 Open Access Article
Open Access ArticleAmplified photocatalytic performance of UiO-66-NH2/BiOI@α-Bi2O3 ternary heterojunctions towards Congo red degradation and H2O2 production
Anubhav
Naik
a,
Kundan Kumar
Das
b,
Prakash Chandra
Sahoo
a and
Rashmi
Acharya
 *a
*a
aDepartment of Chemistry, I.T.E.R, Siksha ‘O’ Anusandhan Deemed to be University, Bhubaneswar, Odisha-751030, India. E-mail: drrashmiacharya75@gmail.com; rashmiacharya@soa.ac.in; Fax: +91-674-2350642; Tel: +91-674-2351777
bCentre for Nano Science and Nano Technology, ITER, Siksha ‘O’ Anusandhan Deemed to be University, Bhubaneswar, Odisha-751030, India
First published on 5th January 2026
Abstract
Designing an efficient photocatalytic system that achieves a broad visible-light absorption window and a minimal recombination rate has been challenging. In this work, we have depicted UiO-66-NH2/BiOI@α-Bi2O3 ternary heterostructures’ (BBUN) fabrication via a simple solvothermal approach. FESEM and TEM studies revealed that BBUN-4 consists of UiO-66-NH2 (UN) nanoparticles, BiOI microspheres (BM), and in situ derived α-Bi2O3 nanorods (BR). The morphology of BM and BR was manipulated by varying the BM and N,N-dimethylformamide (DMF) ratio. It was observed that BM microspheres and BR nanorods were obtained when the BM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMF ratio was maintained at 7.5
DMF ratio was maintained at 7.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Besides, the interaction of DMF incorporated abundant oxygen vacancies (Ov) in BM and BR. The introduction of abundant oxygen vacancies (Ov) markedly broadened the light absorption edge up to 665 nm. The existence of an Ov–Bi–N interfacial charge transport channel momentously improved the charge transfer and separation rate, as evidenced from PL, EIS, and LSV studies. XPS results, Mott–Schottky analysis, and scavenging tests collectively corroborated the formation of a double Z-scheme BBUN heterojunction. The photocatalytic CR degradation rate for BBUN-4 was determined to be 3.05 and 3.43 times greater than that of pristine UN and BM, respectively. BBUN-4 exhibited H2O2 production of 322 µmol L−1, whereas that obtained over BM and UN was only 127 and 164 µmol L−1, respectively.
1. Besides, the interaction of DMF incorporated abundant oxygen vacancies (Ov) in BM and BR. The introduction of abundant oxygen vacancies (Ov) markedly broadened the light absorption edge up to 665 nm. The existence of an Ov–Bi–N interfacial charge transport channel momentously improved the charge transfer and separation rate, as evidenced from PL, EIS, and LSV studies. XPS results, Mott–Schottky analysis, and scavenging tests collectively corroborated the formation of a double Z-scheme BBUN heterojunction. The photocatalytic CR degradation rate for BBUN-4 was determined to be 3.05 and 3.43 times greater than that of pristine UN and BM, respectively. BBUN-4 exhibited H2O2 production of 322 µmol L−1, whereas that obtained over BM and UN was only 127 and 164 µmol L−1, respectively.
1. Introduction
Growing energy demand, gradual depletion of conventional energy fuels, and alarmingly rising environmental issues have led researchers to develop sustainable technologies. Semiconductor-mediated photocatalysis is an environmentally benign avenue that uses inexhaustible sunlight and earth-abundant water for the successful addressing of these challenges.1–3 Nevertheless, inadequate availability of charge carriers, narrow light absorption window, a faster rate of electron–hole pair recombination, low surface area, and weaker redox abilities are some of the inherent features of semiconductor photocatalysts that hinder their practical applications. Designing porous nanostructures with varying dimensionality, selecting suitable semiconductor photocatalysts, creating atomic defects, constructing Z-scheme charge dynamics, and establishing charge transport channels are the crucial aspects to overcome these bottlenecks.Amongst the wide range of investigated semiconductors, metal–organic frameworks (MOFs) have attracted stupendous attention in diversified photocatalytic applications for their intrinsic characteristics, like tunable porous architecture, high surface area, crystalline structure, and stability.4–6 Recently, UiO-66-NH2 nanoparticles have been recognised as a versatile photocatalyst due to their enhanced aqueous stability and their enlarged surface area and porous structure.7 Moreover, the lone pair of electrons on the N atom of NH2 groups can interact with the π*-antibonding orbitals of benzene rings and experience a bathochromic shift in visible light absorption.8 However, it still suffers from a high rate of charge carrier recombination and poor visible light response. Constructing heterojunctions with one or more visible light-responsive semiconductors having preferred band potentials is a promising strategy to hinder the recombination of charge carriers and widen the visible light absorption window.9 BiOI with a small band gap energy (Eg = 1.77–1.92 eV) enables robust photo response in the visible region. Its large positive valence band potential accelerates the water oxidation reaction to generate hydroxyl ions (˙OH), which aids in pollutant degradation.10 Ji et al. fabricated a BiOI nanosheet-anchored UiO-66-NH2 direct Z-scheme heterojunction, which exhibited a faster bisphenol A photodegradation rate as compared to the pristine counterparts.11 However, double Z-scheme heterojunctions constitute a sophisticated class of photocatalytic materials engineered to substantially improve photocatalytic efficiency. In this configuration, three semiconductors are strategically combined to establish two consecutive Z-scheme charge-transfer routes. Electrons generated in the oxidation component are first neutralized by holes in the middle semiconductor, and then another similar recombination happens at the second interface. Through this electron–hole neutralization, only the low-energy carriers are eliminated, while the electrons possessing the highest reduction power and the holes exhibiting the strongest oxidative potential remain spatially isolated. This unique charge-flow pattern effectively minimizes bulk recombination, ensures long-lived carrier separation, and strengthens the intrinsic redox capability, enabling efficient execution of divergent photocatalytic reactions such as water splitting, H2O2 synthesis, advanced pollutant mineralization etc.12
α-Bi2O3 with excellent oxidation properties possesses an appropriate band gap energy (2.5–2.8 eV) for visible light absorption and can be applied for visible light photocatalysis.13 Moreover, it can be derived from BiOI, with the simultaneous formation of BiOI@α-Bi2O3. In situ synthesis of BiOI@α-Bi2O3 not only accumulates the features of both but also makes the process cost-effective. Yan et al. constructed an Ag–AgI/BiOI–Bi2O3 dual Z-scheme heterojunction containing an assembly of BiOI nanosheets, Bi2O3 flakes, and AgI nanoparticles by synthesising BiOI@Bi2O3in situ. They reported that the heterojunction exhibited enhanced photoactivity due to the optimised redox ability, faster charge separation rate, and larger specific surface area. About 95% of the methyl orange was degraded by the heterojunction photocatalyst when exposed to visible light.14 Designing 3D porous BiOI spheres accelerates light harvesting ability through multiple reflections, thereby enriching photoinduced excitons. On the other hand, the fabrication of α-Bi2O3 nanorods can inhibit the recombination rate by demonstrating unidirectional migration of excitons and a short surface charge transfer distance.15 Although Zhang et al. reported the spherical morphology of BiOI in the in situ prepared BiOI@Bi2O3 based double Z-scheme heterojunction through the solvothermal technique, Bi2O3 nanorods were not formed.16 Solvothermal temperature, nature of solvents, solvent to solid ratio, etc., are considered vital to obtain heterojunctions of desired topology for ameliorated photocatalytic performance.
Congo red (CR), an anionic diazo dye, finds extensive applications in the textile, printing, and paper industries. It is highly soluble in water and hard to degrade, making it a persistent pollutant in aquatic ecosystems.17 When discharged into water bodies without adequate treatment, it poses multiple environmental and health risks. Due to its bright colour, CR in water bodies severely affects the aesthetic quality of the environment and blocks sunlight penetration, disrupting the photosynthesis process in aquatic plants. This, in turn, reduces the oxygen level in the water, leading to the death of fish and other aquatic life. Direct exposure to CR or its derivatives can cause skin irritation and respiratory issues, and potentially long-term exposure could lead to cancer.18 The persistence and detrimental effects of CR pose an urgent need for its sustainable degradation into harmless products. Although several studies have reported its photocatalytic destruction, investigations of high CR concentrations with minimal catalytic doses have rarely been reported.
Hydrogen peroxide (H2O2) is an important chemical widely used in energy conversion, chemical synthesis, and environmental remediation.19 The application of conventional synthesis approaches like the electrochemical technique, the anthraquinone method, and the oxidation of alcohol for its manufacture is restricted due to high cost, stringent environmental regulations, and significant energy consumption. In contrast, photocatalytic H2O2 production through two-electron dioxygen reduction has emerged as an environmentally benevolent and energy-inexpensive approach.20,21 In the context of the lethality of CR in the environment and the importance of H2O2 production in mitigating the energy crisis as well as curbing environmental pollution, it is indispensable to develop a cost-effective and viable photocatalytic system.
Herein, we have explored the fabrication of UiO-66-NH2/BiOI@α-Bi2O3 ternary heterojunction photocatalysts through a two-step solvothermal approach. At first, BM microspheres were synthesised by treating a mixture of Bi(NO3)3 and KI in ethylene glycol at 150 °C for 12 hours. In the second step, BM microspheres of a known amount were treated with a mixture of ZrCl4, 2-amino terephthalic acid (2-ATP), and DMF at 120 °C for 24 hours in a Teflon-lined autoclave to obtain the desired products. FESEM and TEM images demonstrated that the integration of UN nanoparticles, BM microspheres, and in situ produced BR nanorods resulted in the successful formation of a ternary BBUN heterojunction. The elevated temperature and pressure prevailed in the reactor, followed by the interaction of DMF and 2-ATP with the BM, resulting in its partial decomposition into α-Bi2O3 nanorods. The morphologies of BM and BR in the heterostructure were controlled by the weight-to-volume ratio between BM and DMF. The interaction of DMF with BM and BR also promoted the incorporation of Ov, which extended the light absorption towards the red end of the visible region, as confirmed by UV-Vis-DRS spectra. The anchoring of UiO-66-NH2 nanoparticles through the coordination of NH2 groups with Bi3+ of the BM microspheres and BR nanorods resulted in the emergence of Bi–N interfacial bonds, thereby establishing the Ov–Bi–N interfacial charge transport channels. Based on the results obtained from XPS studies, band potential measurements, and radical scavenging tests, a double Z-scheme charge transfer mechanism has been suggested. The heterostructure prepared with 600 mg BM (BBUN-4) demonstrated superior photocatalytic activity for the remediation of CR from aqueous solution and H2O2 production. This enhancement is ascribed to the construction of a morphology-controlled ternary heterojunction, Ov in BM and BR, as well as Ov–Bi–N interfacial charge transport channels. The current work explores the possibility of designing morphology-regulated oxygen vacancy-rich binary Z-scheme heterojunctions to achieve superior photocatalytic degradation of organic dyes and H2O2 production.
2. Experimental section
Detailed information on the chemicals, synthetic procedures of BR, material characterization techniques, optical and electrochemical measurements, photocatalytic CR degradation efficiency evaluation, and photocatalytic H2O2 production is provided in the SI (S1).2.1 BiOI microsphere synthesis
A solvothermal synthesis approach was followed to fabricate BM, as reported earlier.22 In a typical synthesis, 1.376 mg of Bi(NO3)3·5H2O was solubilised in 40 ml of ethylene glycol (ELG) under continuous stirring for 30 minutes and was labelled as solution A. Solution B was made by dissolving 0.47 mg of KI in 40 ml of ELG under identical conditions. Then B was mixed with A, and the mixture was subjected to stirring for 1 h. After that, the mixture was treated at 150 °C for 12 h in a Teflon-lined autoclave. The orange-coloured mass so formed was washed with double-distilled water repeatedly, followed by washing with ethanol. The resulting mass was heated at 60 °C in a vacuum oven for 12 h to get BM.2.2 Fabrication of a UiO-66-NH2/BiOI@α-Bi2O3 ternary heterojunction
A simple solvothermal technique was employed to fabricate UiO-66-NH2/BiOI@α-Bi2O3 heterojunctions. 1 mmol ZrCl4 and 1 mmol 2-ATP were dissolved in 40 ml of DMF separately. The two solutions were mixed and subjected to stirring for 30 minutes. Then, a predetermined amount of BM was added to the mixture solution, followed by stirring for another 30 minutes. The mixture solution was treated at 120 °C for 24 h in a Teflon-lined autoclave. The autoclave was then cooled to room temperature, and the product was filtered. The obtained solid was repeatedly rinsed with distilled water and ethanol, followed by drying overnight at 80 °C to obtain the desired product. The fabricated photocatalysts were named as BBUN-1, BBUN-2, BBUN-3, BBUN-4, and BBUN-5, depending on the weight of BM as 50 mg, 200 mg, 400 mg, 600 mg, and 800 mg, respectively. The synthesis procedure of the BBUN heterostructure was presented in Scheme 1. A similar procedure was followed to synthesize pristine UN, without the addition of BM.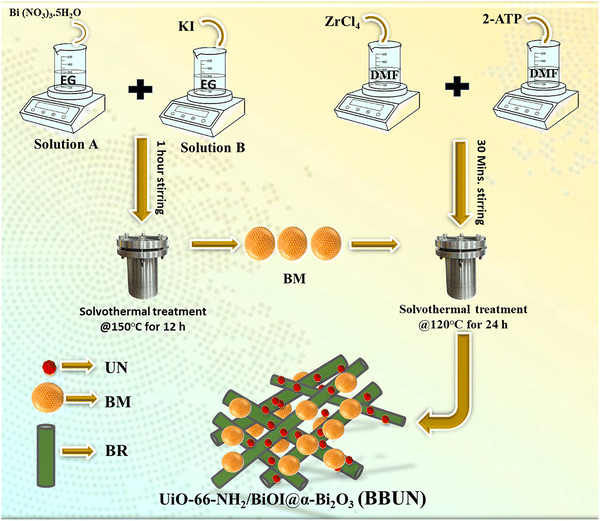 | ||
| Scheme 1 Schematic illustration for the fabrication of UiO-66-NH2/BiOI@α-Bi2O3 (BBUN) ternary heterojunctions. | ||
3. Results and discussion
3.1 Morphology and microstructure
The surface morphology of the synthesised samples was analysed using FESEM. The FESEM images of UN, BM, and BBUN-4 are displayed in Fig. 1. As observed in Fig. 1(a), the fine nanoparticles of UN were joined together to form a sphere-like morphology with a rough surface containing a large number of voids. Such architectures suggested that the UN may possess a high specific surface area with numerous surface-active sites, which are advantageous for the efficient adsorption of reacting species on its surface.23 In Fig. 1(b), BM showed distinct spherical microflowers with particle sizes ranging from 2.5 to 3.8 µm. As shown in the figure, the BM microspheres possessed a porous morphology. In order to validate the formation of the porous structure of the BM microspheres, its enlarged FESEM image is presented in Fig. 1(c). It was revealed from the figure that the two-dimensional (2D) BM sheets were systematically aligned to result in microspheres with interconnected pores. The 3D morphology with an interconnected porous structure is beneficial for increasing the surface area and promoting the charge carriers’ concentration by enhancing visible light absorption efficiency through multiple reflections across the porous channel. Moreover, the interconnected pores can hinder the recombination rate by reducing the charge and mass transfer distance.24 The FESEM image of the BBUN-4 ternary heterojunction is displayed in Fig. 1(d). As observed from the figure, the UN nanoparticles were anchored on the microspheres and nanorods. The appearance of the microsphere confirmed the retention of the 3D morphology with an interconnected porous structure of BM in the composite. However, elevated temperature and high pressure prevailed during the solvothermal treatment, which accelerated the frequency of collisions among the BM microspheres, causing their partial conversion into nanosheets. The electron-donating groups present in the DMF and NH2 groups of 2-ATP present in the reaction medium interact with Bi3+ of the BM nanosheets, while I atoms of BM extend interaction with Zr4+ of the UNH precursor. This leads to the gradual weakening of the Bi–I bonds and their subsequent rupture. As a result, there occurs a rearrangement of Bi–O bonds to form α-Bi2O3. The bulky methyl groups of DMF impart steric hindrance, which restricts isotropic growth and directs α-Bi2O3 to grow in one dimension to obtain nanorods (BR). Further raising the BM/DMF ratio to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in BBUN-5, the smaller number of methyl groups of DMF could not control Bi2O3 growth properly. As a result, Bi2O3 nanoflakes were obtained, as observed in Fig. S1. It was also observed from the figure that the 3D morphology was completely destroyed with the formation of BM nanosheets owing to the vigorous collision among a large amount of BM microspheres.
1 in BBUN-5, the smaller number of methyl groups of DMF could not control Bi2O3 growth properly. As a result, Bi2O3 nanoflakes were obtained, as observed in Fig. S1. It was also observed from the figure that the 3D morphology was completely destroyed with the formation of BM nanosheets owing to the vigorous collision among a large amount of BM microspheres.
The interaction of electron-donating groups of DMF with Bi3+ of BM microspheres and BR nanorods also affects the Bi–O bonds, resulting in the introduction of oxygen vacancies (Ov).25 Incorporated Ovs promotes the coordination of NH2 groups of UN with Bi3+. This facilitates anchoring of UN nanoparticles on the BM microspheres and BR nanorods to form a ternary heterojunction. As shown in the figure, the integration of UN nanoparticles with BR nanorods and BM microspheres resulted in the formation of a BBUN-4 ternary heterojunction along with the existence of numerous voids. All these features amplified the photocatalytic performance of the heterostructure. Fig. 1(e) displays the results of the energy dispersive X-ray spectroscopy (EDS) analysis, and corresponding elemental mapping images presented in Fig. 1(f)–(l) confirm the homogenous distribution of C, Bi, N, O, I, and Zr elements on the surface of the synthesised heterojunction. The existence of carbon (C) might be attributed to the presence of 2-ATP in BBUN-4 and the carbon tapes used in the FESEM analysis.
A transmission electron microscope (TEM) image of BBUN-4 is shown in Fig. 2(a). It was revealed from the figure that BR possessed a rod-shaped structure with an average length and width of 1.2 µm and 0.27 µm, respectively. Octahedral UN nanoparticles, BM microspheres, and BR nanorods are interconnected thoroughly to obtain BBUN-4 ternary heterojunctions. Three distinct phases of BBUN-4 have appeared in the HRTEM image [Fig. 2(b)]. The lattice fringe of 0.27 nm corresponds to the (102) plane of BM, while the (−012) plane of BR is characterised by the lattice spacing of 0.32 nm.26,27 The region with no lattice fringe indicates the UN phase. These results demonstrated that the successful integration of UN with BM and BR during the solvothermal treatment constructed a robust BBUN-4 ternary heterojunction, which is consistent with the observations made in FESEM analysis. Furthermore, Fig. 2(c) illustrates that the defects that appeared in the lattice fringes of BM and BR of the heterostructure may be attributed to the oxygen-rich vacancies incorporated at the interfaces. The selected area electron diffraction (SAED) pattern shown in Fig. 2(d) exhibited polycrystalline rings, reflecting a well-defined crystalline structure and confirming the polycrystalline characteristics of the synthesised BBUN-4 heterojunction.28
 | ||
| Fig. 2 (a) Transmission electron microscopy (TEM) image, (b) and (c) high-resolution TEM (HRTEM) images, and (d) selected area electron diffraction (SAED) pattern of the BBUN-4 heterostructure. | ||
The N2 adsorption–desorption technique was employed to evaluate the specific surface area and pore volume of BM, UN, and BBUN-4. As shown in Fig. 3(a)–(c), the adsorption isotherms of BM, UN, and BBUN-4 revealed type IV isotherms, with a characteristic H3 hysteresis loop indicating the presence of a mesoporous structure.29 The BET surface area and pore volumes of BM, UN, and BBUN-4 are presented in Fig. 3(d) and (e), respectively. Pristine UN exhibited a relatively larger surface area of 502.3 m2 g−1, which can be attributed to its rough and porous morphology, as evident from the FESEM image. Similarly, the 3D morphology with an interconnected porous structure revealed from FESEM studies contributed to the high surface area of 86.3 m2 g−1 for bare BM. The integration of UN, BM, and in situ produced BR enhanced the surface area of BBUN-4 to 525.97 m2 g−1. It was observed from Fig. 3(e) that the pore volume of BBUN-4 was higher than that of its pristine counterparts. The enhanced surface area and pore volume of the ternary composite are responsible for possessing numerous active surface sites, which are advantageous for increased CR adsorption and O2 activation.
 | ||
| Fig. 3 N2 sorption isotherms of (a) BM, (b) UN, and (c) BBUN-4, (d) specific surface area, and (e) pore volume of UN, BM, and BBUN-4. | ||
3.2 Crystal structure and chemical states
X-ray diffraction (XRD) analysis was employed to investigate the structural and crystalline properties of the synthesised photocatalysts. Fig. 4(a) displays the XRD patterns of the UN, BR, and BBUN heterostructures. The diffraction peaks at 2θ = 7.3°, 8.4°, and 25.6°, corresponding to the (111), (200), and (442) planes, confirmed the successful synthesis of pristine UN.30 The characteristic peaks observed at 2θ = 25.7°, 27.3°, 28.0°, 33.0°, 33.2°, 37.6°, and 46.3° can be indexed to the (002), (−120), (012), (121), (200), (112), and (041) planes, respectively, of monoclinic BR (JCPDS no. 14-0699).31 In the BBUN heterojunctions, the presence of UN is evident from the diffraction peak at 2θ = 7.3°, with decreased intensity. This peak intensity decrease may be ascribed to the reduction in crystallinity due to the formation of the composite.32 Furthermore, the appearance of peaks at 2θ = 12.2° (001), 33.0° (102), and 57.6° (212) in the composites corresponds to the tetragonal phase of BM, as per JCPDS no. 85-0863. Notably, the presence of peaks at 2θ = 25.7°, 27.3°, 28.0°, 33.2°, and 46.3° evidenced the presence of BR in the heterojunctions. The peaks corresponding to the (002) and (−120) planes of BR displayed a lower angle shift in the synthesized heterostructures, suggesting lattice distortion due to strong interactions among UN, BR, and BM.33 The sharp and well-defined peaks in the XRD patterns associated with UN, BM, and BR indicate the successful synthesis of BBUN ternary heterojunctions with a high degree of crystallinity.Fourier transform infrared (FTIR) spectroscopy was employed to characterise the surface functional groups in the synthesised samples. Fig. 4(b) illustrates the distinctive absorption peaks for UN, BM, and BBUN-4. The FTIR spectrum of pristine UN displayed a characteristic peak at 3364 cm−1, corresponding to the stretching vibration of N–H bonds present in free NH2 groups. Bands observed at 1651 cm−1 and 1565 cm−1 were attributed to N–H bending in aromatic amines and asymmetric stretching of carboxylic groups, respectively. Additional peaks at 1427 cm−1 and 1385 cm−1 were assigned to C–C stretching and O–C–O symmetric vibrations, correspondingly. A weak absorption band at 1105 cm−1 was indicative of Zr–O stretching vibrations from Zr-oxo clusters, while the broad peaks in the range of 400–800 cm−1 are associated with Zr(–OC) stretching vibrations of UN MOF.34 In the spectrum of BM, the peak that appeared at 1391 cm−1 was assigned to the stretching vibrations of the Bi–I bond, and a distinctive absorption peak of Bi–O stretching vibrations was observed at 503 cm−1. The peaks around 3400 cm−1 and 1603 cm−1 correspond to O–H vibrational frequencies of adsorbed water molecules.35 The presence of characteristic peaks of UN and BM in the BBUN-4 spectrum confirms their presence in the composite. Additionally, the peak that appeared at 893 cm−1 corresponds to the stretching mode of the Bi–O bond present in Bi2O3. Therefore, it was revealed from this evidence that BR was formed in situ during the fabrication of BBUN-4.36 These outcomes corroborated the successful synthesis of the ternary heterojunction, which was also evident from the XRD results. Furthermore, a distinct vibrational band observed at 588 cm−1 can be assigned to the formation of the Bi–N bond.37 The formation of this bond resulted from the interaction between the Bi3+ ions of BM and the –NH2 groups of UN. Bi–N bond formation is indicative of the establishment of an efficient interfacial charge-transfer channel between BM and UN.
X-ray photoelectron spectroscopy (XPS) analysis was performed to investigate the elements' chemical composition and valence states in the prepared samples and the charge transfer dynamics in the ternary heterojunction. The survey spectra of the pristine UN, BM, BR, and BBUN-4 heterostructure are shown in Fig. 5(a). It was evident from the full XPS spectra that the ternary composite contains Zr, N, Bi, O, and I elements, as exhibited by its pristine counterparts. The coexistence of these elements in BBUN-4 was corroborated by elemental mapping and EDS results, which validated the formation of a ternary heterojunction. Fig. 5(b) displays the high-resolution Zr 3d XPS spectra of pristine UN and BBUN-4. The peaks at binding energies 181.7 and 184.0 eV were ascribed to Zr 3d5/2 and Zr 3d3/2 spin orbitals of Zr4+ of UN, respectively.38 In the BBUN-4 spectrum, similar peaks have appeared with a negative shift of binding energies at 181.0 and 183.8 eV. The N 1s spectrum of UN, shown in Fig. 5(c), displayed a single peak at 398.2 eV, which indicates the presence of the –NH2 group.39 However, the N 1s peak was deconvoluted into three peaks at 398.1 eV, 400.3, and 403.1 eV in the BBUN-4 spectrum. The peak at 398.1 eV experienced a shift of 0.1 eV to lower binding energy, corresponding to the presence of the –NH2 group, while the peaks at 400.3 and 403.1 eV might be attributed to the interaction between Bi and N.40 This interaction resulted in the formation of a Bi–N bond between NH2 of UN and Bi of BM, as well as that of BR. The existence of this interfacial bond was evident from the FTIR results. The Bi–N bond formation facilitated the establishment of charge transfer channels among BM, BR, and UN in the ternary heterojunction. The Bi 4f spectra of BM, BR, and BBUN-4 are presented in Fig. 5(d). The pristine BM exhibited two distinct peaks at 158.1 eV and 162.4 eV, attributed to Bi 4f7/2 and Bi 4f5/2 states, respectively, of Bi3+ ions.41 A similar doublet was observed at 157.1 eV and 163.4 eV for BR.43 In the BBUN-4 heterojunction, the peaks ascribed to each of the Bi 4f microstates were deconvoluted into two different peaks. The peak at 158.1 eV representing the Bi 4f7/2 state of BM was relocated at 158.7 eV, whereas that for the Bi 4f5/2 state experienced a shift of 1.6 eV from 162.4 eV. Similarly, the peak corresponding to the Bi 4f7/2 of BR was moved to a higher binding energy of 158.2 eV after a jump of 1.1 eV, and that of Bi 4f5/2 was shifted positively by 0.4 eV to 163.8 eV. Fig. 5(e) represents the O 1s spectrum for BM, BR, and BBUN-4. For BM, the O 1s spectrum was deconvoluted into two peaks at 528.9 eV and 530.3 eV due to the presence of a Bi–O bond and surface hydroxyl groups, correspondingly.42 These two peaks were observed at binding energies of 528.1 eV and 529.1 eV, respectively, in bare BR.43 However, the deconvolution of the O 1s spectrum of BBUN-4 resulted in three peaks. The appearance of a peak at 529.2 eV was ascribed to the Bi–O bond, and that at 531.3 eV corresponded to the presence of surface hydroxyl groups. It is worth noting here that these two peaks experienced a positive shift with respect to pristine BM and BR. The third peak at 530.4 eV is attributed to the presence of oxygen-rich vacancies (Ov) formed during the solvothermal synthesis of BBUN-4. This finding, as well as the formation of interfacial defects revealed from HRTEM analysis, validated the formation of Ov at the interfaces of BBUN-4. Incorporating oxygen vacancies can be beneficial for extending the light absorption range and improving the reactant adsorption ability of BBUN-4.44 Moreover, these vacancies can trap electrons, thereby preventing the recombination of photogenerated electron–hole pairs.45 All these features lead the ternary heterojunction to exhibit higher photocatalytic activity. The I 3d spectrum of BM and BBUN-4 was displayed as a characteristic of I− ions in Fig. 5(f). Two distinct peaks appeared at 617.7 eV and 629.2 eV corresponding to I 3d5/2 and I 3d3/2 states, respectively, for BM.42 In BBUN-4, the peak representing I 3d5/2 was shifted to a higher binding energy by 0.3 eV, while that appearing at 629.2 eV experienced a positive shift of 0.4 eV. As observed, the negative shift of peaks in the Zr 3d and N 1s spectra of BBUN-4 reflected an increased charge density on UN, which acts as an electron acceptor. On the other hand, the positive shift of peaks in the Bi 4f, O 1s, and I 3d spectra indicated a reduced charge density in BM and BR, making them electron donors. Therefore, XPS studies suggested that photoelectrons can be channelized from BM and BR to UN through the heterojunction formed along the interfacial region of the three semiconductors.
 | ||
| Fig. 5 (a) XPS survey spectra of the BM, BR, UN, and BBUN-4 composites, along with high-resolution XPS spectra of (b) Zr 3d, (c) N 1s, (d) Bi 4f, (e) O 1s, and (f) I 3d. | ||
3.3 Optical properties
UV-Vis diffuse reflectance spectroscopy (UV-Vis DRS) was employed to evaluate the optical properties of the synthesised materials over the wavelength range of 200–800 nm. The absorption edges and corresponding band gap energies of the studied semiconductor photocatalysts were determined using this technique. As depicted in Fig. 6(a), the absorption edges of UN and BM were observed at 494 nm and 635 nm, respectively. Interestingly, the DRS spectrum of BBUN-4 displayed a hump in the region of 450 to 530 nm as well as three absorption edges at 473, 538, and 665 nm. The absorption hump in the composite indicated the existence of impurity levels that may be formed by the introduction of Ov in BR and BM.46 It was reported that the absorption maximum for α-Bi2O3 lies around 450 nm, and the corresponding band gap energy is 2.76 eV.47 The absorption edge observed at 473 nm suggested the presence of α-Bi2O3 in BBUN-4 with a bathochromic shift of 23 nm. The spectral band at 665 nm due to BM experienced a 30 nm shift towards the red end of the visible region. This red shift in absorption tail indicated narrowing of the band gaps. As revealed from Fig. 6(b), the band gap energy corresponding to BM in BBUN-4 was decreased from that of its pristine counterpart. The band gap energy corresponding to BR in the composite was found to be lower than that determined in the literature. The lowering of the band gap energy might be attributed to the overlapping of electronic states resulting from the Ovs introduced in BR and BM in the ternary composite with the band edges of the semiconductor.48 The absorption edge corresponding to UN in the composite also experienced a red shift of 44 nm, and the corresponding band gap energy was lower than that of the pristine counterpart. This shift in absorption maximum to longer wavelength and decrease in band gap energy of UN in BBUN-4 might be ascribed to robust interaction of UN with BM and BR, as revealed from FTIR and XPS results. The wide absorption window in the visible region observed for BBUN-4 magnified its ability to harness a large amount of photons from the solar spectrum. Furthermore, the introduction of Ov in BM and BR at the interfaces, overlapping of Ov states with the semiconductor's band edge, and formation of an interfacial Bi–N bond corroborated that there exists an Ov–Bi–N charge transfer channel among BM, BR, and UN at the interface of BBUN-4. The formation of an Ov–Bi–N interfacial charge transfer channel facilitated the separation and migration of photoinduced electron–hole pairs, which in turn suppresses the recombination rate of charge carriers to a considerable extent. | ||
| Fig. 6 (a) UV-Vis DRS spectra, (b) Tauc plots of BM, BR, UN, and BBUN-4, and PL spectra of (c) UN, BM an BBUN-4, and (d) BR. | ||
Photoluminescence (PL) emission in photocatalysts arises due to the recombination of photoinduced excitons. A decrease in PL intensity signifies a reduction in charge carriers’ recombination rates.49 The PL spectra of pure UN, BM, and the BBUN-4 composite under 360 nm excitation are shown in Fig. 6(c), while the PL spectrum of BR obtained under 385 nm excitation is presented in Fig. 6(d). The pristine photocatalysts, UN, BR, and BM, exhibited intense PL signals, indicating substantial charge carrier recombination. In contrast, the BBUN-4 composite demonstrated the lowest PL intensity, suggesting a marked reduction in electron–hole recombination rate. The reduced recombination rate may be ascribed to Ov-introduced impurity levels, which act as an electron shuttle to magnify the charge carriers’ transfer through the Ov–Bi–N interfacial channel.
3.4 Electrochemical analysis
Electrochemical impedance spectroscopy (EIS) was utilized to investigate the charge transfer resistance of the pristine samples and the BBUN-4 heterojunction. The Nyquist plots obtained from the EIS analysis are illustrated in Fig. 7(a). It was revealed from the figure that BBUN-4 exhibited the smallest impedance in comparison to the pristine counterparts, suggesting its lowest resistance to transferring the charge carriers at the interface.50 This indicated that the ternary heterojunction has the fastest charge transfer rate or the highest ability for migration of excitons, and hence, has experienced minimal charge recombination rate as revealed from PL studies. The minimum charge transfer resistance offered by BBUN-4 may be ascribed to the existence of the Ov–Bi–N interfacial charge transport channel. | ||
| Fig. 7 (a) Electrochemical impedance spectroscopy (EIS), (b) linear sweep voltammetry (LSV) plots of UN, BM, BR, and BBUN-4, and Mott–Schottky plots of (c) BR, (d) UN, and (e) BM. | ||
To provide evidence in favour of greater migration of charge carriers in the BBUN-4 heterojunction, linear sweep voltammetry (LSV) was conducted. As illustrated in Fig. 7(b), the LSV analysis demonstrated that UN and BR exhibited minimal anodic current densities of 0.087 mA cm−2 and 0.1 mA cm−2, respectively, while BM displayed a low cathodic current density of −0.27 mA cm−2. The low values of current density observed from LSV plots reveal limited charge mobility and minimal charge carrier separation in the pristine samples.51 Notably, the BBUN-4 heterostructure demonstrated higher cathodic and anodic current responses. At an applied potential of −1.0 V vs. Ag/AgCl, the cathodic current density reached −0.58 mA cm−2, while the anodic current density of 0.17 mA cm−2 was observed at 1.0 V vs. Ag/AgCl. The measured photocurrent densities of BBUN-4 were found to be 2.14, 1.95, and 1.7 times more than those obtained for BM, UN, and BR, respectively. The higher current densities shown by BBUN-4 further validated its superior charge transfer ability and enhanced migration ability owing to the existence of Ov–Bi–N interfacial charge transport channels. These results indicate enhanced photogenerated charge carrier migration within the heterojunction. The integration of UN nanoparticles, BM microspheres, and BR nanorods facilitated efficient charge separation and electron transport, resulting in improved photocurrent generation. Furthermore, these findings align with the photoluminescence (PL) studies, which revealed reduced charge recombination, emphasizing the synergistic effect of the morphology-regulated ternary heterostructure and Ov–Bi–N interfacial charge transfer channel in enhancing photocatalytic performance.
Mott–Schottky (MS) analysis was conducted to evaluate BR, UN, and BM's semiconducting characteristics and flat band potentials. As displayed in Fig. 7(c) and (d), the positive slopes in the MS plots of BR and UN revealed their n-type characteristics, whereas the p-type nature of BM was confirmed from the negative slope in its MS plot shown in Fig. 7(e). The flat band potentials, derived from the intercepts of the linear regions in the MS plots, were determined to be 0.33 V, −0.46 V, and 2.29 V (vs. Ag/AgCl) for BR, UN, and BM, respectively. These values were further converted to the reversible hydrogen electrode (RHE) scale using eqn (1).27
| ERHE = EAg/AgCl − 0.0591 (7 − pH of the electrolyte) + 0.198 | (1) |
At pH 6.8, the flat band potentials on the RHE scale were calculated to be 2.48 V for BM, −0.28 V for UN, and 0.52 V for BR. For p-type semiconductors, the flat band potential is typically located 0.2 eV above the valence band maximum (VBM). Therefore, BM's valence band (VB) position was calculated to be 2.68 V vs. RHE. Its conduction band was evaluated to be 0.74 V (vs. RHE) by using eqn (2). The flat band potential for n-type semiconductors is approximately 0.2 eV below the conduction band minimum (CBM).52 Accordingly, the CBMs for UN and BR were determined to be −0.48 V (vs. RHE) and 0.32 V (vs. RHE), respectively. Following eqn (2), the VBMs of UN and BR were estimated to be 1.99 V (vs. RHE) and 3.08 V (vs. RHE), respectively.
| ECB = EVB − Eg | (2) |
3.5 Evaluation of photocatalytic efficiency
The photocatalytic efficiency of the prepared catalysts was evaluated by the degradation of CR dye and H2O2 production.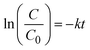 | (3) |
3.6 Photocatalytic reaction mechanism
Appropriate hybridisation of BM microspheres, in situ synthesised BR nanorods, and UN nanoparticles made BBUN-4 possess an extremely large surface area, porous architectures, enormous Ov at the interface, and an Ov–Bi–N interfacial charge transfer channel. As a result, it exhibited a wide range of visible light absorption windows, enriched photo-induced charge carriers, and a fast charge transfer rate, which cumulatively led to amplified photoactivity towards CR degradation and H2O2 production. To further understand the boosted pursuit of the ternary heterojunction, plausible charge transfer dynamics were elucidated. Usually, h+, ˙OH, and ˙O2− are considered the crucial reactive species for the photodegradation of organic compounds. In order to explore the primary reactive species involved in the degradation of CR, radical trapping experiments were carried out. Triethanolamine (TEA), isopropyl alcohol (IPA), and 1,4-benzoquinone (BQ) were used as the quenching agents for h+, ˙OH, and ˙O2−, respectively.60 The CR degradation efficiency of BBUN-4 in the presence of different scavenging agents is presented in Fig. 8(d). It was observed that 97.25% of CR was degraded in the absence of any trapping agent. However, the degradation efficiency sharply declined to 18.7% and 14.3% in the presence of BQ and IPA, respectively. Upon the addition of TEA, the degradation percentage was found to be 76.2%. These results suggested that both ˙O2− and ˙OH are the dominant reactive species driving the photocatalytic CR degradation, while h+ plays a minor role.The formation of ˙O2− and ˙OH can be ascertained if the thermodynamic criteria need to be satisfied by the heterostructured photocatalysts. The thermodynamic requirement for the release of ˙O2− is that the conduction band edge level (ECB) of the semiconductor photocatalyst must be more anodic than the standard reduction potential of the oxygen reduction reaction (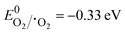 vs. RHE, pH = 7.0). Similarly, the valence band edge position (EVB) should be placed below the standard redox potential of the water oxidation reaction (
vs. RHE, pH = 7.0). Similarly, the valence band edge position (EVB) should be placed below the standard redox potential of the water oxidation reaction (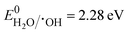 vs. RHE, pH = 7.0) in order to meet the thermodynamic feasibility for the production of ˙OH radicals. Therefore, the CB edge and VB levels of UN, BM, and BR were determined by the Mulliken electronegativity formula presented in eqn (4).
vs. RHE, pH = 7.0) in order to meet the thermodynamic feasibility for the production of ˙OH radicals. Therefore, the CB edge and VB levels of UN, BM, and BR were determined by the Mulliken electronegativity formula presented in eqn (4).
| EVB = X − Ee + 0.5Eg | (4) |
To propose a plausible charge transfer mechanism in the ternary heterojunction for required reactive species production, the type II pathway was first considered. According to it, photogenerated electrons can migrate from the CB of UN with a more negative energy level to that of BM and BR with a less negative potential. Simultaneously, holes are transferred from a more positive VB level of BM and BR to that with a less positive position of UN, as shown in Scheme 2. The CB potentials of BM (0.74 eV) and BR (0.32 eV) are placed below the potential required for the oxygen reduction reaction (ORR). Therefore, the electrons accumulated on the CB of BM and BR possessed inadequate reduction ability to generate ˙O2−. On the other hand, the holes (h+) accumulated on the VB of UN cannot produce ˙OH free radicals as the VB potential is less cathodic than that required for the water oxidation reaction (WOR). However, these outcomes contradict the results obtained from quenching experiments. This suggests that the type II charge transfer mechanism cannot satisfy the thermodynamic requirements for the production of ˙O2− and ˙OH species. In order to validate the feasibility of ˙O2− and ˙OH formation during the photocatalytic reactions, a binary Z-scheme charge transfer dynamics was proposed. In this system, all three semiconductors, UN, BM, and BR, generate electron–hole pairs upon exposure to solar light. According to XPS analysis, UN functions as an electron acceptor while BR and BM serve as electron donors. The photoinduced electrons from the CB of BM and BR with weaker redox potentials are transported through Ov–Bi–N charge transfer channels to recombine with the holes present at the VB of UN, which has inadequate redox ability. As a result, the photogenerated electrons at the CB of UN and holes at the VB of BR and BM with strong redox abilities are preserved for the production of ˙O2− and ˙OH species. Therefore, considering the results of scavenging tests, XPS studies, and band structure analysis, it was corroborated that the photocatalytic CR degradation over BBUN-4 proceeds through the construction of a double Z-scheme heterojunction. Additionally, the appearance of ˙O2− as a primary reactive species suggested that the activation of O2 is facilitated by the introduction of Ov, followed by reduction using the photogenerated electrons with high redox potentials preserved at the CB of UN, resulting in the binary Z-scheme charge transfer dynamics. The generated ˙O2− undergoes a reaction with H+ ions to produce H2O2. Thus, the photocatalytic production of H2O2 by BBUN-4 is likely to proceed through a two-step-one-electron transfer mechanism.61 Initially, oxygen (O2) molecules adsorbed on the BBUN-4 surface are reduced by available photoproduced electrons to form superoxide radicals (˙O2−), which are further reduced to release H2O2 as per eqn (5).62
| ˙O2− + e− + 2H+ → H2O2 | (5) |
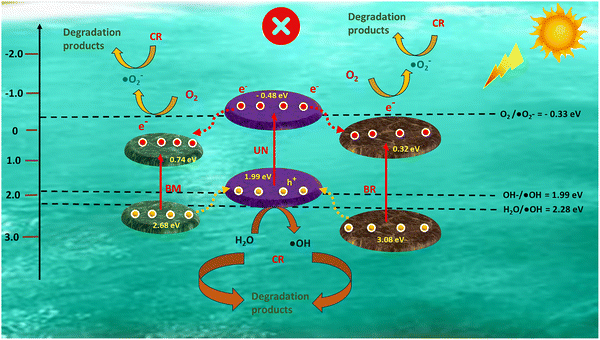 | ||
| Scheme 2 Photocatalytic mechanism of BBUN-4 showing a type II charge transfer pathway for possible CR degradation and H2O2 production. | ||
Following the preceding analysis, the possible mechanism for photocatalytic CR degradation and H2O2 production is presented in Scheme 3.
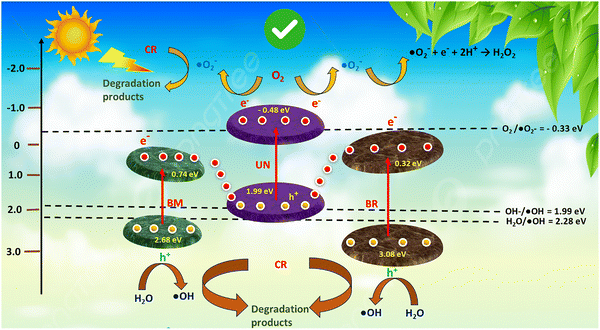 | ||
| Scheme 3 Proposed binary Z-scheme charge transfer process for photocatalytic CR degradation and H2O2 production over BBUN-4. | ||
4. Conclusion
A series of UiO-66-NH2/BiOI@α-Bi2O3 ternary heterojunction photocatalysts were fabricated through a solvothermal route. By controlling the BM and DMF ratio, a ternary BBUN heterojunction with the desired morphology was obtained. When the BM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMF ratio was maintained at 7.5
DMF ratio was maintained at 7.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, a BBUN-4 heterostructure was obtained with UN nanoparticles, BM microspheres, and in situ derived BR nanorods, which were revealed from FESEM and TEM studies. The formation of the ternary heterojunction containing these three components was also evident from UV-Vis DRS results. BBUN-4 possessed many advantageous features like enlarged surface area, mesoporous nature, and interconnecting porous architecture that were found beneficial for its superior photocatalytic performance over the pristine counterparts. The high surface area and mesoporous nature enable it for better dye and O2 adsorption, whereas the interconnecting porous architecture assists in harvesting abundant visible light, which facilitates the enrichment of charge carriers. As revealed from HRTEM and XPS results, abundant Ov were introduced at the interfaces, resulting in a shift of the visible light absorption window to the red end and magnified O2 activation. EIS analysis corroborated that BBUN-4 exhibited the smallest charge transfer resistance, indicating a faster charge transfer rate, which was supported by PL spectra showing reduced charge recombination. LSV confirmed the enhanced photocurrent responses for BBUN-4, further validating its superior charge separation and migration capabilities, attributed to the Ov–Bi–N interfacial charge transfer channel. The binary Z-scheme charge transfer dynamics preserved the photoexcitons with higher redox ability. Overall, BBUN-4 possessed high pollutant adsorption and O2 activation capability, extended visible light absorption characteristics, maximum charge carrier concentration, minimal charge recombination rate, and robust redox ability. As a result, it exhibited an outstanding CR photodegradation efficiency of 97.25% at pH 7, in a 50-ppm solution and a 0.5 g L−1 catalyst dose, outperforming both BM and UN. The photocatalytic H2O2 production was also significantly enhanced by BBUN-4, with a yield of 322 µmol L−1, far exceeding that of BM and UN. This study demonstrates the synergistic effect of the morphology-based ternary heterojunction, Ov–Bi–N interfacial charge transfer channel, and double Z-scheme charge transfer dynamics for efficient photodegradation of CR and H2O2 production.
1, a BBUN-4 heterostructure was obtained with UN nanoparticles, BM microspheres, and in situ derived BR nanorods, which were revealed from FESEM and TEM studies. The formation of the ternary heterojunction containing these three components was also evident from UV-Vis DRS results. BBUN-4 possessed many advantageous features like enlarged surface area, mesoporous nature, and interconnecting porous architecture that were found beneficial for its superior photocatalytic performance over the pristine counterparts. The high surface area and mesoporous nature enable it for better dye and O2 adsorption, whereas the interconnecting porous architecture assists in harvesting abundant visible light, which facilitates the enrichment of charge carriers. As revealed from HRTEM and XPS results, abundant Ov were introduced at the interfaces, resulting in a shift of the visible light absorption window to the red end and magnified O2 activation. EIS analysis corroborated that BBUN-4 exhibited the smallest charge transfer resistance, indicating a faster charge transfer rate, which was supported by PL spectra showing reduced charge recombination. LSV confirmed the enhanced photocurrent responses for BBUN-4, further validating its superior charge separation and migration capabilities, attributed to the Ov–Bi–N interfacial charge transfer channel. The binary Z-scheme charge transfer dynamics preserved the photoexcitons with higher redox ability. Overall, BBUN-4 possessed high pollutant adsorption and O2 activation capability, extended visible light absorption characteristics, maximum charge carrier concentration, minimal charge recombination rate, and robust redox ability. As a result, it exhibited an outstanding CR photodegradation efficiency of 97.25% at pH 7, in a 50-ppm solution and a 0.5 g L−1 catalyst dose, outperforming both BM and UN. The photocatalytic H2O2 production was also significantly enhanced by BBUN-4, with a yield of 322 µmol L−1, far exceeding that of BM and UN. This study demonstrates the synergistic effect of the morphology-based ternary heterojunction, Ov–Bi–N interfacial charge transfer channel, and double Z-scheme charge transfer dynamics for efficient photodegradation of CR and H2O2 production.
Conflicts of interest
There are no conflicts to declare.Data availability
The main article and the supplementary information (SI) include all the data generated or analyzed in this study. Supplementary information is available. See DOI: https://doi.org/10.1039/d5ma01197c.Acknowledgements
The authors sincerely acknowledge the support and encouragement provided by the management of Siksha ‘O’ Anusandhan (Deemed to be University), Bhubaneswar, Odisha, India, which contributed significantly to the successful completion of this work.References
- M. Y. Qi, M. Conte, M. Anpo, Z. R. Tang and Y. J. Xu, Cooperative Coupling of Oxidative Organic Synthesis and Hydrogen Production over Semiconductor-Based Photocatalysts, Chem. Rev., 2021, 121(21), 13051–13085 CrossRef CAS.
- D. Gunawan, J. Zhang, Q. Li, C. Y. Toe, J. Scott, M. Antonietti, J. Guo and R. Amal, Materials Advances in Photocatalytic Solar Hydrogen Production: Integrating Systems and Economics for a Sustainable Future, Adv. Mater., 2024, 36(42), 2404618 CrossRef CAS.
- S. Mansingh, S. Sultana, R. Acharya, M. K. Ghosh and K. M. Parida, Efficient Photon Conversion via Double Charge Dynamics CeO2-BiFeO3 p-n Heterojunction Photocatalyst Promising toward N2 Fixation and Phenol-Cr(VI) Detoxification, Inorg. Chem., 2020, 59(6), 3856–3873 CrossRef CAS.
- Q. Wang and D. Astruc, State of the Art and Prospects in Metal-Organic Framework (MOF)-Based and MOF-Derived Nanocatalysis, Chem. Rev., 2020, 120(2), 1438–1511 Search PubMed.
- A. Jamma, B. Jaksani, C. S. Vennapoosa, S. Gonuguntla, S. Sk, M. Ahmadipour, M. Abraham B, I. Mondal and U. Pal, Defect-rich UiO-66@g-C3N4/Ni frameworks as efficient water splitting photocatalysts, Mater. Adv., 2024, 5, 2785–2796 RSC.
- J. Liu, X. Sun, B. Jiang, M. Liu, Q. Li, X. Xiao, H. Wang, M. Zheng, S. Guo, J. Wu, Y. Zhang, K. Shi and W. Zhou, UiO-66-NH2 Octahedral Nanocrystals Decorated with ZnFe2O4 Nanoparticles for Photocatalytic Alcohol Oxidation, ACS Appl. Nano Mater., 2022, 5, 2231–2240 CrossRef CAS.
- L. Jiang, D. Chen, Z. Hao, D. Cao, R. Liu, J. Cheng, L. Chen, X. Liu, B. Jia and D. Liu, An innovative CuxAg50−x/UiO66-NH2 photocatalyst prepared using a dual ship bottling strategy for photocatalytic CO2 reduction: controlled product selectivity and pathways, Energy Environ. Sci., 2024, 17(21), 8228–8242 RSC.
- Z. Jia, R. Li, P. Ji, Z. Xu, K. P. Homewood, X. Xia, Y. Gao, J. P. Zou and X. Chen, Design and fabrication of a novel 2D/3D ZnIn2S4@Ni/UiO-66-NH2 heterojunction for highly efficient visible-light photocatalytic H2 evolution coupled with benzyl alcohol valorization, Appl. Catal., B, 2024, 357, 124279 CrossRef CAS.
- A. Mehtab and T. Ahmad, Unveiling the Bifunctional Photo/Electrocatalytic Activity of In Situ Grown CdSe QDs on g-C3N4 Nanosheet Z-Scheme Heterostructures for Efficient Hydrogen Generation, ACS Catal., 2024, 14(2), 691–702 CrossRef CAS.
- S. Zhang, J. Guo, S. Deng, R. Zhang, Q. Zhu, Y. Liu, X. Lin and Y. Li, Multifunctional BiOBr/BiOI 3D microspheres for improving visible-light-driven photocatalytic degradation and disinfection efficiency, J. Environ. Chem. Eng., 2024, 12(3), 113017 CrossRef CAS.
- R. Ji, Z. Zhang, L. Tian, L. Jin, Q. Xu and J. Lu, Z- scheme heterojunction of BiOI nanosheets grown in situ on NH2-UiO-66 crystals with rapid degradation of BPA in real water, Chem. Eng. J., 2023, 453, 139897 CrossRef CAS.
- X. Li, H. Sun, Y. Xie, Y. Liang, X. Gong, P. Qin, L. Jiang, J. Guo, C. Liu and Z. Wu, Principles, synthesis and applications of dual Z-scheme photocatalysts, Coord. Chem. Rev., 2022, 467, 214596 CrossRef CAS.
- M. Kempasiddaiah, R. Samanta, R. K. Trivedi, D. Shrivastava, B. Chakraborty and S. Barman, Single-Crystalline α-Bi2O3 Induced by Nitrogen Doping for Enhanced and Selective CO2 Electroreduction to Formate over a Wide Negative Potential Window, ACS Appl. Energy Mater., 2024, 7, 8465–8477 CrossRef CAS.
- Q. Yan, X. Xie, Y. Liu, S. Wang, M. Zhang, Y. Chen and Y. Si, Constructing a new Z-scheme multi-heterojunction photocataslyts Ag-AgI/BiOI-Bi2O3 with enhanced photocatalytic activity, J. Hazard. Mater., 2019, 371, 304–315 CrossRef CAS PubMed.
- Y. C. Liang, Y. H. Chou, B. Y. Chen and W. Y. Sun, Controllable Crystal Growth and Improved Photocatalytic Activity of Porous Bi2O3-Bi2S3 Composite Sheets, ACS Omega, 2023, 8(29), 26055–26064 CrossRef CAS.
- T. Zhang, Y. Wang, X. Xie, Y. Shao, Y. Zeng, S. Zhang, Q. Yan and Z. Li, Dual Z-scheme 2D/3D carbon-bridging modified g-C3N4/BiOI-Bi2O3 composite photocatalysts for effective boosting visible-light-driven photocatalytic performance, Sep. Purif. Technol., 2021, 277, 119443 CrossRef CAS.
- R. R. Wary, M. Narzary, S. K. Nikhil, R. G. Nair, P. Kalita and M. B. Baruah, Rational construction of carbon-rich g-C3N4 wrapped over CePO4 nanorods: Oxygen vacancy mediated 1D/2D Z-scheme photocatalyst towards removal of congo red dye, Colloids Surf., A, 2024, 684, 133142 CrossRef CAS.
- D. Lu, J. Fan, X. Ma, M. Geng, J. Li and T. Xu, Dual-Functional Passivation Agent of Natural Dye Congo Red For Enhanced Carbon-Based Perovskite Solar Cells, ACS Appl. Mater. Interfaces, 2024, 16, 69439–69449 CrossRef CAS.
- L. Acharya, G. Swain, B. P. Mishra, R. Acharya and K. Parida, Development of MgIn2S4 Microflower-Embedded Exfoliated B-Doped g-C3N4 Nanosheets: p-n Heterojunction Photocatalysts toward Photocatalytic Water Reduction and H2O2 Production under Visible-Light Irradiation, ACS Appl. Energy Mater., 2022, 5(3), 2838–2852 CrossRef.
- Y. Hu, Z. Yang, D. Zheng, W. Xing and G. Zhang, Rational synthesis of carbon-rich hollow carbon nitride spheres for photocatalytic H2O2 production and Cr(VI) reduction, Nanoscale, 2025, 17(13), 7856–7864 RSC.
- J. Song, J. M. Yu, J. H. Ahn, H. Cho, J. Oh, Y. S. Kim, J. Kim, M. Ko, S. H. Lee, T. J. Shin, H. Y. Jeong, C. Yang, J. H. Lee, J. W. Jang and S. Cho, Selective, Stable, Bias-Free, and Efficient Solar Hydrogen Peroxide Production on Inorganic Layered Materials, Adv. Funct. Mater., 2022, 32(25), 2110412 CrossRef.
- S. Mishra, L. Acharya, B. Marandi, K. Sanjay and R. Acharya, Boosted photocatalytic accomplishment of 3D/2D hierarchical structured Bi4O5I2/g-C3N4 p-n type direct Z-scheme heterojunction towards synchronous elimination of Cr(VI) and tetracycline, Diamond Relat. Mater., 2024, 142, 110834 CrossRef.
- N. Khosroshahi, M. D. Goudarzi, M. E. Gilvan and V. Safarifard, Collocation of MnFe2O4 and UiO-66-NH2: An efficient and reusable nanocatalyst for achieving high-performance in hexavalent chromium reduction, J. Mol. Struct., 2022, 1263, 132994 CrossRef.
- X. Wang, J. You, Y. Xue, J. Ren, K. Zhang, B. Fu, Q. Xue, J. Tian and H. Zhang, Structural regulation of three-dimensional bismuth vanadate nanochannels for excellent visible light photocatalytic nitrogen fixation, Appl. Catal., B, 2025, 363, 124817 CrossRef.
- L. Chen, C. Li, Y. Zhao, J. Wu, X. Li, Z. Qiao, P. He, X. Qi, Z. Liu and G. Wei, Constructing 3D Bi/Bi4O5I2 microspheres with rich oxygen vacancies by one-pot solvothermal method for enhancing photocatalytic activity on mercury removal, Chem. Eng. J., 2021, 425, 131599 CrossRef.
- D. He, C. Wang, R. Zhao, X. Lu, M. Yang, J. Qiu, K. Wang and C. Wang, BiOX (X = Cl, Br, I)/WO3/Polyacrylonitrile Nanofibrous Membranes for Diagnostic X-Ray Shielding and Visible-Light Photocatalysis, ACS Appl. Nano Mater., 2022, 5(3), 4157–4169 CrossRef.
- S. Padhiari and G. Hota, A Ag nanoparticle functionalized Sg-C3N4/Bi2O3 2D nanohybrid: a promising visible light harnessing photocatalyst towards degradation of rhodamine B and tetracycline, Nanoscale Adv., 2019, 1, 3212 RSC.
- S. K. Nayak, S. K. Pradhan, S. Panda, R. Bariki and B. G. Mishra, MOF derived hierarchical α-Bi2O3-BiVO4-CuFe2O4 multijunction heterostructure with conjugated S-scheme charge mobilization: Photocatalytic decontamination study, toxicity assessment and mechanistic elucidation, Appl. Catal., B, 2025, 360, 124534 CrossRef.
- H. Daneshgar, S. Sojdeh, G. Salehi, M. Edrisi, M. Bagherzadeh and N. Rabiee, Comparative study of synthesis methods and pH-dependent adsorption of methylene blue dye on UiO-66 and NH2-UiO-66, Chemosphere, 2024, 353, 141543 CrossRef PubMed.
- Z. Yang, J. Cao, Y. Chen, X. Li, W. Xiong, Y. Zhou, C. Zhou, R. Xu and Y. Zhang, Mn-doped zirconium metal-organic framework as an effective adsorbent for removal of tetracycline and Cr(VI) from aqueous solution, Microporous Mesoporous Mater., 2019, 277, 277–285 CrossRef.
- S. Bose and M. Kumar, Comparative evaluation of α-Bi2O3/CoFe2O4 and ZnO/CoFe2O4 heterojunction nanocomposites for microwave induced catalytic degradation of tetracycline, Chemosphere, 2024, 364, 143071 CrossRef PubMed.
- C. F. Holder and R. E. Schaak, Tutorial on Powder X-ray Diffraction for Characterizing Nanoscale Materials, ACS Nano, 2019, 13(7), 7359–7365 CrossRef.
- L. Yang, Z. Chen, G. Han, M. Hong, L. Huang and J. Zou, Te-Doped Cu2Se nanoplates with a high average thermoelectric figure of merit, J. Mater. Chem. A, 2016, 4, 9213 RSC.
- K. Chakarova, I. Strauss, M. Mihaylov, N. Drenchev and K. Hadjiivanov, Evolution of acid and basic sites in UiO-66 and UiO-66-NH2 metal-organic frameworks: FTIR study by probe molecules, Microporous Mesoporous Mater., 2019, 281, 110–122 CrossRef.
- I. Ahmad, M. S. Athar, M. Muneer, H. M. Altass, R. F. Felemban and S. A. Ahmed, Graphene oxide decorated BiOI/CdS nanocomposite: An efficient ternary heterostructure for photodegradation and adsorption study of organic pollutants, Surf. Interfaces, 2024, 45, 103819 CrossRef.
- B. Yan, G. Chen, B. Ma, Y. Guo, Y. Zha, J. Li, S. Wang, J. Liu, B. Zhao and H. Xie, Construction of surface plasmonic Bi nanoparticles and α-Bi2O3 co-modified TiO2 nanotube arrays for enhanced photocatalytic degradation of ciprofloxacin: Performance, DFT calculation and mechanism, Sep. Purif. Technol., 2024, 330, 125180 CrossRef.
- P. J. Mafa, M. E. Malefane, F. Opoku, A. O. Oladipo, G. Mamba, T. L. Yusuf, J. F. Nure, S. L. Lebelo, D. Liu, J. Gui, B. B. Mamba and A. T. Kuvarega, Dual Charge Transfer Mechanisms in Intimately Bonded S-scheme Heterojunction Photocatalyst with Expeditious Activity toward Environmental Remediation, Adv. Sustainable Syst., 2025, 9, 2401070 CrossRef.
- Z. Xia, L. Wang, W. Tan, L. Yuan, X. He, J. Wang, L. Chen, S. Zen, S. Lu and Z. Jiao, Visible-Light Photocatalytic Degradation Efficiency of Tetracycline and Rhodamine B Using a Double Z-Scheme Heterojunction Catalyst of UiO-66-NH2/BiOCl/Bi2S3, Inorg. Chem., 2024, 63(31), 14578–14590 CrossRef.
- R. Bariki, S. K. Pradhan, S. Panda, S. K. Nayak, A. R. Pati and B. G. Mishra, Hierarchical UiO-66(–NH2)/CuInS2 S-Scheme Photocatalyst with Controlled Topology for Enhanced Photocatalytic N2 Fixation and H2O2 Production, Langmuir, 2023, 39, 7707–7722 CrossRef.
- T. V. L. Thejaswini, D. Prabhakaran and M. A. Maheswari, Soft synthesis of Bi Doped and Bi-N co-doped TiO2 nanocomposites: A comprehensive mechanistic approach towards visible light induced ultra-fast photocatalytic degradation of fabric dye pollutant, J. Environ. Chem. Eng., 2016, 4(1), 1308–1321 CrossRef.
- Q. Meng, A. Kravchenko, B. Zhang, H. Yang, C. Liu, Y. Li, X. Sheng, L. Fan, F. Li and L. Sun, Remarkable synergy of borate and interfacial hole transporter on BiVO4 photoanodes for photoelectrochemical water oxidation, Mater. Adv., 2021, 2, 4323 RSC.
- Q. Wang, H. Wu, Q. Gao, D. Lin, Y. Fan, R. Duan, Y. Cong and Y. Zhang, Fabrication of visible-light-active Bi/BiOI-Bi2O3 composite with enhanced photocatalytic activity, J. Colloid Interface Sci., 2019, 548, 255–264 CrossRef.
- X. Cheng, X. Xiao, F. Wang, T. Lu and Y. Zhang, Heterojunctions Based on BiOBr Nanosheets Decorated on α-Bi2O3 for Photodegradation of Rhodamine B, ACS Appl. Nano Mater., 2024, 7, 4413–4422 CrossRef.
- S. Mishra, B. L. Tudu, N. Mishra, K. Sanjay and R. Acharya, Fabrication of oxygen vacancy modified 2D–2D g-C3N4/ZnFe2O4 heterostructures for amplifying photocatalytic methyl orange degradation and hydrogen production, Mater. Adv., 2025, 6, 9085 RSC.
- L. Hao, H. Huang, Y. Zhang and T. Ma, Oxygen Vacant Semiconductor Photocatalysts, Adv. Funct. Mater., 2021, 31(25), 2100919 CrossRef.
- D. Cui, L. Wang, K. Xu, L. Ren, L. Wang, Y. Yu, Y. Du and W. Hao, Band-gap engineering of BiOCl with oxygen vacancies for efficient photooxidation properties under visible-light irradiation, J. Mater. Chem. A, 2018, 6, 2193–2199 RSC.
- X. Cheng, X. Xiao, F. Wang, T. Lu and Y. H. Zhang, Heterojunctions Based on BiOBr Nanosheets Decorated on α-Bi2O3 for Photodegradation of Rhodamine B, ACS Appl. Nano Mater., 2024, 7, 4413–4422 CrossRef.
- H. Li, J. Shi, K. Zhao and L. Zhang, Sustainable molecular oxygen activation with oxygen vacancies on the {001} facets of BiOCl nanosheets under solar light, Nanoscale, 2014, 6, 14168 RSC.
- K. A. Tsai, J. C. Chang and Y. C. Pu, Charge Carrier Dynamics at Heterojunction of Semiconductor Nanoheterostructures for Photocatalytic Solar Fuel Generation, Adv. Energy Sustainability Res., 2025, 2400329 CrossRef.
- D. Ping, F. Yi, G. Zhang, S. Wu, S. Fang, K. Hu, B. Bin Xu, J. Ren and Z. Guo, NH4Cl-assisted preparation of single Ni sites anchored carbon nanosheet catalysts for highly efficient carbon dioxide electroreduction, J. Mater. Sci. Technol., 2023, 142, 1–9 CrossRef.
- X. Xiao, S. Li, L. Zuo, R. Li, Z. Li, L. Liu, H. Fan and B. Li, Twin S-Scheme Heterojunction ZnO/UiO-66-NH2@ZnIn2S4 Rhombic Octahedra for Efficient Photocatalytic H2 Evolution, Adv. Funct. Mater., 2025, 2418778 CrossRef.
- S. Mishra, L. Acharya, S. Sharmila, K. Sanjay and R. Acharya, Designing g-C3N4/NiFe2O4 S-scheme heterojunctions for efficient photocatalytic degradation of Rhodamine B and tetracycline hydrochloride, Appl. Surf. Sci. Adv., 2024, 24, 100647 CrossRef.
- S. Mishra and R. Acharya, Recent updates in modification strategies for escalated performance of Graphene/MFe2O4 heterostructured photocatalysts towards energy and environmental applications, J. Alloys Compd., 2023, 960, 170576 CrossRef CAS.
- Y. Tang, J. Qiu, D. Dai, G. Xia, B. Fang, Y. Li and J. Yao, ZnIn2S4/MIL-53-NH2 composite photocatalysts for H2O2 production: Synergistic effect of sulfur vacancy and heterostructure, Sep. Purif. Technol., 2025, 354, 129350 CrossRef CAS.
- X. Yan, G. Yu, C. Xing, Y. Hu, H. Liu and X. Li, Schottky junction with Bi/Bi2O3 core–shell nanoparticle modified g-C3N4 for boosting photocatalytic H2O2 evolution from pure water, Catal. Sci. Technol., 2023, 13, 3094 RSC.
- T. Shan, Y. Wang, D. Luo, Z. Huang, F. Zhang, H. Wu, L. Huang, J. Li, L. Chen and H. Xiao, Extended H-bonds/π-bonds networks for boosting electron transfer over polydopamine-covered nanocellulose/g-C3N4 toward efficient photocatalytic H2O2 production, Appl. Catal., B, 2024, 349, 123872 CrossRef CAS.
- Z. Jiang, Y. Zhang, L. Zhang, B. Cheng and L. Wang, Effect of calcination temperatures on photocatalytic H2O2-production activity of ZnO nanorods, Chin. J. Catal., 2022, 43(2), 226–233 CrossRef CAS.
- Y. Tang, J. Qiu, D. Dai, G. Xia, L. Zhang and J. Yao, Defect-engineered Zr-MOFs with enhanced O2 adsorption and activation for photocatalytic H2O2 synthesis, Catal. Sci. Technol., 2024, 14(1), 83–89 RSC.
- A. Mondal, A. Prabhakaran, S. Gupta and V. R. Subramanian, Boosting Photocatalytic Activity Using Reduced Graphene Oxide (RGO)/Semiconductor Nanocomposites: Issues and Future Scope, ACS Omega, 2021, 6, 8734–8743 CrossRef CAS.
- T. S. Prakash, S. Subudhi, S. Das, M. K. Ghosh, M. Das, R. Acharya, R. Acharya and K. Parida, Hydrolytically stable citrate capped Fe3O4@UiO-66-NH2 MOF: A hetero-structure composite with enhanced activity towards Cr (VI) adsorption and photocatalytic H2 evolution, J. Colloid Interface Sci., 2022, 606, 353–366 CrossRef.
- A. Wang, H. Liang, F. Chen, X. Tian, S. Yin, S. Jing and P. Tsiakaras, Facile synthesis of C3N4/NiIn2S4 heterostructure with novel solar steam evaporation efficiency and photocatalytic H2O2 production performance, Appl. Catal., B, 2022, 310, 121336 CrossRef CAS.
- S. Jing, J. Zhao, A. Wang, Q. Ji, R. Cheng, H. Liang, F. Chen, P. Kannan, A. Brouzgou and P. Tsiakaras, Efficient photocatalytic production of H2O2 and photodegradation of tetracycline by CdS/square tubular g-C3N4 S-scheme heterojunction photocatalyst, Chem. Eng. J., 2024, 479, 147150 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2026 |



