Open Access Article
Open Access ArticleShrimp shell-derived chito-protein nanocomposites for sustainable dye effluent remediation: efficiency, reusability, and environmental safety
Nisrine
Nouj
 *a,
Zineb
Majbar
b,
Ingrid Ioana
Buciscanu
c,
Aboubakr
Ben Hamou
*a,
Zineb
Majbar
b,
Ingrid Ioana
Buciscanu
c,
Aboubakr
Ben Hamou
 a,
Ayoub
Chaoui
a,
Mohamed Rida
Abelouah
d,
Mohamed
Idbella
e,
Abdelaziz
Ait Addi
f,
Nadia
Eladlani
g,
Ali
Zourif
a,
Ayoub
Chaoui
a,
Mohamed Rida
Abelouah
d,
Mohamed
Idbella
e,
Abdelaziz
Ait Addi
f,
Nadia
Eladlani
g,
Ali
Zourif
 h,
Mohamed
Benafqir
h,
Mohamed
Benafqir
 a,
Naima
Hafid
a,
Igor
Cretescu
i,
Amane
Jada
j and
Noureddine
El Alem
a
a,
Naima
Hafid
a,
Igor
Cretescu
i,
Amane
Jada
j and
Noureddine
El Alem
a
aLaboratory of Materials and Environment (LME), Faculty of Sciences, Ibn Zohr University, Agadir, Morocco. E-mail: n.nouj@uiz.ac.ma; nouj.nisrine@gmail.com
bEngineering, Electrochemistry and Modeling Environment Laboratory (LEEME), Faculty of Sciences, Sidi Mohamed Ben Abdellah University, Fez 30000, Morocco
cDepartment of Chemical Engineering in Textiles and Leather, Faculty of Industrial Design and Business Management, “Gheorghe Asachi” Technical University of Iasi, Iasi 700050, Romania
dLaboratory of Aquatic Systems: Marine and Continental Environments, Faculty of Sciences, Ibn Zohr University, Agadir 80000, Morocco
eCollege of Agriculture and Environmental Sciences, AgroBioSciences (AgBS) program, Mohammed VI Polytechnic University, Ben Guerir 43150, Morocco
fPhysical Chemistry & Environment Team, Faculty of Sciences, Ibn Zohr University, Agadir, Morocco
gInterdisciplinary Laboratory in Bio-Resources, Environment and Materials, Higher Normal School, Cadi Ayyad University, 4000 Marrakech, Morocco
hLaboratory of Physical-Chemistry, Materials and Catalysis, Faculty of Sciences Ben M'sick, Hassan II University of Casablanca, Morocco
iDepartment of Environmental Engineering and Management, Faculty of Chemical Engineering and Environmental Protection, “Gheorghe Asachi” Technical University of Iasi, Iasi 700050, Romania
jInstitute of Materials Science of Mulhouse (IS2M), High Alsace University, Mulhouse 68100, France
First published on 23rd December 2025
Abstract
The transformation of seafood processing residues into advanced functional materials offers a dual solution to environmental pollution: mitigating waste streams while addressing water contamination. In this study, shrimp exoskeletons were valorized into a chitin–protein composite (SE-CP) through acid demineralization and thermal activation and evaluated as a biosorbent for the removal of anionic textile dyes Sellacid Red (SR) and Sellaset Blue (SB). The material was characterized using SEM, EDX, FTIR, XRD, BET, DLS, XPS, and PZC analyses, confirming a mesoporous structure (specific surface area = 51.4914 m2 g−1) enriched with amino and hydroxyl groups that favor electrostatic and hydrogen-bonding interactions. Batch adsorption studies showed maximum removal efficiencies of 99.2% for SR at pH = 3 and 98.7% for SB at pH = 4 both around 20 °C and an initial dye concentration of 100 mg L−1. Kinetic data fitted the pseudo-second-order model (R2 > 0.96), and equilibrium was best described by the Freundlich isotherm, with adsorption capacities of 158.43 mg g−1 (SR) and 63.81 mg g−1 (SB). SE-CP retained over 76% of its adsorption capacity after five regeneration cycles, indicating strong stability and reusability. This work demonstrates a low-cost and sustainable biosorbent derived from shrimp waste, with high efficiency, reusability, and green synthesis, positioning SE-CP as a promising candidate for industrial dye wastewater treatment within circular economy principles.
1. Introduction
Global socio-economic development and population growth over the last few decades have led to an increase in the quantity and qualitative complexity of special wastes generated by the industrial sector.1,2 However, the global fishing industry generates over 20 million tons of by-products every year, almost all of which is simply wasted.3,4 After processing, 25% of the total ends up as waste, including fish species that were not intended to be caught or other remnants of industrial fish processing.3,5 These marine by-products are generally discarded without taking into account their precious compositions which can be used as value-added products. A large number of fish processing units generate both a large quantity of co-products (bones, skins, scales, fins and swim bladders, which represent 36%, or even 60%, of the mass of raw materials3,6) and a large quantity of untreated wastewater.7,8 Therefore, recovering this waste and transforming it into valuable products are becoming important ways leading to solid waste remediation and taking advantage of the marine products and bio-resources.It should be noted that chitin is an abundant biological polymer, particularly in marine animals and insects.9,10 The crystalline chitin fiber has good properties that enable it to serve as a load-bearing scaffold for the entire bio-composite material, and the other component is usually a protein.11 The remarkable affinity occurring between chitin and proteins is probably one of the main reasons explaining the presence of such a composite in nature.12 Many biological materials, including the exoskeleton of sponges, the cuticles of crustaceans and insects, the beaks of squid, cuttlefish bones and the fangs of spiders, are made up of chitin and protein complexes.13 These chitin–protein complexes exhibit a variety of mechanical behaviours and perform multiple functions, depending on the species and even the different body parts of the same species.14 The use of the chito-protein complex as a biosorbent remains a challenge, given that chitosan is the main biological substance in these fields. However, chitosan exhibits a little solubility in water, under neutral or alkaline conditions. These factors limit the biopolymer feasibility, in addition to its complicated extraction process.15,16 Hence, there is a need to find alternative ways to minimize the biopolymer extraction process and preserve its contaminant-removal properties, particularly for dyes.
The most widespread contaminants found in the textile industry are the cheapest dyes, which are harmful to human health and contribute to environment degradation.17 This low cost of these dyes results from their easy synthesis process, mainly for azo dyes, and the possibility of obtaining a wide palette of colors and shades.18 Thus, despite their toxicity, little research has been carried out taking into account this issue. Hence, Acid red 337 (Sellacid Red PF) and the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 chromium complex of Acid Blue 349 (Sellaset Blue H) belonging to this group of azo dyes are widely used in the textile industry. Furthermore, Acid red 337 has been registered by the European Chemicals Agency (ECHA) as an organic compound harmful to the aquatic environment, and Acid Blue 349 presents similar risks. Therefore, their treatment by using low-cost biosorbents based on fish waste will reduce the risk to human health and avoid the environmental issue.
2 chromium complex of Acid Blue 349 (Sellaset Blue H) belonging to this group of azo dyes are widely used in the textile industry. Furthermore, Acid red 337 has been registered by the European Chemicals Agency (ECHA) as an organic compound harmful to the aquatic environment, and Acid Blue 349 presents similar risks. Therefore, their treatment by using low-cost biosorbents based on fish waste will reduce the risk to human health and avoid the environmental issue.
In the present work, shrimp shells were modified for their use as a biosorbent to remove hazardous low-cost dyes from aqueous solutions. The focus was on the powder, in order to assess its effectiveness in removing two categories of dyes: low- and high-molecular-weight anionic dyes. The shrimp shell modification consisted of the powder extraction after the raw by-product demineralization process. Regarding the toxic dyes, aqueous solutions containing two different anionic dyes were treated by using the bio-based sorbent, in batch equilibrium biosorption tests. A combination of techniques was used to study biosorption mechanisms.
The aim of the present work is to prepare a value-added, bio-based biosorbent suitable for the dye-contaminated effluent treatment. Thus, a biosorbent from renewable resources, offering excellent dye sorption and desorption performance, even at high dye concentrations, for repeated, high-speed dye removal, was developed. Indeed, the objective of our study falls within the framework of three Sustainable Development Goals (SDGs): No. 6, No. 14 and No. 15: improve wastewater treatment and reuse (No. 6) and protect the aquatic environment (No. 14) and the terrestrial environment (No. 15) from persistent chemical substances present in wastewater after treatment with reclaimed marine waste.
2. Materials and experimental methods
2.1. Chemicals
HCl, NaNO3, HNO3 and NaOH were of analytical grade and purchased from Sigma Aldrich. Sellacid Red PF and Sellaset Blue H were sourced from TFL Ledertechnik GmbH, Germany. Distillated water (DW) was used for all aqueous solutions.2.2. Preparation of the biosorbent
Shrimp (Parapenaeus longirostris) exoskeletons were purchased from a local market in Agadir city, Morocco. The exoskeletons were first washed with tap water and then several times with distilled water to remove any foreign debris. The resulting shells were dried at 220 °C for 15 min, ground and sieved to a size of about 500 µm. Thereafter, the resulting material was treated with 1 M HCl in a shrimp shell to HCl solution ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (w/v) for 30 min to remove the mineral constituent (calcite CaCO3). The obtained biosorbent (SE-CP) was washed and filtered to a neutral pH, then dried at 105 °C to constant weight and finally stored for use.
10 (w/v) for 30 min to remove the mineral constituent (calcite CaCO3). The obtained biosorbent (SE-CP) was washed and filtered to a neutral pH, then dried at 105 °C to constant weight and finally stored for use.
2.3. Sorbate investigation
Two commercial dyestuffs, Sellacid Red PF and Sellaset Blue H (TFL Ledertechnik GmbH, Germany) currently used for wool and leather dyeing, were the adsorbates studied in this paper. The structural formulas, molecular weights, maximum absorbance wavelengths, and commercial and C.I. names of the two acid dyes are given in Table 1. A stock solution of each dye (1000 mg L−1) was prepared and diluted to the initial concentrations required for the sorption and kinetics studies. Hydrochloric acid (0.1 M) and sodium hydroxide (0.1 M) were used to adjust the pH of the solutions. All solutions were prepared with DW, and the handled reagents were of analytical grade.2.4. Biosorbent characterization
Various characterization methods were used to assess the morphology, structure, composition and sorption properties of SE-CP. Thus, the morphological characteristics were obtained using scanning electron microscopy (SEM) (SEM, JEOL, JSM-IT200, Akishima, Tokyo, Japan). The elemental composition was determined using energy dispersive X-ray (EDX) analysis. Fourier transform infra-red (FTIR) spectroscopy spectra were recorded in the 4000–500 (cm−1) range using attenuated total reflection Fourier transform infrared spectroscopy (ATR-FTIR, SHIMADZU, IRAffinity-1S, Paris, France). The crystalline structure of the biosorbent was determined by using X-ray diffraction (Bruker D8 Twin, Jena, Germany). The biosorbent specific surface area and the mean pore volume were assessed by the Brunauer–Emmett–Teller (BET) adsorption method using a Micrometrics analyzer, an ASAP 2020 instrument. X-ray photoelectron spectroscopy (XPS VG SCIENTA, model SES-200) was used to assess the structure and composition of the studied biosorbent.2.5. Properties of aqueous biosorbent suspension
2.6. Batch sorption experiments
Batch equilibrium sorption experiments were performed as a function of various parameters such as the dye concentration, the biosorbent dosages, the aqueous phase pH, the adsorbate–adsorbent contact time, and the suspension temperature.Specifically, the biosorption experiment was conducted in a set of glass vials containing different initial dye concentrations (25, 50, 75, 100, 125, 150, 200, 250, 300, and 350 mg L−1) at different pH values (2–12) using different biosorbent dosages (0.02, 0.04, 0.06, 0.08, 0.1, 0.12, 0.14, 0.16, 0.18, 0.2 g) for periods of 15, 30, 60, 120, and 240 min, and temperature values of 20, 30 and 40 °C. Also, the pH was adjusted to the desired values with 0.1 mol L−1 HCl or 0.1 mol L−1 NaOH solutions. However, the measurements before and after the process were taken using a pH meter (OHAUS Starter 2100 pH meter).
After treatment, the supernatant was withdrawn after 24 h of decantation to measure the residual concentration of both dyes in the solution using an UV-Vis spectrophotometer (U-2910, Hitachi, USA). Standard curves of absorbance and linear regression equations had been previously obtained for both colorants. Each kinetic experiment was performed for each dye solution in triplicate. The biosorption capacity for each time interval was calculated using eqn (1):
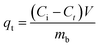 | (1) |
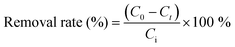 | (2) |
2.7. Biosorption theory
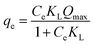 | (3) |
A multilayer formation of the solid sorbate on the sorbent surface requires an application of the Freundlich isotherm. At the same time, the model is formulated in an exponential form as shown in the following equation:21
| qe = KFCe1/n | (4) |
The Temkin isotherm shows whether the sorption phenomenon is based on a linkage factor with an assumption of decreasing biosorption heat with increasing sorbent surface coverage by sorbate particles. The model has a nonlinear form, as presented in the following equation:21
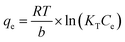 | (5) |
| qt = qe,the(1 − expk1,t) | (6) |
The pseudo-first-order kinetic model is described by a second-order rate equation as specified and represented in the following equation:21
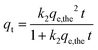 | (7) |
The kinetic model for intra-particle diffusion is formulated based on internal diffusion which is the rate limiting step with an account for external mass transfer effects. The model is described by the following equation:21
| qt = kdt1/2 + Q0 | (8) |
Three thermodynamic parameters were selected using the proper biosorption capacity (qe) and the experimental equilibrium solution concentration (Ce). Gibb's free energy (ΔG), the change in enthalpy (ΔH) and the change in entropy (ΔS) were obtained using the following equations:21
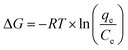 | (9) |
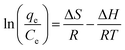 | (10) |
At constant temperature, the ΔG is calculated as a function of the heat of biosorption and the entropy change using the following equation:
| ΔG = ΔH − TΔS | (11) |
2.8. Biosorbent regeneration experiments
Following sorption equilibrium, regeneration of the biomaterial used was carried out with two desorption agents: distilled water (DW) and NaOH (0.1 M), in order to assess their desorption efficiency and the reusability of the dye-loaded biosorbent. The experimental regeneration protocol involves immersing the loaded adsorbent in NaOH or DW (0.1 g/100 mL) for 4 hours, followed by a DW wash. Then, the regenerated SE-CP was dried in an oven at 105 °C. Finally, the regenerated materials (SE-CP@OH and SE-CP@DW) were reused for further biosorption tests. The regeneration process was repeated several times to achieve complete dye removal. SE-CP@OH and SE-CP@DW were thoroughly washed with distilled water to neutral pH and then reused in biosorption experiments four times using the same biosorbents. According to data, sorbent regeneration mass yield was calculated to assess the durability of the material used, which can be calculated using equation:22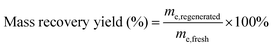 | (12) |
3. Results and discussion
3.1. Full characterization of SE-CP
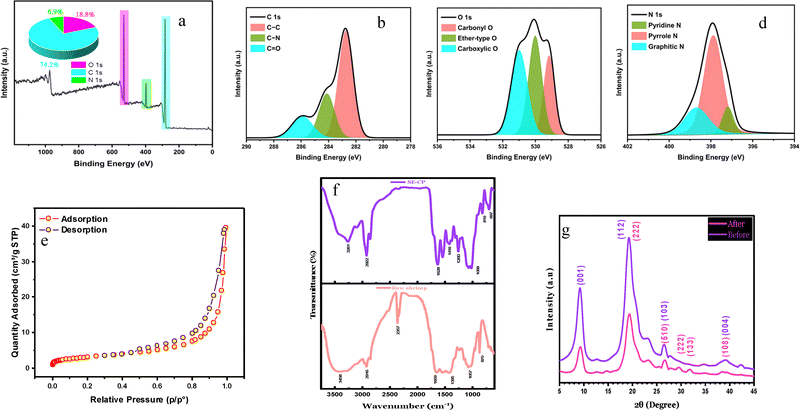
The XPS wide-scan results (Fig. 1(a)) indicate a high carbon content of 74.2%, consistent with chitin being the primary biopolymer in the material. Oxygen and nitrogen were detected at 18.8% and 6.9%, respectively, due to the presence of primary and secondary amides. To further investigate the chemical binding states of these elements, high-resolution XPS spectra were analyzed. The carbon spectrum (Fig. 1(b)) was deconvoluted into three peaks at 284.8 eV, 284.13 eV, and 285.92 eV, corresponding to C–C/C–H, C–N, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, respectively.23 However, we acknowledge the reviewer's concern regarding the calibration of binding energy (BE). While a BE of 284.8 eV is commonly assigned to C–C/C–H bonds, the shift in our data may stem from surface charging effects or the specific nature of the SE-CP material. Similarly, the oxygen spectrum (Fig. 1(c)) exhibited three deconvoluted peaks at 529.21 eV, 530.10 eV, and 531.45 eV, assigned to carbonyl, ether, and carboxyl-type oxygen species, respectively. The nitrogen spectrum (Fig. 1(d)) revealed three peaks at 387.18 eV, 397.88 eV, and 398.96 eV, corresponding to pyridinic-N, pyrrolic-N, and graphitic-N, respectively.24 The presence of these nitrogen configurations suggests structural modifications induced by the acid treatment rather than pyrolysis. Thus, the in situ modification of the biosorbent SE-CP, as described in Section 2.2, leads to generation of pyridinic and pyrrolic nitrogen types associated with oxygen-containing groups. These groups introduce, in turn, more active sites, leading to additional sorption efficiency of the biosorbent SE-CP. As expected, all these functional groups have been attributed to the presence of proteins and polysaccharides.23 It should be noted that the nitrogen present on the surface of the biosorbent SE-CP results from the reaction between the amino group and the hydroxide group during the demineralization step consisting in the mineral layer removal from the raw shrimp exoskeleton. Moreover, the presence of pyridinic nitrogen and pyrrolic nitrogen elements as evidenced by the XPS results (Fig. 1(d)) will play an important role in enhancing the nanoporosity of SE-CP, thereby increasing the number of active sites. In addition, the bond breaking produces long-chain carboxylic acids, ketones and aldehyde groups which react with –NH and –NH2 to generate amino intermediates, which then condense to produce pyridines and pyrimidines. These findings are in good agreement with other reported works confirming that the graphitic nitrogen element present in the SE-CP biosorbent exhibits higher biosorption activity and stability than other nitrogen configurations.
O, respectively.23 However, we acknowledge the reviewer's concern regarding the calibration of binding energy (BE). While a BE of 284.8 eV is commonly assigned to C–C/C–H bonds, the shift in our data may stem from surface charging effects or the specific nature of the SE-CP material. Similarly, the oxygen spectrum (Fig. 1(c)) exhibited three deconvoluted peaks at 529.21 eV, 530.10 eV, and 531.45 eV, assigned to carbonyl, ether, and carboxyl-type oxygen species, respectively. The nitrogen spectrum (Fig. 1(d)) revealed three peaks at 387.18 eV, 397.88 eV, and 398.96 eV, corresponding to pyridinic-N, pyrrolic-N, and graphitic-N, respectively.24 The presence of these nitrogen configurations suggests structural modifications induced by the acid treatment rather than pyrolysis. Thus, the in situ modification of the biosorbent SE-CP, as described in Section 2.2, leads to generation of pyridinic and pyrrolic nitrogen types associated with oxygen-containing groups. These groups introduce, in turn, more active sites, leading to additional sorption efficiency of the biosorbent SE-CP. As expected, all these functional groups have been attributed to the presence of proteins and polysaccharides.23 It should be noted that the nitrogen present on the surface of the biosorbent SE-CP results from the reaction between the amino group and the hydroxide group during the demineralization step consisting in the mineral layer removal from the raw shrimp exoskeleton. Moreover, the presence of pyridinic nitrogen and pyrrolic nitrogen elements as evidenced by the XPS results (Fig. 1(d)) will play an important role in enhancing the nanoporosity of SE-CP, thereby increasing the number of active sites. In addition, the bond breaking produces long-chain carboxylic acids, ketones and aldehyde groups which react with –NH and –NH2 to generate amino intermediates, which then condense to produce pyridines and pyrimidines. These findings are in good agreement with other reported works confirming that the graphitic nitrogen element present in the SE-CP biosorbent exhibits higher biosorption activity and stability than other nitrogen configurations.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds in chitin/chitosan amide I and amide II. This band around 1650 cm−1 was expected due to the presence of chitin oligonucleotides in the extracted powder. However, the ethylene group cleavage band was also observed at 1416 cm−1. Similarly, the absorption bands around 1068 cm−1 and 891 cm−1 confirm the binding of monomers by β-glycosidic bonds.30 The SE-CP spectrum showed the appearance of a new peak at 1260 cm−1 corresponding to α-chitin, which confirms the presence of α-chitin–protein nanofibers in the SE-CP powder.
O bonds in chitin/chitosan amide I and amide II. This band around 1650 cm−1 was expected due to the presence of chitin oligonucleotides in the extracted powder. However, the ethylene group cleavage band was also observed at 1416 cm−1. Similarly, the absorption bands around 1068 cm−1 and 891 cm−1 confirm the binding of monomers by β-glycosidic bonds.30 The SE-CP spectrum showed the appearance of a new peak at 1260 cm−1 corresponding to α-chitin, which confirms the presence of α-chitin–protein nanofibers in the SE-CP powder.
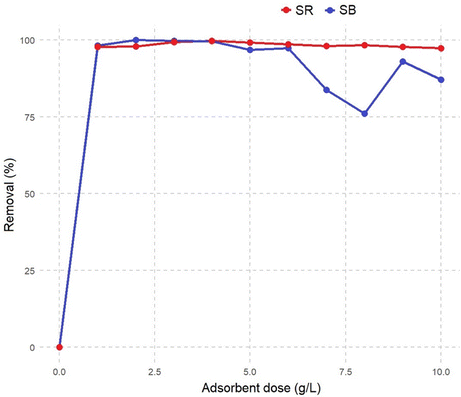
3.2. Batch biosorption studies of SB and SR dyes on the SE-CP surface
3.3. Biosorption kinetics
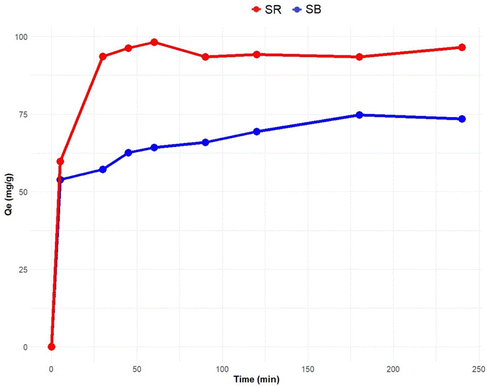
Kinetic analysis of the interactions occurring between red acid and SE-CP is crucial for the design and scale-up studies of the biosorption process.52 Thus, experimental data were compared to three kinetic theoretical models such as the pseudo-first-order, pseudo-second-order and intra-particle diffusion models. Modeling was carried out for experimental biosorption results obtained at pH = 3 for SR and pH = 4 for SB, a stirring speed of 100 rpm, a temperature of 20 °C, a dye concentration of 75 mg L−1, and a biosorbent dosage of 0.08 g for SR and 0.04 g for SB. The results clearly show that the dye biosorption onto the material surface obeys a pseudo-second-order kinetic model. In fact, the correlation coefficients of this model with the experimental data showed the highest R2 value, equal to 0.998 for the optimal feed concentration of 75 mg L−1, as compared with the pseudo-first-order model. However, it was reported elsewhere50 that the experimental biosorption data fitted with the pseudo-first-order model and demonstrated that electron transfer mechanisms were involved in dye binding onto the biomaterial surface.53 The pseudo-second-order kinetic theoretical model as determined for the red acid biosorption onto the biomaterial surface implies a rapid initial rate of biosorption followed by a slower secondary step to reach equilibrium. The characteristics of the pseudo-second-order model are in agreement with the experimental results, as shown in the SI. Finally, the intraparticle diffusion model was also tested to verify the possible influence of mass transfer resistance on SR dye binding to SE-CP. Overall, the tests showed an R2 of 0.961, underlining that this model can also be applied to describe the adsorption kinetics of the SR dye. Kinetic constants were obtained by non-linear regression. As for the removal of the SR dye and based on the correlation coefficients calculated, the pseudo-second-order model was found to be the best suited to explain the adsorption kinetics of blue acid on the material with the R2 value equal to 0.998. Moreover, the intra-particle diffusion model was also tested with an R2 of 0.961. Consequently, the experimental adsorption capacity of SB was very close to the values calculated by the pseudo-secondary order model. The kinetic study showed that the adsorption process occurs in 2 steps. The first one takes few minutes, during which 80% of the equilibrium adsorbed quantity is achieved, whereas in the second step taking several hours of contact, the adsorbed amount remains constant.3.4. Equilibrium biosorption isotherm models
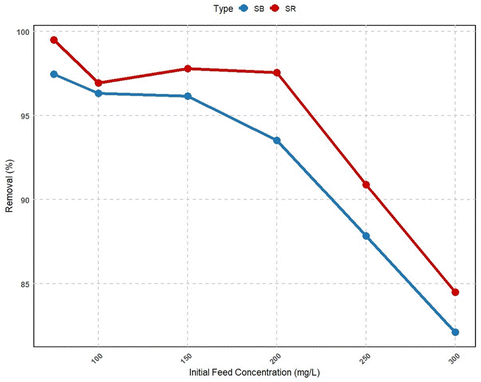
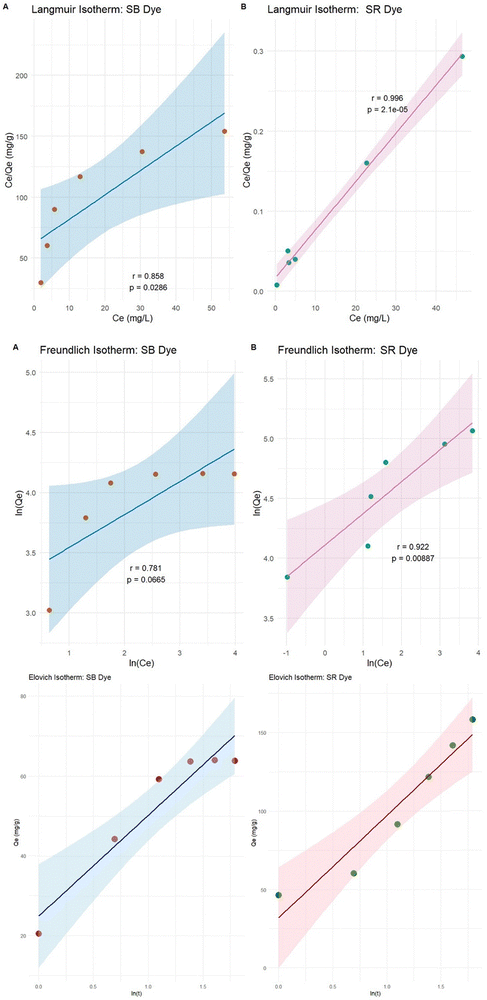
It should be noted that the adsorption models are best determined by non-linear regression analyses, which represent mathematically rigorous methods for determining the adsorption model parameters using equations in their original forms. Hence, the non-linear Langmuir, Freundlich and Elovich models were used in this work to investigate the biosorption equilibrium of the two acid dyes on the material studied (Fig. 7). The overall data indicate a heterogeneous distribution of the material surface active sites, with Freundlich's model being the best for describing adsorption consisting of multilayers when SR is adsorbed onto the SE-CP surface with an R2 of 0.966 (Table 2). On the other hand, in the case of SB on SE-CP, the three models studied Langmuir, Freundlich and Elovich showed R2 values close to 0.858, 0.781 and 0.978, respectively. The reason may lie in the adsorption of the dyes on separate monolayers, and as the best-fitting model is the Freundlich model, the monolayer surface is heterogeneous. In addition, the nature of the adsorption process was investigated by calculating the values of the Freundlich constant ‘1/n’.54 For both dyes, SR and SB, these were less than 1, and they were 0.37 and 0.60, respectively, confirming that biosorption is favorable on SE-CP. Indeed, Freundlich constant values ranging from 0.1 to 0.6 demonstrate the high adsorption capacity. This phenomenon is also attributable to the Langmuir isotherm, since the RL is an important parameter for evaluating the affinity of the binding sites. Setting the RL between 0 and 1 indicates “favorable adsorption” of the dye onto the adsorbent.54 The maximum adsorption quantity Qmax of SB (up to 63.81 mg g−1) is well below that of SR (up to 158.43 mg g−1). Note that the adsorbed quantities during a contact time of 250 minutes, qt, were 45.27 mg g−1 for SR and 34.45 mg g−1 for SB. In comparison with other studies using activated carbon,55–58 the maximum biosorption capacity exhibited by SE-CP in the present study is highly attractive and can even rival those obtained with activated carbon. Indeed, the multilayer biosorption of the biomaterial studied enables it to trap a wide range of pollutants. This biomaterial might be used as an alternative, in the near future, to solve the separation problem and could be marketed as a reference adsorbent for the removal of toxic pollutants.| Langmuir | |||
|---|---|---|---|
| Q m (mg g−1) | K L (L mg−1) | R 2 | |
| SB | 63.815 | 1.370 | 0.858 |
| SR | 158.432 | 0.811 | 0.996 |
| Freundlich | |||
|---|---|---|---|
| K F (mg1−n Ln g−1) | 1/n | R 2 | |
| SB | 16.859 | 0.607 | 0.781 |
| SR | 43.860 | 0.373 | 0.922 |
| Elovich | |||
|---|---|---|---|
| α (mg g min−1) | β (g mg−1) | R 2 | |
| SB | 83.205 | 0.084 | 0.978 |
| SR | 68.771 | 0.612 | 0.843 |
3.5. Thermodynamics of biosorption
Plotting ln(KL) as a function of 1/T for SR and SB dyes produces two straight lines (Fig. 8). Normally, the adsorption capacity increases when the temperature increases. However, in the case of SR, adsorption capacity was found to decrease with increasing temperature. This scenario can be attributed to the fact that as temperature increases, the energy supplied to SR ions to achieve the required activation energy exceeds the desired amount and causes a reverse in the diffusion effect towards the adsorbent active sites available for binding. The associated thermodynamic parameters were also calculated and are presented in Table 3. Negative ΔG values indicate the spontaneous nature of the adsorption process at 20, 30, 40 and 50 °C. If ΔG is negative and ΔH is positive at one temperature, the adsorption process is a spontaneous endothermic reaction and, furthermore, the more negative ΔG is, the more readily the dye is adsorbed by the material. Moreover, ΔS > 0 indicates an increase in the degree of disorder in the adsorbed layer at the solid/solution interface. Taking into account that the heat of adsorption is the negative enthalpy of adsorption, in the current study, the heat of adsorption of both anionic dyes is then negative and less than 40 kJ mol−1, indicating that the adsorption process is mainly physisorption.| Adsorption of Sellacid Red (SR) | |||
|---|---|---|---|
| T (°C) | ΔG (kJ mol−1) | ΔH (kJ mol−1) | ΔS (J k−1 mol−1) |
| 20 | −11.63 | 68.81 | 275.67 |
| 30 | −15.53 | ||
| 40 | −17.09 | ||
| 50 | −13.91 | ||
| Adsorption of Sellaset Blue (SB) | |||
| 20 | −19.11 | 23.84 | 17.04 |
| 30 | −18.36 | ||
| 40 | −18.31 | ||
| 50 | −18.61 | ||
3.6. Reusability and weight stability of SE-CP
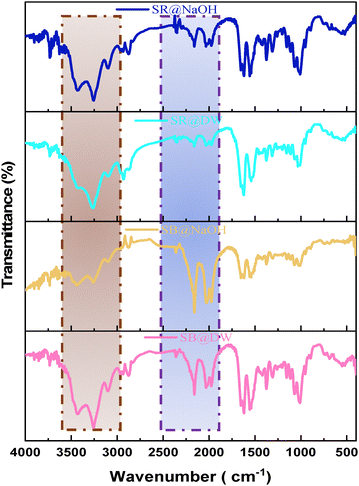
The reuse of the biosorbent and the weight stability of the adsorbed material can be achieved by regenerating the dye-loaded SE-CP. Regeneration of the biomaterial after biosorption is illustrated in the SI, using two solvents: distilled water (DW) and sodium hydroxide (NaOH) for both dyes SR in SR@DW/SR@NaOH and SB in SB@DW/SB@NaOH. After each SE-CP regeneration cycle, weight recovery is illustrated in the SI. Several aspects were monitored during the regeneration process to ensure ease of reuse while minimizing costs. Upon the fifth regeneration cycle with DW, the SB@DW dye exhibited a removal rate higher than that registered for the SR@DW dye, with respective abatement rates of 76.82% and 67.27%. Moreover, the dyes can be desorbed using green solvent such as DW, minimizing regeneration loads, with a significant reduction up to the fifth regeneration cycle, demonstrating the added value of using SE-CP as a biomaterial. After each regeneration cycle, the recovered mass appears to decrease for both dyes, demonstrating the presence of a biosorption phenomenon that generates a loss of mass with each cycle. At the fifth cycle, SB@DW and SR@DW dyes show mass recovery percentages of 77.17% and 62.58%, respectively. This represents good mass recovery after biosorption, confirming that SE-CP is effective in terms of dye removal, even after desorption by DW only. The application of NaOH for SE-CP desorption after biosorption of SR@NaOH and SB@NaOH showed similar efficiencies. Biosorption removal rates reached 83.53% and 82.14% for the fifth regeneration cycle of the SB@NaOH and SR@NaOH dyes, respectively. However, the mass recovered by NaOH is lower than that recovered by DW. Meanwhile, a mass recovery percentage of 33.33% is recorded for the SB dye, while for SR, 59.17% is obtained. This decrease may be due to the sensitivity of the chito-protein complex to alkaline solution (deproteinization process). Finally, the SE-CP seems to be a promising biosorbent and can be considered sustainable, if DW is used as solvent for desorption, due to the low mass loss, which is not very significant.3.7. SEM/EDX analyses of the reusable SE-CP
After five NaOH regeneration cycles, the SE-CP was assumed to be reusable for SR@NaOH dye biosorption (SI). To confirm this hypothesis, we carried out an XDE analysis of the particle, which adheres onto the surface of the material (SI) in comparison with the material surface (SI). The analysis regarding the particle shows a strong contribution of the C and O elements, with traces of Na, Ca and Cu. These elements may be generated after successive interactions with the same material to biosorb the same dye. Furthermore, a section of the same surface shows that the amount of oxygen is greater than that of carbon, with sodium, and traces of Cl and Cu. This is perhaps due to the surface heterogeneity already mentioned (SEM/XDE analysis of SE-CP), where the difference between the amount of carbon and oxygen can be explained by the heterogeneous distribution of active sites on the material surface, resulting in different contents of the particles that make up the pollutant on the material surface. The clear laminate is attributed to the chito-protein complex structure and the NaOH treatment, which partially removes biomaterial proteins with successive treatments. These features can be exploited to extend the biosorption cycles of the SE-CP biomaterial.The results of SB@NaOH regeneration cycles by using the same SE-CP biomaterial are shown in the SI. The coarse surface of a well-porous biomaterial is clear even after several regenerations. However, dye particles are trapped in these pores due to the non-detection of particles on the surface. Closer magnification revealed the layered nature of the SE-CP even after five regeneration cycles (Fig. 9). In addition, XDE analysis (SI) indicates the dominance of carbon and oxygen at the surface, which are the most important particles on the material surface for both the SE-CP (according to SEM/XDE analysis of the SE-CP) and the dye (azo). Evidence of the elements Ca and Cl is visible, proving that biosorption is deeper than the SEM detector was able to record, given that biosorption occurs in separate monolayers (isotherm applied).
3.8. Biosorption mechanism
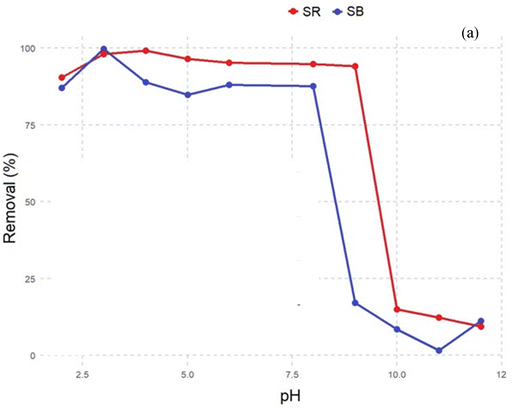
The biosorption of SR and SB dyes onto the SE-CP biomaterial is governed by a combination of physicochemical interactions involving the material's structural components, surface functionality, and the electrostatic nature of the dyes. The characterization of SE-CP revealed the coexistence of chitin and proteins, mainly composed of α-chitin (D-glucosamine units), whose antiparallel molecular arrangement confers high crystallinity and chemical stability.59 The biomaterial surface exhibits abundant hydroxyl, carboxyl, and amino functional groups originating from the chitin–protein matrix, which serve as active sites for dye attachment. The least hydrated and most polarized dye ions are the most strongly adsorbed.Simultaneously, FTIR, SEM and EDX analyses were carried out to further explore the mechanisms of SB and SR biosorption by the SE-CP bio-element. EDX spectra show the adhesion of particles belonging to the SB dye onto the surface, as well as charged macroscopic holes. These results could lead to the involvement of pore filling, which was confirmed by EDX spectra after five regenerations. In the FTIR spectra, the stretching vibration band of (νO–H) and (νN–H) after biosorption of the anionic dyes shifted separately to 3431.5 cm−1 and 3256.2 cm−1, respectively. These results confirm the presence of various functional groups such as –OH, –COOH, and R-OH on the SE-CP surface, leading to the presence of hydrogen bonding between the SE-CP and the dye molecules.60 The amide I and amide II peaks also shifted to lower wavelengths, while the peak at 1619 cm−1 (νC–N) and the appearance of a new peak at 1009.7 cm−1 were attributed to primary amine stretching that could be attributed to π–π interactions. All these changes reflect the involvement of these functional groups in the biosorption process.61 The structures of both SR and SB dyes contain –SO3− groups, which promote electrostatic interactions with the protonated amino groups of chitin at low pH. Under acidic conditions, these –NH2 groups become positively charged (–NH3+), enhancing attraction to the negatively charged dye ions. Consequently, dye uptake by SE-CP increases as pH decreases, confirming that electrostatic interactions play a dominant role in the biosorption process. Furthermore, in accordance with the XPS results, the presence of pyridinic and pyrrolic nitrogen elements was instrumental in enhancing the nanoporosity of SE-CP, thus increasing the number of active sites. In terms of comparison, many studies34,62 have focused on activated carbon derived from shrimp shells for its effectiveness, which undergoes pyrolysis, whereas SE-CP is acid-treated and dried at 220 °C without complete carbonization, making it a promising biomaterial. This difference is likely to influence the carbon's spectral characteristics. Meanwhile, being the origin of pyridinic and pyrrolic nitrogen, these functional groups arise due to the reaction of amino and hydroxyl groups during the demineralization process, which removes the mineral matrix from the shrimp exoskeleton. The observed nitrogen configurations do not necessarily indicate char formation but rather reflect the chemical interactions between chitin-derived functional groups. However, the surface nitrogen species identified by XPS play a crucial role in enhancing the porosity and reactivity of SE-CP. The presence of pyridinic and pyrrolic nitrogen, along with oxygen-containing groups, introduces additional active sites, thereby improving sorption efficiency. These results agree with previous reports showing that graphitic-N improves biosorbent stability and adsorption efficiency. The FTIR spectra further confirm oxygen-containing groups from polysaccharides and proteins that facilitate n–π and electrostatic interactions with anionic dyes. Even after several regeneration cycles (Fig. 10), SE-CP retains its porous structure and remains more effective in removing low-molecular-weight dyes than high-molecular-weight ones.
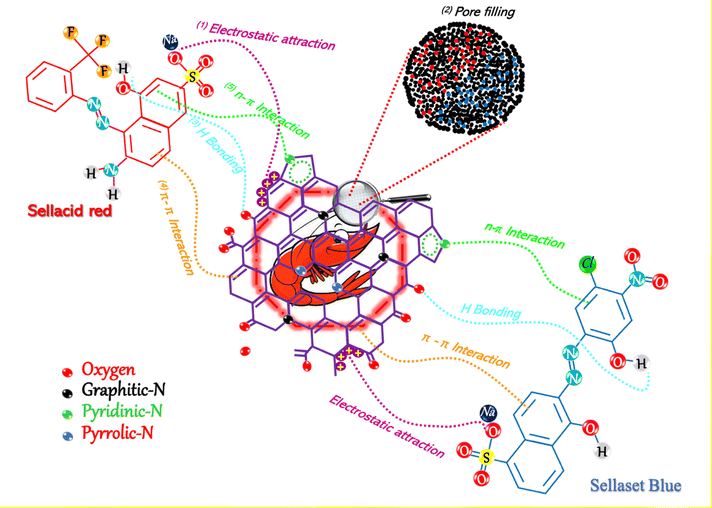 | ||
| Fig. 10 Graphical depiction of the suggested biosorption mechanism during the biosorption process of SR and SB dyes. | ||
4. Study limitations
Despite the promising performance of SE-CP as a biosorbent, several limitations should be noted. First, the current study was conducted under batch laboratory conditions, and the effectiveness of SE-CP in continuous-flow or large-scale industrial systems remains to be validated. Second, adsorption experiments were limited to two model anionic dyes (SR and SB); the performance may differ for other dye classes or complex industrial effluents. Third, while SE-CP showed good regeneration over five cycles, long-term stability and potential fouling effects under repeated usage were not fully assessed. Finally, the influence of competing ions or high ionic strength in real wastewater matrices was not evaluated, which could affect adsorption efficiency. Addressing these aspects in future work will be essential to fully demonstrate the practical applicability of SE-CP.5. Conclusions
In the present work, a modified shrimp exoskeleton (SE-CP) was successfully designed for its use as a biosorbent to remove anionic dyes from aqueous media. The novelty of this new biosorbent is revealed by the function of the chito-protein bio-composite used to remove two anionic dyes from water to reduce liquid and solid (marine by-products) pollution. The SE-CP showed significant removal of the two dyes studied, SR and SB, owing to the heterogeneous distribution of the active sites on the biomaterial surface, leading to multilayer formation. Moreover, the as-prepared biosorbent can be regenerated and reused by using either distilled water or NaOH aqueous solution. Finally, the biosorbent SE-CP offers a high level of efficiency in the removal of dye contaminants from aqueous media, making it a suitable material for sustainable, environmentally friendly remediation.In future work, the scalability and applicability of SE-CP in continuous treatment systems will be explored to support its integration into industrial wastewater management.
Conflicts of interest
There are no conflicts to declare.Data availability
All data supporting the findings of this study are available within the article and its supplementary information (SI). Supplementary information is available on request. See DOI: https://doi.org/10.1039/d5ma01010a.Additional datasets (including raw adsorption data and characterization files) are available from the corresponding author upon reasonable request.
Acknowledgements
Special thanks to all participants from Moroccan, Romanian and French universities for their collaboration. We thank Philippe FIOUX, Cyril VAULOT, Loic VIDAL and Simon GREE from IS2M-CNRS-Mulhouse, France, for XPS, BET, TEM, SEM-EDX, and FTIR analyses of the samples, respectively. The authors sincerely thank the Programme L’Oréal-UNESCO Pour les Femmes et la Science for their support. Special gratitude is extended for the recognition and funding awarded through the 2023 prize, which has greatly contributed to the advancement of this research.References
- O. C. Basurko, M. Aranda, A. Caballero, M. Andres, J. Murua and G. Gabiña, Workshop on the European Green Deal − Challenges and opportunities for EU fisheries and aquaculture Part I: Decarbonisation & circular economy aspects for fisheries STUDY Requested by the PECH Committee Policy Department for Structural and Cohesion Policies Directorate-General for Internal Policies PE, 2023.
- N. Nouj, Z. Majbar, M. R. Abelouah, A. Ben Hamou, A. Chaoui, N. Hafid, M. Benafqir, N. El Alem, A. Jada, H. Ouachtak, A. Ait Addi, I. I. Buciscanu, V. Maier, G. Soreanu and I. Cretescu, Eco-friendly wastewater treatment using a crab shell-based liquid bio-coagulant: Multi-criteria decision analysis related to different pollutants separation, J. Environ. Chem. Eng., 2024, 12(2), 112318, DOI:10.1016/j.jece.2024.112318.
- FAO Aquaculture News, FAO, Rome, 2023. https://www.fao.org/publications.
- M. R. Abelouah, M. Ben-Haddad, S. Hajji, N. Nouj, M. Ouheddou, B. Mghili, G. E. De-la-Torre, L. L. Costa, M. Banni and A. Ait Alla, Exploring marine biofouling on anthropogenic litter in the Atlantic coastline of Morocco, Mar. Pollut. Bull., 2024, 199, 115938, DOI:10.1016/j.marpolbul.2023.115938.
- W. Y. Mo, Y. B. Man and M. H. Wong, Use of food waste, fish waste and food processing waste for China's aquaculture industry: Needs and challenge, Sci. Total Environ., 2018, 613–614, 635–643, DOI:10.1016/j.scitotenv.2017.08.321.
- M. R. Abelouah, M. Idbella, N. Nouj, M. Ben-Haddad, S. Hajji, M. Ouheddou, J. Ourouh, G. Iacomino, R. El Haouti, I. Barra, J. A. Oualid, G. Bonanomi, M. Banni and A. A. Alla, Marine plastic exposure triggers rapid recruitment of plastic-degrading bacteria and accelerates polymer-specific transformations, J. Hazard. Mater., 2025, 137724, DOI:10.1016/j.jhazmat.2025.137724.
- S. Hajji, M. Ben-Haddad, M. R. Abelouah, N. Rangel-Buitrago and A. Ait Alla, Microplastic characterization and assessment of removal efficiency in an urban and industrial wastewater treatment plant with submarine emission discharge, Sci. Total Environ, 2024, 945, 174115, DOI:10.1016/j.scitotenv.2024.174115.
- I. Ahuja, E. Dauksas, J. F. Remme, R. Richardsen and A. K. Løes, Fish and fish waste-based fertilizers in organic farming – With status in Norway: A review, Waste Manage., 2020, 115, 95–112, DOI:10.1016/j.wasman.2020.07.025.
- N. Nouj, N. Hafid, N. El Alem and I. Cretescu, Novel liquid chitosan-based biocoagulant for treatment optimization of fish processing wastewater from a Moroccan plant, Materials, 2021, 14(23), 7133, DOI:10.3390/ma14237133.
- N. Zhou, G. Wei, X. Chen, B. Wu, H. Li, Q. Lu, X. Cao, A. Zhang, K. Chen and P. Ouyang, Self-sufficient biocatalysts constructed using chitin-based microspheres, Chem. Eng. J., 2023, 459, 141660, DOI:10.1016/j.cej.2023.141660.
- N. E. Mushi, T. Nishino, L. A. Berglund and Q. Zhou, Strong and Tough Chitin Film from α-Chitin Nanofibers Prepared by High Pressure Homogenization and Chitosan Addition, ACS Sustainable Chem. Eng., 2019, 7, 1692–1697, DOI:10.1021/acssuschemeng.8b05452.
- C. K. S. Pillai, W. Paul and C. P. Sharma, Chitin and chitosan polymers: Chemistry, solubility and fiber formation, Prog. Polym. Sci., 2009, 34, 641–678, DOI:10.1016/j.progpolymsci.2009.04.001.
- M. Feng, X. Lu, D. Hou and S. Zhang, Solubility, chain characterization, and derivatives of chitin, Handbook of Chitin and Chitosan: Preparation and Properties, Elsevier, 2020, vol. 1, pp. 101–129 DOI:10.1016/B978-0-12-817970-3.00004-3.
- S. Shukla, A. Sharma, G. Rathi, A. Manchanda, A. Khan, M. A. Malik and S. A. Chaudhry, Facile Fabrication of novel Y-doped ZnO biochar-based nanocomposite for efficient removal of Sulfasalazine, Congo red and Bismarck brown-R: Insight into adsorption performance and mechanism, Surf. Interfaces, 2025, 71, 106817, DOI:10.1016/j.surfin.2025.106817.
- N. Nouj, M. R. Abelouah and M. Idbella, et al., Unlocking the Full Potential of Agro-Waste Bio-Coagulants for Industrial Wastewater Treatment using pH-First Optimization, Water, Air, Soil Pollut., 2026, 237, 97, DOI:10.1007/s11270-025-08729-x.
- I. Hamed, F. Özogul and J. M. Regenstein, Industrial applications of crustacean by-products (chitin, chitosan, and chitooligosaccharides): A review, Trends Food Sci. Technol., 2016, 48, 40–50, DOI:10.1016/j.tifs.2015.11.007.
- P. Sharma, H. Kaur, M. Sharma and V. Sahore, A review on applicability of naturally available adsorbents for the removal of hazardous dyes from aqueous waste, Environ. Monit. Assess., 2011, 183, 151–195, DOI:10.1007/s10661-011-1914-0.
- S. Mahmood, A. Bi, S. Shukla, S. Husain, J. Prakash and S. A. Chaudhry, Synthesis of carbon quantum dots decorated titanium disilicide: a novel hybrid solar-driven photocatalyst for sustainable wastewater treatment, J. Mater. Chem. A, 2025, 13(37), 31781–31801, 10.1039/D5TA02427G.
- A. B. Hamou, S. Elamraoui, S. Escudero-Curiel, A. Chaoui, S. Farsad, A. Amjlef and N. El Alem, Enhancing surface area of HNO3-activated digestate-derived biochar for methylene blue adsorption: RSM-CCD optimization, DFT-MD calculations, and mechanistic study, Appl. Surf. Sci., 2025, 709, 163842, DOI:10.1016/j.apsusc.2025.163842.
- A. B. Hamou, A. Amjlef, N. Nouj, A. Chaoui, S. Farsad, F. Morlet-Savary and N. El Alem, Persulfate activation by Fe-rich biochar from sewage sludge digestate for organic pollutants removal: performance and superoxide radical role, J. Environ. Manage., 2025, 390, 126331, DOI:10.1016/j.jenvman.2025.126331.
- H. N. Tran, E. C. Lima, R. S. Juang, J. C. Bollinger and H. P. Chao, Thermodynamic parameters of liquid–phase adsorption process calculated from different equilibrium constants related to adsorption isotherms: A comparison study, J. Environ. Chem. Eng., 2021, 9(6), 106674, DOI:10.1016/j.jece.2021.106674.
- S. Li, Y. Gong, Y. Yang, C. He, L. Hu, L. Zhu, L. Sun and D. Shu, Recyclable CNTs/Fe3O4 magnetic nanocomposites as adsorbents to remove bisphenol A from water and their regeneration, Chem. Eng. J., 2015, 260, 231–239, DOI:10.1016/j.cej.2014.09.032.
- H. Hu, Y. Lin, B. Yang, X. Wen, P. Ma, X. J. Loh, Z. Luo, Z. Li and Y. L. Wu, Biomineralization-inspired functional biomaterials: From principles to practice, Chem. Eng. J., 2025, 504, 158624, DOI:10.1016/j.cej.2024.158624.
- I. Shahzadi, Y. Wu, H. Lin, J. Huang, Z. Zhao, C. Chen, X. Shi and H. Deng, Yeast biomass ornamented macro-hierarchical chitin nanofiber aerogel for enhanced adsorption of cadmium(II) ions, J. Hazard. Mater., 2023, 453, 131312, DOI:10.1016/j.jhazmat.2023.131312.
- B. Focher, A. Naggi, G. Torri, A. Cosani and M. Terbojevich, Structural differences between chitin polymorphs and their precipitates from solutions-evidence from CP-MAS sC.NMR, FT-IR and FT-Raman spectroscopy, Carbohydr. Polym., 1992, 17(2), 97–102 CrossRef.
- K. Mohanrasu, A. C. Manivannan and H. J. R. Rengarajan, et al., Eco-friendly biopolymers and composites: a sustainable development of adsorbents for the removal of pollutants from wastewater, npj Mater. Sustain., 2025, 3, 13, DOI:10.1038/s44296-025-00057-9.
- S. Kumari, P. Rath, A. Sri Hari Kumar and T. N. Tiwari, Extraction and characterization of chitin and chitosan from fishery waste by chemical method, Environ. Technol. Innovation, 2015, 3, 77–85, DOI:10.1016/j.eti.2015.01.002.
- S. Kumari, S. H. Kumar Annamareddy, S. Abanti and P. Kumar Rath, Physicochemical properties and characterization of chitosan synthesized from fish scales, crab and shrimp shells, Int. J. Biol. Macromol., 2017, 104, 1697–1705, DOI:10.1016/j.ijbiomac.2017.04.119.
- K. Suneeta, P. Rath and H. K. A. Sri, Chitosan from shrimp shell (Crangon crangon) and fish scales (Labeorohita): Extraction and characterization, Afr. J. Biotechnol., 2016, 15, 1258–1268, DOI:10.5897/ajb2015.15138.
- E. Muñoz and J. A. García-Manrique, Water absorption behaviour and its effect on the mechanical properties of flax fibre reinforced bioepoxy composites, Int. J. Polym. Sci., 2015, 2015(1), 390275, DOI:10.1155/2015/390275.
- H. S. Jung, M. H. Kim and W. H. Park, Preparation and Structural Investigation of Novel β-Chitin Nanocrystals from Cuttlefish Bone, ACS Biomater. Sci. Eng., 2019, 5, 1744–1752, DOI:10.1021/acsbiomaterials.8b01652.
- G. Cárdenas, G. Cabrera, E. Taboada and S. P. Miranda, Chitin characterization by SEM, FTIR, XRD, and 13C cross polarization/mass angle spinning NMR, J. Appl. Polym. Sci., 2004, 93, 1876–1885, DOI:10.1002/app.20647.
- J. D. Goodrich and W. T. Winter, α-Chitin nanocrystals prepared from shrimp shells and their specific surface area measurement, Biomacromolecules, 2007, 8, 252–257, DOI:10.1021/bm0603589.
- X. Liu, C. He, X. Yu, Y. Bai, L. Ye, B. Wang and L. Zhang, Net-like porous activated carbon materials from shrimp shell by solution-processed carbonization and H3PO4 activation for methylene blue adsorption, Powder Technol., 2018, 326, 181–189, DOI:10.1016/j.powtec.2017.12.034.
- H. El Knidri, R. El Khalfaouy, A. Laajeb, A. Addaou and A. Lahsini, Eco-friendly extraction and characterization of chitin and chitosan from the shrimp shell waste via microwave irradiation, Process Saf. Environ. Prot., 2016, 104, 395–405, DOI:10.1016/j.psep.2016.09.020.
- E. H. Cho and L. C. Lin, Nanoporous Material Recognition via 3D Convolutional Neural Networks: Prediction of Adsorption Properties, J. Phys. Chem. Lett., 2021, 12, 2279–2285, DOI:10.1021/acs.jpclett.1c00293.
- Z. Yu and D. Lau, Molecular dynamics study on stiffness and ductility in chitin–protein composite, J. Mater. Sci., 2015, 50, 7149–7157, DOI:10.1007/s10853-015-9271-y.
- S. M. Dadou, M. I. El-Barghouthi, S. K. Alabdallah, A. A. Badwan, M. D. Antonijevic and B. Z. Chowdhry, Effect of protonation state and N-acetylation of chitosan on its interaction with xanthan gum: A molecular dynamics simulation study, Mar. Drugs, 2017, 15(10), 298, DOI:10.3390/md15100298.
- P. X. Pinto, S. R. Al-Abed and D. J. Reisman, Biosorption of heavy metals from mining influenced water onto chitin products, Chem. Eng. J., 2011, 166, 1002–1009, DOI:10.1016/j.cej.2010.11.091.
- B. Bolto and J. Gregory, Organic polyelectrolytes in water treatment, Water Res., 2007, 41, 2301–2324, DOI:10.1016/j.watres.2007.03.012.
- L. B. Escudero, P. Y. Quintas, R. G. Wuilloud and G. L. Dotto, Recent advances on elemental biosorption, Environ. Chem. Lett., 2019, 17, 409–427, DOI:10.1007/s10311-018-0816-6.
- O. Oprea, D. Gingasu, D. C. Culita, J. M. Calderon Moreno, G. Marinescu and S. Preda, A sustainable approach for the synthesis of magnesium aluminate spinel towards photocatalytic applications, J. Mater. Sci., 2025, 97, 1–21, DOI:10.1007/s10853-025-10946-y.
- A. Laghzal, M. H. Hmamou, B. Boudinar, N. Nouj, H. Ighnih, F. Salmoun and Y. Tligui, Valorizing Moroccan crab shells to purify water from Orange G dye: Exploring equilibrium, kinetics, and thermodynamics, Desalin. Water Treat., 2024, 320, 100738, DOI:10.1016/j.dwt.2024.100738.
- R. M. Jain, K. H. Mody, J. Keshri and B. Jha, Biological neutralization and biosorption of dyes of alkaline textile industry wastewater, Mar. Pollut. Bull., 2014, 84, 83–89, DOI:10.1016/j.marpolbul.2014.05.033.
- K. Rambabu, J. AlYammahi, G. Bharath, A. Thanigaivelan, N. Sivarajasekar and F. Banat, Nano-activated carbon derived from date palm coir waste for efficient sequestration of noxious 2,4-dichlorophenoxyacetic acid herbicide, Chemosphere, 2021, 282, 131103, DOI:10.1016/j.chemosphere.2021.131103.
- A. Spoiala, C. I. Ilie, G. Dolete, A. M. Croitoru, V. A. Surdu, R. D. Truscă and L. M. Ditu, Preparation and characterization of chitosan/TiO2 composite membranes as adsorbent materials for water purification, Membranes, 2022, 12, 804, DOI:10.3390/membranes12080804.
- K. Rambabu, G. Bharath, F. Banat and P. L. Show, Biosorption performance of date palm empty fruit bunch wastes for toxic hexavalent chromium removal, Environ. Res., 2020, 187, 109694, DOI:10.1016/j.envres.2020.109694.
- A. Zakaria, J. Amane and E. A. Noureddine, Core–shell architecture based on bio-sourced porous carbon: The shape formation mechanism at the solid/liquid interface layer, RSC Adv., 2019, 9, 25544–25553, 10.1039/c9ra04869c.
- M. Benafqir, Z. Anfar, M. Abbaz, R. El Haouti, S. Lhanafi, Y. Azougarh, A. Ait El Fakir, M. Ez-zahery and N. El Alem, Hematite–titaniferous sand as a new low-cost adsorbent for orthophosphates removal: Adsorption, mechanism and Process Capability study, Environ. Technol. Innovation, 2019, 13, 153–165, DOI:10.1016/j.eti.2018.10.009.
- G. D. Değermenci, N. Değermenci, V. Ayvaoğlu, E. Durmaz, D. Çakır and E. Akan, Adsorption of reactive dyes on lignocellulosic waste; characterization, equilibrium, kinetic and thermodynamic studies, J. Cleaner Prod., 2019, 225, 1220–1229, DOI:10.1016/j.jclepro.2019.03.260.
- Y. Zhou, L. Ge, N. Fan and M. Xia, Adsorption of Congo red from aqueous solution onto shrimp shell powder, Adsorpt. Sci. Technol., 2018, 36, 1310–1330, DOI:10.1177/0263617418768945.
- K. Rambabu, G. Bharath, F. Banat, P. L. Show and H. H. Cocoletzi, Mango leaf extract incorporated chitosan antioxidant film for active food packaging, Int. J. Biol. Macromol., 2019, 126, 1234–1243, DOI:10.1016/j.ijbiomac.2018.12.196.
- P. Krishnamoorthy, D. G. Dixon, Z. Ren, N. Mora and C. W. Chao, Modeling the distribution of an adsorbing solute in a catalyzed column, Miner. Eng., 2022, 182, 107556, DOI:10.1016/j.mineng.2022.107556.
- J. Chang, Z. Shen, X. Hu, E. Schulman, C. Cui, Q. Guo and H. Tian, Adsorption of Tetracycline by Shrimp Shell Waste from Aqueous Solutions: Adsorption Isotherm, Kinetics Modeling, and Mechanism, ACS Omega, 2020, 5, 3467–3477, DOI:10.1021/acsomega.9b03781.
- A. Chaoui, A. Imgharn, A. C. Estrada, A. B. Hamou, S. Farsad, N. Nouj, M. Ez-zahery, T. Trindade, A. Albourine and N. E. Alem, Chromium(VI) remediation via biochar@polyaniline composite: Advancing water treatment using biogas residue digestate, Adv. Compos. Hybrid Mater., 2025, 8(4), 312, DOI:10.1007/s42114-025-01345-7.
- R. Haounati, H. Ighnih, R. E. Malekshah, N. Nouj, H. Ouachtak, B. Šljukić and A. Ait Addi, Advanced contaminant removal through synergistic interactions of montmorillonite, CoFe2O4 nanoparticles, and surfactants: Combining theoretical and experimental approaches, J. Mol. Liq., 2025, 428, 127490, DOI:10.1016/j.jcis.2008.08.066.
- X. Q. Qiao, F. C. Hu, F. Y. Tian, D. F. Hou and D. S. Li, Equilibrium and kinetic studies on MB adsorption by ultrathin 2D MoS2 nanosheets, RSC Adv., 2016, 6, 11631–11636, 10.1039/c5ra24328a.
- N. El Messaoudi, A. El Mouden, M. El Khomri, A. Bouich, Y. Fernine, Z. Ciğeroğlu, J. H. P. Américo-Pinheiro, N. Labjar, A. Jada, M. Sillanpää and A. Lacherai, Experimental study and theoretical statistical modeling of acid blue 25 remediation using activated carbon from Citrus sinensis leaf, Fluid Phase Equilib., 2022, 563, 113585, DOI:10.1016/j.fluid.2022.113585.
- J. A. Rippon and D. J. Evans, Improving the properties of natural fibres by chemical treatments, Handbook of Natural Fibres: Processing and Applications, Elsevier Inc., 2020, vol. 2, pp. 245–321 DOI:10.1016/B978-0-12-818782-1.00008-0.
- A. Ben Hamou, M. Enneiymy, S. Farsad, A. Amjlef, A. Chaoui, N. Nouj, A. Majdoub, A. Jada, M. Ez-Zahery and N. El Alem, Novel chemically reduced cobalt-doped g-C3N4 (CoCN-x) as a highly heterogeneous catalyst for super-degradation of organic dyes via peroxymonosulfate activation, Mater. Adv., 2024, 5(5), 1960–1976, 10.1039/D3MA00818E.
- A. Chaoui, S. Farsad, A. Ben Hamou, A. Amjlef, N. Nouj, M. Ezzahery and N. El Alem, Reshaping environmental sustainability: Poultry by-products digestate valorization for enhanced biochar performance in methylene blue removal, J. Environ. Manage., 2024, 351, 119870, DOI:10.1016/j.jenvman.2023.119870.
- V. T. Le, M. U. Dao, H. S. Le, D. L. Tran, V. D. Doan and H. T. Nguyen, Adsorption of Ni(II) ions by magnetic activated carbon/chitosan beads prepared from spent coffee grounds, shrimp shells and green tea extract, Environ. Technol., 2020, 41, 2817–2832, DOI:10.1080/09593330.2019.1584250.
| This journal is © The Royal Society of Chemistry 2026 |