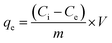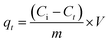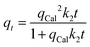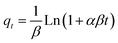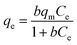Open Access Article
Open Access ArticleEco-inspired synthesis of a melamine formaldehyde-reinforced onion peel biochar/alginate composite for efficient lead ion removal from wastewater
Sarah A.
Felemban
 *a and
Alaa O.
Baryyan
*a and
Alaa O.
Baryyan
 b
b
aDepartment of Chemistry, Faculty of Science, Umm Al-Qura University, 21955 Makkah, Saudi Arabia. E-mail: safelemban@uqu.edu.sa
bDepartment of Pharmaceutical Sciences, Faculty of Pharmacy, Umm Al-Qura University, 21955 Makkah, Saudi Arabia
First published on 19th January 2026
Abstract
The present study focuses on the fabrication of a highly efficient, eco-friendly solid adsorbent, namely melamine formaldehyde-reinforced onion peel biochar/alginate composite (MCAg), along with onion peel-based biochar (C) and biochar/alginate composite beads (CAg). The synthesized adsorbents were characterized using various instrumental techniques. MCAg exhibited a surface area of 309.4 m2 g−1, a point of zero charge at pH 6.6, and the presence of multiple surface chemical functional groups as identified by FTIR analysis. The fabricated adsorbents were applied for the batch adsorption of Pb2+ ions under different operating conditions, in addition to desorption, reusability, and real-sample studies. MCAg showed the highest Langmuir adsorption capacity (348.9 mg g−1) at pH 6, a solid dosage of 2.5 g L−1, 20 °C, and 50 min of shaking time. The presence of coexisting ions in real polluted samples reduced the adsorption capacity for Pb2+ ions by an average of approximately 30%. Ethylenediaminetetraacetic acid was found to be the most effective desorbing agent for Pb2+ ions from the adsorbent surfaces. Kinetic and thermodynamic studies confirmed the applicability of the pseudo-first order and van’t Hoff models, indicating a physisorption mechanism and a spontaneous adsorption process. Column adsorption of Pb2+ ions was evaluated at three different bed heights (2, 3, and 4 cm) using an initial concentration of 80 mg L−1 and a flow rate of 15 mL min−1, achieving a maximum adsorption capacity of 232.7 mg g−1 at a 4 cm bed height, with the experimental data fitting well with the Yoon–Nelson and Thomas models. The effective adsorption of Pb2+ by MCAg is mainly related to the synergistic action of surface functional groups enabling ion exchange, electrostatic attraction, and complexation with Pb2+ ions, highlighting the composite's strong potential for polluted water treatment.
1. Introduction
Environmental pollution caused by heavy metals has become a serious concern due to its impact on ecosystem sanitation and human health. Lead ions (Pb2+), the second most toxic metal contaminant in water after arsenic, severely affect aquatic bodies.1,2 Lead ions have no beneficial biological function but are neurotoxic and damage multiple organ systems in animals. Lead causes nervous system diseases and developmental disorders in children, and damages bones, liver, kidneys, brain, and heart. The World Health Organization (WHO) has set permissible Pb2+ levels in drinking water at 10 µg L−1.3 Lead pollution is problematic because lead is non-degradable and undergoes bioaccumulation through food webs, which amplifies its toxic effects across trophic levels. Sources of Pb2+ pollution include mining, metallurgy, battery production, electroplating, and fossil fuel combustion.4 Adsorption is considered favorable due to its low cost, high efficiency, and ease of use with possible regeneration, but commercial implementation awaits. Widespread adoption depends on creating efficient, low-cost, sustainable adsorbents that meet regulatory requirements.5–7The materials employed in this study combine aspects of sustainable biomass utilization for eco-inspired composite engineering, with each having unique advantages and disadvantages.1 Onion peel biochar valorizes agricultural waste and offers several adsorption benefits. Its advantages include high carbon content (typically 60–80%), porous structure with surface area between 200 and 800 m2 g−1, and oxygen-containing functional groups (e.g., carboxyl, hydroxyl, and phenolic), which promote metal ion binding.5 Onion peel biochar is cost-efficient, sustainable, and chemically stable across varying pH values. The hydrophobic nature of biochar may reduce water dispersibility and active site accessibility for ion adsorption.8,9 Sodium alginate is a naturally occurring biopolymer from brown seaweed with features relevant to environmental applications. Biopolymer compatibility and biodegradability raise disposal concerns, while its gel-forming ability enables separation from treated water. Sodium alginate contains various chemical functional groups such as carboxyl and hydroxyl that show high affinity for metal ions through ionic interactions. However, limitations include poor mechanical stability in aqueous environments, which leads to swelling; sensitivity to pH, restricting its use to near-neutral conditions; and a lower surface area compared to carbon-based materials, thereby limiting its adsorption applications.10–12 Melamine-formaldehyde is a key matrix-forming component that provides properties essential for composite stability. Formaldehyde leaching can introduce harmful organic pollutants into water bodies, which can be dangerous even at low concentrations due to their cytotoxic and genotoxic properties. Such leakage may harm aquatic species, disturb vital microbial ecosystems in wastewater treatment systems, and perhaps endanger human health if contaminated water is reused. In our situation, formaldehyde is not employed as a freestanding solid adsorbent; rather, it is integrated into a highly stable composite matrix, considerably reducing the possibility of its release. Nonetheless, monitoring and minimizing potential formaldehyde leaching is critical to ensuring the environmental safety of the developed material. The composite system combines three components that reinforce mutual weaknesses and enhance advantages. Biochar's surface area and functional groups, sodium alginate's gelation and metal-binding abilities, and melamine's mechanical reinforcement create a composite with enhanced properties and operational stability.13,14 Several diverse adsorbents have been developed for Pb2+ ion removal, with different methodologies showing positive results. Recent studies demonstrate materials with high potential for Pb2+ ion removal applications. Gao et al. produced an alginate/melamine/chitosan gel composite achieving a Pb2+ ion uptake of 1331.6 mg g−1. The composite showed excellent Pb2+ ion selectivity and retained 60% capacity after eight sorption–desorption cycles.15 A sepiolite-polyethylenimine/sodium alginate composite demonstrated a Pb2+ ion adsorption capacity of 1094.9 mg g−1, following the Langmuir isotherm model. The adsorption mechanism was governed by ion exchange and electrostatic interactions, following pseudo-second order kinetics.16 He et al. prepared a molybdenum disulfide biochar/calcium carbonate/alginate composite with an adsorption capacity of 909.1 mg g−1, combining biochar's surface area with alginate's metal-binding properties.17 Liu et al. developed a sodium alginate melamine@zeolitic imidazolate framework-67 composite with a Pb2+ ion adsorption capacity of 635.0 mg g−1, removing lead through electrostatic interactions and complexation.18 Wang et al. created a calcium alginate/biochar composite achieving a Pb2+ ion removal of 664.6 mg g−1, demonstrating synergy between biopolymers and biomaterials.19 Blending biochar, biopolymers, and synthetic reinforcing agents offers a promising pathway for next-generation materials, overcoming individual performance limitations with good cost-effectiveness and environmental sustainability. Although MF-based composites have been described, this work is unique in combining melamine-formaldehyde with alginate and onion-peel-derived biochar, with the last component being an eco-friendly, heteroatom-rich feedstock that has not been employed before in such ternary systems. Our easy and ecologically friendly synthesis method produces a composite with increased mechanical strength, numerous active sites, and high reusability. This technique improves conventional MF-based adsorbents by including sustainable materials, simplifying manufacture, and improving performance for effective Pb2+ removal. Synergistic integration would yield superior adsorption capacity, mechanical stability, and regeneration efficiency.
This study addresses Pb2+ ion contamination by developing low-cost adsorbents from agricultural waste, supporting environmental protection, public health, and circular economy goals through sustainable water treatment solutions. The methodology involves eco-inspired synthesis, characterization, and performance testing through pyrolytic conversion of onion peel waste to biochar, melamine-formaldehyde resin preparation, and composite formulation in an alginate matrix. Physicochemical techniques were utilized for comprehensive characterization of thermal, chemical, and textural properties. The adsorption of Pb2+ ions was investigated under varied conditions such as adsorbent dosage, initial solution pH, contact time, temperature, and initial pollutant concentration, using batch (static) adsorption experiments. Real water samples were also tested. Additionally, dynamic fixed-bed adsorption experiments were conducted using the most efficient adsorbent material.
2. Materials and methods
2.1. Materials
Sodium alginate (>99.5%), melamine (99%), ethylenediaminetetraacetic acid (99.5%), cysteine (>98%), and lead nitrate (≥99.5%) were obtained from Sigma Aldrich, Czechia. Sodium hydroxide (≥98%), hydrochloric acid (37%), nitric acid (70%), and potassium carbonate (96%) were purchased from El-Nasr for pharmaceutical and chemical industries Co., Egypt. Formaldehyde (%), methanol (≥99.5%), anhydrous calcium chloride (97%), and sodium chloride (>99%) were obtained from Loba Chemie, Mumbai, India.2.2. Solid adsorbent preparation
2.3. Characterization of the prepared solid adsorbents
Thermal, chemical, and textural characterization is essential for the investigation of solid adsorbent materials. The textural properties of the synthesized materials (C, CAg, and MCAg) were characterized using N2 gas adsorption–desorption analysis performed on a NOVA 3200e gas sorption analyzer (Quantachrome Corp., USA). Prior to measurements, all solid samples were degassed under vacuum (10−4 Torr) at 120 °C for 15 h to remove adsorbed contaminants. The analysis aimed to determine key structural parameters, including specific surface area (SBET, m2 g−1), total pore volume (Vp, cm3 g−1), and average pore diameter (nm).X-ray diffraction analysis was conducted to determine the crystalline composition of samples C, CAg, and MCAg using a D8 Advance diffractometer (Bruker, Germany). The instrument operated with Cu-Kα radiation (λ = 1.5406 Å) at 40 kV and 40 mA. Scans were performed over a 2θ range of 10° to 50° with a scanning rate of 1° min−1.
Thermogravimetric analysis (TGA) was performed on the solid adsorbents (C, CAg, and MCAg) using an SDT Q600 thermoanalyzer (TA Instruments, V20.9 Build 20) under a nitrogen atmosphere (20 mL min−1 flow rate). The samples were heated from ambient temperature to 900 °C to evaluate their thermal stability and decomposition behavior.
The surface chemical functional groups of C, CAg, and MCAg were detected using attenuated total reflectance Fourier transform infrared spectroscopy (ATR-FTIR) utilizing a ZnSe crystal on a Nicolet Impact 400 D spectrometer, operating at a resolution of 5 cm−1 and a scan range of 4000–400 cm−1.
The pHPZC values of C, CAg, and MCAg as solid adsorbents were evaluated using the salt addition method. For each sample, 0.2 g of dried material was dispersed in 50 mL of 0.1 M NaCl solution (prepared with deoxygenated distilled water) in sealed containers. The initial pH (pHinitial) was systematically adjusted from 2 to 12 using 0.05 mol L−1 HCl or NaOH solutions. Following 4 h of agitation, the equilibrium pH (pHfinal) was measured using a calibrated HI 2020 pH meter (Hanna Instruments). pHPZC was identified as the pH value where ΔpH (pHfinal − pHinitial) = 0.
The surface morphology of C, CAg, and MCAg particles was investigated by scanning electron microscopy (SEM). Prior to imaging, sample fragments were sputter-coated with a 10 nm gold layer to enhance conductivity. High-resolution micrographs were obtained using a JEOL JSM-6510LV analytical SEM (Japan) operating at an acceleration voltage of 10 kV.
The nanostructure features of all materials were characterized using a JEOL JEM-2100 transmission electron microscope (Japan). For sample preparation, the solids were first dispersed in ethanol via 20-min ultrasonication, and then deposited onto carbon-coated copper TEM grids. This preparation protocol ensured optimal particle distribution for high-resolution imaging.
2.4. Adsorption procedures of Pb2+ ions
Different nonlinear kinetic and equilibrium adsorption models were applied to evaluate key adsorption parameters. The kinetics of Pb2+ adsorption onto C, CAg, and MCAg were analyzed using nonlinear formulations of the pseudo-first order (eqn (3)), pseudo-second order (eqn (5)), Elovich (eqn (6)), and Weber–Morris intraparticle diffusion models (eqn (7)). The amount of Pb2+ adsorbed at a specific time (qt, mg g−1) was calculated using eqn (4). In addition, the equilibrium adsorption data (as summarized in Table 1) were interpreted using several nonlinear isotherm models, including Langmuir (eqn (8)), Freundlich (eqn (10)), Temkin (eqn (11)), and Dubinin–Radushkevich (eqn (12)). The dimensionless separation factor (RL), which indicates the favorability of adsorption, was calculated using eqn (9). Furthermore, eqn (13) was employed to estimate the mean free energy of adsorption.
The effect of temperature on the batch adsorption of Pb2+ ions was examined by conducting experiments with 0.125 g of the adsorbent in 50 mL of 800 mg L−1 Pb2+ solution at pH 6, under shaking for 50 min and 120 rpm. The experiments were performed at varying temperatures (20, 30, 40, and 45 °C). Thermodynamic parameters, including the changes in enthalpy (ΔH°, kJ mol−1), entropy (ΔS°, kJ mol−1 K−1), and Gibbs free energy (ΔG°, kJ mol−1), were calculated using eqn (14)–(16) as presented in Table 1.
To evaluate the reusability of the solid adsorbent, ten successive adsorption/desorption cycles were performed under fixed conditions: an adsorbent dosage of 2.5 g L−1, an initial Pb2+ concentration of 800 mg L−1, a contact time of 50 min, pH 6, and a temperature of 20 °C. After each cycle, the adsorbent was separated by centrifugation, thoroughly washed multiple times with 50 mL of 0.1 mol L−1 EDTA solution to remove any residual Pb2+ and then dried at 100 °C before reuse in the next cycle.
3. Results and discussion
3.1. Solid adsorbent characterization
The textural characterization of the synthesized adsorbents, namely C, CAg and MCAg, was determined by displaying their nitrogen adsorption/desorption isotherms as shown in Fig. 1a. According to the IUPAC classification, all synthesized adsorbents exhibit type IV adsorption isotherms, confirming that they are mesoporous materials.21 Moreover, the isotherms of all the adsorbents show distinct hysteresis loops of H3-type, starting at 0.4 P/P°, associated with the capillary condensation phenomenon occurring within the pores. These hysteresis loops indicate the presence of slit-like pores of adsorbents.22,23 The specific surface area, the total pore volume, and the average pore diameter of all adsorbents were analyzed based on the Brunauer–Emmett–Teller (BET) theory, as shown in Table 2. The C, CAg, and MCAg adsorbents exhibit specific surface areas of 466.3, 134.6, and 309.4 m2 g−1, respectively, with total pore volumes of 0.8153, 0.2749, and 0.3908 cm3 g−1, showing a consistent pattern following the order of C > MCAg > CAg. The MCAg adsorbent exhibits a greater specific surface area and total pore volume than CAg by 2.29 and 1.42 times, respectively, demonstrating the successful combination of melamine formaldehyde with the CAg adsorbent. Furthermore, the average pore diameters of C, CAg and MCAg are 6.98, 8.16, and 5.06 nm, respectively. Barrett–Joyner–Halenda (BJH) pore size distribution analysis, as presented in Fig. S1a, revealed distinct maxima for samples C, CAg, and MCAg at 6.98 nm, 8.16 nm, and 5.06 nm, respectively, indicating their mesoporous structures and differences in pore development among the synthesized adsorbents.24 The pore size distribution of the MCAg composite predominantly falls within the mesoporous range, according to IUPAC classification, serving as efficient transport pathways for pollutants. The enhancement of the surface area, total pore volume and average pore diameter of the MCAg composite provides more active sites for adsorption with effective performance, making it a potential candidate for lead ion adsorption. | ||
| Fig. 1 Nitrogen adsorption (a), TGA (b), ATR-FTIR (c), and pH at point of zero charge (d) for C, CAg, and MCAg. | ||
| Properties | C | CAg | MCAg |
|---|---|---|---|
| S BET (m2 g−1) | 466.3 | 134.6 | 309.4 |
| V P (cm3 g−1) | 0.8153 | 0.2749 | 0.3908 |
| Average pore diameter (nm) | 6.98 | 8.16 | 5.06 |
| pHPZC | 6.1 | 6.4 | 6.6 |
The thermal stability of the prepared adsorbents was assessed using thermogravimetric analysis, and the results are presented in Fig. 1b. All the prepared adsorbents are thermally stable up to 300 °C before undergoing decomposition. At 175 °C, the C, CAg, and MCAg adsorbents show mass reductions of 1.5, 7.2 and 7.7%, respectively, due to the evaporation of adsorbed water from their surfaces.25 The C adsorbent exhibits a lower mass loss compared to CAg and MCAg adsorbents, due to its higher carbon content resulting from pyrolysis at 500 °C. Also, biochar exhibited fewer active functional sites compared to CAg and MCAg adsorbents. Between 250 and 400 °C, all three adsorbents started to decompose significantly. The biochar adsorbent (C) undergoes a notable mass loss of 11.9% at 350 °C, indicating the decomposition of the residual volatile components, whereas the beads of CAg and MCAg composites lose considerable masses of 31.1 and 24.4%, respectively, which is related to the thermal degradation of polysaccharide chains and destruction of melamine formaldehyde resin. At 900 °C, the residual mass percentages of C, CAg and MCAg are 73.7, 37.4, and 44.4%, respectively. Such high residual masses of all the prepared adsorbents are owing to the substantial carbon content of the biochar component. The order of thermal stability of all the prepared adsorbents is C > MCAg > CAg. The MCAg composite shows good thermal stability, indicating a successful combination of biochar with adsorbents derived from alginate and melamine formaldehyde.
The XRD patterns shown in Fig. S1b give information on the structural properties of the synthesized materials. The biochar sample (C) shows a broad diffraction peak between approximately 12.9° and 26.3° (2θ), reflecting its largely amorphous structure with minimal graphitic order, which is typical of carbon-based materials produced from biomass.26 The sharper and more intense peak at 27.4° observed in the CAg composite (biochar–alginate) suggests greater structural order, likely arising from the interactions between biochar and alginate, as well as the incorporation of silver species. This reflects a noticeable improvement in the crystallinity compared to pure biochar. The XRD pattern of the MCAg composite (melamine-formaldehyde/biochar/alginate) shows peaks at 12.9° and 23.8°, as well as larger characteristics that indicate a more heterogeneous and semi-crystalline structure. The peak at 23.8° is most likely connected with the presence of melamine-formaldehyde resin,27 indicating that it was successfully incorporated into the biochar-alginate matrix. These structural changes are significant because they can impact the physicochemical characteristics of the material and perhaps improve its adsorption effectiveness.
The ATR-FTIR spectral data of C, CAg, and MCAg adsorbents are depicted in Fig. 1c. Different characteristic peaks appear in the FTIR spectra of the biochar adsorbent; the broad peak at 3333.1 cm−1 confirms the intermolecular and intramolecular stretching vibrations of –OH groups. The characteristic peak at 1592.1 cm−1 is caused by stretching vibrations of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups. A strong peak appears at 1024.7 cm−1 due to the C–O stretching vibrations of different functional groups, including alcohols, phenols and carboxylic acids, on the surface of the biochar adsorbent. A weak peak at 2093.8 cm−1 was noted, which is typically related to C
O groups. A strong peak appears at 1024.7 cm−1 due to the C–O stretching vibrations of different functional groups, including alcohols, phenols and carboxylic acids, on the surface of the biochar adsorbent. A weak peak at 2093.8 cm−1 was noted, which is typically related to C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C or C
C or C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N stretching vibrations; such groups can appear in small magnitudes during the pyrolysis of lignocellulosic biomass. The C–H group causes multiple peaks, including one in the 2800–2900 cm−1 range, caused by stretching vibrations. Additionally, peaks at 1430.2, 1324.3, 883.1, and 815.9 cm−1 are attributed to bending vibrations of the aliphatic and aromatic C–H group. The IR spectrum of the CAg adsorbent has nearly the same characteristic peaks as adsorbent C, indicating the presence of similar functional groups in both adsorbents, with slight variations. For example, the peak of the –OH group slightly broadens and shifts to 3364.7 cm−1 because of the overlapping of –OH peaks from both C and CAg adsorbents, as well as hydrogen bond formation between both adsorbents. In addition, the noticeable peaks of alginate (CAg) are observed at 1592.1 and 1412.4 cm−1, which correspond to symmetric and asymmetric stretching vibrations of the C
N stretching vibrations; such groups can appear in small magnitudes during the pyrolysis of lignocellulosic biomass. The C–H group causes multiple peaks, including one in the 2800–2900 cm−1 range, caused by stretching vibrations. Additionally, peaks at 1430.2, 1324.3, 883.1, and 815.9 cm−1 are attributed to bending vibrations of the aliphatic and aromatic C–H group. The IR spectrum of the CAg adsorbent has nearly the same characteristic peaks as adsorbent C, indicating the presence of similar functional groups in both adsorbents, with slight variations. For example, the peak of the –OH group slightly broadens and shifts to 3364.7 cm−1 because of the overlapping of –OH peaks from both C and CAg adsorbents, as well as hydrogen bond formation between both adsorbents. In addition, the noticeable peaks of alginate (CAg) are observed at 1592.1 and 1412.4 cm−1, which correspond to symmetric and asymmetric stretching vibrations of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group.18 When combining melamine formaldehyde with biochar and alginate to form the MCAg composite, a roughly similar pattern to that of C and CAg adsorbents appears, with some variations in peaks, including their shapes, intensities and positions.13 The lower intensity peak appears at 3388.6 cm−1, which could result from the overlapping signals of –OH and –NH derived from the different prepared adsorbents. A sharp peak with a slight widening is seen at 1575.1 cm−1, which could be related to the interactions between vibrations of functional groups of all three adsorbents (asymmetric stretching of C–O derived from CAg and C
O group.18 When combining melamine formaldehyde with biochar and alginate to form the MCAg composite, a roughly similar pattern to that of C and CAg adsorbents appears, with some variations in peaks, including their shapes, intensities and positions.13 The lower intensity peak appears at 3388.6 cm−1, which could result from the overlapping signals of –OH and –NH derived from the different prepared adsorbents. A sharp peak with a slight widening is seen at 1575.1 cm−1, which could be related to the interactions between vibrations of functional groups of all three adsorbents (asymmetric stretching of C–O derived from CAg and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N vibrations). Moreover, a low intensity peak disappears and a noticeably wider peak with a small shoulder appears at 1162.3 and 1059.5 cm−1, indicating the overlapping absorption peaks of different functional groups (stretching vibrations of C–O and C–N groups, the former arising from C and Ag adsorbents, and the latter arising from the MF adsorbent). Overall, all observations support the successful integration of the three adsorbents.
N vibrations). Moreover, a low intensity peak disappears and a noticeably wider peak with a small shoulder appears at 1162.3 and 1059.5 cm−1, indicating the overlapping absorption peaks of different functional groups (stretching vibrations of C–O and C–N groups, the former arising from C and Ag adsorbents, and the latter arising from the MF adsorbent). Overall, all observations support the successful integration of the three adsorbents.
The zeta potential of the adsorbent has a significant impact on the ion adsorption process. The pHPZC of C, CAg and MCAg adsorbents was determined and is displayed in Fig. 1d and Table 2. The graph shows a sharp decline in the zeta potentials of all prepared adsorbents as the pH values increase, with the pHPZC values being 6.1, 6.4, and 6.6, respectively. As the pH rises, the protonation of functional groups on the surface of adsorbents reduces until the pH value exceeds pHPZC, at which point the adsorbent surface becomes entirely negatively charged. Oppositely, when the pH level decreases below pHpzc, the protonation of the functional groups on the adsorbent surface increases, resulting in an entirely positively charged surface.28 Furthermore, it can be seen from the pHPZC results that the higher zeta potential value of the MCAg composite indicates that it has a greater number of functional groups than C and CAg adsorbents.
Scanning electron microscopy (SEM) is used to determine the surface properties and morphology of the C, CAg and MCAg adsorbents as shown in Fig. 2a–c, respectively. The surface of the C adsorbent features rectangular, plate-like structures arranged vertically with a rough texture. In addition, large, darker regions corresponding to pore holes are observed, proving the highly intrinsic nature of biochar. The SEM of the CAg adsorbent shows large, rectangular, stacked, sheet-like structures. The surfaces of these particles are granular with characteristic brighter edges, confirming the variability of adsorbents’ surface composition. Lower pore holes compared to the C adsorbent are also observed. The SEM image of the MCAg composite shows features like both C and CAg adsorbents, with agglomerations of granular particles being densely packed; however, it reveals some different features. Firstly, granules are packed loosely, creating narrow channels between them. In addition, the surface of the MCAg composite presents clear, distinct, and well-defined grooves and ridges with varying sizes. Such specific features of the MCAg composite result from the interactions of functional groups of all three adsorbents, which contribute effectively to enhancing surface porosity and prove the successful combination of MF with C and Ag adsorbents.
Transmission electron microscopy (TEM) is also used to examine the internal structures of the C, CAg and MCAg adsorbents at the nanoscale, giving 2D images as shown in Fig. 2c–e, respectively. The C adsorbent shows a folded structure of stacked tube-like particles with dense masses that resemble clouds, confirming the intrinsic behaviour of carbon-based biochar adsorbents. Open-structure clusters, including small spherical particles and large cloud-like structures with large voids, are observed in the TEM of the CAg adsorbent. The appearance of these two features indicates the successful insertion of alginate into biochar, as small spherical particles correspond to alginate, while cloud-like structures are attributed to biochar. The TEM image of the MCAg composite shows clusters composed of multiple chains of small, monodisperse spherical particles, characterised by a combination of transparent and darker spots, penetrated by multiple voids that are homogeneously distributed. Such homogeneity of particles and voids indicates the well-controlled synthesis of the MCAg composite. The dark and transparent regions are evidence of variation in composition, confirming the effective integration of the three prepared adsorbents. The larger number of spherical particles could be linked to the increased number of functional groups present in the MF adsorbent.29
3.2. Batch adsorption of Pb2+ ions
| Models | Parameters | C | CAg | MCAg |
|---|---|---|---|---|
| PFO | q cal (mg g−1) | 166.3 | 221.8 | 333.8 |
| k 1 (min−1) | 0.0451 | 0.0631 | 0.0800 | |
| R 2 | 0.9913 | 0.9777 | 0.9914 | |
| χ 2 | 0.1184 | 0.4716 | 0.3265 | |
| PSO | q cal (mg g−1) | 221.44 | 275.56 | 375.95 |
| k 2 (g mg−1 min−1) × 10−4 | 1.7702 | 2.3103 | 2.3388 | |
| R 2 | 0.9797 | 0.9674 | 0.9825 | |
| χ 2 | 0.2754 | 0.6878 | 0.4849 | |
| Elovich | α (mg g−1 min−1) | 10.3726 | 24.4715 | 52.0193 |
| β (g mg−1) | 0.0149 | 0.0139 | 0.0112 | |
| R 2 | 0.9639 | 0.9468 | 0.9706 | |
| χ 2 | 0.4899 | 1.1234 | 1.1130 | |
| Weber and Morris intraparticle diffusion | k diff (mg g−1 min−1/2) | 21.7535 | 26.8936 | 35.9692 |
| C | 1.7820 | 14.5918 | 46.6761 | |
| R 2 | 0.9263 | 0.8877 | 0.8922 | |
| χ 2 | 0.9998 | 2.3707 | 4.0850 |
The findings clarify that all models well demonstrated Pb2+ adsorption by C, CAg, and MCAg supported by the high values of regression coefficients (R2), ranging from 0.8877 to 0.9914, in addition to declining values of the reduced chi-square (χ2), ranging from 0.1184 to 4.0850. The PFO model is a more optimal model, with a better fit of the Pb2+ adsorption data by C, CAg, and MCAg compared to the PSO model, supported by higher R2 (0.9913, 0.9777, and 0.9914 by C, CAg, and MCAg, respectively), accompanied by lower χ2 (0.1184, 0.4716, and 0.3265, respectively). The adsorption capacities (qcal, mg g−1) obtained with PFO (166.25, 221.75, and 333.82 mg g−1) are much closer to those relative to the Langmuir model with a reduced average deviation of 6.1%. The PFO model exhibits a range of root means square error (RMSE) values from 0.3441 to 0.5714. The highest rate constant value (k1, 0.0800 min−1) was achieved by the MCAg composite, demonstrating the highest rate of Pb2+ adsorption due to its high surface area with a variety of functional groups on its surface. The PFO model is more suitable for describing the adsorption of Pb2+ ions onto all the synthesized adsorbents (C, CAg, and MCAg) than the PSO model. This conclusion is supported by the higher R2 values, lower χ2 values, and smaller deviations between the experimental and calculated adsorption capacities observed for the PFO model compared to those of the PSO model. In the Elovich model, the β values follow the order C (0.0149) > CAg (0.0139) > MCAg (0.0112), suggesting the quickest surface coverage of MCAg by Pb2+ ions. The most outstanding α value (52.0193 mg g−1 min1/2) has been obtained with MCAg, indicating the fastest initial rate of Pb2+ adsorption onto its surface among other prepared adsorbents. The superior C value of 46.6761 calculated by the Weber and Morris model has been reached by the MCAg adsorbent, confirming that the interactions of Pb2+ ions with the outer surface of pores, besides their diffusion into pores, significantly affect the Pb2+ adsorption rate. Moreover, MCAg with the highest intraparticle diffusion rate constant (kdiff, 35.9692 mg g−1 min1/2) suggests the enhanced diffusion of Pb2+ particles into internal pores of such adsorbent, resulting in quicker adsorption.20
| Models | Parameters | C | CAg | MCAg |
|---|---|---|---|---|
| Langmuir | q m (mg g−1) | 184.5 | 236.4 | 348.9 |
| b (L mg−1) | 0.0881 | 0.1606 | 0.1860 | |
| K L | 0.0409 | 0.0148 | 0.0080 | |
| R 2 | 0.9760 | 0.9873 | 0.9462 | |
| χ 2 | 0.2193 | 0.1734 | 2.5603 | |
| Freundlich | 1/n | 0.3385 | 0.2786 | 0.2938 |
| K F(L1/n mg1−1/n g−1) | 38.2563 | 69.6008 | 103.2916 | |
| R 2 | 0.8534 | 0.9285 | 0.7894 | |
| χ 2 | 1.3419 | 0.9757 | 7.3066 | |
| Temkin | b T (J mol−1) | 61.3284 | 54.3202 | 35.1652 |
| K T (L g−1) | 1.0271 | 2.1388 | 2.2000 | |
| R 2 | 0.9568 | 0.9761 | 0.9436 | |
| χ 2 | 0.3950 | 0.3257 | 2.0380 | |
| Dubinin–Radushkevich | q DR (mg g−1) | 158.2 | 209.5 | 304.4 |
| E DR (kJ mol−1) | 0.2382 | 0.5127 | 0.5466 | |
| R 2 | 0.9599 | 0.9166 | 0.9287 | |
| χ 2 | 0.3669 | 1.9580 | 2.5549 |
All isotherm models show a notable increase in adsorption capacity (qe, mg g−1) at lower equilibrium concentrations (Ce < 30 mg L−1) of Pb2+, resulting from the availability of more accessible binding sites on the surfaces of the C, CAg, and MCAg adsorbents. As the Pb2+ concentration increases, qe also increases due to the occupation of additional binding sites until the adsorbent surfaces become saturated. Consequently, the rate of increase of qe becomes nearly constant, even at higher concentrations (Ce > 45 mg L−1) of Pb2+, due to the progressive depletion of available binding sites for adsorption.37
The Langmuir isotherm provided the best match for C and CAg, with high correlation coefficients (R2 = 0.9760 and 0.9873, respectively) and low reduced chi-square (χ2) values (0.2193 and 0.1734, respectively). This shows that the adsorption of Pb+ ions on these two materials happens mostly as monolayer coverage on a homogeneous surface. The predicted maximum adsorption capacities (qm, mg g−1) demonstrate the effectiveness of these materials, increasing from 184.5 mg g−1 (C) to 236.4 mg g−1 (CAg). The CAg composite has a lesser surface area (134.6 m2 g−1) than C (466.3 m2 g−1), but the composite performs better due to greater affinity from alginate functional groups. The MCAg composite, constructed from melamine formaldehyde, onion peel biochar, and alginate, had the greatest Langmuir qm value (348.9 mg g−1). However, its R2 value (0.9462) was somewhat lower than that for C and CAg. This finding can be explained by the extensive surface heterogeneity and different functional groups seen in MCAg, as indicated by ATR-FTIR studies. These functional groups improve Pb2+ binding efficiency through various interactions (e.g., electrostatic, coordination). However, the larger χ2 value (2.5603) indicates a weaker Langmuir model fit, potentially due to departure from idealized monolayer behaviour.38 MCAg demonstrates the highest adsorption rate and the most favorable affinity toward Pb2+ ions, as evidenced by its Langmuir constant (b = 0.1860 L mg−1), indicating stronger interactions with the target metal ions.39 The separation factor (KL) values for Pb2+ adsorption onto C, CAg, and MCAg (0.0409, 0.0148, and 0.0080, respectively) all lie between 0 and 1, confirming that the adsorption process is favorable for all three adsorbents, with MCAg showing superior performance.40 Additionally, the 1/n values obtained from the Freundlich model for C (0.3385), CAg (0.2786), and MCAg (0.2938) also fall within the range of 0 to 1, reinforcing the favorable nature of Pb2+ adsorption.41 These findings agree with the corresponding KL values, further validating the efficiency of the prepared adsorbents.
Adsorption on heterogeneous surfaces is considered using the Freundlich model, which demonstrated a moderate correlation for CAg (R2 = 0.9285) but a rather poor match for MCAg (R2 = 0.7894) and C (R2 = 0.8534). Furthermore, this model's higher χ2 values, especially for MCAg (7.3066), suggest that it falls short in describing the adsorption process for these substances. However, the Freundlich constant (KF) gradually increased from C (38.26) to MCAg (103.29), indicating that greater material complexity is associated with a higher adsorption capacity and surface affinity.
The Temkin model further confirmed that all materials exhibited favorable adsorption behaviour, with decreasing bT values (heat of adsorption) from C (61.33 J mol−1) to MCAg (35.17 J mol−1) and R2 values ranging from 0.9436 to 0.9761. Because of either a wider spread of binding sites on the modified surface or the saturation of high-energy sites, the lower bT for MCAg suggests that Pb2+ adsorption entails less strong interactions.
The Dubinin–Radushkevich (D–R) model offered new information on the adsorption process. The calculated average adsorption energy (EDR) values for all materials were less than 1 kJ mol−1, indicating that physisorption is the primary process. MCAg had the greatest EDR value (0.5466 kJ mol−1) and qDR (304.4 mg g−1), showing improved adsorption performance driven by both surface textural aspects and the presence of active functional groups.38 Despite having a modest surface area (309.4 m2 g−1), MCAg outperformed C and CAg in terms of Pb2+ removal capability. The plentiful and diverse surface functional groups on MCAg, as revealed by ATR-FTIR analysis, played an important role in improving its adsorption behaviour.
Overall, the isothermal results show that surface chemistry and functionalization, especially in MCAg, are important factors that determine adsorption capacity, even if surface area is a major factor. The difference in model fitting further shows that because of its structural complexity, Pb2+ adsorption onto MCAg could not precisely adhere to ideal monolayer or homogeneous site assumptions.
The adsorption of Pb2+ onto the created MCAg composite as a solid adsorbent sample is regulated by a succession of physicochemical interactions arising from its heterogeneous surface chemistry. The melamine-formaldehyde resin, alginate matrix, and biochar have oxygen- and nitrogen-bearing functional groups (e.g., –OH, –COOH, –NH, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) that promote significant electrostatic attraction and surface complexation with Pb2+ ions. Additionally, Pb2+ in solution undergoes ion-exchange reactions with exchangeable cations on alginate and charcoal surfaces. MCAg's porous structure improves Pb+ absorption by facilitating adsorption and intraparticle diffusion. Electrostatic attraction, ion exchange, and inner-sphere complexation work together contributing to MCAg's high affinity for Pb2+.
O) that promote significant electrostatic attraction and surface complexation with Pb2+ ions. Additionally, Pb2+ in solution undergoes ion-exchange reactions with exchangeable cations on alginate and charcoal surfaces. MCAg's porous structure improves Pb+ absorption by facilitating adsorption and intraparticle diffusion. Electrostatic attraction, ion exchange, and inner-sphere complexation work together contributing to MCAg's high affinity for Pb2+.
| Samples | R 2 | ΔH° (kJ mol−1) | ΔS° (kJ mol−1 K−1) | −ΔG° at 25 °C (kJ mol−1) |
|---|---|---|---|---|
| C | 0.9843 | 12.73 | 80.49 | 11.25 |
| CA | 0.9725 | 10.11 | 75.60 | 12.42 |
| MCAg | 0.9647 | 7.58 | 71.13 | 13.61 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kst) of around 18.0, the Pb2+–EDTA complex exhibits a strong chelation interaction that greatly increases the desorption efficiency from the adsorbent surface.45 To prevent secondary contamination from the Pb-EDTA complex, post-treatment alternatives include chemical precipitation (Ca2+/Fe3+), advanced oxidation to break down EDTA, and membrane/ion-exchange separation for safe handling of the desorption effluent.
Kst) of around 18.0, the Pb2+–EDTA complex exhibits a strong chelation interaction that greatly increases the desorption efficiency from the adsorbent surface.45 To prevent secondary contamination from the Pb-EDTA complex, post-treatment alternatives include chemical precipitation (Ca2+/Fe3+), advanced oxidation to break down EDTA, and membrane/ion-exchange separation for safe handling of the desorption effluent.
As shown in Fig. 6d, ten successive adsorption/desorption cycles were carried out to assess the produced adsorbents' durability and reusability. Following the tenth cycle, a little decrease in adsorption capacity was noted (19, 14, and 16 mg g−1 for C, CAg, and MCAg, respectively); this was probably brought on by the slow loss or obstruction of active sites throughout the washing and filtration procedures.46 All adsorbents, however, maintained a high level of performance, exhibiting outstanding regeneration capability and validating their appropriateness, effectiveness, and stability for recurring Pb2+ adsorption applications.
The MCAg sample retrieved after ten adsorption–desorption cycles was analyzed by SEM and ATR-FTIR to evaluate its structural stability (Fig. S2). SEM images showed no noticeable change in surface morphology, with only slight particle aggregation. The ATR-FTIR spectra displayed the same characteristic functional groups, with a minor decrease in peak transmittance, likely linked with the repeated adsorption–desorption cycles. These results prove that MCAg maintains its structural reliability and demonstrates exceptional reusability.
3.3. Column adsorption of Pb2+ ions
| Parameters | BH (cm) | ||
|---|---|---|---|
| 2.0 | 3.0 | 4.0 | |
| t e (min) | 174 | 187 | 208 |
| t b (min) | 90 | 115 | 133 |
| q o (mg g−1) | 360.5 | 285.9 | 232.7 |
| Yoon-Nelson model | |||
| τ exp (min) | 147 | 165 | 178 |
| τ cal (min) | 146 | 163 | 176 |
| K YN (min−1) | 0.0864 | 0.0854 | 0.0842 |
| R 2 | 0.9881 | 0.9902 | 0.9962 |
| χ 2 | 0.0018 | 0.0013 | 0.0004 |
| Thomas model | |||
| q Th (mg g−1) | 18.9 | 14.0 | 11.4 |
| K Th (L mg−1 min−1) × 10−3 | 1.0542 | 1.0412 | 1.0370 |
| R 2 | 0.9881 | 0.9901 | 0.9962 |
| χ 2 | 0.0018 | 0.0013 | 0.0004 |
The breakthrough times (tb, min) were found to be 90, 115, and 133 min for the bed heights of 2.0, 3.0, and 4.0 cm, respectively, while the exhaust times (te, min) were 174, 187, and 208, respectively. It is noticed that tb and te increased proportionally by 1.27 and 1.15, and by 1.07 and 1.11 with the increasing bed height of the column from 2.0 to 3.0, then to 4.0 cm, confirming the higher availability of active sites on the longer path of the adsorbent.47 In addition, the values of column adsorption capacity of MCAg (qo, mg g−1) were enhanced by 1.54, as the height of the bed column decreased from 4 to 2 cm because of the limitation of adsorption sites caused by solid packing.48
The empty bed residence time (EBRT) for the column adsorption equals (πr2/µ)BH, where r represents the inner column radius (cm), while the bed mass transfer zone (MTZ) equals BH(1−tb/te). The EBRT values for the applied 2, 3, and 4 cm bed heights were computed as 0.236, 0.353, and 0.471 min, respectively, signifying that increasing the bed height spreads the contact time between Pb2+ and the MCAg active adsorption sites. This enhanced residence time endorses better diffusion and more efficient application of the adsorbent, consistent with fixed-bed adsorption principles where higher beds support enhanced metal uptake before breakthrough. Similarly, the MTZ values increased from 0.966 to 1.155 and 1.442 cm as the bed height increased, reflecting the development of a broader mass transfer region due to the accessibility of more active sites for adsorption. Despite the larger MTZ at higher beds, the decreasing MTZ-to-bed-height ratio indicates more effective bed utilization, amended adsorption kinetics, and enhanced interaction between Pb2+ and the MCAg surface in deeper beds. Collectively, these results prove that the 4 cm bed provides the most favorable working conditions for Pb2+ column removal under the applied flow rate.
3.4. Comparison of MCAg capability with other materials
Table 7 shows the greatest adsorption capacity for batch and column adsorption when using the synthesized MCAg composite compared to other solid adsorbents.51–56 Overall, MCAg appears to be a viable adsorbent for treating lead ion-contaminated wastewater.| Adsorbents | Conditions | Adsorption capacity (mg g−1) | Ref. |
|---|---|---|---|
| Batch adsorption | |||
| Polyaniline alginate nanofibers | pH = 5, t = 10 min, T = 25 °C | 202.00 | 51 |
| Araucaria gum/calcium alginate composite beads | pH = 5, t = 10 min, T = 25 °C | 149.95 | 52 |
| Cellulose nanoparticles/chitosan composite | pH = 6.5, t = 120 min, T = 25 °C | 221.10 | 53 |
| GO/Ch/FeOOH | pH = 5.5, t = 70 min, T = 40 °C | 111.11 | 54 |
| MCAg | pH = 6, t = 50 min, T = 20 °C | 348.9 | This study |
| Column adsorption | |||
| MgAl@AC | C i = 36 mg L−1, µ = 30 mL min−1, pH = 5 | 37.5 | 55 |
| Palm shell activated carbon | C i = 50 mg L−1, µ = 33.33 mL min−1, pH = 5 | 92.6 | 56 |
| CR11 | C i = 225 mg L−1, µ = 10 mL min−1, pH = 6.5 | 161.1 | 52 |
| MCAg | C i = 80 mg L−1, µ = 15 mL min−1, pH = 6 | 285.9 | This study |
4. Conclusion
Adsorption, as an efficient procedure for pollutant removal, requires a tailored solid adsorbent with specific characteristics such as sustainability, eco-friendliness, low cost, high adsorption capacity, and easy modifiability. Herein, a melamine formaldehyde-reinforced onion peel biochar/alginate composite (MCAg) was fabricated for the removal of Pb2+ ions from aqueous media. The fabricated solid adsorbent exhibited a considerable surface area (309.4 m2 g−1) and a neutral pHPZC, and was rich in active chemical functional groups. Batch adsorption of Pb2+ ions reached 348.9 mg g−1 at pH 6 after 50 min, while column adsorption reached 232.7 mg g−1 at a bed height of 4 cm, an initial pollutant concentration of 80 mg L−1, and a solution flow rate of 15 mL min−1. The combination of biochar, melamine formaldehyde, and alginate produced a mechanically and thermally stable material with a higher surface area and mesoporosity, along with various channels that facilitate pollutant ion penetration and adsorption. Additionally, the diverse surface chemical functional groups originating from the material combination can readily attract pollutant ions. It is recommended that the developed material can serve as a solid adsorbent for various organic and inorganic pollutants. Furthermore, after modification with semiconductor catalysts, it could be employed for the degradation of organic pollutants. In conclusion, the authors state that the fabricated MCAg material is a promising candidate for environmental applications.Author contributions
Sarah A. Felemban and Alaa O. Baryyan: conceptualization and methodology, Alaa O. Baryyan: formal analysis and investigation, Sarah A. Felemban and Alaa O. Baryyan: writing – original draft preparation, data handling, and analysis of data. Sarah A. Felemban: investigation and revision of the final article.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this work.Data availability
The data supporting this article are provided in the supplementary information (SI) and are also presented in the tables and figures of the main manuscript. Supplementary information is available. See DOI: https://doi.org/10.1039/d5ma01050k.References
- F. C. P. Tonelli and F. M. P. Tonelli, in Bioremediation and Biotechnology, Persistent and Recalcitrant Toxic Substances, ed. R. A. Bhat, K. R. Hakeem and N. B. Saud Al-Saud, Springer International Publishing, Cham, 2020, vol. 3, pp. 15–23 Search PubMed.
- T. A. Al-Sabbagh and S. Shreaz, Int. J. Environ. Res. Public Health, 2025, 22, 1–30 CrossRef PubMed.
- World Health Organization, WHO Housing and health guidelines, 2018 Search PubMed.
- C. Filote, M. Roşca, R. M. Hlihor, P. Cozma, I. M. Simion, M. Apostol and M. Gavrilescu, Processes, 2021, 9, 1–38 CrossRef.
- N. A. A. Qasem, R. H. Mohammed and D. U. Lawal, npj Clean Water, 2021, 4, 36 CrossRef CAS.
- C. Saka, Ö. Şahin and M. M. Küçük, Int. J. Environ. Sci. Technol., 2012, 9, 379–394 CrossRef CAS.
- A.-A. Ciobanu, I. Michalak and L. Bulgariu, in Bio-organic Amendments for Heavy Metal Remediation, ed. A. Husen, M. Iqbal, A. Ditta, S. Mehmood, M. Imtiaz and M. S. Tu, Elsevier, 2024, pp. 69–84 Search PubMed.
- P. Granados, S. Mireles, E. Pereira, C. L. Cheng and J. J. Kang, Appl. Sci., 2022, 12, 5040 CrossRef CAS.
- M. Prelac, I. Palčić, D. Cvitan, D. Anđelini, M. Repajić, J. Ćurko, T. K. Kovačević, S. Goreta Ban, Z. Užila, D. Ban and N. Major, Antioxidants, 2023, 12, 1697 CrossRef CAS PubMed.
- J. Kurowiak, A. Kaczmarek-Pawelska, A. G. Mackiewicz and R. Bedzinski, Processes, 2020, 8, 304 CrossRef CAS.
- B. Wang, Y. Wan, Y. Zheng, X. Lee, T. Liu, Z. Yu, J. Huang, Y. S. Ok, J. Chen and B. Gao, Crit. Rev. Environ. Sci. Technol., 2019, 49, 318–356 CrossRef CAS PubMed.
- A. Wierzbicka, M. Bartniak, J. Waśko, B. Kolesińska, J. Grabarczyk and D. Bociaga, Gels, 2024, 10, 491 CrossRef CAS PubMed.
- A. F. Hassan and M. A. Khoj, Environ. Sci. Pollut. Res., 2024, 31, 35233–35248 CrossRef CAS PubMed.
- M. X. Tan, Y. N. Sum, J. Y. Ying and Y. Zhang, Energy Environ. Sci., 2013, 6, 3254–3259 RSC.
- C. Gao, X.-L. Wang, Q.-D. An, Z.-Y. Xiao and S.-R. Zhai, Carbohydr. Polym., 2021, 256, 117564 CrossRef CAS PubMed.
- X. Wu, Y. Song, P. Yin, Q. Xu, Z. Yang, Y. Xu, X. Liu, Y. Wang, W. Sun and H. Cai, Appl. Clay Sci., 2022, 228, 106647 CrossRef CAS.
- Y. He, X. Jia, S. Zhou, J. Chen, S. Zhang, X. Li, Y. Huang, T. Wågberg and G. Hu, Sep. Purif. Technol., 2022, 303, 122212 CrossRef CAS.
- X. Zhang, Z. Li, T. Zhang, J. Chen, W. Ji and Y. Wei, Environ. Sci. Pollut. Res., 2023, 30, 18364–18379 CrossRef CAS PubMed.
- L. Gao, Z. Li, W. Yi, L. Wang, N. Song, W. Zhang, G. Li, S. Wang, N. Li and A. Zhang, J. Environ. Chem. Eng., 2023, 11, 109074 CrossRef CAS.
- N. A. Alamrani, Int. J. Biol. Macromol., 2025, 318, 145023 CrossRef CAS PubMed.
- J. C. P. Broekhoff, in Preparation of Catalysts II, ed. B. Delmon, P. Grange, P. Jacobs and G. Poncelet, Elsevier, 1979, vol. 3, pp. 663–684 Search PubMed.
- J. Wang, H. Zhang, N. Wang, H. Mo, Z. Yang, Y. Dong, Q. Liu, X. Huang and B. Han, Polymers, 2025, 17, 826 CrossRef CAS PubMed.
- J. Zuo, W. Li, Z. Xia, T. Zhao, C. Tan, Y. Wang and J. Li, Coatings, 2023, 13, 1884 CrossRef CAS.
- A. F. Hassan, G. A. El-Naggar, G. Esmail and W. A. Shaltout, Appl. Surf. Sci. Adv., 2023, 13, 100388 CrossRef.
- Y. Chun, K. R. Kim, H. R. Kim, S. K. Lee, J. H. Lee, J. H. Lee, C. Park, H. Y. Yoo and S. W. Kim, J. Polym. Environ., 2022, 30, 2037–2049 CrossRef CAS.
- M. M. M. Madkhali, R. Ghubayra, A.-N. M. A. Alaghaz, A. F. Hassan and A. G. Braish, Int. J. Biol. Macromol., 2024, 281, 136451 CrossRef CAS PubMed.
- M. Rashid, M. M. Islam, H. Minami, M. Aftabuzzaman, M. A. Rahman, M. M. Hossain, S. M. Hoque, M. A. Alam and H. Ahmad, Mater. Chem. Phys., 2020, 249, 122957 CrossRef CAS.
- M. Khajavian, S. Ismail and M. Afshari, Sep. Purif. Technol., 2025, 373, 133611 CrossRef CAS.
- M. A. Khoj, RSC Adv., 2024, 14, 24322–24334 RSC.
- A. S. Eltaweil, H. A. Mohamed, E. M. Abd El-Monaem and G. M. El-Subruiti, J. Mol. Liq., 2024, 408, 125275 CrossRef CAS.
- V. Dhanya and N. Rajesh, Groundw. Sustain. Dev., 2024, 26, 101209 CrossRef.
- C. Zhang, J. Sui, J. Li, Y. Tang and W. Cai, Chem. Eng. J., 2012, 210, 45–52 CrossRef CAS.
- J. Li, G. Lin, F. Tan, L. Fu, B. Zeng, S. Wang, T. Hu and L. Zhang, J. Colloid Interface Sci., 2023, 651, 659–668 CrossRef CAS PubMed.
- R. Ghubayra, I. Mousa, M. M. M. Madkhali, A. N. M. A. Alaghaz and A. F. Hassan, RSC Adv., 2024, 14, 37859–37870 RSC.
- R. E. K. Billah, M. A. Islam, M. A. Khan, M. K. Nazal, L. Bahsis, M. Achak, A. E. D. Mahmoud, M. A. Aziz and B.-H. Jeon, Mater. Chem. Phys., 2025, 332, 130249 Search PubMed.
- D. Xiang, R. Zhu, Y. Chen, M. Zhu, S. Wang, Y. Wu, J. Luo and L. Fu, Chem. Eng. J., 2024, 492, 152292 CrossRef CAS.
- B. Moghtaderi-Esfahani, K. Ghanemi and Y. Nikpour, Desalin. Water Treat., 2025, 321, 100921 CrossRef CAS.
- W. A. Shaltout, A. F. Hassan, M. S. Elsayed and H. Hafez, Mater. Adv., 2025, 6, 4418–4437 RSC.
- C. H. C. Tan, S. Sabar and M. H. Hussin, S. Afr. J. Chem. Eng., 2018, 26, 11–24 Search PubMed.
- R. Vinayagam, S. Ganga, G. Murugesan, G. Rangasamy, R. Bhole, L. C. Goveas, T. Varadavenkatesan, N. Dave, A. Samanth, V. Radhika Devi and R. Selvaraj, Chemosphere, 2023, 310, 136883 Search PubMed.
- S. Mustafa, H. N. Bhatti, M. Maqbool, A. Khan, A. M. Alraih and M. Iqbal, Sustainable Chem. Pharm., 2024, 39, 101577 CrossRef CAS.
- J. N. Naat, S. Suyanta and N. Nuryono, Mater. Adv., 2025, 6, 3220–3236 RSC.
- R. S. El Shenawy, E. A. El Fadaly and A. F. Hassan, Environ. Sci. Pollut. Res., 2025, 32, 13982–14001 CrossRef PubMed.
- M. B. Đolić, V. N. Rajaković-Ognjanović, J. P. Marković, L. J. Janković-Mandić, M. N. Mitrić, A. E. Onjia and L. V. Rajaković, Appl. Surf. Sci., 2015, 324, 221–231 CrossRef.
- T. P. Nguyen, V. D. Nguyen, M. T. Trinh, P. L. Han, T. H. Nguyen, M. T. Nguyen and A.-T. Vu, J. Hazard. Mater. Adv., 2025, 19, 100770 CAS.
- A. F. Hassan, A. Elhassanein, W. A. Shaltout and G. H. G. Ahmed, Int. J. Biol. Macromol., 2025, 294, 139465 CrossRef CAS PubMed.
- I. S. Aldabagh and K. K. Hummadi, Environ. Adv., 2025, 19, 100606 CrossRef CAS.
- A. Samanth, R. Vinayagam, T. Varadavenkatesan and R. Selvaraj, Environ. Res., 2024, 261, 119696 CrossRef CAS PubMed.
- A. H. Jawhari, H. Y. Otaif, N. Amri and Y. E. Mukhrish, Int. J. Biol. Macromol., 2025, 318, 145075 CrossRef CAS PubMed.
- W. A. Shaltout, H. Hafez, M. S. Elsayed and A. F. Hassan, RSC Adv., 2025, 15, 16901–16920 RSC.
- N. Jiang, Y. Xu, Y. Dai, W. Luo and L. Dai, J. Hazard. Mater., 2012, 215–216, 17–24 CrossRef CAS PubMed.
- M. A. Khoj, A. F. Hassan, N. S. Awwad, H. A. Ibrahium and W. A. Shaltout, Int. J. Biol. Macromol., 2024, 255, 128234 CrossRef CAS PubMed.
- E. A. Matter, A. F. Hassan, N. M. Elfaramawy and G. Esmail, Biomass Convers. Biorefinery, 2025, 15, 10961–10976 Search PubMed.
- S. Sheshmani, M. Akhundi Nematzadeh, S. Shokrollahzadeh and A. Ashori, Int. J. Biol. Macromol., 2015, 80, 475–480 CrossRef CAS PubMed.
- A. K. A. Khalil, I. W. Almanassra, A. Chatla, I. Ihsanullah, T. Laoui and M. Ali Atieh, Chem. Eng. Sci., 2023, 281, 119192 Search PubMed.
- G. Issabayeva, M. K. Aroua and N. M. Sulaiman, J. Hazard. Mater., 2008, 155, 109–113 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2026 |