 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Recent advances in hollow and core–shell intrinsically conducting polymers for applications in electromagnetic interference shielding/microwave absorption, removal of metal ions/dyes and supercapacitors
Suneel Kumar
Srivastava

Department of Chemistry, Indian Institute of Technology, Kharagpur-721302, India. E-mail: suneel@chem.iitkgp.ac.in; suneelchemkgp@gmail.com
First published on 4th November 2025
Abstract
In recent years, considerable attention has been paid to hollow micro–nano-structured intrinsically conducting polymers (ICPs), like polyaniline, polypyrrole, polythiophene and poly(3,4-ethylenedioxythiophene). The availability of the shells and inner voids in the hollow ICP accounts for their tunable physical/chemical properties, offers low density, a high surface area, reduced length for both mass and charge transport, and offers promising applications in environmental remediation and energy. In view of this, the present review article is focused on the synthesis methods used in the fabrication of hollow intrinsically conducting polymers by means of hard and soft template approaches including template-free methods, synthesis of double-shelled hollow spheres of ICP, hollow ICP nanocomposites and core–shell structural materials of ICP. Subsequently, the review highlights the application of the hollow intrinsically conducting polymers, their nanocomposites and core–shell structured materials for applications in electromagnetic interference shielding/microwave absorption, removal of heavy metal ions and different dyes from wastewater, and as efficient electrode materials in supercapacitors. Finally, the review ends with a summary and future perspective on hollow-structured and core–shell conducting polymers and their applications in the above fields of application.
1. Introduction
Intrinsically conducting polymers (ICPs), comprising polyaniline (PANI), polypyrrole (PPy), polythiophene (PT), and poly(3,4-ethylenedioxythiophene) (PEDOT), are considered a special class of polymers due to their light weight, ease of fabrication, good environmental stability, low cost, high mechanical flexibility, and good biocompatibility.1Fig. 1 (inset) shows the schematic chemical structures of different monomers of all these ICPs.2 They exhibit tunable surface area, electrical conductivity, optical properties, unique redox tenability, and high electrochemical response.3 As a result, ICPs find their applications in various advanced fields, such as sensing,4 solar cell rechargeable,5 battery,6 supercapacitor,7 bioelectronic applications,8 electromagnetic interference shielding and microwave absorption,9 healthcare monitoring,10 photocatalysis and opportunities for artificial intelligence,11 biomedical applications,12 drug delivery,13 removal of water pollutants.14,15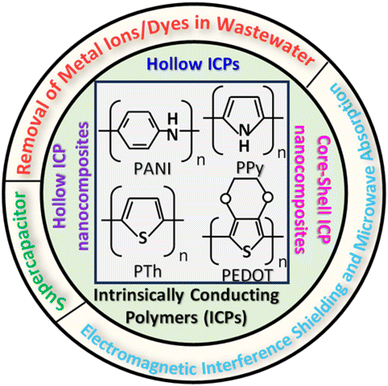 | ||
| Fig. 1 Hollow and core–shell intrinsically conducting polymers (ICPs) and their applications (Inset: structures of common ICPs.2 Reproduced with permission from Elsevier). | ||
The physical properties of PANI, PPy, PTH and PEDOT are influenced by their morphology.16 In this regard, PANI, compared with other ICPs, has been studied extensively for various reasons to date considering different morphologies, such as granular structure,17 nanoparticles,17 spongy,18 dendrite,19 nano-neddles,20 nanocables,21 nanotubes,21,22 nanofiber,23 nanowire,24 nanobelts,25 nanorods,26 nanostick,27 nanoflakes,28 microspheres,29 nanocapsules,30 flower-like nanosheet clusters,31 coral-like,32 honeycomb,33 worm-like interlinked structures,34 tetragonal star like,35 nanospheres36etc. Polypyrrole, polythiophene and PEDOT also exist in many of these morphologies. It is well established that manipulation of the morphology can lead to enhancement of the physicochemical properties and the performance of conducting polymers.37,38 In this regard, ‘hollow structure’ ICPs with void space inside a distinct shell and dimensions in the micrometer-/nanometer range have been receiving a considerable amount of attention in recent years.39–50 The synthesis of such hollow materials with a single or double shells of various compositions and morphologies has been reported following hard-templating, soft-templating and template-free methods.49
Hollow structures can be of different shapes, as schematically illustrated in Fig. 2.41 From this perspective, hollow spheres are considered to be more advantageous than their corresponding solid counterparts due to the presence of a large fraction of empty space inside and intact shell(s).50 The interior geometry, surface functionality, high specific surface area, and high conductivity, as well as the controllable chemical and physical properties of the hollow ICP nanostructures make them the most promising candidates in several of the applications stated earlier.6,51 In addition, much attention has also been paid to nanotubes, characterized by their unique hollow tubular structure.52 Furthermore, the introduction of nanomaterial(s) into these conducting polymers can introduce unique properties such as high surface area, high electrical conductivity and other features that could enhance their performance in multifaceted applications.49–53
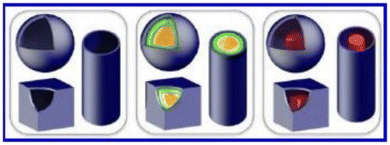 | ||
| Fig. 2 Schematic illustration showing various hollow structures: (left) hollow spheres/boxes/tubes; (middle) multi-shelled hollow spheres/boxes/tubes; (right) yolk–shell, cube-in-box, and wire-in-tube structures.41 Reproduced with permission from ACS. | ||
The microwave (MW) radiation emitted from various electrical and electronic devices adversely degrades their performance9,54 and is very harmful to human health.55 Therefore, electromagnetic interference (EMI) shielding and microwave absorption materials are essentially needed for mitigating such electromagnetic pollution.56–59 In this context, hollow conducting polymers combined with fillers (such as magnetic nanoparticles) can also facilitate enhanced EMI shielding and microwave absorption due to the dielectric loss owing to the intrinsic conductivity from the polymer and magnetic loss arising from the magnetic filler, respectively. The unique hollow morphology of conducting polymers with increased surface area prevents secondary electromagnetic pollution due to reflection. Furthermore, the presence of multiple internal reflections and the trapping of EM waves contribute to their excellent performance in EMI shielding and microwave absorption. It may be noted that hollow ICPs can interact with EM waves and convert the energy of the microwaves into heat and dissipate it.9 In addition, hollow PANI and PPy have been extensively studied as adsorbents in the removal of heavy metals and dyes in water.60–64 The excellent adsorption performance of hollow conducting polymers in the removal of metals and dyes is ascribed to their large specific surface area and its tunable surface properties. The available functional binding sites in the conducting polymers readily interact with pollutants through various mechanisms, accounting for their effectiveness as adsorbents. Recently, hollow ICPs have also been used in supercapacitor applications due to their low cost and properties.7 It may be noted that the unique hollow spherical morphology of ICPs accounts for their larger surface area, significantly lower density, and high loading capacity, and shorter ion diffusion paths facilitate achieving high-power density, fast charge and discharge rates, and long cycle life.65 In addition, hollow conducting polymers also improve cycling stability by allowing for controlled volume changes during redox reactions.53
In recent years, core–shell-structured materials formed by the judicious tuning of the core as well as the shell(s) have received considerable attention due to their applications in a variety of fields.66–68 In this regard, core–shell materials exhibiting enhanced magnetic and dielectric loss have the ability to perform well in electromagnetic interference shielding and microwave absorption.67,69 In addition, impedance matching in the core–shell materials creates numerous interfaces for multiple reflections and facilitates attenuation of microwaves due to multiple reflections.9 Core–shell materials formed by combining a functional conducting polymer shell with a stable core are also receiving more attention as adsorbents for the removal of pollutants in water.68 The choice is mainly guided by the unique properties of both the core and the shell in achieving high adsorption capacity, separation efficiency, and reusability compared with conductive polymers exhibiting low solubility, limited active sites, poor mechanical strength, and separation difficulties. Most importantly, a magnetic core can facilitate the complete separation of the core–shell adsorbents from water by applying an additional magnetic field.70,71 The core–shell approach has also been used in fabricating ICP-based electrode materials with excellent electrochemical performance in supercapacitor applications.7 This attractive choice is motivated by the synergistic combination of the core and shell materials leading to a high specific surface area for abundant reaction sites, mechanical stability, good electrical conductivity for fast charge transfer, faster diffusion kinetics, high specific capacitance, and longer cyclability.72,73
In view of this, several review articles have appeared in recent years on intrinsically conducting polymers focused on the electromagnetic interference shielding and microwave absorption,2,9,54,74–80 removal of heavy metal ions60–64,81–85 and organic dyes60–64,86–91 in wastewater, and supercapacitors,7,65,72,73,92–98 and several others are also referred to in subsequent sections. However, considering the rapid developments, there exists a lack of a comprehensive review highlighting the novel contribution of hollow and core–shell intrinsically conducting polymers with their applications in mitigating electronic/environmental pollution and their applications in energy storage.
Motivated by this, the present review aims to highlight the novel contribution made by intrinsically conducting polymers in this regard by focusing exclusively on hollow and core–shell morphologies across three distinct application fields (EMI shielding, adsorption, supercapacitors). Accordingly, the present article is focused on the preparation of hollow intrinsically conducting polymers (PANI, PPy, PTP, PEDOT) and core–shell materials comprising ICP through different approaches. This is followed by their applications in electromagnetic interference shielding/microwave absorption, removal of heavy metal ions/dyes in water, and supercapacitors (Fig. 1). Finally, the review article ends with a future perspective and summary. Overall, the fabrication of the unique hollow and core–shell morphology of important intrinsically conducting polymers and the role they play in the multifaceted applications reviewed in this article clearly complements the existing literature.
2. Intrinsically conducting polymers
Considerable interest has been aroused in intrinsically conducting polymers (ICPs) during the past decades due to their many advantages, such as easy modification, chemical diversity, corrosion resistance, morphology, tunable conductivity and applications.99–101 The different properties of these polymers are related to the conjugated chains with alternating single and double bonds and the delocalized π-electrons.96 The conducting polymers have been doped by different methods in order to achieve high conductivities.101–104 In general, conductivity increases with increasing doping level and becomes saturated at high doping levels for most of the conducting polymers.105 The doping is introduced in ICPs through the backbone of the polymer chain by neutral dopants (I2, Br2, AsF2, H2SO4, FeCl3etc.), ionic dopants (LiClO4, FeClO4, CF3SO3Na, BuNClO4etc.), organic dopants (CF3COOH, CF3SO3Na etc.) and polymeric dopants such as poly(styrene sulphonic acid), poly(vinyl sulphonic acid), and poly(acrylic acid).106,107The electrical properties of conducting polymers are modified by p- and n-type doping.105 The delocalization of the charge carriers over the polymer chain accounts for their electronic conductivity.102 Generally, the negatively charged carriers in n-doping are not as stable as positively charged ones. This makes p-type doping more attractive in academic research as well as for all practical application purposes.102,108,109Fig. 3(a) describes the electronic and chemical structures of polythiophene as a representative conducting polymer subjected to p-type doping and n-type doping.102 The electronic bands and chemical structures illustrating undoped, polaron, bipolaron and fully doped states of polypyrrole are also described in Fig. 3(b).102
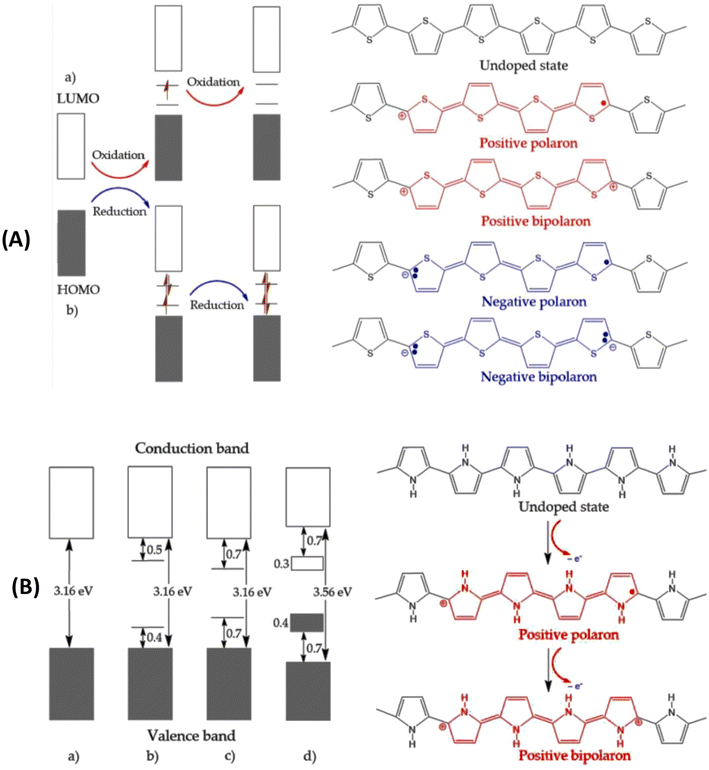 | ||
| Fig. 3 (A) The electronic band and chemical structures of polythiophene (PTh) with (a) p-type doping and (b) n-type doping, and (B) electronic bands and chemical structures illustrating (a) undoped; (b) polaron; (c) bipolaron; and (d) fully doped states of polypyrrole.102 Reproduced with permission from MDPI. | ||
3. Synthesis of intrinsically conducting polymers
3.1 Chemical methods
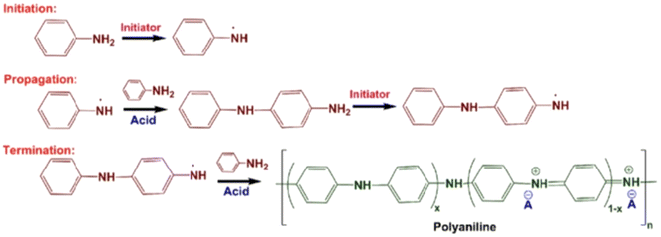 | ||
| Fig. 4 Chemical polymerization mechanisms of polyaniline. Chemical polymerization of polyaniline is carried out in acidic medium by using a common initiator such as ammonium persulfate and potassium persulfate.99 Reproduced with permission from ACS. | ||
The mechanism of pyrrole monomer polymerization has also received a considerable amount of attention.127–131 The most widely accepted polymerization mechanism of PPy is displayed in Fig. 5.127,128 The oxidation of a pyrrole monomer yields a radical cation of the monomer in the initiation step (Fig. 5A). Coupling of the two generated radical cations then deprotonation produces a bipyrrole.130 This bipyrrole in the propagation step is oxidized again and couples with another oxidized segment in the propagation step Fig. 5(B). Further continuation of the re-oxidation, coupling, and deprotonation ultimately leads to the formation of polypyrrole.
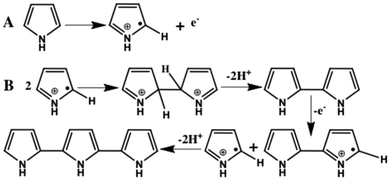 | ||
| Fig. 5 The oxidation of a pyrrole monomer (A) and formation of dimer and trimer of pyrrole (B).128 Reproduced with permission from Elsevier. | ||
Another chemical oxidative polymerization mechanism has also been proposed. According to this, the radical cation in the propagation step of this mechanism reacts with a neutral monomer.131 The dimer formed in this manner is oxidized to a dimeric radical cation that subsequently attacks another neutral monomer to form a trimer (Fig. 6(A)),128 and polymer chains grow by the repeated process to ultimately form polypyrrole. Recently, Tan and Ghandi127 observed that a commonly used mechanism (Fig. 5B) for the polymerization of pyrrole is not well suited for the formation of PPy in aqueous medium. Instead, the polymerization mechanism of polypyrrole follows a different mechanism, as schematically shown in Fig. 6.128
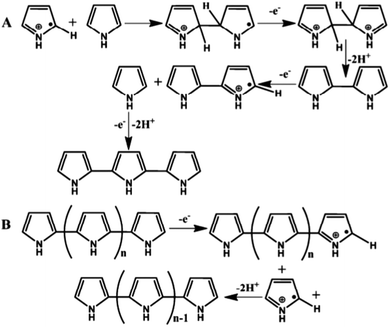 | ||
| Fig. 6 The formation of dimer and trimer of pyrrole (A) and further formation of the PPy (B).128 Reproduced with permission from Elsevier. | ||
3.2 Electrodeposition methods
The electrodeposition method has been proved to be a versatile route for growing conducting polymers on a substrate for small-scale synthesis within a short reaction time under mild reaction conditions.145 This technique is simple, rapid, cost effective and ensures good control of the micro/nanostructured polymer morphology, achieved with accurate process control, compared with other techniques.146–153 The process is usually performed using a constant current or voltage approach, typically in a three-electrode (reference, working, and counter) cell containing the electrolyte and the monomer solution. The monomer of the conducting polymer undergoes electrochemical polymerization and is subsequently deposited on the surface of the substrate (Ti,147 Mg,149 Pt,150 stainless steel,150 carbon cloth,151 glassy C,152 indium tin oxide,153 graphite sheet154etc). The effect of counter ions on the physical properties of polypyrrole electrochemically deposited on a Pt electrode has been reported.155 The codeposition method has also been reported, similar to electrode coating, by dissolving the insulating polymer (host) in the electrolyte solution that also contains the monomer of the conductive polymer.156Fig. 7 describes the electrochemical polymerization mechanisms of polyaniline carried out in the electrolyte solution of aniline and acid through application of a potential difference between the working and counter electrodes.99
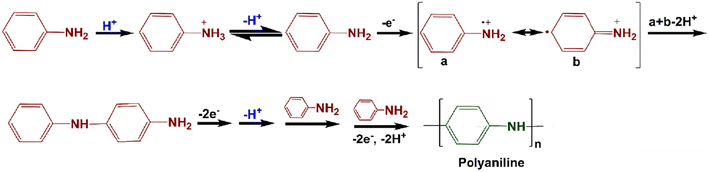 | ||
| Fig. 7 Electrochemical polarization mechanism of polyaniline (electro-polymerization is carried out in the electrolyte solution of aniline and acid through applying a potential difference between the working and counter electrode).99 Reproduced with permission from ACS. | ||
3.3 Other methods
In addition, several other preparative methods for conducting polymers have been used, such as photopolymerization,157 radio-frequency plasma polymerization,158 organometallic cross-coupling reaction159 and vacuum vapour phase polymerization.160,161The properties of the conducting polymers are influenced by oxidant, monomer molar ratio, type of oxidizing agent, pH, polymerization temperature, time, concentration, electrolyte concentration, and degree of doping.162–165 The electrical conductivity of most conducting polymers increases with dopant concentration and becomes saturated at a high doping level.104 Temperature is another parameter playing a significant role in the synthesis of ICPs. The selection of solvent is also an important factor. Generally, the polymerization of PANI and PPy is reported using solvents like water, MeOH, THF, DMF, DMSO acetonitrile, propylene carbonate and sea water.41,166–168 However, aqueous medium as a solvent is most desirable due to its relatively low cost, ease of handling and nontoxicity.
4. Synthesis of hollow and core–shell ICPs
The synthesis of hollow polymeric micro/nanospheres and micro/nanotubes of ICPs have been receiving much attention in recent years due to their promising applications in electromagnetic interference shielding and microwave absorption, environmental remediation and energy storage devices. In view of this, ICPs exhibiting multifaceted types of hollow morphology, such as hollow micro/nanospheres and micro/nanotubes, have been achieved through different approaches. These include direct and template-directed synthesis, the core–shell approach, self-assembly and electrospinning, and are described below.4.1 Hard template method for single-shell hollow ICPs
Recently, template-assisted synthesis has attracted much attention in the fabrication of materials of well-defined morphology in terms of shape and size ranging from nanometer to micrometer range.169–172 In this regard, template-assisted methods are considered as one of most accepted approaches for the synthesis of hollow-structured intrinsically conducting polymer materials. These templates can be classified into two types, namely hard and soft templates, based on the difference in their structure (Fig. 8(a)).173 Hard templates are rigid structures in contrast to the soft templates characterized by flexible structures, with each approach guided by its own advantages and disadvantages.174 The synthesis of materials using different types of templates is schematically shown in Fig. 8(b).175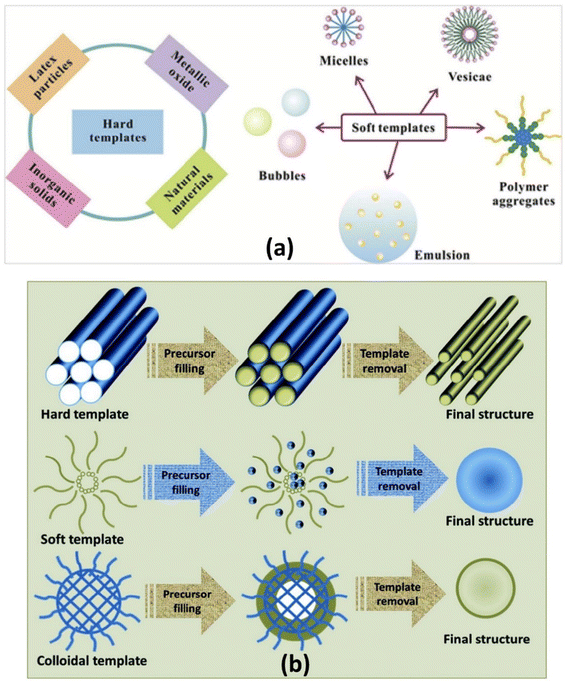 | ||
| Fig. 8 (a) Representative materials that can be used as templates.173 Reproduced with permission from Elsevier, and (b) schematic representation of the synthesis of materials using different types of templates.175 Reproduced with permission from RSC. | ||
However, the main challenge in the preparation of hollow morphology, such as spheres, using template methods lies in the availability of suitable templates of definite size/shape, surface property, and production availability, and their subsequent removal through chemical or thermal means as an additional step to produce a void space.176 In this regard, the hard template has great advantages, such as adjustable pore structure and morphology; nevertheless, the hard template method also has disadvantages.171 It usually requires a core surface modification to ensure successful coating of shell substances.173 Hollow structures can also be prepared by a soft template method as a viable option.175,176 Although the hard template has high reproducibility and stability, its separation may be troublesome and may damage the structure of the desired materials with hollow morphology.171 On the other hand, with the soft template it is easier to prepare nanomaterials of various sizes and shape due to the simplicity of the process involved, and its easy removal than the hard template.171 In addition, soft templates are sensitive to solution environments (pH, solvents, ionic strength etc.), thereby limiting the application of the soft-core template processes.177 The hollow morphology achieved by the hard template procedure is guided by its adjustable pore structure and morphology. As a result, several hollow nanostructures can be fabricated considering the availability of the wide range of such hard templates.178
Cheng et al.184 prepared highly uniform and ordered polypyrrole nanotube arrays with the help of porous anodic aluminium oxide following chemical oxidation polymerization for 2 hours. Functional polypyrrole nanotubes have been fabricated using the anodized aluminium oxide membrane as template in liquid phase polymerization conditions.185 In another method, Jang et al.186 adopted one-step vapour deposition polymerization to synthesize a highly uniform surface and tuneable wall thickness in polypyrrole nanotubes using anodic aluminium oxide template membranes soaked in ferric chloride aqueous solution.
Quasi-polyaniline hollow nanotubes (outer dia: 230 nm) were prepared by a dipping method based on the highly ordered porous anodic alumina membrane.187 Poly(3, 4-ethylenedioxythiophene) tosylate film was grown in confined anodized aluminium oxide (nanopores) transferred onto cleaned ITO glass substrates following the chemical polymerization of the mixture comprising the solution of EDOT, iron(III)-tosylate in butanol and pyridine.188 Liu et al.189 electrochemically synthesized PEDOT nanotube arrays in the cylindrical pores of an alumina template membrane using acetonitrile solution of 20 mM EDOT.
Fu et al.191 initially prepared silica nanoparticles with surface-grafted polymer of 4-vinylaniline (SiO2-g-PVAn) according to the scheme displayed in Fig. 9(a). Subsequent surface oxidative graft copolymerization of aniline using the aniline moieties of PVAn and deprotonation followed by exposure to HF produced hollow nanospheres of P(VAn-graft-PANI) (thickness of shell: ∼15–40 nm, core void dia: ∼25 nm). A mesoporous polyaniline hollow nanosphere with average diameter and thickness of 330 nm shell 78 nm, respectively, has been prepared using SiO2@resorcinol-formaldehyde/SiO2 as substrate followed by in situ polymerization of aniline on its surface and subsequent expulsion of the silica shell by dispersing it in HF at room temperature.192 Li et al.193 prepared Pt/PPy hollow hybrid microspheres by using NH2-functionized SiO2 as template decorated with H2PtCl6, Fig. 9(b). In another study, SiO2/polymethacrylic acid (PMAA) microspheres were used as templates to synthesize hollow polypyrrole microspheres (Fig. 9(c)).194
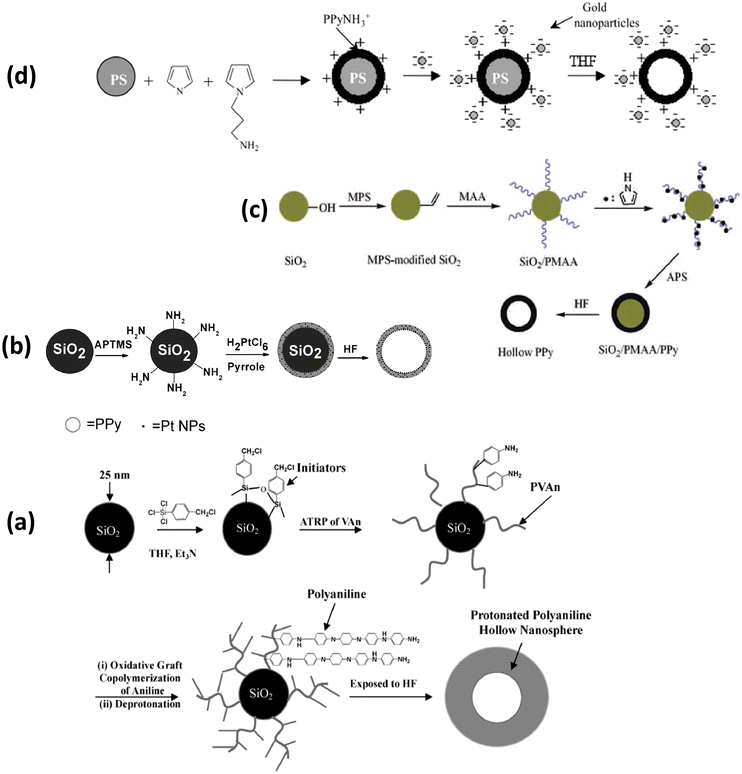 | ||
| Fig. 9 (a) Preparation of conductive poly(4-vinylaniline-graft-polyaniline), or P(VAn-g-PANI), hollow nanospheres via surface-initiated atom transfer radical polymerization (ATRP) and oxidative graft copolymerization.191 Reproduced with permission from ACS. (b) The illustration of the formation of Pt/PPy hollow spheres.193 Reproduced with permission from Wiley. (c) Formation of SiO2/PMAA/PPy nanocomposite and PPy hollow spheres.194 Reproduced with permission from Wiley. (d) Schematic representation of the assembly of negatively charged gold nanoparticles on the surface of positively charged core–shell polypyrrole–polystyrene latex particles bearing surface-protonated N-propylamino groups.206 Reproduced with permission from ACS. | ||
4.1.3.1 Hollow polyaniline. Among several hard templates, polystyrene microspheres have attracted much attention in synthesizing spherically shaped particles, spherical core–shell and hollow structures.195–199 Bai et al.200 prepared colloidal hollow spheres of conducting polymers (PANI and PPy) by using sulfonated polystyrene beads as a templating agent. In another study, hollow PANI and PPy microspheres were prepared by oxidative chemical oxidation of the respective monomers using sulfonated polystyrene microspheres as template (size: 2.2–3.4 μm).201 Niu et al.202 prepared PANI capsules and hollow PANI spheres with controlled shell thickness and cavity size using sulfonated polystyrene as a template. Sulfonated polystyrene particles have been used as template to synthesize hollow polyaniline203 and polypyrrole204 by emulsion polymerization of the individual monomers in acid solution and ammonium persulphate (oxidant). Saraf et al.205 prepared hollow microspheres of PANI doped with styrene sulfonic acid (size: 0.5–1 μm) through a chemical route by maintaining the dopant
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) monomer
monomer![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) oxidant ratio as 1
oxidant ratio as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
Mangeney et al.206 used polystyrene latex particles (dia: 1.33 μm) to prepare PPy-coated PS latex polystyrene bearing surface-protonated N-propyl amino functional groups in aqueous solution by copolymerization of pyrrole and N-aminated pyrrole (pyrrole-NH2) using FeCl3. Furthermore, the fabricated (PS-PPyNH2) particles were subsequently decorated with citrate-stabilized gold nanoparticles, as shown in Fig. 9(d). Uniform polyaniline thin shells and hollow capsules were fabricated using polyelectrolyte-coated microspheres as templates.207 Monodisperse hollow polyaniline nanospheres with controlled surface smoothness were synthesized by in situ polymerization of aniline monomers adsorbed on a carboxyl-functionalized polystyrene surface.208 In another study, Sung et al.209 synthesized submicron-size hollow PANI dicarboxylate salt to study the influences of alkyl chain length, functional group and stable dispersion on its electrorheological performance.
Polyaniline/Au composite hollow spheres were successfully synthesized using polystyrene/sulfonated polystyrene as the templates.210 The thickness of the PANI shell can be well controlled by adjusting the amount of aniline monomer. Fig. 10(a–d) shows the morphology of PANI (A: SEM; C: TEM) and PANI/Au (B: SEM; D: TEM) composite hollow spheres under specified preparative conditions. The possible formation of hollow PANI spheres and its Au composites are presented in Fig. 11. Gao et al.211 fabricated hollow polyaniline microspheres in the presence of Cu rings as template using H4SiW12O40 and ammonium persulfate as dopant and oxidant, respectively. Zhang and Liu212 synthesized hollow polyaniline nanoparticles via the chemical oxidative polymerization of aniline using γ-Fe2O3 nanoparticles as the reactive templates in the presence of hydrochloric acid. Their studies have shown that reacting temperature played a vital role in the formation of the hollow nanoparticles. Halloysite was used as a hard template to prepare polyaniline–polypyrrole binary composite nanotubes.213 Gao and coworkers214 demonstrated the fabrication of polyaniline nanotubes using the inner eggshell membrane as a template. Their investigations revealed the key role played by the pore size of the template in the formation of polyaniline nanotubes.
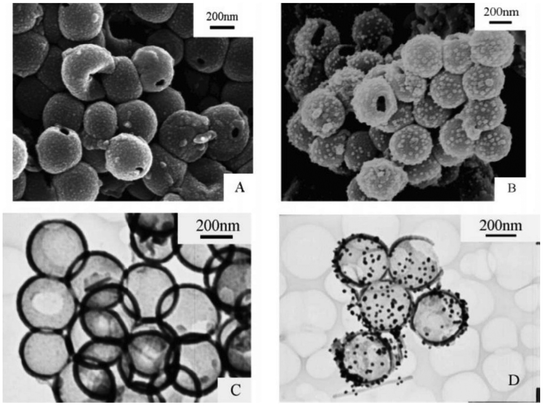 | ||
| Fig. 10 SEM and TEM images of PANI (A: SEM; C: TEM) and PANI/Au (B: SEM; D: TEM) composite hollow spheres (Synthetic conditions: aniline, 1 mmol, APS, 1 mmol, concentration of PANI in the Au colloid, 1.0 mg mL−1).210 Reproduced with permission from ACS. | ||
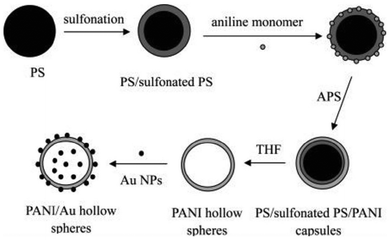 | ||
| Fig. 11 Scheme of the preparation of PANI and PANI/Au hollow spheres.210 Reproduced with permission from ACS. | ||
Zhu et al.215 reported the double surfactant-layer of polyvinylpyrroldine (PVP) and sodium dodecyl sulfate (SDS)-assisted oxidative polymerization using monodisperse metal oxides (CuO, Fe2O3, In2O3) as templates (Fig. 12(a)). Following this, hollow PANI nanocapsules were prepared by dissolving metal oxides in acid solution. The method has also been used to prepare nanocomposites of CuO/PANI, Fe2O3/PANI, In2O3/PANI and Fe2O3/Si O2/PANI. Further investigations revealed the formation of well-controlled core/shell metal oxides/PANI nanocomposites and PANI capsules. Hollow octahedral PANI nanocapsules have been fabricated using Cu2O (octahedral) as template in the presence of H3PO4.216,217 It may be noted that the removal of the Cu2O template is not required, compared with other conventional methods, due to its reaction with ammonium persulfate (oxidant) to form a soluble Cu2+ salt during the process of the polymerization. The synthesis of nanorings and flat hollow capsules of polyaniline was also reported via the chemical oxidative polymerization of aniline using VOPO4 2H2O nanoplates acting as oxidant and sacrificial template.218
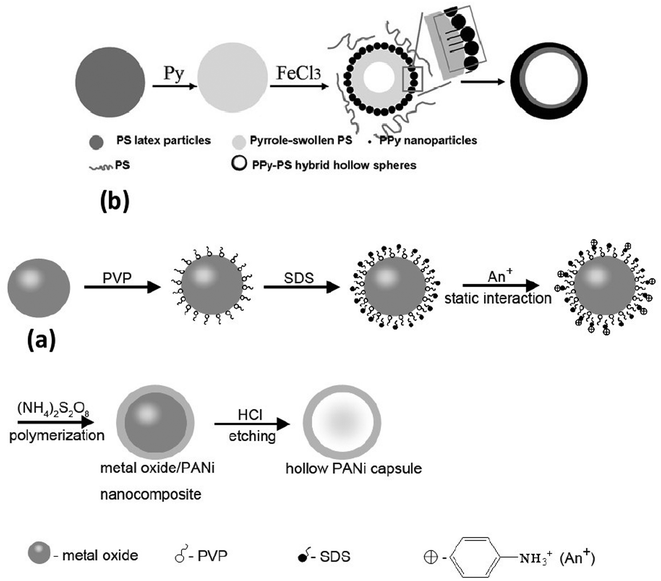 | ||
| Fig. 12 (a) Schematic illustration for the formation of well-controlled core/shell metal oxides/PANi nanocomposites and PANi capsules.215 Reproduced with permission from IOP. (b) The fabrication process of PPy-PS hybrid hollow spheres.225 Reproduced with permission from Elsevier. | ||
4.1.3.2 Hollow polypyrrole. The most common synthetic approach for the fabrication of the hollow polypyrrole microsphere was followed by a core (polystyrene, PS)/shell (polypyrrole) approach. This involved the in situ chemical oxidative copolymerization of the pyrrole monomer on the surface of a sulfonated PS microsphere followed by the extraction of the PS cores in a suitable solvent like tetrahydrofuran.219–224 Zhang et al.225 prepared polypyrrole-polystyrene (PPy-PS) hybrid hollow spheres by oxidative polymerization of pyrrole FeCl3 6H2O in an emulsion of PS latex, as shown in Fig. 12(b). It was suggested that the formation of PPy-PS hybrid hollow spheres could be induced by capillary forces that exist among the PPy nanoparticles (granular) deposited on the surfaces of PS latex at the start of the reaction. Hollow polypyrrole microcapsules (dia: 527 nm, shell thickness: 20 nm) have been reported, by the cosolvent approach using a polystyrene core as a template.226 Marinakos et al.227 used Au nanoparticles as templates in synthesizing nanometer-sized hollow PPy nanocapsules. Mesoporous hollow polypyrrole spheres have been fabricated by a chemical polymerization method using silica spheres as hard templates.228 Su et al.229 reported the synthesis of polypyrrole hollow nanospheres using poly(methyl methacrylate) nanospheres as templates. Chang et al.230 carried out in situ polymerization of pyrrole in the presence of polystyrene (PS) latex particles. Subsequent removal of the core (PS) resulted in the formation of hollow spherical polypyrrole balls.
According to Qu and Shi,231 the direct electrochemical oxidation of pyrrole in an aqueous solution of poly(styrene sulfonic acid) resulted in microstructures with hollow interiors comprising microspheres, microcrocks, microbowls, and micropumpkins. A stepwise electropolymerization process has been adopted in producing the nanostructured arrays of hollow polypyrrole with a conical shape.232 The pores of nanoporous polycarbonate membrane were used as templates to chemically synthesize polypyrrole nanotubules.233 Kros et al.234 carried out the polymerization of monomers (e.g., pyrrole, thiophenes) inside the pores of track-etched polymeric membranes. The hollow tubules formed in this manner exhibited relatively enhanced electrical properties compared with their respective bulk analogues. In another work, a ZSM-5 molecular sieve was used as a template to synthesize hollow pyrrole–platinum complex spheres following the chemical polymerization of pyrrole with potassium hexachloroplatinate(IV) as oxidant.235 Dubal et al.236 synthesized polypyrrole nanotubes using MnO2 as a sacrificial template in the presence of pyrrole 1 M HCl and K2Cr2O7. Hollow nanotubes of polypyrrole have been prepared rapidly by chemical oxidative polymerization of pyrrole in the presence of V2O5 nanofibers (sacrificial template) and FeCl3 as oxidant. This was followed by the removal of the template by dissolving it in aq. 1.0 M HCl.237 In another study, MnO2 powder was selected for a simultaneous dual role as oxidizing agent and sacrificial template in the chemical synthesis of hollow sea urchin-shaped polypyrrole.238 In another study, polystyrene beads were used as the sacrifice template to prepare hollow polypyrrole nanoparticles.239 Capsular PPy hollow nanofibers were fabricated by polymerizing pyrrole monomer on hollow V2O5 fibers (template) and subjected to acid etching to remove the V2O5 template.240 In addition, Fe3O4@polypyrrole hollow capsules241 and Fe3O4@PPy yolk/shell composites242 were also prepared by a hard-template method.
4.1.3.3 Hollow poly(3,4 ethylenedioxythiophene) (PEDOT). Rehmen et al.243 carried out vapor deposition of tosylate-doped PEDOT in fabricating hollow nanosphere coatings using polystyrene as template on carbon paper electrodes. In another study, PEDOT hollow nanospheres were successfully synthesized from SiO2/PEDOT core/shell nanospheres by subjecting SiO2 to chemical oxidative polymerization followed by etching of SiO2 by hydrofluoric acid.244 Luo et al.245 coated functionalized PEDOT on a polystyrene core in aqueous solutions. The subsequent removal of this core by dissolving it in an appropriate organic solvent produced hollow PEDOT particles with single holes and PEDOT capsules. According to Zhang et al.,246 PEDOT hollow spheres were fabricated using sulfonated polystyrene sphere template-assisted interfacial polymerization and introduced into the MXene film as an attractive flexible electrode for energy storage. ZnO microflower arrays have been used as a template to synthesize hollow microflower arrays of PEDOT with several two-dimensional hollow nanopetals on each microflower.247 Cheng et al.248 electrodeposited poly(3,4-ethylenedioxythiophene) hollow microflower film onto a fluorine-doped tin oxide glass substrate using a film of ZnO microflowers as the template.
4.2 Soft template method and template-free approach for single-shelled ICPs
The choice of soft template and template free approach in synthesis of conducting polymers are guided by its multifaceted advantages such as the simplicity of the process, good repeatability and no requirement for its removal. This method is invariably used in the synthesis of conducting polymers nanotubes, hollow spheres and yolk–shell sphere-type materials.173,249–253 In this regard, several structural directing agents are used, such as surfactants, block copolymers, amino acids, urea, methyl orange etc. The presence of these molecules aligns the monomers of the conducting polymers in solution and facilitates the overall polymerization process into the desired nanostructures like tubes or spheres by forming micelles or self-assembled structures around the monomers.Han et al.256 synthesized polyaniline nanotubes by the oxidative polymerization of aniline in dilute solution in the presence of cetyltrimethylammonium bromide. Self-assembled PANI nanotubes with a rectangular cross-sectional shape have been synthesized by in situ chemical oxidation polymerization in the presence of citric acid as the dopant.257 In another study, Zhang et al.258 prepared polyaniline nanotubes by a self-assembly process using carboxylic acids (propionic acid, lactic acid, succinic acid, malonic acid, tartaric acid, and citric acid) as dopants. They also investigated the effect of hydrogen bonding on the formation of nanotubes and aggregated dendrites of polyaniline. Highly crystalline polyaniline nanotubes and nanofibers have been synthesized in the presence of dicarboxylic acids (oxalic acid, malonic acid, succinic acid, glutaric acid, and adipic acid) (acting as dopants).259 Zhang et al.260 fabricated nanotubular polyaniline (self-assembled) following the chemically synthesized by ammonium persulfate oxidation of aniline in the presence of amino acid. The role played by the amino acids in this study is guided by their effect on the initial soft template for the growth of nanotubes based on the formation of micelles or oligomeric species during the initial stage of aniline oxidation. Rana et al.261 used a soft template method to prepare PANI nanotubes of almost uniform diameter by selecting benzene 1,2,4,5-tetracarboxylic acid, acting simultaneously as dopant acid as well as structure-directing agent. Huang and Wan262 investigated the influence of the molecular structure of sulfonic acids on the tubular morphology of the doped PANI. According to this, β-NSA-doped PANI exhibited tubular morphology by preparing it using an in situ doping polymerization method. Hollow PEDOT spheres (dia: 1.7–4.6 μm) were synthesized by aqueous chemical polymerization using self-assembled membrane of poly(3,4-ethylenedioxythiophene) doped with acetic acid at room temperature and ammonium persulfate as oxidant.263
Alternatively, synthesis of self-assembled polyaniline nanotubes has also been reported using itaconic acid,264 camphor sulfonic acid,265 polymeric acids: poly(4-styrenesulfonic acid), poly(acrylic acid), poly(methyl vinyl ether-alt-maleic acid),266 malic acid, succinic acid, citric acid, and tartaric acid267 as dopants. Mu et al.268 reported the fabrication of self-assembled polyaniline nanotubes doped with D-tartaric acid for high-performance supercapacitor applications. Zhang and Wan269 synthesized chiral polyaniline nanotubes following the self-assembly process using (1R)-(−)-10-camphorsulfonic acid (L-CSA) and (1S)-(+)-10-camphorsulfonic acid (D-CSA). It was noted that the formation yields and the size of the doped polyaniline nanotubes were guided by the molar ratio of CSA to aniline. Panigrahi and Srivastava270 reported the synthesis of polyaniline hollow microspheres by the ultrasound-assisted emulsion polymerization technique using polystyrene microspheres as a template.
Zhang et al.271 reported a self-assembly process for the syntheses of polyaniline nanotubes (dia:130–250 nm) doped with α-naphthalene sulfonic acid, β-naphthalene sulfonic acid, and 1,5-naphthalene disulfonic acid. According to Sun and Deng,272DL-tartaric acid played an important role as a dopant in determining the morphology of polyaniline prepared by interfacial oxidation polymerization of aniline using APS as the initiator. These findings led to the formation of spherical mushroom-like morphology and nanotubes of polyaniline corresponding to the DL-tartaric acid concentration of ∼0.02 M and ∼0.04 M, respectively.
Qiu et al.273 synthesized polyaniline nanotubes (dia:100–300 nm, length: up to 2 µm) through a template-free polymerization using (NH4)2S2O8 as an oxidant and a protonic acid dopant (C60-(OSO3H)6 or PAMAM4.0 [naphthyl (SO3H)2]24). The formation of oriented arrays of polyaniline nanotubes (60 to 150 nm in diameter) were also reported by hydrogen-bonding directionality in the presence of a crown ether derivative and ammonium persulfate in HCl solution.274 Poly(2-acrylamido -2-methylpropane sulfonic acid) has been used as a dopant as well as soft template for the synthesis of uniform hollow microspheres of PANI (dia: 410 nm, shell thickness: 72 nm) in aqueous solution following in situ polymerization of aniline in the presence of ammonium persulfate.275 Huang et al.276 reported a template-free method following inversed microemulsion polymerization in fabricating polyaniline hollow microspheres (outer dia: 4–6 mm, shell thickness: 150–250 nm) using β-naphthlene sulfoinic acid as dopant. Zhu et al.277 prepared superhydrophobic rambutan-like hollow spheres of polyaniline by a self-assembly method in the presence of perfluorooctane sulfonic acid (dopant and soft template).
Zhang and Wan278 reported transformation of self-assembled polyaniline from 1 D nanotubes (dia: ∼109–150 nm) to hollow microspheres by changing the molar ratio of the dopant (salicylic acid) to monomer (aniline). The freeze-fracture electron microscopy studies revealed the role played by hollow spherical micelles comprising salicylic acid/aniline as templates in the formation of nanotubes/hollow spheres. The driving force in the self-assembly of hollow microspheres might be due to be the hydrogen bonding of the –OH group (salicylic acid) and amine group (polyaniline). Liang et al.279 synthesized polypyrrole nanotube aerogels by using the weakly interconnected network of self-assembled nanotubes of lithocholic acid as a soft template.
Hollow nanospheres of methyl-substituted polyaniline280 and poly(m-methylaniline)281 microspheres were prepared through the self-assembly processes in the presence of ammonium persulfate. Tavandashti et al.282 studied the transition of polyaniline from nanotubes to nanospheres following a soft template route, as schematically displayed in Fig. 13(a and b). According to this, the fabrication of polyaniline nanospheres was carried out via the oxidative polymerization of aniline in the presence of β-naphthalenesulfonic acid (β-NSA) as both surfactant and dopant, and ammonium persulfate as oxidant at 2–5 °C. Furthermore, control of the morphology of polyaniline was achieved by changing the reaction conditions. Ding et al.283 prepared PANI nanotubes with diameters of 100–150 nm of single nanotubes by carrying out direct oxidation with APS in the absence of hard templates and acidic dopants. During this, the formation of the hollow spheres at the initial stage was accompanied by the micelles (soft template) formed by the aniline monomer in aqueous solution. Furthermore, investigations revealed a decrease in pH with increasing polymerization time resulted in a change in the morphology from hollow spheres to short and long tubes. Triton X-100 has been selected as a soft template to fabricate poly(aniline-copyrrole) hollow nanospheres via oxidative polymerization of aniline.284 In a micelle-mediated phase transfer method, perfluorooctanoic acid/aniline acted as soft templates to form hollow nano/microspheres of polyaniline with mesoporous brain-like convex-fold shell structures.285
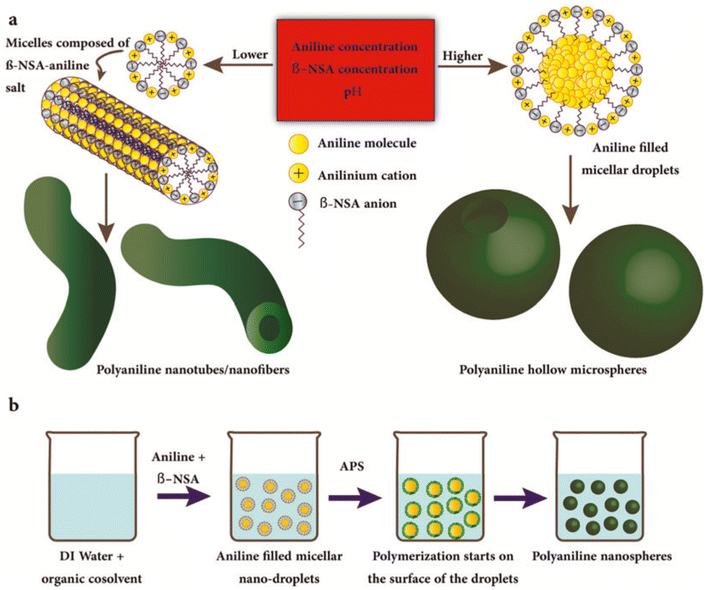 | ||
| Fig. 13 The schematics of the formation mechanisms of (a) PANI nanotubes/nanofibres and hollow microspheres and (b) PANI nanospheres.282 Reproduced with permission from Wiley. | ||
Vitamin C upon the addition of aniline monomer ([Vitamin C]/[Aniline] = 0.25) produced polyaniline nanotubes (dia.: 80–120 nm, length extending to several micrometers) via the oxidative polymerization method.22 Ren and others286 adopted a soft template method in the fabrication of a polyaniline microtube acidic solution using methyl orange as dopant in the presence of aniline monomer and ammonium peroxydisulfate. According to this, methyl orange is self-assembled into supramolecular aggregates and acts as templates in the formation of PANI microtubes. Urea has also been used as a soft template in the synthesis of polyaniline nanotubes by in situ chemical oxidative polymerization of aniline monomer.287 Orellana and Roberts288 used a simple approach for preparing polypyrrole microtubes without the need for a solution or substrate-based template. Wang et al.289 synthesized polypyrrole nanotubes of ∼50 nm diameter and a length extending to several micrometers using pyrrole, FeCl3·6H2O, and methyl orange as monomer, initiator, and soft template, respectively.
A self-assembly method has been used to synthesize polyaniline nanotubes in the excess of (NH4)2S2O8 oxidant.290 This could be ascribed to the formation of aniline dimer cation-radicals acting as effective surfactants. Zhang et al.291 fabricated polyaniline hollow spheres with controllable size and shell thickness through the oxidation–reduction reaction-driven approach under hydrothermal conditions in the absence of any sacrificial templates and organic surfactants and using H2O2 and Fe3+ as oxidant and catalyst, respectively. Hollow polyaniline microsphere were also prepared by polymerization of aniline in aqueous medium using K3[Fe(CN)6] as oxidant.292 Wei and Wan293 synthesized hollow microspheres of PANI (dia: 450–1370 nm) using aniline emulsion template doped with β-naphthalene sulfonic acid at −10 °C in the presence of ammonium persulfate acting as the oxidant. Investigations are also reported on the synthesis of PANI nanotubes, nanotubes with rectangular and circular cross-section hollow microspheres294 and 3D hollow microspheres assembled from 1D PANI nanowires.295
Liu et al.296 reported a template-free method to synthesize polyaniline film (thickness: 100 nm) embedded with PANI nanotubes without any surfactant or organic acid. Their studies revealed that oligomers with certain structures are responsible for the growth of the nanotubes. A micelle soft-template method was used in fabricating PANI nanotubes (external dia: 110 internal dia:, 10 nm, length: several μm) in the presence of oxalic acid as a dopant.297 Tajima et al.298 used nanobubble soft templates formed by ultrasonic irradiation in the synthesis of hollow polypyrrole spheres.
Xia et al.302 used poly(vinylpyrr1olidone) (PVP) as a soft template in order to fabricate PEDOT exhibiting hollow spheres (size: 130–820 nm) via a self-assembly method. Bian et al.303 used star-like unimolecular micelles as templates for the controlled syntheses of hollow nanostructures of polythiophene nanoparticles. A reverse emulsion polymerization was described using sodium bis(2-ethylhexyl) sulfosuccinate cylindrical micelles as the template at room temperature for the synthesis of PEDOT nanotubes (dia: 50–100 nm, length: 10–20 mm).304 The synthesis of self-assembled 3D hierarchical PEDOT micro/nanostructures including hollow spheres and double-layer bowls was carried by a template-free method in the presence of perfluorosebacic acid as the dopant and FeCl3 6H2O as the oxidant.305 Ali et al.306 synthesized PEDOT/Au hollow nanospheres by an in situ polymerization method comprising the ratio of EDOT and HAuCl4 from 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. In another study, a surfactant-free Ouzo emulsion method was used to synthesize hollow or partially collapsed bowls of PEDOT using FeCl3·6H2O, acetone, and toluene as oxidant, solvent, and anti-solvent, respectively (Fig. 14(a)).307 This method has also been extended in successfully synthesizing PANI, PPy and PTh. Fig. 14(b–d) show the SEM images of PEDOT under different experimental conditions. Ni et al.308 used the chemical polymerization of EDOT to synthesize hollow PEDOT microsphere in the presence of ammonium persulfate (oxidant) and PVP (micelles). Sui et al.309 synthesized hollow microspheres of PEDOT (size: 0.5 to 10 μm) by the chemical oxidative polymerization of EDOT using ammonium persulfate in a catanionic surfactant solution comprising a mixture of cetyltrimethylammonium bromide and sodium dodecylbenzenesulfonate. Recently, Ge et al.195 prepared honeycomb-shaped photothermal polypyrrole by electropolymerization.
2. In another study, a surfactant-free Ouzo emulsion method was used to synthesize hollow or partially collapsed bowls of PEDOT using FeCl3·6H2O, acetone, and toluene as oxidant, solvent, and anti-solvent, respectively (Fig. 14(a)).307 This method has also been extended in successfully synthesizing PANI, PPy and PTh. Fig. 14(b–d) show the SEM images of PEDOT under different experimental conditions. Ni et al.308 used the chemical polymerization of EDOT to synthesize hollow PEDOT microsphere in the presence of ammonium persulfate (oxidant) and PVP (micelles). Sui et al.309 synthesized hollow microspheres of PEDOT (size: 0.5 to 10 μm) by the chemical oxidative polymerization of EDOT using ammonium persulfate in a catanionic surfactant solution comprising a mixture of cetyltrimethylammonium bromide and sodium dodecylbenzenesulfonate. Recently, Ge et al.195 prepared honeycomb-shaped photothermal polypyrrole by electropolymerization.
 | ||
| Fig. 14 (a) Schematic illustration for the oxidative chemical polymerization of PEDOT hollow spheres/bowls. (b–d) SEM images of the synthesized PEDOT nanostructures, (b) PEDOT nanoparticles before wash-removal of oxidant, (c) after the removal of oxidant by ethanol washing, and (d) collapsed hollow bowls after the washing and drying.307 Reproduced with permission from Elsevier. | ||
4.3 Electrospinning method
Electrospinning is relatively inexpensive, simple and versatile method used in fabricating hollow (and core–shell) polymer fibers for a variety of applications.310,311 The electrospun poly(amic acid) fiber membrane has been used as a template to fabricate hollow polyaniline nanofibers by in situ polymerization of aniline.312 The formation of highly aligned PEDOT nano- and microscale fibers and tubes has been reported based on this technique and oxidative chemical polymerization.313 The electrospinning preparation methods of several other hollow conducting polymers are described in subsequent sections under applications.4.4 Synthesis of double-shelled hollow spheres of ICPs
In comparison with single-shell hollow spheres, hollow spheres with multi-shell structures receive extra advantages from inner structures.314 The permeable shell and void space between each shell in the unique hollow structure accounts for interesting properties, such as a sequential absorbing/scattering characteristic, and the shortening of the diffusion distance for mass and charge transport for various applications. In this regard, very limited research has been reported on the preparation of intrinsically conducting polymers exhibiting the double-shell type of morphology. Bei and Xia315 reported the synthesis of double-shelled polypyrrole hollow particles with a structure similar to that of a thermal bottle, using polystyrene hollow spheres (templates) containing a hole on the surface as templates. The choice of such template facilitated the diffusion of the monomer and acted as an initiator to form uniform PPy coatings on the inner and outer surfaces. Subsequently, selective removal of PS by dissolving in tetrahydrofuran (solvent) resulted in the formation of double-shelled polypyrrole hollow particles with a structure resembling that of a thermal bottle. Niu et al.316 used iron oxide hollow microspheres as both the sacrificial template and initiator in acidic solution to prepare double-shelled polypyrrole hollow microspheres. According to Gu et al.,317 MnO2 nanorods selected as a self-sacrifice template formed double-shelled hollow polypyrrole nanotubes by in situ polymerization of the pyrrole monomer in the presence of hydrochloride acid and sodium p-styrene sulfonate.4.5 Synthesis of hollow nanocomposites of ICPs and core–shell structures
Recently, hollow and core@shell composites have been receiving considerable attention due to their effective combination and interesting properties, with great promise for a broad range of applications in many fields.67 In this regard, intrinsically conducting polymer-derived composites have gained significant recognition due to their unique properties, such as environmental stability, processability, and less corrosive nature with tunability.72,73 In addition, such ICP-based binary and ternary core–shell nanocomposites offer advantages of their properties due to the combination of the core and the shell.75 Core–shell nanostructured materials can be prepared by physical and chemical methods. However, the chemical synthesis of hollow and core–shell ICP nanostructures is better recognised compared with physical methods. This is mainly due to their greater versatility, precise control, and ability to use templates, unlike physical methods which invariably require complex equipment. In view of this, preparative methods for these hollow and core–shell ICP composites are described in the subsequent sections under their applications in the fields of electromagnetic interference shielding/microwave absorption, removal of metal ions/dyes and as supercapacitors.Recently, electrodeposition has become a promising method for fabricating ICP-based core–shell materials as superior electrodes in supercapacitors, though its potential is yet to be harnessed.145,318 This is ascribed to the superior control over morphology, enhanced performance through binder-free synthesis, and improved interface between the polymer and substrate. Chen et al.319 synthesized polypyrrole (binder free film) on carbon cloth by the electrodeposition method. Core–shell nanorod arrays with polyaniline were successfully electrodeposited into a mesoporous NiCo2O4 support.320 Wu et al. reported321 polypyrrole-coated low-crystallinity Fe2O3 supported on carbon cloth by a combination of chemical reduction and electrodeposition methods. The preparation of a free-standing graphene/polyaniline/MnO2 ternary composite by sequential electrodeposition on carbon cloth as working electrodes was reported.322 In another study, electrodeposition of PANI on the surface of GO/α-MnO2 was used to prepare hierarchical GO/α-MnO2/PANI composites.323 This electrodeposition technique has also been used in fabricating (CoCrFeMnNi)3O4@CC-PPy,324 LaMnO3@CC-PPy,325 and porous PPy/black phosphorus oxide composites electrodeposited on CNT.326 In another approach, Fauzi et al.327 used oxidative chemical vapor deposition to deposit a submicrometer-thick layer of PPy on the carbon fabric.
5. Application of hollow ICPs and their core–shell nanocomposites
5.1 Electromagnetic interference shielding and microwave absorption
In recent decades, the growing and extensive use of devices has contributed to electronic pollution due to electromagnetic interference, limiting their applications and also threatening human life (Fig. 15).328 In this regard, the development of electromagnetic shielding and microwave-absorbing materials has recently been receiving considerable attention When an electromagnetic (EM) wave perforates through the shielding material, it involves primarily three processes, namely reflection, absorption, and multiple reflection.2 The shielding performance of a material is expressed by term electromagnetic interference shielding effectiveness (EMI SE). The total EMI SE (SET) measures the ability of a material to block EM waves and is expressed in decibels (dB) as a function of the logarithm of the ratio of the power P, electric E, or magnetic H field intensities before and after EM attenuation, i.e.,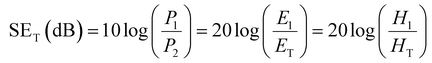 | (1) |
 | ||
| Fig. 15 Electromagnetic radiation hazards and application of EWAMs around daily life.328 Reproduced with permission from Elsevier. | ||
Furthermore, SET is the sum of shielding efficiency originating from absorption (SEA), reflection (SER) and multiple reflections (SEM),9i.e.,
| SET = SER + SEA + SEM | (2) |
The factors affecting the shielding effectiveness include permeability, permittivity, skin depth and thickness, external physical properties, eddy current loss, magnetic loss, dielectric loss, size and morphology etc.54 The impedance matching ratio, |ZMZ0| (Zin: input impedance, Z0; free space impedance) is also another important factor that provides information about EM waves entering the shield. It may be noted that the reflection loss (RL) of a shielding material is closely related to the input impedance (Zin) and free space impedance (Z0). According to the transmission line theory, both Zin and RL are expressed as shown below.9
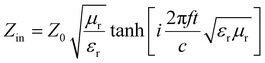 | (3) |
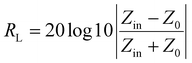 | (4) |
The methods commonly used to measure the electromagnetic shielding efficiency (also referred to as effectiveness) of a material can be based on the space transmission, shield box, shield room and coaxial transmission line approaches.75
In this regard, much interest has been focused recently on developing electromagnetic wave (EMW) shielding materials undergoing absorbing mechanisms.9 This is because EMWs, following the reflection mechanism, produce secondary pollution due to their reflection to the environment. Accordingly, conductive polymers have been receiving considerable attention guided by their conductivity, light weight, corrosion resistance and processibility.9 In this regard, the morphology of the intrinsically conducting polymer materials is considered a vital parameter in their functioning as EMW absorbers. This is ascribed to successive internal reflection (multiple internal reflections of incident EM waves).204,329–333 Hollow microhemispheres like polypyrrole and carbon dielectric materials could act as promising high-performance microwave absorbers with strong reflection loss and wide absorption frequency bandwidth.332
According to the available literature, electromagnetic wave absorption materials have proved to be very effective at mitigating electromagnetic pollution and interference.9 The choice of an ideal microwave absorber material is guided by light weight, good thermal stability, antioxidation capability, multi-interfaces, impedance matching, synergistic effects, strong absorption properties and wide bandwidth simultaneously.54,333–335 In addition, other important features that affect the absorption of microwaves include complex permittivity/permeability, impudence matching and morphology of these materials.9 In such studies, the choice of ICPs is guided by the low cost of synthesis and great environmental stability of hollow morphology, to account for the improved microwave absorbing properties. In addition, hollow ICP composites and ICP based core–shell materials have also been fabricated, integrating magnetic and dielectric losses to facilitate the absorption of EM waves.334
Among these, Fe3O4 has drawn a great deal of attention because of its low cost, easy synthesis, saturation magnetization value and high Curie temperature.9 However, several drawbacks of Fe3O4, such as ease of oxidation, high density, dramatic decrease of permeability in the high-frequency range due to the Snoek's limit, weak interfacial compatibility and substrate dispersion difficulty need to be addressed.9,334,335 In view of this, combining ICPs with magnetic materials could be more effective at enhancing the electromagnetic wave attenuation synergistically.329 Furthermore, outstanding performance can also be achieved by combining dielectric and magnetic components together.336 Accordingly, most studies are focused on Fe3O4 in the form of binary composites with ICP or its ternary nanocomposites involving ICPs and carbonaceous materials. It may be noted that the hollow structures of Fe3O4 nanospheres and PANI reduce the weight of the composites. In addition, they also extend the transmission path of microwaves as result of multiple reflection and scattering loss.
Guo et al.339 studied electromagnetic wave absorption of the hollow polypyrrole nanorods fabricated by using the self-assembly template that can be easily removed. These findings have shown that tuning the wall thickness facilitates control of the dielectric constant of HPPy. The variation of reflection loss with frequency in the range of 2–18 GHz with filler loading of 10 wt% indicated the minimum reflection of –54.94 dB with the largest effective absorption bandwidth of 7.36 GHz (thickness: 3.4 mm). The possible EMA mechanism of HPPy nanorods could be ascribed to impedance matching, multiple reflection, a good conductive network and enhancement of polarization loss. In another method, hollow or partially collapsed bowls of polyaniline, polypyrrole, polythiophene and poly(3,4-ethylenedioxythiophene) have successfully been synthesized by surfactant-free Ouzo emulsion.307 Subsequently, the EMI shielding performance of the three samples PEDOT sphere/bowl film (thickness: 6.5 μm), composite film of PEDOT hollow spheres/bowls film (thickness: 6.3 μm) with infiltrated PH1000 and pure PH1000 film (thickness: 6.2 μm) has been investigated in X-band (8–12 GHz). It was noted that EMI SE of the corresponding samples over the X-band follow the order: PEDOT hollow spheres/bowls film with infiltrated PH1000 (∼75 dB) > PEDOT:PSS with infiltrated PH1000 film (∼70 dB) > PEDOT hollow spheres/bowls film (very low). The low electrical conductivity of pure PEDOT hollow spheres/bowls film accounts for their inferior EMI performance. In contrast, the composite comprising the PEDOT hollow spheres/bowls film with infiltrated PH1000 exhibited enhanced EM absorption, as evident from the total EMI SE (SET), due to the larger contribution from the absorption compared with the reflection. Ni et al.308 observed a maximum RL of −24 dB at 15.9 GHz for a sample (thickness: 2 mm) of PEDOT hollow microspheres.
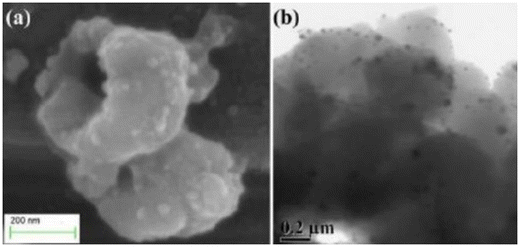 | ||
| Fig. 16 (a) SEM image and (b) TEM image of HPPy/Ag-10.204 Reproduced with permission from Nature Publication. | ||
 | ||
| Fig. 17 (a) Variation of EMI shielding of PPy, HPPy, HPPy/Ag-2, HPPy/Ag-5, HPPy/Ag-10 with varying frequency at 0.5–8 GHz, and (b) trapping mechanism of EM wave through enhanced internal reflection in HPPy/Ag: An anticipated scheme.204 Reproduced with permission from Nature Publication. | ||
Microwave absorption of milled carbon fiber (4 wt%) and hollow polyaniline spheres (1 wt%) impregnated in the epoxy matrix (thickness: 1.8 mm) exhibited maximum absorption (−49.3 dB) and the effective bandwidth of 1.7 GHz–10 dB in the X band region.344 The observed reflection loss of EM waves in the given frequency range was attributed to the improvement in impedance matching, multiple reflections and scattering of EM waves. According to Wang et al.,345 hollow polyaniline-derived N/S co-doped carbon nanoflakes possessed RminL of −53.5 dB (10.2 GHz) and a corresponding effective absorption band of 4.5 GHz at 2.9 mm. This could be explained on the basis of conductivity loss, interfacial polarization relaxation and the impedance matching from the hollow structure synergistically, resulting in excellent microwave absorption performance.
Chu et al.346 fabricated a well-designed structure of sandwich-like composite films based on hollow polyaniline and cellulose nanofiber (CNF) in the surface layers and MXene/CNF in the intermediate layer. This displayed an EMI SE of 35.3 dB for a proportion of MXene and PANI achieved at a lower filler loading compared with many others. It was suggested that the formation of such sandwich structure effectively reduced the reflection of the EM wave and made the absorption more dominant. Hollow polyaniline/Fe3O4 microsphere (7.33 wt%) composites showed the maximum reflection loss of −15.6 dB and maximum bandwidth of 8.0 GHz over −10 dB in the frequency range of 2–18 GHz.347 Double-shelled hollow polypyrrole nanotubes were synthesized by using the reactive MnO2 template.317 They exhibited the optimal reflection loss of −50.4 dB and a wide EAB of 7.7 GHz in the presence of 5 wt% in a paraffin wax matrix. Such performance was ascribed to the synergistic effects of interfacial polarization and conduction loss.
5.1.4.1 Binary core@shell nanocomposites. Guo et al.348 observed maximum reflection loss (RmaxL) and EAB values of −52.01 dB and 2.72 GHz in the hollow core–shell-structured Fe3O4@Polypyrrole (Fe3O4: 60.0 wt%) synthesized by an in situ polymerization method. Such performance was attributed to the synergistic effect on account of dielectric as well as magnetic losses. The broadband electromagnetic wave absorption properties of Fe3O4/PPy double-carbonized core–shell-like composites (thickness: 1.6 mm) exhibited RminL and EAB of −26 dB and 4.64 GHz, respectively.349 The observed performance was ascribed to the synergistic effects of conductive loss, dielectric loss, magnetic loss, and multiple reflection loss. Tang et al.350 synthesized Fe3O4@PPy with hollow core–shell structures by a solvothermal process followed by in situ polymerization. For this purpose, 170 μL, 180 μL, 190 μL of pyrrole were taken and the corresponding nanocomposites, referred to as FP-170, FP-180 and FP-190, acted as an efficient microwave absorber as indicated by their observed performance from a minimum reflection loss of −63.82 dB (4.55 mm), −84.92 dB (3.87 mm), and −71.25 dB (2.64 mm) at low frequencies, respectively. The maximum effective absorption bandwidth values of the corresponding composites were found to be 3.48 GHz (2.39 mm), 4.20 GHz, 2.38 mm and 4.96 GHz (2.16 mm). The excellent microwave absorption performance of hollow core–shell Fe3O4@PPy could be due to the good impedance matching, strong magnetic loss, including natural resonance and eddy current loss, excellent conductivity, defects and polar groups in h-Fe3O4 and PPy acting as polarization centers, the presence of abundant heterogeneous interfaces and interfacial polarization. Furthermore, the presence of the hollow structure of Fe3O4 in the nanocomposite contributed to the multiple reflection and scattering losses and also enhanced the impedance matching.
Promlok et al.351 reported the formation of the hollow magnetic polyaniline by in situ polymerization in one pot for EMI applications. In another study, Fe3O4–polyelectrolyte modified polyaniline (Fe3O4–PE@PANI) was prepared by the self-assembly approach.352 TEM imaging of the product clearly established the formation of hollow Fe3O4-Polyelectrolyte (PE)@PANI nanocomposites (average size ∼500 nm), with Fe3O4 nanoparticles tightly and completely attached to the PANI hollow sphere surfaces. The 50 wt% of Fe3O4−PE@PANI loaded in paraffin exhibited a minimum reflection loss of −6.5 dB (14.3 GHz) and the frequency bandwidth (<–5 dB) from 12.5 to 15 GHz, and ascribed it to the impedance matching and dielectric/magnetic loss abilities. Hou et al.353 prepared hollow-structure Fe3O4/PANI microspheres based on three steps, namely the preparation of Fe3O4 nanoparticles, hollow PANI microspheres and Fe3O4/PANI microspheres. Subsequently, they investigated the effects of the mass ratio of aniline/PS (template) on their EMW absorption properties. Fe3O4/PANI microspheres corresponding to the mass ratio of aniline/PS of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 and thickness of 1.5 mm attained RminL of −24.3 dB, and the bandwidths below −10 dB corresponded to 4.64 GHz (11.04–15.68 GHz) for 2 mm thickness of the sample. It was suggested that dielectric loss, magnetic loss (2–7 GHz) and eddy current loss (7–18 Ghz) played important roles. In addition, hollow Fe3O4/PANI microspheres acted as a potential absorber in the absorption of microwaves due to strong destructive interference and eddy current loss. Hollow poly(aniline-co-pyrrole)–Fe3O4 (0.06 g) composite nanospheres were prepared via the oxidative polymerization of a mixture of aniline and pyrrole in the presence of a magnetic fluid.354 The reflection loss calculations showed the best microwave-absorbing property between 0.5–10 GHz for the hollow poly(aniline-co-pyrrole) filled with 0.06 g of Fe3O4. These studies also revealed that both dielectric and magnetic loss significantly affect the efficiency of microwave absorption.
6 and thickness of 1.5 mm attained RminL of −24.3 dB, and the bandwidths below −10 dB corresponded to 4.64 GHz (11.04–15.68 GHz) for 2 mm thickness of the sample. It was suggested that dielectric loss, magnetic loss (2–7 GHz) and eddy current loss (7–18 Ghz) played important roles. In addition, hollow Fe3O4/PANI microspheres acted as a potential absorber in the absorption of microwaves due to strong destructive interference and eddy current loss. Hollow poly(aniline-co-pyrrole)–Fe3O4 (0.06 g) composite nanospheres were prepared via the oxidative polymerization of a mixture of aniline and pyrrole in the presence of a magnetic fluid.354 The reflection loss calculations showed the best microwave-absorbing property between 0.5–10 GHz for the hollow poly(aniline-co-pyrrole) filled with 0.06 g of Fe3O4. These studies also revealed that both dielectric and magnetic loss significantly affect the efficiency of microwave absorption.
In another study, a Fe3O4/PANI355 composite was fabricated with hollow Fe3O4 nanospheres and polyaniline nanotubes by the hydrothermal treatment and chemical oxidative polymerization, and its micro absorption properties were studied by varying the ratio of PANI and the loading amount of the composites in paraffin. These findings have shown remarkable performances (RminL: −55.03 dB, EAB: 4.88 GHz) for its thickness of 1.84 mm in the frequency range of 2–18 GHz. This excellent performance was attributed to the interfacial polarization loss, multiple reflection scattering loss and the synergistic effect of hollow Fe3O4 (magnetic) and 1D PANI (dielectric) material. Fe3O4 microsphere core (size: 300 nm)/PANI shell (thickness: 100 nm) synthesized by in situ polymerization reached RmaxL of −37.4 dB at 15.4 GHz.356 Such enhanced microwave absorption properties arise due to the improved impedance, dielectric loss, interfacial loss and synergistic effect. Core–shell Fe3O4−PEDOT microspheres (EDOT)/(Fe3O4 molar ratio = 20) were also prepared by a two-step method in the presence of polyvinyl alcohol (stabilizer) and p-toluenesulfonic acid (dopant) (Fig. 18(a)).357 TEM studies, displayed in Fig. 18(b) established Fe3O4 microspheres coated by PEDOT, and the thickness of the shell corresponded to 90 nm. The variation of reflection losses of Fe3O4/PEDOT with frequency in Fig. 18(c) showed excellent microwave absorbing property (RminL: –30 dB at 9.5 GHz, thickness: 4 mm) due to the impact of layer thickness, volume fraction and conductivity.
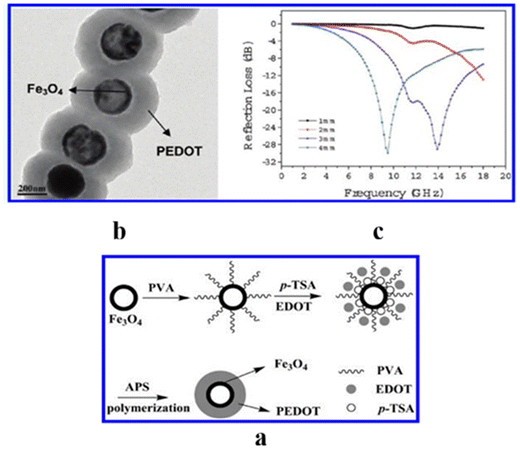 | ||
| Fig. 18 (a) Formation mechanism of Fe3O4@PEDOT core–shell microspheres, (b) TEM images of Fe3O4@PEDOT core–shell microspheres prepared with (EDOT)/(Fe3O4) ratios: 20, and (c) reflection losses in different thickness of Fe3O4@PEDOT composites with (EDOT)/(Fe3O4) = 20.357 Reproduced with permission from ACS. | ||
Fe3O4-polyaniline,358 Fe3O4@Polypyrrole,359,360 and BaFe12O19@PANIn361 core–shell structured materials have also been studied for their electromagnetic wave absorption. Hosseini et al.362 used a core–shell approach for fabricating polythiophene nanofiber coated on MnFe2O4/Fe3O4 through the combined co-precipitation and in situ polymerization methods. The micro absorption properties of the 1.5 mm nanocomposite showed minimum RL of −21 dB (12 GHz) in the frequency range 8.0–12.0 GHz. CoSe2@polythiophene363 and rGO/Ni0.5Co0.5Fe2O4@PEDOT364 core–shell composites were also synthesized and studied for their electromagnetic, microwave absorbing properties. Fe3O4 microspheres @PPy anchored on 3D graphene aerogel (GO to Fe3O4@Ppy wt ratio: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) of 2.5 mm thickness reached a minimum reflection loss of −40.53 dB at 6.32 GHz and the effective bandwidth of 5.12 GHz (11.12–16.24 GHz).365 Such enhanced microwave absorption properties of the ternary composite could be explained on the basis of abundant interfaces, enlarged dielectric properties, enhanced conductivity and synergistic effect.
3) of 2.5 mm thickness reached a minimum reflection loss of −40.53 dB at 6.32 GHz and the effective bandwidth of 5.12 GHz (11.12–16.24 GHz).365 Such enhanced microwave absorption properties of the ternary composite could be explained on the basis of abundant interfaces, enlarged dielectric properties, enhanced conductivity and synergistic effect.
Excellent microwave absorption performance has been shown by PANI decorated on prism-shaped hollow carbon (thickness: 2.5 mm) synthesized by in situ polymerization.366 It showed the minimum reflection loss of −64.0 dB (11.1 GHz) and EAB corresponding to 5.0 GHz (9.5–14.5 GHz) attributed to high impedance matching, dielectric loss and geometric effect. Gai et al.367 constructed PPy nanotubes@MoS2 core–shell and observed the optimal RL of −49.1 dB at 6.1 GHz and the widest bandwidth up to 6.4 GHz from 11.5 to 17.5 GHz (RL < −10 dB) corresponding to its thickness of 2.5 mm. Such enhanced microwave absorption properties of PPy@MoS2 composite were ascribed to the morphology, attenuation capacity and the impedance matching.
Tian et al.368 fabricated PPy@PANI of tunable shell thickness (30–120 nm) by direct polymerization of aniline on the surface of PPy microspheres. The fabricated PPy@PANI composite showed a maximum reflection loss of −34.8 dB at 13.9 GHz and bandwidths exceeding −10 dB corresponding to 11.9–16.6 GHz (4.7 GHz). In another study, hollow ZnxFe3−xO4@Polyaniline369 core–shell composites acted as high-performance microwave absorbers with high reflection loss, respectively. In situ polymerized-grown polyaniline nanorod arrays on the surface of carbon (C@PANI) microspheres exhibited a waxberry-like shape.370 Subsequently, their microwave absorption performance was evaluated and displayed considerable reflection loss (samples thickness: 2.2 mm), impedance matching ratio of samples and 3 D reflection loss with different thickness, as shown in Fig. 19(a, b and c), respectively. It was noted that C@PANI exhibited superior microwave absorption performance (sample thickness: 2.2 mm), RminL: −59.6 dB at 15.5 GHz, effective bandwidth for RL < −10 dB: 5.4 GHz (12.6 to 18 GHz) compared with pure PANI and carbon microspheres. It was suggested that dielectric loss ability, synergistic effect and defects present in the C microspheres contributed to this high microwave absorption performance of C@PANI microspheres. In addition, relatively low electrical conductivity owing to the amorphous structure of C@PANI could be favourable in the impedance matching. The variation of 3 D reflection loss of C@PANI microspheres with the sample thickness in the range of 2–3 mm showed the shifting of the RminL peak from the high to low-frequency region. Based on the observations, the absorption mechanism of C@PANI microsphere has also been proposed. In another study, PANI shell fabricated on the surfaces of CNTs (CNT/aniline mass ratio = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) acted as an excellent microwave absorber (RminL: ∼41.5 dB at 9.5 GHz and EB for RL ≤ –10 dB: 5.1 GHz for 2 mm thickness) as a result of the microwave dissipation ability of CNTs and good impedance matching.371
2) acted as an excellent microwave absorber (RminL: ∼41.5 dB at 9.5 GHz and EB for RL ≤ –10 dB: 5.1 GHz for 2 mm thickness) as a result of the microwave dissipation ability of CNTs and good impedance matching.371
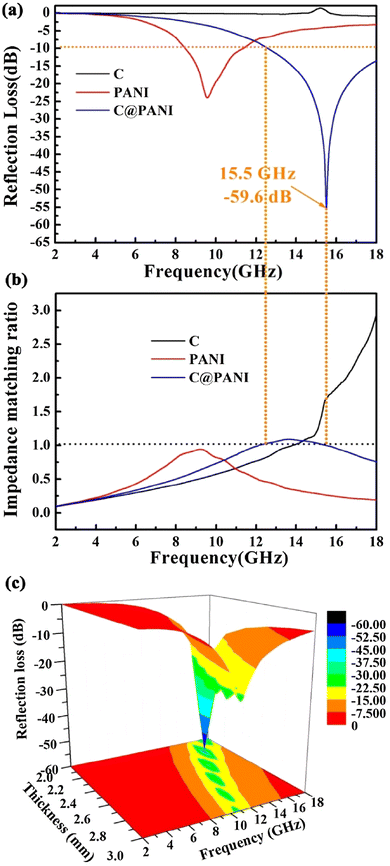 | ||
| Fig. 19 (a) Reflection loss of samples with the thickness of 2.2 mm; (b) impedance matching ratio of samples; (c) three-dimensional reflection loss of C@PANI micro-spheres with different thickness.370 Reproduced with permission from Elsevier. | ||
Liu et al.372 reported excellent microwave absorption performance (RmaxL: −38.1 dB at 11.6 GHz) in the frequency range of the X-band for the pyrrole as the shell on the core of carbon microspheres. The microwave absorption studies were made on core shell-type PEDOT (outer shell) nanocomposite with barium ferrite (center) synthesized by in situ emulsion polymerization in the frequency range of 12.4–18 GHz.373 The subsequent findings on microwaves have shown its absorption value (SEA) of 22.5 dB (>99% attenuation) owing to the higher dielectric and magnetic loss contributions. The effective electromagnetic shielding performance (EMI SE: 28.8 dB) has also been achieved in a core–shell heterostructure comprising polyaniline-coated bagasse fiber.374 According to Saini et al.,375 TEM studies established the coating of PANI deposited via in situ polymerization on the surface of individual MWCNT that contributed towards the absorption-dominated total shielding effectiveness (−27.5 to −39.2 dB) in the frequency range of (12.4–18.0 GHz). In another study, Polyaniline@Helical CNTs with dual chirality exhibited enhanced microwave absorption synergistically.376
5.1.4.2 Ternary and quaternary core@shell nanocomposites. Zhang et al.377 used a spray-dry method to fabricate a ternary composite comprising hollow microspheres of PPy@Fe3O4/CNTs by combining the conductive PPy, strongly magnetic Fe3O4 and high-conductivity CNT components. It showed the maximum reflection loss of −51.8 dB (8.8 GHz) at the thickness of 2.38 mm. Such performance of PPy@Fe3O4/CNTs as an excellent microwave absorber was attributed to the dielectric, magnetic and conductive loss achieving synergistic absorption. Hollow polypyrrole/Ni/PVDF microspheres prepared by spray phase inversion acted as an efficient electromagnetic microwave absorber (RminL: −47.2 dB at 25.36 GHz) and (RminL: −39.8 dB at 31.30 GHz).378 Further studies on the variation in the absorber thickness (1.0–3.5 mm) resulted in the tuning of the effective absorption bandwidth in the range of 18–40 GHz. PPy@FeCo@PPy nanotubes exhibited RminLof –50.5 dB and an EAB of 5.7 GHz at a thickness of 2.0 mm.379 Excellent microwave absorption performance was also reported in the trilaminar composite comprising double-shell PPy@Air@MnO2 nanotubes,380 double-shell hollow poly(acrylonitrile) microspheres@polyaniline@Ag.381 Ge et al.382 prepared hollow-spherical composites of PANI/CoS/(carbon nanodots) CDS under the applied magnetic field of 0.5 T and observed strong electromagnetic wave absorbing characteristics (RmaxL: −24 dB at 14 GHz). According to this, magnetic field-induced ferromagnetic nanodomains of Co2+clusters greatly enhance Maxwell–Wagner relaxation as well as ionic orientation polarization in the composite, leading to the dielectric loss. This in combination with magnetic loss contributed to EMW absorption of PANI/CoS/CDs-0.5T in the low-frequency range of 2–12.5 GHz.
Manna and Srivastava,383 motivated by the role played by the dual interface in microwave absorption and shielding performance, reported the fabrication of Fe3O4@C@PANI according to the scheme in Fig. 20(a) by varying the aniline![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe3O4@C as 9
Fe3O4@C as 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 8
1, 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 7
2, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 with the aniline monomer under identical reaction conditions, and the products were designated as PFC-10, PFC-20 and PFC-30, respectively. HRTEM imaging of the trilaminar PFC-10 composite in Fig. 20(b) clearly shows the formation of the highest outermost thickness of nonmagnetic polyaniline shell (∼30 nm) on Fe3O4@C corresponding to the aniline
3 with the aniline monomer under identical reaction conditions, and the products were designated as PFC-10, PFC-20 and PFC-30, respectively. HRTEM imaging of the trilaminar PFC-10 composite in Fig. 20(b) clearly shows the formation of the highest outermost thickness of nonmagnetic polyaniline shell (∼30 nm) on Fe3O4@C corresponding to the aniline![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe3O4@C ratio of 9
Fe3O4@C ratio of 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Further investigations revealed the decrease in complex permittivity and increase in complex permeability on encapsulation of Fe3O4@C by PANI due to impedance matching. Fig. 20(c) shows the highest shielding efficiency for the PFC-10 composite predominantly due to absorption (SEA: ∼47 dB) rather than reflection (SER: ∼15 dB) in the frequency range of 2–8 GHz. Such findings in Fe3O4@C@PANI are unique owing to the dual interfaces compared with Fe3O4@C and Fe3O4/PANI due to their applicability limited to a discrete frequency. In addition, such remarkable EM wave attenuation in the Fe3O4@C by PANI trilaminar core@shell composite was also supported by impedance matching and dielectric and magnetic loss.
1. Further investigations revealed the decrease in complex permittivity and increase in complex permeability on encapsulation of Fe3O4@C by PANI due to impedance matching. Fig. 20(c) shows the highest shielding efficiency for the PFC-10 composite predominantly due to absorption (SEA: ∼47 dB) rather than reflection (SER: ∼15 dB) in the frequency range of 2–8 GHz. Such findings in Fe3O4@C@PANI are unique owing to the dual interfaces compared with Fe3O4@C and Fe3O4/PANI due to their applicability limited to a discrete frequency. In addition, such remarkable EM wave attenuation in the Fe3O4@C by PANI trilaminar core@shell composite was also supported by impedance matching and dielectric and magnetic loss.
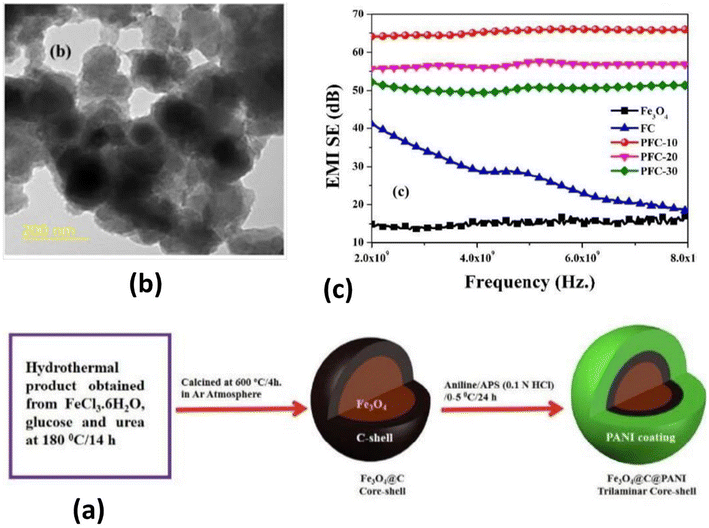 | ||
Fig. 20 (a) Schematic presentation of the fabrication of Fe3O4@C@PANI ternary composite (aniline![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe3O4@C (FC) = 9 Fe3O4@C (FC) = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 8 1, 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 7 2, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 with aniline monomer under identical reaction conditions and procedure and designated as PFC-10, PFC-20 and PFC-30, respectively), (b) HRTEM images of PFC-10, and (c) frequency vs. EMISE of PFC composites.383 Reproduced with permission from ACS. 3 with aniline monomer under identical reaction conditions and procedure and designated as PFC-10, PFC-20 and PFC-30, respectively), (b) HRTEM images of PFC-10, and (c) frequency vs. EMISE of PFC composites.383 Reproduced with permission from ACS. | ||
Motivated by this, they extended their work on core@shell@shell-type Fe3O4@SiO2@PPy, anticipating that the aggregation of Fe3O4 in the PPy matrix could be prevented by introducing SiO2 as first shell (dielectric) between the interfaces of Fe3O4 and PPy in comparison to Fe3O4 alone.384 Their findings indicated Fe3O4@SiO2@PPy (Fe3O4@SiO2/pyrrole wt/wt = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) exhibited the highest total shielding efficiency (SET: ∼32 dB) in the frequency range of 2–8 GHz (Fig. 21(a)) following reflection as the dominant shielding mechanism. This was attributed to role played by dual interfaces, poor impedance matching between the PPy (conducting)/SiO2 (insulating) and high electrical conductivity of Fe3O4@SiO2@PPy and interfacial polarization, and reflection/scattering of EM waves. These findings clearly established that switching of the dominating shielding mechanism from absorption to reflection could be achieved by tuning C@ PANI compared with SiO2@PPy shells in Fe3O4@C@PANI and Fe3O4@SiO2@PPy trilaminar composites, respectively (Fig. 21(b)). Such switching over of the shielding mechanism was also supported by the impedance mismatch and impedance matching in Fe3O4@ SiO2@PPy and Fe3O4@C@PANI.
9) exhibited the highest total shielding efficiency (SET: ∼32 dB) in the frequency range of 2–8 GHz (Fig. 21(a)) following reflection as the dominant shielding mechanism. This was attributed to role played by dual interfaces, poor impedance matching between the PPy (conducting)/SiO2 (insulating) and high electrical conductivity of Fe3O4@SiO2@PPy and interfacial polarization, and reflection/scattering of EM waves. These findings clearly established that switching of the dominating shielding mechanism from absorption to reflection could be achieved by tuning C@ PANI compared with SiO2@PPy shells in Fe3O4@C@PANI and Fe3O4@SiO2@PPy trilaminar composites, respectively (Fig. 21(b)). Such switching over of the shielding mechanism was also supported by the impedance mismatch and impedance matching in Fe3O4@ SiO2@PPy and Fe3O4@C@PANI.
 | ||
Fig. 21 (a) Plot of frequency versus SET of Fe3O4@SiO2@PPy (PFS) nanocomposites (Pyrrole/Fe3O4@SiO2 (PS) = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 8 1, 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, and 7 2, and 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) with the aniline monomer under identical reaction conditions and procedures, and the samples were designated as (PFS-10, PFS-20, and PFS-30, respectively), and (b) tuning of shells in trilaminar Fe3O4@SiO2@PPy and Fe3O4@C@PANI core@shell nanocomposites in controlling electromagnetic interference through switching of the shielding mechanism.384 Reproduced with permission from ACS. 3) with the aniline monomer under identical reaction conditions and procedures, and the samples were designated as (PFS-10, PFS-20, and PFS-30, respectively), and (b) tuning of shells in trilaminar Fe3O4@SiO2@PPy and Fe3O4@C@PANI core@shell nanocomposites in controlling electromagnetic interference through switching of the shielding mechanism.384 Reproduced with permission from ACS. | ||
Ji et al.385 synthesized hollow γ-Fe2O3@PEDOT (FP) and γ-Fe2O3@SiO2@PEDOT (FSP) core–shell nanocomposites. The corresponding SEM images of FSP and FP clearly established the removal of the SiO2 layer from γ-Fe2O3@SiO2@EDOT to form hollow γ-Fe2O3@PEDOT. Further investigations on the frequency (2–18 GHz) dependence of reflection loss revealed remarkable microwave absorption properties of hollow γ-Fe2O3@PEDOT (RminL: −44.7 dB: 12.9 GHz, EAB: 4.3 GHz in 10.8–15.1 GHz range) compared with the γ-Fe2O3@SiO2@PEDOT (RminL: −21.3 dB: 14.1 GHz, EAB: 3.8 GHz: 12.6–16.4 GHz range) for thickness 2.0 mm. This was ascribed to the synergistic effect between the magnetic and dielectric components and the core–shell structure. Reports are also available on enhanced electromagnetic wave absorption of Co@Hollow carbon nanospheres @PANI386 and FeNi@C@PANI.387 Panigrahi and Srivastava388 fabricated rubber (EPDM, NBR, and NR)@Polystyrene@Polyaniline blends and observed high shielding efficiency (>30 dB:1–8 GHz). Such high performance of rubber blends was attributed to the trapping of EM waves through enhanced internal reflection due to the typical core–shell morphology of PS@PANI. PPy nanotubes/NR/NBR389 core–shell composites also displayed excellent electromagnetic wave absorption properties.
γ-Fe2O3/(SiO2)2−SO3H/PPy composite (thickness: 2.0 mm) core/shell/shell microspheres displayed substantially improved microwave absorption properties (RmaxL: −43.1 dB) (15.1 GHz), EAB: 6.1 GHz (11.9–18.0 GHz) (Fig. 22(a)).390 This was ascribed to impedance matching, unique core/shell/shell structures, synergistic interaction, dielectric loss (SiO2 and PPy layers) and magnetic loss (γ-Fe2O3). A model has been proposed in Fig. 22(b) to account for the effects of core/shell/shell structures on microwave absorption. Core/shell/shell-structured Ni/C/PANI nanocapsules prepared by a two-step process involving modified arc-discharge and chemical polymerization exhibited an optimal RL value of −9.3 dB at 6.2 GHz (thickness: 3 mm) with broad −5 dB (3.4–18 GHz) bandwidth.391 Wang et al.392 prepared hierarchical Fe3O4@Graphene@PANI following hydrothermal and in situ polymerization. The composite exhibited favourable microwave absorption properties, as evident from RmaxL of –43.7 dB (at 10.7 GHz) and the EAB <10 dB of 5.4 GHz (6.8–12.2 GHz) with a matching thickness of 3 mm. These findings were explained on the basis of impedance matching, enhanced interfacial polarization and orientation polarization.
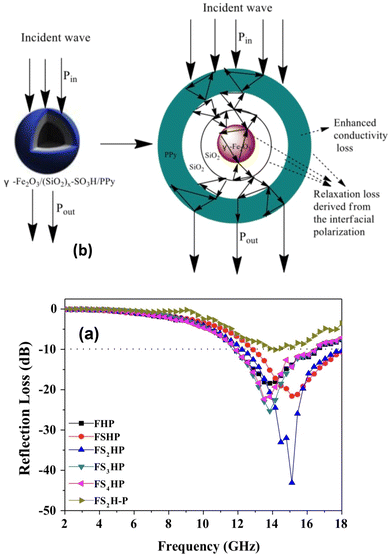 | ||
| Fig. 22 (a) Variation of RL of γ-Fe2O3/(SiO2)x–SO3H/polypyrrole (referred to as FSxHP, where x = 0,1,2,3,4) core/shell/shell microspheres and FS2H-P (physical blend of FS2H and PPy) with the frequency (d = 2 mm), and (b) physical model of the effects of core/shell/shell structures on the microwave absorption.390 Reproduced with permission from Springer. | ||
Ding et al.393 fabricated core@shell hierarchical cable-like TiO2@Fe3O4@PPy (Thickness: 3.2 mm) and observed maximum reflection loss of −61.8 dB owing to the magnetic–dielectric synergy. CoNi@SiO2@Polypyrrole nanocomposites displayed enhanced microwave absorbing capacity as evident from their minimum reflection loss of −34.19 dB at 9.59 GHz (thickness: 2.12 mm) and the EAB with RL < −10 dB in the entire X-band.394 In another study, core–shell SiCNWs@MnO2@PPy prepared through a multistep process showed the minimum reflection loss of −50.59 dB (matching thickness: 2.41 mm) and the effective absorption bandwidth of 6.64 GHz (matching thickness: 2.46 mm).395 This excellent electromagnetic wave absorption performance was ascribed to the advantage of the interfacial polarization and dipole polarization displayed by core–shell SiCNWs@MnO2@PPy. Several other ternary composites have also been reported as efficient microwave absorbers, such as Ni/PANI/RGO,396 Fe3O4@PEDOT microspheres/RGO,397 NiCo2O4 (hollow)@PPy nanofibers/RGO,398 encapsulation of γ-Fe2O3-decorated RGO in PANI core–shell tubes,399 RGO/Fe3O4/PANI,56 N-doped Graphene@PANI nanorod modified by Fe3O4 nanoclusters,400 polyaniline/graphene oxide/Fe3O4,401 magnetite nanoparticles decorated CNT/PANI,402 PANI/CIP/Fe3O4,403 and Fe3O4@SiO2@PPy.404 Shukla405 prepared dual core–shell structured Fe3O4/C/PPy (Fe3O4/C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PPy:2
PPy:2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 wt/wt) composites via a hydrothermal and chemical oxidative polymerization method and observed their absorption-dominated excellent EMI shielding efficiency (>28) dB in the range of 1 to 8.5 GHz (Fig. 23(a)). The probable mechanism in Fig. 23(b) shows the attenuation of electromagnetic waves by the trilaminar Fe3O4/C/PPy composite. It was also suggested that spin motion plays a decisive role in this performance. In addition, excellent electromagnetic absorption properties have also been reported in PANI@Natural graphite flakes (NGF)/MWCNT406 and PEDOT/RGO/Co3O4.407
8 wt/wt) composites via a hydrothermal and chemical oxidative polymerization method and observed their absorption-dominated excellent EMI shielding efficiency (>28) dB in the range of 1 to 8.5 GHz (Fig. 23(a)). The probable mechanism in Fig. 23(b) shows the attenuation of electromagnetic waves by the trilaminar Fe3O4/C/PPy composite. It was also suggested that spin motion plays a decisive role in this performance. In addition, excellent electromagnetic absorption properties have also been reported in PANI@Natural graphite flakes (NGF)/MWCNT406 and PEDOT/RGO/Co3O4.407
 | ||
Fig. 23 (a) Plots of frequency versus SET Fe3O4/C/PPy core/shell composites with pyrrole: (Fe3O4/C = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 8 1, 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2,7 2,7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) with pyrrole monomer under similar reaction situations, referred to as10, 20 and 30, respectively, and (b) schematic diagram of EMI attenuation mechanism.405 Reproduced with permission from Springer. 3) with pyrrole monomer under similar reaction situations, referred to as10, 20 and 30, respectively, and (b) schematic diagram of EMI attenuation mechanism.405 Reproduced with permission from Springer. | ||
According to the available literature, a very limited amount of work has been reported on quaternary core–shell composites in general, or their applications in microwave absorption. In one such study quaternary MWCNT/CuO/Fe3O4/PANI nanocomposites following the weight ratio of CuO/Fe3O4/PANI to MWCNT in the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 1
3, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 and 1
4 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 showed minimum reflection losses −45.7, −85.4, and −87.4 dB, respectively.408 The corresponding absorption bandwidths (RL ≤ −10 dB) of MWCNT/CuO/Fe3O4/PANI nanocomposites were found to be 6, 7.6, and 6 GHz. The higher value of loss constant (α) of MWCNT/CuO/Fe3O4/PANI nanocomposites indicated both magnetic and dielectric loss tangent playing key roles in influencing the microwave absorption efficiency.
5 showed minimum reflection losses −45.7, −85.4, and −87.4 dB, respectively.408 The corresponding absorption bandwidths (RL ≤ −10 dB) of MWCNT/CuO/Fe3O4/PANI nanocomposites were found to be 6, 7.6, and 6 GHz. The higher value of loss constant (α) of MWCNT/CuO/Fe3O4/PANI nanocomposites indicated both magnetic and dielectric loss tangent playing key roles in influencing the microwave absorption efficiency.
Wang et al.409 fabricated a 3D heterostructure of Graphene@Fe3O4@WO3@PANI using a hydrothermal method and chemical oxidation polymerization and studied its performance in microwave absorption. In addition, the presence of WO3 decreased permittivity, facilitating better impedance matching. The synthesized quaternary nanocomposite displayed a maximum RL value (−46.7 dB) at 9.4 GHz and the maximum absorbing bandwidth exceeding −10 dB at 1.8 GHz (12.4 to 14.2 GHz) corresponding to the thickness of 4 mm and 1.5 mm, respectively. The EM wave absorption mechanism involved contributions originating from enhanced interfacial polarization, better impedance matching, multiple reflection, and a synergistic effect. In another study, Liu et al.410 investigated the electromagnetic wave absorption properties of graphene (GN@Fe3O4@PANI) decorated with TiO2 prepared by a hydrothermal method and in situ polymerization, as described in Fig. 24(a). The corresponding TEM image in Fig. 24(b and c) indicated TiO2 nanosheets oriented perpendicular to GN@Fe3O4@PANI and TiO2 nanosheets forming hierarchical structures. The EM wave absorption properties of graphene@Fe3O4@PANI@TiO2 nanosheets loaded in 50 wt% paraffin are displayed in Fig. 24(d). According to this, GN@Fe3O4@PANI@TiO2 nanosheets showed RmaxL of −41.8 dB at 14.4 GHz (thickness: 1.6 mm) and absorption bandwidth of RL < −10 dB at ∼3.5 GHz. The attenuation of EM waves was attributed to the interfacial polarization and improved impedance matching of the nanocomposites. Table 1 records the microwave absorbing properties of hollow ICPs, hollow ICP nanocomposites and ICP based core–shell nanocomposites.
 | ||
| Fig. 24 (a) Schematic illustration of the fabrication of the GN@Fe3O4@PANI@TiO2 nanosheets, (b and c) TEM images of graphene GN@Fe3O4@PANI@TiO2 nanosheets, and (d) reflection loss curves of GN@Fe3O4@PANI@TiO2 nanosheets.410 Reproduced with permission from Elsevier. | ||
| Conducting polymer/nanocomposites | Method of preparation | Filling (wt%) in the matrix selected | Frequency range | Shielding performance (sample thickness) | Maximum bandwidth (<10 dB) (GHz) | Ref. |
|---|---|---|---|---|---|---|
| Hollow polyaniline/Ag | Emulsion polymerization | — | 100 kHz–20 GH | SET: 19.5 dB | — | 203 |
| Hollow polypyrrole/Ag | Via in situ chemical oxidative copolymerization | — | 100 kHz–20 GHz | SET: 34.5 dB | — | 204 |
| Fe3O4 (core: ∼100 nm)/PPy (shell: ∼70 nm) | In situ polymerization | 50 wt% in paraffin | 2–18 GHz | R minL: −22.4 dB at 12.9 GHz (thickness: 2.3 mm) | ∼5 GHz in the range of 4–18 GHz (thickness: 1.5–5.0 mm) | 329 |
| Fe3O4 microsphere@PANI | Two step oxi. polymerization | 50 wt% in paraffin wax bn | 2–16 GHz | R maxL: −31.3 dB at 9.0 GHz (thickness: 3 mm) | 4.0–18 GHz (thickness: 1.5 to 5.0 mm) | 330 |
| Graphene@Fe3O4@SiO2@PANI | Dilute polymerization | 25 wt% in paraffin wax | 2–18 GHz | R maxL: −40.7 dB at 12.5 GHz (thickness: 2.5 mm) | 5.8 GHz (10.5–16.3 GHz) | 331 |
| 3D helical hollow superstructure of PANI | Co-self-assembly process combined with emulsion droplets | 20 wt% in paraffin | 2–18 GHz | R minL: −51.60 dB at 13.95 GHz (thickness:2.0 mm) | 5.12 GHz (12.03–17.15 GHz) | 337 |
Hollow PPy nanofibers based self-assembled aerogel (seeds and Py proportion = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5) 0.5) |
Self-assembly | 8% in paraffin | 2–18 GHz | R minL: −58.73 dB at 16.48 GHz (thickness: 2.30 mm) | 7.28 GHz (thickness: 2.69 mm) | 338 |
| Hollow PPy nanorods | Via self-assembly template sacrificial strategy | 10 wt% in paraffin | 2–18 GHz | R minL: −54.94 dB at 13.2 GHz (thickness: 3.4 mm) | 7.36 GHz | 339 |
| PANI microtubes | Via a self-assembly process assisted by excess APS | — | 0 to 6000 MHz. | R L: −15.5 dB | — | 342 |
| PANI hierarchical microtubes | SDS/HCl (7.5 mM) assisted oxidative polymerization method | 50% in paraffin | 2–18 GHz | −43.6 dB: absorption (thickness: 1.55 mm) | 5.84 GHz | 343 |
| Polyaniline 3D hollow spheres integrated milled carbon fibers (MCF) | PANI 3D hollow spheres are synthesized by removing the polystyrene (PS) core from PS/PANI composites | 4 wt% MCF + 1 wt% PANI in epoxy | 8–12 GHz | Max. absorption −49.3 dB (thickness: 1.8 mm) | 1.7 GHz (thickness: 1.8 mm) | 344 |
| Polyaniline-mediated N and S co-doped carbon nanoflakes | In situ polymerization method and carbonization process with oxygen limitation | Paraffin wax (80 wt%) and samples (20 wt%) | 2–18 GHz | R minL: −53.5 dB at 10.2 GHz (thickness: 2.9 mm) | 4.5 GHz at 2.9 mm | 345 |
| Hollow PANI /CNF in the surface layers and MXene/CNF in the intermediate layer | Alternating vacuum-assisted filtration method | MXene: 8 wt% and Hollow PANI : 24 wt% in CNF matrix | 8–12.4 GHz | EMI SE: 35.3 dB | — | 346 |
| Hollow Fe3O4 (7.33 wt%)@PANI (magnetic fluid: 15 ml) | Hollow PANI prepared by using PS microspheres as hard template and decorating Fe3O4 on its surface. | Paraffin filled with with a sample (loaded with 7.33 wt% Fe3O4) content of 75% | 2–18 GHz | R maxL: −15.6 dB | 8.0 GHz | 347 |
| Fe3O4@PPy | In situ polymerization method | 60 wt% of composites blended with paraffin wax | 2–18 GHz | R maxL: −52.01 dB in 25 wt% of paraffin wax loaded sample of 3.1 mm thickness | 2.72 GHz (thickness: 3.1 mm) | 348 |
| Fe3O4/PPy double-carbonized core–shell-like composite | Rapid microwave assisted carbonization process | — | 2–18 GHz | R minL: −26 dB at 16 GHz (thickness: 1.6 mm) | 4.64 GHz (thickness: 1.6 mm) | 349 |
| Hollow core–shell Fe3O4@PPy | Solvothermal process, followed by in situ polymerization (aniline monomer: 170 μL) | 50% of nanocomposite by wt in paraffin | 2–18 GHz | R minL: −84.92 dB at 3.87 mm | 4.20 GHz at 2.38 mm | 350 |
| Fe3O4–PE@PANI hollow sphere | Electrostatic self-assembly approach | 50 wt% in paraffin | 0.5–15 GHz | R minL: −6.5 dB at 14.3 GHz | Frequency bandwidth at less than −5 dB (12.5 to 15 GHz) | 352 |
Hollow structure Fe3O4/PANI microspheres (aniline![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PS = 1 PS = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) 6) |
Three-step synthesis 1.5 and 2.0 mm, the bandwidth below and 4.64 GHz (11.04–15.68 GHz) | 50 wt% in paraffin | 2–18 GHz | R minL: −24.3 dB at 18 GHz (thickness: 1.6 mm) | 2.48 GHz (15.52–18 GHz) for 1.5 mm thickness, 4.64 GHz (11.04–15.68 GHz) for 2 thickness | 353 |
| Hollow poly(aniline-co-pyrrole)–(0.6 g Fe3O4) | Oxidized polymerization of aniline and pyrrole in the presence of Fe3O4 (0.06 g) using non-ionic surfactant as a template | 50 wt% in paraffin | 0.5–10 GHz | R minL : −3 dB at 9 GHz and 10 GHz (thickness: 2 mm) | — | 354 |
| Fe3O4/PANI (hollow 0D/1D) | Hydrothermal treatment and chemical oxidative polymerization | 40% in wax | 2–18 GHz | R minL: −55.03 dB (thickness: 1.84 mm) | 4.88 GHz (thickness: 1.84 mm) | 355 |
| Fe3O4/polyaniline core/shell microspheres (PANI shell thickness: 100 nm) | In situ polymerization method | 50 wt% in paraffin | 1–18 GHz, | R maxL: −37.4 dB at 15.4 GHz | — | 356 |
| Fe3O4-PEDOT microspheres (EDOT/Fe3O4 vol. fraction: 20%) | Two-step method | Paraffin wax | 2–18 Ghz | R minL: ∼−30 dB at 9.5 GHz (thickness: 4 mm) | — | 357 |
| Core–shell Fe3O4−polyaniline | In situ polymerization of aniline in the presence of dodecyl benzenesulfonic acid | 40% Fe3O4−polyaniline in paraffin wax | 0.03–18 GHz | R optimalL: −35.1 dB at 16.7 GHz (thickness: 1.7 mm) | — | 358 |
| Core–shell Fe3O4@Polypyrrole | Process comprising etching (time: 5 min), polymerization and replication | 50 wt% filler in paraffin | 2–18 GHz | R maxL: −41.9 dB (99.99%) at 13.3 GHz (thickness: 2.0 mm) | 6.0 GHz (12.0–18.0 GHz). | 359 |
| Core–shell Fe3O4@Polypyrrole microspheres (pyrrole/Fe3O4 ratio = 20) | Chemical oxidative polymerization in the presence of polyvinyl alcohol and p-toluenesulfonic acid | 50% filler in paraffin wax | 2–18 GHz | R maxL: −31.5 dB (99.99%) at 15.5 GHz (thickness: 2.5 mm) | 5.2 GHz (12.8–18 GHz) | 360 |
| Core–shell structure BaFe12O19@PANI | 30 vol% paraffin wax and 70 vol% powdered filler | In situ polymerization method (aniline: 400 μL) | 2–18 GHz | RmaxL: −65.354 dB at 17.28 GHz (thickness: 1.47 mm.) | 2.3 GHz | 361 |
| Polythiophene nanofibers coated on MnFe2O4/Fe3O4 (core–shell) | Co-precipitation and in situ polymerization | — | 8.0–12.0 GHz | R minL: −21 dB at 12 GHz (thickness: 1.5 mm) | Whole X-band | 362 |
| CoSe2@Polythiophene core–shell | Hydrothermal followed by in situ polymerization | 10 wt% mixed with PVDF | 2–18 GHz | R minL: −55.40 dB (thickness: 1.76 mm) | 5.8 GHz (thickness: 1.76 mm) | 363 |
| rGO/Ni0.5Co0.5Fe2O4@PEDOT | In situ oxidative polymerization | — | 12.4–18.0 GHz | SET: 38.79 dB | Broad absorption bandwidth | 364 |
| 3D graphene-supported Fe3O4-coated by polypyrrole | One-step chemical reduction method (GO to Fe3O4@PPy) wt ratio = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
Composites soaked into molten paraffin | 2–18 GHz | R minL: −40.53 dB at 6.32 GHz (thickness:2.5 mm) | 5.12 GHz (thickness: 2.5 mm) | 365 |
| Prism-shaped hollow carbon decorated with polyaniline | Carbonization and in situ polymerization | 30 wt% filler in wax | 2–18 GHz | RminL: −64.0 dB at 11.1 GHz (thickness: 2.5 mm) | 5.0 GHz (9.5–14.5 GHz) | 366 |
| Core–shell PPy@MoS2 | Combining chemical oxidative polymerization and hydrothermal process | 40 wt% filler in paraffin | 2–18 GHz | R optimalL: −49.1 dB at 6.1 GHz (thickness: 2.5 mm) | 6.4 GHz (11.5–17.5 GHz) at 2.5 mm | 367 |
| PPy@PANI | Polymerization of aniline on the surface of PPy microspheres | 50 wt% filler in paraffin | 2–18 GHz | R maxL: −34.8 dB at 13.9 GHz (thickness: 2 mm) | 4.7 GHz (11.9–16.6 GHz) | 368 |
| Hollow ZnxFe3−xO4@Polyaniline | Solvothermal followed by in situ chemical oxidation polymerization (ZnxFe3−xO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) aniline ratio = 0.5 aniline ratio = 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
50 wt% filler in paraffin wax | 2–18 GHz | R minL: −59.44 dB at 11.04 GHz (thickness: 2.31 mm) | 4.65 GHz (13.35–18.0 GHz) for thickness of 1.72 mm. | 369 |
| Waxberry-like Carbon@Polyaniline microspheres | Via dilute polymerization | 30 wt filler in paraffin wax | 2–18 GHz | R minL: −59.6 dB at 15.5 GHz (thickness : 2.2 mm) | 5.4 GHz (12.6 to 18 GHz) for thickness of 2.2 mm. | 370 |
| CNTs/polyaniline (shell) | Hybrid powders with wax at 10 wt% loading |
In situ polymerization (mass ratio of CNTs/PANI:1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
2–18 GHz | R minL: −41.5 dB at 9.5 GHz (thicknesses: f 2 mm) | 5.1 GHz at thicknss of 2.0 mm. | 371 |
| PPy (shell)@Carbon microsphere (CMC) | In situ polymerization | 40 wt% of composite corresponding to 0.6 g of CMC in paraffin wax | 2–18 GHz. | R maxL: −38.1 dB at 11.6 GHz (thickness: 3.00 mm) | 11.17 to 12.26 GHz (thickness of 3.00 mm) | 372 |
| Core shell PEDOT/barium ferrite | In situ emulsion polymerization | — | 12.4–18 GHz | SEA: 22.5 dB at 15 GHz with minimal reflection loss of 2 dB | — | 373 |
| PANI-coated bagasse fiber (BF) core–shell heterostructure | One-step in situ polymerization of aniline in the dispersed system of BF | Mass ratio of BF/PANI to paraffin = 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 60 |
8.2–12.4 GHz | EMI SE: 28.8 dB (thickness: 0.4 mm) | — | 374 |
| Polyaniline-coated MWCNT | In situ polymerization | — | 12.4–18.0 GHz | SET: −27.5 to −39.2 dB | — | 375 |
| Polyaniline@Helical-CNTs with dual chirality | In situ polymerization | Mass ratio of the samples to wax: 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
2–18 GHz. | R maxL: −32.5 dB at 8.9 GHz (thickness: 3.7 mm) | 5.1 GHz (from 7.1 to 11.2 GHz). | 376 |
| Hollow PPy microspheres@Fe3O4/CNTs | Spray-dry method | Mass ratio of powder with paraffin: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
2–16 GHz | R maxL: −51.8 dB at 8.8 GHz (thickness: 2.38 mm) | 2.24 GHz (7.76 GHz to 10 GHz) | 377 |
| Hollow PPy/Ni/PVDF microspheres | Spray phase inversion method | Mixing samples with paraffin wax in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 wt ratio 8 wt ratio |
18–40 GHz | R minL: −47.2 dB at 25.36 GHz and −39.8 dB at 31.30 GHz | 18–40 GHz (thickness: 1.0–3.5 mm) | 378 |
| PPy@FeCo@PPy nanotubes | Combination of electroless plating and oxidative polymerization | Mass fraction of 15 wt% dispersed in the paraffin | 2–18 GHz | R minL: −50.5 dB (thickness: 2.0 mm) | 5.7 GHz (thickness: 2.0 mm) | 379 |
| 1D double-shell PPy@Air@MnO2 nanotubes | 50 wt% in paraffin | Dispersion and self-assembly method | 2–18 GHz | R maxL: −52.49 dB at 8.88 GHz (thickness: 2.94 mm) | 3.84 GHz (thickness: 2.94 mm) | 380 |
Hollow-spherical composites of PANI/CoS/carbon nanodots (molar ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
30 wt% in paraffin wax | Via in situ polymerization in the presence of magnetic field | 2–18 GHz | R maxL: −24 dB at 14 GHz under external applied field of 0.5 T (thickness: 3 mm) | 1.92 GHz | 382 |
Fe3O4@Carbon@PANI (Fe3O4@C: Aniline = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 wt/wt) 9 wt/wt) |
Multiple steps | — | 2–8 GHz | RL: ∼33 dB SET: ∼65 dB | — | 383 |
| Fe3O4@SiO2@PPy | Multistep | Mass ratio of products and paraffin = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
2–18 GHz | SET: ∼32 dB (thickness: 1 mm) | — | 384 |
| γ-Fe2O3@PEDOT | Reaction of hydrofluoric acid and and γ-Fe2O3@SiO2@PEDOT | Mass ratio of γ-Fe2O3@PEDOT and paraffin = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
2–18 GHz | R minL: −44.7 dB at 12.9 GHz with a matching layer thickness of 2.0 mm | 4.3 GHz (10.8–15.1 GHz) | 385 |
| Co@Hollow carbon nanospheres@Polyaniline | Mixed with paraffin in mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
Soft template, switching liquid phase transport and in situ polymerization method | 2–18 GHz | R minL: −43.63 dB (matching thickness: 2.8 mm) | 9.75 GHz (8–17.5 GHz) at a matched thickness of 2.8 mm | 386 |
| FeNi@C@Polyaniline | Combining the arc-discharge process and an in situ chemical oxidative polymerization reaction | FeNi@C@Polyaniline (40 wt%) mixed with paraffin | 2–18 GHz | R minL: −49.2 dB at 16.6 GHz for a thickness of 1.3 mm | 5 GHz (13–18 GHz) for the thickness of 1.4 mm | 387 |
| PS@PANI | Solution mixing | — | 100 kHz–20 GHz | EMI SE: ∼32 dB at 8 GHz (thickness: 0.5 mm) | — | 388 |
| PPy nanotubes/NR/NBR (90/10) | 5.24 wt% in paraffin | Mixing technology | 8.2–12.4 GHz | RminL: −56.67 dB (thickness:2.9 mm) | 3.7 GHz (thickness:2.9 mm) | 389 |
| γ-Fe2O3/(SiO2)x–SO3H/polypyrrole core/shell/shell microspheres | Sol–gel process and an in situ polymerization | 70% in paraffin wax | 2–18 GHz | R maxL: −43.1 dB (15.1 GHz), (thickness: 4 mm) | 6.1 GHz (11.9–18.0 GHz) | 390 |
| Core/shell/shell-structured Ni/C/polyaniline | Modified arc discharge method and a chemical polymerization method | 40 wt% in paraffin | 2–18 GHz | R optimalL: −9.3 dB at 6.2 GHz (thickness: 3 mm) | 5 dB (3.4–18 GHz) | 391 |
| Fe3O4 microsphere@Graphene nanosheets@PANI nanorods | Hydrothermal and in situ polymerization methods | 30% in paraffin | 2–18 GHz | R maxL: −43.7 dB at 10.7 GHz with a thickness of 3 mm | 5.4 GHz (from 6.8 to 12.2 GHz) | 392 |
| TiO2@Fe3O4@PPy | Sequential process of solvothermal treatment and polymerization | — | 2–18 GHz | R maxL: −61.8 dB (thickness: 3.2 mm) | 6.0 GHz (X and Ku band) at 2.2 mm thickness | 393 |
| CoNi@SiO2@PPy | Three-step reaction: | — | 8.2 to 12.4 GHz | R minL: −34.19 dB at 9.59 GHz (thickness 2.12 mm) | Entire X-band (8.2–12.4 GHz) | 394 |
| SiCNWs@MnO2@PPy heterostructures | Chemical vapor deposition and two-step electrodeposition process | — | 2–18 Ghz | R minL: −50.59 dB (Matching thickness: 2.41 mm) | 6.64 GHz (matching thickness: 2.46 mm) | 395 |
| Ni/PANI/RGO | Hydrothermal followed by in situ polymerization | — | 2–18 GHz | R maxL: −51.3 dB at 4.9 GHz with 3.5 mm thickness | 3.1 GHz (3.3 to 6.4 GHz) | 396 |
| Fe3O4@PEDOT microspheres/RGO | Multiple steps | 50 wt% in paraffin | 2.0–18.0 GHz | R maxL: −48.8 dB at 9.12 GHz (matching thickness:2.9 mm) | 4.32 GHz (matching thickness:2.9 mm) | 397 |
| Core–shell NiCo2O4@Polypyrrole nanofibers/RGO | 50 wt% in paraffin | Combination of multiple steps | 2.0–18.0 GHz | R minL: −61.20 dB at 14.26 GHz a (thickness:1.56 mm) | 4.90 GHz at 1.57 mm | 398 |
| γ-Fe2O3 decorated RGO in PANI core–shell tubes | Aniline to Fe2O3-decorated rGO weight ratios = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
Multiple steps | 8.2–12.8 GHz | SET: ∼51 dB) (thickness: 2.5 mm) | — | 399 |
| N-doped graphene@PANI nanorod arrays modified with Fe3O4 | Hydrothermal reaction | 25 wt% of the sample in paraffin | 2–18 GHz | R maxL: −40.8 dB at 14.8 GHz with thickness of 2.7 mm | 5.1 GHz (10.4 to 15.5 GHz) | 400 |
| PANI/GO/Fe3O4 | In situ polymerization | 50% in paraffin wax | 2–18 GHz | R maxL: −53.5 dB at 7.5 GHz (thickness: 3.91 mm) | 2.8 GHz (thickness: 3.91 mm) | 401 |
| PANI/carbonyl iron powder (CIP)/Fe3O4 | Mechanical mixing PANI/CIP composite with PANI/Fe3O4 composite with mass ratio of 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
— | 0.5–18 GHz | R maxL: −48.3 dB at 9.6 GHz (thickness: 1.76 mm) | — | 403 |
| Fe3O4@SiO2@PPy | Microemulsion polymerization method | Composites mixed paraffin with 15 wt% | 2–18 Ghz | R minL: −40.9 dB at 6 GHz (thickness: 5 mm) | 6.88 GHz (11.12–18 GHz) | 404 |
Fe3O4/C/PPy (Fe3O4/C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PPy: 2 PPy: 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 wt/wt) 8 wt/wt) |
Hydrothermal and chemical oxidative polymerization method | — | 1–8.5 GHz | EMI SE: >28 dB (thickness: 0.8 mm) | — | 405 |
| PANI@Natural graphitic flakes (NGF)/MWCNT | In situ by ball milling | 10 wt% of MWCNTs | 12.4–18.0 GHz | EMI SE: −98 dB | — | 406 |
| PEDOT@RGO/Co3O4 | Two-step method | 50 wt% of paraffin | 2–18 GHz | R maxL: −51.1 dB (10.7 GHz) at thickness of 2.0 mm | 3.1 GHz (9.4–12.5 GHz) | 407 |
| MWCNT/CuO/Fe3O4/PANI (wt ratios of CuO/Fe3O4/PANI to MWCNT = 1.5) | Step by-step approach | 25% filler in paraffin | 8.2–18 GHz | R minL: −87.4 dB (thickness: 3 mm) | 6 GHz (12–18 GHz) | 408 |
| Graphene@Fe3O4@WO3@PANI | Simple hydrothermal method and chemical oxidation polymerization | Composite blended with 70 wt% wax | 2–18 GHz | R maxL: −46.7 dB at 9.4 GHz (thickness: 4 mm) | 1.8 GHz (12.4–14.2 GHz) at thickness of 1.5 mm | 409 |
| Graphene@Fe3O4@PANI@TiO2 | Hydrothermal method and in situ polymerization | 50 wt% in paraffin | 2–18 GHz | R maxL: −41.8 dB at 14.4 GHz (thickness: 1.6 mm) | 3.5 GHz | 410 |
5.2 Removal of heavy metals in water
Heavy metals have atomic weights between 63.5 and 200.6, and a specific gravity greater than 5.0.411 These are considered as extremely important toxic environmental pollutants on their discharge from tanneries, fertilizers, mining, metal plating, batteries, pesticides, paper industries and many more, into lakes, rivers, and oceans or into open environments, endangering human life and other living systems owing to their toxicity. Some heavy metals such as Cu, Zn, Fe, Mn are essential for the working of the human metabolic system, but can lead to harmful effects when their concentrations are very high.62Table 2 highlights the sources as well as the health effects of heavy metals on human beings.412| Heavy metal | Sources | Health effects | |
|---|---|---|---|
| Essential heavy metal | Zinc (Zn) | Oil refining plumbing, brass manufacturing | Gastrointestinal disorders, kidney and liver abnormal functioning |
| Copper (Cu) | Copper polishing plating, printing | Abdominal disorders, metabolic activity abnormalities | |
| Iron (Fe) | High intake of iron supplements & oral consumption | Vomiting, diarrhea, abdominal pain, dehydration & lethargy | |
| Cobalt (Co) | Hip alloy replacement case | Haematological, cardiovascular hepatic, endocrine | |
| Non essential heavy metal | Chromium (Cr) | Steel fabrication, electroplating textile | Lung disorders, (bronchitis, cancer), renal and reproductive system |
| Lead (Pb) | Batteries, coal combustion, paint industry | Serious effects on mental health (Alzheimer's disease), nervous system | |
| Arsenic (As) | Atmospheric deposition, mining, pesticides | Highly effects dermal region (cancer), brain & cardiac problems | |
| Mercury (Hg) | Coal combustion, fish, mining, paint industry, paper industry, volcanic eruption | Sclerosis, blindness, Minamata disease, deafness, gastric problems, renal disorders | |
| Cadmium (Cd) | Plastic, fertilizers, pesticides | Osteo related problems, prostate cancer, lung diseases, renal issues |
In view of this, heavy metal ions can be removed from wastewaters/aqueous solution by using different adsorbents. Intrinsically conducting polymers, especially polyaniline and polypyrrole, have played an excellent role as adsorbents in the removal of different heavy metal ions from contaminated aqueous solutions.81–85,413 This is ascribed to their several advantages, such as simple synthesis, excellent environmental stability, chemical versatility, biocompatibility, low cost, the presence of functional groups that facilitate their modification, and functionalization by doping and composite formation to favor the adsorption process by tuning their properties.85,414 Furthermore, the hollow morphology of ICPs, especially polyaniline, can be effective for the adsorption of various pollutants such as heavy metals and dyes due to a large surface area. In addition, hollow ICP-based composites and core–shell nanomaterials have been reported as good adsorbents for removing heavy metal pollutants from wastewater due to their unique physical and chemical properties.
5.2.1.1 Hollow ICPs. Recently, hollow spherical polyaniline synthesized using a poly(styrene-co-acrylic acid) sphere as the template showed high adsorption of Cr(VI), as evident from values of 601.3, 347.8 and 235 mg g−1 at pH corresponding to 1, 2, and 3, respectively.416 Furthermore, the adsorption of Cr(VI) followed a pseudo second-order equation and Redlich–Peterson isotherm models, including excellent regeneration. According to Wu et al.,417 hollow polyaniline micro/nanospheres (10 mg) achieved a maximum removal capacity of 127.88, 43.20 and 25.6 mg g−1 at the 1.2 mmol L−1 initial concentration of Cr(VI), corresponding to the solution pH of 3, 4 and 5, respectively. The kinetic studies fitted well with the second-order model, and the removal of Cr(VI) could be described by the Langmuir isotherm. 1D-polyaniline nanowire/tubes removed Cr(VI) rapidly and effectively from aqueous solution over a suitable pH range and the adsorbent could be easily regenerated for reuse.418 A hollow tubular structure comprising the amino acid-doped polyaniline by in situ chemical polymerization method exhibited a removal capacity toward Cr(VI) (60.0 mg g−1).419 Li et al.420 prepared bamboo-like polypyrrole nanotubes following the reactive-template vapor phase polymerization method using electrospun V2O5 nanofibers acting as templates as well as the oxidant. These findings indicated the maximum adsorption capacity of 9.281 mmol g−1 for Cr(VI) in aqueous solution, with the adsorption data fitting well with the Langmuir model and following pseudo-second-order kinetics (k: 0.0031 g mmol−1 min−1).
5.2.1.2 Binary core–shell composites. A polyaniline/polystyrene core–shell nanocomposite showed the ∼95% removal of Cr(VI) corresponding to the initial concentration of Cr(VI), adsorbent dose, volume of the medium and pH of 100 mg L−1, 250 mg, 50 mL and 2, respectively.421 The maximum adsorption capacity (qm) of PANI/PS for Cr(VI) was found to be 19 mg g−1. The possible mechanism involved the complexation between Cr(VI) ions and the N atoms of the –N
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– groups through sharing of their lone pair of electrons. In addition, polyacrylonitrile/polyaniline core/shell nanofiber mat,422 sulfonated poly(arylene ether nitrile)/polypyrrole core/shell nanofibrous mat,423 Ag-P/Ppy core–shell composite,424 polypyrrole-wrapped oxidized MWCNTs,425 polyaniline-coated ethyl cellulose,426 and polypyrrole-coated halloysite nanotube clay nanocomposite427 have also been used as adsorbents in the efficient removal of toxic Cr(VI) from aqueous solution.
C– groups through sharing of their lone pair of electrons. In addition, polyacrylonitrile/polyaniline core/shell nanofiber mat,422 sulfonated poly(arylene ether nitrile)/polypyrrole core/shell nanofibrous mat,423 Ag-P/Ppy core–shell composite,424 polypyrrole-wrapped oxidized MWCNTs,425 polyaniline-coated ethyl cellulose,426 and polypyrrole-coated halloysite nanotube clay nanocomposite427 have also been used as adsorbents in the efficient removal of toxic Cr(VI) from aqueous solution.
Fe3O4/PANI microspheres were fabricated through the interfacial polymerization and used as adsorbent in removing about 94% of Cr(VI) ions from water.428 The adsorption isotherm followed the Langmuir isotherm mode (qm: 200 mg g−1), and pseudo-second-order kinetics. Fe3O4/PANI microspheres. In addition, Fe3O4/PANI has been evaluated for its regenerability and reusability to remove adsorbed Cr(VI) ions using NaOH aqueous solution. These studies have shown the adsorption capacity of Fe3O4/PANI microspheres retained 90% of the initial value after reusing five times. It may be interesting to mention that the regeneration of Fe3O4/PANI microspheres using hydrochloric acid is not possible as it degrades the polyaniline and dissolves the Fe3O4. In another study, Fe3O4/polypyrrole nanotubes prepared by a one-pot process exhibited a Cr(VI) adsorption capacity of ∼451.45 mg g−1.429 This suggested that the adsorption process takes place due to ion exchange and chelation. Furthermore, the existence of −NH+ on the Fe3O4/PPy nanotubes partially reduced Cr(VI) to Cr(III). Fe3O4/PPy nanotubes also retained about 90% of the initial removal efficiency after 5 adsorption/desorption cycles. According to this, adsorption of Cr(VI) on the Fe3O4/PPy nanotubes involved ion exchange and chelation. As a result, Cr(VI) was partially reduced to Cr(III) due to the existence of −NH+ on the Fe3O4/PPy nanotubes.
In another study, Fe3O4-coated polypyrrole (initial concentration: 200 mg L−1) showed 100% adsorption for 200 mg L−1 Cr(VI) aqueous solution corresponding to pH 2.430 The proposed mechanism for the removal of Cr(VI) was guided by ion exchange and reduction on the surface of the nanocomposite. The kinetics studies indicated a pseudo-second-order rate model and Langmuir model predicted from the fitting of isotherm data. The maximum adsorption capacity of Polypyrrole/Fe3O4 magnetic nanocomposite increased from 169.4 to 243.9 mg g−1 corresponding to the temperature change from 25 °C to 45 °C, respectively. The possible mechanism was elucidated based on XPS studies. According to this, the possible mechanism for Cr(VI) removal by the PPy/Fe3O4 could be due to ion exchange and reduction on the surface. Several other adsorbents comprising magnetite arginine-functionalized polypyrrole,431 magnetic particle-coated PPy and PANI,432 polypyrrole-coated Fe3O4,433 polypyrrole-modified natural corncob-core sponge,434 polyaniline-coated protonic titanate nanobelt,435 and MnO2-coated polyaniline nanofibers436 also successfully removed hexavalent chromium from water.
Du et al.437 synthesized core–shell polypyrrole/hollow mesoporous SiO2 particles by in situ polymerization to study the removal of Cr(VI) from aqueous solution that exhibited the maximum adsorption capacity of Cr(VI) of ∼322 mg g−1 at 25 °C following the quasi-second-order kinetic model and the Langmuir isotherm model. In another study, deposition of PANI on the surface of ThO2 has been validated by TEM analysis.438 Further investigations revealed the adsorption of Cr(VI) on this core shell composite to be dependent on the solution pH. The kinetic model and adsorption process fitted well with the pseudo-second-order and Langmuir model (qm: 141 mg g−1). Polyacrylonitrile/polypyrrole core/shell nanofiber mat exhibited high selectivity for Cr(VI) compared with the Ni(II) and Cu(II) ions in the solution.439 The high removal efficiency of hexavalent chromium has also been reported on L-cystine-doped glucose carbon spheres (GCS)@PPy,440 PANI@Nano hollow carbon spheres,441 Polypyrrole@Attapulgite,442 copper slag@polyaniline,443 polyaniline@Ni(OH)2,444 PPy@MgFe2O,445 polypyrrole/graphene oxide,446 and PANI@Almond shell biocomposite.447
5.2.1.3 Ternary core–shell composites. Dutta et al.448 prepared polypyrrole–polyaniline-coated rice husk ash (termed PPy PANI@RHA) by in situ polymerization and used it in the removal of Cr(VI) from aqueous solution, and the corresponding findings are shown in Fig. 25(a–d). It revealed about 98% of Cr(VI) removal at room temperature (303 K) under optimum conditions (adsorbent dose: 0.8 g L−1, adsorbate concentration: 50 mg L−1, pH of ∼2, contact time: 300 min). The adsorption studies indicated Elovich kinetics and the results are better described using the Freundlich isotherm model with a maximum adsorption capacity of 769.15 mg g−1. The possible Cr(VI) adsorption mechanism by the PPy–PANI@ RHA adsorbent has been described in Fig. 26 on the basis of ion-exchange, strong electrostatic attraction and reduction of Cr(VI). Their findings also indicated the removal efficiency of Cr(VI) remains more or less unaltered in the presence of moderate concentrations of co-existing ions (Ca2+, Na+, Mg2+, and Cl−, NO3−, PO43−). The regenerated adsorbent subjected to adsorption of Cr(VI) for 5 desorption/adsorption cycles showed a removal efficiency of 94% and 80% in the first and second cycles, respectively.
 | ||
| Fig. 25 (a) Effect of initial concentration of Cr(VI) at a different temperature (PPY PANI@RHA dose: 0.8 g L−1; contact time: 300 min; agitation speed: 200 rpm; pH∼2), (b) effect of adsorbent dose at pH 2 and without pH (∼6.8) adjustment (initial Cr(VI) concentration: 50 mg L−1; contact time: 300 min; agitation speed: 200 rpm; temperature: 303 K), (c) effect of initial solution pH on % removal of Cr(VI) by PPY–PANI@RHA, PPY–PANI and RHA adsorbents (Initial Cr(VI) concentration: 50 mg L−1; contact time: 300 min; agitation speed: 200 rpm; temperature: 303 K), and (d) variation of zeta potential of PPY–PANI@RHA at different pH.448 Reproduced with permission from RSC. | ||
 | ||
| Fig. 26 Representation of possible Cr(VI) adsorption by PPY–PANI@RHA.448 Reproduced with permission from RSC. | ||
In another study, the adsorption of Cr(VI) in aqueous solution on a polypyrrole-decorated graphene-silica (Graphene/SiO2@Polypyrrole) composite exhibited the maximum adsorption capacity corresponding to 429.2 mg g−1 (298 K) at pH 2.449 The adsorption of Cr(VI) on graphene/SiO2@ polypyrrole fitted well with the pseudo-second-order kinetic and Langmuir isotherm model. The simultaneous removal of methyl orange and Cr(VI) from water has also been evaluated based on PPy@Magnetic chitosan adsorbent.450 Da Rocha et al.451 reported the formation of a layer of PPy chains on the PMMA/rice husk ash (RHA) fibers and observed a Cr(V) removal capacity qe of 363.63 mg g−1 (after 150 min) at pH 2. In another study, core–shell PS/PANI-Fe3O4 adsorbent removed 100% Cr(VI) corresponding to pH: 2, adsorbent: 0.05 g, initial concentration of Cr(VI): 5 mg L−1, total volume: 30 mL and 120 min, respectively.452 The removal of hexavalent chromium from water has also been studied using a polyaniline/wood sawdust/polyethylene glycol composite.453 Yao et al.454 prepared Fe3O4@Polypyrrole nanospheres with a hierarchical porous structure anchored on graphene nanosheets and noted the excellent adsorption capability in Cr(VI) removal (qm: ∼348.4 mg g−1) due to the synergic effect between graphene and Fe3O4@polypyrrole. The adsorption kinetics was explained on the basis of pseudo-second-order kinetics. In addition, the fabricated adsorbent was found to be stable and retained ∼72.2% removal efficiency after six cycles.
The large specific surface area of γ-Fe2O3@Chitosan@PPy accounted for the maximum Cr(VI) adsorption capacity of 301.2 mg g−1.455 The magnetic nanocomposite removed 100% Cr(VI) in aqueous solution, and this was described as based on exchange and chelation as the dominant interaction mechanisms.
5.2.2.1 Hollow ICPs. Han et al.456 fabricated a hollow structure comprising PANI nanospheres with incontinuous multicavities by chemical polymerization using chloroaurate acid as oxidant and citric acid as dopant. Subsequently PANI nanospheres exhibiting incontinuous multicavities were formed by dissolving Au particles in excess saturated KI/I2 solution. The adsorption capacity and adsorptivity values of PANI in the removal of Pb in water correspond to 1589 mg g−1 and 93%, respectively.
5.2.2.2 Binary and ternary core–shell composites. γ-Fe2O3 coated with proton acid doping polyaniline nanocomposites (γ-Fe2O3@PANI) showed high adsorption capacity for arsenic(V) removal in water (pH: 4 to 11), and this was explained based on the electrostatic interaction and hydrogen bonding.457 Mehdinia et al.458 investigated the effects of the transformation of core–shell Fe3O4@PPy to its rattle type (yolk shell) on removal of heavy metals Pb2+ and Cu2+ from water. PANI/TiO2 adsorbent prepared by the chemical oxidative polymerization of aniline on the surface of TiO2 hydrate showed adsorption capacities of Cr(VI) in water to be 394.43 mg g−1 with excellent reusability.459 Furthermore, the adsorption of Cr(VI) oxyanions involved electrostatic attraction, hydrogen bonding and anion-π interactions.
Vatani and Eisazadeh460 coated polythiophene on polystyrene and poly(vinyl chloride) to investigate the role they play as adsorbents in the removal of Pb(II) from aqueous solution. They observed the higher removal efficiency of PTh/PVC compared with PTh/PS nanocomposites under the optimum conditions (pH: 2, initial concentration of cations 100 mg−1, equilibrium time: 30 min). Chen et al.461 prepared a PTh/MnO2 composite with MnO2 as the core and PTh as the shell for the selective adsorption towards Pb2+, Zn2+ and Cu2+ from industrial wastewater in the aqueous medium. These findings showed the adsorption capacities of Pb2+, Zn2+ and Cu2+ (within 30 min) were found to be 82.10, 30.72 and 60.79 mg g−1, respectively. These findings have been explained on the basis of a synergistic effect between PTh and MnO2.
Binary composites have been prepared following the coating of polyaniline for their applications in the removal of Pb(II) ion in water. Fe3O4 particles coated with polyaniline showed the maximum lead adsorption capacity of Pb(II) as 114.9 mg g−1.462 Pb(II) ions were found to be almost completely removed under the optimum conditions (initial concentration of lead(II): 50 ppm, adsorbent dosage: 3 mg, pH: 9.3).
According to Shakhsari et al.,463 CoFe2O4@PANI removed 98% of Pb at 25 °C from water under the optimum condition of initial concentration of metal ions (17 mg L−1), adsorbent dose (1 g), pH (7) and time (120 minutes). The adsorption kinetics followed the quasi-first-order model, and data fitted well with the Freundlich isotherm. Polypyrrole-coated ZnO-NiO nanocomposites have also been investigated as adsorbents for enhanced removal of Pb(II) from aqueous solution and wastewater.464 The possible mechanism of Pb(II) adsorption on the PPy/ZnO-NiO adsorbent has been explained based on ion exchange, electrostatic attraction, and the formation of metal complexes. The reusability and regeneration experiments after six adsorption–desorption cycles have shown a decrease in the adsorption and desorption of Pb(II) from 98.7% to 77.8% and 92% to 67%, respectively. Naphthalene sulfonic acid-doped polyaniline@Ni0 composite (0.5 g L−1) achieved 90.9% removal efficiency of Pb(II) ions in aqueous solution (pH: 5).465 The findings based on the isotherm data supported the Langmuir isotherm model, with maximum Pb(II) removal capacity of 414.6 mg g−1 at 25 °C. Fe3O4/polyaniline hollow microspheres have been fabricated and used as adsorbent in the uptake of Pb2+ from aqueous solution.466 It followed the pseudo-second-order kinetics and adsorption data agreed well with the Langmuir isotherm.
Zare et al.467 used a poly(aniline-co-3-aminobenzoic acid)-based magnetic core–shell nanocomposite and observed the maximum adsorption capacity of Pb(II) to be 138.31 mg g−1 in an aqueous solution. The findings were also found to be in agreement with the pseudo-first order and Freundlich isotherm. Pomegranate-like MnO2@PANI using 0.1 mol L−1 of KMnO4 has been successfully synthesized according to the procedure described in Fig. 27(a).468 Subsequently, the variation of removal ratios (q) of Pb(II) ions with PANI nanoflowers (NF), commercial MnO2, PANI spheres prepared with cavities and pomegranate-like MnO2@PANI spheres as adsorbents has been studied, and the findings are displayed in Fig. 27(b and c). It is noted that respective removal ratios (q) values correspond to 36.1%, 54.9%, 71.2%, and 99.2%, respectively. MnO2@PANI showed the maximum sorption capacity of 309.6 mg g−1. Such excellent adsorbability of MnO2@PANI spheres was ascribed to the presence of mesoporous structures of PANI spheres with more exposed adsorption sites towards Pb(II) ions. Alternatively, enhancing the sorption ability of Pb(II) ions could be ascribed to the interaction (chelation/physisorption) between MnO2 and PANI contributing synergistically. The removal ratios of different heavy metal ions on PANI and PANI@MnO2 as adsorbents were also tested for Ni(II), Cd(II), Cu(II), Zn(II) heavy metal ions. Subsequent findings revealed higher removal ratios of these heavy metals for the MnO2@PANI hybrids compared with PANI nanospheres and also agreed well with the earlier results on Pb(II) ion adsorption. Chemically modified polythiophene with copper nanoparticles and polyvinylpyrolidine-sulfonic acid469 and polypyrrole-iron oxide–seaweed470 exhibited the adsorption capacity of lead from aqueous solution of 111 mg g−1 and 333.33 mg g−1, respectively.
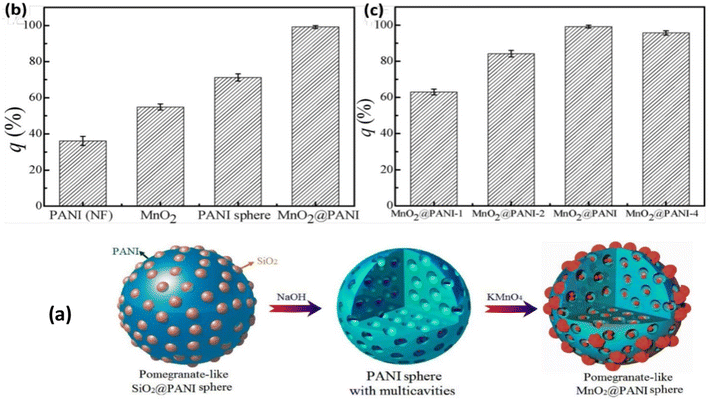 | ||
| Fig. 27 (a) Schematic representation of the preparation of pomegranate-like MnO2@PANI sub-microspheres, (b) removal ratios of Pb(II) ions with PANI(NF), MnO2, PANI sphere and MnO2@PANI as adsorbent, (c) removal ratios of Pb(II) ions with MnO2@PANI-1, to MnO2@PANI-2, MnO2@PANI and MnO2@PANI-4 as adsorbent. Adsorption conditions: [Pb(II)] = 60 mg L−1, [adsorbent] = 0.5 g L−1, pH = 5.0 ± 0.1, T = 25 °C, 12 h.468 Reproduced with permission from Elsevier. | ||
PANI/Jute fiber composites exhibited effective Cr(VI) and Cd(II) ion decontamination from water.471 The core/shell like structure of the PTh/SiO2 composite exhibited superior selectivity to separate and recycle Zn2+ among multiple ions (Pb2+, Zn2+, Cu2+) in wastewater due to the synergistic effect.472 Sun et al.473 studied the adsorption of Cu(II), Pb(II) and Ni(II) on PANI@Aminopropyltriethoxysilane (APTS)-Fe3O4/Attapulgite composite and observed the maximum adsorption capacity of 189.03, 270.27 and 142.86 mg g−1, respectively. The reusability performance of PANI@APTS-Fe3O4/Attapulgite after 5 times of use showed a slight decrease for all the heavy metals (Cu(II): 84% to 79%, Pb(II): 87% to 81%, Ni(II): 63% to 56%). SiO2-coated magnetic graphene oxide modified with a pyrrole-thiophene copolymer showed a maximum adsorption capacity for Cu(II), Pb(II), Zn(II), Cr(III) and Cd(II) in aqueous solution corresponding 201, 230, 125, 98 and 80 mg g-1, respectively.474 High-temperature hydrothermally prepared polypyrrole-derived N-doped carbon nanotubes decorated with fish scale-like MoS2 nanosheets showed a significantly much higher removal capacity for Pb(II) (qm: 381.87 mg g−1) in waste water.475 Based on XPS studies, the following possible mechanism for the adsorption of Pb(II) on the C-Ppy@MoS2 has been proposed:
| Mo(IV) + 2H+ + 4e− + 4H2O (aq) → 5H2 (g) + MoO42−(VI) |
| MoO42−(VI) + Pb2+ + S → PbMoO4−xSx (s) |
It is believed that the protons produced in the above reaction may have a driving effect on the adsorption process. The unique structure of the fish scale-like MoS2 nanosheets on the C-PPy nanotubes accounts for the adsorption of Pb(II).
 | ||
| Fig. 28 (a) Effect of adsorbent dose (Experimental conditions: C0: 1000 μg L−1; pH∼7; contact time: 240 min; T: 300 ± 3 K), (b) effect of contact time (Experimental conditions: adsorbent dose: 1 g L−1; C0: 1000 μg L−1; pH ∼7; T: 300 ± 3 K), (c) influence of initial solutions pH (Experimental conditions: adsorbent dose: 1 g L−1; C0: 1000 μg L−1; contact time: 240 min; T: 300 ± 3 K) on As(III) and As(V) removal efficiency using PNHM/Fe3O4-40, (d) ζ-potential of PNMH/Fe3O4-40 under various pH conditions.476 Reproduced with permission from Nature Publication. | ||
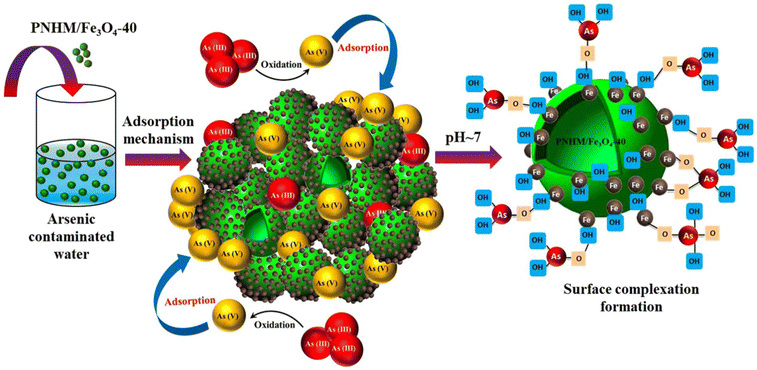 | ||
| Fig. 29 Schematic representation of arsenic adsorption mechanism in aqueous solution.476 Reproduced with permission from Nature Publication. | ||
Further studies have shown an increase in the adsorption of As(III) and As(V) on the surface of PNHM/Fe3O4-40 on increasing the temperature from 293 K to 313 K.477 In another study, Fe3O4/PANI was prepared by the chemical polymerization method.478 SEM studies confirmed the formation of PANI on the surface of Fe3O4 grains. This core–shell structure of Fe3O4/5%PANI exhibited the highest arsenic adsorption ability at pH 7 and 300 K. According to Muhammad et al.,479 the Fe3O4/PANI core–shell composite can be applied as an effective adsorbent for the removal of hexavalent chromium and divalent nickel from water. Polyaniline/Fe0 (ref. 480) and iron-functionalized polythiophene (PTh@Fe)481 were also reported as excellent adsorbents for the removal of arsenic from aqueous solutions. In addition, heavy metals from aqueous solution have also been removed by polyaniline coated on sawdust,482 Fe3O4-embedded poly(thiophene),483 PAN/PANI-Nylon,484 2-aminopyridine functionalized magnetic core–shell Fe3O4@Polypyrrole composite,485 and Fe3O4/SiO2/PANI-SDBS nanocomposite.486 Fe3O4@PPy core–shell functionalized with tetrakis (4-carboxyphenyl) porphyrin (TCCP) prepared in multiple steps displayed 100% efficiency for Hg(II) removal in water.487 Investigations have also been reported on the adsorptive removal of Hg2+ by polyaniline/attapulgite.488 Ren et al.489 prepared Fe3O4@Polypyrrole@Sodium dodecyl sulfate core@shell composite and observed the selective removal of Mn(VII) and other dyestuffs from wastewater (Table 3).
| Metal ions | Adsorbent used | Water type | Preparative method | Experimental conditions on removal of metal ions | q m (mg g−1) | Removal isotherm fitted to | Kinetics data fitted to (k) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Cr(VI) | Hollow PANI micro/nano sphere | Waste water | Monomer polymerization in alk solution with Triton X-100 as soft templates | Cr(VI): 1.2 m mol L−1 (20 mL), dosage: 10 mg, pH 3.0, qe (time): ∼79 mg g−1 (180 min) | 127.88 | Langmuir | Pseudo sec. order | 417 |
| Cr(VI) | Amino acid-doped PANI nanotubes | Aqueous solutions | In situ chemical polymerization | Temp.: 25 °C Cr(VI): 30 mg L−1 (10 mL), dosage: 0.25 g, pH: 7, removal (time): ∼100% (120 min) | 60 | Langmuir | Pseudo sec. order | 419 |
| Cr(VI) | Bamboo-like PPy nanotubes | Aqueous solution | Reactive-template vapor phase polymerization method | Cr(VI): 1.95 mmol L−1 (20 mL), dosage: 3 mg, pH: 3, adsorption capacity (time): ∼9 mmol g−1 (1400 min) | 9.281 mmol g−1 | Langmuir | Pseudo sec. order (0.0031 g mmol−1 min−1) | 420 |
| Cr(VI) | Core–shell polyaniline/polystyrene | Aqueous solutions | Microemulsion polymer of polystyrene followed by in situ polymer of aniline monomer | Temp.: 20 °C Cr(VI): 100 mg L−1 (50 mL), dosage: 250 mg, pH: 2, removal (time): ∼95% (30 min) | 19 | Temkin | Pseudo sec. order (k: 6.27 × 10−3 mg L−1 min−1) | 421 |
| Cr(VI) | Polyacrylonitrile/polyaniline core/shell nanofiber mat | Aqueous solution | Via electrospinning followed by in situ polymerization | Temp.: 25 °C, Cr(VI): 207 mg L−1 (30 mL), dosage: 100 mg, pH: 2, qt (time): 53.4 mg g−1 (12 h) | 71.28 | Langmuir | Pseudo sec. order (k: 7.20 × 10−3 g mg−1 min−1) | 422 |
| Cr(VI) | Sulfonated poly(arylene ether nitrile)/polypyrrole core/shell | Aqueous solution | Electrospinning technique followed by in situ polymerization | Temp.: RT, Cr(VI): 50 ppm (50 mL), dosage: 20 mg, pH: 2, removal (time): 56.5% (4 h) | 165.3 | Langmuir | Pseudo-second order (k: 6.9 × 10−4 g mg−1 min−1) | 423 |
| Cr(VI) | Ag-P/PPy core–shell | Water | In situ chemical oxidative polymerization | Temp.: RT, Cr(VI): 50 mg L−1 (50 mL), dosage: 0.05 g, pH: 2, removal (time): 99.4% (55 min) | 138.5 | Temkin and Redlich Peterson | Pseudo-second order | 424 |
| Cr(VI)C | Polypyrrole-wrapped oxidized MWCNTs | Aqueous solution | In situ chemical polymerization | Temp.: 25 °C Cr(VI): 200 mg L−1 (50 mL), dosage: 0.5 g L−1, pH: 2, removal (time): 100% (300 min) | 294 | Langmuir | Pseudo sec. order (k: 0.0028 g mg−1 min−1) | 425 |
| Cr(VI) | PPy-coated Halloysite nanotube (HNT) clay | Deionized water | In situ polymerization of pyrrole in the dispersion of HNTs | Temp.: 25 °C, Cr(VI): 100 mg L−1 (50 mL), dosage: 1.5 g L−1, pH: 2.0, removal (time); ∼100% (24 h) | 149.25 | Langmuir | Pseudo sec. order (k: 0.019 g mg−1 min−1) | 427 |
| Cr(VI) | Core–shell Fe3O4/PANI microspheres | Water | Interfacial polymerization | Temp.: RT, Cr(VI): 100 mg g−1 (60 mL), dosage: 0.1 g 1, pH: 2, removal (time); ∼86% (180 min) | 200 | Langmuir isotherm | Pseudo sec. order | 428 |
| Cr(VI) | Fe3O4/PPy nanotubes | Aqueous solution | One-pot process | Temp.: 25 °C, Cr(VI): 20 mg L−1 (50 mL), dosage: 0.01 g, pH:1, removal (time); 94.62% (24 h) | 451.45 | Langmuir | Pseudo sec. order | 429 |
| Cr(VI) | Fe3O4-coated PPy | Aqueous solution | In situ polymerization of pyrrole monomer | Temp.: 25 °C, Cr(VI): 200 mg L−1 (50 mL), dosage: 2 g L−1, pH: 2, removal (time); 100% (24 h) | 169.5 | Langmuir | Pseudo-sec. order (k: 0.037 g mg−1 min−1) for [Cr(VI)]: 150 mg L−1 | 430 |
| Cr(VI) | Fe3O4@ arginine-functionalized PPy | Deionised water | In situ polymerization | Temp.: 25 °C. Cr(VI): 200 mg L−1 (50 mL), dosage: 0.05 g, pH: 2, removal (time); ∼100% (1 h) | 322.58 | Langmuir | Pseudo sec. order (k: 6.67 × 10−3 g mg−1 min−1) | 431 |
| Cr(VI) | PPy/γ-Fe2O3 | Aqueous media | Emulsion polymerization | Temp.: RT, Cr(VI): 2.5 mg L−1 (10 mL), dosage: 2 mg, pH: 2, removal (time); ∼100% (1 h) | 208.8 | Langmuir | Pseudo sec. order (k: 2.0 × 10−2 g mg−1 min−1) | 432 |
| Cr(VI) | PANI/γ-Fe2O3 | Aqueous media | Emulsion polymerization | Temp.: RT, Cr(VI): 2.5 mg L−1 (10 mL), dosage: 2 mg, pH: 2, removal (time); ∼100% (1 h) | 195.7 | Langmuir | Pseudo sec. order (k: 7.5 × 10−4 g mg−1 min−1) | 432 |
| Cr(VI) | PPy-modified corncob-core sponge | Aqueous solution | Solution polymerization | Temp.: 25 °C, Cr(VI): 100 mg L−1, dosage: 3 g L−1, pH: 3.5, removal, (time);∼100% (180 min) | 84.7 | Langmuir | Pseudo sec. order (k: 0.0023 g mg−1 min−1): [Cr(VI)]: 50 mg L−1 | 434 |
| Cr(VI) | MnO2-coated polyaniline nanofibers | Aqueous solution | In situ oxidative polymerization | Temp.: 298 K, Cr(VI): 10 mg L−1 (50 mL), dosage: 10 mg, pH: 1, removal (time); ∼96% (60 min) | 158.2 | Freundlich | Pseudo-sec. order (k: 0.0751 g mg−1 min−1) | 436 |
| Cr(VI) | PPy/hollow mesoporous silica | Aqueous solution | In situ polymerization | Temp.: 25 °C, Cr(VI): 400 mg L−1 (25 mL), dosage: 80 mg, pH: 2, removal (time);∼100% (24 h) | 322 | Langmuir | Quasi-second-order | 437 |
| Cr(VI) | Polyacrylonitrile/PPy core/shell nanofiber mat | Aqueous solution | Electrospinning followed by in situ polymerization of pyrrole monomer | Temp.: 25 °C Cr(VI): 200 ppm (30 mL), dosage: 0.20 g, pH: 2, removal (time); 84.5% (12 h) | 61.80 | Langmuir | Pseudo sec. order (k: 1.77 × 10−3 g mg−1 min−1) | 439 |
| Cr(VI) | L-Cystine-doped glucose carbon sphere @PPy | Water | In situ growth method | Temp.: RT, Cr(VI): 100 mg L−1 (100 mL), dosage: 0.050 g, pH: 1, adsorption capacity, qe (time); 209.18 mg g−1, (24 h) | 108.41 | Langmuir | Pseudo sec. order (k: 0.0014 g mg−1 min−1) | 440 |
| Cr(VI) | PANI@Nano hollow carbon sphere | Wastewater | In situ polymerization method | Temp.: 298 K, Cr(VI): 100 mg L−1 (25 mL), dosage: 10 mg, pH: 1, removal (time);100% (300 min) | 250 | Langmuir | Pseudo sec. order (k: 0.284 × 10−3 g mg−1 min−1) | 441 |
| Cr(VI) | PPy/attapulgite core–shell | Aqueous solutions | In situ polymerization on the surface of attapulgite | Temp.: 298 K Cr(VI): 50 mg L−1 (100 mL), dosage: 0.2 g, pH: 3, removal (time): 99.27% (10 min) | 48.45 | Langmuir | Pseudo sec. order (k: 1069 × 10−5 mg−1 s−1) | 442 |
| Cr(VI) | Cu slag@PANI | Aqueous solution | In situ polymerization | Temp.: 303 K Cr(VI): 300 mg L−1 (20 mL), dosage: 0.01 g, pH: 2, absorption capacity (time): 357.68 mg g−1 (24 h) | 343.23 (303 K) | Langmuir | Pseudo sec. order (k: 0.00409 g mg−1 min−1) | 443 |
| Cr(VI) | PANI nanotubes@Ni(OH)2 | Aqueous solution | By depositing Ni(OH)2 on 2-naphthalene sulfonic acid-doped PANI nanotubes surface | Temp.: 25 °C, Cr(VI): 100 mg L−1 (50 mL), dosage: 0.03–0.05 g (50 mL), pH: 4.0, removal (time): ∼100% (24 h) | 625 (25 °C) | Langmuir | Pseudo-sec. order (k: 0.03008 g mg−1 min−1) | 444 |
| Cr(VI) | PPy@5% MgFe2O4 | Water and wastewater | Oxidation method | Temp.: 25 °C., Cr(VI): 100 mg L−1 (10 mL), dosage: 1 g L−1, pH: 3–6, removal (time): ∼93 mg g−1 (600 min) | 138.6 | Langmuir | Pseudo sec order (0.00 21 g mg-1 min−1) | 445 |
| Cr(VI) | PANI@Almond shell | Aqueous solutions | In situ chemical polymerization | Temp.: 298 K, Cr(VI): 50 mg L−1, dosage: 0.5 g L−1, pH: 7, removal (time): 95.86% (120 min) | 335.25 | Freundlich | Pseudo sec. order (k: 0.0273 mg g−1 min−1) | 447 |
| Cr(VI) | PPy–PANI-coated rice husk ash | Aqueous solutions | In situ chemical polymerization | Temp.: RT, Cr(VI): 50 mg L−1 (100 mL), dosage: 0.8 g L−1, pH: 2, removal (time): ∼98% (300 min) | 769.15 | Freundlich | Pseudo sec. order (k: 0.009 mg g−1 min−1) | 448 |
| Cr(VI) | Graphene/SiO2@PPy | Deionized water | In situ polymerization | Temp.: 298 K, Cr(VI): 100 mg L−1 (250 mL), dosage: 100 mg, pH: 2, qe (time): ∼240 mg g−1 (5000 min) | 429.2 | Langmuir | Pseudo sec. order (k: 1.852 × 10−5 min−1) | 449 |
| Cr(VI) | PPy/Fe3O4/chitosan | Water | Co-precipitation followed by in situ polymerization | Temp.: 25 °C, Cr(VI): 100 mg L−1 (50 mL), dosage: 100 mg, pH: 2, q (time): ∼84% (40 min) | 105 | Langmuir | — | 450 |
| Cr(VI) | PMMA/rice husk/PPy membrane | Water | Electrospinning and chemical polymerization | Temp.: 283 K, Cr(VI):10 mg L−1, dosage (membrane): 0.02, mx0.02 m (wt: 0.6 mg), pH: 2.0, removal (time): ∼95% (24 h) | 363.63 | Langmuir | Pseudo sec. order (k: 49.90 mg g−1 min−1) | 451 |
| Cr(VI) | PS/PANI/Fe3O4 | Water | Simultaneous chem. oxidation polymerization and precipitation | Temp.: 303 K, Cr(VI): 5 mg L−1 (30 mL), dosage: 0.05 g, pH: 2.0, efficiency (time): 100% (120 min) | 23.753 | Freundlich | Pseudo sec. order | 452 |
| Cr(VI) | PANI/wood sawdust/PEG | Water | Oxidation polymerization | Temp.: RT, Cr(VI): 50 ppm (50 mL), dosage: 40 g L−1, pH: 5, removal (time): ∼98% (30 min) | 3.2 | Langmuir | — | 453 |
| Cr(VI) | γ-Fe3O4/chitosan/PPy | Aqueous media. | Coprecipitation method + in situ polymerization | Temp.: RT, Cr(VI): 10 mg L−1 (10 mL), dosage: 2 mg, pH: 2, removal (time): 100% (720 min) | 301.2 | Freundlich | Pseudo sec. order (k: 7.12 × 10−5 min−1) | 455 |
| Cr(VI) | PANI/jute fiber | Deionized water | In situ polymerization | Temp.: 25 °C, Cr(VI): 100 mg L−1 (200 mL), dosage: 1.0 g, pH: 2, removal (time): 98% (120 min) | 50 | Langmuir | Pseudo sec order (k: 0.0023 mg g−1 min−1) | 471 |
| Pb(II) | Amino acid-doped PANI nanotubes | Aqueous solutions | In situ chemical polymerization | Temp.: 25 °C [Pb(II):]:30 mg L−1 (10 mL), dosage:0.25 g, pH: 7, removal (time): ∼68% (120 min) | — | — | — | 419 |
| Pb(II) | Hollow polyaniline nanosphere | Deionized water | Chemical polymerization | Temp.: 30 °C, Pb(II): 20 mg L−1 (25 mL), dosage: 20 mg, pH: 7, absorptivity (time): 97% (24 h) | 1589 | Freundlich | — | 456 |
| Pb(II) | Fe3O4/@Py yolk–shell | Water | Multi step | Pb(II): 1 mg L−1 (100 mL), dosage:15 mg, pH: 6, removal (time): ∼96% (30 min) | 65.093 | Langmuir, Freundlich | Pseudo sec. order (k: 0.004 g mg−1 h−1) | 458 |
| Pb(II) | PTh coated on PS | Deionized water | Chemical oxidative polymerization | Temp.: RT, Pb(II): 100 mg L−1 (25 mL), dosage: 0.25 mg, pH: 2, removal (time): ∼85% (30 min) | — | — | — | 460 |
| Pb(II) | PTh coated on PVC | Deionized water | Chem. oxi. polymerization | Temp.: RT, Pb(II): 100 mg L−1 (25 mL), dosage: 0.25 mg, pH: 2, removal (time): ∼85% (30 min) | — | — | — | 460 |
| Pb(II) | PANI/Fe3O4 | Wastewater | In situ polymerization | Temp.: 25 °C, Pb(II): 50 ppm (100 mL), dosage: 3 mg, pH: 9, removal (time): 93% (60 min) | 114.9 | Langmuir | Pseudo sec. order (k: 0.0074 g g−1 min−1) | 462 |
| Pb(II) | CoFe2O4@PANI | Water | Aniline polymerization | Temp.: 25 °C, Pb(II): 17 mg L−1 (50 mL), dosage: 1 g, pH: 7, removal (time): 98% (120 min) | 23.31 | Langmuir | Quasi first order | 463 |
| Pb(II) | PPy-coated ZnO-NiO | Wastewater | Chemical polymerization | Temp.: 298 K, Pb(II): 100 mg L−1 (100 mL), dosage: 25 mg, pH: 5, removal (time): 98.7% (240 min) | 436.48 | Langmuir | Pseudo sec. order (k: 0.00013 mg g−1 min−1) | 464 |
| Pb(II) | PANI nanotubes/Ni0 | Aqueous solution | Immobilisation | Temp.: 25 °C, Pb(II): 100 mg L−1 (20 mL), dosage: 0.5 g L−1, pH: 5.1, removal (time): ∼90% (24 h) | 414.6 | Langmuir | Pseudo sec. order (k: 0.00798 mg g−1 min−1) | 465 |
| Pb(II) | MnO2@PANI | Water | Oxidation polymerization | Temp.: 25 °C, Pb(II): 60 mg L−1 (20 mL), dosage: 0.5 g L−1, pH: 5, removal (time): ∼98% (12 h) | 309.6 | Langmuir | Pseudo sec. order (k: 17.52 g mg−1 min−1) | 468 |
| Pb(II) | PTP/polyvinylpyrolidine (PVP)/sulfonic acid/Cu | Aqueous solution | In situ co-polymerization | Temp.: 298 K, Pb(II): 30 mg L−1 (10 mL), dosage: 3.0 g L−1, pH: 7, removal (time): ∼28 mg g−1 (180 min) | 111.11 | Langmuir | Pseudo sec. order (k: 3.62 × 10−4 g g−1 min−1) | 469 |
| Pb(II) | PPy/Fe3O4/seaweeds | Aqueous solution | In situ chemical oxidative polymerization | Temp.: 40 °C, Pb(II): 100 mg L−1 (50 mL), dosage: 10 mg, pH: 5, removal (time): 97.25% (20 min) | 333.33 | Langmuir | Pseudo sec. order (k: 0.005535 g mg−1 min−1) | 470 |
| Pb(II) | Carbon–PPy@MoS2 | Wastewater | Template method involving five steps | Temp.: 25 °C, Pb(II): 54.26 ppm (100 mL), dosage: 50 mg, removal (time): 100% (10 min) | 381.87 | Langmuir | Pseudo sec order (k: 1.75 × 10−4 g mg−1 min−1) | 475 |
| Pb(II) | Fe3O4/SiO2/PANI-SDBS | Wastewater | Polymerization of aniline | Temp.: 30 °C. Pb(II): 15 mg L−1 (50 mL). dosage: 30 mg, pH: 7.0, removal (time): 94.1% (120 min) | 72.20 | Freundlich model | Pseudo sec. order | 486 |
| As(V) | γ-Fe2O3@PANI | Deionized water | In situ polymerization | Temp.: 298 K, As(V): 20 mg L−1 (100 mL), adsorbent: 0.5 g L−1, pH: 5, qe (time): 0.354 mg g−1 (5 h) | 37.61 | Langmuir | Pseudo sec. order (k: 1.733 g mmol−1 min−1) | 457 |
| As(III) | Hollow PANI microsphere-Fe3O4 (Fe3O4: 40 wt%) | Water | Multiple steps | Temp.: 300 K, As(III): 1000 mg L−1 (100 mL), dosage: 5 g L−1, pH:7, removal (time): 98% (240 min) | 28.27 | Freundlich | Pseudo sec order (k: 0.363 g mg−1 min−1) | 476 |
| As(V) | Hollow polyaniline microsphere/Fe3O4 (Fe3O4: 40 wt%) | Water | Multiple steps | Temp.: 300 K, As(V): 1000 mg L−1 (100 mL), dosage: 2 g L−1, pH:7, removal (time): 100% (240 min) | 83.078 | Freundlich | Pseudo sec order (k: 0.733 g mg−1 min−1) | 476 |
| As(V) | PANI/Fe0 | Aqueous solutions | Polyaniline nanofiber in polymerization media + NaBH4 | Temp.: RT, As(V): 1 mg L−1 (20 mL), dosage: 1 mg L−1, pH: 7, removal (time): 100% (60 min) (24 h) | 227.3 | Langmuir isotherm | Pseudo sec. order (k: 0.01343–0.01714 g mg−1 min−1) | 480 |
| As(III) | PANI/Fe0 | Aqueous solutions | Polyaniline nanofiber in polymerization media + NaBH4 | Temp.: RT, As(III): 1 mg L−1 (20 mL), dosage: 1 g L−1, pH: 7, removal (time): 100% (60 min) | 232.5 | Langmuir isotherm | Pseudo sec. order (k: 0.0332 g mg−1 min−1) | 480 |
| Cd(II) | PANI/jute fiber | Deionized water | In situ polymerization | Temp.: 25 °C, Cd(II): 100 mg L−1 (200 mL) (750 mL), dosage: 1.0 g, pH: 5, removal (time): 99% (120 min) | 140 | Langmuir | Pseudo sec. order (k: 0.0039 mg g−1 min−1) | 471 |
| Cd(II) | PANI-coated sawdust | Wastewater | Mixing method | Temp.: 20.5 °C, Cd(II): 40 mg L−1 (750 mL), dosage: 0.75 g, pH: 6, qe (time): ∼500 mg g−1 (35 min) | 430 | Freundlich | Pseudo sec. order | 482 |
| Cd(II) | Fe3O4/SiO2/PANI-SDBS | Waste water | Polymerization of aniline | Temp.: 30 °C, Cd(II): 15 mg L−1 (50 mL), dosage: 30 mg, pH: 7.0, removal (time): 77.47% (120 min) | 67.84 | Freundlich model | Pseudo sec. order | 486 |
| Mn(VII) | Functionalized-Fe3O4@PPy | Aqueous solution | In situ polymerization of pyrrole monomer on Fe3O4 | Temp.: 20 °C, Mn(VII): 150 mg L−1 (50 mL), dosage: 20 mg, pH: 2, contact time: ∼90% (240 min) | 781.25 | Freundlich | Pseudo sec. order (k: 4.8 × 10−4 g mg−1 min−1) | 485 |
| Mn(VII) | Core@Shell Fe3O4@Polypyrrole@Sodium Dodecyl Sulphate | Complex wastewater | Hydrothermal + in situ polymerization methods | Temp.: 20 °C, Mn(VII): 60 mg L−1 (50 mL), dosage: 10 mg, pH: 7, removal (time): ∼93.14% (60 min) | 175.75 | Langmuir | Pseudo sec. order (k: 2.93 × 10−5 g mg−1 min−1) | 489 |
| Cu(II) | PPy/γ-Fe2O3 | Aqueous media | Emulsion polymerization | Temp.: RT, Cu(II): 2.5 mg L−1 (10 mL), dosage: 2 mg, pH: 2, qe (time): ∼165 mg g−1 (2 h) | 170 | Langmuir | Pseudo sec. order (k: 4.7 × 10−2 g mg−1 min−1) | 432 |
| Cu(II) | PANI/γ-Fe2O3 | Aqueous media | Emulsion polymerization | Temp.: RT, Cu(II) 2.5 mg L−1 (10 mL), dosage: 2 mg, pH: 2, qe (time): ∼165 mg g−1 (2 h) | 106.8 | Langmuir | Pseudo sec. order (k: 6.1 × 10−3 g mg−1 min−1) | 432 |
| Cu(II) | Fe3O4@PPy yolk–shell | Water | Multistep | Cu(II): 1 mg L−1 (100 mL), dosage:15 mg, pH:6, removal (time): ∼95% (30 min) | 25.179 | Langmuir, Freundlich | Pseudo sec. order (k: 0.035 g mg−1 h−1) | 458 |
| Ni(II) | Amino acid-doped PANI nanotubes | Aqueous solutions | In situ chemical polymerization | Temp.: 25 °C Ni(II):30 mg L−1 (10 mL), dosage:0.25 g, pH: 7, removal (time): ∼68% (120 min) | — | — | — | 419 |
5.3. Removal of dyes
These dyes are primarily used by the leather, paint, varnish, pharmaceutical, textile, printing, pulp and paper, food and several other industries.60–64Fig. 30 depicts the sources and pathways of various dye pollutants in water bodies.88 However, the discharge of these highly toxic, carcinogenic and non-biodegradable toxic dyes from these sources, including their inappropriate disposal on agricultural land without suitable treatment, even in extremely small amounts, is dangerous to aquatic life, microorganism, and human health.61 The details have already been described about the classification, examples, applications, solubility in water, and ecotoxicological effects of dyes on living organisms.88 Therefore, the issue of toxic dyes present in wastewater remains a major challenge to existing conventional water treatment systems. As a consequence, the environmental remediation of the presence of these water-soluble dyes in wastewater is mandatory before they are released into water bodies, to avoid negative effects. In view of this, the adsorption process, among the different available technologies, is considered as one of the most studied. | ||
| Fig. 30 Sources and pathways of dyes in the environment.88 Reproduced with permission from RSC. | ||
In this regard, polyaniline has been reported as one of most studied and cost-effective adsorbents in the decontamination processes of wastewater treatment, owing to its easy synthesis with a possibility of doping, exhibiting environmental stability, mechanical flexibility and good physicochemical properties and the presence of amine and imine groups.64,489 However, conventional PANI powder has a low surface area that limits its ability to adsorb dye. In this regard, hollow and core–shell-structured conducting polymers, especially polyaniline, have been used for the effective adsorption of dyes owing to their high surface area and other properties.
From this perspective, hollow-structure polyaniline is more advantageous for the removal of dye due to its high surface area and porous structure. In addition, core–shell-structured polyaniline also offers several advantages that include enhanced adsorption capacity, improved selectivity, better stability, and achieving increased surface area and the ability to incorporate different materials for specific interactions with the dye molecule.64,68 Accordingly, the adsorption performance of hollow conducting polymers, hollow conducting polymer composites and conducting polymer-based core–shell composites has been reviewed below for the removal of different dyes from water.490–550
5.3.1.1 Hollow ICPs. Wu et al.491 used a functionalized polystyrene sphere as the template to fabricate hollow spherical polyaniline (average dia: 300 nm, shell thickness: 100 nm) and observed its high adsorption capacity for RhB (61.75 mg g−1). According to Chen et al.492 hollow polyaniline helical nanobelts have been prepared through the generation of hollow oligoaniline helical nanobelts acting as sacrificial templates during the chemical oxidation method. It was inferred that the presence of the large number of adsorption sites comprising several amino and imino groups in hollow PANI helical nanobelts facilitated the effective adsorption of rhodamine 6G.
5.3.1.2 Binary core–shell composites. Investigations have also been made on the removal of rhodamine B dye in aqueous solutions by using polyaniline coated on filter papers,493 PANI/Sawdust,494 and PANI@Carbonized tea waste material.495 Rachna et al.496 studied the removal of RHB dye using PANI@Zn ferrite and observed maximum removal at pH 2. The adsorbent was also found to be effective even after desorption. Thermodynamics studies indicated the adsorption process to be spontaneous and exothermic in nature. Dhanavel et al.497 prepared α-MoO3/polyaniline by the chemical oxidative polymerization method. Furthermore, these findings showed the maximum adsorption capacity of 36.36 mg g−1 and 76.22 mg g−1 corresponding to RhB and CR dyes, respectively. Ovando-Medina et al.498 coated polypyrrole (dia: 200–300 nm) on coffee grounds waste (CGD) by in situ pyrrole polymerization using potassium persulfate as oxidant. Subsequent studies have shown that the removal of the RhB dye from aqueous solution is favoured by basic pH due to its adsorption on CGD/PPy. It was also noted that the adsorption isotherm followed the Redlich–Peterson and Langmuir models with qm of 50.59 mg g−1.
5.3.1.3 Ternary core–shell composites. Ren et al.499 used Fe3O4@polypyrrole@4-vinylpyridine composites for the removal of rhodamine B and some other dyes (methylene blue, malachite green, alizarin red). The investigations on the adsorption kinetics and isotherm studies of rhodamine B fitted well with the pseudo-second-order model and Langmuir model (qm: 58.72 g mg−1), respectively. The removal of RhB after five adsorption–desorption cycles of rhodamine B was found to be to 97.87%. According to the proposed mechanism, the adsorption of different dyes on the ternary composite could be related to the synergistic effect of electrostatic interaction, hydrogen-bonding interaction, and π–π interaction. Thermodynamic studies indicated spontaneous endothermic behavior for all four dyes. Xu et al.500 used polyaniline/attapulgite-supported nanoscale Fe0 to study the removal property and degradation mechanism for rhodamine B, in addition to alizarin yellow R, methyl red, chrome black T, methyl orange and methylene blue. Their findings indicated relatively better removal performance of the composite adsorbent for azo dyes compared with non-azo dyes. Polyaniline-modified magnetic nanocomposites coated with dicationic ionic liquid was used to remove rhodamine B from a water sample.501 The overall findings indicated best fitting of the kinetic data and adsorption studies to the pseudo-second order and Temkin's models, respectively.
In another study, the maximum adsorption capacity values of rhodamine B, methylene blue, malachite green, and crystalline violet present on Fe3O4@polypyrrole@2-acrylamido-2-methyl-1-propanesulfonic acid composite in aqueous solution were found to be 215.054 mg g−1, 183.486 mg g−1, 144.718 mg g−1 and 194.175 mg g−1, respectively.502 The trilaminar composite maintained a removal efficiency exceeding 95% after going through 5 cycles. The kinetic, adsorption isotherm and thermodynamic studies indicated pseudo-second-order, Langmuir model and an endothermic spontaneous adsorption process, respectively. In addition, a mechanism has also been proposed on the adsorption of dyes by Fe3O4@PPy@AMPS composite based on the electrostatic interactions between dyes and adsorbent, formation of hydrogen bonding involving O (in AMPS) and H (dyes), π–π interactions between the benzene rings (AMPS) and in dye including the hydrophobic interactions. Sayed et al.503 prepared a PANI/C hybrid by an in situ chemical oxidation method under ultrasonic vibration. Scanning electron microscopy of the composite revealed the PANI network formation of intersecting nanorods. They also reported that the uptake of rhodamine B by the free form of the employed polyaniline took place due to hydrogen bonding, electrostatic interactions, and pi–pi interaction.
Varghese et al.504 prepared a PEG-capped PANI/TiO2/CuO composite by in situ chemical oxidative polymerization to study its adsorption performance in the removal of rhodamine B and other organic dyes in water. TEM studies of the trilaminar composite confirmed the incorporation of TiO2/CuO into the tubular-shaped fibrous network of PANI. The adsorption efficiency of MB, MG, CR, RhB, and CV on PANI/TiO2/CuO after 120 minutes corresponded to the removal efficiency of 98%, 67.7%, 95.1%, 64.4%, and 97.3%, respectively (Fig. 31(a)). The effect of a variety of materials on the adsorption of RhB dye has also been studied and the corresponding findings are displayed in Fig. 31(b). These findings revealed the superior removal performance by PANI/TiO2/CuO (PTC) (89.7%) compared with PANI, (64.9%) TiO2 (63.9%), CuO (44.3%), PANI/TiO2 (PT): (72.9%), PANI/CuO (PC): 60.4%, and TiO2/CuO (TC): 52.7% in 240 min due to its more negative zeta potential value. Fig. 31(c) shows the effect of adsorbent (PANI/TiO2/CuO) amounts on the adsorption of RhB for 120 min. According to this, the maximum adsorption efficiency was inevitable at 0.2 g of adsorbent. The variation of initial dye concentration in Fig. 31(d) indicated the maximum removal efficiency of 89.7% (adsorbent: 0.2 g, dye: 5 mg L−1). The temperature dependence of the adsorption studies in Fig. 31(e) showed the removal efficiency decreasing from 89.7% (25 °C) to 78% (50 °C). The dependence of the pH, displayed in Fig. 31(f), showed the removal efficiency of RhB increasing from 78.5% to 89.7% on increasing pH from 4 to 6. Furthermore, the adsorption process followed the Langmuir adsorption isotherm (qm: 3.53 mg g−1) and pseudo-second-order kinetics. Fig. 32 shows the effect of pH on the adsorption efficiency of PANI/TiO2/CuO.
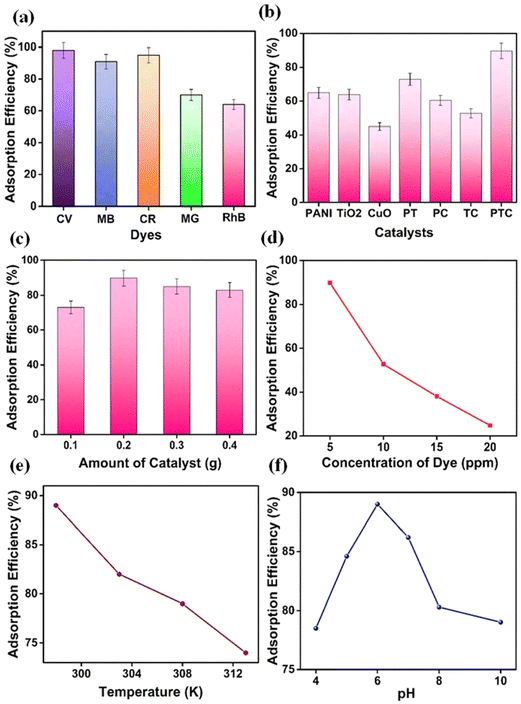 | ||
| Fig. 31 (a) Adsorption efficiency of various dyes using PANI/TiO2/CuO at 120 min, (b) efficiency of RhB using different catalysts at 240 min, (c) effect of amount of adsorbent on RhB adsorption using PANI/TiO2/CuO for 120 min, (d) RhB adsorption at different dye concentrations using PANI/TiO2/CuO for 240 min, (e) effect of RhB adsorption using PANI/TiO2/CuO at different temperatures, and (f) effect of pH on the adsorption of RhB using PANI/TiO2/CuO.504 Reproduced with permission from Elsevier. | ||
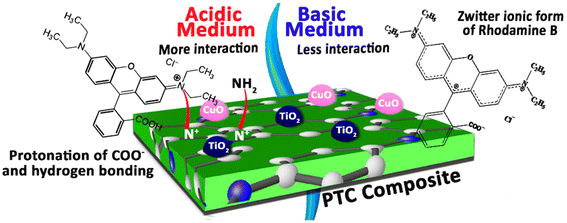 | ||
| Fig. 32 The effect of pH on the adsorption efficiency of PANI/TiO2/CuO.504 Reproduced with permission from ACS. | ||
5.3.2.1 Hollow ICPs. A natural amino acid-doped polyaniline hollow tubular morphology419 and hollow spherical polyaniline shell491 exhibited a removal capacity for Congo red from aqueous solution corresponding to 955.6 mg·g−1 and 60 mg g−1, respectively. Benhaddad et al.505 synthesized hollow sea urchin-shaped polypyrrole in acidic medium using nanostructured MnO2 powder acting as oxidizing agent and sacrificial template. Subsequently, they studied its comparative performance in the adsorption of Congo red and methylene blue in aqueous solutions. These studies indicated that adsorption and removal of Congo red and methylene blue was favoured under acidic and basic pH conditions, respectively. The adsorption followed Langmuir isotherm and pseudo-second-order kinetics for both the dyes. Mondal et al.506 prepared a network of polyaniline nanotubes by in situ polymerization of aniline in the presence of ammonium persulfate and different aromatic carboxylic acids acting as a dopant (referred to as B4CA). Subsequent investigations on its application in wastewater treatment led to the following maximum adsorption capacity order for CR and other dyes: indigo carmine (300 mg g−1) ≥ Eriochrome Black T (288 mg g−1) > methyl orange (285 mg g−1) > Congo red (194 mg g−1) > N,N′-bis(4-benzosulfonic acid)-perylene-3,4,9,10-tetracarboxylbisimide (192 mg g−1). It may be noted that the adsorption of the dye is guided here by the electrostatic interaction between dye molecules and the PANI surface.
5.3.2.2 Binary core–shell composites. PANI@ZnO nanocomposites were synthesized by the oxidation chemical process and its time-dependent performance evaluated for the adsorption of Congo red from aqueous solution, keeping pH: 5.0, C0: 150 mg L−1, and adsorbent dose: 10 mg at 298 K.507 These findings suggested 81.37% removal of CR at 60 min. The kinetic model and isotherm data fitted well with the pseudo-second-order model (k: 0.0004 g mg−1 min−1) and Langmuir (qm: 76.92 mg g−1), respectively. The regeneration efficiency of PANI@ZnO was found to be adequate even after five repeated cycles. Tanweer et al.508 prepared 3D-polyaniline/activated silica gel by in situ polymerization and used it as potential adsorbent in the successful removal of Congo red, brilliant green, crystal violet, and methyl orange. In another approach, hollow electrospun Polypyrrole@Cellulose fibrous membrane was prepared by electrospinning followed by a dip-coating approach, and achieved 99.4% rejection of anionic Congo red.509
Teng et al.510 observed 89.62% removal of CR from aqueous solution on PANI-coated Fe3O4 nanoparticles (PANI/Fe3O4: 70/30). These studies also indicated slightly enhanced dye removal by PANI/Fe3O4 compared with PANI alone. The adsorption of CR on the PANI/Fe3O4 adsorbent could be ascribed to the possible bond formation between the –OH functional group (Fe3O4) and CR, electrostatic interactions between the –NH+ of PANI (emeraldine salt form) and anionic sulfonic group of CR dye, and hydrogen bonding interaction between CR and the PANI/Fe3O4 surface. The regeneration studies showed 77.4% removal retained after the fifth cycle of adsorption–desorption. Singh et al.511 confirmed the coverage of in situ-prepared PANI over the zinc titanate surface by FESEM. This showed dramatically enhanced Congo red adsorption (qm: 64.51 mg g−1) compared with that of PANI and zinc titanate.
5.3.2.3 Ternary core–shell composites. Recently, several works have been reported on ternary core–shell composites comprising Fe3O4 as a magnetic material. In one such work, Fe3O4/polypyrrole/carbon black nanocomposite fabricated by encapsulating Fe3O4 nanoparticles in PPy/Carbon black exhibited 96.9% removal of Congo red under given experimental conditions.512 The findings matched well with the Langmuir isotherm (qm: 500 mg g−1) and pseudo-second order kinetics. Dutta et al.513 fabricated polyaniline hollow microsphere (PNHM)/MnO2/Fe3O4 composites by in situ deposition of MnO2 and Fe3O4 nanoparticles on the surface of PNHM. Subsequently, they used it as adsorbent to study the removal of methyl green and Congo red in water. In view of this, the effect of pH on MG and CR dye removal efficiency (inset: change of wavelength of the dye solutions at different solution pH) are presented in Fig. 33(a and b), respectively. In addition, the variation of the zeta potential of PNHM/MnO2/Fe3O4 with the variation of pH, the effect of adsorbent dose, contact time, and initial dye concentration on MG and CR dye removal efficiency are, respectively, described in Fig. 33(c–f). It is noted that the ternary composite showed 98% and 88% adsorption efficiency in the removal of CR and MG under optimum conditions. The adsorption mechanism of MG and CR dye removal on the surface of PNHM/MnO2/Fe3O4 at pH ∼6.75 is schematically displayed in Fig. 34. This has been explained on the basis of electrostatic interaction, ion exchange and the formation of covalent bonds.
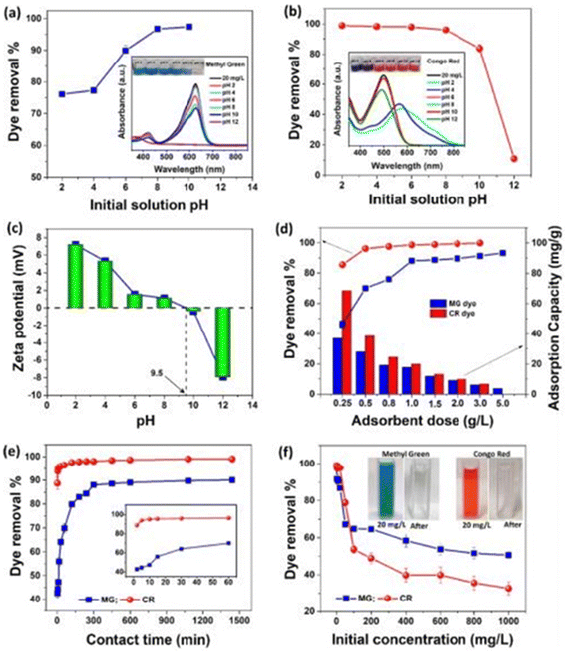 | ||
| Fig. 33 Effect of pH on (a) MG and (b) CR dye removal efficiency (inset: change of wavelength of the dye solutions at different solution pH); (c) zeta potential of PNHM/MnO2/Fe3O4 with the variation of pH, effect of (d) adsorbent dose, (e) contact time and (f) initial dye concentration on MG and CR dye removal efficiency.513 Reproduced with permission from ACS. | ||
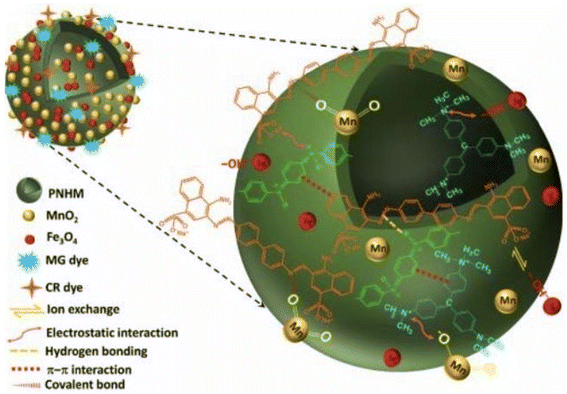 | ||
| Fig. 34 Schematic representation of plausible adsorption mechanism of MG and CR dye on the surface of PNHM/MnO2/Fe3O4 at pH ∼6.75.513 Reproduced with permission from ACS. | ||
A PANI@ZnO-SiO2 hybrid material displayed good adsorption efficacy for CR (83.82 mg g−1) and MB (71.19 mg g−1) in aqueous solution.514 According to Weng et al.,515 ultrathin Mg silicate nanosheets were grown on Fe3O4@PPy and subsequently used as adsorbent to remove Congo red from aqueous solution. These findings revealed the applicability of the Langmuir isotherm model with maximum monolayer adsorption capacities of 540.5 mg−1 and kinetic data fitting to the pseudo-second-order model (k: 0.9030 g mg−1 min−1). Adsorptive removal of CR on L-cysteine/rGO/PANI nanocomposite was done at room temperature to find out the optimum conditions.516 These findings indicated the data fitted well with the Langmuir model (qm: 56.57 mg g−1) and kinetics validating pseudo-second-order. Furthermore, the adsorption process was found, guided via intra-particle diffusion and thermodynamic studies that indicated the adsorption process of CR was endothermic and spontaneous.
5.3.3.1 Hollow ICPs. Contamination by toxic, carcinogenic, and non-biodegradable methylene blue in water remains a threat to human health as well as environmental safety and needs urgent attention.517 Ayad et al.518 observed the adsorption capacity of PANI nanotubes to be about 8 times higher compared with PANI powder. In addition, the adsorption data of MB best fitted with the Langmuir isotherm (monolayer saturation capacity: 9.21 mg g−1) and followed pseudo-second-order kinetics (k: 0.03595 g mg−1 min−1). In another study, polyaniline hollow nanotubes (external dia: 50–60 nm, internal dia: 5–10 nm) have been in situ synthesized by using acid green crystal as structure-directing agent as well as template, and were used in the adsorption of methylene blue from aqueous media.519 The experimental data supported fitting of a pseudo-first-order kinetic model (k: 0.001 mon−1) and maximum monolayer of 69.4 mg g−1. Amer et al.520 reported the acid-free synthesis of polyaniline nanotubes via the aniline oxidation using ammonium peroxydisulfate as an oxidant in the presence of methyl orange as a structure-guiding agent in the dual removal of MB and AG dyes from aqueous solution. The kinetic studies confirmed the second-order model, while, adsorption data fitted well to the Langmuir isotherm. Furthermore, the calculated values of the maximum monolayer capacity for MB and AG were found to 91.1 and 58 mg g−1, respectively.
5.3.3.2 Binary core–shell composites. PPy@MoS2 hollow microtubes prepared through the combination of multiple steps successfully removed CR and MB from aqueous solution, with kinetic data fitting well the pseudo-second order.521 Furthermore, the adsorption process followed the Langmuir model and Freundlich model for MB (qm: 121.3 mg g−1) and CR (qm: 598.7 mg g−1). The observed performance of dye removal was ascribed to its 1D hierarchical hollow structure and the synergistic effect between the MoS2 nanoflakes and PPy coating. Ayad et al.522 observed complete adsorption of methylene blue displayed by the polyaniline nanotubes base/silica composite (0.05 g) in 10 min corresponding to the 0.95 mg L−1 initial concentration of the dye. According to their findings, rate of adsorption increased in the following order: PANI NTs base/silica composite > PANI NTs base > conventional PANI base/silica composite > conventional PANI base. The experimental data were applied to study the kinetics (pseudo-first order, pseudo-second order) and the intraparticle diffusion models. Furthermore, MB adsorption data fitted best with the Langmuir isotherm. PANI partially covering the TiO2 hydrate in water (pH 3–11) exhibited a high maximum adsorption capacity (458.10 mg g−1).523 Further investigations have shown its 99% regeneration capability achieved after 10 cycles. It was suggested that the adsorption performance of MB onto PANI/TIO2 is guided by hydrogen bonding and electrostatic interaction on amino sites, π–π stacking, a hydrophobic effect on the benzene ring site and a synergistic effect.
El-Sharkaway et al.524 synthesized a transparent layered and wrinkled wavy structure of GO covered with PANI and used it subsequently in the preparation of PANI-RGO, and used this to study the adsorption ability for MB in water. The kinetic studies of the PANI/GO and PANI/RGO indicated pseudo-first-order kinetics and the adsorption data best suited the Langmuir model. The estimated maximum dye adsorption capacity values of MB on PANI/GO and PANI/RGO adsorbents were found to be 14.2 and 19.2 mg g−1, respectively. These findings affirmed PANI/RGO to be more effective compared with PANI/GO for the removal of MB. Ayad et al.525 prepared a coating of polypyrrole on a cotton textile by in situ oxidative polymerization of pyrrole monomer and subsequently used this as adsorbent of methylene blue in alkaline solutions. The adsorption process followed the Freundlich isotherm model and the adsorption kinetics fitted well with pseudo-second order. They also extended their work on the removal of Acid Green 25 in acidic medium and observed some affinity.
5.3.3.3 Ternary core–shell composites. Fe3O4@Polypyrrole@Sulfamic acid composite prepared by the hydrothermal method and in situ polymerization has been evaluated for its performance in the adsorption of MB and other dyes (MG, CV, and RhB).526 The adsorption kinetics and adsorption isotherms of all the dyes were found to fit with pseudo-second-order and Langmuir adsorption isotherm, respectively. The thermodynamic study of the adsorption process indicated a spontaneous heat absorption behavior. Wang et al.527 fabricated a core–shell Fe3O4@Polypyrrole@Sodium dodecyl benzene sulfonate (SDBS) composite following the combination of different methods and studied the effect of different parameters on the adsorption of MB and MG from aqueous solutions. These findings indicated kinetics and adsorption following the pseudo-second order and Langmuir isotherm (qMBm: 124.07 mg g−1, qMGm: 73.10 mg g−1), respectively. Furthermore, the cycling stability of the adsorbent was also assessed by carrying out an adsorption–desorption experiment for five cycles. These findings have shown the remarkable removal efficiencies of MB (80%) and MG (90%) following the five desorption–adsorption cycles. The excellent adsorption performance of dye on the ternary core–shell composite was assigned to the synergistic effect of the electrostatic interactions, hydrogen bonding, and π-π interactions mediated by PPy and SDBS.
Polypyrrole/GO@Fe3O4 has been prepared by a one-step method and used as magnetic adsorbent in the removal of methylene blue dye from aqueous solution (pH: 8).528 This adsorbent exhibited an adsorption capacity of 323.2 mg g−1 for MB. A super-paramagnetic architecture comprising Fe3O4@PPy@RGO ternary nano-adsorbents was fabricated to remove methylene from wastewater.529 The choice of Fe3O4 hollow spheres, polypyrrole layers and graphene sheets was guided by the role they play in contributing towards the abundant hydrophilic groups, protecting hollow spheres from acid corrosion/surface oxidation and in enhancing the removal performance of Fe3O4@PPy. HRTEM studies of Fe3O4@PPy@RGO established the formation of a ternary nano-architecture comprising wrapping of Fe3O4@PPy by RGO sheets. Fig. 35(a) shows Fe3O4@PPy@RGO exhibiting a superior removal efficiency for MB compared with Fe3O4 and Fe3O4@PPy in neutral solution (pH: 7). Furthermore, Fe3O4@PPy@RGO retained about 96% removal efficiency after five cycles of the adsorption–desorption process Fig. 35(b). In addition, the removal efficiency of these adsorbents in acidic solution (pH: 2) and alkaline solution (pH 10) are also displayed in Fig. 35(c and d), respectively. Graphene-modified magnetic polypyrrole530 and Fe3O4/polypyrrole/phytic acid531 also efficiently removed methylene blue from aqueous solution.
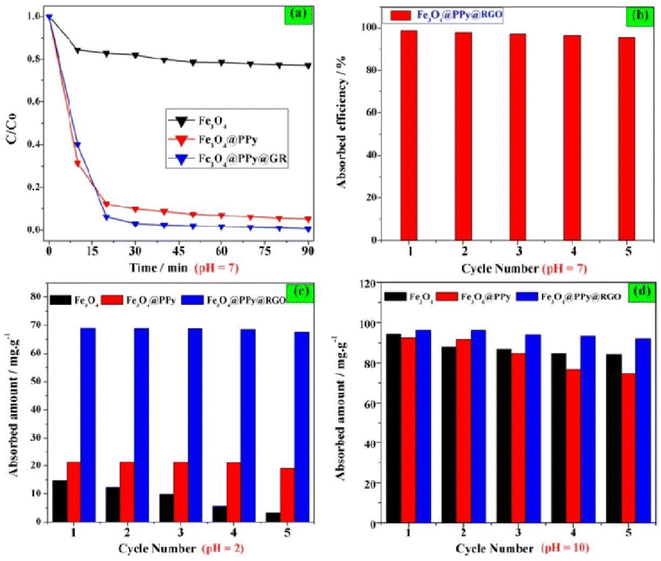 | ||
| Fig. 35 (a) Removal efficiency of magnetic adsorbents in neutral solution (pH 7); (b) removal efficiency of Fe3O4@PPy@RGO in five cycles, neutral solution (pH 7); (c) removal efficiency of magnetic adsorbents in acidic solution (pH 2) and (d) alkaline solution (pH 10).529 Reproduced with permission from Elsevier. | ||
5.3.4.1 Hollow ICPs and their composites. Very limited research has been reported on the removal of methyl orange using hollow spheres and one-dimensional ICPs as adsorbents. According to Zhao et al.,419 amino acid-doped polyaniline nanotubes exhibited the following order towards the adsorption capacity of different dyes: Congo red (112.0 mg g−1) > orange yellow (69.2 mg g−1) ≥ indigo carmine (66.8 mg g−1) > methyl orange (54.8 mg g−1) > crystalline violet (50.0 mg g−1). Yildirim et al.534 carried out the removal of methyl orange using a polyaniline nanotube-filled sodium alginate bio-composite to study the effect of adsorbent dose, pH, time, and concentration of MO. Based on the applicability of the Langmuir isotherm, maximum adsorption capacity (qm) was found to be 370.4 mg g−1 at 25 °C under optimum conditions.
5.3.4.2 Binary and ternary core–shell composites. TEM studies of a PANI-MWCNTs (4 wt%) composite prepared by in situ oxidative polymerization showed spherical PANI covering the tubular structure of MWCNT nonuniformly.535 The adsorption studies of methyl orange using PANI-MWCNTs as adsorbent agreed with the second-order kinetics (k: 5.265 × 10−4 g mg−1 min−1 at 30 °C) and Langmuir equilibrium model (qm: 149.25 mg g−1). Polyaniline (skin)/polyamide 6 (core) composite fiber prepared via a facile in situ oxidation polymerization was tested for the removal of methyl orange.536 These findings revealed the adsorption/desorption kinetics and isotherms following the pseudo-second-order and Langmuir models (qm: 58.7 mg g−1), respectively. A waterborne poly vinyl pyrrolidone-stabilized polyaniline core (dia: 85–90 nm)–shell (dia: 20–22 nm) composite also effectively removed MO in aqueous solution.537
Zhang et al.538 fabricated halloysite nanotubes/polypyrrole nanocomposites by in situ polymerization of pyrrole monomer in the presence of HNTs. Subsequently, its adsorption efficiency was evaluated in the removal of methyl orange as a function of adsorbent dose, contact time, initial concentration MO, and temperature on the adsorption efficiency. The adsorption kinetics fitted the pseudo-second-order and adsorption isotherms validated by Langmuir (qm: 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 146 mg g−1) and Freundlich models. The thermodynamic investigations pointed to an adsorption process of methyl orange onto HNTs/PPy that was spontaneous and exothermic. Furthermore, regeneration experiments have shown the reusability of HNTs/PPy nanocomposites at least three times in removing methyl orange in aqueous solution.
146 mg g−1) and Freundlich models. The thermodynamic investigations pointed to an adsorption process of methyl orange onto HNTs/PPy that was spontaneous and exothermic. Furthermore, regeneration experiments have shown the reusability of HNTs/PPy nanocomposites at least three times in removing methyl orange in aqueous solution.
Yao et al.539 prepared core–shell Fe3O4@C@polyaniline composite microspheres as a separable adsorbent for the removal of MO dye from aqueous solution. The TEM images of the product confirmed the core–shell structure of the Fe3O4@C@PANi composite microspheres. The adsorption isotherms and kinetics data supported the Langmuir and pseudo-second-order models, respectively. The trilaminar core–shell composite also exhibited excellent adsorption capability (qm: 120.2 mg g−1), and retained excellent adsorption capability (∼81%) after five adsorption–desorption cycles.
Table 4 presents the removal of the various dyes in aqueous solution based on hollow intrinsically conducting polymers, nanocomposites comprising hollow intrinsically conducting polymers and core–shell based ICP nanocomposites as adsorbents.
| Dye | Adsorbent | Water type | Preparative method | Experimental conditions and findings | q m (mg g−1) | Removal isotherm fitted to | Kinetics data fitted to | Ref. |
|---|---|---|---|---|---|---|---|---|
| a q m: maximum adsorption capacity. AG: acid green, AR: acid red, AR1: acid red 1, AYR: alizarin yellow R, BB: brilliant blue, BG: brilliant green, CR: Congo red, CV: crystal violet, E102 (tartrazine: TTz), EB: Evans blue, EY: eosin Y, OG: orange green, IC: indigo carmine, MG: malachite green, MO: methyl orange, MB: methylene blue, MR: methyl red, OG: orange green, Rh: rhodamine, RhB: rhodamine B, Rh6B: rhodamine 6B. Rh6G: rhodamine 6G, SY: sunset yellow. | ||||||||
| CR | Amino acid-doped PANI nanotubes | Aqueous solutions | In situ chemical polymerization | Temp.: 25 °C CR:30 mg L−1 (10 mL), dosage:0.25 g, pH: 7, removal (time): ∼110 mg g−1 (90 min) | 112 | Langmuir | Pseudo first-order | 419 |
| OG | PANI@Almond shell | Aqueous solutions | In situ chemical polymerization | Temp.: 298 K, OG: 50 mg L−1, dosage: 0.5 g L−1, pH: 5, removal (time): 94.69% (120 min) | 190.98 | Freundlich | Pseudo sec. order (k: 0.0008 mg g−1 min−1) | 447 |
| MO | PPy/Fe3O4/chitosan | Water | Co-precipitation and in situ polymerization | Temp.: 25 °C, MO: ∼100 mg L−1 (50 mL), adsorbent: 100 mg, pH: 3, removal efficiency (time): 67 mg g−1 (40 min) | 95 | Langmuir | — | 450 |
| IC | PMMA/rice husk/PPy membrane | Water | Electrospinning and chemical polymerization | Temp.: 283 K, IC: 30 mg L−1, dosage (membrane): 0.02m × 0.02 m (wt: 0.6 mg), pH:2.0, qe (time): 142.9 mg g−1 (70 min) | 144.93 | Langmuir | Pseudo sec. order (k: 0.002 g mg−1 min−1) | 451 |
| E102 | PMMA/rice husk/PPy membrane | Water | Electrospinning and chemical polymerization | Temp.: 283 K, TZ:60 mg L−1, dosage (membrane): 0.02 m × 0.02 m2 (wt: 0.6 mg), pH: 2.0, qe (time): 165.7 mg g−1 (60 min) | 171.23 | Langmuir | Pseudo sec. order (k: 0.002 min−1) | 451 |
| RhB | Fe3O4@PPy@Sodium dodecyl sulphate | Complex wastewater | Combination of a hydrothermal method in situ polymerization | Temp.: 20 °C, RhB:40 mg L−1 (50 mL), adsorbent: 10 mg, pH: 7, removal (time): 94.83% (7 h) | 127.55 | Langmuir | Pseudo sec. order (k: 3.027 × 10−3 g mg−1 min−1) | 489 |
| CR | Fe3O4@PPy@Sodium dodecyl sulphate | Complex wastewater | Combination of hydrothermal method and in situ polymerization | Temp.: 20 °C, CR:40 mg L−1 (50 mL), adsorbent: 10 mg, pH: 7, removal (time): 96.45% (7 h) | 101.63 | Langmuir | Pseudo sec. order (k: 2.75 × 10−4 g mg−1 min−1) | 489 |
| MB | Fe3O4@PPy@Sodium dodecyl sulphate | Complex wastewater | Combination of a hydrothermal method in situ polymerization | Temp.: 20 °C, MB:40 mg L−1 (50 mL), adsorbent: 10 mg, pH: 7, removal (time): 93.27% (400 min) | 135.50 | Langmuir | Pseudo sec. order (k: 1.71 × 10−4 g mg−1 min−1) | 489 |
| MG | Fe3O4@PPy@Sodium dodecyl sulphate | Complex wastewater | Three-step process | Temp.: 20 °C, MG:40 mg L−1 (50 mL), adsorbent: 10 mg, pH: 7, removal (time): 96.03% (7 h) | 182.82 | Langmuir | Pseudo sec. order (k: 8.93 × 10−4 g mg−1 min−1) | 489 |
| AR1 | Fe3O4@PPy@Sodium dodecyl sulphate | Complex wastewater | Combination of a hydrothermal method in situ polymerization | Temp.: 20 °C, AR1:40 mg L−1 (50 mL), adsorbent: 10 mg, pH: 2, removal (time): 92.99% (7 h) | 181.16 | Langmuir | Pseudo sec. order (k: 2.66 × 10−4 g mg−1 min−1) | 489 |
| RhB | Hollow spherical PANI | Aqueous solution | In situ polymerization on functionalized polystyrene template | RhB: 100 mg L−1 (50 mL), adsorbent: 5 mg, qe (time): 61.75 mg g−1 (72 h) | 61.75 | — | — | 491 |
| CR | Hollow spherical PANI | Aqueous solution | Chemical polymerization | CR: 5 mg (50 mL), Adsorbent: 100 mg L−1, qe (time): 46.91 (72 h) | 46.91 | — | — | 491 |
| Rh6G | Hollow PANI helical nanobelts | Aqueous solution | Chemical oxidation of aniline | Temp.: RT, Rh6G: 50 mg (100 mL), adsorbent: 479 mg L−1, removal (time): ∼249 mg L−1 (120 min) | 0.42 mg mg−1 | Langmuir | Pseudo sec. order (k: 1.28 mg mg−1 min−1) | 492 |
| RhB | Coating of PANI onto carbonized tea waste | Aqueous solutions | Coating of polyaniline onto carbonized tea waste material | Temp.: 30 °C, RhB: 50 mg L−1 (100 mL), adsorbent: 100 mg, pH: 8.0, removal (min): 95.21% (60 min) | 34.93 | Langmuir | Pseudo-second-order (k: 0.0035 g mg−1 min−1) | 495 |
| RhB | α-MoO3/PANI | Water | Chemical oxidative polymerization using camphor sulfonic acid as dopant | Temp.: 20 °C, RhB: 2.1 × 10−5 mol L−1 (50 mL), adsorbent: 50 mg, pH:3, Removal (time): 91% (60 min) | 36.36 | Langmuir | — | 497 |
| CR | α-MoO3/PANI | Water | Chemical oxidative polymerization using camphor-10 sulfonic acid as dopant | Temp.: 15 °C, CR: 1.5 × 10−5 mol L−1 (1000 mL), adsorbent: 50 mg, pH: 5, removal (time): 94.6 (60 min) | 76.22 | Langmuir | — | 497 |
| RhB | PPy/coffee grounds waste | Aqueous solution | Pyrrole polymerization | Temp.: 15 °C, RhB: 200 mg L−1 (150 mL), adsorbent: 125 mg, pH: 9, removal (time): 32 mg g−1 (80 min) | 50.597 | Redlich–Peterson and Langmuir | — | 498 |
| RhB | Fe3O4@PPy@4-Vinylpyridine | Wastewater | Multiple steps | Temp.: 20 °C, RhB: 50 mg L−1 (30 mL), adsorbent: 10 mg, pH = 7, removal (time): ∼97% (500 min) | 58.72 | Langmuir | Pseudo sec. order (k: 9.728 × 10−4 g mg−1 min−1) | 499 |
| MB | Fe3O4@PPy@4 vinylpyridine | Wastewater | Multiple steps | Temp.: 20 °C, MB: 50 mg L−1 (30 mL), adsorbent: 10 mg, pH = 7, removal (time): ∼100% (500 min) | 85.98 | Langmuir | Pseudo sec. order (k: 3.704 × 10−3 g mg−1 min−1) | 499 |
| MG | Fe3O4@PPy@4-vinylpyridine | Wastewater | Multiple steps | Temp.: 20 °C, MG: 50 mg L−1 (30 mL), adsorbent: 10 mg pH = 7, removal (time): ∼98% (600 min) | 36.114 | Langmuir | Pseudo sec. order | 499 |
| AR | Fe3O4@PPy@4-vinylpyridine | Wastewater | Multiple steps | Temp.: 20 °C, AR: 200 mg L−1 (30 mL), adsorbent: 10 mg pH = 7, removal (time): ∼90% (600 min) | 55.90 | Freundlich | Pseudo sec. order (k: 2.270 × 10−4 g mg−1 min−1) | 499 |
| MB | PANI/attapulgite-supported nanoscale zero-valent Fe | Aqueous solution | Liquid-phase chemical reduction method | Temp.: 298 K., MB: 60 mg L−1 (50 mL), adsorbent: 0.05 g, pH: 11, removal (time): 30% (30 minutes) | 23 | Langmuir | — | 500 |
| AYR | PANI/attapulgite-supported nanoscale zero-valent Fe | Aqueous solution | Liquid-phase chemical reduction method | Temp.: 298 K, AYR: 60 mg L−1 (50 mL), adsorbent: 0.05 g, pH:3, removal (time): 99.56% (10 min) | 72 | Langmuir | — | 500 |
| RhB | Polyaniline magnetic nanoparticles/dicationic ionic liquid | Tap/industrial and lake water | Coating aniline and dicationic ionic liquids on magnetic nanoparticle | Temp.: 298 K, RhB:10 mg g−1 (10 mL), adsorbent: 20 mg, pH: 1, removal (time); ∼94% (60 min) | 109.90 | Temkin | Pseudo sec. order (k: 0.4172 g mg−1 min−1) | 501 |
| RhB | Magnetite@PPy@2-acrylamido-2-methyl-1-propanesulfonic acid microspheres | Aqueous solutions | Two-step process | Temp.: 293 K., RhB: 60 mg L−1, adsorbent: 7 mg, pH: 7, removal (time): >90% (300 min) | 215.054 | Langmuir | Pseudo sec. order (k: 2.441 × 10−3 g mg−–1 min−1) | 502 |
| MB | Magnetite@PPy@2-acrylamido-2-methyl-1-propanesulfonic acid microspheres | Aqueous solutions | Two-step process | Temp.: 293 K. MB: 50 mg L−1, adsorbent: 7 mg, pH: 7, removal (time): ∼100% (240 min) | 183.486 | Langmuir | Pseudo sec. order | 502 |
| CV | Magnetite@PPy@2-acrylamido-2-methyl-1-propanesulfonic acid microspheres | Aqueous solutions | Two-step process | Temp.: 293 K, CV: 50 mg L−1, adsorbent: 7 mg, pH: 7, removal (time): ∼100% (300 min) | 194.175 | Langmuir | Pseudo sec. order (k: 3.156 × 10−3 g mg−1 min−1) | 502 |
| RhB | PEG capped PANI/TiO2/CuO | Water | In situ polymerization of aniline in the presence of TiO2/CuO | Temp.: 300 K. RhB: 5 mg L−1 (50 mL), adsorbent: 0.2 g, pH: 6, removal (time): 89.7% (240 min) | 3.53 | Langmuir | Pseudo sec. order (k: 0.809 g. mg−1 min−1) | 504 |
| CR | PANI@ZnO | Deionized water | In situ oxidation chemical process | Temp.: 298 K, CR: 150 mg L−1 (25 mL), adsorbent: 10 mg, pH: 5, Removal (time):∼70% (60 min) | 76.92 | Langmuir | Pseudo sec. order (0.0004 g mg−1 min−1) | 507 |
| MO | 3D PANI@Activated SiO2 gel | Aqueous solution | In situ polymerization | Temp.: 298.15 K, MO: 50 mg L−1 (30 mL), adsorbent: 0.25 g, pH: 3, removal efficiency (time): ∼100% (200 min) | 161.29 | Langmuir | Pseudo sec. order 0.036 g mg−1 min−1 | 508 |
| BG | 3D PANI@Activated SiO2 gel | Aqueous solution | In situ polymerization | Temp.: 298.15 K. BG: 50 mg L−1 (30 mL), adsorbent: 0.025 g, pH: 8, removal (time): 100% (200 min) | 136.98 | Langmuir | Pseudo sec. order (k: 1.34 g mg−1 min−1) | 508 |
| CR | PANI-ZnTiO3 | Aqueous solutions | Polymerization of aniline in the suspensions of ZTO | Temp.: RT, CR:50–150 ppm (100 mL), adsorbent: 200 mg, pH: natural pH, removal (time): 90% (15 min) | 64.51 | Langmuir | Pseudo sec. order (k: 0.000509 g s−1 mg−1) | 511 |
| CR | Fe3O4/PPy/carbon black | Aqueous solution | Encapsulating Fe3O4 nanoparticles in PPy/carbon black | CR: 120 mg L−1 (40 mL), adsorbent: 0.5 g L−1, pH: 7, removal (time): 95% (240 min) | 500 | Langmuir | Pseudo sec. order (k: 0.007 × 10−2 g mg−1 min−1) | 512 |
| MB | Fe3O4/PPy/carbon black | Aqueous solution | Encapsulating Fe3O4 nanoparticles in PPy/carbon black | MB: 40 mg L−1 (40 mL), adsorbent: 0.5 g L−1, pH: 7, removal (time): 95.9% (120 min) | 90.9 | Langmuir | Pseudo sec. order (k: 0.14 × 10−2 g.mg−1 min−1) | 512 |
| CR | PANI microsphere/MnO2/Fe3O4 | Aqueous solution | In situ deposition | Temp.: 303 ± 3 K, CR: 20 mg L−1, adsorbent: 1 g L−1, pH: ∼6.75, removal (time): 98% (1500 min) | 599.49 | Elovich | Pseudo sec. order (k: 0.25 g mg−1 min−1) | 513 |
| MG | PANI microsphere/MnO2/Fe3O4 | Aqueous solution | In situ deposition | Temp.: 303 ± 3 K, MG: 20 mg L−1, adsorbent: 1 g L−1, pH: ∼6.75, removal (time ): 88% (1500 min) | 1142.13 | Freundlich | Pseudo sec. order (k: 0.012 g mg−1 min−1) | 513 |
| CR | L-Cysteine/rGO/PANI | Aqueous solution | rGO + 0.1M L-cysteine solution + aniline monomer + APS | Temp.: RT, CR: 30 mg L−1 (10 mL), adsorbent: 0.025 g, pH: neutral, removal (time): 98% (10 min) | 56.57 | Langmuir | Pseudo sec. order | 516 |
| MB | Polyaniline nanotubes | Aqueous solutions | In situ polymerization | Temp.: 25 °C, MB: 3.1 mg L−1 (100 mL), adsorbent: 0.05 g, pH: qe/time: ∼0.6 mg g−1 (150 min) | 9.21 | Langmuir | Pseudo sec. order (k: 0.03595 g mg−1 min−1) | 518 |
| MB | Polyaniline hollow nanotubes | In situ synthesis | In situ polymerization | Temp.: RT, MB: 6.2 mg L−1 (100 mL), Adsorbent: 10 mg, pH: 11, Removal/time: ∼92% (600 min) | 69.4 | Langmuir | Pseudo sec. order (k: 0.001 g mg−1 min−1) | 519 |
| AG | PANI hollow nanotubes | Aqueous media | Using acid green as a structure-directing agent and soft template | Temp.: 298 K, AG:6.1 mg L−1 (100 mL), adsorbent: 10 mg, pH: 3.0, removal (time): 52% (6 h) | 57.8 | Langmuir | Pseudo sec. order (k: 8.9 × 10−4 g mg−1 min−1) | 519 |
| MB | Polyaniline nanotubes | Aqueous medium | Aniline oxidation in the presence of methyl orange | Temp.: 298 K, MB = 8.8 mg L−1 (0.1 L), adsorbent: 20 mg, pH: 9, removal (time): > 90% (300 min) | 91.1 | Langmuir | Pseudo sec. order (k: 0.00117 g mg−1 min−1) | 520 |
| AG | PANI nanotubes | Aqueous medium | Green approach via the aniline, oxidation | Temp.: 25 °C, AG: 20 mgL−1 (100 ml), adsorbent: 8 mg L−1, pH: 3, removal (time): 67% (320 min) | 58 | Langmuir | Pseudo sec.-order (k: 0.002 g mg−1 min−1) | 520 |
| CR | PPy@MoS2 hollow microtubes | Aqueous medium | Hydrothermal process, in situ polymerization and sulfidation | Temp.: RT, CR: 60 mg L−1 (5 mL), adsorbent: 50 mg L−1, pH: neutral, removal (time): 84,14% (120 min) | 598.7 | Freundlich | Pseudo sec. order (k: 0.0030 g mg−1 min−1) | 521 |
| MB | PPy@MoS2 hollow microtubes | Aqueous medium | Hydrothermal process and in situ polymerization and sulfidation | Temp.: RT, MB:150 mg L−1 (5 mL), adsorbent: 50 mg L−1, pH: neutral, removal (time): 41.1% (120 min) | 121.3 | Langmuir | Pseudo sec. order (k: 0.0003 g mg−1 min−1) | 521 |
| MB | PANI nanotubes base/silica | Distilled water | In situ polymerization | Temp.: 25 °C, MB: 0.95 mg L−1 (100 mL), adsorbent: 0.05 g, removal (time): 100% (10 min) | 10.31 | Langmuir | Pseudo sec. order (k: 0.09 g mg−1 min−1) | 522 |
| MB | PANI/TiO2 hydrate | Distilled water | One-pot chemical oxidative polymerization | Temp.: 298 K, MB:100–200 mg L−1 (10 mL), adsorbent: 2 g L−1, pH: 3–11, removal (time): ∼100% (12 h) | 458.10 | Freundlich model | Pseudo sec. order (k: 0.0009 g mg−1 min−1) | 523 |
| MB | PPy-coated cotton textile | Aqueous solution | In situ oxidative polymerization of cotton textiles | Temp.: 25 °C, MB: 3.9 mg L−1 (50 mL), adsorbent: 0.05 g, pH: 7, removal (time): 96% (24 h) | 6.83 | Freundlich | Pseudo sec. order (k: 0.083 g mg−1 min−1) | 525 |
| MB | Fe3O4@PPy@SDBS | Aqueous solutions | Through hydrothermal, in situ polymerization, and surface modification | Temp.: 20 °C, MB: 20–30 mg L−1 (30 mL), adsorbent: 10 mg, pH: 7, removal (time): ∼97% (500 min) | 124.07 | Langmuir | Pseudo sec. order (1.213 × 10−3 g mg−1 min−1) | 527 |
| MG | Fe3O4@PPy@SDBS | Aqueous solutions | Through hydrothermal, in situ polymerization, and surface modification | Temp.: 20 °C, MG: 20 mg L−1 (30 mL), adsorbent: 8 mg, pH: 7, removal (time): ∼96% (500 min) | 73.10 | Langmuir | Pseudo sec. order (1.445 × 10−3 g mg−1 min−1) | 527 |
| MB | PPy/GO@Fe3O4 | Aqueous solutions | One step | Temp.: RT, MB: 100 mg L−1 (40 mL), adsorbent: 10 mg, pH: 8, removal (time): ∼80% (140 min) | 323.2 | Langmuir | Pseudo second, order (k: 0.00172 g mg−1 min−1) | 528 |
| MB | Fe3O4@PPy/RGO | Aqueous solutions | Chemical route | Temp.: 30 °C. MB:100 mg L−1 (30 mL), adsorbent: 0.333 g L−1, pH: natural, removal (time): ∼95% (60 min) | 270.3 | Langmuir isotherm | Pseudo-second-order (k: 0.0154 g mg−1 min−1) | 530 |
| MB | Fe3O4/polypyrrole/phytic acid | Water | In situ polymerization | Temp.: 35 °C, MB: 100 ppm (50 mL), adsorbent: 80 mg, pH: 10, removal (time): ∼86.4% (120 min) | 153.84 | Langmuir | Pseudo sec. order (k: 0.001 g mg−1 min−1) | 531 |
| CV | Fe3O4/polypyrrole/phytic acid | Water | In situ polymerization | Temp.: 35 °C, CV: 100 ppm (50 mL), adsorbent: 80 mg, pH: 10, removal (time): ∼85% (120 min) | 181.82 | Langmuir | Pseudo sec. order (k: 0.001 g mg−1 min−1) | 531 |
| MO | PANI nanotube-filled sodium alginate | Distilled water | Mixing of PANI nanotubes in water + CaCl2 | Temp.: 35 °C, MO: 20 mg L−1 (50 mL), adsorbent: 0.05 g, pH: 2, removal (time): ∼76% (90 min) | 370.4 | Langmuir | Pseudo sec. order (k: 0.001 g mg−1 min−1) | 534 |
| MO | PANI-MWCNT | Water | In situ oxidative polymerization | Temp.: 30 °C, MO: 30 mgL−1 (100 ml), adsorbent: 8 mg L−1, pH: neutral, removal (time): ∼94% (60 min) | 149.25 | Langmuir | Pseudo sec. order (k: 5.265 × 10−4 g mg−1 min−1) | 535 |
| MO | PANI (skin)/polyamide 6 (core) | Aqueous solution | In situ oxidation polymerization | Temp.: 298 K, MO:10 ppm, adsorbent: 0.03g, pH: 6, removal (time): 58.7 mg g−1 (120 min) | 58.7 | Langmuir | Pseudo sec. order | 536 |
| MO | Waterborne poly vinyl pyrrolidone-stabilized PANI core–shell | Tap water | Waterborne PVP-stabilized PANI core–shell | Temp.: 28 °C, MO: 32.73 mg L−1 (20 ml), adsorbent: 5 mg, pH 7.02, removal (time): 100% (15 min) | — | Langmuir | — | 537 |
| MO | Halloysite nanotubes/PPy | Aqueous solution | In situ polymerization | Temp.: 25 °C, MO: 90 mg L−1 (50 mL), adsorbent: 0.15 g, pH: natural, removal (time): 98.6% (120 min) | 214.6 | Langmuir and Freundlich | Pseudo sec. order (k: 0.0037 g mg−1 min−1) | 538 |
| RB | PANI@TiO2 | Deionized water | Sonochemical method | Temp.: 28 °C. RB: 100 mg L−1 (100 mL), adsorbent: 50 mg, pH: 6.7, qt (time): ∼80 mg g−1 (120 min) | — | Langmuir and Freundlich | First order (k: 0.007 min−1), pseudo sec. order (k: 0.00008 g mg−1 min−1/2) | 540 |
| RB | PANI@SiO2 | Deionized water | Sonochemical method | Temp.: 28 °C. RB: 100 mg L−1 (100 mL), adsorbent: 50 mg, pH: 6, qt (time): ∼30 mg g−1 (120 min) | — | Langmuir and Freundlich | First order (k: 0.024 min−1), pseudo sec. order (k: 0.00029 g mg−1 min−1/2) | 540 |
| R6G | PANI@TiO2 | Deionized water | Sonochemical method | Temp.: 28 °C. R6G: 50 mg L−1 (100 mL), adsorbent: 5 mg, pH: 6.7, qt (time): ∼90 mg g−1 (120 min) | 94 | Langmuir and Freundlich | First order (k: 0.023 min−1), pseudo sec. order (k: 0.00 069 g mg−1 min−1/2) | 540 |
| R6G | PANI@SiO2 | Deionized water | Sonochemical method | Temp.: 28 °C. R6G: 5 mg L−1 (100 mL), adsorbent: 5 mg, pH: 6.7, qt (time): ∼60 mg g−1 (120 min) | 61 | Langmuir and Freundlich | First order (k: 0.011 min−1), pseudo sec. order (k: 0.00 006 g mg−1 min−1/2) | 540 |
| CR | PANI@TiO2 | Deionized water | Sonochemical method | Temp.: 28 °C. CR: 100 mg L−1 (100 mL), adsorbent: 5 mg, pH: 6.7, qt (time): ∼93 mg g−1 (120 min) | 93 | Langmuir and Freundlich | First order (k: 0.020 min−1), pseud sec. order (k: 0.00048 g mg−1 min−1/2) | 540 |
| CR | PANI@SiO2 | Deionized water | Sonochemical method | Temp.: 28 °C. CR: 100 mg L−1 (100 mL), adsorbent: 5 mg, pH: 6.7, qt (time): ∼70 mg g−1 (120 min) | 71 | Langmuir and Freundlich | First order (k: 0.0253 min−1), pseudo sec. order (k: 0.00034 g mg−1 min−1/2) | 540 |
| MB | PANI@TiO2 | Deionized water | Sonochemical method | Temp.: 28 °C. MB: 100 mg L−1 (100 mL), adsorbent: 50 mg, pH: 6.7, qt (time): ∼90 mg g−1 (120 min) | 89 | Langmuir and Freundlich | Pseudo first order (k: 0.024 min−1), pseudo sec. order (k: 0.00050 g mg−1 min−1/2) | 540 |
| MB | PANI@SiO2 | Deionized water | Sonochemical method | Temp.: 28 °C, MB: 50 mg L−1 (100 mL), adsorbent: 50 mg, pH: 6.7, qt (time): ∼73 mg g−1 (120 min) | 74 | Langmuir and Freundlich | First order (k: 0.037 min−1), pseudo sec. order (k: 0.00056 g mg−1 min−1/2) | 540 |
| EB | PANI@TiO2 | Deionized water | Sonochemical method | Temp.: 28 °C. EB: 100 mg L−1 (100 mL), adsorbent: 5 mg, pH: 6.7, qt (time) ∼55 mg g−1 (120 min) | — | Langmuir and Freundlich | First order (k: 0.008 min−1) pseudo sec. order (k: 0.00011 g mg−1 min−1/2) | 540 |
| EB | PANI@SiO2 | Deionized water | Sonochemical method | Temp.: 28 °C. EB: 100 mg L−1 (100 mL), adsorbent: 5 mg, pH: 6.7, qt (time) ∼55 mg g−1 (120 min) | — | Langmuir and Freundlich | First order (k: 0.013 min−1) pseudo sec. order (k: 0.00012 g mg−1 min−1/2) | 540 |
| BB | PANI@TiO2 | Deionized water | Sonochemical method | Temp.: 28 °C. BB: 100 mg L−1 (100 mL), adsorbent: 5 mg, pH: 6.7, qt (time):∼42 mg g−1 (120 min) | — | Langmuir and Freundlich | Pseudo first order (k: 0.011 min−1), pseudo sec. order (k: 0.00013 g mg−1 min−1/2) | 540 |
| BB | PANI@SiO2 | Deionized water | Sonochemical method | Temp.: 28 °C. BB: 100 mg L−1 (100 mL), adsorbent: 5 mg, pH: 6.7, qt (time): ∼42 mg g−1 (120 min) | 86 | Langmuir and Freundlich | Pseudo first order (k: 0.038 min−1), pseudo sec. order (k: 0.00060 g mg−1 min−1/2) | 540 |
| MG | Graphene/Fe3O4/PANI | Aqueous solutions | Multiple steps | Temp.: 25 °C, MG: 16 mg L−1 (50 mL), adsorbent: 30 mg, pH: 6.5, removal (after cycle 1): 97.72% | 196.10 | Langmuir | Pseudo sec. order (k: 0.0022 g mg−1 min−1) | 541 |
| AR1 | Graphene/Fe3O4/PANI | Aqueous solutions | Multiple steps | Temp.: 25 °C, AR1: 16 mg L−1 (50 mL), adsorbent: 30 mg, pH: 6.5, removal efficiency (after cycle 1): 97.013% | 150.27 | Langmuir | Pseudo sec. order (k: 0.0021 g mg−1 min−1) | 541 |
| MG | CNT/PANI | Aqueous solution | Static interfacial polymerization technique | Temp.: 20 °C, MG: 8–12 mg L−1 (100 mL), adsorbent: 0.1 g, pH: 7, removal (time): ∼95% (120 min) | 15.45 | Langmuir | Pseudo sec- order (k: 5.0 × 10−3 mg g−1 min−0.5) | 542 |
| AR1 | PPy/Mn0.8Zn0.2Fe2O4/GO (PMG50) | Wastewater | In situ Py polymerization | Temp.: 298 K, AR1:10 mg L−1 (10 mL), adsorbent: 100 mg, pH: 2, removal (time): 98.8% (120 min) | — | — | Pseudo sec. order (0.320 g mg−1 min−1) | 545 |
| BG | Cross-linked PANI/chitosan-graphene oxide-oxidized SWCNT | Aqueous solution | Chemical oxidative copolymerization | Temp.: 22 °C, BG: 5 mg L−1 (25 mL), adsorbent: 12.5 mg, pH 6, removal efficiency (time): ∼98.4% % (120 min) | 21.27 | — | Pseudo-second-order (k: 9.2 × 10−4 mg g−1 min-0.5) | 546 |
| AR1 | Cross-linked PANI/chitosan-graphene oxide-oxidized SWCNT | Aqueous solution | Chemical oxidative copolymerization | Temp.: 22 °C, AR1: 20 mg L−1 (25 mL), adsorbent: 12.5 mg, pH 2, removal efficiency (time): 99.7% (120 min) | 90.91 | — | Pseudo-second-order (k: 3.4 × 10−4 mg g−1 min−0.5) | 546 |
| BG | Core–shell polythiophene/ZnO/MWCNTs | Aqueous solutions | In situ chemical polymerization (two-way method) | Temp.: 25 °C. BG: 5 mg L−1 (20 mL), adsorbent: 30 mg, pH: 6, removal efficiency (time): 94% (90 min) | 8.3 | — | Pseudo second-order (k: 1.9 × 10–3 mg g−1 min−1) | 547 |
5.4. Supercapacitor applications of hollow ICPs
Supercapacitors are considered as energy storage devices, storing and releasing energy through the movement of ions within the electrolyte.549,550 Furthermore, carbonaceous materials, conducting polymers, and transition metal oxides have attracted much attention as electrode material in this application. A comparison of their merit/demerits is highlighted in Table 5. These supercapacitors are regarded as more promising candidates for having very high-power density, long cycling stability and excellent durability. The supercapacitors can be classified into electrical double-layer capacitors (EDLCs), pseudocapacitors (PCs), and hybrid supercapacitors, as schematically based on the mechanism involved in energy storage (Fig. 36).551 EDLCs are non-faradaic supercapacitors or ultracapacitors involving the storage of the charge taking place in the electrical double layer formed at the electrode/electrolyte interface without any changes in the chemical properties of the electrode materials. In the case of PC, charge is stored electrochemically through highly reversible redox reactions at the electrode–electrolyte interface, and they offer more power and charge density compared with EDLCs. A hybrid supercapacitor, on the other hand, is a combination of an EDLC and a pseudocapacitor and allows for both faradaic and non-faradaic charge storage.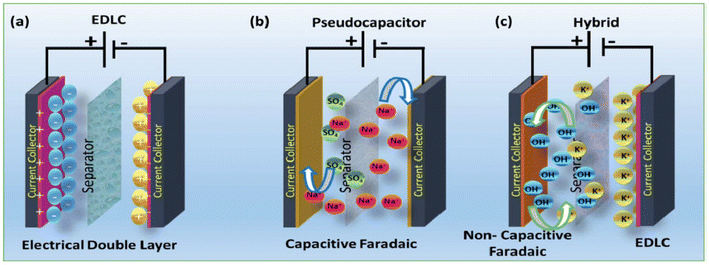 | ||
| Fig. 36 Charge storage mechanism of supercapacitors, (a) EDLCs, (b) pseudocapacitors and (c) hybrid supercapacitors.551 Reproduced with permission from RSC. | ||
| Materials | Advantages | Disadvantages |
|---|---|---|
| Carbonaceous materials | High specific surface area | Low energy density |
| High electrical conductivity | Poor cyclability | |
| Inexpensive | ||
| Eco-friendly | ||
| High electrochemical stability | ||
| Conducting polymers | High specific capacitance | Low conductivity |
| Tunable electrical conductivity Unique solution processability | Poor electrochemical stability | |
| High flexibility | ||
| Easy fabrication | ||
| Transition metal oxide | High specific capacitance | Low conductivity |
| Wide potential window | Poor electrochemical stability | |
| High energy density |
In this regard, intrinsically conducting polymers are favoured in supercapacitor applications due to their simple synthesis, tunable conductivity, large surface area, high flexibility, and pseudocapacitive properties.7 These features enable them to store and release energy quickly, thereby making conducting polymers an ideal candidate for high-power applications. The superior electrochemical performance of these ICPs is greatly guided by their morphology. In view of this, hollow-structured material electrodes can be better alternatives by offering higher specific surface area to facilitate the large electrode/electrolyte interface favorable for the fast transport of charges and ions as well as act as the ion reservoir to benefit the accumulation of ions. Therefore, ICPs are considered as promising electrode materials due to their low production cost and their ability to possess redox pseudocapacitance and double-layer capacitance. However, the application of ICPs is limited due to their inferior stability; therefore, ICP is combined with other active materials to overcome this intrinsic disadvantage. Accordingly, the performance of ICP for hollow-structured ICPs, their composites and core–shell-based materials is discussed below.
Polyaniline hollow microspheres (dia: 410 nm, thickness: 72 nm) doped by (poly(2-acrylamido-2-methylpropane sulfonic acid)) (PAMPS) synthesized by a self-assembly method have shown excellent promise in the field of supercapacitors.554 Li et al.555 fabricated Ce3+-doped polyaniline hollow microspheres by a self-assembly method; it exhibited a high specific capacitance of 248.2 F g−1 (1 mA cm−2) due to its enhanced conductivity compared with PANI (201.6 F g−1). In another approach, hollow PANI nanospheres prepared by in situ chemical oxidative polymerization of aniline in the presence of uniform poly(methyl methacrylate-butyl methacrylate-methacrylic acid) latex microspheres (self-sacrificial template) demonstrated specific capacitance (485.5 F g−1 at 1 A g−1) and performance after 500 cycles (69%).556
Polypyrrole hollow nanospheres prepared using polystyrene (PS) as a template that was subsequently removed by dissolving it in DMF displayed high specific capacitances (350 F g−1 at 1 A g−1).557 The corresponding symmetric supercapacitor has shown the maximum energy density of 40 Wh kg−1 at a power density of 490 W kg−1. Electrochemical studies were also performed on hollow polypyrrole films prepared by depositing polypyrrole on a 3D colloidal crystal template.558 According to Li et al.,559 hollow capsular polypyrrole nanofibers with porous capsule acted as a flexible, binder-free, self-supported supercapacitor electrode exhibiting specific capacitance of 203 F g−1 at 2 mV s−1 and excellent capacitance retention (>90% after 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 charge–discharge cycles @10 A g−1). This was attributed to the availability of enough free space in the capsular walls of the hollow PPy film to facilitate the volume variation during doping/de-doping. Zhang et al.244 synthesized PEDOT hollow nanospheres by expulsion of SiO2 from the SiO2@PEDOT nanospheres by dissolving it hydrofluoric acid. The PEDOT hollow sphere film fabricated in this manner showed a high specific capacitance (121.6 F g−1) at the current density of 0.5 A g−1 and sustained 65.7% of its initial specific capacitance at a current density of 8.0 A g−1.
000 charge–discharge cycles @10 A g−1). This was attributed to the availability of enough free space in the capsular walls of the hollow PPy film to facilitate the volume variation during doping/de-doping. Zhang et al.244 synthesized PEDOT hollow nanospheres by expulsion of SiO2 from the SiO2@PEDOT nanospheres by dissolving it hydrofluoric acid. The PEDOT hollow sphere film fabricated in this manner showed a high specific capacitance (121.6 F g−1) at the current density of 0.5 A g−1 and sustained 65.7% of its initial specific capacitance at a current density of 8.0 A g−1.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles). This suggested that the carbon-coated PANI electrode acted as a constraint layer to prevent the tubular-structured PANI from expanding outward during the charge–discharge process. ZnO nanorod arrays (sacrificial templates) were used to fabricate polyaniline nanotube arrays and their electrochemical polymerization was studied for supercapacitor applications (Fig. 37(b)).561 Jyothibasu and Lee562 fabricated polypyrrole tubes using curcumin (template), as schematically shown in Fig. 37(c) and combined it with functionalized carbon nanotubes (f-CNTs) to form freestanding electrodes (referred to as PPyC3T2/f-CNT). It showed excellent areal capacitance of 11
000 cycles). This suggested that the carbon-coated PANI electrode acted as a constraint layer to prevent the tubular-structured PANI from expanding outward during the charge–discharge process. ZnO nanorod arrays (sacrificial templates) were used to fabricate polyaniline nanotube arrays and their electrochemical polymerization was studied for supercapacitor applications (Fig. 37(b)).561 Jyothibasu and Lee562 fabricated polypyrrole tubes using curcumin (template), as schematically shown in Fig. 37(c) and combined it with functionalized carbon nanotubes (f-CNTs) to form freestanding electrodes (referred to as PPyC3T2/f-CNT). It showed excellent areal capacitance of 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 830.4 mF cm−2 at a current density of 2 mA cm−2 in a thick freestanding PPyC3T2/f-CNT-electrode at a high mass loading of 30 mg cm−2. Furthermore, a symmetric supercapacitor assembled by using the PPyC3T2/f-CNT displayed an areal capacitance, cycling stability, high energy density and maximum power density of 2732 mF cm−2 at 2 mA cm−2, 118.18% retention after 12
830.4 mF cm−2 at a current density of 2 mA cm−2 in a thick freestanding PPyC3T2/f-CNT-electrode at a high mass loading of 30 mg cm−2. Furthermore, a symmetric supercapacitor assembled by using the PPyC3T2/f-CNT displayed an areal capacitance, cycling stability, high energy density and maximum power density of 2732 mF cm−2 at 2 mA cm−2, 118.18% retention after 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 cycles, 242.84 μW h cm−2 and 129.35 mW cm−2, respectively.
500 cycles, 242.84 μW h cm−2 and 129.35 mW cm−2, respectively.
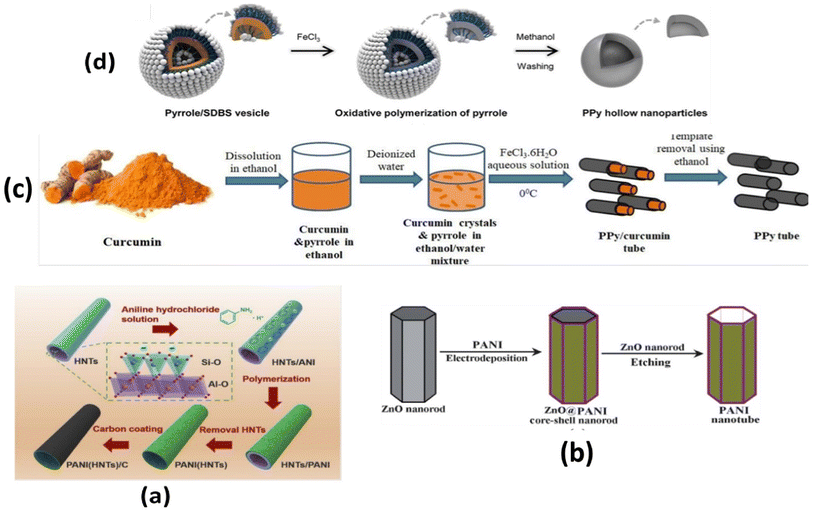 | ||
| Fig. 37 (a) Schematic presentation on the preparation procedure of PANI(HNTs) and PANI(HNT)s/C.560 Reproduced with permission from Elsevier. (b) The illustration for the formation of PANI nanotubes via a sacrificial ZnO nanorods template route.561 Reproduced with permission from RSC. (c) Schematic representation of the preparation of polypyrrole tubes using curcumin as template.562 Reproduced with permission from RSC. (d) Schematic diagram of the synthesis of PPy hollow nanospheres.565 Reproduced with permission from RSC. | ||
Hollow PANI nanotubes (inner dia: 80 nm, outer dia: 180 nm) prepared via one-step polymerization attained specific capacitance of 436 F g−1 (0.5 A g−1) in H2SO4 (1 mol L−1) solution with good cycling stability (89.2%: 500 cycles at a current density of 0.5 A g−1).563 Crystalline tetragonal hollow polyaniline nanotubes prepared by using methyl orange as self-degrading template showed a specific capacitance of 590 ± 36 F g−1 at a scan rate of 5 mV s−1.564 The assembled symmetrical device exhibited maximum energy density and power density of 14.56 Wh kg−1 and 250 W kg−1, respectively. Ahn et al.565 prepared PPy hollow nanoparticles with controlled diameters through surfactant-templated chemical oxidation polymerization (Fig. 37(d)). The specific capacitance of PPy hollow nanoparticles (326 F g−1) fabricated in this manner was found to be approximately twice as large as that of solid PPy nanospheres. High-performance supercapacitor performance has also been displayed by PANI nanofibers, nanotubes, and nanospheres,566 and hollow polyaniline nanofibers fabricated by electrospinning.312 Hryniewicz and Vidotti567 for the first time electrodeposited PEDOT nanotubes onto stainless steel mesh electrodes in the presence of methyl orange template and observed enhanced supercapacitive and electrocatalytic properties. Wan et al.568 observed high specific capacitance (290 F g−1 at 1 A g−1) and outstanding cyclic stability (capacitance retention: 83% even after 1000 cycles) for the polyaniline hollow nanofibers supercapacitor electrode exhibiting 3D porous network architecture.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 10 A g−1) and energy density (20.4 Wh kg−1) at a power density of 194 W kg−1. Wang and others570 investigated the electrochemical performance of mesoporous MnO2/polyaniline composite with a unique morphology of hierarchical hollow submicron spheres synthesized by an interfacial approach. Electrochemical studies revealed its superior electrochemical properties, as indicated by its specific capacitance (262 F g−1) and higher capacitance retention (93% of its initial capacitance after 800 cycles) owing to its microstructure and polyaniline/MnO2 coexisting together.
000 cycles at 10 A g−1) and energy density (20.4 Wh kg−1) at a power density of 194 W kg−1. Wang and others570 investigated the electrochemical performance of mesoporous MnO2/polyaniline composite with a unique morphology of hierarchical hollow submicron spheres synthesized by an interfacial approach. Electrochemical studies revealed its superior electrochemical properties, as indicated by its specific capacitance (262 F g−1) and higher capacitance retention (93% of its initial capacitance after 800 cycles) owing to its microstructure and polyaniline/MnO2 coexisting together.
3D hollow balls of graphene–polyaniline hybrids were prepared through the self-assembly of graphene oxide and PMMA followed by the polymerization of polyaniline, and demonstrated high specific capacitance and good cycling stability.571 Luo et al.572 used layer-by-layer assembly for the preparation of a graphene–polyaniline hollow microsphere for supercapacitor application. It displayed excellent specific capacitance (381 F g−1: 4.0 A g−1) and good cycling stability (83% after 1000 cycles) in 1 M H2SO4 solution due to a synergistic effect. The hierarchical Ti3C2/PANI nanotubes have shown excellent specific capacitance performance (596 F g−1 at 0.1 A g−1) with 94.7% retention after 5000 cycles (at 1 A g−1).573 Furthermore, a symmetric supercapacitor device based on this showed excellent performance (energy density: 25.6 Wh kg−1 at 153.2 W kg−1, power density: 1610.8 W kg−1 at 13.2 Wh kg−1, capacitance retention 81.1% after 4000 cycles).
Devi and Kumar574 prepared nanocomposites of reduced graphene oxide/polyaniline nanotubes in the presence as well as the absence of irradiated high energetic ions and performed electrochemical investigations as a supercapacitor. They observed the relatively enhanced specific capacitance (482 F g−1 at 0.5 A g−1) and cycling stability (92%) compared with the unirradiated nanocomposite. This was ascribed to the increased surface energy and porosity as a result of irradiation. Yang et al.575 tested the electrochemical performance of a polypyrrole hollow nanosphere intercalated graphene-based flexible supercapacitor and observed its area specific capacitance and capacitance retention of 381.54 mF cm−2 (at 2 mA cm−2) and 93.94% after 1000 cycles, respectively. Graphene–polypyrrole hollow sphere,576 hollow polypyrrole nanosphere embedded in N-doped graphene layers,577 3D-arrayed polyaniline hollow nanospheres encaging RuO2 nanoparticles,578 polyaniline hollow fibers decorated by graphene,579 graphene–polypyrrole nanotubes,580 3D metal−organic frameworks with conductive polypyrrole tubes,581 and hollow PPy nanospheres decorated on the CNT582 have been synthesized and also used as electrode materials for electrochemical supercapacitor applications.
5.4.4.1 Binary core–shell nanocomposites. Tian et al.583 wrapped polyaniline nanowires on polypyrrole hollow nanotubes by the chemical method and observed a high specific capacitance (∼765 F g−1 at a scan rate of 10 mV s−1) and the capacitance loss of ∼13.7% after 1000 charging/discharging cycles. This performance was explained on the basis of the synergistic effect of PANI and PPy. Core–shell polyaniline-functionalized carbon quantum dots exhibited high specific capacity of 264.6 F g−1 (2.5 A g−1) and high stability indicated by 5000 charge–discharge cycles.584 The sea urchin-like polyaniline grown on the surface of polypyrrole by dilute solution polymerization (referred to as PPy@PANI) displayed a core–shell structure, according to Fig. 38(a).585 The variation of the specific capacitance versus the scan rate is displayed in Fig. 38(b). According to this, the specific capacitance of PPy@PANI corresponds to 510 F g−1 (scan rate: 10 mV s−1) compared with the PPy (87 F g−1) electrode and the pure PANI (256 F g−1) electrodes. PPy@PANI also showed good cycling stability as evident from 12.4% loss of specific capacitance after 1000 charge/discharge, as seen in Fig. 38(c). This electrochemical performance of the PPy@PANI electrode has been ascribed to the synergistic effect between the core PPy and shell PANI layer.
 | ||
| Fig. 38 (a) Schematic diagram for the synthesis of core–shell PPy@PANI nanospheres. (b) SC values’ plot of the scan rates and (c) charge/discharge cycling of the core–shell PPy@PANI-0.008, individual PPy, and the PANI electrode carried out at 5 A g−1 in H2SO4 aqueous electrolyte (1 M). The insert is the SEM images of the core–shell@PANI-0.008 before and after 1000 cycles.585 Reproduced with permission from ACS. | ||
Li et al.586 fabricated hollow microsphere (MS) and microtubes (MT) of PPy using polystyrene microspheres and methyl orange as hard and soft templates, respectively. Subsequently, they prepared composites of MS and MT with poly(1,5-diaminoanthraquinone) (referred as PDAAQ) and observed the following order for specific capacitance at a current density of 1 A g−1:
PPy@PDAAQ MTs (533 F g−1) > PPy@PDAAQ MSs (471 F g−1) > PDAAQ, (348 F g−1) >PPy MTs (322 F g−1) > PPy (MSs 235 F g−1).
The hollow morphology of PPy could account for the observed high specific capacitance of PPy@PDAAQ MTs.
The specific capacitance retention after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles followed the following trend:
000 cycles followed the following trend:
PDAAQ (146.0%) > PPy@PDAAQ MTs (107.4%), PPy@PDAAQ MSs (98.0%), PPy MTs (43.6%), and PPy MSs (27.3%).
Fan et al.587 prepared a core–shell hybrid comprising PS@PANI, polyaniline hollow spheres (PANI-HS)@GO and PANI-HS@ ERGO (electrochemically reduced graphene oxide) according to the procedure as illustrated in Fig. 39(a). The morphology and structure of the PANI-HS36@GO (36: shell thickness of PANI-HS) hybrid was studied by SEM and is displayed in Fig. 39(b). The PANI-HS36@ERGO hybrid indicated its significantly high specific capacitance corresponding to 614 F g−1 at 1 A g−1 current density (Fig. 39(c)) and maintained 90% after 500 charging/discharging cycles at a current density of 1 A g−1 (Fig. 39(d)) due the synergistic effect.
 | ||
| Fig. 39 (a) Schematic illustration on the preparation of PANI-HS36@ERGO hybrids (36 here refers to shell thickness in nm), (b) SEM images of PANI-HS36@GO hybrids, (c) galvanostatic charge−discharge curves of ERGO, PANIHS36, and PANI-HS36@ERGO hybrids within a potential window of 0–0.80 V at a current density of 1 A g−1 (modified), and (d) plots of specific capacitance for PANI-HS36 and PANI-HS36@ERGO hybrids at various current densities (modified).587 Figure reproduced with permission from ACS. | ||
Du et al.588 used MnO2 nanotubes as the reactive template for the preparation of PANI nanotubes using an in situ polymerization process, that exhibited excellent specific capacitance (455.7 F g−1 at 0.5 A g−1), rate capability of 63.2% even up to 30 A g−1. However, it showed poor cycling stability due to the swelling and shrinking of PANI during intercalating/deintercalating. Therefore, they fabricated hierarchical carbon layer-encapsulated PANI nanotubes by a hydrothermal method and observed its superior performance compared with the PANI nanotubes. In addition, the assembled symmetric supercapacitors based on this delivered high energy density (42.32 Wh kg−1) and power density (16.44 kW kg−1) and had good cycling stability. Mini and Devendrappa589 prepared a core/shell polyaniline/NiO nanocomposite and studied its electrochemical performance as an electrode in supercapacitor applications. These findings revealed its specific capacitance, energy density, power density and coulombic efficiency corresponding to 362 F g−1 (1 A g−1), 50.2 Wh kg−1, 0.50 kW kg−1 and 99% (at 4 Ag−1), respectively. The enhanced specific capacitance performance was ascribed to the nanosized effect of NiO and the synergic effect between NiO and PANI.
Polypyrrole nanosphere (dia: ∼70 nm)/graphene oxide synthesized via in situ surface-initiated polymerization exhibited specific capacitance of 370 F g−1@0.5 A g−1, and capacitance retention of 91.2% even after 4000 cycles owing to the core–shell structure and synergistic effect.590 Qi et al.591 reported the preparation of core–shell-structured tubular graphene nanoflake-coated polypyrrole nanotubes and observed its large capacitance, high capacitance retention and stable cycling performance. The energy density and cycling stability of the symmetric supercapacitor device based on this correspond to 11.4 μWh cm−2 at 720 μW cm−2 and over 80% capacitance retention after 5000 cycles, respectively.
In recent years, there has been considerable interest in studying the capacitive properties of MoS2 for its application in supercapacitors.592–597 However, despite many advantages, the aggregation, poor electrical conductivity and specific capacitance of MoS2 limit its practical application in energy storage electrode materials.592 As a result, MoS2 is combined with conducting polymers to overcome this problem. In view of this, Chen et al.593 prepared urchin-like MoS2@PANI (MoS2: 25 wt%) by a hydrothermal method and observed its maximum capacitance of 645 F g−1 at 0.5 A g−1 due to the synergistic effect. In addition, excellent cycling stability (89% capacitance retention) was observed after 2000 cycles at a current density of 10 A g−1.
Zhang et al.594 fabricated core/shell microspheres comprising hollow MoS2/PANI-5 (5 represents the mass ratio of aniline to hollow MoS2 microspheres). The electrochemical studies of this electrode material displayed the specific capacitance of 633 F g−1 @0.5 A g−1 and retention of the capacitance up to 86.0% after 1000 cycles at 10 A g−1, benefitting from polyaniline confined firmly on the microspheres of MoS2. In addition, in the MoS2/PANI-5 composite the energy density could deliver 31.7 W h kg−1 at the power density of 0.3 kW kg−1.
A template-assisted method was employed to synthesize the MoS2/PANI (mass ratio = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) hollow microsphere and used as a promising electrode for a supercapacitor.595 SEM images clearly established the coating of PANI on the surface of the hollow structure of MoS2 microspheres (dia: ∼2 μm). The specific capacitance was found to be 299 F g−1 at a scan rate: 10 A g−1 and was attributed to the synergistic effect of MoS2 core and PANI coating shell. MoS2/PANI microsphere electrode materials also delivered excellent cycling stability, as evident from its retention of 84.3% after 8000 cycles. The investigations also indicated the energy density reaching 23.1 Wh kg−1 at a power density of 8320 W kg−1. The specific capacitances of the fabricated symmetric supercapacitor based on MoS2/PANI microspheres electrodes corresponded to 231, 139, 97, 79 F g−1 at the current density of 0.2 A g−1. Furthermore, the assembled symmetric supercapacitor also exhibited high cycling stability (80.4% after 5000 cycles at 1 A g−1). These findings highlighted the potential application of MoS2/PANI hollow microspheres for supercapacitors.
2) hollow microsphere and used as a promising electrode for a supercapacitor.595 SEM images clearly established the coating of PANI on the surface of the hollow structure of MoS2 microspheres (dia: ∼2 μm). The specific capacitance was found to be 299 F g−1 at a scan rate: 10 A g−1 and was attributed to the synergistic effect of MoS2 core and PANI coating shell. MoS2/PANI microsphere electrode materials also delivered excellent cycling stability, as evident from its retention of 84.3% after 8000 cycles. The investigations also indicated the energy density reaching 23.1 Wh kg−1 at a power density of 8320 W kg−1. The specific capacitances of the fabricated symmetric supercapacitor based on MoS2/PANI microspheres electrodes corresponded to 231, 139, 97, 79 F g−1 at the current density of 0.2 A g−1. Furthermore, the assembled symmetric supercapacitor also exhibited high cycling stability (80.4% after 5000 cycles at 1 A g−1). These findings highlighted the potential application of MoS2/PANI hollow microspheres for supercapacitors.
Ansari et al. reported596 the formation of a mechanically exfoliated MoS2 sheet coupled with polyaniline. TEM studies of the product indicated PANI was coated on the MoS2 sheet and displayed the specific capacitance of 510.12 F g−1 at a current of 1 A g−1 due to the synergistic effect and interfacial interaction. The hierarchical PEDOT@MoS2 showed high specific capacitance (2540 mF cm−2 at 1 mA cm−2) and excellent capacitance retention (98.5%) after 5000 cycles at a high current density of 100 mA cm−2.597 This was ascribed to the hierarchical core/shell structure of the electrode material and the synergic effect. In addition, the assembled asymmetric supercapacitor delivered a high energy density (937 Wh m−2) at 6500 W m−2 and excellent cycling stability with capacitance retention of 100% (5000 cycles).
According to Liang et al.,598 V2O5@PPy displayed high conductivities and the synergic effect accounted for the specific capacitance of 307 F g−1 and good cycling life (82% capacity retention up to 1000 cycles). Such performance of V2O5@PPy benefited from the enhanced conductivity, synergistic effect, and the stability of the composite. The symmetric V2O5/PPy device exhibited a maximum energy density of 37 Wh Kg−1 at a power density of 161 W kg−1. In addition, polyaniline-carbon nanotube core–shell,599 pistil-like MnCo2O4.5@PANI,600 and high-performance electrodes for supercapacitors.601 Ni ferrite@Polypyrrole,602 core/sheath structured ultralong MnOx/PPy nanowires,603 and CuS@PANI604 also displayed superior performance as electrodes in supercapacitor applications. The performance of MWCNT coated with PANI (core dia: ∼80–150 nm) has also been evaluated for supercapacitor application.605 The findings showed maximum specific capacitance (552.11 F g−1 at 4 mA cm−2) for PANi/MWCNT (10 wt%) in 1 M H2SO4.
Xia et al.606 prepared a core–shell PANI/CNTs nanostructured hybrid by chemical vapor and electrochemical deposition methods according to Fig. 40. Subsequent findings based on galvanostatic charge/discharge (GCD) curves of CNTs and PANI/CNTs with and without the addition of 0.02 M Fe3+/Fe2+ are displayed in Fig. 41(a). It was inferred that discharge time was extended on the addition of the Fe3+/Fe2+ redox couple. The following order for discharge time was observed: PANI/CNTs (0.02 M Fe3+/Fe2+) > PNI/CNTs > CNT (0.02 M Fe3+/Fe2+) > CNTs. GCD curves of PANI/CNTs at different current density in 1 M H2SO4 in Fig. 41(b) confirmed the ideal capacitive mechanism of the core–shell hybrid. GCD curves of PANI/CNTs in 1 M H2SO4/0.02 M Fe3+/Fe2+ electrolyte at different current densities are displayed in Fig. 41(c). These findings affirmed a non-ideal triangular pattern corresponding to the redox reaction behavior of the Fe3+/Fe2+ redox couple. The specific capacitance curves of PANI/CNTs at different current densities in Fig. 41(d) showed improvement in the specific capacitance of PANI/CNTs (1005 F g−1) on adding 0.02 M Fe3+/Fe2+ (1547 F g−1) at 2 A g−1 in 1 M H2SO4. This superior performance was attributed to the synergistic effect originating from the contribution of the Fe3+/Fe2+ redox couple and the PANI/CNTs core–shell structure. In addition, the specific capacitance of the assembled symmetric architecture device was calculated and found to be 412 F g−1 at 0.5 A g−1. In addition, the energy density (22.9 Wh kg−1) at a power density of 700.1 W kg−1 and the capacitance retention of 97% (2000 charge–discharge cycles) was reached using PVA/H2SO4/Fe3+/Fe2+ gel as redox electrolyte. These findings supported the role played by the core–shell PANI/CNT hybrid electrode and Fe3+/Fe2+ redox additive electrolyte in achieving this enhanced electrochemical performance.
 | ||
| Fig. 40 Illustrations of the fabrication process for PANI/CNTs on carbon cloth (CC).606 Reproduced with permission from Springer. | ||
 | ||
| Fig. 41 (a) GCD curves of CNTs and PANI/CNTs at 1.5 mA cm−2 in 1 M H2SO4 with and without the addition of 0.02 M Fe3+/Fe2+. (b) GCD curves of PANI/CNTs testing in 1 M H2SO4 electrolyte at different current densities. (c) GCD curves of PANI/CNTs testing in 1 M H2SO4/0.02 M Fe3+/Fe2+ electrolyte at different current densities. (d) Specific capacitance curves of PANI/CNTs at different current densities.606 Reproduced with permission from Springer. | ||
An enhanced electrochemical performance has been reported for the hierarchical NiCo2S4@PANI core–shell nanowires grown on a carbon filter.607 The specific capacitance of the electrode fabricated in this manner showed enhanced areal capacitance of 4.74 F cm−2 (1823 F g−1) at 2 mA cm−2 and a capacitive retention of 86.2% (5000 cycles) compared with NiCo2S4/CF due to the availability of the more electrochemically active sites and faster ionic diffusion. The excellent cycling stability of NiCo2S4@PANI/CF was suggested to be induced by the presence of the PANI shell. The assembled asymmetric supercapacitor (positive electrode: NiCo2S4@PANI/CF, negative electrode: graphene/CF) delivered a high energy density of 64.92 Wh kg−1 at a power density of 276.23 W kg−1. This was found to be significantly higher compared with the asymmetric and symmetric supercapacitors based on NiCo2S4 and was ascribed to the core/shell heterostructure of NiCo2S4@PANI/CF.
Pan et al.608 displayed more surface capacitive contribution and enhanced electrochemical performance benefiting from the unique heterostructure in NiCo2O@Polyaniline nanotubes anchored on carbon. Their finding showed a specific capacitance of 720.5 C g−1 at 1 A g−1, found to be much better than that of NiCo2O4. In addition, cycling performance and coulombic efficiency was also investigated for the NiCo2O4@PANI electrode. These findings showed the sample retaining 99.64% of its initial gravimetric capacity following 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles with 100% coulombic efficiency due to the high efficiency of rapid electron transfer for charge storage and delivery. They also suggested that unique coaxial structure of NiCo2O4@Polyaniline increases the interface area of the electrode/electrolyte and facilitates a shorter diffusion path for ions and electrons.
000 cycles with 100% coulombic efficiency due to the high efficiency of rapid electron transfer for charge storage and delivery. They also suggested that unique coaxial structure of NiCo2O4@Polyaniline increases the interface area of the electrode/electrolyte and facilitates a shorter diffusion path for ions and electrons.
Mu et al.609 used polystyrene sulfonate microspheres as a template to fabricate graphene/polyaniline hybrid hollow microspheres in two steps involving the layer-by layer assembly technique followed by in situ oxidative chemical polymerization. Subsequent studies have indicated a specific capacitance of graphene/polyaniline hybrid hollow microspheres of ∼633 F g−1 in a 1.0 M H2SO4 electrolyte as well as excellent cycling stability (with 92%) of its original specific capacitance, in all probability due to the synergistic effect. Alternatively, the contribution of the unique structure facilitating the reduced transport lengths for both mass and charge transport also cannot be ruled out. The energy density has also been calculated and found to be 382.97 Wh kg−1 at a current density of 10 mA cm−2.
In another study, the hierarchical polyaniline/NiCo-layered double hydroxide (PANI/NiCo-LDH) core–shell composite nanofiber network was prepared by a two-step strategy (in situ oxidative polymerization and electrochemical deposition) on carbon cloth.610 The choice of a LDH nanosheet shell grown on the PANI network in this work was guided by the role it plays in facilitating ion and electron transport and also in relieving the strain change of the electrode during redox reaction. The electrode fabricated in this manner delivered a specific capacitance of 1845 F g−1 at 0.5 A g−1 and excellent cycling stability (82% after 5000 cycles at 10.0 A g-1), benefiting from rapid electron transport and ion diffusion. In addition, the assembled asymmetric device (positive electrode: PANI/NiCo-LDH, negative electrode: activated carbon) displayed excellent energy density (46.0 Wh kg−1) at a power density of 351.6 W kg−1 and good cycling performance. Polyaniline/graphene nanosheets,611 Co(OH)2–polyaniline,612 polyaniline/NiCo2S4,613 carbon nanotube@polypyrrole core–shell,614,615 and a Janus-type α-Fe2O3/PEDOT nanoparticles core/shell616 also functioned as high-performance supercapacitive electrode materials.
5.4.4.2 Ternary and quaternary core–shell ICP nanocomposites. Vellakkat and Hundekkal617 reported the formation of a chitosan-mediated synthesis of core/double shell ternary polyaniline/chitosan/cobalt oxide nano composite for their application as a high energy storage electrode material in supercapacitors. In another study, in situ chemically prepared polyaniline film-wrapped Ag-decorated MnO2 nanorod (PANI/Ag@MnO2) showed a specific capacitance of 1028.66 F g−1 at a current density of 1 A g−1 synergistically.618 Furthermore, the assembled asymmetric supercapacitor (PANI/Ag@MnO2//AC) device in 1 M H2SO4 displayed high energy density (49.77 Wh kg−1) at a power density of 1599.75 W kg−1. According to Iqbal et al.,619 a ternary composite comprising porous Polyaniline@CNT-MnO2 nanorods prepared by a hydrothermal method and in situ oxidative polymerization of aniline in 0.1 M KOH electrolyte achieved specific capacity, cycle life, energy density and power density of 143.26 C g−1at 3 mV s−1, 119% (3500 cycles) at 0.3 A g−1, 27.17 Wh kg−1 and 298 W kg−1, respectively. MWCNT@MnO2@PPy supercapacitor electrodes attained specific capacitance of 272.7 F g−1 and reasonable cycling performance.620 Ho et al.621 synthesized a PPy/multilayer graphene-wrapped copper nanoparticles (MLG-Cu NPs) composite by a two-step process on a flexible carbon cloth substrate. This displayed excellent specific capacitance performance (845.38 F g−1) at the current density of 1 A g−1.
Moreno et al.622 recorded the cyclic voltammetry (CV) of vertically aligned ZnO@CuS@PEDOT core@shell nanorod arrays decorated with MnO2 nanoparticles at different scan rates in 1 M LiClO4 aqueous electrolyte. The appearance of a more-or-less quasi-rectangular with symmetric shape of CV indicated the fast reversible reaction and an ideal capacitor-type behaviour. Furthermore, ZnO@CuS@PEDOT exhibited excellent electrochemical performance, as evident from its high specific areal capacitance of 19.85 mF cm−2, good rate capability and cycling stability. This promising capacitive behavior was attributed to the unique hierarchical core–shell hybrid nanorod configuration and synergistic effects. A core@shell hollow Bi2O3−x@Carbon fiber@PEDOT electrode fabricated through a multistep process exhibited specific capacitance of 460 F g−1 (1 A g−1) and reasonably good cycling stability.623 It was also noted that energy density, power density and remarkable cycling performance in the assembled symmetric supercapacitor corresponded to 16.4 Wh kg−1, 500.34 W kg−1 and 99% capacitance retention after 8500 cycles.
The coaxial core–sheath shaped supercapacitor based on polypyrrole-functionalized graphene/carbon nanotubes hollow fibers exhibited ultrahigh length specific capacitance and energy density.624 According to Ghosh et al.,625 an asymmetric supercapacitor comprising 3-D urchin shaped coaxial MnO2@PANI composite and a self-assembled 3-D pillared graphene foam exhibited an energy density of 37 Wh kg−1 at a power density of 386 W kg−1 and stable cycling performance. In another report, graphene carbon sphere@PANI@RGO composites reached specific capacitance of 446.19 F g−1 (scan rate: 5 mV s−1) in 1 M H2SO4 solution with 93.4% capacitance retentions after 1000 cycles.626 Wang et al.627 prepared a core–shell MoS2/PPy/PANI ternary hybrid with a ‘pizza-like’ nanostructure. It achieved a high specific capacitance (1273 F g−1 at 0.5 A g−1) with good cycling performance (83% after 3000 charge/discharge cycles). This was ascribed to the synergistic effect, improved electrical conductivity and enhanced electrolyte/electrode interaction. Recently, Liu et al.628 synthesized poly(3,4-propylenedioxythiophene)-OH/CoNi-SeS@Hollow carbon sphere via a sulfurization/selenization ion exchange method and in situ oxidation, and showed excellent electrochemical performance (mass specific capacity: 775.1 C g−1 at 1 A g−1). The assembled asymmetric supercapacitor device possessed a good energy density (84.8 Wh kg−1) and excellent power density (8000 W kg−1), and the capacity retention rate of 82.85% at 4 A g−1 (after 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles). It was suggested that a synergistic effect of the conductive polymer, the hollow structure, and modified electronic structure contributed to achieving the better capacitance performance.
000 cycles). It was suggested that a synergistic effect of the conductive polymer, the hollow structure, and modified electronic structure contributed to achieving the better capacitance performance.
Polypyrrole-coated low-crystallinity Fe2O3 supported on carbon cloth,321 graphene/polyaniline/MnO2,322 GO/α-MnO2/PANI,323 (CoCrFeMnNi)3O4@CC-PPy,324 LaMnO3@CC-PPy,325 PPy/black phosphorus oxide/CNT,326 PANI/GO/CuFe2O4629 and PANI-coated CuO–ZnO–MnO630 have also been investigated as electrodes for their supercapacitor performance.
Table 6 shows the electrochemical performance of various supercapacitors fabricated based on polyaniline electroactive material.
| Electrode material | Method of preparation | Electrolyte | Specific capacitance (current density) | Cycling stability | Energy density (ED) | Power density (PD) | Ref. |
|---|---|---|---|---|---|---|---|
| Ppy1%/DBSA2%/NiO97%-GS | Electrodeposition at 4 mA cm−2 for 10 min | 0.1 M LiClO4 | 679 at 1 A g−1 | 83.9% (1000 cycle) at 1 A g−1 | 94.4 Wh kg−1 | 94.4 W kg−1 | 154 |
| MnO2@PPy | Hard template (PS) method. | 1 M Na2SO4 | 295 F g−1 at of 1 A g−1 | 100% (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles) 10 A g−1 000 cycles) 10 A g−1 |
42 Wh kg−1 | 1100 W kg−1 | 239 |
| Core–shell nanorod arrays with PANI deposited into NiCo2O4 | Electrochemical polymerization | 1 M H2SO4 | 901 F g−1 at1 A g−1 | 91% (3000 cycles) at 10 A g−1 | 81.77 Wh kg−1 at a PD of 399.3 W kg−1 | — | 320 |
| D-Fe2O3@PPy/CC | Chemical reduction and electrodeposition methods | 1 M Na2SO | 640 F g−1 at 1 mA cm-1 | 79.3% of its (10 mA cm-2) after 5000 cycles | — | — | 321 |
| (CoCrFeMnNi)3O4@CC-PPy | Two-step electrodeposition | 2 M H2SO4 | 791 F g−1 at 0.5 A g−1 | 63% (5000 cycles) at 10 A g−1 | 49.2 Wh kg−1 (PD: 800 W kg−1) | — | 324 |
| LaMnO3@CC-PPy | Two-step electrodeposition | 862 F g−1 at 1 A g−1 | 66% (3000 cycles) at 10 A g−1 | 73 Wh k−1 | — | 325 | |
| Hollow polyaniline helical nanobelts | By chemical oxidation of aniline | — | 688 F g−1 at 5 mV s−1 | 81.6% (1000 cycles) | 14.37 Wh kg−1 at PD of 500 W kg−1 | — | 492 |
| Hollow polyaniline microspheres | In situ chemical oxidative polymerization with SPS spheres as the template | 1 M H2SO4 | 421 F g−1 | 45% (500 cycles) at 10 mA cm−2 | — | — | 552 |
| Hollow polyaniline nanocapsules | Interfacial polymerization method | 1 M H2SO4 | 502 F g−1 at 5 mA cm−2 | 83.1% (1000 cycles) at 10 mA cm−2 | — | — | 553 |
| Ce3+-doped polyaniline hollow microspheres | Self-assembly method | H2SO4 | 248.2 F g−1 at 1 mA cm−2 | 41.6% (5000 cycles) at 5 mA cm−2 | — | — | 555 |
| Hollow polyaniline nanospheres | Self-sacrificial templates and emulsion polymerization | 1 M HCl | 485.5 F g−1 at 1 A g−1 | 69% (500 cycles) at 5 A g−1 | — | — | 556 |
| Polypyrrole hollow nanospheres | Hard template method | 1 M H2SO4 | 350 at 1A g−1 | — | 40 Wh kg−1 | 490 W kg−1 | 557 |
| Hollow polypyrrole films | Electrochemical oxidative polymerization of pyrrole | ∼300 F g−1 at 3 A g−1 | No decrease in capacitance (1000) | — | — | 558 | |
| Hollow capsular polypyrrole nanofiber | Vapor-phase polymerization | 1 M H2SO4 | 203 F g−1 at 2 mV sec−1 | >90% (11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles) at 10 A g−1 000 cycles) at 10 A g−1 |
— | — | 559 |
| Nanotubular-polyaniline | Using natural tubular halloysite as hard template | 1 M H2SO4 | 654 F g−1 at 1 A g−1 | 120% (until 2000 cycles) and 87% (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) at 100 mV s−1 000) at 100 mV s−1 |
— | — | 560 |
| Polyaniline nanotube arrays | Electrochemical polymerization using ZnO nanorod arrays as sacrificial templates | 1 M H2SO4 | 675 F g−1 at 50 mV sec−1 | Decrease in ∼30% of Csp value (100) at 50 mV s−1 | — | — | 561 |
| Polyaniline nanotubes (inner dia: 80 nm, outer dia: 180 nm) | One-step polymerization and acrylic acid in aqueous solution | 1 mol L−1 H2SO4 | 436 F g−1 at 0.5 A g−1 | 89.2% (500 cycles) at 0.5 A g−1 | — | — | 563 |
| Crystalline tetragonal hollow PANI nanotubes | MO (self-sacrificial template) in acidic solutions to facilitate the growth of PANI nanotubes | 1 mol L−1 H2SO4 | ∼590 ± 36 1 F g−1 at 5 mV s−1 | Capacitance loss of 49.6% (1000 cycles) at 10 A g−1 | 14.56 Wh kg−1 at PD of 250 W kg−1 | — | 564 |
| PPy hollow nanoparticles | Surfactant-templated chemical oxidation polymerization | 1 M Na2SO4 | 326 F g−1 at A g−1 | 86% (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles) 000 cycles) |
— | — | 565 |
| Hollow polyaniline nanofiber | Chemical oxidation polymerization | 1 M H2SO4 | 290 F g−1 at 1 A g−1 | 83% (1000 cycles) at 3 A g−1 | — | — | 568 |
| MnO2/polyaniline hollow sphere | Interfacial synthesis | 0.5 M Na2SO4 | 262 F g−1 at 1.5 mA cm−1 | 93% (800 cycles) at 9 mA cm−1 | — | — | 570 |
| 3D-hollow balls of graphene-polyaniline | Self-assembly method | 1 M H2SO4 | 331 F g−1 at 1 A g−1 | 14% loss of capacitance (500 cycles) at 1 A g−1 | — | — | 571 |
| 3D-graphene–polyaniline hybrid hollow spheres | Layer-by-layer assembly | 1 M H2SO4 | 381 F g−1 at 4.0 A g−1 | 83% (1000 cycles) at 0.5A g−1 | — | — | 572 |
| Hollow polyaniline nanotubes supported on Ti3C2 MXene | By exfoliating Ti3C2 followed by in situ polymerization | 1 M H2SO4 | 596.6 F g−1 at 0.1 A g−1 | 94.7% (5000 cycles) at 1 A g−1 | 25.6 Wh kg−1 at PD of 153.2 W kg−1 | 1610 Wkg−1 at ED of 13.2 Wh kg−1 | 573 |
| RGO/polyaniline nanotubes | In situ reduction | 1 M-H2SO4 | Unirradiated: 448 F g−1 at 0.5 A g−1 | Unirradiated: 89% (1000) | Unirradiated: 30.52 Wh kg−1 | Unirradiated: 174.96 W kg−1 | 574 |
| RGO/polyaniline nanotubes | In situ reduction | 1 M-H2SO4 | Irradiated: 482 F g−1 at 0.5 A g−1 | Irradiated: 92% (1000 cycles) | Irradiated: 32.81 Wh kg−1 | Irradiated: 174.98 W kg−1 | 574 |
| Graphene–polypyrrole hollow sphere | Pickering emulsion polymerization | 1 M H2SO4 | 238 F g−1 at 1 A g−1 | 90.7% (1500 cycles) at 1 A g−1 | — | — | 576 |
| N-doped graphene (NG)/hollow PPy | Hollow PPy nanospheres prepared by using a sacrificial template and embedded in NG layers | 1 M HCl | 575 F g−1 at a current density of 1 A g−1 | 90.1 (500 cycles) at 1 A g−1. | 47.92 Wh kg−1 at 1 A g−1 | — | 577 |
| Polyaniline hollow nanospheres encaging RuO2 nanoparticles | Polymerizing aniline monomers on 3D-arrayed PS nanospheres | 1.0 M aqueous HClO4 | 1570 F g−1 at 10 mV s−1 | 77.6% (1000 cycles) at 10 mV s−1 | — | — | 578 |
| Polyaniline hollow fibers (PANI-HF) | In situ polymerization of aniline in the presence of electrospun PAN nanofibers | 1 M H2SO4 | 425 F g−1 at 20 mV s−1 | 72% (500 cycles) at 100 mV s−1 | — | — | 579 |
| PANI-hollow fiber decorated by RGO | Self-assembling of GO sheets on PANI-HF followed by electrochemical reduction of GO | 1 M H2SO4 | 449 F g−1 at 20 mV s−1 | 91% (500 cycles) at 100 mV s−1 | — | — | 579 |
| Graphene–polypyrrole nanotube | Multi steps | 2 M H2SO4 aqueous solution | 324 F g−1 at 1.5 A g−1 | 88% (200 cycles) at 1.5 A g−1 | — | — | 580 |
MOF-PPy tubes (mass ratio % of PPy![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28) 28) |
Dispersion and mixing method | 1 M Na2SO4 | 554.4 F g−1 at 0.5 A g−1 | 90.7% (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles) at 20 A g−1 000 cycles) at 20 A g−1 |
0.0113 mW h cm−2 with PD of 0.12 mW cm−2 | — | 581 |
| Hollow PPy nanospheres decorated on CNTs | Via in situ chem. oxid. emulsion interfacial polymerization | 1.0 M aqueous NaNO3 | 33.9 F g−1 at 20 mV s−1 | 71% (500 cycles) | — | — | 582 |
| Polyaniline nanowires wrapped on the polypyrrole nanotubes | Chemical synthesis method | 1 M H2SO4 | 765 F g−1 at a scan rate of 10 mV s–1 | 86.3% (1000 cycles) at 10 mV s−1 | — | — | 583 |
| Core–shell polyaniline-functionalized carbon quantum dots (CQDs) | Via adsorption of CQDs on PANI to be produced PANI | 1 M H2SO4 | 264.6 F g−1 at 2.5 A g−1 | High stability (5000 cycles) | — | — | 584 |
| PPy@PANI nanosphere | By dilute solution polymerization | H2SO4 | 510 F g−1 at 10 mV s−1 | 87.6% (1000 cycles) at 5 A g−1 | — | — | 585 |
| Polypyrrole@poly(1,5-diaminoanthraquinone) | Dispersion and sonication | 1.0 M H2SO4 | 533 F g−1 at 1 A g−1 | 107.4% (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles) at 100 mV s−1 . 000 cycles) at 100 mV s−1 . |
7.5 Wh kg−1 (at a PD of 96.1 W kg−1) | 1124.9 W kg−1 (at ED of 4.0 Wh kg−1) | 586 |
| Graphene-wrapped polyaniline hollow spheres | Solution-based coassembly process | 1 M H2SO4 | 614 F g−1 at 1 A g−1 | 90% (500 cycles) 1 A g−1 | — | — | 587 |
| Carbon layer-encapsulated polyaniline nanotubes | In situ polymerization + hydrothermal method | 1 M H2SO4 | 410.5 F g−1 at 1 A g−1 | 63% (2000 cycles) | 42.32 Wh kg−1 | 16.44 kW kg−1 | 588 |
| Polyaniline/nickel oxide core/shell | In situ polymerization in the presence of NiO nanoparticles | 1 M H2SO4 | 372 F g−1 at 20 mV s−1 | — | 50.2 Wh Kg−1 at 1 Ag−1 | 0.50 kW Kg−1 at 1 Ag−1 | 589 |
| Core–shell nanospherical polypyrrole/graphene oxide | In situ surface-initiated polymerization method | 1.0 M H2SO4 | 370 F g−1 at 0.5 A g−1 | 91.2% (4000 cycles) | — | — | 590 |
| MoS2@PANI (wt% of MoS2 in: 71.2% for sample react 24 h) | Chemical polymerization | 0.5 M H2SO4 | 669 F g−1 at 1 A g−1 | 91% (4000 cycles) at 10 A g−1 | 106 Wh kg −1 at a power density of 106 kW kg−1 | — | 592 |
| MoS2@Polyaniline (with 25 wt% MoS2) | One-pot hydrothermal method, energy density was 25.7 Wh kg | 1 M H2SO4 | 645 F g−1 at 0.5 A g−1 | 89% (2000 cycles) at 10 A g−1 | 25.7 Wh kg−1 at PD of 779.9 W kg−1 | — | 593 |
| Hollow MoS2/PANI core/shell microsphere | In situ oxidative polymerization | 1 M H2SO4 | 633 F g−1 at 0.5 A g−1 | 86.0% (1000 cycles) at 10 A g−1 | 31.7 W h kg−1 at 0.3 kW kg−1 | — | 594 |
| MoS2/polyaniline hollow microsphere | Template-assisted method | 1 M H2SO4 | 364 F g−1 at a scan rate of 5 mV s−1 | 84.3% (8000 cycles) at 10 A g−1 | 32 Wh kg−1 at PD of 320 W kg−1 | — | 595 |
| Mechanically exfoliated MoS2 sheet coupled with polyaniline | In situ chemical oxidative polymerization | KOH | 510.12 F g−1 at 1 A g−1 | ∼80% (2500 cycles) | — | — | 596 |
| PEDOT@MoS2 | Electrochemical co-deposition of EDOT and MoS2 | 1 M H 2SO4 | 2540 mF cm−2 at 1 mA cm−2 | 98.5% (5000 cycles) at 100 mA cm−2 | 937 Wh m−2 at PD of 6500 W m−2 | — | 597 |
V2O5@Polypyrrole (V2O5 Sol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pyrrole Pyrrole![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SDBS = 40 ml SDBS = 40 ml![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 ml 0.1 ml![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 mg) 20 mg) |
Sol–gel with in situ polymerization method | 1 M Na2SO4 | 307 F g−1 at 1 A g−1 | ∼60% (2000 cycles) at 3 A g−1 | 37 Wh Kg−1 at PD of 161 W kg−1 | — | 598 |
| 3D core–shell pistil-like MnCo2O4/polyaniline | Electrochemical deposition polymerization | 2 M KOH | 1098 F g−1 at 1 A g−1 | 83.2% (5000 cycles) at 10 A g−1 | — | — | 600 |
| Nickel ferrite/polypyrrole core–shell | In situ chemical oxidation containing sodium dodecyl sulfate | 0.1 N H2SO4 | 721.66 F g−1 at 1 A g−1 | No significant change (1–1000 cycles) | 51.95 Wh kg−1 | 6.18 kW kg−1 | 602 |
| Core/sheath-structured ultralong MnOx/polypyrrole nanowires | In situ polymerization | 1.0 M Na2SO4 | 1091.4 F g−1 at 1 A g−1 | 97.4% (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles) at 10 A g−1 000 cycles) at 10 A g−1 |
144 Wh kg−1 at PD of 1100 W kg−1 | — | 603 |
| CuS@Polyaniline microspheres | Chemical oxidative polymerization | Li2SO4 | 308.1 F g−1 at 0.5 A g−1 | 71.6% (1000 cycles) at 1 A g−1 | __— | — | 604 |
| Polyaniline/CNT core–shell | Chemical vapor deposition and electrochemical deposition | 1 M H2SO4 electrolyte | 823 F g−1 at 5.0 A g−1 | — | 22.9 Wh kg−1 at PD of 700.1 W kg−1 | — | 606 |
| Hierarchical NiCo2S4@Polyaniline grown on carbon fiber | Hydrothermal method and potentiostatic deposition | 6 M KOH | 1823 F g−1 at 2 mA cm−2 | 86.2% (5000 cycles) | 64.92 Wh kg−1 at PD of 276.23 W kg−1 | — | 607 |
| NiCo2O4@PANI nanotubes anchored on C | Electrodeposition method | — | 720.5 C g−1 at 1 A g−1 | 99.64% (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles) 000 cycles) |
— | — | 608 |
| Graphene/polyaniline hybrid hollow microspheres | Combination of layer-by layer assembly and in situ chemical oxidative polymerization | 1.0 M H2SO4 | 633 F g−1 at 10 mA cm−2 | 92% (1000 cycles) at at 80 mV s−1 | 382.97 Wh kg−1 at 10 mA cm−2 | — | 609 |
| PANI/NiCo-LDH core–shell composite | Electrochemical deposition method | 2.0 M KOH | 1845 F g−1 at 0.5 A g−1 | 82% (5000 cycles) at 0.5 A g−1 | 46.0 Wh kg−1 at PD of 351.6 W kg−1 | — | 610 |
| Graphene nanosheets coating with polyaniline | In situ polymerization | 6 M KOH | 261.4 F g−1 at 100 mA g−1 | Capacitance decreased to 161.2 F g−1 after 500 cycles at 0.1 A g−1 | — | — | 611 |
| Polyaniline-coated NiCo2S4 | Chemical oxidative polymerization | PVA-KOH gel | 1879 F g−1 at 1 A g−1 | 91.1% (2000 cycles) at 8 A g−1 | 54.06 Wh kg−1 at PD of 0.79 kW kg−1 | 27.1 Kw kg−1 at ED of 15.9 Wh kg−1 | 613 |
| Hierarchical core/shell Janus-type a-Fe2O3/PEDOT | Multistep process | PVA–H2SO4 Hydrogel | 252.8 F g−1 at 0.1 A g−1 | 92% (1000) at 0.6 A g−1 | 136.3 Wh kg−1 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 526 W kg−1 526 W kg−1 |
616 |
| Core/double shell PANI/chitosan/cobalt oxide | In situ chemical oxidation method | 1 M H2SO4 | 1253 F g−1 at 5 mV s−1 | 91% (5000) at of 1 A g−1 | 95.42 Wh kg−1 at 1 A g−1 | 1549 W kg−1 at 3 A g−1 | 617 |
| Polyaniline-Ag-MnO2 nanorod | Two-step process | 2 M KOH | 1028.6 F g−1 at 1 A g−1 | 93.7% (5000 cycles) at 10 A g−1 | 49.77 Wh kg−1 | 1599.75 W kg−1 | 618 |
| Polyaniline@CNTs–MnO2 | Hydrothermal and in situ oxidative polymerization of aniline | 0.1 M KOH | 143.26 C g−1 at 3 mV s−1 | 119% (3500 cycles) at 3.0 A g−1 | 27.17 Wh kg−1 at 0.3 A g−1 | 298.00 W kg−1 at 0.3 A g−1 | 619 |
| MWCNTs@MnO2@Polypyrrole | Multiple steps | — | 272.7 F g−1 | 60% after 300 cycles | — | — | 620 |
| ZnO NRs@CuS@PEDOT@MnO2 | Multiple steps | 1 M LiClO4 | 19.85 mF cm−2 at 5 mV s−1 | 18% loss of initial value (1500) at 100 mV s−1 | — | — | 622 |
| Hollow Bi2O3@Carbon fibre@PEDOT | Electrospinning technique using stabilization, pyrolyzation and polymerization | PVA-KOH g | 460 F g−1 at 1 A g−1 | 93.6% (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles) at 2 A g−1 000 cycles) at 2 A g−1 |
16.4 Wh kg−1 | 500.34 W kg−1 | 623 |
| Hierarchical graphene/polyaniline hollow microsphere | Multiple steps | 1 M H2SO4 | 446.19 F g−1 at 5 mV s−1 | 93.4% (1000 cycles) at 2 A g−1, 8.7% (5000 cycles) at 2 A g−1 | — | — | 626 |
| “Pizza-like” MoS2/polypyrrole/polyaniline architecture | Multistep process | 0.5 m H2SO4 | 1273 F−1 g at 0.5 A g−1 | ∼83% (3000 cycles) at 2 A g−1 | — | — | 627 |
6. Future scope and perspectives
Conducting polymers, in spite of promising cost-effectiveness and tunable properties, have certain limitations such as lower conductivity compared with metals, and poor mechanical strength, processability, solubility and environmental stability remain the key issues to be addressed in their high-demand applications.1,2 These challenges are very critical to future ongoing research in order to harness the full potential of conducting polymers in different applications. Furthermore, advancements through innovative synthesis methods including new materials compositions and material design are likely to play key roles in developing scalable and cost-effective hollow conducting polymers, their nanocomposites and core–shell materials with significantly enhanced performance in their applications in electrical/environmental remediation and energy storage devices. Recently, research is also emerging on the electrodeposition technique as a promising approach for developing effective electrode materials in supercapacitor applications.154,321–325 In this regard, the unique insight gained by focusing exclusively on hollow and core–shell morphologies in the present review article across the three distinct applications (EMI shielding, adsorption, supercapacitors) complements the existing literature.Electromagnetic interference creates electronic pollution that is very harmful to human health and the functioning of electronic devices and remains a critical challenge for researchers.9 The practical application of electromagnetic interference/microwave absorption materials is limited by complex synthetic procedures and expensive raw materials. In this regard, lightweight hollow ICPs and their binary and ternary composites and core–shell structured materials are reported in trapping/absorbing electromagnetic waves to enhance dielectric loss, multiple internal reflection, and scattering of EM waves.9 However, challenges require researchers to develop thermally and mechanically stable hollow-structured ICPs, their composites, and core–shell-based materials with superior electrical conductivity, dielectric/magnetic, and corrosion-resistance properties, for applicability to a wide range of frequencies and absorption bandwidth.54 The conducting blends with simultaneously enhanced mechanical properties could also be interesting for future applications in EMI shielding and microwave absorption.388
The removal of heavy metals and organic dyes in wastewater remains a critical challenge for researchers.84,88 In this regard, polyaniline and polypyrrole have attracted considerable attention as promising adsorbents in the separation of dyes and metal ions from wastewater. In view of this, hollow conducting polymers60–64 and core–shell materials66,67 hold great promise in the adsorptive removal of metal ions from wastewater due to their enhanced surface area, tunability, and potential for synergistic effects.88 Challenges exist in developing low-cost, high-performing adsorbents with significantly enhanced activity and long-term stability for the separation of the mixture of individual dyes, metal ions, dye/metal ions and other types of pollutants present in wastewater. Furthermore, regeneration of the spent adsorbents is still challenging and needs more attention in developing cost-effective regeneration methods for their reusability with high economic viability and environmental sustainability.631 Furthermore, safe disposal or reuse of spent/exhausted adsorbents also needs to be considered.
Hollow ICPs have emerged as a promising electrode material in supercapacitor applications. Their larger surface area and shorter ion diffusion path facilitate faster charging and discharging.72,73 However, the future scope of hollow conducting polymers as an electrode material is limited due to their poor structural stability, which results in lower cyclability and capacity retention of the assembled supercapacitor.632 This problem can be mitigated by fabricating nanocomposites by integrating different components, such as carbonaceous, metal oxides, metal chalcogenides, and MXene base materials through an inexpensive and facile synthesis approach. Attention is also focused on achieving enhanced mechanical stability of hollow ICPs to prevent degradation during repeated charging and discharging cycles. In addition, the fabrication of hollow copolymers of ICPs remains another area not well addressed so far.633 In addition, core–shell nanostructured electrode materials fabricated by the coating of ICPs on a conducting core have been very promising due to the short ion transport pathways and abundant active sites. Recently, the electrodeposition method has also received more attention, though there exist several challenges in real-world applications.
Future research also needs to be focused on the development of new cost-effective synthetic core–shell methods. This can be realized by utilizing naturally driven green and sustainable biomass,634 plant waste,635 marine bio-waste,636 marine plastic waste,637 biomass waste,638–642 cotton,643 fly ash of coal waste644 and agricultural by-products645 as a source of carbon in realizing the fabrication of conducting polymer-coated core–shell materials across the three distinct application fields.
7. Summary and conclusion
The hollow conducting polymers (such as hollow microspheres, nanotubes, etc.) have attracted great attention for their wide and multifaceted applications guided by their properties. In this regard polyaniline and, to some extent polypyrrole, have received much attention owing to good processability, excellent environmental stability, and unique properties, such as controllable internal structures, low density, high surface areas, permeability including surfaces and interfaces, properties controllable by oxidation and protonation states, and their ability to exist in a number of intrinsic redox states. In view of this, the present article has dealt with the general methods reported on the preparation of conducting polymers and fabrication of hollow (PANI < PPy, PTh and PEDOT) structures and their mechanism. This was followed by a review of the synthetic strategies for the fabrication of their hollow morphologies, such as nanotubes, nanocapsules, nano and microspheres etc. by utilizing hard templates (Al2O3, SiO2, polystyrene etc.), soft templates, sacrificial templates and template-free methods. The article further describes the formation of nanocomposites comprising hollow and core–shell structures of ICPs and their applications in the protection of the environment and harnessing of energy. The choice of hollow morphology of conducting polymers is considered a novel approach for the trapping of microwave radiation through enhanced internal reflection, and their remarkable performance has been reviewed. In addition, a review of hollow-structured conducting polymers has demonstrated their great structural advantages as efficient adsorbents in the removal of toxic metal ions and dyes present in waste water. The review finally concludes with future perspectives on hollow and core–shell ICPs for their application as microwave absorbers, adsorbents and electrode materials in the absorption of electromagnetic waves and removal of metals ions/dyes in wastewater. It is also anticipated that the present review will provide a significant inspiration to researchers working on hollow and core–shell ICP for their application several other fields.Conflicts of interest
There are no conflict of interests to declare.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Acknowledgements
The author, formerly a Professor in the Department of Chemistry at the Indian Institute of Technology (IIT) Kharagpur, remains very appreciative for making this effort possible and gratefully acknowledge. Author also thanks to his former research scholars Dr Ritwick Panigrahi, Dr. Kunal Manna, Dr Soumi Dutta and Dr Rakesh Manna for their valuable contributions in this field. Thanks are also extended to Professor Ashok Kumar Gupta, Dr Brahma Gupta, Mr Akash Rawat, Mr Adarsh Singh, Department of Civil Engneering, IIT Kharagpur, Dr. Tapas Kuila, Senior Principal Scientist and Mr Anjan Chakraorty, CSIR-Central Mechanical Engineering Research Institute, Durgapur, India for their assistance at various stages of preparing this article.References
- A. Varghese, K. R. P. Sunajadevi and D. Pinheiro, Energy Adv., 2025, 4, 743 RSC.
- S. K. Srivastava, Nanostructured Materials for Environmental Applications, ed. S. Balakumar, V. Keller and M. V. Shankar, Springer, 2021, pp. 167–215 Search PubMed.
- M. B. Hasan, M. M. Parvez, A. Y. Abir and M. F. Ahmad, Heliyon, 2015, 11, e42375 CrossRef.
- H. H. Al-Refai, A. A. Ganash and M. A. Hussein, Mater. Today Commun., 2021, 26, 101935 CrossRef.
- M. A. H. Bayan, F. A. Taromi, M. Lanzi and F. Pierini, Sci. Rep., 2021, 11, 21144 Search PubMed.
- H. Zhang, X. Wang, H. Ma and M. Xue, Adv. Energy Sustainable Res., 2021, 2, 2100088 CrossRef.
- E. Dhandapani, S. Thangarasu, S. Ramesh, K. Ramesh, R. Vasudevan and N. Duraisamy, J. Energy Storage, 2022, 52, 104937 CrossRef.
- X. Gao, Y. Bao, Z. Chen, J. Lu, T. Su, L. Zhang and J. Ouyang, Adv. Electron. Mater., 2023, 9, 2300082 CrossRef.
- S. K. Srivastava and K. Manna, J. Mater. Chem. A, 2022, 10, 7431–7496 RSC.
- C. Van Le and H. Yoon, Int. J. Mol. Sci., 2024, 25, 1564 CrossRef.
- H. Kazim, M. Sabri, A. AlOthman and M. Tawalbeh, Nano-Struct. Nano-Objects, 2024, 40, 101371 CrossRef.
- T. Nezakati, A. Seifalian, A. Tan and A. M. Seifalian, Chem. Rev., 2018, 118, 6766–6843 CrossRef.
- A. Puiggalí-Jou, L. J. del Valle and C. Alemán, J. Controlled Release, 2019, 309, 244–264 CrossRef.
- S. K. Srivastava, RSC Appl. Interfaces, 2024, 1, 340–429 RSC.
- J. Kumari, A. Singh, A. Rawat, S. K. Srivastava, A. K. Gupta and R.SC. Sustain, Submitted, 2025.
- M. S. Tamboli, A. A. Gupta, H. S. Avchat, S. S. Bhosale and D. S. Shrikant, Webology, 2020, 17, 761–771 Search PubMed.
- M. Ayad, G. El-Hefnawy and S. Zaghlol, Chem. Eng. J., 2013, 217, 460–465 CrossRef CAS.
- M. Aleahmad, H. G. Taleghani and H. Eisazadeh, Asian J. Chem., 2010, 22, 7353–7360 Search PubMed.
- Y. Gao, Z.-H. Kang, X. Li, X.-J. Cui and J. Gong, CrystEngComm, 2011, 13, 3370–3372 RSC.
- E. Saracino, S. Zuppolini, V. Guarino, V. Benfenati, A. Borriello, R. Zamboni and L. Ambrosio, RSC Adv., 2021, 11, 11347–11355 RSC.
- K. Huang, Y. Zhang, Y. Long, J. Yuan, D. Han, Z. Wang, L. Niu and Z. Chen, Chem. – Eur. J., 2006, 12, 5314–5319 CrossRef PubMed.
- M. M. Sk and C. Y. Yue, J. Mater. Chem. A, 2014, 2, 2830 RSC.
- S. Virji, J. X. Huang, R. B. Kaner and B. H. Weiller, Nano Lett., 2004, 4, 491–496 CrossRef.
- E. Song and J.-W. Choi, Nanomaterials, 2013, 3, 498–523 CrossRef PubMed.
- G. Li, H. Peng, Y. Wang, Y. Qin, Z. Cui and Z. Zhang, Macromol. Rapid Commun., 2004, 25, 1611–1614 Search PubMed.
- J. Jang, J. Bae and K. Lee, Polymer, 2005, 46, 3677–3684 CrossRef.
- X.-G. Li, A. Li and M.-R. Huang, Chem. – Eur. J., 2008, 14, 10309–10317 CrossRef PubMed.
- H. Wang and Y. Lu, Synth. Met., 2012, 162, 1369–1374 CrossRef.
- Q. Zhang, W. Li, X. Liu, J. Ma, Y. Gu, R. Liu and J. Luo, ACS Appl. Mater. Interfaces, 2024, 16, 1461–1473 CrossRef PubMed.
- D. Zhang, X. Fan, X. Hao and G. Dong, Ind. Eng. Chem. Res., 2019, 58, 1906–1913 CrossRef.
- H. Wang, Y. Liu, L. Kong, S. Yin, X. Shen and S. Premlatha, Colloids Surf., A, 2023, 665, 131234 Search PubMed.
- M. Shi, Y. Zhang, M. Bai and B. Li, Synth. Met., 2017, 233, 74–78 CrossRef.
- C. Yu, J. Zhai, X. Gao, M. Wan, L. Jiang, T. Li and Z. Li, J. Phys. Chem. B, 2004, 108, 4586–4589 CrossRef.
- B. Butoi, A. Groza, P. Dinca, A. Balan and V. Barna, Polymers, 2017, 9, 732 CrossRef PubMed.
- S. J. T. Rezaei, Y. Bide and M. R. Nabid, Synth. Met., 2011, 161, 1414–1419 CrossRef.
- G. M. Neelgund and A. Oki, Polym. Int., 2011, 60, 1291–1295 CrossRef PubMed.
- H.-W. Park, T. Kim, J. Huh, M. Kang, J. E. Lee and H. Yoon, ACS Nano, 2012, 6, 7624–7633 CrossRef PubMed.
- K. Namsheer, V. B. Reneesha and C. S. Rout, Synthesis and Morphology of Conducting Polymers, ACS Symposium Series, 2023, Vol. 1438, Ch. 2, pp. 9–27, DOI:10.1021/bk-2023-1438.ch002.
- L. Yu, X. Y. Yu and X. W. Lou, Adv. Mater., 2018, 30, 1800939 CrossRef PubMed.
- L. Yu, H. Hu, H. B. Wu and X. W. Lou, Adv. Mater., 2017, 29, 1604563 Search PubMed.
- X. Wang, J. Feng, Y. Bai, Q. Zhang and Y. Yin, Chem. Rev., 2016, 116, 10983–11060 Search PubMed.
- P. Liu and L. Zhang, Crit. Rev. Solid State Mater. Sci., 2009, 34, 75–87 Search PubMed.
- Y. Xue, S. Chen, J. Yu, B. R. Bunes, Z. Xue, J. Xu, B. Lu and L. Zang, J. Mater. Chem. C, 2020, 8, 10136–10159 Search PubMed.
- I. Hussain, S. Sahoo, M. S. Sayed, M. Ahmad, M. S. Javed, C. Lamiel, Y. Li, J.-J. Shim, X. Ma and K. Zhang, Coord. Chem. Rev., 2022, 458, 214429 CrossRef.
- X. W. Lou, L. A. Archer and Z. Yang, Adv. Mater., 2008, 20, 3987–4019 CrossRef.
- K. An and T. Hyeon, Nano Today, 2009, 4, 359–373 CrossRef.
- M. Zhu, Y. Cheng, Q. Luo, M. El-khateeb and Q. Zhang, Mater. Chem. Front., 2021, 5, 2552–2587 RSC.
- L. Yang, H. Xu, G. He and H. Chen, Dalton Trans., 2022, 51, 13559–13572 RSC.
- J. Qi, X. Lai, J. Wang, H. Tang, H. Ren, Y. Yang, Q. Jin, L. Zhang, R. Yu, G. Ma, Z. Su, H. X. Zhao and D. Wang, Chem. Soc. Rev., 2015, 44, 6749–6773 RSC.
- K. Zhang, K. Song and K. Clays, Nanophotonics, 2018, 7, 693–671 CrossRef.
- X. Zhu, J. Tang, H. Huang, T. Lin, B. Luo and L. Wang, Sci. Bull., 2020, 65, 496–512 CrossRef.
- A. Venkataraman, E. V. Amadi, Y. Chen and C. Papadopoulos, Nanoscale Res. Lett., 2019, 14, 220 CrossRef PubMed.
- G. Ma, Z. Wen, J. Jin, Y. Lu, X. Wu, M. Wu and C. Chen, J. Mater. Chem. A, 2014, 2, 10350–10354 RSC.
- C. Kuila, A. Maji, N. C. Murmu, T. Kuila and S. K. Srivastava, Carbon, 2023, 210, 118075 CrossRef.
- R. Manna, K. Ghosh and S. K. Srivastava, Langmuir, 2021, 37, 7430–7441 CrossRef PubMed.
- R. Manna and S. K. Srivastava, ACS Omega, 2021, 6, 9164–9175 Search PubMed.
- D. Xu, M. Zhang, C. Wang, Z. Shen, M. Wang, J. Zhang, Z. Han, L. Li, X. Xiong and P. Chen, Polymer, 2025, 317, 127900 CrossRef.
- K. Manna and S. K. Srivastava, J. Phys. Chem. C, 2018, 122, 19913–19920 CrossRef.
- S. Kumari, J. Dalal, A. Kumar, R. Pal, R. Chahal and A. Ohlan, RSC Adv., 2024, 14, 662–676 RSC.
- S. Senguttuvan, P. Senthilkumar, V. Janaki and S. Kamala-Kannan, Chemosphere, 2021, 267, 129201 CrossRef PubMed.
- E. N. Zare, A. Motahari and M. Sillanpää, Environ. Res., 2018, 162, 173–195 CrossRef.
- P. Rana, B. Kaur, K. Poonia, V. Soni, P. Singh, S. Thakur, C.-W. Huang, V.-H. Nguyen and P. Raizada, Inorg. Chem. Commun., 2025, 172, 11365 Search PubMed.
- A. Taghizadeh, M. Taghizadeh, M. Jouyandeh, M. K. Yazdi, P. Zarrintaj, M. R. Saeb, E. C. Lima and V. K. Gupta, J. Mol. Liq., 2020, 312, 113447 Search PubMed.
- S. Dutta, Fabrication od sustainable nano adsorbents in removal of toxic pollutants from contaminated water, Ph.D Thesis, Indian Institute of Technology, Kharagpur, 2022 Search PubMed.
- X. Y. Lai, J. E. Halpert and D. Wang, Energy Environ. Sci., 2012, 5, 5604–5618 RSC.
- C. F. Jones, L. Resina, F. C. Ferreira, P. Sanjuan-Alberte and T. Esteves, J. Phys. Chem. C, 2024, 128, 11083–11100 CrossRef.
- Y. Bhattacharjee and S. Bose, ACS Appl. Nano Mater., 2021, 4, 949–972 CrossRef.
- M. K. Goswami, A. Srivastava, R. K. Dohare, A. K. Tiwari and A. Srivastav, Environ. Sci. Pollut. Res., 2023, 30, 73031–73060 CrossRef.
- L. Wang, X. Li, X. Shi, M. Huang, X. Li, Q. Zeng and R. Che, Nanoscale, 2021, 13, 2136 RSC.
- L. Liu, S. Liu, L. Zhao, G. Su, X. Liu, H. Peng, J. Xue and A. Tang, J. Mol. Liq., 2020, 313, 113593 CrossRef.
- A. Muhammad, A.-U. H. Ali Shah, S. Bilal and G. Rahman, Materials, 2019, 12, 1764 CrossRef.
- C. Xia, W. Chen, X. Wang, M. N. Hedhili, N. Wei and H. N. Alshareef, Adv. Energy Mater., 2015, 1401805 Search PubMed.
- D. Khalafallah, D. E. E. Refaay, X. Gu, A. M. A. Henaish and Q. Zhang, Energy Storage Mater., 2025, 78, 104284 CrossRef.
- N. Maruthi, M. Faisal and N. Raghavendra, Synth. Met., 2021, 272, 116664 CrossRef.
- B. G. Soares, G. M. O. Barra and T. Indrusiak, J. Compos. Sci., 2021, 5, 173 CrossRef.
- M. Zahid, R. Anum, S. Siddique, H. M. F. Shakir and Z. A. Rehan, J. Thermoplast. Compos. Mater., 2021, 36, 1717–1761 Search PubMed.
- X. Su, Y. Liu, Z. Liao, Y. Bi, M. Ma, Y. Chen, Y. Ma, F. Wan and K. L. Chung, Synth. Met., 2022, 291, 117190 Search PubMed.
- N. Biçer, I. T. Çelik and I. A. Kariper, J. Ind. Text., 2022, 51, 36S–64S CrossRef.
- A. Kausar and I. Ahmad, J. Compos. Sci., 2023, 7, 240 Search PubMed.
- J. Wang, Q. Sun, J. Li, Y. Guo, W. Tian, Y. Liu, B. Wu, L. Deng, N. Mahmood and X. Jian, Mater. Today Phys., 2023, 31, 100981 CrossRef.
- H. Hajjaoui, A. Soufi, W. Boumya, M. Abdennouri and N. Barka, J. Compos. Sci., 2021, 5, 233 CrossRef.
- A. Samadi, M. Xie, J. Li, H. Shon, C. Zheng and S. Zhao, Chem. Eng. J., 2021, 418, 129425 Search PubMed.
- S. Jadoun, J. P. Fuentes, B. F. merUrbano and J. Yáñez, J. Environ. Chem. Eng., 2023, 11, 109226 Search PubMed.
- A. Samadi, Z. Wang, S. Wang, S. K. Nataraj, L. Kong and S. Zhao, Chem. Eng. J., 2023, 478, 147506 CrossRef.
- M. M. Talukder, M. M. R. Khan and M. K. Amin, S. Afr. J. Chem. Eng., 2023, 44, 276–282 Search PubMed.
- A.-N. Chowdhury, S. R. Jesmeen and M. M. Hossain, Polym. Adv. Technol., 2004, 15, 633–638 Search PubMed.
- A. Nasar and F. Mashkoor, Environ. Sci. Pollut. Res. Int., 2019, 26, 5333–5356 CrossRef.
- S. Dutta, B. Gupta, S. K. Srivastava and A. K. Gupta, Mater. Adv., 2021, 2, 4497–4531 RSC.
- R. Rehman, A. Raza, F. Yasmeen, A. Dar, Z. T. Al-thagaf and Z. Meraf, Adsorpt. Sci. Technol., 2022, 7047832, DOI:10.1155/2022/7047832.
- J. Stejskal, Polymers, 2022, 14, 4243 CrossRef PubMed.
- K. M. Z. Hasan, N. A. Khan, M. Yoon and S. H. Jhung, J. Ind. Eng., Chem., 2025, 152, 330–339 CrossRef.
- Y. Han and L. Dai, Macromol. Chem. Phys., 2019, 1800355, DOI:10.1002/macp.201800355.
- L. Fu, Q. Qu, R. Holze, V. V. Kondratiev and Y. Wu, J. Mater. Chem. A, 2019, 7, 14937–14970 RSC.
- M. I. Ul Hoque and R. Holze, Polymers, 2023, 15, 730 CrossRef PubMed.
- S. Ahmed, A. Ahmed, D. B. Basha, S. Hussain, I. Uddin and M. A. Gondal, Synth. Met., 2023, 295, 117326 CrossRef.
- M. G. Tadesse, A. S. Ahmmed and J. F. Lübben, J. Compos. Sci., 2024, 8, 53 CrossRef.
- V. J. V. Vinayak, K. Deshmukh, V. R. K. Murthy and S. K. K. Pasha, J. Energy Storage, 2024, 100, 113551 CrossRef.
- O. B. Okafor, A. P. I. Popoola, O. M. Popoola and S. O. Adeosun, Next Mater., 2025, 6, 100389 Search PubMed.
- E. N. Zare, P. Makvandi, B. Ashtari, F. Rossi, A. Motahari and G. Perale, J. Med. Chem., 2019, 63, 1–22 CrossRef PubMed.
- J. Cui, F.-F. Xing, H. Luo, J.-Q. Qin, Y. Li, Y. Zhong, F. Wei, J. Fu, C. Jing, J. Cheng, Z.-S. Wu and S. Liu, J. Energy Chem., 2021, 62, 145–152 Search PubMed.
- K. Z. Mousaabadi, A. A. Ensafi, R. Fazel-Zarandi and A. Vahabi, J. Iran. Chem. Soc., 2024, 21, 1769–1794 CrossRef.
- T.-H. Le, Y. Kim and H. Yoon, Polymers, 2017, 9, 150 CrossRef.
- A. G. MacDiarmid, R. J. Mammone, R. B. Kaner, S. J. Porter, R. Pethig, A. J. Heeger; and D. R. Rosseinsky, Philos. Trans. R. Soc., A, 1985, 314, 3–15 CrossRef.
- J. V. Yakhmi, V. Saxena and D. K. Aswal, Functional Materials Preparation, Processing and Applications, Elsevier, 2012, pp. 61–110, DOI:10.1016/B978-0-12-385142-0.00002-7.
- A. K. Bakhshi, Bull. Mater. Sci., 1995, 18, 469–495 CrossRef.
- P. C. Maity, M.Tech. Thesis, Indian Institute of Technology, Hyderabad, 2016. https://raiithold.iith.ac.in/2718/1/MS14METCH11003.pdf.
- C.-C. Hung and T.-C. Wen, J. Taiwan Inst. Chem. Eng., 2011, 42, 371–376 CrossRef.
- S. Qian, H. A. Lin, Q. Pan, S. Zhang, Y. Zhang, Z. Geng, Q. Wu, Y. He and B. Zhu, Bioact. Mater., 2023, 26, 24–51 Search PubMed.
- H. Tang, H. Cai, H. Zhao, Z. Liu, R. Tan and F. Huang, CCS Chem., 2023, 5, 2534–2544 CrossRef.
- G. G. Wallace, G. M. Spinks, L. A. P. Kane-Maguire and P. R. Teasdale, Conductive. Electroactive Polymers, 3rd edn, CRC Press, 2008 Search PubMed.
- S. Goswami, S. Nandy, E. Fortunato and R. Martins, J. Solid State Chem., 2023, 317, 123679 CrossRef.
- K. Namsheer and C. S. Rout, RSC Adv., 2021, 11, 5659–5697 Search PubMed.
- B. Qiu, J. Wang, Z. Li, X. Wang and X. Li, Polymers, 2020, 12, 310 Search PubMed.
- S. P. Armes and J. F. Miller, Synth. Met., 1988, 22, 385–393 CrossRef.
- J. Stejskal, A. Riede, D. Hlavatá, J. Prokeš, M. Helmstedt and P. Holler, Synth. Met., 1998, 96, 55–61 CrossRef.
- R. Manna, Functionalized Graphene Based Nanocomposites as Flexible Dielectric Materials and Microwave Absorber, Ph.D Thesis, Indian Institute of Technology, Kharagpur, 2021 Search PubMed.
- J. Yang, Y. Ding, G. Chen and C. Li, Eur. Polym. J, 2007, 43, 3337–3343 CrossRef.
- S. Budi, Yusmaniar, A. Juliana, U. Cahyana, A. Purwanto, A. Imaduddin and E. Handoko, IOP Conf. Ser.:J. Phys.: Conf. Ser., 2018, 983, 012162, DOI:10.1088/1742-6596/983/1/012162.
- A. H. Majeed, L. A. Mohammed, O. G. Hammoodi, S. Sehgal, M. A. Alheety, K. K. Saxena, S. A. Dadoosh, I. K. Mohammed, M. M. Jasim and N. U. Salmaan, Int. J. Polym. Sci., 2022, 9047554, DOI:10.1155/2022/9047554.
- J. Mahmood, N. Arsalani, S. Naghash-Hamed, Z. Hanif and K. E. Geckeler, Sci. Rep., 2024, 14, 11653 Search PubMed.
- J. C. Thieblemont, M. F. Planche, C. Petrescu, J. M. Bouvier and G. Bidan, Synth. Met., 1993, 59, 81–96 CrossRef.
- Y. Sood, K. Singh, H. Mudila, P. E. Lokhande, L. Singh, D. Kumar, A. Kumar, N. M. Mubarak and M. H. Dehghani, Heliyon, 2024, 10, e33643 CrossRef CAS.
- M. Omastová, J. Pionteck and M. Trchová, Synth. Met., 2003, 135–136, 437–438 CrossRef.
- Y. Kudoh, Synth. Met., 1996, 79, 17–22 CrossRef CAS.
- Y. Kudoh, K. Akami and Y. Matsuya, Synth. Met., 1998, 95, 191–196 CrossRef CAS.
- A. Yussuf, M. Al-Saleh, S. Al-Enezi and G. Abraham, Int. J. Polym. Sci., 2018, 4191747, DOI:10.1155/2018/4191747.
- Y. Tan and K. Ghandi, Synth. Met., 2013, 175, 183–191 CrossRef CAS.
- A. Kausaite-Minkstimiene, V. Mazeiko, A. Ramanaviciene and A. Ramanavicius, Colloids Surf., A, 2015, 483, 224–231 CrossRef CAS.
- V. Bocchi, L. Chierici, G. P. Gardini and R. Mondelli, Tetrahedron, 1970, 26, 4073–4082 CrossRef CAS.
- T. V. Vernitskaya and O. N. Efimov, Russ. Chem. Rev., 1997, 66, 443–457 CrossRef.
- A. F. Diaz and J. Bargon, Handbook of Conducting Polymers, Marcel Dekker, NewYork, 1986 Search PubMed.
- T. P. Kaloni, P. K. Giesbrecht, G. Schreckenbach and M. S. Freund, Chem. Mater., 2017, 29(24), 10248–10283 CrossRef CAS.
- D. Thanasamy, D. Jesuraj, S. K. K. Kannan and V. Avadhanam, Polymer, 2019, 175, 32–40 CrossRef CAS.
- M. Jaymand, M. Hatamzadeh and Y. Omidi, Prog. Polym. Sci., 2015, 47, 26–69 Search PubMed.
- S. Das, D. P. Chatterjee, R. Ghosh and A. K. Nandi, RSC Adv., 2015, 5, 20160 Search PubMed.
- S. Nie, Z. Li, Y. Yao and Y. Jin, Front. Chem., 2021, 9, 803509 CrossRef CAS PubMed.
- G. Heywang and F. Jonas, Adv. Mater., 1992, 4, 116–118, DOI:10.1002/adma.19920040213.
- Y. Jiang, T. Liu and Y. Zhou, Adv. Funct. Mater., 2020, 2006213 Search PubMed.
- R. Corradi and S. Armes, Synth. Met., 1997, 84, 453–454 CrossRef CAS.
- N. Paradee and A. Sirivat, Polym. Int., 2014, 63, 106–113 Search PubMed.
- S. G. Im, D. Kusters, W. Choi, S. H. Baxamusa, M. C. M. Van de Sanden and K. K. Gleason, ACS Nano, 2008, 2, 1959–1967 Search PubMed.
- D. Bhattacharyya, R. M. Howden, D. C. Borrelli and K. K. Gleason, J. Polym. Sci., Part B: Polym. Phys., 2012, 50, 1329–1351 CrossRef CAS.
- Y.-H. Ha, N. Nikolov, S. K. Pollack, J. Mastrangelo, B. D. Martin and R. Shashidhar, Adv. Funct. Mater., 2004, 15, 615–622 Search PubMed.
- M. A. Ali, H. Kim, C. Lee, H. Nam and J. Lee, Synth. Met., 2011, 161, 1347–1352 CrossRef CAS.
- J. M. Bone and J. L. Jenkins, J. Chem. Educ., 2023, 100(10), 4062–4071 CrossRef CAS PubMed.
- S. A. Kumar, S. Sahoo, G. K. Laxminarayana and C. S. Rout, Small, 2024, 20, 2402087 CrossRef CAS PubMed.
- J. Liao, S. Wu, Z. Yin, S. Huang, C. Ning, G. Tan and P. K. Chu, ACS Appl. Mater. Interfaces, 2014, 6, 10946–10951 Search PubMed.
- S. A. Campbell, Y. Li, S. Breakspear, F. C. Walsh and J. R. Smith, Trans. IMF, 2007, 85, 237–244 Search PubMed.
- X. Luo and X. T. Cui, Acta Biomater., 2011, 7, 441–446 Search PubMed.
- J. Tietje-Girault, C. Ponce de Leó and F. C. Walsh, Surf. Coat. Technol., 2007, 201, 6025–6034 CrossRef CAS.
- J. Li, L. Zhao and P. Liu, Langmuir, 2023, 39(40), 14297–14307 CrossRef CAS PubMed.
- K. K. Shiu, F.-Y. Song and K.-W. Lau, J. Electroanal. Chem., 1999, 476, 109–117 CrossRef CAS.
- S. S. Shah, M. A. Aziz, A.-R. Al-Betar and W. Mahfoz, Arabian J. Chem., 2022, 15, 104058 Search PubMed.
- J. E. Nady, A. Shokry, M. Khalil, S. Ebrahim, A. M. Elshaer and M. Anas, Sci. Rep., 2022, 3611, DOI:10.1038/s41598-022-07483-y.
- K. M. Cheung, D. Bloor and G. C. Stevens, J. Mater. Sci., 1990, 25, 3814–3837 Search PubMed.
- R. Menon and A. K. Mukherjee, Encycl. Nanosci. Nanotechnol., 2004, 8, 715–729 CAS.
- S. V. J. Siti, A. Ulianas and S. Aini, J. Phys.:Conf. Ser., 2021, 1788(1), 012004, DOI:10.1088/1742-6596/1788/1/012004.
- X. Gong, L. Dai, A. W. H. Mau and H. J. Griesser, J. Polym. Sci., Part A: Polym. Chem., 1998, 36, 633–643 CrossRef CAS.
- T. Yamamoto, J. Organomet. Chem., 2002, 653, 195–199 CrossRef CAS.
- M. Mueller, M. Fabretto, D. Evans, P. Hojati-Talemi, C. Gruber and P. Murphy, Polymer, 2012, 53, 2146–2151 CrossRef CAS.
- R. Brooke, P. Cottis, P. Talemi, M. Fabretto, P. Murphy and D. Evans, Prog. Mater. Sci., 2017, 86, 127–146 CrossRef CAS.
- S. P. Armes, Synth. Met., 1987, 20, 365–371 Search PubMed.
- Y. Cao, A. Andreatta, A. J. Heeger and P. Smith, Polymer, 1989, 30, 2305–2311 CrossRef CAS.
- Y. Li and J. Yang, J. Appl. Polym. Sci., 1997, 65, 2739–2744 CrossRef CAS.
- S. Sinha, S. Bhadra and D. Khastgir, J. Appl. Polym. Sci., 2009, 112, 3135–3140 CrossRef CAS.
- T. Yonehara, K. Komaba and H. Goto, Polymers, 2020, 12, 375 CrossRef CAS.
- C. A. Ferreira, S. Aeiyach, M. Delamar and P. C. Lacaze, J. Electroanal. Chem., 1990, 284, 351–369 CrossRef CAS.
- G. Ciric-Marjanovic, M. Trchova and J. Stejskal, J. Raman Spectrosc., 2008, 39, 1375–1387 Search PubMed.
- A. Kaur, B. Bajaj, A. Kaushik, A. Saini and D. Sud, Mater. Sci. Eng. B, 2022, 286, 116005 CrossRef CAS.
- S. Zhang, B. Cheng, Z. Jia, Z. Zhao, X. Jin, Z. Zhao and G. Wu, Adv. Compos. Hybrid Mater., 2022, 5, 1658–1698 Search PubMed.
- Y. Xie, D. Kocaefe, C. Chen and Y. Kocaefe, J. Nanomater., 2016, 2302595, DOI:10.1155/2016/2302595.
- Q. Zhang, W. Wang, J. Goebl and Y. Yin, Nano Today, 2009, 4, 494–507 CrossRef CAS.
- Y. Bao, C. Shi, T. Wang, X. Li and J. Ma, Microporous Mesoporous Mater., 2016, 227, 121–136 Search PubMed.
- A.-G. Schiopu, D. M. Iordache, M. Oproescu, L. M. Cursaru and A.-M. Iota, Crystals, 2024, 14, 899 Search PubMed.
- R. R. Poolakkandy and M. M. Menamparambath, Nanoscale Adv., 2020, 2, 5015–5045 RSC.
- Y. Boyjoo, M. Wang, V. K. Pareek, J. Liu and M. Jaroniec, Chem. Soc. Rev., 2016, 45, 6013–6047 RSC.
- T. He, D. Chen, X. Jiao, Y. Xu and Y. Gu, Langmuir, 2004, 20, 8404–8408 CrossRef CAS PubMed.
- T. Cheng, Y. Xiang, X. He, J. Pang, W. Zhu, L. Luo, Y. Cao and R. Pei, J. Mater. Chem. B, 2025, 13, 4739–4769 RSC.
- A. Bai, C.-C. Hu, Y.-F. Yang and C.-C. Lin, Electrochim. Acta, 2008, 53, 2258–2264 CrossRef CAS.
- C. Mijangos and J. Martin, Polymers, 2023, 15, 525 CrossRef CAS PubMed.
- S. Xiong, Q. Wang and H. Xia, Mater. Res. Bull., 2004, 1569–1580 CrossRef CAS.
- B. H. Kim, D. H. Park, J. Joo, S. G. Yu and S. H. Lee, Synth. Met., 2005, 150, 279–284 CrossRef CAS.
- L. Liu, C. Zhao, Y. Zhao, N. Jia, Q. Zhou, M. Yan and Z. Jiang, Eur. Polym. J., 2005, 41, 2117–2121 CrossRef CAS.
- F.-L. Cheng, M.-L. Zhang and H. Wang, Sensors, 2005, 5, 245–249 CrossRef CAS.
- N. Esman and J.-P. Lellouche, Polym. Chem., 2010, 1, 158–160 RSC.
- J. Jang and J. H. Oh, ChemComm, 2004, 882–883 RSC.
- S. Chuanyu and W. Yu, Optoelectron. Adv. Mater., Rapid Commun., 2012, 6, 1037–1040 Search PubMed.
- S. Das, A. Kumar and K. S. Narayan, Phys. Rev. Mater., 2022, 6, 025602 CrossRef CAS.
- R. Liu, S. I. Cho and S. B. Lee, Nanotechnology, 2008, 19, 215710 CrossRef PubMed.
- P. P. Pednekar, S. C. Godiyal, K. R. Jadhav and V. J. Kadam, Chapter 23 - Mesoporous silica nanoparticles: a promising multifunctional drug delivery system, Micro Nano Technol, ed. A. Ficai and A. M. B. T.-N. for C. T. Grumezescu, Elsevier, 2017, pp. 593–621 Search PubMed.
- G. D. Fu, J. P. Zhao, Y. M. Sun, E. T. Kang and K. G. Neoh, Macromolecules, 2007, 40, 2271–2275 CrossRef CAS.
- Y. Hao, L. Song and Y. Zhang, Prog. Org. Coat., 2022, 168, 106912 CrossRef CAS.
- Y. Li, Y. Chang, M. Jin, Y. Liu and G. Han, J. Appl. Polym. Sci., 2012, 126, 1316–1321 CrossRef CAS.
- W. Wang, L. Lu, T. Chen and M. Rao, J. Appl. Polym. Sci., 2012, 126, 974–979 CrossRef CAS.
- J. Ge, L.-H. Rong, X. Cheng, Y. Tang, D. J. Pochan, E. B. Caldona and R. C. Advincula, Macromolecules, 2025, 58, 3289–3297 CrossRef CAS.
- M. N. Gorsd, M. N. Blanco and L. R. Pizzio, Procedia Mater. Sci., 2012, 1, 432–438 CrossRef CAS.
- A. B. D. Nandiyanto, A. Suhendi, T. Ogi, R. Umemotoa and K. Okuyama, Chem. Eng. J., 2014, 256, 421–430 CrossRef CAS.
- Y.-C. Kuo, S.-S. Wang, K.-C. Chang, H. Chen and J. Thermoplast, Compos. Mater., 2013, 28, 1091–1109 Search PubMed.
- S.-J. Ding, C.-L. Zhang, M. Yang, X.-Z. Qu, Y.-F. Lu and Z.-Z. Yang, Polymer, 2006, 47, 8360–8366 CrossRef CAS.
- M.-Y. Bai, Y.-J. Cheng, S. A. Wickline and Y. Xia, Small, 2009, 5, 1747–1752 CrossRef CAS PubMed.
- Y. Yang, Y. Chu, F. Yang and Y. Zhang, Mater. Chem. Phys., 2005, 92, 164–171 CrossRef CAS.
- Z. Niu, Z. Yang, Z. Hu, Y. Lu and C. C. Han, Adv. Funct. Mater., 2003, 13, 949–954 CrossRef CAS.
- R. Panigrahi and S. K. Srivastava, Mater. Res. Bull., 2015, 64, 33–41 CrossRef CAS.
- R. Panigrahi and S. K. Srivastava, Sci. Rep., 2015, 5, 7638 CrossRef PubMed.
- M. Saraf, A. Deep and A. Sharma, Conference:EMSI-2014, University of Delhi. DOI:10.13140/2.1.4400.0009.
- C. Mangeney, S. Bousalem, C. Connan, M.-J. Vaulay, S. Bernard and M. M. Chehimi, Langmuir, 2006, 22, 10163–10169 CrossRef CAS PubMed.
- X. Shi, A. L. Briseno, R. J. Sanedrin and F. Zhou, Macromolecules, 2003, 36, 4093–4098 CrossRef CAS.
- C. Sun and D. Sheng, Chem. Lett., 2011, 40, 153–155 CrossRef CAS.
- B. H. Sung, Y. G. Ko and U. S. Choi, Colloids Surf., A, 2007, 292, 217–223 CrossRef CAS.
- X. Feng, C. Mao, G. Yang, W. Hou and J.-J. Zhu, Langmuir, 2006, 22, 4384–4389 CrossRef CAS PubMed.
- Y. Gao, F. Wang, J. Gong, Z. Su and L. Qu, J. Nanosci. Nanotechnol., 2008, 8, 5972–5976 CrossRef CAS PubMed.
- L. Zhang and P. Liu, Mater. Lett., 2010, 64, 1755–1757 CrossRef CAS.
- S. Zuo, W. Liu, C. Yao, X. Li, Y. Kong, X. Liu, H. Mao and Y. Li, Chem. Eng. J., 2013, 228, 1092–1097 CrossRef CAS.
- Y. Gao, X. Li, J. Gong, B. Fan, Z. Su and L. Qu, J. Phys. Chem. C, 2008, 112, 8215–8222 CrossRef CAS.
- C.-L. Zhu, S.-W. Chou, S.-F. He, W.-N. Liao and C.-C. Chen, Nanotechnology, 2007, 18, 275604 CrossRef.
- Z. Zhang, J. Deng, J. Sui, L. Yu, M. Wan and Y. Wei, Macromol. Chem. Phys., 2006, 207, 763–769 CrossRef CAS.
- Z. Zhang, J. Sui, L. Zhang, M. Wan, Y. Wei and L. Yu, Adv. Mater., 2005, 17, 2854–2857 CrossRef CAS.
- G. Li, Y. Li, Y. Li, H. Peng and K. Chen, Macromolecules, 2011, 44, 9319–9323 CrossRef CAS.
- W. Liu, A. L. Cholli, R. Nagarajan, J. Kumar, S. t Tripathy, F. F. Bruno and L. Samuelson, J. Am. Chem. Soc., 1999, 121, 11345–11355 CrossRef CAS.
- S.-R. Yun, G.-O. Kim, C. W. Lee, N.-J. Jo, Y. Kang and K.-S. Ryu, J. Nanomater., 2012, 894539, DOI:10.1155/2012/894539.
- A. Madani, B. Nessark, R. Brayner, H. Elaissari, M. Jouini, C. Mangeney and M. M. Chehimi, Polymer, 2010, 51, 2825–2835 CrossRef CAS.
- C. H. Chang, P. S. Son, J.-A. Yoon and S.-H. Choi, J. Nanomater., 2010, 168025, DOI:10.1155/2010/168025.
- S. Li, M. Zhou, Y. Zhang, X. Zhang and L. Ding, J. Macromol. Sci., Part A:Pure Appl. Chem., 2018, 55, 98–105 CrossRef CAS.
- K. Manna, Fabrication of Carbonaceous/Magnetic Nanomaterial filled Polymer Nanocomposites in Electromagnetic Interference Shielding Applications, Ph. D Thesis, Indian Institute of Technology Kharagpur, 2020 Search PubMed.
- J. Zhang, T. Qiu, S. Ren, H. Yuan, L. He and X. Li, Mater. Chem. Phys., 2012, 134, 1072–1078 CrossRef CAS.
- T.-L. Hsieh, P.-S. Hung, C.-J. Wang, Y.-S. Chou and P. W. Wu, SN Appl. Sci., 2019, 1, 319 Search PubMed.
- S. M. Marinakos, D. A. Shultz and D. L. Feldheim, Adv. Mater., 1999, 11, 34–37 CrossRef CAS.
- F. Yin, D. Wang, Z. Zhang, C. Zhang and Y. Zhang, Mater. Lett., 2017, 207, 225–229 CrossRef CAS.
- D. Su, J. Zhang, S. Dou and G. Wang, ChemComm, 2015, 51, 16092–16095 RSC.
- C. H. Chang, P. S. Son, J.-A. Yoon and S.-H. Choi, J. Nanomater., 2010, 168025, DOI:10.1155/2010/168025.
- L. Qu and G. Shi, J. Polym. Sci., Part A: Polym. Chem., 2004, 42, 3170–3177 CrossRef CAS.
- J. Huang, B. Quan, M. Liu, Z. Wei and L. Jiang, Macromol. Rapid Commun., 2008, 29, 1335–1340 CrossRef CAS.
- L. Piraux, V.-A. Antohe, E. Ferain and D. Lahem, RSC Adv., 2016, 6, 21808–21813 RSC.
- A. Kros, R. J. M. Nolte and N. A. J. M. Sommerdijk, Adv. Mater., 2002, 14, 1779–1782 CrossRef CAS.
- X. Lixin, L. Guangye, L. Jingfu and S. Mengtao, Kinet. Catal., 2011, 52, 716–722 CrossRef.
- D. P. Dubal, Z. Caban-Huertas, R. Holze and P. Gomez-Romero, Electrochim. Acta, 2016, 191, 346–354 CrossRef.
- X. Zhang and S. K. Manohar, J. Am. Chem. Soc., 2005, 127, 14156–14157 CrossRef PubMed.
- L. Benhaddad, M.-C. Bernard, C. Deslouis, L. Makhloufi, B. Messaoudi, A. Pailleret and H. Takenouti, Synth. Met., 2013, 175, 192–199 CrossRef.
- C. Wang, Z. Liu, Q. Wang, J. Guo, Q. Zhao and Y. Lu, J. Electroanal. Chem., 2021, 901, 115780 CrossRef.
- J. Zhao, Z. Li, J. Wang, Q. Li and X. Wang, J. Mater. Chem. A, 2015, 3, 15124–15132 RSC.
- J. Wu, Y. Liu and L. Bao, Chem. Lett., 2015, 44, 557–559 CrossRef.
- T. Yao, T. Cui, X. Fang, J. Yu, F. Cui and J. Wu, Chem. Eng. J., 2013, 225, 230–236 CrossRef.
- J. Rehmen, T. Pathirana, L. Garcia-Quintana, R. Kerr, P. C. Howlett, K. Zuber, C. Pozo-Gonzalo and D. R. Evans, ACS Appl. Nano Mater., 2020, 3, 3820–3828 CrossRef.
- S. Zhang, J. Ren, Y. Zhang, H. Peng, S. Chen, F. Yang and Y. Cao, Org. Electron., 2020, 77, 105497 CrossRef.
- S.-C. Luo, H.-H. Yu, A. C. A. Wan, Y. Han and J. Y. Ying, Small, 2008, 4, 2051–2058 CrossRef PubMed.
- X. Zhang, D. Xin, Z. Yu, J. Sun, Q. Li, X. He, Z. Liu and Z. Lei, J. Colloid Interface Sci., 2025, 677, 472–481 CrossRef PubMed.
- C.-W. Kung, Y.-H. Cheng, H.-W. Chen, R. Vittal and K.-C. Ho, J. Mater. Chem. A, 2013, 10693–10702 RSC.
- Y.-H. Cheng, C.-W. Kung, L.-Y. Chou, R. Vittal and K.-C. Ho, Sens. Actuators, B, 2014, 192, 762–768 CrossRef.
- M. Wan, Adv. Mater., 2008, 20, 2926–2932 CrossRef.
- F.-L. Li and H.-J. Zhang, Materials, 2017, 10, 995 CrossRef PubMed.
- L. Pan, H. Qiu, C. Dou, Y. Li, L. Pu, J. Xu and Y. Shi, Int. J. Mol. Sci., 2010, 11, 2636–2657 CrossRef PubMed.
- Z. Zhang, T. Sun, M. Shao and Y. Zhu, in Functional Nanomaterials: Synthesis, Properties, and Applications, ed. W.-Y. Wong and Q. Dong, Wiley, Ch. 8, 2022, pp. 303–336. DOI:10.1002/9783527828562.ch8.
- A. Ali, R. Jamal and T. Abdiryim, RSC Adv., 2021, 11, 33425–33430 RSC.
- Z. Zhang, Z. Wei and M. Wan, Macromolecules, 2002, 35, 5937–5942 CrossRef.
- E. N. Konyushenko, M. Trchová, J. Stejskal and I. Sapurina, Chem. Pap., 2010, 64, 56–64, DOI:10.2478/s11696-009-0101-z.
- J. Han, J. Dai, C. Zhou and R. Guo, Polym. Chem., 2013, 4, 313–321 RSC.
- G. H. Lim and H. J. Choi, J. Ind. Eng. Chem., 2017, 47, 51–55 CrossRef.
- L. Zhang, Y. Long, Z. Chen and M. Wan, Adv. Funct. Mater., 2004, 14, 693–698 CrossRef.
- Z. Zhang, M. Wan and Y. Wei, Adv. Funct. Mater., 2006, 16, 1100–1104 CrossRef.
- L. Zhang, H. Peng, Z. D. Zujovic, P. A. Kilmartin and J. Travas-Sejdic, Macromol. Chem. Phys., 2007, 208, 1210–1217 CrossRef.
- U. Rana, K. Chakrabarti and S. Malik, J. Mater. Chem., 2012, 22, 15665–15671 RSC.
- J. Huang and M. Wan, J. Polym. Sci., Part A: Polym. Chem., 1999, 37, 1277–1284 CrossRef.
- J. Wu, Y. Li and W. Feng, Synth. Met., 2007, 157, 1013–1018 CrossRef.
- X. Wu, Y. Wang, Y. Xiao, Y. Han, T. Li and Y. Ma, Synth. Met., 2022, 291, 117212 CrossRef.
- Y. Long, Z. Chen, N. Wang, Y. Ma, Z. Zhang, L. Zhang and M. Wan, Appl. Phys. Lett., 2003, 83, 1863–1865 CrossRef.
- L. Zhang, H. Peng, J. Sui, P. A. Kilmartin and J. Travas-Sejdic, Curr. Appl. Phys., 2008, 8, 312–315 CrossRef.
- W. Wu, D. Pan, Y. Li, G. Zhao, L. Jing and S. Chen, Electrochim. Acta, 2015, 152, 126–134 CrossRef.
- J. Mu, G. Ma, H. Peng, J. Li, K. Sun and Z. Lei, J. Power Sources, 2013, 242, 797–802 CrossRef.
- L. Zhang and M. Wan, Thin Solid Films, 2005, 477, 24–31 CrossRef.
- R. Panigrahi and S. K. Srivastava, RSC Adv., 2013, 3, 7808–7815 RSC.
- Z. Zhang, Z. Wei, L. Zhang and M. Wan, Acta Mater., 2005, 53, 1373–1379 CrossRef.
- Q. Sun and Y. Deng, Mater. Lett., 2008, 62, 1831–1834 CrossRef.
- H. Qiu and M. Wan, Macromolecules, 2001, 34, 675–677 CrossRef.
- H. Xia, D. Cheng, P. Lam and H. S. O. Chan, Nanotechnology, 2006, 17, 3957–3961 CrossRef.
- Y. Li, Z. Li and F. Zheng, Mater. Lett., 2015, 148, 34–36 CrossRef CAS.
- K. Huang, X.-H. Meng and M. Wan, J. Appl. Polym. Sci., 2006, 100, 3050–3054 CrossRef CAS.
- Y. Zhu, D. Hu, M. X. Wan, L. Jiang and Y. Wei, Adv. Mater., 2007, 19, 2092–2096 CrossRef CAS.
- L. Zhang and M. Wan, Adv. Funct. Mater., 2003, 13, 815–820 CrossRef CAS.
- W. Liang, S. Rhodes, J. Zheng, X. Wang and J. Fang, ACS Appl. Mater. Interfaces, 2018, 10, 37426–37433 CrossRef CAS PubMed.
- M. Liu, C. Luo, R. Huang, H. Peng, Y. Wang and J. Travas-Sejdic, Int. J. Polym. Mater. Polym. Biomater., 2014, 63, 602–608 CrossRef CAS.
- L. Zhang, H. Ping, J. Sui, C. Soellar, P. A. Kilmartin and J. Travas-Sejdic, J. Phys. Chem. C, 2009, 113, 9128–9134 CrossRef CAS.
- N. P. Tavandashti, M. Ghorbani and A. Shojaei, Polym. Int., 2015, 64, 88–95 CrossRef.
- H. Ding, J. Shen, M. Wan and Z. Chen, Macromol. Chem. Phys., 2008, 209, 864–871 Search PubMed.
- C. Zhou, J. Han, G. Song and R. Guo, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 3563–3572 Search PubMed.
- R. Yuan, H. Wang, T. Ji, L. Mu, L. Chen, Y. Zhu and J. Zhu, J. Mater. Chem. A, 2015, 3, 19299–19303 RSC.
- L. Ren, K. Li and X. Chen, Polym. Bull., 2009, 63, 15–21 Search PubMed.
- S. Pang, W. Chen, Z. Yang, Z. Liu, X. Fan and D. Fang, Polymers, 2017, 9, 510 Search PubMed.
- K. P. D. Orellana and M. E. Roberts, Meet. Abstr., 2014, MA2014-01, 266. https://iopscience.iop.org/issue/2151-2043/MA2014-01/2.
- S. Wang, Q. Lu, Y. K. Kwon and H. J. Choi, J. Mol. Liq., 2025, 437, 128554 CrossRef CAS.
- N.-R. Chiou, L. J. Lee and A. J. Epstein, Chem Mater., 2007, 19, 3589–3591 CrossRef CAS.
- Y.-S. Zhang, W.-H. Xu, W.-T. Yao and S.-H. Yu, J. Phys. Chem. C, 2009, 113, 8588–8594 Search PubMed.
- H. Zhang, Y. Li, X. Wang, J. Li and F. Wang, Polymer, 2011, 52, 4246–4252 CrossRef CAS.
- Z. Wei and M. Wan, Adv. Mater., 2002, 14, 1314–1317 Search PubMed.
- C. Gao, M. Ai, X. Li and Z. Xu, J. Polym. Sci., Part A: Polym. Chem., 2011, 49, 2173–2182 CrossRef CAS.
- Y. Zhu, G. Ren, M. Wan and L. Jiang, Macromol. Chem. Phys., 2009, 210, 2046–2051 CrossRef CAS.
- P. Liu, Y. Zhu, J. Torres, S. H. Lee and M. Yun, J. Polym. Sci., Part A: Polym. Chem., 2017, 55, 3973–3979 CrossRef CAS.
- B. Sim and H. J. Choi, RSC Adv., 2015, 5, 11905 RSC.
- A. Tajima, Y. Ogawa, K. Nakabayashi and M. Atobe, Proceedings of the Annual Meeting of the Japan Society of Sonochemistry, 2015, 24, Session ID P10, Pages 39–40, 2017. DOI:10.20577/pamjss.24.0_39.
- D. P. Bhattarai, A. P. Tiwari, B. Maharjan, B. Tumurbaatar, C. H. Park and C. S. Kim, J. Colloid Interface Sci., 2019, 534, 447–458 CrossRef CAS PubMed.
- X. Yang, Z. Zhu, T. Dai and Y. Lu, Macromol. Rapid Commun., 2005, 26, 1736–1740 CrossRef CAS.
- J. Wang, Y. Xu, F. Yan, J. Zhu and J. Wang, J. Power Sources, 2011, 196, 2373–2379 CrossRef CAS.
- Y. Xia, M. Wei and Y. Lu, Synth. Met., 2009, 159, 372–376 CrossRef CAS.
- Y. Bian, C. Yang, Q. Gu, X. Zhu, Y. Wang and X. Zhang, J. Polym. Sci., Part A:Polym. Chem., 2019, 57, 1550–1555 Search PubMed.
- X. Zhang, J.-S. Lee, G. S. Lee, D.-K. Cha, M. J. Kim, D. J. Yang and S. K. Manohar, Macromolecules, 2005, 39, 470–472 Search PubMed.
- Z. Guo, Y. Qiao, H. Liu, C. Ding, Y. Zhu, M. Wan and L. Jiang, J. Mater. Chem., 2012, 22, 17153–17158 RSC.
- A. Ali, R. Jamal, T. Abdiryim and X. Huang, J. Electroanal. Chem., 2017, 787, 110–117 CrossRef CAS.
- J. Lee, S.-B. Cho, K. C. Dimitrov, Y. Lee and D.-Y. Khang, Eur. Polym. J., 2024, 206, 112771 CrossRef CAS.
- X. Ni, X. Hu, S. Zhou, C. Sun, X. Bai and P. Chen, Polym. Adv. Technol., 2011, 22, 532–537 Search PubMed.
- J. Sui, L. Zhang, J. Travas-Sejdic and P. A. Kilmartin, Macromol. Symp., 2010, 290, 107–114 CrossRef CAS.
- M. Acosta, M. D. Santiago and J. A. Irvin, Materials, 2022, 15, 8820 CrossRef CAS PubMed.
- X. Karagiorgis, S. Sandhu, P. J. Skabara and R. Dahiya, Polym. Int., 2025 DOI:10.1002/pi.70026.
- Y.-E. Miao, W. Fan, D. Chen and T. Liu, ACS Appl. Mater. Interfaces, 2013, 5, 4423–4428 Search PubMed.
- Z.-Q. Feng, J. Wu, W. Cho, M. K. Leach, E. W. Franz, Y. I. Naim, Z.-Z. Gu, J. M. Corey and D. C. Martin, Polymers, 2013, 54, 702–708 CrossRef CAS PubMed.
- M. Zhu, J. Tang, W. Wei and S. Li, Mater. Chem. Front., 2020, 4, 1105–1149 Search PubMed.
- M.-Y. Bai and Y. Xia, Macromol. Rapid Commun., 2010, 31, 1863–1868 Search PubMed.
- C. Niu, B. Zou, Y. Wang, L. Chen, H. Zheng and S. Zhou, Chem. Commun., 2015, 51, 5009–5012 RSC.
- H. Gu, J. Huang, N. Li, H. Yang, G. Chen, C. Dong, C. Gong and H. Guan, J. Mater. Sci. Technol., 2023, 146, 145–153 CrossRef CAS.
- H. Lv, Q. Pan, Y. Song, X.-X. Liu and T. Liu, Nano-Micro Lett., 2020, 12, 118 CrossRef CAS PubMed.
- Z. Chen, X. Zhao, R. Lu, R. Hong and X. Yang, Synth. Met., 2023, 296, 117378 CrossRef CAS.
- N. Jabeen, Q. Xia, M. Yang and H. Xia, ACS Appl. Mater. Interfaces, 2016, 8, 6093–6100 CrossRef CAS.
- C. Wu, Z. Pei, M. Lv, D. Huang, Y. Wang and S. Yuan, Molecules, 2023, 28, 434 CrossRef CAS PubMed.
- A. Xu, Y. Yu, W. Li, Y. Zhang, S. Ye, Z. Zhao and Y. Qin, Electrochim. Acta, 2022, 435, 141378 CrossRef CAS.
- D. Liu, L. Zhou, Y. Liu, C. Xia, J. Ouyang and A. A. Adesina, J. Environ. Chem. Eng., 2025, 12, 113450 CrossRef.
- X. Hu, Y. Duan, Z. Hao, Z. Meng, B. Wang, Z. Kang, S. Liu and H. Tian, J. Alloys Compd., 2024, 1009, 176851 CrossRef CAS.
- X. Sun, Z. Hao, F. Zeng, J. Xu, H. Nan, Z. Meng, J. Yang, W. Shi, Y. Zeng, X. Hu and H. Tian, J. Colloid Interface Sci., 2022, 610, 601–609 CrossRef CAS PubMed.
- Z. Lv, Y. Tang, Z. Zhu, J. Wei, W. Li, H. Xia, Y. Jiang, Z. Liu, Y. Luo, X. Ge, Y. Zhang, R. Wang, W. Zhang, X. J. Loh and X. Chen, Adv. Mater., 2018, 13, 1805468 CrossRef PubMed.
- F. Fauzi, Y. Di, D. M. Morales and R. K. Bose, ACS Appl. Energy Mater., 2025, 8, 4656–4668 Search PubMed.
- P. Yin, D. Lan, C. Lu, Z. Jia, A. Feng, P. Liu, X. Shi, H. Guo, G. Wu and J. Wang, J. Mater. Sci. Technol., 2025, 204, 204–223 CrossRef CAS.
- Y. Li, G. Chen, Q. Li, G. Qiu and X. Liu, J. Alloys Compd., 2011, 509, 4104–4107 CrossRef CAS.
- C. Cui, Y. Du, T. Li, X. Zheng, X. Wang, X. Han and P. Xu, J. Phys. Chem. B, 2012, 116, 9523–9531 CrossRef CAS PubMed.
- L. Wang, J. Zhu, H. Yang, F. Wang, Y. Qin, T. Zhao and P. Zhang, J. Alloys Compd., 2015, 634, 232–238 CrossRef CAS.
- Z. Wan, H. Ma, S. Hou, Y. Wang, J. Ho and H. Ge, J. Mater. Sci.: Mater. Electron., 2021, 32, 10991–11003 CrossRef CAS.
- S. Chhetri and T. Kuila, RSC Appl. Polym., 2024, 2, 507–533 RSC.
- S. K. Srivastava and V. Mittal, Hybrid Nanomaterials: Developments in Energy, Environments and Polymer Nanocomposites, ed. S. K. Srivastava and V. Mittal, Scrivener-Wiley, 2017, pp. 241–320 Search PubMed.
- R. Che, J. Gu, J. Kong, W. Lu, Y. Huang, H. Lv, X. Liu, X. i. Qi, G. Wu and H. Wu, Cell Rep. Phys. Sci., 2025, 6, 102502 CrossRef.
- P. Saini, V. Choudhary, N. Vijayan and R. K. Kotnala, J. Phys. Chem. C, 2012, 116, 13403–13412 CrossRef CAS.
- Y. Yang, J. Zhang, W. Zou, S. Wu, F. Wu, A. Xie and Z. Wei, Macromol. Rapid Commun., 2018, 39, 1700591 Search PubMed.
- C. Xu, F. Wu, A. Xie, L. Duan, Z. Yang, Y. Xia, M. Sun and Z. Xiong, Ind. Eng. Chem. Res., 2020, 59, 7604–7610 CrossRef CAS.
- R. Guo, L. Wu, J. Shi, F. Wu and A. Xie, Synth. Met., 2022, 290, 117161 Search PubMed.
- M. Wan, J. Li and S. Li, Polym. Adv. Technol., 2001, 12, 651–657 CrossRef CAS.
- R. Moučka, M. Sedlačík, H. Kasparyan, J. Prokeš, M. Trchová, F. Hassouna and D. Kopecký, Int. J. Mol. Sci., 2020, 21, 8814 CrossRef PubMed.
- Y. Sun, G. Guo, B. Yang, M. He, Y. Tian, J. C. Cheng and Y. Liu, J. Mater. Res., 2012, 27, 457–462 CrossRef CAS.
- X. Yang, B. Fan, X. Wang, X. Tang, J. Wang, G. Tong, X. Wang and W. Tian, J. Environ. Chem. Eng., 2021, 9, 105672 CrossRef CAS.
- S. K. Singh, M. J. Akhtar and K. K. Kar, Ind. Eng. Chem. Res., 2020, 59, 9076–9084 CrossRef CAS.
- H. Wang, K. Zhang and Y. Zhu, J. Mater. Sci.: Mater. Electron., 2024, 35, 1063 CrossRef CAS.
- Q. Chu, W. Tao, H. Lin, M. Ma, S. Chen, Y. Shi, H. He and X. Wang, Ind. Crops Prod., 2023, 194, 116299 CrossRef CAS.
- C. Yang, H. Li, D. Xiong and Z. Cao, React. Funct. Polym., 2009, 69, 137–144 CrossRef CAS.
- J. Guo, Y. Sun, X. Li, S. Xi, M. M. Ibrahim, H. Qiu, G. A. M. Mersal, Z. M. El-Bahy, V. Murugadoss, W. Abdul, F. Zhou, J. Ren, Z. Guo and J. Zhu, Compos. Sci. Technol., 2024, 258, 110917 CrossRef CAS.
- A. Elhassan, X. Lv, I. Abdalla, J. Yu, Z. Li and B. Ding, Polymers, 2024, 16, 1160 CrossRef CAS PubMed.
- P. Tang, J. Du, M. Li, X. Zhao, H. Ren, X. Zhang, G. Wu and X. Wang, ACS Appl. Nano Mater., 2024, 7, 10325–10337 CrossRef CAS.
- D. Promlok, W. Wichaita, S. Phongtamrug, C. Kaewsaneha, P. Sreearunothai, T. Suteewong and P. Tangboriboonrat, Prog. Org. Coat., 2024, 186, 108002 CrossRef CAS.
- Y.-F. Zhu, Q.-Q. Ni, Y.-Q. Fu and T. Natsuki, J. Nanopart. Res., 2013, 15, 1988 CrossRef PubMed.
- J. Hou, L. Zhang, H. Qiu, W. Duan, X. Wang, X. Wan and X. Du, J. Mater. Sci.:Mater. Electron., 2017, 28, 9279–9288 CrossRef CAS.
- Y.-F. Zhu, L. Zhang, T. Natsuki, Y.-Q. Fu and Q.-Q. Ni, Synth. Met., 2012, 162, 337–343 CrossRef CAS.
- P. Tang, M. Li, J. Du, Y. Gong, X. Zhang, G. Wu and X. Wang, Appl. Surf. Sci., 2024, 649, 159183 CrossRef CAS.
- B. Zhang, Y. Du, P. Zhang, H. Zhao, L. Kang, X. Han and P. Xu, J. Appl. Polym. Sci., 2013, 130, 1909–1916 Search PubMed.
- W. Zhou, X. Hu, X. Bai, S. Zhou, C. Sun, J. Yan and P. Chen, ACS Appl. Mater. Interfaces, 2011, 3, 3839–3845 CrossRef CAS PubMed.
- Y. Sun, F. Xiao, X. Liu, C. Feng and C. Jin, RSC Adv., 2013, 3, 22554–22559 RSC.
- Z. Wu, D. Tan, K. Tian, W. Hu, J. Wang, M. Su and L. Li, J. Phys. Chem. C, 2017, 121, 15784–15792 CrossRef CAS.
- M. Qiao, X. Lei, Y. Ma, L. Tian, K. H. Su and Q. Zhang, Ind. Eng. Chem. Res., 2016, 55, 6263–6275 CrossRef CAS.
- M. Wang, Y. Lin, Y. Liu and H. Yang, J. Mater. Sci. Mater. Electron., 2019, 30, 14344–14354 CrossRef CAS.
- S. H. Hosseini, A. Moghimi and M. Moloudi, Mater. Sci. Semicond. Process., 2014, 24, 272–277 CrossRef CAS.
- C. Dong, D. Li, H. Wang, B. Cai, Y. Xin, H. Peng, Y. Zhao, N. Wang, Z. Cui and G. Wang, Carbon, 2023, 215, 118459 CrossRef CAS.
- S. Kumari, J. Dalal, A. Kumar and A. Ohlan, Adv. Eng. Mater. A, 2022, 24, 2200635 CrossRef CAS.
- J. Li, H. Ji, Y. Xu, J. Zhang and Y. Yan, J. Mater. Res. Technol., 2020, 9, 762–772 CrossRef.
- X. Li, L. Yu, W. Zhao, Y. Shi, L. Yu, Y. Dong, Y. Zhu, Y. Fu, X. Liu and F. Fu, Chem. Eng. J., 2020, 379, 122393 CrossRef.
- L. Gai, Y. Zhao, G. Song, Q. An, Z. Xiao, S. Zhai and Z. Li, Composites, Part A, 2020, 136, 105965 CrossRef.
- C. Tian, Y. Du, P. Xu, R. Qiang, Y. Wang, D. Ding, J. Xue, J. Ma, H. Zhao and X. Han, ACS Appl. Mater. Interfaces, 2015, 7, 20090–20099 CrossRef PubMed.
- Q. Shang, H. Feng, J. Liu, Q. Lian, Z. Feng, N. Chen, J. Qiu and H. Wu, J. Colloid Interface Sci., 2021, 584, 80–91 CrossRef PubMed.
- L. Yu, Y. Zhu and Y. Fu, Appl. Surf. Sci., 2018, 427, 451–457 CrossRef.
- Y.-Y. Wang, W.-J. Sun, H. Lin, P.-P. Gao, J.-F. Gao, K. Dai, D.-X. Yan and Z.-M. Li, Composites, Part B, 2020, 199, 108309 CrossRef.
- J. Liu, Z. Wang, S. Rehman and H. Bi, RSC Adv., 2017, 7, 53104–53110 RSC.
- A. Ohlan, K. Singh, A. Chandra and S. K. Dhawan, ACS Appl. Mater. Interfaces, 2010, 2, 927–933 Search PubMed.
- Y. Zhang, M. Qiu, Y. Yu, B. Y. Wen and L. Cheng, ACS Appl. Mater. Interfaces, 2016, 9, 809–818 CrossRef PubMed.
- P. Saini, V. Choudhary, B. P. Singh, R. B. Mathur and S. K. Dhawan, Mater. Chem. Phys., 2009, 113, 919–926 Search PubMed.
- X. Tian, F. Meng, F. Meng, X. Chen, Y. Guo, Y. Wang, W. Zhu and Z. Zhou, ACS Appl. Mater. Interfaces, 2017, 9, 15711–15718 Search PubMed.
- M. Zhang, X. Qian, Q. Zeng, Y. Zhang, H. Cao and R. Che, Carbon, 2021, 175, 499–508 Search PubMed.
- F. Liu, J. Sui, G. I. N. Waterhouse, W. Zhou, X. Jiang, Z. Zhang and L. Yu, J. Mater. Sci., 2022, 57, 7570–7586 Search PubMed.
- J. Shu, L. Wang, Y. Dai, A. Chen, X. Wang and Z. Deng, J. Alloys Compd., 2024, 1001, 175030 Search PubMed.
- L. Yang, M. Liu, G. Liang, X. Xiong, W. You, H. Cheng and R. Che, J. Mater. Chem. C, 2024, 12, 15501–15509 RSC.
- B. Zhang, J. Wang, J. Peng, J. Sun, X. Su, Y. Zou and Y. Zhou, J. Mater. Sci.: Mater. Electron., 2019, 30, 9785–9797 CrossRef.
- C. Ge, X. Zhang, J. Liu, F. Jin, J. Liu and H. Bi, Appl. Surf. Sci., 2016, 378, 49–56 CrossRef.
- K. Manna and S. K. Srivastava, ACS Sustainable Chem. Eng., 2017, 5, 10710–10721 CrossRef.
- K. Manna and S. K. Srivastava, Langmuir, 2020, 36, 4519–4531 Search PubMed.
- S. Ji, C. Li, Z. Zhang, X. Jiang and L. Yu, Synth. Met., 2018, 239, 59–65 Search PubMed.
- L. Meng, J. Li, X. Li, Z. Wang and W. Zhou, J. Alloys Compd., 2023, 966, 171528 Search PubMed.
- D. Han, N. Xiao, H. Hu, B. Liu, G. Song and H. Yan, RSC Adv., 2015, 5, 97944–97950 Search PubMed.
- R. Panigrahi, S. K. Srivastava and J. Pionteck, Rubber Chem. Technol., 2018, 91, 97–119 Search PubMed.
- H. Yang, A. Wang, X. Feng, H. Dong, T. Zhuang, J. Sui, S. Zhao and C. Sun, Polymers, 2023, 15, 1866 CrossRef PubMed.
- C. Li, Y. Zhang, S. Ji, X. Jiang, Z. Zhang and L. Yu, J. Mater. Sci., 2018, 53, 5270–5286 CrossRef.
- X. Liu, S. W. Or, C. M. Leung and S. L. Ho, J. Appl. Phys., 2014, 115, 17A507 CrossRef.
- Y. Wang, X. Wu, W. Zhang, C. Luo, J. Li, Q. Wang and Q. Wang, Mater. Chem. Phys., 2018, 209, 23–30 CrossRef.
- J. Ding, L. Wang, Y. Zhao, L. Xing, X. Yu, G. Chen, J. Zhang and R. Che, Small, 2019, 15, 1902885 CrossRef PubMed.
- J. Zhang and X. Wang, J. Mater. Sci.: Mater. Electron., 2018, 29, 1592–1599 CrossRef.
- M. Zhang, L. Zhao, W. Zhao, T. Wang, L. Yuan, Y. Guo, Y. Xie, T. Cheng, A. Meng and Z. Li, Nano Res., 2023, 16, 3558–3569 Search PubMed.
- Y. Wang, X. Wu, W. Zhang and S. Huang, Synth. Met., 2015, 210, 165–170 CrossRef.
- X. Liu, X. Zhao, J. Yan, Y. Huang, T. Li and P. Liu, Carbon, 2021, 178, 273–284 CrossRef.
- H. Wang, S. Feng, M. Sun, X. Li, C. Wang, Z. Lin, M. Ma, T. Li and Y. Ma, J. Colloid Interface Sci., 2024, 658, 889–902 CrossRef.
- A. P. Singh, M. Mishra, P. Sambyal, B. K. Gupta, B. P. Singh, A. Chandra and S. K. Dhawan, J. Mater. Chem. A, 2014, 2, 3581–3593 RSC.
- L. Wang, Y. Huang, C. Li, J. Chen and X. Sun, Synth. Met., 2014, 198, 300–307 Search PubMed.
- J. Zhao, J. Lin, J. Xiao and H. Fan, RSC Adv., 2015, 5, 19345–19352 RSC.
- D. Zhang, J. Cheng, X. Yang, B. Zhao and M. Cao, J. Mater. Sci., 2014, 49, 7221–7230 CrossRef.
- Z. He, Y. Fang, X. Wang and H. Pang, Synth. Met., 2011, 161, 420–425 Search PubMed.
- T. Liu, N. Liu, S. Zhai, S. Gao, Z. Xiao, Q. An and D. Yang, J. Alloys Compd., 2019, 779, 831–843 CrossRef.
- V. Shukla, J. Mater. Sci., 2020, 55, 2826–2835 CrossRef.
- T. K. Gupta, B. P. Singh, R. h B. Mathur and S. R. Dhakate, Nanoscale, 2014, 6, 842–851 RSC.
- P. B. Liu, Y. Huang and X. Sun, ACS Appl. Mater. Interfaces, 2013, 5, 12355–12360 CrossRef PubMed.
- S.-S. Afzali, S. H. Hekmatara, J. Seyed-Yazdi and S. M. B. M. Hosseini, Sci. Rep., 2022, 12, 9590 CrossRef PubMed.
- Y. Wang, X. Wu, W. Zhang, C. Luo, J. Li and Q. Wang, Synth. Met., 2017, 231, 7–14 CrossRef.
- P. Liu, Y. Huang, Y. Yang, J. Yan and X. Zhang, J. Alloys Compd., 2016, 662, 63–68 CrossRef.
- D. Lakherwal, International Journal of Environmental Research and Development, 2014, 4, 41–48 Search PubMed.
- N. Rama Jyothi, Heavy Metal Sources and Their Effects on Human Health, in Heavy Metals - Their Environmental Impacts and Mitigation, ed. M. K. Nazal and H. Zhao, IntechOpen, 2021, Ch. 2, DOI:10.5772/intechopen.95370.
- M. Chigondo, B. Nyamunda, M. Maposa and F. Chigondo, Water Sci. Technol., 2022, 85, 1600–1619 CrossRef PubMed.
- M. I. Khan, M. K. Almesfer, A. Elkhaleefa, I. Shigidi, M. Z. Shamim, I. H. Ali and M. Rehan, Polymers, 2021, 13, 3810 CrossRef.
- A. Olad and R. Nabavi, J. Hazard. Mater., 2007, 147, 845–851 CrossRef PubMed.
- H. Chang, Q. Meng, D. Liu, Y. Wu, Z. Yang, B. Sun, F. Liu and Y. Liu, J. Appl. Polym. Sci., 2022, 139, e52822 CrossRef.
- H. Wu, Q. Wang, G. T. Fei, S. H. Xu, X. Guo and L. D. Zhang, Nanoscale Res. Lett., 2018, 13, 401 CrossRef PubMed.
- X. Guo, G. T. Fei, H. Su and L. D. Zhang, J. Phys. Chem. C, 2011, 115, 1608–1613 CrossRef.
- Z. Zhao, Y. Yang, L. Xu, Z. Qiu, Z. Wang, Y. Luo and K. Du, J. Chem., 2022, 2041512, DOI:10.1155/2022/2041512.
- S. Li, X. Lu, X. Li, Y. Xue, C. Zhang, J. Lei and C. Wang, J. Colloid Interface Sci., 2012, 378, 30–35 CrossRef PubMed.
- M. S. Lashkenari, B. Davodi, M. Ghorbani and H. Eisazadeh, High Perform. Polym., 2012, 24, 345–355 CrossRef.
- J. Wang, K. Pan, E. P. Giannelis and B. Cao, RSC Adv., 2013, 3, 8978–8987 RSC.
- Y. Zhan, X. Wan, S. He and Y. He, J. Chem. Technol. Biotechnol., 2018, 93, 1432–1442 CrossRef.
- A. H. Bhat and T. N. Chisti, J. Chem. Eng., 2023, 11, 110664 Search PubMed.
- M. Bhaumik, S. Agarwal, V. K. Gupta and A. Maity, J. Colloid Interface Sci., 2016, 470, 257–267 CrossRef PubMed.
- B. Qiu, C. Xu, D. Sun, H. Yi, J. Guo, X. Zhang, H. Qu, M. Guerrero, X. Wang, N. Noel, Z. Luo, Z. Guo and S. Wei, ACS Sustainable Chem. Eng., 2014, 2, 2070–2080 CrossRef.
- N. Ballav, H. J. Choi, S. B. Mishra and A. Maity, Appl. Clay Sci., 2014, 102, 60–70 CrossRef.
- X. Han, L. Gai, H. Jiang, L. Zhao, H. Liu and W. Zhang, Synth. Met., 2013, 171, 1–6 CrossRef.
- W. Zhang, Y. Wang, Y. Fei, Y. Wang, Z. Zhang, M. Kou, Q. Feng, S. Wang and X. Du, Adv. Mater. Sci. Eng., 2021, 7068003, DOI:10.1155/2021/7068003.
- M. Bhaumik, A. Maity, V. V. Srinivasu and M. S. Onyango, J. Hazard. Mater., 2011, 190, 381–390 CrossRef.
- M. Chigondo, H. K. Paumo, M. Bhaumik, K. Pillay and A. Maity, J. Mol. Liq., 2019, 275, 778–791 CrossRef.
- A. E. Chávez-Guajardo, J. C. Medina-Llamas, L. Maqueira, C. A. S. Andrade, K. G. B. Alves and C. P. de Melo, Chem. Eng. J., 2015, 281, 826–836 CrossRef.
- A. M. Muliwa, T. Y. Leswifi, M. S. Onyango and A. Maity, Sep. Purif. Technol., 2016, 158, 250–258 Search PubMed.
- J. Zhang, H. Chen, Z. Chen, J. He, W. Shi, D. Liu, H. Chi, F. Cui and W. Wang, RSC Adv., 2016, 6, 59292–59298 RSC.
- T. Wen, Q. Fan, X. Tan, Y. Chen, C. Chen, A. Xu and X. Wang, Polym. Chem., 2016, 7, 785–794 RSC.
- K. V. Brungesh, B. M. Nagabhushana, M. N. K. Harish and R. H. Krishna, J. Environ. Anal. Toxicol., 2017, 7, 1000442 Search PubMed.
- L. Du, P. Gao, Y. Liu, T. Minami and C. Yu, Nanomaterials, 2020, 10, 686 CrossRef PubMed.
- S. Sahu, U. K. Sahu and R. K. Patel, J. Chem. Eng. Data, 2019, 64, 1294–1304 CrossRef.
- J. Wang, K. Pan, Q. He and B. Cao, J. Hazard. Mater., 2013, 244–245, 121–129 CrossRef PubMed.
- Y. Li, I. Peng, J. Guo and Z. Chen, Langmuir, 2020, 36, 11508–11516 CrossRef PubMed.
- F. Wang, Y. Zhang, Q. Fang, Z. Li, Y. Lai and H. Yang, Chemosphere, 2021, 263, 128109 CrossRef PubMed.
- Y. Chen, H. Xu, S. Wang and L. Kang, RSC Adv., 2014, 4, 17805–17811 Search PubMed.
- Y. Wu, H. Li, Z. Zhao, X. Yi, D. Deng, L. Zheng, X. Luo, Y. Cai, W. Luo and M. Zhang, J. Alloys Compd., 2021, 851, 156741 CrossRef.
- M. Bhaumik, V. K. Gupta and A. Maity, J. Environ. Chem. Eng., 2018, 6, 2514–2527 CrossRef.
- P. Karthikeyan, S. S. Elanchezhiyan, S. Meenakshi and C. M. Park, J. Hazard. Mater., 2021, 408, 124892 CrossRef PubMed.
- S. Li, X. Lu, Y. Xue, J. Lei, T. Zheng and C. Wang, PLoS One, 2012, 7, e43328 CrossRef PubMed.
- A. Hsini, A. Essekri, N. Aarab, M. Laabd, A. A. Addi, R. Lakhmiri and A. Albourine, Environ. Sci. Pollut. Res. Int., 2020, 27, 15245–15258 CrossRef PubMed.
- S. Dutta, S. K. Srivastava and A. K. Gupta, Mater. Adv., 2021, 2, 2431–2443 RSC.
- W. Fang, X. Jiang, H. Luo and J. Geng, Chemosphere, 2018, 197, 594–602 CrossRef PubMed.
- N. S. Alsaiari, A. Amari, K. M. Katubi, F. M. Alzahrani, F. B. Rebah and M. A. Tahoon, Processes, 2021, 9, 576 Search PubMed.
- H. D. da Rocha, E. S. Reis, G. P. Ratkovski, R. J. da Silva, F. D. S. Gorza, G. C. Pedro and C. P. de Melo, J. Taiwan Inst. Chem. Eng., 2020, 110, 8–20 Search PubMed.
- M. K. Debnath, M. A. Rahman, H. Minami, M. M. Rahman, M. A. Alam, M. K. Sharafat, M. K. Hossain and H. Ahmad, J. Appl. Polym. Sci., 2019, 136, 47524 Search PubMed.
- M. R. Samani and D. Toghraie, J. Environ. Health Sci. Eng., 2019, 17, 53–62 CrossRef.
- W. Yao, T. Ni, S. Chen, H. Li and Y. Lu, Compos. Sci. Technol., 2014, 99, 15–22 CrossRef.
- E. da S. Reis, F. D. S. Gorza, G. da C. Pedro, B. G. Maciel, R. J. da Silva, G. P. Ratkovski and C. P. de Melo, J. Environ. Chem. Eng., 2021, 9, 104893 CrossRef.
- J. Han, P. Fang, J. Dai and R. Guo, Langmuir, 2012, 28, 6468–6475 CrossRef PubMed.
- Y. Zhang, D.-B. Jiang, Y. Wang, T. C. Zhang, G. Xiang, Y.-X. Zhang and S. Yuan, Ind. Eng. Chem. Res., 2020, 59, 7554–7563 CrossRef.
- A. Mehdinia, R. Niroumand and A. Jabbari, Int. J. Environ. Sci. Technol., 2020, 17, 2721–2730 CrossRef.
- N. Wang, J. Feng, W. Yan, L. Zhang, Y. Liu and R. Mu, Front. Environ. Sci. Eng., 2022, 16, 105 CrossRef.
- Z. Vatani and H. Eisazadeh, Polym. Plast. Technol., 2013, 52, 1621–1625 CrossRef.
- J. Chen, R. Dong, S. Chen, D. Tang, X. Lou, C. Ye, T. Qiu and W. Yan, J. Cleaner Prod., 2022, 338, 130536 CrossRef.
- A. S. Rad, J. Water Wastewater, 2020, 31, 169–183 Search PubMed.
- P. Shakhsari, P. A. Azar, F. Z. Hargalani and M. H. Givianrad, IOSRJEN, 2021, 11, 06–15 Search PubMed.
- L. I. A. Ali, H. F. Alesary, H. K. Ismail, W. H. Hassan, A. A. Kareem and B. K. Nile, J. Water Process Eng., 2024, 64, 105589 CrossRef.
- M. Bhaumik, A. Maity and H. G. Brink, Chem. Eng. J., 2021, 417, 127910 CrossRef.
- F. Wang, L. Huang, J. Li, L. Lin, Z. Liu and Z. Dong, Polym. Chem., 2014, 5, 4332–4338 RSC.
- E. N. Zare, M. M. Lakouraj and A. Ramezani, New J. Chem., 2016, 40, 2521–2529 RSC.
- C. Cui, X. Sun, C. Zhou, Y. Liu, H. Xiong, Y. Li and J. Han, Colloids Surf., A, 2021, 616, 126336 CrossRef.
- M. Karegar and M. M. Khodaei, J. Appl. Polym. Sci., 2022, 139, 51489 CrossRef.
- G. Sarojini, S. Venkateshbabu and M. Rajasimman, Chemosphere, 2021, 278, 130400 CrossRef PubMed.
- Q. Huang, D. Hu, M. Chen, C. Bao and X. Jin, J. Mol. Liq., 2019, 285, 288–298 CrossRef.
- J. Chen, J. Zhu, N. Wang, J. Feng and W. Yan, Chem. Eng. J., 2019, 360, 1486–1497 CrossRef.
- P. Sun, W. Zhang, B. Zou, X. Wang, L. Zhou, Z. Ye and Q. Zhao, Appl. Clay Sci., 2021, 209, 106151 CrossRef.
- K. Molaei, H. Bagheri, A. A. Asgharinezhad, H. Ebrahimzadeh and M. Shamsipur, Talanta, 2017, 167, 607–616 CrossRef PubMed.
- H. Zhang, X. Ding, S. Wang, Y. Huang, X.-F. Zeng, S. Maganti, Q. Jiang, M. Huang, Z. Guo and D. Cao, Eng. Sci., 2022, 18, 320–328 Search PubMed.
- S. Dutta, K. Manna, S. K. Srivastava, A. K. Gupta and M. K. Yadav, Sci. Rep., 2020, 10, 4982 CrossRef.
- S. Dutta, A. K. Gupta, S. K. Srivastava and M. K. Yadav, Arsenic in the Environment: Bridging Science to Practice for Sustainable Development As2021, Proceedings of the 8th International Congress and Exhibition, 2021, The Netherlands, CRC Press.
- V. Q. Trung, N. T. H. Trang, T. M. Thi, K. Vorayuth, N. M. Nghia and M. A. Tuan, Mater. Trans., 2018, 59, 1095–1100 Search PubMed.
- A. Muhammad, A. u. H. A. Shah and S. Bilal, Appl. Sci., 2020, 10, 2882 CrossRef.
- M. Bhaumik, C. Noubactep, V. K. Gupta, R. I. McCrindle and A. Maity, Chem. Eng. J., 2015, 271, 135–146 Search PubMed.
- R. Chetia, S. Devi, N. Shukla, A. Hazarika, S. Bordoloi, B. Pokhrel, B. K. Saikia, A. Gogoi and S. Konwer, ACS Omega, 2024, 9, 37012–37024 CrossRef.
- M. S. Mansour, M. E. Ossman and H. A. Farag, Desalination, 2011, 272, 301–305 CrossRef.
- Y. Sadeghipour, F. Mojoudi and G. Behbudi, Adv. Appl. NanoBio-Technol., 2020, 1, 20–27 Search PubMed.
- A. Almasian, M. Giahi, G. C. Fard, S. A. Dehdast and L. Maleknia, Chem. Eng. J., 2018, 351, 1166–1178 CrossRef.
- M. Zheng, Y. Wei, J. Ren, B. Dai, W. Luo, M. Ma, T. Li and Y. Ma, Sep. Purif. Technol., 2021, 277, 119455 CrossRef.
- M. M. Youssif and M. Wojnicki, Materials, 2025, 18, 2083 CrossRef PubMed.
- J. Shokraiyan, M. Asadi, V. Jahed, T. Sahraeian and M. Rabbani, Desalin. Water Treat., 2021, 231, 244–253 CrossRef.
- H. Cui, Y. Qian, Q. Li, Q. Zhang and J. Zhai, Chem. Eng. J., 2012, 211–212, 216–223 CrossRef.
- J. Ren, C. Wang, H. Zhang, X. Liu, T. Yang, W. Zheng, T. Li and Y. Ma, Langmuir, 2023, 39, 10098–10111 CrossRef PubMed.
- W. Wang, Y. Lv, H. Liu and Z. Cao, Sep. Purif. Technol., 2024, 330, 125265 CrossRef.
- Y. Wu, H. Chang, J. Peng, Y. Liu, B. Sun, Z. Yang, S. Gao and F. Liu, Polym. Bull., 2023, 80, 3675–3688 Search PubMed.
- S. Chen, D. Xu, L. Chen, Y. Zhang, C. Hu, L. Zhang and J. Chen, ACS Appl. Polym. Mater., 2023, 5, 10303–10314 CrossRef.
- S. Majumdar, U. Saikia and D. Mahanta, J. Chem. Eng. Data, 2015, 60, 3382–3391 CrossRef CAS.
- M. H. Fekri, M. B. Keivani, M. R. Mehr and B. Akbari-adergani, J. Mazandaran Univ. Med. Sci., 2019, 29, 166–179 Search PubMed.
- P. L. Meena, J. K. Saini, A. K. Surela, K. Poswal and L. K. Chhachhia, Biomass Convers. Biorefin., 2024, 14, 1711–1730 CrossRef.
- K. Rachna, A. Agarwal and N. B. Singh, Environ. Nanotechnol. Monit. Manage., 2018, 9, 154–163 Search PubMed.
- S. Dhanavel, E. A. K. Nivethaa, K. Dhanapal, V. K. Gupta, V. Narayanan and A. Stephen, RSC Adv., 2016, 6, 28871–28886 RSC.
- V. M. Ovando-Medina, N. E. Dávila-Guzmán, N. V. Pérez-Aguilar, H. Martínez-Gutiérrez, I. D. Antonio-Carmona, S. Y. Martínez-Amador and A. Dector, Iran. Polym. J., 2018, 27, 171–181 CrossRef CAS.
- J. Ren, C. Wang, J. Ding, T. Li and Y. Ma, ACS Appl. Polym. Mater., 2022, 4, 9449–9462 CrossRef CAS.
- H. Xu, Y. Zhang, Y. Cheng, W. Tian, Z. Zhao and J. Tang, Adsorpt. Sci. Technol., 2019, 37, 217–235 CrossRef CAS.
- M. S. Shahriman, N. N. M. Zain, S. Mohamad, N. S. A. Manan, S. M. Yaman, S. Asmand and M. Raoov, RSC Adv., 2018, 8, 33180–33192 RSC.
- C. Wang, B. Liang, H. Gao, T. Yang, T. Li, Y. Ma, H. M. Abo-Dief, G. Roymahapatra, J. Zhang, K. M. Abualnaja, Z. M. El-Bahy and Z. Guo, Colloids Surf., A, 2024, 700, 134659 CrossRef CAS.
- M. A. Sayed, A. Mohamed, S. A. Ahmed, A. M. El-Sherbeeny, W. A. Zoubi and M. R. Abukhadra, ACS Omega, 2023, 8, 47210–47223 CrossRef PubMed.
- A. Varghese, S. Devi and K. R. D. Pinheiro, Mater. Today Commun., 2023, 35, 105739 CrossRef CAS.
- L. Benhaddad, N. Belhouchat, A. Gueddouri, M. L. Hammache and H. Saighi, Russ. J. Gen. Chem., 2023, 93, 2378–2392 CrossRef CAS.
- S. Mondal, U. Rana, P. Das and S. Malik, ACS Appl. Polym. Mater., 2019, 1, 1624–1633 Search PubMed.
- I. Toumi, H. Djelad, F. Chouli and A. Benyoucef, J. Inorg. Organomet. Polym. Mater., 2021, 32, 112–121 CrossRef.
- M. S. Tanweer, Z. Iqbal and M. Alam, Langmuir, 2022, 38, 8837–8853 CrossRef CAS PubMed.
- C. Ao, B. Zhang, L. Yuan, J. Li, D. Wu, R. Xu and B. Pan, J. Membr. Sci., 2024, 708, 123020 CrossRef CAS.
- X. P. Teng, M. Y. K. Bryan, P. V. Chai and J. Y. Law, Mater. Today Proc., 2021, 46, 1875–1881 CrossRef CAS.
- S. Singh, S. Perween and A. Ranjan, J. Environ. Chem. Eng., 2021, 9, 105149 CrossRef CAS.
- H. Ali and A. M. Ismail, J. Polym. Environ., 2023, 31, 976–998 Search PubMed.
- S. Dutta, S. K. Srivastava, B. Gupta and A. K. Gupta, ACS Appl. Mater. Interfaces, 2021, 13, 54324–54338 Search PubMed.
- I. Benchikh, S. Lahreche, Y. Benmimoun and A. Benyoucef, Res. Square, preprint, 2022, DOI:10.21203/rs.3.rs-1479466/v1.
- Y. Weng, Z. Jin, S. Xie and M. Zhang, Colloids Surf., A, 2024, 681, 132745 CrossRef CAS.
- S. Razzaq, M. Akhtar, S. Zulfiqar, S. Zafar, I. Shakir, P. O. Agboola, S. Haider and M. F. Warsi, J. Taibah Univ. Sci., 2021, 15, 50–62 CrossRef.
- I. Khan, K. Saeed, I. Zekker, B. Zhang, A. H. Hendi, A. Ahmad, S. Ahmad, N. Zada, H. Ahmad and L. A. Shah, et al. , Water, 2022, 14, 242 CrossRef CAS.
- M. M. Ayad and A. A. El-Nasr, J. Phys. Chem. C, 2010, 114, 14377–14383 CrossRef CAS.
- W. A. Amer, M. M. Omran, A. F. Rehab and M. M. Ayad, RSC Adv., 2018, 8, 22536–22545 Search PubMed.
- W. A. Amer, M. M. Omran and M. M. Ayad, Colloids Surf., A, 2019, 562, 203–212 CrossRef CAS.
- Y. Zhang, S. He, Y. Zhang, Y. Feng, Z. Pan and M. Zhang, Colloids Surf., A, 2022, 632, 127765 CrossRef CAS.
- M. M. Ayad, A. Abu El-Nasr and J. Stejskal, J. Ind. Eng. Chem., 2012, 18, 1964–1969 Search PubMed.
- N. Wang, J. Chen, J. Wang, J. Feng and W. Yan, Powder Technol., 2019, 347, 93–102 CrossRef CAS.
- E. A. El-Sharkaway, R. M. Kamel, I. M. El-Sherbiny, S. S. Gharib, E. A. El-Sharkaway, R. M. Kamel, I. M. El-Sherbiny and S. S. Gharib, Environ. Technol., 2019, 41, 2854–2862 CrossRef PubMed.
- M. M. Ayad, W. A. Amer, S. Zaghlol, I. M. Minisy, P. Bober and J. Stejskal, Chem. Pap., 2018, 72, 1605–1618 CrossRef CAS.
- Y. Wang, J. Appl. Organomet. Chem., 2025, 39, e7977 CrossRef CAS.
- C. Wang, B. Zhang, X. Sun, Y. Zhang, W. Li, T. Yang, Y. Ma, Z. Sun and T. Li, Sep. Purif. Technol., 2024, 329, 125140 CrossRef CAS.
- A. A. Nezhad, M. Alimoradi and M. Ramezani, Mater. Res. Express, 2018, 5, 025508 CrossRef.
- W. Zhang, L. Hao, Y. Wang, Q. Lin, C. Lu and Z. Xu, J. Environ. Chem. Eng., 2016, 4, 882–890 CrossRef CAS.
- L. Bai, Z. Li, Y. Zhang, T. Wang, R. Lu, W. Zhou, H. Gao and S. Zhang, Chem. Eng. J., 2015, 279, 757–766 CrossRef CAS.
- N. Mohanty, S. Behera and B. N. Patra, Ind. Eng. Chem. Res., 2025, 64, 2274–2282 CrossRef CAS.
- K. O. Iwuozor, J. O. Ighalo, E. C. Emenike, L. A. Ogunfowora and C. A. Igwegbe, Curr. Res. Green Sustainable Chem., 2021, 4, 100179 CrossRef CAS.
- S. Radoor, J. Karayil, A. Jayakumar, J. Parameswaranpillai and S. Siengchin, Colloids Surf., A, 2021, 611, 125852 CrossRef CAS.
- S. Yildirim, B. Isik and V. Ugraskan, Mater. Chem. Phys., 2023, 307, 128083 CrossRef CAS.
- S. Pete, R. A. Kattil and L. Thomas, Diam. Relat. Mater., 2021, 117, 108455 CrossRef CAS.
- Y. Xia, T. Li, J. Chen and C. Cai, Synth. Met., 2013, 175, 163–169 CrossRef CAS.
- A. R. Prasad and A. Joseph, RSC Adv., 2017, 7, 20960 RSC.
- M. Zhang, L. Chang and Z. Yu, Desalin. Water Treat., 2018, 110, 209–218 CrossRef CAS.
- W. Yao, C. Shen and Y. Lu, Compos. Sci. Technol., 2013, 87, 8–13 CrossRef CAS.
- M. Maruthapandi, L. Eswaran, J. H. T. Luong and A. Gedanken, Ultrason. Sonochem., 2020, 62, 104864 CrossRef CAS PubMed.
- N. Sadegh, H. Haddadi, F. Sadegh and A. Asfaram, Polyhedron, 2024, 248, 116671 CrossRef CAS.
- Y. Zeng, L. Zhao, W. Wu, G. Lu, F. Xu, Y. Tong, W. Liu and J. Du, J. Appl. Polym. Sci., 2013, 127, 2475–2482 CrossRef CAS.
- Z. Tang, M. Ai and J. Peng, J. Comput. Theor. Nanosci., 2017, 14, 1965–1969 CrossRef CAS.
- F. Huang, X. Tian, W. Wei, X. Xu, J. Li, Y. Guo and Z. Zhou, J. Cleaner Prod., 2022, 344, 131100 CrossRef CAS.
- M. A. Gabal, E. A. Al-Harthy, Y. M. Al Angari, M. Abdel Salam, A. Awad, A. A. Al-Juaid and A. Saeed, Catalysts, 2022, 12, 1624 CrossRef CAS.
- H. K. Alzahrani and D. F. Katowah, Nanocomposites, 2023, 9, 183–202 CrossRef CAS.
- S. A. Alqarni, Nanocomposites, 2022, 8, 47–63 CrossRef CAS.
- Z. Miri, S. Elhami, V. Zare-Shahabadi and H. J. Jahromi, Spectrochim. Acta, Part A, 2021, 262, 120130 CrossRef CAS PubMed.
- P. Roy and S. K. Srivastava, Nanomaterials for Electrochemical Energy Storage Devices, Scrivener Publishing, 2020 Search PubMed.
- S. Raj, S. K. Srivastava, P. Kar and P. Roy, RSC Adv., 2016, 6, 95760–95767 RSC.
- N. Swain, B. Saravanakumar, M. Kundu, L. Schmidt-Mende and A. Ramadoss, J. Mater. Chem. A, 2021, 9, 25286–25324 RSC.
- Y.-T. Tan, F. Ran, L.-R. Wang, L.-B. Kong and L. Kang, J. Appl. Polym. Sci., 2012, 127, 1544–1549 CrossRef.
- W. Yang, Z. Gao, N. Song, Y. Zhang, Y. Yang and J. Wang, J. Power Sources, 2014, 272, 915–921 Search PubMed.
- Z. Li, Y. Shen and Y. Li, J. Electrochem. Soc., 2018, 165, G75–G79 Search PubMed.
- Z. Li, J. Hu, Y. Li and J. Liu, ChemistrySelect, 2018, 3, 6737–6742 CrossRef CAS.
- Z. Wang, H. Qiang, C. Zhang, Z. Zhu, M. Chen, C. Chen and D. Zhang, J. Polym. Res., 2018, 25, 129 CrossRef.
- R. Hong, X. Zhao, R. Lu, M. You, X. Chen and X. Yang, Molecules, 2024, 29, 2331 CrossRef CAS PubMed.
- M. S. Kim, J. H. Moon, P. J. Yoo and J. H. Park, J. Electrochem. Soc., 2012, 159, A1052–A1056 CrossRef CAS.
- Z. Li, J. Cai, P. Cizek, H. Niu, Y. Du and T. Lin, J. Mater. Chem. A, 2015, 3, 16162–16167 RSC.
- P. Fan, S. Wang, H. Liu, L. Liao, G. Lv and L. Mei, Electrochim. Acta, 2020, 331, 135259 CrossRef CAS.
- Z.-L. Wang, R. Guo, G.-R. Li, H.-L. Lu, Z.-Q. Liu, F.-M. Xiao, M. Zhang and Y.-X. Tong, J. Mater. Chem., 2012, 22, 2401–2404 RSC.
- J. P. Jyothibasu and R.-H. Lee, J. Mater. Chem. A, 2020, 8, 3186–3202 RSC.
- H.-H. Huang, Y.-H. Wu, M.-Z. Wang and X.-W. Ge, Chin. J. Chem. Phys., 2018, 31, 827–832 CrossRef CAS.
- D. Xu, A. Chen, Q. Sheng, G. Hu, L. Chen, Y. Zhang, S. Chen and C. Hu, J. Power Sources, 2025, 638, 236627 CrossRef CAS.
- K.-J. Ahn, Y. Lee, H. Choi, M.-S. Kim, K. Im, S. Noh and H. Yoon, Sci. Rep., 2015, 5, 14097, DOI:10.1038/srep14097.
- S. Xiong, Y. Zhang, Y. Wang, B. Wu, J. Chu, X. Wang, R. Zhang, M. Gong, Z. Li and Z. Chen, High Perform. Polym., 2019, 32, 600–608 CrossRef.
- B. M. Hryniewicz and M. Vidotti, ACS Appl. Nano Mater., 2018, 1, 3913–3924 CrossRef CAS.
- Y. Wang, X. Wu, Y. Xiao, Y. Han, T. Li and Y. Ma, Synth. Met., 2023, 299, 117483 CrossRef CAS.
- X. Zhang, J. Zhao, T. Xia, Q. Li, C. Ao, Q. Wang, W. Zhang, C. Lu and Y. Deng, Energy Storage Mater., 2020, 31, 135–145 CrossRef.
- J.-G. Wang, Y. Yang, Z.-H. Huang and F. Kang, J. Power Sources, 2012, 204, 236–243 CrossRef CAS.
- N. B. Trung, T. V. Tam, H. R. Kim, S. H. Hur, E. J. Kim and W. M. Choi, Chem. Eng. J., 2014, 255, 89–96 CrossRef CAS.
- J. Luo, Q. Ma, H. Gu, Y. Zheng and X. Liu, Electrochim. Acta, 2015, 173, 184–192 CrossRef CAS.
- W. Wu, C. Wang, C. Zhao, D. Wei, J. Zhu and Y. Xu, J. Colloid Interface Sci., 2020, 580, 601–613 CrossRef CAS PubMed.
- M. Devi and A. Kumar, Polym. Compos., 2020, 41, 653–667 CrossRef CAS.
- Y. Yang, P. Zhu, L. Zhang, T. Li, Q. Wang, R. Sum and C. Wong, 19th International Conference on Electronic Packaging Technology (ICEPT), Shanghai, China, 2018, pp. 270–274, DOI:10.1109/ICEPT.2018.8480452.
- S. Wu, J. Tian, X. Yin and W. Wu, Nano, 2019, 14, 1950056 CrossRef CAS.
- Z. Wang, C. Zhang, C. Xu, Z. Zhu and C. Chen, Ionics, 2016, 23, 147–156 CrossRef.
- H. Kwon, D. Hong, I. Ryu and S. Yim, ACS Appl. Mater. Interfaces, 2017, 9, 7412–7423 CrossRef CAS PubMed.
- F. A. Tabar, F. Sharif and S. Mazinani, Polymer, 2018, 154, 80–89 CrossRef.
- J. Liu, J. An, Y. Ma, M. Li and R. Ma, J. Electrochem. Soc., 2012, 159, A828–A833 CrossRef.
- X. Xu, J. Tang, H. Qian, S. Hou, Y. Bando, Md. S. A. Hossain, L. Pan and Y. Yamauchi, ACS Appl. Mater. Interfaces, 2017, 9, 38737–38744 CrossRef PubMed.
- P. Liu, X. Wang and H. Li, Synth. Met., 2014, 189, 173–176 CrossRef.
- D. Tian, H. Cheng, Q. Li, C. Song, D. Wu, X. Zhao, S. Hu, S. Chen and C. Hu, Electrochim. Acta, 2021, 398, 139328 CrossRef.
- K. Kakaei, S. Khodadoost, M. Gholipour and N. Shouraei, J. Phys. Chem. Solids, 2021, 148, 109753 CrossRef.
- S. Chen, H. Cheng, D. Tian, Q. Li, M. Zhong, J. Chen, C. Hu and H. Ji, ACS Appl. Energy Mater., 2021, 4, 3701–3711 CrossRef.
- J. Li, X. Li, W. Wei, D. Wang and P. Liu, Electrochim. Acta, 2021, 395, 139193 CrossRef.
- W. Fan, C. Zhang, W. W. Tjiu, K. P. Pramoda, C. He and T. Liu, ACS Appl. Mater. Interfaces, 2013, 5, 3382–3391 CrossRef PubMed.
- P. Du, W. Wei, D. Liu, H. Kang and P. Liu, Chem. Eng. J., 2018, 335, 373–383 CrossRef.
- V. Mini and H. Devendrappa, Mater. Today Proc., 2018, 5, 23148–23155 CrossRef.
- W. Wu, L. Yang, S. Chen, Y. Shao, L. Jing, G. Zhao and H. Wei, RSC Adv., 2015, 5, 91645–91653 RSC.
- K. Qi, R. Hou, S. Zaman, B. Y. Xia and H. Duan, J. Mater. Chem. A, 2018, 6, 3913–3918 RSC.
- J. Zhu, W. Sun, D. Yang, Y. Zhang, H. H. Hoon, H. Zhang and Q. Yan, Small, 2015, 11, 4123–4129 CrossRef PubMed.
- Q. Chen, F. Xie, G. Wang, K. Ge, H. Ren, M. Yan, Q. Wang and H. Bi, Ionics, 2021, 27, 4083–4096 CrossRef.
- X. Zhang, L. Ma, M. Gan, G. Fu, M. Jin and Y. Zhai, Appl. Surf. Sci., 2018, 460, 48–57 CrossRef.
- S. Zhang, X. Song, S. Liu, F. Sun, G. Liu and Z. Tan, Electrochim. Acta, 2019, 312, 1–10 CrossRef.
- S. A. Ansari, H. Fouad, S. G. Ansari, M. P. Sk and M. H. Cho, J. Colloid Interface Sci., 2017, 504, 276–282 CrossRef PubMed.
- Y. Cai, L. Xu, H. Kang, W. Zhou, J. Xu, X. Duan, X. Lu and Q. Xu, Electrochim. Acta, 2021, 370, 137791 CrossRef.
- Y. Liang, Z. Wei, X. Zhang and R. Wang, ES Energy Environ., 2022, 18, 101–110 Search PubMed.
- D. Thanasamy, D. Jesuraj, V. Avadhanam, K. Chinnadurai, S. Kumar and K. Kannan, J. Energy Storage, 2022, 53, 105087 CrossRef.
- Z. Li, Y. Sui, J. Qi, F. Wei, Y. He, Q. Meng, Y. Ren, X. Zhang, Z. Zhan and Z. Sun, Compos. Interfaces, 2019, 27, 631–644 CrossRef.
- I. Chakraborty, N. Chakrabarty, A. Senapati and A. K. Chakraborty, J. Phys. Chem. C, 2018, 122, 27180–27190 CrossRef.
- S. S. Scindia, R. B. Kamble and J. A. Kher, AIP Adv., 2019, 9, 055218 CrossRef.
- J. Fu, Y. Zhang, H. Zhao, R. Jiang and R. Zhang, J. Colloid Interface Sci., 2020, 559, 39–44 CrossRef PubMed.
- C. Chen, Q. Zhang and C. Peng, Polym. Polym. Compos., 2017, 25, 483–488 Search PubMed.
- A. Imani and G. Farzi, J. Mater. Sci.: Mater. Electron., 2015, 26, 7438–7444 CrossRef.
- C. Xia, M. Leng, W. Tao, Q. Wang, Y. Gao and Q. Zhang, J. Mater. Sci.: Mater. Electron., 2019, 30, 4427–4436 CrossRef.
- X. Liu, Z. Wu and Y. Yin, Chem. Eng. J., 2017, 323, 330–339 CrossRef.
- C. Pan, Z. Liu, W. Li, Y. Zhuang, Q. Wang and S. Chen, J. Phys. Chem. C, 2019, 123, 25549–25558 CrossRef.
- B. Mu, W. Zhang and A. Wang, J. Nanopart. Res., 2014, 16, 2432 CrossRef.
- X. Ge, Y. He, T. Plachy, N. Kazantseva, P. Saha and Q. Cheng, Nanomaterials, 2019, 9, 527 CrossRef PubMed.
- X. Li, H. Song, Y. Zhang, H. Wang, K. Du, H. Li, Y. Yuan and J. Huang, Int. J. Electrochem. Sci., 2012, 7, 5163–5171 CrossRef.
- J. H. Shendkar, M. Zate, K. Tehare, V. V. Jadhav, R. S. Mane, M. Naushad, J. M. Yun and K. H. Kim, Mater. Chem. Phys., 2016, 180, 226–236 Search PubMed.
- X. He, Q. Liu, J. Liu, R. Li, H. Zhang, R. Chen and J. Wang, Chem. Eng. J., 2017, 325, 134–143 CrossRef.
- P. Li, E. Shi, Y. Yang, Y. Shang, Q. Peng, S. Wu, J. Wei, K. Wang, H. Zhu, Q. Yuan, A. Cao and D. Wu, Nano Res., 2014, 7, 209–218 Search PubMed.
- Y. Yesi, I. Shown, A. Ganguly, T. T. Ngo, L.-C. Chen and K.-H. Chen, ChemSusChem, 2016, 9, 370–378 CrossRef PubMed.
- J. W. Park, W. Na and J. Jang, J. Mater. Chem. A, 2016, 4, 8263–8271 Search PubMed.
- M. Vellakkat and D. Hundekkal, Mater. Res. Express, 2016, 3, 015502 CrossRef.
- M. B. Poudel, M. Shin and H. J. Kim, Int. J. Hydrogen Energy, 2021, 46, 474–485 CrossRef.
- J. Iqbal, M. O. Ansari, A. Numan, S. Wageh, A. Al-Ghamdi, M. G. Alam, P. Kumar, R. Jafer, S. Bashir and A. H. Rajpar, Polymers, 2020, 12, 2918 CrossRef PubMed.
- A. H. P. de Oliveira, M. L. F. Nascimento and H. P. de Oliveira, Mater. Res., 2016, 19, 1080–1087 CrossRef.
- H.-Y. Ho, H.-I. Chu, Y.-J. Huang, D.-S. Tsai and C.-P. Lee, Nanotechnology, 2023, 34, 125401 Search PubMed.
- J. Rodríguez-Moreno, E. Navarrete-Astorga, E. A. Dalchiele, R. Schrebler, J. R. Ramos-Barrado and F. Martín, Chem. Commun., 2014, 50, 5652–5655 Search PubMed.
- Y. Huang, J. Wang, X. Ju, S. Zhang and X. Sun, J. Energy Storage, 2023, 72, 108460 CrossRef.
- R. Li, P. Song, Z. Ji, H. Zhou, Y. Xue, L. Kong and X. Shen, Appl. Surf. Sci., 2024, 649, 159188 CrossRef.
- K. Ghosh, C. Y. Yue, M. M. Sk and R. K. Jena, ACS Appl. Mater. Interfaces, 2017, 9, 15350–15363 CrossRef PubMed.
- L. Liu, Y. Wang, Q. Meng and B. Cao, J. Mater. Sci., 2017, 52, 7969–7983 CrossRef.
- K. Wang, L. Li, Y. Liu, C. Zhang and T. Liu, Adv. Mater. Interfaces, 2016, 3, 1600665 CrossRef.
- Y. Liu, X. Wang, T. Abdiryim, R. Jamal, A. Abdurexit, F. Xu, N. Fan, K. Song and H. Yang, J. Energy Storage, 2024, 94, 112530 CrossRef.
- M. Wang, Y. Lin, Y. Liu and H. Yang, J. Mater. Sci. Mater. Electron., 2019, 30, 14344–14354 CrossRef CAS.
- N. Boutaleb, G. M. Al-Senani, S. D. Al-Qahtani, A. Benyoucef and B. D. Alkoudsi, Colloids Surf., A, 2025, 718, 136867 CrossRef CAS.
- A. V. Baskar, N. Bolan, S. A. Hoang, P. Sooriyakumar, M. Kumar, L. Singh, T. Jasemizad, L. P. Padhye, G. Singh, A. Vinu, B. Sarkar, M. B. Kirkham, J. Rinklebe, S. Wang, H. Wang, R. Balasubramanian and K. H. M. Siddique, Total Environ., 2022, 822, 153555 CrossRef CAS PubMed.
- O. B. Okafor, A. P. I. Popoola, O. M. Popoola, U. O. Uyor and V. E. Ogbonna, Polym. Bull., 2024, 81, 189–246 Search PubMed.
- N. Singh and U. Riaz, Polym. Bull., 2022, 79, 10377–10408 CrossRef CAS.
- S. Rawat, R. K. Mishra and T. Bhaskzar, Chemisphere, 2022, 2, 131961 CrossRef PubMed.
- G. Jiang, R. A. Senthil, Y. Sun, T. R. Kumar and J. Pan, J. Power Sources, 2022, 520, 230886 CrossRef CAS.
- A. T. S. C. Brandão, S. State, R. Costa, P. Potorac, J. A. Vazquez, J. Valcarcel, A. F. Silva, L. Anicai, M. Enachescu and C. M. Pereira, ACS Omega, 2023, 8, 18782–18798 CrossRef PubMed.
- D. Tashima, T. Kashio, T. Eguchi, S. Kumagai, T. Tsubota and J. D. W. Madden, Mater. Lett., 2023, 18, 100193 CAS.
- P. Manasa, S. Sambasivam and F. Ran, J. Energy Storage, 2022, 54, 105290 CrossRef.
- K. Mensah-Darkwa, C. Zequine, P. K. Kahol and R. K. Gupta, Sustainability, 2019, 11, 414 CrossRef CAS.
- X. Zhu, Y. Zeng, X. Zhao, D. Liu, W. Lei and S. Lu, EcoEnergy, 2025, 3, e70000, DOI:10.1002/ece2.70000.
- B. R. Holla, M. Kanthraj, Vaishali, Namitha and K. A. Vishnumurthy, J. Ind. Chem. Soc., 2025, 102, 101948 CrossRef CAS.
- P. H. Patil and S. A. Jadhav, RSC Appl. Interfaces, 2024, 1, 624 RSC.
- N. V. Challagulla, M. Vijayakumar, D. S. Rohita, G. Elsa, A. B. Sankar, T. N. A. Rao and M. Karthik, Energy Technol., 2020, 8, 2000417 CrossRef CAS.
- S. N. Karri, S. P. Ega, V. Perupogu and P. Srinivasan, ChemistrySelect, 2021, 6, 2576–2589 CrossRef CAS.
- S. Rajput, V. Tyagi, Sonika, R. Nayak and S. K. Verma, Energy Technol., 2025, 13, 2401977 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2026 |

