Enabling the recirculation of leukocytes in a high-throughput microphysiological system (MPS) to study immune cell-vascular tissue interactions
Tyler
Gerhardson
 ,
Nerses J.
Haroutunian
,
Nerses J.
Haroutunian
 ,
Ryan
Dubay
,
Ryan
Dubay
 ,
Joseph N.
Urban
,
Joseph N.
Urban
 ,
Anthony
Quinnert
,
Anthony
Quinnert
 ,
Brett C.
Isenberg
,
Brett C.
Isenberg
 ,
Samuel H.
Kann
,
Halee
Kim
,
Robert
Gaibler
,
Samuel H.
Kann
,
Halee
Kim
,
Robert
Gaibler
 ,
Hesham
Azizgolshani
,
Elizabeth L.
Wiellette
,
Hesham
Azizgolshani
,
Elizabeth L.
Wiellette
 and
Corin
Williams
and
Corin
Williams
 *
*
Draper, Cambridge, MA, USA. E-mail: cwilliams@draper.com
First published on 6th January 2026
Abstract
Microphysiological systems (MPS) are promising technologies that can enhance the drug development pipeline and fill gaps in identifying medical countermeasures for a variety of public health contexts. The integration of immune cells with MPS is increasingly recognized as a critical element for accurately modeling inflammatory responses in disease, injury, and infection. Specifically, the recruitment of circulating leukocytes to the vascular endothelium is an important first step in the inflammatory cascade. However, developing an MPS that supports physiologically relevant immune cell circulation poses significant biological and engineering challenges due to the delicate, short-lived nature of immune cells and the physical stresses imparted by many pumping systems. Here we present advancements to a previously established high-throughput MPS platform, PREDICT96, to enable recirculation of neutrophil-rich flow within microfluidics-based vascular tissue models. To maintain cells in suspension during recirculation, density adjustments to the culture media were made. Hardware and software controls were integrated to develop a pumping strategy that reduced the peak velocity and acceleration on the recirculating cells, maintaining high viability (90%) and minimal activation of neutrophils for up to 24 hours of continuous recirculation through vascular tissue models. Additionally, an analytical model was developed that mapped pump configuration changes to altered flow characteristics through the system. These technical advancements will enable more accurate modeling of immune cell interactions with tissues in a high-throughput testing platform, which will enhance the understanding of and ability to respond to a range of human health threats.
1. Introduction
Microphysiological systems (MPS) have emerged as powerful technologies to enhance the study of the natural history of disease progression and injury response to various human health threats, and fill gaps in the development and identification of medical countermeasures.1–5 MPS are intended to overcome the limitations of traditional tissue culture to more accurately recapitulate relevant aspects of the in vivo environment, such as biophysical cues and interactions between multiple cell types. The need for sophisticated in vitro models of human disease and injury arises from limitations in the ability to directly study tissue-level responses in vivo. While animal models are leveraged as a substitute for human in vivo responses they can fail to capture important aspects of human physiology, leaving robust solutions to studying some pathological conditions elusive.6,7 The limitations of animal models become especially apparent in the context of inflammatory responses.8 Non-human primates offer the highest alignment of genetic, anatomical and physiological composition with humans but are limited by ethical considerations, cost and availability.9,10 In contrast, MPS offers the potential to be designed to incorporate the appropriate level of complexity to capture human physiology and integrated into platforms that enable longitudinal studies with high temporal resolution and more replicates. Recognizing the potential for in vitro models to address the limitations of animal testing, legislators recently passed the FDA Modernization Act 2.0, which allows alternatives to animal models to support investigational new drugs.11Increased physiological fidelity can be achieved in MPS through the incorporation of cellular and biophysical components that underlie tissue function and responses to perturbations in vivo. As the immune response plays a critical role in injury, infection, and disease, the integration of immune components is a major step toward enhancing MPS capabilities. Of particular interest, circulating leukocytes are first responders of the immune system, providing a mechanism to monitor, detect and eliminate pathogens and aid in tissue response to damage.12,13 One of the first steps required for the initiation of an immune response is leukocyte recruitment to the vascular endothelium. Modeling of endothelial cell-leukocyte interactions is highly dependent on accurate physiological fluid flow.14 Several groups have modeled transendothelial migration in vitro by incorporating immune cells into platforms with flow characteristics.15–22 Although these models have allowed focused study of the migration of immune cells across endothelial monolayers under fluid shear stress (FSS) in the range of 0.25–10 dyn cm−2, they are confined to short duration (∼30 minutes), single pass exposure of tissues to leukocyte-rich flow.
Developing continuous immune cell recirculation with physiological relevance in MPS poses significant biological and engineering challenges due to the delicate nature of immune cells and the mechanically harsh environment developed in many pumping systems. For one, immune cell death begins to occur in static culture within a relatively short time after isolation (∼24 h).23 Additionally, pumps that are advantageous for continuous fluid recirculation (e.g., peristaltic) can have an adverse effect on cell viability which is hypothesized to be due to periodic exposure to very high shear as cells pass through the rollers.24 While syringe pumps and gravitationally-driven flow methods offer gentler mechanisms for establishing flow, the need to replenish the inflow reservoir is at odds with closed-loop recirculation. Cell adhesion, aggregation and settling are additional factors that make recirculation of cells challenging with any mechanism for pumping. Further, the ability to prescribe the correct FSS for a given leukocyte, tissue type and disease state requires the ability to readily modify and predict flow within the MPS.
Here we present advancements to our previously established high-throughput MPS platform, PREDICT96,25 to enable continuous recirculation of immune cell-rich fluid flow. The PREDICT96 plate comprises 96 individual microfluidic devices in a standard culture plate format, allowing its integration with common life sciences tools. Individual devices are a bilayer design comprised of two microfluidic channels separated by a semi-permeable membrane with the capability to support a range of cell types in mono- or co-culture.26–32 Fluid flow is established in the device channels through a custom pneumatic micropump lid and controller. In this manuscript, we demonstrate refined pump hardware for immune cell handling, specifically neutrophils, and present tools to predict and enable precise flow control for immune cell recirculation through the entire system, including: (a) methods for validating continuous cell recirculation and viability through the system; (b) development of hardware and software to improve control of flow dynamics through the system for continuous cell recirculation; (c) development of a system-level model to enable highly controllable cell flow dynamics through the pump and microfluidic tissue models; (d) short-term (2 h) and long-term recirculation (24 h) of primary human neutrophils through the platform, including feasibility studies with vascular tissue models. Altogether, these developments advance the state-of-art MPS technology to enable immune cell-rich flow in a high-throughput platform, opening opportunities to more accurately model disease progression and injury response to various human health threats.
2. Experimental
2.1. Overview of PREDICT96 for leukocyte recirculation
Our custom PREDICT96 platform fully described in Azizgolshani et al. was used to produce controllable flow within microfluidic-based tissue models.25 The PREDICT96 system is composed of a pump controller, a pneumatically actuated pump, and a plate containing 96 microfluidic culturing devices (Fig. 1A). The system was controlled by commands sent to a pump controller via a computer and custom software interface. The user input consisted of commands that set a target flow rate (Q) and average stroke volume (SV) achieved through calibration of the pump.25 The specific pump design used for this work was a pneumatically actuated “high flow” pump that is placed on top of the plate and can impart physiologically relevant FSS up to 7–10 dyn cm−2 on endothelial cells in vascular tissue models.33The pump controller contained a series of six solenoid valves, which converted static pressure and vacuum sources into a series of square pressure waveforms that were routed to the pneumatic pump. The six valves were organized into two groups of three to independently control the left and right half of the pump (Fig. 1B). The pressure waveforms were routed from the controller to the pump via pneumatic tubing and distributed at the pump through pneumatic manifolds to 48 discrete “high flow” micro-pumps (SV ∼12 μL) per side (96 micro-pumps total per pump). Three distinct waveforms were produced to deflect three diaphragm membranes that comprised a single micro-pump: an inlet valve, pump membrane, and outlet valve (Fig. 1B, inset). The membranes reacted to the pressure waveforms by opening in the presence of vacuum and closing in the presence of pressure. The pump membrane waveform set the stroke volume through the pump while the valves were timed to ensure that fluid flowed in one direction through the pump (Fig. S1). The period of a pump cycle (T) which moved one stroke volume through the pump began by opening the inlet valve and ended at closing the outlet valve and was defined as T = SV/Q. Stainless-steel tubes attached to the inlet and outlet ports of each pump extended into the inlet and outlet wells of the device channel when the pump lid was placed atop the PREDICT96 plate, completing the fluidic circuit (Fig. 1A–D). Note that the pneumatic lines that controlled pump operation were separated from the hydraulic compartments of the pump and plate by the pump diaphragm membranes (Fig. 1D).
In these studies, flow was imparted in the basal (bottom) channels of the devices, while the apical (top) channels contained static leukocyte-free media (Fig. 1C). Each channel was a rectangular duct of approximately 7.5 mm in length with a width of 1 mm and height of 250 μm. To achieve leukocyte recirculation at the target flow rate, the pump established a height differential in leukocyte-rich media between the inlet and outlet wells of the basal channels, producing gravitationally-driven flow through the channel (Fig. 1D). To establish leukocyte recirculation in a vascular tissue model, fibroblasts were cultured on the apical side of the semi-permeable membrane and endothelial cells were cultured on the basal side of the membrane within the device channels where leukocytes were recirculated (Fig. 1E). Details on establishment of the vascular model are provided below.
2.2. Fabrication of PREDICT96 hardware
The PREDICT96 pump lid, pump controllers, and microfluidic culture plates were fabricated in-house using previously described methods.25 Plates tops were machined from polyetherimide and bonded to a series of layers that formed the microfluidic channels. The microfluidic channel layers were laser cut (LPKF, ProtoLaser U4) from 188 μm-thick cyclic olefin polymer (COP, ZF14-188: Zeon Corp., Tokyo Japan) and 28 μm-thick cyclic olefin copolymer (COC, 8007 COC: Tekni-plex, Wayne, PA, USA). A 22 μm-thick track-etched polycarbonate membrane with a pore size of 1 μm and open area of 4% was thermally laminated between the microchannel layers in a hydraulic press at 120 °C and 1 MPa for 30 minutes. Plates and pumps were sterilized by ethylene oxide gas prior to use in cell culture experiments described below.2.3. Priming and preparation of the PREDICT96 plate and pump
After sterilization and immediately prior to use with cell culture experiments, PREDICT96 plates and pumps underwent a series of priming steps. First, the plates were exposed to a 5 minute oxygen plasma treatment (March Instruments, PX-250), followed by one rinse with 70% ethanol (Decon Labs, 04-355-305), two rinses with distilled water, and one rinse with phosphate buffered saline (PBS). The priming steps consisted of establishing a gravitational pressure gradient across the inlet and outlet wells of each microfluidic channel by adding 100 μL and 15 μL of fluid, respectively, and subsequently aspirating the fluid out of the wells after the volumes had equilibrated. Pumps were prepared by a series of priming steps that consisted of running different fluids through the pump: first, one 15 minute prime with 70% ethanol followed by three 5 minute rinses with distilled water, at a flow rate of 140 μL min−1. The plates and pumps then underwent a final priming step with density modified media (described below). For the plate, 100 μL and 20 μL of neutrally buoyant media was added to the device inlet and outlet ports, respectively. The fluid was aspirated, and then fresh neutrally buoyant media was added three times to ensure the plate was primed with the target concentration of neutrally buoyant media. Similarly, the pump was primed with three 5 minute rinses of the neutrally buoyant media. Prior to experiments, the pump was calibrated according to methods described previously.25 The measured average stroke volume produced for a single pump cycle was used to set flow rates in the pump software.2.4. Isolation and preparation of neutrophils
All human blood products were collected offsite by a vendor (Charles River Laboratories) and delivered overnight for the isolation of human neutrophils. Neutrophils from multiple donors (Table S1) were isolated from heparinized human whole blood using a negative selection kit (STEMCELL Technologies, EasySep Direct Human Neutrophil Isolation Kit). The neutrophils were suspended in a density-modified media that was comprised of a density gradient media (OptiPrep™, Serumwerk Bernburg AG, 1893) and either Hank's balanced salt solution (HBSS) or EGM-2MV media (Lonza). The ratios of the two fluids were tuned to achieve neutral buoyancy of neutrophils (see Experimental section 2.5). Neutrophil suspensions were used for experiments immediately following isolation and preparation. 45 μL of neutrophil suspension was added to the devices that were primed with 120 μL of neutrally buoyant media to produce an in-device concentration of 2 × 106 neutrophils per mL for a total of 330![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 neutrophils per device.
000 neutrophils per device.
2.5. Density matching of media for cell and particle suspensions
To verify that media modifications could maintain particles in suspension, neutrally buoyant media was initially developed using particles with a well-defined density (polystyrene microparticles, 1.05 g mL−1). Suspensions of equal parts of 8–12.9 μm and 13–17.9 μm polystyrene (PS) microparticles (Spherotech, PPS-5 and PPS-6) in either 1× PBS and 0.05% Tween 20 or a neutrally buoyant media (1× PBS, OptiPrep™, and 0.05% Tween 20) were made at a concentration of ∼1.5 × 106 particles per mL. Note that Tween 20 was added to minimize aggregation of the microbeads and was not used for cell experiments. Aliquots of 100 μL were pipetted into 15 wells of a standard 384 well plate (Corning, CLS3702). The topmost 20 μL was sampled from N = 3 wells at five timepoints over 2 h, where a new well was used at each timepoint such that the volume was never depleted below 80 μL. The concentration of the extracted topmost 20 μL at each timepoint was measured using an automated cell counter (Nexcelom Bioscience LLC., Cellometer).To establish neutrally buoyant media for neutrophil recirculation, experiments were performed to identify the optimal density for neutrophils. Neutrophils were fluorescently stained and suspended in HBSS solutions mixed with different concentrations of Optiprep™. The density of the solutions was measured using a densitometer (Anton Paar, DMA1001). A series of five solutions were made with a range of densities that bracketed the cell density reported in literature. Neutrophils were suspended in the solutions at a concentration of 5 × 104 cells per mL. 100 μL aliquots of each sample were pipetted into 5 adjacent wells of an optically transparent 384 well plate. The plate was imaged on a confocal microscope with a motorized, programmable positioning stage (Zeiss, LSM 780 Confocal). A two-hour time series was set up in the microscope software (Zeiss, Zen Microscopy Software) to acquire an image of the bottom of each well every two minutes. Time series confocal images were analyzed to calculate the total area covered by cells at each timepoint. Plots of area coverage vs. time were used to infer the degree of cell settling over time. Upon completion of the two-hour study, to assess if cells floated in each solution, the top 40 μL of suspension from each well was pipetted into new wells by placing 20 μL aliquots into two distinct wells. The plate was then imaged on an image cytometer (Nexcelcom, Celigo).
2.6. Establishing the vascular tissue model
For a sub-set of experiments, a co-culture of human intestinal microvascular endothelial cells (HIMEC) and fibroblasts (FB) was established in PREDICT96 according to previously described methods28 prior to introduction of neutrophils. HIMECs were seeded in the basal channel on day −1 at a density of 2 × 106 cells per mL using a volume differential of 35 μL in the inlet port and 15 μL in the outlet port to drive the cells into the channel. Once the volume equilibrated, the plate was then flipped upside down for 3–4 h to allow the cells to settle on the basal side of the membrane. FB were seeded in the apical channel on day 0 at a density of 5 × 105 cells per mL and allowed to adhere to the apical side of the membrane. Afterward, FSS was ramped up over a 24 hour period to 7 dyn cm−2 in the HIMEC-containing channel. The tissues grew and stabilized until day 6, with a media change every other day. For activated conditions, HIMECs were dosed with TNFα (3 ng ml−1) on day 6 for 24 hours prior to introduction of neutrophils. For recirculation experiments, fluorescently labeled neutrophils were recirculated in a fluorescently labeled endothelium compartment for 2 to 24 hours at 37 °C, and adhesion was assessed across conditions via live imaging as described in Experimental section 2.10.2.7. Fluorescent labeling of neutrophils and endothelial cells
For live imaging studies, neutrophils were fluorescently labeled (Invitrogen, CellTracker Green CMFDA) by incubating the cells with 1 μM stain in low-serum media for 30 minutes at 37 °C. The dye was subsequently washed out via centrifugation for 9 minutes at 300×g and the neutrophils were resuspended in density matched media for use in experiments. HIMEC were fluorescently labeled (Invitrogen, CellTracker Red CMTPX) by incubating the cells with 1 μM stain in low-serum media for 30 minutes at 37 °C. Excess dye was subsequently washed out with fresh media prior to introducing the neutrophils for recirculation studies.2.8. Neutrophil viability measurements
The viability of neutrophils was measured using an imaging cytometer (Nexcelom, Celigo). Neutrophil suspensions were sampled and mixed with acridine orange and propidium iodide (AOPI) (Revvity, CS2-0106-25ML) and imaged. The cytometer's software processed the images to count the number of dead and live cells. Viability was reported as the percentage of live cells.2.9. Flow cytometry
Flow cytometry was used to assess the purity and activation state of the leukocytes. Aliquots of samples that corresponded to 3 × 105 cells were treated with an Fc blocker for 10 minutes at room temperature (Biolegend, Human TruStain FcX) and stained with a specific antibody cocktail (Biolegend) at a final dilution of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 at 4 °C for 30 minutes. The antibody cocktail used depended on the analysis type (Table S2). Stained cells were washed twice with buffer. Dead cells were stained with nucleic acid stain (Invitrogen, Sytox Green) or membrane permeable stain (Biolegend, Zombie NIR) at a 1
100 at 4 °C for 30 minutes. The antibody cocktail used depended on the analysis type (Table S2). Stained cells were washed twice with buffer. Dead cells were stained with nucleic acid stain (Invitrogen, Sytox Green) or membrane permeable stain (Biolegend, Zombie NIR) at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5000 final dilution for 15 minutes at room temperature. The final cell suspension was analyzed on a flow cytometer (Thermo Fisher, Attune NxT Flow Cytometer). Data were analyzed using FlowJoV10 and gates were determined via fluorescent minus one (FMO) controls.
5000 final dilution for 15 minutes at room temperature. The final cell suspension was analyzed on a flow cytometer (Thermo Fisher, Attune NxT Flow Cytometer). Data were analyzed using FlowJoV10 and gates were determined via fluorescent minus one (FMO) controls.
2.10. In-plate imaging of particle and neutrophil recirculation
In-plate imaging of particle and neutrophil recirculation was performed to measure flux and velocity through the pump and microfluidic channels of the PREDICT96 plate. A CMOS camera (Hamamatsu Photonics, C11440-22CU with ORCA-flash4.0) was mounted to an inverted microscope (Zeiss, AXIO Observer Z1 Inverted Microscope) with a 5× objective. The microscope was set up to image several devices across the plate. For a given device, the field of view and the imaging focal plane were placed at the center of the bottom channel. The location of the stage for each device was stored in the software (Zeiss, ZEN Microscopy Software) to enable rapid, automated switching between devices for time series imaging studies. The bottom channel of each device to be imaged was then seeded with 15 μm fluorescent polystyrene particles (Bang Labs, FSDG009) or fluorescently labeled neutrophils suspended in neutrally buoyant media. 45 μL of particle or neutrophil suspension was added to the devices to produce an in-device concentration of 1 × 106 particles per mL or 2 × 106 neutrophils per mL. Once the devices were loaded with particles or neutrophils, the pump was mated with the plate such that the intake and dispensing ports of the micropumps were respectively placed in the outlet and inlet wells of the bottom channels of devices. Flow was established in each device by running the pump controller software for a range of flow conditions.2.11. Imaging flow dynamics of the PREDICT96 pump
To understand how modifications to the pneumatic circuit directly impacted flow dynamics of the pump itself, flow measurements of the PREDICT96 pump in the absence of the plate were performed. To allow imaging of the flow field, a 100 mm long 0.9 × 0.9 mm square glass capillary tube (Vitrocom, 8290) was connected at the end of a stainless-steel pump outlet port using platinum tubing (New England Small Tube Corp) as a fluidic seal. A 1.5 mL centrifuge tube (Eppendorf, EP022363531) was used as the inlet reservoir by drilling a small hole through the wall of the tube and connecting to the inlet port with a series of tubing and sealant. All connections to the pump ports were kept as short as possible while closely matching the ID of the pump ports to minimize added fluidic resistance and capacitance to the circuit. The glass capillary tube was laid atop a z-height adjustable stage with black backing. The capillary tube was aligned to the field of view of an upright microscope (Zeiss, AxioZoom V16). The imaging focal plane was placed at the midplane of the capillary tube and a camera (Teledyne Photometrics, Prime BSI Express) connected to the microscope was configured to capture images at >800 frames per second. The inlet reservoir was filled with a suspension of 15 μm diameter fluorescent polystyrene particles (Bang Labs, FSDG009) in neutrally buoyant media at a concentration of 1 × 104 particles per mL. Flow was established in each device by running the pump controller software for a range of flow conditions.2.12. Image analysis of flow dynamics measurements
Image analysis for the flow dynamics experiments described above was performed via a custom image processing pipeline using open-source particle tracking software (ImageJ 1.54f, TrackMate).34 Within ImageJ, a custom Jython (https://www.jython.org) script was used to automate both pre-processing and particle tracking of videos acquired for different devices, pump conditions, and timepoints. Pre-processing consisted of: (1) cropping images to isolate regions of interest (ROIs), (2) subtraction of the minimum intensity time-projection to remove background signal, and (3) denoising using a Gaussian blur. Pre-processed images were passed to the TrackMate module where particle detection and tracking were performed. Particles were detected using the built-in Laplacian of Gaussian detector, for which the Quality Threshold and Particle Radius parameters were manually tuned for each experiment to provide adequate discrimination of particles from image noise. Next, linking of particles between frames was performed with the Advanced Kalman Tracker using a 700 μm Initial Search Radius, 100 μm Search Radius, and 5 frame Max Frame Gap; rigorous definitions of each parameter are available in the TrackMate documentation.34 Additionally, all flows were laminar, producing unidirectional, linear particle motion through the flow channel which allowed a feature penalty to be imposed on the transverse displacement of particles which further improved track detection fidelity. To enable application of this feature penalty, care was taken to position the flow channel during imaging experiments such that the longitudinal axis of the channel (and accordingly the direction of particle motion) was laterally aligned with the camera field of view. From the tracking datasets, a separate custom Python script was used to calculate flux and velocity waveforms for neutrophils and polystyrene particles.2.13. Design and integration of pneumatic connection modifications
In the standard configuration of the PREDICT96 operation, a square pressure waveform generated by the pump controller actuates the membranes in the pump that generate flow. The waveform is transmitted from the pump controller to the pump via pneumatic tubing that preserves the square shape of the waveform. To reduce instances of high flow during pump actuation that may be harmful to recirculating cells, the waveform shape was changed by modifying the pneumatic tubing connection. Specifically, a pneumatic resistor–capacitor (RC) connection between the controller and pump was devised, modeled (see Experimental section 2.14) and fabricated. A small internal diameter (ID) tube was implemented to add hydrodynamic resistance, and a larger ID tube was placed in parallel with the pump membrane to add compliance (pneumatic capacitance). The RC connection was used to modulate the risetime of the pressure waveform at the pump membrane.To implement this connection for different flow rates, the resistor was kept fixed while the size of the capacitor was changed. A set of equations was defined to generalize the relationship between the flow rate, capacitance and size of the capacitor. The relationship between capacitance and the internal volume of the capacitor (i.e., internal tubing volume) is described as
 | (1) |
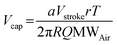 | (2) |
To accommodate the changes to the pressure waveform risetime at the pump membrane and ensure liquid pumping occurred in one direction, the actuation timing of the pressure waveforms applied to the inlet and outlet valves was also modified (Table S3). This was necessary to ensure that (1) the inlet valve was open and the outlet valve was closed prior to a pump intake event and (2) the outlet valve was open and the inlet valve was closed prior to a pump expulsion event (Fig. S1).
2.14. Finite element modeling of pneumatic RC connection
To verify that the proposed RC connection would modify the pressure waveform applied at the pump, finite element modeling of the pneumatic RC connection was performed in COMSOL (COMSOL Multiphysics 5.6). A 2D axisymmetric, laminar flow model was implemented in a geometry comprising a 60 inch length of tubing. The inner diameter (ID) of the tubing was set to either 1/16 inch or 0.02 inch. For the 0.02 inch tubing, a capacitor was modeled by adding a 26 inch long, 0.25 inch ID tube to the outlet of the 60 inch length of tubing. The material properties were set using the built-in properties for air. All boundaries except the inlet were set to wall boundary conditions. The inlet was set to a pressure condition that consisted of a square pressure waveform with an amplitude of 12 psi and frequency of 0.2 Hz. To compare the simulation results with experimental results, the pressure at the inlet and outlet were measured for two tubing sets that matched simulation inputs. One set consisted of a 60 inch long, 1/16 inch ID tube with the outlet was capped with a 1/16″ barbed Luer lock (McMaster-Carr, 51525K291) and Luer lock end plug (McMaster-Carr, 51525K371). The second set consisted of a 60 inch long, 0.02 inch ID tube (resistive tube) with a 26 inch long 0.25 inch ID tube (capacitive tube) connected via a t-connector at the outlet. The capacitor outlet and resistor outlet were both capped with barbed Luer locks (McMaster-Carr, 51525K286 & 51525K291) and Luer lock end plugs.2.15. Measurement of pressure waveforms
To confirm that the pneumatic connection changes produced the desired changes to the pressure waveform, pressure measurements were made between the pneumatic tubing lines and ports on the pump. Pressure measurements were collected using a data acquisition system (BioPAC, Inc., MP150) connected to a pressure sensor (PREPS-N-000, PendoTech) and amplifier (BioPAC, Inc., DA100C). The system was calibrated using a pressure controller (Alicat Scientific, PCD-100PSIF-D/5P).2.16. System-level modeling of flow dynamics within PREDICT96
In order to establish a theoretical basis for experimental results and test a framework for predicting flow dynamics within the pump and plate given an arbitrary pressure waveform at the pump membrane, a system-level model for flow dynamics through the pump and plate was devised. The radial displacement profile of the pump chamber membrane was calculated from a modified version of the deflection for a clamped circular plate under uniform applied pressure given by35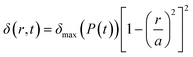 | (3) |
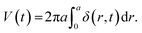 | (4) |
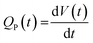 | (5) |
| QPI(t) = QP(t) > 0 | (6) |
| QPE(t) = −QP(t) < 0 | (7) |
 | (8) |
The simulated results were validated for a range of input conditions using fluorescent particle tracking methods described above (experimental sections 3.10, 3.11 and 3.12).
2.17. Statistical analysis
By default, a one-way analysis of variance (ANOVA) test was employed to compare cell viability across recirculation conditions, including static controls, at the final timepoint. In the event that a condition was not normally distributed, non-parametric tests (e.g., Kruskal Wallis) were employed. Either Tukey's HSD (honestly significant difference) or Dunn's post hoc tests were used for pairwise comparisons. A Bonferroni correction factor was used to reduce type I errors for Tukey's HSD and Dunn's tests. A p-value of <0.05 was used to indicate statistical significance unless otherwise specified. Sample sizes of at least N = 3 devices were used, and in some cases more, depending on the number of neutrophils available after isolation from whole blood.3. Results and discussion
3.1. Establishing the immune cell recirculation workflow
To optimize a neutrally buoyant media formulation for human primary neutrophils, we developed a method to optically track cell settling in different ratios of HBSS and OptiPrep™, with densities ranging 1.008–1.09 g mL−1 (Video S1). Fig. 2C and D shows the settling over time for neutrophils in media with different densities. Flat curves (e.g., 1.090 g mL−1) indicated little to no settling while curves that increased and then plateaued (e.g., 1.008 g mL−1) indicated settling over time. Due to minimal neutrophil settling or floating (Fig. S3), we identified 1.080 g mL−1 as the optimal density for neutrophils, which was 0.072 g mL−1 greater than the density of the unmodified media and had a dynamic viscosity of 0.8 mPa s at 37 °C.
Neutrally buoyant media provides a simple way to keep cells suspended during recirculation without hardware or exertion of forces on cells. While other density modifiers exist, we chose OptiPrep™ as it is non-ionic, non-toxic and iso-osmotic. Although preparing media of a specific density is a straightforward calculation using the rule of mixtures, choosing the matching density to ensure cells do not sink or float throughout the duration of an experiment requires accurate knowledge of the cell density. By directly measuring settling over time, our method provides the ability to tailor media formulations to meet the requirements for specific cell types and study duration. For neutrophils, we identified a media density that aligned well with reported literature values that ranged from 1.079–1.083 g mL−1.36 We note that the method can also be extended to other cell types. For example, in a subset of experiments, we identified a media density of 1.070 g mL−1 for monocytes (Fig. S4) which also aligned well with literature.37 While the agreement with literature validates our methods, the reliance on literature may not be suitable for every immune cell recirculation application. For instance, density matching prior to each experiment can provide the ability to account for donor-to-donor variability in cell density, differentiate between activated and inactivated leukocytes, and provide the potential to optimize media density for circulating mixed populations of immune cells.36,38
The reduced viability associated with disruptions in flow may be due to the mechanical response of neutrophils to flow versus static environments.39,40 During frequent sampling, neutrophils experienced durations of no shear followed by periods of shear, which may have resulted in sudden changes to neutrophil response that ultimately resulted in the reduced viability we observed.41 This aligns with many studies that report that neutrophils respond uniquely to the magnitude and duration of applied shear.42,43 Another hypothesis for the reduced viability could be reduced nutrient and oxygen transport to cells within the channels in the absence of flow. This could be explained by longer and more frequent static periods in PREDICT96 channels which were much smaller volume than the wells of a 96 well plate. In either case, the improved viability of neutrophils under constant flow prompted efforts to minimize the number of intermittent samples taken in subsequent experiments.
3.2. The pneumatic resistor–capacitor (RC) connection modulated pressure at the pump
Although circulating neutrophils through PREDICT96 without stopping flow showed high viability (∼85%, Fig. 2F), we hypothesized that slowing the rate of intake and expulsion of cell-rich media within the pump could further improve viability. To this end, we added a pneumatic RC connection between the pump controller and the pump (Fig. 3A and B). The RC connection allowed us to modulate the time required to transition from positive to negative pressure for a given target flow rate. We will henceforth refer to this time as “risetime”.For the standard connection, the risetime was on the order of 200 ms, generating a square waveform (Fig. 3C, left). Initial implementation of the pneumatic RC connection showed potential to extend the risetime by roughly 18-fold to create a triangle waveform (Fig. 3C, right), with good agreement between experimental and simulation data (Fig. 3C, green vs. black curves). For flow applications, the maximum risetime was set by the target flow rate and stroke volume, which set the stroke period. For example, for a flow rate of 140 μL min−1, a capacitance of 1.55 mg psi−1 (23.5″ long, 0.122″ ID tube) achieved a risetime of 2.4 s, approximately a 10-fold increase compared to the standard connection.
The capacitance of the system was set by the compressibility of air and was proportional to the volume of the added chamber and gas properties (eqn (1), Experimental section 2.13). To keep the size of the capacitor manageable for pumping rates of interest, we increased the resistance of the 60″ long pneumatic connection by reducing the tubing ID from 0.066″ to 0.02″. The pneumatic RC connection was implemented to reduce the pulsatory flow through the PREDICT96 system associated with rapid transitions (∼200 ms) in the pump membrane. While pulse dampeners are commonly used in microfluidics44 to smooth flow, they tend to add pneumatic capacitance downstream of the pump and interface directly with the liquid flow path of the pumping circuit and biological compartments. Because our system uses pneumatic actuation, we implemented an upstream solution that directly modulated the actuation of the pump while preserving the target flow rate, without interacting with the biological compartments. Although the capacitor used in this study was chosen to accommodate the stroke rate required to achieve a nominal flow rate of 140 μL min−1 (2 dyn cm−2), accommodating higher or lower flow rates can be achieved by simply changing the capacitor volume as indicated in eqn (2) (Experimental section 2.13).
3.3. Pump control modifications reduced hardware-associated forces, improved viability and limited activation of neutrophils recirculated through PREDICT96
The reduction in flow-associated forces in the PREDICT96 platform correlated with improvements in neutrophil viability. The higher neutrophil acceleration in S-ST correlated with a 25% reduction in neutrophil viability after 2 h of circulation compared to input viability at T = 0 h (Fig. 4D). In contrast, neutrophils recirculated using the S-MT and RC-MT, which had substantially lower accelerations during a pump cycle, exhibited a much smaller drop in viability (∼3% decrease relative to T = 0). In fact, the viability of neutrophils in static suspension controls and recirculated with S-MT and RC-MT were statistically equivalent (p-value = 0.99). These findings suggested that lengthening tI2E was the major factor in improving the viability of recirculating neutrophils.
Although the plate channels served as a convenient viewing window to neutrophil flow during recirculation, the channel dynamics measurements were buffered from the pump dynamics by the capacitive effect of the inlet and outlet wells in the PREDICT96 plate. Still, the peak acceleration through the channel served as a relative indicator of the force that neutrophils experienced during recirculation and a practical metric for comparison. FSS is another important parameter to carefully control and monitor to minimize the degree of flow-induced damage or activation on circulating cells.45 PREDICT96 is designed to apply a time-averaged wall shear stress to tissue by prescribing a target flow rate within the microfluidic culture channels.25 In this experiment, the time-averaged FSS was 2 dyne cm−2 for all pumping configurations. While changes to the flow pulsations for each pump configuration changed the peak dynamic FSS (Table S4), the values for the largest peaks were at least two orders of magnitude below those typically reported to cause damage to circulating cells.45,46
The surface markers were chosen due to their association with neutrophil activation. CD11b and CD62L are both upregulated during stages of diapedesis.49,50 CD177 is associated with neutrophil activation due to its role in vascular migration.51 CD16 and CD66b are both common neutrophil markers associated with activation states.52–54 The lack of overlap between the PMA-activated positive controls and pump configurations provided evidence that recirculated neutrophils were not activated by the PREDICT96 hardware. This was a critical finding, as it suggests that neutrophils will not respond adversely to PREDICT96 hardware, allowing us to isolate neutrophil activation to changes within the tissue environment (e.g., injury or disease states).
While both S-MT and RC-MT showed high neutrophil viability and minimal activation, we continued to use RC-MT for our subsequent recirculation studies due to its additional 2- to 3-fold reduction in plate channel acceleration (Fig. 4C). Additionally, the increased risetime of RC-MT changed the intake and expulsion flow rate through the pump which could impact the viability of recirculated cells for different applications. This was not examined in the neutrophil recirculation comparisons of Fig. 4 due to the need to decouple the pump and plate for pump measurements, but is explored in greater detail in subsequent sections of the paper (see Results and discussion section 3.5).
3.4. Recirculated neutrophils maintained phenotype and function in PREDICT96 vascular tissue models
To demonstrate the performance of the system in a biologically relevant context, we tested neutrophil recirculation in a vascular tissue model. First, we established a mature vascular co-culture model in PREDICT96, comprised of primary human intestinal microvascular endothelial cells (EC) and intestinal fibroblasts, and then introduced recirculating neutrophils into the EC-containing device channels for 24 h. Neutrophil viability under flow with the RC-MT configuration remained high (∼90%) throughout 24 h of continuous recirculation (Fig. 6A). We also assessed the phenotypic composition of the neutrophil population over time, which showed changes between static and flow conditions (Fig. 6B). Neutrophils started predominantly as CD16high/CD66b (normal phenotype) and showed a shift toward CD16dim/CD66b (indicating early signs of apoptosis) over time.55 This phenotype shift was predominant in static conditions, where the majority of the population (79%) was CD16dim/CD66b at 24 h. For neutrophils under flow, the trend toward CD16dim/CD66b was present but dramatically reduced (44%) compared to static neutrophils. This finding highlighted the importance of flow to neutrophil stability over time and suggested that flow better maintains neutrophil health in culture.Considering that neutrophils are “first responders” of the immune system, we next tested their ability to be recruited to inflamed endothelial cells in the PREDICT96 vascular model after 2 h of recirculation. EC were first stimulated with TNFα for 24 h, and then neutrophils were introduced and recirculated for 2 h. There was little neutrophil attachment to control (non-stimulated) EC, while significantly more neutrophils (p < 0.0001) adhered to TNFα-stimulated EC (Fig. 6C and D). This result demonstrated the ability of our model to capture important elements of leukocyte–endothelial cell interactions in response to perturbations.
The high viability and improved phenotype of neutrophils sustained over 24 h of recirculation within PREDICT96 is an exciting finding that shows promise for studying longer-term biological questions, such as leukocyte extravasation and down-stream effects associated with tissue damage, inflammation, or infection. Neutrophils recirculated through PREDICT96 in these experiments had a round-trip recirculation cycle time of approximately 83 s which corresponded to 1041 cycles through the system in 24 h. While the viability remained high after 24 h of recirculation (∼90%) there was still a statistically significant drop from the viability at 0 h and a shift towards a more pre-apoptotic state. This may be explained by the inherent lifespan of neutrophils. In one study, autologous human neutrophils reinjected into subjects were reported to have a half-life of 6–8 h.56 While this is much shorter than the 24 h presented here, a primary factor in the reported half-life in vivo is removal by the reticuloendothelial system, which is notably absent in our vascular-immune in vitro model. A study on the in vitro storage of porcine neutrophils showed a similar 24 h viability (∼90%) suggesting our results align with literature.23 While there is limited data in literature directly comparing neutrophil phenotype under flow versus static conditions, the more drastic shift towards an apoptotic state in static neutrophils may be associated with the absence of FSS or reduced nutrient and oxygen transport (i.e., reduced convective mass transfer) in static samples. It will be of interest to further study the effects of flow on neutrophil phenotype and function in future work.
While leukocyte recruitment in other in vitro vascular tissue models under flow has been demonstrated,15–22 there are differences compared to our platform. Many of these systems have similar characteristics to PREDICT96, such as microfluidic flow channels and porous membrane bilayers. However, previous studies tend to be limited to short durations (3–30 minutes), likely due to limitations of platforms designed for single pass flow of leukocytes. In single pass systems, longer duration studies would require larger and potentially prohibitive numbers of leukocytes. Additionally, single pass systems prevent the buildup of important paracrine signaling factors, as they are removed during single pass flow. By leveraging the closed loop, recirculating flow control of PREDICT96, we are able to recirculate the same population of neutrophils for at least 24 h, enabling the ability to measure important soluble factors secreted by all cell types in the system. We note that analysis of secreted factors was beyond the scope of the current work but would be valuable to study in the future for more in-depth characterization of the vascular-immune model. Additionally, while we only assessed neutrophil recruitment to vascular tissue after 2 h of recirculation, the high viability of neutrophils after 24 h enables the potential to study time dependent effects of vascular recruitment in future studies.
While the current study focused on establishing the feasibility of neutrophil recirculation within PREDICT96 and incorporation with vascular tissue models, there is considerable potential to develop additional or even higher fidelity immune-integrated tissue models within PREDICT96. For instance, a pilot study of monocyte recirculation in vascular tissue yielded a similarly high viability of monocytes after 24 h of continuous recirculation (Fig. S8). Additionally, the platform has been leveraged to study disease progression, therapeutics, and countermeasures across a range of tissue types including lung, kidney, intestine, and vascular.26–32 By establishing a method to recirculate leukocytes within PREDICT96, we have the potential to integrate important immune physiology into a range of in vitro tissue models, which will enhance the ability to capture important aspects of human physiology and disease progression within MPS.
3.5. System-level model predicted characteristics of pump and channel flow dynamics in PREDICT96
Given the dependence of flow dynamics and recirculating immune cell viability on the pump actuation configuration, we sought to establish a method to predict flow dynamics through PREDICT96. Our system-level model for flow dynamics through the pump and plate established a theoretical basis for experimental results and a framework for predicting flow dynamics within the pump and plate for arbitrary applied pump pressure waveforms. Simulated flow characteristics of the pump and plate for single pump cycles across two system configurations were in good agreement with empirical flow dynamics measured during flow experiments using fluorescent beads (Fig. 7). The model accurately captured the reduced (∼3.6-fold) peak particle velocity achieved by implementing the RC-MT configuration when compared with the S-MT configuration (Fig. 7A). For flow dynamics within the plate channels, the model captured the dual peaks associated with the intake and expulsion events of a pump cycle (Fig. 7B). While the contours of the plate velocity profiles showed general agreement with the measured velocity distributions across the S-MT and RC-MT configurations, there was some noticeable misalignment in the decay curvature of the second peak (pump expulsion) of the S-MT configuration. For the RC-MT configuration the model showed improvements when compared to the measured distribution, capturing the ramps preceding the peaks and better capturing the decay curvature following the peaks. While the modeled peaks did not fall within ±1 standard deviation of the mean measured peaks for all configurations, much of this discrepancy can be attributed to the large spikes associated with the peak of the pump expulsion. We hypothesized that this was a result of jetting particles directly from the pump outlet into the plate channel inlet, which were ∼2 mm in proximity, and found that reducing the amplitude of the pressure waveform that actuated the pump removed these features and resulted in better agreement between the model and measurements (Fig. S9). The model was compared to measurements for 16 unique pumping configurations and produced peak velocities that were within ±1 standard deviation of the mean measured peak velocities for 12 out of the 16 configurations (Table S5). While the model has some discrepancies with measured data, it provides a good basis for estimating the flow dynamics that neutrophils or other potential recirculating cell types may experience for different pumping regimes within the pump and plate of the PREDICT96 system.The pump expulsion velocity and the reduction produced by the RC-MT pump configuration highlighted the critical function of the RC connection. The lengthening of the pressure waveform risetime for the same stroke period drastically reduced the velocity of particles through the pump. Despite the substantial difference in pump expulsion velocities between the two configurations (432 mm s−1 for S-MT and 120 mm s−1 for RC-MT, ratio of 3.6), the corresponding plate velocities were nearly identical (14.9 mm s−1 for S-MT and 13.3 mm s−1 for RC-MT, ratio of 1.1). The key advantage of implementing the RC-MT configuration is that it produces flow characteristics, such as time averaged and peak FSS, that are equivalent through physiologically functional portions of the fluid circuit (i.e., the tissue culture channels) while substantially reducing high flow features within the pump as recirculating cells pass through it. The ability to modify the capacitance of the RC connection for different target flow rates or adjust the degree of ramping (eqn (2), Experimental section 2.13) as well as pressure waveform amplitude, provides the ability to optimize system settings for a wide range of applications.
Developing and validating a model for predicting flow dynamics through MPS platforms is an important tool for advancing cell recirculation applications. The analytical model in combination with pneumatic controls developed in this work provides a framework for designing channel flow profiles to achieve target flow through tissues while minimizing potentially damaging factors to flow-sensitive recirculating cells, such as high acceleration or shear stress. While the model presented is designed specifically for PREDICT96, aspects of the model can translate to other MPS platforms. For instance, other groups have proposed similar analytical approaches to solving fluid flow in the context of fluid and cell recirculation through MPS.57 For their MicroHeart MPS, Offeddu et al. presented a fluid equivalent circuit that produced a system of three differential equations that were solved using a numerical solver. They reported simulated data that predicted channel velocities often within ±1 standard deviation of the mean measured velocity. Additionally, many MPS applications leverage liquid reservoirs and pressure heads to drive flow through fluidic channels where tissue cultures are established.58–60 While we modeled a pneumatically driven membrane pump as the source of flow to and from the reservoirs, the model can be easily adapted to different sources of flow.
4. Conclusion
We have presented advancements to a previously established high-throughput MPS platform, PREDICT96, to enable recirculation of viable, functional leukocytes within microfluidic-based tissue models under physiological flow conditions. Hardware and software controls were used to develop a pumping strategy that maintained high viability (90%) and minimal activation of neutrophils for up to 24 hours of continuous recirculation through vascular tissue models. An analytical model was developed and validated that mapped pump controls to flow characteristics through the system that can be used to tune recirculation conditions for other cell types and flow rates. Future work will focus integrating circulating leukocytes into disease and injury models within PREDICT96 and advancing pump controls for a wider range of flow rates. Overall, we expect that advancements in integrating immune cells into MPS will enable more accurate modeling of human (patho)physiology in a variety of contexts related to inflammation, disease, infection, and injury. Immune-integrated human tissue models can be leveraged to enhance our understanding of a range of human health threats, and subsequently, our ability to respond to and treat them.Author contributions
Tyler Gerhardson: conceptualization; data curation; formal analysis; investigation; methodology; validation; visualization; writing – original draft. Nerses J. Haroutunian: investigation; methodology; validation; data curation; software; visualization; writing – review & editing. Ryan Dubay: conceptualization; data curation; formal analysis; investigation; methodology; project administration; supervision; visualization; writing – review & editing. Joseph N. Urban: data curation; investigation; methodology; software; writing – review & editing. Anthony Quinnert: investigation; methodology; validation. Brett C. Isenberg: conceptualization; software; writing – review & editing. Samuel H. Kann: investigation, methodology; validation; data curation; writing – review & editing. Halee Kim: investigation; methodology; validation. Robert Gaibler: conceptualization; investigation; methodology; validation; data curation; project administration; supervision; visualization, writing – review & editing. Hesham Azizgolshani: conceptualization; methodology; writing – review and editing. Elizabeth L. Wiellette: conceptualization; funding acquisition; project administration; supervision; writing – review & editing. Corin Williams: conceptualization; funding acquisition; project administration; supervision; writing – review & editing.Conflicts of interest
The authors have filed a provisional patent on this work. There are no other conflicts to declare.Data availability
All data supporting this article have been included in the main manuscript and supplementary information (SI).Supplementary information is available. See DOI: https://doi.org/10.1039/d5lc01001b.
Acknowledgements
This project has been funded in whole or in part with federal funds from the U.S. Department of Health and Human Services; Administration for Strategic Preparedness and Response; Biomedical Advanced Research and Development Authority, under OT number: 75A50123C00042. The authors also thank Vishal Tandon and Katie Hulse for their discussions and feedback on drafts of the manuscript.References
- L. A. Low and D. A. Tagle, Lab Chip, 2017, 17, 3026–3036 Search PubMed.
- L. A. Low and D. A. Tagle, Expert Opin. Orphan Drugs, 2016, 4, 1113–1121 CrossRef.
- V. K. Singh and T. M. Seed, Expert Opin. Drug Discovery, 2022, 17, 865–878 Search PubMed.
- K. L. Howard, Human Organ-On-A-Chip: Technologies Offer Benefits Over Animal Testing but Challenges Limit Wider Adoption, United States Government Accountability Office, 2025 Search PubMed.
- D. E. Ingber, Nat. Rev. Genet., 2022, 23, 467–491 Search PubMed.
- H. Olson, G. Betton, D. Robinson, K. Thomas, A. Monro, G. Kolaja, P. Lilly, J. Sanders, G. Sipes, W. Bracken, M. Dorato, K. Van Deun, P. Smith, B. Berger and A. Heller, Regul. Toxicol. Pharmacol., 2000, 32, 56–67 CrossRef.
- M. Leist, ALTEX, 2013, 30, 227–230 Search PubMed.
- A. Radbruch and J. Isaacs, Eur. J. Immunol., 2009, 39, 1991–1993 CrossRef PubMed.
- The Scientific Consulting Group, Inc., Human Resources Research, Nonhuman Primate Evaluation and Analysis, The Scientific Consulting Group, Inc., 2024, https://orip.nih.gov/sites/default/files/NHP-Evaluation-and-Analysis-Final-Report-Revised-508.pdf Search PubMed.
- C. Carvalho, A. Gaspar, A. Knight and L. Vicente, Animals, 2018, 9, 12 CrossRef PubMed.
- P.-J. H. Zushin, S. Mukherjee and J. C. Wu, J. Clin. Invest., 2023, 133, e175824 CrossRef.
- M. Leick, V. Azcutia, G. Newton and F. W. Luscinskas, Cell Tissue Res., 2014, 355, 647–656 CrossRef.
- W. N. Andrade, M. G. Johnston and J. B. Hay, Blood, 1998, 91, 1653–1661 Search PubMed.
- W. A. Muller, Vet. Pathol., 2013, 50, 7–22 CrossRef PubMed.
- A. T. Salminen, Z. Allahyari, S. Gholizadeh, M. C. McCloskey, R. Ajalik, R. N. Cottle, T. R. Gaborski and J. L. McGrath, Front. Med. Technol., 2020, 2, 600616 CrossRef PubMed.
- C. E. Green, D. N. Pearson, R. T. Camphausen, D. E. Staunton and S. I. Simon, J. Immunol., 2004, 172, 7780–7790 CrossRef PubMed.
- T. S. Khire, A. T. Salminen, H. Swamy, K. S. Lucas, M. C. McCloskey, R. E. Ajalik, H. H. Chung, T. R. Gaborski, R. E. Waugh, A. J. Glading and J. L. McGrath, Cell. Mol. Bioeng., 2020, 13, 125–139 CrossRef PubMed.
- S. K. Shaw, P. S. Bamba, B. N. Perkins and F. W. Luscinskas, J. Immunol., 2001, 167, 2323–2330 CrossRef PubMed.
- L. Yang, R. M. Froio, T. E. Sciuto, A. M. Dvorak, R. Alon and F. W. Luscinskas, Blood, 2005, 106, 584–592 CrossRef PubMed.
- A. Mossu, M. Rosito, T. Khire, H. Li Chung, H. Nishihara, I. Gruber, E. Luke, L. Dehouck, F. Sallusto, F. Gosselet, J. L. McGrath and B. Engelhardt, J. Cereb. Blood Flow Metab., 2019, 39, 395–410 CrossRef PubMed.
- A. T. Salminen, J. Zhang, G. R. Madejski, T. S. Khire, R. E. Waugh, J. L. McGrath and T. R. Gaborski, Small, 2019, 15, 1804111 CrossRef PubMed.
- O. Steiner, C. Coisne, R. Cecchelli, R. Boscacci, U. Deutsch, B. Engelhardt and R. Lyck, J. Immunol., 2010, 185, 4846–4855 CrossRef PubMed.
- M. C. Bonilla, L. Fingerhut, A. Alfonso-Castro, A. Mergani, C. Schwennen, M. Von Köckritz-Blickwede and N. De Buhr, Biomedicines, 2020, 8, 278 CrossRef PubMed.
- S. Connolly, K. McGourty and D. Newport, Sci. Rep., 2020, 10, 9759 CrossRef PubMed.
- H. Azizgolshani, J. R. Coppeta, E. M. Vedula, E. E. Marr, B. P. Cain, R. J. Luu, M. P. Lech, S. H. Kann, T. J. Mulhern, V. Tandon, K. Tan, N. J. Haroutunian, P. Keegan, M. Rogers, A. L. Gard, K. B. Baldwin, J. C. De Souza, B. C. Hoefler, S. S. Bale, L. B. Kratchman, A. Zorn, A. Patterson, E. S. Kim, T. A. Petrie, E. L. Wiellette, C. Williams, B. C. Isenberg and J. L. Charest, Lab Chip, 2021, 21, 1454–1474 RSC.
- A. L. Gard, R. J. Luu, C. R. Miller, R. Maloney, B. P. Cain, E. E. Marr, D. M. Burns, R. Gaibler, T. J. Mulhern, C. A. Wong, J. Alladina, J. R. Coppeta, P. Liu, J. P. Wang, H. Azizgolshani, R. F. Fezzie, J. L. Balestrini, B. C. Isenberg, B. D. Medoff, R. W. Finberg and J. T. Borenstein, Sci. Rep., 2021, 11, 14961 CrossRef PubMed.
- L. Lopez Quezada, F. Mba Medie, R. J. Luu, R. B. Gaibler, E. P. Gabriel, L. D. Rubio, T. J. Mulhern, E. E. Marr, J. T. Borenstein, C. R. Fisher and A. L. Gard, Adv. Biol., 2024, 8, 2300511 CrossRef.
- M. T. Rogers, A. L. Gard, R. Gaibler, T. J. Mulhern, R. Strelnikov, H. Azizgolshani, B. P. Cain, B. C. Isenberg, N. J. Haroutunian, N. E. Raustad, P. M. Keegan, M. P. Lech, L. Tomlinson, J. T. Borenstein, J. L. Charest and C. Williams, Sci. Rep., 2021, 11, 12225 CrossRef.
- E. E. Marr, T. J. Mulhern, M. Welch, P. Keegan, C. Caballero-Franco, B. G. Johnson, M. Kasaian, H. Azizgolshani, T. Petrie, J. Charest and E. Wiellette, Sci. Rep., 2023, 13, 8922 CrossRef PubMed.
- A. L. Gard, R. J. Luu, R. Maloney, M. H. Cooper, B. P. Cain, H. Azizgolshani, B. C. Isenberg, J. T. Borenstein, J. Ong, J. L. Charest and E. M. Vedula, Commun. Biol., 2023, 6, 92 CrossRef PubMed.
- S. H. Kann, E. M. Shaughnessey, X. Zhang, J. L. Charest and E. M. Vedula, Analyst, 2023, 148, 3204–3216 RSC.
- E. M. Shaughnessey, S. H. Kann, H. Azizgolshani, L. D. Black, J. L. Charest and E. M. Vedula, Sci. Rep., 2022, 12, 13182 CrossRef PubMed.
- R. J. Luu, B. C. Hoefler, A. L. Gard, C. R. Ritenour, M. T. Rogers, E. S. Kim, J. R. Coppeta, B. P. Cain, B. C. Isenberg, H. Azizgolshani, O. R. Fajardo-Ramirez, G. García-Cardeña, M. P. Lech, L. Tomlinson, J. L. Charest and C. Williams, Front. Mol. Biosci., 2023, 10, 1160851 CrossRef PubMed.
- D. Ershov, M.-S. Phan, J. W. Pylvänäinen, S. U. Rigaud, L. Le Blanc, A. Charles-Orszag, J. R. W. Conway, R. F. Laine, N. H. Roy, D. Bonazzi, G. Duménil, G. Jacquemet and J.-Y. Tinevez, Nat. Methods, 2022, 19, 829–832 CrossRef PubMed.
- S. P. Timošenko and S. Woinowsky-Krieger, Theory of plates and shells, McGraw-Hill, Auckland Hamburg, 2nd edn, 1959, https://www.cap-recifal.com/ccs_files/articles/cuveaqua1_denisio/Timoshenko_-_Theory_of_plates_and_shells.pdf Search PubMed.
- M. Hassani, P. Hellebrekers, N. Chen, C. Van Aalst, S. Bongers, F. Hietbrink, L. Koenderman and N. Vrisekoop, J. Leukocyte Biol., 2020, 107, 809–818 CrossRef PubMed.
- A. Zipursky, E. Bow, R. Seshadri and E. Brown, Blood, 1976, 48, 361–371 CrossRef PubMed.
- R. E. Waugh, E. Lomakina, A. Amitrano and M. Kim, Front. Bioeng. Biotechnol., 2023, 11, 1175570 CrossRef PubMed.
- S. Baratchi, H. Danish, C. Chheang, Y. Zhou, A. Huang, A. Lai, M. Khanmohammadi, K. M. Quinn, K. Khoshmanesh and K. Peter, Nat. Commun., 2024, 15, 7023 CrossRef.
- A. Makino, H. Y. Shin, Y. Komai, S. Fukuda, M. Coughlin, M. Sugihara-Seki and G. W. Schmid-Schönbein, Biorheology, 2007, 44, 221–249 Search PubMed.
- E. Pérez-Figueroa, P. Álvarez-Carrasco, E. Ortega and C. Maldonado-Bernal, Front. Immunol., 2021, 12, 631821 CrossRef.
- C. S. Lewis, N. Z. Alsmadi, T. A. Snyder and D. W. Schmidtke, Cell. Mol. Bioeng., 2018, 11, 279–290 CrossRef.
- M. S. Shive, M. L. Salloum and J. M. Anderson, Proc. Natl. Acad. Sci. U. S. A., 2000, 97, 6710–6715 CrossRef.
- J. Abello, Y. Y. Yien and A. N. Stratman, BioRxiV, 2021, preprint, DOI:10.1101/2021.10.17.464751.
- Y. B. Bae, H. K. Jang, T. H. Shin, G. Phukan, T. T. Tran, G. Lee, W. R. Hwang and J. M. Kim, Lab Chip, 2016, 16, 96–103 RSC.
- L. B. Leverett, J. D. Hellums, C. P. Alfrey and E. C. Lynch, Biophys. J., 1972, 12, 257–273 CrossRef.
- M. J. Mitchell, K. S. Lin and M. R. King, Biophys. J., 2014, 106, 2243–2253 CrossRef PubMed.
- H. L. Damascena, W. A. A. Silveira, M. S. Castro and W. Fontes, Cells, 2022, 11, 2889 CrossRef.
- W. A. Muller, Annu. Rev. Pathol.: Mech. Dis., 2011, 6, 323–344 CrossRef PubMed.
- M. Jafari, E. I. Cardenas, S. Ekstedt, J. Arebro, M. Petro, A. Karlsson, E. Hjalmarsson, D. Arnarson, M. Ezerskyte, S. Kumlien Georén and L. O. Cardell, Clin. Transl. Allergy, 2024, 14, e12347 CrossRef.
- M. Bai, R. Grieshaber-Bouyer, J. Wang, A. B. Schmider, Z. S. Wilson, L. Zeng, O. Halyabar, M. D. Godin, H. N. Nguyen, A. Levescot, P. Cunin, C. T. Lefort, R. J. Soberman and P. A. Nigrovic, Blood, 2017, 130, 2092–2100 CrossRef CAS.
- F. S. Lakschevitz, S. Hassanpour, A. Rubin, N. Fine, C. Sun and M. Glogauer, Exp. Cell Res., 2016, 342, 200–209 CrossRef CAS PubMed.
- D. A. Moulding, C. A. Hart and S. W. Edwards, J. Leukocyte Biol., 1999, 65, 875–882 CrossRef CAS.
- K. M. Skubitz, K. D. Campbell and A. P. N. Skubitz, J. Leukocyte Biol., 1996, 60, 106–117 CrossRef CAS PubMed.
- I. Dransfield, A. M. Buckle, J. S. Savill, A. McDowall, C. Haslett and N. Hogg, J. Immunol., 1994, 153, 1254–1263 CrossRef CAS.
- C. Summers, S. M. Rankin, A. M. Condliffe, N. Singh, A. M. Peters and E. R. Chilvers, Trends Immunol., 2010, 31, 318–324 CrossRef CAS.
- G. S. Offeddu, J. C. Serrano, S. W. Chen, S. E. Shelton, Y. Shin, M. Floryan and R. D. Kamm, J. Biomech., 2021, 119, 110330 CrossRef PubMed.
- M. B. Esch, H. Ueno, D. R. Applegate and M. L. Shuler, Lab Chip, 2016, 16, 2719–2729 RSC.
- H. Li, F. Cheng, Z. Wang, W. Li, J. A. Robledo-Lara and Y. S. Zhang, Mol. Syst. Des. Eng., 2022, 7, 1538–1548 RSC.
- Y. I. Wang and M. L. Shuler, Lab Chip, 2018, 18, 2563–2574 Search PubMed.
| This journal is © The Royal Society of Chemistry 2026 |







