Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Sustainable mechanochemical synthesis of functionalisable fluorinated scaffolds for drug discovery using green LAG
Adam G. Buchanana,
Elizabeth T. Areolaa,
Maryam Farrukh Butta,
Yan Kiu Lee a,
Jasper Murphyb,
Annie E. Taylor
a,
Jasper Murphyb,
Annie E. Taylor b,
Avninder S. Bhambrab and
George W. Weaver
b,
Avninder S. Bhambrab and
George W. Weaver *a
*a
aDepartment of Chemistry, Loughborough University, Epinal Way, Loughborough, Leicestershire LE11 3TU, UK. E-mail: g.w.weaver@lboro.ac.uk
bLeicester School of Allied Health Sciences, De Montfort University, The Gateway, Leicester, LE1 9BH, UK
First published on 18th December 2025
Abstract
Fluoroarenes have become widely recognised as useful building blocks in medicinal chemistry, and manipulation of these compounds can be achieved readily using nucleophilic aromatic substitution (SNAr) to introduce a diverse range of functionality for drug development. A more sustainable mechanochemical approach to SNAr of fluoroarenes using planetary ball milling with a range of aliphatic and aromatic amines as nucleophiles has been investigated with 20 examples described. An efficient set of milling conditions using liquid assisted grinding (LAG) employing the bio-solvent Cyrene or water, and short reaction times (30 minutes) has been developed. Yields were consistently higher when using Cyrene or water as LAG agent rather than DMF. The method provides a useful alternative to the dipolar aprotic solvents DMF and DMSO and high temperatures commonly used in SNAr. Ethyl acetate is employed in the extractive work-up, but is recyclable and considered a green solvent. The method reduces or obviates bulk reaction solvent and aqueous waste streams containing dipolar aprotic solvents.
Introduction
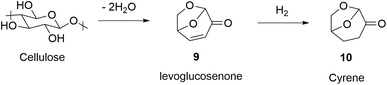
As part of our ongoing programme to develop new compounds active against a number of neglected tropical diseases we required fluorinated arenes substituted with amine or nitrogen heterocyclic groups.1 Such compounds can be elaborated into drug scaffolds by manipulation of the initial arene substituent and/or further substitution of fluorine by SNAr reaction, for example by ortho substitution and cyclocondensation onto aldehyde, ketone or nitrile groups.2 This approach offers an alternative route to fluorinated drug scaffolds which are more usually prepared by late-stage fluorination or by incorporation of reactants containing strategically placed fluorine atoms. Fluorine's small size, high electronegativity, and electrostatic interactions frequently improves drug performance making it key to modern drug development,3 while its leaving-group ability allows for further synthetic manipulation of fluorinated scaffolds enabling unique substitution patterns to be achieved. Fluorine can be introduced either by electrophilic or nucleophilic reactions, but the former often involve the use of highly reactive or expensive fluorinating agents.4 Per- and poly-fluoroarenes can now synthesised using nucleophilic fluorination using fluoride ion (halex reaction) without the need for fluorine gas or reactive reagents derived from F2.5 We have thus exploited SNAr reactions of per- or poly-fluorinated arenes in the construction of novel heterocycles and drug scaffolds.6 SNAr allows the addition of nucleophiles that have proven to be ideal structural motifs in the synthesis of pharmaceutical compounds without the need for transition metal catalysis.7 However, these reactions often require the use of harmful dipolar aprotic solvents, and with the introduction of stricter legislation in recent times with regards to the handling of these solvents, there is an increasing need for a more environmentally friendly way of conducting these reactions. The aim of this work was to investigate the viability of ball milling to promote SNAr reactions, whilst moving away from the use of harmful solvents and towards more sustainable methods using green solvents for liquid-assisted grinding (LAG). Mechanochemistry has become key to recent approaches to develop more sustainable chemical synthesis.8 Recent mechanochemical approaches to SNAr include Andersen and Starbuck's9 work using ball milling to promote SNAr in halonitrobenzenes 1 with amines to form 3 (Scheme 1) but showed temperatures up to 100 °C were required, while Leitch, Smallman and Browne10 reported a study on the synthesis of the organophotocatalyst 1,2,3,5-tetrakis(carbazol-9-yl)-4,6-dicyanobenzene (4CzIPN) 6 using tetrafluoroisophthalonitrile 4 and carbazole 5. They showed that substitution of the four fluorines in 4 with carbazole occurred with yields of up to 92% when using NaOtBu as base, and pre-milling the NaOtBu and carbazole before the addition of the tetrafluoroisophthalonitrile 4. The reaction employed THF for LAG which leads to a higher convection rate and better mass transport often improving the yield of reactions. SNAr reactions are conventionally conducted using toxic dipolar aprotic solvents such as dimethylformamide (DMF) or dimethyl sulfoxide (DMSO), that add significant disposal costs to synthetic protocols. We wished to explore the use of water, or bio-based solvents, such as Cyrene11 10 to promote greener SNAr transformations. Cyrene is synthesised (Scheme 2) by hydrogenation of levoglucosenone 9, a dehydration product of cellulose derived glucose, and whilst renewable hydrogen generation is not currently economically viable, it is possible, meaning that Cyrene is classified as a bio-solvent, and a viable substitute for toxic and non-renewable petrochemical-derived solvents. We also wished to develop lower energy demanding SNAr reactions that can be readily conducted in a short time, rather than the prolonged high temperature reaction commonly encountered (typically >12 h, ∼100 °C). The target compounds were chosen for their desirable properties as building blocks for medicinal scaffolds. Although per- and poly-fluoroalkyl substances (PFAS) are considered environmentally persistent and harmful, the corresponding fluoroarenes are more readily degraded due to their propensity to undergo easy SNAr reaction. Recent work by Gouverneur12 has highlighted how mechanochemical processing with potassium phosphate can be used to degrade PFAS materials while recovering fluoride as KF and K2PFO3 for reuse.Results and discussion
Initial studies using a YKL-0.4L planetary ball mill (Fig. 1a) with reactions conducted in 100 mL stainless steel jars found the use of steel ball bearings of different sizes: 5.68 g (3), 3.28 g (2) and 2.14 g (5) led to the best recovery of material, so these conditions were employed in the SNAr reactions using a 30-minute reaction time and a rotational frequency of 600 rpm. Method optimisation experiments are described in the SI. Three different solvents were employed: Cyrene or water would be compared to DMF as greener aprotic and protic solvents for LAG. Use of an aqueous solvent would allow partial dissolution of the sodium carbonate base employed to neutralise the HF formed as a by-product in the reaction. Reactions were conducted on a 5 mmol scale with 0.25 mL of LAG agent used in each case.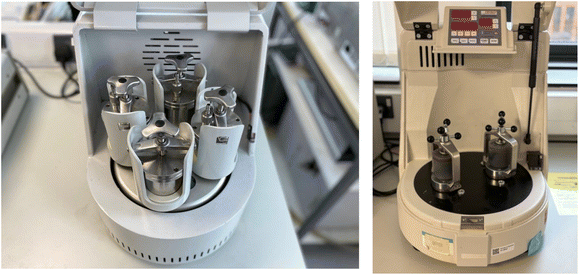 | ||
| Fig. 1 Planetary ball mills used in this study: Changsha Yonglekang YKL-0.4L mill (left) and Fritsch Pulverisette 7 micro mill (right). | ||
Perfluorinated aromatic compounds 7a–e (Scheme 3) with electron-withdrawing substituents were chosen to increase the likelihood of para substitution in the SNAr reaction,13 and to test if poly-substitution would occur under ball milling as often multiple substitution can occur under conventional SNAr methods. Three nitrogen based nucleophiles were used in the study, two aliphatic and one aromatic amine. Morpholine was chosen as a typical aliphatic secondary amine which is often present in drug molecules as a protonatable water solubilising substituent and is an effective nucleophile in SNAr.14 Benzimidazole and cyclopentylamine were chosen to test the lower nucleophilicity of a nitrogen heterocycle compared to a primary amine, and for their pharmaceutically desirable properties. Morpholine was reacted with five different perfluorinated aromatics with electron withdrawing substituents 7a–e, or with pentafluoropyridine 11 as a heterocyclic substrate, with the three chosen solvents (Scheme 2). The results of the reaction set are shown in row 1, with all reactions giving the desired mono-substituted para isomers 8(a–e)a and 12a as confirmed by 19F NMR spectrscopy, except for the formation of compound 12a when using DMF as LAG agent. There was no evidence of di-substitution occurring. All reactions were conducted on a synthetically useful 5 mmol scale with reproducible results (minimum of two repetitions or reactions repeated by different coworkers). The lack of reaction of pentafluoropyridine 11 in DMF was repeatable, and it is not clear why this substrate failed to react with morpholine in the presence of DMF. A control reaction with 7a and morpholine was conducted with no LAG agent. This showed the reaction still to proceed, but not to completion in the same time. Nitrile 8aa was formed in a lower 52% yield after purification.
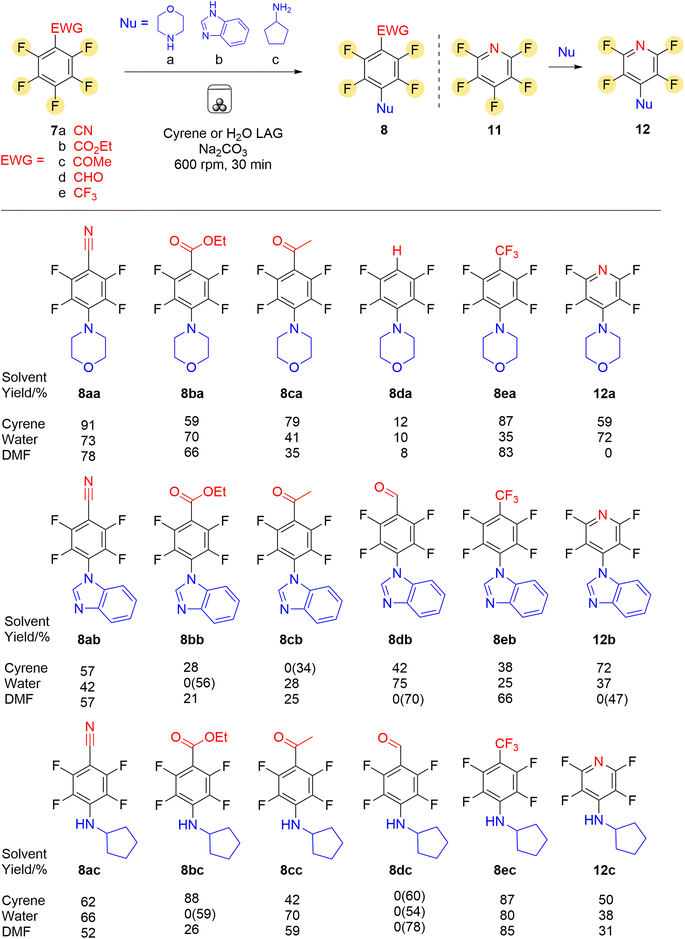 | ||
| Scheme 3 Mechanochemical SNAr reaction of perfluoroarenes with products formed and LAG agent % yields (isolated yields reported; values in parenthesis refer to starting material recovered). | ||
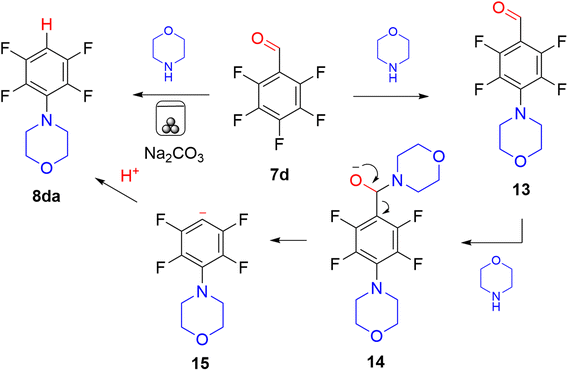
None of the compounds 8(a–e)a and 12a have been previously reported synthesised by mechanochemical methods. Compounds 8aa15 and 12a16 have been prepared in solution, but required prolonged reactions times (e.g. 8aa, 60 h in refluxing THF or 12 h in boiling MeCN). We found 8ba and 8ca were formed in solution in THF in only 36% and 52% respectively after 75 h at room temperature. The ball milling method here afforded 8aa in 91% yield and 12a in 70% in only 30 minutes. 8ea has been prepared previously in 60% isolated yield in THF using a magnesium amide reagent under Schlenk conditions.17 Milling with Cyrene as LAG agent and only sodium carbonate as both base and grinding agent gave 8ea in 87% yield with no need for inert atmosphere conditions. Compounds 8ba and 8ca were synthesised for the first time, with water and Cyrene giving the highest yields, 70% and 79% respectively, as LAG agents. In the reaction of pentafluorobenzaldehyde 7d the expected 4-substituted aldehyde product was not obtained, and 2,3,5,6-tetrafluorophenyl morpholine 8da was isolated instead. Addition of morpholine at the para position was confirmed by the two signals of an AA′BB′ spin pattern in the 19F NMR spectrum but no aldehyde proton signal was visible in the 1H NMR spectrum. A triplet of triplets signal at 6.70 (J 10 and 3 Hz) indicated a single aromatic proton coupling to two pairs of fluorine atoms. Additionally no carbonyl signal was present in the IR spectrum and a mass ion was detected at m/z 236 corresponding to a molecular formula of C10H9F4NO for the decarbonylated compound 8da. This likely formed (Scheme 4) by addition of morpholine at the para position of 7d as first expected, forming 13.
This however undergoes further reaction at the aldehyde centre by addition of a second molecule of morpholine, to form an intermediate 14 which then undergoes Haller Bauer type cleavage18 to form a fluorine stabilised aromatic anion 15 which protonates to give 8da. We have observed similar diacylation reaction of perfluorobenzaldehydes previously.2
After the initial success with the morpholine set of reactions, benzimidazole was then used to investigate whether aromatic nitrogen heterocycles could be introduced by mechanochemical SNAr. Often strong bases such NaH are required to form the heterocyclic anion to ensure sufficient nucleophilicity, but all reactions in the ball mill were successful with both Cyrene or water proving effective as LAG agents. The reactions produced the mono-substituted para derivatives in moderate to good yields (Scheme 3: 8(a–e)b and 12b). Reaction with pentafluorobenzaldehyde 7d was successful with a good yield of 8db (75%) (δH 10.35 for the aldehydic proton) obtained with water as LAG agent. Use of Cyrene afforded a moderate yield of 42%, but none of the deacylated product was detected. Expect in the case of 8eb yields were better using Cyrene or water rather than DMF as solvent. No instances of di-substitution were observed which is often an issue in solution based SNAr chemistry. Compounds 8ab, 8eb and 12b have been synthesised by conventional solution methods previously19 as part of a study on polar crystal engineering exploiting π–π interactions, but used bulk THF as solvent and long reaction times (48 h). Aldehyde 8db was prepared previously by us as a precursor to biologically active benzothiophenes.1c
Cyclopentylamine was the third nucleophile to be investigated for mechanochemial reaction with the set of fluoroarenes. Due to the compounds role as drug scaffold precursors, amines were prioritised as being desirable nucleophiles. The novel compounds 8ac, 8bc, 8cc, 8ec and 12c were successfully synthesised in moderate to high yields with Cyrene or water outperforming DMF as LAG agents in terms of yield. The reaction between pentafluorobenzaldehyde 7d and cyclopentylamine was unsuccessful with all three solvents investigated, with two repetitions attempted for each solvent. TLC analysis of the crude products showed complex mixtures that could not be separated using column chromatography. None of the product 8dc was isolated. Possible side reactions could have occurred between the amine and the aldehyde such as imine formation or deacylation. With DMF as LAG agent a high recovery (78%) of 7d was recovered indicating a slow reaction rate in this solvent. There was no indication of imine formation between the amine and the ketone group of Cyrene.
Considering the Kamlet–Abboud–Taft (KAT) parameters20 of the solvents used, both Cyrene and water have greater π* values (0.93 and 1.08 respectively) than DMF (0.88), and should better stabilise dipolar interactions in the transition state for addition of the nucleophile. These reactions are likely to proceed by a two-step addition–elimination mechanism, although there is increasing evidence that many SNAr reactions are concerted processes.7 Hydrogen Bond Accepting (HBA) solvent Cyrene can also interact with the NH bond of the amines increasing nucleophilicity, while water being also a hydrogen bond donor (HBA-D) solvent should also assist in stabilising the departing fluoride ion. Alcohols, such as bio-ethanol, could also be effective LAG agents, but we did not investigate these as we have found ethanol can add to perfluoroarenes under basic conditions to form ethoxy substituted by-products. Additionally Cyrene (b.p. 227 °C) is stable up to 195 °C and is non-toxic and biodegradable. DMF (b.p 153 °C) begins to decompose above 100 °C forming carbon monoxide and dimethylamine, and this is likely to occur under the high frictional forces of the ball mill. We have previously detected dimethylamino containing by-products in SNAr reactions using DMF.
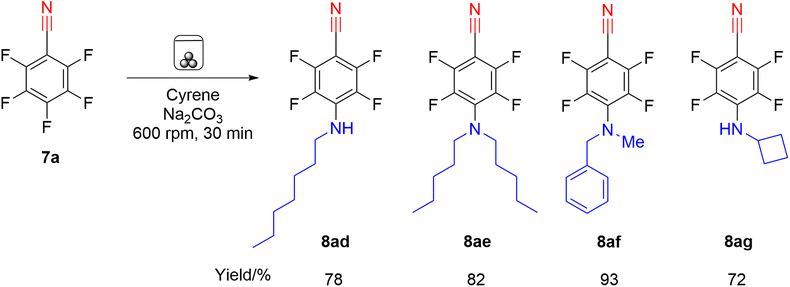
With the optimised conditions employing Cyrene as LAG agent we then investigated reactions of nitrile 7a with more hindered primary and secondary amines (Scheme 5) and also employed agate milling jars and balls in a Fritsch Pulverisette 7 planetary micro mill (Fig. 1b) as an alternative to steel which has the advantage of not introducing potentially harmful metal particulates into the drug precursors. These amines also reacted successfully in high yield (72–93%) on a 2 mmol scale.
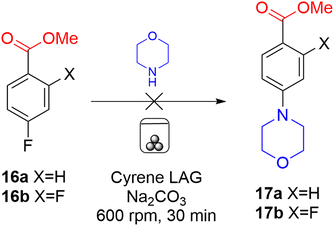
With perfluorinated arenes 7a–e shown to react successfully under milling conditions, mono- and di-fluorinated benzoates 16a and 16b (Scheme 6) were investigated as less reactive arenes with morpholine as nucleophile. Cyrene was employed for LAG but both reactions were unsuccessful, with only starting material recovered in each case. No evidence for the formation of 17a or 17b, or the corresponding ortho isomers, was obtained. No reaction was also observed between morpholine and 4-fluorobenzonitrile. Chloronitrobenzene 1, discussed above (Scheme 1), required a reaction temperature of 100 °C, and even though fluorine as nucleofuge21 in 16 should increase reaction rate relative to chlorine, it appears the ester group is not sufficiently activating to allow reaction under the current milling conditions. The temperature of the jars in the ball mill increase by around 10 °C due to friction, but this did not influence reaction rate sufficiently to allow reaction.
We also studied the potential reaction of a fluoroarene 7f bearing an electron donating group (t-butylphenoxy) with morpholine (Scheme 7), but no reaction occurred under the conditions successful for arenes 7a–e. Only starting material 7f was isolated in a high recovery of 76%. This result matches solution phase reactions of perfluoroarenes with electron donating substituents that require more forcing conditions to undergo SNAr and which, in some cases, lead to meta substitution.13 Further investigations into reactions with less activated substrates, and the effects of temperature are in progress.
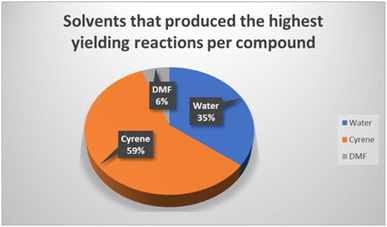
An estimation of the energy consumption of the milling method compared to conventional heating was made. The YKL0.4L ball mill used operated at 750 W and allowed four reactions to be completed simultaneously in 30 minutes. Energy consumption was determined to be approximately 0.09 kWh per reaction compared to 6.6 kWh for conventional thermal reactions (reflux for 12 h). The method developed reduces energy usage as well as minimising solvent waste and disposal costs. The green solvent Cyrene was shown to be the most effective LAG agent affording the highest yields (Fig. 2) in 59% of reactions, with water also proving superior to DMF.
The simplicity of the method should allow easy translation to continuous production methods such as twin-screw extrusion (TSE).22
Conclusions
In conclusion, a more sustainable mechanochemical approach to conduct SNAr of fluorinated aromatics with nitrogen nucleophiles has been developed. Cyrene or water were shown to be more effective LAG agents than DMF and the method provides a viable alternative to conventional solution phase SNAr reactions using dipolar aprotic solvents. Ethyl acetate is employed in the extractive work-up, but is recyclable and considered a green solvent. The method reduces or removes bulk reaction solvent waste and aqueous streams contaminated with dipolar aprotic solvents. A library of twenty (fifteen novel) functionalisable scaffolds has been prepared with the compounds being used in the synthesis of new screening candidates for drug development.Author contributions
AGB and ETA developed the experimental method, synthesised compounds and obtained characterisation data, MFB, CL, JM, and AET synthesised compounds and obtained characterisation data. ASB supervised the project and designed target compounds. GWW conceived and supervised the project and wrote the manuscript. All authors have reviewed the manuscript.Conflicts of interest
There are no conflicts to declare.Data availability
Supplementary information (SI): all supporting experimental data, including synthetic procedures, analytical and spectroscopic characterisation data, and copies of 1H, 19F and 13C NMR spectra. See DOI: https://doi.org/10.1039/d5mr00100e.Acknowledgements
We thank the Royal Society of Chemistry (Research Enablement Grant E21-3753888107) and both Lougborough and De Montfort Universities for support.References
- (a) L. B. Tulloch, H. Tawell, A. E. Taylor, M. L. Lima, A. Dawson, S. Carvalho, R. J. Wall, V. Corpas-Lopez, G. Dey, J. Duggan, L. G. Magalhaes, L. S. Torrie, L. Frame, D. Robinson, S. Patterson, M. Tinti, G. W. Weaver, W. J. Robinson, M. Cal, M. Kaiser, P. Maeser, P. Sjö, B. Perry, M. Kelly, A. F. Francisco, A. S. Bhambra and S. Wyllie, Sci. Transl. Med., 2025, 17, eadu4564, DOI:10.1126/scitranslmed.adu4564; (b) E. Taylor, M. Hering, M. R. J. Elsegood, S. J. Teat, G. W. Weaver, R. R. J. Arroo, M. Kaiser, P. Maeser and A. S. Bhambra, Bioorg. Med. Chem. Lett., 2024, 109, 129825 CrossRef PubMed; (c) A. S. Bhambra, M. Edgar, M. R. J. Elsegood, Y. Li, G. W. Weaver, R. R. J. Arroo, V. Yardley, H. Burrell-Saward and V. Krystof, Eur. J. Med. Chem., 2016, 108, 347–353 CrossRef CAS PubMed.
- H. W. Tawell, W. Robinson, Y. Li, G. J. Tizzard, S. J. Coles, A. S. Bhambra, M. Edgar and G. W. Weaver, RSC Adv., 2025, 15, 12843 RSC.
- (a) E. P. Gillis, K. J. Eastman, M. D. Hill, D. J. Donnelly and N. A. Meanwell, J. Med. Chem., 2015, 58, 8315–8359 CrossRef CAS; (b) K. Müller, C. Faeh and F. Diederich, Science, 2007, 317, 1881 CrossRef.
- (a) N. Rozatian and D. R. W. Hodgson, Chem. Commun., 2021, 57, 683 RSC; (b) R. Szpera, D. F. J. Moseley, L. B. Smith, A. J. Sterling and V. Gouverneur, Angew. Chem., Int. Ed., 2019, 58, 14824–14848 CrossRef CAS.
- A. Pleschkea, A. Marholda, M. Schneidera, A. Kolomeitsevb and G.-V. Röschenthaler, J. Fluorine Chem., 2004, 125, 1031–1038 CrossRef.
- (a) S. Riaz, M. Edgar, M. R. J. Elsegood, L. Horsburgh, S. J. Teat, T. G. Warwick and G. W. Weaver, Cryst. Growth Des., 2019, 19, 5237–5248 CrossRef CAS; (b) J. Ponce González, M. Edgar, M. R. J. Elsegood and G. W. Weaver, Org. Biomol. Chem., 2011, 9, 2294–2305 RSC.
- (a) M. Shigeno, K. Hayashi, O. Sasamoto, R. Hirasawa, T. Korenaga, S. Ishida, K. Nozawa-Kumada and Y. Kondo, J. Am. Chem. Soc., 2024, 146, 32452–32462 CrossRef CAS; (b) L. Finck and M. Oestr, Chem.–Eur. J., 2021, 27, 11061–11064 CrossRef CAS; (c) S. Rohrbach, A. J. Smith, J. Hao Pang, D. L. Poole, T. Tuttle, S. Chiba and J. A. Murphy, Angew. Chem., Int. Ed., 2019, 58, 16383–16388 Search PubMed.
- (a) X. Liu, Y. Li, L. Zeng, X. Li, N. Chen, S. Bai, H. He, Q. Wang and C. Zhang, Adv. Mater., 2022, 34, 2108327 CrossRef CAS PubMed; (b) R. T. O'Neill and R. Boulatov, Nat. Rev. Chem., 2021, 5, 148–167 CrossRef PubMed; (c) T. Friščić, C. Mottillo and H. Titi, Angew. Chem., Int. Ed., 2019, 59, 1018–1029 CrossRef; (d) J. L. Howard, Q. Cao and D. L. Browne, Chem. Sci., 2018, 9, 3080–3094 RSC.
- J. M. Andersen and H. F. Starbuck, J. Org. Chem., 2021, 86, 13983–13989 CrossRef CAS.
- J. Leitch, H. Smallman and D. Browne, J. Org. Chem., 2021, 86, 14095–14101 CrossRef CAS.
- (a) N. A. Stini, P. L. Gkizis and C. G. Kokotos, Green Chem., 2022, 24, 6435 RSC; (b) T. Brouwer and B. Schuur, ACS Sustain. Chem. Eng., 2020, 8, 14807–14817 CrossRef CAS; (c) F. Gao, R. Bai, F. Ferlin, L. Vaccaro, M. Li and Y. Gu, Green Chem., 2020, 22, 6240–6257 Search PubMed; (d) J. E. Camp, ChemSusChem, 2018, 11, 3048–3055 Search PubMed.
- L. Yang, Z. Chen, C. A. Goult, T. Schlatzer, R. S. Paton and V. Gouverneur, Nature, 2025, 640, 100–106 CrossRef CAS PubMed.
- (a) R. D. Chambers, Fluorine in Organic Chemistry, Blackwell, Oxford, 2004 CrossRef; (b) G. M. Brooke, J. Fluorine Chem., 1997, 86, 1–76 CrossRef; (c) R. D. Chambers and C. R. Sargent, Adv. Heterocycl. Chem., 1981, 28, 1–71 CrossRef CAS.
- J. Alarcón-Espósito, R. Tapia, R. Contreras and P. Campodónico, RSC Adv., 2015, 5, 99322–99328 RSC.
- M. Cargill, K. Linton, G. Sandford, D. Yufit and J. Howard, Tetrahedron, 2010, 66, 2356–2362 Search PubMed.
- G. Podolan, P. Jungk, D. Lentz, R. Zimmer and H. Reissig, Adv. Synth. Catal., 2015, 357, 3215–3228 CrossRef CAS.
- L. J. Bole, L. Davin, A. R. Kennedy, R. McLellan and E. Hevia, Chem. Commun., 2019, 55, 4339–4342 RSC.
- (a) A. Haller and E. Bauer, Comptes Rendus, 1908, 147, 824 Search PubMed; (b) G. Mehta and R. V. Venkateswaran, Tetrahedron, 2000, 56, 1399–1422 CrossRef CAS; (c) A. K. Barbour, M. W. Buxton, P. L. Coe, R. Stephens and J. C. Tatlow, J. Chem. Soc., 1961, 808–817 Search PubMed.
- H. Althagbi, J. Lane, G. Saunders and S. Webb, J. Fluorine Chem., 2014, 166, 88–95 CrossRef CAS.
- (a) J. Sherwood, J. Granelli, C. R. McElroy and J. H. Clark, Molecules, 2019, 24, 2209 CrossRef CAS PubMed; (b) P. G. Jessop, D. A. Jessop, D. Fu and L. Phan, Green Chem., 2012, 14, 1245–1259 RSC; (c) M. J. Kamlet, J. L. Abboud and R. W. Taft, J. Am. Chem. Soc., 1977, 99, 6027–6038 CrossRef CAS.
- N. A. Senger, B. Bo, Q. Cheng, J. R. Keeffe, S. Gronert and W. Wu, J. Org. Chem., 2012, 77, 9535–9540 CrossRef CAS PubMed.
- (a) J. A. Leitch, P. Richardson and D. L. Browne, Chimia, 2023, 77, 339–345 CrossRef CAS; (b) J. Andersen, H. Starbuck, T. Current, S. Martin and J. Mack, Green Chem., 2021, 23, 8501–8509 RSC.
| This journal is © The Royal Society of Chemistry 2026 |