Open Access Article
Open Access ArticleShaping coordination polymers by ball milling
Giorgio Cagossi a,
Beatrice Piombo
a,
Beatrice Piombo a,
Andrea Daolio
a,
Andrea Daolio a,
Paolo P. Mazzeo
a,
Paolo P. Mazzeo a,
Alessia Bacchi
a,
Alessia Bacchi a and
Paolo Pelagatti
a and
Paolo Pelagatti *ab
*ab
aDepartment of Chemical Sciences, Life Science and Environmental Sustainability, University of Parma, Parco Area delle Scienze 17/A, 43124 Parma, Italy. E-mail: paolo.pelagatti@unipr.it
bInteruniversity Consortium of Chemical Reactivity and Catalysis (C.I.R.C.C.), Via Celso Ulpiani 27, 70126 Bari, Italy
First published on 4th December 2025
Abstract
Selective syntheses of known 1D-coordination polymers derived from the combination of 4,4′-bipyridine (bipy) or 1,2-bis-(4-pyridyl)ethylene (dpe) with Zn(OAc)2·2H2O were achieved under mechanochemical conditions by carefully controlling the mechanochemical parameters, including reaction stoichiometry and the nature of the solvent used in liquid assisted grinding. Ligand exchange reactions performed under grinding conditions showed the conversion of the dpe-containing polymers into bipy-containing polymers, whereas the reverse reactions proved unfeasible. A computational rationale for the observed reactivity is provided. The dimensionality growth of the dpe-containing 1D-coordination polymers into a three-dimensional pillared metal–organic framework was achieved by reaction with terephthalic acid. The same 3D-MOF was also obtained by a one-pot procedure, involving dpe, Zn(OAc)2·2H2O and terephthalic acid simultaneously ground in the presence of a small aliquot of N,N-dimethylformamide. All the reactions occurred in high yields affording pure products with a favorable environmental profile, as evidenced by the environmental factor (EF) and reaction mass efficiency (RME) calculated for selective reactions. This work highlights how mechanochemistry not only allows the efficient synthesis of coordination polymers but also their post-synthetic modifications by environmentally benign protocols.
Introduction
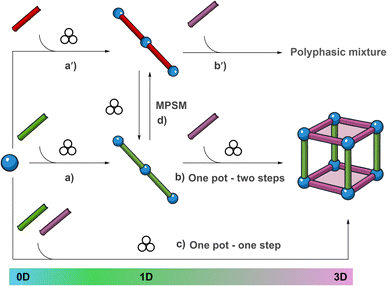
Mechanochemistry represents a unique way of conducting a chemical reaction based on the grinding of solid reagents.1–3 This is usually performed using a ball-mill apparatus, where solid particles are ground inside a rigid container by means of accelerated balls.4 Since it removes the need of a solvent, mechanochemistry is intrinsically green as it produces virtually no waste.5,6 Moreover, under these conditions, so-called “impossible reactions”7 – reactions involving highly insoluble reagents – are promoted. Hence, the popularity of mechanochemistry is raised among the synthetic chemists, although there is often low understanding of the nature of the events that govern the reactions under milling conditions.8–10 Among the porous cutting-edge functional materials, Metal–Organic-Frameworks (MOFs) have a prominent role.11–13 MOFs are coordination polymers (CPs) derived from the combination of metal-containing secondary-building-units (SBUs) interconnected by organic linkers. Their importance in fields where porosity plays a crucial role is well-known and extensively reviewed.14–16 Despite the huge number of structurally characterized MOFs, examples of their industrial applications are scarce.17 One of the main drawbacks that hamper MOF implementation is the low scalability of their synthesis, often based on solvothermal reactions. Although the solvothermal route makes possible the isolation of X-ray quality single crystals, fundamental for the full understanding of their hosting capacity, the use of high boiling and toxic solvents, long reaction times and, often, unsatisfactory yields negatively impact the cost-effectiveness and viability of the final materials. It follows that the mechanochemical synthesis of MOFs is highly attractive as shown by the number of publications appeared in the last few years, with some dedicated reviews.18–20 Among the different types of MOFs reported in the literature, a pivotal role is played by mixed-ligand MOFs (ML-MOFs).21,22 Often, ML-MOFs are made by square planes containing SBUs connected by dicarboxylate linkers, then pillared by neutral ones such as bis-pyridines,23–25 giving rise to porous frameworks labeled pillared-MOFs (PL-MOFs). The presence of two different linkers allows for high degrees of functionalization that translate into highly versatile crystalline materials.26,27 Although step-wise constructions of PL-MOFs in solution28,29 and on planar surfaces were successfully performed,30–32 the single reactive steps that lead to the assembly of the final 3D architecture are poorly understood. The mechanochemical synthesis of PL-MOFs counts a limited number of examples,33–37 but efforts in this direction are highly desirable. In particular, the stepwise construction of PL-MOFs under mechanochemical conditions is of particular relevance for demonstrating the feasibility of well-controlled and selective processes in non-conventional reactive environments. The number of reports addressing the stepwise mechanochemical construction of PL-MOFs is scarce.34,38,39 Likewise, the number of papers dealing with mechanochemical post-synthetic modifications (MPSMs) of CPs involving linker exchange is also limited.40,41 Therefore, efforts in this direction are highly desirable. In our laboratory we have solid experience in the mechanochemical synthesis of different types of materials such as organic co-crystals containing active pharmaceutical ingredients,42–45 hybrid materials derived from the combination of lignin and inorganic nanocrystals,46–48 as well as metal complexes49 and coordination polymers.50 Contributing to the development and understanding of the mechanochemical preparation of MOFs, we decided to build PL-MOFs through a stepwise, bottom-up approach, whose experimental design is depicted in Fig. 1. It involves a first step dedicated to the assembly of 1D-CPs derived from the reaction of rigid bis-pyridine-containing linkers, in particular 4,4′-bipyridine (bipy) and 1,2-bis-(4-pyridyl)ethylene (dpe) with Zn(OAc)2·2H2O (Fig. 1, path a or a′). 1D-CPs are subsequently reacted with terephthalic acid (H2ta) to form the target PL-MOFs (Fig. 1, path b or b′) via substitution of the acetate anion with ta2− dianions. Finally, a one-pot procedure involving the simultaneous grinding of the three reagents (metal salt, bis-pyridine-linker and H2ta) is also performed (Fig. 1, path c), and the results are compared with those coming from paths a and b. The progress of each reaction was monitored by ex situ powder X-ray diffraction analysis (PXRD), comparing the diffractograms of the ground materials with those calculated from the known crystalline structures deposited in the CCDC databank. Moreover, MPSMs were also performed to assess the conversion of one 1D-CP into another by linker exchange, as depicted in Fig. 1 (path d). The two pyridine linkers, bipy and dpe, were chosen based of the following considerations: (i) solution syntheses of 1D-CPs derived from the reaction of the two linkers with Zn(OAc)2 are known; (ii) different crystalline structures are reported, whose formation depends on the adopted experimental conditions (vide infra); (iii) the structures differ in framework topology and type of SBUs contained in the polymeric chains.Similarly, the dicarboxylate linker ta2− was chosen on the basis of the following considerations: (i) H2ta is expected to replace the basic AcO− anions as AcOH, which, owing to its volatility, can easily leave the reaction medium, thus avoiding the use of an external base that would inevitably lead to the formation of a salt byproduct; (ii) ML-MOFs containing ta2−, bipy or dpe, and Zn2+ obtained by solution syntheses are known; (iii) their structures present different crystalline frameworks and SBUs (vide infra). The adopted mechanochemical approach is particularly attractive since it allows evaluation of the convergence of the different reactive pathways reported in Fig. 1 and, at the same time, to compare the results obtained with solution syntheses. Given the different reactive environments, mechanochemical and wet reactions can lead to different products, an aspect that is of particular interest.
Results and discussion
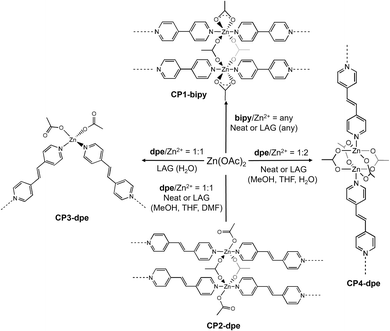
Structural background from CPs synthesized in solution
The linearity of the two ligands bipy and dpe is ideal for the construction of CPs of different dimensionality and examples of 1D and 3D polymers can be found in the literature. A structural search on the Cambridge Structural Database (CSD) revealed the presence of two 1D-CPs containing bipy, Zn2+ and acetate anions. In one case, here referred to as CP1-bipy, the polymer has a ladder structure with a SBU of formula [Zn2(µ-OAc)2(κ2-OAc)2(py)4] (Fig. 2, CSD Refcode: ALUPUS), wherein each Zn2+ ion is octahedrally surrounded by two oxygen atoms belonging to two bridging acetate anions, two oxygens of a κ2-chelating acetate anion and two pyridine rings. The formula of the polymer is [Zn2(OAc)4(bipy)2]n. The polymer was synthesized either under hydrothermal conditions51 or at room temperature in organic solvents52 (ethanol/dichloromethane mixture) with a bipy![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zn molar ratio of 1
Zn molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. A different framework, composed by zig–zag chains of formula [Zn2(OAc)4(bipy)]n, here referred to as CP0-bipy (Fig. 2, CSD Refcode: EYELID), was obtained at room temperature in methanol when an excess of metal was used.53 The SBU is less symmetrical that the one found in CP1-bipy, its formula being [Zn2(µ-OAc)2(κ2-OAc)(κ2/µ-OAc)(py)2]. The two metals display different coordination environments, octahedral and trigonal bipyramidal. In the CSD, there are three structurally known 1D-CPs containing dpe, Zn2+ and acetate anions. One structure, here referred to as CP2-dpe (Fig. 2, CSD Refcode: MANMEU), contains the same SBU found in CP1-bipy, with which it is isoreticular, its formula being [Zn2(OAc)4(µ-dpe)2]n.54 It was obtained by buffering a MeOH solution of the salt and a THF solution of the linker with ACN, using a 1
1. A different framework, composed by zig–zag chains of formula [Zn2(OAc)4(bipy)]n, here referred to as CP0-bipy (Fig. 2, CSD Refcode: EYELID), was obtained at room temperature in methanol when an excess of metal was used.53 The SBU is less symmetrical that the one found in CP1-bipy, its formula being [Zn2(µ-OAc)2(κ2-OAc)(κ2/µ-OAc)(py)2]. The two metals display different coordination environments, octahedral and trigonal bipyramidal. In the CSD, there are three structurally known 1D-CPs containing dpe, Zn2+ and acetate anions. One structure, here referred to as CP2-dpe (Fig. 2, CSD Refcode: MANMEU), contains the same SBU found in CP1-bipy, with which it is isoreticular, its formula being [Zn2(OAc)4(µ-dpe)2]n.54 It was obtained by buffering a MeOH solution of the salt and a THF solution of the linker with ACN, using a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dpe
1 dpe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) salt molar ratio. The same polymer was also obtained by slow evaporation of a methanol solution containing equimolar amounts of the reagents. The same reaction conducted in DMF and then in hot water led instead to a 1D-CP where dpe bridges tetrahedrally coordinated Zn2+ ions (CP3-dpe, Fig. 2, CSD Refcode: BUJDES), containing infinite 1D zig–zag chains with SBUs of the type [Zn(OAc)2(py)2]. Each metal coordinates two κ1-monodentate acetate anions, for a whole polymer formula corresponding to [Zn(OAc)2(µ-dpe)]n. The same framework was also isolated from a reaction between Zn(NO3)2·6H2O, dpe and NaOAc in a H2O/EtOH mixture at room temperature.55 Finally, a polymer of formula [Zn2(OAc)4(µ-dpe)]n was obtained when a methanol solution of Zn(OAc)2·2H2O and a THF solution of the ligand were buffered by a methanol layer.54 In this case, linear 1D chains containing paddle-wheel SBUs of formula [Zn2(OAc)4(py)2] bridged by dpe were found (CP4-dpe, Fig. 2, CSD Refcode: MANLUJ). The SBUs contained in the frameworks of the PL-MOFs derived from the combination of ta2−, Zn2+ and bipy/dpe are shown in Fig. 3. Two different structures containing bipy are known (Fig. 3, left). One case corresponds to a two-fold interpenetrated cubic lattice composed by squares containing paddle-wheel SBUs bridged by dicarboxylate anions and pillared by bipy linkers (CSD Refcode: EDADIX).56
salt molar ratio. The same polymer was also obtained by slow evaporation of a methanol solution containing equimolar amounts of the reagents. The same reaction conducted in DMF and then in hot water led instead to a 1D-CP where dpe bridges tetrahedrally coordinated Zn2+ ions (CP3-dpe, Fig. 2, CSD Refcode: BUJDES), containing infinite 1D zig–zag chains with SBUs of the type [Zn(OAc)2(py)2]. Each metal coordinates two κ1-monodentate acetate anions, for a whole polymer formula corresponding to [Zn(OAc)2(µ-dpe)]n. The same framework was also isolated from a reaction between Zn(NO3)2·6H2O, dpe and NaOAc in a H2O/EtOH mixture at room temperature.55 Finally, a polymer of formula [Zn2(OAc)4(µ-dpe)]n was obtained when a methanol solution of Zn(OAc)2·2H2O and a THF solution of the ligand were buffered by a methanol layer.54 In this case, linear 1D chains containing paddle-wheel SBUs of formula [Zn2(OAc)4(py)2] bridged by dpe were found (CP4-dpe, Fig. 2, CSD Refcode: MANLUJ). The SBUs contained in the frameworks of the PL-MOFs derived from the combination of ta2−, Zn2+ and bipy/dpe are shown in Fig. 3. Two different structures containing bipy are known (Fig. 3, left). One case corresponds to a two-fold interpenetrated cubic lattice composed by squares containing paddle-wheel SBUs bridged by dicarboxylate anions and pillared by bipy linkers (CSD Refcode: EDADIX).56
The formula of the SBU is [Zn2(COO)4(py)2], while the formula of the framework is [Zn2(ta)2(bipy)]n. The crystals grew under solvothermal conditions, in a DMF/EtOH mixture, using Zn(NO3)2·6H2O as the salt. A second two-fold interpenetrated prismatic framework was obtained by conducting the solvothermal reaction in pyridine (CSD Refcode: LOTXOH).57 In this case, the SBU has the formula [Zn2(µ-COO)2(κ1-COO)2(py)2], where each Zn ion has trigonal bipyramidal coordination, satisfied by two oxygens of two bridging carboxylates, one oxygen of a κ1-monodentate carboxylate and two pyridine rings. In this case, the formula of the framework is [Zn2(ta)2(bipy)2]n.
Three ML-MOFs containing dpe, ta2− and Zn2+ have been reported in the CSD (Fig. 3, right). In one case (CSD Refcode: AWUVIZ), the not-interpenetrated cubic net has the typical pillared structure, with paddle-wheel SBUs of the type [Zn2(COO)4(py)2] and a framework formula corresponding to [Zn2(ta)2(dpe)]n. It was isolated from an unexpected thermal decomposition of a bis-phenyl tetracarboxylic ligand in DMF at 105 °C.58 A different framework was isolated through an elaborate layered crystallization, where dpe (MeOH) and Zn(NO3)2·6H2O (water) reacted with a mixture of H2ta and triethylamine (EtOH) in the presence of a buffer of MeOH and DMF. In this case, a doubly interpenetrated PL-MOF was isolated, whose framework has the formula [Zn2(ta)2(dpe)2]n. Here, planes containing SBUs similar to those found in CP2-dpe are pillared by dpe linkers (CSD Refcode: SUJQUK in Fig. 3, right).59 Finally, a third structure was obtained reacting equimolar amounts of Zn(NO3)2·6H2O, dpe and H2ta under hydrothermal conditions.60 Here, tetrahedral SBUs where Zn2+ is surrounded by two pyridines and two κ1-monodentate acetates are contained in a diamondoid net (CSD Refcode: CUYKEN in Fig. 3, right), for a whole framework formula [Zn(ta)(dpe)]n.
Background of mechanochemically synthesized CPs containing bipy and dpe
The number of 1D-CPs mechanochemically synthesized containing bipy is very limited compared with those derived from solution syntheses. To the best of our knowledge, there is only one report dealing with the mechanochemical reaction involving bipy and Zn(OAc)2.61 The reaction was conducted by manually grinding equimolar amounts of the two reagents, and led to CP1-bipy. A sonochemical synthesis of the same polymer conducted in ethanol has also been published.62 A slightly higher number of reports can be found regarding other transition metal salts, such as MX2 (M = Co, Zn, Pt; X = Cl or Br).63–65 Finally, a series of nitrate-containing polymers with different transition metals have been prepared by ball-milling and twin-screw extrusion.66 In most cases, the PXRD traces were indicative of the formation of the same products isolated in solution. Similarly, there are only two reports dealing with the mechanochemical synthesis of 1D-CPs containing dpe. In one case, the linker bridges heteronuclear Fe3SCu2 clusters in 2D-assemblies,67 obtained by grinding the dianion [Fe3S]2− with [Cu(ACN)4]+ and dpe under Liquid Assisted Grinding (LAG) conditions. The other CP derives instead from the grinding of a diphosphonic acid, dpe and Cu(OAc)2 under LAG conditions.68 In both cases, the structures were confirmed by PXRD analysis by comparison with the known single-crystal deposited data.A rather limited number of examples dealing with the mechanochemical synthesis of ML-MOFs containing bipy can be found. In combination with H2ta and ZnO under LAG conditions, the two-fold interpenetrated PL-MOF [Zn(ta)(bipy)]n (CSD Refcode: LOTXOH) was isolated (Fig. 3, left).34 Other mechanochemically synthesized pillared MOFs contain fumarate,34 diphosphonate37 and bent dicarboxylate linkers.69 In the only report regarding PL-MOFs containing dpe, the product was obtained grinding fumaric acid, ZnO and dpe under LAG conditions.34 Based on PXRD characterization, it was assumed to be a two-fold interpenetrated cubic framework analogous to that found for the structurally characterized homologous Cu-MOF,70 containing paddle-wheel SBUs.
Synthesis of the 1D-CPs conducted in this work
The mechanochemical syntheses of the 1D-CPs are depicted in Scheme 1. The experimental conditions adopted for each reaction are provided in Table 1. When equimolar amounts of Zn(OAc)2·2H2O and bipy were ground using an agate mortar and pestle, the quantitative formation of CP1-bipy was observed within 60 minutes, as previously reported (Table 1-entry 1 and Scheme 1).61 To transfer the process to ball milling, we carried out an optimization of the reaction conditions, selecting this synthesis as a model reaction and using MeOH as the LAG agent. Ex situ PXRD monitoring at different reaction times revealed the selective formation of the polymer within the first minute of grinding, with 60 minutes required to ensure complete reagent consumption and, importantly, a high degree of crystallinity (Table 1-entry 1 and Fig. 4, orange trace). Fig. S1 collects the PXRD traces acquired at different grinding times. Repeating the same reaction under neat grinding conditions again led to the complete and selective formation of CP1-bipy, though with significantly lower crystallinity (Table 1, entry 1 and Fig. S2 for PXRD traces). We further investigated the influence of several milling parameters. Increasing the milling frequency from 20 Hz to 30 Hz did not lead to significant differences in the outcome (Fig. S3). Likewise, the use of milling balls with different diameters (7 mm or 10 mm) had no appreciable effect. Finally, a range of solvents were tested as LAG additives (η = 0.3), such as DCM, ACN, THF, DMF and H2O, but none of them affected neither the selectivity and yield of the reaction nor the crystallinity of the final product (Table 1-entry 1 and Fig. 4; see Fig. S4 for PXRD traces). In an attempt to direct the synthesis towards CP0-bipy, the reaction was repeated using a two-fold excess of the metal precursor and MeOH as the LAG solvent (η = 0.3), consistently with the conditions used in the solution-phase synthesis of CP0-bipy.53 However, after 60 minutes of grinding, PXRD analysis showed the exclusive formation of CP1-bipy alongside unreacted Zn(OAc)2·2H2O (Table 1, entry 1 and Fig. S5 for PXRD traces). Additional tests using various stoichiometric ratios of bipy and Zn(OAc)2·2H2O similarly resulted in no products other than CP1-bipy and the unreacted starting material (Table 1, entry 1). These results suggest that, under mechanochemical conditions, the formation of the ladder motif is strongly favoured. From literature data referring to solution syntheses,54 it appears that the type of 1D-CP that forms from the reaction between dpe and Zn(OAc)2 strongly depends on the solvent used, regardless of the stoichiometry of the reaction. To test if the same solvent effect could be observed under mechanochemical conditions, parallel reactions between equimolar amounts of dpe and Zn(OAc)2·2H2O were conducted using liquid-assisted grinding with the solvents employed in the solution syntheses. In all cases, the parameter η was set to 0.3. In solution, CP2-dpe forms in pure MeOH or by a layering of MeOH, THF and ACN. Under grinding, this polymer forms when MeOH, THF or DMF are used as LAG agents (Table 1-entry 2 and Scheme 1), as evidenced by PXRD analysis (Fig. 4). The use of either ACN or DCM was instead unsuccessful, leaving the reagents practically intact. The use of water as the LAG agent led instead to CP3-dpe (Table 1-entry 3, Scheme 1 and Fig. 4, purple trace), in line with what is observed in solution.54,55 It follows that the use of equimolar amounts of reagents leads to the formation of the frameworks featured by the same Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dpe ratio, where the type of SBU is dictated by the solvent. In no case the formation of the paddle-wheel containing polymer, CP4-dpe, was observed. Actually, this polymer corresponds to a Zn
dpe ratio, where the type of SBU is dictated by the solvent. In no case the formation of the paddle-wheel containing polymer, CP4-dpe, was observed. Actually, this polymer corresponds to a Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dpe ratio of 2
dpe ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and in solution it forms by layering MeOH and THF solutions of the reagents.54 Then, to promote the formation of CP4-dpe, the reaction was repeated in either MeOH or THF using a Zn
1, and in solution it forms by layering MeOH and THF solutions of the reagents.54 Then, to promote the formation of CP4-dpe, the reaction was repeated in either MeOH or THF using a Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dpe molar ratio of 2
dpe molar ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Table 1-entry 4 and Scheme 1).
1 (Table 1-entry 4 and Scheme 1).
| Entry | Ligand | Ligand/Zn2+ molar ratio | LAG additive (h = 0.3) | Product |
|---|---|---|---|---|
| a Reaction conditions: ball-mill frequency: 20 or 30 Hz; grinding time: 60 minutes; ball ∅ = 7 or 10 mm. | ||||
| 1 | bipy | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1 1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 or 1 2 or 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
Neat, MeOH, DCM, ACN, THF, DMF | CP1-bipy [Zn2(OAc)4(µ-bipy)2]n |
| 2 | dpe | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Neat, MeOH, THF, DMF | CP2-dpe [Zn2(OAc)4(µ-dpe)2]n |
| 3 | dpe | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
H2O | CP3-dpe [Zn(OAc)2(µ-dpe)]n |
| 4 | dpe | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
Neat, MeOH, THF, H2O | CP4-dpe [Zn2(OAc)4(µ-dpe)]n |
Under these conditions, the formation of CP4-dpe was observed, as indicated by PXRD analysis (Fig. 4, green trace). It is worth noting that the use of H2O as the LAG agent did not affect the reaction, provided the same Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dpe molar ratio (Table 1-entry 4 and Fig. 4). Notably, in all cases, the complete conversion of the reagents was observed within 60 minutes of grinding. Fig. S6 collects all the PXRD traces obtained with different LAG agents and stoichiometries.
dpe molar ratio (Table 1-entry 4 and Fig. 4). Notably, in all cases, the complete conversion of the reagents was observed within 60 minutes of grinding. Fig. S6 collects all the PXRD traces obtained with different LAG agents and stoichiometries.
Finally, the neat grinding of equimolar amounts of the reagents led to the formation of CP2-dpe, while the same reaction repeated using a Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dpe molar ratio of 2
dpe molar ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 led to CP4-dpe (Table 1-entries 2 and 4).
1 led to CP4-dpe (Table 1-entries 2 and 4).
On the base of the synthetic results, the following considerations can be traced back: (1) regardless of the solvent used as the LAG agent, bipy leads exclusively to the ladder polymer CP1-bipy, even when a two-fold excess of metal, expected to favor the formation of CP0-bipy, is applied; (2) dpe shows a higher structural variability that can be tuned by a judicious selection of reaction stoichiometry and liquid used as the LAG agent. In particular, the chosen reaction stoichiometry selects the type of CP that forms. When a Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dpe = 1
dpe = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio is applied, CP2-dpe forms irrespective to the solvent used as LAG agent, with the exception of water that leads to CP3-dpe. Conversely, when a Zn
1 molar ratio is applied, CP2-dpe forms irrespective to the solvent used as LAG agent, with the exception of water that leads to CP3-dpe. Conversely, when a Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dpe = 2
dpe = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio is chosen, the paddle-wheel containing CP4-dpe forms, irrespective to the liquid used as the LAG agent, water included. The solvent induced selectivity does not seem ascribable to a template effect of the solvent molecules, since all the isolated products were devoid of traces of solvents, as indicated by FTIR spectroscopy (see Fig. S14–S18).
1 molar ratio is chosen, the paddle-wheel containing CP4-dpe forms, irrespective to the liquid used as the LAG agent, water included. The solvent induced selectivity does not seem ascribable to a template effect of the solvent molecules, since all the isolated products were devoid of traces of solvents, as indicated by FTIR spectroscopy (see Fig. S14–S18).
Rationalization of reaction selectivity and formation of 1D-CPs
In silico simulations were conducted to obtain additional insights into the performed reactions (summarized in Fig. 5). A preliminary assessment of the relative stabilities of the possible zinc coordination polymers was performed through the program Gaussian16.71 The free energy of reaction (schematized in Fig. 5A, above) of minimal SBUs composed of zinc metals and acetate and pyridine (py) capping ligands was computed in vacuum and in the three solvents (SMD method) H2O, MeOH and DMF (Fig. 5A), considering the ligand arrangements derived from the complete substitution of the two molecules of water contained in the starting salt. The SBU contained in CP0-bipy is labelled as “D-PW”, the SBU contained in CP1-bipy and CP2-dpe as “Ld”, the SBU contained in CP3-dpe as “Tet” and, finally, the SBU contained in CP4-dpe as “PW”. When a Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) py molar ratio of 1
py molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 is considered, corresponding to a Zn
1 is considered, corresponding to a Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) linker ratio of 2
linker ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (linker = bipy or dpe), the paddlewheel-type SBU is always preferred, with the SBU featuring CP0-bipy being much less stable during the optimization process. This is in line with the experimentally observed tendency to produce CP4-dpe regardless of the solvent used as the LAG agent. When instead a Zn
1 (linker = bipy or dpe), the paddlewheel-type SBU is always preferred, with the SBU featuring CP0-bipy being much less stable during the optimization process. This is in line with the experimentally observed tendency to produce CP4-dpe regardless of the solvent used as the LAG agent. When instead a Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) py ratio of 1
py ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 is considered, corresponding to a Zn
2 is considered, corresponding to a Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) linker ratio of 1
linker ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the “Ld” complex (corresponding to the isolated CP1-bipy or CP2-dpe) is often preferred to “Tet” (in line with the experimental analysis) with the exception of water solvation. In this solvent, the “Ld” coordination is disfavored, so that the “Tet” one (corresponding to the isolated CP3-dpe) is the most stable. Although the milling experiments were conducted under LAG conditions and not in solution, the calculations strongly suggest that the medium polarization induced by the solvent during the formation of the SBU might be a prime factor in the formation of a specific crystalline phase, also in line with the solution experiments already performed.54 Considering the coordination environment of Zn in the different SBUs, it turns out that “Tet” is favored when water is involved. Additional information regarding the relative stability of the crystalline phases can be retrieved from periodic simulations by means of the program CASTEP.72 Simulating the reaction enthalpy of the performed experiments (Fig. 5B), the results show that the formation of CP0-bipy is thermodynamically disfavored, while CP1-bipy is strongly stabilized. It follows that, in the formation of the two 1D-CPs containing bipy, the marked difference in stability of the two possible crystalline phases plays a crucial role, likely sufficient to promote the formation of CP1-bipy in any of the solvent analyzed, even in water, where the formation of the SBU by itself is thermodynamically disfavored, or when different stoichiometric ratios are employed.
1, the “Ld” complex (corresponding to the isolated CP1-bipy or CP2-dpe) is often preferred to “Tet” (in line with the experimental analysis) with the exception of water solvation. In this solvent, the “Ld” coordination is disfavored, so that the “Tet” one (corresponding to the isolated CP3-dpe) is the most stable. Although the milling experiments were conducted under LAG conditions and not in solution, the calculations strongly suggest that the medium polarization induced by the solvent during the formation of the SBU might be a prime factor in the formation of a specific crystalline phase, also in line with the solution experiments already performed.54 Considering the coordination environment of Zn in the different SBUs, it turns out that “Tet” is favored when water is involved. Additional information regarding the relative stability of the crystalline phases can be retrieved from periodic simulations by means of the program CASTEP.72 Simulating the reaction enthalpy of the performed experiments (Fig. 5B), the results show that the formation of CP0-bipy is thermodynamically disfavored, while CP1-bipy is strongly stabilized. It follows that, in the formation of the two 1D-CPs containing bipy, the marked difference in stability of the two possible crystalline phases plays a crucial role, likely sufficient to promote the formation of CP1-bipy in any of the solvent analyzed, even in water, where the formation of the SBU by itself is thermodynamically disfavored, or when different stoichiometric ratios are employed.
Post-synthetic modifications of 1D-CPs (MPSMs)
In the attempt to enlarge the number of CPs containing bipy mechanochemically synthesized, we directed our attention to the possibility of exchanging dpe with bipy from CPs of the first, through MPSMs (Scheme 2). Provided that no change of the SBU occurs during grinding, this approach could, in principle, lead to new bipy-containing CPs.73 To test the possibility of a simple linker exchange, we first reacted CP2-dpe with an equimolar amount of bipy, in the presence of a small amount of MeOH (η = 0.3).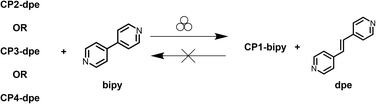 | ||
| Scheme 2 Mechanochemical post-synthetic modifications (MPSMs) highlighting the irreversible conversion of dpe-containing 1D-CPs to CP1-bipy. | ||
The expected product was CP1-bipy. After 60 minutes of grinding, the solid was washed with MeOH to remove the dpe eventually formed. The PXRD trace of the ground material corresponded exactly to that of CP1-bipy. Notably, the PXRD analysis conducted on the crude product showed the peaks corresponding to CP1-bipy and free dpe, indicating that the washing step does not have any influence on the reaction outcome (Fig. S7). Corroborated by the successful preservation of the SBU, we extended the same procedure to the other two CPs CP3-dpe and CP4-dpe. Unexpectedly, in both cases the conversion to CP1-bipy was again observed, although with CP3-dpe some traces of CP2-dpe were detected. In this case, the complete conversion into CP1-bipy could be achieved using a two-fold excess of bipy. The results indicate that the linker exchange reactions do not occur by a simple linker replacement, but by a substantial structural modification of the SBU, which must occur by partial acetate detachment caused by the replacement of the linker in the coordination sphere of the metal. This can be understood on the basis of the coordinative lability of the acetate group, well proved by its known coordination isomerism. The recurrent formation of CP1-bipy confirms, once again, the stability of the ladder structure under mechanochemical conditions when bipy is involved. The inverse reaction, corresponding to bipy replacement by dpe in CP1-bipy, was also tested. CP1-bipy was then ground in the presence of an equimolar amount of dpe using MeOH or water as LAG agents. In both cases, the PXRD analysis of the products evidenced the failure of the reactions (Scheme 2), with the peaks characteristic of the starting materials still clearly visible, pointing out the high stability of the polymer framework of CP1-bipy.
Rationalization of the MPSMs of 1D-CPs
While we do not possess a singular explanation of the MPSMs observed in this work, calculations can help to partially rationalize the behavior observed experimentally on the basis of thermodynamic considerations. As depicted in Fig. 5B, CP1-bipy is more stable than CP2-dpe and CP3-dpe. Hence, provided that no new unreported crystalline phases are accessible, the reactions of CP2-dpe and CP3-dpe with bipy are expected to bring to the formation of CP1-bipy, as experimentally confirmed. Moreover, the energy difference between CP1-bipy and CP4-dpe is negligible, suggesting that the conversion of CP4-dpe to CP1-bipy is still obtainable, especially if aided by additional factors such as the presence of MeOH as a LAG agent, which was not considered in periodic calculations. Factors such as the inertness of CP1-bipy or its relative solubility in MeOH compared to CP4-dpe might be invoked but were not experimentally tested. Lastly, it must be stressed that calculations on CP4-dpe were necessarily performed in the P21/c space group to remove the disorder of the dpe ligand (the original space group is C2/c) and the presence of a plethora of different ligand arrangements might slightly impact the final stability of the computed structure.Dimensionality growth: from 1D-CPs to PL-MOFs
The synthetic step responsible for the framework dimensionality growth is based on the replacement of the acetate anion by bridging terephthalate anions (Fig. 1). This means that the intervention of H2ta on the chosen 1D-CP is expected to lead to the elimination of acetic acid. Among the known ML-MOFs containing dpe, [Zn2(ta)2(dpe)2]n (CSD Refcode: SUJQUK)59 (Fig. 3) can be considered as derived from the simple substitution of the κ2-chelating acetate ions in PC2-dpe with κ1-monodentate bridging terephthalate dianions, each connecting two adjacent SBUs in the square planes. Hence, we first reacted PC2-dpe with an equimolar amount of H2ta (Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2ta molar ratio = 1
H2ta molar ratio = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in the presence of a small aliquot of DMF (η = 0.5), the same solvent used for the solution synthesis of the MOF. The reaction led, after 60 minutes of grinding, to the smooth formation of the expected compound, as can be inferred from the comparison of the experimental and calculated PXRD patterns in Fig. 6 (top and Fig. S8).
1) in the presence of a small aliquot of DMF (η = 0.5), the same solvent used for the solution synthesis of the MOF. The reaction led, after 60 minutes of grinding, to the smooth formation of the expected compound, as can be inferred from the comparison of the experimental and calculated PXRD patterns in Fig. 6 (top and Fig. S8).
Interested to see if it was possible to transfer a geometrically different SBU from a 1D-CP to a PL-MOF, the same reaction was repeated with CP3-dpe. Once again, the formation of SUJQUK was observed (Fig. 6, top and Fig. S9), indicating that the simple transfer of the intact tetrahedral SBU in the new framework was unsuccessful. We then focused our attention on CP4-dpe, which, upon simple replacement of the bridging acetate ions of the paddle-wheel with ta2− anions, would, in principle, yield the known PL-MOF [Zn2(ta)2(dpe)]n (CSD Refcode: AWUVIZ, Fig. 3, right). In this case, the reaction was performed using a Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2ta molar ratio of 1
H2ta molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 to ensure the complete substitution of the acetate anions. The reaction gave a mixture of SUJQUK and a second phase lacking dpe, with formula [Zn4(ta)3(OAc)2(DMF)4]n (CSD Refcode: URUZOZ, Fig. S10).74 No trace of free dpe was visible in the powder diffractogram (Fig. 6, bottom). It is worth noting that the homoleptic polymer was first isolated under solvothermal conditions using a 3D-printed polypropylene autoclave, employing a reactant solution intended to yield the iconic MOF-5 of formula [Zn4O(ta)3].75,76 In that case, its formation was attributed to a partial degradation of the plastic autoclave. In our case, its formation is likely attributable to a mismatched reaction stoichiometry. CP4-dpe and SUJQUK present a Zn
2 to ensure the complete substitution of the acetate anions. The reaction gave a mixture of SUJQUK and a second phase lacking dpe, with formula [Zn4(ta)3(OAc)2(DMF)4]n (CSD Refcode: URUZOZ, Fig. S10).74 No trace of free dpe was visible in the powder diffractogram (Fig. 6, bottom). It is worth noting that the homoleptic polymer was first isolated under solvothermal conditions using a 3D-printed polypropylene autoclave, employing a reactant solution intended to yield the iconic MOF-5 of formula [Zn4O(ta)3].75,76 In that case, its formation was attributed to a partial degradation of the plastic autoclave. In our case, its formation is likely attributable to a mismatched reaction stoichiometry. CP4-dpe and SUJQUK present a Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dpe ratio of 2
dpe ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively. Hence, in the mechanochemical reaction, the amount of ligand dpe was insufficient to achieve the right stoichiometry. In fact, the same reaction involving polymers featured by a Zn
1, respectively. Hence, in the mechanochemical reaction, the amount of ligand dpe was insufficient to achieve the right stoichiometry. In fact, the same reaction involving polymers featured by a Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dpe ratio of 1
dpe ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, such as PC2-dpe and PC3-dpe, led to the complete formation of SUJQUK.
1, such as PC2-dpe and PC3-dpe, led to the complete formation of SUJQUK.
Finally, the feasibility to synthesize via a one-step procedure, following the reaction pathway c depicted in Fig. 1, was tested. Grinding equimolar amounts of Zn(OAc)2·2H2O, dpe and H2ta in the presence of DMF as the LAG agent led to the smooth formation of SUJQUK within 60 minutes of grinding, as can be inferred from Fig. 5 (Fig. S11). Notably, repeating the same reaction with a Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dpe
dpe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2ta ratio of 2
H2ta ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 resulted in a mixture of SUJQUK and URUZOZ, as expected. This outcome confirms that the formation of URUZOZ occurs under conditions where dpe is deficient in the jar. Rather unexpectedly, the mechanochemical reaction between PC1-bipy and H2ta, carried out using DMF as the LAG agent, did not yield a single, well-defined product. Despite multiple attempts to optimize the reaction conditions—including variations in stoichiometric ratios, choice and volume of the LAG solvent, and milling time (tested up to 90 minutes)—the outcome consistently resulted in a complex mixture of unidentified phases. A one-step reaction involving Zn(OAc)2·2H2O, H2ta and bipy was also tested, resulting in the same mixtures of unidentified products. A complete overview of the mechanochemical transformations described in this work is depicted Scheme 3.
2 resulted in a mixture of SUJQUK and URUZOZ, as expected. This outcome confirms that the formation of URUZOZ occurs under conditions where dpe is deficient in the jar. Rather unexpectedly, the mechanochemical reaction between PC1-bipy and H2ta, carried out using DMF as the LAG agent, did not yield a single, well-defined product. Despite multiple attempts to optimize the reaction conditions—including variations in stoichiometric ratios, choice and volume of the LAG solvent, and milling time (tested up to 90 minutes)—the outcome consistently resulted in a complex mixture of unidentified phases. A one-step reaction involving Zn(OAc)2·2H2O, H2ta and bipy was also tested, resulting in the same mixtures of unidentified products. A complete overview of the mechanochemical transformations described in this work is depicted Scheme 3.
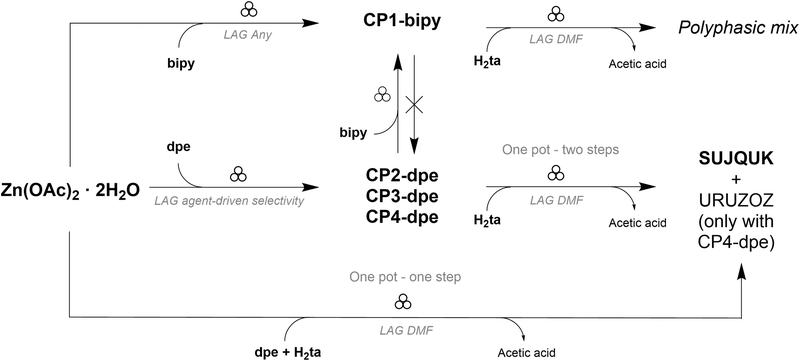 | ||
| Scheme 3 Complete reaction pathway scheme showing the syntheses of the 1D-CPs, their post-synthetic modifications and the dimensionality growth leading to the PL-MOF SUJQUK. | ||
Green metrics
To evaluate the environmental benefit coming from mechanochemistry, the environmental factor (EF) and reaction mass efficiency (RME) of selected mechanochemical reactions were calculated and compared with those of the corresponding solution-based syntheses. The latter were inferred from the experimental details reported in the respective cited references. As representative reactions, we selected the synthesis of CP1-bipy and the one-pot procedure for the synthesis of SUJQUK. Further details of the performed calculations can be found in the SI. Notably, for the synthesis of CP1-bipy, a significant reduction of the E factor is found, from 114.40 in the wet procedure to ≤0.5 in the mechanochemical one. A similar trend is seen for the synthesis of SUJQUK, where the E factor decreases from 121 (solution synthesis) to 1.34 (mechanochemical synthesis). The RME factors also show substantial improvements: from 35.12% to 73.86% for CP1-bipy and from 41.61% to 77.71% for SUJQUK, respectively.Experimental section
General methods
4,4′-Bipyridine (bipy), 1,2-bis(4-pyridyl)ethylene (dpe), terephthalic acid (ta) and Zn(OAc)2·2H2O were commercially available and used as received. The solvents used for LAG were used without any pre-treatment. The solvents used were methanol (MeOH), dichloromethane (DCM), acetonitrile (ACN), N,N-dimethylformamide (DMF), tetrahydrofurane (THF) and distilled water (H2O).PXRD characterization
PXRD data were collected in Bragg–Brentano geometry with Cu Kα radiation on a Rigaku SmartLab XE diffractometer equipped with a solid-state Hypix3000 2D detector. The samples were placed on glass supports and exposed to radiation (1.5° ≤ 2θ ≤ 50°) with a scan of 10° min−1. A length-limiting slit of 15 mm was used to exploit the maximum loading of the sample holder; 5° Soller slits were employed to improve the peak profile and limit the overlapping of reflections.Computational methods
Mechanochemical syntheses
All the reactions were conducted by means of a vibrating ball-mill Retsch MM400 using a 5 mL stainless steel jar equipped with a ball of the same material having a diameter of 7 mm and a weight of 3 g. Where necessary, a small aliquot of solvent was added by means of a micropipette, keeping the ratio µL solvent per mg solids (η value) between 0 and 2 µL mg−1. The jar was shacken at 20 Hz, unless otherwise stated, for the desired time, and the solid withdrawn was vacuum dried and analyzed by FTIR and PXRD analysis. When necessary, the ground solid was subjected to washing with a selected solvent prior to analysis. Structural matching was determined by comparing the experimental PXRD traces with those calculated from the single-crystal structure of the target compound.[Zn2(OAc)4(µ-bipy)2]n (CP1-bipy). Zn(OAc)2·2H2O (164.62 mg, 0.75 mmol) and bipy (117.13 mg, 0.75 mmol) were used. For the reactions conducted under LAG conditions, the solvents used (100 µL) were MeOH, DCM, ACN, DMF, THF and H2O. The reaction was conducted at 20 Hz. In all cases, the complete conversion of the reagents into CP1-bipy was observed (CSD Refcode: ALUPUS). The reaction conducted under neat conditions contained a higher amount of amorphous phase, as evidenced by PXRD analysis.
FTIR (cm−1): 3075, 3043, 3989, 3924, 1602, 1488, 1418, 1407, 1332, 1238, 1217, 1067, 1044, 1005, 932, 884, 818, 731, 672, 649, 622, 464. Elemental analysis: calcd (%) for C28H28N4O8Zn2: C, 49.51; H, 4.15; N, 8.25. Found (%): C, 49.21; H, 4.17; N, 8.01.
[Zn2(OAc)4(µ-dpe)2]n (CP2-dpe). Zn(OAc)2·2H2O (164.6 mg, 0.75 mmol), dpe (136.67 mg, 0.75 mmol) and 100 µL of solvent (MeOH, DMF and THF) were used. The reaction was conducted at 20 Hz. In all cases, the complete conversion of the reagents into CP2-dpe was observed (CCDC Refcode: MANMEU).
FTIR (cm−1): 3067, 3012, 1600, 1503, 1419, 1381, 1326, 1250, 1207, 1072, 1014, 988, 832, 666, 650, 617, 552. Elemental analysis: calcd (%) for C32H32N4O8Zn2: C, 52.55; H, 4.41; N, 7.66. Found (%): C, 52.59; H, 4.40; N, 7.49.
{[Zn(OAc)2(µ-dpe)](H2O)}n (CP3-dpe). As for CP1-dpe but adding 100 µL of H2O. The complete conversion of the reagents into CP3-dpe was observed (CCDC Refcode: BUJDES).
FTIR (cm−1): 3533, 3012, 2925, 1607, 1581, 1507, 1434, 1391, 1329, 1077, 1027, 986, 927, 832, 670, 619, 550. Elemental analysis: calcd (%) for C16H18N2O5Zn: C, 50.08; H, 4.73; N, 7.30. Found (%): C, 50.59; H, 4.40; N, 7.49.
[Zn2(OAc)4(µ-dpe)]n (CP4-dpe). Zn(OAc)2·2H2O (219.51 mg, 1 mmol), dpe (31.11 mg, 0.5 mmol) and 100 µL of solvent (MeOH, THF and H2O) were used. The reaction was conducted at 20 Hz. In all cases, the complete conversion of the reagents into CP4-dpe was observed (CCDC Refcode: MANLUJ).
FTIR (cm−1): 3063, 3046, 2971, 2924, 1626, 1613, 1509, 1423, 1346, 1296, 1251, 1229, 1080, 1030, 990, 842, 665, 622, 571, 554. Elemental analysis: calcd (%) for C20H22N2O8Zn2: C, 43.74; H, 4.04; N, 5.10. Found (%): C, 43.49; H, 4.02; N, 4.91.
General procedure for post-synthetic modifications (MPSMs)
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ligand = 1
ligand = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio was added, followed by 100 µL of solvent (MeOH or H2O). The jar was closed and shaken at 20 Hz for 60 minutes. The resulting solid was removed from the jar, washed with MeOH to remove the undesired side products, vacuum dried and analyzed by PXRD analysis.
1 molar ratio was added, followed by 100 µL of solvent (MeOH or H2O). The jar was closed and shaken at 20 Hz for 60 minutes. The resulting solid was removed from the jar, washed with MeOH to remove the undesired side products, vacuum dried and analyzed by PXRD analysis.Reaction involving CP2-dpe: H2ta (83.07 mg, 0.50 mmol), CP2-dpe (183.86 mg, 0.25 mmol), complete conversion of the reagents to give a mixture of SUJKUK and URUZOZ.
Reaction involving CP3-dpe: H2ta (49.84 mg, 0.30 mmol), CP3-dpe (110.31, 0.30 mmol), complete conversion to SUJKUK.
Reaction involving CP4-dpe: H2ta (83.07 mg, 0.50 mmol), CP4-dpe (122.27, 0.25 mmol), complete conversion to SUJKUK.
Direct synthesis. Zn(OAc)2·2H2O (109.75 mg, 0.5 mmol), H2ta (83.07 mg, 0.5 mmol) and dpe (91.11 mg, 0.5 mmol) were introduced in the jar together with 200 µL of DMF. The jar was shaken for 60 minutes. The product was removed from the jar, eventually washed with DMF, vacuum dried and analyzed by PXRD analysis. Complete conversion into SUJKUK was observed.
All the attempts aimed at converting PC1-bipy into a PL-MOF failed, leading to a complex polyphasic mixture.
Conclusions
In this work, we successfully employed mechanochemistry for the selective synthesis of various one-dimensional coordination polymers derived from the combination of two pyridyl-containing linkers (bipy and dpe) with Zn(OAc)2·2H2O. By carefully selecting the reagent ratios and the type of solvent used as a LAG agent, we selectively obtained different crystalline frameworks containing different secondary building units (SBUs), reproducing products previously synthesized by solution-based methods, but with higher yields and significantly greener protocols. Post-synthetic modifications via linker exchange reactions were also carried out under mechanochemical conditions, demonstrating the facile and complete transformation of dpe-based polymers into a bipy-based polymer. A computational approach provided insights into the observed selectivity by comparing the relative stability of the involved SBUs and frameworks. Finally, the efficient synthesis of a three-dimensional pillared metal–organic framework was accomplished through two alternative and convergent strategies: (i) a stepwise approach involving the dimensional expansion of dpe-containing 1D polymers via grinding with terephthalic acid in the presence of DMF as a LAG agent; and (ii) a one-pot synthesis where all components were simultaneously ground under similar conditions. The set of mechanochemical reactions described is reported in Scheme 3. This work highlights not only the effectiveness of mechanochemistry as a green tool for the selective construction of coordination polymers, but also its efficacy in well controlled post-synthetic modifications and in promoting framework dimensionality growth. Considering the important role that coordination polymers, particularly MOFs, are expected to play in the future, the development of green end efficient protocols for their shaping is of real importance.Author contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information: PXRD traces, FTIR spectra, green metrics calculation, and computational details. See DOI: https://doi.org/10.1039/d5mr00106d.Acknowledgements
The authors thank the Laboratorio di Strutturistica Chimica M. Nardelli of the University of Parma for X-ray data collections. GC acknowledges the Italian Ministry of University and Research (MUR) for funding the PhD scholarship in Chemical Sciences under DM 118/2023. This work has benefited from the equipment and framework of the COMP-R (2023–2027) Initiatives, funded by the Italian Ministry for Education, University and Research program for the “Departments of Excellence”. This work also benefited from the 2020 and 2022 calls of the PRIN: Research Projects of Relevant Interest program of the Italian Ministry for University and Research PRIN 2020Y2CZJ2 (NICE-Natural Inspired Crystal Engineering) and PRIN 202224KAX8 (FLIPPER: FLuorinated PePtidEs for Resumption).Notes and references
- T. Friščić, C. Mottillo and H. M. Titi, Mechanochemistry for Synthesis, Angew. Chem., Int. Ed., 2020, 59, 1018–1029 CrossRef PubMed.
- J. L. Howard, Q. Cao and D. L. Browne, Mechanochemistry as an emerging tool for molecular synthesis: what can it offer?, Chem. Sci., 2018, 9, 3080–3094 RSC.
- J. Batteas, K. G. Blank, E. Colacino, F. Emmerling, T. Friščić, J. Mack, J. Moore, M. E. Rivas and W. Tysoe, RSC Mechanochem., 2025, 2, 10–19 RSC.
- D. V. N. Prasad and J. Theuerkauf, Effect of grinding media size and chamber length on grinding in a spex mixer mill, Chem. Eng. Technol., 2009, 32, 1102–1106 CrossRef CAS.
- P. Sharma, C. Vetter, E. Ponnusamy and E. Colacino, Assessing the Greenness of Mechanochemical Processes with the DOZN 2.0 Tool, ACS Sustainable Chem. Eng., 2022, 10, 5110–5116 CrossRef CAS.
- K. J. Ardila-Fierro and J. G. Hernández, Sustainability Assessment of Mechanochemistry by Using the Twelve Principles of Green Chemistry, ChemSusChem, 2021, 14, 2145–2162 CrossRef CAS PubMed.
- F. Cuccu, L. De Luca, F. Delogu, E. Colacino, N. Solin, R. Mocci and A. Porcheddu, Mechanochemistry: New Tools to Navigate the Uncharted Territory of “Impossible” Reactions, ChemSusChem, 2022, 15, e202200362 CrossRef CAS PubMed.
- A. A. L. Michalchuk, M. Trestman, S. Rudic, P. Portius, P. T. Fincham, C. R. Pulham and C. A. Morrison, Predicting the reactivity of energetic materials: an: ab initio multi-phonon approach, J. Mater. Chem. A, 2019, 7, 19539–19553 RSC.
- M. Senna and A. A. L. Michalchuk, RSC Mechanochem., 2025, 2, 351–369 RSC.
- G. Rothenberg, A. P. Downie, C. L. Raston and J. L. Scott, J. Am. Chem. Soc., 2001, 123, 8701–8708 CrossRef CAS PubMed.
- R. Freund, et al., The Current Status of MOF and COF Applications, Angew. Chem., Int. Ed., 2021, 60, 23975–24001 CrossRef CAS PubMed.
- A. Kirchon, L. Feng, H. F. Drake, E. A. Joseph and H. C. Zhou, From fundamentals to applications: a toolbox for robust and multifunctional MOF materials, Chem. Soc. Rev., 2018, 47, 8611–8638 RSC.
- L. Gagliardi and O. M. Yaghi, Three Future Directions for Metal-Organic Frameworks, Chem. Mater., 2023, 35, 5711–5712 CrossRef CAS.
- T. Ghanbari, F. Abnisa and W. M. A. Wan Daud, A review on production of metal organic frameworks (MOF) for CO2 adsorption, Sci. Total Environ., 2020, 707, 135090 CrossRef CAS PubMed.
- Z. Ji, H. Wang, S. Canossa, S. Wuttke and O. M. Yaghi, Pore Chemistry of Metal–Organic Frameworks, Adv. Funct. Mater., 2020, 30, 2000238 CrossRef CAS.
- J. Wang, et al., A comprehensive transformer-based approach for high-accuracy gas adsorption predictions in metal-organic frameworks, Nat. Commun., 2024, 15, 1–14 Search PubMed.
- R. Oktavian, et al., Gas adsorption and framework flexibility of CALF-20 explored via experiments and simulations, Nat. Commun., 2024, 15, 1–10 Search PubMed.
- F. Afshariazar and A. Morsali, The unique opportunities of mechanosynthesis in green and scalable fabrication of metal-organic frameworks, J. Mater. Chem. A, 2022, 10, 15332–15369 RSC.
- S. Głowniak, B. Szczęśniak, J. Choma and M. Jaroniec, Mechanochemistry: toward green synthesis of metal–organic frameworks, Mater. Today, 2021, 46, 109–124 CrossRef.
- Y. Li, et al., Synthesis and shaping of metal-organic frameworks: a review, Chem. Commun., 2022, 58, 11488–11506 RSC.
- I. Senkovska, V. Bon, L. Abylgazina, M. Mendt, J. Berger, G. Kieslich, P. Petkov, J. L. Fiorio, J.-O. Joswig, T. Heine, L. Schaper, C. Bachetzky, R. Schmid, R. A. Fischer, A. Poppl, E. Brunner and S. Kaskel, Understanding MOF Flexibility: An Analysis Focused on Pillared Layer MOFs as a Model System, Angew. Chem., Int. Ed., 2023, 62, e202218076 CrossRef CAS PubMed.
- S. Pullen and G. H. Clever, Mixed-Ligand Metal-Organic Frameworks and Heteroleptic Coordination Cages as Multifunctional Scaffolds - A Comparison, Acc. Chem. Res., 2018, 51, 3052–3064 CrossRef CAS PubMed.
- D. Balestri, P. P. Mazzeo, C. Carraro, N. Demitri, P. Pelagatti and A. Bacchi, Stepwise Evolution of Molecular Nanoaggregates Inside the Pores of a Highly Flexible Metal–Organic Framework, Angew. Chem., Int. Ed., 2019, 58, 17342–17350 CrossRef CAS PubMed.
- D. Balestri, P. P. Mazzeo, R. Perrone, F. Fornari, F. Bianchi, M. Careri, A. Bacchi and P. Pelagatti, Deciphering the Supramolecular Organization of Multiple Guests Inside a Microporous MOF to Understand their Release Profile, Angew. Chem., Int. Ed., 2021, 10194–10202 CrossRef CAS PubMed.
- D. Giovanardi, P. P. Mazzeo, P. Pelagatti and A. Bacchi, Guest Molecules Play Tug of War in a Breathing MOF: The Stepwise Monitoring of an Elastic Framework Deformation via SC-SC Transformations, Cryst. Growth Des., 2023, 23, 8726–8734 CrossRef CAS.
- L. Liu, Z. Li, B. Wang, L. Wang, X. Meng and Z. He, Polymeric frameworks constructed from bulky carboxylates and 4,4′-bipyridine linkages: synthesis, crystal structures, and properties, Cryst. Growth Des., 2009, 9, 5244–5258 CrossRef CAS.
- M. Du, C. P. Li, C. S. Liu and S. M. Fang, Design and construction of coordination polymers with mixed-ligand synthetic strategy, Coord. Chem. Rev., 2013, 257, 1282–1305 CrossRef CAS.
- M. L. Mortensen, J. N. Vo, M. U. Hyder, A. Shrivasta, G. T. McCandless and K. J. Balkus Jr, Transformation of a copper complex to a 1-D coordination polymer to 3-D metal-organic frameworks, Polyhedron, 2023, 242, 116512 CrossRef CAS.
- B. J. Burnett and W. Choe, Stepwise pillar insertion into metal-organic frameworks: a sequential self-assembly approach, CrystEngComm, 2012, 14, 6129–6131 RSC.
- K. Otsubo, T. Haraguchi, O. Sakata, A. Fujiwara and H. Kitagawa, Step-by-step fabrication of a highly oriented crystalline three-dimensional pillared-layer-type metal-organic framework thin film confirmed by synchrotron X-ray diffraction, J. Am. Chem. Soc., 2012, 134, 9605–9608 CrossRef CAS PubMed.
- B. J. Burnett, P. M. Barron, C. Hu and W. Choe, Stepwise synthesis of metal - organic frameworks: replacement of structural organic linkers, J. Am. Chem. Soc., 2011, 133, 9984–9987 CrossRef CAS PubMed.
- R. Haldar and C. Wöll, Hierarchical assemblies of molecular frameworks—MOF-on-MOF epitaxial heterostructures, Nano Res., 2021, 14, 355–368 CrossRef CAS.
- Y. Chen, H. Wu, Y. Youan, D. Lv, Z. Qiao, D. An, X. Wu, H. Liang, Z. Li and Q. Xia, Highly rapid mechanochemical synthesis of a pillar-layer metal-organic framework for efficient CH4/N2 separation, Chem. Eng. J., 2020, 385, 123836 CrossRef CAS.
- T. Friscić and L. Fábián, Mechanochemical conversion of a metal oxide into coordination polymers and porous frameworks using liquid-assisted grinding (LAG), CrystEngComm, 2009, 11, 743–745 RSC.
- B. Parmar, Y. Rachuri, K. K. Bisht and E. Suresh, Syntheses and Structural Analyses of New 3D Isostructural Zn(II) and Cd(II) Luminescent MOFs and Their Application towards Detection of Nitroaromatics in Aqueous Media, ChemistrySelect, 2016, 1, 6308–6315 CrossRef CAS.
- T. Gao, H.-J. Tang, S.-Y. Zhang, J.-W. Cao, Y.-N. Wu, J. Chen, Y. Wang and K.-J. Chen, Mechanochemical synthesis of three-component metal-organic frameworks for large scale production, J. Solid State Chem., 2021, 303, 122547 CrossRef CAS.
- M. Rautenberg, B. Bhattacharya, C. Das and F. Emmerling, Mechanochemical Synthesis of Phosphonate-Based Proton Conducting Metal-Organic Frameworks, Inorg. Chem., 2022, 61, 10801–10809 CrossRef CAS PubMed.
- M. Rautenberg, B. Bhattacharya, J. Witt, M. Jain and F. Emmerling, In situ time-resolved monitoring of mixed-ligand metal-organic framework mechanosynthesis, CrystEngComm, 2022, 24, 6747–6750 RSC.
- W. Yuan, T. Friščić, D. Apperley and S. L. James, High Reactivity of Metal–Organic Frameworks under Grinding Conditions: Parallels with Organic Molecular Materials, Angew. Chem., Int. Ed., 2010, 49, 3916–3919 CrossRef CAS PubMed.
- Z. Jiang, W. Xue, H. Huang, H. Zhu, Y. Sun and C. Zhong, Mechanochemistry-assisted linker exchange of metal-organic framework for efficient kinetic separation of propene and propane, Chem. Eng. J., 2023, 454(1), 140093 CrossRef CAS.
- M. Carcelli, A. Bacchi, P. Pelagatti, G. Rispoli, D. Rogolino, T. W. Sanchez, M. Sechi and N. Nemati, Ruthenium arene complexes as HIV-1 integrase strand transfer inhibitors, J. Inorg. Biochem., 2013, 118, 74–82 CrossRef CAS PubMed.
- P. P. Mazzeo, M. Prencipe, T. Feiler, F. Emmerling and A. Bacchi, On the Mechanism of Cocrystal Mechanochemical Reaction via Low Melting Eutectic: A Time-Resolved in Situ Monitoring Investigation, Cryst. Growth Des., 2022, 22, 4260–4267 CrossRef CAS PubMed.
- F. Fornari, N. Riboni, C. Spadini, C. S. Cabassi, M. Iannarelli, C. Carraro, P. P. Mazzeo, A. Bacchi, S. Orlandini, S. Furlanetto and M. Careri, Development of novel cocrystal-based active food packaging by a Quality by Design approach, Food Chem., 2021, 347, 129051 CrossRef PubMed.
- F. Montisci, P. P. Mazzeo, C. Carraro, M. Prencipe, P. Pelagatti, F. Fornari, F. Bianchi, M. Careri and A. Bacchi, Dispensing Essential Oil Components through Cocrystallization: Sustainable and Smart Materials for Food Preservation and Agricultural Applications, ACS Sustainable Chem. Eng., 2022, 10, 8388–8399 CrossRef CAS.
- P. P. Mazzeo, S. Canossa, C. Carraro, P. Pelagatti and A. Bacchi, Systematic coformer contribution to cocrystal stabilization: energy and packing trends, CrystEngComm, 2020, 42, 7341–7349 RSC.
- V. Sinisi, P. Pelagatti, M. Carcelli, A. Migliori, L. Mantovani, L. Righi, G. Leonardi, S. Pietarinen, C. Hubsch and D. Rogolino, A Green Approach to Copper-Containing Pesticides: Antimicrobial and Antifungal Activity of Brochantite Supported on Lignin for the Development of Biobased Plant Protection Products, ACS Sustainable Chem. Eng., 2019, 7(3), 3213–3221 CrossRef CAS.
- C. Gazzurelli, A. Migliori, P. P. Mazzeo, M. Carcelli, S. Pietarinen, G. Leonardi, A. Pandolfi, D. Rogolino and P. Pelagatti, Making Agriculture More Sustainable: An Environmentally Friendly Approach to the Synthesis of Lignin@Cu Pesticides, ACS Sustainable Chem. Eng., 2020, 8, 14886–14895 CrossRef CAS.
- C. Gazzurelli, M. Carcelli, P. P. Mazzeo, C. Mucchino, A. Pandolfi, A. Migliori, S. Pietarinen, G. Leonardi, D. Rogolino and P. Pelagatti, Exploiting the Reducing Properties of Lignin for the Development of an Effective Lignin@Cu2O Pesticide, Adv. Sustainable Syst., 2022, 6(8), 2200108 CrossRef CAS.
- G. Cagossi, P. P. Mazzeo and P. Pelagatti, Mechanochemistry comparison between mechanochemical and solution synthesis of Zn and Cu complexes containing pyridine and p-halogen substituted, RSC Mechanochem., 2025, 2, 475–481 RSC.
- C. Gazzurelli, M. Solzi, F. Cugini, P. P. Mazzeo, A. Bacchi and P. Pelagatti, Comparison of different synthetic approaches for the fabrication of a bio-inspired 1D-coordination polymer: from solution chemistry to mechanochemistry, Inorg. Chim. Acta, 2022, 539, 121010 CrossRef CAS.
- B. Conerney, P. Jensen, P. E. Kruger, B. Moubaraki and K. S. Murray, Synthesis and structural characterisation of two coordination polymers (molecular ladders) incorporating [M(OAc)2]2 secondary building units and 4,4′-bipyridine [M = Cu(II), Zn(II)], CrystEngComm, 2003, 5, 454–458 RSC.
- J. Kim, U. Lee and K. K. Bon, One-dimensional network of zinc(II) coordination polymer with 4,4′-bipyridine (4,4′-bipy), Bull. Korean Chem. Soc., 2006, 27, 918–920 CrossRef CAS.
- T. W. Lee, J. P. K. Lau and W. T. Wong, Synthesis and characterization of coordination polymers of Zn(II) with 1,3-bis(4-pyridyl)propane and 4,4′-pyridine ligands, Polyhedron, 2004, 23, 999–1002 CrossRef CAS.
- L. T. Ni, M. Nagarathinam and J. J. Vittal, Topochemical photodimerization in the coordination polymer [{(CF3CO2)(µ-O2CCH3)Zn}2(µ-bpe)2]n through single-crystal to single-crystal transformation, Angew. Chem., Int. Ed., 2005, 44, 2237–2241 CrossRef PubMed.
- P. Phuengphai, S. Youngme, N. Chaichit and J. Reedijk, New 3D supramolecular networks built from 1D and 2D frameworks via π-π and H-bonding interactions: topology and catalytic properties, Inorg. Chim. Acta, 2013, 403, 35–42 CrossRef CAS.
- B. Chen, C. Liang, J. Yang, D. S. Contreras, Y. L. Clancy, E. B. Lobkovsky, O. M. Yaghi and S. Dai, A microporous metal-organic framework for gas-chromatographic separation of alkanes, Angew. Chem., Int. Ed., 2006, 45, 1390–1393 CrossRef CAS PubMed.
- J. Tao, M. L. Tong and X. M. Chen, Hydrothermal synthesis and crystal structures of three-dimensional co-ordination frameworks constructed with mixed terephthalate (tp) and 4,4′-bipyridine (4,4′-bipy) ligands: [M(tp)(4,4′-bipy)] (M = CoII, CdII or ZnII), J. Chem. Soc., Dalton Trans., 2000, 3669–3674, 10.1039/b005438k.
- B. Liu, H.-F. Zhou, Z.-H. Guan, L. Hou, B. Cui and Y.-Y. Wang, Cleavage of a C-C σ bond between two phenyl groups under mild conditions during the construction of Zn(II) organic frameworks, Green Chem., 2016, 18, 5418–5422 RSC.
- M. H. Mir, L. L. Koh, G. K. Tan and J. J. Vittal, Single-crystal to single-crystal photochemical structural transformations of interpenetrated 3D coordination polymers by [2+2] cycloaddition reactions, Angew. Chem., Int. Ed., 2010, 49, 390–393 CrossRef CAS PubMed.
- D. Liu, H. X. Li, Y. Chen, Y. Zhang and J. P. Lang, Hydrothermal synthesis and structural characterization of two zinc coordination polymers of 1,2-di(4-pyridyl)ethylene and benzenedicarboxylate, Chin. J. Chem., 2008, 26, 2173–2178 CrossRef CAS.
- A. C. Tella, G. Mehlana, L. O. Alimi and S. A. Bourne, Solvent-Free Synthesis, Characterization and Solvent-Vapor Interaction of Zinc(II) and Copper(II) Coordination Polymers Containing Nitrogen-Donor Ligands, Zeitschrift fur Anorganische und Allgemeine Chemie, 2017, 643, 523–530 CrossRef CAS.
- A. Scano, E. Mereu, V. Cabras, G. Mannias, A. Garau, M. Pilloni, G. Orrù, A. Scano and G. Ennas, Green Preparation of Antimicrobial 1D-Coordination Polymers: [Zn(4,4′-bipy)Cl2]∞ and [Zn(4,4′-bipy)2(OAc)2]∞ by Ultrasonication of Zn(II) Salts and 4,4′-Bipyridine, Molecules, 2022, 27(19), 6677 CrossRef CAS PubMed.
- C. J. Adams, H. M. Colquhoun, P. C. Crawford, M. Lusi and A. G. Orpen, Solid-state interconversions of coordination networks and hydrogen-bonded salts, Angew. Chem., Int. Ed., 2007, 46, 1124–1128 CrossRef CAS PubMed.
- C. J. Adams, M. F. Haddow, M. Lusi and A. G. Orpen, Crystal engineering of lattice metrics of perhalometallate salts and MOFs, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 16033–16038 CrossRef CAS PubMed.
- A. Pichon and S. L. James, An array-based study of reactivity under solvent-free mechanochemical conditions - insights and trends, CrystEngComm, 2008, 10, 1839–1847 RSC.
- S. Darwish, S. Q. Wang, D. M. Croker, G. M. Walker and M. J. Zaworotko, Comparison of Mechanochemistry vs Solution Methods for Synthesis of 4,4′-Bipyridine-Based Coordination Polymers, ACS Sustainable Chem. Eng., 2019, 7, 19505–19512 CrossRef CAS.
- M. C. Hsu, R. Y. Lin, T. Y. Sun, Y. X. Huang, M.-S. Li, Y.-H. Li, H.-L. Chen and M. Shieh, Inorganic-organic hybrid Cu-dipyridyl semiconducting polymers based on the redox-active cluster [SFe3(CO)9]2−: filling the gap in iron carbonyl chalcogenide polymers, Dalton Trans., 2024, 53, 7303–7314 RSC.
- G. C. Laredo, P. M. Vega-Merino, J. A. Montoya-de la Fuente, R. J. Mora-Vallejio, E. Meneses-Ruiz, J. J. Castillo and B. Zapata-Rendon, Comparison of the metal-organic framework MIL-101 (Cr) versus four commercial adsorbents for nitrogen compounds removal in diesel feedstocks, Fuel, 2016, 180, 284–291 CrossRef CAS.
- M. Y. Masoomi, A. Morsali and P. C. Junk, Rapid mechanochemical synthesis of two new Cd(ii)-based metal-organic frameworks with high removal efficiency of Congo red, CrystEngComm, 2015, 17, 686–692 RSC.
- B. Chen, S. Ma, F. Zapata, F. R. Fronczek, F. B. Lobkovsky and H. C. Zhou, Rationally designed micropores within a metal-organic framework for selective sorption of gas molecules, Inorg. Chem., 2007, 46(4), 1233–1236 CrossRef CAS PubMed.
- M. J. Frisch, et al., Gaussian16, preprint at 2016 Search PubMed.
- S. J. Clark, M. D. Segall, C. J. Pickard, P. J. Hasnip, M. I. J. Probert, K. Refson and M. Payne, First principles methods using CASTEP, Z. Kristallogr.–Cryst. Mater., 2005, 220(5–6), 567–570 CrossRef CAS.
- C. Jin, S. Shi, S. Liao, S. Liu, S. Xia, Y. Luo, S. Wang, H. Wang and C. Chen, Post-Synthetic Ligand Exchange by Mechanochemistry: Toward Green, Efficient, and Large-Scale Preparation of Functional Metal-Organic Frameworks, Chem. Mater., 2023, 35, 4489–4497 CrossRef CAS.
- G. L. Denisov, P. V. Primakov and Y. V. Nelyubina, A New Metal-Organic Framework: Product of Solvothermal Synthesis in 3D-Printed Autoclaves, Russ. J. Coord. Chem., 2021, 47, 253–260 CrossRef CAS.
- M. S. Biserčić, B. Mirjanovic, B. N. Vasiljevic, S. Mentus, B. A. Zasonska and G. Ciric-Marjanovic, The quest for optimal water quantity in the synthesis of metal-organic framework MOF-5, Microporous Mesoporous Mater., 2019, 278, 23–29 CrossRef.
- F. Containing and L. Rectangular, Framework containing large rectangular, J. Am. Chem. Soc., 1995, 117, 10401–10402 CrossRef.
- J. P. Perdew and M. E. Kieron Burke, Generalized Gradient Approximation Made Simple, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS PubMed.
- A. Tkatchenko and M. Scheffler, Accurate molecular van der Waals interactions from ground-state electron density and free-atom reference data, Phys. Rev. Lett., 2009, 102, 6–9 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2026 |