Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Low temperature green synthesis of red emitting Pb-free CsMnBr3 perovskite films
Saurabh
Singh
 *a,
Xiyu
Wen
b and
Fuqian
Yang
*a,
Xiyu
Wen
b and
Fuqian
Yang
 a
a
aMaterials Program, Department of Chemical and Materials Engineering, University of Kentucky, Lexington, KY 40506, USA. E-mail: ssi303@uky.edu
bCenter for Aluminium Technology, University of Kentucky, Lexington, KY 40506, USA
First published on 8th December 2025
Abstract
Lead halide perovskites (APbX3) have demonstrated exceptional opto-electronic properties, but their inherent toxicity and environmental hazards hindered their practical deployment in display technologies such as liquid crystal display (LCD) backlights. Herein, we report for the first time a facile, water-mediated synthesis of red-emitting CsMnBr3 thin films from an aqueous solution of CsBr and MnBr2 precursors at a low temperature of ∼50 °C. Unlike traditional synthesis routes reported for synthesis of CsMnBr3 powders or nanocrystals, relying heavily on toxic solvents, high temperatures, or inert atmospheres, the green approach utilizes water as a benign medium to facilitate the [MnBr6] octahedral coordination assembly, yielding continuous red films with strong photoluminescence (λ: ∼644 nm, FWHM: ∼75 nm). The as-synthesized CsMnBr3 films exhibit remarkable optical quality with an ultra-wide color gamut coverage (∼132% of NTSC 1953 and ∼186% of sRGB color standards), making them a promising alternative for traditional red phosphors in LCD backlight applications. The characterization of electrical and photo-responses reveals a negative photoconductivity under UV irradiation, attributed to the powdered microstructure and hygroscopic nature of MnBr2 under ambient air conditions. The photo-response of the red-emissive CsMnBr3 films exhibits a power-law dependence on high-energy irradiation under ambient conditions at ∼18 °C and a relative humidity of ∼65%, along with faster self-recovery behavior, highlighting the complex role of defect-mediated charge transport. This green, low-cost, and scalable synthesis route offers a promising pathway toward sustainable and lead-free phosphor materials for next-generation wide-color-gamut display technologies.
1. Introduction
Metal halide perovskites have shown great promise over the past decade to revolutionize next-generation energy and optoelectronic devices such as light-emitting diodes (LEDs), and display technologies, due to their tunable band gaps, high defect tolerance, high thermal resilience, strong light absorption, high photoluminescence quantum yields (PLQY), and facile solution processability.1–3 Among a wide family of perovskites, all-inorganic halide perovskite systems (such as CsBX3, B = Pb, Sn, Mn, and X = Cl, Br, I) overpower hybrid organic–inorganic systems owing to their superior thermal and environmental stability while maintaining their excellent opto-electronic properties including higher color purity and higher defect tolerance, making them particularly attractive for device integration and optimization.4,5There are serious concerns, such as lead toxicity and their long-term environmental hazards, which have hindered further growth and inclusivity of lead-based halide perovskites (APbX3) in high-performance photovoltaic and display technologies. Also, common synthesis approaches for producing lead halide perovskites (APbX3), such as hot injection,6 LARP,7 ultrasonic,8 solvothermal9 and microwave assisted,10 further pose greater challenges, hindering their large-scale commercialization, due to the involvement of high temperature, high vacuum and inert environments, high cost, toxic polar and non-polar solvents of DMF, DMSO, hexane and toluene, low reproducibility, excessive external energies, and complex synthesis processes.9,11–13 Therefore, the need to explore alternative cations such as Sn, Mn, Bi, Sb, and double perovskites, and develop eco-friendly processes has become imperative, although their stability and toxicity trade-offs remain significant.14
Manganese-based halide perovskites (e.g., CsMnCl3 and CsMnBr3) have attracted a significant amount of attention, given that Mn2+ is relatively less toxic than Pb2+ cations and exhibits a bright and efficient photoluminescence (PL) red emission arising from the spin- and parity-forbidden d–d (4T1 to 6A1) transitions within the [MnBr6] octahedra of the CsMnBr3 lattice.15–17 This red PL emission is particularly valuable for display technologies, where deep-red emitters are required for wide gamut color reproduction for backlight units (BLUs) in liquid crystal displays (LCDs).18 However, conventional red phosphors such as rare-earth nitride phosphors, Mn4+ fluorides, and cadmium quantum dots, often suffer from high synthesis costs,19 limited tunability,20 synthesis complexity,19 environmental impact,21 low color purity,20 and poor thermal or moisture stability,22 whereas Mn-based perovskites such as CsMnBr3 offer low-cost, lead-free, and tunable alternatives for these traditional phosphor materials.15,23,24
Despite these lucrative advantages, synthesizing high-quality red-emitting CsMnBr3 films remains challenging to date as most synthesis methods typically involve solid-state synthesis, microwave-assisted synthesis, or colloidal hot-injection routes, which predominantly yield red-emitting powders or nanocrystals rather than continuous red-emitting thin films suitable for device integration.17,25–27 Moreover, these approaches typically involve toxic solvents, surfactants, elevated temperatures, and inert environments, which limit the scalability and compatibility with low-cost device fabrication.17,25–27 For instance, Almutlaq et al.15 reported a hot-injection method to produce red-emitting CsMnBr3 nanocrystals, requiring high temperature (∼170 °C), an inert environment, and toxic solvents such as octadecene (ODE) and hexane, thereby raising significant environmental concerns. Interestingly, Sahu et al.27 employed a microwave-assisted approach to synthesize red-emitting CsMnBr3 crystals. However, this approach is limited by demands of high vacuum and inert environments for the preparation of a Cs-oleate precursor, complicated synthesis conditions, and a non-stoichiometric excess of MnBr2 (an optimized molar ratio of Cs2CO3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MnBr2 was 1
MnBr2 was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7). Gao et al.16 reported a solid-state synthesis of CsMnBr3 powders exhibiting bright red emission at ∼650 nm, but is challenged by non-uniform film formation and poor substrate adhesion issues.
7). Gao et al.16 reported a solid-state synthesis of CsMnBr3 powders exhibiting bright red emission at ∼650 nm, but is challenged by non-uniform film formation and poor substrate adhesion issues.
Recent advances in halide-based luminescent materials further highlight the importance of film or composite morphologies for functional opto-electronic applications.28,29 For instance, Cai et al.29 demonstrated the potential of CsPbBr3/PS composites for ultrawide-range wearable temperature sensors, while Cai et al.28 incorporated CsPbBr3/PDMS nanospheres for multi-dimensional sensing and interactive displays. However, continuous emissive films of pure CsMnBr3 remain unexplored, leaving a clear gap for device-compatible, red-emitting lead-free halide films. Therefore, fabricating a continuous, red emissive CsMnBr3 films is crucial as unlike powders or nanocrystals, continuous CsMnBr3 films would allow direct device integration, ensure uniform optical properties, and enable charge transport and photoconductivity characterization, which are inaccessible in non-film morphologies. Additionally, solution-processable film deposition approaches can improve scalability, reduce reliance on toxic solvents, and expand compatibility with standard thin-film optoelectronic fabrication techniques.30
In this work, we report for the first time a simple, low-cost, and facile green synthesis of red-emitting CsMnBr3 thin films at a low temperature of ∼50 °C via thermal evaporation of an aqueous solution containing CsBr and MnBr2 precursors in a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. Unlike conventional toxic solvents (e.g., ODE, hexane, and DMF/DMSO), water acts as green and low-cost medium enhancing ionic transport and facilitating the assembly of [MnBr6] octahedral coordination, yielding red-emissive CsMnBr3 films (λ: ∼644 nm, FWHM: ∼75 nm) directly in aqueous solutions. The as-synthesized CsMnBr3 films exhibited an ultra-wide gamut coverage area of ∼132% of NTSC 1953 and ∼186% of sRGB color standards, thereby demonstrating their strong potential as an alternative to conventional red phosphors used in wide color gamut display applications (such as LCD backlights). This simple water-mediated synthesis route to produce red-emissive CsMnBr3 films opens up new avenues for scalable, low-toxicity, easily processable, reproducible, and device-compatible phosphor materials, addressing both the sustainability and performance needs of next-generation display technologies.
2. Unlike conventional toxic solvents (e.g., ODE, hexane, and DMF/DMSO), water acts as green and low-cost medium enhancing ionic transport and facilitating the assembly of [MnBr6] octahedral coordination, yielding red-emissive CsMnBr3 films (λ: ∼644 nm, FWHM: ∼75 nm) directly in aqueous solutions. The as-synthesized CsMnBr3 films exhibited an ultra-wide gamut coverage area of ∼132% of NTSC 1953 and ∼186% of sRGB color standards, thereby demonstrating their strong potential as an alternative to conventional red phosphors used in wide color gamut display applications (such as LCD backlights). This simple water-mediated synthesis route to produce red-emissive CsMnBr3 films opens up new avenues for scalable, low-toxicity, easily processable, reproducible, and device-compatible phosphor materials, addressing both the sustainability and performance needs of next-generation display technologies.
2. Experimental details
2.1. Synthesis of CsMnBr3 films
CsBr (99.9%, Beantown Chemical) and MnBr2 (≥97%, Strem Chemicals Inc.) powders were mixed in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio in 2 mL of DI water to obtain a precursor solution. 100 µL of this precursor solution was drop-casted on a cleaned Cu substrate, which was vacuum-dried at ∼50 °C to obtain crystallized films, as shown in Fig. 1, for further characterization.
2 molar ratio in 2 mL of DI water to obtain a precursor solution. 100 µL of this precursor solution was drop-casted on a cleaned Cu substrate, which was vacuum-dried at ∼50 °C to obtain crystallized films, as shown in Fig. 1, for further characterization.
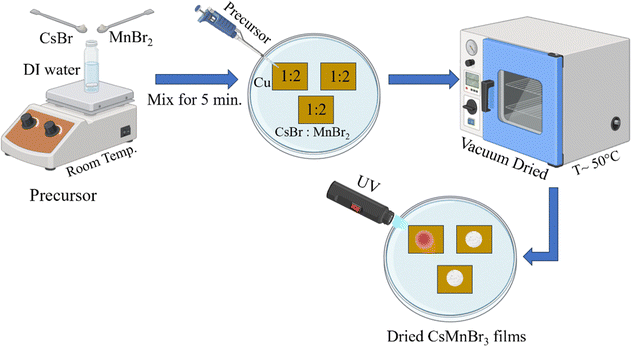 | ||
Fig. 1 Schematic depicting the synthesis route of CsMnBr3 films via an aqueous solution of CsBr and MnBr2 in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio. 2 molar ratio. | ||
2.2. Synthesis of CsPbBr3-PMMA films for LCD backlights
CsPbBr3 films used as green convertor film in white LCD backlights were prepared via the standard LARP method, where CsBr (0.4 mmol) (99.9%, Beantown Chemical) and PbBr2 (0.4 mmol) (>98%, Strem Chemicals Inc.) were mixed in 10 mL of DMF (VWR) with 1 mL of oleic acid (Ward's Science) and 0.5 mL of oleylamine (>50%, TCI America). The resulting mixture was stirred at 20 °C overnight to form a precursor solution. 1 mL of this prepared precursor solution was quickly dripped in 10 mL of toluene (VWR) under vigorous stirring at 20 °C to form CsPbBr3 nanocrystals. The precursor solution was added to a PMMA solution (formed via dissolving PMMA powders (Mw = 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) (Fisher Scientific) in toluene in 0.02 g mL−1 concentration). The formed PMMA solution was coated on glass slides of 0.80 × 6.66 × 72.62 mm3 in dimensions, ultimately forming CsPbBr3-PMMA films
000) (Fisher Scientific) in toluene in 0.02 g mL−1 concentration). The formed PMMA solution was coated on glass slides of 0.80 × 6.66 × 72.62 mm3 in dimensions, ultimately forming CsPbBr3-PMMA films
2.3. Materials characterization
The morphological and chemical compositional analyses were conducted on a scanning electron microscope (SEM) (JEOL JSM-5900lLV) equipped with an energy-dispersive X-ray spectroscope (EDS). The structural characterization of the as-prepared CsMnBr3 films was performed on an X-ray diffractometer (XRD) (Siemens D500) with CuKα radiation (λ = 1.5406 Å). X-ray photoelectron spectroscopy (XPS) (Thermo Scientific K-alpha X-ray photoelectron spectrometer) was utilized to analyze the chemical composition of the as-prepared CsMnBr3 films. The Photoluminescence studies of the as-prepared CsMnBr3 films were carried out using a spectrometer (Ocean optics, FLAME-S-VIS-NIR-ES, Ocean Optics) under a UV excitation wavelength of 365 nm. Absolute photoluminescence quantum yield (PLQY) measurements of CsMnBr3 films were conducted on a Horiba Scientific Fluoromax Plus-C fluorometer in which a solid sample was mounted on a sample stage and placed in an integrating sphere. An excitation wavelength of 365 nm was used for PLQY measurements with a 1 nm slit width and a 0.1 s integration time. The Raleigh scattering peak of the excitation and the PL from these CsMnBr3 films were extracted via scanning the emission in a range of 345–710 nm. A blank copper substrate without specimen was also scanned in the same scanning range of 345–710 nm for reference. Furthermore, PLQY calculations were performed using Horiba Scientific FluorEssence™ software with a factory acquired integrating sphere correction defined as PLQY = ((PB − PA)/(LA − LB)) × 100%, where (PB − PA) is the number of emitted photons, (LA − LB) is the number of absorbed photons, respectively. The lifetime decay studies were conducted using a DeltaHub™ high throughput time correlated single photon counting (TCSPC) controller. The electrical responses (I–V) of the as-prepared CsMnBr3 films were investigated using a Keithley 2400 source-meter (Keithley, Solon, OH, USA) under white and UV irradiation.3. Results
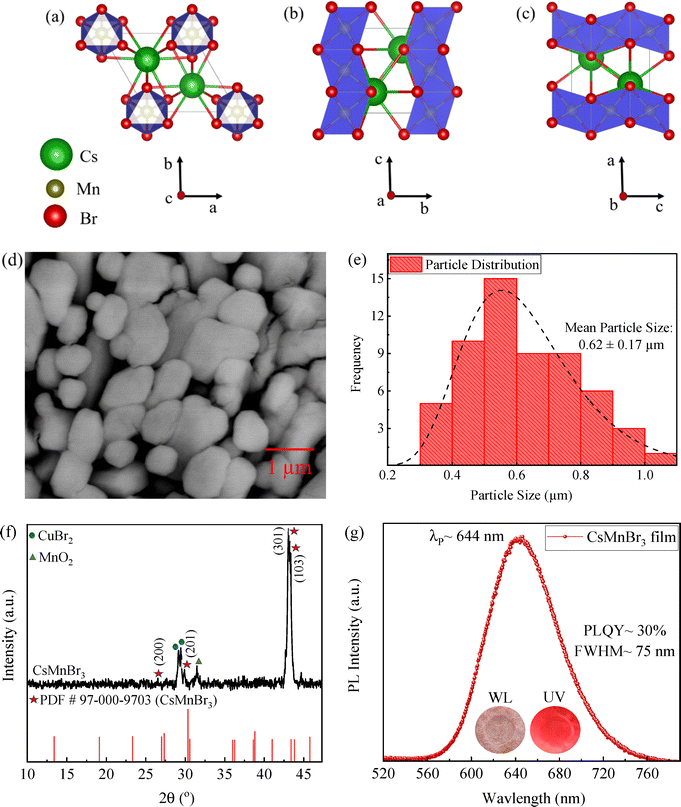
Fig. 2a–c depicts schematically the crystal structure of CsMnBr3 along the a, b, and c crystallographic axes, respectively, highlighting strongly coupled one-dimensional connectivity of the [MnBr6] octahedra for red photoluminescence of the material.15,17,26,31Fig. 2d presents the surface morphology (SEM micrograph) of the as-prepared CsMnBr3 films with a densely packed granular structure, suggesting successful formation of CsMnBr3 microcrystals at ∼50 °C. The microcrystals are irregularly shaped but interconnected, which is favorable for charge transport in optoelectronic applications. The analysis of the size distribution (Fig. 2e) demonstrates a near-normal distribution with a mean size of 0.62 ± 0.17 µm. The EDS analysis, as shown in Fig. S1 (SI), confirms a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 atomic ratio of Cs
3 atomic ratio of Cs![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn
Mn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Br in the as-prepared films, consistent with the stoichiometric CsMnBr3.
Br in the as-prepared films, consistent with the stoichiometric CsMnBr3.
The XRD pattern of the as-deposited CsMnBr3 films is presented in Fig. 2f. A set of diffraction peaks are observed around ∼26.58°, 29.95°, 43.1° and 43.36°, corresponding to the (200), (201), (301), and (103) planes of the hexagonal phase of CsMnBr3 with a space group of P63/mmc (PDF #97-000-9703). Using Bragg's law (eqn (S1)32 in the SI) and the hexagonal system's d-spacing equation (eqn (S2)33 in the SI), the lattice constants are calculated to be a ∼7.63 Å and c ∼6.97 Å, which are in good accordance with the reported results on the CsMnBr3 structure.16,31 However, all the observed Bragg peaks exhibit a left shift toward lower diffraction angles compared to the standard pattern, indicating lattice expansion as explained by Bragg's Law32 (eqn (S1)). Such shifts are well reported for perovskite materials.34–39 The leftward shift with some peak broadening likely can be attributed to transient hydration or intercalation of water during crystallization37,40 and defect-induced micro-strain. Note that moderate secondary peaks related to CuBr2 (∼17 wt%) and MnO2 (∼10 wt%) were also detected (Fig. 2f) in addition to the dominant phase CsMnBr3 (∼73 wt%), likely arising from the residues of precursors. These values were estimated from the relative intensities of representative phase reflections, likely representing an upper bound for impurity contents. Although, these impurity phases may introduce localized non-radiative trap states or scattering centers at grain boundaries, potentially reducing photoluminescence quantum yield or charge-carrier mobility,41–43 a relatively moderate fraction of such impurities suggests that the film's opto-electronic performance is likely governed by the dominant red-emissive CsMnBr3 phase, with only slight quenching from these impurity phases, respectively.
Fig. 2g shows the PL spectrum of the CsMnBr3 film deposited on a Cu substrate under UV excitation of ∼365 nm, depicting a broad peak (FWHM ∼75 nm) centered at ∼644 nm and exhibiting photoluminescence quantum yield (PLQY) of ∼30%, respectively. This emission arises from the characteristic d–d transitions of octahedrally coordinated Mn2+ ions, successfully excited under a high-energy UV source (λ ∼ 365 nm) and is consistent with the reports in the literature.15,44,45 For instance, Almutlaq et al.15 reported a broad PL spectrum (FWHM: ∼78 nm) for colloidal CsMnBr3 centered at ∼643 nm arising due to d-d transitions of Mn2+ ions in octahedral coordination from a low energy excited state (4T1) to the corresponding ground state (6A1). The insets in Fig. 2g display the optical images of the as-prepared CsMnBr3 films, exhibiting bright red emission under UV irradiation (λ: ∼365 nm) and validating the effectiveness of the high energy UV light in exciting the Mn2+ transitions responsible for red emission.46,47 Furthermore, the Cu substrate was explicitly chosen for deposition of CsMnBr3 films owing to its high electrical conductivity and compatibility with our experimental setup. Although copper is known to be chemically active and may tend to react with halide species to form copper halides or other by-products,48,49 several factors might limit such interactions under our synthesis conditions namely rapid solvent (water) evaporation during deposition, brief halide–substrate contact, and the absence of any acidic or strongly oxidative species to promote Cu halide formation. Moreover, X-ray diffraction spectra (Fig. 2f) also revealed minor peaks corresponding to CuBr2 and MnO2, indicating trace impurity formation, while preserving the dominant red emitting CsMnBr3 phase. Importantly, photoluminescence measurements conducted on both copper (Cu) and glass substrates (Fig. S3) exhibited similar PL emission spectra, confirming that the observed optical properties (Fig. 2g) are intrinsic to the CsMnBr3 phase and are not significantly affected by the utilization of the Cu substrate.
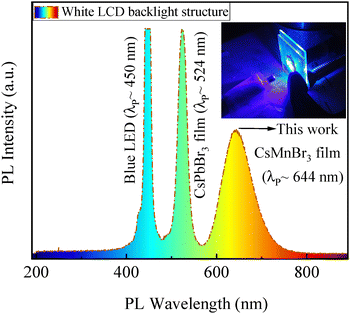
Thereafter, the as-prepared CsMnBr3 films were also exposed to blue LED light of ∼450 nm in wavelength. The PL spectrum is presented in Fig. 3a together with the one under UV light of ∼365 nm in wavelength. The films exhibit the nearly same red PL emission with a peak centered around ∼644 nm, supporting that the observed characteristic luminescence originates from the intrinsic d–d transition of Mn2+ ions, i.e., the spin- and parity-forbidden transitions [excited 4T1 state to ground 6A1 state] within the octahedral [MnBr6] coordination environment.15,26,50,51 The nearly same red emission under different excitation wavelengths highlights the robust optical response of the as-prepared CsMnBr3 films via the green route and further validates their suitability as stable red emitters in optoelectronic applications.
The photo-emission characteristics of CsMnBr3 films were evaluated using the CIE chromaticity diagram (Fig. 3b). The CsMnBr3 films exhibit CIE color coordinates of (∼0.60, ∼0.30), placing them within the red region of the standard CIE 1931 color space. This is well in accord with the PL emission peak at ∼644 nm. Such chromaticity positions are highly desirable for opto-electronic applications as they align closely with the red color gamut requirements of different standard color spaces such as NTSC and sRGB.52–55
The long-term stability of CsMnBr3 films was examined under both vacuum and ambient conditions (T: ∼17 °C, relative humidity (RH): ∼65%), as shown in Fig. 3c–h, respectively. Fig. 3c depicts the time-dependent PL spectra of the CsMnBr3 films in a vacuum environment, monitored over a period of 12 days. A gradual decrease in PL intensity with a small blue shift in PL wavelength was observed with respect to increasing time. This is in contrast to the PL spectra observed for the CsMnBr3 films under ambient conditions (T: ∼17 °C, RH: ∼65%), as shown in Fig. 3d, which experienced a faster degradation in PL intensity and a significant blue shift of PL wavelength and limited stability to several hours in ambient environments.
The time dependent PL intensities and PL wavelengths are extracted from Fig. 3c and d and plotted independently for both the vacuum and ambient conditions, as shown in Fig. 3e–h. The PL intensity of CsMnBr3 films exhibits slower decay under the vacuum conditions (Fig. 3e) in contrast to rapid decay under ambient conditions, as shown in Fig. 3f, respectively. Both the decay behavior follows exponential dependence on time, as illustrated by the fitting curves. The enhanced PL stability of the as-prepared CsMnBr3 films in the vacuum environment might be attributed to the absence of extrinsic degradation pathways, such as oxygen and moisture-induced halide oxidation, and/or hydrolysis reactions, which generally accelerate the non-radiative recombination processes for faster PL quenching over time.56–61
The peak emission wavelength of CsMnBr3 films experiences a small linear blue shift from ∼644 nm to ∼641 nm within 12 days under the vacuum conditions (Fig. 3g), in contrast to a rapid exponential blue shift under ambient conditions (T: ∼17 °C, RH: ∼65%) from ∼644 nm to ∼612 nm within 3 hours, as shown in Fig. 3h. This further highlights the slower structural and/or surface-related relaxation processes, such as minor lattice contraction and/or defect reconfiguration, experienced by the as-synthesized CsMnBr3 films in the vacuum environment.56–61
The observed unstable PL characteristics of CsMnBr3 films under ambient conditions (Fig. 3f and h) can be attributed to an array of factors such as moisture-induced halide migration and structural lattice distortions56–61 and inherent hygroscopic and deliquescent nature of MnBr2 (utilized in the precursor formation), which is highly soluble in water62–65 and hence readily absorbs atmospheric moisture, especially in low temperature and high humid environments. This accelerates the hydrolysis process of Mn–Br bonds and ultimately destabilizes the [MnBr6] octahedra present inside the CsMnBr3 lattice, facilitating some lattice distortions and creating corresponding non-radiative recombination centers resulting in faster PL decay profiles (Fig. 3f) observed in such ambient environments.62–65
These experimental findings (Fig. 3c–h) highlight the strong susceptibility of CsMnBr3 films to ambient conditions (involving moisture and oxygen) and underscores the necessity of encapsulation or passivation strategies to enable long-term device functionality of these films.66–70 We also emphasize that these PL measurements were conducted without incorporating any surface coating or encapsulation strategies, and hence, they only reflect the unprotected, intrinsic stability of the as-fabricated CsMnBr3 films under ambient conditions (T: ∼17 °C, RH: ∼65%). Similar degradation pathways have been extensively reported for different halide perovskites, such as MAPbI3 and CsPbBr3, where water ingress and subsequent hydrate formation induce structural collapse and severe luminescence quenching behavior.71,72 However, several strategies have been previously reported for similar halide perovskite systems to enhance their long-term ambient durability.73,74 For instance, silica (SiO2) coatings have been widely utilized to shield perovskite nanocrystals from moisture- and oxygen-induced degradations,75 while amidinium-based molecular passivation layers were recently employed for perovskite systems to dramatically enhance the environmental robustness, showing ∼10-fold reduction in ligand deprotonation and ∼2-fold increase in retention of the photoluminescence quantum yield under ambient conditions.76 Overall, such encapsulation strategies can offer realistic pathways to enhance the long-term stability of the as-synthesized CsMnBr3 films for practical optoelectronic and display applications.73,74
The charge-carrier recombination dynamics of the as-prepared CsMnBr3 films was probed using time-resolved photoluminescence spectroscopy (TRPL). Fig. 4 presents the temporal profile of TRPL for the CsMnBr3 films with the PL emission peak centered around ∼644 nm. The temporal profile of TRPL is fitted using a tri-exponential decay equation given as:77,78
| ITRPL(t) = A1e−t/τ1 + A2e−t/τ2 + A3e−t/τ3 | (1) |
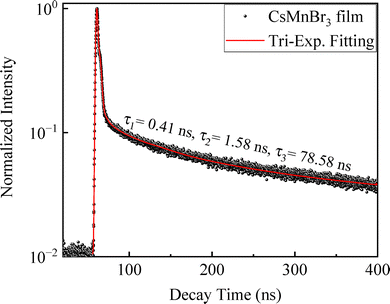 | ||
| Fig. 4 Transient PL decay curves of the as-prepared CsMnBr3 films fitted with the tri-exponential decay equation. | ||
here, ‘Ai’ (i = 1, 2 and 3) represents the relative amplitudes, and ‘τi’ represents the characteristic lifetime of the ith component, respectively.
The curve fitting reveals three distinct characteristic lifetimes of 78.58 ns, 1.58 ns and 0.41 ns, corresponding to radiative recombination, surface-mediated recombination and trap-mediated recombination processes, respectively.78 The longer radiative carrier lifetime component of ∼78 ns for the CsMnBr3 films, compared to previously reported decay components for different perovskite systems such as CsPbBr3 and MAPbI3,4,15,79,80 indicates strong suppression of non-radiative recombination channels that are typically introduced via surface defects or trap states,78 and points towards an effective defect passivation within these films. Additionally, the longer radiative lifetime components in halide perovskite systems, such as CsMnX3, CsPbX3 and MAPbX3, are generally associated with higher internal quantum yield (QY) and improved device performance in opto-electronic applications, such as LEDs and other photovoltaics systems.81,82 The mechanism behind such prolonged radiative decays is usually ascribed to an array of factors, including low defect density, reduced electron–phonon coupling, and possible charge carrier localization.83,84
The temperature dependence of photoluminescence was investigated for the as-prepared CsMnBr3 films, using the PL spectra shown in Fig. 5a, under ambient conditions and ∼365 nm UV irradiation. The temperature was slowly increased from ∼20 °C to ∼120 °C. A decrease in PL intensity with increasing temperature was observed (Fig. 5b), which can be ascribed to enhanced exciton–phonon dissociation85 from a higher degree of scattering at elevated temperatures, resulting in alteration of charge carrier's effective masses and ultimately reducing the radiative recombination rates.85 Such thermal quenching of photoluminescence is a common phenomenon for most luminescent materials, including perovskites of CsPbX34,86,87 and CsMnX3.44,85,88,89 The temperature-dependent PL behavior can be approximately described using the following equation,85,88,89
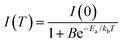 | (2) |
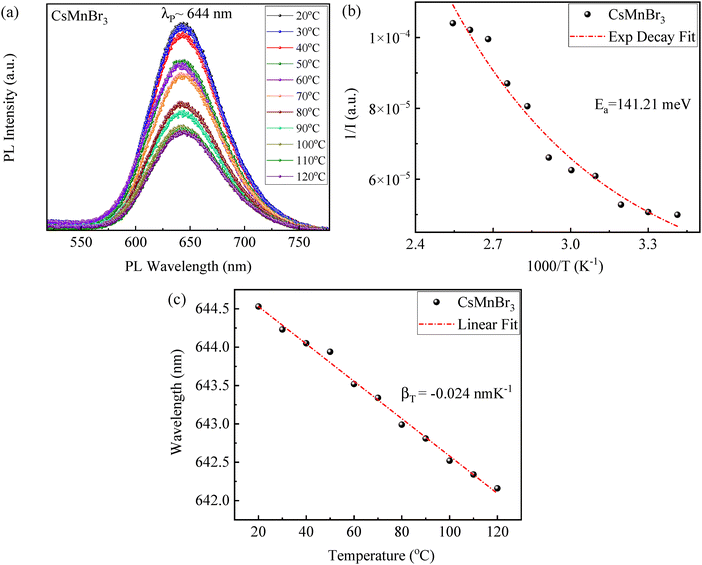 | ||
| Fig. 5 (a) PL spectra of the as-prepared CsMnBr3 films at different temperatures, (b) temperature dependence of PL peak intensity, and (c) temperature dependence of PL peak wavelength. | ||
Using eqn (2) to curve-fit the data in Fig. 5b yields a thermal activation energy of ∼141 meV, which is significantly higher than some previously reported values for halide perovskite systems, including CsPbBr3 (∼14–75 meV),90,91 MAPbX3 (∼6–53 meV),92 Cs3BiBr6 (∼41 meV),93 and MAPb(BrxI1−x)3 (∼20–38 meV).94 A higher energy barrier reduces an overall thermal accessibility of non-radiative decay pathways, suggesting stronger confinement of the emitting centers and fewer trap-mediated recombination losses. This behavior aligns with the localized nature of Mn2+ d–d characteristic transitions in [MnBr6] octahedra present inside the CsMnBr3 lattice, making them less susceptible to phonon-mediated thermal scattering compared to the delocalized band-edge excitons in Pb-based perovskite systems.95
Having a Br-rich environment during the synthesis of CsMnBr3 films may facilitate surface passivation via improving surface coordination by saturating under-coordinated metal sites, increasing the thermal energy barrier for non-radiative recombination channels.96,97 For instance, Ghimire et al.96 demonstrated enhancement in luminescence behavior (such as PLQY of ∼98%) of CsPbBr3 perovskite by filling Br-vacancies with NaBr, KBr and CsBr halides, resulting in a reduction of non-radiative decay rates. Similarly, Jing et al.97 developed a Br-rich passivation layer via selective etching in mixed halide CsPb(BrxI1−x)3 perovskite, enhancing the PL intensity by ∼103 times and suppressing non-radiative decay pathways through this passivation layer.
The temperature-dependent PL spectrum of the CsMnBr3 films shows a linear monotonous blue shift in the PL emission peak with increasing temperature in accordance with eqn (S3) in the SI85. The decreasing rate of the PL peak wavelength with respect to temperature is ∼0.024 nm K−1 from the linear regression fitting. This blue shift is usually attributed to an interplay of lattice expansion, exciton–phonon coupling, and possible modifications in self-trapped exciton (STE) states, which effectively shift the dominant PL emission to higher energy states as temperature increases.64–67 A similar behavior has already been observed for many halide perovskite materials, including CsMnBr3 and CsPbBr3.
The electrical response of the as-prepared CsMnBr3 films was evaluated under different illumination conditions, including dark, white light, and UV light (λ: ∼365 nm) at varying excitation intensities, as shown in Fig. 6a–d, respectively. Fig. 6a shows the current–voltage (I–V) characteristics of these films under white, dark and UV excitations at a scan rate of 0.55 V s−1. The linear I–V response points towards ohmic contacts between electrodes and the film, enabling the extraction of conductance using eqn (S4) shown in the SI.98,99 The conductance values for these films decrease progressively under dark (G: ∼2.06 × 10−9 S) to white light (G: ∼1.69 × 10−9 S) and to UV light (G: ∼9.6 × 10−10 S) illumination conditions, with the lowest conductance value (G ∼9.6 × 10−10 S) under the UV irradiation. This indicates the presence of negative photoconductivity (NPC)100–102 inside the as-synthesized CsMnBr3 films and is consistent with some recent reports on other perovskite materials, including CsPbX3, Cs3Bi2Br9, and Cs3Bi2Cl9.100–102
For instance, Tailor et al.101 observed a linear I–V response for the Cs3Bi2Br9 crystal, whose conductivity decreases under light exposure in comparison to the ‘dark’ state, exhibiting negative photoconductivity (NPC) due to trap of photo-generated charge carriers at charged defect states and an internal electric field opposite to the externally applied field, leading to a decrease of the net current under light illumination. Similarly, the time-resolved current–time (I–t) measurements conducted on these films show a linear I–t behavior with the change rate of current (r) (extracted via eqn (S5) in the SI) exhibiting a similar decreasing behavior from the dark (r: ∼1.21 × 10−9 A s−1) to white light (r: ∼9.95 × 10−10 A s−1) to UV light (r: ∼5.6 × 10−10 A s−1) (Fig. S2a in the SI). The lowest change rate is ∼5.6 × 10−10 A s−1 under UV light, consistent with the suppressed carrier transport observed in the I–V curves shown in Fig. 6a.
To further investigate the behavior of negative photoconductivity (NPC), the electrical response of the as-prepared films was observed under varying UV intensities of 0.01 W cm−2 (UV1), 0.03 W cm−2 (UV2), 0.14 W cm−2 (UV3), and 0.57 W cm−2 (UV4), as shown in Fig. 6b. Interestingly, the electrical conductance exhibits an intensity-dependent suppression (from ∼2.06 × 10−9 S under dark to ∼5.82 × 10−10 S under UV), with the highest UV illumination intensity yielding the lowest conductance (∼5.82 × 10−10 S). Correspondingly, the I–t curves under the corresponding UV illumination intensities (UV1 to UV4) (Fig. 6c) reveal a decreasing trend in the change rate of current (r) with increasing UV intensity, consistent with the I–V results. The observed trends are consistent with trap-mediated mechanisms,100–103 where higher excitation intensities (such as UV irradiation) can promote carrier capture at surface defects, grain boundaries, and other photoactive sites within the micron-sized, powder-like CsMnBr3 films, thereby reducing free carrier density and transport efficiency, leading to NPC behavior. However, we note that direct dynamic measurements, such as time-resolved photoluminescence (TRPL), temperature-dependent carrier transport, or power-dependent lifetime studies, would be required to unambiguously confirm this NPC mechanism, however, similar NPC responses, attributed to trap activation, space-charge buildup, and light-induced defect occupations, have been widely reported in other halide perovskite systems including CsPbX3, Cs3Bi2Br9, and Cs3Bi2Cl9, respectively.100–103
Fig. S2b in the SI shows the I–V response of the as-prepared CsMnBr3 films during ON–OFF alternate cycling under UV illumination (λ: ∼365 nm) at a scan rate of 0.25 V·s−1. Electric current exhibits an approximately exponential rise when transitioning to the dark (‘OFF’) state and a corresponding exponential decay under UV (‘ON’) illumination. These effects become more pronounced at higher applied voltages. Correspondingly, Fig. 6d displays the I–t response for the ON–OFF cycles, showing well-defined transient behavior. Fitting these I–t curves with a single-exponential equation (eqn (S6)104 in the SI) yields a rise characteristic time (τrise) of ∼4.07 s and a decay characteristic time (τdecay) of ∼2.54 s. Interestingly, despite the larger value of τrise than the τdecay, the overall ‘dark’ current after each ‘OFF’ cycle recovered to progressively higher values than the previous baseline, indicating an effective and relatively faster self-recovery process, as shown in Fig. 6d. This is in contrast to other NPC materials, for instance, Tailor et al.101 reported slower I–t response curves with an overall decaying current for Cs3Bi2Br9 during ON–OFF cycling under highly intense light exposure (100 mW cm−2).
The practical applicability of the as-prepared CsMnBr3 films was demonstrated by constructing a white-emitting backlight structure from the red-emitting CsMnBr3 and green-emitting CsPbBr3 films with a commercial blue LED source (λ: ∼450 nm), as schematically illustrated in Fig. S4 in the SI. Upon excitation, the backlight structure exhibits distinct and well-separated PL emission peaks corresponding to blue (∼450 nm), green (∼524 nm), and red (∼644 nm) components, as shown in Fig. 7. The inset in Fig. 7 depicts an optical image of the constructed structure under dark, confirming the production of bright white light through color mixing of RGB components. Such clear spectral resolution of RGB components is highly beneficial for achieving wide color gamut coverage and superior color rendering in display applications (discussed later in this paper), and aligns with the recent drive toward perovskite-based backlighting technologies for next-generation optoelectronic devices.105,106
4. Discussion
In this work, CsMnBr3 films were deposited on the Cu substrate at ∼50 °C from an aqueous solution of CsBr and MnBr2. The XRD pattern (Fig. 2f) reveals small traces of impurities of CuBr2 and MnO2, suggesting the following reaction| Cu + CsBr + 2MnBr2 + O2 → CsMnBr3 + CuBr2 + MnO2 | (3) |
In principle, water plays a crucial and active role in the synthesis of CsMnBr3 instead of just serving as a passive solvent. The presence of even small amounts of water significantly enhances ionic mobility and cation–halide (Mn2+–Br−) interaction, facilitating the formation of well-defined [MnBr6] octahedral coordination, and accelerating perovskite formation. This effect has been demonstrated by Tsvetkov et al.107 in the mechano-chemical synthesis of similar halide perovskite materials, such as CsPbI3, where water was demonstrated to strongly promote the reaction kinetics of CsPbI3 from CsI and PbI2 mixtures via increased mobility of the constituting ionic species. Additionally, Xiao et al.108 demonstrated a nuanced role of water in crystallization of the MAPbI3 perovskite. They reported that a suitable water content can facilitate high-quality perovskite film growth by enabling intermediate hydrate formation such as MAPbI3·H2O, facilitating the conversion process from precursors to perovskite structures.
In the CsMnBr3 lattice, the Mn2+ ion is octahedrally coordinated with Br− anions to form [MnBr6] octahedra (Fig. 8), which is responsible for the observed red PL emission (∼644 nm) (Fig. 2g), owing to the d–d transition of Mn2+ cations from 4T1 (4G) to 6A1 energy states (Fig. 8) and this is further verified by exciting the lattice with two different high-energy sources (∼365 nm and ∼450 nm) (Fig. 3a) resulting in similar red PL emission (∼644 nm with FWHM of ∼7 nm), consistent with previous reports on CsMnBr3.46,47 Almutlaq et al.15 concluded that the red PL emission (∼643 nm) of CsMnBr3 is indeed due to 4T1 to 6A1 (d–d) transitions of Mn2+ in [MnBr6] octahedra after extracting a similar red PL emission (∼643 nm with FWHM of ∼78 nm) under three different excitation wavelengths (∼380 nm, ∼45 nm, and ∼540 nm). Furthermore, a relatively modest photoluminescence quantum yield (PLQY ∼30%) in our CsMnBr3 films (particle size ∼0.6 µm), compared to CsMnBr3 nanocrystals (PLQY ∼54%) reported by Almutlaq et al.15 may be attributed to an array of factors including strong light scattering and re-absorption arising from the micron-sized powder morphology compared to the nanocrystalline systems reported in past literature,15,17 residual impurity phases and surface/bulk defect states, acting as nonradiative recombination centers, and partial oxidation of Mn2+ cations under ambient air, overall leading to a quenched photoluminescence behavior in Mn-based halide materials.109
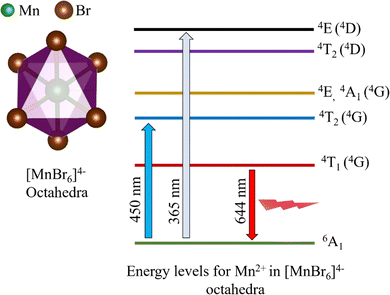 | ||
| Fig. 8 Energy level diagram of octahedrally coordinated Mn2+ ions in CsMnBr3, depicting d–d transitions and the photoluminescence pathway for red emission (∼644 nm). | ||
Fig. 9 shows the temperature dependence of the band gap of the as-prepared CsMnBr3 films, which follows the Bose–Einstein model110 given as:
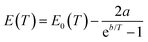 | (4) |
 | ||
| Fig. 9 Temperature dependence of photon energy for CsMnBr3 films, fitted via the Bose–Einstein model. | ||
The blue shift of the band gap with increasing temperature indicates higher electron–phonon activity and thermal lattice expansion at elevated temperatures, likely reducing the antibonding overlap between 3d Mn and 4p Br orbitals and ultimately widening the band gap. A similar behavior is reported for different halide perovskites, such as CsPbBr3. Higher temperatures lead to enhanced electron–phonon scattering, resulting in lower antibonding overlap between 6s Pb and 4p Br, thereby shifting the valence band maxima (VBM) to lower energy values, and ultimately leading to a shift of PL emission peaks to higher energy values.86,95,111–114
The correlations between electronic conductance (G) and irradiance (I) and between photo-resistance (ΔRph) and UV-light intensity can be expressed as:
| G = G0Ia and ΔRph = R0Ib | (5) |
The power-law dependence of electronic conductance and photo-resistance indicates that a strong UV irradiation enhances the trapping and/or recombination of charge carriers, limiting photocarrier migration, consistent with the observed NPC phenomena (Fig. 6a–d). Although direct defect characterization (such as EPR or XPS) or humidity-dependent photoconductivity studies were not conducted in present work, the observed NPC phenomena can plausibly be attributed to an array of defect-mediated charge-trapping mechanisms including the hygroscopic nature of MnBr2 facilitating rapid moisture uptake115 (via formation of hydrates or other bromide complexes) and activating new deep trap states or ionic defects which can trap the charge carriers,57,116 higher surface area or powdered morphology exhibited by our as-synthesized CsMnBr3 films, which may enhance the recombination ‘hot spots’ for charge carriers, and UV activation of meta-stable trap states that may capture the photogenerated carriers, thereby impeding the overall carrier transport.100–102,117–119
Some related studies showed that in trap-rich systems, photoconductivity often transitions from linear to sub-linear behavior depending on trap-state density. For instance, Yi et al.120 demonstrated that the photoconductivity of hybrid and all inorganic perovskite crystals exhibited a crossover in power law dependence with respect to excitation intensities between power exponents 1 (linear) and 1/2 (sublinear). This behavior was observed in all compounds of cation type (organic or inorganic) or crystallographic phases.
The photocurrent–voltage (ΔIph–V) curves exhibit an initial linear region followed by a nonlinear threshold (VNL) that shifts to higher voltage values with increasing irradiance, as shown in Fig. 10c. The slopes of the linear region of these ΔIph–V curves show a decaying trend with increasing excitation irradiance, as fitted via the following equation:121
| ΔIph = cV + d | (6) |
The analysis of the change rates of current (r) and photocurrent (Δrph), as shown in Fig. 10d and e, demonstrates an irregular decaying trend of the change rate of current with excitation irradiance. The change rate of photocurrent (Δrph) follows a power-law decaying model (Δrph = r0Iα)120 with α ∼ 0.2, implying diminishing gains at higher photon flux levels. The photocurrent at VNL (onset of non-linear region) continues to decline with excitation intensity following a power-law model (ΔIph = s0Iβ),120 as shown in Fig. 10f, with β ∼ 0.19, consistent with other perovskite materials of CH3NH3PbBr3 and CsPbBr3, where photocurrent exhibited a power dependence with incident excitation intensities with a crossover between power exponents of 1 and 1/2, respectively.120 These results highlight the trapping of photo-generated charge carriers via different trap states, leading to NPC behavior of the CsMnBr3 films.
The as-constructed white emitting backlight structures (Fig. 7) exhibit a ‘colder’ white light with a higher color coordinated temperature (CCT) of ∼8455 K and a wider color gamut coverage area (extracted through Commission Internationale de l’Eclairage (CIE) color coordinates) spanning 132% of NTSC 1953 and ∼186% of sRGB color standards, as calculated via eqn (S7) and (S8)85 and depicted in Fig. 11, respectively. Although the spectral overlaps between emission bands were not separately integrated, it was inherently represented in the measured combined PL spectrum for these stacked LCD backlight structures (Fig. 7). Furthermore, these wide gamut coverage values exceed those commonly reported for red-emitting phosphor films18,85,122 (commonly used in white backlight structures), underscoring not only the superior spectral purity but also the color rendering capability of the CsMnBr3 films. When coupled with the environmentally benign aqueous synthesis route, it further highlights the promise of these green-fabricated CsMnBr3 films for next-generation display technologies.
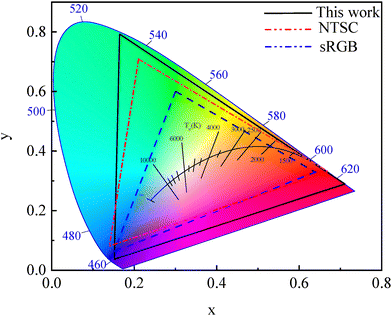 | ||
| Fig. 11 CIE color gamut coverage of white emitting backlight structures from the as-prepared CsMnBr3 films (λP ∼ 644 nm). | ||
Comparison among different red emitting phosphors,123–136 utilized in white LCD backlight structures, is made in terms of the CIE color gamut coverage area with respect to the NTSC standard, as shown in Fig. 12. It is evident that the red-emitting CsMnBr3 films outperform other red-emitting phosphors reported, making them excellent candidates for next generation display applications.
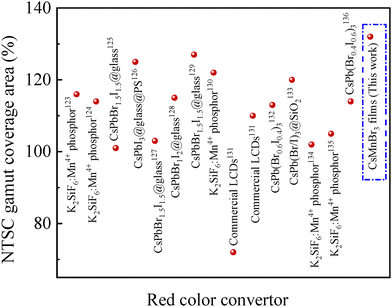 | ||
| Fig. 12 Relative NTSC gamut coverage areas of white-emitting backlight structures with different green emitting films. | ||
4. Conclusions
In summary, we have demonstrated a sustainable, facile, and low-cost approach to synthesize red-emitting CsMnBr3 films of high quality by using water as the only solvent. This low temperature green route overcomes the limitations of conventional methods that rely on toxic solvents, high temperatures, and/or high inert atmospheres, and directly yields emissive thin films rather than powders or colloidal nanocrystals. The as-fabricated CsMnBr3 films exhibit efficient deep-red photo-luminescence (λ ∼ 644 nm) and an exceptionally wide color gamut (spanning ∼132% of NTSC 1953 and ∼186% of sRGB color standards), underscoring their suitability as next-generation backlight materials for wide-color displays, which majorly rely on red phosphors to date. These films exhibit an intriguing NPC behavior and power-law dependent photo-responses, which provide new insights into the charge transport mechanism of Pb-free Mn-based halide perovskites. Overall, this work offers fresh insights for scalable, environment-friendly, and high-performance phosphor films for optoelectronic and display technologies.Conflicts of interest
The authors declare no competing financial interest.Data availability
All possible experimental and analysed results have been included in this manuscript and supplementary information (SI) explicitly. No other new data have been generated by any further experiments/analyses. Supplementary information is available. See DOI: https://doi.org/10.1039/d5ma01256b.Acknowledgements
FY is grateful for the support by the NSF through the CBET-2018411 monitored by Dr Nora F Savage.References
- B. Conings, J. Drijkoningen, N. Gauquelin, A. Babayigit, J. D'Haen, L. D'Olieslaeger, A. Ethirajan, J. Verbeeck, J. Manca and E. Mosconi, Intrinsic thermal instability of methylammonium lead trihalide perovskite, Adv. Energy Mater., 2015, 5(15), 1500477 CrossRef.
- Y. Dong, T. Qiao, D. Kim, D. Parobek, D. Rossi and D. H. Son, Precise control of quantum confinement in cesium lead halide perovskite quantum dots via thermodynamic equilibrium, Nano Lett., 2018, 18(6), 3716–3722 CrossRef CAS PubMed.
- A. Tiwari, N. S. Satpute, C. M. Mehare and S. Dhoble, Challenges, recent advances and improvements for enhancing the efficiencies of ABX3-based PeLEDs (perovskites light emitting diodes): A review, J. Alloys Compd., 2021, 850, 156827 CrossRef CAS.
- S. Singh, X. Tang and F. Yang, Low-temperature synthesis of highly stable blue-emitting perovskite CsPbBr3 quantum dots, J. Phys. Chem. C, 2025, 129(7), 3942–3950 CrossRef CAS.
- K. Chen, C. Wang, Z. Peng, K. Qi, Z. Guo, Y. Zhang and H. Zhang, The chemistry of colloidal semiconductor nanocrystals: From metal-chalcogenides to emerging perovskite, Coord. Chem. Rev., 2020, 418, 213333 CrossRef CAS.
- Y. Bai, M. Hao, S. Ding, P. Chen and L. Wang, Surface chemistry engineering of perovskite quantum dots: strategies, applications, and perspectives, Adv. Mater., 2022, 34(4), 2105958 CrossRef CAS PubMed.
- F. Zhang, H. Zhong, C. Chen, X.-G. Wu, X. Hu, H. Huang, J. Han, B. Zou and Y. Dong, Brightly luminescent and color-tunable colloidal CH3NH3PbX3 (X = Br, I, Cl) quantum dots: potential alternatives for display technology, ACS Nano, 2015, 9(4), 4533–4542 CrossRef CAS PubMed.
- Y. Tong, E. Bladt, M. F. Aygüler, A. Manzi, K. Z. Milowska, V. A. Hintermayr, P. Docampo, S. Bals, A. S. Urban and L. Polavarapu, Highly luminescent cesium lead halide perovskite nanocrystals with tunable composition and thickness by ultrasonication, Angew. Chem., Int. Ed., 2016, 55(44), 13887–13892 CrossRef CAS PubMed.
- M. Chen, Y. Zou, L. Wu, Q. Pan, D. Yang, H. Hu, Y. Tan, Q. Zhong, Y. Xu and H. Liu, Solvothermal synthesis of high-quality all-inorganic cesium lead halide perovskite nanocrystals: from nanocube to ultrathin nanowire, Adv. Funct. Mater., 2017, 27(23), 1701121 CrossRef.
- Q. Pan, H. Hu, Y. Zou, M. Chen, L. Wu, D. Yang, X. Yuan, J. Fan, B. Sun and Q. Zhang, Microwave-assisted synthesis of high-quality “all-inorganic” CsPbX3 (X= Cl, Br, I) perovskite nanocrystals and their application in light emitting diodes, J. Mater. Chem. C, 2017, 5(42), 10947–10954 RSC.
- Z. Long, H. Ren, J. Sun, J. Ouyang and N. Na, High-throughput and tunable synthesis of colloidal CsPbX 3 perovskite nanocrystals in a heterogeneous system by microwave irradiation, Chem. Commun., 2017, 53(71), 9914–9917 RSC.
- L. Rao, Y. Tang, C. Song, K. Xu, E. T. Vickers, S. Bonabi Naghadeh, X. Ding, Z. Li and J. Z. Zhang, Polar-solvent-free synthesis of highly photoluminescent and stable CsPbBr3 nanocrystals with controlled shape and size by ultrasonication, Chem. Mater., 2018, 31(2), 365–375 CrossRef.
- J. Zou, M. Li, X. Zhang and W. Zheng, Perovskite quantum dots: synthesis, applications, prospects, and challenges, J. Appl. Phys., 2022, 132(22), 220901 CrossRef CAS.
- M. H. Miah, M. U. Khandaker, M. J. Hossen, N. E. Ashrafi, I. Jahan, M. Shahinuzzaman, M. Nur-E-Alam, M. Y. Hanfi, M. H. Ullah and M. A. Islam, Lead-free alternatives and toxicity mitigation strategies for sustainable perovskite solar cells: a critical review, Mater. Adv., 2025, 6(9), 2718–2752 RSC.
- J. Almutlaq, W. J. Mir, L. Gutiérrez-Arzaluz, J. Yin, S. Vasylevskyi, P. Maity, J. Liu, R. Naphade, O. F. Mohammed and O. M. Bakr, CsMnBr3: Lead-free nanocrystals with high photoluminescence quantum yield and picosecond radiative lifetime, ACS Mater. Lett., 2021, 3(3), 290–297 CrossRef CAS.
- P. Gao, S. Cheng, J. Liu, J. Li, Y. Guo, Z. Deng, T. Qin and A. Wang, Facile synthesis of highly emissive all-inorganic manganese bromide compounds with perovskite-related structures for white LEDs, Molecules, 2022, 27(23), 8259 CrossRef CAS PubMed.
- G. Xu, C. Wang, Y. Li, W. Meng, G. Luo, M. Peng, B. Xu and Z. Deng, Solid-state synthesis of cesium manganese halide nanocrystals in glass with bright and broad red emission for white LEDs, Chem. Sci., 2023, 14(20), 5309–5315 RSC.
- J. H. Oh, H. Kang, M. Ko and Y. R. Do, Analysis of wide color gamut of green/red bilayered freestanding phosphor film-capped white LEDs for LCD backlight, Opt. Express, 2015, 23(15), A791–A804 CrossRef CAS PubMed.
- S. Li, R.-J. Xie, T. Takeda and N. Hirosaki, Critical review—narrow-band nitride phosphors for wide color-gamut white LED backlighting, ECS J. Solid State Sci. Technol., 2017, 7(1), R3064 CrossRef.
- L. Wang, X. Wang, T. Kohsei, K.-I. Yoshimura, M. Izumi, N. Hirosaki and R.-J. Xie, Highly efficient narrow-band green and red phosphors enabling wider color-gamut LED backlight for more brilliant displays, Opt. Express, 2015, 23(22), 28707–28717 CrossRef CAS PubMed.
- D. Mo, L. Hu, G. Zeng, G. Chen, J. Wan, Z. Yu, Z. Huang, K. He, C. Zhang and M. Cheng, Cadmium-containing quantum dots: properties, applications, and toxicity, Appl. Microbiol. Biotechnol., 2017, 101(7), 2713–2733 CrossRef CAS PubMed.
- T. Zhao, S. Zhang and D. Zhu, A Novel Zero-Thermal-Quenching Red Phosphor with High Quantum Efficiency and Color Purity, Inorganics, 2023, 11(10), 406 CrossRef CAS.
- M. Li, W. Li and H. Wei, Unique Optoelectronic Properties and Applications of Lead-Free Perovskites and Derivatives, Adv. Photon. Res., 2025, 6(2), 2400095 CrossRef CAS.
- P. Han, X. Zhang, C. Luo, W. Zhou, S. Yang, J. Zhao, W. Deng and K. Han, Manganese-doped, lead-free double perovskite nanocrystals for bright orange-red emission, ACS Cent. Sci., 2020, 6(4), 566–572 CrossRef CAS PubMed.
- T. W. Kang, E. J. Choi, Y. J. Park, J. Hwang, B. Bae and S. W. Kim, Phase-selective synthesis of lead-free CsMnBr3 and Cs3MnBr5 nanocrystals dependent on solvent concentration, Opt. Lett., 2022, 47(7), 1806–1809 CrossRef CAS PubMed.
- H. Bahmani Jalali, A. Pianetti, J. Zito, M. Imran, M. Campolucci, Y. P. Ivanov, F. Locardi, I. Infante, G. Divitini and S. Brovelli, Cesium manganese bromide nanocrystal sensitizers for broadband Vis-to-NIR downshifting, ACS Energy Lett., 2022, 7(5), 1850–1858 CrossRef CAS PubMed.
- P. Sahu and S. P. Mukherjee, Rapid and efficient microwave-assisted synthesis of Mn-doped cesium bromide to phase engineered cesium manganese bromide nanocrystals with color-tunable RGB emission, J. Mater. Chem. C, 2025, 13(19), 9465–9473 RSC.
- J. Cai, X. Zhang, Y. Chen, W. Lai, Y. Ye, S. Xu, Q. Yan, T. Guo, J. Luo and E. Chen, Neuron-inspired CsPbBr3/PDMS nanospheres for multi-dimensional sensing and interactive displays, Light: Sci. Appl., 2025, 14(1), 55 CrossRef CAS PubMed.
- J. Cai, W. Lai, Y. Chen, Y. Ye, S. Xu, T. Guo and E. Chen, Ultrawide-Range Wearable Temperature Sensor Utilizing Reversible Luminescence of CsPbBr3/PS Composites, Nano Energy, 2025, 111152 CrossRef CAS.
- I. López-Fernández, D. Valli, C. Y. Wang, S. Samanta, T. Okamoto, Y. T. Huang, K. Sun, Y. Liu, V. S. Chirvony and A. Patra, Lead-free halide perovskite materials and optoelectronic devices: progress and prospective, Adv. Funct. Mater., 2024, 34(6), 2307896 CrossRef.
- H. J. Seifert and E. Dau, Über die Systeme Alkalimetallbromid/Mangan (II)-bromid, Z. Anorg. Allg. Chem., 1972, 391(3), 302–312 CrossRef CAS.
- G. Jauncey, The scattering of X-rays and Bragg's law, Proc. Natl. Acad. Sci. U. S. A., 1924, 10(2), 57–60 CrossRef CAS PubMed.
- H. P. Klug and L. E. Alexander, X-ray diffraction procedures: for polycrystalline and amorphous materials, 1974.
- H. Liang, W. Yang, J. Xia, H. Gu, X. Meng, G. Yang, Y. Fu, B. Wang, H. Cai and Y. Chen, Strain effects on flexible perovskite solar cells, Adv. Sci., 2023, 10(35), 2304733 CrossRef CAS PubMed.
- M. Wang, Z. Ni, X. Xiao, Y. Zhou and J. Huang, Strain engineering in metal halide perovskite materials and devices: Influence on stability and optoelectronic properties, Chem. Phys. Rev., 2021, 2(3), 031302 CrossRef.
- X. Huang, X. Tang, X. Wen, S. Singh, Y. C. Lu and F. Yang, Blue-emitting CsI thin films, J. Lumin., 2025, 280, 121126 CrossRef CAS.
- S. Mazumdar, Y. Zhao and X. Zhang, Stability of perovskite solar cells: Degradation mechanisms and remedies, Front. Electron., 2021, 2, 712785 CrossRef.
- B. Yang, D. Bogachuk, J. Suo, L. Wagner, H. Kim, J. Lim, A. Hinsch, G. Boschloo, M. K. Nazeeruddin and A. Hagfeldt, Strain effects on halide perovskite solar cells, Chem. Soc. Rev., 2022, 51(17), 7509–7530 RSC.
- S. Singh, X. Wen and F. Yang, Effect of NaCl on the luminescent behavior of CsI thin films, RSC Adv., 2025, 15(44), 36993–37005 RSC.
- I. Poli Improving the stability of halide perovskites for photovoltaic applications and solar fuel generation. University of Bath, 2019.
- S. Macpherson, T. A. Doherty, A. J. Winchester, S. Kosar, D. N. Johnstone, Y.-H. Chiang, K. Galkowski, M. Anaya, K. Frohna and A. N. Iqbal, Local nanoscale phase impurities are degradation sites in halide perovskites, Nature, 2022, 607(7918), 294–300 CrossRef CAS PubMed.
- H. Jin, E. Debroye, M. Keshavarz, I. G. Scheblykin, M. B. Roeffaers, J. Hofkens and J. A. Steele, It's a trap! On the nature of localised states and charge trapping in lead halide perovskites, Mater. Horiz., 2020, 7(2), 397–410 RSC.
- M. Othman, Q. Jeangros, D. A. Jacobs, M. H. Futscher, S. Zeiske, A. Armin, A. Jaffrès, A. G. Kuba, D. Chernyshov and S. Jenatsch, Alleviating nanostructural phase impurities enhances the optoelectronic properties, device performance and stability of cesium-formamidinium metal–halide perovskites, Energy Environ. Sci., 2024, 17(11), 3832–3847 RSC.
- Q. Kong, B. Yang, J. Chen, R. Zhang, S. Liu, D. Zheng, H. Zhang, Q. Liu, Y. Wang and K. Han, Phase engineering of cesium manganese bromides nanocrystals with color-tunable emission, Angew. Chem., Int. Ed., 2021, 60(36), 19653–19659 CrossRef CAS PubMed.
- D. Liang, H. Xiao, W. Cai, S. Lu, S. Zhao, Z. Zang and L. Xie, Mn2+ -based luminescent metal halides: syntheses, properties, and applications, Adv. Opt. Mater., 2023, 11(15), 2202997 CrossRef CAS.
- J. Lin, Q. Zhang, L. Wang, X. Liu, W. Yan, T. Wu, X. Bu and P. Feng, Atomically precise doping of monomanganese ion into coreless supertetrahedral chalcogenide nanocluster inducing unusual red shift in Mn2+ emission, J. Am. Chem. Soc., 2014, 136(12), 4769–4779 CrossRef CAS PubMed.
- J. Orive, J. L. Mesa, R. Balda, J. N. Fernandez, J. S. Rodriguez Fernandez, T. Rojo and M. A. I. Arriortua, Enhancement of the luminescent properties of a new red-emitting phosphor, Mn2 (HPO3) F2, by Zn substitution, Inorg. Chem., 2011, 50(24), 12463–12476 CrossRef CAS PubMed.
- S. Park, T. Rhodin and L. Rathbun, Halide formation and etching of Cu thin films with Cl2 and Br2, J. Vac. Sci. Technol., A, 1986, 4(2), 168–172 CrossRef CAS.
- R. Krumpolec, T. Homola, D. C. Cameron, J. Humlíček, O. Caha, K. Kuldová, R. Zazpe, J. Přikryl and J. M. Macak, Structural and optical properties of luminescent copper (I) chloride thin films deposited by sequentially pulsed chemical vapour deposition, Coatings, 2018, 8(10), 369 CrossRef.
- Q. Kong, X. Xu, Z. Wang, J. Chen and Y. Feng, Multi-Stimuli-Responsive photoluminescence of cesium manganese bromide by temperature and solvent-induced structural phase transitions, Chem. Eng. J., 2024, 485, 149741 CrossRef CAS.
- M. Zeng, L. Wei, J. Hu, X. Zeng, G. Li, X. Zhang, Y. Hu, H. Gu and Y. Li, Achieving high transparency and stable luminescence in CsMnBr3 nanocrystals embedded glass, J. Eur. Ceram. Soc., 2025, 45(4), 117029 CrossRef CAS.
- H. Zhang and J. A. Rogers, Recent advances in flexible inorganic light emitting diodes: from materials design to integrated optoelectronic platforms, Adv. Opt. Mater., 2019, 7(2), 1800936 CrossRef.
- B. Xu, J. Zhou, C. Zhang, Y. Chang and Z. Deng, Research Progress on Quantum Dot-Embedded Polymer Films and Plates for LCD Backlight Display, Polymers, 2025, 17(2), 233 CrossRef CAS PubMed.
- G. J. Lee, J.-G. Lee, Y. Kim, T. Park, Y. W. Ko and J.-H. Ko, The effect of the reflective property of a reflection film on the performance of backlight units with quantum-dot films for LCD applications, J. Inf. Disp., 2021, 22(2), 55–61 CrossRef CAS.
- Z. Luo, D. Xu and S.-T. Wu, Emerging quantum-dots-enhanced LCDs, J. Disp. Technol., 2014, 10(7), 526–539 Search PubMed.
- S. Hu, X. Yan, Y. Zhang, B. Yang, H. Li and C. Sheng, Light-induced photoluminescence quenching and degradation in quasi 2D perovskites film of (C6H5C2H4NH3) 2 (CH3NH3) 2 [Pb3I10], Appl. Sci., 2021, 11(6), 2683 CrossRef CAS.
- N. A. Manshor, Q. Wali, K. K. Wong, S. K. Muzakir, A. Fakharuddin, L. Schmidt-Mende and R. Jose, Humidity versus photo-stability of metal halide perovskite films in a polymer matrix, Phys. Chem. Chem. Phys., 2016, 18(31), 21629–21639 RSC.
- Z. Song, A. Abate, S. C. Watthage, G. K. Liyanage, A. B. Phillips, U. Steiner, M. Graetzel and M. J. Heben, Perovskite solar cell stability in humid air: partially reversible phase transitions in the PbI2-CH3NH3I-H2O system, Adv. Energy Mater., 2016, 6(19), 1600846 CrossRef.
- H.-C. Chen, A. Shabir, C. M. Tan, P. Singh and J.-H. Lin, Degradation dynamics of quantum dots in white LED applications, Sci. Rep., 2021, 11(1), 24153 CrossRef CAS PubMed.
- H. Moon, C. Lee, W. Lee, J. Kim and H. Chae, Stability of quantum dots, quantum dot films, and quantum dot light-emitting diodes for display applications, Adv. Mater., 2019, 31(34), 1804294 CrossRef PubMed.
- S. Huang, Z. Li, B. Wang, N. Zhu, C. Zhang, L. Kong, Q. Zhang, A. Shan and L. Li, Morphology evolution and degradation of CsPbBr3 nanocrystals under blue light-emitting diode illumination, ACS Appl. Mater. Interfaces, 2017, 9(8), 7249–7258 CrossRef CAS PubMed.
- C. Balarew, D. Rabadjieva, S. Tepavitcharova, C. Christov and O. Angelova, Thermodynamic study of the aqueous rubidium and manganese bromide system, J. Solution Chem., 1999, 28(7), 949–958 CrossRef CAS.
- Q.-C. Yang, D.-F. Qiu, Y.-L. Dang, Z.-P. Qiao and Z.-W. Xu, Phase equilibrium systems of CsX+ MnX2+ H2O (X = Cl, Br) at 298.15 K and standard molar enthalpy of formation of CsMnX3·2H2O and Cs2MnX4·2H2O, J. Chem. Thermodyn., 2021, 161, 106541 CrossRef CAS.
- W. M. Haynes, CRC handbook of chemistry and physics, CRC press, 2016 Search PubMed.
- H. Konieczna, D. Lundberg and I. Persson, Solvation and coordination chemistry of manganese (II) in some solvents. A transfer thermodynamic, complex formation, EXAFS spectroscopic and crystallographic study, Polyhedron, 2021, 195, 114961 CrossRef CAS.
- Y. Liu, L. Zhang, X. Long, P. Jiang, C. Geng and S. Xu, Ultra-stable CsPbBr 3 nanocrystals with lead-carboxylate/SiO 2 encapsulation for LED applications, J. Mater. Chem. C, 2021, 9(37), 12581–12589 RSC.
- J. Park, K. Y. Jang, S. H. Lee, D.-H. Kim, S.-H. Cho and T.-W. Lee, Stable orthorhombic CsPbBr3 light emitters: encapsulation-assisted in situ synthesis, Chem. Mater., 2023, 35(16), 6266–6273 CrossRef CAS.
- F. Boussoufi, M. Pousthomis, A. Kuntzmann, M. D’amico, G. Patriarche and B. Dubertret, Spray-drying polymer encapsulation of CsPbBr3 perovskite nanocrystals with enhanced photostability for LED downconverters, ACS Appl. Nano Mater., 2021, 4(7), 7502–7512 CrossRef CAS.
- A. Ghosh, L. Gerenser, C. Jarman and J. Fornalik, Thin-film encapsulation of organic light-emitting devices, Appl. Phys. Lett., 2005, 86(22), 223503 CrossRef.
- Q. Lu, Z. Yang, X. Meng, Y. Yue, M. A. Ahmad, W. Zhang, S. Zhang, Y. Zhang, Z. Liu and W. Chen, A review on encapsulation technology from organic light emitting diodes to organic and perovskite solar cells, Adv. Funct. Mater., 2021, 31(23), 2100151 CrossRef CAS.
- M. A. A. Kazemi, N. Folastre, P. Raval, M. Sliwa, J. M. V. Nsanzimana, S. Golonu, A. Demortiere, J. Rousset, O. Lafon and L. Delevoye, Moisture-Induced Non-Equilibrium Phase Segregation in Triple Cation Mixed Halide Perovskite Monitored by In Situ Characterization Techniques and Solid-State NMR, Energy Environ. Mater., 2023, 6(2), e12335 CrossRef CAS.
- C. X. Zhang, T. Shen, D. Guo, L. M. Tang, K. Yang and H. X. Deng, Reviewing and understanding the stability mechanism of halide perovskite solar cells, InfoMat, 2020, 2(6), 1034–1056 CrossRef CAS.
- J. Li, R. Xia, W. Qi, X. Zhou, J. Cheng, Y. Chen, G. Hou, Y. Ding, Y. Li and Y. Zhao, Encapsulation of perovskite solar cells for enhanced stability: Structures, materials and characterization, J. Power Sources, 2021, 485, 229313 CrossRef CAS.
- B. McKenna, J. R. Troughton, T. M. Watson and R. C. Evans, Enhancing the stability of organolead halide perovskite films through polymer encapsulation, RSC Adv., 2017, 7(52), 32942–32951 RSC.
- Q.-F. Li, J.-T. Wang and Z. Wang, Improving the stability of perovskite nanocrystals via SiO 2 coating and their applications, RSC Adv., 2024, 14(2), 1417–1430 RSC.
- Y. Yang, H. Chen, C. Liu, J. Xu, C. Huang, C. D. Malliakas, H. Wan, A. S. Bati, Z. Wang and R. P. Reynolds, Amidination of ligands for chemical and field-effect passivation stabilizes perovskite solar cells, Science, 2024, 386(6724), 898–902 CrossRef CAS PubMed.
- E. V. Péan, S. Dimitrov, C. S. De Castro and M. L. Davies, Interpreting time-resolved photoluminescence of perovskite materials, Phys. Chem. Chem. Phys., 2020, 22(48), 28345–28358 RSC.
- Y. Sun, Y. Wang, H. Zhu, N. Jin, A. Mohammad, N. Biyikli, O. Chen, K. Chen and J. Zhao, Excitation wavelength-dependent photoluminescence decay of single quantum dots near plasmonic gold nanoparticles, J. Chem. Phys., 2022, 156(15), 154701 CrossRef CAS.
- S. Wei, J. Hu, C. Bi, K. Ren, X. Wang, N. H. de de Leeuw, Y. Lu, M. Sui and W. Wang, Strongly-Confined CsPbBr3 Perovskite Quantum Dots with Ultralow Trap Density and Narrow Size Distribution for Efficient Pure-Blue Light-Emitting Diodes, Small, 2024, 20(36), 2400885 CrossRef CAS PubMed.
- L.-Y. Wang, L.-L. Deng, X. Wang, T. Wang, H.-R. Liu, S.-M. Dai, Z. Xing, S.-Y. Xie, R.-B. Huang and L.-S. Zheng, Di-isopropyl ether assisted crystallization of organic–inorganic perovskites for efficient and reproducible perovskite solar cells, Nanoscale, 2017, 9(45), 17893–17901 RSC.
- F. Staub, H. Hempel, J.-C. Hebig, J. Mock, U. W. Paetzold, U. Rau, T. Unold and T. Kirchartz, Beyond bulk lifetimes: insights into lead halide perovskite films from time-resolved photoluminescence, Phys. Rev. Appl., 2016, 6(4), 044017 CrossRef.
- S. Bera, A. Saha, S. Mondal, A. Biswas, S. Mallick, R. Chatterjee and S. Roy, Review of defect engineering in perovskites for photovoltaic application, Mater. Adv., 2022, 3(13), 5234–5247 RSC.
- X. Guan, Y. Li, Y. Meng, K. Wang, K. Lin, Y. Luo, J. Wang, Z. Duan, H. Liu and L. Yang, Targeted elimination of tetravalent-Sn-induced defects for enhanced efficiency and stability in lead-free NIR-II perovskite LEDs, Nat. Commun., 2024, 15(1), 9913 CrossRef CAS PubMed.
- T. Kirchartz, T. Markvart, U. Rau and D. A. Egger, Impact of small phonon energies on the charge-carrier lifetimes in metal-halide perovskites, J. Phys. Chem. Lett., 2018, 9(5), 939–946 CrossRef CAS PubMed.
- X. Tang, Y. Zhang, N. L. Kothalawala, X. Wen, D. Y. Kim and F. Yang, MAPbBr3 nanocrystals from aqueous solution for poly (methyl methacrylate)-MAPbBr3 nanocrystal films with compression-resistant photoluminescence, Nanotechnology, 2022, 33(23), 235605 CrossRef CAS.
- N. Phung, A. Mattoni, J. A. Smith, D. Skroblin, H. Köbler, L. Choubrac, J. Breternitz, J. Li, T. Unold and S. Schorr, Photoprotection in metal halide perovskites by ionic defect formation, Joule, 2022, 6(9), 2152–2174 CrossRef CAS.
- J. Shi, M. Wang, Z. Da, C. Zhang, J. Wang, Y. Ding, Y. Xu and N. V. Gaponenko, Studies on the optical stability of CsPbBr 3 with different dimensions (0D, 1D, 2D, 3D) under thermal environments, Nanoscale, 2023, 15(26), 11190–11198 RSC.
- S. Kahmann, O. Nazarenko, S. Shao, O. Hordiichuk, M. L. Kepenekian, J. Even, M. V. Kovalenko, G. R. Blake and M. A. Loi, Negative thermal quenching in FASnI3 perovskite single crystals and thin films, ACS Energy Lett., 2020, 5(8), 2512–2519 CrossRef CAS.
- Q. Zhang, M. He, Q. Wan, W. Zheng, M. Liu, C. Zhang, X. Liao, W. Zhan, L. Kong and X. Guo, Suppressing thermal quenching of lead halide perovskite nanocrystals by constructing a wide-bandgap surface layer for achieving thermally stable white light-emitting diodes, Chem. Sci., 2022, 13(13), 3719–3727 RSC.
- M. Anni, A. Cretí, M. L. De Giorgi and M. Lomascolo, Local morphology effects on the photoluminescence properties of thin CsPbBr3 nanocrystal films, Nanomaterials, 2021, 11(6), 1470 CrossRef CAS PubMed.
- G. Xiang, Y. Zhou, W. Peng, J. Zhang, Y. Liu, J. Zhang, Z. Yue, X. Zhang, C. Song and B. Ding, Vacuum-deposited perovskite CsPbBr3 thin-films for temperature-stable Si based pure-green all-inorganic light-emitting diodes, Ceram. Int., 2023, 49(13), 21624–21633 CrossRef CAS.
- A. Xie, T. H. Nguyen, C. Hettiarachchi, M. E. Witkowski, W. Drozdowski, M. D. Birowosuto, H. Wang and C. Dang, Thermal quenching and dose studies of X-ray luminescence in single crystals of halide perovskites, J. Phys. Chem. C, 2018, 122(28), 16265–16273 CrossRef CAS.
- Y. Tang, L. Gomez, M. Van Der Laan, D. Timmerman, V. Sebastian, C.-C. Huang, T. Gregorkiewicz and P. Schall, Room temperature synthesis and characterization of novel lead-free double perovskite nanocrystals with a stable and broadband emission, J. Mater. Chem. C, 2021, 9(1), 158–163 RSC.
- H. S. Bawazir; S. M. Qaid; H. M. Ghaithan; K. K. AlHarbi; A. F. Bin Ajaj and A. S. Aldwayyan, Phase state influence on photoluminescence of MAPb (BrxI1−x)3 perovskites towards optimized photonics applications, Photonics, MDPI, 2022, vol. 10, p. 21 Search PubMed.
- Y. Xu, J. Li, F. Zhao, Y. Gao, R. Chen and T. He, Optical Properties of a CsMnBr3 Single Crystal, ACS Omega, 2022, 7(33), 29415–29419 CrossRef CAS PubMed.
- S. Ghimire, M. F. Khatun, B. M. Sachith, T. Okamoto, J. Sobhanan, C. Subrahmanyam and V. Biju, Highly Luminescent and Stable Halide Perovskite Nanocrystals by Interfacial Defect Passivation and Amphiphilic Ligand Capping, ACS Appl. Mater. Interfaces, 2023, 15(34), 41081–41091 CrossRef CAS PubMed.
- Q. Jing, M. Zhang, X. Huang, X. Ren, P. Wang and Z. Lu, Surface passivation of mixed-halide perovskite CsPb (BrxI1−x)3 nanocrystals by selective etching for improved stability, Nanoscale, 2017, 9(22), 7391–7396 RSC.
- J. Wiley, Introduction to solid state physics, New York, 1986, 185.
- G. Horowitz, Organic field-effect transistors, Adv. Mater., 1998, 10(5), 365–377 CrossRef CAS.
- N. K. Tailor, C. A. Aranda, M. Saliba and S. Satapathi, Negative photoconductivity: bizarre physics in semiconductors, ACS Mater. Lett., 2022, 4(11), 2298–2320 CrossRef CAS.
- N. K. Tailor, P. Maity and S. Satapathi, Observation of Negative Photoconductivity in Lead-Free Cs3Bi2Br9Perovskite Single Crystal, ACS Photonics, 2021, 8(8), 2473–2480 CrossRef CAS.
- N. K. Tailor, P. Maity, M. I. Saidaminov, N. Pradhan and S. Satapathi, Dark self-healing-mediated negative photoconductivity of a lead-free Cs3Bi2Cl9 perovskite single crystal, J. Phys. Chem. Lett., 2021, 12(9), 2286–2292 CrossRef CAS PubMed.
- S. X. Li, Y. S. Xu, C. L. Li, Q. Guo, G. Wang, H. Xia, H. H. Fang, L. Shen and H. B. Sun, Perovskite single-crystal microwire-array photodetectors with performance stability beyond 1 year, Adv. Mater., 2020, 32(28), 2001998 CrossRef CAS.
- B. Sreelakshmi and R. Thamankar, Investigation of the transient photo-response and switching window of an Al/indigo/Al device: unveiling negative photoconductivity and the photo-enhanced memory window, Mater. Adv., 2024, 5(14), 5912–5921 RSC.
- M. Wang, S. Wang, R. Chen, M. Zhu, Y. Liu, H. Ding, J. Ren, T. Xuan and H. Li, Highly Efficient and Stable CsPbBr3-Alginic Acid Composites for White Light-Emitting Diodes, Coatings, 2023, 13(6), 1062 CrossRef CAS.
- W. Yue, Y. Liu, C. Heyu, L. Chunyang, L. Weizhen, X. Haiyang, Z. Cen, W. Zhongqiang and L. Yichun, White LED based on CsPbBr3 nanocrystal phosphors via a facile two-step solution synthesis route, Mater. Res. Bull., 2018, 104, 48–52 CrossRef.
- D. Tsvetkov, M. Mazurin, I. Ivanov, D. Malyshkin, V. Sereda and A. Zuev, Crucial role of water in the mechanosynthesis of CsPbI3 and other ABX3 halides, Chem. - Eur. J., 2020, 26(55), 12549–12552 CrossRef CAS PubMed.
- S. Xiao, K. Zhang, S. Zheng and S. Yang, Good or evil: what is the role of water in crystallization of organometal halide perovskites?, Nanoscale Horiz., 2020, 5(8), 1147–1154 RSC.
- X. Shen, K. Kang, Z. Yu, W. H. Jeong, H. Choi, S. H. Park, S. D. Stranks, H. J. Snaith, R. H. Friend and B. R. Lee, Passivation strategies for mitigating defect challenges in halide perovskite light-emitting diodes, Joule, 2023, 7(2), 272–308 CrossRef CAS.
- L. Gupta, S. Rath, S. Abbi and F. Jain, Temperature dependence of the fundamental band gap parameters in cadmium-rich Zn x Cd 1-x Se using photoluminescence spectroscopy, Pramana, 2003, 61, 729–737 CrossRef CAS.
- A. Merdasa, M. Bag, Y. Tian, E. Kallman, A. Dobrovolsky and I. G. Scheblykin, Super-resolution luminescence microspectroscopy reveals the mechanism of photoinduced degradation in CH3NH3PbI3 perovskite nanocrystals, J. Phys. Chem. C, 2016, 120(19), 10711–10719 CrossRef CAS.
- S. Meloni, G. Palermo, N. Ashari-Astani, M. Grätzel and U. Rothlisberger, Valence and conduction band tuning in halide perovskites for solar cell applications, J. Mater. Chem. A, 2016, 4(41), 15997–16002 RSC.
- L. Zhang, W. Geng, C.-J. Tong, X. Chen, T. Cao and M. Chen, Strain induced electronic structure variation in methyl-ammonium lead iodide perovskite, Sci. Rep., 2018, 8(1), 7760 CrossRef PubMed.
- J. Li, X. Yuan, P. Jing, J. Li, M. Wei, J. Hua, J. Zhao and L. Tian, Temperature-dependent photoluminescence of inorganic perovskite nanocrystal films, RSC Adv., 2016, 6(82), 78311–78316 RSC.
- E. De Lyle The magnetic anisotropies of manganese-bromide and ferrous-chloride. The University of Chicago, 1964.
- S. Wu, L. Yuan, G. Chen, C. Peng and Y. Jin, All-inorganic Mn 2+ -doped metal halide perovskite crystals for the late-time detection of X-ray afterglow imaging, Nanoscale, 2023, 15(33), 13628–13634 RSC.
- J. Tang, W. Tian, C. Zhao, Q. Sun, C. Zhang, H. Cheng, Y. Shi and S. Jin, Imaging the moisture-induced degradation process of 2D organolead halide perovskites, ACS Omega, 2022, 7(12), 10365–10371 CrossRef CAS PubMed.
- J.-S. Park, J. Calbo, Y.-K. Jung, L. D. Whalley and A. Walsh, Accumulation of deep traps at grain boundaries in halide perovskites, ACS Energy Lett., 2019, 4(6), 1321–1327 CrossRef CAS.
- M. A. Haque, J. L. Li, A. L. Abdelhady, M. I. Saidaminov, D. Baran, O. M. Bakr, S. H. Wei and T. Wu, Transition from positive to negative photoconductance in doped hybrid perovskite semiconductors, Adv. Opt. Mater., 2019, 7(22), 1900865 CrossRef CAS.
- H. T. Yi, P. Irkhin, P. P. Joshi, Y. N. Gartstein, X. Zhu and V. Podzorov, Experimental demonstration of correlated flux scaling in photoconductivity and photoluminescence of lead-halide perovskites, Phys. Rev. Appl., 2018, 10(5), 054016 CrossRef CAS.
- K. M. Tenny and M. Keenaghan Ohms Law. 2017.
- T. Yata, Y. Miyamoto and K. Ohmi, Emissive Liquid-Crystal Display Panels Consisting of Red–Green–Blue Patterned Phosphor Layers and Near-Ultraviolet Light-Emitting-Diode Backlight, Jpn. J. Appl. Phys., 2012, 51(2R), 022202 CrossRef.
- Y.-L. Tong, Y.-W. Zhang, K. Ma, R. Cheng, F. Wang and S. Chen, One-step synthesis of FA-directing FAPbBr3 perovskite nanocrystals toward high-performance display, ACS Appl. Mater. Interfaces, 2018, 10(37), 31603–31609 CrossRef CAS PubMed.
- Y. Zu, J. Xi, L. Li, J. Dai, S. Wang, F. Yun, B. Jiao, H. Dong, X. Hou and Z. Wu, High-brightness and color-tunable FAPbBr3 perovskite nanocrystals 2.0 enable ultrapure green luminescence for achieving recommendation 2020 displays, ACS Appl. Mater. Interfaces, 2019, 12(2), 2835–2841 CrossRef PubMed.
- Y. Lu, Y. Xu, S. Chen, J. Lin, J. Zhu, S. Wang, Y. Zheng, F. Huang and D. Chen, Ultra-narrowband emitting and highly stable CsPbX3@ glass@ PDMS (X3 = Br3, Br1. 5I1. 5) monolithic composite film for backlit displays, J. Lumin., 2022, 248, 118952 CrossRef CAS.
- H. Ye, L. Huang, X. Liu, M. Yang, W. Chen, Y. Chen, W. Xiang, S. Pan and X. Liang, Opportunities and challenges in CsPbX3@ Glasses@ Film with High Quality for LCD by Process Optimization towards commercialization, Ceram. Int., 2023, 49(6), 9010–9016 CrossRef CAS.
- J. Lin, Y. Lu, X. Li, F. Huang, C. Yang, M. Liu, N. Jiang and D. Chen, Perovskite quantum dots glasses based backlit displays, ACS Energy Lett., 2021, 6(2), 519–528 CrossRef CAS.
- J. Lin, Z. Zhou, J. Lai, W. Ye, T. Pang, X. Li, L. Zeng, L. Lei, D. Yu and D. Chen, CsPbX3@ Glass (X= Cl, Br, I) Nanocomposites with 1 Whole-Family High Absorption Efficiency above 75% for Backlit Display, Laser Photon. Rev., 2025, 19(4), 2401649 CrossRef CAS.
- S. Chen, J. Lin, S. Zheng, Y. Zheng and D. Chen, Efficient and stable perovskite white light-emitting diodes for backlit display, Adv. Funct. Mater., 2023, 33(18), 2213442 CrossRef CAS.
- C. X. Guo, J. H. Chen, Z. L. He, J. H. Wei and D. B. Kuang, In Situ Growth of MAPbBr3 Quantum Dots in Crosslinked PMMA for Wide-Gamut Display Backlights, Small, 2025, 21(42), e09037 CrossRef CAS PubMed.
- F. Zhang, J. Song, B. Han, T. Fang, J. Li and H. Zeng, High-Efficiency Pure-Color Inorganic Halide Perovskite Emitters for Ultrahigh-Definition Displays: Progress for Backlighting Displays and Electrically Driven Devices, Small Methods, 2018, 2(10), 1700382 CrossRef.
- H. C. Wang, S. Y. Lin, A. C. Tang, B. P. Singh, H. C. Tong, C. Y. Chen, Y. C. Lee, T. L. Tsai and R. S. Liu, Mesoporous silica particles integrated with all-inorganic CsPbBr3 perovskite quantum-dot nanocomposites (MP-PQDs) with high stability and wide color gamut used for backlight display, Angew. Chem., Int. Ed., 2016, 55(28), 7924–7929 CrossRef CAS PubMed.
- C. Sun, Y. Zhang, C. Ruan, C. Yin, X. Wang, Y. Wang and W. W. Yu, Efficient and stable white LEDs with silica-coated inorganic perovskite quantum dots, Adv. Mater., 2016, 28(45), 10088–10094 CrossRef CAS PubMed.
- X. Zhang, H.-C. Wang, A.-C. Tang, S.-Y. Lin, H.-C. Tong, C.-Y. Chen, Y.-C. Lee, T.-L. Tsai and R.-S. Liu, Robust and stable narrow-band green emitter: an option for advanced wide-color-gamut backlight display, Chem. Mater., 2016, 28(23), 8493–8497 CrossRef CAS.
- K. Ma, X.-Y. Du, Y.-W. Zhang and S. Chen, In situ fabrication of halide perovskite nanocrystals embedded in polymer composites via microfluidic spinning microreactors, J. Mater. Chem. C, 2017, 5(36), 9398–9404 RSC.
- J. Y. Sun, F. T. Rabouw, X. F. Yang, X. Y. Huang, X. P. Jing, S. Ye and Q. Y. Zhang, Facile two-step synthesis of all-inorganic perovskite CsPbX3 (X = Cl, Br, and I) zeolite-Y composite phosphors for potential backlight display application, Adv. Funct. Mater., 2017, 27(45), 1704371 CrossRef.
| This journal is © The Royal Society of Chemistry 2026 |