A strategy of H-bond confinement catalysis for efficient degradation of polyethylene glycol into glycol diester over an OH-functionalized ionic liquid
Xiaoqian
Chang
 *ac,
Yunpeng
Xu
b,
Chunliang
Hou
a,
Ruihan
Wang
*ac,
Yunpeng
Xu
b,
Chunliang
Hou
a,
Ruihan
Wang
 ac and
Xiaoyang
Chang
d
ac and
Xiaoyang
Chang
d
aCollege of Chemical Engineering, Hebei Normal University of Science and Technology, Qinhuangdao, Hebei 066004, China. E-mail: cxqxyp@163.com
bHebei Normal University of Science and Technology, Qinhuangdao, Hebei 066004, China
cLow Carbon Conversion and Innovation Research Center, Chemical Engineering College, Hebei Normal University of Science and Technology, Qinhuangdao, Hebei 066004, China
dCollege of Optical and Electronic Technology, China Jiliang University, Hangzhou, Zhejiang 311222, China
First published on 20th November 2025
Abstract
Upgrading plastic waste into high-value chemicals and achieving recycling is of great significance for sustainable development. Herein, we report a green, mild, solvent- and metal-free catalytic approach that uses hydroxyl-functionalized ionic liquids to induce the cleavage of C–O and O–H bonds in polyethylene glycol (PEG), successfully converting PEG into ethylene glycol diester. Through experiments, NMR analysis and DFT calculations, we find that the anions and cations of [HO-EtMIm][OTf] can respectively act as hydrogen bond acceptors and donors to activate the O–H and C–O bonds in PEG, as well as the C=O double bonds in benzoic anhydride. Subsequently, the activated hydroxyl O atom in PEG can attack the carbonyl C atom in benzoic anhydride through nucleophilic action, and the activated O atom in benzoic anhydride can also attack the C atom in PEG through nucleophilic action, thereby undergoing depolymerization and forming new C–O bonds, ultimately generating the target product. This mechanism clearly demonstrates the importance of the unique confinement effect of ionic liquids, which is achieved through spatial confinement, electrostatic stabilization, directional activation, and hydrogen bonding synergy, providing corresponding innovative support for future research.
Green foundation1. Our work introduces a novel hydrogen-bond-driven catalytic system using [HO-EtMIm][OTf] for PEG depolymerization, replacing conventional metal catalysts and harsh conditions with a mild (90 °C), energy-efficient approach. The IL's unique cation–anion synergy activates PEG and benzoic anhydride through H-bonding, enabling selective C–O/O–H bond cleavage without toxic byproducts.2. Adhering to the catalysis principle, this green method employs a recyclable ionic liquid [HO-EtMIm][OTf] for synthesizing ethylene glycol dibenzoate. It proceeds efficiently at 90 °C, far below the >150 °C required by conventional catalysts, and achieves a 97% yield with almost no loss in activity over five cycles. 3. Future greening enhancements include developing bio-derived ionic liquids from renewable resources, and expanding applications to mixed plastic waste through tailored hydrogen-bond networks; for instance, selectively binding specific polymers or regulating the compatibility of plastics through hydrogen bonds. |
Introduction
Polyethylene glycol (PEG) is an important polymer material with wide applications in industrial production and daily life. However, the problems of environmental accumulation and degradation of PEG have become a global concern. In recent years, with increasingly strict environmental protection requirements, significant progress has been made in the degradation research of PEG, especially in fields such as biological degradation (such as microbial degradation and enzyme-catalyzed degradation),1–4 photocatalytic oxidation,5 physical–chemical combined degradation,6 and chemical catalysis7–11 (Scheme 1). However, the PEG biodegradation process has significant limitations. For instance, the natural degradation rate process is usually slow and highly dependent on specific environmental conditions (such as suitable microbial communities, temperature, pH value, and oxygen content).1–4 Photocatalytic oxidation requires high energy input, and the photon utilization efficiency is generally low.5 The physical–chemical combined degradation usually relies on external energy inputs such as ultrasound, microwave, and high-voltage electric fields,6 which are expensive and have high operation and maintenance costs. This makes the chemical catalytic degradation technology a research hotspot. Examples include copper slag catalytic ozonation,7 alcoholysis degradation of PEG derivatives,8 and converting PEG into high-value-added chemicals (i.e. ethanol, chloroesters, organosilicon compounds, etc.)9–11 (Scheme 1 and SI Table S1). However, in these reports, metal-based catalysts, reductants and stringent reaction conditions are usually required. Therefore, finding ways to improve degradation efficiency, reduce energy consumption, and realize the resource utilization of degradation products still remains the main challenge currently faced.Ionic liquids (ILs) are molten salts composed of organic cations and inorganic/organic anions, typically in a liquid state at room temperature or close to it. Compared to traditional solvents, ionic liquids possess unique properties such as extremely low vapor pressure, high thermal stability, a wide electrochemical window and controllable solubility. Their physical and chemical properties (such as viscosity, polarity, and catalytic activity) can be precisely controlled by altering the structures of cations and anions.12 Therefore, they have extensive applications in green chemistry, electrochemistry, catalysis, separation technologies, energy materials, etc.13–17 The properties of ionic liquids are determined by their complex intermolecular interactions. Strong electrostatic interactions dominate the stability of ionic pairs, hydrogen bonds and π–π stacking influence the local structure and solubility, and van der Waals forces and solvation effects regulate viscosity and phase behavior.18,19 The synergistic action of these forces makes ionic liquids designable “functional liquids”, and their design flexibility makes them an important tool for achieving sustainable chemical processes,20 such as catalyzing the degradation of polymers into N-succinimide, amides, carboxylic acids and hydrocarbons, alcohols, carbonates, etc.21–27
In our previous studies, it was found that the cations and anions of ionic liquids could, respectively, act as hydrogen bond donors (HBD) and hydrogen bond acceptors (HBA), enabling reactions such as the ring dehydration of diols, the trimerization of aliphatic diethers, the dehydration and double decomposition of aromatic alcohols with sulfur ethers, and the benzylation of aromatic alcohols with alkanes.28–33 Therefore, during our continuous efforts on the IL catalytic reaction, in this study, we proposed a strategy to catalyze the degradation of PEG using the cation–anion hydrogen bond confinement synergy in ionic liquids under metal- and solvent-free, and green mild conditions. We found that the anions and cations in the functionalized ionic liquids [HO-EtMIm][OTf] could, respectively, act as hydrogen bond acceptors and donors to activate the O–H/C–O bonds of PEG and the C=O double bonds of benzoic anhydride through the cooperative confinement effect of hydrogen bonds. Subsequently, the activated hydroxyl O atom in PEG attacked the anhydride group C atom of benzoic anhydride through nucleophilic reaction, and the single bond O atom of the activated benzoic anhydride nucleophilically attacked the ether oxygen C atom in PEG, thereby causing depolymerization, forming new C–O ester bonds, and ultimately obtaining the target diester product.
Results and discussion
Initially, taking tetraethylene glycol as the model substrate of polyethylene glycol, a series of depolymerization reactions with benzoic anhydride were carried out to obtain product 2 (ethylene glycol dibenzoate) in various ionic liquids, as shown in Fig. 1. Obviously, the results indicated that the reaction did not occur without ionic liquid catalysts, and product 2 could not form either. When the anions were all [OTf]−, the reaction involving [EtMIm]+ as the cation hardly occurred. However, when [HO-EtMMIm]+, [HO-EtN111]+ and [HO-EtMIm]+ were used as the cations, the depolymerization could proceed almost smoothly. Particularly, the [HO-EtMIm][OTf] delivered a significantly higher catalytic performance among them, with a product yield of up to 97% and approximately 93% of the selectivity (Fig. 1 and SI Fig. S2). The above results indicated that the hydroxyl-functionalized cations in ionic liquids may be effective for this reaction.Furthermore, we also investigated the influence of ionic liquids with different anions on the performance of this reaction. When the ionic liquid anions were [OTf]−, [OTs]−, and [HSO4]− with [HO-EtMIm]+ as cations, the depolymerization could perform to a certain extent except for [N(CN)2]−, [Cl]−, [BF4]−, [PF6]−, [ClO4]−, [NTf2]− and [NO3]−. In contrast, when [OTf]− was used as the anionic component of the ionic liquid, the yield obtained was the highest (97%). The above results indicated that the role of the ionic liquid anion [OTf]− in the depolymerization reaction was more crucial than that of other anions. Moreover, it can be deduced that the hydroxyl group of the cation [HO-EtMIm]+ and the anion [OTf]− in the ionic liquid all played crucial roles in this reaction according to the above experimental results, which might synergically catalyze the depolymerization of the substrate.
The [HO-EtMIm][OTf] ionic liquid catalyst is at the forefront in the field of sustainable catalysis. Compared to traditional catalysts such as H2SO4, this IL demonstrated comprehensive green chemical advantages and had superior sustainability indicators. Waste control: the E-factor was only 0.18, which was 97% lower than that of traditional catalysts (4.7), significantly reducing reaction waste; atomic utilization: the atom economy reached 89% (traditional method: 68%), maximizing the utilization of reaction raw materials; material efficiency: the process quality intensity (PMI) was as low as 4.3, saving 77% of material input compared to traditional processes (18.9); low carbon characteristics: the carbon footprint was 31 g of CO2-eq per mol, only 24% of that of traditional catalysts (127 g CO2-eq per mol); environmental friendliness: the water ecological toxicity was Class 1 (>100 mg L−1), and its safety was much better than that of traditional acid catalysts of Class 3; energy saving: the energy intensity was 2.4 kWh mol−1, reducing energy consumption by 72% (traditional method 8.7 kWh mol−1) (SI Table S2).34–37 This ionic liquid achieved a dual breakthrough in terms of economy and sustainability through a synergistic mechanism of efficient catalysis and low-consumption cycling while maintaining high catalytic activity.
Subsequently, with [HO-EtMIm][OTf] as the optimal IL catalyst, the influences of various parameters, including reaction temperature, time, and amount of IL on this reaction were investigated in detail (Fig. 2). It can be obviously seen that the reaction temperature had a significant influence on the yield of product 2. The reaction could be carried out after 50 °C, and the yield rose with the increase of temperature, reaching the maximum value of 97% at 90 °C (Fig. 2A), after which the yield remained almost unchanged. The effect of reaction time on the yield of product showed that the yield of the reaction showed a stable upward trend at 90 °C, reaching the highest yield of 97% at 8 hours (Fig. 2B), and after that, the yield remained almost constant. Therefore, under the optimal reaction time and temperature conditions mentioned above, the effects of the amount of IL on this reaction were researched (Fig. 2C). The results obviously showed that the reaction did not occur without an ionic liquid catalyst. When the amount of IL was increased continuously, the yield of product 2 increased rapidly, reaching the maximum value of 97% at 1.2 mmol, and remained basically unchanged thereafter.
 | ||
| Fig. 2 Effects of reaction parameters: (A) temperature, (B) time, (C) amount of IL and (D) the IL recycling tests. | ||
In addition, the recyclability of the ionic liquid was also explored. After extraction from the reaction solution and drying under vacuum, the IL was subsequently recycled and used for the next reaction. As shown in Fig. 2D, after reusing [HO-EtMIm][OTf] five times, the yields of product 2 basically remained unchanged, indicating that this IL was stable and recoverable under the same experimental conditions. Moreover, as shown in SI Fig. S3A and B, 1H and 19F NMR spectra of recovered [HO-EtMIm][OTf] remained unchanged before and after the reaction, indicating that the IL had good recyclability and stability.
Inspired by the excellent catalytic effect of the model substrate 1, based on the aforementioned optimized conditions, we carried out a large-scale depolymerization reaction of different average molecular weights (average Mn: 200–4000) of PEG using the ionic liquid [HO-EtMIm][OTf]. We found that the yields of product 2 were all above 79%, and could reach up to 95% (Fig. 3A and SI Table S3). These indicated that [HO-EtMIm][OTf] had good tolerance to PEG with different degrees of polymerization and had a relatively wide range of applications. Furthermore, this ionic liquid was also applicable to different average molecular weights of polypropylene glycols (PPG). Among them, the yield of product 3 (1,2-propanediol bisphthalate) was above 58% in all cases, with the highest reaching 87% (Fig. 3B, SI Fig. S5 and Table S3). Overall, from Fig. 3A and B, it could be seen that the yields of PEG were higher than those of PPG. The high yield of PEG was attributed to its flexible chain structure (reducing steric hindrance) and good solubility/diffusion ability, while PPG experienced a slight decrease in reaction efficiency due to the steric hindrance of side chains and high viscosity/low solubility. These various effects worked together, making it easier for PEG to form the diester products. In addition, the acetic anhydride was used instead of benzoic anhydride to carry out the extension reaction with PEG (average Mn: 200–4000) under similar conditions. The results showed that the yields of product 4 (ethylene glycol diacetate) were all above 63% (Fig. 3C, SI Fig. S6 and Table S3). The yields of benzoic anhydride were generally higher than that of acetic anhydride. This was mainly because the strong electron-withdrawing effect of the benzene ring enhanced the electrophilicity of the carbonyl carbon, and the benzoate radical leaving group was more stable, promoting the reaction equilibrium to shift towards the product; while the methyl electron-donating effect of acetic anhydride reduced the activity of the carbonyl group, and the acetoate radical was less stable. Therefore, the reaction efficiency of benzoic anhydride was much better. Furthermore, compared with other depolymerization reactions of PEG, this ionic liquid catalyst demonstrated excellent catalytic performance, a green and mild process, sustainability, and a wide range of applicability (SI Tables S1 and S3).
In order to explore the interaction and reaction mechanism between [HO-EtMIm][OTf] and the substrate, 1H, 17O and 19F NMR spectra were recorded (Fig. 4). It is well known that both the electronegative O and F atoms could form H-bonds with H atoms. It could be clearly seen from Fig. 4A and C that the 1H and 17O NMR spectra of tetraethylene glycol and IL had corresponding changes before and after mixing, indicating that [HO-EtMIm][OTf] could activate tetraethylene glycol and there was some kind of interaction between them, while the 19F NMR spectra had no significant changes. The results indicated that the O atom in the IL anion [OTf]− might form a hydrogen bond with the H atom in tetraethylene glycol, rather than the F atom. Here, the chemical shift changed in the spectra of the pure substance (tetraethylene glycol, IL) and the mixture (R/IL) could be attributed to the strong hydrogen bond interaction between them (Fig. 4A and C). In the 17O NMR spectra, it can be observed that the resonance band of the O atom in [OTf]− changed from 162.44 ppm to 161.67 ppm (Fig. 4C), while the signal of the F atom in [OTf]− hardly changed (Fig. 4B). Meanwhile, the resonance band of the hydroxyl H atom in tetraethylene glycol moved from 4.54 ppm to 4.86 ppm when tetraethylene glycol mixed with IL (Fig. 4A). These results showed that the O atoms in [OTf]− were more inclined to form hydrogen bonds with tetraethylene glycol than F atoms, thus activating the O–H bond of tetraethylene glycol. In addition, in the 1H NMR spectra, it can be observed that the resonance peak of hydroxyl hydrogen atoms in [HO-EtMIm]+ shifted from 5.59 ppm to 4.86 ppm before and after mixing (Fig. 4A), moving to a higher field. At the same time, it can be clearly seen that when tetraethylene glycol was mixed with the ionic liquid, the resonance peak of the oxygen atom located at about −3.53 ppm in tetraethylene glycol significantly weakened, and shifted to −5.28 ppm (Fig. 4C). These results indicated that the hydroxyl H atoms in the cation of IL could form hydrogen bonds with the oxygen atoms in tetraethylene glycol, thereby activating the C–O bonds of tetraethylene glycol.
The calculation results regarding hydrogen bonds in Fig. 4D indicated that the hydroxyl H atom of tetraethylene glycol could form hydrogen bonds with the O atom in [OTf]− of the [HO-EtMIm][OTf] (with a hydrogen bond length of 1.90 Å); meanwhile, the hydroxyl H atom of the cation [HO-EtMIm]+ in the [HO-EtMIm][OTf] could also form hydrogen bonds with the O atoms in the ether oxygen bonds of tetraethylene glycol (the hydrogen bond length was 1.83 Å). Furthermore, Fig. 4E presents the electrostatic potential (ESP) distribution of [HO-EtMIm][OTf] and tetraethylene glycol. It could be clearly observed that the electrostatic potential of –OH in tetraethylene glycol was positive, with relatively low electron density, making it more likely to attract negatively charged particles (red area). In contrast, the O atom in [OTf]− exhibited a negative electrostatic potential in [HO-EtMIm] [OTf], forming an electron-rich region (blue area). These two regions overlapped with each other, indicating that the H-bond formed between [HO-EtMIm][OTf] and tetraethylene glycol also had electrostatic attraction. Similarly, in the region where hydrogen bonds formed, the ether oxygen bonds in tetraethylene glycol had a negative electrostatic potential and a relatively high electron density, making them more capable of attracting positively charged particles (blue area). Correspondingly, in [HO-EtMIm][OTf], the hydroxyl H atom of [HO-EtMIm]+ had a positive electron potential, which was an electron-deficient area, and was more likely to attract negatively charged particles (red area). These two regions overlapped, indicating that the O atom in the ether oxygen bonds of tetraglycol and the [HO-EtMIm]+ in [HO-EtMIm][OTf] also had electrostatic attractions. In addition, the RDG analysis diagram used multi-dimensional data visualization to reveal the non-covalent interaction characteristics of the tetraethylene glycol and [HO-EtMIm][OTf] composite system. As shown in Fig. 4F, the molecular structure model in the lower left corner clearly indicated the precise location of hydrogen bonds (corresponding to Fig. 4D), showing that the hydroxyl H of tetraethylene glycol with the O atom of the [OTf]− anion in ionic liquid [–CF3(SO2)O⋯H–(OCH2CH2)4–OH] and the O atom in the ether oxygen bond of tetraethylene glycol with the hydroxyl H atom of [HO-EtMIm]+ in ionic liquid [HO–(CH2CH2O)3CH2CH2(H)O⋯HO–EtMIm] all formed typical H-bonds (O–H⋯O). This strong, attractive effect was represented by a dense blue cluster in the right scatter plot within the range of −0.05 to −0.03 a.u. It is worth noting that the scatter plot showed a significant aggregation near sign(λ2)ρ ≈ 0 (green area), and the RDG values were concentrated in the range of 0.5–1.5, indicating the existence of extensive van der Waals interactions in the system. This might be due to the hydrophobic interaction between the alkyl chain of tetraethylene glycol and the imidazole ring, as well as the weak attraction of π electron clouds. The sparse scattered points in the red area of the color scale (λ2 > 0) suggested that there was local spatial repulsion between the imidazole ring cation and the [OTf]− anion, which was alleviated through molecular conformational adjustment and this tetraethylene glycol/[HO-EtMIm][OTf] composite system ultimately could form a stable composite structure dominated by hydrogen bonds and assisted by van der Waals forces. To sum up, the above NMR analysis and calculation results showed that the IL anion ([OTf]−) can form H-bonds with hydroxyl H atoms of tetraethylene glycol as a hydrogen bond acceptor (HBA) and activate O–H bonds. The IL cation ([HO-EtMIm]+) could act as the hydrogen bond donor (HD) and form hydrogen bonds with the oxygen atoms of tetraethylene glycol, thereby activating the C–O bonds, providing relevant evidence for the subsequent mechanism.
More specifically, we conducted corresponding calculations on the NPA charges of tetraethylene glycol (Fig. 5, Table 1, SI Fig. S7 and Table S4). The differences in NPA charges of the O and C atoms of tetraethylene glycol are shown in Fig. 5 and Table 1. Among them, the charge differences could be compared as follows: 55/44 > 59/41 > 59/38 > 58/35. According to previous research reports,38,39 the larger the difference in NPA charges of the O and C atoms in tetraethylene glycol, the easier the bond-breaking reaction was to proceed. Therefore, the atoms marked as 55/44 and 59/41 were more likely to undergo reactions, which provided certain evidence support for the subsequent mechanism speculation.
 | ||
| Fig. 5 The differences in NPA charges of C and O atoms of tetraethylene glycol and benzoic anhydride. | ||
| Substrate | Atom type | Atom number | NPA charge |
|---|---|---|---|
| Tetraethylene glycol | O/C | 55/44 | −0.7445/−0.01912 |
| O/C | 59/41 | −0.6164/−0.02079 | |
| O/C | 59/38 | −0.6164/−0.03811 | |
| O/C | 58/35 | −0.5903/−0.03247 | |
| Benzoic anhydride | O/C | 54/31 | −0.6149/0.86864 |
| O/C | 29/31 | −0.57587/0.86864 | |
| O/C | 29/30 | −0.57587/0.86095 | |
At the same time, in order to explore the interaction and research mechanism between [HO-EtMIm][OTf] and benzoic anhydride, experiments with 1H, 17O and 19F NMR spectra were conducted (Fig. 6A–C). The experimental results clearly showed that the 1H and 17O NMR spectra of benzoic anhydride and IL changed correspondingly before and after mixing (Fig. 6A and C), indicating that this ionic liquid can activate benzoic anhydride and there was also an interaction between them, while the 19F NMR spectra did not change significantly (Fig. 6B). In the 1H NMR spectra, the resonance band of the H atom of OH in [HO-EtMIm]+ moved obviously towards the higher field from 5.19 ppm to 4.64 ppm (Fig. 6A). Meanwhile, the resonance band of the oxygen atom in benzoic anhydride significantly weakened (approximately at 375 ppm) (Fig. 6C), while the O atom of anion [OTf]− in IL did not shift. These findings provided evidence that the H atom of –OH in the IL cation [HO-EtMIm]+ could form a hydrogen bond with the O atom in benzoic anhydride, thus activating the C–O bond of benzoic anhydride.
The calculation results in Fig. 6D also indicate that the carbonyl O atom of benzoic anhydride could form a hydrogen bond with the hydroxyl H atom in the IL cation [HO-EtMIm]+ (the hydrogen bond distance was 1.89 Å). Moreover, Fig. 6E shows the electrostatic potential (ESP) distribution of [HO-EtMIm][OTf] and benzoic anhydride. It could be clearly observed that in the region where hydrogen bonds were formed, the O atom of the carbonyl group exhibited a negative electrostatic potential (ESP < 0) due to its lone pair of electrons and strong electronegativity, indicating an electron-rich area (an electrophilic site) (blue area). In contrast, in [HO-EtMIm][OTf], [HO-EtMIm]+ presented a positive electrostatic potential (ESP > 0), indicating an electron-deficient area (a nucleophilic site) (red area). These two areas overlapped, suggesting that there was also electrostatic attraction in the field of hydrogen-bond formation. Fig. 6F shows the RDG analysis diagram of benzoic anhydride and ionic liquid [HO-EtMIm][OTf]. This figure clearly demonstrates the non-covalent interaction characteristics between benzoic anhydride and [HO-EtMIm][OTf]. In the molecular structure model located in the lower left corner, the blue arrow clearly indicates the formation site of hydrogen bond, namely the H-bond formed between the carbonyl O atom of benzoic anhydride and the hydroxyl H atom of the cation [HO-EtMIm]+ in [HO-EtMIm][OTf] [O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(Ph)–O–(Ph)C
C(Ph)–O–(Ph)C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯HO-EtMIm]. This corresponds to the dense blue point cluster in the negative region of the sign(λ2)ρ on the right RDG scatter plot (−0.05 to −0.03 a.u.), confirming that this is a highly localized electronic density interaction indicative of a strong attractive interaction. The green area in the middle of the scatter plot (sign(λ2)ρ ≈ 0) reflected the van der Waals forces between molecules, possibly coming from the hydrophobic stacking or π–π interaction between the benzene ring and the imidazole ring. The few red scattered points on the right (sign(λ2)ρ > 0) suggest local spatial steric hindrance, such as the steric repulsion between the large [OTf]− in the ionic liquid and the aromatic ring of benzoic anhydride. The color scale bar (from blue to red) further quantified the distribution of electronic density (ρ), with the blue area corresponding to high electronic density (i.e., hydrogen bond sites) and the red area showing the electron-sparsely repelled regions. Overall, it indicated that the stability of this benzoic anhydride/[HO-EtMIm][OTf] composite system was mainly driven by hydrogen bonds and van der Waals forces. The above NMR analysis and calculation results showed that the H atom of –OH in the IL cation [HO-EtMIm]+ could be used as hydrogen bond donors to form a H-bond with the O atom of benzoic anhydride, thereby activating the C–O bond in benzoic anhydride.
O⋯HO-EtMIm]. This corresponds to the dense blue point cluster in the negative region of the sign(λ2)ρ on the right RDG scatter plot (−0.05 to −0.03 a.u.), confirming that this is a highly localized electronic density interaction indicative of a strong attractive interaction. The green area in the middle of the scatter plot (sign(λ2)ρ ≈ 0) reflected the van der Waals forces between molecules, possibly coming from the hydrophobic stacking or π–π interaction between the benzene ring and the imidazole ring. The few red scattered points on the right (sign(λ2)ρ > 0) suggest local spatial steric hindrance, such as the steric repulsion between the large [OTf]− in the ionic liquid and the aromatic ring of benzoic anhydride. The color scale bar (from blue to red) further quantified the distribution of electronic density (ρ), with the blue area corresponding to high electronic density (i.e., hydrogen bond sites) and the red area showing the electron-sparsely repelled regions. Overall, it indicated that the stability of this benzoic anhydride/[HO-EtMIm][OTf] composite system was mainly driven by hydrogen bonds and van der Waals forces. The above NMR analysis and calculation results showed that the H atom of –OH in the IL cation [HO-EtMIm]+ could be used as hydrogen bond donors to form a H-bond with the O atom of benzoic anhydride, thereby activating the C–O bond in benzoic anhydride.
To be more specific, we also conducted corresponding calculations on the NPA charges of benzoic anhydride (Fig. 5, Table 1, SI Fig. S8 and Table S5). The differences in NPA charges between the O and C atoms in benzoic anhydride are shown in Fig. 5 and Table 1. It could be seen that the order of charge differences from largest to smallest was 54/31 > 29/31 > 29/30. The greater the difference in NPA charges between the O and C atoms in benzoic anhydride, the easier the bond-breaking reaction would be. Therefore, the atoms marked as 54/31 and 29/31 were more likely to react. However, the charge difference of the former was significantly larger than that of the latter.
During the process of constructing the molecular structure through DFT calculations, the hydrogen bond structure formed between [HO-EtMIm][OTf] and the O atom labeled as 29, as well as the subsequent bond-breaking structure between 29 and 31, could not be maintained well. They could only maintain the hydrogen bond structure formed with the double bond oxygen (labeled as 54), which was the bond-breaking reaction between 54 and 31 that occurred preferentially. This might be due to the insufficient space for the formation of hydrogen bonds by a single bond oxygen (as both sides had a benzene ring), and the steric hindrance effect prevented this hydrogen bond from being maintained. These demonstrated the confinement effect of the [HO-EtMIm]+ in the ionic liquid, which could provide corresponding evidence support for the subsequent mechanism speculation.
Due to the formation of hydrogen bonds between the above-mentioned tetraethylene glycol, benzoic anhydride and ionic liquid, the NPA charge of the C atom (labeled 41) in tetraethylene glycol changed from −0.0256 in pure tetraethylene glycol to −0.0208 in the mixed system (tetraethylene glycol/[HO-EtMIm][OTf]), indicating a decrease in electron cloud density and an enhancement in the electrophilicity of this C atom; meanwhile, the NPA charge of the O atom (labeled 55) in tetraethylene glycol transformed from −0.691 in pure tetraethylene glycol to −0.745 in the mixed system, indicating an increase in electron cloud density and an enhancement in the nucleophilicity of this O atom (Fig. 4D, SI Fig. S7 and Table S4). Moreover, the NPA charge of the C atom (labeled 31) in benzoic anhydride converted from 0.821 in pure benzoic anhydride to 0.869 in the mixed system (benzoic anhydride/[HO-EtMIm][OTf]), indicating a decrease in electron cloud density and an enhancement in the electrophilicity of this C atom; at the same time, the NPA charge of the O atom (labeled 29) in benzoic anhydride changed from −0.523 in pure benzoic anhydride to −0.576 in the mixed system, indicating an increase in electron cloud density and an enhancement in the nucleophilicity of this O atom (Fig. 6D, SI Fig. S8 and Table S5). Therefore, during the reaction, the O atom in the mixed tetraethylene glycol/IL system might attack the carbonyl C atom of the anhydride group in benzoic anhydride nucleophilically to form a phenylacetyl cation and a phenylformate anion (Ph–C+=O and Ph–CO–O−), and the activated O atom in the mixed benzoic anhydride/IL system might attack the C atom in tetraethylene glycol nucleophilically, ultimately forming product 2.
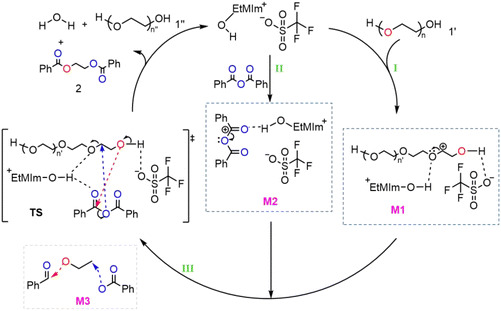
Based on the above experimental results, NMR analysis, and DFT calculation results, the following possible reaction mechanism pathways could be inferred, as shown in Scheme 2. Firstly, in Path I, the oxygen atom in the anion [OTf]− of [HO-EtMIm][OTf] and the hydroxyl hydrogen atom in the cation [HO-EtMIm]+ could, respectively, activate the O–H bonds and C–O bonds of PEG through hydrogen bonding, thereby forming the transition state M1. At the same time, in Path II, the hydroxyl H atom in the cation [HO-EtMIm]+ of [HO-EtMIm][OTf] could activate the C=O double bonds in benzoic anhydride through hydrogen bonding, forming the transition state M2. Subsequently, in Path III, the activated hydroxyl O atom in 1′ could attack the anhydride group C atom in benzoic anhydride through nucleophilic action (as indicated by the red arrow), and the activated oxygen atom in benzoic anhydride could also attack the C atom in PEG through nucleophilic action (as indicated by the blue arrow) (forming a transition state structure similar to M3), thereby undergoing a depolymerization reaction, forming new C–O bonds, and ultimately generating the target product 2, releasing the ionic liquid into the next cycle. This reaction mechanism demonstrated the unique characteristics of ionic liquids in the polymer degradation reaction. Firstly, there was the spatial confinement effect. In Scheme 2, the anion and cation of the ionic liquid confined the reactants PEG, benzoic anhydride, and intermediates M1 and M2 within the specific spatial range through electrostatic forces or spatial steric hindrance, resulting in a stable electrically neutral environment for the entire system. Secondly, there was the directional activation effect. For example, the cation of the ionic liquid could prompt the substrate benzoic anhydride to approach the active site in a specific orientation (such as the O atom of the C=O double bond in the anhydride group rather than the single bond O atom in O=C–O), thereby stabilizing the reaction structure and inhibiting the formation of by-products. Thirdly, there was the hydrogen bond cooperative catalysis effect. The cation and anion of the ionic liquid could, respectively, act as hydrogen bond acceptors and donors to form corresponding H-bonds with the substrates, activating the corresponding C–O bonds/C=O double bonds and O–H bonds, and thus cooperatively catalyzing the reaction to generate the target product. In conclusion, this mechanism clearly demonstrated the significance of the confinement effect of the ionic liquid on the reaction through spatial constraints, electrostatic stabilization, directional activation, and hydrogen-bond synergy, providing innovative and corresponding support for future research and application of ionic liquids.
From a more specific and deeper perspective, in order to further analyze and understand the hydrogen-bond interaction formed by the cations and anions in ILs and the interactions of the reaction substrates during the reaction process, we conducted DFT calculations to obtain more information about the HB interactions between tetraethylene glycol and different anions ([N(CN)2]−, [OTs]−, [Cl]−, [BF4]−, [PF6]−, and [NTf2]−) or between benzoic anhydride and different cations ([HO-EtMMIm]+ and [HO-EtN111]+). The results shown in Fig. 7 indicate that the hydrogen bond distances formed between the ILs and tetraethylene glycol were approximately 1.95 Å/1.91 Å, 1.92 Å/1.90 Å, 2.08 Å/1.99 Å, 1.97 Å/1.93 Å, 2.02 Å/1.98 Å, and 1.97 Å/1.90 Å, respectively. Overall, these distances were greater than the H-bond distance formed between [HO-EtMIm][OTf] and tetraethylene glycol (1.90 Å/1.83 Å, Fig. 4D). Among them, [HO-EtMIm][OTf] and [HO-EtMIm][OTs] could form HBs with the substrate at shorter distances than the other ILs, which to some extent indicated that they could form stronger HBs with tetraethylene glycol, and [HO-EtMIm][OTf] had the strongest ability to form hydrogen bonds with the corresponding highest product yield (97%, Fig. 1). In addition, the cations in ILs and benzoic anhydride might form hydrogen bonds during the reaction process and play a corresponding role in activating benzoic anhydride. Clearly, the H-bond distances between the carbonyl O atom of benzoic anhydride and the hydroxyl H atom of [HO-EtMMIm]+ and [HO-EtN111]+ were approximately 1.94 Å and 1.93 Å, respectively, which were greater than the H-bond distance formed between [HO-EtMIm]+ and the carbonyl O atom of benzoic anhydride (1.89 Å, Fig. 6D). This indicated that the hydroxyl H atom in [HO-EtMIm]+ acted as a hydrogen bond donor and had a stronger ability to form HBs with the carbonyl O atom of benzoic anhydride, thereby improving the reaction efficiency. These results were basically consistent with the experimental results shown in Fig. 1, where the yield was the highest (97%) when the ionic liquid was [HO-EtMIm][OTf]. These effective cations and anions in ionic liquids could form hydrogen bonds with substrates and activate the substrates during the reaction. Under the confinement effect and the synergistic catalytic effect of hydrogen bonds between the anions/cations of ionic liquids and the substrates, the reaction proceeded smoothly and ultimately generated the target product.
Building upon the experimental findings of high catalytic efficiency (97%), excellent selectivity (∼93%), and successful ionic liquid recyclability over five cycles, a life cycle assessment (LCA) (SI) was conducted to quantitatively evaluate the environmental sustainability of this H-bond confinement catalysis strategy. The remarkable recyclability of the [HO-EtMIm] [OTf] catalyst was identified as the foundation for minimizing the environmental footprint. The LCA results revealed that by allocating the initial burden of IL synthesis across multiple uses, the process transitions from being potentially unsustainable to one with a significant green advantage. However, with the catalyst issue mitigated, the assessment shifted the focus to new environmental hotspots: ethyl acetate, as the extractant, became the main factor causing toxicity effects; the energy consumption required for the 8 hour reaction at 90 °C and the excessive use of benzoic anhydride (which affected the overall atom economy) also imposed a significant burden. In addition, this quantitative analysis confirmed that the unique confinement effect and H-bond synergy could efficiently activate the substrates at the molecular level, not only enabling the high-yield (97%) conversion of PEG into value-added diesters under mild conditions but also laying the foundation for a sustainable process by significantly reducing the catalyst-related impact. Furthermore, the comprehensive green chemistry assessment of this process, based on LCA, quantified the key green chemistry indicators. The results indicated that although the reaction itself had a relatively high carbon efficiency (∼90.11%) and the recycling of the catalyst demonstrated the advantages of green design, the high E-factor (3.23) and PMI (4.23) (both mainly driven by solvent usage) as well as significant energy consumption and a slightly lower atom economy (88.73%) were the key weaknesses that affected its overall environmental footprint. These indicators aligned with the LCA findings that the solvent use, reactant ratio and energy consumption were the key areas for improvement. Therefore, the LCA and the green chemistry metrics provided a clear direction for future optimization, suggesting that further greening of this technology should target process engineering aspects, such as implementing solvent recovery, optimizing reactant stoichiometry and reducing energy consumption, to fully utilize the environmental advantages inherent in this catalytic system.
Conclusions
In conclusion, we proposed a strategy to achieve hydrogen bonding confinement and cooperative catalytic effects between the cations and anions in ionic liquids, successfully inducing the cleavage and degradation of C–O/O–H bonds in polyethylene glycol and converting it into diester compounds with a yield of up to 97% and a selectivity of about 93%. Mechanistic studies showed that the anions and cations in [HO-EtMIm][OTf] could activate the O–H/C–O bonds of PEG and the C=O double bonds of benzoic anhydride through hydrogen bonds. Subsequently, the activated hydroxyl H atom in PEG nucleophilically attacked the carbonyl C atom of benzoic anhydride, and the activated O atom in benzoic anhydride could nucleophilically attack the C atom in PEG, resulting in the degradation of PEG and formation of the final target product. This research provides a simple, environmentally friendly, and efficient method for converting PEG into high-value-added chemicals based on the unique properties of ionic liquids, and also offers a green, clean approach and some theoretical guidance for the production of more complex and diverse high-value-added chemicals with different functions, which may have broad application prospects.Materials and methods
Materials
The ILs including [EtMIm][OTf] (99%), [HO-EtMIm][OTf] (99%), [HO-EtMMIm][OTf] (99%), [HO-EtN111][OTf] (99%), [HO-EtMIm][BF4] (99%), [HO-EtMIm][PF6] (99%), [HO-EtMIm][NTf2] (99%), [HO-EtMIm][Cl] (99%), [HO-EtMIm][OTs] (99%), [HO-EtMIm][N(CN)2] (99%), [HO-EtMIm][ClO4] (99%), [HO-EtMIm][NO3] (99%), and [HO-EtMIm][HSO4] (99%) were purchased from the Centre of Green Chemistry and Catalysis, Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences. The ILs were subjected to freeze-drying treatment before use, and their chemical structures are shown in SI Fig. S1. Tetraethylene glycol (99%) and benzoic anhydride (99%) were purchased from Beijing InnoChem Science and Technology Co., Ltd. Acetic anhydride (99%) was purchased from Tianjin Damao Chemical Reagent Factory. Polyethylene glycol (PEG, average Mn: 200, 400, 600, 800, 1000, 1500, 2000 and 4000) and polypropiol (PPG, average Mn: 200, 400, 600, 1000, 2000, 3000, and 4000) were obtained from Shanghai Macklin Biochemical Technology Co., Ltd. Mesitylene and dimethyl sulfoxide-d6 (DMSO-d6, 99.8 atom % D) were purchased from Shanghai Aladdin Biochemical Technology Co., Ltd.General procedures for depolymerization of polyethylene glycol
The depolymerization reactions were conducted in a thick-walled pressure tube with an internal volume of about 16 mL equipped with a magnetic stirrer. In a typical experiment, the IL, PEG and benzoic anhydride were successively loaded into the reactor under a nitrogen atmosphere, and then the sealed reactor was placed in an oil bath at the desired temperature (e.g. 90 °C). Subsequently, after the required reaction time was over, it was taken out and allowed it cool naturally. Quantitative analysis of the product was performed by 1H NMR spectroscopy using mesitylene as the internal standard.Recycling test of the IL catalyst
The reusability of [HO-EtMIm][OTf] was tested using the benchmark reaction of depolymerization. After the reaction, the reaction solution was extracted with ethyl acetate to remove the product. Subsequently, the water in the IL solution was removed by vacuum drying. Finally, the recovered [HO-EtMIm][OTf] was reused directly for the next run.NMR measurements
Liquid 1H NMR and 13C spectra at normal temperature were recorded on a Bruker Avance III 600 Hz spectrometer. 1H, 17O, and 19F NMR spectra at 333.15K were recorded on n AVANCE III 500 WB NMR spectrometer. Chemical shifts are given in parts per million (ppm) relative to tetramethylsilane. To eliminate the effect of solvent, Wilmad coaxial insert NMR tubes were used for 1H, 19F, and 17O NMR analysis. DMSO-d6 was added in the inner tube, and the sample was added in the outer tube.HPLC characterization
The HPLC analysis was performed on an Agilent 1290II-6460 system. Separation was achieved using an Agilent SB-C18 column (2.1 × 100 mm, 1.8 µm) maintained at 40 °C. The mobile phase consisted of (A) 0.1% formic acid in water and (B) acetonitrile, delivered at a flow rate of 0.2 mL min−1. A gradient elution program was employed: initially holding at 10% B for 1 minute, then increasing linearly to 90% B by 10 minutes, holding until 15 minutes, followed by a rapid return to the initial 10% B condition at 15.01 minutes. The system was then re-equilibrated under the initial conditions for a 4 minute post-run time.DFT calculations
The Density Functional Theory (DFT) calculations were performed using the Gaussian 16 software.40 The B3LYP functional41 was adopted for all calculations in combination with the D3BJ dispersion correction.42 For geometry optimization and frequency calculations, the 6-31G(d,p) basis set was used for all atoms.43,44 The Natural Bond Orbital (NBO) calculation was performed at the B3LYP-D3BJ/6-311G(d,p) level.45Conflicts of interest
There are no conflicts to declare.Author contributions
Xiaoqian Chang: methodology, investigation, formal analysis, funding acquisition, project administration, supervision, resources, writing – original draft, and writing – review & editing. Yunpeng Xu: writing – review & editing, formal analysis, resources, and data curation. Chunliang Hou: investigation and formal analysis. Ruihan Wang: supervision and resources. Xiaoyang Chang: investigation and data curation.Data availability
All data generated or analysed during this study are included in this article and its supplementary information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5gc05259a.Acknowledgements
This work is supported by the Science Research Fund of Hebei Normal University of Science & Technology (2025YB035) for the financial support.References
- S. B. Borrelle, J. Ringma and K. L. Law, et al., Predicted growth in plastic waste exceeds efforts to mitigate plastic pollution, Science, 2020, 369, 1515–1518 CrossRef CAS PubMed.
- W. Ali, H. Jeong and J. S. Lee, et al., Biodegradable microplastics interaction with pollutants and their potential toxicity for aquatic biota: a review, Environ. Chem. Lett., 2024, 22, 1185–1220 CrossRef CAS.
- F. Kawai, T. Kawabata and M. Oda, Microbial degradation of polyethylene glycol (PEG) and its derivatives: Current knowledge and future perspectives, Appl. Microbiol. Biotechnol., 2018, 102, 2067–2076 Search PubMed.
- Y. Zhang, X. Li and C. Wang, et al., Magnetic PEGDA-chitosan inverse opal hydrogel immobilized laccase for efficient degradation of bisphenol A and PEG-based polymers, Chem. Eng. J., 2021, 405, 126647–126658 CrossRef.
- Y. Wu, C. Fan and X. Zhang, et al., Photocatalytic degradation of polyethylene glycol using TiO2 nanoparticles: Kinetics and mechanism study, Appl. Catal., B, 2024, 342, 123456–123467 Search PubMed.
- R. Wang, S. Zhang and X. Li, et al., Plasmonic catalysis for polymer degradation: From localized heating to bond cleavage, Sci. Adv., 2022, 8, eabn1034 Search PubMed.
- W. Z. Zhong, W. C. Hao and S. H. Liang, et al., Catalytic ozonation of polyethylene glycol in aqueous solution by copper slag: Efficiency, active substances and mechanisms, J. Water Process Eng., 2024, 59, 104958–104960 CrossRef.
- W. Zhong, S. Liang and S. Luo, et al., Glycolysis of PEG-modified polyisocyanurate using ethylene glycol/diethylene glycol systems: Reaction mechanisms and recycled polyol characterization, Polym. Degrad. Stab., 2023, 218, 110567–110578 CrossRef.
- Z. L. Zhao, G. Gao and Y. J. Xi, et al., Inverse ceria-nickel catalyst for enhanced C-O bond hydrogenolysis of biomass and polyether, Nat. Commun., 2024, 15, 8444–8453 CrossRef CAS.
- S. Enthaler, Application of fatty acid chlorides in the iron-catalyzed depolymerization of polyethers, Eur. J. Lipid Sci. Technol., 2013, 115, 239–245 CrossRef CAS.
- E. Feghali and T. Cantat, Room temperature organocatalyzed reductive depolymerization of waste polyethers, polyesters, and polycarbonates, ChemSusChem, 2015, 8, 980–984 CrossRef CAS.
- S. Zhang, N. Sun and X. He, et al., Physical properties of ionic liquids: Database and evaluation, J. Phys. Chem. Ref. Data, 2006, 35, 1475–1517 CrossRef CAS.
- T. Welton, Ionic liquids: A brief history, Chem. Rev., 2018, 118, 4631–4639 CrossRef PubMed.
- J. P. Hallett and T. Welton, Room-temperature ionic liquids: Solvents for synthesis and catalysis, Chem. Rev., 2011, 111, 3508–3576 CrossRef CAS PubMed.
- Q. Zhang, X. Liu and L. Yin, et al., Designing ionic liquid electrolytes for Li-S batteries with high coulombic efficiency and energy density, Adv. Mater., 2023, 35, 2300125–2300136 Search PubMed.
- F. Jérôme and K. De Oliveira Vigier, Catalytic upgrading of biomass-derived compounds with ionic liquids, Chem. Soc. Rev., 2021, 50, 10023–10043 Search PubMed.
- G. Xu, Y. Wang and X. Lian, et al., Brønsted acidic ionic liquids for sustainable biomass valorization: From dissolution to catalytic conversion, Green Chem., 2021, 23, 1375–1390 Search PubMed.
- K. Fumino and R. Ludwig, Analyzing the interaction energies between cations and anions in ionic liquids: The subtle balance between coulomb forces and hydrogen bonding, Phys. Chem. Chem. Phys., 2014, 16, 21903–21929 RSC.
- A. M. Smith, K. R. J. Lovelock and S. Perkin, Quantifying the role of hydrogen bonding in ionic liquid clusters, Science, 2019, 363, 776–779 Search PubMed.
- P. Wasserscheid and W. Keim, Ionic liquids-new ‘solutions’ for transition metal catalysis, Angew. Chem., Int. Ed., 2000, 39, 3772–3789 CrossRef CAS.
- F. T. Wu, Y. P. Wang and Y. F. Zhao, et al., Upcycling poly(succinates) with amines to N-substituted succinimides over succinimide anion-based ionic liquids, Nat. Commun., 2024, 15, 712–720 CrossRef CAS PubMed.
- X. L. Qu, G. Y. Zhou and R. Wang, et al., Synergistic catalysis of imidazole acetate ionic liquids for the methanolysis of spiral poly(ethylene 2,5-furandicarboxylate) under a mild condition, Green Chem., 2021, 23, 1871–1882 RSC.
- F. T. Wu, Y. P. Wang and Y. F. Zhao, et al., Lactate anion catalyzes aminolysis of polyesters with anilines, Sci. Adv., 2023, 9, eade7971 CrossRef CAS PubMed.
- R. Salas, R. Villa and F. Velasco, et al., Ionic liquids in polymer technology, Green Chem., 2025, 27, 1620–1651 RSC.
- W. Zeng, Y. F. Zhao and F. T. Zhang, et al., A general strategy for recycling polyester wastes into carboxylic acids and hydrocarbons, Nat. Commun., 2024, 15, 160–167 CrossRef.
- M. S. Liu, J. Guo and Y. Q. Gu, et al., Versatile imidazole-anion-derived ionic liquids with unparalleled activity for alcoholysis of polyester wastes under mild and green conditions, ACS Sustainable Chem. Eng., 2018, 6, 15127–15134 CrossRef CAS.
- Y. Chen, Z. Wang and J. Zhang, et al., Efficient chemical recycling of polyethylene terephthalate (PET) using ionic liquid catalysts, Nat. Commun., 2023, 14, 5123–5136 CrossRef.
- H. Wang, Y. F. Zhao and F. T. Zhang, et al., Hydrogen-bonding catalyzed ring-closing C-O/C-O metathesis of aliphatic ethers over ionic liquid under metal-free conditions, Angew. Chem., Int. Ed., 2020, 59, 11850–11855 CrossRef CAS PubMed.
- H. Wang, Z. H. Zhao and Y. F. Zhao, et al., Cation-anion confined hydrogen-bonding catalysis strategy for ring-closing C-O/O-H metathesis of alkoxy alcohols under metal-free conditions, Green Chem., 2023, 25, 8791–8797 RSC.
- X. Q. Chang, Y. P. Wang and Y. F. Zhao, et al., Hydrogen bonding-catalyzed synthesis of 1,4-dioxanes from dehydrative cyclization of vicinal diols in ionic liquids, New J. Chem., 2023, 47, 7299–7304 RSC.
- H. Wang, Y. F. Zhao and F. T. Zhang, et al., Hydrogen-bond donor and acceptor cooperative catalysis strategy for cyclic dehydration of diols to access O-heterocycles, Sci. Adv., 2021, 7, eabg0396 CrossRef CAS PubMed.
- F. T. Wu, Y. P. Wang and Y. Qian, et al., A green route to benzyl phenyl sulfide from thioanisole and benzyl alcohol over dual functional ionic liquids, Chem. – Asian J., 2023, 18, e202201078 CrossRef CAS PubMed.
- F. T. Wu, Y. F. Zhao and Y. P. Wang, et al., Hydrogen-bonding and acid cooperative catalysis for benzylation of arenes with benzyl alcohols over ionic liquids, Green Chem., 2022, 24, 3137–3142 RSC.
- Y. Zhang, J. H. Clark and V. L. Budarin, et al., Green metrics for ionic liquids: Evaluation of sustainable catalytic processes, Green Chem., 2023, 25, 3319–3332 Search PubMed.
- B. M. Trost, The atom economy-A search for synthetic efficiency, Science, 1991, 254, 1471–1477 CrossRef CAS PubMed.
- Z. Wang, D. S. Alessi and D. Allen, et al., Ecotoxicity assessment of functionalized ionic liquids in aquatic systems, Environ. Sci. Technol., 2022, 56, 5437–5445 Search PubMed.
- Y. Gu, S. Zhang and Y. Deng, et al., Life cycle assessment of hydroxyl-functionalized ionic liquids: from synthesis to application, J. Cleaner Prod., 2022, 380, 134921–134935 CrossRef.
- J. D. Figueroa-Villar and A. A. Vieira, Nuclear magnetic resonance and molecular modeling study of exocyclic carbon-carbon double bond polarization in benzylidene barbiturates, J. Mol. Struct., 2013, 1034, 310–317 CrossRef CAS.
- Z. W. Zhang, W. Q. Mao and K. L. Wang, et al., Highly efficient and reversible carbon dioxide capture by carbanion-functionalized ionic liquids, ChemSusChem, 2024, 17, e202401111 CrossRef CAS PubMed.
- M. J. Frisch and G. W. Trucks, et al., Gaussian 16, Revision A.01, Gaussian, Inc., Wallingford CT, 2016. https://gaussian.com Search PubMed.
- P. J. Stephens, F. J. Devlin and C. F. Chabalowski, et al., Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields, J. Chem. Phys., 1994, 98, 11623–11627 CrossRef CAS.
- S. Grimme, J. Antony and S. Ehrlich, et al., A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu, J. Chem. Phys., 2010, 132, 154104 CrossRef.
- P. C. Hariharan and J. A. Pople, The influence of polarization functions on molecular orbital hydrogenation energies, Theor. Chim. Acta, 1973, 28, 213–222 CrossRef CAS.
- W. J. Hehre, R. Ditchfield and J. A. Pople, Self-consistent molecular orbital methods. XII. Further extensions of Gaussian-type basis sets for use in molecular orbital studies of organic molecules, J. Chem. Phys., 1972, 56, 2257–2261 CrossRef CAS.
- R. B. J. S. Krishnan, J. S. Binkley and R. Seeger, et al., Self–consistent molecular orbital methods. XX. A basis set for correlated wave functions, J. Chem. Phys., 1980, 72, 650–654 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2026 |