Fluorinated pyrimidine 5-carboxamides as potential tools for MERTK targeted fluorine-18-PET-radioligand development
Ramesh
Mudududdla†
 a,
Siu Wai
Wong†
a,
Nghi
Nguyen
a,
Lian
Xue
a,
Michael A.
Stashko
b,
Xiaodong
Wang
b,
Lucy
Vivash
a,
Siu Wai
Wong†
a,
Nghi
Nguyen
a,
Lian
Xue
a,
Michael A.
Stashko
b,
Xiaodong
Wang
b,
Lucy
Vivash
 c,
Michele D.
Binder
c,
Michele D.
Binder
 d,
Uwe
Ackermann
e,
Ylva E.
Bozikis
a,
Trevor J.
Kilpatrick
*f and
Jonathan B.
Baell
d,
Uwe
Ackermann
e,
Ylva E.
Bozikis
a,
Trevor J.
Kilpatrick
*f and
Jonathan B.
Baell
 *a
*a
aMedicinal Chemistry, Monash Institute of Pharmaceutical Sciences, Monash University, Parkville, Victoria 3052, Australia. E-mail: jbaell29@gmail.com
bCenter for Integrative Chemical Biology and Drug Discovery, Division of Chemical Biology and Medicinal Chemistry, Eshelman School of Pharmacy, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, USA
cDepartment of Neuroscience, School of Translational Medicine, Monash University, Melbourne, Victoria 3010, Australia
dFlorey Institute of Neuroscience and Mental Health, Parkville, Victoria 3010, Australia
eDepartment of Nuclear Medicine and Centre for PET, Austin Health, Heidelberg, Victoria 3084, Australia
fThe Florey Department of Neuroscience and Mental Health, University of Melbourne, Parkville, Victoria 3010, Australia. E-mail: tkilpat@florey.edu.au
First published on 3rd December 2025
Abstract
MER tyrosine kinase (MERTK) is highly expressed on the protective and reparative phenotype of microglia, which is in response to neuroregeneration following the neuronal damage induced by multiple sclerosis (MS). A specific imaging tool, which can differentiate anti-inflammatory and immunosuppressive responses of microglia, could be highly beneficial for the early detection and clinical management of MS. To identify potential 18F-radiotracers to image anti-inflammatory responses of microglia, herein a series of fluorinated pyrimidine-5-carboxamide derivatives were prepared from a database of MERTK ligands. Several potent MERTK ligands were discovered with promising selectivity profiles over other off-targets (AXL, TYRO3 and FLT3). A cell-based assay was employed to assess cellular inhibitory MERTK potency, which may be regarded as being particularly relevant to an in vivo imaging situation. This study reports the discovery of several new, potent, and selective fluorinated compounds against MERTK, paving the way for PET tracer development to image protective microglial phenotype in MS patients.
Introduction
Multiple sclerosis (MS) is a chronic autoimmune disorder of the central nervous system (CNS), characterized by lesions in the brain and spinal cord that arise from demyelination and neurodegeneration.1,2 The track record of curative drug development for MS is very poor and a critical factor is the lack of precision imaging tools to inform patient response to clinically trialed drugs in the organ which matters the most – the brain.3,4 For example, microglial activation is a hallmark during disease progression.5–7 Activated microglia can contribute to either disease pathogenesis through the production of a wide range of pro-inflammatory mediators including TNF-α, interleukin 1 beta (IL-1β), and interleukin 6 (IL-6). On the other hand, microglia can also be involved in protective and reparative mechanisms, prompting anti-inflammatory and immunosuppressive responses.8–10 Clearly, the predominant microglial phenotype that is present within the diseased microenvironment is critical knowledge for assessment of any given patient's disease status and the need for therapeutic intervention, whether experimental or FDA-approved.11–15 Yet there is currently no imaging tool available to distinguish between microglia of these various phenotypes, and those microglial imaging tools currently available target the translocator protein (TSPO) and are not subtype specific.16,17The TAM receptor tyrosine kinase family consists of TYRO3, AXL and mer tyrosine kinases and both MERTK and AXL are shown to be abundant in microglia.18 Recent studies have shown that MERTK is highly expressed in microglia with a predominantly reparative phenotype, in particular by promoting efferocytosis and hence the capacity for remyelination to occur.19–22 As such, there is increasing interest in the development of PET radiotracers that target MERTK.23 Such tracers would enable physicians to distinguish between pathogenic processes that underpin disease pathogenesis and reparative activity, allowing stratification of patients. This heightened ability to gauge individual MS patient microglial activity at any stage of the disease would be beneficial in guiding clinical trials, follow-up treatments, pathological studies and patient response.
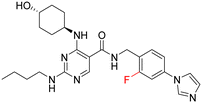
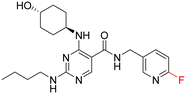
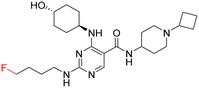
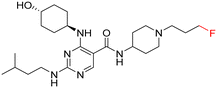
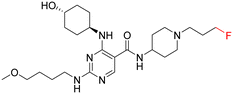
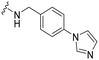
As part of our initial investigations to develop radiotracers for assessing microglial phenotype, we have discovered [18F]MIPS15692 (Fig. 1), a proof-of-concept MERTK-selective PET radiotracer that provides an imaging signal in brain slices of a murine model of MS.24 However, suboptimal properties of this and other recently reported UNC5293-based MERTK 18F-PET-radiotracers (Fig. 1) are driving the search for new MERTK radioligands with better brain pharmacokinetics and CNS permeability.25 To discover the next generation 18F-PET-radiotracers assessing microglial phenotype, it is essential to conduct a deep SAR on the existing database of MERTK inhibitors. This involves incorporating a specific fluorine atom as an atomic surrogate for a subsequent 18F-substituent and examining resultant bioactivity and selectivity profiles against both TAM and FLT3 kinases. For this purpose, we have synthesized a series of fluorinated pyrimidine-5-carboxamide derivatives and demonstrated their in vitro and cellular potency in targeting MERTK. Additionally, these compounds showed selectivity over other TAM kinases, including AXL and TYRO3, as well as FLT3, a potential off-target kinase in microglia-specific 18F-PET-neuroimaging.
Access to a database of MERTK inhibitors that has been established by researchers at the University of North Carolina for other indications allowed us to select alternative scaffolds for exploration, these being the three pyrimidine 5-carboxamides UNC2881 (1), UNC2580 (2), and UNC2876 (3) (Fig. 2).26–28 Compounds 1–3 exhibit favorable kinase inhibitory activities against MERTK (<6 nM) along with promising selectivity profiles over AXL (>60-fold), TYRO3 (>41-fold), and FLT3 (>12-fold), which makes them useful starting points for our SAR study. Herein, we report our SAR investigation focused on fluorinated derivatives of these scaffolds to gauge their potential for PET radiotracer development. We have classified the structural modifications of compounds 1–3 into two groups, A and B, as shown in Fig. 1. These modifications introduce a fluorine atom to improve the compounds' ease of radiolabeling and enhance their overall suitability for radiotracer development.
 | ||
| Fig. 2 MERTK ligands UNC2881 (1), UNC2580 (2), and UNC2876 (3) and proposed sites (A and B) for fluorine placement. | ||
Results and discussion
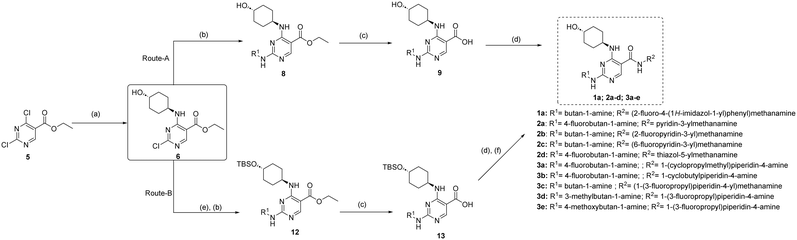
Synthesis of fluorinated pyrimidine 5-carboxamides 1a, 2a–d & 3a–e follows Scheme 1. In the first step, ethyl 2,4-dichloropyrimidine-5-carboxylate 5 was reacted with trans-4-aminocyclohexanol to give compound 6, which was then subjected to a second nucleophilic attack with an alkyl amine 7 following route A to yield 2,4-diamino-pyrimidine carboxylic acid ethyl ester 8. Compound 9 was obtained after basic hydrolysis of 8 using LiOH, which was then subjected to EDCI couplings with various right-hand side amine handles to give the desired final fluorinated pyrimidine 5-carboxamides (2a–d). Similarly, intermediate 12 was obtained following route B. Compound 6 was subjected to TBS protection followed by nucleophilic attack by aliphatic amines (refer to SI for structures). The carboxylic acid 13 was obtained after basic hydrolysis and was then engaged in EDCI amide couplings followed by silyl deprotection under an acidic medium, yielding the desired fluorinated pyrimidine 5-carboxamides 1a and 3a–e in excellent yields.Compound 1 was reported as a potent MERTK inhibitor for the treatment of thrombosis with IC50 values against MERTK and FLT3 of 4.3 nM and 55 nM, respectively.26 It was envisaged that a fluorine substituent at the ortho-position of the phenyl ring benzylamine ring on compound 1 (site A in Fig. 2) could form a six-membered ring with the NH-function of carboxamide by forming the intramolecular hydrogen bond and encourage CNS penetrance. Thus, compound 1a was prepared and tested in a kinase inhibition assay (Table 1). Notably, fluorinated analogue 1a exhibited good to excellent activity and selectivity profiles against MERTK, TYRO3, and FLT3 kinases when compared with its parent compound 1. The IC50 of compound 1a against MERTK was 20 nM, which is slightly weaker than that reported for the parent compound 1. We speculate that this difference in IC50 may be attributed to the distinct assays used. Our compounds were screened using a radiometric protein kinase assay, while the activity of the parent compound 1 reported by UNC was measured using the microfluidic capillary electrophoresis (MCE) assay. Therefore, directly comparing our compound with those reported in the literature is not meaningful. The MCE assay was primarily conducted to characterize our compounds internally and provide consistent comparative data within our study, rather than to directly compare with values found in the literature. Fluorinated compound 1a maintains a good level of potency against MERTK and demonstrates a favourable selectivity profile for MERTK over other TAM receptors, exhibiting a selectivity index (SI) >10 against both AXL and TYRO3 kinases. Furthermore, the selectivity for MERTK over FLT3 relative to 1 has been significantly enhanced, achieving an SI exceeding 40-fold. This illustrates that the addition of a fluorine atom at the ortho-position of the benzylamine ring is indeed beneficial in maintaining the potency for MERTK and increasing the selectivity over FLT3.
| Compound | R | IC50 (nM) | |||
|---|---|---|---|---|---|
| MERTK | AXL | TYRO3 | FLT3 | ||
| a Reported IC50 values by UNC. b IC50 values are the mean of two independent radiometric protein kinase assays (SD = ±0.5 nM), Selectivity Index values are listed in brackets. | |||||
| UNC2881 (1) |
|
4.3a | 360a (84) | 250a (58) | 55a (13) |
| 1a |
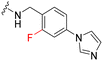
|
20b | 290b (15) | 1100b (55) | 810b (41) |
UNC2580 (2) was reported as a potent mer-selective kinase inhibitor with a reported IC50 value of 5.8 nM,27 in our hands being 11 nM. The calculated CNS multiparameter optimization score (CNS MPO) for 2 is 3.9, an improvement over compound 1 (CNS MPO 2.7).29 Considering its high CNS permeability score and relatively low molecular weight, we selected 2 for further SAR investigation around sites A & B (Fig. 2). Compound 2 was resynthesized to serve as our positive control and subsequently evaluated in TAM and FLT3 kinase assays to directly compare the activity strengths with the newly synthesized fluorinated derivatives. The activity profiles observed are detailed in Table 2, entry 1. The determined IC50 values were 11 nM for MERTK, 15 nM for AXL, 910 nM for TYRO3, and 1200 nM for FLT3, respectively. Four new fluorinated analogues (2a–d) were prepared and profiled in TAM and FLT3 kinase assays: compounds 2a and 2d with the fluorine atom on the n-butylamine side chain, and 2b and 2c with fluorine on the pyridine ring. As shown in Table 2, the new analogue 2a with a fluorine atom on the butylamine side chain inhibited MERTK with an IC50 of 51 nM, representing a 4.6-fold loss in potency compared with its parent compound 2 (IC50 = 11 nM). Thus, 2a retains measurable activity but is substantially less potent than the parent molecule. Gratifyingly, 2a also maintained favourable selectivity profiles over AXL (∼13-fold), TYRO3 (∼57-fold), and FLT3 (∼47-fold). This fluorination strategy was explored on the N-(thiazol-2-ylmethyl)pyrimidine-5-carboxamide core, as exemplified by analogue 2d bearing a 4-fluorobutylamine substituent at the C2 position. Kinase profiling showed that 2d retained notable activity against MERTK (IC50 = 38 nM) and demonstrated a favorable selectivity index for MERTK over AXL (∼11-fold), TYRO3 (∼42-fold), and FLT3 (∼39-fold). A similar selectivity profile was observed for compound 2b, which features a fluorine atom at the C2-position of the pyridine ring; however, the potency against MERTK was significantly diminished (IC50 = 68 nM) in comparison to parent compound 2. Subsequently, the fluorine atom was repositioned from the C-3 position to the C-6 position of the pyridine ring, leading to the synthesis of compound 2c. This novel 6-fluoropyridine derivative 2c demonstrated significant potency against MERTK, with an IC50 value of 26 nM, which represents only a minor 2.3-fold reduction in activity compared to its parent compound 2. Furthermore, this compound exhibits a significantly greater SI value for MERTK compared to AXL (∼15-fold), TYRO3 (∼73-fold), and FLT3 (∼54-fold) kinases. Its substantial MERTK activity, combined with elevated SI values and a favourable CNS MPO score of 3.5, positions this compound as a compelling candidate for future investigation.
| Compound | R1 | R2 | IC50a in nM | |||
|---|---|---|---|---|---|---|
| MERTK | AXL | TYRO3 | FLT3 | |||
| a IC50 values are the mean of two independent radiometric protein kinase assays (SD = ±0.5 nM), selectivity index values are listed in brackets. | ||||||
| UNC2580 (2) |
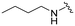
|
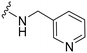
|
11 | 150 (14) | 910 (83) | 1200 (110) |
| 2a |

|
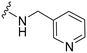
|
51 | 680 (13) | 2900 (57) | 2400 (47) |
| 2b |
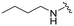
|
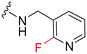
|
68 | 940 (14) | 3100 (46) | 2700 (40) |
| 2c |
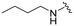
|
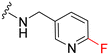
|
26 | 390 (15) | 1900 (73) | 1400 (54) |
| 2d |

|
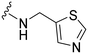
|
38 | 400 (11) | 1600 (42) | 1500 (39) |
UNC2876 (3) also belongs to the pyrimidine 5-carboxamide family and is reported as a potent inhibitor of MERTK.27 This compound exhibits strong inhibitory activity against MERTK (IC50 of 3.6 nM) with good selectivity over AXL, TYRO3, and FLT3 kinases.27 Moreover, compound 3 structurally resembles our recently reported 18F-labelled MERTK radioligand (MIPS15692).24 Due to its high potency and selectivity, we selected 3 as our third lead for further SAR investigation focusing on fluorine atom installations. Its CNS MPO score of 2.3 is relatively low (below the recommended threshold of ≥4 for CNS permeability), however, and points to the likely need for improvement during future lead optimisation. As depicted in Fig. 2 and Table 3, our SAR modifications on compound 3 are focused on modifying sites A and B. Compound 3 was resynthesized as our positive control along with our previous fluorinated MERTK ligand, MIPS15692, and evaluated in TAM and FLT3 kinase inhibition assays to compare with its new derivatives. The observed TAM and FLT3 activity profiles of compound UNC2876 (3) and MIPS15692 are detailed in Table 3, entries 1 & 2. As previously reported, both compounds UNC2876 (3) and MIPS15692 potently inhibit MERTK (IC50 = 9.8 nM and 21 nM, respectively) while showing reduced activity against AXL, TYRO3, and FLT3, indicating strong selectivity for MERTK. However, their IC50s are a little weaker than the reported values. As indicated previously, we speculate that this difference in IC50 may be attributed to the distinct assays used. Initial modifications of compound 3 were primarily focused on modifying the n-butylamine side chain at C2 of the pyrimidine. Two fluorinated compounds, 3a and 3b, were prepared and evaluated for their TAM and FLT3 potency using a kinase inhibition assay. The kinase assay produced variable outcomes concerning n-butyl amine modification, which incorporated a 4-fluorobutylamine side chain at the C2 position of the pyrimidine 5-carboxamide core (Table 3). Compound 3a demonstrated moderate MERTK inhibitory activity with reduced off-target activity against AXL, TYRO3, and FLT3 kinases.
| Compound | R1 | R2 | IC50a (nM) | |||
|---|---|---|---|---|---|---|
| MERTK | AXL | TYRO3 | FLT3 | |||
| a IC50 values are the mean of two independent radiometric protein kinase assays (SD = ±0.5 nM), selectivity index values are listed in brackets. b Reported IC50 values by UNC. | ||||||
| UNC2876 (3) |
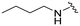
|
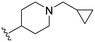
|
9.8 | 92 (9) | 880 (90) | 4000 (408) |
| 3.6b | 218b | 150b | 49b | |||
| MIPS15692 |
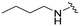
|
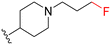
|
21 | 270 (13) | 1400 (67) | 7000 (333) |
| 4.0b | 170b | 400b | 110b | |||
| 3a |

|
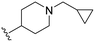
|
160 | 960 (6) | 4900 (31) | >10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (>63) 000 (>63) |
| 3b |

|
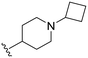
|
47 | 440 (9) | 3200 (68) | 9400 (200) |
| 3c |
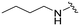
|
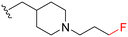
|
95 | 360 (4) | 3300 (35) | 4500 (47) |
| 3d |
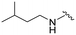
|
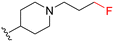
|
56 | 370 (7) | 3800 (68) | 8700 (155) |
| 3e |
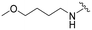
|
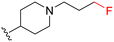
|
190 | 990 (5) | 9700 (51) | >10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (>53) 000 (>53) |
When comparing compound 3a to its parent compound 3, a 16-fold loss of activity was observed for MERTK, yielding an IC50 value of 160 nM. In contrast, the activities for AXL, TYRO3, and FLT3 demonstrated further reductions, with declines of ∼10-fold, ∼5.6-fold, and >2.5-fold, respectively. Nonetheless, the elevated IC50 values for MERTK led to a diminished SI, particularly concerning the AXL kinase when compared to compound 3. Interestingly, our new 4-fluorobutylamine pyrimidine 5-carboxamide analogue 3b with a slight modification on piperidine ring, has retained activity against MERTK with an IC50 of 47 nM, and decreased AXL (∼4.5-fold), TYRO3 (∼3.6-fold), and FLT3 (∼2.5-fold) kinase activity profiles compared with its parent compound 3. Furthermore, this compound has demonstrated satisfactory SI values against AXL (∼9-fold), TYRO3 (∼68-fold), and FLT3 (∼200-fold) kinases. Further modifications of compound 3 were carried out on both n-butylamine and piperidine sides of the pyrimidine core. Three new analogues 3c–3e were prepared by engaging several alkyl and alkoxy amine linkers at the C2 position of the pyrimidine while keeping either the 4-(3-fluoropropyl)piperidin-4-amine or (1-(3-fluoropropyl)piperidin-4-yl)methanamine on the pyrimidine 5-carboxamide side. The kinase assay data revealed >10-fold loss of MERTK potency for 3c and 3e, while compound 3d partially retained MERTK activity (IC50 = 56 nM) and selectivity, being over ∼7-fold for AXL, over ∼68-fold for TYRO3, and ∼155-fold for FLT3. Overall, this SAR study on compound 3 has produced two fluorinated ligands of MERTK (3b and 3d), among them 3b with the most merit for continued characterisation and development.
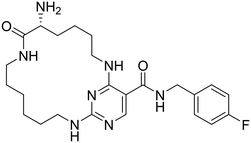
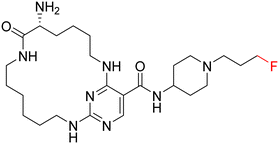
Macrocycles have gained a great deal of attention in drug discovery over the past decade. Macrocyclization of kinase inhibitors can improve physicochemical properties such as CNS penetration, on-target efficacy, and selectivity.30 Macrocycle formation can help restrain molecular flexibility and facilitate target binding by means of reducing the entropic energy penalty on binding.30 Recently, Mclver et al. reported UNC2541 (4) as a potent (IC50 = 4.4 nM) and selective macrocyclic MERTK inhibitor.31 Based on 4 and MIPS15692, a new macrocyclic hybrid (4a) was designed, replacing the 4-fluoro benzylamine group of 4 by a 4-(3-fluoropropyl)piperidin-4-amine that is a feature of MIPS15692. The synthesis of new macrocyclic compound 4a was achieved by a similar synthetic route described in the literature for 4 but with slight modifications (SI).31 TAM and FLT3 kinase profiling of 4a, the potency and selectivity profile for MERTK, is summarized in Table 4. The new macrocyclic analogue, 4a, shows significant MERTK bioactivity with an IC50 of 52 nM, compared to its parent macrocycle, 4, which has an IC50 of 4.4 nM. This represents approximately a 12-fold loss in activity. This difference is believed to be due to the various assay screenings used, specifically the kinase inhibition assay versus the MCE. Moreover, 4a also maintained good selectivity over AXL (∼4-fold) and superior selectivity over TYRO3 (∼60-fold) and FLT3 (>192-fold). This result firmly confirms macrocyclization with 4-(3-fluoropropyl)piperidin-4-amine linker on pyrimidine carboxylic acid side as a viable strategy for abrogating FLT3 bioactivity. Despite the encouraging kinase activity profile, the macrocyclic variant 4a has exhibited a low CNS MPO score of 2.5 that has emerged from its large molar mass (506.3 g mol−1) and high topological polar surface area (136.2 Å2). Many macrocycles, however, have been known to retain favourable permeability properties despite being mismatched by their physicochemical metrics, in part because of intramolecular burial of solvent exposed surfaces, a phenomenon not necessarily recognized by MPO, a parameter based on regression analyses not derived from a macrocyclic training set. In summary, macrocyclic ligand 4a is a potent and selective MERTK ligand, warranting further investigation of molecular simplification and assessment of cellular activity and permeability properties.
It is apparent that in order to understand SAR trends, we have used biochemical IC50, and this is a convenient and reasonable approach. More traditionally, PET radiotracer development assesses affinity rather than biochemical inhibition, but for a ligand that competes with ATP at its binding site, the biochemical IC50 value could be viewed as more relevant to gauge future PET radiotracer potential. However, even more relevant is potency in a cellular context. Therefore, several leading compounds were selected for the assessment of cellular activity against MERTK. The cell-based assay measured the extent of MERTK phosphorylation that occurs intracellularly.31 It is a reasonable assumption that more potent inhibitors with lower cellular IC50 values represent ligands with greater occupancy at the ATP-binding site in an intracellular context. On this basis and from the perspective of the needs for radiotracer development, the cell-based functional inhibition of MERTK may be considered to be more relevant than the biochemical assay (IC50). The cellular activities of selected ligands against MERTK are summarized in Table 5. All MERTK ligands, except for 2c, showed a sub-micromolar phosphorylation bioactivity against pMERTK in these cell-based assays. Compound 3b, bearing the fluoro butylamine substituent, was the strikingly most active compound in this pMERTK ELISA assay with an IC50 of 16 nM. Most of the other ligands exhibited a decrease in MERTK bioactivity by 10- to 20-fold in comparison with their biochemical IC50 values. The shift between non-cellular (IC50) and cellular (IC50) bioactivity was not unexpected since cellular permeability is a variable that can negatively influence cell-based activity, but in particular higher intracellular concentrations of competing substrate, ATP, than deployed in the biochemical assay.31 Compounds 3b, 3d and 3e were shown to have better cellular activity compared with that of fluorophenyl imidazole analogue 1a, despite being less active against MERTK in the biochemical kinase inhibition assay, correlating to the drop of CNS MPO score from 3.5 (3d), 3.1 (3b) to 2.4 (1a) as seen in Table 5. The large variation in the activity of 2c in terms of the large drop from the kinase inhibition assay to the pMERTK ELISA assay remains unexplained, although it could be speculated that 2-fluoropyridyl electrophilic reactivity could interfere in the cellular assay.
Overall, the introduction of fluorine at the butyl side chain resulted in good activities against MERTK in both MCE and pMERTK ELISA assays. Nevertheless, the variations from compound to compound in the ratio between the pMERTK ELISA IC50 and MCE MERTK IC50 are marked, and if the former value is more representative of PET radiotracer utility, this observation is an important consideration for ranking and prioritisation of leading compounds. Overall, our aim in this work was to establish a robust SAR foundation, particularly exploring fluorination and other modifications to optimize MERTK affinity and CNS permeability. While radiolabeling studies are part of our longer-term plans, they were beyond the scope of the present manuscript.
Conclusions
In summary, a series of fluorinated and non-fluorinated pyrimidine-5-carboxamide derivatives and related compounds were prepared. Potency and selectivity for MERTK for all compounds were evaluated by a kinase inhibition assay. Several potent compounds against MERTK were discovered with good selectivity profiles over other off-targets (AXL, TYRO3, and FLT3), and compounds 1a, 2a, 2c, 2d, 3b, and 3d especially displayed two-digit nanomolar potency for MERTK with >5-fold selectivity over other TAM enzymes. Importantly, compounds 1a, 2c, and 3b revealed that a fluorine substituent can be installed in specific positions on both sides of the pyrimidine carboxamide core without significantly compromising MERTK potency and selectivity. Macrocyclic variant 4a was shown to be potent against MERTK with superior selectivity over FLT3. Our cellular (pMERTK IC50) bioactivity of selected MERTK ligands revealed that compound 3b was the strikingly most active compound. Overall, the most promising pyrimidine carboxamide derivatives—compounds 1a, 2a, 2c, 3b, and 3d—warrant further investigation as potential 18F-labeled MERTK radioligands for MS imaging. However, additional optimisation of CNS permeability will likely be necessary.Author contributions
R. M. and S. W. W. contributed equally to this work. This manuscript was written through the contributions of all authors. All authors have approved the final version of the manuscript.Conflicts of interest
X. W. is equity holders in Meryx, Inc.Data availability
The synthesis and characterization data for all compounds in this article are included in the supporting data of the supplementary information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5md00695c.Acknowledgements
The authors thank the National Health and Medical Research Council of Australia (NHMRC) for research grant and fellowship for J. B. B. (1117602 and 1142814) and Investigator grant for T. J. K. (1175775). Both J. B. B. and T. J. K. thank MS Australia for research grant (20-0119).References
- D. S. Reich, C. F. Lucchinetti and P. A. Calabresi, Multiple Sclerosis, N. Engl. J. Med., 2018, 378(2), 169–180 CrossRef CAS PubMed.
- A. J. Thompson, S. E. Baranzini, J. Geurts, B. Hemmer and O. Ciccarelli, Multiple sclerosis, Lancet, 2018, 391(10130), 1622–1636 CrossRef PubMed.
- W. J. Brownlee, T. A. Hardy, F. Fazekas and D. H. Miller, Diagnosis of multiple sclerosis: progress and challenges, Lancet, 2017, 389(10076), 1336–1346 CrossRef PubMed.
- P. Wildner, M. Stasiołek and M. Matysiak, Differential diagnosis of multiple sclerosis and other inflammatory CNS diseases, Mult. Scler. Relat. Disord., 2020, 37, 101452 CrossRef PubMed.
- J. W. Prineas, E. E. Kwon, E. S. Cho, L. R. Sharer, M. H. Barnett, E. L. Oleszak, B. Hoffman and B. P. Morgan, Immunopathology of secondary-progressive multiple sclerosis, Ann. Neurol., 2001, 50(5), 646–657 CrossRef CAS PubMed.
- C. F. Lucchinetti, B. F. G. Popescu, R. F. Bunyan, N. M. Moll, S. F. Roemer, H. Lassmann, W. Brück, J. E. Parisi, B. W. Scheithauer and C. Giannini, et al., Inflammatory Cortical Demyelination in Early Multiple Sclerosis, N. Engl. J. Med., 2011, 365(23), 2188–2197 CrossRef CAS PubMed.
- M. H. Barnett and J. W. Prineas, Relapsing and remitting multiple sclerosis: pathology of the newly forming lesion, Ann. Neurol., 2004, 55(4), 458–468 CrossRef PubMed.
- S. Guo, H. Wang and Y. Yin, Microglia Polarization From M1 to M2 in Neurodegenerative Diseases, Front. Aging Neurosci., 2022, 14, 1–16 Search PubMed.
- J. D. Cherry, J. A. Olschowka and M. K. O'Banion, Neuroinflammation and M2 microglia: the good, the bad, and the inflamed, J. Neuroinflammation, 2014, 11(1), 98 CrossRef PubMed.
- V. Salvi, F. Sozio, S. Sozzani and A. Del Prete, Role of Atypical Chemokine Receptors in Microglial Activation and Polarization, Front. Aging Neurosci., 2017, 9, 148 CrossRef PubMed.
- L. T. Remington, A. A. Babcock, S. P. Zehntner and T. Owens, Microglial Recruitment, Activation, and Proliferation in Response to Primary Demyelination, Am. J. Pathol., 2007, 170(5), 1713–1724 CrossRef PubMed.
- M. M. Hiremath, Y. Saito, G. W. Knapp, J. P. Y. Ting, K. Suzuki and G. K. Matsushima, Microglial/macrophage accumulation during cuprizone-induced demyelination in C57BL/6 mice, J. Neuroimmunol., 1998, 92(1), 38–49 CrossRef CAS PubMed.
- E. J. McMahon, K. Suzuki and G. K. Matsushima, Peripheral macrophage recruitment in cuprizone-induced CNS demyelination despite an intact blood–brain barrier, J. Neuroimmunol., 2002, 130(1), 32–45 CrossRef CAS PubMed.
- P. G. E. Kennedy, W. George and X. Yu, The Possible Role of Neural Cell Apoptosis in Multiple Sclerosis, Int. J. Mol. Sci., 2022, 23(14), 7584 CrossRef CAS PubMed.
- S. Hisahara, J. Yuan, T. Momoi, H. Okano and M. Miura, Caspase-11 Mediates Oligodendrocyte Cell Death and Pathogenesis of Autoimmune-Mediated Demyelination, J. Exp. Med., 2001, 193(1), 111–122 CrossRef CAS PubMed.
- E. L. Werry, F. M. Bright, O. Piguet, L. M. Ittner, G. M. Halliday, J. R. Hodges, M. C. Kiernan, C. T. Loy, J. J. Kril and M. Kassiou, Recent Developments in TSPO PET Imaging as A Biomarker of Neuroinflammation in Neurodegenerative Disorders, Int. J. Mol. Sci., 2019, 20(13), 1–21 Search PubMed.
- W. Beaino, B. Janssen, D. J. Vugts, H. E. de Vries and A. D. Windhorst, Towards PET imaging of the dynamic phenotypes of microglia, Clin. Exp. Immunol., 2021, 206(3), 282–300 CrossRef PubMed.
- G. Tondo, D. Perani and C. Comi, TAM Receptor Pathways at the Crossroads of Neuroinflammation and Neurodegeneration, Dis. Markers, 2019, 2019, 2387614 Search PubMed.
- G. Zizzo, B. A. Hilliard, M. Monestier and P. L. Cohen, Efficient clearance of early apoptotic cells by human macrophages requires M2c polarization and MerTK induction, J. Immunol., 2012, 189(7), 3508–3520 CrossRef CAS PubMed.
- M. D. Binder, A. D. Fox, D. Merlo, L. J. Johnson, L. Giuffrida, S. E. Calvert, R. Akkermann, G. Z. M. Ma, Anzgene and A. A. Perera, et al., Common and Low Frequency Variants in MERTK Are Independently Associated with Multiple Sclerosis Susceptibility with Discordant Association Dependent upon HLA-DRB1*15:01 Status, PLoS Genet., 2016, 12(3), e1005853 CrossRef PubMed.
- G. Z. M. Ma, J. Stankovich, A. The, C. New Zealand Multiple Sclerosis Genetics, T. J. Kilpatrick and M. D. Binder, Field, J. Polymorphisms in the Receptor Tyrosine Kinase MERTK Gene Are Associated with Multiple Sclerosis Susceptibility, PLoS One, 2011, 6(2), e16964 CrossRef CAS PubMed.
- K. Shen, M. Reichelt, R. V. Kyauk, H. Ngu, Y. A. Shen, O. Foreman, Z. Modrusan, B. A. Friedman, M. Sheng and T. J. Yuen, Multiple sclerosis risk gene Mertk is required for microglial activation and subsequent remyelination, Cell Rep., 2021, 34(10), 108835 CrossRef CAS PubMed.
- H. Andrew, D. Robert and P. Martin, [18F]JHU16907 for PET Imaging of MER tyrosine kinase (MERTK), J. Nucl. Med., 2017, 58(supplement 1), 209 Search PubMed.
- S. W. Wong, L. Vivash, R. Mudududdla, N. Nguyen, S. J. Hermans, D. M. Shackleford, J. Field, L. Xue, A. Aprico and N. C. Hancock, et al., Development of [18F]MIPS15692, a radiotracer with in vitro proof-of-concept for the imaging of MER tyrosine kinase (MERTK) in neuroinflammatory disease, Eur. J. Med. Chem., 2021, 226, 113822 CrossRef CAS PubMed.
- L. Wang, Y. Zhou, X. Wu, X. Ma, B. Li, R. Ding, M. A. Stashko, Z. Wu, X. Wang and Z. Li, The Synthesis and Initial Evaluation of MerTK Targeted PET Agents, Molecules, 2022, 27(5), 1460 CrossRef CAS PubMed.
- W. Zhang, A. L. McIver, M. A. Stashko, D. DeRyckere, B. R. Branchford, D. Hunter, D. Kireev, M. J. Miley, J. Norris-Drouin and W. M. Stewart, et al., Discovery of Mer Specific Tyrosine Kinase Inhibitors for the Treatment and Prevention of Thrombosis, J. Med. Chem., 2013, 56(23), 9693–9700 CrossRef CAS PubMed.
- X. Wang, W. Zhang and S. Frye, Therapeutic uses of selected pyrrolopyrimidine compounds with anti-mer tyrosine kinase activity, WO2015/157127A1, 2015.
- M. Hoque, S. Wai Wong, A. Recasens, R. Abbassi, N. Nguyen, D. Zhang, M. A. Stashko, X. Wang, S. Frye and B. W. Day, et al., MerTK activity is not necessary for the proliferation of glioblastoma stem cells, Biochem. Pharmacol., 2021, 186, 114437 CrossRef CAS PubMed.
- T. T. Wager, X. Hou, P. R. Verhoest and A. Villalobos, Central Nervous System Multiparameter Optimization Desirability: Application in Drug Discovery, ACS Chem. Neurosci., 2016, 7(6), 767–775 CrossRef CAS PubMed.
- A. Poulsen and B. Dymock, CHAPTER 5 Small Molecule Macrocyclic Kinase Inhibitors, In Kinase Drug Discovery: Modern Approaches, The Royal Society of Chemistry, 2019, pp. 97–127 Search PubMed.
- A. L. McIver, W. Zhang, Q. Liu, X. Jiang, M. A. Stashko, J. Nichols, M. J. Miley, J. Norris-Drouin, M. Machius and D. DeRyckere, et al., Discovery of Macrocyclic Pyrimidines as MerTK-Specific Inhibitors, ChemMedChem, 2017, 12(3), 207–213 CrossRef CAS PubMed.
Footnote |
| † R. M. and S. W. W. contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2026 |