Open Access Article
Open Access ArticleHigh-refractive-index copolymers produced by radical copolymerization of aromatic heterocyclic monomers in deep eutectic solvents
Yuji Tsuharaa,
Kento Satoa,
Sadaki Samitsu b and
Hideharu Mori
b and
Hideharu Mori *a
*a
aDepartment of Organic Materials Science, Graduate School of Organic Materials Science, Yamagata University, 4-3-16, Jonan, Yonezawa, 992-8510, Japan. E-mail: h.mori@yz.yamagata-u.ac.jp
bNational Institute for Materials Science, 1-2-1, Sengen, Tsukuba, 305-0047, Japan
First published on 14th January 2026
Abstract
The development of a greener and more efficient methodology for simultaneously achieving polymers with a high refractive index, high Abbe number, good transparency, and high thermal stability remains a challenge, particularly for sustainable production using environmentally friendly solvents. Herein, we report the green production of high-refractive-index copolymers in a deep eutectic solvent (DES) by the radical copolymerization of aromatic heterocyclic S-vinyl and N-vinyl monomers, namely benzothiazoyl vinyl sulfide (BTVS) and N-vinyl carbazole (NVC), combined with methyl methacrylate (MMA). Three hydrophobic natural DESs (NADESs; Thy/Cou, Thy/Men, and Tdc/Men) prepared from naturally derived compounds (Thy: thymol, Men: (±)-menthol, Cou: coumarin, and Tdc: 1-tetradecanol) and two hydrophilic DESs prepared from choline chloride (ChCl) and zinc chloride (ZnCl2) with urea (ChCl/urea and ZnCl2/urea) were used for the radical copolymerization of MMA/BTVS, MMA/NVC, and BTVS/NVC. An increase in both the polymer yield and molecular weight of the resulting copolymers was observed, depending on the nature of the DESs and the polymerization conditions. P(BTVS-co-NVC)s prepared at a constant feed ratio (100/100) in the DESs exhibited high refractive indices (1.6738–1.7359 at 589 nm), which were higher than those of P(MMA-co-BTVS)s (1.6076–1.6794) and P(MMA-co-NVC)s (1.5922–1.6349). Three series of the copolymers showed good transparency (>96% at 400 nm) and high thermal stability (Td5 = 378 °C, 249 °C, and 246 °C for MMA/NVC, MMA/BTVS, and BTVS/NVC copolymers, respectively). P(BTVS-co-NVC) prepared at a suitable BTVS/NVC (150/50) feed ratio in ZnCl2/urea showed the highest refractive index (1.7528) with a relatively high Abbe number (21.1), which differs from the general tendency for a trade-off between refractive index and Abbe number. This work demonstrates the great potential of DESs for the sustainable production of next-generation high-refractive-index polymers and provides new opportunities for their applications in related fields.
Introduction
High-refractive-index polymers are indispensable for a prosperous society with a wide range of applications, such as modern lenses, image sensors, antireflective coatings, microlens arrays, and optical guides.1–8 The structural design and practical synthesis of polymers with high refractive indices, particularly those higher than 1.7, have attracted significant interest, and generally rely on building blocks with high molar refraction and small molar volume, based on the Lorentz–Lorenz equation.1–3,7,9 For instance, sulfur atoms have been incorporated in polymeric materials using various modern methods, such as thiol–ene10–16 and thiol–yne17,18 reactions, ring-opening polymerization,19 and inverse-vulcanization.7,20 Other intriguing examples involve the incorporation of high molar refraction units (e.g., phosphorus units21), highly polarizable atoms (e.g., Ge and Sn),22 metal (e.g., Zn23 and Zr24), and semi-metal (e.g., Se11,25–27). The utilization of charge-transfer complexation28 and hydrogen bonds29 has recently emerged as an efficient tool for enhancing the refractive index.Despite continuous progress in synthetic methodologies for high-refractive-index polymers, they are generally produced by polymerization in harmful and volatile organic solvents. Owing to the increasing demand for sustainable production with environmentally friendly systems, the development of a greener process is highly desirable to produce high-refractive-index polymers, in which the environmental impact can be reduced by using fewer organic solvents while achieving high polymerization efficacy (high atom economy). Deep eutectic solvents (DESs) have recently emerged as practical green solvents with unique and attractive properties (e.g., low volatility, non-toxicity, cost effectiveness, thermal stability, low flammability, tunable polarity and solubility).30–33 A variety of DESs, usually obtained by mixing two or more solids corresponding to a hydrogen bond acceptor and donor (HBA and HBD), have been employed as green solvents in various polymerization systems.34–36 The increased viscosity and polarity of DESs has been reported to enhance the polymerization rate, compared to conventional solvents (e.g., aqueous or organic solvents).37,38 Among DESs with a wide range of HBA/HBD combinations and structural diversity, natural DESs (NADESs) consisting of natural components (e.g., sugars, organic acids, and amino acids)33,39,40 have emerged as promising green solvents in various fields, including polymer science.41 A variety of studies have been reported on the effect of DESs on the polymerization of methyl methacrylate (MMA).42–44 For instance, Serra et al. reported homogeneous radical polymerization of MMA and other hydrophobic monomers in DL-menthol/1-tetradecanol NADES,42 and block copolymer synthesis using MMA in L-menthol/thymol NADES.43 Yu et al. demonstrated photo-induced reversible addition–fragmentation chain transfer (RAFT) polymerization of MMA in tetrabutylammonium chloride/ethylene glycol DES in air,44 and the potential of DESs for the production of ultrahigh molecular weight PMMA.45 However, DESs have rarely been used to produce high-refractive-index polymers.
In this study, we investigated the effect and usefulness of DESs for the sustainable production of high-refractive-index polymers via the radical copolymerization of the aromatic heterocyclic S-vinyl and N-vinyl monomers, benzothiazoyl vinyl sulfide (BTVS) and N-vinyl carbazole (NVC), which were selected to enhance the refractive index (Fig. 1a). As a comonomer, MMA was also selected, owing to its use as a starting monomer for the production of PMMA thermoplastic known as organic glass or acrylate glass (refractive index = 1.49 and Abbe number = 58).3,6 Remarkably high molar refraction of the –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–C– group (4.10), compared to that of the –C
N–C– group (4.10), compared to that of the –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–C– group (1.73),46 enables aromatic heterocycles to act as efficient building blocks to enhance the refractive index of synthetic polymers, e.g., triazines,47–49 pyridazines,50 pyrimidines50–52 and carbazole16 units in the backbone chains, as well as carbazole,6,53 thiophene,54,55 and thiadiazole1,56 units in the side chains. We previously reported the effectiveness of S-vinyl sulfides with aromatic heterocycles (e.g., BTVS) for the production of high-refractive-index polymers,57,58 owing to their high sulfur content, –C
C–C– group (1.73),46 enables aromatic heterocycles to act as efficient building blocks to enhance the refractive index of synthetic polymers, e.g., triazines,47–49 pyridazines,50 pyrimidines50–52 and carbazole16 units in the backbone chains, as well as carbazole,6,53 thiophene,54,55 and thiadiazole1,56 units in the side chains. We previously reported the effectiveness of S-vinyl sulfides with aromatic heterocycles (e.g., BTVS) for the production of high-refractive-index polymers,57,58 owing to their high sulfur content, –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– bonds, and aromatic rings in the monomer units. However, synthetic difficulties were occasionally encountered in achieving high molecular weight products, complete monomer conversion (high polymer yield), and a limited range of suitable comonomers because of the relatively low polymerizability of S-vinyl monomers. For instance, radical polymerization of BTVS, which has two sulfur atoms, one –C
N– bonds, and aromatic rings in the monomer units. However, synthetic difficulties were occasionally encountered in achieving high molecular weight products, complete monomer conversion (high polymer yield), and a limited range of suitable comonomers because of the relatively low polymerizability of S-vinyl monomers. For instance, radical polymerization of BTVS, which has two sulfur atoms, one –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–C– group, and one benzene ring, afforded PBTVS with a high refractive index (1.7432, Abbe number = 17.0),57 whereas the monomer conversion and molecular weights were limited even in bulk polymerization.59 Block and random copolymers consisting of BTVS with 2-vinylnaphthalene prepared by RAFT polymerization in dimethyl formamide (DMF) allowed for the improvement of transparency while maintaining a high refractive index (1.72–1.66).57 Nevertheless, a greener and more efficient methodology enabling simultaneous achievement of high-refractive-index polymers with improved productivity (e.g., higher conversion) and other essential properties (e.g., high Abbe number, high transparency) is still required.
N–C– group, and one benzene ring, afforded PBTVS with a high refractive index (1.7432, Abbe number = 17.0),57 whereas the monomer conversion and molecular weights were limited even in bulk polymerization.59 Block and random copolymers consisting of BTVS with 2-vinylnaphthalene prepared by RAFT polymerization in dimethyl formamide (DMF) allowed for the improvement of transparency while maintaining a high refractive index (1.72–1.66).57 Nevertheless, a greener and more efficient methodology enabling simultaneous achievement of high-refractive-index polymers with improved productivity (e.g., higher conversion) and other essential properties (e.g., high Abbe number, high transparency) is still required.
In this study, three hydrophobic NADESs (Thy/Cou (1/1), Thy/Men (1/1), and Tdc/Men (1/2)) prepared from naturally derived compounds (Thy: thymol, Men: (±)-menthol, Cou: coumarin, and Tdc: 1-tetradecanol) were selected to verify the usefulness of radical (co)polymerization of aromatic heterocyclic monomers (Fig. 1). Two hydrophilic DESs prepared from choline chloride (ChCl) and zinc chloride (ZnCl2) (ChCl/urea (1/2) and ZnCl2/urea (1/3.5)) acting as the HBA, combined with urea as the HBD, were also selected. The effects of these hydrophobic NADESs and hydrophilic DESs on the thermal copolymerization of MMA/BTVS, MMA/NVC, and BTVS/NVC were investigated for their polymerization behavior (improved productivity with higher polymer yield and enhanced molecular weights), optical properties (refractive index, Abbe number, and transparency), and other essential properties (thermal stability and film-forming ability) of the resulting copolymers. Photopolymerization was performed to confirm the extensibility of the DES-based green process. The comonomer combinations and their compositions were optimized to afford targeted high-refractive-index copolymers with reasonable Abbe numbers by tuning the contents of three cardinal units (sulfur, –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–, and aromatic units) in the copolymers, while higher molecular weight products with high polymer yield (high productivity) were achieved by suitable selection of the nature of DESs (hydrophobic NADESs and hydrophilic DESs) and polymerization systems (thermal- and photo-induced systems). This study is the first example of the productive integration of DESs and radical copolymerization of aromatic heterocyclic S-vinyl and N-vinyl monomers toward green and efficient production of high-refractive-index copolymers with improved productivity (polymer yield) and high molecular weights, as well as enhanced refractive indices and Abbe numbers.
N–, and aromatic units) in the copolymers, while higher molecular weight products with high polymer yield (high productivity) were achieved by suitable selection of the nature of DESs (hydrophobic NADESs and hydrophilic DESs) and polymerization systems (thermal- and photo-induced systems). This study is the first example of the productive integration of DESs and radical copolymerization of aromatic heterocyclic S-vinyl and N-vinyl monomers toward green and efficient production of high-refractive-index copolymers with improved productivity (polymer yield) and high molecular weights, as well as enhanced refractive indices and Abbe numbers.
Results and discussion
Copolymer synthesis in deep eutectic solvents (DESs)
Three hydrophobic NADESs (Thy/Cou (1/1), Thy/Men (1/1), and Tdc/Men (1/2)) with relatively low viscosities (<100 mPa s)60 were prepared from naturally derived compounds (Thy, Men, Cou, and Tdc) at suitable feed ratios, as reported previously.60,61 Two hydrophilic DESs (ChCl/urea (1/2)30 and ZnCl2/urea (1/3.5)62) were also prepared from ChCl and ZnCl2, acting as HBA, combined with urea as HBD, which have relatively high viscosities (750 and 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 340 mPa s for ChCl/urea (1/2) and ZnCl2/urea (1/3.5) at 25 °C (ref. 32)). Liquid MMA was miscible with all the DESs, whereas liquid BTVS was miscible only with Thy/Cou and Thy/Men. Solid NVC was dissolved only in Thy/Cou and Thy/Men. All homopolymers (PMMA, PBTVS, and PNVC) were soluble in Thy/Cou and Thy/Men but insoluble in other DESs (Tdc/Men, ChCl/urea, and ZnCl2/urea), as shown in Table S1. These hydrophobic NADESs and hydrophilic DESs were tested for the radical copolymerization of MMA/BTVS, MMA/NVC, and BTVS/NVC, allowing the production of targeted copolymers with predetermined structures and compositions by selecting comonomer combinations and their feed ratios.
340 mPa s for ChCl/urea (1/2) and ZnCl2/urea (1/3.5) at 25 °C (ref. 32)). Liquid MMA was miscible with all the DESs, whereas liquid BTVS was miscible only with Thy/Cou and Thy/Men. Solid NVC was dissolved only in Thy/Cou and Thy/Men. All homopolymers (PMMA, PBTVS, and PNVC) were soluble in Thy/Cou and Thy/Men but insoluble in other DESs (Tdc/Men, ChCl/urea, and ZnCl2/urea), as shown in Table S1. These hydrophobic NADESs and hydrophilic DESs were tested for the radical copolymerization of MMA/BTVS, MMA/NVC, and BTVS/NVC, allowing the production of targeted copolymers with predetermined structures and compositions by selecting comonomer combinations and their feed ratios.
Initially, three hydrophobic NADESs were tested for the thermal radical copolymerization of MMA and BTVS (Table 1). When liquid BTVS was mixed with MMA in the presence of 2,2′-azobis(isobutyronitrile) (AIBN) at [AIBN]0/[MMA]0/[BTVS]0 = 1/100/100 in hydrophobic NADESs (conc. = 50 wt%), a transparent solution was obtained, and this transparency was maintained during the copolymerization at 60 °C for 24 h (Fig. S4). The resulting mixture was precipitated in hexane, followed by filtration, yielding P(MMA-co-BTVS). 1H NMR spectroscopy of the resulting P(MMA-co-BTVS) showed no detectable peaks attributable to NADESs or residual monomers (MMA and BTVS), implying efficient purification (Fig. S3). The polymer yields of P(MMA-co-BTVS) prepared in the hydrophobic NADESs (32–55%) were comparable to or higher than those prepared in bulk (34%), suggesting the feasibility of improving productivity using the naturally derived green solvents. Size-exclusion chromatography (SEC) measurements of P(MMA-co-BTVS)s revealed a broad unimodal peak with a molecular weight range (number average molecular weight (Mn) = 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–19
000–19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, Mw/Mn = 1.91–2.94) lower than that prepared in bulk (Mn = 44
000, Mw/Mn = 1.91–2.94) lower than that prepared in bulk (Mn = 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, Mw/Mn = 2.86). The copolymer structures evaluated using 1H NMR spectroscopy displayed broad peaks corresponding to the aromatic rings of BTVS at 6.5–8.0 ppm and the methoxy group of MMA at approximately 3.5 ppm. The BTVS content in P(MMA-co-BTVS)s determined by elemental analysis was 32–44%, suggesting the preferred insertion of MMA. This tendency is attributed to the reactivities of MMA (Q = 0.74, e = 0.40 (ref. 63)) and the BTVS derivative (Q = 0.32, e = −1.4 (ref. 64) for phenyl vinyl sulfide). These results are consistent with our preliminary experimental results of thermal homopolymerization in hydrophobic NADESs (Tables S3 and S5, Fig. S1 and S2), showing the formation of high molecular weight PMMA with relatively high conversion (e.g., Mn = 120
000, Mw/Mn = 2.86). The copolymer structures evaluated using 1H NMR spectroscopy displayed broad peaks corresponding to the aromatic rings of BTVS at 6.5–8.0 ppm and the methoxy group of MMA at approximately 3.5 ppm. The BTVS content in P(MMA-co-BTVS)s determined by elemental analysis was 32–44%, suggesting the preferred insertion of MMA. This tendency is attributed to the reactivities of MMA (Q = 0.74, e = 0.40 (ref. 63)) and the BTVS derivative (Q = 0.32, e = −1.4 (ref. 64) for phenyl vinyl sulfide). These results are consistent with our preliminary experimental results of thermal homopolymerization in hydrophobic NADESs (Tables S3 and S5, Fig. S1 and S2), showing the formation of high molecular weight PMMA with relatively high conversion (e.g., Mn = 120![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, yield = 64%), whereas in the case of BTVS there was a lower molecular weight and conversion (e.g., Mn = 5400, yield = 20%) in Tdc/Men.
000, yield = 64%), whereas in the case of BTVS there was a lower molecular weight and conversion (e.g., Mn = 5400, yield = 20%) in Tdc/Men.
| Entry | Monomer (M1/M2) | [I]0/[M1]0/[M2]0 | DES | Yieldb (%) | Mn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c (SEC) c (SEC) |
Mw/Mn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c (SEC) c (SEC) |
n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) md md |
|---|---|---|---|---|---|---|---|
| a Copolymerization with AIBN in DES at 60 °C for 24 h.b Hexane insoluble part.c Measured by SEC using PSt standard in DMF.d Evaluated from sulfur content determined by elemental analysis.e Methanol insoluble part.f Evaluated from nitrogen content determined by elemental analysis. | |||||||
| CP1-1 | MMA/BTVS | 1/100/100 | Bulk | 34 | 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
2.86 | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
| CP1-2 | Thy/Cou | 32 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.91 | 68![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 32 32 |
||
| CP1-3 | Thy/Men | 38 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
2.19 | 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 40 |
||
| CP1-4 | Tdc/Men | 55 | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
2.94 | 56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 44 44 |
||
| CP1-5 | ChCl/urea | 67e | 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
2.73 | 54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 46 46 |
||
| CP1-6 | ZnCl2/urea | 59e | 51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
2.75 | 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 56 56 |
||
| CP2-1 | MMA/NVC | 1/100/100 | Bulk | 76 | 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
4.39 | 46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 54f 54f |
| CP2-2 | Thy/Cou | 60 | 56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
3.07 | 69![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 31f 31f |
||
| CP2-3 | Thy/Men | <5% | — | — | — | ||
| CP2-4 | Tdc/Men | 82 | 59![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
2.80 | 54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 46f 46f |
||
| CP2-5 | ChCl/urea | 85e | 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
2.82 | 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 65f 65f |
||
| CP2-6 | ZnCl2/urea | 61e | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
2.52 | 58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 42f 42f |
||
| CP3-1 | BTVS/NVC | 1/100/100 | Bulk | 13 | 680 | 1.82 | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 91 91 |
| CP3-2 | Thy/Cou | 21 | 750 | 1.61 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99 99 |
||
| CP3-3 | Tdc/Men | 14 | 3900 | 1.89 | 83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 17 |
||
| CP3-4 | ChCl/urea | 50e | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
2.29 | 69![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 31 31 |
||
| CP3-5 | ZnCl2/urea | 33e | 5500 | 4.33 | 58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 42 42 |
||
| CP3-6 | 1/50/150 | ZnCl2/urea | 34e | 3300 | 7.71 | 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 66 66 |
|
| CP3-7 | 1/75/125 | ZnCl2/urea | 38e | 4300 | 6.67 | 51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 49 49 |
|
| CP3-8 | 1/150/50 | ZnCl2/urea | 32e | 8400 | 3.57 | 89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 11 |
|
When hydrophilic DESs (ChCl/urea and ZnCl2/urea) were used for the copolymerization of MMA and BTVS under the same conditions ([AIBN]0/[MMA]0/[BTVS]0 = 1/100/100, conc. = 50 wt%), it was difficult to achieve a miscible state with BTVS in DES (Table S1), leading to heterogeneous polymerization at 60 °C for 24 h (Fig. S4). Using an efficient purification process by reprecipitation into methanol, the hydrophilic DESs and unreacted monomers (MMA and BTVS) could be removed, as confirmed by 1H NMR spectroscopy (Fig. S3). Under these conditions, copolymerization proceeded efficiently to afford P(MMA-co-BTVS)s as white solids with higher yields (59–67%) and molecular weights (Mn = 51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–85
000–85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, Mw/Mn = 2.75–2.73) than those in bulk. This may be due to the higher viscosity of the hydrophilic DESs, which leads to a relatively slow diffusion of radical species, and therefore a decrease in chain transfer and termination reactions, resulting in higher molecular weights and polymer yields. Another hypothesis is the protected radical effect, by which small monomer domains are formed with DES, like the case of ionic liquids, to reduce the termination rate, resulting in increased molecular weights, and increase the concentration of radicals, leading to the increased polymer yields.44,65 The copolymerization at higher temperature (80 °C) led to a slight decrease in the molecular weights (Mn = 40
000, Mw/Mn = 2.75–2.73) than those in bulk. This may be due to the higher viscosity of the hydrophilic DESs, which leads to a relatively slow diffusion of radical species, and therefore a decrease in chain transfer and termination reactions, resulting in higher molecular weights and polymer yields. Another hypothesis is the protected radical effect, by which small monomer domains are formed with DES, like the case of ionic liquids, to reduce the termination rate, resulting in increased molecular weights, and increase the concentration of radicals, leading to the increased polymer yields.44,65 The copolymerization at higher temperature (80 °C) led to a slight decrease in the molecular weights (Mn = 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–29
000–29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, Table S7).
000, Table S7).
Photopolymerization of MMA and BTVS was also attempted using hydrophobic NADESs (Table S8). A homogeneous mixture was obtained from liquid MMA and BTVS in the presence of a photoinitiator (2,2-dimethoxy-2-phenylacetophenone (DMPA)) at [DMPA]0/[MMA]0/[BTVS]0 = 1/100/100 in hydrophobic NADESs (50 wt%). The copolymerization was performed using UV light (λ = 365 nm, 330 mW cm−2 for LED light) at room temperature in air. Copolymerization proceeded homogeneously in Thy/Cou and Thy/Men, whereas heterogeneous copolymerization occurred for Tdc/Men. P(MMA-co-BTVS)s with relatively low yields (30–36%) and molecular weights (Mn = 8200–8400, Mw/Mn = 2.21–4.62) were obtained, regardless of the NADESs, suggesting a limited effect of the nature of the NADESs on the photocopolymerization behavior. A similar tendency was also observed in photohomopolymerization in hydrophobic NADESs (yield = 14–34% for BTVS and 6–16% for MMA, Tables S4 and S6). Photocopolymerization in hydrophilic DESs is impossible because of insufficient solubility of the monomers (MMA and BTVS) in hydrophilic DESs at room temperature (Table S1). Nevertheless, these results imply the feasibility of using nonvolatile DESs for the photopolymerization of aromatic heterocyclic monomers, which can extend the possibility of producing high-refractive-index polymers for a broad range of potential applications such as nanoimprinting and 3D printing under ambient conditions.
Solid N-vinyl monomer (NVC) was copolymerized with liquid MMA in DESs (Table 1). Under copolymerization conditions of [AIBN]0/[MMA]0/[NVC]0 = 1/100/100, solid NVC was dissolved in liquid MMA at room temperature prior to carrying out bulk copolymerization. The results were compared to copolymerization in DESs (Table 1). When the copolymerization was conducted in the hydrophobic NADESs (Thy/Cou and Tdc/Men) with AIBN at 60 °C for 24 h, P(MMA-co-NVC)s with higher yields (60–82%) and molecular weights (Mn = 56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–59
000–59![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, Mw/Mn = 2.80–3.07) were obtained, which were comparable to those in the bulk copolymerization (yield = 76%, Mn = 60
000, Mw/Mn = 2.80–3.07) were obtained, which were comparable to those in the bulk copolymerization (yield = 76%, Mn = 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, Mw/Mn = 4.39). A remarkably high molecular weight P(MMA-co-NVC) (Mn = 250
000, Mw/Mn = 4.39). A remarkably high molecular weight P(MMA-co-NVC) (Mn = 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) was obtained in ZnCl2/urea, and a relatively high molecular weight product with high yield (Mn = 95
000) was obtained in ZnCl2/urea, and a relatively high molecular weight product with high yield (Mn = 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, yield = 85%) was obtained in ChCl/urea, implying that the nature of the hydrophilic DES can contribute to an increase in the molecular weight and polymer yield. The photocopolymerization of MMA and NVC afforded P(MMA-co-NVC) with moderate molecular weight and polymer yield (Mn = 11
000, yield = 85%) was obtained in ChCl/urea, implying that the nature of the hydrophilic DES can contribute to an increase in the molecular weight and polymer yield. The photocopolymerization of MMA and NVC afforded P(MMA-co-NVC) with moderate molecular weight and polymer yield (Mn = 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100, yield = 61%) in Thy/Men (Table S8). This behavior was distinct from that of thermal copolymerization, showing no significant P(MMA-co-NVC) was obtained with AIBN at 60 °C (yield < 5%, Table 1). These results imply the feasibility of using both thermo- and photo-induced systems, depending on the nature of the aromatic heterocyclic monomers and constitutional components of the DESs, for the production of targeted high-refractive-index copolymers.
100, yield = 61%) in Thy/Men (Table S8). This behavior was distinct from that of thermal copolymerization, showing no significant P(MMA-co-NVC) was obtained with AIBN at 60 °C (yield < 5%, Table 1). These results imply the feasibility of using both thermo- and photo-induced systems, depending on the nature of the aromatic heterocyclic monomers and constitutional components of the DESs, for the production of targeted high-refractive-index copolymers.
The effects of hydrophobic NADESs and hydrophilic DESs on the copolymerization of BTVS and NVC with AIBN were studied under the same conditions (Table 1). Even when solid NVC was dissolved in liquid BTVS, bulk copolymerization of BTVS and NVC at 60 °C afforded only oligomers (Mn < 1000) with a low yield (13%). A similar low molecular weight P(BTVS-co-NVC) was obtained in hydrophobic NADESs. A remarkable increase in the polymer yield and molecular weight of P(BTVS-co-NVC) was achieved by copolymerization in hydrophilic DESs; the highest molecular weight P(BTVS-co-NVC) (Mn = 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, Mw/Mn = 2.29) was obtained with high yield (50%) in ChCl/urea, and a relatively high molecular weight product with broad molecular weight distribution (Mn = 5500, Mw/Mn = 4.33) was obtained in ZnCl2/urea at 60 °C. The resulting BTVS/NVC copolymers were insoluble in the hydrophilic DESs, and therefore liquid BTVS and solid NVC monomers were suspended during polymerization. Similar to emulsion polymerization, the polymerization rate (Rp) is substantially higher in the hydrophilic DESs (heterogeneous system) than in the hydrophobic NADESs (homogeneous system), which is probably attributed to compartmentalization effect. The segregation of the propagating radicals in a compartmentalized system may lead to a reduced termination rate. Such behaviors may affect the improved yields and molecular weights. The copolymerization of BTVS and NVC at higher temperature (80 °C) led to a slight decrease in the molecular weight (Mn < 10
000, Mw/Mn = 2.29) was obtained with high yield (50%) in ChCl/urea, and a relatively high molecular weight product with broad molecular weight distribution (Mn = 5500, Mw/Mn = 4.33) was obtained in ZnCl2/urea at 60 °C. The resulting BTVS/NVC copolymers were insoluble in the hydrophilic DESs, and therefore liquid BTVS and solid NVC monomers were suspended during polymerization. Similar to emulsion polymerization, the polymerization rate (Rp) is substantially higher in the hydrophilic DESs (heterogeneous system) than in the hydrophobic NADESs (homogeneous system), which is probably attributed to compartmentalization effect. The segregation of the propagating radicals in a compartmentalized system may lead to a reduced termination rate. Such behaviors may affect the improved yields and molecular weights. The copolymerization of BTVS and NVC at higher temperature (80 °C) led to a slight decrease in the molecular weight (Mn < 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), regardless of the hydrophobic NADESs and hydrophilic DESs (Table S7).
000), regardless of the hydrophobic NADESs and hydrophilic DESs (Table S7).
To find a suitable composition in terms of the polymerization behavior (polymer yield and molecular weights) and optical properties (high refractive index, high Abbe number, film-forming ability, and transparency), copolymerization was conducted at 60 °C in hydrophilic ZnCl2/urea at different feed ratios ([BTVS]0/[NVC]0 = 50/150, 75/125, and 150/50). The SEC traces of the copolymers showed bimodal peaks or a broad peak with a tailing at lower molecular weight region (Fig. S14a). Nevertheless, P(BTVS-co-NVC)s with high weight average molecular weights (Mw > 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) and different comonomer compositions were obtained (Table 1) depending on the feed ratio. Hence, the copolymerization strategy in DESs affords a greener and more efficient methodology to overcome the inherent synthetic drawbacks (low productivity and insufficient molecular weights) of copolymers consisting of aromatic heterocyclic S-vinyl and N-vinyl monomers.
000) and different comonomer compositions were obtained (Table 1) depending on the feed ratio. Hence, the copolymerization strategy in DESs affords a greener and more efficient methodology to overcome the inherent synthetic drawbacks (low productivity and insufficient molecular weights) of copolymers consisting of aromatic heterocyclic S-vinyl and N-vinyl monomers.
Correlation between refractive indices and Abbe numbers
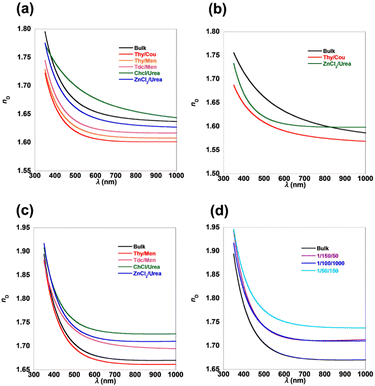
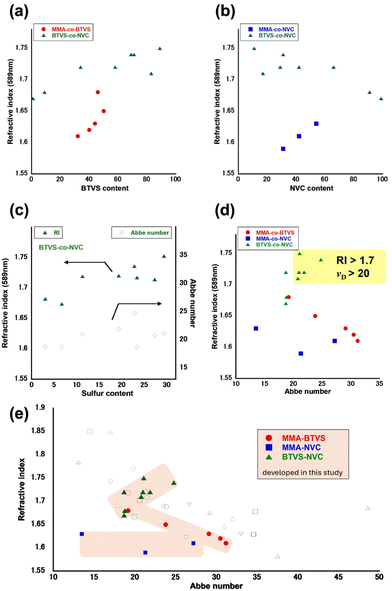
To evaluate the differences between the three series of MMA/BTVS, MMA/NVC, and BTVS/NVC copolymers, their sulfur and nitrogen contents, refractive indices, and Abbe numbers are summarized in Table 2. All the copolymers, P(MMA-co-BTVS), P(MMA-co-NVC), and P(BTVS-co-NVC), exhibit good solubility in common solvents (e.g., CHCl3 and DMF, Table S10), affording good processability and film-forming ability, such as the formation of smooth thin films via a spin-coating process. This good solubility likely originates from the molecular design of the aromatic heterocyclic monomers, which have flexible C–S bonds in BTVS and C–N bonds in NVC, leading to free rotation between the C–C backbone chain and heterocycle-substituted pendant groups in the copolymers. Uniform thin films of P(MMA-co-BTVS), P(MMA-co-NVC), and P(BTVS-co-NVC) were prepared by spin coating CHCl3 solutions (conc. = 1.0 wt%, 1500 rpm, 90 s), which were used for ellipsometry measurements. Fig. 2 compares the wavelength-dependent refractive indices of P(MMA-co-BTVS), P(MMA-co-NVC), and P(BTVS-co-NVC) prepared at a constant feed ratio (100/100) in hydrophobic NADESs, hydrophilic DESs, and bulk. The P(MMA-co-BTVS)s with high molecular weights prepared in hydrophilic DESs show high refractive indices (1.6794 for ChCl/urea and 1.6443 for ZnCl2/urea), which are comparable to those prepared in bulk (1.6488) and higher than those prepared in hydrophobic NADESs (<1.63). Similarly, P(MMA-co-NVC) prepared in ZnCl2/urea shows a higher refractive index (1.6065) than that prepared in Thy/Cou, while the copolymer prepared by bulk polymerization has a higher NVC content and therefore a higher refractive index (1.6349). P(MMA-co-BTVS)s show slightly higher refractive indices in the 1.6076–1.6794 range than P(MMA-co-NVC)s (1.5922–1.6349), which is likely because of the higher refractive index of BTVS over that of NVC (1.74 for PBTVS,57 1.69 for PNVC,66 and 1.49 for PMMA3). Among the three series of copolymers MMA/BTVS, MMA/NVC, and BTVS/NVC, P(BTVS-co-NVC)s prepared in hydrophilic DESs show higher refractive indexes (1.7359–1.7204). Both BTVS and NVC contain aromatic heterocycles; consequently, these copolymers exhibit excellent refractive indices.| Entryb | S cont.c (%) | N cont.d (%) | Film thickness (nm) | nD (589 nm) | nF (486 nm) | nd (587 nm) | nc (656 nm) | νD |
|---|---|---|---|---|---|---|---|---|
| a Film thickness, refractive indices (nD, nF, nd, and nc) and Abbe number (νD) were determined by ellipsometry.b See Table 1 and Table S7 for detailed copolymer samples. CP1, CP2, and CP3 correspond to P(MMA-co-BTVS), P(MMA-co-NVC), and P(BTVS-co-NVC), respectively.c Sulfur content.d Nitrogen content. | ||||||||
| CP1-1 | 21.70 | 4.76 | 131.5 | 1.6488 | 1.6696 | 1.6491 | 1.6423 | 23.8 |
| CP1-2 | 15.74 | 5.17 | 96.6 | 1.6076 | 1.6233 | 1.6077 | 1.6039 | 31.2 |
| CP1-3 | 18.63 | 4.04 | 106.2 | 1.6159 | 1.6320 | 1.6160 | 1.6118 | 30.5 |
| CP1-4 | 19.87 | 4.30 | 107.5 | 1.6253 | 1.6425 | 1.6255 | 1.6210 | 29.1 |
| CP1-5 | 20.60 | 4.68 | 191.9 | 1.6794 | 1.7044 | 1.6797 | 1.6690 | 19.2 |
| CP1-6 | 23.45 | 5.07 | 114.5 | 1.6443 | 1.6663 | 1.6446 | 1.6376 | 22.5 |
| CP2-1 | — | 5.03 | 178.5 | 1.6349 | 1.6679 | 1.6353 | 1.6209 | 13.5 |
| CP2-2 | — | 3.39 | 136.2 | 1.5922 | 1.6124 | 1.5924 | 1.5846 | 21.3 |
| CP2-6 | — | 4.22 | 263.0 | 1.6065 | 1.6244 | 1.6067 | 1.6021 | 27.2 |
| CP3-1 | 3.04 | 7.20 | 97.6 | 1.6822 | 1.7115 | 1.6825 | 1.6752 | 18.8 |
| CP3-2 | 0.37 | 6.72 | 101.0 | 1.6738 | 1.7028 | 1.6741 | 1.6668 | 18.7 |
| CP3-3 | 27.35 | 6.87 | 86.1 | 1.7141 | 1.7407 | 1.7144 | 1.7063 | 20.8 |
| CP3-4 | 22.83 | 7.51 | 109.2 | 1.7359 | 1.7598 | 1.7361 | 1.7301 | 24.8 |
| CP3-5 | 19.36 | 7.20 | 101.5 | 1.7204 | 1.7471 | 1.7206 | 1.7141 | 21.9 |
| CP3-6 | 11.32 | 7.97 | 114.1 | 1.7193 | 1.7476 | 1.7195 | 1.7134 | 21.0 |
| CP3-8 | 29.40 | 7.29 | 117.1 | 1.7528 | 1.7811 | 1.7531 | 1.7454 | 21.1 |
| CP3-11 | 23.42 | 7.19 | 113.8 | 1.7172 | 1.7465 | 1.7175 | 1.7081 | 18.7 |
As shown in Fig. 3a and b, the refractive index tends to increase with increasing BTVS and NVC content in P(MMA-co-BTVS)s and P(MMA-co-NVC)s, respectively. The Abbe numbers of MMA-containing copolymers vary substantially in the 19–32 range for P(MMA-co-BTVS)s and 13–28 for P(MMA-co-NVC)s, depending on the MMA content. These results are consistent with the general tendency that an increase in the refractive index leads to a decrease in the Abbe number. However, achieving high refractive indices while maintaining a high Abbe number is challenging; a lower Abbe number corresponds to higher dispersion.1,2 The refractive indices of the P(BTVS-co-NVC)s with different comonomer compositions increase substantially with increasing BTVS content (Fig. 3a). The contents of three cardinal units (sulfur, –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–, and aromatic rings) in the P(BTVS-co-NVC)s were determined by tuning the BTVS feed ratio, and refractive indices of P(BTVS-co-NVC)s increased from 1.6738 to 1.7528, while maintaining Abbe numbers greater than 18. As shown in Fig. 3c, the Abbe numbers of P(BTVS-co-NVC)s (18–25) are less sensitive to the sulfur content. This is attributed to the similar characteristics of BTVS and NVC, which are aromatic heterocyclic vinyl monomers with S and N atoms that are inherently distinct from conventional monomers containing only carbon and oxygen atoms (e.g., MMA). Notably, P(BTVS-co-NVC)s having suitable comonomer compositions (BTVS/NVC = 89/11–34/66) exhibit refractive indices of higher than 1.7 and Abbe numbers greater than 20, which differs from the general tendency for a trade-off between the refractive index and Abbe number (Fig. 3d). Comparison between aromatic heterocycle-containing copolymers developed in this work and previously reported polymers also indicates unique tendency of P(BTVS-co-NVC)s (Fig. 3e and Table S11), suggesting the possibility to go beyond a limitation line for the trade-off between the refractive index and Abbe number. Particularly, P(BTVS-co-NVC) prepared with [BTVS]0/[NVC]0 = 150/50 in ZnCl2/urea shows the highest refractive index (1.7528) with a relatively high Abbe number (21.1). In contrast, a typical trade-off between the refractive index and Abbe number is observed for P(MMA-co-BTVS)s and the refractive indexes of P(MMA-co-NVC)s are less sensitive to the Abbe numbers (Fig. 3e). In addition to the high molar fractions of both BTVS and NVC, which have aromatic heterocycles, the high refractive indices of the P(BTVS-co-NVC)s can be attributed to low molar volumes governed by polymerization shrinkage. Generally, a polymer exhibits a higher refractive index than that of the corresponding monomer because of denser packing.6 In our system, two vinyl monomers were copolymerized radically, which led to the loss of a double bond for each monomer, resulting in shrinkage. The decrease in the molar volume via shrinkage during copolymerization may contribute to the high refractive indices of P(BTVS-co-NVC)s, and the degree of the shrinkage is probably affected by the comonomer feed ratio and polymerization conditions (e.g., employment of DESs). Because the Abbe number is determined by the refractive index in addition to the molar refraction and molar dispersion,1,2 shrinkage during copolymerization may affect the Abbe number. The unique characteristics of DESs, their ability to form hydrogen bonds, and other specific interactions with monomers (e.g., thiazoyl group in BTVS, carbazole unit in NVC, and ester group in MMA), may contribute to improving the polymer yield and molecular weights, and to solving the tendency for a trade-off between refractive index and Abbe number.
N–, and aromatic rings) in the P(BTVS-co-NVC)s were determined by tuning the BTVS feed ratio, and refractive indices of P(BTVS-co-NVC)s increased from 1.6738 to 1.7528, while maintaining Abbe numbers greater than 18. As shown in Fig. 3c, the Abbe numbers of P(BTVS-co-NVC)s (18–25) are less sensitive to the sulfur content. This is attributed to the similar characteristics of BTVS and NVC, which are aromatic heterocyclic vinyl monomers with S and N atoms that are inherently distinct from conventional monomers containing only carbon and oxygen atoms (e.g., MMA). Notably, P(BTVS-co-NVC)s having suitable comonomer compositions (BTVS/NVC = 89/11–34/66) exhibit refractive indices of higher than 1.7 and Abbe numbers greater than 20, which differs from the general tendency for a trade-off between the refractive index and Abbe number (Fig. 3d). Comparison between aromatic heterocycle-containing copolymers developed in this work and previously reported polymers also indicates unique tendency of P(BTVS-co-NVC)s (Fig. 3e and Table S11), suggesting the possibility to go beyond a limitation line for the trade-off between the refractive index and Abbe number. Particularly, P(BTVS-co-NVC) prepared with [BTVS]0/[NVC]0 = 150/50 in ZnCl2/urea shows the highest refractive index (1.7528) with a relatively high Abbe number (21.1). In contrast, a typical trade-off between the refractive index and Abbe number is observed for P(MMA-co-BTVS)s and the refractive indexes of P(MMA-co-NVC)s are less sensitive to the Abbe numbers (Fig. 3e). In addition to the high molar fractions of both BTVS and NVC, which have aromatic heterocycles, the high refractive indices of the P(BTVS-co-NVC)s can be attributed to low molar volumes governed by polymerization shrinkage. Generally, a polymer exhibits a higher refractive index than that of the corresponding monomer because of denser packing.6 In our system, two vinyl monomers were copolymerized radically, which led to the loss of a double bond for each monomer, resulting in shrinkage. The decrease in the molar volume via shrinkage during copolymerization may contribute to the high refractive indices of P(BTVS-co-NVC)s, and the degree of the shrinkage is probably affected by the comonomer feed ratio and polymerization conditions (e.g., employment of DESs). Because the Abbe number is determined by the refractive index in addition to the molar refraction and molar dispersion,1,2 shrinkage during copolymerization may affect the Abbe number. The unique characteristics of DESs, their ability to form hydrogen bonds, and other specific interactions with monomers (e.g., thiazoyl group in BTVS, carbazole unit in NVC, and ester group in MMA), may contribute to improving the polymer yield and molecular weights, and to solving the tendency for a trade-off between refractive index and Abbe number.
Optical and thermal properties
The simultaneous achievement of high refractive index, high Abbe number, good transparency, and high thermal stability is still a target of interest in the field of high-refractive-index polymers. The transparency of the representative copolymers, P(MMA-co-BTVS), P(MMA-co-NVC), and P(BTVS-co-NVC), with similar comonomer compositions, was evaluated using UV-vis spectrophotometry. The combination-dependent transparency and thermal stability of the comonomers are compared in Table 3. Copolymer films produced by drop-casting from a CHCl3 solution (1.0 wt%) were used for evaluation, and the results are shown in Fig. 4a. Three copolymers show good transparency in the visible region (>96% at 400 nm), regardless of the comonomer combination (MMA/BTVS, MMA/NVC, or BTVS/NVC). As expected, radical copolymerization of MMA with the aromatic heterocyclic monomers afforded copolymers with good transparency, film-forming ability, and high thermal stability originating from MMA incorporation while maintaining a relatively high refractive index. Furthermore, structural design of pendant type copolymers with free rotation between the C–C backbone chain and aromatic heterocycle-substituted pendant groups can efficiently reduce interactions between the aromatic heterocycles and delocalized π-electrons. The incorporation of suitable aromatic heterocycles into the side chain through flexible C–S and C–N bonds impart good solubility and film-forming ability under mild conditions, in addition to an enhanced refractive index and Abbe number. No significant effect of the yellow color on the interpretation of optical measurements was detected for P(BTVS-co-NVC)s, while the cause of the yellow coloration is unknown. Further studies to clarify the cause of the coloring and to provide a suitable method for preventing yellow coloration are now in progress and will be communicated separately.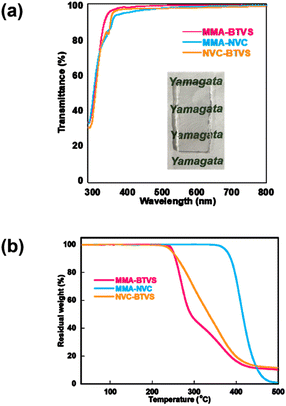 | ||
| Fig. 4 (a) UV-vis transmittance spectra and photographs of representative copolymer films and (b) TGA curves of the representative copolymers, P(MMA-co-BTVS), P(MMA-co-NVC), and P(BTVS-co-NVC). | ||
| Entry | Sample | Mn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b (Mw/Mn) b (Mw/Mn) |
n/mc | Sulfur cont.c | Film thicknessd (nm) | RId | Abbe numberd | Td5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) e (°C) e (°C) |
|---|---|---|---|---|---|---|---|---|
| a See Table 1 for detailed copolymer samples.b Measured by SEC.c Determined by elemental analysis.d Determined by ellipsometry.e The temperature at 5% weight loss (Td5). | ||||||||
| CP1-5 | P(MMA-co-BTVS) | 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (2.73) 000 (2.73) |
54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 46 46 |
20.60 | 191.9 | 1.6794 | 19.2 | 249 |
| CP2-6 | P(MMA-co-NVC) | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (2.52) 000 (2.52) |
58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 42 42 |
— | 263.0 | 1.6065 | 27.2 | 378 |
| CP3-5 | P(BTVS-co-NVC) | 5500 (4.33) | 58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 42 42 |
19.36 | 101.5 | 1.7204 | 21.9 | 246 |
Thermogravimetric analysis (TGA) of P(MMA-co-BTVS) and P(BTVS-co-NVC) reveals a gradual and/or stepwise thermolysis process in the range of 240–400 °C, whereas P(MMA-co-NVC) shows single-step decomposition in the range of 370–460 °C, as shown in Fig. 4b. The Td5 of P(MMA-co-NVC) is 378 °C, which is significantly higher than those of P(MMA-co-BTVS) and P(BTVS-co-NVC) (Td5 = 249 °C and 246 °C, respectively). Glass transition temperature (Tg) values of representative P(MMA-co-NVC)s were 153–158 °C, which were determined by differential scanning calorimetry (DSC). The values were apparently higher than those of BTVS-containing copolymers (Tg = 87–103 °C for P(MMA-co-BTVS)s and 88–90 °C for P(BTVS-co-NVC)s, Fig. S15). The thermal properties (Td5 and Tg values) could be manipulated by the chemical structure of the monomers, their composition, and molecular weights, which are crucial for further investigations (e.g., mechanical properties measurement). High thermal stability and practical optical transparency of P(MMA-co-NVC), while maintaining relatively high refractive indices (1.59–1.64), are attributed to suitable incorporation of MMA in the copolymers. P(BTVS-co-NVC)s synthesized from two aromatic heterocyclic monomers in DESs exhibit sufficient thermal stability (Td5 > 240 °C), highest refractive index (>1.7), relatively high Abbe number (>20), and high transparency (>96% at 400 nm), which make them promising candidates for future green production of high-refractive-index copolymers.
Conclusion
Three series of copolymers were synthesized by radical copolymerization of aromatic heterocyclic monomers (BTVS and NVC), combined with MMA, in hydrophobic NADESs and hydrophilic DESs. Compared with a conventional strategy using organic solvents or in bulk, radical copolymerization in DES has the following advantages: (i) increase in productivity (e.g., higher polymer yields of P(MMA-co-BTVS) prepared in ChCl/urea and Tdc/Men, and P(BTVS-co-NVC) prepared in ChCl/urea, compared to those in bulk), (ii) increase in molecular weight (e.g., highest molecular weights of P(MMA-co-NVC) prepared in ZnCl2/urea, and P(MMA-co-BTVS) and P(BTVS-co-NVC) prepared in ChCl/urea), and (iii) increased refractive index (e.g., higher refractive index of P(BTVS-co-NVC) prepared in ChCl/urea and ZnCl2/urea). Advantages (i) and (ii) are related to the increased viscosity and polarity of the DESs, depending on their nature. Advantage (iii) is related to the comonomer composition (e.g., BTVS/NVC) and the degree of shrinkage during copolymerization, which are likely affected by the nature of the DESs used as green polymerization solvents. P(BTVS-co-NVC) prepared in ZnCl2/urea showed the highest refractive index (>1.75) and a relatively high Abbe number (>21). The green synthetic approach using DESs involves the integration of aromatic heterocyclic S-vinyl and N-vinyl monomers to increase the refractive index and MMA to achieve good transparency and polymerizability. Suitable combinations of building blocks with aromatic heterocycles and DESs endow high-refractive-index copolymers with high Abbe numbers, high transparency, and increased productivity.Author contributions
Yuji Tsuhara: investigation, methodology, writing – original draft; Kento Sato: investigation; Sadaki Samitsu: investigation, validation, writing – review & editing; Hideharu Mori: conceptualization, supervision, validation, writing – review & editing.Conflicts of interest
There are no conflicts of interest to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information: experimental details, preliminary polymerization results in DESs (homopolymerization, copolymerization at higher temperature, and photocopolymerization), photographs of representative samples, and material characterization data (1H NMR spectra, SEC traces, solubility/miscibility, elemental analysis, and DSC results), and comparison data. See DOI: https://doi.org/10.1039/d5lp00343a.Acknowledgements
This work was financially supported by a JSPS KAKENHI Grant-in-Aids for Scientific Research (B) (23H02007). This work was supported by “Advanced Research Infrastructure for Materials and Nanotechnology in Japan (ARIM)” of the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Proposal Number JPMXP1224NM5511.References
- J.-g. Liu and M. Ueda, J. Mater. Chem., 2009, 19, 8907–8919 RSC.
- T. Higashihara and M. Ueda, Macromolecules, 2015, 48, 1915–1929 CrossRef CAS.
- G. S. Jha, G. Seshadri, A. Mohan and R. K. Khandal, e-Polym., 2007, 120 CrossRef.
- H. Ma, A. K. Y. Jen and L. R. Dalton, Adv. Mater., 2002, 14, 1339–1365 CrossRef CAS.
- D. Helmut, Angew. Chem., Int. Ed. Engl., 1979, 18, 49–59 CrossRef.
- T. Badur, C. Dams and N. Hampp, Macromolecules, 2018, 51, 4220–4228 CrossRef CAS.
- T. S. Kleine, R. S. Glass, D. L. Lichtenberger, M. E. Mackay, K. Char, R. A. Norwood and J. Pyun, ACS Macro Lett., 2020, 9, 245–259 CrossRef CAS PubMed.
- K. Mazumder, B. Voit and S. Banerjee, ACS Omega, 2024, 9, 6253–6279 CrossRef CAS PubMed.
- E. K. Macdonald and A. P. Shaver, Polym. Int., 2015, 64, 6–14 CrossRef CAS.
- X. Chen, L. Fang, J. Wang, F. He, X. Chen, Y. Wang, J. Zhou, Y. Tao, J. Sun and Q. Fang, Macromolecules, 2018, 51, 7567–7573 CrossRef CAS.
- Y. Su, E. B. D. S. Filho, N. Peek, B. Chen and A. E. Stiegman, Macromolecules, 2019, 52, 9012–9022 CrossRef CAS.
- M. D. Alim, S. Mavila, D. B. Miller, S. Huang, M. Podgorski, L. M. Cox, A. C. Sullivan, R. R. McLeod and C. N. Bowman, ACS Mater. Lett., 2019, 1, 582–588 CrossRef CAS.
- K. Nakabayashi, S. Sobu, Y. Kosuge and H. Mori, J. Polym. Sci., Part A: Polym. Chem., 2018, 56, 2175–2182 CrossRef CAS.
- S. Iino, S. Sobu, K. Nakabayashi, S. Samitsu and H. Mori, Polymer, 2021, 224, 123725 CrossRef CAS.
- K. Nakabayashi, T. Imai, M.-C. Fu, S. Ando, T. Higashihara and M. Ueda, Macromolecules, 2016, 49, 5849–5856 CrossRef CAS.
- Z. Dou, J. Li, Z. Shi, J. Sun and Q. Fang, ACS Appl. Polym. Mater., 2025, 7, 5302–5311 CrossRef CAS.
- Q. Wei, R. Poetzsch, X. Liu, H. Komber, A. Kiriy, B. Voit, P.-A. Will, S. Lenk and S. Reineke, Adv. Funct. Mater., 2016, 26, 2545–2553 CrossRef CAS.
- S. Gazzo, G. Manfredi, R. Poetzsch, Q. Wei, M. Alloisio, B. Voit and D. Comoretto, J. Polym. Sci., Part B:Polym. Phys., 2016, 54, 73–80 CrossRef CAS.
- M. Luo, X.-H. Zhang and D. J. Darensbourg, Acc. Chem. Res., 2016, 49, 2209–2219 CrossRef CAS PubMed.
- J. J. Griebel, R. S. Glass, K. Char and J. Pyun, Prog. Polym. Sci., 2016, 58, 90–125 CrossRef CAS.
- Z. Tan, C. Wu, M. Zhang, W. Lv, J. Qiu and C. Liu, RSC Adv., 2014, 4, 41705–41713 RSC.
- S. D. Bhagat, J. Chatterjee, B. Chen and A. E. Stiegman, Macromolecules, 2012, 45, 1174–1181 CrossRef CAS.
- S. Nagayama and B. Ochiai, Polym. J., 2016, 48, 1059–1064 CrossRef CAS.
- S. D. Bhagat, E. B. D. S. Filho and A. E. Stiegman, Macromol. Mater. Eng., 2015, 300, 580–585 CrossRef CAS.
- Q. Li, Y. Zhang, Z. Chen, X. Pan, Z. Zhang, J. Zhu and X. Zhu, Org. Chem. Front., 2020, 7, 2815–2841 RSC.
- Y. Tokushita, A. Watanabe, A. Torii, K. Nakabayashi, S. Samitsu and H. Mori, React. Funct. Polym., 2021, 165, 104960 CrossRef CAS.
- Y. Tokushita, S. Furuya, S. Nobe, K. Nakabayashi, S. Samitsu and H. Mori, Polymer, 2021, 237, 124346 CrossRef CAS.
- N. Huo and W. E. Tenhaeff, Macromolecules, 2023, 56, 2113–2122 CrossRef CAS PubMed.
- S. Watanabe, H. Nishio, T. Takayama and K. Oyaizu, ACS Appl. Polym. Mater., 2023, 5, 2307–2311 CrossRef CAS.
- A. P. Abbott, G. Capper, D. L. Davies, R. K. Rasheed and V. Tambyrajah, Chem. Commun., 2003, 1, 70–71 RSC.
- Q. Zhang, K. D. O. Vigier, S. Royer and F. Jerome, Chem. Soc. Rev., 2012, 41, 7108–7146 RSC.
- E. L. Smith, A. P. Abbott and K. S. Ryder, Chem. Rev., 2014, 114, 11060–11082 CrossRef CAS PubMed.
- A. Paiva, R. Craveiro, I. Aroso, M. Martins, R. L. Reis and A. R. C. Duarte, ACS Sustainable Chem. Eng., 2014, 2, 1063–1071 CrossRef CAS.
- D. Carriazo, M. C. Serrano, M. C. Gutierrez, M. L. Ferrer and F. del Monte, Chem. Soc. Rev., 2012, 41, 4996–5014 RSC.
- L. I. N. Tome, V. Baiao, W. da Silva and C. M. A. Brett, Appl. Mater. Today, 2018, 10, 30–50 CrossRef.
- J. D. Mota-Morales, R. J. Sanchez-Leija, A. Carranza, J. A. Pojman, F. del Monte and G. Luna-Barcenas, Prog. Polym. Sci., 2018, 78, 139–153 CrossRef CAS.
- K. F. Fazende, D. P. Gary, J. D. Mota-Morales and J. A. Pojman, Macromol. Chem. Phys., 2020, 221, 1900511 CrossRef CAS.
- M. Isik, F. Ruiperez, H. Sardon, A. Gonzalez, S. Zulfiqar and D. Mecerreyes, Macromol. Rapid Commun., 2016, 37, 1135–1142 CrossRef CAS PubMed.
- Y. H. Choi, J. van Spronsen, Y. Dai, M. Verberne, F. Hollmann, I. W. C. E. Arends, G.-J. Witkamp and R. Verpoorte, Plant Physiol., 2011, 156, 1701–1705 Search PubMed.
- Y. Dai, J. van Spronsen, G.-J. Witkamp, R. Verpoorte and Y. H. Choi, Anal. Chim. Acta, 2013, 766, 61–68 Search PubMed.
- A. V. Gomez, A. Biswas, C. C. Tadini, R. F. Furtado, C. R. Alves and H. N. Cheng, J. Braz. Chem. Soc., 2019, 30, 717–726 Search PubMed.
- V. A. Pereira, T. C. Rezende, P. V. Mendonca, J. F. J. Coelho and A. C. Serra, Green Chem., 2020, 22, 6827–6835 RSC.
- V. A. Pereira, P. V. Mendonca, J. F. J. Coelho and A. C. Serra, Polymer, 2022, 242, 124586 CrossRef CAS.
- C.-Y. Li and S.-S. Yu, Macromolecules, 2021, 54, 9825–9836 CrossRef CAS.
- Y.-T. Chou, W.-R. Lee and S.-S. Yu, Macromolecules, 2024, 57, 9241–9249 CrossRef CAS.
- Chemical Handbook Basic II, Maruzen, 1979 Search PubMed.
- Y. Nakagawa, Y. Suzuki, T. Higashihara, S. Ando and M. Ueda, Macromolecules, 2011, 44, 9180–9186 CrossRef CAS.
- M.-C. Fu, Y. Murakami, M. Ueda, S. Ando and T. Higashihara, J. Polym. Sci., Part A: Polym. Chem., 2018, 56, 724–731 CrossRef CAS.
- N.-H. You, T. Higashihara, Y. Oishi, S. Ando and M. Ueda, Macromolecules, 2010, 43, 4613–4615 CrossRef CAS.
- N.-H. You, Y. Nakamura, Y. Suzuki, T. Higashihara, S. Ando and M. Ueda, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 4886–4984 CrossRef CAS.
- K. Nakabayashi, T. Imai, M.-C. Fu, S. Ando, T. Higashihara and M. Ueda, J. Mater. Chem. C, 2015, 3, 7081–7087 RSC.
- N. Oh, K.-H. Nam, M. Goh, B.-C. Ku, J. G. Kim and N.-H. You, Polymer, 2019, 165, 191–197 CrossRef CAS.
- R. A. Minns and R. A. Gaudiana, J. Macromol. Sci., Pure Appl. Chem., 1992, 29, 19–30 CrossRef.
- Y. C. An and G.-i. Konishi, J. Appl. Polym. Sci., 2012, 124, 789–795 CrossRef CAS.
- T. Matsuda, Y. Funae, M. Yoshida and T. Takaya, J. Macromol. Sci., Pure Appl. Chem., 1999, 36, 1271–1288 CrossRef.
- Y. Tang, S. Cabrini, J. Nie and C. Pina-Hernandez, Chin. Chem. Lett., 2020, 31, 256–260 CrossRef CAS.
- Y. Sato, S. Sobu, K. Nakabayashi, S. Samitsu and H. Mori, ACS Appl. Polym. Mater., 2020, 2, 3205–3214 CrossRef CAS.
- I. Watanabe, K. Morikawa, S. Samitsu and H. Mori, Macromol. Chem. Phys., 2023, 224, 2300289 CrossRef CAS.
- K. Nakabayashi, A. Matsumura, Y. Abiko and H. Mori, Macromolecules, 2016, 49, 1616–1629 CrossRef CAS.
- D. J. G. P. van Osch, C. H. J. T. Dietz, J. van Spronsen, M. C. Kroon, F. Gallucci, M. v. S. Annaland and R. Tuinier, ACS Sustainable Chem. Eng., 2019, 7, 2933–2942 CrossRef CAS.
- S. Yuki, R. Shinohe, Y. Tanaka and H. Mori, Polym. Chem., 2024, 15, 3629–3640 RSC.
- A. P. Abbott, J. C. Barron, K. S. Ryder and D. Wilson, Chem. – Eur. J., 2007, 13, 6495–6501 CrossRef CAS PubMed.
- J. Brandrup and E. H. Immergut, Polymer Handbook, Wiley Publisher, New York, 1991 Search PubMed.
- C. C. Price and H. Morita, J. Am. Chem. Soc., 1953, 75, 4747–4750 CrossRef CAS.
- K. J. Thurecht, P. N. Gooden, S. Goel, C. Tuck, P. Licence and D. J. Irvine, Macromolecules, 2008, 41, 2814–2820 CrossRef CAS.
- J. E. McGrath, L. Rasmussen, A. R. Shultz, H. K. Shobha, M. Sankarapandian, T. Glass, T. E. Long and A. J. Pasquale, Polymer, 2006, 47, 4042–4057 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2026 |