Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Sustainable TiO2 photocatalysts modified with hollyhock-derived carbon dots and natural dye for enhanced visible-light degradation of Congo red: a comparative study
Govar H.
Hamasalih
a,
Sewara J.
Mohammed
 *b and
Shujahadeen B.
Aziz
*b and
Shujahadeen B.
Aziz
 *c
*c
aDepartment of Physics, College of Education, University of Sulaimani, Old Campus, Kurdistan Regional Government, Sulaymaniyah 46001, Iraq
bDepartment of Chemistry, College of Science, University of Sulaimani, Qlyasan Street, Kurdistan Regional Government, Sulaymaniyah 46001, Iraq. E-mail: sewara.mohammed@univsul.edu.iq
cTurning Trash to Treasure Laboratory (TTTL), Research and Development Center, University of Sulaimani, Qlyasan Street, Kurdistan Regional Government, Sulaymaniyah 46001, Iraq. E-mail: shujahadeenaziz@gmail.com
First published on 8th December 2025
Abstract
This study introduces a sustainable method for valorizing Alcea rosea (hollyhock) floral waste by developing two novel TiO2-based photocatalysts modified with biomass-derived materials: a natural dye (HH dye) and carbon dots (HHCDs). The HHCDs were synthesized via a one-pot hydrothermal process at 180 °C, yielding oxygen-rich, amorphous carbon dots. TiO2 nanoparticles were prepared by a sol–gel method and subsequently modified with either HH dye or HHCDs through environmentally benign procedures. Comprehensive characterization (FTIR, XRD, UV-vis, and FE-SEM) confirmed the successful incorporation of both modifiers and their interaction with the TiO2 surface. Optical analysis indicated a significant reduction in the bandgap for both composites, with HH dye@TiO2 (∼2.67 eV) exhibiting a lower bandgap than HHCDs@TiO2 (∼2.89 eV). Electrochemical measurements revealed that HHCDs@TiO2 facilitated more effective charge carrier separation, whereas HH dye@TiO2 demonstrated superior light-harvesting capabilities due to its anthocyanin content. In photocatalytic degradation experiments under visible light, HHCDs@TiO2 demonstrated superior performance, achieving 97.1% degradation of Congo red dye within 80 minutes, compared to 96.8% in 120 minutes for HH dye@TiO2. Both composites exhibited remarkable long-term stability, retaining over 95% of their efficiency after 180 days of storage. Optimal degradation conditions were identified at mildly acidic to neutral pH using 0.04 g of HHCDs@TiO2 and 0.06 g of HH dye@TiO2. This work presents a novel, dual-approach strategy for fabricating efficient and eco-friendly photocatalysts, highlighting their significant potential for solar-driven water purification and environmental remediation.
1. Introduction
The extensive use of synthetic dyes in industries such as textiles, paper, and plastics has led to the discharge of large volumes of dye-contaminated wastewater, posing significant threats to aquatic ecosystems and public health.1 Among these pollutants, azo dyes such as Congo red (CR) are particularly concerning due to their complex aromatic structure, high solubility, and resistance to natural biodegradation, coupled with their potential carcinogenic properties.2,3 The intense color of CR can significantly impede photosynthesis by reducing light penetration in aquatic ecosystems.4 Moreover, CR may degrade into benzidine, a known carcinogen, leading to long-term ecological and biological harm.5,6 These adverse characteristics establish CR as a critical model pollutant for evaluating advanced wastewater treatment technologies.7Conventional treatment methods, including adsorption, coagulation, and flocculation, are often ineffective, as they primarily transfer contaminants between phases rather than degrading them.8 In contrast, advanced oxidation processes (AOPs), particularly heterogeneous photocatalysis, offer a more sustainable approach by utilizing light energy to generate reactive oxygen species (ROS), which mineralize organic pollutants into harmless end products.9,10 Titanium dioxide (TiO2) has been widely used as a photocatalyst due to its non-toxicity, low cost, and chemical stability. However, its practical application is hampered by two primary limitations: a wide bandgap (∼3.2 eV for anatase and ∼3.0 eV for rutile), which restricts its photoactivity to the ultraviolet region (only ∼4% of the solar spectrum), and the rapid recombination of photogenerated electron–hole pairs, which drastically reduces quantum efficiency.11–13
To overcome these limitations, TiO2 has been modified through various strategies, including metal/non-metal doping, coupling with narrow-bandgap semiconductors, and sensitization with organic dyes or carbon-based nanomaterials.14,15 Among these, natural dyes and carbon dots (CDs) derived from plant biomass have emerged as promising, green, and cost-effective modifiers for enhancing the visible-light response of TiO2.16,17 Natural dyes, rich in anthocyanins and flavonoids, are abundant, biodegradable, and exhibit excellent light-harvesting capabilities in the visible spectrum.18,19 Hollyhock (Alcea rosea), an ornamental plant, produces vividly pigmented flowers that are typically discarded after blooming. This floral waste represents a valuable and untapped source of natural dyes with inherent electron-donating and photosensitizing properties suitable for photocatalysis.20
Carbon dots, a class of zero-dimensional, carbon-based nanomaterials, have also gained significant attention ascribed to their tunable photoluminescence, high surface area, biocompatibility, and ability to facilitate electron transfer.21–24 CDs can improve charge separation, broaden light absorption, and contribute to up-conversion luminescence.25,26 When derived from plant-based dyes, they offer a dual advantage, converting biomass waste into functional nanomaterials while enhancing photocatalyst performance. Nevertheless, scalable, low-cost methods for preparing CD-based photocatalysts remain a challenge, limiting their widespread application.27,28
Compared to synthetic modifiers, which often involve complex, energy-intensive processes and potentially toxic precursors, biomass-derived materials offer a compelling green alternative. Their advantages include abundance, biodegradability, low cost, and inherent surface functional groups that enhance pollutant adsorption and facilitate semiconductor anchoring. While challenges in scalability and precise control of properties persist, the valorization of agricultural waste into functional photocatalytic materials represents a significant stride towards a circular economy.29–31 Recent research has demonstrated the potential of biomass-derived CDs as eco-friendly photocatalyst modifiers.32 However, few studies have directly compared the performance of TiO2 modified with natural dyes and their corresponding CDs derived from the same biomass source. Such comparisons are essential for understanding structure–activity relationships and optimizing green photocatalyst design for practical and industrial use.
This study demonstrates a sustainable approach by synthesizing and comparatively evaluating two TiO2-based photocatalysts modified with hollyhock-derived natural dye (HH dye) and hollyhock-derived carbon dots (HHCDs), both sourced from the same floral waste. The photocatalysts were thoroughly characterized to understand their structural, morphological, optical, and electrochemical properties. Their performance was evaluated through the degradation of Congo red under visible light irradiation. This work not only contributes to the development of efficient, waste-derived photocatalysts for water purification but also provides fundamental insights into the distinct mechanistic roles of a natural dye and its corresponding carbon dots in enhancing TiO2 photocatalysis. The findings align with the principles of waste valorization and support broader efforts towards achieving the United Nations Sustainable Development Goals (SDGs) for clean water and sustainable production.
2. Experimental section
2.1 Materials
Hollyhock flowers (Alcea rosea), used for extracting natural dye (HH dye) and synthesizing hollyhock dye-derived carbon dots (HHCDs), were collected from the garden of the University of Sulaimani's Research and Development Center (Sulaymaniyah, Kurdistan Region, Iraq). Titanium(IV) isopropoxide (Ti[OC(CH3)2]4 (TTIP), ≥97%, Sigma-Aldrich) served as the titanium precursor. Ethanol (C2H5OH, 99.9%), hydrochloric acid (HCl, 37%, Merck), and deionized (DI) water were used for sol–gel synthesis. Congo red (CR, C32H22N6Na2O6S2, 99.5%, Merck) was employed as the model pollutant. All chemicals were used as received without further purification.2.2 Instrumentations
Fourier transform infrared (FT-IR) spectroscopy (Vmax in cm−1) with KBr pellet methodon a PerkinElmer spectrophotometer (Waltham, MA, USA) was used to find the characteristic functional groups. The 1H and 13C nuclear magnetic resonance (NMR) spectra were obtained using a 600 MHz Bruker BioSpin spectrometer (Rheinstetten, Germany) using DMSO-d6 as the solvent. Photocatalytic degradation was monitored by recording UV-visible absorption spectra using a UV 6100 double beam spectrophotometer with a wavelength range of 190–1100 nm, while diffuse reflectance spectra (DRS) were collected on a Varian Cary 100 UV-Vis spectrophotometer equipped with a DRA-CA-30I integrating sphere to determine the optical band gap. X-ray diffraction (XRD) was performed using an Angstrom Advance ADX 2700 diffractometer (Massachusetts, USA) under Cu Kα radiation (λ = 1.5406 Å), which scanned the 10–80° 2θ range with a step size of 0.1°. Photoluminescence (PL) spectra were measured with a Cary Eclipse fluorescence spectrophotometer (Agilent Technologies, USA). HRTEM was carried out using an FEI Tecnai G2 F30. At the same time, the surface chemical composition and elemental states were analyzed using X-ray photoelectron spectroscopy (XPS) with a Thermo Fisher ESCALAB 250Xi instrument. Morphological features were examined by field-emission scanning electron microscopy (FE-SEM) coupled with energy-dispersive X-ray spectroscopy (EDS) using a TESCAN MIRA3 system (Czechia) and photoreactor 2 × 30 W flood blue light LED lamps.2.3 Methodologies
The suspension was subjected to sonication using a UIP500hdT ultrasonic processor (20 kHz, 250 W), with the probe immersed directly in the solution. During the 10-minute sonication, the temperature naturally increased from 20 °C to 65 °C due to cavitation effects. After allowing it to settle for 10 minutes, the extract was decanted. The residual plant material was then re-extracted twice using 150 mL of ethanol under identical sonication conditions to maximize pigment recovery.
The combined extracts were purified by sequential filtration through Whatman No. 40 filter paper to remove coarse particulates, followed by 0.22 µm syringe filtration to remove finer debris. The filtrate was concentrated using a rotary evaporator, and the resulting dye was further dried in a silica gel desiccator. The final dry yield of the HH dye was 2.25 g (∼28.1%), which was stored at 4 °C for subsequent characterization and application, as shown in Scheme 1a.
The solution was filtered twice through Whatman No. 40 filter paper (Whatman International Ltd, Kent, UK) to remove coarse particulates and then centrifuged at 5000 rpm for 10 min to separate larger carbonaceous aggregates. The supernatant was further purified by filtration through a 0.22 µm PTFE syringe filter. The solvent was evaporated under reduced pressure using a rotary evaporator, and the residue was redissolved in chloroform to facilitate the removal of residual polar impurities.
Liquid–liquid extraction was performed three times using a chloroform/water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) system. The chloroform phase was collected and concentrated under reduced pressure, yielding 0.5 g (50% yield) of purified brownish-yellow HHCDs. The final product was stored at 4 °C for subsequent characterization and photocatalytic studies (Scheme 1b illustrates the synthesis procedure of the HHCDs).
1 v/v) system. The chloroform phase was collected and concentrated under reduced pressure, yielding 0.5 g (50% yield) of purified brownish-yellow HHCDs. The final product was stored at 4 °C for subsequent characterization and photocatalytic studies (Scheme 1b illustrates the synthesis procedure of the HHCDs).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DI water
DI water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOH
EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HCl = 1
HCl = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1.
0.1.
Solution A was prepared by mixing 30 mL of ethanol with 1.82 mL of deionized water and 870 µL of HCl, followed by stirring for 10 min. Solution B was prepared by combining 30 mL of TTIP with 30.86 mL of ethanol in a round-bottom flask, maintained at 10 °C using a cooling bath, and stirred continuously.
Solution A was added dropwise to solution B at a rate of 1 drop every 5 s under stirring at 10 °C, resulting in a transparent solution. Stirring was continued for an additional 10 min, after which the mixture was aged for 24 h to form a thick gel. The gel was transferred to a glass Petri dish and dried at room temperature for 48 h, yielding pale yellow TiO2 materials. These were ground into a fine powder using a mortar and pestle, then calcined at 500 °C for 4 h in a muffle furnace to obtain white TiO2NPs, as illustrated in Scheme 1c.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in 150 mL of ethanol. The mixtures were stirred at 25 °C in the dark for 30 minutes, during which the color of TiO2 changed from white to dark blue in the dye-doped system (HH dye@TiO2) and became brown in the CD-doped system (HHCDs@TiO2), confirming successful composite formation (Scheme 2a and b).
1) in 150 mL of ethanol. The mixtures were stirred at 25 °C in the dark for 30 minutes, during which the color of TiO2 changed from white to dark blue in the dye-doped system (HH dye@TiO2) and became brown in the CD-doped system (HHCDs@TiO2), confirming successful composite formation (Scheme 2a and b).
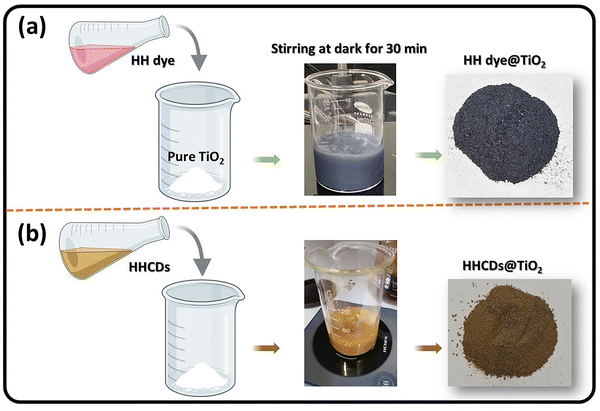 | ||
| Scheme 2 Illustration of the fabrication process and the photographs of the resulting (a) HH dye@TiO2 and (b) HHCDs@TiO2 nanocomposites. | ||
The resulting precipitates were washed 3–4 times with distilled water to remove unbound dye or carbon dots, then dried overnight at 50 °C in an oven. The final composites were stored in amber vials to prevent photodegradation before characterization and photocatalytic testing.
Before irradiation, 0.02–0.07 g of photocatalyst was dispersed in 50 mL of CR solution (10 ppm) and stirred in the dark for 30 min to achieve adsorption–desorption equilibrium. The mixture was then exposed to visible light under constant stirring, with an electric fan maintaining the temperature at 25 °C. At intervals of 30, 60, 90, and 120 min, 5 mL aliquots were withdrawn, centrifuged to remove the catalyst, and analyzed by UV-vis spectrophotometry at λmax = 497 nm to monitor CR degradation. The recovered photocatalysts were washed with distilled water, dried at 60 °C, and reused for recyclability studies.
The effects of irradiation time (30–120 min), catalyst dosage (0.02–0.07 g), initial dye concentration (5–20 ppm), and solution pH (4.17, 5.75, and 8.70) were systematically investigated. Unless otherwise specified, a fixed irradiation time of 60 min was applied for all experiments except those assessing time-dependent degradation or recyclability. Photocatalyst stability was evaluated over five consecutive cycles, with washing and drying between runs to ensure reproducibility.
The degradation efficiency (%) was calculated as:
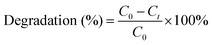 | (1) |
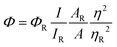 | (2) |
3. Results and discussion
3.1 HH dye characterization
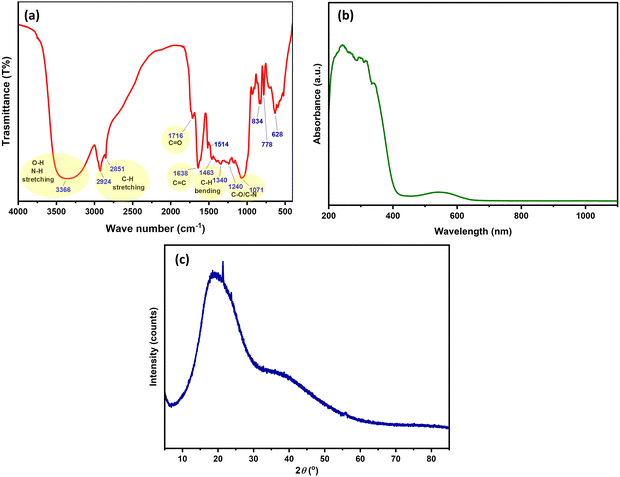
The FTIR spectrum of the HH dye (Fig. 1a) reveals the presence of various functional group characteristics of polyphenolic and flavonoid-rich plant extracts.38,39 A broad absorption band at 3366 cm−1 corresponds to O–H and N–H stretching vibrations, indicating the presence of hydroxyl and amine groups typically found in natural pigments such as anthocyanins,40 while the peaks at 2924 cm−1 and 2851 cm−1 are attributed to aliphatic C–H stretching.41,42 A strong band at 1716 cm−1 is assigned to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching (carbonyl groups), likely from anthocyanins or other conjugated systems, and the band at 1638 cm−1 corresponds to aromatic C
O stretching (carbonyl groups), likely from anthocyanins or other conjugated systems, and the band at 1638 cm−1 corresponds to aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibrations.43 Additional peaks at 1463 cm−1 and 1340 cm−1 are associated with C–H bending of aliphatic carbons.44 Absorptions between 1240 and 1071 cm−1 correspond to C–O and C–N stretching, indicating the presence of ether and amine functionalities.45–47 These functional groups suggest strong potential for interfacial interactions with TiO2 surfaces.
C stretching vibrations.43 Additional peaks at 1463 cm−1 and 1340 cm−1 are associated with C–H bending of aliphatic carbons.44 Absorptions between 1240 and 1071 cm−1 correspond to C–O and C–N stretching, indicating the presence of ether and amine functionalities.45–47 These functional groups suggest strong potential for interfacial interactions with TiO2 surfaces.
The UV-vis absorbance spectrum of the HH dye (Fig. 1b) exhibits strong absorption in the UV and visible regions, with a distinct band at ∼550 nm, attributed to an n–π* electron transition.48 This suggests its efficient visible-light harvesting capability, which is crucial for enhancing the photocatalytic activity of TiO2 under visible light. Dyes are enriched with colorants and anthocyanins, and thus their absorption will cover the UV to visible region of electromagnetic radiation.
The XRD analysis, as shown in (Fig. 1c), confirms the amorphous nature of the HH dye. This is evidenced by a broad diffraction band centered within the 2θ range of approximately 16° to 24°, characteristic of amorphous structural features.49
3.2 Structural and morphological characterization of HHCDs
The HHCDs were synthesized via an eco-friendly hydrothermal method using the HH dye as a dual carbon and nitrogen source. The synthesized HHCDs were characterized using multiple techniques, including FTIR, 1H-NMR, 13C-NMR, XPS, UV-vis spectroscopy, photoluminescence (PL), HR-TEM, and XRD. These techniques were employed to elucidate the chemical structure, optical properties, and morphology of the HHCDs, clarifying their role in improving the visible-light photocatalytic activity of sustainable TiO2 systems.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O functionalities.51 Notably, the band at 1655 cm−1 is assigned to C
O functionalities.51 Notably, the band at 1655 cm−1 is assigned to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching, suggesting nitrogen incorporation, possibly in the form of an imine or pyridinic structure.52 Graphitic C
N stretching, suggesting nitrogen incorporation, possibly in the form of an imine or pyridinic structure.52 Graphitic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C vibrations appear around 1624 cm−1 and 1608 cm−1, indicating retention of conjugated systems.53,54 A C–H bending peak is observed at 1371 cm−1. Peaks at 1241 cm−1 and 1173 cm−1 correspond to C–O and C–N bonds, which contribute to surface polarity and potential interaction with TiO2.55,56
C vibrations appear around 1624 cm−1 and 1608 cm−1, indicating retention of conjugated systems.53,54 A C–H bending peak is observed at 1371 cm−1. Peaks at 1241 cm−1 and 1173 cm−1 correspond to C–O and C–N bonds, which contribute to surface polarity and potential interaction with TiO2.55,56
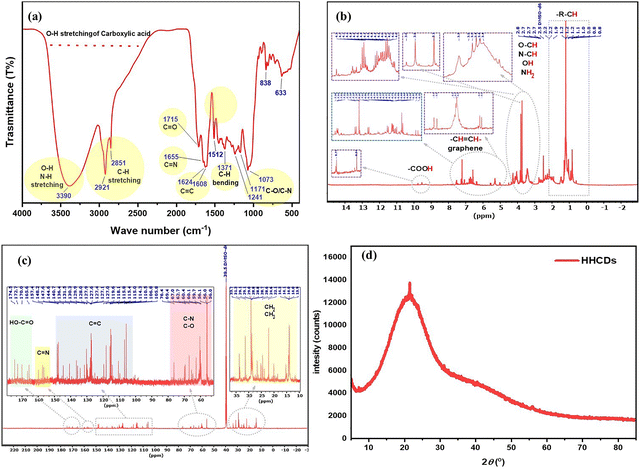 | ||
| Fig. 2 (a) FT-IR spectrum, (b) 1H-NMR spectrum (DMSO-d6), (c) 13C-NMR spectrum (DMSO-d6), and (d) XRD pattern of HHCDs. | ||
Collectively, these results verify that the carbonization process not only preserves but also enhances key functional groups on the HHCDs, including hydroxyl, carbonyl, and nitrogen-containing species. These groups are critical for promoting interfacial charge transfer and boosting photocatalytic activity. The incorporation of CDs with such a rich functional group landscape is crucial for improving the light–matter interactions of TiO2, thereby enhancing its overall photocatalytic performance. Further insights into the role of CDs within the TiO2 composite structure are discussed in the subsequent sections.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), indicating a graphitic carbon framework and retained sp2-hybridized structures derived from the HH dye precursor.58 The 4.3–3.4 ppm region shows protons adjacent to electronegative atoms (O–CH, N–CH, –OH, –NH2), further supporting nitrogen and oxygen incorporation, and suggesting increased polarity and potential electron-donating capabilities.58 Finally, signals at 2.8–0.8 ppm correspond to aliphatic protons (–CH3, –CH2–), reflecting residual hydrocarbon chains that contribute to the amphiphilic nature and dispersibility of HHCDs in aqueous systems.59,60
CH–), indicating a graphitic carbon framework and retained sp2-hybridized structures derived from the HH dye precursor.58 The 4.3–3.4 ppm region shows protons adjacent to electronegative atoms (O–CH, N–CH, –OH, –NH2), further supporting nitrogen and oxygen incorporation, and suggesting increased polarity and potential electron-donating capabilities.58 Finally, signals at 2.8–0.8 ppm correspond to aliphatic protons (–CH3, –CH2–), reflecting residual hydrocarbon chains that contribute to the amphiphilic nature and dispersibility of HHCDs in aqueous systems.59,60
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), verifying the presence of nitrogen elements, a key feature for enhancing electron density and surface reactivity.61 The regions belonging to 148–105 ppm reveal the prominent sp2-hybridized carbon (C
N), verifying the presence of nitrogen elements, a key feature for enhancing electron density and surface reactivity.61 The regions belonging to 148–105 ppm reveal the prominent sp2-hybridized carbon (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) signals, indicating a graphitic core that facilitates light absorption and charge transfer.62 Furthermore, the peaks between 76 and 56 ppm are attributed to C–O and C–N bonds, suggesting hydroxyl and amine functional groups, which improve aqueous stability and HHCD interactions.63 Finally, signals at 13–33 ppm arise from aliphatic carbons (–CH3, –CH2–), likely from residual hydrocarbon chains during synthesis.63 These 13C-NMR results are in excellent agreement with the FTIR and 1H-NMR findings, confirming the presence of diverse surface functionalities, including carboxyl, hydroxyl, amine, and aromatic moieties, which collectively enhance the photocatalytic performance of the HHCD-modified TiO2 system.
C) signals, indicating a graphitic core that facilitates light absorption and charge transfer.62 Furthermore, the peaks between 76 and 56 ppm are attributed to C–O and C–N bonds, suggesting hydroxyl and amine functional groups, which improve aqueous stability and HHCD interactions.63 Finally, signals at 13–33 ppm arise from aliphatic carbons (–CH3, –CH2–), likely from residual hydrocarbon chains during synthesis.63 These 13C-NMR results are in excellent agreement with the FTIR and 1H-NMR findings, confirming the presence of diverse surface functionalities, including carboxyl, hydroxyl, amine, and aromatic moieties, which collectively enhance the photocatalytic performance of the HHCD-modified TiO2 system.
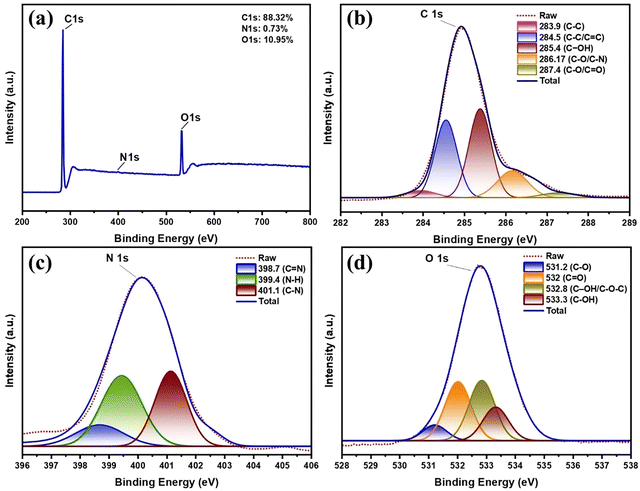
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C/C–C graphitic carbon),69 285.4 eV (C–OH hydroxyl groups),70 286.17 eV (C–O/C–N ether/amine linkages),71 and 287.4 eV (C
C/C–C graphitic carbon),69 285.4 eV (C–OH hydroxyl groups),70 286.17 eV (C–O/C–N ether/amine linkages),71 and 287.4 eV (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O carbonyl groups).72 This distribution confirms the presence of both aromatic domains and oxygenated functional groups, consistent with our FTIR and NMR results. The N 1s spectrum (Fig. 3c) further demonstrates nitrogen incorporation through three characteristic components at 398.7 eV (pyridinic N, C
O carbonyl groups).72 This distribution confirms the presence of both aromatic domains and oxygenated functional groups, consistent with our FTIR and NMR results. The N 1s spectrum (Fig. 3c) further demonstrates nitrogen incorporation through three characteristic components at 398.7 eV (pyridinic N, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 399.4 eV (pyrrolic N, N–H), and 401.1 eV (graphitic N, C–N), which are known to enhance charge carrier density and surface reactivity.73,74
N), 399.4 eV (pyrrolic N, N–H), and 401.1 eV (graphitic N, C–N), which are known to enhance charge carrier density and surface reactivity.73,74
Complementary information comes from the O 1s spectrum (Fig. 3d), where deconvolution yields four components at 531.2 eV (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O carbonyl), 532.0 eV (C–O epoxy/ether), 532.8 eV (C–OH/C–O–C hydroxyl/ester), and 533.3 eV (adsorbed water/oxygen species).75–77 The predominance of oxygen-containing functional groups explains the excellent aqueous dispersibility of HHCDs observed during synthesis and application. These XPS findings collectively demonstrate that HHCDs possess an ideal surface chemistry for photocatalytic applications, combining conjugated sp2 carbon domains for charge transport, nitrogen dopants for enhanced electron density, and oxygen functional groups for improved interfacial interactions with TiO2.
O carbonyl), 532.0 eV (C–O epoxy/ether), 532.8 eV (C–OH/C–O–C hydroxyl/ester), and 533.3 eV (adsorbed water/oxygen species).75–77 The predominance of oxygen-containing functional groups explains the excellent aqueous dispersibility of HHCDs observed during synthesis and application. These XPS findings collectively demonstrate that HHCDs possess an ideal surface chemistry for photocatalytic applications, combining conjugated sp2 carbon domains for charge transport, nitrogen dopants for enhanced electron density, and oxygen functional groups for improved interfacial interactions with TiO2.
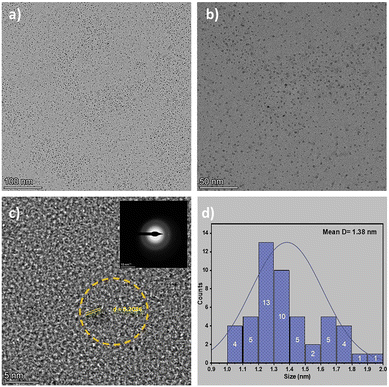
High-magnification HR-TEM images (Fig. 4c) reveal well-defined lattice fringes with an interplanar spacing of 0.208 nm. This measurement corresponds to the (100) crystallographic plane of graphitic carbon and aligns well with the value of 0.21 nm reported by Guo et al.78 This observation confirms the presence of sp2-hybridized carbon domains within the HHCD structure, indicating a partially ordered graphitic framework embedded in an amorphous carbon matrix.79 This structure is further corroborated by the selected-area electron diffraction (SAED) pattern (Fig. 4c inset), which displays characteristic diffuse rings.
This unique nanoarchitecture is pivotal to the optoelectronic properties of the HHCDs.80,81 The embedded graphitic domains facilitate efficient charge carrier transport via π–π* electronic transitions, while the surrounding amorphous regions host abundant surface functional groups that enhance photoluminescence and allow for facile surface modification. Furthermore, the narrow particle size distribution of 5–10 nm (Fig. 4d) is optimal for enhancing visible-light absorption and promoting charge separation. These structural attributes are highly advantageous for photocatalysis. They facilitate improved interfacial electron transfer within the HHCD–TiO2 composite, ultimately leading to the enhanced degradation of Congo red dye under visible light irradiation.
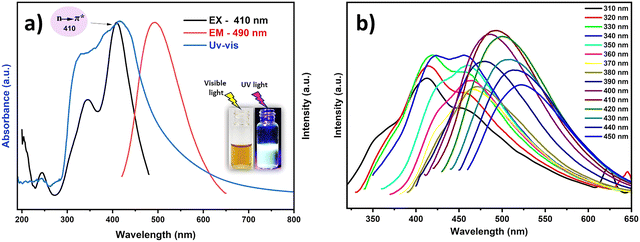
PL analysis revealed excitation-dependent emission behavior, as evidenced by the series of emission spectra collected from 310 to 450 nm excitation (Fig. 5b). The maximum emission intensity occurs at 490 nm when excited at 410 nm, which corresponds to the absorption maximum observed in the UV-vis spectrum. This excitation–emission relationship was further confirmed by the excitation spectrum monitored at 490 nm (Fig. 5a), verifying 410 nm as the optimal excitation wavelength. The relatively narrow Stokes shift (80 nm) between absorption and emission maxima suggests efficient radiative recombination processes in the HHCDs.
The absolute quantum yield (QY) of the HHCDs was determined to be 4.49% using fluorescein (QY = 95%) as a reference standard. This QY value is moderate compared to some synthetic carbon dots and sufficient for photocatalytic applications.83 The observed fluorescence properties stem from the combination of quantum confinement effects in the sp2 carbon domains and surface state emissions from the functional groups, as evidenced by FTIR and NMR results.
3.3 Characterization of the TiO2 nanocomposites (HH dye@TiO2, HHCDs@TiO2)
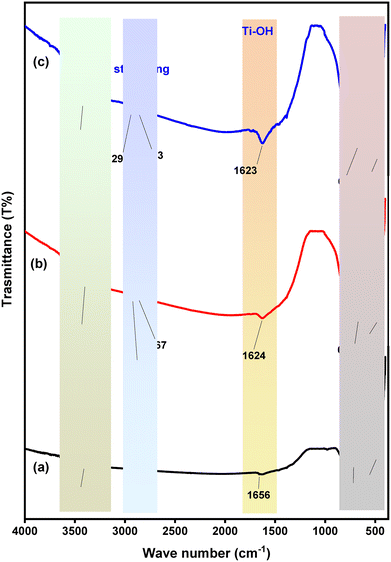 | ||
| Fig. 6 FTIR spectra of (a) pure TiO2, (b) HH dye@TiO2 nanocomposites, and (c) HHCDs@TiO2 nanocomposites. | ||
The HH dye@TiO2 nanocomposite (Fig. 6b) shows modified spectral features indicating successful hybridization. The O–H stretching shifts to 3398 cm−1, suggesting hydrogen bonding between TiO2 surface groups and HH dye molecules.86 New peaks at 2923 cm−1 and 2867 cm−1 correspond to aliphatic C–H stretching vibrations from the HH dye components. The 1624 cm−1 region represents overlapping contributions from Ti–OH bending and aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching from the dye's conjugated system.87,88 The Ti–O vibrations shift slightly to 667 cm−1 and 470 cm−1, demonstrating structural interactions between TiO2 and HH dye functional groups without phase alteration.89,90 More pronounced modifications appear in the HHCDs@TiO2 spectrum (Fig. 6c). The O–H stretching intensifies and shifts to 3420 cm−1, indicating enhanced surface hydroxylation.91 Stronger C–H vibrations (2935 cm−1, 2863 cm−1) reflect greater organic surface coverage by HHCDs. The maintained anatase structure is evidenced by Ti–O vibrations at 669 cm−1 and 471 cm−1,92 while the more intense 1623 cm−1 band suggests reinforced interfacial interactions between TiO2 and HHCD surface groups.
C stretching from the dye's conjugated system.87,88 The Ti–O vibrations shift slightly to 667 cm−1 and 470 cm−1, demonstrating structural interactions between TiO2 and HH dye functional groups without phase alteration.89,90 More pronounced modifications appear in the HHCDs@TiO2 spectrum (Fig. 6c). The O–H stretching intensifies and shifts to 3420 cm−1, indicating enhanced surface hydroxylation.91 Stronger C–H vibrations (2935 cm−1, 2863 cm−1) reflect greater organic surface coverage by HHCDs. The maintained anatase structure is evidenced by Ti–O vibrations at 669 cm−1 and 471 cm−1,92 while the more intense 1623 cm−1 band suggests reinforced interfacial interactions between TiO2 and HHCD surface groups.
These spectral changes collectively verify successful nanocomposite formation. The enhanced intensity and shifted peaks in HHCDs@TiO2 suggest stronger interfacial coupling compared to HH dye@TiO2, which should facilitate improved visible-light absorption and charge carrier separation, crucial for enhancing Congo red photodegradation efficiency.
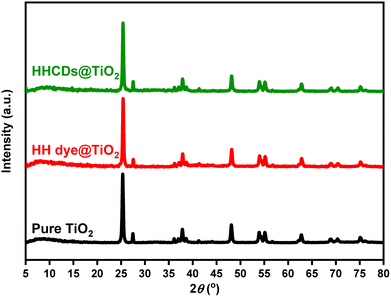 | ||
| Fig. 7 XRD patterns comparing unmodified TiO2 nanoparticles with HH dye@TiO2, and HHCDs@TiO2 nanocomposites, demonstrating the intact crystalline structure after surface modification. | ||
Pure TiO2 nanoparticles display characteristic peaks at 2θ values of 25.3°, 37.9°, 48.3°, 54.1°, 55.1°, 62.8°, 69.2°, 70.3°, and 75.4°, corresponding to the (101), (004), (200), (105), (211), (204), (116), (220), and (215) crystallographic planes of the tetragonal anatase phase, respectively.94 The sharpness and high intensity of these peaks demonstrate the excellent crystallinity of the starting TiO2 material. Both modified nanocomposites show nearly identical diffraction patterns to the unmodified TiO2, with no detectable peak shifts or additional phases present.
The absence of distinct diffraction peaks attributable to either HH dye or HHCDs, despite the evidence of graphitic domains in HHCDs from HR-TEM analysis, is attributed to two primary factors.95,96 First, the modifiers were loaded at a low weight ratio (10%) and are highly dispersed as a surface layer on the TiO2 nanoparticles. Consequently, the X-ray scattering from these nanoscale components is overwhelmed by the intense diffraction from the bulk crystalline TiO2 phase. Second, the broad, low-intensity (002) graphitic hump characteristic of HHCDs (centered around 22°) is effectively masked by the sharp, high-intensity peaks of the anatase and rutile phases. This is a common phenomenon when a small quantity of a semi-crystalline or amorphous material is composited with a highly crystalline substance, rendering the weaker signals undetectable.97 This structural preservation confirms that the modification process does not alter the fundamental crystalline architecture of TiO2 while still enabling surface functionalization.98
The maintained crystallinity is particularly advantageous for photocatalytic applications as it ensures structural stability during photoreactions and preserves the intrinsic charge transport properties of TiO2. Meanwhile, the surface modifications provide the additional benefit of enhanced visible-light absorption through the incorporated organic components, creating an optimal combination of crystalline stability and improved light harvesting capability for Congo red degradation.
EDX analysis provides quantitative evidence of the surface modifications. The pure TiO2 spectrum (Fig. 8a) shows only titanium and oxygen signals, confirming the absence of impurities. In contrast, the HH dye@TiO2 spectrum (Fig. 8b) reveals trace carbon content, while the HHCDs@TiO2 spectrum (Fig. 8c) displays distinct peaks for carbon and nitrogen in addition to the primary Ti and O signals. As summarized in Table 1, the carbon content increases significantly from <0.0 wt% in pure TiO2 to approximately 1.8 wt% in HH dye@TiO2 and 8.74 wt% in HHCDs@TiO2. Nitrogen is also detected, with concentrations of 1.3 wt% in the HH dye@TiO2 and 7.91 wt% in HHCDs@TiO2. This progressive increase in carbon content, particularly the substantial rise in HHCDs@TiO2, confirms the effective surface deposition of organic components.100
| Catalyst | Element | Weight% | Atomic% |
|---|---|---|---|
| TiO2 | O | 38.79 | 65.49 |
| Ti | 61.21 | 34.51 | |
| Total | 100 | 100 | |
| HH dye@TiO2 | C | 1.8 | 4.14 |
| N | 1.3 | 2.86 | |
| O | 43.6 | 57.12 | |
| Ti | 53.3 | 35.88 | |
| Total | 100 | 100 | |
| HHCDs@TiO2 | C | 8.74 | 5.4 |
| N | 7.91 | 3.32 | |
| O | 34.1 | 54.04 | |
| Ti | 49.25 | 37.24 | |
| Total | 100 | 100 |
The morphological and compositional changes observed through FE-SEM and EDX correlated well with the enhanced photocatalytic performance as can be seen in a later section. The increased carbon content provides more active sites for pollutant adsorption, while the nitrogen incorporation from HHCDs may introduce additional charge carrier trapping centers. The compact surface morphology of HHCDs@TiO2 suggests improved interfacial contact between the carbon dots and TiO2, which should facilitate more efficient charge transfer during photocatalysis. These structural modifications are expected to significantly enhance the visible-light photocatalytic degradation of Congo red compared to unmodified TiO2.
3.4 Optical characterization of the TiO2 nanocomposites
The analysis utilizes the Tauc relation:
| (αhν)n = B(hν − Eg) | (3) |
| F(R) = (1 − R)2/2R | (4) |
Fig. 9 presents the DRS spectra plotted as F(R) versus wavelength. Modified TiO2 samples showed reduced reflectance compared to pure TiO2, particularly in the visible region, indicating enhanced light absorption.104 The Tauc plots derived from these spectra (Fig. 10a and b) reveal distinct optical transitions. Linear extrapolation yields direct OBG values of 3.25 eV (pure TiO2), 3.08 eV (HH dye@TiO2), and 2.89 eV (HHCDs@TiO2), and indirect OBG values of 2.93 eV, 2.67 eV, and 1.3 eV for pure and doped TiO2 samples, respectively (Table 2).
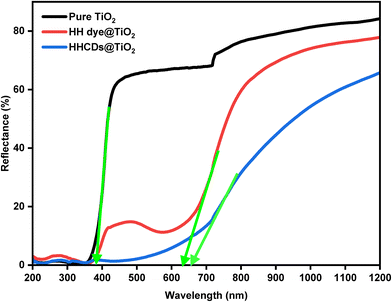 | ||
| Fig. 9 UV-vis diffuse reflectance spectra (Kubelka–Munk function) of pure and modified TiO2 photocatalysts, showing enhanced visible-light absorption for the nanocomposites. | ||
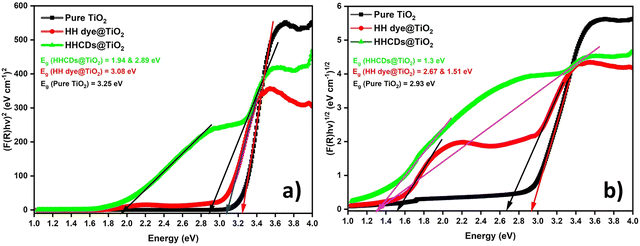 | ||
| Fig. 10 Tauc plots for (a) direct (n = 2) and (b) indirect (n = 1/2) bandgap determination through linear extrapolation. | ||
| Transition | Catalysts | Trapping level (eV) | OBG energy (eV) |
|---|---|---|---|
| (F(R) × hν)2 | Pure TiO2 | — | 3.25 |
| HH dye@TiO2 | — | 3.08 | |
| HHCDs@TiO2 | 1.94 | 2.89 | |
| (F(R) × hν)1/2 | Pure TiO2 | — | 2.93 |
| HH dye@TiO2 | 1.51 | 2.67 | |
| HHCDs@TiO2 | 1.3 | 1.3 |
The progressive OBG narrowing demonstrates successful modification of TiO2's electronic structure. The 0.36 eV reduction in direct bandgap for HHCDs@TiO2 confirms improved visible-light absorption, while the exceptionally low indirect bandgap (1.3 eV) suggests the introduction of mid-gap states that facilitate charge carrier generation.105 These defect states, evidenced by trapping levels between 1.3 and 1.94 eV, act as “stepping stones” inside the gap, which separates the VB from the CB and thus will enhance the photocatalytic efficiency under visible light.
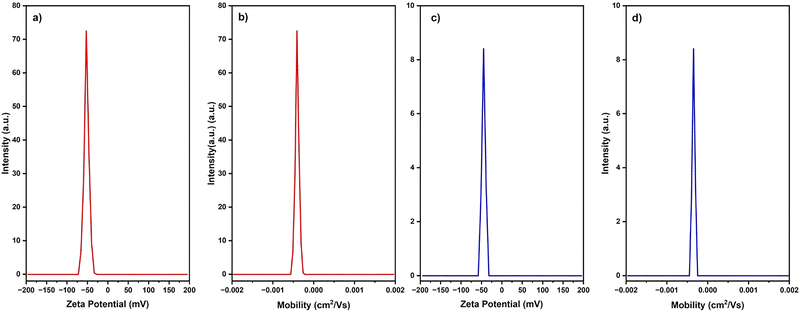 | ||
| Fig. 11 (a) and (c) The zeta potential and (b) and (d) the electrophoretic mobility of HH dye@TiO2 and HHCDs@TiO2, respectively. | ||
Complementary electrophoretic mobility measurements yielded values of −0.000404 cm2 V−1 s−1 and −0.000349 cm2 V−1 s−1 for HH dye@TiO2 and HHCDs@TiO2, respectively (Fig. 11b and d). These negative values confirm the overall negative surface charge of the photocatalysts, with the modest magnitude indicating controlled migration under applied electric fields. The surface charge originates primarily from anionic functional groups introduced through modification, including carboxyl (–COOH) groups from hollyhock-derived components, surface hydroxyl (–OH) moieties, and amino (–NH2) groups present in the carbon dot structures. These functional groups collectively enhance both surface charge density and aqueous dispersibility.107
These results demonstrate that both nanocomposites exhibit high negative zeta potentials and superior colloidal stability, indicating enhanced surface coverage by the adsorbed dye molecules compared to unmodified TiO2. This improved stability is particularly advantageous for photocatalytic applications as it maintains uniform dispersion of active sites, prolongs interaction with target pollutants, prevents performance-degrading aggregation, and facilitates efficient light absorption throughout the reaction medium. The robust colloidal stability demonstrated by these measurements confirms the successful surface modification of TiO2 and predicts favorable performance in aqueous photocatalytic degradation of Congo red.
3.5 Photocatalytic activity of TiO2 nanocomposites for CR degradation
 | ||
| Fig. 12 Photodegradation efficiency of CR (5 ppm) under visible light (60 min) as a function of HH dye@TiO2 and HHCDs@TiO2 dosage. | ||
Beyond these optimal loadings, distinct behaviors emerged between the two catalysts. HH dye@TiO2 exhibited reduced efficiency at higher dosages. Various interpretations have been introduced in the literature to explain this phenomenon, such as excessive catalyst loading increases suspension opacity, significantly limiting light penetration and photon absorption. Second, catalyst agglomeration at elevated concentrations reduces the availability of active sites for both dye adsorption and surface reactions. Third, light scattering effects become more pronounced, with aggregated particles potentially deactivating excited molecules through collisions with ground-state species.
In contrast, HHCDs@TiO2 maintained stable photocatalytic performance even at higher dosages. This remarkable stability suggests that the carbon dot modification effectively enhances catalyst dispersibility and optimizes light utilization, thereby mitigating the common limitations observed with conventional modified TiO2. These findings align well with established literature reporting optimal TiO2 loadings in the range of 400–500 mg L−1,109,110 while simultaneously highlighting the superior performance characteristics of our carbon dot-modified photocatalyst under visible light conditions.
The observed performance differences between the two catalysts underscore the significant advantages conferred by carbon dot modification. The HHCDs@TiO2 nanocomposite not only achieves higher maximum degradation efficiency (>99.9% vs. 97%) but also demonstrates greater stability across a wider range of catalyst loadings. This enhanced performance profile suggests that the carbon dot modification successfully addresses key limitations typically associated with TiO2-based photocatalysts, particularly regarding light absorption efficiency and particle dispersion at higher concentrations.
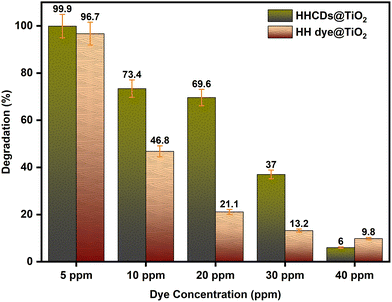 | ||
| Fig. 13 Photodegradation efficiency of HH dye@TiO2 and HHCDs@TiO2 as a function of initial CR concentration under visible-light irradiation (60 min, unadjusted pH, optimum catalyst dosages). | ||
The superior performance of HHCDs@TiO2, particularly evident at intermediate concentrations (10–20 ppm), suggests that the carbon dot modification enhances the catalyst's ability to maintain active site accessibility and light absorption efficiency even in the presence of competing dye molecules.
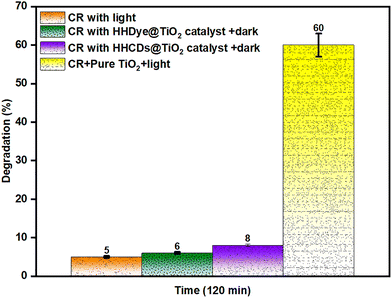
To assess the adsorption capacity of the photocatalysts, experiments were conducted in complete darkness using optimal dosages of HH dye@TiO2 and HHCDs@TiO2 with 10 ppm CR solutions. Both catalysts showed minimal activity, with degradation efficiencies of just 6% and 8%, respectively, after 120 minutes, demonstrating that adsorption plays a negligible role in the overall degradation process.
The performance of pure TiO2 under visible light irradiation resulted in 60% CR degradation, confirming that the TiO2 efficiency remains limited. The dark control experiment establishes that the observed dye removal is primarily due to photocatalytic degradation rather than adsorption.
The HH dye@TiO2 system achieved 98.6% degradation after 140 minutes, with the reaction kinetics showing substantial slowing beyond 120 minutes (96.8% degradation). In striking contrast, the HHCDs@TiO2 nanocomposite exhibited markedly faster degradation kinetics, reaching 97.1% efficiency in 80 minutes. The accompanying UV-vis spectra (Fig. 15 inset) corroborate these temporal trends, showing progressive diminution of the characteristic CR absorption peaks.
This dramatic enhancement in the photocatalytic performance of the HHCDs@TiO2 nanocomposite can be attributed to the synergistic effects of an expanded visible light absorption range, improved charge carrier separation efficiency, and an increased number of active surface sites.114,115 The HHCDs@TiO2 composite degrades the CR dye in approximately half the time required by the HH dye@TiO2 composite. This accelerated kinetics underscores the significant advantage of incorporating CDs into photocatalytic materials, which is particularly critical for time-sensitive wastewater treatment applications that demand rapid pollutant removal.
The photocatalytic systems exhibited maximum efficiency in strong acidic conditions (pH 4.17), achieving remarkable degradation rates of 99% for HH dye@TiO2 and 93.3% for HHCDs@TiO2 nano-composites. The enhanced performance of dye-doped TiO2 could be attributed to two reasons: (1) the protonation of TiO2 surface groups in acidic media creates a positive surface charge that promotes electrostatic attraction and subsequent adsorption of anionic CR molecules, and (2) the abundance of H+ ions facilitates the generation of reactive oxygen species, thereby accelerating the degradation process.116,117
As the pH increased towards alkaline conditions, we observed a substantial decrease in degradation efficiency, with minimum values of 12% (HH dye@TiO2) and 32.1% (HHCDs@TiO2) at pH 8.7. This dramatic reduction results from surface deprotonation of the photocatalysts, leading to negative surface charges that electrostatically repel the anionic dye molecules. Meanwhile, a slight increase in degradation was noted in the alkaline region, potentially due to hydroxyl radical-mediated oxidation.118 This effect was relatively insignificant compared to the dominant electrostatic interactions.
These findings demonstrate that the solution pH is a critical parameter controlling photocatalytic efficiency, with acidic to near-neutral conditions (pH 4.17–7) proving most favorable for anionic dye degradation. The superior performance of both photocatalysts in acidic media underscores the importance of surface charge characteristics and reactive species generation in the photocatalytic mechanism.
As demonstrated in Fig. 17, both photocatalysts exhibited excellent operational stability throughout the testing period. HHCDs@TiO2 showed particularly remarkable durability, with only a minimal reduction in degradation efficiency from 99% to 97% after five cycles. Similarly, HH dye@TiO2 maintained good stability, with its efficiency decreasing modestly from 97% to approximately 90% over the same number of cycles.
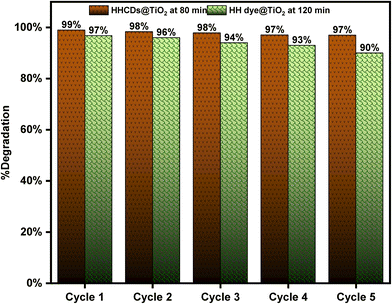 | ||
| Fig. 17 Cyclic stability test of the HH dye@TiO2 and HHCDs@TiO2 photocatalysts for CR degradation over five consecutive uses under visible light irradiation. | ||
The slight decline in performance observed for both materials can be attributed to several factors:119,120 (1) inevitable physical loss of the catalyst material during recovery and washing processes between cycles, (2) potential accumulation of degradation byproducts on active sites, and (3) possible gradual structural changes in the photocatalysts with repeated use. Notably, the superior retention of activity by HHCDs@TiO2 suggests that the carbon dot modification may provide enhanced protection against these degradation mechanisms.
These results confirm that both modified photocatalysts, particularly the carbon dot-enhanced HHCDs@TiO2, possess the durability required for sustainable water treatment applications. The maintained high performance over multiple cycles indicates excellent potential for long-term use in practical environmental remediation scenarios.
3.6 Mechanistic insights into photocatalytic degradation pathways
The photocatalytic degradation mechanism of Congo red was systematically investigated through radical scavenging experiments using both HH dye@TiO2 and HHCDs@TiO2 nanocomposites under visible light irradiation. Initial dark control experiments confirmed the essential light dependence of the process, with minimal degradation observed without illumination. To identify the active species responsible for dye degradation, we employed a series of specific scavengers targeting different reactive oxygen species and charge carriers (Table 3).| Reactive species | Scavengers | Equivalent | Degradation (%) using HH dye@TiO2 at 120 min | Degradation (%) using HHCDs@TiO2 at 90 min |
|---|---|---|---|---|
| Standard (no scavengers) | 97 | 99 | ||
| Dark | Trace | Trace | ||
| e− | AgNO3 | 2 | 33 | 27.5 |
| h+ | KI | 1 | 89.4 | 88.97 |
| 1O2 | L-Histidine | 1 | 27.9 | 36.6 |
| ˙OH | VC | 1 | 4.1 | 15.6 |
| ˙O2− | p-Bq | 1 | Trace | Trace |
The scavenging experiments revealed distinct patterns of reactivity for each photocatalyst system. When the electron scavenger (AgNO3) was introduced,121 the degradation efficiency dropped significantly to 33% for HH dye@TiO2 and 27% for HHCDs@TiO2, demonstrating the crucial role of photogenerated electrons in both systems. The addition of a hole scavenger (KI) caused more moderate reductions to 89.4% and 89.0%, respectively, indicating that while holes contribute to the process, their role is less dominant compared to electrons. These results collectively establish that charge separation and subsequent electron transfer represent key steps in the photocatalytic mechanism.
The investigation of reactive oxygen species provided particularly valuable insights into the degradation pathways. The dramatic suppression of degradation efficiency observed with hydroxyl radical scavenger (vitamin C), reducing activity to just 4.1% for HH dye@TiO2 and 15.6% for HHCDs@TiO2, unequivocally identifies ˙OH as the predominant oxidative species. This finding is complemented by the significant but less severe inhibition observed with singlet oxygen scavenger (L-histidine), which decreased degradation to 27.9% and 36.6%, respectively, confirming a secondary role for 1O2 in the process. In contrast, the minimal effect of superoxide scavenger (p-benzoquinone) suggests that ˙O2− radicals contribute negligibly to the overall degradation mechanism.
These experimental results support a proposed mechanism where visible light excitation generates electron–hole pairs in both photocatalyst systems. The photogenerated electrons participate in limited oxygen reduction, while the holes either directly oxidize dye molecules or react with surface hydroxyl groups/water to produce highly reactive ˙OH radicals.122 The HHCDs@TiO2 appears to facilitate a more balanced contribution from multiple reactive species (˙OH, e−, and 1O2), explaining its enhanced photocatalytic efficiency compared to HH dye@TiO2, where ˙OH radicals dominate the oxidation process. The superior performance of HHCDs@TiO2 likely stems from improved charge separation and additional reactive pathways enabled by the carbon dot modification.
Fig. 18 illustrates the proposed mechanistic pathways, beginning with light-induced excitation of electrons from the valence band (VB) to the conduction band (CB) or mid-gap states, generating electron–hole pairs. The photogenerated electrons participate in limited oxygen reduction to form ˙O2−, while the holes either directly oxidize dye molecules or react with surface-adsorbed water/hydroxyl groups to produce ˙OH radicals. These reactive species, along with 1O2, collectively mediate the oxidative degradation of Congo red into CO2 and H2O. The photocatalytic degradation of CR is a complex process that proceeds through the cleavage of the azo bonds, generating intermediate organic compounds such as benzidine derivatives, followed by further ring opening and eventual mineralization to CO2, H2O, and inorganic ions.123,124 The collective evidence from these experiments provides a robust foundation for understanding the enhanced photocatalytic behavior of these modified TiO2 systems and their potential applications in wastewater treatment.
4. Conclusion
In conclusion, this study successfully demonstrates the development of sustainable TiO2 nanocomposites modified with hollyhock-derived natural dye (HH dye) and carbon dots (HHCDs) for the efficient visible-light degradation of Congo red (CR). Comprehensive characterization confirmed the successful formation of the composites and their enhanced optoelectronic properties. A critical finding was the significant narrowing of the direct optical bandgap from 3.25 eV for pure TiO2 to 3.08 eV for HH dye@TiO2 and further to 2.89 eV for HHCDs@TiO2, which directly correlated with enhanced visible-light absorption. Photocatalytic performance under visible light revealed the superior activity of the HHCDs@TiO2 composite, which achieved 97.1% degradation of 10 ppm CR in just 80 minutes, outperforming the HH dye@TiO2 composite that required 120 minutes to reach 96.8% degradation.Systematic optimization identified the optimal catalyst dosage to be 0.06 g for HH dye@TiO2 and a lower, more efficient loading of 0.04 g for HHCDs@TiO2. The degradation efficiency was highly dependent on solution pH, with strongly acidic conditions (pH 4.17) proving most favorable, yielding remarkable degradation rates of 99% and 93.3% for HH dye@TiO2 and HHCDs@TiO2, respectively. Furthermore, both photocatalysts exhibited excellent operational stability, with the HHCDs@TiO2 nanocomposite showing exceptional reusability by retaining over 97% of its initial efficiency after five consecutive cycles. Mechanistic investigations through scavenging experiments unequivocally identified hydroxyl radicals as the predominant oxidative species, with the carbon dot modification in HHCDs@TiO2 facilitating more effective charge separation.
This work establishes a green protocol for valorizing hollyhock floral waste into high-performance photocatalysts. The direct comparison reveals that carbon dot modification offers superior benefits over the natural dye alone, particularly in enhancing reaction kinetics and charge separation. The HHCDs@TiO2 composite, with its rapid degradation kinetics, high efficiency, and remarkable stability, emerges as a highly promising and sustainable candidate for solar-driven wastewater treatment, advancing the principles of a circular economy and green chemistry.
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Data availability
Data will be made available on request.Acknowledgements
The authors thank the University of Sulaimani for its scientific support in accomplishing this research.References
- L. Wang, L. Zhao, D. Si, Z. Li, H. An, H. Ye, Q. Xin, H. Li and Y. Zhang, Sep. Purif. Technol., 2024, 331, 125571 CrossRef CAS.
- S. I. Siddiqui, E. S. Allehyani, S. A. Al-Harbi, Z. Hasan, M. A. Abomuti, H. K. Rajor and S. Oh, Processes, 2023, 11, 807 CrossRef CAS.
- V. Vaiano and I. De Marco, Separations, 2023, 10, 230 CrossRef CAS.
- A. Thakur, A. Kumar and A. Singh, Carbon, 2024, 217, 118621 CrossRef CAS.
- R. Ahmad and R. Kumar, Appl. Surf. Sci., 2010, 257, 1628–1633 CrossRef CAS.
- E. D’Souza, A. B. Fulke, N. Mulani, A. Ram, M. Asodekar, N. Narkhede and S. N. Gajbhiye, Environ. Earth Sci., 2017, 76, 721 CrossRef.
- S. Wafiroh, A. Abdulloh and A. A. Widati, Chem. Chem. Technol., 2021, 15, 291–298 CrossRef CAS.
- A. Ahmad, S. H. Mohd-Setapar, C. S. Chuong, A. Khatoon, W. A. Wani, R. Kumar and M. Rafatullah, RSC Adv., 2015, 5, 30801–30818 RSC.
- F. A. Zahrandika, S. Adityosulindro, S. N. Felia and K. Kusrestuwardhani, E3S Web Conf., 2024, 485, 02006 CrossRef CAS.
- R. A. El-Salamony, E. Amdeha, A. M. El Shafey and A. M. Al Sabagh, Int. J. Environ. Anal. Chem., 2023, 103, 868–883 CrossRef CAS.
- J. Zhang, P. Zhou, J. Liu and J. Yu, Phys. Chem. Chem. Phys., 2014, 16, 20382–20386 RSC.
- T. Chen, W.-L. Chen, B. J. Foley, J. Lee, J. P. C. Ruff, J. Y. P. Ko, C. M. Brown, L. W. Harriger, D. Zhang, C. Park, M. Yoon, Y.-M. Chang, J. J. Choi and S.-H. Lee, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, 7519–7524 CrossRef CAS PubMed.
- Q. Wang, J. Qiao and J. Zhou, ECS J. Solid State Sci. Technol., 2014, 3, Q157–Q161 CrossRef CAS.
- J. Wang, Z. Wang, W. Wang, Y. Wang, X. Hu, J. Liu, X. Gong, W. Miao, L. Ding, X. Li and J. Tang, Nanoscale, 2022, 14, 6709–6734 RSC.
- M. Ghosh, P. Chowdhury and A. K. Ray, Catalysts, 2020, 10, 917 CrossRef CAS.
- A. H. Zyoud, F. Saleh, M. H. Helal, R. Shawahna and H. S. Hilal, J. Nanomater., 2018, 2018, 1–14 CrossRef.
- C. Lin, Q. Xia, K. Li, J. Li and Z. Yang, J. Korean Phys. Soc., 2018, 72, 1307–1312 CrossRef CAS.
- G. H. Hamasalih, S. J. Mohammed and S. B. Aziz, J. Sol-Gel Sci. Technol., 2025, 116, 1142–1166 CrossRef CAS.
- Y. Tanaka, N. Sasaki and A. Ohmiya, Plant J., 2008, 54, 733–749 CrossRef CAS.
- S. B. Aziz, D. M. Aziz, D. S. Muhammad, P. O. Hama, D. Q. Muheddin, S. Al-Zangana, A. M. Huseein, G. H. Hamasalih, A. H. A. Darwesh and O. G. Abdullah, J. Inorg. Organomet. Polym. Mater., 2025, 35, 2109–2125 CrossRef CAS.
- Z. Ma, H. Ming, H. Huang, Y. Liu and Z. Kang, New J. Chem., 2012, 36, 861 RSC.
- W. Wang, L. Cheng and W. Liu, Sci. China: Chem., 2014, 57, 522–539 CrossRef CAS.
- D. Bhattacharya, M. K. Mishra and G. De, J. Phys. Chem. C, 2017, 121, 28106–28116 CrossRef CAS.
- A. Emanuele, S. Cailotto, C. Campalani, L. Branzi, C. Raviola, D. Ravelli, E. Cattaruzza, E. Trave, A. Benedetti, M. Selva and A. Perosa, Molecules, 2019, 25, 101 CrossRef.
- B. Zhang, H. Maimaiti, D.-D. Zhang, B. Xu and M. Wei, J. Photochem. Photobiol., A, 2017, 345, 54–62 CrossRef CAS.
- H. Zhang, H. Huang, H. Ming, H. Li, L. Zhang, Y. Liu and Z. Kang, J. Mater. Chem., 2012, 22, 10501 RSC.
- C. Praharaj, Saloni, G. K. Patel and S. Nara, Ind. Crops Prod., 2025, 230, 121074 CrossRef CAS.
- S. Raja, G. T. S. T. da Silva, S. Anbu, C. Ribeiro and L. H. C. Mattoso, Biomass Convers. Biorefin., 2024, 14, 21925–21937 CrossRef CAS.
- H. Radi, K. F. El-Nemr, S. M. Elmesallamy and E. Amdeha, Pigm. Resin Technol., 2025, 54, 561–570 CrossRef CAS.
- E. Amdeha, Topics in Mining, Metallurgy and Materials Engineering, Springer Science and Business Media Deutschland GmbH, 2021, pp. 385–417 Search PubMed.
- E. Amdeha, Diversity and Applications of New Age Nanoparticles, IGI Global, 2023, pp. 112–154 Search PubMed.
- J. Fan, L. Kang, X. Cheng, D. Liu and S. Zhang, Nanomaterials, 2022, 12, 4473 CrossRef CAS PubMed.
- G. Náthia-Neves, Á. L. Santana, J. Viganó, J. Martínez and M. A. A. Meireles, Processes, 2021, 9, 1435 CrossRef.
- A. Ghorbani, G. Eghlima, M. Farzaneh and A. Rezghiyan, BMC Plant Biol., 2025, 25, 478 CrossRef CAS PubMed.
- N. F. S. Daud, F. M. Said, M. Ramu and N. M. H. Yasin, IOP Conf. Ser.:Mater. Sci. Eng., 2020, 736, 022084 CAS.
- K. Saeed, I. Khan, T. Gul and M. Sadiq, Appl. Water Sci., 2017, 7, 3841–3848 CrossRef CAS.
- M. Grabolle, M. Spieles, V. Lesnyak, N. Gaponik, A. Eychmüller and U. Resch-Genger, Anal. Chem., 2009, 81, 6285–6294 CrossRef CAS.
- N. A. Abdel-salam, N. M. Ghazy, S. M. Sallam, M. M. Radwan, A. S. Wanas, M. A. ElSohly, M. A. El-Demellawy, N. M. Abdel-Rahman, S. Piacente and M. L. Shenouda, Nat. Prod. Res., 2018, 32, 702–706 CrossRef CAS PubMed.
- A. Safari, M. Rahimi, A. Sonboli, H. Behboudi and S. Nejad Ebrahimi, Ind. Crops Prod., 2024, 222, 119944 CrossRef CAS.
- A. M. Babatimehin, O. E. Ogunbamowo, G. O. Ajayi, A. El Gamal, T. Bin Emran, E. A. Ofudje and M. Hefnawy, BioResources, 2025, 20, 6948–6965 CAS.
- S. B. Aziz, D. S. Muhammad, S. J. Mohammed, D. Q. Muheddin, S. Al-Zangana, A. M. Hussein, A. R. Murad, G. H. Hamasalih, S. M. Hamad and D. Shaikhah, J. Mater. Sci.:Mater. Eng., 2025, 20, 86 Search PubMed.
- A. Ebrahiminezhad, V. Varma, S. Yang, Y. Ghasemi and A. Berenjian, Nanomaterials, 2015, 6, 1 CrossRef PubMed.
- Y. Zhang, L. Jin, Q. Chen, Z. Wu, Y. Dong, L. Han and T. Wang, Fitoterapia, 2015, 102, 7–14 CrossRef CAS PubMed.
- A. Ebrahiminezhad, Y. Barzegar, Y. Ghasemi and A. Berenjian, Chem. Ind. Chem. Eng. Q., 2017, 23, 31–37 CrossRef CAS.
- M. A. Hasan, I. M. M. Rahman, M. R. Hossain and F. I. Chowdhury, Chem. Phys. Impact, 2025, 10, 100777 CrossRef.
- S. Pandit, P. Behera, J. Sahoo and M. De, ACS Appl. Bio Mater., 2019, 2, 3393–3403 CrossRef CAS.
- A. Ebrahiminezhad, Y. Barzegar, Y. Ghasemi and A. Berenjian, Chem. Ind. Chem. Eng. Q., 2017, 23, 31–37 CrossRef CAS.
- D. M. Aziz, S. J. Mohammed, P. A. Mohammed, S. Al-Zangana, S. B. Aziz, D. S. Muhammad, R. T. Abdulwahid, A. H. A. Darwesh and S. A. Hussein, Spectrochim. Acta, Part A, 2025, 325, 125142 CrossRef CAS.
- O. K. Hamaamin, H. O. Ghareeb and S. J. Mohammed, Sci. Rep., 2025, 15, 28891 CrossRef CAS PubMed.
- M. A. Mousa, H. H. Abdelrahman, M. A. Fahmy, D. G. Ebrahim and A. H. E. Moustafa, Sci. Rep., 2023, 13, 12863 CrossRef CAS PubMed.
- B. De and N. Karak, RSC Adv., 2013, 3, 8286 RSC.
- B. de Campos Vidal and M. L. S. Mello, Micron, 2011, 42, 283–289 CrossRef.
- F. Dai, Q. Zhuang, G. Huang, H. Deng and X. Zhang, ACS Omega, 2023, 8, 17064–17076 CrossRef CAS PubMed.
- M. Boumediene, B. Haddad, A. Paolone, M. A. Assenine, D. Villemin, M. Rahmouni and S. Bresson, J. Mol. Struct., 2020, 1220, 128731 CrossRef CAS.
- L. Yang, P. W. May, L. Yin, J. A. Smith and K. N. Rosser, J. Nanopart. Res., 2007, 9, 1181–1185 CrossRef CAS.
- A. F. Shaikh, M. S. Tamboli, R. H. Patil, A. Bhan, J. D. Ambekar and B. B. Kale, J. Nanosci. Nanotechnol., 2019, 19, 2339–2345 CrossRef CAS PubMed.
- A. A. Nerantzaki, C. G. Tsiafoulis, P. Charisiadis, V. G. Kontogianni and I. P. Gerothanassis, Anal. Chim. Acta, 2011, 688, 54–60 CrossRef CAS.
- B. Zghari, P. Doumenq, A. Romane and A. Boukir, J. Mater. Environ. Sci., 2017, 8, 4496–4509 CAS.
- E. Alexandri, R. Ahmed, H. Siddiqui, M. Choudhary, C. Tsiafoulis and I. Gerothanassis, Molecules, 2017, 22, 1663 CrossRef.
- S. Upadhyayula, D. Bao, B. Millare, S. S. Sylvia, K. M. M. Habib, K. Ashraf, A. Ferreira, S. Bishop, R. Bonderer, S. Baqai, X. Jing, M. Penchev, M. Ozkan, C. S. Ozkan, R. K. Lake and V. I. Vullev, J. Phys. Chem. B, 2011, 115, 9473–9490 CrossRef CAS.
- R. Munir, N. Javid, M. Zia-ur-Rehman, M. Zaheer, R. Huma, A. Roohi and M. M. Athar, Molecules, 2021, 26, 4908 CrossRef CAS.
- B. De and N. Karak, RSC Adv., 2013, 3, 8286 RSC.
- Z. O. Oyman, W. Ming and R. van der Linde, Eur. Polym. J., 2006, 42, 1342–1348 CrossRef CAS.
- Y. Yang, J. Hou, D. Huo, X. Wang, J. Li, G. Xu, M. Bian, Q. He, C. Hou and M. Yang, Microchim. Acta, 2019, 186, 259 CrossRef.
- M. Vedamalai, A. P. Periasamy, C.-W. Wang, Y.-T. Tseng, L.-C. Ho, C.-C. Shih and H.-T. Chang, Nanoscale, 2014, 6, 13119–13125 RSC.
- K. F. Kayani and C. N. Abdullah, J. Fluoresc., 2024, 35, 1125–1137 CrossRef.
- S. J. Mohammed, F. E. Hawaiz, S. B. Aziz and S. H. Al-Jaf, Opt. Mater., 2024, 149, 115014 CrossRef CAS.
- A. McEnroe, E. Brunt, N. Mosleh, J. Yu, R. Hailstone and X. Sun, Talanta Open, 2023, 7, 100236 CrossRef.
- F. R. U. Cortes, E. Falomir, J. Lancis and G. Mínguez-Vega, Appl. Surf. Sci., 2024, 665, 160326 CrossRef CAS.
- H. Liu, X. Lv, C. Li, Y. Qian, X. Wang, L. Hu, Y. Wang, W. Lin and H. Wang, Nanoscale, 2020, 12, 10956–10963 RSC.
- H. Yu, Y. Xiang, K. Wu, D. He, X. Chai, L. Xu, Y. Cheng, X. Duan and W. Li, LWT, 2024, 208, 116744 CrossRef CAS.
- Y.-F. Kang, Y.-H. Li, Y.-W. Fang, Y. Xu, X.-M. Wei and X.-B. Yin, Sci. Rep., 2015, 5, 11835 CrossRef.
- K. F. Kayani and C. N. Abdullah, J. Fluoresc., 2024, 35, 1125–1137 CrossRef.
- Y. Hao, Y. Song, T. Li, Y. Tuo, M. Tian and F. Chai, J. Environ. Chem. Eng., 2023, 11, 109863 CrossRef CAS.
- A.-M. Alam, B.-Y. Park, Z. K. Ghouri, M. Park and H.-Y. Kim, Green Chem., 2015, 17, 3791–3797 RSC.
- J. V. Rojas, M. Toro-Gonzalez, M. C. Molina-Higgins and C. E. Castano, Mater. Sci. Eng., B, 2016, 205, 28–35 CrossRef CAS.
- H. Miao, Y. Wang and X. Yang, Nanoscale, 2018, 10, 8139–8145 RSC.
- H. Guo, Y. Lu, Z. Lei, H. Bao, M. Zhang, Z. Wang, C. Guan, B. Tang, Z. Liu and L. Wang, Nat. Commun., 2024, 15, 4843 CrossRef CAS.
- B. Murugesan, J. Sonamuthu, N. Pandiyan, B. Pandi, S. Samayanan and S. Mahalingam, J. Photochem. Photobiol., B, 2018, 178, 371–379 CrossRef CAS PubMed.
- M. Vedamalai, A. P. Periasamy, C.-W. Wang, Y.-T. Tseng, L.-C. Ho, C.-C. Shih and H.-T. Chang, Nanoscale, 2014, 6, 13119–13125 RSC.
- K. Jiang, S. Sun, L. Zhang, Y. Wang, C. Cai and H. Lin, ACS Appl. Mater. Interfaces, 2015, 7, 23231–23238 CrossRef CAS.
- N. Kim, J. Lee, M. Gu and B. Kim, Carbon Energy, 2021, 3, 590–614 CrossRef CAS.
- M. Tavan, Z. Yousefian, Z. Bakhtiar, M. Rahmandoust and M. H. Mirjalili, Ind. Crops Prod., 2025, 231, 121207 CrossRef CAS.
- M. H. Zare and A. Mehrabani-Zeinabad, Sci. Rep., 2022, 12, 10388 CrossRef CAS.
- S. El-Sherbiny, F. Morsy, M. Samir and O. A. Fouad, Appl. Nanosci., 2014, 4, 305–313 CrossRef CAS.
- S. Hosseinpour, F. Tang, F. Wang, R. A. Livingstone, S. J. Schlegel, T. Ohto, M. Bonn, Y. Nagata and E. H. G. Backus, J. Phys. Chem. Lett., 2017, 8, 2195–2199 CrossRef CAS PubMed.
- S. Pasieczna-Patkowska, M. Cichy and J. Flieger, Molecules, 2025, 30, 684 CrossRef CAS.
- L. S. Chougala, M. S. Yatnatti, R. K. Linganagoudar, R. R. Kamble and J. S. Kadadevarmath, J. Nano- Electron. Phys., 2017, 9, 04005-1–04005-6 CrossRef.
- N. P. Lata, M. S. Hussain, M. Abdulla-Al-Mamun, T. U. Rashid and S. M. Shamsuddin, Heliyon, 2024, 10, e29255 CrossRef CAS.
- E. A. Al-Oubidy and F. J. Kadhim, Opt. Quantum Electron., 2019, 51, 23 CrossRef.
- B.-C. Bui, N.-N. Vu, H.-E. Nemamcha, H. T. Nguyen, V.-A. Nguyen and P. Nguyen-Tri, J. Water Process Eng., 2025, 70, 106904 CrossRef.
- S. S. Ghumro, B. Lal and T. Pirzada, ACS Omega, 2022, 7, 4333–4341 CrossRef CAS PubMed.
- A. I. Kontos, I. M. Arabatzis, D. S. Tsoukleris, A. G. Kontos, M. C. Bernard, D. E. Petrakis and P. Falaras, Catal. Today, 2005, 101, 275–281 CrossRef CAS.
- R. Liu, H. Li, L. Duan, H. Shen, Y. Zhang and X. Zhao, Ceram. Int., 2017, 43, 8648–8654 CrossRef CAS.
- R. M. S. Sendão, M. Algarra, J. Lázaro-Martínez, A. T. S. C. Brandão, A. Gil, C. Pereira, J. C. G. Esteves da Silva and L. Pinto da Silva, Colloids Surf., A, 2025, 713, 136475 CrossRef.
- X. Zhang, S. Lu, D. He, M. Chai, Z. Wu, X. Yao and Y. Yang, Trans. Nonferrous Met. Soc. China, 2023, 33, 2395–2405 CrossRef CAS.
- H. Huang, H. Ouyang, T. Han, H. Wang and X. Zheng, RSC Adv., 2019, 9, 3532–3541 RSC.
- X. Zeng, Z. Wang, N. Meng, D. T. McCarthy, A. Deletic, J. Pan and X. Zhang, Appl. Catal., B, 2017, 202, 33–41 CrossRef CAS.
- A. Mozdbar, A. Nouralishahi, S. Fatemi and F. S. Talatori, J. Water Process Eng., 2023, 51, 103465 CrossRef.
- J. Chen, J. Shu, Z. Anqi, H. Juyuan, Z. Yan and J. Chen, Diamond Relat. Mater., 2016, 70, 137–144 CrossRef CAS.
- J. Tauc, R. Grigorovici and A. Vancu, Phys. Status Solidi B, 1966, 15, 627–637 CrossRef CAS.
- M. M. A. El Raheem, M. M. Wakkad, H. A. Mohamed, N. A. Hamed and H. F. Mohamed, J. Mater. Sci.:Mater. Eng., 2025, 20, 95 Search PubMed.
- P. Kubelka and F. Munk, Z. Tech. Phys., 1931, 12, 193 Search PubMed.
- R. López and R. Gómez, J. Sol-Gel Sci. Technol., 2012, 61, 1–7 CrossRef.
- H. F. Haneef, A. M. Zeidell and O. D. Jurchescu, J. Mater. Chem. C, 2020, 8, 759–787 RSC.
- K. Jarzynska, K. Ciura, X. J. Gao, A. Mikolajczyk, X. Gao and T. Puzyn, Nano Today, 2025, 64, 102783 CrossRef CAS.
- D. A. Kader, S. A. Abdalla, S. J. Mohammed, D. M. Aziz, D. D. Ghafoor, T. M. Abdullah, N. N. M. Agha, F. S. Mustafa and S. A. Hassan, J. Photochem. Photobiol., A, 2025, 462, 116253 CrossRef CAS.
- S. Albukhaty, L. Al-Bayati, H. Al-Karagoly and S. Al-Musawi, Anim. Biotechnol., 2022, 33, 864–870 CrossRef CAS PubMed.
- N. Daneshvar, D. Salari and A. R. Khataee, J. Photochem. Photobiol., A, 2003, 157, 111–116 CrossRef CAS.
- I. K. Konstantinou and T. A. Albanis, Appl. Catal., B, 2004, 49, 1–14 CrossRef CAS.
- A. Rafiq, M. Ikram, S. Ali, F. Niaz, M. Khan, Q. Khan and M. Maqbool, J. Ind. Eng. Chem., 2021, 97, 111–128 CrossRef CAS.
- M. B. K. Suhan, M. R. Al-Mamun, N. Farzana, S. M. Aishee, M. S. Islam, H. M. Marwani, M. M. Hasan, A. M. Asiri, M. M. Rahman, A. Islam and M. R. Awual, Nano-Struct. Nano-Objects, 2023, 36, 101050 CrossRef CAS.
- N. Ramesh, C. W. Lai, M. R. Bin Johan, S. M. Mousavi, I. A. Badruddin, A. Kumar, G. Sharma and F. Gapsari, Heliyon, 2024, 10, e40998 CrossRef CAS.
- N. Kim, J. Lee, M. Gu and B. Kim, Carbon Energy, 2021, 3, 590–614 CrossRef CAS.
- F. Zhao, Y. Rong, J. Wan, Z. Hu, Z. Peng and B. Wang, Catal. Today, 2018, 315, 162–170 CrossRef CAS.
- C. Guillard, H. Lachheb, A. Houas, M. Ksibi, E. Elaloui and J.-M. Herrmann, J. Photochem. Photobiol., A, 2003, 158, 27–36 CrossRef CAS.
- K. Chen, W. Dong, Y. Huang, F. Wang, J. L. Zhou and W. Li, J. Environ. Chem. Eng., 2025, 13, 117529 CrossRef CAS.
- I. K. Konstantinou and T. A. Albanis, Appl. Catal., B, 2004, 49, 1–14 CrossRef CAS.
- J. Trakulmututa, C. Chuaicham, A. Srikhaow and K. Sasaki, Sustainable Mater. Technol., 2024, 42, e01129 CrossRef CAS.
- Y. Lin, D. Li, J. Hu, G. Xiao, J. Wang, W. Li and X. Fu, J. Phys. Chem. C, 2012, 116, 5764–5772 CrossRef CAS.
- M. Umair, C. M. Pecoraro, F. Di Franco, M. Santamaria, L. Palmisano, V. Loddo and M. Bellardita, Sustainable Mater. Technol., 2025, 43, e01188 CrossRef CAS.
- N. Ramesh, C. W. Lai, M. R. Bin Johan, S. M. Mousavi, I. A. Badruddin, A. Kumar, G. Sharma and F. Gapsari, Heliyon, 2024, 10, e40998 CrossRef CAS PubMed.
- E. Palma Soto, C. A. Rodriguez Gonzalez, P. A. Luque Morales, H. Reyes Blas and A. Carrillo Castillo, Catalysts, 2024, 14, 589 CrossRef CAS.
- G. E. Quintanilla-Villanueva, A. Sicardi-Segade, D. Luna-Moreno, R. E. Núñez-Salas, J. F. Villarreal-Chiu and M. M. Rodríguez-Delgado, Catalysts, 2025, 15, 84 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2026 |