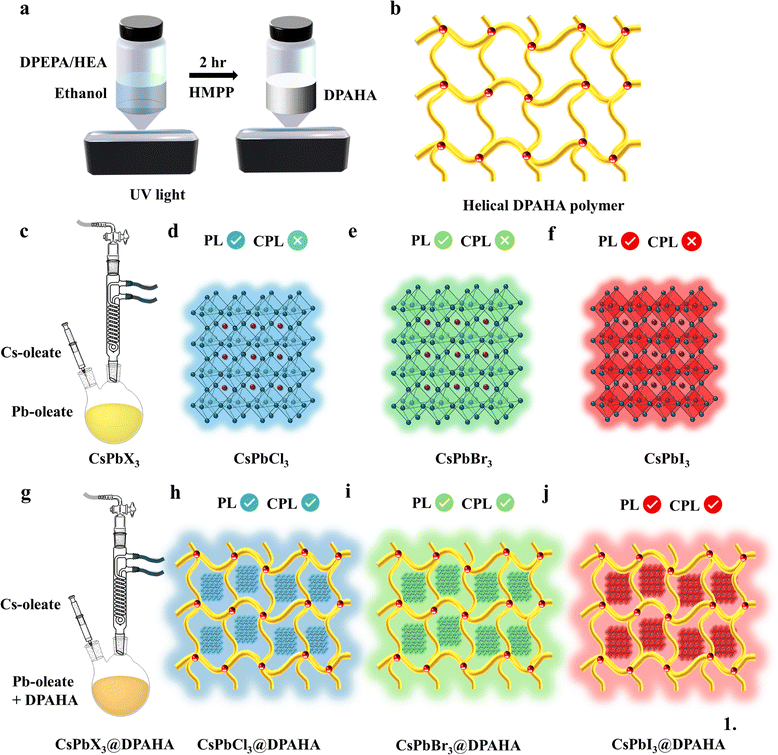
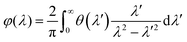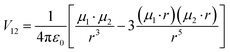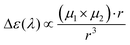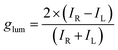Photopolymerized helical acrylate networks enable stable and full-color circularly polarized luminescence in perovskite nanocrystals
Atanu
Jana
 *a,
Deblina
Das
*a,
Deblina
Das
 a,
Sourav
Mal
a,
Sourav
Mal
 a,
Tarak Nath
Mandal
a,
Tarak Nath
Mandal
 b,
Youngsin
Park
b,
Youngsin
Park
 *c and
Sangeun
Cho
*c and
Sangeun
Cho
 *a
*a
aDivision of System Semiconductor, Dongguk University, Seoul 04620, Republic of Korea. E-mail: atanujana@dongguk.edu; sangeun.c@dongguk.edu
bDepartment of Chemistry, SRM Institute of Science and Technology, Kattankulathur, Tamil Nadu 603203, India
cDepartment of Chemistry, College of Natural Science, Ulsan National Institute of Science and Technology, Ulsan 44919, Republic of Korea. E-mail: ysinpark@unist.ac.kr
First published on 11th December 2025
Abstract
Photopolymerized chiral polymer frameworks offer an unexplored strategy to couple structural confinement with optical activity in hybrid materials. Here, we introduce a confinement-driven design in which a photopolymerized hydrophobic helical acrylate network (DPAHA) simultaneously stabilizes and imparts chirality to all-inorganic CsPbX3 (X = Cl, Br, I) perovskite nanocrystals. The polymer forms rigid, non-centrosymmetric cavities that asymmetrically confine the nanocrystals, generating pronounced circular dichroism and circularly polarized luminescence (CPL) spanning the full visible spectrum. Unlike ligand or supramolecular approaches limited by desorption or fragility, photopolymerized confinement ensures durable chiral organization and long-term stability (>6 months). Remarkably, compositional tuning enables handedness inversion and emission-color control from blue to red. This study establishes a general principle for chirality transfer through asymmetric confinement, transforming achiral perovskite emitters into robust, full-color CPL sources. The strategy introduces photopolymerized chiral networks as a versatile platform for scalable, stable, and tunable chiroptical materials for photonic and spintronic applications.
New conceptsCircularly polarized luminescence (CPL) materials promise transformative advances in quantum displays, photonic encryption, and spin-based optoelectronics, yet most existing perovskite systems rely on fragile chiral ligands or supramolecular assemblies. This work pioneers a confinement-driven approach that uses a photopolymerized helical acrylate network (DPAHA) to endow achiral CsPbX3 nanocrystals with durable and tunable chiroptical activity. The rigid, non-centrosymmetric polymer cavities simultaneously stabilize and break symmetry in the embedded nanocrystals, producing bright CPL with color-tunable emission and exceptional environmental robustness. By merging photopolymerization chemistry with chiral confinement, this design establishes a new paradigm for scalable fabrication of stable, full-color CPL-active materials. |
1. Introduction
Circularly polarized luminescence (CPL) has attracted significant attention as a key photonic functionality for advanced technologies such as quantum dot displays,1 light-emitting diodes,2,3 lasers,4 and spintronic devices.5 Metal halide perovskite nanocrystals (NCs) are promising candidates for CPL generation because of their high photoluminescence quantum yields, narrow emission bandwidths, and facile bandgap tunability across the visible spectrum.6,7 However, the intrinsically achiral nature of the perovskite lattice poses a fundamental barrier, as efficient CPL emission requires breaking of inversion symmetry.8 The grand challenge is therefore twofold: imparting robust chirality to achiral perovskite NCs while simultaneously ensuring long-term structural and environmental stability.Several approaches have been developed to address this challenge, yet each suffers from intrinsic limitations.8,9 Surface functionalization with chiral organic ligands is conceptually straightforward, but ligand desorption and surface defect formation often erode both chiroptical activity and material stability.10 Incorporation into chiral liquid crystals or supramolecular assemblies provides cooperative chirality but relies on fragile long-range order that is difficult to maintain under operational conditions.11–16 Chiral polymer hosts have recently emerged as a promising alternative, but most examples rely on weak noncovalent interactions with the NC surface, yielding modest CPL activity and limited stabilization.17–19
These limitations raise several unresolved questions central to advancing the field: (i) how can intrinsically achiral perovskite NCs be endowed with durable and tunable chiroptical activity beyond fragile ligand or supramolecular approaches? (ii) is it possible to achieve stabilization and CPL induction within a single platform? and (iii) can confinement within a helical chiral polymer framework enable spectral control, including handedness inversion of CPL across the visible range? Addressing these questions requires a robust design principle integrating structural stability, efficient chirality induction, and spectral tunability.
The radical photocrosslinking of acrylates is a fast, efficient, and controllable process that proceeds under ambient conditions without the need for high temperatures or harsh catalysts.13,20–22 Acrylate derivatives contain highly reactive vinyl groups (–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–C(
CH–C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)–), that undergo fast photopolymerization in the presence of an initiator.23,24 Upon ultraviolet (UV) or visible light irradiation, radicals generated from the initiator attack the vinyl groups, leading to bond opening and chain propagation; when di- or multifunctional acrylates are used, polymer chains crosslink at multiple sites to form robust three-dimensional networks, which are ultimately stabilized through radical recombination or disproportionation.25 The degree of crosslinking can be precisely tuned by monomer functionality and irradiation conditions, providing structural and mechanical control. Because the reaction occurs under mild conditions, it is compatible with sensitive functional groups and nanomaterials, enabling in situ encapsulation without degradation.22 The resulting crosslinked polymer matrices combine high mechanical strength, chemical resistance, and structural rigidity, making them highly versatile hosts capable of stabilizing NCs and tailoring their local environment for advanced photonic applications.
O)–), that undergo fast photopolymerization in the presence of an initiator.23,24 Upon ultraviolet (UV) or visible light irradiation, radicals generated from the initiator attack the vinyl groups, leading to bond opening and chain propagation; when di- or multifunctional acrylates are used, polymer chains crosslink at multiple sites to form robust three-dimensional networks, which are ultimately stabilized through radical recombination or disproportionation.25 The degree of crosslinking can be precisely tuned by monomer functionality and irradiation conditions, providing structural and mechanical control. Because the reaction occurs under mild conditions, it is compatible with sensitive functional groups and nanomaterials, enabling in situ encapsulation without degradation.22 The resulting crosslinked polymer matrices combine high mechanical strength, chemical resistance, and structural rigidity, making them highly versatile hosts capable of stabilizing NCs and tailoring their local environment for advanced photonic applications.
In this work, we present a confinement-driven strategy based on a branched crystalline acrylate polymer (DPAHA) synthesized via photopolymerization (Scheme 1). The DPAHA polymer was characterized by solid-state NMR spectroscopy. The rigid three-dimensional helical network creates non-centrosymmetric cavities that encapsulate CsPbX3(X = Cl, Br, I) NCs during synthesis. This strategy achieves two goals simultaneously: (1) long-term stabilization of perovskite NCs (more than 6 months) against degradation through chemical passivation and physical encapsulation, and (2) induction of chirality through asymmetric confinement, leading to pronounced circular dichroism and CPL across the visible spectrum.
Remarkably, this approach also affords spectral tunability and handedness inversion of CPL emission depending on halide composition, a feature rarely achieved in polymer-based chiral perovskite systems. By merging stability enhancement with chiroptical functionality in a single materials platform, our method represents a significant step toward practical CPL-active perovskite emitters (Table S1). A small, non-centrosymmetric chiral monomer (DPEPA/HEA) featuring a high-energy UV absorption peak serves as an ideal molecular building block for constructing a helical polymer (DPAHA). Although DPEPA/HEA is intrinsically achiral, its flexible backbone and multiple acrylate units can adopt twisted, asymmetric conformations that self-organize into chiral supramolecular arrangements, where exciton coupling between closely spaced chromophores produces strong circular dichroism signal in UV region (200–275 nm) even in the absence of stereogenic centers (Fig. S2).26,27 Upon polymerization, the resulting supramolecular helical structure exhibits a red-shifted absorption extending into the visible region, attributed to chiral molecular packing and intermolecular interactions rather than extended π-conjugation. This hierarchical design effectively transfers chirality from the molecular to the polymer level, enabling strong optical activity and efficient chiral energy transfer across the visible spectrum. When the helical polymer encapsulates halide perovskite NCs, confined interfacial interactions induce chiral organization within the inorganic lattice. This chiral confinement results in pronounced CPL. Overall, this molecular-to-polymer strategy provides a rational and tunable approach for generating CPL emission across the visible region by leveraging supramolecular chirality and excitonic coupling between the polymer host and perovskite guest.
2. Methodology
2.1. Materials
Oleylamine (OLA, 70%), cesium carbonate (Cs2CO3, 99.9%), octadecene (ODE, 90%), oleic acid (OA, 90%), lead(II) chloride (PbCl2, 98%), lead(II) bromide (PbBr2, ≥98%), lead(II) iodide (PbI2, 99%), trioctylphosphine (TOP, 97%), dipentaerythritol penta-/hexa-acrylate (DPEPA/HEA, contains ≤650 ppm MEHQ as inhibitor), 2-hydroxy-2-methylpropiophenone (HMPP, 97%), and ethanol (≥99.5%) were purchased from Sigma Aldrich.2.2. Synthesis of dipentaerythritol penta-/hexa-acrylate polymers (DPAHA) through photopolymerization of DPEPA/HEA
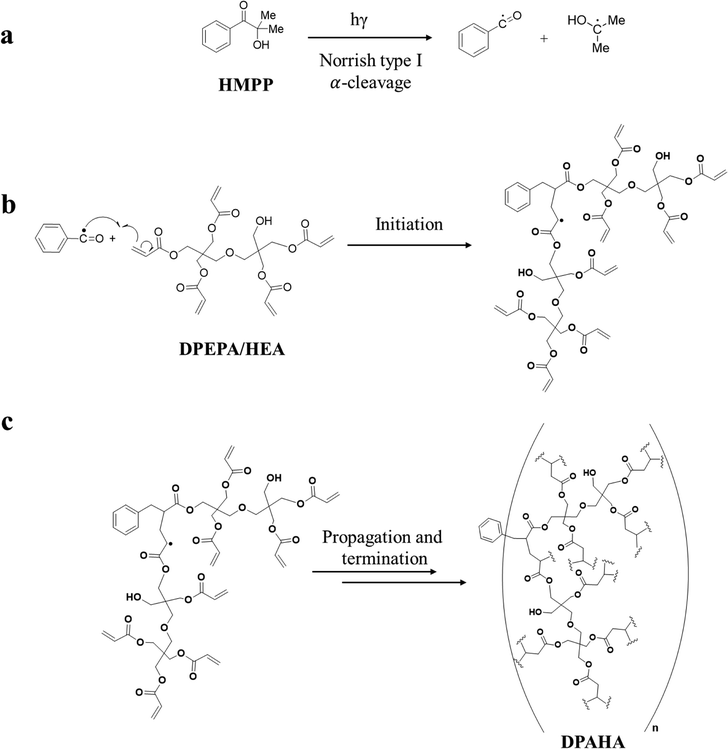
The branched polymer, DPAHA was prepared through a UV-induced photopolymerization process using DPEPA/HEA as the monomer. Initially, 0.1 g of the monomer was dissolved in 2 mL of ethanol, and 20 µL of HMPP was added as the photoinitiator. This mixture was then exposed to 365 nm ultraviolet light for 2 hours to trigger polymerization. After the reaction was complete, the obtained white solid was carefully washed with ethanol to remove any residual reactants and impurities. Finally, the product was dried in an oven at 60 °C for 12 hours, resulting in a clean, dry branched polymer material.2.3. Synthesis of perovskite NCs
Detailed synthetic procedure of pristine CsPbX3 (X = Cl/Br/I) NCs were provided in SI.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 minutes. The supernatant was discarded, and the precipitate was redispersed in toluene.
000 rpm for 10 minutes. The supernatant was discarded, and the precipitate was redispersed in toluene.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 minutes, the supernatant was discarded, and the pellet was redispersed in toluene for further use.
000 rpm for 10 minutes, the supernatant was discarded, and the pellet was redispersed in toluene for further use.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 minutes, the supernatant was removed, and the NCs were redispersed in toluene for further use.
000 rpm for 10 minutes, the supernatant was removed, and the NCs were redispersed in toluene for further use.
2.4. Characterization methods
A spectroflurometer (model: JASCO FP-8500) was used to analyze the PL spectra of the samples. A spectrophotometer (model: JASCO V-770) was used to obtain the UV-Vis spectra of the samples, while a CPL spectrometer (model: CPL-300) was used to obtain CPL spectra, and a CD spectrometer (model: J-1500-150) was used to obtain CD and ORD spectra. TEM images were taken on a JEOL JEM-2100F electron microscope at 200 kV to analyze the morphology and size distribution of the perovskite materials. Samples were prepared on 200-mesh carbon-coated Cu grids using dilute nanowire solutions, which were then allowed to evaporate. Crystallographic analysis of specimens was conducted using an XRD analyzer with Cu Kα radiation. Powder XRD was performed using Cu Kα radiation (λ = 1.5406 Å). The measurements were taken in a 2θ range of 5° to 50° to identify the crystal structure of the perovskite materials. Water contact angle (WCA) measurements were performed using a pendant drop tensiometer (DSA100, KRÜSS, Germany). The elemental valence states within the samples were identified using XPS (Versaprobe II), while the microstructural characteristics of the samples were examined using field-emission SEM (CLARA LMH). FTIR spectroscopy analysis was conducted using a spectrometer (Nicolet iS50). NMR analysis was conducted using a 500 MHz FT-NMR spectrometer.3. Results and discussion
3.1. Synthesis of mechanism of DPAHA and CsPbX3@DPAHA composites
The branched acrylate polymer DPAHA was prepared through a photoinitiated crosslinking of dipentaerythritol penta-/hexa-acrylate (DPEPA/HEA) as the monomer in three steps (Scheme 2 and Fig. S1 and S2). Upon UV irradiation, the photoinitiator HMPP undergoes Norrish type I α-cleavage to generate benzoyl and tert-butyl radicals,28,29 which initiate polymerization through attack on vinyl groups. The multifunctional acrylate structure facilitates extensive branching, while residual vinyl groups enable additional crosslinking, ultimately yielding a rigid three-dimensional network. The experimental details of the synthesis are described in the SI. Solid-state 1H NMR spectroscopy confirms the formation of DPAHA polymer (Fig. S3). The acrylate vinyl peaks at δ = 5.8–6.5 ppm in the DPEPA/HEA monomer vanish completely in DPAHA polymer, demonstrating full consumption of the double bonds.30,31 In the monomer, the signal at δ = 4.2–4.3 ppm, attributed to esterified O–CH2 groups adjacent to the acrylate carbonyl, is absent in the polymer, reflecting the loss of vinyl deshielding and subsequent overlap of these protons with the broad aliphatic resonances of the polymer backbone (He, Hf), while the upfield signals at δ = 3.3–3.6 ppm corresponds to ether/bridge O–CH2 protons of the dipentaerythritol scaffold, reflecting reduced deshielding in the absence of neighboring carbonyl groups. These observations confirm incorporation of ester linkages and preservation of the dipentaerythritol backbone within the cross-linked network. A weak signal around δ ≈ 2.5 ppm is attributed to methylene protons adjacent to residual hydroxyl groups.223.2. Structural characterizations
The CsPbX3@DPAHA NCs were then synthesized via a hot-injection method. In a typical procedure, octadecene (ODE), DPAHA and the corresponding lead halide precursor (PbX2; X = Cl, Br, I) were loaded into a two-neck flask and dried under vacuum at elevated temperature. Subsequently, pre-dried oleylamine (OLA) and oleic acid (OA) were introduced under a nitrogen atmosphere. After complete dissolution of the lead halide salt, the solution temperature was raised, and a preheated Cs-oleate solution in ODE was rapidly injected. The reaction was quenched after a short duration by cooling in an ice-water bath. The resulting NCs were isolated by centrifugation, discarding the supernatant, and redispersed in toluene for further characterization and use. Beyond perovskite NCs, this confinement–polymerization concept can be employed to other luminescent systems such as CdSe quantum dots, rare-earth NCs, or organic emitters for durable and tunable CPL-active materials. We have also tried to synthesize the CsPbBr3@DPAHA via in situ method in two different solvents, ethanol and toluene (Fig. S4). However, perovskite NCs loose the luminescence properties due to degradation of its crystal structure in both the cases. Details of the control experiments were provided in SI.X-ray diffraction (XRD) patterns of pristine and polymer-encapsulated CsPbX3 NCs are shown in Fig. 5a–c. Pristine CsPbCl3 NCs exhibit sharp reflections at 2θ ≈ 15°, 21°, 31°, 35°, and 45°, which can be indexed to the (100), (110), (200), (211), and (220) planes of cubic CsPbCl3, confirming phase-pure crystallinity.32 Upon incorporation into the amorphous DPAHA polymer matrix, the diffraction peaks remain at the same positions, indicating structural retention, but display reduced intensity and slight broadening, consistent with smaller crystallite size and polymer-interactions. Importantly, no secondary phases such as CsCl or PbCl2 are detected. A similar trend is observed for CsPbBr3 NCs, which show intense reflections at 2θ ≈ 15°, 21°, 31°, 35°, 38°, and 45°, corresponding to the (100), (110), (200), (210), (211), and (220) planes of cubic CsPbBr3.33 After polymer encapsulation, the diffraction pattern of CsPbBr3@DPAHA displays broadened and weaker peaks, suggesting a decrease in crystallinity while preserving the perovskite phase. In the case of CsPbI3, multiple reflections are observed at 2θ ≈ 21.5°, 22°, 25.2°, 25.5°, 26°, 27.5°, 29.7°, 31.5°, 32.4°, 37.6°, 39.2°, 41.1°, and 44.2°, characteristic of the black phase. The additional peaks at 9.75° and 13° indicate yellow phase34 is also present along with the black phase. The corresponding CsPbI3@DPAHA composite exhibits identical peak positions, confirming that the polymer encapsulation stabilizes the framework without inducing phase transformation.
Fourier-transform infrared (FTIR) spectroscopy further verified to probe the chemical interactions between CsPbX3 NCs and DPAHA (Fig. S5d). The broad band at 3497 cm−1 is assigned to O–H stretching vibrations, likely arising from residual hydroxyl groups.35 The bands at 2920 and 2850 cm−1 correspond to the asymmetric and symmetric stretching of –CH2 groups in the aliphatic polymer backbone of DPAHA. A strong FTIR peak at 1734 cm−1 is attributed to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching of ester functionalities, confirming successful acrylate polymerization.36 The peak at 1457 cm−1 is ascribed to CH2 bending, whereas the bands at 1155 and 1053 cm−1 arise from C–O–C stretching in ester linkages. The FTIR spectra of both the pristine polymer and the CsPbX3@DPAHA composites exhibit prominent absorption bands at 3497, 2920, 2850, 1734, 1457, 1155, and 1053 cm−1.23 The preservation of these characteristic FTIR peaks of DPAHA indicates that the polymer network remains chemically intact upon NC incorporation. Minor shifts and intensity changes in the C
O stretching of ester functionalities, confirming successful acrylate polymerization.36 The peak at 1457 cm−1 is ascribed to CH2 bending, whereas the bands at 1155 and 1053 cm−1 arise from C–O–C stretching in ester linkages. The FTIR spectra of both the pristine polymer and the CsPbX3@DPAHA composites exhibit prominent absorption bands at 3497, 2920, 2850, 1734, 1457, 1155, and 1053 cm−1.23 The preservation of these characteristic FTIR peaks of DPAHA indicates that the polymer network remains chemically intact upon NC incorporation. Minor shifts and intensity changes in the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–O–C regions suggest coordination between polymer carbonyl groups and surface Pb2+ or Cs+ ions, corroborating effective embedding and interaction of CsPbX3 NCs within the polymer matrix.37
O and C–O–C regions suggest coordination between polymer carbonyl groups and surface Pb2+ or Cs+ ions, corroborating effective embedding and interaction of CsPbX3 NCs within the polymer matrix.37
Scanning electron microscope (SEM) images do not reveal distinct particle morphology due to the small size of the NCs (Fig. S6); however, all constituent elements are clearly confirmed by SEM-energy dispersive spectroscopy (EDS) analysis. The C, O, and N signals in all CsPbX3 NCs originate from (i) ligand capping molecules (oleic acid, oleylamine), which contain long-chain alkyl groups with C, N, and O atoms. These species produce detectable elemental mapping signals along with all-inorganic elemental compositions (Cs, Pb, X) (Fig. S6). The halide/Pb ratios deviated from the ideal value of 3 for pristine NCs (1.92 for Cl, 6.01 for Br, and 3.86 for I), reflecting surface effects (Fig. S7–S9).38 Upon DPAHA modification, the halide/Pb ratios decrease 4.69 (Br) and 2.64 (I), consistent with surface passivation where DPAHA preferentially binds Pb sites, partially displacing surface halides and reducing their detected signal. These results confirm that DPAHA modifies the surface stoichiometry without affecting the bulk composition, highlighting its role in stabilizing the NCs through Pb-site coordination.
Transmission electron microscopy (TEM) was employed to investigate the morphology of the fabricated materials (Fig. 1). Pristine CsPbX3 NCs exhibit a continuous and well-dispersed distribution, indicative of uniform size and arrangement.38 The size distribution histogram is shown in Fig. S10. For CsPbCl3 NCs (a), the particle sizes are centered around 10 nm, with most particles distributed between 9.5 and 11 nm. In the case of CsPbBr3 NCs (b), the histogram peaks near 15 nm, spanning mainly from 14 to 16 nm. For CsPbI3 NCs (c), particle sizes are primarily concentrated around 13 nm, distributed between 12 and 14 nm. Upon encapsulation within DPAHA, the composites adopt a nanosheet-like morphology, in which individual NCs are not distinctly resolved due to their embedding within the continuous polymer network. High-resolution TEM (HR-TEM) was further used to determine the lattice spacings (d-spacings) of the NCs. For both CsPbCl3 and CsPbBr3, the d-spacing remains ≈0.29 nm, corresponding to (002) planes of cubic phase structure in pristine and polymer-encapsulated forms,39 while CsPbI3 and its composite exhibit a slightly larger spacing of ≈0.32 nm.40 These observations confirm that the intrinsic crystal lattice of CsPbX3 NCs is preserved after polymer encapsulation.
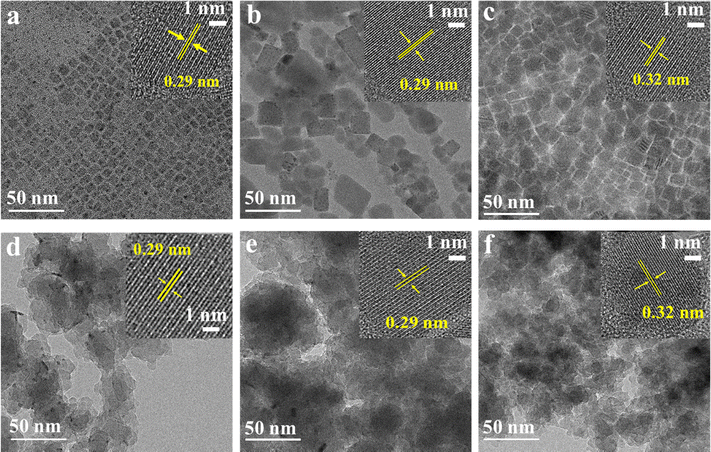 | ||
| Fig. 1 TEM of CsPbX3 NCs and hybrids. TEM images (with HRTEM insets) of (a) CsPbCl3, (b) CsPbBr3, (c) CsPbI3, (d) CsPbCl3@DPAHA, (e) CsPbBr3@DPAHA, and (f) CsPbI3@DPAHA. | ||
3.3. Surface chemical analysis
X-ray photoelectron spectroscopy (XPS) was employed to investigate the surface chemistry of pristine CsPbX3 NCs (X = Cl, Br, I) and their composites (Fig. S11–S13). XPS analysis was conducted on CsPbBr3 NCs and their composites encapsulated with DPAHA to investigate surface chemical states and electronic structure modifications induced by polymer encapsulation. In the C 1s region, pristine CsPbBr3 exhibits peaks at 284.3 eV and 284.9 eV, corresponding to C–C and C–N bonds, respectively, arising from residual surface ligands.41 Upon encapsulation, these peaks shift to 285.3 eV (C–C) and 287.4 eV (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), indicating incorporation of carbonyl functionalities and electron-withdrawing effects from the acrylate matrix. The O 1s spectrum of pristine NCs shows a peak at 530.7 eV, attributed to Pb–O interactions,35 whereas encapsulated NCs display peaks at 533.0 eV and 535.0 eV, assigned to C
O), indicating incorporation of carbonyl functionalities and electron-withdrawing effects from the acrylate matrix. The O 1s spectrum of pristine NCs shows a peak at 530.7 eV, attributed to Pb–O interactions,35 whereas encapsulated NCs display peaks at 533.0 eV and 535.0 eV, assigned to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–O bonds within the polymer, reflecting effective surface passivation. Cs 3d peaks of pristine NCs appear at 723.7 eV (3d5/2) and 737.6 eV (3d3/2), shifting to 725.0 eV and 739.17 eV upon encapsulation, indicative of reduced electron density around Cs+ ions and interfacial interactions with the polymer matrix, accompanied by a slight increase in spin–orbit splitting. Pb 4f spectra of pristine NCs exhibit peaks at 137.8 eV (4f7/2) and 142.7 eV (4f5/2), which shift negatively to 137.2 eV and 142.1 eV after encapsulation, suggesting stabilization of Pb2+ in a relatively more electron-rich environment without altering the oxidation state, as confirmed by unchanged spin–orbit splitting. The Br 3d region displays peaks at 67.9 eV (3d5/2) and 68.9 eV (3d3/2) for pristine NCs, which shift to 68.4 eV and 69.6 eV in the composite, indicating reduced electron density around bromide ions due to electrostatic interactions with polymer functional groups, along with a slight increase in spin–orbit splitting from 1.0 eV to 1.2 eV. Variations in intensity ratios across Cs, Pb, and Br core levels further suggest attenuation of photoelectron signals and partial surface masking by the polymer overlayer. Collectively, these observations demonstrate that polymer encapsulation effectively modifies the surface chemistry of CsPbBr3 NCs, stabilizes the NCs against oxidation, and alters the local electronic environment around Cs+, Pb2+, and Br− ions, highlighting the role of the acrylate matrix in surface passivation and electronic modulation. A detailed analysis of the XPS spectra of CsPbX3 NCs and corresponding composites is provided in the SI.
O and C–O bonds within the polymer, reflecting effective surface passivation. Cs 3d peaks of pristine NCs appear at 723.7 eV (3d5/2) and 737.6 eV (3d3/2), shifting to 725.0 eV and 739.17 eV upon encapsulation, indicative of reduced electron density around Cs+ ions and interfacial interactions with the polymer matrix, accompanied by a slight increase in spin–orbit splitting. Pb 4f spectra of pristine NCs exhibit peaks at 137.8 eV (4f7/2) and 142.7 eV (4f5/2), which shift negatively to 137.2 eV and 142.1 eV after encapsulation, suggesting stabilization of Pb2+ in a relatively more electron-rich environment without altering the oxidation state, as confirmed by unchanged spin–orbit splitting. The Br 3d region displays peaks at 67.9 eV (3d5/2) and 68.9 eV (3d3/2) for pristine NCs, which shift to 68.4 eV and 69.6 eV in the composite, indicating reduced electron density around bromide ions due to electrostatic interactions with polymer functional groups, along with a slight increase in spin–orbit splitting from 1.0 eV to 1.2 eV. Variations in intensity ratios across Cs, Pb, and Br core levels further suggest attenuation of photoelectron signals and partial surface masking by the polymer overlayer. Collectively, these observations demonstrate that polymer encapsulation effectively modifies the surface chemistry of CsPbBr3 NCs, stabilizes the NCs against oxidation, and alters the local electronic environment around Cs+, Pb2+, and Br− ions, highlighting the role of the acrylate matrix in surface passivation and electronic modulation. A detailed analysis of the XPS spectra of CsPbX3 NCs and corresponding composites is provided in the SI.
3.4. Optical behavior and stability
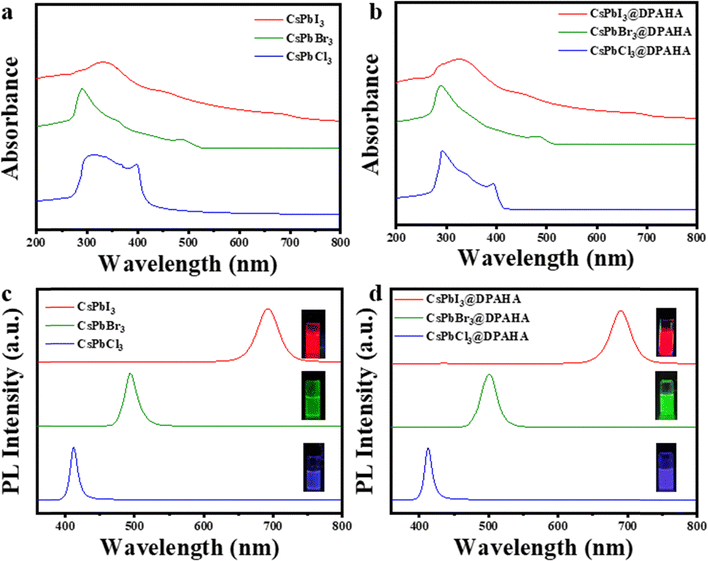
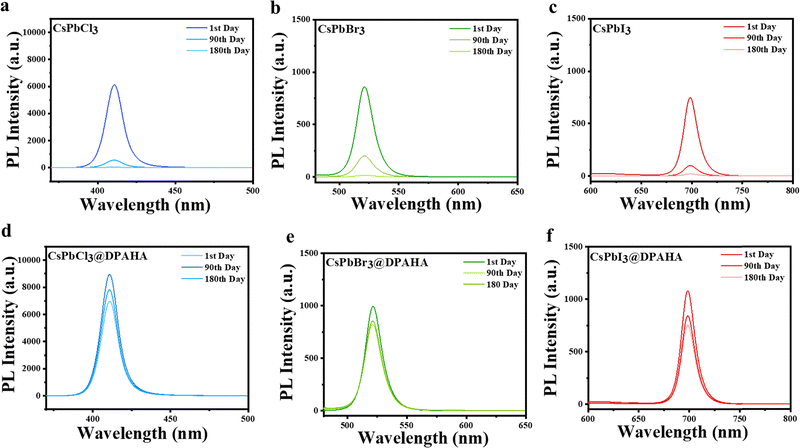
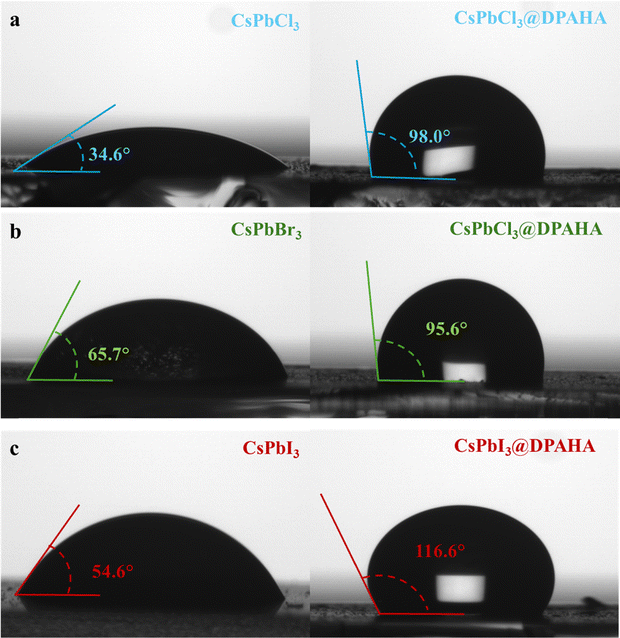
UV-vis absorption and photoluminescence (PL) spectra of the CsPbX3 (X = Cl, Br, I) NCs and their DPAHA-capped composites were measured to assess the influence of the polymer matrix on their optical properties (Fig. 2). Both pristine and CsPbX3@DPAHA exhibit strong fluorescence under UV light (Fig. S14). Pristine CsPbCl3 NCs exhibit an absorption maximum at 397.4 nm, with PL emission at 406 nm, corresponding to a Stokes shift of 8.6 nm; the CsPbCl3@DPAHA composite shows absorption at 393 nm and emission at 402 nm (Stokes shift ≈9 nm). CsPbBr3 NCs display absorption at 483 nm and emission at 492 nm (Stokes shift 9 nm), while the CsPbBr3@DPAHA composite shows 486.4 nm absorption and 495 nm emission (Stokes shift 8.6 nm). CsPbI3 exhibits absorption at 685 nm and emission at 695 nm (Stokes shift 10 nm), and the CsPbI3@DPAHA composite shows 681 nm absorption and 691 nm emission (Stokes shift 10 nm). These Stokes shifts arise from relaxation of excited carriers to band-edge states and potential surface-trap-mediated recombination. The minor spectral shifts after encapsulation indicate that the intrinsic optical properties of CsPbX3 NCs are largely preserved within the DPAHA matrix. The absorbance and photoluminescence excitation (PLE) spectra for both pristine CsPbX3 NCs and their respective polymer composites were shown in Fig. S15. For the pristine NCs, the absorbance maxima are observed at 402 nm (CsPbCl3), 512 nm (CsPbBr3), and 638 nm (CsPbI3). The PLE spectra of pristine and composites show a well-defined peak at a wavelength lower than the absorbance maximum, indicating that the emissive states are selectively excited via higher-energy absorption transitions rather than directly at the absorbance maxima.The stability of the composites was next assessed relative to pristine NCs (Fig. 3). Pristine CsPbCl3 and CsPbI3 degrade almost completely within 180 days, losing emission intensity and exhibiting severe spectral broadening, whereas CsPbBr3 retains 70–80% of its initial PL, reflecting its higher intrinsic stability. In contrast, the DPAHA-modified samples display substantially improved durability: CsPbCl3@DPAHA and CsPbI3@DPAHA preserve about 70–80% and 60–70% of their emission, respectively, while CsPbBr3@DPAHA maintains nearly 90% of its initial intensity with negligible broadening. These findings confirm the pivotal role of DPAHA in mitigating surface degradation, most notably in the iodide system, and establish bromide NCs as the most robust members of the series. The influence of DPAHA on the wettability and stability of CsPbX3 NCs was evaluated through contact angle measurements (Fig. 4 and Table S2). Pristine CsPbCl3, CsPbBr3, and CsPbI3 exhibit contact angles of 34.6°, 65.7°, and 54.6°, respectively, confirming their hydrophilic character and susceptibility to water-induced degradation. After surface modification with DPAHA, the contact angles increased to 98.0°, 95.6°, and 116.6°, corresponding to enhancements of 63.4°, 29.9°, and 62.0°, respectively. These results clearly demonstrate a transformation from hydrophilic to hydrophobic surfaces, with the most pronounced improvements observed for CsPbCl3 and CsPbI3. This hydrophobic polymeric coating forms a protective barrier which significantly enhances the stability of perovskite NCs.
3.5. Chiroptical properties of DPAHA and CsPbX3@DPAHA composites
To confirm the induction of chirality, circular dichroism (CD) and optical rotatory dispersion (ORD) measurements were performed (Fig. 5). The UV-vis absorption spectrum of DPAHA exhibits a sharp peak around 282 nm, which is characteristic of π → π* transitions associated with the acrylate C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds in the polymer backbone (Fig. S16a). Although these acrylate groups are not extensively conjugated, their localized π electrons absorb strongly in the near-UV region. Additionally, a broad, weak absorption tail extending from approximately 350 to 800 nm is observed, likely arising from n → π* transitions of the carbonyl (C
C double bonds in the polymer backbone (Fig. S16a). Although these acrylate groups are not extensively conjugated, their localized π electrons absorb strongly in the near-UV region. Additionally, a broad, weak absorption tail extending from approximately 350 to 800 nm is observed, likely arising from n → π* transitions of the carbonyl (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) groups in the acrylate esters.42 These n → π* transitions are inherently weaker than the π → π* transitions, giving rise to a low-intensity shoulder in the longer-wavelength UV-vis region. Overall, the spectrum is dominated by the π → π* absorption at ∼282 nm, with the higher-wavelength tail reflecting weaker carbonyl-related transitions. The CD spectrum of DPAHA correlates closely with its UV-vis absorption features (Fig. S16b) and it is CPL-inactive (Fig. S16c). The pronounced CD signal at 347 nm corresponds to the strong π → π* absorption of the acrylate C
O) groups in the acrylate esters.42 These n → π* transitions are inherently weaker than the π → π* transitions, giving rise to a low-intensity shoulder in the longer-wavelength UV-vis region. Overall, the spectrum is dominated by the π → π* absorption at ∼282 nm, with the higher-wavelength tail reflecting weaker carbonyl-related transitions. The CD spectrum of DPAHA correlates closely with its UV-vis absorption features (Fig. S16b) and it is CPL-inactive (Fig. S16c). The pronounced CD signal at 347 nm corresponds to the strong π → π* absorption of the acrylate C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds in the polymer backbone. The significant difference between the UV-vis absorption peak (∼282 nm) and the CD peak (∼347 nm) arises because UV-vis measures total light absorption by a chromophore, while CD specifically detects the difference in absorption of left- and right-circularly polarized light, which depends on the chiral environment. In the case of DPAHA, although both peaks originate from π → π* transitions of the acrylate C
C double bonds in the polymer backbone. The significant difference between the UV-vis absorption peak (∼282 nm) and the CD peak (∼347 nm) arises because UV-vis measures total light absorption by a chromophore, while CD specifically detects the difference in absorption of left- and right-circularly polarized light, which depends on the chiral environment. In the case of DPAHA, although both peaks originate from π → π* transitions of the acrylate C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C groups, the helical chiral conformation of the polymer perturbs these electronic transitions, causing the CD signal to appear at a different (red-shifted) wavelength. The weaker, broad CD signal extending into the 350–800 nm region aligns with the low-intensity n → π* absorption tail of the carbonyl (C
C groups, the helical chiral conformation of the polymer perturbs these electronic transitions, causing the CD signal to appear at a different (red-shifted) wavelength. The weaker, broad CD signal extending into the 350–800 nm region aligns with the low-intensity n → π* absorption tail of the carbonyl (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) groups, reflecting subtle chiral perturbations in the carbonyl electronic transitions. Thus, the CD spectrum mirrors the main π → π* absorption peak and the weaker carbonyl-related transitions, providing insight into the chiroptical activity of both the acrylate and ester functional groups in DPAHA.
O) groups, reflecting subtle chiral perturbations in the carbonyl electronic transitions. Thus, the CD spectrum mirrors the main π → π* absorption peak and the weaker carbonyl-related transitions, providing insight into the chiroptical activity of both the acrylate and ester functional groups in DPAHA.
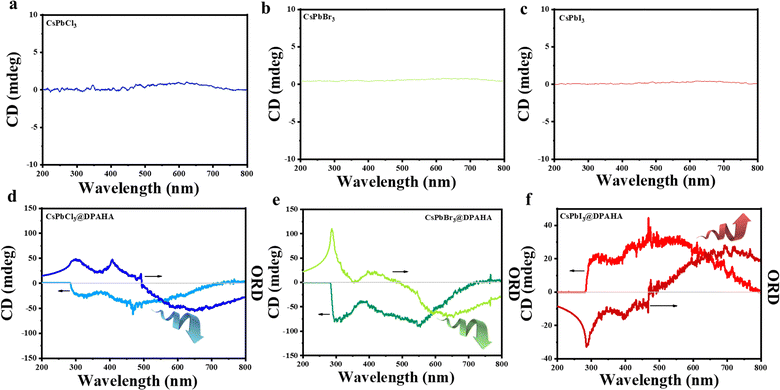
Pristine CsPbX3 (X = Cl, Br, I) NCs exhibit no detectable CD signals, consistent with their achiral nature (Fig. 5b–d). Upon encapsulation within the giant three-dimensional polymer structure of DPAHA, the CsPbX3@DPAHA composites display broad CD responses, with peaks at ≈468 nm (CsPbCl3, left-handed rotation), ≈554 nm (CsPbBr3, left-handed rotation), and ≈560 nm (CsPbI3, right-handed rotation), slightly shifted relative to the corresponding absorption maxima (Fig. 5e–g). The opposite rotational sense observed for CsPbI3 likely arises from subtle differences in surface structure, lattice parameters, and exciton–polymer interactions, which alter the sign of the electronic transition dipole moments under the chiral field of DPAHA. All polymer-encapsulated NCs exhibit an additional CD feature at 315–330 nm, absent in the pristine NCs, originating from π → π* electronic transitions in DPAHA. The CD peaks are broad in all cases due to helical chiral DPAHA which has also very broad CD signals, which create a distribution of chiral microenvironments and slightly different excitonic transition energies. Interestingly, ORD spectra of all composites show a prominent peak around 492 nm, irrespective of halide composition. The ORD peak arises from the same excitonic transitions responsible for the CD signals, as CD and ORD are intrinsically related through the Kramers–Kronig relations; the wavelength-dependent optical rotation observed in ORD corresponds to the integrated differential absorption measured in CD. ORD and CD spectra are intrinsically linked because both arise from the same electronic transitions in chiral molecules. Their relationship is formally expressed by the reciprocal Kramers–Kronig relation, which connects the dispersive and absorptive components of optical activity.
The consistent ORD peak at 492 nm indicates the dominant influence of the polymer-induced chiral field on the perovskite excitons, providing a complementary verification of induced chirality (Fig. 5e–g). The combination of CD and ORD measurements is therefore critical, as CD probes the magnitude and sign of chiroptical absorption while ORD provides information on the dispersive optical rotation, together offering a comprehensive understanding of the chiral electronic environment.
The exciton–chiral coupling parameter (k) quantifies the degree of chirality transfer from DPAHA to CsPbX3 NCs.43
The sign and magnitude of k determine the chiroptical response: k > 0 corresponds to a CD signal of one handedness (e.g., right-handed), k < 0 corresponds to the CD signal of opposite handedness (e.g., left-handed), and k = 0 indicates no CD signal.
Pristine CsPbX3 NCs are intrinsically achiral. Their exciton transitions are primarily electric-dipole allowed (µ ≠ 0), but the magnetic transition dipole (m) is negligible or randomly oriented, leading to a near-zero exciton–chiral coupling parameter, k ≈ 0, and consequently, there is no detectable CD. In contrast, the chiral polymer DPAHA possesses helicity that impose a fixed spatial arrangement on the electric and magnetic transition dipoles of the acrylate or ester chromophores, generating a nonzero kDPAHA and an intrinsic CD signal. Upon encapsulation of NCs within DPAHA, the polymer's chiral field perturbs the exciton states of the NCs: the electric dipoles (µNC) partially align with the polymer-induced chiral environment, and a magnetic dipole component (mNC) is induced via spin–orbit coupling and exciton–phonon or exciton–polymer interactions. This structured alignment produces a finite, nonzero exciton–chiral coupling parameter (kNC@DPAHA), whose magnitude reflects the efficiency of chirality transfer, and whose sign determines the handedness of the induced CD: CsPbCl3 and CsPbBr3 display k > 0 (right-handed rotation), whereas CsPbI3 exhibits k < 0 (left-handed rotation), likely due to subtle differences in surface reconstruction, lattice parameters, and exciton–polymer interactions. While pristine NCs are achiral and show no CD, DPAHA provides an intrinsic chiral scaffold, and the encapsulated NCs acquire a finite k, demonstrating effective transfer of chiral information from the polymer to the excitonic states of the perovskite NCs, thereby generating well-defined, handed chiroptical activity.
Beyond the local intra-exciton chirality captured by k, inter-excitonic interactions between coupled chromophores or excitons near chiral interfaces can further enhance or modulate the CD signal. The interaction energy between two transition dipoles, such as excitonic states at the perovskite–polymer interface, is given by the dipole–dipole coupling formula:43,44
µ
1 and µ2 are the transition dipole moments of interacting excitons, and r is the displacement vector between them. In chiral environments, this interaction becomes chiroptically active, and the differential CD signal intensity is proportional to the triple scalar product:
This term captures the chiral arrangement of interacting dipoles: a nonzero triple product indicates non-coplanar dipoles in a chiral geometry, consistent with the helical organization imposed by DPAHA. Thus, both the local exciton–chiral coupling parameter k and the long-range exciton–exciton coupling V12 contribute to the observed chiroptical activity, enabling enhanced and tunable CPL across the visible spectrum.
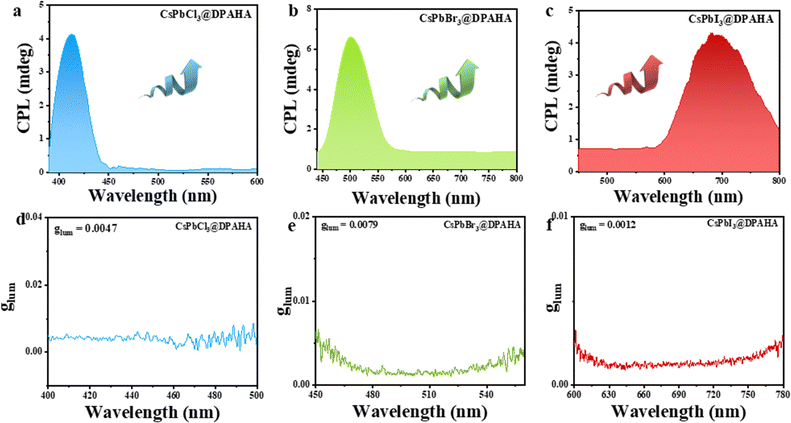
CPL measurements further investigated the chiroptical properties of both pristine and polymer-encapsulated CsPbX3 (X = Cl, Br, I) NCs (Fig. 6). Pristine CsPbCl3, CsPbBr3, and CsPbI3 NCs show no CPL (Fig. S17), while CsPbCl3@DPAHA, CsPbBr3@DPAHA, CsPbI3@DPAHA display strong CPL at 412, 501, and 689 nm, respectively, all right-handed. These results demonstrate that the DPAHA polymer matrix induce CPL activity across all halide compositions by breaking symmetry at the NC surface and lattice through asymmetric confinement. Interestingly, CsPbI3@DPAHA exhibits a left-handed CD signal but a right-handed CPL signal, indicating CD–CPL inversion. In contrast, CsPbBr3@DPAHA and CsPbCl3@DPAHA show consistent handedness across CD and CPL, reflecting differences in lattice rigidity and exciton dynamics. The smaller halides (Br−, Cl−) produce more rigid lattice frameworks (∼0.29 nm spacing), resulting in similar perturbations of the ground- and excited-state transition dipoles under the chiral polymer field, so the handedness of CPL emission aligns with the CD signal. By comparison, CsPbI3@DPAHA contains the larger I− ion, which leads to an expanded lattice (∼0.32 nm), higher polarizability, and altered exciton relaxation pathways. These structural and electronic differences, together with stronger asymmetric surface interactions from the polymer, result in a decoupling of ground- and excited-state chiroptical responses, causing the CPL emission to exhibit right-handed rotation while the ground-state CD signal remains left-handed. In the ground state, the CD signal arises from the perturbation of the electronic transition dipoles of the CsPbI3 NCs by the chiral electric fields imposed by the helicoidal DPAHA polymer. This results in a left-handed induced CD response, reflecting the sign of the chiral perturbation acting on the band-edge absorption transition. However, upon photoexcitation, the exciton in iodide-based perovskites undergoes substantial lattice relaxation due to their large spin–orbit coupling, soft lattice, and strong exciton–phonon interactions. This excited-state reconstruction alters the relative orientation between the electric (µ) and magnetic (m) transition dipole moments that determine the CPL sign. In CsPbI3, the excited-state structural relaxation slightly reorients the magnetic dipole moment such that the resulting µ·m product acquires the opposite sign from that in the ground state, leading to a right-handed CPL signal despite a left-handed CD signal.14,45–49 Thus, while all polymer-encapsulated NCs exhibit CPL activity, the CD–CPL inversion is unique to CsPbI3 due to its distinct lattice, exciton dynamics, and enhanced polymer-induced asymmetry. CPL and CPL-optical rotatory dispersion (CPL-ORD) are closely related chiroptical phenomena that provide complementary insights into excited-state chirality. While CPL reports the asymmetry in emission intensity, CPL-ORD captures the dispersive response, revealing subtle interactions between the chiral polymer environment and the excitonic states of the NCs. In our system, the CPL-ORD peaks closely match the CPL maxima (e.g., 409, 500, and 686 nm for CsPbCl3@DPAHA, CsPbBr3@DPAHA, and CsPbI3@DPAHA, respectively) (Fig. S18), confirming that the induced polymer chirality uniformly influences both the intensity and rotational properties of the emitted light. To quantitatively evaluate the degree of chiral emission, the luminescence dissymmetry factor (glum) was calculated for all samples following the equation:6
| IR = intensity of right-circularly polarized emitted light (detected at the receiver) |
| IL = intensity of left-circularly polarized emitted light. |
Polymer-encapsulated composites showed markedly enhanced glum values: CsPbBr3@DPAHA reached 0.0079, CsPbCl3@DPAHA 0.0047, and CsPbI3@DPAHA 0.0012. The dramatic enhancement in CPL activity can be fundentally attributed to the asymmetric confinement and interfacial surface interactions imposed by the helical polymer matrix DPAHA on the intrinsically achiral perovskite NCs. The helical architecture of DPAHA forms non-centrosymmetric nanoscale cavities that impose a chiral spatial arrangement on the encapsulated NCs. This confinement breaks the inherent structural symmetry of the NCs, thereby enabling the induction of chirality in their electronic excited states, which is critical for CPL emission. At the molecular level, the polymer's chiral environment perturbs the excitonic states of the perovskite NCs by altering the orientation and coupling of their electric and magnetic transition dipole moments. The interfacial coupling between the perovskite NC surface atoms and the polymer's ester and acrylate groups facilitates chiral exciton–phonon and exciton–polymer interactions, which induce a finite magnetic dipole transition moment in the NCs. This magnetic dipole component, coupled with the electric dipole transitions, produces nonzero rotational strength for the excited states, thus promoting chiral radiative recombination pathways that manifest as enhanced CPL signals. Furthermore, the hierarchical helical structure of DPAHA provides a supramolecular chiral field that extends beyond individual molecules, ensuring that the induced chirality is not localized but rather collectively propagated through the composite material. This cooperative effect amplifies the chiral asymmetry of the luminescent states, further boosting glum. The luminescence dissymmetry factors (glum) of our composites reach 0.0079 (CsPbBr3@DPAHA), significantly higher than reported polymer-based systems (typically 10−4–10−3).18,19
The variation in the luminescence dissymmetry factors (glum) among the DPAHA-encapsulated perovskite NCs, CsPbBr3@DPAHA (0.0079), CsPbCl3@DPAHA (0.0047), and CsPbI3@DPAHA (0.0012) can be attributed to fundamental differences in their structural and electronic properties induced by halide substitution. These differences influence key parameters such as exciton binding energy, lattice constants, and surface chemistry, all of which play critical roles in governing the extent of chiral exciton coupling within the helical DPAHA matrix. CsPbBr3@DPAHA composite, which exhibit intermediate bandgaps (∼2.55 eV) and lattice constants, are particularly well-suited for effective exciton–polymer coupling. The balance between strong optical transitions and moderate dielectric screening facilitates efficient transfer of chirality from the DPAHA host to the perovskite excitons, resulting in the highest glum among the three compositions. In contrast, CsPbCl3@DPAHA composite possesses a wider bandgap (∼3.15 eV) and a more compact lattice, which may reduce orbital overlap and exciton delocalization, thus limiting the strength of exciton–chiral field interactions and yielding a slightly lower glum. On the other end of the spectrum, CsPbI3@DPAHA composite exhibits the smallest bandgap (∼1.82 eV) and a more ionic, polarizable lattice structure. While this enhances light absorption in the red region, the softer lattice and higher defect density typically associated with iodide-based perovskites can weaken the exciton–polymer interface and reduce the coherence of chiral excitonic states, leading to a lower glum. Moreover, subtle variations in surface termination and ligand binding across the halide series further modulate the degree of chiral perturbation experienced by the NCs. These observations indicate the importance of halide composition as a tunable parameter for modulating chiroptical performance in polymer-encapsulated perovskites, offering a pathway toward customized CPL materials across the visible spectrum.
Moreover, the composites retain up to ∼90% of their emission intensity after 180 days, whereas ligand-stabilized analogues typically degrade within weeks.50,51 The dense polymer network acts as a physical barrier against environmental degradation pathways, including moisture ingress and oxidative damage. Simultaneously, the rigid helical polymer prevents NC aggregation and surface defect formation, which are common causes of non-radiative recombination and photoluminescence loss. These results demonstrate the dual advantages of confinement-induced chirality and long-term stability, positioning DPAHA-encapsulated perovskites as robust and practical CPL-active emitters.
To evaluate the generality of the chiral confinement effect of DPAHA, we performed additional experiments using rhodamine B, a widely used achiral organic dye as a model emitter incorporated into the same DPAHA polymer matrix. Interestingly, under identical polymerization and measurement conditions, no detectable CPL signal was observed for rhodamine B@DPAHA (Fig. S19). This result suggests that the chiral confinement provided by the DPAHA network is not universally transferable to all emitters and that efficient chirality induction likely requires specific types of excitonic states or stronger electronic coupling between the chiral host and the emissive species. Hence, the judicious design of chiral molecules is necessary to induce strong and stable CPL activity in achiral fluorophores.
4. Conclusion
In summary, we have demonstrated a confinement-driven strategy to simultaneously stabilize and induce chirality in intrinsically achiral perovskite NCs by encapsulating them within a photopolymerized branched acrylate network (DPAHA). The rigid, helicoidal polymer framework creates non-centrosymmetric hydrophobic cavities that impose asymmetric spatial confinement, thereby breaking inversion symmetry and enabling pronounced circular dichroism and CPL activity across the visible spectrum. Unlike ligand- or supramolecular-based approaches, this strategy provides long-term stability exceeding six months, demonstrating the dual role of DPAHA as both a protective scaffold and a chiral inducer. Importantly, compositional tuning of CsPbX3 (X = Cl, Br, I) within the polymer matrix not only allows spectral control but also enables inversion of CPL handedness, showcasing the versatility of confinement engineering for tailoring excited-state chirality. By uniting stability enhancement and chiroptical functionality in a single platform, this work advances a robust design principle for CPL-active perovskite emitters. More broadly, it establishes photopolymerized crystalline polymers as a generalizable route for transforming achiral luminescent nanomaterials into durable, tunable chiral light sources, opening pathways toward practical integration in chiral optoelectronic and quantum photonic devices.Conflicts of interest
The authors declare no conflict of interest.Data availability
The data supporting this article have been included as part of the supplementary information (SI). The supplementary information contains additional experimental details, characterization data, and supporting figures. See DOI: https://doi.org/10.1039/d5mh02011e.Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant (grant no: RS-2025-24683196, BrainLink RS-2023-00236798, and 2021R1A2C1006113).References
- M. Yuan, X. Wen, X. Jiang, L. Polavarapu, G. Zhou and X. Hu, Laser Photon. Rev., 2025, 02178, 1–23 Search PubMed.
- D.-W. Zhang, M. Li and C.-F. Chen, Chem. Soc. Rev., 2020, 49, 1331–1343 RSC.
- R. Chowdhury, M. D. Preuss, H.-H. Cho, J. J. P. Thompson, S. Sen, T. K. Baikie, P. Ghosh, Y. Boeije, X. W. Chua, K.-W. Chang, E. Guo, J. van der Tol, B. W. L. van den Bersselaar, A. Taddeucci, N. Daub, D. M. Dekker, S. T. Keene, G. Vantomme, B. Ehrler, S. C. J. Meskers, A. Rao, B. Monserrat, E. W. Meijer and R. H. Friend, Science, 2025, 387, 1175–1181 CrossRef CAS PubMed.
- P. Stachelek, L. MacKenzie, D. Parker and R. Pal, Nat. Commun., 2022, 13, 553 CrossRef CAS PubMed.
- Y. Lu, Q. Wang, L. Han, Y. Zhao, Z. He, W. Song, C. Song and Z. Miao, Adv. Funct. Mater., 2024, 34, 1–30 Search PubMed.
- D. Das, Y. Park, S. Mal, K. Kyhm, R. A. Taylor, A. Jana and S. Cho, Adv. Funct. Mater., 2025, e14790 CrossRef.
- H. Liu, A. S. Portniagin, B. Tang, K. Vighnesh, Y. Li, Y. Wu, D. A. Rusanov, L. Ke, Y. Wang, D. Zhu, D. Chen, K.-C. Law, M. V. Babak, E. Ushakova and A. L. Rogach, ACS Nano, 2025, 19, 17774–17784 CrossRef CAS PubMed.
- C. He, Z. Li, H. Jiang, S. Luo, W. Zhang and X. Qin, Adv. Mater., 2025, 07400, 1–26 Search PubMed.
- S. Basu and N. Amdursky, ACS Nano, 2025, 19, 33717–33733 CrossRef CAS PubMed.
- Y. H. Kim, Y. Zhai, E. A. Gaulding, S. N. Habisreutinger, T. Moot, B. A. Rosales, H. Lu, A. Hazarika, R. Brunecky, L. M. Wheeler, J. J. Berry, M. C. Beard and J. M. Luther, ACS Nano, 2020, 14, 8816–8825 CrossRef CAS PubMed.
- D. Han, G. Chen and L. Peng, Chem. Synth., 2024, 75 CAS.
- S. Liu, X. Liu, Y. Wu, D. Zhang, Y. Wu, H. Tian, Z. Zheng and W.-H. Zhu, Matter, 2022, 5, 2319–2333 CrossRef CAS.
- H. Yamamoto, T. Inagaki, J. Park, S. Yoshida, K. Kaneko, T. Hanasaki and K. Akagi, Macromolecules, 2021, 54, 8977–8986 CrossRef CAS.
- K. Fu and G. Liu, ACS Nano, 2024, 18, 2279–2289 CrossRef CAS PubMed.
- M. Zhou, Y. Sang, X. Jin, S. Chen, J. Guo, P. Duan and M. Liu, ACS Nano, 2021, 15, 2753–2761 CrossRef CAS PubMed.
- J. Liang, P. Guo, X. Qin, X. Gao, K. Ma, X. Zhu, X. Jin, W. Xu, L. Jiang and P. Duan, ACS Nano, 2020, 14, 3190–3198 CrossRef CAS PubMed.
- B. Zhao, X. Gao, K. Pan and J. Deng, ACS Nano, 2021, 15, 7463–7471 CrossRef CAS PubMed.
- Y. Shi, P. Duan, S. Huo, Y. Li and M. Liu, Adv. Mater., 2018, 30, 1–7 Search PubMed.
- P. Liu, W. Chen, Y. Okazaki, Y. Battie, L. Brocard, M. Decossas, E. Pouget, P. Müller-Buschbaum, B. Kauffmann, S. Pathan, T. Sagawa and R. Oda, Nano Lett., 2020, 20, 8453–8460 CrossRef CAS PubMed.
- X. Zhu, H. Dong, J. Chen, J. Xu, Z. Li, F. Yuan, J. Dai, B. Jiao, X. Hou, J. Xi and Z. Wu, Adv. Funct. Mater., 2022, 32, 1–8 CAS.
- Y. Tang, X. Zhang, K. Liao, L. Qiu, N. Du, L. Xiao, J. Ma, B. Wu, Z. Wu and G. Wang, Adv. Opt. Mater., 2025, 13, 1–10 CAS.
- X. Jin, K. Ma and H. Gao, J. Am. Chem. Soc., 2022, 144, 20411–20420 CrossRef CAS PubMed.
- M. Dhar, A. Das, D. Parbat and U. Manna, Angew. Chem., Int. Ed., 2022, e202116763 CAS.
- A. Shome, A. Das, A. Borbora, M. Dhar and U. Manna, Chem. Soc. Rev., 2022, 51, 5452–5497 RSC.
- M. Chen, M. Zhong and J. A. Johnson, Chem. Rev., 2016, 116, 10167–10211 CrossRef CAS PubMed.
- S. Huang, H. Yu and Q. Li, Adv. Sci., 2021, 8, 1–20 Search PubMed.
- X. Cheng, T. Miao, Y. Qian, Z. Zhang, W. Zhang and X. Zhu, Int. J. Mater. Sci., 2020, 21, 6186 CAS.
- R. Al Nuumani, G. Bolognesi and G. T. Vladisavljević, Langmuir, 2018, 34, 11822–11831 CrossRef CAS PubMed.
- Y. Sanai, S. Kagami and K. Kubota, Polym. J., 2020, 52, 375–385 CrossRef CAS.
- R. Wang, F. Damanik, T. Kuhnt, A. Jaminon, S. Hafeez, H. Liu, H. Ippel, P. J. Dijkstra, N. Bouvy, L. Schurgers, A. T. ten Cate, A. Dias, L. Moroni and M. B. Baker, Adv. Healthcare Mater., 2023, 12, 1–13 Search PubMed.
- C. F. Yu, S. P. Rwei and Y. C. Shu, RSC Adv., 2023, 13, 2331–2338 RSC.
- S. Cho, S. Kim, J. Kim, Y. Jo, I. Ryu, S. Hong, J.-J. Lee, S. Cha, E. B. Nam, S. U. Lee, S. K. Noh, H. Kim, J. Kwak and H. Im, Light: Sci. Appl., 2020, 9, 156 CrossRef PubMed.
- M. Pegu, H. Roshan, C. Otero-Martínez, L. Goldoni, J. Zito, N. Livakas, P. Rusch, F. De Boni, F. Di Stasio, I. Infante, L. De Trizio and L. Manna, ACS Energy Lett., 2025, 10, 2268–2276 CrossRef CAS PubMed.
- F. Ke, C. Wang, C. Jia, N. R. Wolf, J. Yan, S. Niu, T. P. Devereaux, H. I. Karunadasa, W. L. Mao and Y. Lin, Nat. Commun., 2021, 12, 461 CrossRef CAS PubMed.
- A. Jana and K. S. Kim, ACS Energy Lett., 2018, 3, 2120–2126 CrossRef CAS.
- E. Yara-Varón, J. Eras Joli, M. Balcells, M. Torres and R. Canela-Garayoa, RSC Adv., 2012, 2, 9230 RSC.
- Sanjayan C. G., J. Mannekote Shivanna, J. D. Schiffman, S. Mohan, S. Budagumpi and R. G. Balakrishna, ACS Appl. Mater. Interfaces, 2022, 14, 38471–38482 CrossRef CAS PubMed.
- A. Jana, Q. Ba and K. S. Kim, Adv. Funct. Mater., 2019, 29, 1900966 CrossRef.
- L. C. Schmidt, A. Pertegás, S. Gonzalez-carrero, O. Malinkiewicz, S. Agouram, G. M. Espallargas, H. J. Bolink, R. E. Galian, J. Pérez-prieto and S. González-carrero, J. Am. Chem. Soc., 2014, 136, 850–853 CrossRef CAS PubMed.
- S. Ma, S. H. Kim, B. Jeong, H. Kwon, S. Yun, G. Jang, H. Yang, C. Park, D. Lee and J. Moon, Small, 2019, 15, 1–12 Search PubMed.
- C. Rocks, V. Svrcek, P. Maguire and D. Mariotti, J. Mater. Chem. C, 2017, 5, 902–916 RSC.
- R. W. Newberry and R. T. Raines, Acc. Chem. Res., 2017, 50, 1838–1846 CrossRef CAS PubMed.
- N. S. S. Nizar, M. Sujith, K. Swathi, C. Sissa, A. Painelli and K. G. Thomas, Chem. Soc. Rev., 2021, 50, 11208–11226 RSC.
- T. N. Mandal, S. Mal, D. Das, S. Cho and A. Jana, Mater. Today Phys., 2025, 59, 101885 CrossRef CAS.
- Y. Ru, B. Zhang, X. Yong, L. Sui, J. Yu, H. Song and S. Lu, Adv. Mater., 2023, 35, 2207265 CrossRef CAS PubMed.
- K. Fu, D. H. Qu and G. Liu, J. Am. Chem. Soc., 2024, 146, 33832–33844 CrossRef CAS PubMed.
- S. Zheng and G. Liu, CCS Chem., 2024, 6, 2198–2209 CrossRef CAS.
- Z. Long, S. Zheng, W. Zhou and G. Liu, Chem. Commun., 2024, 60, 9054–9057 RSC.
- K. Horie, Y. Fujita, K. Kaneko, T. Hanasaki and K. Akagi, ACS Appl. Mater. Interfaces, 2025, 17, 15988–15999 CrossRef CAS PubMed.
- X. Jin, K. Ma, J. Chakkamalayath, J. Morsby and H. Gao, ACS Energy Lett., 2022, 7, 610–616 CrossRef CAS.
- X. Yang, C. Valenzuela, X. Zhang, Y. Chen, Y. Yang, L. Wang and W. Feng, Matter, 2023, 6, 1278–1294 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2026 |