Open Access Article
Open Access ArticleCost-effective oxygen flask combustion and electrothermal vaporization capacitively coupled plasma microtorch optical emission spectrometry as a green and white method for multielemental determination in food
Augustin Catalin
Mot
 a,
Adrian-Ioan
Dudu
a,
Adrian-Ioan
Dudu
 ab,
Tiberiu
Frentiu
ab,
Tiberiu
Frentiu
 ac,
Dorin
Petreus
d,
Erika-Andrea
Levei
e,
Zamfira
Stupar
ac,
Dorin
Petreus
d,
Erika-Andrea
Levei
e,
Zamfira
Stupar
 e,
Maria
Frentiu
e and
Eniko
Covaci
e,
Maria
Frentiu
e and
Eniko
Covaci
 *ac
*ac
aBabeş-Bolyai University, Faculty of Chemistry and Chemical Engineering, Arany Janos 11, 400028 Cluj-Napoca, Romania. E-mail: eniko.covaci@ubbcluj.ro
bEnzymology and Applied Biocatalysis Research Center, Faculty of Chemistry and Chemical Engineering, Babeş-Bolyai University, Arany Janos 11, 400028 Cluj-Napoca, Romania
cBabeş-Bolyai University, Research Center for Advanced Analysis, Instrumentation and Chemometrics, Arany Janos 11, 400028 Cluj-Napoca, Romania
dTechnical University of Cluj-Napoca, Faculty of Electronics, Telecommunications and Information Technology, Gheorghe Baritiu 26-28, 400027 Cluj-Napoca, Romania
eNational Institute for Research and Development of Optoelectronics INOE 2000, Research Institute for Analytical Instrumentation Subsidiary, Donath 67, 400293 Cluj-Napoca, Romania
First published on 18th November 2025
Abstract
The study presents the analytical characterization of a cost-effective green and white method for the simultaneous determination of Cd, Pb, Zn, Cu, Hg, Se, and As in food using sample dissolution assisted by oxygen flask combustion and simultaneous detection by small-sized electrothermal vaporization capacitively coupled plasma microtorch optical emission spectrometry (OFC-SSETV-µCCP-OES). Attractiveness, effectiveness and cost-efficiency of the novel method were demonstrated through the greenness and whiteness degrees evaluated using the AGREEprep software and RGB 12 algorithm, compared to traditional high-pressure microwave-assisted wet digestion and determination by inductively coupled plasma optical emission spectrometry (HP-MAWD-ICP-OES), graphite furnace atomic absorption spectrometry and thermal desorption atomic absorption spectrometry methods. Signal integration over 5 pixels along the spectral line profile provided an improvement of 2–3 fold of the limits of detection in the range of 0.01 mg kg−1 (Hg, Cd, Zn) to 1.20 mg kg−1 (Se), compared to the measured signal corresponding to the pixel of the emission line maximum. Validation by analysis of certified reference materials proved that the method is not affected by the non-spectral matrix effects, with recoveries and extended uncertainties in the range of 90–113% and 9–25% (k = 2), respectively. Tukey's statistical test (p < 0.05 statistical significance) and z scores revealed no bias between the results obtained by (OFC)-SSETV-µCCP-OES, with both external calibration and the standard addition method, and those obtained by (HP-MAWD)-ICP-OES with external calibration. The applicability of the method was demonstrated through the analysis of fish tissue, mushroom, and dietary supplement samples, with precision ranging from 4.9% to 14.5%. The (OFC)-SSETV-µCCP-OES method presented a greenness score of 77%, evaluated by the AGREEprep metric, while the redness, greenness, blueness and whiteness scores, evaluated by the RGB 12 algorithm, were 86%, 94%, 98% and 93%. The scores are related to the combination of representative features of sample preparation by OFC, namely virtually energy-free processing, reduced reagents consumption and generated waste, along with the potential of simple and cost-effective miniaturized instrumentation for the simultaneous determination of trace elements in a low power (15 W) and low Ar consumption (150 mL min−1) microplasma source, which are essential for greater chemical sustainability in analytical procedures compared to conventional methods.
Introduction
Sample preparation techniques for elemental analysis, selected according to the type of matrix and the analytes, should ensure their conversion into a form readily accessible to the employed spectrometric method, in order to achieve the best analytical performance.1–3 In the case of solid samples, the development of quicker and easier preparation strategies, such as direct sampling from the solid matrix, as well as various time- and energy-efficient extraction procedures that maintain comparable effectiveness to traditional methods based on organic matrix destruction coupled with quantification using more sensitive detectors in miniaturized instrumentation, has emerged as alternatives to traditional approaches generally based on high-pressure microwave-assisted wet digestion (HP-MAWD) using concentrated acids.4,5 However, alternative dissolution methods, such as microwave-induced combustion and oxygen flask combustion (OFC), have been employed together with multielemental spectrometric techniques based on inductively coupled plasma optical emission spectrometry (ICP-OES), inductively coupled plasma mass spectrometry, atomic absorption spectrometry, and atomic fluorescence spectrometry for the determination of trace metals and nonmetals with or without chemical vapor generation in organic matrices, such as biological samples, foodstuffs of vegetable and animal origin, graphitic and organic polymeric materials, fuels, and even environmental samples with high carbon content.6–20 Microwave induced combustion and OFC procedures offer significant advantages for sample preparation, such as: (i) the use of small amounts of additional reagents alongside oxygen for combustion; (ii) low volumes and concentrations of reagents used for the absorption and dissolution of analytes released during combustion, typically between 0.1 and 1 mol L−1 HNO3, HCl, NH4OH, etc.; (iii) low waste generation; (iv) reduced energy consumption and a fast combustion process; and (v) simple sample handling. Such approaches could be a support in the development of multielemental analytical techniques with high greenness, redness, blueness, and whiteness performance metrics, by coupling with cost-effective instrumentation based on a microplasma source and a low-resolution microspectrometer for the determination of both priority hazardous elements and essential trace elements in food. To the best of our knowledge, such an approach has not yet been reported in the literature.A comprehensive evaluation of the analytical method colours, which includes not only the greenness related to sample preparation, but also the redness reflecting figures of merit or analytical performance, and the blueness encompassing indicators of practical applicability, cost-effectiveness and functional features, is a crucial approach in modern analytical chemistry. The evaluation of the whiteness degree of a new analytical method represents a balance between the colours of the method, depending on the specific characteristics of the method, as certain criteria associated with one colour may be more prominent than others. Nonetheless, all contribute to the overall whiteness score, expressed on a scale up to 100%, which reflects whether the evaluated method is fit-for-purpose across a broad spectrum of parameters included in the proposed model.21–29
We have demonstrated that coupling diffusive gradients in thin films passive sampling, performed in situ for surface waters and ex situ for soil samples, with small-sized electrothermal vaporization capacitively coupled plasma microtorch optical emission spectrometry (SSETV-µCCP-OES), not only improves the detection limits of the elements' labile fractions in water and soil, but also enables the development of simultaneous multielement methods characterized by a high degree of greenness and whiteness. These advantages are especially significant when compared to traditional techniques commonly used in environmental monitoring laboratories, such as graphite furnace atomic absorption spectrometry (GFAAS) and thermal desorption atomic absorption spectrometry (TDAAS).30,31
The aim of this study was to develop and validate a cost-effective method by coupling the OFC sample preparation procedure with simultaneous multielemental detection using SSETV-µCCP-OES for the determination of several elements relevant to food monitoring and human health, such as Cd, Pb, Zn, Cu, Hg, Se and As. The (OFC)-SSETV-µCCP-OES method was validated based on limits of detection (LODs), accuracy, and precision, assessed through the analysis of certified reference materials (CRMs) and comparison with traditional techniques, namely the single element determination of Hg by TDAAS, the sequential GFAAS, and the simultaneous determination by ICP-OES, the latter applied with chemical vapor generation for As, Hg, and Se under previously established prereduction and derivatization conditions.32 The influence of the pixel number for signal integration across the episodic spectral line profile was evaluated in terms of method sensitivity, calibration curve linearity, and LODs, in comparison with signal integration based on a single pixel corresponding to the spectral line maximum. The optimal number of pixels for signal integration along the line profile yielding the best LODs, evaluated according to the signal-to-background ratio and relative standard deviation of the background (SBR–RSDB) procedure, was established.33,34 The advantages of the new (OFC)-SSETV-µCCP-OES method in terms of analytical performance, utility, and applicability were highlighted through its greenness, evaluated by the AGREEprep metric, and its whiteness, assessed via the RGB 12 algorithm.21,22
Materials and methods
Instrumentation
A schematic representation of the (OFC)-SSETV-µCCP-OES experimental setup is shown in Fig. 1. The functional details of the SSETV-µCCP-OES experimental setup have been previously described.30 The system consists of a capacitively coupled plasma microtorch (Babeş-Bolyai University, Cluj-Napoca, Romania) operated at 15 W and 150 mL min−1 Ar, powered by a miniaturized 13.56 MHz radiofrequency generator (Technical University of Cluj-Napoca, Romania), interfaced with a Maya2000 Pro microspectrometer (Ocean Optics, Dunedin, USA) purged with high-purity N2 6.0 covering a spectral range of 165–309 nm with a full width at half maximum of 0.35 nm.35,36 The vapour generated via the miniaturized SSETV device (Babeş-Bolyai University, Cluj-Napoca, Romania) from a 10 µL liquid microsample was introduced into the microplasma source by electrothermal evaporation from a Rh microfilament with 250 µm diameter and 99,9% purity (Goodfellow, Cambridge, UK).35 The microfilament was heated using the TENMA 72-13360 programmable power supply (TENMA Inc., China) at 80 °C (0.25 V and 1.93 A) for 180 s for sample drying and at 1500 °C (2.3 V and 4.56 A) for 10 s for sample vaporization. These conditions enabled multielement evaporation and simultaneous recording of 3D episode emission spectra (signal–wavelength–time) with an integration time of 100 ms per episodic spectrum. Radial spectroscopic observation of the microplasma was performed through a collimating lens with 10 mm focal length without fibre optics, at a height of 0.8 mm above the Mo microelectrode tip. The observation height was adjusted with an increment of 100 µm using a 3D translator on which the microspectrometer was mounted. Thermal calibration of the Rh microfilament was carried out over two temperature ranges of 50–600 °C and 800–1600 °C by measuring the filament temperature using the 3ML-CF1 and 1MH1-CF3 IR sensors (Optris GmbH & Co.KG, Berlin, Germany).37 The TENMA 72-13360 power supply enabled a temperature control of the microfilament with a precision better than ±15 °C and an accuracy of at least ±20 °C, at a target value of 1500 °C for 7 s after the filament reached the set temperature in approximately 3 seconds from the start of heating.30 A SMCEVT307-5 DZ-01 F-Q two-way valve powered by the HY3003 Mastech supply (Premier Farnell, Leeds, UK) was inserted into the argon flow path to control its route during the drying and vaporization stage. These operating conditions ensured highly reproducible evaporation of the microsample, as well as recording of episodic emission spectra for all elements.A Spectro CIROSCCD simultaneous ICP-OES spectrometer (Spectro, Kleve, Germany) was used for Cu, Zn, Cd and Pb determination in samples prepared by HP-MAWD under measurement conditions previously described.37 For the determination of Hg, As and Se by chemical vapor generation, a HGX-200 hydride/cold vapor generator (Teledyne CETAC Technologies, Omaha, Nebraska, USA) was coupled to the ICP spectrometer.32
A Berghof MW3 S+ digester (Berghof, Germany) was employed for sample preparation, according to the previously reported HP-MAWD procedure.32
Total carbon (TC), total organic carbon (TOC) and total inorganic carbon (TIC) fractions were quantified using a Multi N/C 2100S Analyzer (Analytik Jena, Jena, Germany) in accordance with ISO 20236:2024.38
Reagents, standard solutions, certified reference materials and food samples
All reagents and standard solutions used in this study for sample preparation were purchased from Merck (Darmstadt, Germany). Single-element standard solutions of Cd, Cu, Pb, Zn, Hg, As and Se (1000 mg L−1) were used to prepare the multielement calibration standards in 2% (v/v) HNO3 over the concentration range of 0.01–0.10 mg L−1 Cd, Hg and Zn, 0.10–1.00 mg L−1 Cu and Pb, and 0.10–2.00 mg L−1 As and Se. Certified reference materials, namely Tort-3 lobster hepatopancreas (National Research Council Canada, Ottawa, Ontario, Canada), CE278k mussel tissue (Institute for Reference Materials and Measurements – IRMM, Geel, Belgium), CS-M-3 dried mushroom powder (Boletus edulis) (Institute of Nuclear Chemistry and Technology, Warsaw, Poland), GBW 10011 wheat (Institute of Geophysical and Geochemical Exploration, Langfang, China) and SRM 3280 multivitamin/multielement tablets (National Institute of Standards and Technology, Gaithersburg, USA) were used to check the accuracy (recovery and precision) of the (OFC)-SSETV-µCCP-OES method. Ultrapure water was produced in the laboratory using a Milli-Q system (Millipore, Bedford, USA). Solution of 0.1 mol L−1 HNO3 was used as absorbing medium and sample dissolution by combustion. A solution of 0.1 mol L−1 HCl was used for the decomposition of TIC in solid samples. All glassware was decontaminated by soaking for 12 hours in 10% (v/v) HNO3 and subsequently rinsed with ultrapure water. The combustion flask and the platinum sample holder were sequentially washed between combustions with 10% (v/v) HNO3, ultrapure water and acetone, whereas after each working day the platinum basket was kept overnight in concentrated HNO3 for decontamination. Fish samples and dietary supplements were purchased from supermarkets and pharmacies in Cluj-Napoca, Romania, while mushroom samples were collected from forests in the surrounding area.Sample preparation by OFC-assisted dissolution
The combustion of samples was carried out using a 500 mL or 1000 mL quartz Schöniger-type Erlenmeyer flask manufactured by Exeter Analytical (Coventry, UK), equipped with a platinum basket (20 × 7 mm), manufactured by Elemental Microanalysis Ltd (Okehampton, Devon, UK) (Fig. 1). Amounts of 50 mg homogenized powdered CRMs and food samples were weighed directly onto 3 cm × 2 cm pieces of Whatman® Grade 542 hardened ashless quantitative filter paper, wrapped and placed in the platinum basket attached to the Erlenmeyer flask stopper. A volume of 10 mL of 0.1 mol L−1 HNO3 absorbent solution was introduced into the flask to retain the gases generated during combustion. The flask was then purged with 5.0 grade oxygen for 5 minutes at a flow rate of 1 L min−1. The filter paper containing the sample was manually ignited and immediately inserted into the Erlenmeyer flask through the stopper and hermetically sealed. Combustion was carried out with the flask positioned upside down to prevent gas losses. The resulting gases were absorbed over a 10-minute period into the nitric acid solution by manually stirring the flask every 2 minutes. Multielement determinations were performed directly in the absorbent solution after centrifugation at 3000 rpm for 10 min. A blank sample was prepared by combusting a sample-free filter paper. The sample amount of 50 mg and the 500 mL or 1000 mL combustion flask were selected based on the combustion capacity of the system, which depends on the amount of oxygen required for efficient combustion of carbon-rich matrices such as food samples.7(OFC)-SSETV-µCCP-OES method validation
The (OFC)-SSETV-µCCP-OES method was validated based on LODs, accuracy, and precision. Instrumental LODs were evaluated based on the SBR–RSDB approach (LOD = 3 × 0.01 × RSDB × c/SBR).33,34 The RSDB is the relative standard deviation of the background (%) calculated from the background signal of 11 episodic spectra recorded before the appearance of the analyte signal, considering 1, 3, 5 and 7 pixels similar to the number of pixels used in the integration of the analyte signal over the spectral line profile. The SBR is the signal-to-background ratio for an analyte concentration within the calibration range. The analyte signals used in the calibration curve plotting were obtained by 3D integration (signal–wavelength–time), according to the number of pixels used to integrate the signal over the spectral line profile (CP ± n) from each episodic spectrum. CP corresponds to the pixel of the emission line maximum, while n = 1, 2, 3 correspond to the pixels on the wings of the line profile relative to CP. The dependence of the calibration curve slope, RSDB, SBR and LODs versus the number of integrating pixels of the signal was evaluated, and the number of pixels that yielded the best LODs was selected. The LODs obtained by (OFC)-SSETV-µCCP-OES were compared with values obtained in our laboratory using traditional spectrometric methods, such as GFAAS, ICP-OES with chemical vapor generation for Hg, As, and Se, and TDAAS for Hg. The linearity of the calibration curves was checked using Mandel's test.39Analyte recovery in the CRM samples was evaluated against the certified concentration and the expanded uncertainty at a coverage factor of k = 2, corresponding to a 95% confidence level. Additionally, the z-score was calculated according to the European Guidelines for the validation of analytical methods.40 The magnitude of non-spectral matrix interferences in the (OFC)-SSETV-µCCP-OES method, which may cause bias from the target value, was assessed by comparing the results obtained for CRMs using external calibration and the standard addition method. The results obtained in CRM samples by the (OFC)-SSETV-µCCP-OES method were also validated by comparison with HP-MAWD in a HNO3–H2O2 mixture and determination by ICP-OES. Tukey's statistical test41 (p < 0.05 statistical significance) was used to check any possible bias between the results obtained with (OFC)-SSETV-µCCP-OES and (HP-MAWD)-ICP-OES. A study of the combustion efficiency (%) of the samples, according to the volume of the employed flask, was carried out. Combustion efficiency was evaluated based on the TOC weight in the solid sample, the absorbing solution, and any potential unburned carbon residue. Total carbon in solid was measured directly by combustion at 1100 °C, while TOC was determined after removal of TIC by treating the solid sample with 0.1 mL of 1 mol L−1 HCl for 30 min directly in the sample boat. In liquid samples potentially containing particles in suspension, the determinations were performed by catalytic oxidation at 800 °C. Sample homogeneity was ensured by initial vortexing for 2 min, followed by continuous stirring (speed level 8) during sampling.
Greenness and whiteness evaluation by the AGREEprep metric and RGB 12 algorithm
The AGREEprep metric is based on 10 assessment steps corresponding to the green sample preparation principles.21 This metric was selected because it more effectively accounts for the environmental impact of sample preparation and enables a more accurate evaluation of energy consumption and waste generation, compared to other metrics in which these aspects are poorly defined and difficult to quantify. The AGREEprep metric was carried out using the open-access software available through the link indicated in the paper.21 The software generates a pictogram through which the performance of the method is intuitively visualised and allows rapid identification of the strengths and weaknesses of the method.The RGB 12 metric includes 12 criteria, divided into four for each of the red, green, and blue components using the Excel worksheet provided as the SI to the paper.22 Therefore, the RGB 12 model was used because it is an evidence-based method, sufficiently flexible, which demands the evaluator to rigorously adapt all 12 individual parameters in assessing the whiteness degree.
The performance indicators of (OFC)-SSETV-µCCP-OES, highlighted by the colours, were compared with those of (HP-MAWD)-SSETV-µCCP-OES and (HP-MAWD)-ICP-OES with chemical vapor generation for As, Se, and Hg, GFAAS and TDAAS for Hg, respectively. This ensured a structured evaluation and enabled a clear identification of advantages compared to the reference methods.
Results and discussion
Efficiency of sample combustion
The concentrations of TC, TIC and TOC in the analysed food samples and absorbing liquid potentially containing particles in suspension, for the combustion of a 50 mg sample in a 500 mL flask, are presented in the SI (Table S1). The combustion efficiency, presented in the same table, was calculated from the mass balance of the TOC fraction in the solid sample, and the residual TOC in absorbing liquid containing any remaining suspended matter. The TOC fraction in the analysed food was predominant, ranging from 45% to 99%, with lower weights corresponding to the dietary supplements. In the absorbing liquid, TOC concentrations ranged from 8.2 to 50.2 µg mL−1, whereas TIC represented the largest fraction (39–89%), due to the absorbed CO2 resulting from the combustion. This observation is consistent with the results obtained for liquid samples purged with nitrogen, in which the TIC fraction was below the LOD of the method (1 µg mL−1), with only the dissolved organic fraction being determined.The combustion efficiency for 50 mg of GBW 10011 (Wheat) and Tort-3 (Lobster hepatopancreas) CRMs versus flask volume (250, 500 and 1000 mL) is presented in the SI (Fig. S1). The combustion efficiency was 90.1 ± 7.3% for GBW 10011 and 96.3 ± 7.2% for Tort-3 in the case of the 250 mL flask. The incomplete combustion was evidenced by the residual material remaining in the platinum basket. Therefore, the flask volume was increased to 500 and 1000 mL, yielding an increase in the combustion efficiency to 98.1 ± 6.8% and 99.9 ± 6.7% for Tort-3, and 99.6 ± 6.2% and 99.9 ± 7.1% for GBW 10011. The same phenomenon was observed in the case of other samples, as indicated in the SI (Table S1). Increasing the flask volume to 500 mL effectively prevented incomplete combustion, with residues ranging from 0.2 to 20 mg. The higher quantities corresponded to the dietary supplements with low TOC content, which was also highlighted in the remaining residue. In the case of the 1000 mL flask, combustion was virtually complete, with no detectable residue on the platinum basket. However, a 500 mL vessel was considered sufficient for the combustion of the food samples under study.
Element vaporization from the Rh microfilament
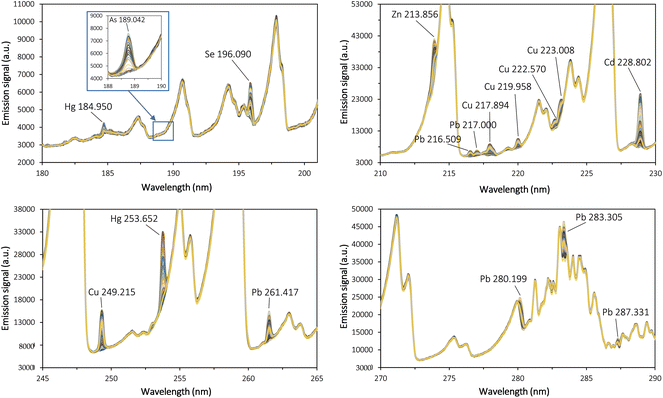
The operating conditions of the miniaturized SSETV and µCCP-OES tandem should ensure both efficient and high flow rate of the microsample vapor into the microplasma by instantaneous vaporization, so that simultaneous analysis is feasible.31Fig. 2 presents the episodic emission spectra recorded by evaporation of a 10 µL microsample containing 0.1 mg L−1 Cd, Zn, and Hg; 1 mg L−1 Cu and 2 mg L−1 Se, from a Rh filament heated to 1500 ± 20 °C (2.3 V, 4.56 A) for 10 s, interfaced with microplasma operated at 15 W, 150 mL min−1 Ar flow, and 0.8 mm spectroscopic observation height above the Mo tip microelectrode. The emission spectrum of arsenic was recorded separately using a single element solution of 2 mg L−1 as a result of spectral interference of the As 228.812 nm line with that of Cd at 228.802 nm. The operating parameters of the experimental set-up were selected based on previously reported results in the analysis of food and environmental samples prepared by the HP-MAWD procedure.37Fig. 3 presents the transient signals of the analytes at the most sensitive spectral lines resulting from the episodic emission, recorded with an integration time of 100 ms at CP ± 2 pixels. The shape of the emission spectra, with maxima at 1.8 s (Hg), 3.6 s (Se), 4.5 s (Cd), 4.9 s (Pb), 5.0 s (As), 5.2 s (Zn) and 5.5 s (Cu), indicates a temporal evaporation behaviour dependent on the nature of the elements, during the 10 s heating time of the filament. A selective evaporation of Hg is evident, but no separation of the Cd 228.802 nm emission from that of As at 228.812 nm was achieved.
Calibration curves, sensitivity and LODs dependence on the integrating pixels of the signal over the spectral line profile
The relationship between the calibration curves obtained by the SSETV-µCCP-OES method for different numbers of pixels for signal integration over the spectral line profile is illustrated in the SI (Fig. S2). The same dependence for SBR, RSDB and LODs is presented in the SI (Fig. S3–S5).According to Fig. S2, an enhancement of sensitivity of 4.2 to 4.7 times, depending on the element, was observed by increasing the number of integrating pixels to 7. The determination coefficients (R2) of the calibration curves were in the range of 0.9987 to 0.9994 for signal integration over 1 to 7 pixels along the spectral line profile. However, the Mandel statistical test confirmed linearity over the studied calibration ranges, regardless of the pixel number, with experimental Fexp values of up to 4.42, which were lower than the tabulated Ftab,(95%,1,n−3) = 10.13. A dependence according to a second-degree equation for the SBR was obtained, and consequently, an increase of 3.7 to 4 times for a number of 5 pixels compared to the SBR resulted at the CP (SI, Fig. S3) was obtained. The RSDB ranged between 0.5% and 6.0%, showing an increase with the number of pixels considered for background signal averaging (SI, Fig. S4). The increase is attributed to the variation of the background signal with wavelength, due to the line position of As 189.042 nm and Se 196.090 nm on the line-wings of molecular O2 emission (175–205 nm, B3Σu− − X3Σg−, Schuman–Runge transition), and Zn 213.856 nm and Hg 253.652 nm on the line wings of molecular NO emission (200–300 nm, X2Π → A2Σ+ transition). Considering the dependence of SBR and RSDB versus the number of pixels, the best LODs were achieved when integrating the signal over 5 pixels along the spectral line profile (SI Fig. S5). The instrumental LODs obtained by the SBR–RSDB approach for the SSETV-µCCP-OES method with analytical signal integration over 5 pixels, together with those obtained in the analysis of food samples subjected to OFC, are presented in Table 1. The LODs obtained by ICP-OES with pneumatic nebulization and chemical vapor generation for As, Se and Hg, and those obtained by GFAAS and TDAAS, are illustrated for comparison.
| Element | SSETV-µCCP-OES | ICP-OES (mg kg−1) | GFAAS | |||
|---|---|---|---|---|---|---|
| µg L−1 | mg kg−1 | pg | mg kg−1 | pg | ||
| a Obtained by CV(HG)-ICP-OES. b Obtained by TDAAS. c Chemical modifiers: As: 0.1% Pd(NO3)2 + 0.06% Mg(NO3)2; Pb and Cd: 1% NH4H2PO4 + 0.06% Mg(NO3)2. | ||||||
| Hg | 0.05 | 0.010 | 0.5 | 0.04a | 0.004b | 0.0008 |
| Cu | 0.14 | 0.030 | 1.4 | 0.62 | 0.030 | 3.0 |
| Zn | 0.04 | 0.010 | 0.4 | 0.04 | 0.020 | 1.6 |
| Pb | 0.35 | 0.070 | 3.5 | 0.85 | 0.030c | 3.0 |
| Cd | 0.05 | 0.010 | 0.5 | 0.06 | 0.006c | 0.6 |
| Se | 6.0 | 1.20 | 60 | 0.05a | — | — |
| As | 5.0 | 1.00 | 50 | 0.06a | 0.020c | 2.0 |
The instrumental LODs of the SSETV-µCCP-OES method for 5 pixels were in the range 0.04 µg L−1 (Zn) to 6.0 µg L−1 (Se). The analytical signal integration over 5 pixels along the spectral line profile resulted in an improvement of LODs generally 2–3 times compared to those obtained in the case of the CP signal corresponding to the emission line maximum. In the case of Zn, the improvement was only 1.25-fold due to the significant increase in RSDB values, rising from 0.8% for a single pixel to 2.4% and 3.2% for 5 and 7 pixels, respectively, despite achieving an SBR enhancement of 3.7 and 4.2 times. The significant increase of RSDB in the case of Zn 213.856 nm is due to the position of the analytical line on the line-wing of 214.91 nm associated with the NO molecular emission band (5.45 eV, X2Π → A2Σ+ transition), which causes a significant variation of the background signal, as shown previously. The (OFC)-SSETV-µCCP-OES method provides LODs of 0.01 mg kg−1 (Hg, Cd, Zn); 0.030 mg kg−1 (Cu); 0.070 mg kg−1 (Pb); 1.00 mg kg−1 (As) and 1.20 mg kg−1 (Se) in foodstuffs, for 50 mg samples subjected to OFC and dissolution in 10 mL 0.1 mol L−1 HNO3. The LODs are better for Zn, Cd, Cu, and Pb compared to sample preparation by HP-MAWD and ICP-OES measurement with pneumatic nebulization, and similar to the LOD for Hg achieved by CV-ICP-OES. The LODs obtained for Se and As by the (OFC)-SSETV-µCCP-OES method without chemical vapor generation are significantly poorer compared to HG-ICP-OES (0.05 and 0.06 mg kg−1). A LOD of 1.4 µg L−1 and 0.5 mg kg−1 for As was previously achieved by the HG-µCCP-OES method.42 The LODs are similar for Cu, and apparently, about two times better for Zn, and two times poorer for Pb and Cd compared to GFAAS. However, it is important to consider the microsample volume of 10 µL for SSETV-µCCP-OES versus 20 µL in GFAAS. Therefore, the absolute LODs (pg) in the (OFC)-SSETV-µCCP-OES method were in the range of 0.4 (Zn) to 3.5(Pb), comparable with those obtained by GFAAS. The LOD was much poorer for As compared to GFAAS, but differences in instrumental components, which largely determine method sensitivity, should also be considered. The LOD for Hg in the SSETV-µCCP-OES method is four times poorer compared to TDAAS, but the sample amount should also be considered, namely 200 mg for TDAAS versus 50 mg dissolved in 10 mL absorption solution for the SSETV-µCCP-OES method.
Accuracy of the (OFC)-SSETV-µCCP-OES method
Table 2 presents the results for the CRMs (mean and expanded uncertainty, k = 2) obtained by SSETV-µCCP-OES using both external calibration and the standard addition method, compared to the certified values.| CRM | Calibration | Cu | Zn | Cd | Pb | Hg | Se | As | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Certified value ± UCRMa (mg kg−1) | Found value ± Ulabb (mg kg−1) | Certified value ± UCRMa (mg kg−1) | Found value ± Ulabb (mg kg−1) | Certified value ± UCRMa (mg kg−1) | Found value ± Ulabb (mg kg−1) | Certified value ± UCRMa (mg kg−1) | Found value ± Ulabb (mg kg−1) | Certified value ± UCRMa (mg kg−1) | Found value ± Ulabb (mg kg−1) | Certified value ± UCRMa (mg kg−1) | Found value ± Ulabb (mg kg−1) | Certified value ± UCRMa (mg kg−1) | Found value ± Ulabb (mg kg−1) | ||
| a U CRM is the extended uncertainty from the certificate (k = 2, 95% confidence level). b U lab is the extended uncertainty in the laboratory (k = 2, 95% confidence level, n = 3 repeated measurements). c |z| score calculated according to the Eurachem guide.40 | |||||||||||||||
| Tort-3 (lobster hepatopancreas) | Ext. calib. | 497 ± 22 | 515 ± 63 | 136 ± 6 | 146 ± 16 | 42.3 ± 1.8 | 39.6 ± 5.5 | 0.225 ± 0.018 | 0.249 ± 0.044 | 0.292 ± 0.022 | 0.328 ± 0.046 | 10.9 ± 1.0 | 11.9 ± 2.4 | 59.5 ± 3.8 | 53.7 ± 8.4 |
| Std. add. | 524 ± 66 | 147 ± 17 | 41.5 ± 3.8 | 0.238 ± 0.047 | 0.319 ± 0.052 | 12.0 ± 3.2 | 63.5 ± 12.1 | ||||||||
| CE278k (mussel tissue) | Ext. calib. | 5.98 ± 0.27 | 6.35 ± 0.98 | 71 ± 4 | 66 ± 6 | 0.336 ± 0.025 | 0.357 ± 0.064 | 2.18 ± 0.18 | 2.09 ± 0.46 | 0.071 ± 0.007 | 0.072 ± 0.018 | 1.62 ± 0.12 | <3.96 (LOQ) | 6.7 ± 0.4 | 6.5 ± 1.2 |
| Std. add. | 5.77 ± 1.36 | 73 ± 11 | 0.371 ± 0.048 | 2.10 ± 0.50 | 0.079 ± 0.020 | <3.96 (LOQ) | 6.9 ± 1.6 | ||||||||
| CS-M-3 (dried mushroom powder) | Ext. calib. | 18.73 ± 0.70 | 18.72 ± 2.81 | 113.30 ± 3.28 | 106.05 ± 16.23 | 1.229 ± 0.110 | 1.195 ± 0.291 | 1.863 ± 0.108 | 1.943 ± 0.330 | 2.849 ± 0.104 | 3.137 ± 0.445 | 17.43 ± 1.36 | 18.69 ± 3.74 | 0.651 ± 0.026 | <3.30 (LOQ) |
| Std. add. | 20.83 ± 4.15 | 105.63 ± 18.21 | 1.285 ± 0.325 | 1.691 ± 0.343 | 2.796 ± 0.448 | 17.56 ± 4.25 | <3.30 (LOQ) | ||||||||
| GBW 10011 (wheat) | Ext. calib. | 2.7 ± 0.2 | 2.5 ± 0.4 | 11.6 ± 0.7 | 12.1 ± 2.3 | 18 ± 4 | 19 ± 5 | 0.065 ± 0.024 | <0.23 (LOQ) | 1.6 | 1.7 ± 0.2 | 0.053 ± 0.007 | <1.20 (LOD) | 0.031 ± 0.005 | <3.30 (LOQ) |
| Std. add. | 2.7 ± 0.6 | 12.2 ± 2.6 | 17 ± 4 | <0.23 (LOQ) | 1.5 ± 0.4 | <1.20 (LOD) | <3.30 (LOQ) | ||||||||
| SRM 3280 (multi-vitamin tablets) | Ext. calib. | 1400 ± 170 | 1309 ± 215 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 150 ± 810 150 ± 810 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 170 ± 1179 170 ± 1179 |
0.08015 ± 0.00086 | 0.09067 ± 0.02295 | 0.2727 ± 0.0024 | 0.2963 ± 0.0718 | — | — | 17.42 ± 0.45 | 17.67 ± 3.77 | 0.132 ± 0.044 | <1.00 (LOD) |
| Std. add. | 1374 ± 249 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 140 ± 1300 140 ± 1300 |
0.08519 ± 0.01952 | 0.2894 ± 0.0679 | — | 17.58 ± 3.64 | <1.00 (LOD) | ||||||||
| Pooled recovery (%) | Ext. calib. | 99 ± 15 | 100 ± 14 | 103 ± 22 | 105 ± 18 | 108 ± 17 | 106 ± 18 | 94 ± 17 | |||||||
| Std. add. | 102 ± 20 | 102 ± 16 | 103 ± 20 | 100 ± 20 | 103 ± 22 | 104 ± 21 | 105 ± 21 | ||||||||
| Precision (%) | Ext. calib. | 6.1–8.2 | 4.6–9.5 | 4.9–13.2 | 6.8–12.1 | 5.9–12.5 | 10.0–10.7 | 7.8–9.2 | |||||||
| Std. add. | 6.1–12.0 | 5.8–10.7 | 4.6–12.6 | 8.5–11.9 | 8.0–13.3 | 10.3–13.3 | 9.5–11.6 | ||||||||
| |z| scorec | Ext. calib. | 0.1–1.7 | 0.1–1.8 | 0.3–1.3 | 0.4–1.4 | 0.1–1.8 | 0.1–1.0 | 0.4–1.8 | |||||||
According to Table 2, there is good agreement between the found values obtained from both the external calibration and the standard addition method, compared to the certified values. The recoveries were within the range of 90–113%, with a trueness of 9–25% (k = 2) for external calibration and 91–110% with a trueness of 9–27% for standard addition, respectively. This demonstrates that the (OFC)-SSETV-µCCP-OES method is not affected by the non-spectral interferences from the sample matrix, and external calibration can be reliably used. According to the Eurachem Guide, the z-scores calculated for the found results using external calibration fell within the range of 0.1–1.8, lower than 2, which indicates a satisfactory performance without generating any signal of concern.40
In terms of As and Cd determination by SSETV-µCCP-OES, it was previously shown that the determination of Cd at 228.802 nm cannot be performed in the presence of As, due to spectral interference from the As 228.812 nm line. This spectral line of As is approximately 25 times lower than that of Cd, and consequently, the LOD for As at 228.812 nm was 2.8 µg L−1, compared to 0.12 µg L−1 for Cd when integrating the transient signal only at the CP. Under these conditions, a positive bias occurs in the determination of Cd at concentrations higher than 8.5 µg L−1 As.31 Considering the signal integration over 5 pixels on the spectral line profile, a LOD of 2 µg L−1 As and a quantification limit of 7 µg L−1 As are obtained, which could introduce a positive bias in the determination of Cd. This value is similar to the LOD of 5 µg L−1 As at the 189.042 nm line. Therefore, in the present study, the determination of Cd at 228.802 nm and As was carried out using the following procedure. Two calibration curves were drawn for As at 189.042 nm and 228.812 nm using single-element solutions. A calibration curve was performed for Cd at 228.802 nm, using solutions in the absence of As. The As concentration in the sample was determined at 189.042 nm, and based on the calibration curve drawn at 228.812 nm, the emission signal was calculated when the As concentration exceeded 5 µg L−1. A signal correction of the sample was performed at 228.802 nm by subtracting the As signal from the total signal, thus obtaining the net signal of Cd in the sample. After correction, the concentration of Cd was determined based on the calibration curve drawn at Cd 228.802 nm.
Table 3 presents the results obtained for the determination of elements in the CRM samples using the ICP-OES method and HP-MAWD procedure and the generation of chemical vapor for Hg, As, and Se.
| CRM | Cu | Zn | Cd | Pb | Hg | Se | As | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Certified value ± UCRMa (mg kg−1) | Found value ± Ulabb (mg kg−1) | Certified value ± UCRMa (mg kg−1) | Found value ± Ulabb (mg kg−1) | Certified value ± UCRMa (mg kg−1) | Found value ± Ulabb (mg kg−1) | Certified value ± UCRMa (mg kg−1) | Found value ± Ulabb (mg kg−1) | Certified value ± UCRMa (mg kg−1) | Found value ± Ulabb (mg kg−1) | Certified value ± UCRMa (mg kg−1) | Found value ± Ulabb (mg kg−1) | Certified value ± UCRMa (mg kg−1) | Found value ± Ulabb (mg kg−1) | |
| a U CRM is the extended uncertainty from the certificate (k = 2, 95% confidence level). b U lab is the extended uncertainty in the laboratory (k = 2, 95% confidence level, n = 3 repeated measurements). c |z| score calculated according to the Eurachem guide.40 | ||||||||||||||
| Tort-3 (lobster hepatopancreas) | 497 ± 22 | 460 ± 61 | 136 ± 6 | 128 ± 23 | 42.3 ± 1.8 | 42.6 ± 4.9 | 0.225 ± 0.018 | <0.85 (LOD) | 0.292 ± 0.022 | 0.296 ± 0.043 | 10.9 ± 1.0 | 10.5 ± 1.6 | 59.5 ± 3.8 | 53.7 ± 7.8 |
| CE278k (mussel tissue) | 5.98 ± 0.27 | 5.77 ± 1.36 | 71 ± 4 | 75 ± 11 | 0.336 ± 0.025 | 0.306 ± 0.084 | 2.18 ± 0.18 | <2.81 (LOQ) | 0.071 ± 0.007 | <0.13 (LOQ) | 1.62 ± 0.12 | 1.80 ± 0.47 | 6.7 ± 0.4 | 6.5 ± 1.2 |
| CS-M-3 (dried mushroom powder) | 18.73 ± 0.70 | 20.83 ± 4.15 | 113.30 ± 3.28 | 110.30 ± 21.5 | 1.229 ± 0.110 | 1.232 ± 0.306 | 1.863 ± 0.108 | <2.81 (LOQ) | 2.849 ± 0.104 | 2.662 ± 0.474 | 17.43 ± 1.36 | 16.91 ± 2.35 | 0.651 ± 0.026 | 0.662 ± 0.095 |
| GBW 10011 (wheat) | 2.7 ± 0.2 | 2.5 ± 0.4 | 11.6 ± 0.7 | 12.1 ± 1.8 | 18 ± 4 | 19 ± 5 | 0.065 ± 0.024 | <0.85 (LOD) | 1.6 | 1.7 ± 0.3 | 0.053 ± 0.007 | <0.16 (LOQ) | 0.031 ± 0.005 | <0.06 (LOD) |
| SRM 3280 multi-vitamin tablets | 1400 ± 170 | 1470 ± 260 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 150 ± 810 150 ± 810 |
9870 ± 1460 | 0.08015 ± 0.00086 | <0.20 (LOQ) | 0.2727 ± 0.0024 | <0.85 (LOD) | — | 17.42 ± 0.45 | 17.73 ± 1.64 | 0.132 ± 0.044 | <0.20 (LOQ) | |
| Pooled recovery (%) | 100 ± 18 | 100 ± 16 | 99 ± 23 | 100 ± 17 | 102 ± 17 | 96 ± 16 | ||||||||
| Precision (%) | 6.6–11.8 | 7.3–9.7 | 5.8–13.7 | 7.3–8.9 | 4.6–13.1 | 7.2–9.2 | ||||||||
| |z| scorec | 0.3–1.8 | 0.3–1.0 | 0.1–0.8 | 0.2–0.8 | 0.4–0.8 | 0.3–1.8 | ||||||||
Data in Table 3 indicate recoveries (k = 2) in the range of 91–111% by the (HP-MAWD)-ICP-OES method using pneumatic nebulization for Cu, Zn, Cd and Pb, and 90–111% for Hg, Se and As assisted by chemical vapor generation.
The Tukey statistical test showed that there is no significant difference (p < 0.05) between the found values by the (OFC)-SSETV-µCCP-OES method and those obtained by the reference ICP-OES method and sample preparation by HP-MAWD.41
Applicability of the (OFC)-SSETV-µCCP-OES method in real food samples
The results obtained for the determination of elements in fish tissue and mushrooms are presented in Table 4, while those for dietary supplements in Table 5. In the case of dietary supplements, the found concentration for the essential elements (Cu, Zn and Se) was compared to the targeted values declared on the label. The concentration of toxic elements (Cd, Pb, Hg, and As) was below the LOD of the method. The results indicated a precision of 4.9–14.5% for the (OFC)-SSETV-µCCP-OES method, based on the combined uncertainty, which accounted for errors in the preparation of calibration standards and samples, calibration curve fitting, and aliquot analysis.| Sample | Mean concentration ± Ulaba (mg kg−1) | ||||||
|---|---|---|---|---|---|---|---|
| Cu | Zn | Cd | Pb | Hg | Se | As | |
| a U lab is the extended uncertainty in the laboratory (k = 2, 95% confidence level, n = 3 repeated measurements). | |||||||
| Fish tissue 1 | 3.51 ± 0.44 | 22.5 ± 3.2 | <0.01 (LOD) | <0.07 (LOD) | <0.01 (LOD) | <1.20 (LOD) | <1.00 (LOD) |
| Fish tissue 2 | 0.835 ± 0.125 | 8.00 ± 1.30 | <0.01 (LOD) | <0.07 (LOD) | <0.01 (LOD) | <1.20 (LOD) | <1.00 (LOD) |
| Mushroom 1 | 54.0 ± 11.5 | 118.4 ± 17.8 | 0.318 ± 0.044 | 0.423 ± 0.071 | 0.062 ± 0.018 | <3.96 (LOQ) | <1.00 (LOD) |
| Mushroom 2 | 42.4 ± 7.5 | 129.8 ± 15.8 | 0.124 ± 0.019 | 0.794 ± 0.125 | 0.671 ± 0.157 | <3.96 (LOQ) | <1.00 (LOD) |
| Mushroom 3 | 71.8 ± 7.0 | 116.0 ± 15.2 | 0.263 ± 0.057 | <0.231 (LOQ) | 0.506 ± 0.073 | <3.96 (LOQ) | <1.00 (LOD) |
| Mushroom 4 | 41.3 ± 9.2 | 85.8 ± 12.4 | 0.192 ± 0.024 | 0.345 ± 0.082 | 0.209 ± 0.042 | <3.96 (LOQ) | <1.00 (LOD) |
| RSD (%) | 4.9–11.1 | 6.1–8.1 | 6.3–10.8 | 7.9–11.9 | 7.2–14.5 | — | — |
| Sample | Cu | Zn | Se | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Declared value (mg per capsule) | Found value ± Ulaba (mg per capsule) | Recovery ± Ulaba (%) | Declared value (mg per capsule) | Found value ± Ulaba (mg per capsule) | Recovery ± Ulaba (%) | Declared value (µg per capsule) | Found value ± Ulaba (µg per capsule) | Recovery ± Ulaba (%) | |
| a U lab is the extended uncertainty in the laboratory (k = 2, 95% confidence level, n = 3 repeated measurements). b |z| score calculated according to the Eurachem guide.40 | |||||||||
| Supplement 1 | 0.500 | 0.490 ± 0.062 | 98 ± 13 | 5 | 4.64 ± 0.61 | 93 ± 13 | 45 | 49.1 ± 6.0 | 109 ± 12 |
| Supplement 2 | 2 | 2.16 ± 0.30 | 108 ± 14 | 25 | 26.7 ± 3.3 | 107 ± 12 | — | — | |
| Supplement 3 | 1.67 | 1.78 ± 0.28 | 107 ± 16 | 6.7 | 6.58 ± 0.69 | 98 ± 10 | — | — | |
| Pooled recovery ± Ulaba (%) | 104 ± 14 | 99 ± 12 | 109 ± 12 | ||||||
| Precision (%) | 6.3–7.9 | 5.2–6.6 | 6.1 | ||||||
| |z| scoreb | 0.4–1.2 | 0.4–1.2 | 1.8 | ||||||
Colours of the (OFC)-SSETV-µCCP-OES method
The whiteness of the (OFC)-SSETV-µCCP-OES method was evaluated based on the comprehensive RGB 12 algorithm, which incorporates the redness, greenness, and blueness components.22 The red component was assessed in accordance with the intended application and analytical performance, including LODs, precision, and accuracy. The AGREEprep metric was used for the evaluation of the green component, considering the reagent toxicity, amount of reagents, waste generated, energy consumption during sample preparation and analysis, and operator safety. The blue component considered cost- and time-efficiency, instrument miniaturization and operational simplicity, degree of automation, on-line applicability, portability, and potential for on-site measurements. The overall whiteness score was calculated as the average of the individual RGB scores. The greenness of the (OFC)-SSETV-µCCP-OES method, compared to (OFC)-ICP-OES, (HP-MAWD)-SSETV-µCCP-OES and (HP-MAWD)-ICP-OES, evaluated by the AGREEprep metric is presented in the SI (Fig. S6). Inputs used to assign AGREEprep scores for the evaluated methods are presented in the SI (Table S2). The colours corresponding to the (OFC)-SSETV-µCCP-OES method, in comparison with (HP-MAWD)-GFAAS, (HP-MAWD)-ICP-OES and TDAAS methods for determination in foods, evaluated according to the RGB 12 algorithm, are presented in the SI (Fig. S7). Evaluation tables by the RGB 12 algorithm for these methods are presented in the SI (Table S3). The green score of the (OFC)-SSETV-µCCP-OES method was evaluated at 77%, and attributed both to the virtually energy-free sample preparation by OFC (criterion 8), and the use of simple miniaturized instrumentation based on a very low energy and Ar consumption for microplasma generation and multielemental determination (criterion 9). Additionally, the OFC preparation method demonstrates a high degree of greenness due to the low sample consumption, minimal waste generation, and enhanced operator safety (criteria 4, 5 and 10). The green score decreases to 64% when analysis is performed using (OFC)-ICP-OES, due to the complexity of the instrumentation and the high energy and Ar consumption of the plasma source. The green score drops to 40% for (HP-MAWD)-SSETV-µCCP-OES, even if the analysis is performed on a simple microplasma-based instrument. This decrease is attributed to the significantly higher consumption of HNO3, larger sample and waste volumes generated, the high energy demand of HP-MAWD, and safety concerns arising from the potential risk of explosion of the digestion vessels (criteria 2, 4, 5, 8 and 10). The green score decreases to 26% when sample preparation and analysis involve the (HP-MAWD)-ICP-OES method and chemical vapor generation for Hg, As, and Se determination. The red score of the (OFC)-SSETV-µCCP-OES method is 86%, slightly lower than the 94% of the GFAAS method, due to the poorer LODs associated with the microplasma source, despite its capability for simultaneous multielement determination. The SSETV-µCCP-OES method offers a slightly higher red score due to its multielemental determination capability, compared to TDAAS (red score of 82%) exclusively designed for Hg determination.The other performance parameters considered in the red score evaluation of the (OFC)-SSETV-µCCP-OES method, namely precision and recovery, were considered comparable to traditional methods, such as ICP-OES and GFAAS. The highest blue score (98%), obtained for the (OFC)-SSETV-µCCP-OES method, is attributed to the miniaturization and low-cost of the microplasma source and low-resolution microspectrometer, as well as the relatively high speed of sample preparation by OFC and simultaneous analysis (minimum 6 samples/hour). The green score of 94% for the microplasma-based analytical method coupled with OFC sample preparation is higher than that of GFAAS (81%) and ICP-OES with chemical vapor generation (69%) and is consistent with the results obtained through AGREEprep evaluation presented earlier. Although TDAAS is used only for Hg determination, it was evaluated to a green score of 98% in the RGB 12 algorithm owing to its ability to perform direct solid analysis without any reagent consumption. Overall, the whiteness score of the (OFC)-SSETV-µCCP-OES method, evaluated by the RGB 12 algorithm, was 93%, compared to 86%, 82% and 76% for TDAAS, GFAAS and ICP-OES, respectively.
Conclusions
It has been demonstrated that the coupling of fully miniaturized SSETV-µCCP-OES instrumentation with sample dissolution assisted by OFC is a cost-effective approach and enables simultaneous multielemental determination in food, including priority hazardous elements (Cd, Pb and Hg). The method achieved the highest green score in the evaluation by the AGREEprep metric compared to ICP-OES coupled with the same sample preparation procedure, but especially when HP-MAWD was used. The RGB 12 algorithm also highlighted the attractiveness, effectiveness, and cost-efficiency of sample preparation by OFC with simultaneous analysis of microsamples using SSETV-µCCP-OES. These critical analytical performances were highlighted by the high red and green scores, as well as the highest blue and white scores, in comparison with commercially available traditional spectrometric methods, such as GFAAS, TDAAS and ICP-OES. A substantial improvement in the SSETV-µCCP-OES method sensitivity was obtained by integrating the analytical signal over the spectral line profile, generated by the CCD detector pixels of the microspectrometer, along with time-based integration of the episodic spectra signals, compared to transient signals integrated only on the central pixel of the analytical line. A significant improvement in LODs was obtained by integrating the signal over the spectral line profile on 5 pixels, this being a compromise between enhanced sensitivity and the increase in background signal noise, both associated with the number of pixels. The signal correction applied for Cd 228.802 nm, due to the spectral interference with the adjacent As 228.812 nm line, and using As determination at two wavelengths proved to be effective for Cd and As determination in food when a low-resolution microspectrometer is used. Supplementary studies are needed to improve the sensitivity for Se and As determination in foods by the (OFC)-SSETV-µCCP-OES method through preconcentration after sample combustion. It is expected that the performance parameters of the method associated with the red, blue, green, and white scores to be enhanced, while the spectral interference between Cd and As at the most sensitive Cd 228.802 nm line to be avoided.Author contributions
Augustin Catalin Mot: writing – original draft; methodology, investigation, formal analysis, data curation, validation. Adrian-Ioan Dudu: methodology, investigation, formal analysis, data curation. Tiberiu Frentiu: supervision, project management, funding acquisition, writing – review & editing. Dorin Petreus: investigation, formal analysis. Erika-Andreea Levei: investigation, data curation. Zamfira Stupar: investigation, formal analysis. Maria Frentiu: investigation, formal analysis. Eniko Covaci: writing – review & editing, data curation, visualization, software, resources.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included within the article and supplementary information (SI), while those mentioned by way of a simple summary statement are available from the corresponding author upon reasonable request.Supplementary information is available. See DOI: https://doi.org/10.1039/d5ja00297d.
Acknowledgements
This work was supported by a grant of the Romanian Ministry of Research, Innovation and Digitization, CNCS/CCCDI-UEFISCDI, contract no. 15PED/2025, project number PN-IV-P7-7.1-PED-2024-0091, within PNCDI IV. This work was also supported by a grant of the Romanian Ministry of Research Innovation and Digitization through the Core Program within the National Research Development and Innovation Plan 2022–2027, carried out with the support of MCID, project no. PN 23 05.References
- E. H. Evans, J. Pisonero, C. M. M. Smith and R. N. Taylor, J. Anal. At. Spectrom., 2025, 40, 1136–1157 RSC.
- M. Patriarca, N. Barlow, A. Cross, S. Hill, A. Robson and J. Tyson, J. Anal. At. Spectrom., 2024, 39, 624–698 RSC.
- J. R. Bacon, O. T. Butler, W. R. L. Cairns, O. Cavoura, J. M. Cook, C. M. Davidson and R. Mertz-Kraus, J. Anal. At. Spectrom., 2024, 39, 11–65 RSC.
- M. Ibourki, O. Hallouch, K. Devkota, D. Guillaume, A. Hirich and S. Gharby, J. Food Compos. Anal., 2023, 120, 105330 CrossRef CAS.
- J. Sneddon, C. Hardaway, K. K. Bobbadi and A. K. Reddy, Appl. Spectrosc. Rev., 2006, 41, 1–14 CrossRef CAS.
- E. M. M. Flores, J. S. Barin, M. F. Mesko and G. Knapp, Spectrochim. Acta, Part B, 2007, 62, 1051–1064 CrossRef.
- E. M. M. Flores and R. S. Picoloto, Sample Dissolution for Elemental Analysis/Oxygen Flask Combustion, 110-117, in Encyclopedia of Analytical Science, ed. P. Worsfold, C. Poole, A. Townshend and M. Miro, Academic Press, 3rd edn, 2019 Search PubMed.
- P. C. Crestani, T. C. Pereira, E. T. F. Larruscain, C. R. Laureano, E. M. M. Flores and F. A. Duarte, Food Chem., 2023, 429, 136916 CrossRef CAS.
- L. S. F. Pereira, G. D. Iop, M. S. Nascimento, L. O. Diehl, C. A. Bizzi, J. S. Barin and E. M. M. Flores, J. Braz. Chem. Soc., 2016, 27, 526–533 CAS.
- J. S. F. Pereira, L. S. F. Pereira, L. Schmidt, C. M. Moreira, J. S. Barin and E. M. M. Flores, Microchem. J., 2013, 109, 29–35 CrossRef CAS.
- F. S. Rondan, G. S. Coelho Junior, R. M. Pereira, A. S. Henn, E. I. Muller and M. F. Mesko, Food Chem., 2019, 285, 334–339 CrossRef CAS PubMed.
- V. H. Cauduro, C. M. A. C. Alves, M. S. Nascimento, G. T. Druzian, F. P. Balbinot, M. F. Mesko and E. M. M. Flores, Anal. Bioanal. Chem., 2024, 11, 2859–2870 CrossRef.
- G. Gohlke, T. C. Pereira, A. P. F. Padilha, C. R. Andriolli, A. S. Henn, R. S. Picoloto, P. A. Mello and E. M. M. Flores, J. Food Compos. Anal., 2025, 144, 107704 CrossRef CAS.
- J. P. Souza, C. Cerveira, T. M. Miceli, D. P. Moraes, M. F. Mesko and J. S. F. Pereira, Food Chem., 2020, 321, 126715 CrossRef CAS.
- G. S. Coelho Junior, V. P. Souza, J. Kratzer, J. Dedina and E. M. M. Flores, Spectrochim. Acta, Part B, 2024, 221, 107055 CrossRef CAS.
- J. P. Souza, K. Kellermann, M. S. Camargo, D. P. Moraes, D. Pozebon and J. S. F. Pereira, J. Anal. At. Spectrom., 2018, 33, 1284–1291 RSC.
- J. P. Souza, J. L. Boff, L. F. Rodrigues, D. P. Moraes and J. S. F. Pereira, Anal. Methods, 2022, 14, 1285–1290 RSC.
- W. Geng, T. Furuzono, T. Nakajima, H. Takanashi and A. Ohki, J. Hazard. Mater., 2010, 176, 356–360 CrossRef CAS.
- W. Geng, T. Nakajima, H. Takanashi and A. Ohki, Fuel, 2008, 87, 559–564 CrossRef CAS.
- Y. Y. Ma, K. Zeng and T. C. Duan, Microchem. J., 2019, 148, 743–747 CrossRef CAS.
- W. Wojnowski, M. Tobiszewski, F. Pena-Pereira and E. Psillakis, Trends Anal. Chem., 2022, 149, 116553 CrossRef CAS.
- P. M. Nowak, R. Wietecha-Posluszny and J. Pawliszyn, Trends Anal. Chem., 2021, 138, 116223 CrossRef CAS.
- P. M. Nowak, Green Chem., 2023, 25, 4625–4640 RSC.
- P. M. Nowak, W. Wojnowski, N. Manousi, V. Samanidou and J. Plotka-Wasylka, Green Chem., 2025, 27, 5546–5553 RSC.
- P. M. Nowak, Green Chem., 2025, 27, 6699–6710 RSC.
- N. Manousi, W. Wojnowski, J. Plotka-Wasylka and V. Samanidou, Green Chem., 2023, 25, 7598–7604 RSC.
- P. M. Nowak and F. Arduini, Green Anal. Chem., 2024, 10, 100120 CrossRef.
- C. M. Hussain, C. G. Hussain and R. Kecili, Trends Anal. Chem., 2023, 159, 116905 CrossRef CAS.
- F. A. Esteve-Turrillas, S. Garrigues and M. de la Guardia, Trends Anal. Chem., 2024, 170, 117464 CrossRef CAS.
- S. B. Angyus, M. Senila, T. Frentiu, M. Ponta, M. Frentiu and E. Covaci, Talanta, 2023, 259, 124551 CrossRef CAS PubMed.
- S. B. Angyus, M. Senila, E. Covaci, M. Ponta, M. Frentiu and T. Frentiu, J. Anal. At. Spectrom., 2024, 39, 141–152 RSC.
- B. D. Szeredai, T. Frentiu, N. Muntean, A. I. Dudu and E. Covaci, J. Anal. At. Spectrom., 2025, 40, 942–953 RSC.
- P. W. J. M. Boumans, Spectrochim. Acta, Part B, 1991, 46, 431–445 CrossRef.
- P. W. J. M. Boumans, J. C. Ivaldi and W. Slavin, Spectrochim. Acta, Part B, 1991, 46, 641–665 CrossRef.
- T. Frentiu, M. Ponta, E. Darvasi, S. Butaciu, S. I. Cadar, M. Senila, A. Mathe, M. Frentiu, D. M. Petreus, R. Etz, F. Puskas and D. Sulea, Miniaturized analyzer with Rhodium-filament evaporator for simultaneous determination of elements from liquid microsamples by optical emission spectrometry, Romanian Pat., RO 131066 B1, State Office for Inventions and Trademarks, Bucharest, 2020 Search PubMed.
- D. Petreus, E. Plaian, A. Grama, E. Cordos and S. Cadar, Low power plasma generator at atmospheric pressure, Romanian Pat., RO128077-B1, State Office for Inventions and Trademarks, Bucharest, 2016 Search PubMed.
- T. Frentiu, S. Butaciu, E. Darvasi, M. Ponta, M. Frentiu and D. Petreus, Chem. Pap., 2017, 71, 91–102 CrossRef CAS.
- ISO 20236:2024, Water quality – Determination of total organic carbon (TOC), dissolved organic carbon (DOC), total bound nitrogen (TNb) and dissolved bound nitrogen (DNb) after high temperature catalytic oxidative combustion, International Organization for Standardization, Geneva, 2024.
- J. Mandel, The Statistical Analysis of Experimental Data, Dover Publications, New York, 1964, pp. 272–309 Search PubMed.
- Eurachem Guide: Selection, Use and Interpretation of Proficiency Testing (PT) Schemes, ed. B. Brookman and I. Mann, 3rd edn, 2021, EPT_2021_P1_EN.pdf, accessed 17 July 2025 Search PubMed.
- J. M. Tukey, Biometrics, 1949, 5, 99–114 CrossRef CAS.
- A. I. Mihaltan, T. Frentiu, M. Ponta, D. Petreus, M. Frentiu, E. Darvasi and C. Marutoiu, Talanta, 2013, 109, 84–90 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2026 |