Open Access Article
Open Access ArticleNumerical investigation of high-performance bilayer tin-based perovskite solar cells with SCAPS-1D
Hariharan
Rajasekaran
 ,
Thangaraji
Vasudevan
and
Lung-Chien
Chen
,
Thangaraji
Vasudevan
and
Lung-Chien
Chen
 *
*
Department of Electro-Optical Engineering, National Taipei University of Technology, Taipei City 10608, Taiwan, Republic of China. E-mail: rhariharan318@gmail.com; orgthangaraji@gmail.com; ocean@ntut.edu.tw; Tel: (+886)-2-2771-2171
First published on 3rd January 2026
Abstract
A comprehensive simulation-based investigation was conducted on an advanced, lead-free perovskite solar cell (PSC) design. This cell achieved high performance through its novel absorber architecture, which utilized a dual-layer configuration made of tin-based perovskite materials (CsSnI3 and CsSnCl3). Simulations were carried out to determine device performance and stability limits by employing the SCAPS-1D software tool. The device structure was designed to enable bandgap alignment with CsSnI3, which was used as a narrow bandgap material to act as a light harvester, and CsSnCl3, which was used as a wider bandgap material to act as a charge and defect passivation layer. Prior to the simulations, necessary material parameter details, such as the orbital components forming the band edges and bandgap widening, were thoroughly verified. RIGOROUS simulations on SCAPS-1D revealed a maximum power conversion efficiency (PCE) value of 30.02% (FF = 88.56%, Jsc = 32.09 mA cm−2, and Voc = 1.05 V) when optimal parameter inputs were used. Important stability constraints on various PSC devices were obtained by precisely modelling the defects, which resulted in PCE failure when either the interface defect density value and/or the respective bulk density value of either CsSnI3/CsSnCl3 layer exceeded 1 × 1015 cm−3. Therefore, high-quality materials are mandatory. In addition, thermal stability analysis indicated that the PCE value is inversely related to temperature. Importantly, the analysis indicates that the voltage component Voc influences the PCE value predominantly.
1. Introduction
Perovskite solar cells (PSCs) have attracted considerable attention owing to their high power conversion efficiency (PCE), which reaches 26%, and their very low-cost fabrication process.1–3 Moreover, their tunable bandgap, long carrier diffusion ranges, and solution processability render them promising for future solar cells. Recently, nanocrystals of all-inorganic caesium lead halide perovskites (PVSKs) (CsPbY3, wherein Y is a halogen atom) have been synthesized, and they exhibit exceptional optoelectronic attributes as well as easy solution-processing.4,5 Although their brilliant performance has attracted great attention, their toxicity due to the presence of environmentally hazardous lead is a very important concern that is yet to be properly alleviated.6 To alleviate such toxicity, recent attempts have been directed towards exploring alternatives, more environmentally friendly materials, and tin-containing PVSKs (CsSnY3) have recently emerged as very promising materials.7–9 Although tin- and lead-containing PVSKs possess remarkable optoelectronic attributes owing to their identical electronic configuration, their toxicity is yet to be properly alleviated. In tin-containing PVSKs, the high instabilities of Sn2+ towards oxidation to Sn4+ under ambient conditions (which cause the formation of p-type self-doped materials) and the high recombination rate of charge carriers significantly impede device stability and efficiency.10–12 Although CsSnY3 PVSKs offer great benefits owing to their optimal bandgap, high carrier mobility, and less adverse effects on the environment compared with those of their counterpart Cd- and Pb-based materials,13 their further utilization is impeded by their instabilities.Among Sn-based halide PVSKs, CsSnI3 has become a reassuring light-harvesting material because of its direct bandgap (∼1.3 eV), strong optical absorption in the visible to near-infrared regions, and robust defect-independent electronic band structure.14,15 CsSnI3 exhibits low stability under atmosphere ambient, which restricts its applications involving long lifetimes. However, a promising route to optimizing efficiency and robustness simultaneously involves the design of bilayer and graded bandgap absorber designs by incorporating CsSnI3 and wide-bandgap PVSKs, such as CsSnCl3.16 As CsSnCl3 has a wider bandgap of ∼2.3 eV, it acts satisfactorily as a ‘window’ layer, increasing the band match and blocking recombination at interfaces.17 The CsSnCl3/CsSnI3 bilayer structure has several beneficial attributes: (i) dual-band optical absorption because of the wide and narrow bandgap materials, which augment light and carrier concentration simultaneously;18 (ii) the graded band profile enables efficient carrier separation and hinders recombination, and (iii) CsSnCl3 acts as an oxidation passivation agent and protects CsSnI3 layers against oxidation, which degrades device stability.19 This design takes inspiration from band engineering methods adopted by high-efficiency Pb-based tandems and PSCs but is applicable to a completely lead-free structure.
Further optimization of device performance requires the selection of appropriate charge transport layers. PCBM (phenyl-C61-butyric acid methyl ester) is commonly employed as an ETL because of its high electron mobility, good energy-level alignment with the conduction bands of PVSKs, and ability to reduce interfacial recombination.20,21 On the other hand, Cu2SnS3 (CFTS) has recently attracted attention as a suitable HTL material because of its earth-abundant components, chemical stability, proper work function, and compatibility with the absorbers of PVSKs.22–24 The employment of CFTS as a nontoxic and stable HTL opens up a more environmentally friendly pathway over conventional organic HTL materials, such as Spiro-OMeTAD, which are restricted because of their high cost and poor long-term stability.25 As a result, the device architecture PCBM/CsSnCl3/CsSnI3/CFTS represents a totally lead-free inorganic PSC that has optimized interfaces for efficient charge transport coupled with enhanced stability during device operation. This device architecture not only minimizes the toxicity issues but also inherits the synergistic advantages of bilayer absorbers, inorganic stability, and environmentally benign transport layers.26,27 Recent simulations and experimental investigations have pointed out that the critical optimization of the thickness of bilayer absorbers is highly necessary to balance the competing processes of light absorption and charge transport, which directly influence the Voc, Jsc, and FF.28–30,39,40
This research focuses on a fully lead-free solar cell structure (PCBM/CsSnCl3/CsSnI3/CFTS) and uses one-dimensional semiconductor simulation (SCAPS-1D) to explore the impact of the thickness of the absorber layer on the overall device efficiency and operation. Although most studies have focused on either single-absorber CsSnY3-based devices or Pb-based bilayer architectures, our effort exceptionally probes the synergistic CsSnCl3/CsSnI3 bilayer as well as the ecologically friendly charge transport layers. We report critical insights into the rational design of stable and highly efficient lead-free PSCs through thickness-dependent changes in the PCE, Jsc, FF, and Voc. The findings underscore that the strategic engineering of the bilayer, in concert with ecologically friendly transport materials, can offer a realistic pathway toward next-generation, sustainable photovoltaic devices.
2. Methodology and modelling
2.1. Density functional theory calculations
Density functional theory (DFT) calculations were used to examine the electronic characteristics of CsSnI3 and CsSnCl3 with the Quantum ESPRESSO package (v7.4.1).31 The Perdew–Burke–Ernzerhof (PBE) functional was employed, which is a type of generalized gradient approximation (GGA), to effectively model the exchange and correlation effects governing electron–electron interactions. Scalar-relativistic standard-preserving pseudopotentials were utilized for Cs, Sn, I, and Cl. To achieve sufficient precision and ensure the convergence of the total energy, the simulation employed a plane-wave cutoff energy set at 60 Rydberg (Ry). Additionally, the Brillouin zone was sampled using a 6 × 6 × 6 Monkhorst–Pack mesh, which successfully limited the residual error in the total energy calculation to less than 10−6 Ry. The cubic crystal structures of CsSnI3 and CsSnCl3 were fully optimized such that the forces affecting every atom were less than 10−3 Ry Bohr−1. Subsequently, the density of states and electronic band structures were computed based on the relaxed geometries, with vacuum-level alignment applied to reference energy levels relative to the Fermi energy. All the simulations were performed utilizing Quantum ESPRESSO, a publicly available plane-wave DFT software package.312.2. Governing laws and equations in SCAPS-1D modeling
SCAPS-1D, a simulator application that was first designed and developed at Ghent University under the guidance of Professor M. Burgelman, has been extensively employed for modelling diverse solar cell architectures.32 In this research, we conducted numerical simulations using SCAPS-1D (version 3.3.12). The software allows the incorporation of up to seven layers, along with front and back contacts, as input parameters. Key device metrics, such as the PCE (η), Jsc, FF, current–voltage (J–V), and spectral response (QE) characteristics, can be evaluated. The simulations are principally controlled by the interaction of two fundamental physical laws: the distribution of the internal electric field is explained by Poisson's eqn (1), while the transport and conservation of electrons and holes are described by the continuity eqn (2).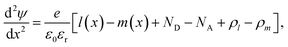 | (1) |
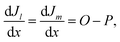 | (2) |
The drift and diffusion equations are given by eqn (3) and (4), respectively:
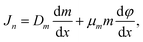 | (3) |
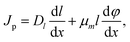 | (4) |
To complement the atomic-scale insights obtained from DFT, the solar energy efficiency of the CsSnI3/CsSnCl3 bilayer solar cell was investigated through device-level simulations using SCAPS-1D (solar cell capacitance simulator). SCAPS-1D is a one-dimensional modeling application widely utilized for thin-film photovoltaic cells. This structure facilitates the in-depth examination of the electrical, optical, and recombination processes within multilayer device structures. The bilayer absorber was constructed by stacking CsSnI3 and CsSnCl3 with thicknesses optimized according to material parameters derived from DFT calculations and previous reports. Key inputs included bandgap energies, electron affinities, dielectric constants, effective density of states, carrier mobilities, and defect densities. Attention was devoted toward interface defect density and band alignment, extracted from DFT electrostatic potential analysis, to realistically capture charge transport and recombination dynamics at the heterojunction.
To maintain model integrity, the numerical input parameters for the SCAPS-1D simulation, specifically the dielectric constants, carrier effective masses, and mobilities, were sourced from established theoretical literature and experimental details (as detailed in Table 1) rather than directly from the DFT calculations. While DFT was employed in this study to offer a qualitative understanding of the electronic structure and orbital hybridization of the bilayer interface, the SCAPS-1D framework utilizes literature-validated values to maintain consistency with recognized benchmarks. Furthermore, the adoption of a 1.52-eV bandgap for the CsSnCl3 layer, rather than its bulk value of ∼3.0 eV, is a strategic choice representing an optimized Cl-rich mixed-halide interface layer (SnI3−xClx). This value is consistent with high-performance Sn-halide heterojunction simulations23,26,37,39,40 and ensures the ideal graded band alignment required to maximize charge extraction while suppressing interfacial recombination.
| Parameter | FTO | PCBM | CsSnI3 | CsSnCl3 | CFTS |
|---|---|---|---|---|---|
| Thickness (µm) | 0.5 | 0.05 | 0.1–1.5 | 0.15 (varied) | 0.1 |
| E g (eV) | 3.5 | 2 | 1.3 | 1.52 | 1.3 |
| X (eV) | 4 | 3.9 | 3.6 | 3.90 | 3.3 |
| ε r | 9 | 3.9 | 9.93 | 29.4 | 9 |
| N c (cm−3) | 2.2 × 1018 | 2.5 × 1021 | 1 × 1019 | 1 × 1019 | 2.2 × 1018 |
| N v (cm−3) | 1.8 × 1019 | 2.5 × 1021 | 1 × 1018 | 1 × 1019 | 1.819 |
| μ n (cm2 V−1 s−1) | 20 | 0.2 | 1.5 × 103 | 2 | 21.98 |
| μ h (cm2 V−1 s−1) | 10 | 0.2 | 5.85 × 102 | 2 | 21.98 |
| N A (cm−3) | 0 | 0 | 1021 | 1 × 1015 | 1 × 1018 |
| N D (cm−3) | 1 × 1021 | 2.93 × 1017 | 0 | 0 | 0 |
| N t (cm−3) | 1 × 1015 | 1 × 1015 | 1 × 1013 | 1 × 1013 | 1 × 1015 |
The parameters for the material and interface used in the simulations are summarized in Tables 1 and 2. FTO was treated as a wide-bandgap degenerate semiconductor with high donor concentration to ensure transparency and conductivity, while PCBM was assigned a 2.0-eV bandgap with low carrier mobilities, consistent with its role as the ETL. The CsSnI3 absorber was modelled with a tunable thickness (0.1–1.5 µm), a 1.3-eV direct bandgap, and high carrier mobilities. Conversely, CsSnCl3 was represented with a fixed thickness of 150 nm (varied in bilayer configurations), a slightly larger bandgap of 1.52 eV, a high dielectric constant (εr = 29.4), and comparatively lower mobilities, reflecting its transport limitations.
| Interface | Defect charge state | Electron/hole capture cross section (cm2) | Energetic distribution | Defect energy level reference | Integrated defect density (cm−2) |
|---|---|---|---|---|---|
| a Single. b Above the VB maximum. | |||||
| CFTS/CsSnI3 | Neutral | 1 × 10−19 | 1 × 10−10 | ||
| CsSnI3/CsSnCl3 | Neutral | 1 × 10−19 | 1 × 10−10 | ||
| CsSnCl3/PCBM | Neutral | 1 × 10−19 | 1 × 10−10 | ||
CFTS, employed as the hole transport material, was defined with balanced carrier mobilities (∼22 cm2 V−1 s−1) and a moderate electron affinity (3.3 eV), ensuring efficient hole extraction. To account for recombination effects, defect densities were set to 1013 cm−3 for the bulk layers, and neutral interfacial trap states with a capture cross-section of 10−19 cm2 and 1010 cm−2 for density were introduced at the CFTS/CsSnI3, CsSnI3/CsSnCl3, and CsSnCl3/PCBM interfaces. These carefully chosen parameters enabled the simulations to reproduce trends consistent with those in the existing literature and reveal the performance improvements achieved in this work.
Illumination was modelled under typical AM1.5G solar spectrum conditions. The solution process involved solving Poisson's equation and the continuity equations (for both electrons and holes) concurrently and iteratively (self-consistently) throughout the entire simulated device structure. From the simulated J–V characteristics, key solar cell measurements like the FF, PCE, Voc, and Jsc were determined. A thickness-dependent study was performed by altering the depth of the active material layer from 0.3 to 1.5 µm to assess its influence on light absorption, carrier collection, and recombination processes. The results confirmed the DFT-based hypothesis that the graded bandgap bilayer facilitates efficient charge separation while suppressing recombination losses, resulting in the best possible device performance at a 1.5-µm absorber thickness.
A sensitivity analysis was conducted regarding the assumptions of the interface layer properties. The performance of the device was notably affected by the alignment of the bandgap at the CsSnI3/CsSnCl3 heterojunction. An increase in the interface bandgap beyond 1.55 eV causes a drop in the Jsc due to increased extraction barriers; reducing it below 1.50 eV led to higher interfacial recombination. This confirms that the selection of 1.52 eV representing an optimized mixed-halide interface is the critical 'sweet spot' for achieving the peak efficiency of 30.02%.
The combination of DFT and SCAPS-1D enables a deep understanding of how the material properties interact with device architecture to provide proper guidance for the rational design of efficient and lead-free PSCs.
3. Results and discussion
3.1. Charge density and electrostatic potential analysis of CsSnI3 and CsSnCl3
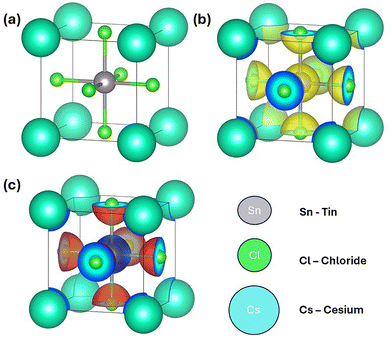
In Fig. 1(a), the crystal structure of CsSnI3 is depicted, where Cs atoms occupy the cub-octahedral sites and Sn atoms are octahedrally coordinated by I atoms. This characteristic perovskite (PVSK) lattice emphasizes the robust Sn–I framework that underpins both light absorption and charge transport. The charge density distribution shown in Fig. 1(b) reveals the strong localization of electronic density around iodine atoms, consistent with their high electronegativity. The pronounced intersection of Sn-5s and I-5p orbitals indicates mixed ionic-covalent bonding, which not only stabilizes the lattice but also enables defect tolerance, which is one of the defining attributes of halide PVSKs. Negligible charge accumulation at the Cs sites further confirms their role as electrostatic stabilizers rather than direct contributors to the frontier electronic states.The electrostatic potential profile shown in Fig. 1(c) displays oscillations arising from the periodic arrangement of cations and anions. Deep potential wells appear at iodine sites, while shallower regions are associated with Cs atoms. The overall smooth variation of potential across the lattice reflects effective charge screening and reduced internal transport barriers. This stable electrostatic environment facilitates efficient charge separation and suppresses recombination when CsSnI3 is integrated into device architectures. Overall, the charge density and electrostatic potential analyses underscore the electronic robustness of CsSnI3. The mixed ionic-covalent bonding ensures structural stability, while the smooth electrostatic landscape promotes efficient carrier extraction, reinforcing its suitability as an efficient layer that absorbs light for lead-free PSCs.
In Fig. 2(a), the cubic PVSK lattice of CsSnCl3 is shown, where Cs ions occupy the central A-site positions and Sn atoms are octahedrally coordinated by Cl atoms. Compared to CsSnI3, the presence of smaller and more electronegative Cl ions leads to shorter Sn–Cl bonds, which significantly influence the material's electronic configuration and structural stability. The charge density distribution shown in Fig. 2(b) demonstrates strong localization around Cl atoms, reflecting their dominant role in bond formation. The overlap between Sn-5s and Cl-3p orbitals confirms mixed ionic-covalent bonding with a stronger ionic character than the Sn–I framework. This bonding nature reduces electronic polarizability, thereby widening the bandgap and enhancing the material's chemical robustness. As in CsSnI3, the Cs atoms show negligible charge accumulation, serving mainly as electrostatic stabilizers.
The electrostatic potential profile shown in Fig. 2(c) reveals deeper potential wells at the Cl sites compared to the I sites in CsSnI3, consistent with their higher electronegativity. The smooth potential distribution across the lattice indicates minimal internal barriers for charge transport, and the well-defined potential landscape contributes to defect tolerance by mitigating charge trapping and recombination at lattice imperfections. Overall, CsSnCl3 exhibits a wide-bandgap electronic structure characterized by strong ionic bonding and stable electrostatic potential distribution. While this makes it less suitable as a primary light absorber, it is highly effective as a passivating and stabilizing capping layer in bilayer PSCs, complementing the visible-NIR absorption of CsSnI3 and suppressing interfacial recombination.
The charge density and electrostatic potential analyses of CsSnI3 and CsSnCl3 underscore their complementary roles in photovoltaic applications. CsSnI3, characterized by strong Sn–I orbital hybridization and a relatively smooth electrostatic potential profile, exhibits efficient charge transport and broad absorption across the visible-NIR region. Thus, it is an excellent primary light-harvesting layer. Nevertheless, the comparatively weaker Sn–I bonding and limited chemical stability of CsSnI3 lead to rapid degradation under ambient conditions. In contrast, CsSnCl3 exhibits stronger ionic bonding and deeper electrostatic potential wells associated with Cl atoms, which impart a wider bandgap and significantly improved structural stability. Although its visible-light absorption is intrinsically limited, the stable potential landscape and defect-tolerant nature of CsSnCl3 render it highly effective for suppressing interfacial recombination and passivating the CsSnI3 surface. At the atomic level, the CsSnCl3 capping layer provides field-effect passivation. Its wider bandgap and specific conduction band alignment create a potential barrier that prevents holes from reaching the electron-selective interface, thereby significantly reducing surface recombination velocities. Furthermore, the chloride-rich environment at the heterojunction can passivate iodine vacancies (VI) in the CsSnI3 layer a common trap state by forming stronger ionic Sn–Cl bonds that reduce the density of dangling bonds and suppress trap-mediated recombination.
Accordingly, in a bilayer configuration, CsSnCl3 functions as a protective and electronically complementary layer that enhances open-circuit voltage and device stability, while CsSnI3 remains the primary absorber responsible for photocurrent generation. This synergistic interplay between the narrow-bandgap, high-efficiency absorber (CsSnI3) and the wide-bandgap, stabilizing partner (CsSnCl3) provides a suitable foundation for the superior performance of the bilayer device compared with either constituent material alone.
3.2. Band structures of CsSnI3 and CsSnCl3
Fig. 3(a) presents the computed electronic band structure of CsSnI3 across the high-symmetry directions (Γ–X–M–Γ–R–X|M–R) in the material's Brillouin zone. At 0 eV, the Fermi level is set and shown with a dashed horizontal line. The band dispersion reveals the semiconducting nature of CsSnI3. The valence band maximum (VBM) and the conduction band minimum (CBM) are situated at the identical point, specifically the Γ point in reciprocal space, confirming a straight bandgap of around 1.2–1.3 eV. This bandgap value is consistent with those in previous reports for halide PVSKs and falls within the optimal range for photovoltaic applications. The conduction band displays significant curvature (dispersion), which suggests that the electrons have a small effective mass and, consequently, exhibit high mobility. Conversely, the relatively flat shape of the valence band implies that the holes are characterized by a heavier effective mass. Such electron–hole asymmetry is a hallmark of CsSnI3-based PVSKs and plays an essential part in defining their charge movement characteristics.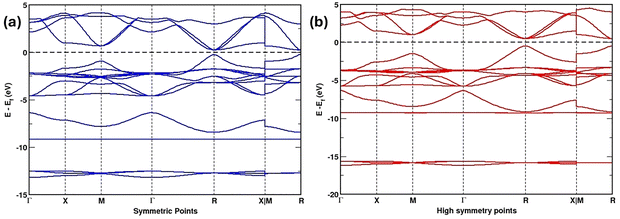 | ||
| Fig. 3 Calculated electronic band structures of (a) CsSnI3 and (b) CsSnCl3 along high-symmetry paths of the material's Brillouin zone. | ||
The orbital-resolved contributions further clarify the bonding and electronic structure. The deeper bands, located near 10 eV, arise primarily from iodine 5s states, while the valence band edge is dominated by hybridization among the I 5p and Sn 5s orbitals. Conversely, the states of the conduction band primarily originate from Sn 5p orbitals. This strong orbital interaction imparts covalent bonding characteristics and accounts for the significant band dispersion near the Fermi level. Overall, the results confirm that CsSnI3 is a semiconductor that has a straight bandgap, effective band dispersion and favourable optoelectronic properties, reinforcing its potential as a highly promising lead-free absorber for PSC and related optoelectronic applications.
Fig. 3(b) illustrates the calculated electronic band structure of CsSnCl3 along the high-symmetry directions (Γ–X–M–Γ–R–X|M–R) of the Brillouin zone, with the Fermi energy set to 0 eV (dashed line). The results reveal that CsSnCl3 is a straight bandgap semiconductor that has a calculated bandgap of 2.2–2.4 eV within the GGA-PBE framework, slightly smaller than the experimentally reported value (∼3.0 eV). The VBM and CBM are situated at the identical location, specifically the Γ point in reciprocal space, which verifies the direct bandgap nature of CsSnCl3. The CBM, primarily composed of Sn-5p states, shows noticeable dispersion, indicative of light electron effective masses and efficient electron transport. By contrast, the valence band, largely dominated by Cl-3p states, is more localized, leading to heavier hole effective masses than those observed in CsSnI3. The asymmetry is consistent with other wide-bandgap halide PVSK.
The deeper electronic states, spanning from approximately 10 eV to 15 eV, originate from strongly bound Sn–Cl orbitals that remain well separated from the frontier bands and contribute minimally to optical transitions. The flat nature of these deeper states underscores the stronger ionic bonding character of Sn–Cl compared to Sn–I. Collectively, these features highlight CsSnCl3 as a chemically stable wide-bandgap PVSK with favourable electron transport properties and intrinsic transparency in the visible range. Owing to such attributes, it is a suitable wide-bandgap partner in bilayer device configurations, where it effectively passivates and stabilizes CsSnI3 while complementing the strong visible NIR absorption of CsSnI3.
3.3. Electronic band structure analysis of CsSnI3 and CsSnCl3via partial density of states (PDOS)
The electronic structure of the CsSnI3 and CsSnCl3 perovskites was investigated using the PDOS, with the results presented in Fig. 4. For the CsSnI3 layer (Fig. 4(a)), the VBM is predominantly formed by I 5p-orbital and Sn 5p-orbital hybridization, with a minor contribution from the Sn 5s-orbital. The CBM, on the other hand, is primarily composed of the Sn 5s-orbital, contributing significantly to the low electron effective mass and the favourable charge transport properties of CsSnI3. The Cs 5s- and 5p-orbitals are located deeper within the valence band, confirming their inert role in the VBM and CBM formation.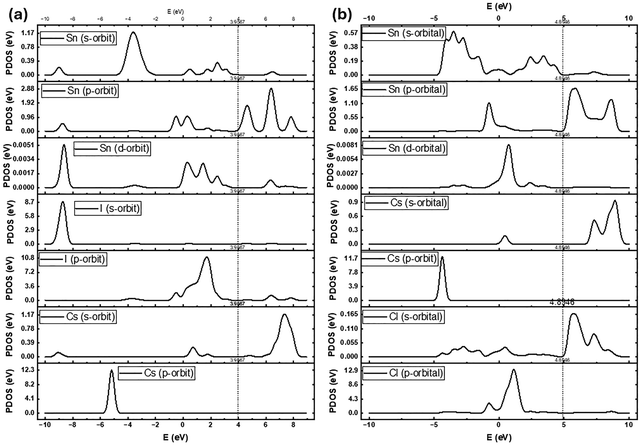 | ||
| Fig. 4 Partial density of states (PDOS) for (a) CsSnI3 and (b) CsSnCl3, highlighting the orbital contributions to the VBM and CBM. The dashed vertical line indicates the Fermi level. | ||
In the CsSnCl3 material (Fig. 4(b)), a similar contribution pattern is observed but with clear differences reflecting the wider bandgap introduced by the lighter halide element, Cl. The VBM is mainly derived from the hybridization of the Sn 5p-orbital and Cl 3p-orbital, again with some involvement from the Sn 5s-orbital. The CBM is still primarily dominated by the Sn 5s-orbital. The primary difference lies in the energy distribution: the VBM and CBM states are shifted to lower and higher energy values, respectively, relative to CsSnI3. This shift leads to a larger bandgap for CsSnCl3, which is essential for its function as a hole-blocking layer in the bilayer device. The substantial contribution of the Sn 5s and 5p states to the band edges in both compounds underscores the importance of the Sn cation oxidation state and coordination geometry in determining the overall electronic properties of these lead-free tin-based perovskites.
3.4. Energy band alignment of single and bilayer CsSnI3/CsSnCl3 absorbers
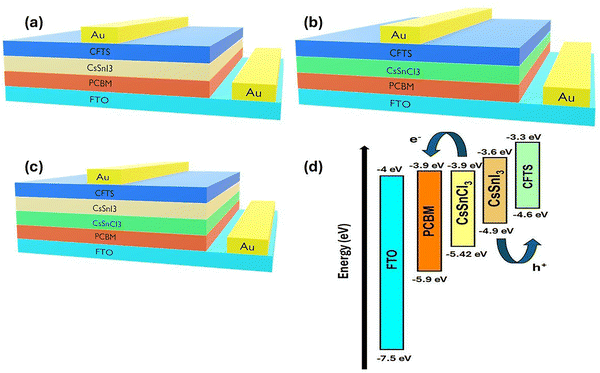
Fig. 5(a)–(c) illustrates the device architecture, where PCBM serves as the electron ETL; CsSnI3, CsSnCl3, or their bilayer acts as the PVSK absorbers; and CFTS functions as the HTL, using gold as the reverse connection and FTO as the fore contact. The corresponding energy band diagram (Fig. 5d) shows well-aligned conduction levels of CsSnI3 (−3.6 eV) and CsSnCl3 (−3.9 eV) with the CBM of PCBM (−3.9 eV), facilitating efficient electron extraction toward FTO. The valence bands of the absorbers (−4.9 eV for CsSnI3 and −5.42 eV for CsSnCl3) align favourably with CFTS (−4.6 eV), enabling effective hole transport to the Au electrode. Importantly, the bilayer configuration introduces a graded band alignment that couples the strong light absorption of CsSnI3 with the enhanced stability and higher open-circuit voltage (Voc) potential of CsSnCl3, thereby suppressing recombination and improving charge separation.Fig. 6 depicts the impact of band alignment on device performance for single and bilayer absorbers. In the CsSnI3-based device (Fig. 6a), the positioning of the valence band peak of CsSnI3 with the highest occupied state of CFTS enables effective hole extraction, although the relatively small conduction band offset at the CsSnI3/PCBM interface may promote back-electron recombination and reduce charge selectivity. In contrast, the CsSnCl3 device (Fig. 6b) benefits from a wider bandgap and higher CBM, which enhance electron blocking on the HTL side. However, the larger mismatch with CFTS impedes hole transport, accounting for its poor efficiency when paired with CFTS. The bilayer configuration (Fig. 6c) effectively integrates the advantages of both absorbers: CsSnI3 contributes strong visible-light absorption, while the CsSnCl3 capping layer improves alignment at the ETL interface, suppresses electron backflow, and enables graded band alignment. This synergistic arrangement promotes smoother carrier transport, reduces interfacial recombination, and highlights bilayer heterostructures as a promising route toward high-performance, lead-free PSCs.
 | ||
| Fig. 6 Simulated energy band diagrams for (a) CsSnI3, (b) CsSnCl3, and (c) the bilayer CsSnI3/CsSnCl3 PVSK absorber integrated with CFTS and PCBM. | ||
3.5. Impact of interface defect density on CsSnI3/CsSnCl3 solar cell performance
The electrical characteristics of the CsSnI3/CsSnCl3 PSC, including the PCE, FF, Voc, and Jsc, exhibit a strong dependency on the interface defect density, as shown in Fig. 10. A minimal impact is observed in the low defect density regime (1 × 1010 to 1 × 1014 cm−2), where Voc (approx. 1.05 V), Jsc (approx. 32.07 mA cm−2), and FF (approx. 86.5%) remain stable, resulting in a high PCE (29.16% to 26.54%). However, as the defect density increases beyond 1 × 1014 cm−2, a sharp degradation occurs across all performance metrics. Crucially, the Voc experiences a significant drop (Fig. 10(a)), falling sharply from 1.01 V at 1 × 1013 cm−2 to 0.84 V at 1 × 1016 cm−2 and saturating at 0.80 V above 1 × 1018 cm−2.Similarly, the Jsc (Fig. 10(c)) remains high until 1 × 1015 cm−2 (31.86 mA cm−2) and then sharply plummets to 15.45 mA cm−2 at 1 × 1020 cm−2. This severe reduction in the Voc and Jsc confirms that these interface defects act as efficient recombination centers, severely limiting carrier extraction and collection. This synergistic degradation leads to a monotonic fall in the PCE (Fig. 10(d)), which experiences its steepest decline between 1 × 1016 cm−2 and 1 × 1018 cm−2 (dropping from 18.99% to 10.14%) and eventually stabilizes at approximately 9.86% for defect densities exceeding 1 × 1020 cm−3, demonstrating the critical need for defect passivation in the CsSnI3/CsSnCl3 device architecture.
3.6. Impact of bulk defect density in the CsSnCl3 layer on bilayer solar cell performance
The photovoltaic performance of the CsSnI3/CsSnCl3 bilayer solar cell, with a fixed low defect density in the CsSnI3 layer (1 × 1013 cm−3), is critically dependent on the bulk defect density within the CsSnCl3 layer, as shown in Fig. 11. At low CsSnCl3 bulk defect densities (1 × 1010 to 1 × 1015 cm−3), the device exhibits outstanding stability and high performance: the Voc remains stable at approximately 1.05 V (Fig. 11(a)), the Jsc maintains its maximum value of approx. 32.09 mA cm−2 (Fig. 11(c)), and the FF remains high at approx. 88% (Fig. 11(b)).This robust performance translates to a peak PCE of 30.03% (Fig. 11(d)). However, the performance undergoes a severe collapse as the CsSnCl3 defect density increases beyond 1 × 1015 cm−3. It should be noted that the degradation in the Voc and FF is the primary driver of this failure. The Voc starts to drop at 1 × 1016 cm−3 (1.02 V) and falls sharply to 0.40 V at 1 × 1020 cm−3. Simultaneously, the FF plunges dramatically from 86.51% at 1 × 1015 cm−3 to only 21.54% at 1 × 1020 cm−3, indicating a significant increase in bulk recombination losses and internal resistance within the electron transport layer. This leads to a catastrophic reduction in the PCE, which plummets from 25.43% at 1 × 1016 cm−3 to a marginal 0.23% at 1 × 1020 cm−3, demonstrating the extreme sensitivity of the overall device efficiency to the crystalline quality of the wide-bandgap CsSnCl3 layer.
3.7. Photovoltaic characteristics and thickness-dependent studies
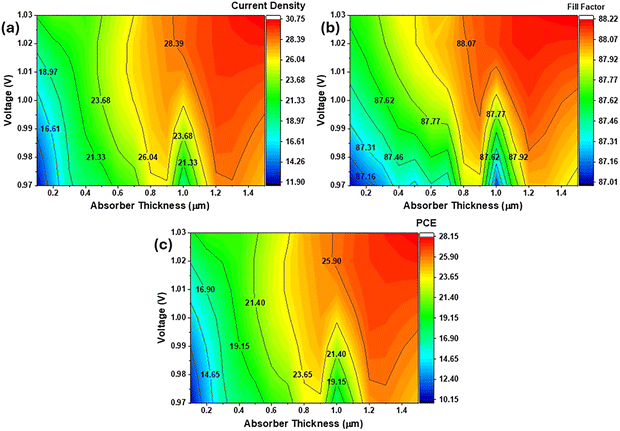
The comparative analysis clearly establishes the superiority of the CsSnI3/CsSnCl3 bilayer architecture over its single-layer counterparts, achieving the highest PCE of 30.02% (Fig. 12(c)). This performance significantly surpasses those of the single-layer CsSnI3 and CsSnCl3 cells of 28.12% and 14.12%, respectively. The CsSnI3 layer provides the necessary narrow bandgap for strong light absorption, resulting in a high Voc of 1.05 V and a high Jsc of 32.09 mA cm−2, substantially better than those of the CsSnCl3 cell of 0.74 V and 24.89 mA cm−2, respectively (Fig. 12(a) and (b)). Importantly, the introduction of the CsSnCl3 layer into the bilayer structure enhances the performance metrics beyond those of the pure CsSnI3 cell, boosting the Jsc from 30.73 to 32.09 mA cm−2 and maintaining an exceptional FF of 88.54%.The analysis of the PCE curves shown in Fig. 12(d) strongly supports the CsSnI3/CsSnCl3 bilayer as the optimal structure, particularly in its ability to maintain a superior PCE across all tested thicknesses. When the thickness of absorber is 0.1 µm, the bilayer structure already exhibits a superior PCE of 18.19%, which is significantly higher than those of the CsSnI3 and CsSnCl3 cells at 10.16% and 10.71%, respectively. The single-layer CsSnCl3 cell rapidly reaches its maximum PCE of 15.75% around 0.9 µm, and this PCE begins to decrease to 15.54% at 1.5 µm, demonstrating clear saturation and performance degradation in thicker films. In stark contrast, the bilayer and CsSnI3 cells continue their growth: at 1.5 µm, the CsSnI3 cell reaches 28.12%, while the bilayer maintains its lead, achieving the overall maximum efficiency of 30.02%. The fact that the bilayer PCE consistently increases up to the final measured thickness of 1.5 µm confirms its structural advantage in combining high photogeneration with efficient charge collection through minimized recombination. Thus, it is the highest performing and most promising configuration for further optimization beyond the simulated thickness range.
3.8. Analysis of quantum efficiency for single and bilayer PSCs
The wavelength-dependent external quantum efficiency (EQE) of CsSnI3, CsSnCl3, and their bilayer configuration is shown in Fig. 13, revealing distinct absorption behaviors for each device type. The CsSnI3 single absorber exhibits a strong photo response beginning at ∼310 nm, with the EQE rising to a maximum of ∼95% around 360–380 nm and remaining above 90% until ∼600 nm. Beyond this range, the EQE gradually decreases, falling below 80% near 800 nm and reaching ∼74% at 900 nm, reflecting its narrow bandgap (∼1.3 eV) that allows efficient visible-light absorption but limits response in the near-infrared. In contrast, the CsSnCl3 single absorber shows a much weaker response across the spectrum, peaking at ∼44% within the range of 300–330 nm and declining steadily to ∼14% at 900 nm. This limited performance is consistent with its wide bandgap (∼3 eV), which confines absorption primarily to the UV region.The bilayer CsSnI3/CsSnCl3 device, however, effectively combines the strong visible-light absorption of CsSnI3 with the UV response of CsSnCl3, producing a broader and enhanced EQE spectrum. In this configuration, the EQE exceeds 98% in the 350–600 nm range, surpassing the performance of both individual absorbers, remaining above 90% even at longer wavelengths (∼700–800 nm), where the CsSnI3 absorber begins to decline, and the CsSnCl3 absorber contributes minimally. These results demonstrate that the bilayer architecture leverages the complementary absorption properties of both materials, capturing UV photons via CsSnCl3 and visible photons via CsSnI3, which leads to superior photon-to-electron conversion. This synergistic effect accounts for the higher Jsc and overall PCE observed in the bilayer solar cell compared to those of the single-absorber devices.
3.9. Temperature-dependent photovoltaic performance of bilayer CsSnI3/CsSnCl3 PSCs
The temperature-dependent performance of the bilayer CsSnI3/CsSnCl3 PSC was systematically evaluated within the range of 300–500 K using SCAPS-1D simulations. As shown in Fig. 14, all the photovoltaic parameters exhibit a monotonic degradation with an increase in temperature. The Jsc (Fig. 14a) shows only a marginal decline, suggesting that the photogeneration of carriers remains largely unaffected. In contrast, the Voc (Fig. 14c) decreases sharply because of enhanced nonradiative recombination and a reduction in the built-in potential, while the FF (Fig. 14d) decreases progressively, reflecting increased series resistance and transport losses at elevated temperatures. Consequently, the PCE (Fig. 14b) undergoes a pronounced reduction of nearly 40% across the investigated range, primarily governed by the Voc decline, followed by FF losses, with the Jsc contributing minimally. These results underscore the intrinsic thermal instability of the CsSnI3/CsSnCl3 bilayer architecture and highlight the importance of defect passivation and interface optimization to mitigate recombination pathways and ensure stable operation under practical device conditions.3.10. Performance comparison of single and bilayer PSC based on SCAPS-1D simulations
The simulated PSC parameters presented in Table 3 emphasize the relative performance of CsSnI3, CsSnCl3, and their bilayer configuration alongside previously reported Sn- and Pb-based PSCs. Devices based on CsSnI3 demonstrate strong photovoltaic characteristics, with the simulation yielding the following: PCE = 28.12%, FF = 88.22%, Jsc = 30.73 mA cm−2, and Voc = 1.03 V, which are competing with or superior to those of earlier CsSnI3-based architectures. In contrast, CsSnCl3 alone delivers a much weaker performance (FF = 75.66%, Voc = 0.74 V, Jsc = 24.89 mA cm−2), resulting in a low PCE of 14.12%, confirming its limited suitability as a primary absorber. Remarkably, the bilayer CsSnI3/CsSnCl3 device achieves the following: PCE = 30.02%, FF = 88.54%, Jsc = 32.09 mA cm−2, and Voc = 1.05 V, surpassing those of both single absorbers and most reported structures. These findings underscore the synergistic advantage of bilayer engineering, where CsSnCl3 functions as an efficient passivation and stabilizing interfacial layer, thereby enhancing the Voc and overall device efficiency well beyond the values achieved in previous studies.| Device architecture | V oc (V) | J sc (mA cm−2) | FF (%) | PCE (%) | Ref. |
|---|---|---|---|---|---|
| FTO/TiO2/CsPbI3/FaPbI3/spiro/Au | 1.10 | 24.50 | 74.00 | 19.94 | 33 |
| ITO/TiO2/CsSnI3/CsSnCl3/spiro-OMeTAD/Au | 0.96 | 28.55 | 85.87 | 24 | 34 |
| ITO/PCBM/CsSnI3/CFTS/Se | 0.87 | 33.99 | 83.46 | 24.73 | 27 |
| ITO/PCBM/CsSnI3/CuI/Au | 0.91 | 14.24 | 78.11 | 10.11 | 35 |
| FTO/PCBM/CsSnCl3/PTAA/Au | 1.3 | 15.34 | 89.90 | 17.93 | 36 |
| ITO/TiO2/CsSnCl3/CBTS/Au | 1.01 | 26.22 | 82.03 | 21.75 | 26 |
| FTO/PCBM/CsSnI3/CFTS/Au | 1.03 | 30.73 | 88.22 | 28.12 | This work |
| FTO/PCBM/CsSnCl3/CFTS/Au | 0.74 | 24.89 | 75.66 | 14.12 | This work |
| FTO/PCBM/CsSnCl3/CsSnI3/CFTS/Au | 1.05 | 32.09 | 88.54 | 30.02 | This work |
While the simulated PCE of 30.02% represents a near-ideal theoretical limit, practical implementation presents several experimental hurdles. The main obstacle is the swift oxidation of Sn2+ into Sn4+ under ambient conditions,39,40 resulting in significant p-type self-doping and deep-level traps that cause intense non-radiative recombination. Achieving the high performance predicted here would require stringent oxygen-free processing, advanced encapsulation, and the use of chemical additives to stabilize the Sn2+ oxidation state and maintain the low defect densities (1013 cm−3) assumed in our optimal model.
4. Conclusion
In the final analysis, the CsSnI3/CsSnCl3 bilayer solar cell numerical investigation, conducted mainly through the SCAPS-1D simulation tool, confirms unambiguously its very high potential for serving as a lead-free and high-efficiency option. First, the initial DFT analysis confirmed the electronic properties, which facilitated the structural design, especially in confirming the beneficial Sn s- and p-orbital dominance at the band edges and the widening of the bandgap introduced by the CsSnCl3 layer. The resulting graded bandgap design yielded a compelling peak PCE of 30.02% under optimal conditions. However, these simulations also provided critical insights that this performance is highly vulnerable to material defects: the PCE collapsed catastrophically when either the interface defect density surpassed 1 × 1014 cm−2 or the bulk defect density in each layer exceeded 1 × 1015 cm−3. These losses were driven essentially by enhanced non-radiative recombination and subsequent Voc and FF degradation. Thirdly, the thermal stability analysis showed that the device efficiency was negatively correlated to the increasing operating temperature, with Voc loss as the major thermal degradation mechanism. Consequently, to ensure that the theoretical 30% efficiency of this promising CsSnI3/CsSnCl3 architecture is practicable, rigorous defect passivation techniques and effective thermal management methods are absolutely required.Author contributions
Hariharan Rajasekaran contributed to writing, software, data curation, and formal analysis. Thangaraji Vasudevan handled writing, review, editing, and data curation. Lung-Chien Chen contributed to supervision, project administration, conceptualization, and funding. All authors approved the manuscript.Conflicts of interest
The authors state that they have no financial conflicts of interest or personal relationships that could have affected the research presented in this paper.Data availability
Supplementary information (SI) is available. See DOI: https://doi.org/10.1039/d5ma01437a.Data cannot be shared openly but are available on request from the authors.
Acknowledgements
This research was supported by the Ministry of Science and Technology (MOST), Taiwan (Contract No. 111-2221-E-027-040-MY3), and the National Taipei University of Technology-Industry Joint Research Program, Taiwan (Contract No. 211A171).References
- X. Jiang, S. Qin, L. Meng, G. He, J. Zhang, Y. Wang, Y. Zhu, T. Zou, Y. Gong, Z. Chen, G. Sun, M. Liu, X. Li, F. Lang and Y. Li, Nature, 2024, 635, 860–866 CrossRef PubMed.
- J. Zhou, L. Tan, Y. Liu, H. Li, X. Liu, M. Li, S. Wang, Y. Zhang, C. Jiang, R. Hua, W. Tress, S. Meloni and C. Yi, Joule, 2024, 8, 1691–1706 CrossRef CAS.
- Z. He, T. Luan, S. Zhang, Q. Wei, D. Huang, L. Wang, Y. Wang, P. Li and W. W. Yu, Adv. Mater., 2024, 36, 2410363 Search PubMed.
- F. Palazon, F. Di Stasio, Q. A. Akkerman, R. Krahne, M. Prato and L. Manna, Chem. Mater., 2016, 28, 2902–2906 CrossRef CAS PubMed.
- Y. Wang, J. Wan, J. Ding, J.-S. Hu and D. Wang, Angew. Chem., Int. Ed., 2019, 58, 9414–9418 Search PubMed.
- B. Saparov, J.-P. Sun, W. Meng, Z. Xiao, H.-S. Duan, O. Gunawan, D. Shin, I. G. Hill, Y. Yan and D. B. Mitzi, Chem. Mater., 2016, 28, 2315–2322 Search PubMed.
- S. J. Lee, S. S. Shin, Y. C. Kim, D. Kim, T. K. Ahn, J. H. Noh, J. Seo and S. I. Seok, J. Am. Chem. Soc., 2016, 138, 3974–3977 CrossRef CAS PubMed.
- F. Hao, C. C. Stoumpos, D. H. Cao, R. P. H. Chang and M. G. Kanatzidis, Nat. Photonics, 2014, 8, 489–494 CrossRef CAS.
- H. Tsai, W. Nie, J.-C. Blancon, C. C. Stoumpos, R. Asadpour, B. Harutyunyan, A. J. Neukirch, R. Verduzco, J. J. Crochet, S. Tretiak, L. Pedesseau, J. Even, M. A. Alam, G. Gupta, J. Lou, P. M. Ajayan, M. J. Bedzyk, M. G. Kanatzidis and A. D. Mohite, Nature, 2016, 536, 312–316 CrossRef CAS PubMed.
- N. K. Noel, S. D. Stranks, A. Abate, C. Wehrenfennig, S. Guarnera, A.-A. Haghighirad, A. Sadhanala, G. E. Eperon, S. K. Pathak, M. B. Johnston, A. Petrozza, L. M. Herz and H. J. Snaith, Energy Environ. Sci., 2014, 7, 3061–3068 RSC.
- Y. Shao, Y. Yuan and J. Huang, Nat. Energy, 2016, 1, 15001 CrossRef CAS.
- W. Meng, B. Saparov, F. Hong, J. Wang, D. B. Mitzi and Y. Yan, Chem. Mater., 2016, 28, 821–829 CrossRef CAS.
- G. K. Gupta, A. Garg and A. Dixit, J. Appl. Phys., 2018, 123, 013101 CrossRef.
- I. Chung, J.-H. Song, J. Im, J. Androulakis, C. D. Malliakas, H. Li, A. J. Freeman, J. T. Kenney and M. G. Kanatzidis, J. Am. Chem. Soc., 2012, 134, 8579–8587 CrossRef CAS PubMed.
- M. H. Kumar, S. Dharani, W. L. Leong, P. P. Boix, R. R. Prabhakar, T. Baikie, C. Shi, H. Ding, R. Ramesh, M. Asta, M. Graetzel, S. G. Mhaisalkar and N. Mathews, Adv. Mater., 2014, 26, 7122–7127 Search PubMed.
- K. Sun, J. Huang, J. Li, C. Yan and X. Hao, Sci. China: Phys., Mech. Astron., 2022, 66, 217302 Search PubMed.
- J. Shamsi, D. Kubicki, M. Anaya, Y. Liu, K. Ji, K. Frohna, C. P. Grey, R. H. Friend and S. D. Stranks, ACS Energy Lett., 2020, 5, 1900–1907 Search PubMed.
- M. Jeong, I. Choi, E. Go, Y. Cho, M. Kim, B. Lee, S. Jeong, Y. Jo, H. Choi, J. Lee, J.-H. Bae, S. K. Kwak, D. Kim and C. Yang, Science, 2020, 369, 1615–1620 CrossRef CAS PubMed.
- B. Li, H. Di, B. Chang, R. Yin, L. Fu, Y. Zhang and L. Yin, Adv. Funct. Mater., 2021, 31, 2007447 Search PubMed.
- J. You, L. Meng, T.-B. Song, T.-F. Guo, Y. (Michael) Yang, W.-H. Chang, Z. Hong, H. Chen, H. Zhou, Q. Chen, Y. Liu, N. De Marco and Y. Yang, Nat. Nanotechnol., 2016, 11, 75–81 CrossRef CAS PubMed.
- D. Jeong, G.-U. Kim, D. Lee, S. Seo, S. Lee, D. Han, H. Park, B. Ma, S. Cho and B. J. Kim, Adv. Energy Mater., 2022, 12, 2201603 CrossRef CAS.
- R. Garai, R. K. Gupta, M. Hossain and P. K. Iyer, J. Mater. Chem. A, 2021, 9, 26069–26076 RSC.
- H. Song, X. Meng, Z. Wang, H. Liu and J. Ye, Joule, 2019, 3, 1606–1636 Search PubMed.
- L. Wang, S. Yang, Q. Han, F. Yu, X. Cai, F. Liu, C. Zhang and T. Ma, ACS Appl. Energy Mater., 2020, 3, 9724–9731 CrossRef CAS.
- P. Gao, M. Grätzel and M. K. Nazeeruddin, Energy Environ. Sci., 2014, 7, 2448–2463 Search PubMed.
- M. K. Hossain, G. F. I. Toki, A. Kuddus, M. H. K. Rubel, M. M. Hossain, H. Bencherif, Md. F. Rahman, Md. R. Islam and M. Mushtaq, Sci. Rep., 2023, 13, 2521 CrossRef CAS PubMed.
- M. K. Hossain, M. S. Uddin, G. F. I. Toki, M. K. A. Mohammed, R. Pandey, J. Madan, Md. F. Rahman, Md. R. Islam, S. Bhattarai, H. Bencherif, D. P. Samajdar, M. Amami and D. K. Dwivedi, RSC Adv., 2023, 13, 23514–23537 RSC.
- J. Balasubramaniyan, T. Vasudevan, G. Rajamanickam and L.-C. Chen, Sol. Energy, 2025, 302, 113976 Search PubMed.
- G. F. I. Toki, M. K. Hossain, M. Shihab Uddin, A. M. Tawfeek, S. Rabhi, M. A. Darwish, R. Haldhar, D. K. Dwivedi, J. Madan and R. Pandey, Inorg. Chem. Commun., 2024, 165, 112439 Search PubMed.
- M. Burgelman, K. Decock, S. Khelifi and A. Abass, Thin Solid Films, 2013, 535, 296–301 Search PubMed.
- P. Giannozzi, S. Baroni, N. Bonini, M. Calandra, R. Car, C. Cavazzoni, D. Ceresoli, G. L. Chiarotti, M. Cococcioni, I. Dabo, A. Dal Corso, S. de Gironcoli, S. Fabris, G. Fratesi, R. Gebauer, U. Gerstmann, C. Gougoussis, A. Kokalj, M. Lazzeri, L. Martin-Samos, N. Marzari, F. Mauri, R. Mazzarello, S. Paolini, A. Pasquarello, L. Paulatto, C. Sbraccia, S. Scandolo, G. Sclauzero, A. P. Seitsonen, A. Smogunov, P. Umari and R. M. Wentzcovitch, J. Phys.: Condens. Matter, 2009, 21, 395502 CrossRef PubMed.
- M. Burgelman, P. Nollet and S. Degrave, Thin Solid Films, 2000, 361–362, 527–532 Search PubMed.
- A. Hajjiah, M. Gamal, I. Kandas, N. E. Gorji and N. Shehata, Sol. Energy Mater. Sol. Cells, 2022, 248, 112026 CrossRef CAS.
- S. Baruah, J. Borah and S. Maity, IEEE J. Quantum Electron., 2025, 1 Search PubMed.
- M. K. Hossain, G. F. I. Toki, D. P. Samajdar, M. Mushtaq, M. H. K. Rubel, R. Pandey, J. Madan, M. K. A. Mohammed, Md. R. Islam, Md. F. Rahman and H. Bencherif, ACS Omega, 2023, 8, 22466–22485 CrossRef CAS PubMed.
- S. Srivastava, A. K. Singh, P. Kumar and B. Pradhan, J. Appl. Phys., 2022, 131, 175001 CrossRef CAS.
- O. Saidani, A. Yousfi, D. P. Samajdar, X. Xu, T. Biniyam Zemene, S. Bhattarai, M. K. Hossain and G. S. Sahoo, Sol. Energy Mater. Sol. Cells, 2024, 277, 113122 Search PubMed.
- S. Sharma, M. Kumar, D. V. Singh, O. R. Herrera and J. M. Siqueiros, Mater. Lett., 2024, 366, 136472 CrossRef CAS.
- G. Venkateswarlu, C. V. M. Chaturvedi, U. Nanda, J. Bhaskara Rao, E. Sampad and N. Bodasingi, Opt. Quantum Electron., 2025, 57, 634 CrossRef.
- G. Venkateswarlu, E. Sampad, U. Nanda, J. Bhaskara Rao, C. V. M. Chaturvedi and N. Bodasingi, J. Electron. Mater., 2026, 55, 873–890 CrossRef.
| This journal is © The Royal Society of Chemistry 2026 |