Open Access Article
Open Access ArticleFabrication of low-fouling reverse osmosis membranes by grafting poly(2-methoxyethyl acrylate) via surface-initiated atom transfer radical polymerization method
Ines
Haddar†
 a,
Tomoki
Kato
a,
Shin-ichi
Nakao
a,
Xiao-lin
Wang
ac,
Kazumi
Tsukamoto
b,
Takahiro
Kawakatsu
b and
Kazuki
Akamatsu
a,
Tomoki
Kato
a,
Shin-ichi
Nakao
a,
Xiao-lin
Wang
ac,
Kazumi
Tsukamoto
b,
Takahiro
Kawakatsu
b and
Kazuki
Akamatsu
 *a
*a
aDepartment of Environmental Chemistry and Chemical Engineering, School of Advanced Engineering, Kogakuin University, 2665-1 Nakano-machi, Hachioji-shi, Tokyo 192-0015, Japan. E-mail: akamatsu@cc.kogakuin.ac.jp; Tel: +81 42 628 4584
bKurita Innovation Hub, Kurita Water Industries Ltd., 1-4-1 Daikanyama, Akishima-shi, Tokyo 196-0005, Japan
cDepartment of Chemical Engineering, Tsinghua University, Beijing 100084, People's Republic of China
First published on 24th December 2025
Abstract
The surfaces of polyamide-based low-pressure reverse osmosis (RO) membranes were modified with poly(2-methoxyethyl acrylate) (PMEA) via surface-initiated atom transfer radical polymerization for the first time to achieve low-fouling characteristics. The successful grafting of PMEA was demonstrated by attenuated total reflectance Fourier-transform infrared spectroscopy and zeta potential measurements. The grafting amount could be tuned from 0.020 to 0.23 mg cm−2 by changing the grafting time. The modified membranes maintained their salt rejection performances with slight reductions of pure water permeability especially when the grafting amount was smaller than 0.05 mg cm−2. This result indicated that the grafted PMEA had almost no effect on salt rejection but slightly increased the permeation resistance. Compared with unmodified membranes, the modified membranes were found to exhibit low fouling against a variety of organic substances, such as lysozyme, guar gum and tetraethylene glycol monooctyl ether. The results indicate that surface modification of a low-pressure RO membrane with PMEA is a feasible method to obtain a membrane with low-fouling characteristics.
1. Introduction
The escalating global water crisis necessitates the urgent development of solutions to meet challenges arising from population growth, expanded agriculture, economic development and the looming threat of climate change.1,2 The United Nations estimates that severe water scarcity will affect over 2.7 billion people by 2025, particularly in North African and Middle Eastern countries.3 To address this crisis and reach sustainable development goals, unconventional water sources are being explored, where gold standard thin film composite (TFC) polyamide membranes are employed for various membrane technology applications.4–6Reverse osmosis (RO) technology has emerged as a versatile and energy-efficient solution. This technology offers high flux together with high selectivity for several applications such as seawater desalination, ultrapure water production and wastewater treatment, which contributes to sustainability.7,8 To date, most commercial TFC RO membranes are formed as flat sheets with an ultrathin aromatic polyamide film coating. The polyamide layer is formed by interfacial polymerization with amine monomers and acyl chloride monomers.9,10 These membranes perform well and are already in widespread production. However, persistent fouling issues, influenced by factors such as the membrane material, zeta potential of the membrane surface, and operational conditions, pose considerable challenges.11,12 Organic fouling remains a critical concern because of its detrimental impact on membrane performance and necessitates frequent chemical cleaning, which increases operational costs and decreases the membrane lifespan.13 Major fouling substances include proteins, polysaccharides and surfactants.14–17 One possible approach to assessing fouling behavior in both laboratory settings and practical operations involves the use of model substances, such as lysozyme for proteins, guar gum for polysaccharides and tetraethylene glycol monooctyl ether (TEGMO) for surfactants.
One effective strategy for mitigating membrane fouling has been to modify the membrane surface with hydrophilic polymers, either through physical deposition or covalent immobilization.18–22 This approach appeared pivotal because it can prevent the hydrophobic interactions between the polyamide membrane and certain organic foulants that largely enable membrane fouling. Previous studies have highlighted the efficacy of specific polymers, such as poly[(2-methacryloyloxy)ethyl]dimethyl[3-sulfopropyl]ammonium hydroxide (pMEDSAH),23 poly(carboxybetaine acrylic acetate)24 and poly(2-methacryloyloxyethyl phosphorylcholine),25 in preventing biofouling. Additionally, it has been demonstrated that poly(2-methoxyethyl acrylate) (PMEA) is an effective surface modifier for the fabrication of low-fouling microfiltration membranes.26–28 Unlike other low-fouling polymers mentioned above, PMEA is insoluble in water. PMEA has been extensively investigated for use in biomaterials and has excellent biocompatibility. Tanaka et al. demonstrated that “intermediate water” can effectively prevent protein adsorption onto membrane surfaces.29–31 At hydrated polymers, three types of water exist, “free water”, “intermediate water” (i.e. freezing bound water) and “non-freezing water”, which can be classified using differential scanning calorimetry. The strong correlation between the amount of the intermediate water and the blood compatibility of the biomaterial polymers were demonstrated.32 Furthermore, Nagumo et al. demonstrated that the hydrated PMEA prohibits the adsorption of organic substances by using molecular dynamic simulations.33 Based on these publications, PMEA is one of the potential polymers for surface modification to achieve low-fouling properties. Surface-initiated atom transfer radical polymerization (SI-ATRP) is a promising alternative to conventional modification methods.34–36 Unlike techniques such as redox reactions and electrostatic coating, SI-ATRP offers precise control over the length of the grafted polymers, overcoming limitations such as low surface density and poor stability. This method holds considerable potential for advancing membrane technology, addressing key challenges and enhancing performance and longevity.
Our objective in this study was to fabricate low-fouling RO membranes with enhanced resistance against organic fouling by harnessing the benefits of grafted PMEA. To tailor the membrane properties precisely, we controlled the grafting amount through varying the polymerization time. The surface properties of the membranes were analyzed by attenuated total reflectance Fourier-transform infrared (ATR-FTIR) spectroscopy and zeta potential measurement. To demonstrate the effectiveness of our modified membranes in real-world scenarios, we conducted filtration and immersion tests using three different aqueous solutions containing lysozyme as a model protein-like foulant, guar gum as a model polysaccharide-like foulant and TEGMO as a model surfactant-like foulant. The meticulous control strategy enabled us to fine-tune the surface characteristics of the RO membrane and optimize the performance towards enhanced organic fouling resistance.
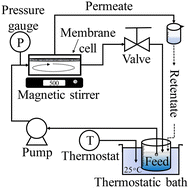
2. Experimental
2.1. Materials
As the base membrane, a polyamide-based low-pressure RO membrane, ES20, which was purchased from Nitto Denko Corporation, Japan, was used. Hexane, 3-aminopropyltrimethoxysilane, L(+)-ascorbic acid, tris(2-pyridylmethy)amine (TPMA), copper(II) bromide (CuBr2), methanol, 2-methoxyethyl acrylate (MEA), sodium chloride (NaCl) and lysozyme were purchased from Fujifilm Wako Pure Chemical Corp., Japan. In addition, α-bromoisobutyryl bromide (BIBB) and TEGMO were purchased from Sigma-Aldrich and guar gum (F50, 5000 cps) was purchased from San-ei Yakuhin Boeki Co., Ltd., Japan. In some cases, microfiltration membranes whose average pore size was 60 nm were used to pretreat the feed solution to remove undissolved substances. The MEA monomer was distilled before use, whereas all other chemicals were used as received.2.2. Surface modification via SI-ATRP
First, the RO membrane was pretreated using a procedure reported elsewhere.23 Two wooden plates, each measuring 12 cm × 12 cm, and eight double clips were employed to securely hold the membrane during the process. A 1 vol% 3-aminopropyltrimethoxysilane aqueous solution was poured on the top surface of the RO membrane. After 10 min, the membrane surface was washed thoroughly with pure water. Second, a 3 wt% BIBB–hexane solution was poured on the top surface of the membrane and left for 1 minute to immobilize the brominated initiator. Finally, ATRP was conducted by using 1.81 wt% MEA in a methanol solvent and adding 0.35 g of ascorbic acid, 0.02 g of CuBr2 and 0.06 g of TPMA. The ATRP reaction was conducted at room temperature, and the reaction time ranged from 5 min to 48 h. The MEA-treated membranes were thoroughly washed with pure water, followed by storage in the refrigerator. Prior to being subjected to the ATRP reaction, the membranes were precisely cut to a diameter of 37 mm for use in membrane performance evaluation tests and a diameter of 10 mm for use in surface characterization.2.3. Characterization of the membranes modified with PMEA
The 10 mm diameter modified membranes were vacuum-dried at 30 °C for 30 min. Next, each membrane was weighed thrice to ensure the grafting amount was determined precisely. The grafting amount indicates the degree of grafting and was calculated with: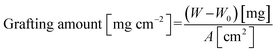 | (1) |
SEM (JCM-7000; JEOL, Japan) was used to observe the surface structure. The surface chemical composition was determined via ATR-FTIR (FT/IR-4200; JASCO Corporation, Japan). The zeta potential of the membrane surface was analyzed using a zeta potential and particle size analyzer (ELSZ-2KH, Otsuka Electronics Co., Ltd., Japan).
2.4. Evaluation of the membrane performance
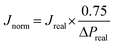 | (2) |
The foulant concentration at the surface of the membrane mounted on the cell was estimated using a mass transfer coefficient that was estimated using the following empirical equation (obtained by the osmotic pressure method):38,39
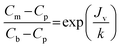 | (3) |
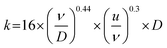 | (4) |
| D = 8.76 × 10−9 × (Mw)−0.48 | (5) |
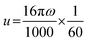 | (6) |
3. Results and discussion
3.1. Impact of the polymerization time on the grafting amount
Fig. 2 shows that the grafting amount increased with the polymerization time because extending the reaction time allowed the polymer chains to grow. As the reaction time increased, more polymerization occurred and the grafting amount increased. It ranged widely from 0.020 to 0.23 mg cm−2.3.2. Membrane characterization
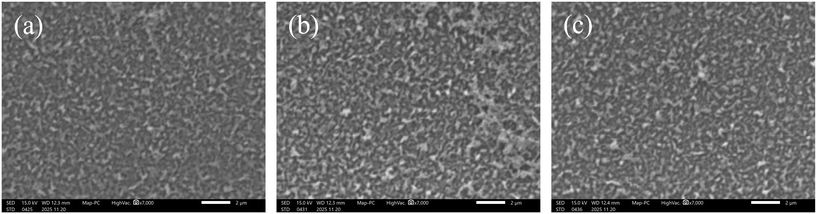
Fig. 3 shows the pictures of the surface of (a) a pristine membrane, (b) the modified membranes with a grafting amount of 0.04 mg cm−2, and (c) that of 0.08 mg cm−2, which were taken by SEM. No clear difference was observed between the pristine membrane and the modified membranes, indicating that the membrane was not damaged at all by the grafting procedure. Fig. 4(a) shows the results of the ATR-FTIR analysis conducted on a pristine membrane and a modified membrane whose grafting amount was 0.15 mg cm−2. A peak at 1660 cm−1 appears in the spectra of both membranes, which corresponds to the amide I band and is characteristic for polyamide-based RO membranes. The peak at approximately 1730 cm−1 appears only in the spectrum of the modified membrane and corresponds to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration of PMEA. The peak intensity generally increases with the grafting amount. Fig. 4(b) shows that the ratio of the PMEA peak to the polyamide peak (I1730/I1660) is positively correlated with the grafting amount. Taken together with the findings in Fig. 2, these results indicate that the peak ratio increased with the polymerization time. Theoretically, the grafting was expected to be performed uniformly because the polymerization step was a rate-determining step in ATRP. The diffusion of the MEA monomer cannot be a rate-determining step. In fact, the concentration of MEA was as high as 1.81 wt% in this study.
O stretching vibration of PMEA. The peak intensity generally increases with the grafting amount. Fig. 4(b) shows that the ratio of the PMEA peak to the polyamide peak (I1730/I1660) is positively correlated with the grafting amount. Taken together with the findings in Fig. 2, these results indicate that the peak ratio increased with the polymerization time. Theoretically, the grafting was expected to be performed uniformly because the polymerization step was a rate-determining step in ATRP. The diffusion of the MEA monomer cannot be a rate-determining step. In fact, the concentration of MEA was as high as 1.81 wt% in this study.
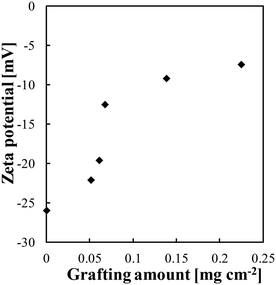
Fig. 5 shows the zeta potential of the surface of the modified membrane in response to the grafting amount. The pristine membrane carried negative charges of up to −26 mV. The charge of the surface grafted with PMEA was less negative, indicating that the grafting process was successful. The negative charge approached neutrality with increasing grafting amount. One contributing factor to this phenomenon was the shielding effect of the grafted polymers, which decreased the exposure of the carboxyl groups on the surface of the polyamide layer. This trend in the zeta potential aligns with the observation of the zeta potential of microfiltration membranes becoming neutral with increasing PMEA grafting amount.40
3.3. Impact of the grafting amount on the pure water permeability and salt rejection
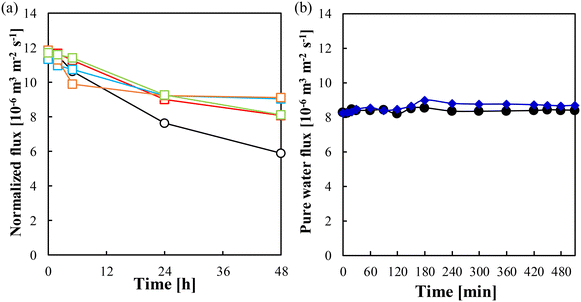
Fig. 6(a) illustrates the relationship between the grafting amount and Lp. Membranes with higher grafting amounts exhibited lower Lp values, resulting in the downward trend in the figure. For instance, compared with the Lp of the pristine membrane, the Lp of the membranes whose grafting amounts were 0.052 mg cm−2 and 0.23 mg cm−2 was lower by 2.7% and 84%, respectively. There was a relatively slight decrease in Lp for membranes whose grafting amounts were smaller than 0.05 mg cm−2. Higher grafting amounts resulted in a more substantial reduction in Lp, which is mainly attributed to the formation of a thick PMEA layer on the membrane surface. Fig. 6(b) shows the relationship between the grafting amount and the observed rejection of NaCl. All the modified membranes exhibited similar salt rejection, which ranged from 97% to 98% at a transmembrane pressure of 1.2 MPa. This result further demonstrates that PMEA did not affect the membrane performance adversely. The consistent and high salt rejection observed across all the modified membranes demonstrates the effectiveness of the ATRP method for introducing PMEA onto the membrane surface without compromising the desalination performance.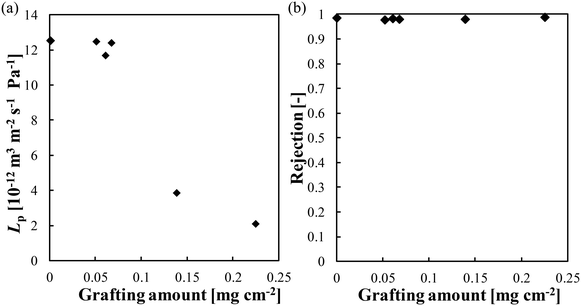 | ||
| Fig. 6 Relationship between the grafting amount and (a) pure water permeability and (b) observed rejection of NaCl. | ||
3.4. Low-fouling properties
These findings lead us to conclude that the optimum PMEA grafting amount for ES20 to achieve the desired performance ranged from 0.02 to 0.04 mg cm−2. Within this range, there was a small reduction in the pure water permeability, the salt rejection performance was maintained, and low fouling by proteins, polysaccharides and surfactants was achieved.
4. Conclusion
The feasibility of surface modification of ES20 with PMEA to achieve low-fouling characteristics was demonstrated in this study. First, we used SI-ATRP to indicate that PMEA was successfully grafted on the ES20 surface and confirmed the results through ATR-FTIR and zeta potential measurements. The grafting amount could be tuned by varying the polymerization time, and the surface became more neutral as the grafting amount increased. The grafted PMEA created permeation resistance in the membrane, such that the pure water permeability decreased with the grafting amount. However, the reduction in the pure water permeability was small for grafting amounts below 0.05 mg cm−2. The salt rejection performance was independent of the grafting amount and remained high for the modified membranes. Importantly, the modified membranes with grafting amounts below 0.04 mg cm−2 exhibited excellent resistance to fouling by lysozyme, guar gum and TEGMO, suggesting successful suppression of fouling by potential foulants. These achievements will contribute to developing effective strategies for mitigating fouling in low-pressure RO membranes.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Data availability
Data will be made available on request.Acknowledgements
We thank Edanz (https://jp.edanz.com/english-editing-c) for editing a draft of this manuscript.References
- C. He, Z. Liu, J. Wu, X. Pan, Z. Fang, J. Li and B. A. Bryan, Future global urban water scarcity and potential solutions, Nat. Commun., 2021, 12, 4667, DOI:10.1038/s41467-021-25026-3.
- H. Shemer, S. Wald and R. Semiat, Future global urban water scarcity and potential solutions, Membranes, 2023, 13, 612, DOI:10.3390/membranes13060612.
- W. Musie and G. Gonfa, Fresh water resource, scarcity, water salinity challenges and possible remedies: A review, Heliyon, 2023, 9, e18685, DOI:10.1016/j.heliyon.2023.e18685.
- D. L. Zhao, S. Japip, Y. Zhang, M. Weber, C. Maletzko and T.-S. Chung, Emerging thin-film nanocomposite (TFN) membranes for reverse osmosis: A review, Water Res., 2020, 173, 115557, DOI:10.1016/j.watres.2020.115557.
- W. J. Lau, S. Gray, T. Matsuura, D. Emadzadeh, J. P. Chen and A. F. Ismail, A review on polyamide thin film nanocomposite (TFN) membranes: History, applications, challenges and approaches, Water Res., 2015, 80, 306–324, DOI:10.1016/j.watres.2015.04.037.
- Z. Yang, H. Guo and C. Y. Tang, The upper bound of thin-film composite (TFC) polyamide membranes for desalination, J. Membr. Sci., 2019, 590, 117297, DOI:10.1016/j.memsci.2019.117297.
- Y. J. Lim, K. Goh, M. Kurihara and R. Wang, Seawater desalination by reverse osmosis: Current development and future challenges in membrane fabrication – A review, J. Membr. Sci., 2021, 629, 119292, DOI:10.1016/j.memsci.2021.119292.
- K. Park, J. Kim, D. R. Yang and S. Hong, Towards a low-energy seawater reverse osmosis desalination plant: A review and theoretical analysis for future directions, J. Membr. Sci., 2020, 595, 117607, DOI:10.1016/j.memsci.2019.117607.
- S. Habib and S. T. Weinman, A review on the synthesis of fully aromatic polyamide reverse osmosis membranes, Desalination, 2021, 502, 114939, DOI:10.1016/j.desal.2021.114939.
- T. Wang, L. Dai, Q. Zhang, A. Li and S. Zhang, Effects of acyl chloride monomer functionality on the properties of polyamide reverse osmosis (RO) membrane, J. Membr. Sci., 2013, 440, 48–57, DOI:10.1016/j.memsci.2013.03.066.
- M. Qasim, M. Badrelzaman, N. N. Darwish, N. A. Darwish and N. Hilal, Reverse osmosis desalination: A state-of-the-art review, Desalination, 2019, 459, 59–104, DOI:10.1016/j.desal.2019.02.008.
- G. Kang and Y. Cao, Development of antifouling reverse osmosis membranes for water treatment: A review, Water Res., 2012, 46, 584–600, DOI:10.1016/j.watres.2011.11.041.
- W. S. Ang, N. Y. Yip, A. Tiraferri and M. Elimelech, Chemical cleaning of RO membranes fouled by wastewater effluent: Achieving higher efficiency with dual-step cleaning, J. Membr. Sci., 2011, 382, 100–106, DOI:10.1016/j.memsci.2011.07.047.
- A. E. Contreras, Z. Steiner, J. Miao, R. Kasher and Q. Li, Studying the Role of Common Membrane Surface Functionalities on Adsorption and Cleaning of Organic Foulants Using QCM-D, Environ. Sci. Technol., 2011, 45, 6309–6315, DOI:10.1021/es200570t.
- J. Wu, Z. Wang, Y. Wang, W. Yan, J. Wang and S. Wang, Polyvinylamine-grafted polyamide reverse osmosis membrane with improved antifouling property, J. Membr. Sci., 2015, 495, 1–13, DOI:10.1016/j.memsci.2015.08.007.
- W. S. Ang, A. Tiraferri, K. L. Chen and M. Elimelech, Fouling and cleaning of RO membranes fouled by mixtures of organic foulants simulating wastewater effluent, J. Membr. Sci., 2011, 376, 196–206, DOI:10.1016/j.memsci.2011.04.020.
- Y.-Q. Xu, Y.-H. Wu, X. Tong, L.-W. Luo and H.-B. Wang, How to select ideal model organic matters for membrane fouling research on water and wastewater treatment, Water Cycle, 2023, 4, 55–59, DOI:10.1016/j.watcyc.2023.02.002.
- V. Freger, J. Gilron and S. Belfer, TFC polyamide membranes modified by grafting of hydrophilic polymers: an FT-IR/AFM/TEM study, J. Membr. Sci., 2002, 209, 283–292, DOI:10.1016/S0376-7388(02)00356-3.
- Y. Guan, S.-L. Li, Z. Fu, Y. Qin, J. Wang, G. Gong and Y. Hu, Preparation of antifouling TFC RO membranes by facile grafting zwitterionic polymer PEI-CA, Desalination, 2022, 539, 115972, DOI:10.1016/j.desal.2022.115972.
- M. Liu, Q. Chen, L. Wang, S. Yu and C. Gao, Improving fouling resistance and chlorine stability of aromatic polyamide thin-film composite RO membrane by surface grafting of polyvinyl alcohol (PVA), Desalination, 2022, 539, 115972, DOI:10.1016/j.desal.2015.03.028.
- N. S. Kandiyote, T. Avisdris, C. J. Arnusch and R. Kasher, Grafted Polymer Coatings Enhance Fouling Inhibition by an Antimicrobial Peptide on Reverse Osmosis Membranes, Langmuir, 2019, 35, 1935–1943, DOI:10.1021/acs.langmuir.8b03851.
- J. Gao, J. Liu, L. Liu, J. Dong, X. Zhao and J. Pan, Multiple Interface Reactions Enabled Zwitterionic Polyamide Composite Reverse Osmosis Membrane for Enhanced Permeability and Antifouling Property, Ind. Eng. Chem. Res., 2023, 62, 2904–2912, DOI:10.1021/acs.iecr.2c04058.
- Z. Yang, D. Saeki and H. Matsuyama, Zwitterionic polymer modification of polyamide reverse-osmosis membranes via surface amination and atom transfer radical polymerization for anti-biofouling, J. Membr. Sci., 2018, 550, 332–339, DOI:10.1016/j.memsci.2018.01.001.
- H. Z. Shafi, Z. Khan, R. Yang and K. K. Gleason, Surface modification of reverse osmosis membranes with zwitterionic coating for improved resistance to fouling, Desalination, 2015, 362, 93–103, DOI:10.1016/j.desal.2015.02.009.
- D. Saeki, T. Tanimoto and H. Matsuyama, Anti-biofouling of polyamide reverse osmosis membranes using phosphorylcholine polymer grafted by surface-initiated atom transfer radical polymerization, Desalination, 2014, 350, 21–27, DOI:10.1016/j.desal.2014.07.004.
- K. Akamatsu, T. Furue, F. Han and S. Nakao, Plasma graft polymerization to develop low-fouling membranes grafted with poly(2-methoxyethylacrylate), Sep. Purif. Technol., 2013, 102, 157–162, DOI:10.1016/j.seppur.2012.10.013.
- K. Akamatsu, T. Saito, H. Ohashi, X. Wang and S. Nakao, Plasma Graft Polymerization and Surface-Initiated Atom Transfer Radical Polymerization: Characteristics of Low-Fouling Membranes Obtained by Surface Modification with Poly(2-methoxyethyl Acrylate), Ind. Eng. Chem. Res., 2021, 60, 15248–15255, DOI:10.1021/acs.iecr.1c02462.
- K. Akamatsu, M. Sano, F. Okada, S. Nakao and X. Wang, Immersion in advanced oxidation water followed by AGET-ATRP to develop low-fouling microfiltration membranes by grafting poly(2-methoxyethyl acrylate), Ind. Eng. Chem. Res., 2023, 62, 10611–10618, DOI:10.1021/acs.iecr.3c01571.
- M. Tanaka, T. Motomura, M. Kawada, T. Anzai, Y. Kasori, T. Shiroya, K. Shimura, M. Onishi and A. Mochizuki, Blood compatible aspects of poly(2-methoxyethylacrylate) (PMEA)—relationship between protein adsorption and platelet adhesion on PMEA surface, Biomaterials, 2020, 21, 1471–1481, DOI:10.1016/S0142-9612(00)00031-4.
- M. Tanaka, T. Motomura, N. Ishii, K. Shimura, M. Onishi, A. Mochizuki and T. Hatakeyama, Cold crystallization of water in hydrated poly(2-methoxyethyl acrylate) (PMEA), Polym. Int., 2000, 49, 1709–1713, DOI:10.1002/1097-0126(200012)49:12<1709::AID-PI601>3.0.CO;2-L.
- M. Tanaka, S. Kobayashi, D. Murakami, F. Aratsu, A. Kashiwazaki, T. Hoshiba and K. Fukushima, Design of Polymeric Biomaterials: The “Intermediate Water Concept”, Bull. Chem. Soc. Jpn., 2019, 92, 2043–2057, DOI:10.1246/bcsj.20190274.
- K. Sato, S. Kobayashi, M. Kusakari, S. Watahiki, M. Oikawa, T. Hoshiba and M. Tanaka, The Relationship Between Water Structure and Blood Compatibility in Poly(2-methoxyethyl Acrylate) (PMEA) Analogues, Macromol. Biosci., 2015, 15, 1296–1303, DOI:10.1002/mabi.201500078.
- R. Nagumo, K. Akamatsu, R. Miura, A. Suzuki, N. Hatakeyama, H. Takaba and A. Miyamoto, A Theoretical Design of Surface Modifiers for Suppression of Membrane Fouling: Potential of Poly(2-mehoxyethylacrylate), J. Chem. Eng. Jpn., 2012, 45, 568–570, DOI:10.1252/jcej.12we110.
- Y. Zhang, Z. Wang, W. Lin, H. Sun, L. Wu and S. Chen, A facile method for polyamide membrane modification by poly(sulfobetaine methacrylate) to improve fouling resistance, J. Membr. Sci., 2013, 446, 164–170, DOI:10.1016/j.memsci.2013.06.013.
- A. J. Blok, R. Chhasatia, J. Dilag and A. V. Ellis, Surface initiated polydopamine grafted poly([2-(methacryoyloxy)ethyl]trimethylammonium chloride) coatings to produce reverse osmosis desalination membranes with anti-biofouling properties, J. Membr. Sci., 2014, 468, 216–223, DOI:10.1016/j.memsci.2014.06.008.
- U. Hirsch, M. Ruehl, N. Teuscher and A. Heilmann, Antifouling coatings via plasma polymerization and atom transfer radical polymerization on thin film composite membranes for reverse osmosis, Appl. Surf. Sci., 2018, 436, 207–216, DOI:10.1016/j.apsusc.2017.12.038.
- S. Ohno, S. Nakao, X. Wang and K. Akamatsu, Morphology and Surface Characteristics of PVDF/Poly(2-methoxyethyl acrylate) Blend Membranes Prepared via NIPS and Their pH-Independent Low-Fouling Properties, Ind. Eng. Chem. Res., 2022, 61, 15326–15335, DOI:10.1021/acs.iecr.2c02271.
- G. Jonsson, Boundary layer phenomena during ultrafiltration of dextran and whey protein solutions, Desalination, 1984, 51, 61–77, DOI:10.1016/0011-9164(84)85053-5.
- G. B. van den Berg, I. G. Rácz and C. A. Smolders, Mass transfer coefficients in cross-flow ultrafiltration, J. Membr. Sci., 1989, 47, 25–51, DOI:10.1016/S0376-7388(00)80858-3.
- K. Akamatsu, M. Sano, F. Okada, S. Nakao and X. Wang, Poly(2-methoxyethyl acrylate)-grafted microfiltration membranes exhibiting low-fouling properties in the presence of salt species, RSC Appl. Interfaces, 2024, 1, 677–683, 10.1039/D4LF00129J.
Footnote |
| † Kurita Water Industries Ltd., Kurita Innovation Hub, 1-4-1 Daikanyama, Akishima-shi, Tokyo 196-0005, Japan. |
| This journal is © The Royal Society of Chemistry 2026 |