Stick to the beads: supercharging medicinal chemistry and methodology development with ChemBeads
Noah P.
Tu
 * and
Ying
Wang
* and
Ying
Wang
AbbVie Inc., 1 N. Waukegan Rd, North Chicago, Illinois 60064, USA. E-mail: noahtu@hotmail.com
First published on 16th October 2025
Abstract
This perspective explores the development, preparation, and widespread application of ChemBeads, a solid reagent delivery platform designed to overcome longstanding challenges in miniaturized and automated chemical experimentation. Originating from innovation at AbbVie, ChemBeads are formed by dry-coating active reagents onto inert carrier beads, transforming poorly flowing powders into uniform, flowable materials compatible with robotic and manual dispensing. Enabled by resonant acoustic mixing (RAM) or alternative techniques like vortex mixing, ChemBeads have streamlined high-throughput experimentation (HTE) and medicinal chemistry workflows. Their applications span a wide range of transformations including photoredox catalysis, cross-electrophile coupling, C–N and C–H functionalizations and late-stage oxidations. Industrial and academic case studies highlight the critical role of ChemBeads in accelerating the development of new synthetic methodologies that would have otherwise taken significantly longer to accomplish. By solving the long standing problem of material handling at a miniaturized scale with efficiency and generality, ChemBead technology formed the foundation of the AbbVie Discovery HTE platform and positioned us as one of the industry leaders in this field. Over the years, we expanded the technology to make it greatly accessible to academic institutions by making the process economically and operationally friendly. In parallel, we extended this technology in other areas, which ultimately promoted industry–academia collaborations. The technology's expansion into biocatalysis (EnzyBeads), solubility assays, and solid form screening further demonstrates its adaptability.
Solid dispensing challenges in modern drug discovery
The evolution of drug discovery has brought about an increasing demand for speed, precision, and scalability in chemical synthesis. A major driver behind this trend is the growing reliance on HTE, structure–activity relationship studies, and direct-to-biology—all of which require reliable, miniaturized workflows capable of performing thousands of reactions in parallel at sub-milligram scales. Under these settings, the ability to dispense small quantities of reagents accurately and reproducibly becomes a key enabler of innovation.While liquid-handling technologies have seen significant advances, including robust pipetting systems and nanolitre-scale droplet generators, the dispensing of solid reagents remains a persistent bottleneck. Traditional solid dosing methods—including manual weighing, spatula-based transfer, or pre-made stock solutions—are labour-intensive, error-prone, and incompatible with automation at microgram scales.1,2 The pharmaceutical industry has widely embraced miniaturization and parallelization to accelerate development timelines and reduce material costs. However, solid reagents introduce unique challenges due to their variable bulk densities, heterogeneous particle sizes, electrostatic properties, and hygroscopicity. These factors hinder consistent material transfer, often resulting in dose variability, cross-contamination, and data irreproducibility, all of which undermine experimental integrity and limit the scalability of high-throughput platforms.
These challenges are not limited to synthetic reactions. In preclinical formulation screening, excipient compatibility studies, thermodynamic solubility profiling and solid form screening, researchers often require solid dosage forms or neat powders to generate representative data.3,4 Yet, few dispensing tools exist that match the precision, automation compatibility, and throughput achieved in liquid-handling workflows.
To overcome these limitations, ChemBeads were developed as a novel solid-handling technology.2,5 Based on coating active reagents onto chemically inert glass beads, ChemBeads transform poorly flowing powders into uniform, flowable, and robotically compatible forms. This innovation enables nanomole to milligram-scale dispensing with high accuracy and reproducibility, making ChemBeads a powerful enabling platform for modern automated and miniaturized experimentation in both academic and industrial drug discovery environments.
This perspective examines the technological underpinnings and automation-enabling features of ChemBeads, highlighting its diverse applications across medicinal chemistry and new chemistry method development. Case studies from industrial R&D settings, notably at AbbVie, demonstrate the scalable deployment of ChemBeads and suggest their usefulness for improving workflows in pharmaceutical R&D.
The pre-ChemBead era: challenges in solid reagent dispensing
Accurate, reproducible dosing is essential in high-throughput experimentation (HTE), where even minor discrepancies in reagent amounts can lead to failed reactions or misleading outcomes.6 Traditional techniques for solid handling lacked the speed, precision and scalability necessary for modern drug discovery.7 As miniaturized synthesis and parallel experimentation gained traction, the demand for reliable microscale solid handling grew. Prior to the introduction of ChemBeads, the microscale handling of solid reagents posed a persistent challenge. Most laboratories relied on manual weighing using microbalances or resorted to the labour-intensive preparation of stock solutions for solid-phase reagents. These methods, while familiar, were inherently inefficient and prone to variability, particularly when working with hygroscopic, air-sensitive, or electrostatically charged materials. Automation solutions were largely confined to liquid handling systems, which necessitated either the use of pre-dissolved solid reagents or the exclusion of poorly soluble compounds. This constraint often required the use of toxic, volatile solvents, introducing safety concerns and potentially compromising reaction scope and compatibility.7To address this, early attempts at automating solid dispensing included precision powder-handling systems developed by manufacturers such as Chemspeed, Mettler-Toledo, Sirius Automation and Unchained Lab.8 While these instruments offered enhanced accuracy, their cost, maintenance demands, and substantial physical footprint limited adoption, particularly in smaller or resource-constrained laboratories.
Other innovations, such as slurry-based dosing, wax encapsulation,9 and capsule and tablet methods10 provided incremental improvements but were hindered by issues with inconsistent particle distribution, clogging, and degradation of sensitive reagents during storage or transfer.8
Origins of ChemBeads and innovation at AbbVie
In early 2017, we, the Advanced Chemistry Technology (ACT) group at AbbVie Discovery, set out to establish in-house capabilities for conducting HTE. Initial attempts to use various robotic instruments for dispensing >100 native solid reagents needed to assemble comprehensive HTE screening plates yielded unsatisfactory results, with weighing errors ranging from a few tenths to several hundredth percent. Moreover, frequent instrument stalls caused by poorly flowing solids significantly hindered the feasibility of unattended operations. We attributed the issue to the intrinsic physical property differences among powders, such as density, flowability, cohesiveness, and static behaviour. Realizing that using a single robotic platform to dispense a wide range of native solids was inherently problematic but at the same time highly desirable. This insight prompted us to search for a solution that could standardize diverse solid material properties while preserving chemical integrity. We drew inspiration from pharmaceutical formulation strategies, where active pharmaceutical ingredients are commonly blended with excipients to enhance handling.11 We applied this principle and developed a novel strategy to unify solid properties and enhance flowability by dry-mixing finely powdered reagents with inert glass beads. This innovative dry particle coating process transformed poorly flowing solids into uniform, free-flowing materials, enabling consistent and reliable automated dispensing across diverse solid-handling robotic platforms as well as manual dispensing devices.The resulting bead-based formulations allowed solid reagents to be dispensed volumetrically and reproducibly, akin to liquids, using standard robotic tools. ChemBeads thus overcame critical barriers in small-scale synthesis, enabling accurate reagent dosing without compromising stability or reaction reproducibility. This approach represented a significant departure from traditional native solid dispense methods, offering a practical and scalable solution for miniaturized synthesis workflows.
A paradigm shift for solid reagent management
The introduction of ChemBeads marked a turning point in the management of solid reagents within HTE, parallel compound library synthesis and other high-throughput environments. ChemBeads addressed longstanding challenges in accuracy, scalability, and compatibility with automation. Unlike prior methods, ChemBeads allowed the physical behaviour of a solid reagent to be decoupled from its chemical reactivity and therefore facilitating robust parallel experimentation with previously incompatible materials.As the demand for data-driven discovery, green chemistry, and miniaturized synthesis continues to grow, innovations like ChemBeads are poised to play an increasingly central role in the design of next-generation drug discovery platforms.
ChemBead preparation steps
The preparation of ChemBeads involves a simple process designed to neutralize the challenging physical properties of solid reagents, transforming them into stable, homogeneous, and flowable forms suitable for both manual and automated dispensing methods. Chemically inert beads serve as carriers for the solid reagents. The critical stages of ChemBead preparation are as follows:Selection of beads
The selection of appropriate beads is crucial, as they serve as carriers for the solid reagents. Beads are chosen from chemically inert materials, ensuring the integrity of the active solid reagent. Glass beads are commonly used due to their chemical inertness, affordability, smooth surface, and transparency, which aid visual inspection. Polystyrene beads are favoured for their affordability, lightweight nature, and chemical stability, which are essential for biochemical assays. The surface area of the beads influences their selection, as it dictates the loading capacity of solid reagents. Beads with higher surface areas provide more adhesion space, making them suitable for handling reagents that require larger loading capabilities. Bead size must be controlled to ensure uniform flow during dispensing and maintain dosing accuracy. Properly sized beads prevent clogging and ensure consistent reagent loading. In addition to serving as carriers for reagents to the reaction vessel, beads can also be integral to experimental design. For instance, in mechanochemistry, coated milling balls not only deliver reagents but also generate high impact forces that initiate chemical reactions.Reagent blending
After selecting the appropriate beads, the solid reagent is combined with the host beads. The active solid reagent is weighed with high precision to ensure consistent dosing across ChemBead batches. The ratio of reagent to inert carrier (loading level) is determined and expressed as a weight-to-weight ratio or mmol g−1 unit. The weight-to-weight ratio typically ranges from 0.5% to 20%, depending on experimental design by the user. In theory, as long as the user can confidently weigh out the active reagent, there is no limitation how low the loading can be. On the other hand, maximum loading ratio depends on factors related to individual solid physical properties, environmental factors such as humidity and the material of the mixing container (e.g., glass vs. plastic). Based on our experience, a loading level of 5% weight-to-weight ratio of reagent to bead yields the most versatile ChemBead, preserving favourable solid properties—such as flowability, homogeneity, and a suitable dispense weight range for automated solid dispensing platforms. For reagents requiring higher loading (such as inorganic bases or reagents needed in stoichiometric quantities), beads can be prepared with increased loading. However, careful attention should be paid to uncoated materials and the risk of deteriorating solid flowability.Dry particle coating
Once the solid reagent is combined with the carrier beads, the dry particle coating technique is applied. This stage utilizes resonant acoustic mixing (RAM) technology for uniform distribution of reagent particles on the carrier beads. In the absence of RAM technology, alternatives like vortex mixers can be used.12The dry particle coating technique is a materials engineering approach that enables fine solid reagents—referred to as guest particles—to adhere to the surface of larger, inert host particles without forming covalent bonds.13 In the case of ChemBeads, glass beads (or polystyrene beads for EnzyBeads) are employed as host materials due to their chemical inertness and compatibility with a wide range of reagents used in synthetic chemistry. Mechanical agitation or mixing provides the energy needed for guest particles to adhere to host surfaces via weak intermolecular forces, such as van der Waals interactions (Fig. 1).2
These non-covalent forces are strong enough to retain the guest material during storage and dispensing, yet weak enough to allow for rapid and complete release when exposed to a compatible solvent during reaction initiation. As a result, solid reagents that would otherwise suffer from poor flow, static charge accumulation, or dose inconsistency can now be dispensed accurately and reproducibly in both manual and robotic settings. This transformation is particularly beneficial in medicinal chemistry, where accurate microscale dosing is essential for reliable HTE and automated synthesis workflows.
Sieving and characterization
After coating, vibratory sieving is performed on the coated beads to remove residual fines or aggregates, promote particle uniformity, and enhance flow properties. This step is critical for automated dispensing, as it prevents jamming and ensures dosing precision with micro-dispensers. The final ChemBead product undergoes characterization to confirm successful reagent loading and ensure batch consistency.Resonant acoustic mixing (RAM)
The dry particle coating technique hinges on the application of controlled mechanical force to facilitate the uniform adhesion of solid reagent particles to chemically inert host beads. RAM is a highly efficient and reproducible technology that plays a pivotal role in this process. RAM utilizes low-frequency, high-intensity acoustic energy to achieve thorough mixing of solids without traditional stirring impellers or blades, making it particularly well-suited for dry particle coating methods such as those used in ChemBead preparation.Operating typically at around 60 Hz, this process creates motion within the vessel's contents and acoustic energy that travels through RAM's base during upward movement and is echoed by the vessel lid during downward movement, effectively acting as transducers.14 This acoustic energy interacts with different materials, facilitating rapid fluidization and dispersion, which results in efficient and thorough mixing. Unlike conventional mixers, RAM ensures optimized surface contact without dead zones or overheating.
Since the mixing container can be kept sealed during the entire mixing process, it preserves the chemical integrity of moisture- or air-sensitive reagents, which also reduce contamination risk and make it highly compatible with pharmaceutical applications where purity and traceability are paramount. This non-invasive mixing environment is essential for coating fine reagent particles onto inert beads without the use of solvents or binders.
A key advantage of RAM is its scalability. Because the acoustic energy is efficiently distributed, RAM systems maintain mixing consistency across a wide range of batch sizes. This property allows parameters optimized on a small laboratory scale to be directly translated to larger scales with minimal revalidation. With our experience in producing over 5000 ChemBeads, we've utilized a single PharmaRam II instrument to accommodate mixing container sizes ranging from 1 mL tubes to 1 L bottles.15
Moreover, the PharmaRam II enables parallel processing by allowing multiple containers to be mixed simultaneously, enhancing efficiency and throughput.
RAM also supports green chemistry principles. Its solvent-free operation eliminates the need for downstream drying, or filtration steps, and reduces the environmental burden associated with solvent disposal. Additionally, RAM parameters—including mixing time, intensity, and bead-to-reagent ratios—can be precisely tuned to meet specific application needs.
While RAM remains the most versatile and scalable technique for preparing ChemBeads, its broad adoption is sometimes limited by high capital investment and lab space requirements. This could be prohibitive for many academic and small-scale research laboratories. Consequently, several alternative methods have been explored that, while less advanced, can still produce functional reagent-coated beads suitable for HTE applications.12,16
Solid dispensing tools
Due to their high flowability and uniform physical solid properties, ChemBeads are remarkably well-suited for distribution using various instruments, whether in fully automated or manual formats. The spherical nature of the glass bead core combined with a consistent and adherent coating enables ChemBeads to flow freely and avoid common issues like bridging, static buildup, or clogging that typically hinder the automation of powder dispensing. This makes them highly compatible with a range of commercially available solid dispensing platforms. Researchers worldwide have successfully demonstrated the adaptation of automated powder dispensing robots, which can deliver multiple ChemBeads into multi-well plates. These robots are most suitable for comprehensive HTE plate setups, offering sub milligram accuracy. Our initial studies using the Chemspeed SWAVE system17 found that ChemBeads can be used to achieve accurate, reproducible volumetric dispensing of solid materials in HTE settings, with relative standard deviations typically below 5% across 96-well formats.2,18 Subsequent reports from our peer groups and from robot manufacturers that utilized different solid dispensing platforms further verified the high performance of ChemBead dispensing. These robotic platforms include Chemspeed Crystal Powderdose,19 Chemspeed Swile,20 Mettler Toledo Quantos21 and Unchain Labs Junior.22,23A unique automated dispensing robot to dose ChemBeads in 96 and 384 well microtiter plates at unprecedented speed is reported.24 The highly customized robot utilizes a novel dosing head design that enables reliable volumetric dosing into both 96- and 384-well microtiter plates. The system also features an intuitive graphical user interface, making the dosing protocol easy and flexible while ensuring traceability.
Manual ChemBead dispensing using calibrated scoops
Recognizing that automated solid dispensing instruments can be resource prohibitive and might limit accessibility, we developed the calibrated ChemBead scoop to promote broad adoption of ChemBead technology (Fig. 2, top). Its simplicity, cost-effectiveness, and ease of use make it possible for anyone, anywhere, to reliably measure and distribute ChemBeads, supporting consistency and expanding the user base. Calibrated scoops are simple, yet highly effective tools designed for reproducible, volumetric manual dispensing of ChemBeads.2,18,25,26 The scoops are designed to dispense a fixed volume of beads, ensuring accurate and consistent delivery of the active compound without the need for microbalance-based weighing. Because the solid content on ChemBeads is uniformly distributed and loading levels are pre-characterized, the amount of active ingredient dispensed can be reliably calculated based on the calibrated scoop volume. This tool enables rapid and user-friendly dosing, particularly valuable in high-throughput environments or early screening stages, where sub-milligram-scale accuracy is needed but robotic systems are unavailable or impractical.27,28 As an extension to the fixed volume scoops, a variable volume scoop has been developed (Fig. 2, bottom), which allows users to change the amount of ChemBead delivery based on experimental design. Users just need to first perform a calibration step, and once the desired scoop volume has been determined, subsequent delivery of ChemBeads can be done without the use of a balance. The calibrated scoops bridge the gap between full automation and manual handling, enhancing accessibility of ChemBead workflows in both research and industrial settings.Volumetric parallel ChemBead dispenser
As described above, the assembly of HTE screening plates using ChemBeads can be achieved through two primary methods: fully automated robotic dispensers and manual calibrated scoops. The automated robotic approach facilitates accurate dispensing of hundreds of solids at small scales through solid dispensing robots. This method offers the advantage of minimal human intervention, although it requires significant capital investment and lab space, and faces potential operation downtime due to instrument malfunctions, which can disrupt operations for an extended period of time.Manual assembly of screening plates, in contrast, involves minimal capital investment, making it more suitable for smaller screening sets due to its low-throughput nature.
To counter these issues, a manual parallel ChemBead dispenser was created, offering a high throughput yet cost-effective alternative (Fig. 3).29 The dispenser comprises three major components: the top plate with 96 compartments for ChemBeads, the middle ChemBead metering plate with 96 calibrated holes for 3 mg of ChemBeads, and the bottom plate for dispensing. The user slides the middle plate to align holes for dispensing ChemBeads into 96 receiving vials, ensuring efficient and consistent dispensing.
 | ||
| Fig. 3 Top: Parallel ChemBead dispenser. Middle and bottom: Schematic and components of a parallel ChemBead dispenser. | ||
This parallel bead dispenser effectively combines the high throughput of robotic dispensing with low–cost, low-maintenance calibrated scoops. It allows consistent weight dispensing with an error margin of ±5%, which typically does not impact the HTE experiment's outcomes.29 For experiments requiring multiple reagents per vial, additional parallel ChemBead dispensers can introduce more reagents, or reagents can be premixed before coating on beads for simultaneous dispensing. As a result, this dispenser provides a rapid, on-demand assembly solution for HTE screening plates without the need for maintaining large inventories of pre-dispensed screening plates.30–32
Practical considerations and limitations of ChemBeads
While ChemBead technology delivers substantial operational advantages, several practical considerations are essential for successful implementation. ChemBeads generally exhibit excellent storage stability under typical laboratory conditions, but their performance can be affected by issues such as elevated humidity and temperatures, recrystallization or reduced reagent loading observed at 40 °C and 75% relative humidity. To maintain optimal quality, ChemBeads should be stored in dry, controlled environments, and additional precautions (e.g., desiccators or purge box storage) may be necessary in humid climates.The most consistent and scalable ChemBead preparation relies on the use of RAM instruments, which provide highly reproducible dry particle coating. However, the cost and spatial requirements of RAM equipment may limit accessibility in resource-constrained settings. Alternative mixing methods such as vortex mixing can produce functional ChemBeads,12 but may yield lower consistency, throughput, or scalability compared to RAM-based approaches.
Achieving reliable ChemBead performance across different laboratories depends on precise control of factors including bead size, reagent physical characteristics, loading ratios, and the environmental conditions during both preparation and storage. Deviations in these parameters may impact flowability, dosing accuracy, and experimental reproducibility. Standardizing preparation protocols and thoroughly characterizing ChemBead batches can help mitigate variability, though complete inter-laboratory uniformity may still be challenging, especially when transitioning between equipment or scaling production.
Importantly, ChemBead preparation does not alter the intrinsic chemical stability or reactivity of the native material: the reagent is physically coated onto the bead surface without chemical modification or covalent bonding. Therefore, ChemBead formulations retain all chemical properties and sensitivities of the original reagent. Researchers should apply equivalent handling, storage, and safety precautions for ChemBeads as they would for the native material to ensure integrity and performance.
Case study
Use of ChemBeads for HTE at AbbVie
The AbbVie Discovery HTE group is uniquely positioned within the chemistry technology group in the Discovery organization. This arrangement allows HTE to have early impacts on the drug discovery process. Modern medicinal chemists are tasked with creating increasingly sophisticated molecules, which are harder to synthesize due to complex molecular designs and additional functional groups affecting reaction conditions. They also face difficulties with limited starting material availability for intermediates, a challenge less prevalent in later stages of discovery or process chemistry. Medicinal chemists must quickly identify viable routes to synthesize desired compounds under tight timelines; if a compound isn't achievable within a couple of weeks, alternative approaches or compounds must be explored to fulfil research objectives. To expedite the identification of usable conditions for compound synthesis, our discovery HTE platform was established in mid-2017. We started with offering CN and Suzuki–Miyaura cross coupling screens to our medicinal chemists. The initial responses were overwhelmingly positive. Since then, we have expanded the screening sets to include 17 different transformations (Fig. 4).Obtaining a small quantity (<1 mg) of the targeted compound for immediate biological testing is the main goal for medicinal chemists to answer structure–activity relationship questions. Our HTE setup is tailored toward providing an initial set of reactions conditions that medicinal chemists can use to produce targeted compounds, usually within 2–3 days. Further reaction optimization, if needed, can be carried out by the medicinal chemists or by our process research group counterpart using their focused HTE screening sets. Since the inception of ChemBead-based HTE at AbbVie Discovery, the platform has demonstrated remarkable productivity and impact in medicinal chemistry. Over 850 screens have been conducted, supporting more than 100 medicinal chemistry projects and involving over 160 requesting chemists. The platform has enabled the development of >400 unique ChemBead reagents across 17 different transformations, with an encouraging 67% average hit rate. Each reaction requires only 0.25–1 mg of material, and results are typically delivered within a 2–4 day turnaround. Collectively, these efforts have generated over 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 data points, which we have used to create different predictive models for reaction conditions (Fig. 5).
000 data points, which we have used to create different predictive models for reaction conditions (Fig. 5).
In addition to serving as the foundation technology for the development of standard reaction screens, the following sections highlight how ChemBead technology has facilitated the swift development of advanced chemical techniques that have gained significant prominence over the last few years at AbbVie.
ChemBeads were employed to deliver solid reagents (e.g., metal porphyrin complex, PIDA, phthaloyl peroxide, NaHCO3, MnO2 and NFSI, etc.) by coating them onto inert glass beads. The resulting ChemBead formulations were dispensed into 96-well plates using a parallel ChemBead dispenser or the calibrated scoops, achieving reaction scales as low as 0.5 μmol per well.
We validated the ChemOx platform using antalarmin as a model substrate and subsequently applied it to a panel of marketed drugs, including ondansetron, rosiglitazone, tolvaptan, and rivaroxaban. The platform yielded a range of oxidized analogs—many not observed in biocatalytic screens—and demonstrated the complementarity of chemical LSO in drug metabolism and SAR development. Notably, 22 oxidized hits were identified using ChemOx between 2020 and 2024 across 21 discovery programs and one clinical program at AbbVie, four of which involved porphyrin-catalyzed transformations. ChemBeads enabled the practical miniaturization and automation of solid reagent delivery in LSO workflows. Their use in the ChemOx platform illustrates the power of this technology to enhance throughput, reduce material requirements, and accelerate the exploration of oxidative transformations for lead diversification.
To overcome challenges like reagent solubility and dispensing accuracy, ChemBeads were used to pre-load and dispense catalysts and ligands efficiently into 96-well PCR plates. This reduced plate setup time and ensured reproducibility for nanomole-scale reactions.
Using this platform, we discovered new reaction conditions, including a previously unreported palladium-catalyzed method that successfully coupled challenging amines to DNA-conjugated aryl halides. Optimization using ChemBeads and automation improved conversions from expanded reaction scope.
The method was also confirmed to preserve DNA integrity via ligation and qPCR analysis, making it suitable for library construction workflows.
In essence, ChemBeads are central to enabling automated, scalable, and DNA-compatible high-throughput screening for C–N couplings, accelerating reaction development for medicinal chemistry applications.
The second approach focuses on the use of commercially available enzyme-containing cell-free lysate powders (E-CFL). These powders present challenges for accurate dosing by weight because they could be flocculent, electrostatic, and non-homogeneous. One solution is to hydrate the powder and then dispense the resulting liquid into screening plates. However, this raises concerns about the stability of the catalyst during storage, as the activity can diminish rapidly, even when kept at −80 °C.21
In collaboration with our Process group, and building on the ChemBead concept, we have developed EnzyBeads to address persistent challenges related to the volumetric dosing and stability of biocatalysts facilitating consistent and automated enzyme delivery for microscale medicinal chemistry applications.21 EnzyBeads tackles these issues by providing enzymes in flowable bead formats that are fully compatible with robotic and manual powder dispensing systems. This innovation enables accurate volumetric delivery into well-plate assays, microreactors, and droplet-based systems. This is essential in structure–activity relationship studies involving enzymatic transformations such as ketoreductase, amine transaminase and lipase reactions. Our comparative studies between EnzyBeads and traditional methods using hydrated E-CFL have shown that the preparation process does not have any deleterious effect on enzyme function. It's important to note that formulating enzymes as EnzyBeads differs significantly from enzyme immobilization, which involves attaching enzymes to a solid support or matrix via adsorption, covalent binding and entrapment, which restricts their movement in a solution.33 In contrast, in the case of EnzyBeads (as well as ChemBeads), the enzyme is fully released from the beads upon contact with a solvent.34
In a notable example, our Drug Development Group implemented a ChemBead-based platform to evaluate thermodynamic solubility across a panel of APIs.35 Using 96-well plates and automated workflows, the team screened compound solubility in various aqueous buffers and co-solvent systems. ChemBeads enabled accurate solid dosing with high consistency in a high throughput setting. This advancement improved overall efficiency and assay reproducibility, while significantly reducing material waste.
ChemBead use cases in the pharmaceutical industry
A central challenge in such multi-component systems is the reproducible delivery of a poorly soluble sodium carbonate base and a Ni(dtbbpy)Cl2 precatalyst in the organic solvents chosen and the hard-to-dose sticky nature of a supersilane derivative aminosupersilane (Si-1). These problems complicated the screening process and raised potential reproducibility issues. The team addressed these operational difficulties with the use of ChemBeads for the accurate measurement of reagents on a small scale (10 μmol) and for dosing reagents that are poorly soluble in organic solvents. With the initial success with ChemBeads, the team expanded their evaluation of ChemBeads on the photoredox screening platform. They tested conditions from their traditional HTE plate, including base, solvent, silane, and photocatalyst. The team opted to validate Na2CO3, Si-1, and Ni(dtbbpy)Cl2 on ChemBeads due to the challenges these reagents pose in terms of accurately weighing and dosing on a small scale. The screen was conducted four times to ensure consistency, mitigating any variability from the photochemistry HTE setup's LED light source. The team observed minimal variability across the replicates and only minor variability (up to 8%) when using up to two ChemBead reagents. The best results emerged when all three reagents (Si-1, Na2CO3, and Ni(dtbbpy)Cl2) were loaded onto the beads, a finding they attribute to the enhanced precision in reagent measurement provided by ChemBeads. This case, among others, underscores the transformative impact of ChemBeads in modern medicinal chemistry, particularly for screening workflows that demand precision, efficiency, and material conservation.
In another study by Piacentini and co-workers, ChemBeads were employed as a key technology for enabling robust and practical photochemical HTE.23 The researchers evaluated five representative reactions to assess the performance of ChemBeads in combination with temperature-controlled reactors. Their results demonstrated that ChemBeads provided reproducible outcomes at reaction scales ranging from 10 to 27 μmol. By enabling accurate and traceable solid dosing across reaction arrays, ChemBeads effectively addressed common challenges in batch photochemistry, such as photocatalyst solubility and variability in reagent delivery. This platform was successfully applied to optimize an Ir/Ni-catalyzed C–N visible-light cross-coupling between aryl halides and fluorinated amines. The optimized conditions were subsequently validated at a 100-fold scale-up using a large-scale batch setup (PHIL photoreactor), confirming the scalability and robustness of the ChemBead-assisted workflow.
Koolma and co-workers integrated ChemBeads into a miniaturized platform for the Liebeskind–Srogl coupling (LSC) of heteroaromatic thioethers, aimed at expanding the reaction's utility in medicinal chemistry.37 ChemBeads enabled accurate and reproducible dosing of microscale reagent quantities—such as 0.1 μmol of palladium precatalyst and 2 μmol of copper(I) thiophenecarboxylate or copper(I) 3-methylsalicylate—within reaction volumes as small as 20 μL. This setup supported HTE and kinetic studies, allowing for efficient parallel synthesis and rapid evaluation of substrate scope. By ensuring reagent uniformity and minimizing cross-contamination, ChemBeads provided a robust and scalable solution for standardized LSC conditions. The platform demonstrated broad applicability across challenging heterocycles, offering orthogonal reactivity and functional group tolerance valuable for library synthesis and lead optimization in drug discovery workflows.
Initially, their HTE process relied on liquid handling in polypropylene microtiter plates, which worked for certain room-temperature reactions but proved limiting for air- and moisture-sensitive reagents due to material compatibility issues. To address this, the team at Astex transitioned to glass reaction vials for enhanced chemical stability, employed micro stir bars for effective mixing, and the use of solid reagents in ChemBead formulation. Reactions are now prepared and stored under nitrogen to improve shelf-life. The workflow has been advanced with semi-automation and standardized procedures that are compatible with various robotic systems, supporting data generation for future machine-learning models.
The team investigated three different glass bead sizes (diameters <106 μm, 212–300 μm and >425 μm) at 3 loading capacities (wt/wt at 1%, 5% and 10%) using an established coating procedure. Consistent with our previous findings, the results indicated that using glass bead sizes of 212–300 μm and at 5% by weight loading produced ChemBeads most suitable for their work. At this combination, scanning electron microscopy confirmed uniform distribution of the reagents and the bead surfaces were uniformly covered without excess unbound powder. This 5% loading capacity, which was consistent across several compounds including nicotinamide, saccharin, Tris, and L-tartaric acid, provided a practical benchmark for screening new compounds. Quantitative UPLC analysis validated the linear relationship between theoretical and actual loadings (slopes = 0.8–1.0, R2 > 0.9), ensuring reliable dosing in screening workflows.
Critically, the surface coating did not impair the flowability of the ChemBeads. Shear cell measurements showed that coated beads maintained high flow function coefficients (FFc = 8.2) nearly identical to that of uncoated beads (FFc = 8.7), and far superior to native powder (FFc = 2.3). This enabled automated dispensing with high accuracy—within ±5% error for 20 mg targets—across 100 consecutive samples.
ChemBeads also demonstrated excellent physical and chemical stability under ambient conditions (25 °C, 60% RH), though elevated temperature and humidity (40 °C, 75% RH) led to crystal ripening and reduced loading due to recrystallization, suggesting the need for dry storage conditions.
The practical utility of ChemBeads was further validated through salt and cocrystal screening. ChemBeads successfully formed identical crystalline phases as traditional powder methods across multiple systems, including salts (maleic acid–DL-methionine, L-tartaric-L-lysine, Tris–indomethacin and Tris–ibuprofen) and cocrystals (nicotinamide–indomethacin, nicotinamide–ibuprofen and saccharin–indomethacin). These results were confirmed using X-ray powder diffraction and differential scanning calorimetry. The presence of glass beads did not affect polymorphic outcomes. Notably, ChemBeads also enhanced crystallization kinetics: in the maleic acid–DL-methionine salt system, complete crystallization was achieved in just 2 hours using ChemBeads, compared to only 60% conversion after 4 hours with traditional powders. The authors suggested the acceleration is likely due to increased mechanical agitation, localized heat, and surface interactions facilitated by bead movement.
Despite the enhanced kinetics, the team found no evidence of polymorphic transformation or new form generation, even for systems known to exhibit polymorphism, such as nicotinamide. This supports the conclusion that ChemBeads provide a reliable and inert medium for solid form generation, suitable for direct characterization without requiring separation from the glass beads.
This work demonstrates that ChemBeads not only replicate the performance of traditional powder-based crystallization methods but offer superior precision, automation compatibility, and material efficiency. Their ability to streamline salt and cocrystal screening workflows, while maintaining phase fidelity, makes ChemBeads a valuable tool for pharmaceutical solid form development, particularly in the context of high-throughput and small-scale applications.
ChemBead use cases in academia
In traditional CEC workflows, accurate dosing of solid zinc powder presents a critical challenge due to its static-prone nature and poor flow properties. To address this, the joint team developed a bead-based solid delivery system using ChemBeads coated with zinc dust (Zn@ChemBead) and ligands. This allowed precise volumetric dosing into 96-well plates under inert conditions—an achievement previously difficult with standard powder-handling methods.
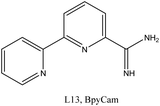
Using this ChemBead-based protocol, the team executed 666 parallel microscale reactions, screening over 200 substrate combinations. This led to the identification of a novel ligand, L13 (BpyCam), which complements conventional bipyridyl ligands like dtbbpy for coupling diverse aryl halides and alkyl halides. The joint team also observed higher reaction conversion using a heater shaker without the use of a micro stir bar, suggesting that using ChemBeads in microscale reactions provides better mixing. The reproducibility and precision of ChemBeads enabled efficient screening, offering a direct route to scalable synthetic protocols with broad substrate scope and improved yields.
The miniaturized screen, facilitated by ChemBeads, permitted the evaluation of reactivity, regioselectivity, and scalability in a rapid and resource-efficient manner. Notably, the single-component iridium complex demonstrated broad functional group tolerance and outperformed traditional precatalyst systems in terms of air stability, speed of activation, and substrate scope. This study underscores the synergistic value of innovative catalyst design and solid reagent handling technologies like ChemBeads in advancing miniaturized, high-throughput functionalization strategies. ChemBeads were instrumental in transforming a technically demanding C–H borylation reaction into a robot-compatible, screening-friendly protocol suitable for modern drug discovery platforms.
Outlook and conclusions
Over the past two decades, the field of medicinal chemistry research has undergone significant transformation. Advances in technologies such as HTE, computational chemistry, direct-to-biology, structural biology, and artificial intelligence/machine learning have enhanced the efficiency of medicinal chemistry research. Embracing the “doing more with less” philosophy, researchers have shifted toward using smaller quantities of chemicals, reducing the consumption of reagents, solvents, and other materials. Miniaturization allows for conducting more experiments in less time—a crucial benefit in drug discovery, where thousands of compounds are synthesized and tested for various properties.The shift from experiments involving grams to sub-milligram quantities necessitated the innovation of innovative technologies. One key challenge was the proper handling of solid materials in microgram quantities for small-scale reaction setups. ChemBead technology has emerged as an effective solution, offering ease of implementation with minimal time and financial investment. This technology has been rapidly adopted by both industry and academia, setting a new standard for assembling small-scale reactions, providing a scalable, accurate, and automation-compatible solution to the persistent bottlenecks of solid dosing. Through innovations in dry particle coating, particularly using RAM, ChemBead enables volumetric dosing of solid reagents with the same ease as liquids. This facilitates efficient high-throughput screening, reduces material waste, and improves experimental reproducibility. Their impact spans a wide spectrum of applications, from photoredox catalysis and cross-electrophile coupling to complex late-stage oxidations and borylation screens.
Industrial adoption by pharmaceutical leaders like AbbVie, Roche, GSK, Boehringer Ingelheim, and Astex, along with academic collaborations, has solidified ChemBeads as a key enabling technology in miniaturized experimentation. Furthermore, the extension of this platform to EnzyBeads, solubility studies, and solid form screens demonstrates its versatility beyond synthetic chemistry. As drug discovery shifts toward automation and reaction miniaturization, ChemBeads are poised to play a central role in shaping the future of chemical innovation.
Technology development, while often challenging, has the potential to create broad and lasting impact, extending far beyond narrowly focused solutions to specific problems. Our work on ChemBead technology exemplifies this, serving as a vital bridge between academia and industry and making these tools more accessible to researchers in academic settings. By offering this technology, we have unlocked new opportunities for collaboration.41 Notably, the development of ChemBead technology has transformed our approach to HTE—a field where we initially lagged but, through innovation, have now become leaders. This journey highlights that while it is relatively easy to devise complex solutions to difficult problems, finding a truly simple and elegant solution is much more challenging and valuable.
Continued development in high-throughput technologies, such as reaction heating in high-density plastic well plates, fast analytical methods, and standardized experimental data capturing tailored for building predictive machine learning models, represents areas of active pursuit that promise further advancements in this transformative landscape.
Author contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.Conflicts of interest
The authors declare no competing financial interest. N. P. T. and Y. W. are employees of AbbVie. The design, study conduct, and financial support for this research were provided by AbbVie. AbbVie participated in the interpretation of data, review, and approval of the publication.Data availability
There are no additional data available.Acknowledgements
The authors would like to extend our deepest gratitude to all members who have contributed to the ChemBead project. Their unwavering dedication, expertise, and collaborative teamwork were instrumental in advancing this research.Notes and references
- J. Comley, Drug Discov. World, 2009, 10, 53 Search PubMed.
- N. P. Tu, A. W. Dombrowski, G. M. Goshu, A. Vasudevan, S. Djuric and Y. Wang, Angew. Chem., Int. Ed., 2019, 58, 7987 CrossRef CAS PubMed.
- R. Chadha and S. Bhandari, J. Pharm. Biomed. Anal., 2014, 87, 82 CrossRef CAS PubMed.
- Y. Gui, Pharm. Res., 2023, 40, 2347 CrossRef CAS.
- A. Dombrowski, N. P. Tu and Y. Wang, US 20190033185A1, 2019.
- A. B. Santanilla, E. L. Regalado, T. Pereira, M. Shevlin, K. Bateman, L.-C. Campeau, J. Schneeweis, S. Berritt, Z.-C. Shi, P. Nantermet, Y. Liu, R. Helmy, C. J. Welch, P. Vachal, I. W. Davies, T. Cernak and S. D. Dreher, Science, 2015, 347, 49 CrossRef PubMed.
- M. Shevlin, ACS Med. Chem. Lett., 2017, 8, 601 CrossRef CAS PubMed.
- N. P. Tu and B. J. Kotecki, The Power of High-Throughput Experimentation, ACS Publications, Washington, DC, 2022 Search PubMed.
- A. C. Sather, H. G. Lee, J. R. Colombe, A. Zhang and S. L. Buchwald, Nature, 2015, 524, 208 CrossRef CAS PubMed.
- D. Petkova, N. Borlinghaus, S. Sharma, J. Kaschel, T. Lindner, J. Klee, A. Jolit, V. Haller, S. Heitz, K. Britze, J. Dietrich, W. M. Braje and S. Handa, ACS Sustainable Chem. Eng., 2020, 8, 12612 CrossRef CAS.
- R. B. Shah, M. A. Tawakkul and M. A. Khan, AAPS PharmSciTech, 2008, 9, 250 CrossRef CAS PubMed.
- A. C. Impastato, J. T. C. Brown, Y. Wang and N. P. Tu, ACS Med. Chem. Lett., 2023, 14, 514 CrossRef CAS PubMed.
- R. Sharma and G. Setia, Powder Technol., 2019, 356, 548 Search PubMed.
- Resodyn company website, https://resodynmixers.com/2020/12/30/what-is-resonantacoustic-mixing/ (accessed 10-6-2025).
- Resodyn company website, https://resodynmixers.com/wp-content/uploads/2024/07/PharmaRAM-II.pdf (accessed 10-6-2025).
- K. Kang, N. L. Loud, T. A. DiBenedetto and D. J. Weix, J. Am. Chem. Soc., 2021, 143, 21484 CrossRef CAS PubMed.
- Chemspeed company website, https://www.chemspeed.com/example-solutions/isynth-swave/ (accessed 10-6-2025).
- M. C. Martin, G. M. Goshu, J. R. Hartnell, C. D. Morris, Y. Wang and N. P. Tu, Org. Process Res. Dev., 2019, 23, 1900 CrossRef CAS.
- Chemspeed company website, https://www.chemspeed.com/media/crystal-powderdose-dispensing-chembeads/ (accessed 10-6-2025).
- Chemspeed company website, https://www.chemspeed.com/products/crystal-swile/ (accessed 8-2-2025).
- J. T. C. Brown, N. P. Tu and R. M. Phelan, Org. Process Res. Dev., 2021, 25, 337 CrossRef CAS.
- J. M. Fordham, P. Kollmus, M. Cavegn, R. Schneider and M. Santagostino, J. Org. Chem., 2022, 87, 4400 CrossRef CAS PubMed.
- P. Piacentini, J. M. Fordham, E. Serrano, L. Hepp and M. Santagostino, Org. Process Res. Dev., 2023, 27, 798 CrossRef CAS.
- V. Porte, P. Hirschle, K. Frank, L. Hepp, P. Kollmus, E. Serrano, C. Ziegler, R. Heinrich, S. Seebacher, C. Weiss, J. M. Fordham and M. Santagostino, Org. Process Res. Dev., 2025, 29, 2124 CrossRef CAS.
- T. Kerackian, G. Chacktas, D. Durand and E. Romero, J. Flow Chem., 2024, 14, 367 CrossRef.
- R. M. Ward, Y. H. Hu, N. P. Tu and J. M. Schomaker, ChemSusChem, 2024, 17, e202300964 CrossRef CAS PubMed.
- M. N. Bahr, D. B. Damon, S. D. Yates, A. S. Chin, J. D. Christopher, S. Cromer, N. Perrotto, J. Quiroz and V. Rosso, Org. Process Res. Dev., 2018, 22, 1500 CrossRef CAS.
- M. N. Bahr, M. A. Morris, N. P. Tu and A. Nandkeolyar, Org. Process Res. Dev., 2020, 24, 2752 CrossRef CAS.
- N. J. Gesmundo, N. P. Tu, K. A. Sarris and Y. Wang, ACS Med. Chem. Lett., 2023, 14, 521 CrossRef CAS.
- A. L. Aguirre, N. L. Loud, K. A. Johnson, D. J. Weix and Y. Wang, Chem. – Eur. J., 2021, 27, 12981 CrossRef CAS PubMed.
- J. Izquierdo-Ferrer, M. E. Bellizzi, N. Tu, A. Bogdan and Y. Wang, ACS Med. Chem. Lett., 2025, 16, 916 CrossRef PubMed.
- K. A. D'Angelo, C. La, B. Kotecki, J. W. Wilson, C. Karmel, R. Swiatowiec, N. P. Tu, S. Shekhar and J. F. Hartwig, J. Am. Chem. Soc., 2024, 146, 32717 CrossRef.
- Y. R. Maghraby, R. M. El-Shabasy, A. H. Ibrahim and H. Mohamed El-Said Azzazy, ACS Omega, 2023, 8, 5184 CrossRef CAS.
- J. S. Chew, T. T. N. Ho and C. L. K. Lee, New J. Chem., 2020, 45, 32 RSC.
- S. Alex, K. Gleason, M. Lipert and N. P. Tu, presented in part at the American Association of Pharmaceutical Science PharmSci360, Orlando Fl, October 2023 Search PubMed.
- M. P. Glogowski, N. Cercizi, T. Lynch-Colameta, L. H. Ridgers, J. P. Phelan, A. M. Rowley and M. P. Rauch, Org. Lett., 2024, 26, 2420 CrossRef CAS.
- V. Koolma, R. Staiger, M. Schühle, A. Bixenmann, E. Bauschatz, M. Schmid, F. M. Miloserdov and B. Herlé, Org. Lett., 2024, 26, 2852 CrossRef CAS.
- C. Townley, D. Branduardi, G. Chessari, B. D. Cons, C. Griffiths-Jones, R. J. Hall, C. N. Johnson, Y. Ochi, S. Whibley and R. Grainger, RSC Med. Chem., 2023, 14, 2699 RSC.
- J. Meng, C. H. Zhou, L. Yin, J. Chen, J. Xi, C. Chen, T. Cai and Y. Gui, Mol. Pharmaceutics DOI:10.1021/acs.molpharmaceut.5c01051 , Article ASAP.
- J. H. A. Schuurmans, T. M. Masson, S. D. A. Zondag, S. Pilon, N. Bragato, M. Claros, T. den Hartog, F. Sastre, J. van den Ham, P. Buskens, G. Fiorani and T. Noel, Chem. Sci., 2024, 15, 19842 RSC.
- A. Vasilopoulos and Y. Wang, ACS Med. Chem. Lett., 2025, 16, 1473 CrossRef CAS PubMed.
Footnote |
| † AbbVie internal unpublished data. |
| This journal is © The Royal Society of Chemistry 2026 |