, different pH conditions, generally in the range of pH 1–12, were used; in general, at pH 7, the surface charges on the MNPs were found to be negative.135–138 Some phytogen@MNPs have been reported to have positive ZPs below pH 4.139 Therefore, the point at which the charges of the MNPs become zero, rendering them electrically neutral, is referred to as the point of zero charge (PZC). Below the PZC, the surfaces of the MNPs acquired a positive charge, thereby attracting negatively charged ions. Conversely, above the PZC, the surface becomes negatively charged, facilitating the adsorption of positively charged ions. The stability of the nanoparticles was determined by ZP analysis. For example, Kovář et al.140 synthesized superparamagnetic MNPs for magnetic resonance imaging. They showed that the ZP remained constant at approximately −39 mV during the two months; hence, it was reported to be stable. Therefore, it can be concluded that a constant ZP charge signifies an increased stability. In general, NPs are considered stable when they possess a surface charge ≥± 30.Nassar et al.139 in their report used an extract from Citrus reticulata (CR, mandarin) peel during MNP synthesis. The surface modification of MNPs through the functional groups of the mandarin extract yielded a ZP value of −44.3 mV at pH 7, indicating the stability of the synthesized Fe3O4 NPs. The ZPs and hydrodynamic sizes are graphically presented in Fig. 4a–c and the PZC was found to be at pH 3.8 (Fig. 4c).
 | ||
| Fig. 4 (a) ZP at neutral pH, (b) histogram of hydrodynamic size, and (c) ZP at various pH values for Citrus reticulata (mandarin) peel inspired magnetite nanoparticles (source: this image is taken from an article written by Nassar et al.139). | ||
According to them, the tendency of the prepared NPs to repel each other reduced the polydispersity index (PDI = 0.419), which, in turn, increased the long-term stability of the NPs in solution. They suggested that in a basic medium (pH ∼11), the deprotonation of the capping biomolecules (e.g., polyphenols, flavonoids, etc.) containing functional groups of the CR-extract was responsible for the significant negative charge on phytogen@MNPs. Therefore, the dispersion of positively charged MNPs was stabilized by CR-extracted biomolecules, preventing their aggregation and agglomeration, and thus, their growth and expansion.
Hence, the better stability of the phytogen@MNPs is attributed to the presence of various coordinating groups containing surface coatings and high surface charges.
4.2 UV-vis spectral study and band gap analysis
Ultraviolet-visible spectroscopy was used to examine the absorbance profiles of the MNPs. In the typical UV absorption spectrum of the MNPs, a continuous decrease in absorbance from 300 to 800 nm was observed. Another piece of information obtained from the UV-vis spectra is the bandgap, which is a measure of the energy gap between the conduction and valence bands. Tauc et al.141,142 applied an expression to determine the optical bandgap of Ge (eqn (4)):| (αhν)1/n = A (hν − Eg) | (4) |
The band gap of a semiconductor (MNPs) varies with size. Abdulla et al.143 conducted a thorough investigation of the relationship between size and band gap. These results suggest that in semiconductors characterized by narrow to moderate band gaps, a reduction in size leads to an increase in the band gap. A similar study was performed by Singh et al.144 using CdSe, CdTe, ZnS, ZnSe, and ZnTe semiconductor compounds, and they found that the optical band gap varied with the size and shape (i.e., spherical nanospheres, nanowires, and nanofilms) of the materials. According to this theory, the bandgap energy increases as the particle size (diameter or length) of semiconductor nanomaterials decreases.
Golthi et al.145 used an aqueous leaf extract of Jatropha podagrica (JP) to synthesize JP-Fe3O4 NPs. Fig. 5a shows the UV-vis absorption spectra of the extract and the JP-Fe3O4 NPs. They reported that JP leaf extract exhibited pronounced absorption bands at 280 and 351 nm, attributable to the composition of various phytochemicals, including phenolic acids, tannins, alkaloids, proanthocyanidins, flavonoids, and glycol flavones. Furthermore, the formation of JP-Fe3O4 NPs was confirmed by the presence of a characteristic absorption band at 341 nm, which was primarily due to the scattering and absorption of light by the nanoparticles. The presence of characteristic peaks from the JP leaf extract at 280 and 351 nm in the JP-Fe3O4 NPs spectra is noteworthy and confirms the capping of leaf extract over NPs. Fig. 5b displays the equivalent Tauc plot, and the value of the direct band gap was 3.25 eV.
 | ||
| Fig. 5 (a) UV-vis spectra and (b) corresponding Tauc plot for the Fe3O4 nanoparticles synthesized using Jatropha podagrica leaf extract (source: this image is taken from an article written by Golthi et al.145); (c) FTIR spectra of magnetite nanoparticles using Thymus schimperi leaf extract (i) after and (ii) before calcination (source: this line is taken from an article written by Geneti et al.131); (d) pXRD of MNPs synthesized using the leaf extract of Azadirachta indica (source: this image is taken from an article written by Zambri et al.150). | ||
From Table 5, it can be concluded that the band gap varies with the type of phytogen present on the phytogen@MNPs. The efficiency of a photocatalytic material is significantly dependent on its optical bandgap. This is because an incoming photon can only cause the electronic state to shift from the valence band to the conduction band if its energy is greater than or equal to the bandgap energy.
| Nanoparticles | Direct band-gap (Eg, eV) | References |
|---|---|---|
| Bare MNPs | 2.00 | 129 |
| Nigella sativa seed extract (aq. alc.)@MNPs | 2.74 | |
| Artemisia herba-alba (ARM)@MNPs | 2.87 | 146 |
| Rosemarinus officinalis (ROS)@MNPs | 2.95 | |
| Matricaria Pubescens (MAT)@MNPs | 2.96 | |
| Juniperus phoenicea (JUN)@MNPs | 2.97 | |
| Murraya paniculata (L) Jack flower extract (aq.)@MNPs | 2.57 | 24 |
| Caralluma acutangulla leaf extract (aq.)@MNPs | 1.94 | 147 |
4.3 FTIR study
Fourier-transform spectroscopy validated the presence of phytogens on the surface of MNPs. In general, the O–H stretching frequency (from water or coating agents such as polyphenol and eugenol) appeared at 3200–3500 cm−1. In the case of the N–H stretching of amides, it appears in the same range as that of O–H. The carbonyl group appeared at approximately 1600–1700 cm−1. The spectral range of 1600 cm−1 corresponds to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibration, whereas the range of 1400–1500 cm−1 is associated with the H–C–H scissoring vibration of the –CH2– group. The range of 1300–1400 cm−1 pertains to N–O bending, the 1200–1300 cm−1 range is indicative of the C–O asymmetric stretching vibration in cyclic polyphenols, and the 1000–1100 cm−1 range corresponds to the C–O stretching vibration.148 Owing to the presence of polyamides in the phytogen@MNPs, the N–H stretching frequency for 2° amines appeared at 2800–2900 cm−1, which is in the same range as the C–H stretching of the alkyl groups. For MNPs, the Fe–O stretching frequency appears at approximately 400–600 cm−1, which includes tetrahedral and octahedral Fe–O sites.149 Based on FTIR analysis, it can be inferred which types of molecules containing coordinating groups may be present on the surface of the MNPs.
C stretching vibration, whereas the range of 1400–1500 cm−1 is associated with the H–C–H scissoring vibration of the –CH2– group. The range of 1300–1400 cm−1 pertains to N–O bending, the 1200–1300 cm−1 range is indicative of the C–O asymmetric stretching vibration in cyclic polyphenols, and the 1000–1100 cm−1 range corresponds to the C–O stretching vibration.148 Owing to the presence of polyamides in the phytogen@MNPs, the N–H stretching frequency for 2° amines appeared at 2800–2900 cm−1, which is in the same range as the C–H stretching of the alkyl groups. For MNPs, the Fe–O stretching frequency appears at approximately 400–600 cm−1, which includes tetrahedral and octahedral Fe–O sites.149 Based on FTIR analysis, it can be inferred which types of molecules containing coordinating groups may be present on the surface of the MNPs.
Geneti et al.131 synthesized MNPs using the leaf extract of Thymus schimperi and recorded the FT-IR spectrum of the calcined NPs, as shown in Fig. 5c(i). Significant absorption bands were identified at 3440 cm−1 (O–H stretching), 1622 cm−1 (N–H bending of amide), 1425 cm−1 (>CH2< bending), 875 cm−1 (C–H bending), and 568 cm−1 (Fe–O stretching). In contrast, Fig. 5c(ii) shows the FTIR spectra of the uncalcined nanoparticles, with absorption bands observed at 3420 cm−1 (O–H stretching), 2959 cm−1 (>CH2< stretching), 1623 cm−1 (N–H bending of amide), 1456 cm−1 (>CH2< stretching), 1377 cm−1 (O–H bending), 1064 cm−1 (C–N stretching), and 600 cm−1 (Fe–O stretching of Fe3O4). According to them, phytochemicals such as thymol and carvacrol were present in the extract; hence, O–H stretching vibration was observed, and the presence of H2O molecules may also contribute to its appearance.
4.4 Powdered X-ray diffractometry study
Powdered X-ray diffraction data generally provide information on the crystallinity and crystal size. The functionalized MNPs with a different surface coating with the same NP core had similar XRD patterns. For example, Bouafia et al.151 synthesized MNPs using an extract from the leaves of Artemisia L., employing a 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 volume ratio of Fe(III) chloride (0.1, 0.01, 0.04, and 0.07 M) to the leaf extract. The XRD patterns of both the synthesized and annealed MNPs (at 500 °C) exhibited sharp and narrow peaks, indicative of their fine particle nature and small crystallite size. Six distinct diffraction peaks were observed at 2θ values of 30.25°, 35.45°, 43.20°, 53.39°, 57.26°, and 62.90°, corresponding to the (220), (311), (400), (422), (511), and (440) crystallographic planes of the Artemisia L@MNPs phase. These reflections are consistent with the cubic structure of magnetite (space group: Fd-3m), indexed to Joint Committee on Powder Diffraction Standards (JCPDS) Card No. 01-075-0449. A progressive increase in peak intensity was observed with increasing ferric chloride concentration, suggesting enhanced crystallinity and a promotion effect on the degree of crystallization. The average crystallite size calculated using the Debye–Scherrer equation ranged from 24.67 to 34.28 nm.
1 volume ratio of Fe(III) chloride (0.1, 0.01, 0.04, and 0.07 M) to the leaf extract. The XRD patterns of both the synthesized and annealed MNPs (at 500 °C) exhibited sharp and narrow peaks, indicative of their fine particle nature and small crystallite size. Six distinct diffraction peaks were observed at 2θ values of 30.25°, 35.45°, 43.20°, 53.39°, 57.26°, and 62.90°, corresponding to the (220), (311), (400), (422), (511), and (440) crystallographic planes of the Artemisia L@MNPs phase. These reflections are consistent with the cubic structure of magnetite (space group: Fd-3m), indexed to Joint Committee on Powder Diffraction Standards (JCPDS) Card No. 01-075-0449. A progressive increase in peak intensity was observed with increasing ferric chloride concentration, suggesting enhanced crystallinity and a promotion effect on the degree of crystallization. The average crystallite size calculated using the Debye–Scherrer equation ranged from 24.67 to 34.28 nm.
After synthesizing MNPs using Azadirachta indica leaf extract, Zambri et al.150 observed an XRD pattern that revealed six distinct Fe3O4 diffraction peaks. With the Fd3ms space group, the sample was indexed to a single-phase cubic structure, and all the diffraction peaks agreed well with JCPDS file No. 19-0629. Using Rietveld refinement, the lattice parameters were determined to be a = b = c = 8.3559 Å, and the unit cell volume was 583.42 Å3.150 The structural planes of Fe3O4 and the Rietveld refinement of the XRD patterns are shown in Fig. 5d. The Debye–Scherrer equation, expressed as D = (0.94λ)/(β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ), was employed to determine the average crystallographic size (D), where λ represents the X-ray wavelength, β denotes the broadening of the peak at half maximum, and the calculated average size was 9.48 nm.
θ), was employed to determine the average crystallographic size (D), where λ represents the X-ray wavelength, β denotes the broadening of the peak at half maximum, and the calculated average size was 9.48 nm.
4.5 FESEM study
Field emission scanning electron microscopy (FE-SEM) is usually used to determine the morphology of NPs, that is, their shape. FESEM usually provides an idea of whether the NPs are surrounded by some type of coating agent or not. In addition to FESEM, energy dispersive spectroscopy can be used to reveal the possible elements present on the coated NPs. Energy Dispersive Spectroscopy (EDS)-elemental mapping, another version of EDS, allows for the colorful mapping of each possible element.Mohamed et al.152 synthesized MNPs using green coffee and reported FESEM images (Fig. 6a and b) along with EDS color mapping (Fig. 6c–f) and found strong evidence for the formation of MNPs coated with green coffee containing molecules. The color mapping images clearly indicate that the NPs contained 25% Fe, 9% C, and 66% O.
 | ||
| Fig. 6 (a and b) FESEM and (c–f) EDS color mapping analysis of the green-synthesized MNPs using green coffee (source: this image is taken from an article written by Dhar et al.152). A typical (g) SEM image and (h) EDS spectrum of MNPs obtained by green synthesis using Lathyrus sativus peel extract (source: this image is taken from an article written by Mohamed et al.153). | ||
The morphology of Lathyrus sativus peel extract-based MNPs, which were irregularly shaped and aggregated, was observed by Dhar et al.153 (Fig. 6g). They speculated that this could be due to the interaction between the phytochemicals and Fe3O4-NPs, as discussed in their study. They stated that agglomeration could be caused by the steric effect attributed to the interaction between the MNPs' magnetic nature and their active sites. Fig. 6h shows the elemental composition of MNPs. Both elemental oxygen and iron were confirmed by the high peak at 6.398 keV and sharp peak at 0.525 keV in the EDS spectrum. Therefore, the unique Fe and oxygen peaks in the EDS image (Fig. 6h) indicate that the Fe3O4-NPs were formed via an eco-friendly and sustainable route.
4.6 HRTEM study
High-resolution transmission electron microscopy (HRTEM) was employed to determine the morphology and size of phytogen@MNPs. HRTEM image analysis confirmed their shape and provided insights into the presence of the coating material. Furthermore, the selected area electron diffraction (SAED) pattern obtained from HRTEM revealed the crystalline nature of the phytogen@MNPs, supporting the findings of the powder XRD analysis.Golthi et al.145 analyzed the size and morphology of Jatropha podagrica-leaf extract synthesized MNPs using TEM and found that the nanoparticles were irregularly shaped (Fig. 7a–d). According to their findings, most of the particles were agglomerated and had an average size of 15.57 nm (Fig. 7e). According to their findings, the crystal planes obtained from SAED results (Fig. 7f) resembled the XRD patterns for MNPs, which supported the XRD data.
 | ||
| Fig. 7 (a–d) HRTEM images under resolutions of 50, 20, 10, and 2 nm, respectively; (e) histogram of size analysis; and (f) SAED pattern for the Jatropha podagrica-leaf extract synthesized MNPs (source: this image is taken from an article written by Golthi et al.145). | ||
4.7 Surface area analysis via the Brunauer–Emmett–Teller method
The Brunauer–Emmett–Teller (BET) method was used to determine the specific surface areas of the obtained materials, which depended on the synthesis method. The nitrogen physisorption isotherms and pore size distribution of each NM were used to determine its surface area. According to the International Union of Pure and Applied Chemistry (IUPAC) classification, nanocrystalline Fe3O4 can be microporous, mesoporous, or macroporous with pore sizes of <2, 2–50, or >50 nm.154Mahlaule-Glory et al.155 synthesized MNPs using M. burkeana extract and used them for the degradation of the sulfisoxazole antibiotic, methylene blue dye (MB), and the removal of microorganisms from real water. They performed BET analysis (Fig. 8a–c) to obtain information regarding the surface properties, such as surface area, pore volume, and size of the green-synthesized Fe3O4 NPs. They obtained a surface area of 12.83 m2 g−1, with a pore size of 35.67 nm, which falls within the range of 2–50 nm, implying that the sorbent is mesoporous. These data clearly indicate that MNPs can be used as photocatalysts for the degradation of various contaminants in wastewater.
 | ||
| Fig. 8 (a) Adsorption–desorption isotherm, (b) pore size, and (c) surface area plot for the M. burkeana extract mediated Fe3O4 NPs (source: this image is taken from an article written by Mahlaule-Glory et al.155). | ||
4.8 TGA study
Thermogravimetric analysis (TGA) was used to measure the thermal stability and percentage of coating on the NP surface. In general, surface-coated MNPs were heated at 25–900 °C under a 10 °C min−1 heat flow in an inert environment. Generally, in TGA, we obtained only a one-step weight loss, but in the case of phytogen@MNPs, only two or three weight loss steps were observed. The first step of weight loss generally occurs because of the evaporation of water or adsorbed ions, such as hydroxyl groups. In the following step, the coating begins to degrade, and the surfaces are exposed, which remain stable even at high temperatures (900 °C). Sometimes, coating materials can have very good coordinating groups, and thus the coated MNPs become thermally stable.Das et al.54 synthesized MNPs from aqueous Terminalia arjuna bark extract, which was characterized using various techniques, including TGA, and used to remove Pb(II) and MB. The experiment was performed in the temperature range of 25–900 °C at a rate of 20 °C min−1, and three stages of degradation were observed (Fig. 9a). In the first stage (12.6%, <180 °C), H2O, OH−, etc. and weakly adsorbed volatile impurities left the surface. In the second stage (26.6%, 180–500 °C), degradation of phytogen occurred from the surface. In the third phase of degradation (4.1%, 500–900 °C), complete removal of the surface coating occurred. Based on these results, the surface of the synthesized MNPs had a coverage of approximately 43.3%.
 | ||
| Fig. 9 (a) TGA analysis of T. arjuna bark extract (aq.) coated MNPs (source: this image is taken from an article written by Das et al.54); analysis of Azadirachta indica leaf extract-mediated MNPs: (b) VSM analysis, (c) before and (d) after magnetic separation (source: this image is taken from an article written by Zambri et al.150). | ||
4.9 Magnetic measurement study
Magnetic measurements were obtained by analyzing the samples using VSM or AGM. In standard magnetic measurements, when the coercivity approaches zero, the curve exhibits an absence of a hysteresis loop, thereby indicating a superparamagnetic nature. The applicability of a material increases with its magnetic moment, specifically its saturation magnetization value. The magnetic moment of MNPs depends on the type of coating material present on the surface and the size of the MNPs. The larger the size of the MNPs, the greater the magnetic moment.156 In brief, as the particle size decreased, the number of magnetic dipoles also decreased. The interactions between the magnetic moments result in the internal coupling of magnetic resistance, which is subsequently reduced by these dipoles.157 For example, bare MNPs had a saturation magnetization value (Ms) of 70 emu g−1, whereas when Cinnamomum tamala leaf extract (aq.) containing molecules were present on the surface, a significant reduction in Ms occurred, which was found to be 33.8 emu g−1.148Zambri et al.150 synthesized MNPs with the help of Azadirachta indica leaf extract and recorded the magnetic moment by a VSM study; the results are shown in Fig. 9b. The absence of a hysteresis loop in their results suggests a superparamagnetic behavior. The maximum saturation magnetization (Ms) of the synthesized MNPs was 73.040 emu g−1, which was lower than that of the bare MNPs (Ms = 92 emu g−1), most likely because of the disordered spin layer on their surfaces. According to their findings, a decrease in the size of the resultant particles significantly increases the ratio of the disordered layer to the radius of the MNPs. Consequently, this surface spin disorder contributed to the reduced Ms of smaller nanoparticles. Therefore, Ms was enhanced by increasing the crystallite size of the magnetic particles. It has been reported that Ms values of 7–22 emu g−1 are suitable for biomedical applications. They also showed the behavior of Fe3O4 nanoparticles before and after the application of an external magnetic field, as shown in Fig. 9c and d.
Table 6 summarizes the information obtained from the characterization techniques and provides additional details.
| Techniques | Information |
|---|---|
| DLS | Size distribution is determined by measuring laser light scattering through a colloidal solution (PDI), indicating nanosized particle formation through hydrodynamic analysis |
| ZP measurement | It determines the surface charge, stability of colloidal suspensions, and isoelectric point.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) The stability of the NPs is enhanced by increased electrostatic repulsion, as indicated by larger magnitude potentials The stability of the NPs is enhanced by increased electrostatic repulsion, as indicated by larger magnitude potentials |
| UV-vis spectroscopy | Detection of optical properties based on size, shape, concentration and agglomeration. The instrument allows precise measurement of solution concentrations including adsorbate, adsorbent, and post-treatment samples through absorbance analysis, and is also employed to estimate the band gap of semiconducting nanomaterials |
| Photoluminescence (PL) spectroscopy | Optical properties can be monitored by this technique. PL spectroscopy investigates biomaterial changes, characterizes nanoparticle conjugation, and assesses encapsulation efficiency and release mechanisms |
| Time resolved fluorescence (TRF) spectroscopy | Measures the decay of fluorescence over time after excitation |
| Cathodoluminescence (CL) | Optical properties can be observed from this technique. It is an emission spectroscopy that measures the light emission spectrum under free electrons |
| pXRD | It monitors crystal structure, composition, oxidation states, electronic structure, grain size, particle size, defects, and growth kinetics |
| FTIR, NIR | It shows functional groups (polymeric, organic, and inorganic materials) present on the surface and molecular interactions between medicines and encapsulating MNPs, which are crucial for tailoring properties and performance in drug delivery systems |
| Raman spectroscopy | It is a powerful and non-destructive tool for characterizing the density of defects and assessing the structural evolution of NPs, including the degree of graphitization |
| Electron microscopy (TEM, SEM, AFM, STEM) | TEM and SEM help to determine purity and exfoliation of material bundles along with size, morphology, crystal structure, dispersion of MNPs, and elemental composition. AFM provides 3D shape and surface evaluation |
| SAED | Crystal structure, diffraction pattern, and orientation can be determined |
| Energy-dispersive X-ray spectroscopy (EDX or EDS) or its mapping analysis | It measures elemental composition and colored mapping of different elements |
| X-ray photoelectron spectroscopy (XPS) | It is used to study the surface of a material, elemental composition, and oxidation states |
| CHNS analysis | It determines the percentage of carbon, hydrogen, nitrogen, and sulfur |
| Differential scanning calorimetry (DSC)/TGA | Compositional purity and thermal stability can be determined |
| BET surface area analysis | It measures the surface area, porosity, and density. Pore volume and pore size can also be determined |
| Magnetic moment measurements (VSM, AGM, SQUID) | They establish the magnetic nature and determine the magnetic moment. SQUID is the most sensitive, and VSM is the least sensitive technique, while AGM lies in between them |
| Atomic emission spectroscopy (AES) | Elemental composition can be measured from this technique |
| Inductively coupled plasma-mass spectrometry (ICP-MS) | It can determine the size, size distribution, and NP concentration, and it identifies and quantifies the elemental composition of samples |
| Static laser light scattering (SLS) | It measures and detects particle sizes ranging from nm to mm, and distributions. It also measures the angular variation of light scattering used to indicate particle size |
| Grazing incidence small-angle X-ray scattering (GISAXS) | It gives information about the structure, morphology, correlations, and mutual orientation |
| Four-point-probe resistivity measurement | Provides quantitative data of conductive properties |
In conclusion, it can be stated that the performance of the synthesized MNPs in pollutant removal is linked to their physicochemical characteristics, particularly particle size, surface area, and surface functionalization. The nanoscale dimensions provide a high surface-to-volume ratio and abundant active sites, enhancing the adsorption of heavy metals, dyes, and pharmaceutical residues. A controlled size distribution is essential for achieving superparamagnetism, which allows magnetic recovery from complex aqueous matrices. Surface modification using phytogenic functional groups improves the colloidal stability, prevents aggregation, and introduces binding sites that promote selective interactions with contaminants. These surface moieties enable strong chemisorption of metal ions and facilitate catalytic pathways for generating ROS, which are essential for the photocatalytic degradation of organic pollutants. The structural and surface attributes of MNPs directly influence their adsorption capacity, degradation kinetics, and recyclability, highlighting the need for rational design to maximize environmental remediation efficiency.
5. Removal of wastewater contaminants
5.1 Wastewater containing contaminants: a deep insight
Organic pollutants in wastewater include dyes, humic substances, phenolic compounds, petroleum, surfactants, pesticides, microplastics, polyfluoro/chloro alkyl/arenes, and pharmaceutical organic compounds.158 Organic contaminants in water can produce harmful compounds during disinfection. According to an estimate, on average, approximately 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 micro- and nanoplastics are ingested annually by every person through seafood.159 Exposure to microplastics in humans can result in oxidative stress, cytotoxicity, neurotoxicity, disruption of the immune system, and translocation to other tissues.160 Aquatic organisms in stream ecosystems may experience both acute and chronic adverse effects such as behavioral changes, tissue accumulation of contaminants, reproductive impairments, and inhibited cellular proliferation resulting from prolonged exposure to low concentrations of complex pharmaceutical wastes. They can also cause carcinogenicity, neurotoxicity, and developmental toxicity.161
000 micro- and nanoplastics are ingested annually by every person through seafood.159 Exposure to microplastics in humans can result in oxidative stress, cytotoxicity, neurotoxicity, disruption of the immune system, and translocation to other tissues.160 Aquatic organisms in stream ecosystems may experience both acute and chronic adverse effects such as behavioral changes, tissue accumulation of contaminants, reproductive impairments, and inhibited cellular proliferation resulting from prolonged exposure to low concentrations of complex pharmaceutical wastes. They can also cause carcinogenicity, neurotoxicity, and developmental toxicity.161
Inorganic pollutants that can be harmful to humans include metallic (mostly heavy metals) and non-metallic ions such as Hg(II), Cu(II), Pb(II), U(VI), Cd(II), As(V/VIVI), Cr(VI), NO3−, PO43−, SO42−, F−, Cl−, and C2O42−.162 These pollutants lead to environmental disruption, adversely affect aquatic life and wildlife, and pose several health risks to humans, including kidney and liver damage, as well as an increased risk of cancer.163,164 Previous studies have reported the impact of heavy metals on cellular organelles and various biological systems. These include the cell membrane, lysosomes, mitochondria, nuclei, endoplasmic reticulum, and several enzymes involved in detoxification, metabolism, and damage repair. It has been reported that metal ions interact with DNA and nuclear proteins within cells, resulting in DNA damage and conformational changes that may influence cell apoptosis, cell cycle progression, or carcinogenesis.165,166
Among the harmful microorganisms, Escherichia coli (E. coli), Staphylococcus aureus (S.aureus), Vibrio cholerae, Shigella dysenteriae, Vibrio parahaemolyticus, Cryptosporidium, Campylobacter, Enterovirus, Giardia, and Rotavirus are most commonly found in water.167 These microorganisms can cause several health problems, such as diarrhea, stomach cramps, fever, vomiting, cough, runny nose, skin rashes, upset stomach, yellow skin or eyes, abdominal pain, and shortness of breath. Some infections manifest in a way that can even result in death; for example, according to an estimate by the World Health Organization (WHO), approximately 485![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 diarrheal deaths occur each year due to contaminated drinking water.
000 diarrheal deaths occur each year due to contaminated drinking water.
5.2 Magnetite nanoparticles towards wastewater purification via adsorption and degradation
Owing to the presence of these contaminants, the future demand for potable water is unlikely to be met, and nearly all water sources are rendered unsuitable for consumption. Different methods have been used to remove various organic and inorganic pollutants from wastewater, such as membrane filtration (nanofiltration, distillation, ultrafiltration, and microfiltration), adsorption, chemical, electric, photocatalytic, chemical transformation, coagulation, flocculation, and biological treatments. Among the available techniques, photocatalytic degradation and adsorption are the two most efficient, simple, and environmentally friendly processes for cleaning contaminated water.168,169 Photocatalytic removal of various organic substances and inorganic ions by NPs can occur via the formation of several ROS, such as singlet oxygen (O21), hydroxyl radicals (OH˙), superoxide ions (O2−), hydrogen peroxide (H2O2), electrons (e−), and holes (h+). ROS, e, and h+ can oxidize or reduce organic and inorganic pollutants to form CO2, H2O, and other non-toxic forms of pollutants. Conversely, adsorption pertains to the interaction between the adsorbent, which consists of pollutants, and the adsorbate, represented by the NPs. This interaction can manifest as either a surface phenomenon, known as physisorption, or a whole-body phenomenon, known as chemisorption, involving NPs. In both processes, NPs can be reused several times, thereby making the process cheaper, which is another advantage of the photocatalytic degradation and adsorption techniques.Adsorption is a water purification method that removes dissolved impurities by adhering substances from liquids or gases to the interface between two phases. It is a low-cost and effective method for removing inorganic pollutants, synthetic dyes, and organic pollutants from polluted waters.171 Adsorption can remove several metallic and non-metallic ions/compounds:
• Many types of pesticides and other synthetic organic chemicals.
• Heavy metals like lead, cadmium, uranium, copper, etc.
• Synthetic dyes.
• Pharmaceutical molecules.
• Other organic pollutants like phenols, nitrobenzene etc.
Adsorption has several advantages over other methods, including a simple design, low investment in terms of the initial cost and area required, and the ability to remove almost all types of pollutants from water. The adsorption process is extensively used for treating industrial wastewater containing organic and inorganic pollutants and has attracted considerable attention from researchers. In recent years, the search for cost-effective adsorbents with pollutant-binding capacities has gained popularity. Eco-friendly and renewable materials, such as natural materials, industrial waste, and agricultural waste, are now used as cost-effective adsorbents. Activated carbon prepared from these materials can be used as an adsorbent for water and wastewater treatment. Various metals, metal oxides, metal sulfides, and nanoparticles have been used for adsorptive removal of contaminants from water. MNPs are highly suitable for application as adsorbents when optimally coated.
Although adsorption is a convenient and successful technique for removing pollutants, it has several limitations, such as high complexity, excessive use of reagents, high operational costs, and stability of adsorbents over time.172 For example, Bankole et al.173 reported that commercial activated carbons are good adsorbents, but their limitation is their high cost. Agglomeration, sludge production, and slowness are other disadvantages of the adsorption techniques.174
Photocatalysis is an important green method for decontamination and detoxification of aquatic environments because its functionality remains consistent regardless of temperature for almost all types of organic contaminants. It has several applications, including CO2 reduction, organic contaminant degradation, toxic and heavy metal ion removal, water splitting, bacterial eradication, and self-cleaning.175 Therefore, the development and design of innovative photocatalyst-semiconductors, specifically heterogeneous magnetic materials, have attracted significant attention. They are simple to use, have low toxicity, are easily separable, and consume significantly less energy during operation. According to Liu et al.,176 a magnetite-carbon nanofiber composite (Fe3O4-NPs@CNF) may be an effective choice for reducing carbamazepine pollution. However, the complex synthetic procedures and high-temperature requirements render this material expensive. Lotfi et al.177 used a polysulfone membrane covered with TiO2 to degrade four steroid hormones in wastewater. The high cost, high temperature, and use of dangerous materials are the major drawbacks in these cases. Shi et al.178 removed acetaminophen using a combination of magnetite nanoparticle-modified cyclodextrin and potassium permanganate; however, the oxidizing agent used and the procedure involved were complex. Awwad et al.179 synthesized carbon-doped ceria-NPs for the degradation of cortisone acetate. The two major issues are lengthy processes and high energy consumption. In addition, Jimenez-Salcedo et al.180 proposed that graphitic carbon nitride nanosheets can efficiently degrade sodium diclofenac; however, they used high temperatures and complicated separation procedures.
To purify wastewater, safer nanoparticles should be used to ensure the safety of both aquatic and land uses. Various types of chemically coated nanoparticles have been previously reported. Chemical modification is also a method to stabilize MNPs and endow them with multifunctionality; however, owing to the high production temperature, high cost, and material toxicity, this process is less frequently used. A schematic representation of the use of phytogen@MNPs for wastewater treatment is shown in Fig. 10.
5.3 Organic and inorganic contaminant removal through adsorption
The entire process was performed over a fixed time interval of a few minutes to hours until equilibrium was reached. The same study was performed by varying the pH and concentrations of adsorbates and adsorbents. Four linear kinetic models were used (eqn (5)–(8)):
Pseudo-first-order:
ln(Qe − Qt) = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Qe − k1t Qe − k1t | (5) |
Pseudo-second-order:
 | (6) |
Elovich kinetic model:
 | (7) |
Intra-particle diffusion:
| Qt = kpt1/2 + X | (8) |
The adsorption capacity (Qt, mg g−1) was calculated using the equation (eqn (9)):
 | (9) |
For instance, Fato et al.183 removed Pb(II), Cd(II), and Cu(II) from river water using ultrafine mesoporous magnetite nanoparticles synthesized using simple co-precipitation techniques. These MNPs were 4–17 nm in size and could efficiently remove these ions from water. The authors reported that a pseudo-second-order kinetic model was followed for both single and competitive adsorption processes. According to them, in the case of single adsorption, R2 for the pseudo-second-order model was >0.99 in every case, and the theoretical adsorption capacities [qe (cal) = 25.06 (for Pb(II)), 21.88 (for Cd(II)), and 22.99 (for Cu(II)) mg g−1] matched well with the experimental values [qe (exp) = 24.4 (for Pb(II)), 21.64 (for Cd(II)), and 22.38 (for Cu(II)) mg g−1]. Because the R2 in every case was very close to unity and the calculated adsorption capacity matched well with the experimental one, they concluded that the sorption process obeyed pseudo-second-order kinetics and not pseudo-first-order kinetics. Andelescu et al.184 used magnetite/carbon nanocomposites to remove Cu(II), Cd(II), and Zn(II) from water using a batch adsorption method. According to their findings, the adsorption mechanism was not fully described by the pseudo-first-order [R2 = 0.84–0.98 including all concentrations of Cu(II), Cd(II), and Zn(II)] and Elovich [R2 = 0.77–0.95 including all concentrations of Cu(II), Cd(II), and Zn(II)] equations (eqn (5) and (7)), as indicated by their weaker correlation coefficients (R2). This indicates that chemical adsorption was the predominant controlling mechanism and that the process was fast. The pseudo-second-order [R2 = 0.993–0.999 for all concentrations of Cu(II), Cd(II), and Zn(II)] equation (eqn (6)) yielded the highest correlation coefficients, indicating that it was the most compatible with the experimental results. In addition, the experimentally obtained equilibrium adsorption capacity values [qe (exp)] using the pseudo-second-order model coincided with the calculated values [qe (calc)].184 The possibility of intraparticle diffusion was investigated using the intraparticle diffusion model represented by the Weber and Morris equation (eqn (8)). The plots were examined for all metal ions and depicted in two stages: the external surface adsorption stage in the first step and the closeness to the equilibrium stage in the second step. The fact that the plot did not pass through the origin suggests that intra-particle diffusion was not the limiting step in the adsorption of Cu(II), Cd(II), and Zn(II).
Langmuir isotherm:
 | (10) |
K L is the Langmuir isotherm constant (in L mg−1) related to the separation factor (RL), which provides information about the favorability of the isotherm model, such as (eqn (11)),
 | (11) |
Freundlich isotherm:
 | (12) |
Temkin isotherm:
Qe = B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KT + B KT + B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce Ce | (13) |
 , where b is the Temkin constant (J mol−1), T is the absolute temperature (K), and R = universal gas constant (J mol−1 K−1)). The Temkin model suggests that adsorption is a multilayer process, considering that indirect interactions between the adsorbate and adsorbent and a linear decrease in adsorption heat with increased surface coverage occur, but are only valid for intermediate concentrations.
, where b is the Temkin constant (J mol−1), T is the absolute temperature (K), and R = universal gas constant (J mol−1 K−1)). The Temkin model suggests that adsorption is a multilayer process, considering that indirect interactions between the adsorbate and adsorbent and a linear decrease in adsorption heat with increased surface coverage occur, but are only valid for intermediate concentrations.
Dubinin-Radushkevich isotherm:
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Qe = ln Qe = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Qmax − β ε2 Qmax − β ε2 | (14) |
 , where ε = polanyi potential (in kJ2 mol−2) =
, where ε = polanyi potential (in kJ2 mol−2) = 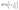 . The Dubinin-Radushkevich isotherm is an empirical model that describes the adsorption on surfaces with varying properties. However, it is only suitable for intermediate concentrations owing to its unrealistic asymptotic behavior. A semi-empirical equation, which assumes a multilayer character, was used to distinguish between the chemical and physical adsorption of metal ions. This depends on the temperature.
. The Dubinin-Radushkevich isotherm is an empirical model that describes the adsorption on surfaces with varying properties. However, it is only suitable for intermediate concentrations owing to its unrealistic asymptotic behavior. A semi-empirical equation, which assumes a multilayer character, was used to distinguish between the chemical and physical adsorption of metal ions. This depends on the temperature.
Elovich isotherm:
 | (15) |
| Aspect | Advantages | Disadvantages |
|---|---|---|
| Langmuir isotherm | ||
| Monolayer coverage | Accurate for single-layer adsorption | Not applicable for multilayer scenarios |
| Surface homogeneity | Ideal for homogeneous sites | Fails for real porous/rough surfaces |
| Simplicity | Convenient to use and understand | Oversimplifies complex systems |
| Adsorbate interactions | Simplified and ignores them | Leads to errors in high coverage or interactive adsorbates |
| Applicability range | Works well at low pressures | Fails at high pressures |
| Temperature dependence | Not required | Neglects temperature effects on adsorption |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Freundlich isotherm | ||
| Surface type | Handles heterogeneous, non-uniform sites | Poor fit for homogeneous, ideal surfaces |
| Adsorption layers | Works for multilayer adsorption | Cannot predict saturation/monolayer coverage at high pressure |
| Mathematical simplicity | Only two empirical constants | Constants lack mechanistic physical meaning |
| Practical application | Widely used for real-world adsorbents | Limited theoretical explanation; purely empirical |
| Range of applicability | Valid at low pressure/concentration | Invalid for high-pressure/saturation regions |
| Parameter dependency | Adaptable to different systems | Constants must be experimentally determined for every system |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Temkin isotherm | ||
| Adsorbate interactions | Considers molecular interactions | Assumes a linear decline, which may not fit all systems |
| Surface type | Useful for moderate heterogeneity | Still based on a homogeneous surface assumption |
| Coverage range | Accurate at moderate coverage | Not valid at very low/high concentrations |
| Mathematical simplicity | Two-parameter model, easy to apply | Parameters lack direct physical meaning |
| Predictive power | Good for catalysis, environmental science | Not suitable for extremes or true saturation |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Dubinin-Radushkevich isotherm | ||
| Micropore adsorption | Accurately models pore filling in microporous materials | Limited accuracy for low pressure/zero loading |
| Surface energy | Handles heterogeneous/Gaussian energy distribution | Not suitable for mesoporous/macroporous materials |
| Physical vs. chemical | Can help distinguish the adsorption mechanism | Parameter determination is empirical |
| Theoretical foundation | Based on polanyi potential theory | Does not model multilayer adsorption |
| Porosity insights | Estimates porosity and energy of adsorbents | Valid only above 15% micropore filling |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Elovich isotherm | ||
| Surface heterogeneity | Models varying site energies, suitable for complex surfaces | Not applicable to homogeneous surfaces |
| Adsorption process | Valid for chemisorption and multilayer scenarios | Less reliable at high coverage or multilayer not present |
| Mathematical utility | Simple to use; fits for kinetic and equilibrium data | Empirical parameters lack deep mechanistic meaning |
| Capacity prediction | Predicts multilayer and dynamic adsorption | Underestimates actual maximum adsorption capacity |
| Practicality | Widely used in environmental, pollutant adsorption | Sometimes exhibits lower fit quality than other models |
In each case, the percentage of removal (including adsorption and degradation) was calculated using the following equation (eqn (16)).
 | (16) |
Using the above equations, the efficiency, mechanism, and optimal conditions of the adsorption techniques for the removal of several contaminants can be evaluated.
For example, Kalantary et al.186 removed nitrate from water using activated carbon-magnetite nanoparticles. After the batch adsorption experiment, the results were plotted in a linear form using the Langmuir, Freundlich, and Temkin isotherm models. The Langmuir isotherm model was found to be the most suitable (R2 = 0.99, close to unity), with a maximum adsorption capacity of 57.1 mg g−1 at pH 3 and contact time of 60 min. Several nanomaterials have been reported to be effective in removing various hazardous contaminants (Table 8).
| Nanomaterials | Waste contaminants | Conditions | Maximum adsorption capacity, Qmax (mg g−1) | Ref. |
|---|---|---|---|---|
| a Denotes g mg−1 min−1 | ||||
| Adsorption | ||||
| Portulaca oleracea extract@MNPs | Cd(II) | 20 ± 2.0 °C, pH 6, 60 min | 177.48 | 187 |
| Pb(II) | 108.22 | |||
| Leaf extract of Thymus schimperi@MNPs | Hg(II) | 25 ± 0.5 °C, pH 5, 90 min | 60.00 | 131 |
| Cr(VI) | 57.37 | |||
| Artocarpus heterophyllus leaf extract coated maghemite@MNPs | Pb(II) | 30 ± 1.0 °C, pH 6.5, 15 min | 108.57 | 188 |
| Sugarcane bagasse extract@MNPs | Carbofuran | rt, pH 7, 720 min | 175.00 | 189 |
| Iprodione | rt, pH 7, 480 min | 119.00 | ||
| Peanut shell extract@MNPs | Carbofuran | rt, pH 7, 480 min | 89.30 | |
| Iprodione | rt, pH 7, 720 min | 2.76 | ||
| Chromolaena odorata aqueous extract@MNPs | Cr(VI) | rt, pH 2, 10 min | 173.12 | 190 |
| Peel extract of jengkol (Archidendron pauciflorum)@MNPs | MB | rt, pH 6, 120 min | 68.49 mol g−1 | 191 |
| Aqueous flower extract of Murraya paniculata (L) Jack@MNPs | Fast sulphon black-F dye | rt, pH 4, 40 min | 6.62 | 24 |
| Parkia Speciosa Hassk. peel extracts@MNPs | pH 4, 120 min | — | 192 | |
| Nanomaterials | Waste contaminants | Conditions | Rate constant, kn [(mg g−1)1−n min−1] | Ref. |
|---|---|---|---|---|
| Photodegradation | ||||
| Nigella sativa seed aqueous extract@MNPs | Hydrocortisone | rt, pH 7, 540 min, 254 nm | k 1 = 0.00271 | 129 |
| Cynara cardunculus leaf aqueous extract @MNPs NPs | MB | rt, pH 7, 80 min | k 2 = 0.00617457a | 193 |
| Andean blackberry leaf extract@MNPs | MB | rt, 120 min, sunlight | k 1 = 0.0105475 | 194 |
| Congo red | rt, 120 min, sunlight | k 1 = 0.0043240 | ||
| Methyl orange (MO) | rt, 120 min, sunlight | k 1 = 0.0028930 | ||
| Calotropis gigantea leaf extract@MNPs | MB | rt, 50 min, sunlight | — | 195 |
| Caralluma acutangula leaf aqueous extract@Fe0/MNPs | MB | 30 °C, 150 min, 254 nm | k 1 = 0.13343 (apparent) | 147 |
| ARM@MNPs | Cresol red | 30 °C, pH 5.25, 60 min, 365 nm | k 1 = 0.00152 | 196 |
| ROS@MNPs | 30 °C, pH 5.05, 60 min, 365 nm | k 1 = 0.00149 | ||
| MAT@MNPs | 30 °C, pH 4.63, 60 min, 365 nm | k 1 = 0.00120 | ||
| JUN@MNPs | 30 °C, pH 3.69, 60 min, 365 nm | k 1 = 0.00100 | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KLvs. 1/T.
KLvs. 1/T.
The Van't Hoff equation is as follows:
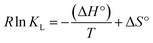 | (17) |
| ΔG° = ΔH°− TΔS° | (18) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KLversus 1/T, and ΔH° is the intercept. Another parameter that can be calculated is the activation energy (Eact), which is a measure of the initiation energy required for adsorption.
KLversus 1/T, and ΔH° is the intercept. Another parameter that can be calculated is the activation energy (Eact), which is a measure of the initiation energy required for adsorption.
The above formulae can be used to plot the thermodynamic curve between ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KL and 1/T, and the slope and intercept of the van't Hoff plots can be used to deduce the thermodynamic parameters. At a specific temperature, the spontaneity and feasibility of the adsorption reaction are indicated by the negative value of ΔG°.198 The feasibility of adsorbate adsorption decreases as the temperature increases, as indicated by the increasing ΔG°value. This process is referred to as physisorption when the ΔH° values fall between −20 and 40 kJ mol−1, and chemisorption when the values fall between – 80 and 400 kJ mol−1.199 In addition, the negative ΔH° value indicates that the adsorption process is exothermic.198 When the adsorbate was adsorbed, there was more unpredictability at the adsorbate–adsorbent interface, as indicated by the positive values of ΔS°.198,199 The thermodynamic parameters are listed in Table 9.
KL and 1/T, and the slope and intercept of the van't Hoff plots can be used to deduce the thermodynamic parameters. At a specific temperature, the spontaneity and feasibility of the adsorption reaction are indicated by the negative value of ΔG°.198 The feasibility of adsorbate adsorption decreases as the temperature increases, as indicated by the increasing ΔG°value. This process is referred to as physisorption when the ΔH° values fall between −20 and 40 kJ mol−1, and chemisorption when the values fall between – 80 and 400 kJ mol−1.199 In addition, the negative ΔH° value indicates that the adsorption process is exothermic.198 When the adsorbate was adsorbed, there was more unpredictability at the adsorbate–adsorbent interface, as indicated by the positive values of ΔS°.198,199 The thermodynamic parameters are listed in Table 9.
| MNPs | Pollutant(s) | T (K) | E act (kcal mol−1) | ΔH° (kcal mol−1) | ΔS° (cal mol−1 K−1) | ΔG° (kcal mol−1) |
|---|---|---|---|---|---|---|
| a kJ mol−1. b J mol−1 K−1. | ||||||
| Terminalia arjuna@MNPs54 | Pb(II) | 290 | — | −20.78a | −66.26b | −1.62a |
| 300 | −0.78a | |||||
| 310 | −0.30a | |||||
| Terminalia arjuna@MNPs54 | MB | 290 | — | −38.60a | −99.69b | −9.74a |
| 300 | −8.68a | |||||
| 310 | −7.75a | |||||
| Artemisia herba-alba @MNPs146 | Evans blue dye | 303.15 | 2.79 | 2.85 | 13.03 | −1.09 |
| 308.15 | −1.18 | |||||
| 313.15 | −1.23 | |||||
| 318.15 | −1.29 | |||||
| Rosemarinus officinalis @MNPs146 | Evans blue dye | 303.15 | 3.21 | 3.32 | 14.04 | −0.93 |
| 308.15 | −1.00 | |||||
| 313.15 | −1.09 | |||||
| 318.15 | −1.14 | |||||
| Matricaria Pubescens @MNPs146 | Evans blue dye | 303.15 | 5.59 | 5.96 | 20.86 | −0.36 |
| 308.15 | −0.47 | |||||
| 313.15 | −0.58 | |||||
| 318.15 | −0.67 | |||||
| Juniperus Phoenicia @MNPs146 | Evans blue dye | 303.15 | 6.29 | 6.54 | 22.70 | −0.35 |
| 308.15 | −0.43 | |||||
| 313.15 | −0.56 | |||||
| 318.15 | −0.66 | |||||
| Artemisia herba-alba@MNPs146 | MO | 303.15 | 3.27 | 3.31 | 13.24 | −0.69 |
| 308.15 | −0.78 | |||||
| 313.15 | −0.83 | |||||
| 318.15 | −0.89 | |||||
| Rosemarinus officinalis @MNPs146 | MO | 303.15 | 3.66 | 3.78 | 14.63 | −0.65 |
| 308.15 | −0.74 | |||||
| 313.15 | −0.81 | |||||
| 318.15 | −0.87 | |||||
| Matricaria Pubescens @MNPs146 | MO | 303.15 | 6.28 | 6.75 | 22.54 | −0.07 |
| 308.15 | −0.19 | |||||
| 313.15 | −0.33 | |||||
| 318.15 | −0.41 | |||||
| Juniperus phoenicea @MNPs146 | MO | 303.15 | 8.45 | 8.67 | 27.27 | +0.16 |
| 308.15 | +0.08 | |||||
| 313.15 | −0.07 | |||||
| 318.15 | −0.24 | |||||
5.4 Photocatalytic degradation of organic and inorganic pollutants
Pseudo-first-order equation:
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ct = −kt + ln Ct = −kt + ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C0 C0 | (19) |
Pseudo-second-order equation:
 | (20) |
Pseudo-zeroth-order equation:
| Ct = −kt + C0 | (21) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ctvs. t, 1/Ctvs. t, and Ctvs. t were plotted to determine whether the degradation of the respective dyes followed pseudo-first-order, pseudo-second-order, or pseudo-zeroth-order kinetics, respectively.
Ctvs. t, 1/Ctvs. t, and Ctvs. t were plotted to determine whether the degradation of the respective dyes followed pseudo-first-order, pseudo-second-order, or pseudo-zeroth-order kinetics, respectively.
During the kinetic experiments, the initial steps were performed in the dark (without irradiation) to determine whether the process involved exclusively photodegradation or adsorption. If adsorption occurred, the experimental solution was kept in the dark, and the absorbance was continuously measured until equilibrium was reached. After adsorption, the experimental solution was exposed to UV or sunlight to initiate photodegradation. Fig. 12 shows the use of phytogen@MNPs for the photodegradation of wastewater contaminants.
Fatimah et al.200 used MNPs synthesized using Parkia speciosa Hassk pod extract and applied them to bromophenol blue (BPB) degradation. From the obtained results, it was found that the R2 values of the pseudo-second-order kinetic model were higher (R2 = 0.98–0.99, i.e., close to unity) than those of the pseudo-first-order kinetic model (R2 = 0.77–0.90, not close enough to unity), so it can be concluded that the photocatalytic degradation of BPB obeyed pseudo-second-order kinetics at all initial concentrations of BPB. Additionally, they proposed that the data fit for the pseudo-second-order model demonstrated how the reaction rate was affected by both the photocatalyst material activity and the role of BPB as a reactant. After examining the kinetic models, the surface process was explained, indicating that the photocatalyst adsorbed BPB. These investigations demonstrated that chemisorption was the primary mechanism governing the interaction of cationic dyes such as BPB with the surface of the synthesized MNPs. A few reported photocatalytic degradations using phytogen@MNPs are presented in Table 8.
The degradation mechanism can be elucidated through scavenging experiments, which provide insights into the types of ROS responsible for the degradation process, such as superoxide anions (˙O2−), hydroxyl radicals (˙OH), and singlet oxygen (1O2). In these experiments, different types of agents (water-soluble compounds) were introduced into the solution containing the target pollutant and phytogen@MNPs. Upon irradiation, pollutant degradation by phytogen@MNPs was either suppressed or inhibited by specific interfering agents. This approach is known as the scavenging experiment. Different interfering agents are known to selectively scavenge specific ROS (Table 10), thereby providing concrete evidence regarding the type of ROS that is actively involved in the degradation process. The degradation mechanisms of the different ROS are shown in Fig. 13.
| ROS | Scavenger | References |
|---|---|---|
| ˙O2− | Methanol, 1,4-benzoquinone, superoxide dismutase | 201–205 |
| ˙OH | Isopropyl alcohol, tert-butyl alcohol, sulphate, monobasic phosphates, carbonate, bicarbonate | 203–207 |
| h+ | Oxalate, sulphite, chloride, iodide, ethylenediamintetraacetic acid disodium, triethanolamine, Formic acid | 201–206 |
| e− | N-phenylaniline, cupric nitrate, dichromate, bromate | 205–207 |
| O21 | Azide, deuterium oxide, L-histidine | 202, 205, and 206 |
| H2O2 | Catalase | 205 |
Suppose that ROS are generated by visible or UV irradiation of phytogen@MNPs and degrade pollutants. When a specific scavenger for a particular ROS was introduced into the solution, the degradation percentage decreased compared with its initial value. The scavenger reacts, thereby reducing the concentration of ROS and subsequently deactivating them in the solution. The deactivation reaction of ROS is represented by eqn (22)–(29):
| H2O2 + catalase = H2O + O2 | (22) |
| ˙O2− + 1,4-benzoquinone = O2 + 1,4-benzoquinone˙− | (23) |
| C2O42− + 2h+ = 2CO2 | (24) |
 | (25) |
| 2Cl− + 2e− = Cl2 | (26) |
| 2I− + 2e− = I2 | (27) |
 | (28) |
 | (29) |
 | (30) |
| MNPs | Pollutant(s) | T (K) | E act (kcal mol−1) | ΔHo (kcal mol−1) | ΔSo (cal mol−1 K−1) | ΔGo (kcal mol−1) |
|---|---|---|---|---|---|---|
| a kJ mol−1 b J mol−1 K−1. | ||||||
| Caralluma acutangula leaf aqueous extract@Fe(0)/MNPs147 | MB | 288.00 | 87.78a | 308.40a | −323.00b | −92.98a |
| 293.00 | −93.96a | |||||
| 298.00 | −95.89a | |||||
| 303.00 | −97.50a | |||||
| Aqueous Nigella sativa seed extract@MNPs129 | Hydrocortisone | 293.00 | 35.40a | — | — | — |
| 303.00 | ||||||
| 313.00 | ||||||
| Artemisia herba-alba @MNPs196 | Cresol red | 303.15 | 3.68 | 4.02 | 15.58 | −0.71 |
| 308.15 | −0.78 | |||||
| 313.15 | −0.85 | |||||
| 318.15 | −0.95 | |||||
| Rosemarinus officinalis @MNPs196 | 303.15 | 3.72 | 4.78 | 17.60 | −0.61 | |
| 308.15 | −0.65 | |||||
| 313.15 | −0.73 | |||||
| 318.15 | −0.82 | |||||
| Matricaria Pubescens @MNPs196 | 303.15 | 5.09 | 5.63 | 19.81 | −0.38 | |
| 308.15 | −0.46 | |||||
| 313.15 | −0.57 | |||||
| 318.15 | −0.65 | |||||
| Juniperus phoenicea @MNPs196 | 303.15 | 6.23 | 6.24 | 21.35 | −0.24 | |
| 308.15 | −0.33 | |||||
| 313.15 | −0.46 | |||||
| 318.15 | −0.55 | |||||
6. Recovery of phytogen@MNPs and reusability study
The recovery of the adsorbent or photocatalyst is an important step because it reduces the overall cost of the process. There are two types of adsorption process: physisorption and chemisorption. In physisorption, adsorbates are bonded to the NP surface via weak interactions, such as van der Waals forces; hence, there is a high probability of reversing the process, that is, the recovery of the adsorbent. In the case of chemisorption, there is a very strong interaction between the adsorbate and the adsorbent; hence, desorption becomes inefficient. Fig. 14 illustrates the regeneration of phytogen@MNPs from contaminants@phytogen@MNPs using various eluents via desorption.The desorption of adsorbates from the surface of the adsorbent can be performed via certain processes, including chemical elution. The desorption efficiency was calculated using eqn (31). Numerous factors affect the efficiency of an eluent, such as the type of adsorbent and adsorbate, interaction between the adsorbate and adsorbent, and desorbing agent used. Acids, alkalis, salts, chelating agents, and solvents (e.g., ethanol, methanol, isopropanol, and acetone) are among the most commonly used desorption agents.208 In addition to elution, degradation of the adsorbate from the surface of the adsorbent is an alternative process for removing adsorbed adsorbates from the adsorbent surface.
 | (31) |
The desorbed and adsorbed concentrations of MB and Pb(II) (mg L−1) are denoted as Cd and Ca, respectively.
The desorption mechanism is similar to that of cation or anion exchange. In general, eluents with small cations such as H+ and Na+ provide better results for desorbing cationic adsorbates. After successive desorption, further adsorption–desorption cycles can be performed.
In the case of the photocatalytic degradation process, reusability can be checked directly by the repeated introduction of MNPs into fresh adsorbate solutions in the presence of irradiation. The percentage of degradation was monitored using a UV spectrophotometer.
7. Stability of nanoparticles after adsorption and degradation
The stability of magnetite nanoparticles (MNPs) coated with plant extracts is a critical factor for their effective application, particularly in environmental remediation and biomedical applications. This stability is influenced by the interaction between the plant extract coating, magnetite core, and surrounding environment, especially after the adsorption of pollutants and degradation processes. After an adsorption–desorption cycle, if phytogen@MNPs do not exhibit any structural changes, they can be used repeatedly until their efficiency decreases. FTIR, powder XRD, TGA, and XPS analyses were used to obtain stability information for the MNPs. Similar (or identical) initial and final data suggest that the MNPs are stable and can be reused.7.1 Stability before adsorption
7.2 Stability after adsorption
The stability of plant extract coated MNPs can be significantly affected by the adsorption of target substances such as pollutants in wastewater treatment or active molecules for drug delivery.214(ii) Oil spill collection: magnetite nanoparticles modified with green hydrophobic biocomponents extracted from Anthemis pseudocotula (AP) were used to collect heavy crude oil spills.215 Surface modification helped fine-tune the MNPs for this application. While these particles showed high efficiency in oil collection and could be recycled using an external magnetic field after 5 min of adsorption, their long-term stability after repeated oil adsorption and recovery cycles requires further investigation to ensure consistent performance and to prevent leaching of the plant extract coating or aggregation of the nanoparticles.215 The thermal stability and magnetic properties of these nanoparticles were characterized, and the influence of the plant extract on dispersion and stability was noted.
7.3 Stability during degradation
The degradation of plant extract coated MNPs can occur owing to various factors, including chemical, biological, or thermal processes, and can lead to a loss of functionality or potential environmental risks.216(ii) Degradation of other organic coatings: although not derived from a plant extract, studies on BSA-coated magnetite nanoparticles demonstrated that thermal characterization using thermogravimetric analysis (TGA) was effective in understanding the degradation behavior of the protein coating.217 The degradation of plant extract coatings follows similar principles, where the organic material breaks down over time, influencing the overall stability and reusability of the nanoparticles. The amount of coating, temperature, and time can significantly affect the stability and degradation of nanoparticles.146
In conclusion, maintaining the stability of plant extract coated magnetite nanoparticles after adsorption and during degradation is paramount for their practical applications.219 Careful selection of plant extracts, optimization of coating methods, and thorough characterization of their behavior under various conditions are crucial to ensure their efficacy, reusability, and environmental safety.210,219
8. Impact of natural ions on adsorption and degradation
Adsorption or degradation in synthetic wastewater can provide a good indication of the potential of MNPs for effective contaminant removal, whereas the same process in natural water bodies can provide specific insights into the real-world use of MNPs. Naturally occurring water bodies contain several cations and anions, such as Ca2+, Na+, F−, SO42−, CO32−, and Cl−. Adsorption is involved in the surface coverage process via adsorbent–adsorbate interactions. When these ions are present in solution, the competition between the adsorbate and these ions varies depending on the nature of the ZP (at the working pH). The effects of water-containing ions on the adsorption process are illustrated in Fig. 15.When an adsorbate possesses a positive charge and is introduced into MNPs, which are typically negatively charged, positive ions such as Ca2+ and Na+ may interfere with the adsorbates. If these interfering ions exhibit a higher charge density than the adsorbate, they initially form surface coverage, followed by adsorbate ions, resulting in a decrease in the adsorption efficiency. During the degradation process, adsorbates are first adsorbed, after which degradation commences via reactive oxygen species (ROS) activity. Consequently, a reduction in degradation efficiency may occur for the same reason.
Guan et al.220 investigated the effects of five cations and five anions on quinolone adsorption by iron-containing minerals and found that K+, Na+, NH4+, Cl−, NO3−, and SO42− exhibited less substantial inhibition of adsorption than Mg2+, Ca2+, HCO3−, and H2PO4−. They also collected samples of naturally occurring surface water, which they then employed as a medium to study the adsorption behavior of quinolones on iron-containing minerals. Maintaining natural water's buffering capacity under circumneutral conditions, they observed that the amount of adsorption was primarily promoted in the goethite system (from 0.56–0.78 µmol g−1 to 0.52–1.43 µmol g−1), but inhibited in the other systems (kaolin: from 1.98–1.99 µmol g−1 to 0.90–1.40 µmol g−1; magnetite: from 1.13–1.33 µmol g−1 to 0.45–0.76 µmol g−1; kaolin: from 1.98–1.99 µmol g−1 to 0.90–1.40 µmol g−1); and hematite: from 0.52–0.65 µmol g−1 to 0.02–0.18 µmol g−1
9. Antibacterial activity study
In addition to the efficiency of removing organic and inorganic contaminants, the antimicrobial efficacy of MNPs against various waterborne microbes is a crucial criterion, as these microbes can cause significant diseases, such as diarrhea and typhoid. Therefore, the elimination of microbes is also important.The antibacterial activity of phytogen@MNPs is primarily enabled by multiple specific mechanisms that effectively target the bacterial cells. One key mechanism is the generation of ROS, such as superoxide and hydroxyl radicals, which cause oxidative stress in bacterial cells, leading to damage to cellular components, including membranes, proteins, and DNA. Oxidative damage disrupts bacterial membrane integrity and metabolism, leading to cell death. Additionally, the physical contact of MNPs with bacterial cell walls results in membrane disruption through direct mechanical interactions and electrostatic forces, as many phytogen@MNPs carry surface charges that facilitate strong binding to negatively charged bacterial membranes. This binding weakens the bacterial wall and increases permeability, causing the leakage of essential intracellular materials. Moreover, phytogen@MNPs can interfere with intracellular processes by penetrating bacterial cells, where they can inhibit protein synthesis and disturb DNA replication, thereby further impairing bacterial survival and reproduction. Some biomodifications involve conjugation with antibacterial agents or molecules that enhance targeting and adhesion to bacteria, thereby amplifying the antimicrobial efficiency. These nanoparticles can also suppress bacterial defense mechanisms, such as efflux pumps and resistance gene expression, which helps overcome antibiotic resistance. The magnetic core facilitates the targeted delivery and concentration of nanoparticles at infection sites using external magnetic fields, improving localized antibacterial action and minimizing side effects. Together, these mechanisms contributed to the broad-spectrum and potent antibacterial activity of phytogen@MNPs against both Gram-positive and Gram-negative bacteria, including drug-resistant strains.
Antibacterial studies are a measure of the toxicity of MNPs towards various types of bacterial samples. Two different methods were used to study the antibacterial activity (Fig. 16).
(A) Measurement of minimum inhibitory concentration (MIC), that is, the concentration at which cell viability is minimal (compared to the reference).
(B) Measurement of the zone of inhibition (ZOI) using the disc diffusion method.
First, the bacterial culture was grown in broth (e.g., Luria broth) by stirring the selected bacteria overnight. After overnight incubation (37 °C), colonies were counted to obtain CFU mL−1 from each plate. Then, it was diluted (a generally accepted CFU lies between 30 and 300) at a particular dilution using the following equation (eqn (32)):
 | (32) |
Below 30 and above 300, the colony counts were very low and very high, respectively; thus, errors may occur. Therefore, these plates should be carefully selected for use in future studies.
Here,
| Total dilution factor = 10sum of serial number of dilutions |
Subsequently, a primary (original) suspension of phytogen@MNPs was prepared and diluted to obtain suspensions with different concentrations. After taking a colony and growing it in broth media, dilution should be performed until an optical density of less than 0.1 is achieved. Different concentrations of phytogen@MNPs were added to the diluted bacterial solution (with an optical density of less than 0.1) and allowed to grow overnight. Thus, the minimum inhibitory concentration (at which the bacterial concentration was the lowest) was measured.
Freshly collected colonies were used to measure the ZOI using the disc diffusion method. Similarly, in this method, different commercial antibiotics and a very dilute suspension of phytogen@MNPs were used on agar plates containing the same dilution of bacterial culture. After one or more days, the size of the zone or well (the circle around which bacteria cannot grow) was measured and compared with that of the control. After comparison, it was concluded that MNPs are efficient candidates (for bacterial removal). Antibacterial processes must be performed at 37 °C, and all equipment, glassware, and tables should be sterilized. Almost in all cases, experiments were performed repeatedly at least three times to obtain data on the reproducibility of the antibacterial analysis.
For example, anthocyanin-rich berry extracts have been used to coat MNPs. Anthocyanin compounds not only improve the stability of nanoparticles but also significantly enhance their antibacterial activity against common bacterial strains, making them promising for biomedical and environmental applications.221,222 In another study, MNPs were synthesized and stabilized using Ipomoea aquatica leaf extract, exhibiting significant antibacterial activity. During the agar well diffusion method, the nanoparticles exhibited a ZOI of 19 mm against Gram-negative E. coli and 14 mm against Gram-positive Bacillus subtilis. This indicates strong antibacterial effects, especially against E. coli, and demonstrates that the bioextract-coated MNPs were effective in biocidal applications against pathogenic bacteria.223 The synthesis of MNPs using Jatropha podagrica leaf extract highlights their potent antibacterial activity. In agar well diffusion tests, 150 µL of these nanoparticles showed ZOIs of 13 mm for Bacillus coagulans, 15 mm for S. aureus, 11 mm for E. coli, and 10 mm for Klebsiella pneumoniae. This study indicates that antibacterial effects occur through oxidative stress via ROS, damaging bacterial membranes and essential biomolecules.145 MNP synthesis using Borassus flabellifer seed coat extract demonstrated significant antibacterial activity against E. coli, S. aureus, Shigella, and B. subtilis. The antibacterial efficacy increased with nanoparticle concentration, showing ZOIs of up to 26 mm for B. subtilis at a concentration of 500 µg mL−1. This enhanced activity results from the synergistic effects of the nanoparticles and bioactive phytochemicals in the seed coat extract, which induce oxidative stress and disrupt bacterial cells. These nanoparticles exhibited strong antioxidant properties and high biocompatibility, supporting their potential biomedical applications.224 Sathishkumar et al.225 synthesized Couroupita guianensis Aubl. fruit extract (CGFE) coated magnetite nanoparticles (CG@MNPs) and applied them to S. aureus MTCC 96, E. coli MTCC 2939, S. typhi MTCC 3917, and K. penumoniae MTCC 530 to evaluate antibacterial efficacy. CG@MNPs and crude CGFE were loaded onto sterile discs at various concentrations (25, 50, and 75 µg mL−1). After a 24 h incubation period at 37 °C, the CG@MNP-loaded discs exhibited dose-dependent inhibition, as shown in Fig. 17a. Compared to Gram-positive S. aureus MTCC 96, CG@MNPs showed the highest zone of inhibition (Fig. 17b) against Gram-negative E. coli MTCC2939, S. typhi MTCC3917, and K. penumoniae MTCC 530. They observed that structural differences in the cell walls of Gram-positive and Gram-negative bacteria accounted for this variation.
 | ||
| Fig. 17 Antibacterial efficacy of CG@MNPs: (a) disc diffusion assay showing ZOIs in a dose-dependent manner against (1) E. coli MTCC 1687, (2) S. typhi MTCC 3917, (3) K. pneumonia MTCC 530, and (4) S. aureus MTCC 96, (A) positive control, (B–D) 25, 50 & 75 µg mL−1 CG@MNPs); (b) ZOIs for the CG@MNPs against the used bacterial samples (source: this image is taken from an artilcle written by Sathishkumar et al.225). | ||
Eldeeb et al.132 used Citrus sinensis peel extract to synthesize MNPs (CS@MNPs) and evaluated their antimicrobial activity against multidrug-resistant pathogens (S. aureus, Streptococcus mutans, Bacillus subtilis, E. coli, Klebsiella pneumoniae, and Candida albicans) using a disk diffusion assay, and found that the maximum ZOI was observed at a concentration of 400 µg mL−1 (Table 12). The MIC of CS@MNPs (using successive dilutions of 50, 25, 12.5, 6.5, 3, and 1 µg mL−1) against the target pathogens was evaluated using a microtiter plate technique. The MICs of green-synthesized CS@MNPs against S. aureus, Streptococcus mutans, Bacillus subtilis, E. coli, Klebsiella pneumonia and Candida albicans were 3, 6.5, 6.5, 12.5, 50, and 25 µg mL−1, respectively. These examples emphasize the versatility and enhanced antibacterial properties imparted by bio-extract coatings on MNPs, which combine natural antimicrobial activity with the physical and magnetic features of the nanoparticle core.
| MNPs | Bacterial strains | Concentration | ZOI | Ref. |
|---|---|---|---|---|
| Citrus sinensis peel extract@MNPs | S. aureus | 400 µg mL−1 | 30 | 132 |
| St. mutans | 37.3 | |||
| B. subtilis | 22.3 | |||
| E. coli | 30 | |||
| K. pneumonia | 14.7 | |||
| C. albicans | 22.6 | |||
| Moringa oleifera@MNPs | A. sobria | — | 21 | 226 |
| Calotropis procera aqueous leaf extract@MNPs | K. pneumonia | — | 7.1 | 127 |
| S. aureus | 22.5 | |||
| B. subtilis | 22.4 | |||
| A. niger | 16.9 | |||
| F. oxysporum | 14.7 | |||
| Glycosmis mauritiana leaf extract@MNPs | B. cereus | 30 µg per disc | 11 ± 1.0 | 227 |
| B. subtilis | 19 ± 2.6 | |||
| E. faecalis | 18 ± 2.0 | |||
| E. coli | 19 ± 1.0 | |||
| K. pneumonia | 12 ± 1.0 | |||
| M. luteus | 16 ± 2.0 | |||
| P. mirabilis | 11 ± 1.0 | |||
| P. vulgaris | 19 ± 1.7 | |||
| P. fluorescence | 18 ± 1.7 | |||
| S. aureus | 16 ± 1.2 | |||
| V. fluvialis | 10 ± 1.5 | |||
| Protoparmeliopsis muralis lichen aqueous extract@MNPs | E. coli | 0.1 M (salt concentration) | 12 ± 0.89 | 228 |
| S. aureus | 15 ± 0.89 | |||
| P. aeruginosa | 13.66 ± 1.36 | |||
| Eichhornia crassipes leaf extract@MNPs | S. aureus | 100 µg mL−1 | 23.3 ± 1 | 229 |
| P. fluorescens | 22.6 ± 1 | |||
| E. coli | 20 ± 1 | |||
| P. aeruginosa | ∼17 | |||
| P. vulgaris | ∼18 | |||
| Zea mays L.ear leaves aqueous extract@MNPs + kanamycin | B. cereus | 25 + 5 µg per disc | 9.87 ± 0.34 | 230 |
| E.coli | 18.86 ± 0.82 | |||
| L. monocytogenes | 13.54 ± 0.30 | |||
| S. aureus | 13.09 ± 0.15 | |||
| S. typhimurium | 13.3 ± 0.47 | |||
| Zea mays L.ear leaves aqueous extract@MNPs + amphotericin b | C. albicans KACC 30003 | 25 + 5 µg per disc | 9.37 ± 0.31 | |
| C. albicans KACC 30062 | 16.69 ± 0.10 | |||
| C. glabrata KBNO 6P00368 | 10.39 ± 0.37 | |||
| C. glabrata KACC 30061 | 15.97 ± 0.58 | |||
| C. saitoana KACC 41238 | 10.59 ± 0.18 | |||
| Leucas aspera aqueous leaf extract@MNPs | E. coli | 50 µg mL−1 | 12 | 231 |
| K. pneumoniae | 10 | |||
| P. mirabilis | 17 | |||
| S. enterica | 21 | |||
| S. flexneri | 20 | |||
| V. cholera | 12 | |||
| P. aeruginosa | 21 | |||
| B. cereus | 0 | |||
| Calotropis procera leaf extract@MNPs | E. coli | 100 mg mL−1 | 0 | 127 |
| K. pneumonia | 7.1 ± 0.28 | |||
| S. aureus | 22.5 ± 0.42 | |||
| B. subtilis | 22.4 ± 0.89 | |||
| A. niger | 16.9 ± 0.67 | |||
| F. oxysporum | 14.7 ± 0.73 | |||
| Qazwan Seeds extract@MNPs | E. coli | 20 mg mL−1 | 18 | 232 |
| A. baumannii | 20 | |||
| P. aeruginosa | 20 | |||
| K. pneumonia | 22 | |||
| E. faecalis | 20 | |||
| C. albicans (fungi) | 10 | |||
| Aqueous flower extract of Murraya paniculata (L) Jack@MNPs | Enterococcus faecalis | 10 µg mL−1 | 11 | 24 |
| Pseudomonas aeruginosa | 10 |
Various phytogen@MNPs have distinct ZOIs against different bacteria, a few of which are listed in Table 12.
The size, charge, and shape of nanoparticles are critical factors in determining their antibacterial mechanisms and efficacy. Smaller nanoparticles possess a larger surface area-to-volume ratio, which enhances their ability to interact with bacterial surfaces and penetrate cells, thereby increasing their antibacterial activity. They are readily taken up by bacteria and can release antimicrobial ions or react more efficiently with cellular components. The nanoparticle surface charge, which is often positive (cationic), facilitates strong electrostatic interactions with the negatively charged bacterial cell membranes. This interaction can destabilize bacterial membranes, causing leakage of contents and cell death, while also increasing the selectivity towards bacteria over mammalian cells.233
This shape influences the interaction of nanoparticles with bacterial cells and biofilms. Non-spherical shapes, such as cubes, discs, or sharp-edged structures, can exhibit higher surface reactivity and improved contact with bacterial membranes, resulting in more potent antibacterial activity than spherical or wire-like nanoparticles. Different shapes also present various crystal facets that influence the surface energy and reactivity. For example, nanocubes with high-energy facets often exhibit greater antibacterial potency than nanospheres. Overall, optimizing the size, charge, and shape of nanoparticles can maximize bacterial cell wall disruption, efficient cellular uptake, biofilm penetration, and targeted delivery of antimicrobial agents, leading to enhanced antibacterial mechanisms and effectiveness.
10. Advantages, safety, toxicity, and lifecycle considerations for Phytogen@MNPs
10.1 Advantages
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio) and stabilizers, enhancing the biocompatibility of the nanoparticles and promoting their biodegradability. Magnetite nanoparticles produced using crude latex from Jatropha curcas and the leaf extract of Cinnamomum tamala have demonstrated promising capabilities in purifying wastewater. These biologically derived nanoparticles effectively eliminate organic contaminants, toxic heavy metals, and pathogenic bacteria, highlighting their potential as eco-friendly water treatment solutions.43 Another study demonstrated the use of widely available and costless mandarin (Citrus reticulata) peels for the synthesis of superparamagnetic MNPs.139
1 ratio) and stabilizers, enhancing the biocompatibility of the nanoparticles and promoting their biodegradability. Magnetite nanoparticles produced using crude latex from Jatropha curcas and the leaf extract of Cinnamomum tamala have demonstrated promising capabilities in purifying wastewater. These biologically derived nanoparticles effectively eliminate organic contaminants, toxic heavy metals, and pathogenic bacteria, highlighting their potential as eco-friendly water treatment solutions.43 Another study demonstrated the use of widely available and costless mandarin (Citrus reticulata) peels for the synthesis of superparamagnetic MNPs.139
10.2 Toxicity and safety of phytogen-coated magnetite nanoparticles
Although green synthesis methods generally lead to less toxic nanoparticles, the safety of phytogen@MNPs still requires rigorous evaluation, especially given their intended use in water purification and potential environmental release.23410.3 Lifecycle assessment of Phytogen@MNPs
A comprehensive life cycle assessment (LCA) is crucial for evaluating the environmental performance of phytogen@MNPs used in wastewater purification, from their green synthesis to eventual disposal or recycling.10.3.1.1 Raw material acquisition. The use of abundant and renewable plant materials for phytosynthesis significantly reduces the environmental burden associated with extraction and processing of synthetic chemicals.
10.3.1.2 Energy consumption. Green synthesis often requires less energy than high-temperature or high-pressure chemical methods, contributing to a lower carbon footprint.
10.3.1.3 Waste generation. Reduced use of hazardous chemicals directly translates to less toxic waste generation during the synthesis phase, aligning with sustainable practices.
10.3.2.1 Efficacy and reusability. Phytogen@MNPs demonstrate high efficiency in removing various pollutants, including heavy metals, organic dyes, and bacteria, from wastewater. Their magnetic properties allow for easy separation and recovery from treated water using external magnets, thereby promoting reusability and reducing operational costs.
10.3.2.2 Reduced secondary pollution. The effective removal of pollutants prevents their discharge into natural water bodies, thereby mitigating environmental contamination.
10.3.2.3 Potential for leaching. Although phytogen coatings enhance stability, the possibility of MNP leaching or degradation during continuous use in wastewater treatment systems requires evaluation. This can release nanoparticles or their breakdown products into purified water or effluents.
10.3.3.1 Disposal. The ultimate disposal of spent phytogen@MNPs, along with adsorbed pollutants, must be managed responsibly. Options include landfilling, incineration, or specialized waste treatment, each with its own environmental implications.
10.3.3.2 Biodegradability. The organic phytogen coating can enhance the biodegradability of nanoparticles, potentially reducing their persistence in the environment compared to synthetic polymer coatings. However, core MNPs are stable and long-lasting.234
• Data gaps regarding the long-term fate, degradation pathways, and ecological impacts of phytogen@MNPs in diverse environmental matrices (e.g., soil, sediment, and aquatic systems) must be addressed.
• Standardized protocols for toxicity testing and environmental risk assessment of these nanoparticles are essential to provide reliable data for regulatory decision-making.
• An LCA should consider the entire supply chain of plant extracts, including cultivation, harvesting, and processing, to ensure that the “green” aspect is maintained throughout the process.
11. Future perspectives
Future prospects for the synthesis of phytogen@MNPs in wastewater purification using plant extracts cover several important topics, with an emphasis on resolving present issues and improving their sustainability and applicability.237–239 Nanoparticles are a viable alternative for wastewater detoxification, which is required due to the rising water pollution caused by ongoing industrialization and urbanization.240Future studies should focus on investigating new plant sources and streamlining the green synthesis process.241 Standardizing biochar production and conducting in-depth field research are necessary to maximize its use in anaerobic biofiltration systems for successful and economical wastewater treatment.239
Therefore, enhancing the effectiveness and repeatability of plant extract-mediated synthesis techniques is essential. This involves gaining a greater understanding of how plant phytochemicals function as capping and reducing agents, enabling improved control over the size, shape, and stability of nanoparticles.151,242 The optimization of these parameters is crucial because they have a considerable impact on the reaction yield. These factors include the reaction temperature, iron precursor concentration, leaf extract concentration, and reaction time.243 Advanced characterization methods will help clarify the characteristics of green-synthesized nanoparticles and guarantee their appropriateness for various wastewater treatment applications.241
Further studies should examine a wider array of plant extracts, including agricultural waste, as capping and bioreducing agents.244 This strategy not only offers a sustainable way to synthesize nanoparticles, but also helps to value waste. The synthesis of magnetite nanoparticles, for example, can be carried out using waste natural resources, such as onion, potato, tea, and moringa wastes, with differing results in terms of efficiency, yield, size, shape, and morphology.
Future research should focus on developing multifunctional phytogen@MNPs to improve their pollutant removal capacity by integrating them into composite structures or mixing them with other materials.241,245 Surface modification with polymers, functional groups, or other NPs may be necessary to target certain contaminants, including heavy metals, dyes, medications, and microplastics.237,246 The magnetic properties of MNPs facilitate their efficient separation from treated wastewater using an external magnetic field, offering significant advantages in terms of recyclability and cost-effectiveness.237
Addressing the challenges associated with scalability, environmental impacts, and long-term performance is vital for the widespread adoption of plant-mediated MNPs in wastewater treatment.240
One of the biggest challenges is the development of cost-effective processes for the large-scale production of plant-mediated MNPs. Although green synthesis techniques are typically less expensive and harmful to the environment than conventional chemical and physical procedures, more studies and improvements are required before these processes can be scaled for industrial use.237,243 The economic feasibility of these techniques is enhanced by utilizing easily accessible and reasonably priced plant extracts.247,248
Even though plant-mediated synthesis is regarded as “green,” further research is necessary to fully understand the long-term toxicity and possible environmental effects of the produced nanoparticles.238 To ensure safe application, studies on ecological implications—such as permanence, toxicity to non-target organisms, and release into the environment, are essential.238 One of the main benefits of green synthesis techniques is the use of non-toxic materials in the synthesis process.249
To construct more complete and effective systems, future studies should focus on integrating plant-mediated MNPs with current wastewater treatment technology.238,250 To enhance the overall treatment efficacy and handle a larger spectrum of pollutants, nanotechnology can be integrated with traditional techniques, including adsorption, photocatalytic degradation, and membrane filtration.240,250 For instance, research has demonstrated that, compared to traditional techniques, MNP-based approaches can improve contaminant concentration and separation efficiency.251
12. Conclusions
The development of phytogen@MNPs represents a significant advancement in sustainable wastewater detoxification. Plant-derived extracts facilitate green synthesis, imparting biocompatibility, stability, and surface multifunctionality to MNPs, which cannot be achieved using conventional methods. Through the analysis of plant sources, synthesis techniques, and material characterization, phytogen@MNPs were found to efficiently adsorb and degrade inorganic ions and organic pollutants, including heavy metals, dyes, pesticides, and pharmaceuticals. Kinetic, isotherm, and thermodynamic studies confirmed their high efficacy, whereas their biocompatibility and lower cost of production highlighted their potential for real-world implementation. However, challenges such as organic coating degradation, iron leaching, scale-up of synthesis protocols, and long-term stability under operational conditions remain, which require further research. Moreover, the standardization of characterization methods and understanding of pollutant–surface interactions should be strengthened to optimize performance and address safety concerns. In summary, phytogen@MNPs represent a promising and versatile solution for next-generation water-purification technologies. Continuous interdisciplinary efforts in scalable production, material optimization, and field-based testing are essential to realize their potential in addressing global water pollution challenges.Author contribution
C. Das: conceptualization, investigation, formal analysis, and writing the original draft. G. Biswas: conceptualization, reviewing and editing.Conflicts of interest
The authors have declared that no competing interests exist.Data availability
No new data were generated in this review article. All data supporting the conclusions of this work are derived from previously published sources, which are fully cited within the manuscript.Acknowledgements
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.References
- K. Saravanakumar, S. De Silva, S. S. Santosh, A. Sathiyaseelan, A. Ganeshalingam, M. Jamla, A. Sankaranarayanan, V. P. Veeraraghavan, D. MubarakAli, J. Lee, G. Thiripuranathar and M.-H. Wang, Chemosphere, 2022, 307, 135593 CrossRef CAS.
- R. L. Bell, J. A. Kase, L. M. Harrison, K. V. Balan, U. Babu, Y. Chen, D. Macarisin, H. J. Kwon, J. Zheng, E. L. Stevens, J. Meng and E. W. Brown, Pathogens, 2021, 10, 1391 CrossRef.
- A. M. Jubb and H. C. Allen, ACS Appl. Mater. Interfaces, 2010, 2, 2804–2812 CrossRef CAS.
- B. Rezaei, P. Yari, S. M. Sanders, H. Wang, V. K. Chugh, S. Liang, S. Mostufa, K. Xu, J. Wang, J. Gómez-Pastora and K. Wu, Small, 2024, 20, 2304848 CrossRef CAS PubMed.
- S. Dutta, R. Dixit and R. K. Sharma, Sep. Sci. Technol., 2022, 97–138 CAS.
- H. Iida, K. Takayanagi, T. Nakanishi and T. Osaka, J. Colloid Interface Sci., 2007, 314, 274–280 CrossRef CAS.
- J. H. Jang and H. B. Lim, Microchem. J., 2010, 94, 148–158 CrossRef CAS.
- M. Arruebo, R. Fernández-Pacheco, M. R. Ibarra and J. Santamaría, Nano Today, 2007, 2, 22–32 CrossRef.
- K. Petcharoen and A. Sirivat, Mater. Sci. Eng. B, 2012, 177, 421–427 CrossRef CAS.
- J. Yang, S. Park, H. Yoon, Y. Huh and S. Haam, Int. J. Pharm., 2006, 324, 185–190 CrossRef CAS.
- B. Markovic, V. Spasojevic, A. Dapcevic, Z. Vukovic, V. Pavlovic, D. Randjelovic and A. Nastasovic, Hem. Ind., 2019, 73, 25–35 CrossRef.
- F. X. Hu, K. G. Neoh and E. T. Kang, Biomaterials, 2006, 27, 5725–5733 CrossRef CAS.
- Z. Ma and H. Liu, China Particuology, 2007, 5, 1–10 CrossRef CAS.
- S. B. Brijmohan and M. T. Shaw, J. Memb. Sci., 2007, 303, 64–71 CrossRef CAS.
- V. Aggarwal, A. Kachore, E. Bala, H. Singh, M. Selvaraj, M. A. Assiri, Saima, R. Kumar and P. K. Verma, J. Phys. Chem. Solids, 2026, 208, 113060 CrossRef CAS.
- H. Singh, Himanshu, A. Kachore, V. Aggarwal, E. Bala, Saima, R. Kumar and P. K. Verma, Langmuir, 2025, 41, 23163–23181 CrossRef CAS.
- H. Singh, A. Kachore, V. Aggarwal, E. Bala, Saima, R. Kumar, P. Kumar and P. K. Verma, ChemistrySelect, 2025, 10(14), e202501004 CrossRef CAS.
- A. Kachore, E. Bala, V. Aggarwal, H. Singh, Saima, M. H. A. Suleiman, M. Selvaraj and P. K. Verma, J. Ind. Eng. Chem., 2025, 146, 494–505 CrossRef CAS.
- T. A. P. Rocha-Santos, TrAC Trends Anal. Chem., 2014, 62, 28–36 CrossRef CAS.
- R. Kumar, B. S. Inbaraj and B. H. Chen, Mater. Res. Bull., 2010, 45, 1603–1607 CrossRef CAS.
- Y. P. Yew, K. Shameli, M. Miyake, N. B. B. Ahmad Khairudin, S. E. B. Mohamad, T. Naiki and K. X. Lee, Arab. J. Chem., 2020, 13, 2287–2308 CrossRef CAS.
- S. Nadaf, G. K. Jena, N. Rarokar, N. Gurav, M. Ayyanar, S. Prasad and S. Gurav, Hybrid Adv., 2023, 3, 100038 CrossRef.
- W. Marimon-Bolivar and N. Toussaint-Jimenez, in 2019 Congreso Internacional de Innovación y Tendencias en Ingenieria (CONIITI), IEEE, 2019, pp. 1–8 Search PubMed.
- J. Laxmi Mangamma and K. Basavaiah, Inorg. Chem. Commun., 2024, 162, 112290 CrossRef CAS.
- M. Bala, K. Pratap, P. K. Verma, B. Singh and Y. Padwad, J. Ethnopharmacol., 2015, 175, 131–137 CrossRef CAS PubMed.
- O. M. Ahmed, in Naturally Occurring Chemicals against Alzheimer's Disease, Elsevier, 2021, pp. 351–358 Search PubMed.
- P. Sharma, B. P. Dwivedee, D. Bisht, A. K. Dash and D. Kumar, Heliyon, 2019, 5, e02437 CrossRef PubMed.
- S. Ghosh, S. Mondol, D. Lahiri, M. Nag, T. Sarkar, S. Pati, S. Pandit, A. A. Alarfaj, M. F. Mohd Amin, H. A. Edinur, M. R. Ahmad Mohd Zain and R. R. Ray, Front. Chem., 2023, 11, 1118454 CrossRef PubMed.
- R. Saini and P. Kumar, Inorg. Chem. Commun., 2023, 156, 111221 CrossRef.
- B. V. Vamsi Krishna, P. Tirupathi Rao, B. Durga Lakshmi, K. Vasudha, S. Esub Basha, B. Putra Kumar, P. S. S. Kiran, K. Shreyas Chandra and R. R K, Next Mater., 2024, 3, 100171 CrossRef.
- M. V. D. Prakash, S. Sampath, K. Amudha, A. Nadeem, B. S. Lopes, B. Durga and S. Muthupandian, Mater. Technol., 2023, 38, 2247908 CrossRef.
- G. Vyas, S. Bhatt and P. Paul, RSC Adv., 2019, 9, 34102–34113 RSC.
- S. Sarkar, R. P. Singh and G. Bhattacharya, 3 Biotech, 2021, 11, 178 CrossRef PubMed.
- S. P. Patil, R. Y. Chaudhari and M. S. Nemade, Talanta Open, 2022, 5, 100083 CrossRef.
- H. J. Berchmans and S. Hirata, Bioresour. Technol., 2008, 99, 1716–1721 CrossRef PubMed.
- N. C. Om Tapanes, D. A. Gomes Aranda, J. W. de Mesquita Carneiro and O. A. Ceva Antunes, Fuel, 2008, 87, 2286–2295 CrossRef.
- O. Osoniyi and F. Onajobi, J. Ethnopharmacol., 2003, 89, 101–105 CrossRef PubMed.
- H. A. Abdelgadir and J. Van Staden, South African J. Bot., 2013, 88, 204–218 CrossRef.
- H. Bar, D. K. Bhui, G. P. Sahoo, P. Sarkar, S. Pyne and A. Misra, Colloids Surf., A, 2009, 348, 212–216 CrossRef.
- N. Satpute, M. K. Ghosh, A. Kesharwani and T. K. Ghorai, Sci. Rep., 2023, 13, 22122 CrossRef PubMed.
- S. Thakur, M. Shandilya and G. Guleria, J. Environ. Chem. Eng., 2021, 9, 104882 CrossRef.
- V. Sharma and L. J. M. Rao, Crit. Rev. Food Sci. Nutr., 2014, 54, 433–448 CrossRef PubMed.
- C. Das, S. Sen, T. Singh, T. Ghosh, S. S. Paul, T. W. Kim, S. Jeon, D. K. Maiti, J. Im and G. Biswas, Nanomaterials, 2020, 10, 1615 CrossRef PubMed.
- W. Hassan and S. N. Zainab Kazmi, J. Nutr. Disord. Ther., 2015, 06, 1000190 Search PubMed.
- A. M. Ealias, J. V. Jose and M. P. Saravanakumar, Environ. Sci. Pollut. Res., 2016, 23, 21416–21430 CrossRef PubMed.
- R. Ribeiro-Santos, M. Andrade, D. Madella, A. P. Martinazzo, L. de Aquino Garcia Moura, N. R. de Melo and A. Sanches-Silva, Trends Food Sci. Technol., 2017, 62, 154–169 CrossRef.
- M. Jalal, S. Ali, M. Ansari, H. Khan and S. Cameotra, Int. J. Adv. Res., 2016, 4, 428–440 CrossRef PubMed.
- M. A. Al Mashud, M. Moinuzzaman, M. S. Hossain, S. Ahmed, G. Ahsan, A. Reza, R. Bin Anwar Ratul, M. H. Uddin, M. A. Momin and M. A. Hena Mostofa Jamal, Heliyon, 2022, 8, e09920 CrossRef PubMed.
- S. Narath, S. K. Koroth, S. S. Shankar, B. George, V. Mutta, S. Wacławek, M. Černík, V. V. T. Padil and R. S. Varma, Nanomaterials, 2021, 11, 1558 CrossRef PubMed.
- S. Mandal, A. Patra, A. Samanta, S. Roy, A. Mandal, T. Das Mahapatra, S. Pradhan, K. Das and D. K. Nandi, Asian Pac. J. Trop. Biomed., 2013, 3, 960–966 CrossRef PubMed.
- R. P. Samy and S. Ignacimuthu, Pharm. Biol., 2001, 39, 417–420 CrossRef.
- M. Seghatoleslam, F. Alipour, R. Shafieian, Z. Hassanzadeh, M. A. Edalatmanesh, H. R. Sadeghnia and M. Hosseini, J. Tradit. Complement. Med., 2016, 6, 262–268 CrossRef PubMed.
- S. Jayaraman, A. Saha and V. Pawar, Indian J. Pharm. Sci., 2012, 74, 339 CrossRef PubMed.
- C. Das, S. Singh, S. Bhakta, P. Mishra and G. Biswas, Chemosphere, 2022, 291, 132673 CrossRef PubMed.
- S. Yallappa, J. Manjanna, B. L. Dhananjaya, U. Vishwanatha, B. Ravishankar, H. Gururaj, P. Niranjana and B. S. Hungund, Nano-Micro Lett., 2016, 8, 120–130 CrossRef PubMed.
- S. Dwivedi and D. Chopra, J. Tradit. Complement. Med., 2014, 4, 224–231 CrossRef PubMed.
- S. Goel and P. Mishra, Mater. Sci. Eng. C, 2019, 104, 109881 CrossRef PubMed.
- H. G. Anlar and M. Bacanli, in Pathology, Elsevier, 2020, pp. 369–378 Search PubMed.
- A. Rajabian and H. Hosseinzadeh, in Nuts and Seeds in Health and Disease Prevention, Elsevier, 2020, pp. 329–355 Search PubMed.
- T. Yuan, P. Nahar, M. Sharma, K. Liu, A. Slitt, H. A. Aisa and N. P. Seeram, J. Nat. Prod., 2014, 77, 2316–2320 CrossRef PubMed.
- B. H. Ali and G. Blunden, Phyther. Res., 2003, 17, 299–305 CrossRef PubMed.
- C. Ezeh, C. Eze, M. U. Dibua, S. Emencheta and C. Ezeh, Biomed. Biotechnol. Res. J., 2022, 6, 400 CrossRef.
- M. Kumar, D. Kaushik, A. Kumar, P. Gupta, C. Proestos, E. Oz, E. Orhan, J. Kaur, M. R. Khan, T. Elobeid, M. Bordiga and F. Oz, Int. J. Food Sci. Technol., 2023, 58, 2883–2892 Search PubMed.
- S. Waseem, Z. U. Nisa, T. Zeeshan, M. D. Ali, T. Begum, Z. N. Kayani, I. Ali and A. Ayub, Nano-Struct. Nano-Objects, 2024, 38, 101212 Search PubMed.
- K. Saravanakumar, S. Shin, A. Sathiyaseelan and M.-H. Wang, Mater. Lett., 2023, 338, 134000 CrossRef CAS.
- M. S. H. Bhuiyan, M. Y. Miah, S. C. Paul, T. Das Aka, O. Saha, M. M. Rahaman, M. J. I. Sharif, O. Habiba and M. Ashaduzzaman, Heliyon, 2020, 6, e04603 CrossRef CAS PubMed.
- B. Malaikozhundan, R. Krishnamoorthi, J. Vinodhini, K. S. N. Nambi and S. Palanisamy, Inorg. Chem. Commun., 2022, 144, 109843 CrossRef CAS.
- A. V. Samrot, S. Saigeetha, C. Y. Mun, S. Abirami, K. Purohit, P. J. J. Cypriyana, T. S. Dhas, L. Inbathamizh and S. S. Kumar, Sci. Rep., 2021, 11, 24511 CrossRef CAS PubMed.
- T. C. Jackson, T. O.-O. Uwah, A. A. Agboke, B. E. Udo and E. M. Udofa, Soft Nanosci. Lett., 2018, 08, 1–7 CrossRef.
- S. Nizamuddin, A. Hymavathi, G. Yaku and U. Umesh Kumar, Mater. Today Proc., 2022, 62, 6854–6856 Search PubMed.
- R. Aswini, K. Jothimani, K. Kannan, R. Pothu, P. Shanmugam, R. Boddula, A. B. Radwan, G. Periyasami, P. Karthikeyan and N. Al-Qahtani, Environ. Geochem. Health, 2024, 46, 334 CrossRef CAS.
- E. Vélez, G. Campillo, G. Morales, C. Hincapié, J. Osorio and O. Arnache, J. Nanomater., 2018, 2018, 1–7 CrossRef.
- B. M. Chandrika, H. C. Manjunatha, L. Seenappa, R. Munirathnam, K. N. Sridhar, S. Manjunatha and A. J. C. Lourduraj, J. Phys. Chem. Solids, 2023, 181, 111538 CrossRef CAS.
- D. Mukherjee, S. Ghosh, S. Majumdar and K. Annapurna, J. Environ. Chem. Eng., 2016, 4, 639–650 CrossRef CAS.
- M. Herlekar, S. Barve and R. Kumar, J. Nanoparticles, 2014, 2014, 1–9 Search PubMed.
- P. J. Burange, M. G. Tawar, R. A. Bairagi, V. R. Malviya, V. K. Sahu, S. N. Shewatkar, R. A. Sawarkar and R. R. Mamurkar, Bull. Natl. Res. Cent., 2021, 45, 181 CrossRef.
- H. Jiang, S. Sathiyavimal, L. Cai, S. Devanesan, S. R. M. Sayed, G. K. Jhanani and J. Lin, Environ. Res., 2023, 236, 116749 CrossRef CAS PubMed.
- A. I. Hussain, S. A. S. Chatha, G. M. Kamal, M. A. Ali, M. A. Hanif and M. I. Lazhari, Int. J. Food Prop., 2017, 20, 1569–1581 CrossRef CAS.
- A. K. Ambedkar, D. Gautam, S. Vikal, M. Singh, A. Kumar, A. Sanger, K. Sharma, B. P. Singh and Y. K. Gautam, ACS Omega, 2023, 8, 29663–29673 CrossRef CAS PubMed.
- P. Zambare, A. Survase and S. Kanase, Int. J. Pharm. Investig., 2022, 13, 106–112 CrossRef.
- K. Baruah, M. Haque, L. Langbang, S. Das, K. Aguan and A. Singha Roy, J. Mol. Liq., 2021, 337, 116422 CrossRef CAS.
- R. R. Multisona, S. Shirodkar, M. Arnold and A. Gramza-Michalowska, Appl. Sci., 2023, 13, 2134 CrossRef CAS.
- D. Khwannimit, R. Maungchang and P. Rattanakit, Int. J. Environ. Anal. Chem., 2022, 102, 5247–5263 Search PubMed.
- N. Shaik, B. Rehana, T. Anitha, R. Vijayalakshmi, K. Sandhya and G. Bandari, Int. J. NANO Dimens., 2025, 16(1–12), 162505 Search PubMed.
- S. Prabhu, T. Daniel Thangadurai, T. Indumathi and P. Kalugasalam, Inorg. Chem. Commun., 2022, 146, 110077 CrossRef CAS.
- H. N. T. Pham, Q. Van Vuong, M. C. Bowyer and C. J. Scarlett, Technologies, 2020, 8, 80 CrossRef.
- A. K. Potbhare, S. Yerpude, A. R. Daddemal-Chaudhary, A. Lambat, A. Mondal, K. M. Dadure, A. R. Rai, A. Abdala and R. G. Chaudhary, Chemosphere, 2024, 359, 142369 CrossRef CAS PubMed.
- M. Kandiah and K. N. Chandrasekaran, J. Nanotechnol., 2021, 2021, 1–18 Search PubMed.
- M. Gupta, R. S. Tomar, S. Kaushik, R. K. Mishra and D. Sharma, Front. Microbiol., 2018, 9, 2030 CrossRef PubMed.
- K. Velayutham, A. A. Rahuman, G. Rajakumar, T. Santhoshkumar, S. Marimuthu, C. Jayaseelan, A. Bagavan, A. V. Kirthi, C. Kamaraj, A. A. Zahir and G. Elango, Parasitol. Res., 2012, 111, 2329–2337 CrossRef PubMed.
- A. Scaria, R. Jayaraj and P. S. Sudheesh, Mater. Today Proc., 2023 DOI:10.1016/j.matpr.2023.11.083.
- M. Mukhopadhyay, M. Shaw and D. Nath, Pharmacogn. Mag., 2017, 13, 216 Search PubMed.
- R. S. T. Suruchi Chaudhary, V. Shrivastava and A. Jyoti, Int. J. Pharm. Phytopharm. Res., 2020, 10, 147–154 Search PubMed.
- S. Khurshid, S. Arif, T. M. Ali, H. M. Iqbal, M. Shaikh, H. Khurshid, Q. Akber and S. Yousaf, Starch/Staerke, 2022, 74, 2100228 CrossRef CAS.
- N. Gautam, K. B. Singh, Snigdha, D. D. Upadhyay and G. Pandey, RSC Adv., 2023, 13, 23181–23196 RSC.
- K. S. Prasad, A. Patra, G. Shruthi and S. Chandan, J. Nanotechnol., 2017, 2017, 1–6 Search PubMed.
- S. S. Majani, S. Sathyan, M. V. Manoj, N. Vinod, S. Pradeep, C. Shivamallu, K. N. Venkatachalaiah and S. P. Kollur, Curr. Res. Green Sustain. Chem., 2023, 6, 100367 CrossRef CAS.
- P. Wei, F. Zhao, Z. Wang, Q. Wang, X. Chai, G. Hou and Q. Meng, Nutrients, 2022, 14, 4079 CrossRef CAS PubMed.
- S. Zafar, A. Ashraf, M. U. Ijaz, S. Muzammil, M. H. Siddique, S. Afzal, R. Andleeb, K. A. Al-Ghanim, F. Al-Misned, Z. Ahmed and S. Mahboob, J. King Saud Univ. - Sci., 2020, 32, 1116–1122 CrossRef.
- A. Manikandan, M. Durka, M. Amutha Selvi and S. Arul Antony, J. Nanosci. Nanotechnol., 2016, 16, 448–456 CrossRef CAS PubMed.
- F. Bano, M. Baber, A. Ali, Z. Shah and S. Muhammad, Pharmacogn. Mag., 2017, 13, 33 Search PubMed.
- J. Khan, A. Sajjad, M. Rukh, K. Khan, S. W. Hassan and M. Zia, Biocatal. Agric. Biotechnol., 2024, 61, 103386 Search PubMed.
- I. Chkhikvishvili, T. Sanikidze, N. Gogia, M. Enukidze, M. Machavariani, N. Kipiani, Y. Vinokur and V. Rodov, Oxid. Med. Cell. Longev., 2016, 2016, 4216285 CrossRef PubMed.
- M. Riaz, R. Ahmad, N. U. Rahman, Z. Khan, D. Dou, G. Sechel and R. Manea, J. Ethnopharmacol., 2020, 255, 112718 CrossRef CAS PubMed.
- K. S. Barnwal, Y. Gupta and N. Jaggi, Surf. Interfaces, 2024, 55, 105326 CrossRef CAS.
- A. Sukhwal, D. Jain, A. Joshi, P. Rawal and H. S. Kushwaha, IET Nanobiotechnology, 2017, 11, 531–537 CrossRef PubMed.
- N. K. Mondal and A. Hajra, J. Mosq. Res., 2016, 6, 1–9 Search PubMed.
- G. Priyadarshana, N. Kottegoda, A. Senaratne, A. de Alwis and V. Karunaratne, J. Nanomater., 2015, 2015, 1–8 Search PubMed.
- D. Maity, S.-G. Choo, J. Yi, J. Ding and J. M. Xue, J. Magn. Magn. Mater., 2009, 321, 1256–1259 CrossRef CAS.
- G. Marchegiani, P. Imperatori, A. Mari, L. Pilloni, A. Chiolerio, P. Allia, P. Tiberto and L. Suber, Ultrason. Sonochem., 2012, 19, 877–882 CrossRef CAS PubMed.
- D. Ghanbari, M. Salavati-Niasari and M. Ghasemi-Kooch, J. Ind. Eng. Chem., 2014, 20, 3970–3974 CrossRef CAS.
- L. Cabrera, S. Gutierrez, N. Menendez, M. P. Morales and P. Herrasti, Electrochim. Acta, 2008, 53, 3436–3441 Search PubMed.
- M. Starowicz, P. Starowicz, J. Żukrowski, J. Przewoźnik, A. Lemański, C. Kapusta and J. Banaś, J. Nanoparticle Res., 2011, 13, 7167–7176 Search PubMed.
- R. Reséndiz-Ramírez, A. Rodríguez-López, J. A. Díaz-Real, H. F. Delgado-Arenas, A. Osornio-Villa, R. Hernández-Leos, V. Vivier and R. Antaño-López, ACS Omega, 2022, 7, 761–772 CrossRef PubMed.
- J. M. M. Silva, P. E. Feuser, R. Cercená, M. Peterson and A. G. Dal-Bó, J. Magn. Magn. Mater., 2023, 580, 170925 CrossRef CAS.
- T. J. Daou, G. Pourroy, S. Bégin-Colin, J. M. Grenèche, C. Ulhaq-Bouillet, P. Legaré, P. Bernhardt, C. Leuvrey and G. Rogez, Chem. Mater., 2006, 18, 4399–4404 CrossRef CAS.
- J. A. López Pérez, M. A. López Quintela, J. Mira, J. Rivas and S. W. Charles, J. Phys. Chem. B, 1997, 101, 8045–8047 Search PubMed.
- M. Salvador, G. Gutiérrez, S. Noriega, A. Moyano, M. C. Blanco-López and M. Matos, Int. J. Mol. Sci., 2021, 22, 427 CrossRef CAS PubMed.
- T. Nanda, A. Rathore and D. Sharma, Front. Mater. Sci., 2020, 14, 387–401 CrossRef.
- H. Reza Ghorbani, H. Pazoki and A. Shokuhi Rad, Biosci. Biotechnol. Res. Asia, 2017, 14, 631–633 Search PubMed.
- S. Dave, S. Dave, A. Mathur and J. Das, in Nanobiotechnology, Elsevier, 2021, pp. 225–234 Search PubMed.
- M. Abdeen, S. Sabry, H. Ghozlan, A. A. El-Gendy and E. E. Carpenter, J. Nanomater., 2016, 2016, 1–7 Search PubMed.
- T. Ahn, J. H. Kim, H.-M. Yang, J. W. Lee and J.-D. Kim, J. Phys. Chem. C, 2012, 116, 6069–6076 CrossRef CAS.
- R. Massart, IEEE Trans. Magn., 1981, 17, 1247–1248 CrossRef.
- R. Blakemore, Science, 1975, 190, 377–379 CrossRef CAS PubMed.
- S. Mørup, M. F. Hansen and C. Frandsen, in Comprehensive Nanoscience and Technology, Elsevier, 2011, pp. 437–491 Search PubMed.
- A. O. Kalu, E. C. Egwim, A. A. Jigam and H. L. Muhammad, Nano Express, 2022, 3, 045004 CrossRef CAS.
- A. Paut, L. Guć, M. Vrankić, D. Crnčević, P. Šenjug, D. Pajić, R. Odžak, M. Šprung, K. Nakić, M. Marciuš, A. Prkić and I. Mitar, Nanomaterials, 2024, 14, 729 Search PubMed.
- C. Das, N. Nath Ghosh, R. Bhardwaj, K. Narula, P. Mishra and G. Biswas, J. Ind. Eng. Chem., 2023, 128, 369–382 Search PubMed.
- M. Dehghani, B. Hajipour-Verdom and P. Abdolmaleki, Front. Chem., 2024, 12 DOI:10.3389/fchem.2024.1413077.
- S. T. Geneti, G. A. Mekonnen, H. C. A. Murthy, E. T. Mohammed, C. R. Ravikumar, B. A. Gonfa and F. K. Sabir, J. Nanomater., 2022, 2022, 1–15 Search PubMed.
- B. A. Eldeeb, W. M. A. El-Raheem and S. Elbeltagi, Sci. Rep., 2023, 13, 19000 CrossRef CAS.
- A. Akbar, S. Riaz, M. Bashir and S. Naseem, IEEE Trans. Magn., 2014, 50, 1–4 Search PubMed.
- R. Etemadifar, A. Kianvash, N. Arsalani and E. Abouzari-Lotf, J. Mater. Sci. Mater. Electron., 2018, 29, 17144–17153 CrossRef CAS.
- P. J. Vikesland, R. L. Rebodos, J. Y. Bottero, J. Rose and A. Masion, Environ. Sci. Nano, 2016, 3, 567–577 RSC.
- J. Zhang, S. Lin, M. Han, Q. Su, L. Xia and Z. Hui, Water, 2020, 12, 446 CrossRef CAS.
- A. Wojciechowska, A. Markowska-Szczupak and Z. Lendzion-Bieluń, Materials, 2022, 15, 1863 CrossRef CAS.
- S. F. Soares, T. Fernandes, T. Trindade and A. L. Daniel-da-Silva, Molecules, 2019, 24, 1958 CrossRef CAS.
- H. N. Nassar, H. R. Ali and N. S. El-Gendy, Fuel, 2021, 294, 120534 CrossRef CAS.
- D. Kovář, A. Malá, J. Mlčochová, M. Kalina, Z. Fohlerová, A. Hlaváček, Z. Farka, P. Skládal, Z. Starčuk, R. Jiřík, O. Slabý and J. Hubálek, J. Nanomater., 2017, 2017, 1–8 CrossRef.
- J. Tauc, R. Grigorovici and A. Vancu, Phys. Status Solidi, 1966, 15, 627–637 Search PubMed.
- P. R. Jubu, F. K. Yam, V. M. Igba and K. P. Beh, J. Solid State Chem., 2020, 290, 121576 CrossRef CAS.
- B. J. Abdullah, Mater. Sci. Semicond. Process., 2022, 137, 106214 CrossRef CAS.
- M. Singh, M. Goyal and K. Devlal, J. Taibah Univ. Sci., 2018, 12, 470–475 CrossRef.
- V. Golthi and J. Kommu, Hybrid Adv., 2023, 4, 100110 CrossRef.
- K. Ahmouda, M. Boudiaf and B. Benhaoua, Nanoscale Adv., 2022, 4, 3250–3271 RSC.
- W. M. Alamier, M. M. El-Moselhy, A. M. Bakry, N. Hasan and A. A. Alamri, Crystals, 2022, 12, 1510 CrossRef CAS.
- C. Das, N. N. Ghosh, V. Pulhani, G. Biswas and P. Singhal, RSC Adv., 2023, 13, 15015–15023 RSC.
- S. Fekri Aval, A. Akbarzadeh, M. R. Yamchi, F. Zarghami, K. Nejati-Koshki and N. Zarghami, Artif. Cells, Nanomedicine, Biotechnol., 2016, 44, 188–193 CrossRef CAS PubMed.
- N. D. S. Zambri, N. I. Taib, F. Abdul Latif and Z. Mohamed, Molecules, 2019, 24, 3803 CrossRef CAS PubMed.
- A. Bouafia, S. E. Laouini, A. Khelef, M. L. Tedjani and F. Guemari, J. Clust. Sci., 2021, 32, 1033–1041 CrossRef CAS.
- P. K. Dhar, P. Saha, M. K. Hasan, M. K. Amin and M. R. Haque, Clean. Eng. Technol., 2021, 3, 100117 CrossRef.
- A. Mohamed, R. R. Atta, A. A. Kotp, F. I. Abo El-Ela, H. Abd El-Raheem, A. Farghali, D. H. M. Alkhalifah, W. N. Hozzein and R. Mahmoud, Sci. Rep., 2023, 13, 7227 CrossRef.
- L. Wu, Y. Li, Z. Fu and B.-L. Su, Natl. Sci. Rev., 2020, 7, 1667–1701 CrossRef.
- L. M. Mahlaule-Glory, S. Mapetla, A. Makofane, M. M. Mathipa and N. C. Hintsho-Mbita, Heliyon, 2022, 8, e10536 CrossRef PubMed.
- C. Caizer, in Handbook of Nanoparticles, Springer International Publishing, Cham, 2016, pp. 475–519 Search PubMed.
- T. Q. Bui, S. N.-C. Ton, A. T. Duong and H. T. Tran, J. Sci. Adv. Mater. Devices, 2018, 3, 107–112 CrossRef.
- S. Chowdhury, N. Khan, G.-H. Kim, J. Harris, P. Longhurst and N. S. Bolan, in Environmental Materials and Waste, Elsevier, 2016, pp. 569–589 Search PubMed.
- J. Hwang, D. Choi, S. Han, S. Y. Jung, J. Choi and J. Hong, Sci. Rep., 2020, 10, 7391 CrossRef.
- M. S. Bhuyan, Front. Environ. Sci., 2022, 10, 827289 CrossRef.
- N. Nassiri Koopaei and M. Abdollahi, DARU J. Pharm. Sci., 2017, 25, 9 CrossRef.
- S. Singh, K. L. Wasewar and S. K. Kansal, in Inorganic Pollutants in Water, Elsevier, 2020, pp. 173–203 Search PubMed.
- M. Kumar, P. Borah and P. Devi, in Inorganic Pollutants in Water, Elsevier, 2020, pp. 33–49 Search PubMed.
- N. A. A. Qasem, R. H. Mohammed and D. U. Lawal, npj Clean Water, 2021, 4, 36 CrossRef.
- P. B. Tchounwou, C. G. Yedjou, A. K. Patlolla and D. J. Sutton, Exp. Suppl., 2012, 101, 133–164 Search PubMed.
- M. Jaishankar, T. Tseten, N. Anbalagan, B. B. Mathew and K. N. Beeregowda, Interdiscip. Toxicol., 2014, 7, 60–72 CrossRef PubMed.
- J. P. S. Cabral, Int. J. Environ. Res. Public Health, 2010, 7, 3657–3703 CrossRef PubMed.
- I. Ali and V. K. Gupta, Nat. Protoc., 2006, 1, 2661–2667 CrossRef PubMed.
- M. N. Chong, B. Jin, C. W. K. Chow and C. Saint, Water Res., 2010, 44, 2997–3027 CrossRef PubMed.
- L. Jiang, Y. Tu, X. Li and H. Li, E3S Web Conf., 2018, 38, 01037 CrossRef.
- M. Al Sharabati, R. Abokwiek, A. Al-Othman, M. Tawalbeh, C. Karaman, Y. Orooji and F. Karimi, Environ. Res., 2021, 202, 111694 CrossRef.
- Z. N. Garba, W. Zhou, I. Lawan, W. Xiao, M. Zhang, L. Wang, L. Chen and Z. Yuan, J. Environ. Manage., 2019, 241, 59–75 CrossRef PubMed.
- D. T. Bankole, A. P. Oluyori and A. A. Inyinbor, Arab. J. Chem., 2023, 16, 104699 CrossRef CAS.
- S. Moosavi, C. W. Lai, S. Gan, G. Zamiri, O. Akbarzadeh Pivehzhani and M. R. Johan, ACS Omega, 2020, 5, 20684–20697 CrossRef CAS PubMed.
- Z. Moradi, S. Z. Jahromi and M. Ghaedi, Interface Sci. Technol., 2021, 557–623 Search PubMed.
- K. Liu, J. C.-C. Yu, H. Dong, J. C. S. Wu and M. R. Hoffmann, Environ. Sci. Technol., 2018, 52, 12667–12674 CrossRef CAS.
- S. Lotfi, K. Fischer, A. Schulze and A. I. Schäfer, Nat. Nanotechnol., 2022, 17, 417–423 CrossRef CAS.
- Y. Shi, Y. Zhang, Y. Cui, J. Shi, X. Meng, J. Zhang and H. He, Carbohydr. Polym., 2019, 222, 114972 CrossRef CAS.
- N. S. Awwad, A. El-Khalafawy, H. A. Ibrahium and M. S. Hamdy, Mater. Res. Express, 2019, 6, 095907 CrossRef CAS.
- M. Jiménez-Salcedo, M. Monge and M. T. Tena, J. Environ. Chem. Eng., 2021, 9, 105827 CrossRef.
- E. Darezereshki, A. khodadadi Darban, M. Abdollahy and A. Jamshidi-Zanjani, Environ. Nanotechnology, Monit. Manag., 2018, 10, 51–62 CrossRef.
- K. O. Yoro, M. K. Amosa, P. T. Sekoai, J. Mulopo and M. O. Daramola, Int. J. Sustain. Eng., 2020, 13, 54–67 CrossRef.
- F. P. Fato, D.-W. Li, L.-J. Zhao, K. Qiu and Y.-T. Long, ACS Omega, 2019, 4, 7543–7549 CrossRef CAS.
- A. Andelescu, M. A. Nistor, S. G. Muntean and M. E. Rădulescu-Grad, Sep. Sci. Technol., 2018, 53, 2352–2364 CrossRef CAS.
- N. Ayawei, A. N. Ebelegi and D. Wankasi, J. Chem., 2017, 2017, 1–11 CrossRef.
- R. Rezaei Kalantary, E. Dehghanifard, A. Mohseni-Bandpi, L. Rezaei, A. Esrafili, B. Kakavandi and A. Azari, Desalin. Water Treat., 2016, 57, 16445–16455 CrossRef CAS.
- P. B. Hassan, R. O. Rasheed and K. Zargoosh, J. Nanomater., 2022, 2022, 1–18 Search PubMed.
- A. Siddiqa, M. H. Khatun and M. G. Mostafa, Desalin. Water Treat., 2025, 321, 100979 CrossRef CAS.
- H. P. Toledo-Jaldin, V. Sánchez-Mendieta, A. Blanco-Flores, G. López-Téllez, A. R. Vilchis-Nestor and O. Martín-Hernández, Environ. Sci. Pollut. Res., 2020, 27, 7872–7885 CrossRef CAS PubMed.
- J. Garvasis, A. R. Prasad, K. O. Shamsheera, T. A. Nidheesh Roy and A. Joseph, Mater. Res. Bull., 2023, 160, 112130 CrossRef CAS.
- M. Rahmayanti, A. Nurul Syakina, I. Fatimah and T. Sulistyaningsih, Chem. Phys. Lett., 2022, 803, 139834 CrossRef CAS.
- A. N. Syakina and M. Rahmayanti, Chem. Data Collect., 2023, 44, 101003 CrossRef CAS.
- Á. de J. Ruíz-Baltazar, S. Y. Reyes-López, M. de L. Mondragón-Sánchez, A. I. Robles-Cortés and R. Pérez, Results Phys., 2019, 12, 989–995 CrossRef.
- B. Kumar, K. Smita, L. Cumbal, A. Debut, S. Galeas and V. H. Guerrero, Mater. Chem. Phys., 2016, 179, 310–315 CrossRef CAS.
- M. S. S. Bhuiya, Md. Anwarul Kabir, Md. Shahnawaz Parvez, J. Nasrin, Md Shoeb, Md A. Rahman, Md. A. Islam and T. Islam, Int. J. Biosci., 2023, 23(4), 110–124, DOI:10.12692/ijb/23.4.110-124.
- K. Ahmouda, M. Boudiaf, D. Barani and B. Benhaoua, J. Photochem. Photobiol. A Chem., 2024, 450, 115442 CrossRef CAS.
- Z.-Y. Yao, J.-H. Qi and L.-H. Wang, J. Hazard. Mater., 2010, 174, 137–143 CrossRef CAS.
- T. M. Budnyak, M. Błachnio, A. Slabon, A. Jaworski, V. A. Tertykh, A. Deryło-Marczewska and A. W. Marczewski, J. Phys. Chem. C, 2020, 124, 15312–15323 CrossRef CAS.
- R. Singh and R. Bhateria, ACS Omega, 2020, 5, 10826–10837 CrossRef CAS.
- I. Fatimah, E. Zunita Pratiwi and W. Prio Wicaksono, Egypt. J. Aquat. Res., 2020, 46, 35–40 CrossRef.
- F. Chen, Q. Yang, X. Li, G. Zeng, D. Wang, C. Niu, J. Zhao, H. An, T. Xie and Y. Deng, Appl. Catal. B Environ., 2017, 200, 330–342 CrossRef CAS.
- S. Dong, L. Cui, Y. Zhao, Y. Wu, L. Xia, X. Su, C. Zhang, D. Wang, W. Guo and J. Sun, Appl. Surf. Sci., 2019, 463, 659–667 CrossRef CAS.
- H. Wang, X. Yuan, Y. Wu, G. Zeng, H. Dong, X. Chen, L. Leng, Z. Wu and L. Peng, Appl. Catal. B Environ., 2016, 186, 19–29 CrossRef CAS.
- F. Chen, Q. Yang, C. Niu, X. Li, C. Zhang, J. Zhao, Q. Xu, Y. Zhong, Y. Deng and G. Zeng, Catal. Commun., 2016, 73, 1–6 CrossRef CAS.
- M. Pelaez, P. Falaras, V. Likodimos, K. O'Shea, A. A. de la Cruz, P. S. M. Dunlop, J. A. Byrne and D. D. Dionysiou, J. Mol. Catal. A Chem., 2016, 425, 183–189 CrossRef CAS PubMed.
- J. T. Schneider, D. S. Firak, R. R. Ribeiro and P. Peralta-Zamora, Phys. Chem. Chem. Phys., 2020, 22, 15723–15733 RSC.
- X. Zhang, T. Guo, X. Wang, Y. Wang, C. Fan and H. Zhang, Appl. Catal. B Environ., 2014, 150–151, 486–495 CrossRef CAS.
- F. Alcalde-Garcia, S. Prasher, S. Kaliaguine, J. R. Tavares and M.-J. Dumont, ACS Eng. Au, 2023, 3, 443–460 CrossRef CAS.
- A. Kumar Das, A. Marwal and R. Verma, Nano Hybrids, 2014, 7, 69–86 Search PubMed.
- D. F. Mercado, P. Caregnato, L. S. Villata and M. C. Gonzalez, J. Inorg. Organomet. Polym. Mater., 2018, 28, 519–527 CrossRef CAS.
- H. M. Fahmy, F. M. Mohamed, M. H. Marzouq, A. B. E.-D. Mustafa, A. M. Alsoudi, O. A. Ali, M. A. Mohamed and F. A. Mahmoud, Bionanoscience, 2018, 8, 491–503 CrossRef.
- M. Alvarado, A. E. Matías Reyes, A. Cruz-Orea, J. Santoyo-Salazar, F. A. Domínguez-Pacheco, C. Hernández-Aguilar and A. A. Duran-Ledezma, Rev. Mex. Física, 2024, 70, 011601 Search PubMed.
- M. A. J. Kouhbanani, N. Beheshtkhoo, S. Taghizadeh, A. M. Amani and V. Alimardani, Adv. Nat. Sci. Nanosci. Nanotechnol., 2019, 10, 015007 CrossRef CAS.
- E. Ghoohestani, F. Samari, A. Homaei and S. Yosuefinejad, Sci. Rep., 2024, 14, 84 CrossRef CAS PubMed.
- M. M. S. Abdullah, A. M. Atta, H. A. Allohedan, H. Z. Alkhathlan, M. Khan and A. O. Ezzat, Nanomaterials, 2018, 8, 855 CrossRef.
- G. Magnacca, A. Allera, E. Montoneri, L. Celi, D. E. Benito, L. G. Gagliardi, M. C. Gonzalez, D. O. Mártire and L. Carlos, ACS Sustain. Chem. Eng., 2014, 2, 1518–1524 CrossRef CAS.
- K. Csach, A. Juríková, J. Miškuf, M. Koneracká, V. Závišová, M. Kubovčíková and P. Kopčanský, Mater. Sci. Forum, 2014, 782, 607–610 CAS.
- S. Jafarirad, P. Hajat Ardehjani and A. Movafeghi, J. Environ. Sci. Heal. Part A, 2019, 54, 516–527 CrossRef CAS PubMed.
- L. S. Ganapathe, M. A. Mohamed, R. Mohamad Yunus and D. D. Berhanuddin, Magnetochemistry, 2020, 6, 68 CrossRef CAS.
- X. Guan, J. Guo, H. Zhang, S. Tao, G. Mailhot, F. Wu and J. Xu, Molecules, 2022, 27, 8037 CrossRef CAS.
- D. Uzunoğlu and A. Özer, ACS Appl. Bio Mater., 2022, 5, 5465–5476 CrossRef.
- P. Shanmugam, S. Boonyuen, Y. Tangjaideborisu, P. Na Nakorn, S. Tantayanon, R. Pothu and R. Boddula, Inorg. Chem. Commun., 2023, 156, 111291 CrossRef.
- M. M. Zaman, M. A. S. Karal, M. N. I. Khan, A. R. M. Tareq, S. Ahammed, M. Akter, A. Hossain and A. K. M. A. Ullah, ChemistrySelect, 2019, 4, 7824–7831 CrossRef.
- J. Sandhya and S. Kalaiselvam, Mater. Res. Express, 2020, 7, 015045 CrossRef.
- G. Sathishkumar, V. Logeshwaran, S. Sarathbabu, P. K. Jha, M. Jeyaraj, C. Rajkuberan, N. Senthilkumar and S. Sivaramakrishnan, Artif. Cells, Nanomedicine, Biotechnol., 2018, 46, 589–598 CrossRef.
- A. N. Abdel Rahman, S. R. Masoud, M. M. S. Fouda, A. A. Abdelwarith, E. M. Younis, S. S. Khalil, H. T. Zaki, E. Mohammed, S. J. Davies and R. E. Ibrahim, Aquac. Reports, 2023, 32, 101692 CrossRef.
- S. Amutha and S. Sridhar, J. Innov. Pharm. Biol. Sci., 2018, 5(2), 22–26 Search PubMed.
- M. Alavi, N. Karimi and T. Valadbeigi, ACS Biomater. Sci. Eng., 2019, 5, 4228–4243 Search PubMed.
- G. Jagathesan and P. Rajiv, Biocatal. Agric. Biotechnol., 2018, 13, 90–94 CrossRef.
- J. K. Patra, M. S. Ali, I.-G. Oh and K.-H. Baek, Artif. Cells, Nanomedicine, Biotechnol., 2017, 45, 349–356 CrossRef PubMed.
- V. Veeramanikandan, G. C. Madhu, V. Pavithra, K. Jaianand and P. Balaji, Int. J. Agric. Innov. Res., 2017, 6(2), 242–250 Search PubMed.
- R. Golabiazar, Iran. J. Chem. Chem. Eng., 2023, 42, 2745–2756 Search PubMed.
- A. Menichetti, A. Mavridi-Printezi, D. Mordini and M. Montalti, J. Funct. Biomater., 2023, 14, 244 Search PubMed.
- S. Feijoo, S. González-García, Y. Moldes-Diz, C. Vazquez-Vazquez, G. Feijoo and M. T. Moreira, J. Clean. Prod., 2017, 143, 528–538 CrossRef.
- M. F. Iannone, M. D. Groppa, M. S. Zawoznik, D. F. Coral, M. B. Fernández van Raap and M. P. Benavides, Ecotoxicol. Environ. Saf., 2021, 211, 111942 CrossRef.
- C. Buzea, I. I. Pacheco and K. Robbie, Biointerphases, 2007, 2, MR17–MR71 CrossRef PubMed.
- A. Lourens, A. Falch and R. Malgas-Enus, J. Mater. Sci., 2023, 58, 2951–2970 CrossRef.
- D. B. Olawade, O. Z. Wada, O. Fapohunda, B. I. Egbewole, O. Ajisafe and A. O. Ige, Front. Nanotechnol., 2024, 6, 1427843, DOI:10.3389/fnano.2024.1427843.
- A. Adenike Adesina, I. Uwanaobong Adeyeye, O. Ebunlomo Okuneye and Y. Temitope Abdulkareem, J. Eng. Technol. Adv., 2025, 22, 097–112 Search PubMed.
- J. Kaur, K. Kaur, K. Kaur, A. S. Matharu and S. K. Mehta, 04, Adv. Environ. Eng. Res., 2023, 1–56 Search PubMed.
- S. Kumar, S. Jain and B. Y. Lamba, Int. J. Environ. Anal. Chem., 2023, 103, 6510–6525 CrossRef.
- J. O. Adeyemi, A. O. Oriola, D. C. Onwudiwe and A. O. Oyedeji, Biomolecules, 2022, 12, 627 CrossRef CAS PubMed.
- A. Ebrahiminezhad, A. Zare-Hoseinabadi, A. K. Sarmah, S. Taghizadeh, Y. Ghasemi and A. Berenjian, Mol. Biotechnol., 2018, 60, 154–168 CrossRef CAS PubMed.
- H. M. Ahmed, M. A. El-khateeb, N. A. Sobhy, M. M. Hefny and F. M. Abdel-Haleem, in The 7th International Electronic Conference on Water Sciences, MDPI, Basel Switzerland, 2023, p. 99 Search PubMed.
- E. C. Nnadozie and P. A. Ajibade, Molecules, 2020, 25, 4110 CrossRef CAS PubMed.
- R. Indhur, I. Amoah, F. Bux and S. Kumari, ACS ES&T Water, 2023, 3, 3741–3754 Search PubMed.
- S. Some, S. Das, R. Mondal, M. Gangopadhyay and G. K. Basak, Int. J. PLANT Environ., 2021, 7, 119–132 CrossRef.
- A. Omidvari, F. Manteghi, B. Sohrabi and Y. Afra, in Proceedings of the 18th International Electronic Conference on Synthetic Organic Chemistry, MDPI, Basel, Switzerland, 2014 Search PubMed.
- A. Sharma, H. Goel, S. Sharma, H. S. Rathore, I. Jamir, A. Kumar, S. C. Thimmappa, K. K. Kesari and B. K. Kashyap, Environ. Sci. Pollut. Res., 2024, 31, 58263–58293 CrossRef PubMed.
- A. Salama, R. Abouzeid, W. S. Leong, J. Jeevanandam, P. Samyn, A. Dufresne, M. Bechelany and A. Barhoum, Nanomaterials, 2021, 11, 3008 CrossRef CAS PubMed.
- N. S. Omar, L. I. Abd Ali, A. F. Qader and R. A. Omer, Rev. Inorg. Chem., 2025, 45(4), 921–932, DOI:10.1515/revic-2024-0117.
| This journal is © The Royal Society of Chemistry 2026 |








