Open Access Article
Open Access ArticleTargeted nanocarriers integrating photodynamic and photothermal therapy: a paradigm shift in rheumatoid arthritis treatment
Sakshi
Priya
,
Dhruv
Sharma
,
Kaushal K.
Jain
,
Sahiba
Chutani
and
Gautam
Singhvi
 *
*
Industrial Research Laboratory, Department of Pharmacy, Birla Institute of Technology and Science, Pilani, Pilani Campus, Vidya Vihar, Pilani, Rajasthan 333031, India. E-mail: gautam.singhvi@pilani.bits-pilani.ac.in
First published on 19th December 2025
Abstract
Rheumatoid Arthritis (RA) is a crippling autoimmune disease characterized by gradual cartilage loss, bone degeneration, and persistent joint inflammation. Widespread adverse effects and ineffective drug distribution hamper the traditional treatment modalities. Recent progress in RA treatment has been advanced by nanocarrier-based phototherapies, including photodynamic therapy (PDT) and photothermal therapy (PTT). These therapies work by inducing necrosis or apoptosis in inflammatory cells through the generation of reactive oxygen species via PDT or localized heat production by PTT. This also leads to a reduction in pro-inflammatory cytokines and modulates macrophage polarization (M1 to M2). This dual approach shows enhanced efficacy by targeting inflammatory cytokines while preserving healthy tissue function, providing site-specific delivery, and improving bioavailability. Preclinical investigations have demonstrated that functionalized nanocarriers for targeting macrophages and synovial fibroblasts show improved drug delivery and therapeutic outcomes. While clinical trials of PDT in refractory RA patients have shown promising results in targeting synovial hyperplasia and inflammatory markers with minimal side effects, the challenges of limited light penetration, hypoxic joint microenvironments, and poor target specificity reduce the efficacy of PDT. This review focuses on multifunctional nanoplatforms that integrate PDT and PTT therapies with nanocarriers, advanced light delivery systems, and phototherapy devices to optimise RA management. These innovations aim to enhance therapeutic precision, reduce symptoms, and improve patient adherence. It also explores cutting-edge advancements in RA treatment strategies, addresses current limitations, and proposes future research directions to bridge the gap between preclinical success and clinical application.
1. Introduction
Rheumatoid Arthritis (RA) is a chronic inflammatory autoimmune disease characterised by cartilage destruction and synovitis, primarily affecting the synovial membranes, synovial bursae of the joints, and tendon sheaths. It presents as joint pain, swelling, functional impairment, and joint deformity. With an aging world population, high prevalence, and increased risk of morbidity and mortality, patients have a poor quality of life and pose a huge economic burden.1 In The Lancet Rheumatology, using data sourced from the Global Burden of Diseases, Injuries, and Risk Factors Study 2021 it was reported that between 1990 and 2020, the global prevalence rate of RA increased by 14.1% and it is projected that between 2020 and 2050, there would be an 80.2% increase in the number of RA cases, by which time 31.7 million individuals will be living with it worldwide.2 Understanding the molecular drivers of RA, particularly the autoimmune process, such as the production of anti-citrullinated protein antibodies (ACPAs), is a highly specific biomarker for RA, with anti-CCP2 (anti-cyclic citrullinated peptide) assays demonstrating a specificity of 88–98%. These autoantibodies often appear years before clinical symptoms, serving as early indicators of disease onset and potential joint erosion. Their production is closely linked to the post-translational modification of proteins by peptidyl-arginine deiminase enzymes, particularly PAD2 and PAD4.3 PAD2 and PAD4 are most strongly linked to RA through both genetic associations and cellular activity, driving the calcium-dependent conversion of arginine into citrulline, a non-standard amino acid. Experimental studies in mouse models of inflammatory arthritis have demonstrated that PAD inhibition can yield therapeutic benefits. In patients, the presence of anti-PAD4 antibodies correlates with more aggressive joint damage, whereas anti-PAD2 antibodies are linked with milder joint and lung involvement. Aberrant PAD activity, together with the generation of citrullination-related autoantibodies, contributes to three central features of RA, such as enhanced citrullination, increased production of pro-inflammatory cytokines, and bone degradation.4 However, applying these mechanistic insights, especially those concerning ACPAs, PAD dysregulation, and citrullination, to develop effective therapies remains difficult, reinforcing the need for more refined and targeted treatment strategies.Despite advances in therapy, current treatments mainly focus on reducing joint inflammation, preventing permanent bone damage, and preserving joint function. These typically include nonsteroidal anti-inflammatory drugs, various categories of disease-modifying antirheumatic drugs (DMARDs), corticosteroids, and targeted cell therapies directed at T cells, B cells, and monocytes/macrophages. However, these treatments are not effective for every patient, and prolonged use can lead to significant side effects.5 Approximately 30–40% of patients receiving biologic DMARDs discontinue treatment due to either lack of effectiveness or adverse reactions.6 Despite early use of conventional synthetic DMARDs, many patients still experience joint deterioration and poor functional outcomes.7 Therefore, there is an urgent need to find new and more effective therapies with minimal or no adverse effects.
Phototherapy, which includes both photodynamic therapy (PDT) and photothermal therapy (PTT), has shown potential as a non-invasive treatment option for inflammatory diseases like RA.8 This approach can directly impact the repair and regeneration of bone, cartilage, and muscle by modulating cellular functions.9 PDT works by using a specific wavelength of light to activate a photosensitizer (PS), which then triggers photochemical reactions that are harmful to nearby tissues. The therapeutic goal is to target the pathological features of RA, such as excessive synoviocyte growth and infiltration of inflammatory cells, by ensuring that the PS accumulates selectively in these cells. This allows for the controlled inhibition of synoviocyte proliferation and inflammatory responses while minimising harm to healthy tissues.10 The PS's pharmacokinetic, photochemical, and photobiological characteristics affect the efficacy of PDT. The majority of potent PSs generally possess an extended delocalized aromatic π electron system, which is a distinctive feature that enables them to efficiently absorb light. They readily assemble and form aggregates in aqueous media due to π–π stacking and hydrophobic interactions.11 Additionally, the tendency of PSs to accumulate in hypermetabolic tissues and cells allows for the selective destruction of overactive inflammatory cells through reactive oxygen species (ROS) generated upon photoactivation. This selective targeting is a key advantage of PDT, and its potential lies in its precise delivery, strong therapeutic effect, and comparatively fewer side effects than traditional treatment methods.10 The evolution of photosensitizers has played a crucial role in advancing PDT in the management of RA. The first generation largely comprised porphyrin mixtures such as photosensin and hematoporphyrin derivatives, but their application was limited by poor solubility, complex composition, and weak cellular selectivity. To address these shortcomings, second-generation photosensitizers, including porphyrin derivatives, metallophthalocyanines, and polycyclic quinones, were introduced. These agents enhanced tissue-targeting capacity, provided deeper penetration by modulating absorption wavelengths, and demonstrated faster systemic clearance with improved therapeutic performance; however, issues of low solubility and limited specificity persisted. Current efforts are focused on third-generation photosensitizers, which involve conjugation with monoclonal antibodies or biologically active molecules (e.g., peptides, lipids, nucleotides, or steroids) to impart selective targeting functions. Such functionalized photosensitizers represent the next step in optimizing PDT for clinical use, offering the precision and efficacy needed to translate phototherapy into practical treatment strategies for RA.12 In parallel, PTT utilizes electromagnetic radiation and works by locally increasing the temperature of tissues through the photoexcitation of materials containing a PS, triggered by exposure to microwaves, radiofrequency waves, and near-infrared (NIR) or visible light.13
Due to their tunable surface properties, nanocarriers (NCs) can efficiently interact with multiple cellular targets, enabling them to deliver therapeutic agents directly to inflamed joint sites, reducing systemic drug exposure and limiting potential side effects.14 When NIR light (750–2500 nm) is used alongside a photothermal agent, there is a potential risk of minor damage to adjacent healthy tissues. Upon irradiation, these nanomaterials absorb photons and dissipate energy through a non-radiative relaxation mechanism. Different types of nanoparticles (NPs), including nanorods, nanoshells, and nanospheres, can enhance the selective targeting of inflammatory cells.13 The development of nano-based co-delivery systems emerged to address the limitations of monotherapy, as these systems can accurately deliver drugs into targeted cells, thereby reducing systemic toxicity and directing treatment toward inflamed areas through surface modifications. Additionally, they help control the drug release rate by encapsulating active agents, enabling higher dosage delivery and minimizing side effects. With current medical advancements, nanocarriers are being increasingly utilized to transport therapeutic agents, thereby improving targeted drug delivery to joints, reducing dosage and toxicity, enabling controlled drug release, and enhancing patient adherence.15
In PTT, when light hits the surface of nanomaterials, and the frequency of the incoming photons aligns with the natural vibration frequency of the NPs, a strong absorption of photon energy occurs, known as the Localized Surface Plasmon Resonance (LSPR) effect. As a result, NPs, especially those made with gold, are excellent candidates for use as photothermal agents due to their high absorption-to-scattering ratios and their ability to efficiently convert light into heat.16 These therapies are triggered to deliver drugs against RA. PDT and PTT localize on inflamed joints and enhance the targeting of inflammatory cells, while still preserving the therapeutic benefits of conventional medications. The drug-delivery system employs a photoactivated targeting mechanism to localize therapeutics to inflammatory sites, requiring only NIR light exposure for sustained symptom management. The combined action of light activation and pharmacological intervention minimizes drug dosage and systemic toxicity while enhancing therapeutic outcomes through synergistic efficacy.15
The objectives of this review are to understand the fundamental mechanisms and therapeutic applications of PDT and PTT in RA management. It aims to evaluate the role of nanocarrier-based delivery systems and their integration with phototherapies for site-specific targeting of inflamed joints, further exploring the synergistic effects of conventional anti-rheumatic drugs with phototherapies, to reduce dosage and minimize systemic toxicity. The review will also highlight advances in nanotechnology for the treatment of RA, the outcomes of preclinical and clinical studies, the challenges associated with them, and future directions.
2. Mechanistic aspects of phototherapy in the management of RA
2.1. Mechanism of photodynamic therapy in RA
Due to a substantial treatment challenge posed by RA, a surgical approach is synovectomy. However, it is invasive and removes only a portion of the diseased synovial tissue. It is also associated with high recurrence rates, postoperative pain, stiffness, and the risk of fractures, often leading to a lengthy recovery period.12 In recent years, arthroscopic synovectomy has gained growing attention due to its minimally invasive nature and reduced immobilization for patients. However, its effectiveness has been limited, as the procedure is primarily applicable to larger joints, reducing its overall utility. Later, laser synovectomy was found to exhibit various shortcomings, like thermal side effects, low ablation rates, and being more time-consuming.17As a newer therapy for RA, PDT relies on the dynamic interaction between a PS, molecular oxygen, and light of a specific wavelength, leading to selective and targeted tissue destruction.18 Compared to steroid injections or arthroscopic synovectomy, PDT is less invasive, offers more precise targeting, and delivers longer-lasting therapeutic effects.10 The procedure involves administering the PS either topically or intravenously, which then preferentially accumulates in the inflamed tissue during a drug-light interval. This is followed by exposure to light, typically in the red wavelength range (λ ≥ 600 nm), to activate the therapeutic effect.18 Researchers are employing PDT to produce ROS that selectively destroy inflammatory cells, including macrophages, synovial fibroblasts, and other immune cells present at RA sites. This targeted cell destruction helps lower the levels of key inflammatory cytokines such as IL-17, TNF-α, IL-1β, and IL-6. Additionally, PDT offers the advantage of precise light delivery, allowing treatment to be focused specifically on the affected joints. The technique is considered safe due to its non-invasive nature and minimal systemic toxicity.12
PDT operates through a specific mechanism as depicted in Fig. 1, where, upon light exposure, the PS transitions from its ground state to an excited singlet state (1PS*). Due to the short lifespan of this state, the PS can either return to its ground state by emitting energy as fluorescence or dissipating it as heat (non-radiative decay). Alternatively, it can undergo intersystem crossing (ISC) to reach a more stable triplet state (3PS*), which has a longer lifetime. In this triplet state, the PS can initiate two types of photochemical reactions: a Type I reaction, where it produces free radicals, or a Type II reaction, where it transfers energy to molecular oxygen (3O2), generating highly reactive singlet oxygen (1O2). Singlet oxygen plays a crucial role in damaging target cells by oxidizing cellular components such as DNA and membrane structures, and by disrupting signalling pathways, ultimately leading to cell necrosis or apoptosis.16 The products generated from both type I and type II reactions contribute to cell death and the therapeutic outcomes of PDT. These reactions can take place simultaneously, and the balance between them is influenced by factors such as the PS, the nature of the substrate, oxygen levels, and the PS's binding affinity to the substrate. However, the Type II reaction is typically predominant in PDT, with singlet oxygen being the main cytotoxic species driving the biological effects.18
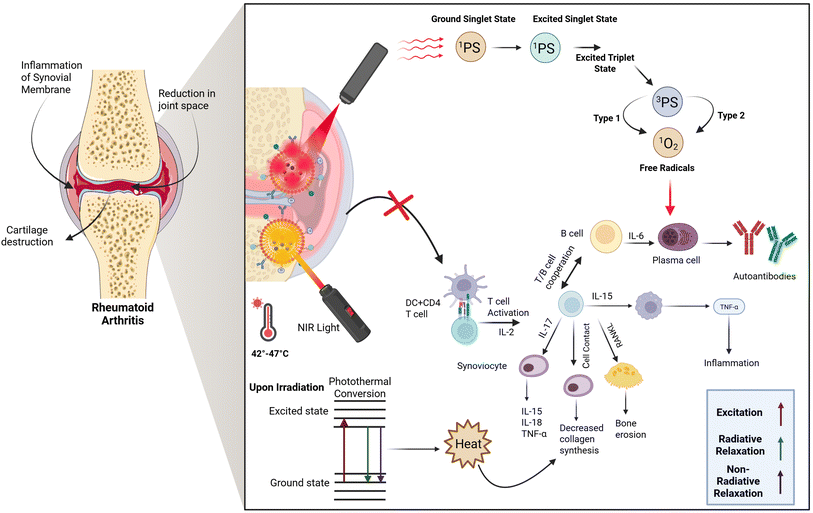 | ||
| Fig. 1 Mechanism of action of photodynamic therapy and photothermal therapy in the treatment of rheumatoid arthritis. Created with https://www.biorender.com/. | ||
2.2. Mechanism of photothermal therapy in RA
One effective approach for treating RA involves the use of PTT to selectively target and destroy pathogenic cells within affected joints. This strategy helps suppress the production of inflammatory mediators and mitigates synovial hyperplasia, as well as the degradation of bone and cartilage. Fibroblast-like synoviocytes (FLSs), known to accumulate extensively in vascularized regions of the joint, adopt an aggressive behavior by directly releasing matrix metalloproteinases (MMPs), which contribute to cartilage breakdown, and by activating the receptor activator of nuclear factor kappa-β ligand (RANKL), which promotes osteoclast formation and bone erosion. In recent years, PTT has demonstrated its potential in selectively eliminating FLSs in animal models of RA, thereby effectively slowing disease progression and positioning itself as a promising therapeutic option.9 At sites of inflammation, PTT not only significantly reduces the number of hyperproliferative inflammatory cells in RA joints but also enhances the catalase-mimicking activity of cerium oxide (ceria), facilitating the breakdown of hydrogen peroxide (H2O2) and boosting oxygen production. This, in turn, helps alleviate inflammation and hypoxia within the diseased microenvironment as depicted in Fig. 1.16 The concerns regarding the toxicity and prolonged retention of conventional inorganic photothermal agents have shifted attention toward biodegradable nanomaterials that provide safer and more effective options for phototherapy. Within this context, graphene oxide quantum dots (GOQDs) stand out as a promising candidate for the treatment of RA. GOQDs, a unique class of 2D nanomaterials, are valued for their excellent biocompatibility, multifunctional bioactivity, and natural degradability. In addition to functioning as efficient drug carriers, they exert therapeutic benefits by modulating immune responses, specifically by promoting the transition of macrophages from the pro-inflammatory M1 state to the anti-inflammatory M2 state, thereby reducing inflammation and aiding tissue recovery.19 Similarly, Black Phosphorus (BP), a layered semiconductor with a folded honeycomb configuration, has attracted considerable interest due to its high drug-loading ability, favourable optical and thermal features, and strong biocompatibility. When exposed to NIR light, BP demonstrates a potent photothermal effect, generating localised heat that eradicates diseased tissue while also producing ROS to further damage abnormal cells. This localised heating additionally improves drug penetration through the skin, an advantage particularly relevant for molecules like rutin. Therefore, BP-rutin conjugates have been incorporated into an HA–PVA hydrogel, forming a composite system with pronounced photothermal activity. This hydrogel ensures sustained drug release in mildly acidic environments, enhances transdermal delivery, and significantly reduces inflammatory mediators associated with RA.20Like tumor tissues, inflamed tissues are characterized by an abundance of blood vessels that retain heat and exhibit lower thermal tolerance compared to healthy tissues. PTT leverages this property by using photothermal agents to convert light energy into localized heat, which induces hyperthermia and selectively destroys inflammatory cells, thereby eliminating diseased tissue. In recent years, PTT has been increasingly used for treating inflammatory conditions and has shown significant promise in managing RA.21 Unlike PDT, PTT does not rely on oxygen to damage targeted cells and tissues. Moreover, it offers high selectivity with minimal side effects, as laser parameters, such as wavelength, intensity, duration, and irradiation site, can be precisely adjusted.16 It's known that PDT requires oxygen for better performance, while PTT destroys target tissues and cells in a hypoxic environment.12
3. Targeted delivery strategies of phototherapeutic agents in the management of RA
Drug targeting refers to the administration of drugs specifically to the site of action or the target tissue, minimizing the exposure of healthy tissues to the drug and reducing side effects. This approach enhances the therapeutic effect of the drug while minimizing its systemic toxicity. Various strategies are employed to achieve targeted drug delivery, including the use of NPs such as liposomes, micelles, and other carrier systems.22–243.1. Macrophages
Macrophages exist in two distinct phenotypes, notably M1 and M2. M1 phenotype macrophages release pro-inflammatory cytokines, such as TNF-α, IL-1β, and IL-6, along with oxygen intermediates and reactive nitrogen species, which stimulate immune cell activation, fibroblast activation, and T cell polarization, thereby fostering inflammation, contributing to articular cartilage degeneration, and ultimately leading to bone erosion.25–27 On the other hand, M2 macrophages predominantly release high levels of anti-inflammatory cytokines, notably IL-4, IL-10, and transforming growth factor-β (TGF-β), which facilitate tissue remodelling, angiogenesis, and wound repair processes.28 In RA, the equilibrium between M1/M2 macrophages becomes disrupted, resulting in an elevated proportion of M1 macrophages.29 Targeting ligands frequently utilized for therapeutic interventions include receptors such as CD44, vasoactive intestinal peptide (VIP) receptors, scavenger receptors (SR), and folate receptors (FR) β, as well as other related receptors that are abruptly overexpressed on the surfaces of activated macrophages.30,31Phototherapy influences cellular processes and signalling pathways within macrophages, resulting in a transition from a pro-inflammatory (M1) phenotype to an anti-inflammatory (M2) phenotype, which leads to reduced inflammation and tissue damage in the joints.32 CD44 and folate receptors are overexpressed in activated macrophages of inflamed joints. Hence, molecules that bind to these receptors are used, leading to endocytosis of the photothermal agent, followed by cell necrosis via singlet oxygen generation.33
Nano-therapy using a quadrilateral ruthenium-based nanotherapy system (QRu-PLGA-RES-DS NPs) to target macrophages and shift them from M1 to M2 phenotype for RA treatment was developed. This system combined resveratrol (RES) to reduce ROS and QRuNPs as photothermal agents. Under an 808 nm laser (0.4 W cm−2), it achieved a temperature of >47 °C with a photothermal efficiency of 13.2%. Compared to RES alone, laser-irradiated NPs showed 3.91-fold and 2.69-fold increases in Arginase-1 and CD206 expression, respectively. This combination also reduced IL-4 (1.75 times), IL-10 (2.13 times), and TGF-β (2.67 times), which is higher than with RES alone.32
A triptolide (TP)-loaded HA hydrogel with gold NPs (TP-PLGA-Au@RGD/HA) for RA treatment was developed, enabling PTT under 808 nm laser irradiation (0.38 W cm−2), reaching 47.2 °C. The hydrogel reduced RA fibroblast-like RA-FLS migration/invasiveness by 54% and 38% inhibition of phosphorylated mTOR and p70S6K, respectively, as shown in Fig. 2. The targeted effects on inflamed joints in vivo, as observed through fluorescence imaging of these hydrogels, showed selective accumulation in the inflamed joint, resulting in reduced pro-inflammatory cytokines and improved bone preservation compared to TP alone.35
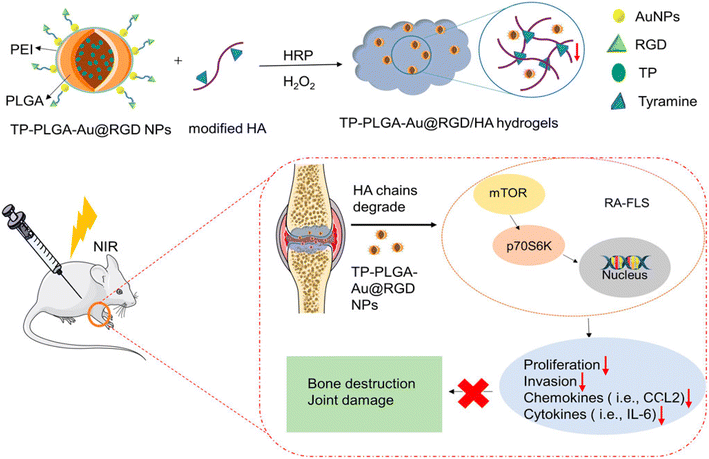 | ||
| Fig. 2 Preparation process of hyaluronic acid hydrogel-loaded RGD-attached gold nanoparticles containing triptolide (TP-PLGA-Au@RGD/HA) hydrogels and schematic illustration of its anti-inflammatory effect in CIA mice. Reproduced with permission from Li et al.35 [Copyright 2022, Frontiers]. | ||
Methotrexate-loaded folic acid functionalized gold nanorods (MTX-FAGMs) were developed to target RA-activated macrophages via the folate receptor β (FRβ). These gold nanorods provided photothermal activity, while folate modification enabled cellular uptake. Under an 808 nm laser (1.0 W cm−2), MTX release reached 63.74% (pH 7.4) and 68.75% (pH 4.5), compared to 22 and 39% without irradiation, respectively. The MTX-FAGMs treated with an NIR laser showed greater cytotoxicity due to the synergistic effects of PTT and chemotherapy. In arthritis-induced rats, treatment with a combination therapy (MTX-FAGMs and laser treatment) reduced paw thickness, TNF-α and IL-6 cytokine levels, and preserved the cartilage structure, thereby limiting the progression of RA.38
3.2. Vasoactive intestinal peptide
VIP is expressed in various cells, including T lymphocytes, macrophages, and inflammatory cells such as synoviocytes. Its anti-inflammatory effects include the reduction of pro-inflammatory agents and induction of inducible nitric oxide synthase (iNOS). Moreover, it inhibits the release of chemokines and encourages the production of anti-inflammatory factors in activated immune cells while promoting negative expression of Toll-like receptor ligands, TLR2 and TLR4. Therefore, the drug can bind to VIP receptors and target the drug at the inflamed site.39,40 NPs conjugated with VIP are engulfed by fibroblasts through endocytosis. In PTT, where irradiation is converted to heat by a photothermal agent, the elevated temperature generated within the NPs induces cell death.Huang et al. developed the nanotherapeutic system, gold nanorods with copper sulphide nanoparticles (Au NR@CuS NPs), targeting the treatment of RA by enhancing oxidative reactions and generation of OH radicals, as shown in Fig. 3. This VIP conjugated nanosystem showed a temperature increase of about 54 °C in 5 min when exposed to a NIR laser (808 nm, 1.0 W cm−2), and an efficiency of 67.2% for photothermal conversion. NR@CuS NPs utilise VIP to become engulfed by fibroblast cells and reach the cytoplasm via endosomes or lysosomes. They exhibit high H2O2 consumption, OH (radical) generation, and higher photocatalytic capability, leading to the death of FLSs, as well as a decrease in the synovial inflammation.41
 | ||
| Fig. 3 Schematic illustration for gold nanorods with copper sulphide nanoparticles (Au NR@CuS NPs) for synthesis and synergistic treatment of RA. Reproduced with permission from Huang et al.41 [Copyright 2021, Elsevier Ltd]. | ||
3.3. Pro-inflammatory cytokines
In RA, TNF-α activates synovial fibroblasts, leading to increased growth of the synovial lining and attracting more inflammatory cells to the affected area.42 IL-17 disturbs bone homeostasis in RA by promoting osteoclast formation while suppressing osteoblast activity. In joints with elevated IL-17 levels, this imbalance enhances osteoclast activity across cortical, trabecular, and subchondral bone, ultimately leading to progressive bone erosion. TNF-α and IL-17 play crucial roles in bone resorption, inducing the differentiation of cells into osteoclasts and contributing to bone damage in RA. Therefore, reducing or blocking the concentrations of both TNF-α and IL-17 shows promising results in improving the condition of RA patients.43 Furthermore, interfering with the signalling pathways (suppression of the phosphorylated nuclear factor kappa B (NF-κB) signalling pathway) can lead to a reduction in the production of proinflammatory cytokines.44 Therefore, nanomaterial-based interventions have been explored to target these inflammatory mediators and restore joint homeostasis in RA.TiO2 nano-whiskers combined with TSPP (TP) for PDT by targeting IL-17 and TNF-α were developed. Upon exposure to visible light (500–550 nm), TP generated singlet oxygen and hydroxyl radicals, causing DNA oxidation and membrane disruption, which led to cell necrosis and/or apoptosis. TP treatment reduced synovial erosion, edema (12.8% on the left foot and 7.64% on the right foot), inflammatory cell infiltration, IL-17 levels (16.53 ± 1.64 pg mL−1 and 21.84 ± 1.128 pg mL−1 in TP-0.4 and TP-0 respectively) and TNF-α levels (249.38 ± 35.30 pg mL−1 and 358.69 ± 3.59 pg mL−1 in TP-0.4 and TP-0 respectively). TIO2 and TSPP generated 10 times more singlet oxygen than TSPP alone, enhancing joint-targeted PDT.45
Arginine–glycine aspartic acid (RGD)-modified palladium (Pd) nanosheets were designed for targeted RA therapy, enabling controlled MTX release via the photothermal effect. Palladium-cysteine@methotrexate@arginine-glycineaspartic acid (Pd-Cys@MTX@RGD) uptake was 6.5 times higher in HUVECs when compared with Pd-Cys@MTX. Upon 808 nm irradiation (0.3 W cm−2, 10 min), Pd-Cys@MTX@RGD nanosheets reached 50.2 °C, significantly reducing cell proliferation from 150% to 45% demonstrating effectiveness. In vivo, this system minimized MTX side effects, inhibited TNF-α and COX-2, and protected cartilage. As shown in Fig. 4, animal imaging confirmed nanosheet accumulation and strong photothermal/photoacoustic signals in Pd-Cys (44.8 °C) and Pd-Cys@MTX@RGD (50.2 °C) groups, while saline showed minimal effects.46
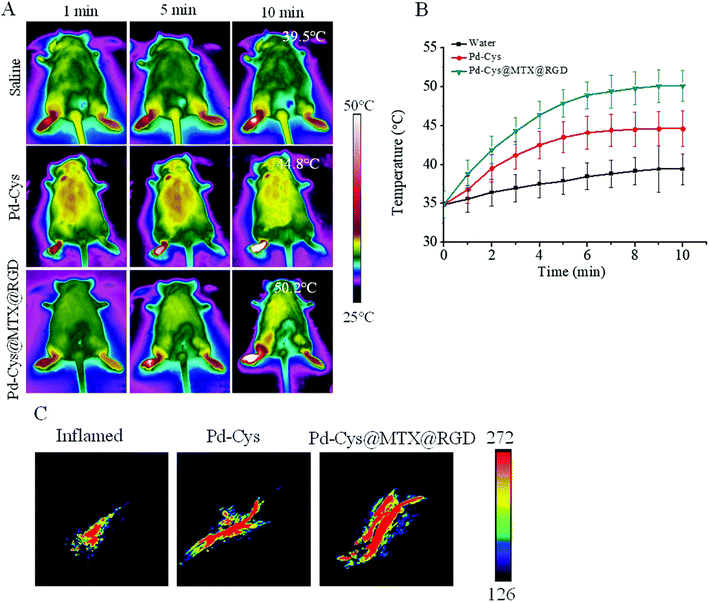 | ||
| Fig. 4 (A) Photothermal images of the palladium-cysteine (Pd-Cys) and palladium-cysteine@methotrexate@arginine-glycineaspartic acid (Pd-Cys@MTX@RGD) nanosheets in RA mice irradiated at 808 nm for 10 min (0.3 W cm−2). (B) Temperature profiles of the Pd-Cys and Pd-Cys@MTX@RGD nanosheets in RA mice irradiated at 808 nm for 10 min. (C) Photoacoustic signal images of the Pd-Cys and Pd-Cys@MTX@RGD nanosheets in RA mice excited with 808 nm irradiation. Reproduced with permission from Chen et al.46 [Copyright 2019, RSC]. | ||
3.4. Synovial fibroblasts
In RA, FLSs show a distinct, pro-pathogenic phenotype and display elevated expression of fibroblast activation protein (FAP), a membrane-bound dipeptidyl peptidase. This heightened FAP expression is associated with increased production of chemokines, cytokines, and matrix metalloproteinases, contributing to inflammation, cartilage, and bone destruction.47 Its elevated levels in RA synovial tissues contribute significantly to the immune evasion mechanism of synovial cells, and research indicates that reducing FAP-positive synovial fibroblasts can mitigate inflammation and improve cartilage preservation in mouse models of arthritis.48PDT involves the administration of a PS to a hyperactive synovial tissue, including synovial fibroblasts. Upon laser light activation, it triggers the production of ROS selectively in these cells, causing cellular damage and oxidative stress, which leads to the destruction or impairment of synovial fibroblasts, potentially alleviating RA-related inflammation and joint damage.47
The effect of hypericin-PDT (HYP-PDT) on treating RA by inducing apoptosis in RASF via the mitochondrial pathway and suppressing NF-κB was studied. HYP enters cells through endocytosis/pinocytosis, binds mitochondria, and generates ROS, leading to caspase-9 and PARP cleavage, caspase-8 downregulation, and apoptosis.49 A core–shell nanostructure gold@ceriumoxide-polyethylene glycol (Au@CeO2-PEG) through a combined photothermal and oxygen-enriched therapy approach was developed. As Au rods exhibit an enhanced LSPR effect, this resulted in a better photothermal effect of Au@CeO2-PEG under NIR irradiation.16
Dorst and his colleagues developed a targeted PDT for RA by eliminating FAP-positive fibroblasts using the PS IRDye700DX. The PS was conjugated with anti-FAP antibody 28H1, diethylenetriaminepentaacetic acid (DTPA) for radio-labelling with 111-indium (111In), forming [111In]In-DTPA-28H1-700DX. Upon 690 nm light excitation, the agent generated ROS, inducing targeted cell death. In AIA and CIA mouse models, this system effectively ablated FAP-expressing fibroblasts, demonstrating precise and efficient therapeutic action.47
3.5. Anti-vascular strategies
In the realm of therapeutic strategies for RA, PDT can be used to induce vascular shutdown on exposure to irradiation.16 It was hypothesized in this case study that hemorrhage and vascular damage would clear out red blood cells (RBCs) from the synovial tissue, leading to a gradual decline in inflammation. Thrombin induces angiogenesis by overproducing vascular endothelial growth factor (VEGF), which contributes to the thickening and excessive vascularization of inflamed synovial tissues.Gabriel and his colleagues developed a thrombin-sensitive polymeric photosensitizer prodrug (T-PS) to inhibit angiogenesis in RA. T-PS consists of pheophorbide units linked to a pegylated poly-L-lysine backbone via thrombin-cleavable peptides. Inactive until activated by thrombin, T-PS showed no toxicity unless irradiated at 665 nm (50 W cm−2). At 25 J cm−2, it induced dose-dependent apoptosis and haemorrhage, with no significant effect at a light dose of 7 J cm−2, thus highlighting its considerable potential for both diagnosing and treating RA.50 The receptor-targeted nanocarrier strategies are schematically presented in Fig. 5.
4. Emerging nanocarrier-based phototherapy for RA management
NCs are materials in the nano-range of 1–100 nm, which improve the delivery of various substances such as photosensitizers, overcoming issues of reduced solubility in water or aggregation tendency, amongst others.51 Stimuli-responsive formulations typically involve an external trigger/stimulus, such as light and sound, to act as drug release switches for the encapsulated NPs, allowing them to release the drug within. Light-triggered therapy involves the use of photothermal agents for PTT and PSs for PDT, and sound-triggered therapy involves acoustic sensitizers for ultrasound therapy.15 Due to reduced systemic toxicity and a non-invasive approach, photothermal therapy is considered comparatively safer. The site of RA is characterised by numerous markers of inflammation, including TNF-α and IL-6, which are produced by macrophages and synovial fibroblasts. These sources are key targets of phototherapeutic agents.52 For this purpose, phototherapeutic agents were modified using NC systems such as liposomes, polymeric NPs, metallic NPs, solid lipid NPs, nanostructured lipid carriers, etc. It is evident from pre-clinical and clinical studies that NCs increased the therapeutic efficacy of phototherapeutic agents. Thus, NCs could be used as a potential delivery system in phototherapy.164.1. Nanocarrier-based photothermal therapy for RA management
PTT involves the introduction of heat to destroy diseased cells/tissues produced by photoexcitation of the material from PSs. Heat is produced from electromagnetic radiation, and the increase in temperature around the microenvironment and the lesioned area of RA enhances the release of nanomedicines, thereby increasing the efficacy of RA treatment. Heat is usually produced by radiofrequency, microwaves, and NIR, or visible light, generating hyperthermia, causing ablation of the lesion by inhibiting the abnormal tissue. During light exposure, it's crucial to control the temperature (41–47 °C) to avoid damage to healthy cells.53–55 Multifunctional nanocomposites are developed to integrate photothermal effects with additional therapeutic capabilities, achieving more targeted and multifunctional RA therapy. The polydopamine (PDA)-coated CeO2-doped zeolitic imidazolate framework-8 (ZIF-8) nanocomposite (denoted as CZP) represents such a therapeutic platform for RA. Under NIR irradiation, the PDA layer efficiently converts light into heat, inducing the destruction of hyperproliferative inflammatory cells within the joint. Concurrently, the acidic microenvironment triggers the degradation of the ZIF-8 framework, enabling the release of CeO2 nanoparticles. These nanoparticles catalyse the decomposition of excess hydrogen peroxide (H2O2), producing oxygen and thereby alleviating hypoxia. Through the integration of photothermal therapy, ROS scavenging, and oxygen generation, the nanocomposite reduces inflammation by downregulating pro-inflammatory cytokines and hypoxia-inducible factor-1α (HIF-1α). Collectively, this synergistic mechanism has shown encouraging potential for improving RA treatment outcomes while avoiding exacerbation of oxidative stress.56 In contrast to the enhanced permeability and retention (EPR) effect observed in tumors, drug retention in RA is primarily driven by the accumulation of inflammatory cells at the diseased site; thus, to enhance the effectiveness of existing RA therapies, various drug carriers have been designed that exploit passive targeting strategies, enabling selective binding to receptors overexpressed in RA-affected joints. This localized enrichment minimizes the requirement for deep light penetration to efficiently activate photothermal agents.57 These systems overcome the limitation of poor light penetration in deep synovial tissues, leading to more effective RA phototherapy.Core–shell palladium@selenium-hyaluronic acid (Pd@Se-HA) NPs integrating selenium (˙OH scavenger) and palladium (photothermal agent) were developed for RA treatment. These NPs exhibited concentration-dependent heating (40.2 °C to 50.4 °C at 10–40 mg mL−1) under an 808 nm laser (0.5 W cm−2, 10 min), with a photothermal efficiency of 34.5%, and were coated with HA for macrophage targeting. Some of the photothermal images and temperature profiles are illustrated in Fig. 6. In vitro studies on RAW 264.7 cells showed 90% viability, indicating negligible toxicity up to 200 µg mL−1 concentrations for the developed NPs, and similar results were observed in HEK293T cells. In vivo, Kunming mice exhibited a reduced inflammation index after 15 days of in situ Pd@Se-HA NP injections. The HA coating on NPs enhanced site-specific targeting and anti-inflammatory effects.58
 | ||
| Fig. 6 (A) The temperature profiles of palladium nanoparticles (Pd NPs; 20.0 mg mL−1), palladium@selenium nanoparticles (Pd@Se NPs; 20.0 mg mL−1), and palladium@selenium-hyaluronic acid nanoparticles (Pd@Se-HA NPs; 20.0 mg mL−1) irradiated at 808 nm (0.5 W cm−2) for 10 min, respectively. (B) The photothermal images of Pd NPs (20.0 mg mL−1), Pd@Se NPs (20.0 mg mL−1), and Pd@Se-HA NPs (20.0 mg mL−1). (C) The temperature profiles of different concentration Pd@Se-HA NPs (40.0, 20.0, 10.0 mg mL−1) irradiated at 808 nm (0.5 W cm−2) for 10 min, respectively. (D) The photothermal images of different concentration Pd@Se-HA NPs (20.0 mg mL−1). (E) The monitored temperature change curves of Pd@Se-HA NPs as irradiated by an NIR laser for 600 s, followed by natural cooling with the laser light turned off, and determination of the time constant for heat transfer from the system using linear regression of the cooling profiles (mean ± SD, n = 3). (F) The linear regression curves of Pd@Se-HA NPs. Reproduced with permission from Zheng et al.58 [Copyright 2021, Elsevier]. | ||
ICAM-1-modified TOF-loaded P(AN-co-AAm)-PEG micelles (AI-TM) were developed to enhance anti-inflammatory effects via microwave-induced hyperthermia. The micelles exhibited high drug loading (4.7 ± 0.2%) and encapsulation efficiency (94.8 ± 4.9%). At 37 °C, 40% drug release was noted at 48 h, while at 43 °C, release increased to 80%, indicating thermal-triggered disintegration. Nile red fluorescence confirmed enhanced cellular uptake under hyperthermia. In RA mice, microwave treatment (8 W, 30 min) post AI-TM injection reduced paw thickness, arthritic scores, and weight loss, proving the formulation's therapeutic efficacy.59
4.2. Nanocarrier-based photodynamic therapy for RA management
PDT involves the transfer of energy from an injected PS, which is excited by irradiation with light of a certain wavelength, to neighbouring molecules, such as cellular oxygen, leading to the formation of ROS, which ultimately results in cell death. A PS remains inactive when administered by the topical or intravenous route until it is exposed to photosensitizing light.51,60 PSs are encapsulated within NPs to improve solubility and also targeted retention and accumulation in desired tissues. Currently, the strategies applied for PDT include the use of inorganic materials with superior chemical and photosensitive properties, as well as the use of bio-responsive NCs for targeted delivery.55Many new platforms are being developed, aiming to reduce or stop the proliferation of FLSs. The retention of the PS can be enhanced by encapsulation in NPs that target RA-FLS, which play a crucial role in the persistent destruction of cartilage and joint damage by producing cytokines that affect the microenvironment of RA synovium.61 Tang et al. developed phase-transition oxygen and indocyanine (ICG) green OI-NPs using PLGA. These NPs offered dual PDT (808 nm) and sonodynamic (1 MHz) therapy (PSDT). ICG showed a sustained release of 71.34% over 24 h, avoiding burst release. The cellular uptake of ICG was 35% higher in OI-NPs than free ICG, indicating enhanced endocytosis. OI-NPs exhibited strong cytotoxicity, reducing MH7A cell viability to 25.05% ± 5.42%. In contrast, blank NP-mediated PSDT showed no obvious cytotoxicity (93.17% ± 1.95% viability).62
The team led by Li developed dissolving microneedles (5-ALA@DMNA) loaded with 5-aminolevulinic acid (5-ALA), a prodrug that forms the PS protoporphyrin-IX (PpIX) to target RA-FLS. Upon 635 nm light irradiation, PpIX generated cytotoxic ROS, leading to dose-dependent cell death. Survival rates dropped from 78% to 12% as 5-ALA concentration increased (20 to 50 µg mL−1). In the presence of N-acetyl-cysteine treatment, the survival rates with the same concentration reduced from 83% to 54%, respectively. The apoptosis increased from 0.45% in controls to 21.73% with 5-ALA + light. 5-ALA@DMNA achieved 4-fold higher drug release than 5-ALA cream, enhancing transdermal delivery and RA targeting.61
Chitosan-based nanogels encapsulating anionic PSs such as tetra-phenyl-porphyrin-tetra-sulfonate (TPPS4), tetra-phenyl-chlorin-tetra-carboxylate (TPCC4), and chlorin e6 (Ce6) to target inflamed articular joints were developed. These nanogels showed prolonged joint retention (up to 7 days), unlike a free PS, which cleared within 24 h. Furthermore, PDT using Ce6-nanogels at 25 J cm−2 in AIA mice resulted in a marked decrease in serum amyloid A (SAA) levels, indicating a robust anti-inflammatory response. This effect was comparable with the reduction seen after prednisolone treatment, indicating comparable therapeutic efficacy highlighting their sustained, macrophage-targeted anti-inflammatory effect in RA.63
PEGylated liposomes containing PS IRDye700DX to target macrophages of inflamed joints were prepared, and at a reduced dose of 10 J cm−2 (690 nm light), RAW 264.7 cell viability for the IRDye700DX-liposome-treated group was found to be 50% compared to the untreated control group. It was reduced to 3% at a higher dose of 50 J cm−2, showing dose-dependent viability. The progression of arthritis was investigated in male DBA/1JRj mice with CIA. Compared to the control groups, the arthritis score was reduced in PDT-treated groups (8.8 and 26.4 J cm−2). Hence, they were successful in providing a localized treatment of RA.64
Nanowhiskers combining tetra-sulfonatophenyl porphyrin (TSPP) with titanium dioxide (TiO2) were developed for theranostic application in RA. These nanowhiskers enabled early fluorescence imaging-based diagnosis by detecting inflamed joints in rats even before clinical symptoms appeared. Therapeutically, TSPP–TiO2 significantly reduced serum IL-17 by about 50% and TNF-α by about 40%. In vivo, the TP-0.4 group exhibited edema reductions of 12.8% (left foot) and 7.64% (right foot), compared to 16.32% and 15.24% in the TP-0 group, confirming both diagnostic and therapeutic potential.45 In the treated mice, a combination of allogenic bone marrow stem cells (BMSCs) with TSPP–TiO2 nanocomposites reduced the arthritic scores and improvements were observed in platelet, RBC, and WBC counts. Microarray data analysis showed that rno-mir-375-3p and rno-mir-196b-3p were up-regulated in the BMSCs of ameliorated RA post-PDT with TSPP–TiO2 nanocomposites.65
4.3. Synergistic photodynamic therapy and photothermal therapy for RA treatment
A combination of PDT and PTT can be an effective treatment, as they act synergistically via different mechanisms. The efficacy of PDT can be enhanced by PTT, which improves the penetration of nanosystems and enhances oxygen perfusion via hyperthermia. Various anti-rheumatic agents can also be combined with PTT to enhance their cellular uptake and membrane permeability via hyperthermia. Alternatively, chemotherapy can improve phototherapy by targeting specific surviving inflammatory cells.16Semiconductor polymer quantum dots (SPs) hybridised with mesoporous silica nanoparticles (MSNs) using PCPDTBT (poly[2,6-(4,4-bis-(2-ethylhexyl)-4H-cyclopenta [2,1-b;3,4-b]dithiophene)-alt-4,7(2,1,3-benzothiadiazole)]) to synthesise PDT and PTT were developed by Li and his group. The NPs were used to house prodrug tirapazamine (TPZ), which gets activated by hypoxia. Cetrimonium bromide (CTAB) was used as a stabilizer for SPs, and the mesoporous structure of silica was functionalized with surface PEG–FA (polyethylene glycol–folic acid) to enhance its circulation under in vivo conditions as well as provide targeting ability to activated macrophages (SMPFs). Mean fluorescence intensity (MFI) was almost 5.4 times greater in cells treated with FITC-SMPFs than FITC-SMs, where FITC represents the fluorescence-producing agent fluorescein isothiocyanate. The MFI of DCF (singlet oxygen, green) and FITC (hypoxia, green) was almost 10 and 7 times greater than that of PBS-treated groups, respectively. This is presented in Fig. 7.66
 | ||
| Fig. 7 (A) Hypoxia and (B) singlet oxygen detection (scale bar, 50 µm), and (C) corresponding mean FITC and (D) DCF fluorescence intensity analysis in activated RAW 264.7 cells with/without a NIR laser (1.0 W cm−2, 5 min) cultured with SMPFs and PBS. The release rate showed a pH-sensitive release of TPZ with a cumulative release rate of 69.7% at pH 5.0 (similar to the pH of the acidic lysosomal environment in activated macrophages), compared to 44.7% at pH 5.0. Under NIR irradiation, the 24 h-cumulative release rate was also higher at pH 5.0 (72.96%) than at pH 7.4 (50.82%).66 Reproduced with permission from Li et al.66 [Copyright 2021, Elsevier]. | ||
Lu and his colleagues developed novel L-cysteine (Cys) assisted copper (Cu)S NPs (termed Cu7.2S4 NPs) to enable combination phototherapy for RA while also promoting osteogenesis and chondrogenesis. Upon 808 nm NIR irradiation (1 W cm−2), temperatures increased to 55 °C (500 µg mL−1) and 41 °C (125 µg mL−1), confirming the photothermal capability. The generation of singlet oxygen was demonstrated using the RNO-ID assay and intracellular DCFH-DA fluorescence, as shown in Fig. 8. In vivo, the Cu7.2S4 + NIR group exhibited the lowest clinical arthritis index and superior preservation of bone, as indicated by high bone mineral density (922.80 mg cm−3) and bone volume/total volume (BV/TV 55.38%). Additionally, the NPs showed antibacterial efficacy against Staphylococcus aureus and Escherichia coli, making them promising agents against bacterial infections during intra-articular injection.67
 | ||
| Fig. 8 In vitro photothermal and photodynamic effects. (a) UV-vis spectra of novel L-cysteine (Cys) assisted copper (Cu)S NPs (Cu7.2S4 NPs) and dd H2O. The NPs showed a strong absorption band in the NIR region (700–900 nm). (b) Quantitative temperature change in Cu7.2S4 NPs with different concentrations under NIR (808 nm, 1 W cm−2) irradiation. (c) The ratio changes of RNO in the Cu7.2S4 (500 µg mL−1) and control group under NIR (808 nm, 1 W cm−2) irradiation for 10 min. The decrease in RNO absorption with time in NP groups suggested the generation of singlet oxygen. (d) Fluorescence images of ROS in L929 cells cocultured with or without the Cu7.2S4 NPs. After 10 min NIR (808 nm, 1 W cm−2) irradiation, much more ROS was produced in the NP group compared with the control group. Reproduced with permission from Lu et al.67 [Copyright 2018, Wiley]. | ||
A tri-modal therapeutic nanoplatform was developed in which MTX was loaded in gold nanorod (Au NR) and copper sulfide (CuS) (Au NR@CuS) octahedral NPs for RA management and coated with HA and VIP (VIP-HA-Au NR@CuS-MTX), overall integrating PTT, PDT, and chemotherapy. The system utilised a gold nanorod core and a copper sulfide octahedral shell to facilitate strong NIR absorption and ROS generation. Functionalization with vasoactive intestinal peptide and hyaluronic acid enhanced synovial targeting. The MTX release was pH- and temperature-sensitive, increasing from 18.3% to 61.9% as the pH decreased from (pH 7.4 to pH 5). The VIP-HA-Au NR@CuS-MTX octahedral NPs (V-HACM NPs) combined with L808 showed a significantly better therapeutic effect in reducing arthritis symptoms compared to V-HAC NPs + L808 and free MTX. Mice treated with this combination had the lowest arthritis index and visibly reduced paw thickness. Joint morphology analysis confirmed better protection of cartilage and joint structure, and histological staining revealed less inflammation and cartilage erosion in the V-HACM NPs + L808 group. Additionally, no major organ damage or hepatorenal toxicity was observed after treatment, as observed in Fig. 9.68
 | ||
| Fig. 9 In each group, the mean clinical index of arthritis (A) and paw thickness (B) was determined within the times indicated. (C) Photo-image of the legs in different treatment groups. (D) The H&E staining method was used to identify the histopathology of the joints in different treatment groups. Reproduced with permission from Huang et al.68 [Copyright 2021, Elsevier]. | ||
Li et al. designed up-conversion NPs (UCNPs) loaded with vasoactive intestinal peptide@SiO2@Pt@CeO2 (V-USPC) for in situ oxygen (O2) production as well as photothermal and photodynamic effects in a study. UCNPs have the advantage of converting NIR into ultraviolet or visible light to achieve PDT in deep tissue. The solution of NPs when irradiated with an 808 nm laser (1.0 W cm−2, 10 min) showed an increase in temperature to 41.7 °C and 44.3 °C for the V-USP (without CeO2) group and the V-USPC group. The decomposition rate of H2O2 was 14% higher in the V-USPC + NIR group compared to that of the V-USPC group alone, in which the decomposition achieved was 69%. In vitro results showed that after exposure to NIR irradiation, cell viability decreased to 67.2% and 41.8% in the V-USP and V-USPC groups, suggesting the involvement of PDT as well as Pt-induced PTT. In vivo results showed that the greatest decrease in paw thickness was observed in the USPC + NIR groups compared to other control groups.69
A black phosphorus nanosheet (BPN)-loaded thermo-responsive hydrogel using PRP–chitosan was developed by Pan and team to eliminate hyperplastic synovial tissue and support osteogenesis. Under 808 nm NIR irradiation (1.0 W cm−2), ROS production was confirmed by peak green fluorescence in L929 cells using the DCFH-DA method at 8 min. CIA mice treated with the BPNs/chitosan/PRP hydrogel and NIR irradiation exhibited the lowest clinical index compared to non-irradiated and control groups, indicating enhanced therapeutic efficacy through triggered, sustained release.70 A table containing the NC systems with the application of phototherapy and the related outcomes is highlighted in Table 1.
| Nanocarrier systems | Route of administration | Delivery target cells/tissues | Drug/therapeutic agent | Phototherapy and PS involved | Achieved results | References |
|---|---|---|---|---|---|---|
| Microneedle (MN) carrying NPs | Transdermal | Inflammatory cytokines (TNF-α, IL-1β, iNOS, JAK2, JAK3, and STAT3) | Loxoprofen (Lox) and Tofacitinib (TOF) | PTT (808 nm), polydopamine | • Enhanced skin penetration and skin retention (3.1 times) greater in the irradiated group [808 + Lox + Tof NPs@MN] compared to the non-irradiated group | 21 |
| Mesoporous silica (MSN)-coated gold nanorods | Intravenous | Activated macrophages | (MTX) | PTT (808 nm) + chemotherapy, gold nanorod | • Reduced clinical scores | 71 |
| • Decreased levels of serum IL-6 and TNF-α | ||||||
| • Reduced paw thickness | ||||||
| PLGA NPs | Intravenous | Fibroblast-like synoviocytes (FLSs) | (MTX) | PTT (808 nm) + chemotherapy, gold (Au) half shells | • Reduction in the dosage upon NIR irradiation | 72 |
| Nano-gold-core multifunctional dendrimer | — | β-folate receptors | MTX | PTT (808 nm) + PDT + chemotherapy, IR780 | • Higher therapeutic efficacy | 73 |
| • Greater release at acidic pH than physiological pH (>4-fold release) | ||||||
| Selenium NPs | — | Activated macrophages | — | Photodynamic effect (560 nm), Rose Bengal (RB) | • Dual targeting from a conjugate of hyaluronic and folic acid | 33 |
| • Imaging is achieved | ||||||
| Liposomes | Intravenous | — | Sinomenine hydrochloride | Microwave | • Enhanced encapsulated drug release (80%) with shorter duration (6 h) | 74 |
| Nanocomposite | Intra-articular | Macrophages | CeO2 NPs | Photothermal (808 nm), polydopamine (PDA) | • Significant downregulation of pro-inflammatory cytokines and hypoxia-inducible factor-1α (HIF-1α) | 56 |
| • Significant mitigation of oxygen to reduce hypoxia (from CeO2 and PDA) |
5. Clinical efficacy trials on phototherapy for RA management
A recent clinical trial has shown that phototherapy using nanocarriers can provide significant therapeutic advantages for treating rheumatoid arthritis. These advanced systems enhance drug delivery and targeting, resulting in improved anti-inflammatory effects and joint protection compared to conventional approaches. Zhao and his colleagues conducted a study outlining the design of a double-blind, randomized, placebo-controlled, prospective trial investigating the efficacy of PDT in addressing synovial hyperplasia in refractory RA patients. Synovial hyperplasia, characterized by abnormal proliferation of synovial tissue in the joints, contributes to joint damage in RA. Patients were divided into three groups: one that received the control, a second that received “PDT once”, and a third that received “PDT twice”. One hundred twenty-six individuals were recruited, with each group comprising 42 individuals. The study recruited individuals diagnosed with refractory RA and confirmed synovial hyperplasia. To the individuals in the control group, 0.9% saline was administered on day zero and on week four by micro-arthroscopic surgery to account for potential psychological effects. The individuals who were to receive PDT once were administered a PS (aminolevulinic acid, 236 mg, added to 5 mL of 0.9% saline) on day zero and 0.9% saline on the fourth week, while those who had to receive PDT twice were injected with a PS on both day zero and the fourth week. In patients receiving the PS, to activate the PS, laser light (630 nm wavelength, 150 mW power, for 20 min) was irradiated via a laser optic fiber into the articular cavity. After the surgery, researchers collected recovery rate and other general data from patients (like clinical assessments, imaging studies, and patient-reported outcomes). Researchers aimed to generate robust evidence regarding the potential efficacy and safety of PDT as a therapeutic intervention for synovial hyperplasia in patients with RA.106. Current challenges and future outlook
In recent years, the development of effective NCs for RA therapy has garnered significant attention. PDT and PTT, characterized by their non-invasiveness, controllability, and precision, have emerged as potential modalities for RA treatment. However, despite their promise, these therapies face challenges such as limited skin penetration, potential thermal damage to normal tissues, and the short-lived diffusion of ROS.57 A conjugated PS exhibits prolonged retention within the body owing to its large molecular mass, leading to its phototoxic effects on healthy tissues over an extended duration.60 In PDT, PSs generate ROS under light stimulation, while in PTT, photothermal conversion agents convert NIR light into heat to target disease regions. For RA treatment, NIR light with a specific penetration depth is crucial to ensure effective action of nanomaterials in PDT and PTT.57 Despite the potential advantages, evidence supporting the efficacy of phototherapy in RA remains limited. In 1995, the first RA-related human PDT study was conducted, employing light activation via arthroscopy on six patients. The limitations, such as side effects, poor pharmacokinetics, and low targeting efficacy of PSs in synovectomy, prompted the exploration of nanosized PSs to enhance treatment. The optimized combination of a PS and nanomaterials showed potential in enhancing the targeting of the drug, minimizing side effects, and improving PDT efficacy for RA treatment. In PDT, optimization is quite challenging because a PS should activate at the correct time and in the correct location; furthermore, the challenge is to select the optimal combination of administration, formulation, localization, irradiation, PS, and release profile.75 In PTT, shape, size, nature, localized surface plasmon resonance, photostability, and biocompatibility (with target tissue) of NPs are some important deciding factors.54PDT and PTT are known for reducing pro-inflammatory events in cancer, as well as in RA treatment. Further investigation is required to understand its immunogenicity in RA. Thus, achieving precise targeting without damaging normal tissues necessitates the development of effective ligands. The limited penetration depth of NIR light, especially in larger joints, poses a challenge and research directions include the development of lasers with enhanced penetrability. The hypoxic microenvironments in RA inflammation sites may decrease the efficacy of PDT. Strategies involving oxygen carriers and reactive oxygen-generating materials need exploration to enhance treatment outcomes.21
While phototherapy-based nano-systems show promise in preclinical studies, challenges persist. The complex nature of RA necessitates further studies, particularly in the rational design of therapeutic nano-systems, the evaluation of biological safety, and the development of multifunctional nano-platforms for clinical applications. In PDT, the laser source is time-consuming, so it was replaced with light-emitting diodes (LEDs), which are a cheaper and less hazardous option. Low-level laser therapy (LLLT) is a type of phototherapy that is not expensive and can be accessed by patients at home. Further research should be conducted to make phototherapy more affordable and accessible to patients, either by modifying the light source or by developing new devices.76
Among the various nanocarrier strategies currently being investigated, metallic nanoparticles (MNPs), including Au and ZnO, are being extensively studied in RA, as they exhibit tunable thermal and optical properties. Although they hold significant potential, concerns regarding their toxicity and ecological impact require careful evaluation. The safety profile of MNPs is largely determined by physicochemical characteristics, including size, shape, surface charge, composition, and surface modifications, as well as by the route and duration of exposure. The adverse effects associated with MNPs may include cytotoxicity, genotoxicity, immunotoxicity, and oxidative stress. Despite ongoing research to clarify these risks, challenges remain in fully understanding their long-term toxicity.77,78 Furthermore, once in the bloodstream, MNPs may accumulate in organs such as the spleen and bone marrow or distribute to other tissues and cells where they are recognized as foreign antigens, triggering immune responses. These NPs can interact with various immune cells, including macrophages, dendritic cells, natural killer cells, and B and T lymphocytes. During such interactions, the NPs are internalised and processed by immune cells, influencing their metabolism, function, and fate.79 Studies have shown that ZnO NPs of differing sizes and surface charges can induce immunotoxicity by promoting inflammation. Au NPs at low concentrations (0.25 ppm) can stimulate immune responses, increasing the expression of pro-inflammatory cytokines such as IL-1β, IL-6, and TNF-α.80 However, at higher concentrations (25 ppm), Au NPs may exhibit pronounced pro-inflammatory or immunotoxic effects, including a sharp decline in lymphocyte proliferation. ZnO NPs have similarly been reported to induce significant immunotoxicity in immune cells and organs, with inflammation and oxidative stress underlying these effects. In male Wistar albino rats, oral administration of ZnO NPs (26.6 nm, 350 mg kg−1) markedly upregulated immune regulatory genes (CD3, CD11b, and HO-1) and inflammatory genes (TLR4 and TLR6), caused DNA strand breaks, increased malondialdehyde levels in the thymus and spleen, and elevated pro-inflammatory cytokines, including IL-10, IL-1β, TNF-α, and IFN-γ.81
Phototherapy holds great potential in RA treatment, offering high therapeutic performance and innovations in NCs and devices bring optimism for effective and targeted RA therapy. However, addressing current limitations and overcoming challenges in immunogenicity, targeting precision, penetration depth, and microenvironment adaptations is imperative for successful clinical translation.
7. Conclusion
Recent advancements in RA management include the emergence of NC-based phototherapy, which offers a non-invasive, patient-centric, and targeted approach. The combination of precise PDT and PTT applications, along with the enhanced delivery capabilities of NCs, effectively addresses the key pathological features of RA, including synovial inflammation, cartilage destruction, and macrophage activation. Extensive preclinical research has demonstrated that these systems can minimise off-target effects while reducing pro-inflammatory cytokines, promoting tissue repair, and modulating immune responses. Though clinical translation is still in its early stages, it has shown significant promise. To prove efficacy and safety, future research should concentrate on creating sophisticated multifunctional nanosystems, refining light delivery methods, and carrying out thorough clinical trials. With continued innovation and interdisciplinary collaboration, NC-based phototherapy could revolutionize RA treatment, providing patients with more effective and safer therapeutic options that preserve joint function and improve quality of life.Author contributions
Sakshi Priya: conceptualization, writing – original draft, writing – review & editing. Dhruv Sharma: writing – original draft, writing – review and editing. Kaushal Kailash Jain: writing – original draft. Sahiba Chutani: writing – review & editing. Gautam Singhvi: conceptualization, supervision, writing – review & editing.Conflicts of interest
The authors declare that they have no financial interests or personal conflicts of interest.Data availability
No primary research results, software or code have been included, and no new data were generated or analysed as part of this review.Acknowledgements
The author, Sakshi Priya, gratefully acknowledges the PhD fellowship support [# IF210090] granted by the Department of Science & Technology – Innovation in Science and Pursuit for Inspired Research (DST-INSPIRE), Government of India. The authors wanted to acknowledge the use of Perplexity AI and Gemini tools for basic literature screening and to identify relevant references. Additionally, Grammarly and QuillBot were employed solely for grammar correction and minor paraphrasing to improve sentence clarity and readability. However, no content was directly copied or generated from them for inclusion in the manuscript. Further, the authors also acknowledge the use of BioRender for enabling the creation of the graphical abstract and schematics under an Academic License.References
- Y. Cao, Y. Yang, Q. Hu and G. Wei, J. Transl. Med., 2023, 21, 1–17 CrossRef PubMed.
- C. S. Lau, Lancet Rheumatol., 2023, 5, e567–e568 CrossRef CAS.
- J. Liu, J. Gao, Z. Wu, L. Mi, N. Li, Y. Wang, X. Peng, K. Xu, F. Wu and L. Zhang, Front. Med., 2022, 8, 1–10 Search PubMed.
- A. M. Curran, P. Naik, J. T. Giles and E. Darrah, Nat. Rev. Rheumatol., 2020, 16, 301–315 CrossRef CAS PubMed.
- R. Ben Mrid, N. Bouchmaa, H. Ainani, R. El Fatimy, G. Malka and L. Mazini, Biomed. Pharmacother., 2022, 151, 113126 CrossRef CAS.
- E. G. Favalli, M. G. Raimondo, A. Becciolini, C. Crotti, M. Biggioggero and R. Caporali, Autoimmun. Rev., 2017, 16, 1185–1195 CrossRef PubMed.
- J. S. Smolen and D. Aletaha, Nat. Rev. Rheumatol., 2015, 11, 276–289 CrossRef PubMed.
- H. Zhao, J. Wei, Y. He, Y. Wu, L. Ge and C. Zheng, Colloids Surf., B, 2024, 239, 113952 CrossRef CAS.
- Z. Zhang, R. Wang, H. Xue, S. Knoedler, Y. Geng, Y. Liao, M. Alfertshofer, A. C. Panayi, J. Ming, B. Mi and G. Liu, Biomater. Res., 2023, 27, 1–36 CrossRef.
- X. Zhao, F. Zuo, E. Chen, Y. Bi, Y. Cao, Y. Yuan, K. Li, Y. Xuan, L. Li, L. Wan, X. Zhang, F. Yan, J. Zhou, K. Yin and C. Xiao, Trials, 2021, 22, 1–8 CrossRef CAS PubMed.
- S. S. Lucky, K. C. Soo and Y. Zhang, Chem. Rev., 2015, 115, 1990–2042 CrossRef CAS PubMed.
- L. Mi, J. Gao, Y. Liu, N. Zhang, M. Zhao, S. Wang and K. Xu, Rheumatol. Autoimmun., 2023, 3, 205–219 CrossRef CAS.
- A. Gadeval, S. Chaudhari, S. P. Bollampally, S. Polaka, D. Kalyane, P. Sengupta, K. Kalia and R. K. Tekade, Drug Discovery Today, 2021, 26, 2315–2328 CrossRef CAS.
- S. Nasra, D. Bhatia and A. Kumar, Nanoscale Adv., 2022, 3479–3494 RSC.
- S. Zhang, M. Zhang, X. Li, G. Li, B. Yang, X. Lu, Y. Gao and F. Sun, Molecules, 2022, 27, 5973 CrossRef CAS.
- Y. Dong, W. Cao and J. Cao, Nanoscale, 2021, 13, 14591–14608 RSC.
- C. Hendrich and W. E. Siebert, Lasers Surg. Med., 1997, 21, 359–364 CrossRef CAS.
- J. H. Correia, J. A. Rodrigues, S. Pimenta, T. Dong and Z. Yang, Pharmaceutics, 2021, 13, 1–16 CrossRef PubMed.
- Y. Lin, Y. Tang, O. Yi, J. Zhu, Z. Su, G. Li, H. Zhou, L. Liu and B. Liu, J. Nanobiotechnol., 2024, 22, 1–22 CrossRef.
- J. R. Hou J and S. Yin, Mater. Today Bio, 2024, 29, 01264 Search PubMed.
- Y. Lu, T. Xiao, R. Lai, Z. Liu, W. Luo, Y. Wang, S. Fu, G. Chai, J. Jia and Y. Xu, Pharmaceutics, 2023, 15, 500 CrossRef.
- A. Tewabe, A. Abate, M. Tamrie, A. Seyfu and E. A. Siraj, J. Multidiscip. Healthc., 2021, 14, 1711–1724 CrossRef PubMed.
- K. Prabahar, Z. Alanazi and M. Qushawy, Indian J. Pharm. Educ. Res., 2021, 55, 346–353 CrossRef CAS.
- M. T. Manzari, Y. Shamay, H. Kiguchi, N. Rosen, M. Scaltriti and D. A. Heller, Nat. Rev. Mater., 2021, 6, 351–370 CrossRef CAS PubMed.
- X. Yang, Y. Chang and W. Wei, Cell Proliferation, 2020, 7, 12854 CrossRef.
- I. Kalashnikova, S. J. Chung, M. Nafiujjaman, M. L. Hill, M. E. Siziba, C. H. Contag and T. Kim, Theranostics, 2020, 10, 11863–11880 CrossRef CAS PubMed.
- Q. Wang and X. Sun, Biomater. Sci., 2017, 5, 1407–1420 RSC.
- S. Tardito, G. Martinelli, S. Soldano, S. Paolino, G. Pacini, M. Patane, E. Alessandri, V. Smith and M. Cutolo, Autoimmun. Rev., 2019, 18, 102397 CrossRef CAS.
- A. Laria, A. Lurati, M. Marrazza, D. Mazzocchi, K. A. Re and M. Scarpellini, J. Inflammation Res., 2016, 9, 1–11 CAS.
- A. Syed and V. K. Devi, J. Drug Delivery Sci. Technol., 2019, 53, 101217 CrossRef CAS.
- S. Xiao, Y. Tang, Z. Lv, Y. Lin and L. Chen, J. Controlled Release, 2019, 316, 302–316 CrossRef CAS.
- X. Chen, X. Zhu, L. Ma, A. Lin, Y. Gong, G. Yuan and J. Liu, Nanoscale, 2019, 11, 18209–18223 RSC.
- K. Y. Lu, P. Y. Lin, E. Y. Chuang, C. M. Shih, T. M. Cheng, T. Y. Lin, H. W. Sung and F. L. Mi, ACS Appl. Mater. Interfaces, 2017, 9, 5158–5172 CrossRef CAS.
- S. Gorantla, G. Gorantla, R. N. Saha and G. Singhvi, Expert Opin. Drug Delivery, 2021, 18, 1553–1557 CrossRef CAS PubMed.
- C. Li, R. Liu, Y. Song, Y. Chen, D. Zhu, L. Yu, Q. Huang, Z. Zhang, Z. Xue, Z. Hua, C. Lu, A. Lu and Y. Liu, Front. Pharmacol, 2022, 13, 849101 CrossRef CAS PubMed.
- D. M. S. H. Chandrupatla, C. F. M. Molthoff, A. A. Lammertsma, C. J. van der Laken and G. Jansen, Drug Delivery Transl. Res., 2019, 9, 366–378 CrossRef CAS PubMed.
- E. Nogueira, A. C. Gomes, A. Preto and A. Cavaco-Paulo, Nanomedicine, 2016, 12, 1113–1126 CrossRef CAS PubMed.
- X. Li, Y. Hou, X. Meng, G. Li, F. Xu, L. Teng, F. Sun and Y. Li, RSC Adv., 2021, 11, 3567–3574 RSC.
- R. P. Gomariz, Y. Juarranz, M. Carrión, S. Pérez-García, R. Villanueva-Romero, I. González-Álvaro, I. Gutiérrez-Cañas, A. Lamana and C. Martínez, Front. Endocrinol., 2019, 10, 1–12 CrossRef PubMed.
- D. Ganea, K. M. Hooper and W. Kong, Acta Physiol., 2015, 213, 442–452 CrossRef CAS.
- R. Huang, C. Zhang, Y. Bu, Z. Li, X. Zheng, S. Qiu, J. O. achwa Machuki, L. Zhang, Y. Yang, K. Guo and F. Gao, Biomaterials, 2021, 277, 121088 CrossRef CAS PubMed.
- D. I. Jang, A. H. Lee, H. Y. Shin, H. R. Song, J. H. Park, T. B. Kang, S. R. Lee and S. H. Yang, Int. J. Mol. Sci., 2021, 22, 1–16 Search PubMed.
- J. Wang, L. He, W. Li and S. Lv, Front. Pharmacol., 2022, 13, 1–10 Search PubMed.
- Y. Li, S. Wei, K. Zhang, Y. Fang, H. Liu, Z. Jin, Q. Guo, J. He, W. Song and F. Zhang, Lasers Med. Sci., 2021, 36, 1411–1419 CrossRef PubMed.
- C. Zhao, F. Ur Rehman, Y. Yang, X. Li, D. Zhang, H. Jiang, M. Selke, X. Wang and C. Liu, Sci. Rep., 2015, 5, 1–11 Search PubMed.
- X. Chen, X. Zhu, T. Xu, M. Xu, Y. Wen, Y. Liu, J. Liu and X. Qin, J. Mater. Chem. B, 2019, 7, 112–122 RSC.
- D. N. Dorst, M. Rijpkema, M. Boss, B. Walgreen, M. M. A. Helsen, D. L. Bos, M. Brom, C. Klein, P. Laverman, P. M. Van Der Kraan, M. Gotthardt, M. I. Koenders and M. Buitinga, Rheumatology, 2020, 59, 3952–3960 CrossRef CAS PubMed.
- A. P. Croft, J. Campos, K. Jansen, J. D. Turner, J. Marshall, M. Attar, L. Savary, C. Wehmeyer, A. J. Naylor, S. Kemble, J. Begum, K. Dürholz, H. Perlman, F. Barone, H. M. McGettrick, D. T. Fearon, K. Wei, S. Raychaudhuri, I. Korsunsky, M. B. Brenner, M. Coles, S. N. Sansom, A. Filer and C. D. Buckley, Nature, 2019, 570, 246–251 CrossRef CAS PubMed.
- K. Zhang, S. Gao, J. Guo, G. Ni, Z. Chen, F. Li, X. Zhu, Y. Wen and Y. Guo, Iran. J. Basic Med. Sci., 2018, 21, 130–137 Search PubMed.
- D. Gabriel, N. Lange, V. Chobaz-Peclat, M. F. Zuluaga, R. Gurny, H. Van Den Bergh and N. Busso, J. Controlled Release, 2012, 163, 178–186 CrossRef CAS PubMed.
- S. W. Yoo, G. Oh, J. C. Ahn and E. Chung, Biomedicines, 2021, 9, 1–27 Search PubMed.
- S. Priya, K. K. Jain, J. Daryani, V. M. Desai, H. Kathuria and G. Singhvi, Nanoscale, 2025, 17, 65–87 RSC.
- J. Zhao, X. Chen, K. Ho, C. Cai, C. Li, M. Yang and C. Yi, Chin. Chem. Lett., 2021, 32, 66–86 CrossRef CAS.
- A. Gadeval, S. Chaudhari, S. P. Bollampally, S. Polaka, D. Kalyane, P. Sengupta and K. Kalia, Drug Discovery Today, 2021, 26, 2315–2328 CrossRef CAS PubMed.
- Y. Han and S. Huang, J. Controlled Release, 2023, 356, 142–161 CrossRef CAS.
- M. W. Chen, Q. J. Lu, Y. J. Chen, Y. K. Hou, Y. M. Zou, Q. Zhou, W. H. Zhang, L. X. Yuan and J. X. Chen, ACS Biomater. Sci. Eng., 2022, 8, 3361–3376 CrossRef CAS PubMed.
- Q. Wang, X. Qin, J. Fang and X. Sun, Acta Pharm. Sin. B, 2021, 11, 1158–1174 CrossRef CAS.
- C. Zheng, A. Wu, X. Zhai, H. Ji, Z. Chen, X. Chen and X. Yu, Acta Pharm. Sin. B, 2021, 11, 1993–2003 CrossRef CAS PubMed.
- Q. Shen, Q. Hu, T. Tang, X. Ying, G. Shu, J. Shen, C. Teng and Y. Du, Biomater. Adv., 2022, 138, 212940 CrossRef CAS PubMed.
- M. Gallardo-Villagrán, D. Y. Leger, B. Liagre and B. Therrien, Int. J. Mol. Sci., 2019, 20, 3339 CrossRef PubMed.
- Y. Li, L. Zheng, W. Cao, X. Yang, Q. Wang, X. Gu, F. Liu, T. Ma, X. Wang and Q. Wang, Biomed. Pharmacother., 2023, 162, 114684 CrossRef CAS PubMed.
- Q. Tang, J. Cui, Z. Tian, J. Sun, Z. Wang, S. Chang and S. Zhu, Int. J. Nanomed., 2017, 12, 381–393 CrossRef CAS.
- F. Schmitt, L. Lagopoulos, P. Käuper, N. Rossi, N. Busso, J. Barge, G. Wagnières, C. Laue, C. Wandrey and L. Juillerat-jeanneret, J. Controlled Release, 2010, 144, 242–250 CrossRef CAS PubMed.
- D. N. Dorst, M. Boss, M. Rijpkema, B. Walgreen, M. M. A. Helsen, D. L. Bos, L. van Bloois, G. Storm, M. Brom, P. Laverman, P. M. van der Kraan, M. Buitinga, M. I. Koenders and M. Gotthardt, Pharmaceutics, 2021, 13, 1–11 CrossRef PubMed.
- F. U. Rehman, C. Zhao, C. Wu, X. Li, H. Jiang, M. Selke and X. Wang, Nano Res., 2016, 9, 3305–3321 CrossRef CAS.
- X. Li, S. Zhang, X. Zhang, Y. Hou, X. Meng, G. Li, F. Xu, L. Teng, Y. Qi, F. Sun and Y. Li, Int. J. Pharm., 2021, 607, 120947 CrossRef CAS.
- Y. Lu, L. Li, Z. Lin, L. Wang, L. Lin, M. Li, Y. Zhang, Q. Yin, Q. Li and H. Xia, Adv. Healthcare Mater., 2018, 7, 1–11 CAS.
- R. Huang, C. Zhang, Y. Bu, Z. Li, X. Zheng, S. Qiu, J. O. achwa Machuki, L. Zhang, Y. Yang, K. Guo and F. Gao, Biomaterials, 2021, 277, 121088 CrossRef CAS PubMed.
- Z. Li, X. Wu, W. Gu, P. Zhou, H. Chen, W. Wang, Z. Cai, S. Cao, K. Guo, X. Zheng and F. Gao, Chem. Eng. J., 2022, 446, 136904 CrossRef CAS.
- W. Pan, C. Dai, Y. Li, Y. Yin, L. Gong, J. O. Machuki, Y. Yang, S. Qiu, K. Guo and F. Gao, Biomaterials, 2020, 239, 119851 CrossRef CAS PubMed.
- X. Li, Y. Hou, X. Meng, G. Li, F. Xu and L. Teng, RSC Adv., 2021, 11, 3567–3574 RSC.
- Y. J. Ha, S. M. Lee, C. H. Mun, H. J. Kim, Y. Bae, J. H. Lim, K. H. Park, S. K. Lee, K. H. Yoo and Y. B. Park, Arthritis Res. Ther., 2020, 22, 1–13 CrossRef PubMed.
- P. K. Pandey, R. Maheshwari, N. Raval, P. Gondaliya, K. Kalia and R. K. Tekade, J. Colloid Interface Sci., 2019, 544, 61–77 CrossRef CAS PubMed.
- Q. Shen, X. Zhang, J. Qi, G. Shu, Y. Du and X. Ying, Int. J. Pharm., 2020, 576, 119001 CrossRef CAS PubMed.
- D. Lee, S. Kwon, S. young Jang, E. Park, Y. Lee and H. Koo, Bioact. Mater., 2022, 8, 20–34 CAS.
- V. A. Wickenheisser, E. M. Zywot, E. M. Rabjohns, H. H. Lee, D. S. Lawrence and T. KathleenTarrant, Curr. Allergy Asthma Rep., 2019, 19, 1–15 CrossRef CAS PubMed.
- S. Devi, D. Anita, B. Madhulika, P. Keshav, K. Sahu and D. Singh, 3 Biotech, 2024, 14, 1–18 CrossRef PubMed.
- H. Agarwal, A. Nakara and V. K. Shanmugam, Biomed. Pharmacother., 2019, 109, 2561–2572 CrossRef CAS PubMed.
- J. Bi, C. Mo, S. Li, M. Huang, Y. Lin, P. Yuan, Z. Liu, B. Jia and S. Xu, Biomater. Sci., 2023, 11, 4151–4183 RSC.
- J. Małaczewska, Pol. J. Vet. Sci., 2015, 18, 181–189 Search PubMed.
- M. A. Abass, S. A. Selim, A. O. Selim, A. S. El-Shal and Z. A. Gouda, IUBMB Life, 2017, 69, 528–539 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2026 |