Open Access Article
Open Access ArticleFunctionalized nanocellulose for efficient removal of ammonium impurities from contaminated water
Priyanka Sharma *a,
Eyad Mohammed A. Alfarsia,
Stanley C. Hicksa,
Miles Anthony Millera,
Saikumar Muddapua,
Abbygayle Razalind Ruggieroa,
William Borgesb,
Julian J. Koebbec,
Michael Kahwajic,
Anindya K. Swarnakard and
James Springsteada
*a,
Eyad Mohammed A. Alfarsia,
Stanley C. Hicksa,
Miles Anthony Millera,
Saikumar Muddapua,
Abbygayle Razalind Ruggieroa,
William Borgesb,
Julian J. Koebbec,
Michael Kahwajic,
Anindya K. Swarnakard and
James Springsteada
aDepartment of Chemical and Paper Engineering, Western Michigan University, Kalamazoo, Michigan 49008, USA. E-mail: priyanka.sharma@wmich.edu; Tel: +1-6315423506
bYale University, New Haven, CT 06520, USA
cRichmond Institute of Design and Innovation at Western Michigan University, Kalamazoo, Michigan 49008, USA
dDepartment of Chemistry, University of Alberta, Alberta, Canada T6G 2G2
First published on 16th January 2026
Abstract
Carboxycellulose nanofibers with carboxylate functionality are a promising material for a multitude of applications, including environmental remediation. In this paper, we present an in-depth systematic study on the application of TEMPO-oxidized CNFs for ammonium removal, demonstrating their versatility against a broad range of ammonium impurities (5–5000 ppm) using both simulated and real contaminated water. The efficiency of CNFs was tested under various conditions, including the amount of CNFs, pH, and time. The results showed that CNFs follow both the Langmuir and the Freundlich isotherm models with good fitting, indicating their monolayer as well as multilayer adsorption mechanism. Furthermore, characterization using FTIR, SEM/EDS, and adsorption and kinetic analyses employing various models reveals that chemisorption is the dominant mechanism of ammonium adsorption by CNFs. The Langmuir isotherm model, when fitted to adsorption data gathered for original ammonium impurities in the range of 5–5000 ppm using CNFs, yielded a maximum adsorption capacity of 303.3 mg g−1. Additionally, this study includes a technical application to remove ammonium impurities from real contaminated fish water, with results indicating an average removal efficiency of between 80% and 90%. We have also developed an in-house flotation remediation system (FRS) to address handling and collection issues related to CNFs and used CNFs. Hence, this study presents a comprehensive and sustainable approach to addressing the most significant ammonium nutrient problem, utilizing the most abundant, scalable, and eco-friendly plant-based material.
Introduction
Several factors, including the ever-increasing human population, expansive industrial activities, and the subsequent rapid development of industries, have been attributed to an increase in the use of anthropogenic ammonia (NH3) and an increase in ammonium ion (NH4+) deposition in aquatic environments. A considerable amount of NH4+ in the aquatic environment originates from human excreta, which contains nitrogenous compounds metabolized by bacteria into NH4+. Additionally, agricultural runoff, particularly from areas with extensive use of nitrogen-based fertilizers, significantly contributes to NH4+ pollution.1 Fertilizers rich in NH4+ and nitrates are applied to crops to enhance growth; however, their significant amounts leach into surface and groundwater, resulting in elevated NH4+ levels in rivers, lakes, and aquifers. Globally, Harmful Algal Blooms (HABs) pose a significant environmental challenge, particularly in nutrient-rich water bodies. These blooms, often caused by cyanobacteria (also known as blue-green algae), thrive in warm, slow-moving waters with high levels of phosphorus and nitrogen.2–4 Excess nutrients, primarily from agricultural runoff, lawn fertilizers, faulty septic systems, and wastewater treatment plants, exacerbate this issue.5 Phosphorus is the primary driver, as it promotes rapid algal growth, while NH4+ acts as an additional nutrient source, sustaining these blooms. HABs disrupt aquatic ecosystems by blocking sunlight, depleting oxygen levels during decomposition, and releasing toxins harmful to humans, animals, and marine life. Despite implementing Total Maximum Daily Loads (TMDLs) to regulate phosphorus inputs, toxic HABs remain a recurring issue. Continued efforts to reduce nutrient pollution, restore natural shorelines, and promote public education are crucial to safeguarding freshwater ecosystems.6,7In industrial settings, NH4+ concentrations in wastewater can vary significantly, with levels often exceeding regulatory limits. For instance, municipal wastewater has NH4+ concentrations ranging from 27 to 100 mg L−1. Fish-processing waste has NH4+ concentrations ranging between 8 and 42 mg L−1. In domestic wastewater, the NH4+ concentration typically ranges from 39 to 60 mg L−1.8 Fertilizer production, food processing facilities, and similar industries frequently have NH4+ concentrations ranging from 5 to 1000 mg L−1.8,9 These values increase depending on the type of processing industry, typically reaching as high as 2945 mg L−1. NH4+ plays a crucial role in both aquatic and terrestrial environments, particularly within the nitrogen cycle.8 It can exist in equilibrium with ammonia (NH3) in aqueous solutions, and the equilibrium between NH4+ and NH3 is highly dependent on pH. At lower pH levels, NH4+ is predominant, while at higher pH levels, NH3 dominates.10 NH4+ in drinking water poses significant health risks. For example, it can react with chlorine during water treatment processes to form chloramines, which can exacerbate asthma symptoms due to excessive exposure to trichloramine.11 Additionally, when NH4+ is oxidized to nitrite in the human body, it can interfere with the oxygen-carrying capacity of hemoglobin, particularly in infants, leading to methemoglobinemia, commonly known as “blue baby syndrome”.12 Therefore, there is an urgent need to address NH4+ in wastewater in a sustainable manner.
It can be removed through various methods, including biological treatment, adsorption techniques,13,14 and chemical precipitation. Adsorption is the most promising technique investigated for removing NH4+ from wastewater. Unfortunately, this technique is often marred by a lack of suitable and viable adsorbent materials. According to Han et al.,15 this challenge is further aggravated by the need to find efficient, affordable, abundant, and sustainable materials. Consequently, different avenues have been explored, including modifying adsorption techniques to accommodate various materials. Wang et al.16 reported that physical activation and chemical modification are the most prominent avenues for enhancing the performance of ion exchange mechanisms based on readily available adsorbent materials. For instance, the authors sought to examine whether physical activation could enhance the adsorption method for removing NH4+ using forestry waste.17,18 Several recent studies have focused on the adsorption of ammonium using activated carbon (AC).18 In addition to this, a few more carbon adsorbents, such as cellulose acetate membrane, AC derived from bacterial cellulose,18 and cellulose sulfate,19 have been used to address the NH4+ problem. To the best of our knowledge, none of the studies have in-depth discussed the use of carboxylated cellulose nanofibers (CNFs) for the remediation of NH4+ pollutants from real and simulated water, and have not provided a practical solution to tackle this problem sustainably. In this study, we have done a systematic in-depth study to apply the CNFs to remediate the NH4+ pollutant from the simulated and real water (from the aquaponic tank), followed by the development of a floatation remediation system (FRS) to ease the handling and to provide the viability of the CNFs adsorbent system for all types of contaminated water sources.
Experimental section
Materials and instruments
TEMPO-oxidized cellulose nanofibers extracted from the forest biomass with a concentration of 1.04 wt% and a carboxylate (COO−) content of 1.15 mmol g−1 were procured from the University of Maine. Analytical ammonium nitrate (ACS reagent, 99%) and sodium bicarbonate (ACS reagent, ≥99.7%) were purchased from Sigma-Aldrich. Sodium hydroxide (ACS reagent, ≥97.0%) pellets and hydrochloric acid (ACS grade, 36.5–38%) were purchased from Fisher Scientific. An ammonium ion-selective electrode was used to measure the concentration of NH4+. The following instruments were used in this study.Fourier transform infrared spectrometry (FTIR): A ThermoFisher instrument (ThermoFisher Scientific, U.S.A.) was used to record the FTIR spectra in transmission mode, between 4000 to 450 cm−1. A total of 12 scans were taken per sample with a resolution of 4 cm−1. The solid samples were recorded in attenuated total reflectance (ATR) mode.
The JEOL 7500F ultra-high-resolution scanning electron microscope, equipped with a cold-field emission emitter, was used to examine the morphology of CNFs. Zeta potential measurements on CNFs samples were performed using MÜtek™ PCD-06, Particle Charge Detector.
Preparation of ammonium solutions
A 5000 ppm NH4+ stock solution was prepared by dissolving 22.22 g of NH4NO3 in 1 L of deionized water. Serial dilutions were made to range from 5000 to 5 ppm for use in adsorption studies.Preparation of cellulose nanofibers (CNFs) suspension
CNFs suspension was prepared using commercial TEMPO-oxidized CNFs gel at an initial concentration of 1.04% (wt%) with carboxylate (COO−) content of 1.15 mmol g−1. The CNFs were diluted to 0.5% (wt%) by adding deionized water under continuous stirring. The suspension was homogenized to ensure an even distribution of the nanofibers. This CNF's suspension was used as an adsorbent for the NH4+ adsorption experiments.Remediation studies using simulated water
The concentration of active NH4+ in the solution was determined using an ion-selective electrode. For the adsorption capacity experiments, 5 mL of CNFs suspension was mixed with 5 mL of NH4+ solution (prepared from ammonium nitrate) in a test tube. In this study, NH4+ concentration is reported as parts per million (ppm) of NH4+ to maintain consistency with other studies that may employ different NH4+ salts. However, the specific NH4+ salt used is noted to account for any potential counter-ion effects.For all experiments except the time study, the solution was stirred for 1 minute and then incubated at room temperature for 24 hours. After incubation, the samples were filtered using a syringe filter with a 0.22 µm pore size, showing effective separation. The filtrate was then analyzed for NH4+ concentration using an ion-selective electrode. To ensure that the syringe material did not contribute to NH4+ adsorption during the experiment, a control test was conducted. A solution of NH4+ with an initial concentration of 500 ppm was prepared and passed through the syringe without any additional materials or treatments inside. The difference in concentration was negligible, confirming that the syringe material did not significantly interfere with the adsorption of NH4+.
pH study
To study the effect of pH on adsorption capacity, the pH of the NH4+ was adjusted to the desired levels using dilute sodium hydroxide or hydrochloric acid. After pH adjustment, the solutions were mixed with a 0.5% CNFs suspension at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. The mixtures were then incubated at room temperature for 24 hours.
1 ratio. The mixtures were then incubated at room temperature for 24 hours.
Time study
Additionally, a study assessed the adsorption capacity over different incubation times ranging from 0.5 to 48 hours using an initial NH4+ concentration of 500 ppm. The NH4+ concentration in the filtrate was measured at each time point to evaluate the effect of contact time on adsorption efficiency.Remediation studies using real water
The contaminated water was collected from the real fish tank located in the Greenhouse Facility at Western Michigan University's Department of Biology. The water was collected directly from the tank before it passed through the in situ filtration. The NH4+ concentration before and after adsorption using a CNFs suspension was determined using an NH4+ test kit, followed by quantitative measurement using UV-adsorption. The details are mentioned in the SI.Floc separation procedure
The floc term refers to the gel material that forms when CNFs interact with NH4+ impurities, causing the suspended CNF fibers to clump together into a lumpy gel. The separation of the floc was performed in the following steps. A 5 mL suspension of 0.5 wt% CNFs having COO− groups was mixed with 5 mL of NH4+ solution prepared from NH4NO3 for simulated water testing at different concentrations from 5000 to 5 ppm (SI) in a test tube. In contrast, the water collected from the fish tank contained its own NH4+ impurities (SI). The solution was gently shaken for 1 minute and then incubated at room temperature for 24 hours to neutralize the charges and initiate the aggregation of CNFs and adsorbed NH4+ into flocs. For separation, the flocs were filtered through a vacuum filtration system equipped with a 0.45 µm membrane filter (Fig. 1). The retained flocs on the membrane were oven-dried and analyzed for further characterization.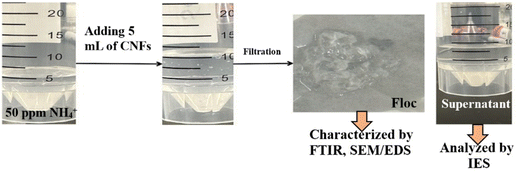 | ||
| Fig. 1 Demonstration of floc preparation and separation of supernatant from the floc for further characterization. | ||
Results and discussion
Chemical and morphological properties
The FTIR analysis (Fig. 2A), conducted at the dry stage, reveals significant changes in the functional groups of CNFs after exposure to NH4+, confirming ion exchange as the dominant interaction mechanism. The broad O–H stretching peak around 3338 cm−1 in CNFs exhibits slight shifts and intensity reductions, which can be attributed to overall hydrogen bonding alteration due to the interaction of NH4+ ions. A notable decrease in the intensity of the (–COO−) stretching peak near 1599 cm−1 in CNFs suggests the involvement of –COO− groups in the binding with NH4+ ions. Additionally, in the floc sample containing CNFs + NH4+, the emergence or intensification of a peak around 1578 cm−1 in the N–H bending vibration provides direct evidence of NH4+ binding to the CNFs. The C–O stretching peak at approximately 1176 cm−1 in CNFs also shows changes in intensity and position, indicative of interactions between NH4+ and ester or carboxyl groups on the CNFs structure. These spectral changes collectively validate that NH4+ ions are adsorbed onto CNFs through an ionic interaction process between the carboxylate and ammonium groups (Fig. 2B), followed by changes in the hydrogen bonding in CNFs as evident by the changes in the O–H stretching vibration at 3338 cm−1.20 The observations emphasize the crucial role of the carboxyl and hydroxyl groups in facilitating effective NH4+ adsorption, making CNFs a promising material for wastewater remediation.21–28 FTIR analysis (Fig. 3) was further supported by the morphological changes that occurred before and after the NH4+ interactions on CNFs.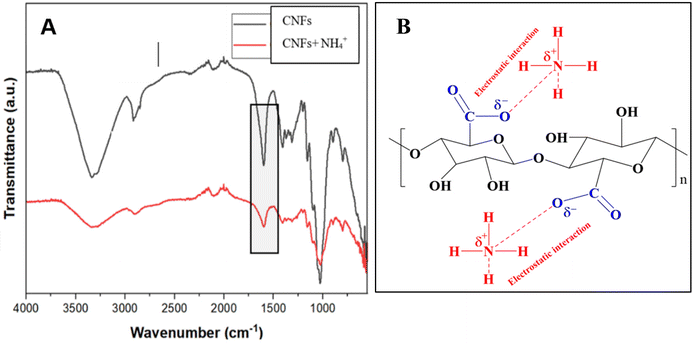 | ||
| Fig. 2 (A) FTIR of CNFs and floc (CNFs and NH4+), (B) schematic of the mechanism of CNFs and NH4+ interaction. | ||
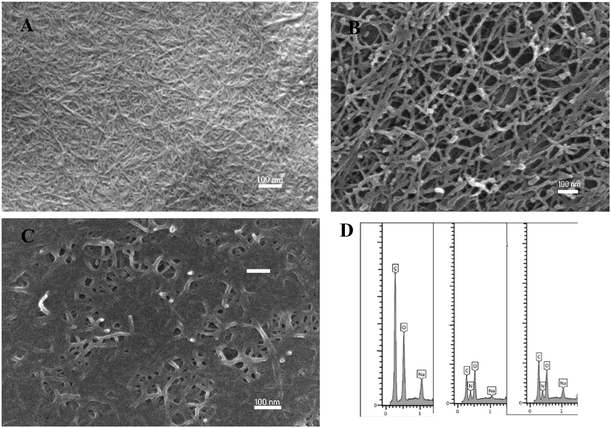 | ||
| Fig. 3 SEM images of CNFs at different NH4+ concentrations, (A) CNFs; (B) CNFs + NH4+ (5 ppm); (C) CNFs + NH4+ (5000 ppm); (D) A, B, C respective EDS spectra. | ||
The morphological analysis conducted via SEM/EDS reveals significant structural changes in CNFs as the concentration of NH4+ varies. One hundred fibers were selected from each image, and their dimensions were measured using ImageJ software to ensure statistical reliability. Pristine CNFs (Fig. 3A and Fig. S1 in SI) exhibited a maximum fiber diameter between 15.4 and 18.1 nm. However, after exposure to 5 ppm NH4+ (Fig. 3B and Fig. S1 in SI), the range increased to 19.7–22.8 nm, indicating swelling of the individual fibers. Notably, when the CNFs were further exposed to a higher concentration of 5000 ppm NH4+ (Fig. 3C and Fig. S1 in SI), the range increased to 21.3 and 24.8 nm, demonstrating substantial structural changes due to the intake of NH4+. The swelling of individual cellulose fibers illustrates the mechanism of NH4+ removal, which can be explained by (i) an ion-exchange mechanism: the size of the NH4+ ion is 140 pm, which is larger than that of Na (95 pm) or H (0.208 nm). The Na+ or H+ is associated with the carboxyl (–COO−) groups on the CNFs. During the interaction of CNFs and NH4+ ions for remediation, the increase in fiber diameter indicates the NH4+ ions replacement with the CNF's carboxyl counterions. These results are consistent with the FTIR results, further supporting ion exchange and indicating ionic interactions. This ion exchange corresponds to the absorption phenomenon. Hence, different isotherm models, as presented in the adsorption studies section, are used to analyze the adsorption efficiency of CNF fibers. (ii) Absorption: Typically, the CNF's fiber width is between 4 and 6 nm. Here, the CNFs we have used have a maximum diameter between 15.4 and 18.1 nm, indicating that CNFs still retain a layered structure, which may have led to the absorption of impurities on the CNFs. The EDS analysis (Fig. 3D) depicts significant changes in the elemental composition of CNFs before and after NH4+ adsorption. Initially, the pristine CNFs sample (Fig. 3D) contains 7.9 wt% of sodium (Na), with no detectable nitrogen (N) present. After exposure to 5 ppm NH4+ (Fig. 3D), the wt% of Na decreases to 3.4 wt%, while nitrogen appears at 21.9 wt%, indicating the successful adsorption of NH4+ ions onto the CNF's surface. This trend changes at 5000 ppm NH4+ (Fig. 3D), where Na increases to 10.1 wt%, and nitrogen decreases to 17.7 wt%. This indicates that at a higher concentration of NH4+, absorption is more dominant than ion exchange. To confirm these results further, elemental analysis is recommended. The indication of nitrogen in the elemental composition strongly supports the intake of NH4+, highlighting CNF's effectiveness as an adsorbent for NH4+ removal in wastewater treatment. We have also performed zeta potential measurements to investigate the change in surface charges of CNFs upon interaction with different NH4+ impurities. The results indicate that the surface charge of CNFs at a concentration of 0.0125 w% showed a charge of −418.5 µg L−1. When 5 ppm NH4+ was added, the charge decreased to −283.8 µg L−1, and further addition of 250 ppm NH4+ reduced the surface charge to −222.6 µg L−1. This trend of decreasing the surface charge of CNFs with an increase in ammonium concentration further evidences the presence of ionic interactions between these two functionalities.
Adsorption studies
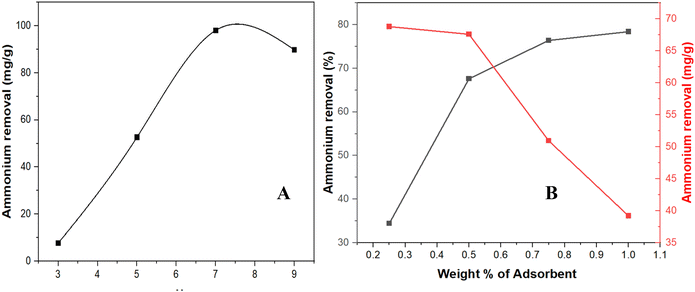 | ||
| Fig. 4 (A) Effect of initial pH on the removal efficiency of NH4+; (B) effect of adsorbent dose on NH4+ removal. | ||
As the pH increased from 3 to 7, the removal efficiency increased, reaching a peak of 98% at pH 7, corresponding to the highest concentration of NH4+ and a favorable interaction between the adsorbent surface and the NH4+. Beyond pH 7, the removal efficiency plateaued at around 85% and slightly decreased due to the deprotonation of NH4+ to NH3, which is neutral and, therefore, less effectively adsorbed onto the adsorbent surface. Additionally, the adsorbent surface began to turn non-ionic (COONa), which decreased the number of COO− groups available for interaction with NH4+, further reducing the removal efficiency. However, at pH 9, it still maintained a removal efficiency of 85%.
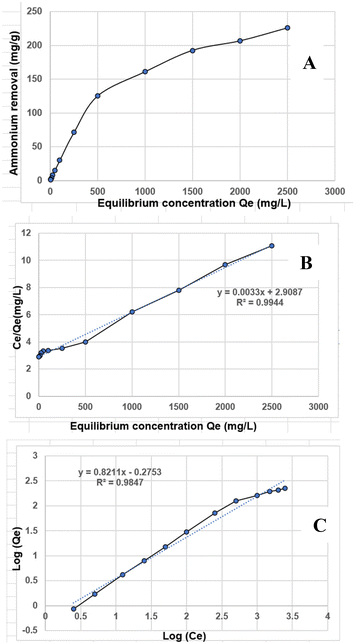 | ||
| Fig. 5 (A) Relationship between NH4+ removal and equilibrium concentration; (B) Langmuir isotherm for NH4+ removal using CNFs; (C) Freundlich isotherm for NH4+ removal using CNFs. | ||
It is also important to emphasize that the increase in removal efficiency begins to stabilize at higher CNF concentrations, as outlined in Fig. 4B and Table S2 of the SI. This indicates that, beyond a certain point, the efficiency is not simply limited by the number of available active sites. Instead, factors such as site saturation, diffusion limitations, and potential agglomeration of CNFs at elevated concentrations can influence effectiveness by reducing the available surface area for interaction. Therefore, after the 1.0% dosage, further increases in CNFs are unlikely to yield significant improvements in removal efficiency, underscoring the importance of optimizing the dosage to achieve excellent results.
| Isotherm model | Parameter | Value |
|---|---|---|
| Langmuir model | Maximum adsorption capacity (Qmax) | 303.03 mg g−1 |
| Langmuir constant (KL) | 0.0033 L mg−1 | |
| R2 | 0.9944 | |
| Freundlich model | Freundlich constant (KF) | 0.302 mg g−1 (L mg−1) 1/n |
| Heterogeneity index (n) | 0.8206 | |
| R2 | 0.9847 |
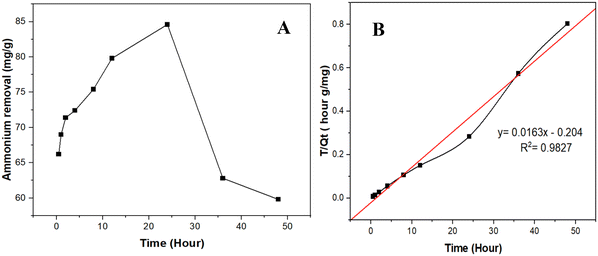 | ||
| Fig. 6 (A) Removal of NH4+ over time using CNFs; (B) Pseudo-second-order kinetics for NH4+ adsorption by CNFs. | ||
The T/Qt versus T plot (Fig. 6B) was used to further assess the adsorption kinetics, fitting the pseudo-second-order model, which generally indicates chemisorption involving valence forces or covalent bonding. The high R2 value of 0.9827 means that the adsorption process is well-described by this model. This suggests that the adsorption of NH4+ is rate-limited by the availability of active adsorption sites, following the pseudo-second-order kinetics.30 The linear fit equation suggests a constant rate of 0.0163 g mg−1 hour, confirming the steady rate of the adsorption process. These findings align with previous studies, which indicate that nanocellulose materials, particularly CNFs, provide efficient active sites for NH4+ adsorption and can be described well using pseudo-second-order kinetics.31 The kinetic analysis confirms that CNFs hold significant promise for removing NH4+ from wastewater, with the initial stages of the adsorption process being particularly crucial. The potential of CNFs for wastewater treatment is a hopeful sign for the future of environmental science, although optimizing contact time is critical to maintaining high efficiency.
| Modification | Removal efficiency | Ref. |
|---|---|---|
| Cellulose nanofibers (CNFs) | 303 mg g−1 | This study |
| Ultrasonically activated forestry waste biochar | 26.28 mg g−1 | 17 |
| Ball-milled biochar | 22.9 mg g−1 | 32 |
| Sulfonated reed waste biochar | 4.20–5.19 mg N g−1 | 33 |
| MgO-biochar composite | 17.5 mg g−1 | 34 |
| Straw biochar modified by soda residue | 3.87 mg g−1 | 35 |
| Reverse osmosis cellulose acetate membranes | 98% | 36 |
| Bacterial cellulose activated carbon | 33.5–221.4 mg g−1 | 18 |
| Nitro-oxidized cellulose nanofibers | 22.7 mg g−1 | 27 |
| Cellulose sulfate nanofibers | 41.1 mg g−1 | 19 |
| Crosslinked sulfonated polymer | 87% | 37 |
| MIEX resin | 98% | 38 |
| Pyridinium-type anion exchange resin | 94.3% | 39 |
Conclusions
In conclusion, this study provides a critical insight into the effectiveness of CNFs in adsorbing ammonium impurities from simulated and real contaminated water. CNFs with a negative surface charge, in the form of carboxylate groups, effectively removed ammonium impurities, achieving a maximum adsorption capacity of 303.3 mg g−1. Additionally, the removal efficiency increased to 98% at a pH of 7, where the ammonium and ammonia both exist at equilibrium. The adsorption capacity of CNFs was evaluated under various conditions, including the amount of adsorbents, pH changes, time variations, and wastewater sources. The remediation experiment, conducted at an ammonium concentration of 500 ppm using a consistent amount of CNFs with 0.5 wt% under various conditions, provided more realistic results and demonstrated the versatility of CNFs in multiple situations. For example, 0.5 wt% of CNFs with 500 ppm ammonium, when mixed without pH alteration, showed a removal efficiency of 71.20%. In contrast, when the pH of the ammonium solution was altered, a 76.3% removal was observed at pH 5 with the same mixing conditions. The CNFs demonstrated the very high efficiency of 86.4–22.5% ammonium removal when tested against a range from 5 to 5000 ppm, respectively. CNFs are a layered structure with abundant negative surface charge, owing to ammonium removal via ion-exchange through the surficial functionality and multilayer adsorption through the layered structure. Therefore, the mechanism obeys both the Langmuir and the Freundlich isotherm models. In summary, CNFs are an eco-friendly, renewable, and sustainable material derived from abundant natural resources, offering tunable functionality and providing an attractive, scalable solution to various environmental remediation studies, with the added benefits of recycling and reusability as shown in this study.Author contributions
EMAA, SCH, MAM, SM, ARR, JJK, PS, and MK designed the experiments. EMAA, SCH, SM, MAM, and JJK acquired and analyzed the data. The manuscript was compiled, written, and revised by PS, JS, AKS, WB, and SM. All authors gave approval to the final version of the manuscript.Conflicts of interest
The authors declare that they have no conflict of interest.Data availability
Data will be made available upon request from the authors. The Supplementary Information file includes Histograms for Figures 3A, B, and C. Effect of pH on ammonium removal efficiency and adsorption. Effect of CNFs dose on ammonium removal efficiency and adsorption capacity. Adsorption efficiency and experimental parameters for NH4+ removal using CNFs. Relationship between the values of Qe and Ce. Effect of time on ammonium removal efficiency and adsorption capacity. Time-dependent adsorption and kinetic parameter values. The initial and final ammonium concentrations of real contaminated fish water tank samples using CNFs in the dilute bag of FRS for remediation. See DOI: https://doi.org/10.1039/d5lp00201j.Acknowledgements
P. R. S. would like to thank the Start-up grant (2023–2026) from the Western Michigan University Department of Chemical and Paper Engineering.References
- V. Yadav, M. Rani, L. Kumar, N. Singh and V. Ezhilselvi, Water, Air, Soil Pollut., 2022, 233, 465 CrossRef CAS.
- K. G. Sellner, G. J. Doucette and G. J. Kirkpatrick, J. Ind. Microbiol. Biotechnol., 2003, 30, 383–406 CrossRef CAS PubMed.
- K. Tewari, Phycology, 2022, 2, 244–253 Search PubMed.
- E. Zohdi and M. Abbaspour, Int. J. Environ. Sci. Technol., 2019, 16, 1789–1806 Search PubMed.
- D. W. Schindler, S. R. Carpenter, S. C. Chapra, R. E. Hecky and D. M. Orihel, Environ. Sci. Technol., 2016, 50, 8923–8929 CrossRef CAS PubMed.
- S. Sonak, K. Patil, P. Devi and L. D'Souza, Environ. Pollut. Prot., 2018, 3, 40–55 Search PubMed.
- D. M. Anderson, E. Fensin, C. J. Gobler, A. E. Hoeglund, K. A. Hubbard, D. M. Kulis, J. H. Landsberg, K. A. Lefebvre, P. Provoost and M. L. Richlen, Harmful Algae, 2021, 102, 101975 CrossRef CAS PubMed.
- A. Omar, F. Almomani, H. Qiblawey and K. Rasool, Sustainability, 2024, 16, 2112 CrossRef CAS.
- B. Thamdrup, Annu. Rev. Ecol. Evol. Syst., 2012, 43, 407–428 CrossRef.
- P. Purwono, H. Hadiyanto and M. Budihardjo, Int. J. Eng., 2023, 36, 565–572 CrossRef CAS.
- G. Wastensson and K. Eriksson, Crit. Rev. Toxicol., 2020, 50, 219–271 CrossRef CAS PubMed.
- J. Berberich, T. Li and E. Sahle-Demessie, in Separation science and technology, Elsevier, 2019, vol. 11, pp. 285–328 Search PubMed.
- M. A. Abdelaziz, M. E. Owda, R. E. Abouzeid, O. Alaysuy and E. I. Mohamed, Int. J. Biol. Macromol., 2023, 225, 1462–1475 CrossRef CAS PubMed.
- Z. Karim, M. Hakalahti, T. Tammelin and A. P. Mathew, RSC Adv., 2017, 7, 5232–5241 RSC.
- B. Han, C. Butterly, W. Zhang, J.-z. He and D. Chen, J. Cleaner Prod., 2021, 283, 124611 CrossRef CAS.
- Y. Wang, L. Chen, Y. Zhu, W. Fang, Y. Tan, Z. He and H. Liao, Environ. Sci. Eur., 2024, 36, 25 CrossRef.
- T. Wang, G. Li, K. Yang, X. Zhang, K. Wang, J. Cai and J. Zheng, Sci. Total Environ., 2021, 778, 146295 CrossRef CAS PubMed.
- A. Khamkeaw, W. Sanprom and M. Phisalaphong, Case Stud. Chem. Environ. Eng., 2023, 8, 100499 CrossRef CAS.
- K. I. Johnson, W. Borges, P. R. Sharma, S. K. Sharma, H.-Y. Chang, M. M. Abou-Krisha, A. G. Alhamzani and B. S. Hsiao, Nanomaterials, 2024, 14, 507 CrossRef CAS PubMed.
- P. R. Sharma, B. Zheng, S. K. Sharma, C. Zhan, R. Wang, S. R. Bhatia and B. S. Hsiao, ACS Appl. Nano Mater., 2018, 1, 3969–3980 CrossRef CAS.
- P. R. Sharma, A. Chattopadhyay, C. Zhan, S. K. Sharma, L. Geng and B. S. Hsiao, Cellulose, 2018, 25, 1961–1973 CrossRef CAS.
- H. Chen, S. K. Sharma, P. R. Sharma, K. Chi, E. Fung, K. Aubrecht, N. Keroletswe, S. Chigome and B. S. Hsiao, Cellulose, 2021, 28, 8611–8628 CrossRef CAS.
- P. R. Sharma, A. Chattopadhyay, S. K. Sharma, L. Geng, N. Amiralian, D. Martin and B. S. Hsiao, ACS Sustainable Chem. Eng., 2018, 6, 3279–3290 CrossRef CAS.
- P. R. Sharma, A. Chattopadhyay, S. K. Sharma and B. S. Hsiao, Ind. Eng. Chem. Res., 2017, 56, 13885–13893 Search PubMed.
- H. Chen, P. R. Sharma, S. K. Sharma, A. G. Alhamzani and B. S. Hsiao, Nanomaterials, 2022, 12, 4156 CrossRef CAS PubMed.
- K. I. Johnson, State University of New York at Stony Brook, 2022 Search PubMed.
- K. I. Johnson, G. Ilacas, R. Das, H.-Y. Chang, P. R. Sharma, C. O. Dimkpa and B. S. Hsiao, Sustainability Sci. Technol., 2024, 1, 014001 CrossRef.
- P. R. Sharma, X. Huang, M. Yang, S. K. Sharma and B. S. Hsiao, Sustainable Separation Engineering: Materials, Techniques and Process Development, 2022, pp. 563–589 Search PubMed.
- F. Bettaieb, M. A. Abdelaziz, I. S. Alatawi, M. A. Aljowni, H. Parveen, S. Mukhtar, N. Omer, R. Jame, S. A. Alshareef, M. E. Owda and Y. A. Bin Jardan, Int. J. Biol. Macromol., 2025, 310, 143354 CrossRef CAS PubMed.
- D. L. Sparks and D. L. Suarez, Rates of Soil Chemical Processes, Soil Science Society of America, Inc., 1991 Search PubMed.
- A. Al Mamun, Y. M. Ahmed, M. a. F. R. AlKhatib, A. T. Jameel and M. A. AlSaadi, Nano, 2015, 10, 1550017 CrossRef CAS.
- Y. Qin, X. Zhu, Q. Su, A. Anumah, B. Gao, W. Lyu, X. Zhou, Y. Xing and B. Wang, Environ. Geochem. Health, 2020, 42, 1579–1587 CrossRef CAS PubMed.
- M. Zhang, R. Sun, G. Song, L. Wu, H. Ye, L. Xu, S. J. Parikh, T. Nguyen, E. Khan and M. Vithanage, Environ. Pollut., 2022, 292, 118412 CrossRef CAS PubMed.
- R. Xiao, H. Zhang, Z. Tu, R. Li, S. Li, Z. Xu and Z. Zhang, Environ. Sci. Pollut. Res., 2020, 27, 7493–7503 CrossRef CAS PubMed.
- L. Liu and M. Zhang, Int. J. Environ. Sci. Technol., 2023, 20, 13783–13798 CrossRef CAS.
- A. Bódalo, J.-L. Gómez, E. Gómez, G. Leon and M. Tejera, Desalination, 2005, 184, 149–155 CrossRef.
- M. G. Torquato, E. C. Corrêa Torquato, A. C. L. Moraes, F. d. A. Buás Campeão, R. José França, M. R. d. C. Marques and L. d. C. Costa, Macromol. Symp., 20224062200052 Search PubMed.
- Y. Yang, Z. Zheng, W. Ji, M. Yang, Q. Ding and X. Zhang, Surf. Interfaces, 2019, 17, 100385 CrossRef CAS.
- L. Fu, J. Zu, H. Wang and X. Pan, J. Radioanal. Nucl. Chem., 2019, 322, 2043–2048 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2026 |