Open Access Article
Open Access ArticleEnhancing circularity of polyolefins via gasification: techno-economic and environmental evaluation of variant processes
Benjamin Caudle a,
Thuy T. H. Nguyen
a,
Thuy T. H. Nguyen ab and
Sho Kataoka
ab and
Sho Kataoka *ab
*ab
aResearch Institute for Chemical Process Technology, National Institute of Advanced Industrial Science and Technology (AIST), Central 5, 1-1-1 Higashi, Tsukuba 305-8565, Ibaraki, Japan. E-mail: s-kataoka@aist.go.jp
bIntegrated Research Center for CCUS Implementation, National Institute of Advanced Industrial Science and Technology (AIST), Central 5, 1-1-1 Higashi, Tsukuba 305-8565, Ibaraki, Japan
First published on 5th December 2025
Abstract
Gasification is often promoted as a primary means of converting plastic waste into a valuable product, advancing the goals of waste reduction and resource conservation. However, the most common form of chemical upcycling – converting plastic waste into other chemicals – results in the continued production of plastics from fossil fuels and requires more energy than conventional methods of disposal. This work models a fully circular system in which plastic waste is gasified to syngas and reconstituted into polyolefins via methanol and olefin synthesis, allowing gasification to be rigorously compared with other polymer recycling technologies. The model is designed to test a range of waste feeds and process conditions with the aim of minimizing cost and carbon footprint while maximizing the circularity. It is found that gasification alone has worse performance in terms of minimum selling price (MSP of $1.85 per kg product) and carbon footprint (CFP of 1.79 kg CO2-equivalent per kg waste) than mechanical and solvent-based recycling methods, and even conventional polyolefin production from fossil fuels followed by disposal via incineration with energy recovery ($1.50 per kg product, 1.33 kg CO2-eq. per kg waste). This is primarily due to the high energy demand of gasification and syngas reforming, the need for additional process inputs such as pure O2, and the relatively low product yield. To address these challenges, novel process modifications are proposed and evaluated to improve both the economic and environmental performance of the gasification process. It is found that integrating gasification with mechanical recycling process results in an overall increase in circularity with an acceptable cost ($0.87 per kg product) and carbon footprint (1.25 kg CO2-eq. per kg waste). This indicates that future gasification systems should be designed to target highly mixed and contaminated, secondary plastic waste streams that would normally be rejected by other methods.
Green foundation1. This work advances green chemistry by critically analyzing gasification, a process proposed as a solution to plastic waste. It covers a wider range of performance indicators (environmental, economic, material) and process configurations than any single previous study. Although gasification does not perform well when used to create circular polymers, a path to success is found though integrating gasification with other recycling processes.2. This work analyzes gasification using detailed, flexible process models with energy integration. Three process metrics are used to compare seven different gasification process configurations for their potential to increase material circularity while decreasing carbon footprint and cost relative to other recycling routes and the conventional polymer lifecycle. 3. Advances in methanol synthesis and methanol-to-olefins processes will increase the efficiency of gasification as a circular solution to plastic waste, but a major hurdle is the cost and availability of green hydrogen. Further research into wholistic plastic recycling systems, including a minimum of noncircular “upcycling”, is the current best direction for a green polymer ecosystem. |
1 Introduction
Plastics have many benefits that make them indispensable to modern society, but their linear lifespan has resulted in a buildup of waste that is causing incalculable damage to the environment and human health.1 While international concern has long focused on the buildup of plastic waste in the environment and depletion of fossil resources, production & disposal of plastic is also a significant source of greenhouse gases, accounting for over 4% of global CO2-equivalent emissions in 2015.2 Recent action to replace landfilling with incineration has significantly reduced the problem of waste entering the environment but further increased the lifetime CO2 emissions of plastics.3 It is vital to move towards a circular ecosystem in which both negative environmental impacts of plastic waste are virtually eliminated through reduction, recycling, and proper disposal.Thermal recovery – burning waste to generate heat or electricity – is the most common form of disposal in Japan, while mechanical recycling is the most common form of recycling, accounting for just over 20% of post-consumer plastic waste – a share that has been increasing in recent years.3 Because physical sorting alone is insufficient to create a stream of pure polymer, the product of mechanical recycling is invariably of lower quality than virgin plastic and follows a downward cascading ‘life spiral’.4,5 Solvent-based recycling, a form of mechanical recycling in which specific plastics are selectively dissolved and precipitated to isolate them from a mixture, is a promising alternative.6–8 While it produces a nearly-virgin-quality product, solvents must be carefully chosen to suit the input waste and target polymer.9 Moreover, the waste feed requires some degree of physical sorting beforehand to remove excessive contamination, and the presence of additives can reduce the effectiveness of this method.10,11 Other options exist but have not yet achieved practical implementation. For example, chemical depolymerization is effective for certain plastics but is not practically applicable to polyolefins.12–14 Depolymerization via supercritical water shows promising initial results but is still in the early stages of investigation.15
Gasification is a well-developed alternative to mechanical recycling and depolymerization. Because it breaks down most plastic waste into small molecules, it has proven effective at converting a variety of waste into a uniform product.16,17 The resulting syngas can be separated to extract H2 and CH4 or used in chemical synthesis.18–20 Prior investigations into gasification of plastic waste are typically limited to producing chemicals other than polymers, so-called chemical “upcycling”. For example, Prifti et al. investigated a process that converts mixed polyethylene (PE) and polypropylene (PP) into methanol via gasification, steam-methane reforming (SMR) and methanol synthesis from syngas.21 They determined that the process is economically feasible, but did not perform an analysis of the greenhouse gas emissions.22 Another potentially limiting factor is the choice of waste feed. Because of material availability constraints, Prifti et al. were limited to PE and PP in their reactor feed, which will have a different syngas product from that produced from mixed plastic waste. The effect of this discrepancy on circular polyolefin production is one subject of this work. Other researchers, most notably Kuusela et al., have analyzed the process of converting captured CO2 into polyolefins via methanol synthesis, a methanol-to-propylene (MTP) reactor, and polymerization of the isolated ethylene and propylene.23,24 Their lifecycle analysis indicates that the process provides a net sink for captured CO2, but the process would operate at a loss without subsidies or significantly higher credits for CO2 capture. Analyzing the processes separately misses the large potential for integration between gasification, a net endothermal process, and methanol and olefin synthesis, which are net exothermal. Other factors, such as the variability of the waste plastic feed, sources of raw materials such as oxygen and hydrogen, and potential options for optimizing the process should be explored in depth. To give gasification a thorough investigation as a potential route to circular polymers, it is necessary to investigate the entire process, from waste to product, under a variety of configurations.
This work looks at this part of the circular plastic ecosystem, analyzing the potential for plastic recycling via gasification, with a thorough investigation of process designs and a look at integration with other recycling methods. The target waste is a mix of plastics commonly found in post-consumer waste in Japan, including polyethylene (PE), polypropylene (PP), polystyrene (PS), and polyethylene-terephthalate (PET) and the target products are simple polyolefins (PE and PP). Because anything short of a circular process results in continuing production of plastics from fossil fuels at increasing rates, the products of the process are the polyolefins polypropylene and polyethylene. The entire process consumes considerable energy and a significant amount of the original mass is lost. To counteract these effects, variations on the process design and operation are investigated with the goal of minimizing the carbon footprint and process cost while maximizing the circularity.
2 Methods
2.1 Gasification process design
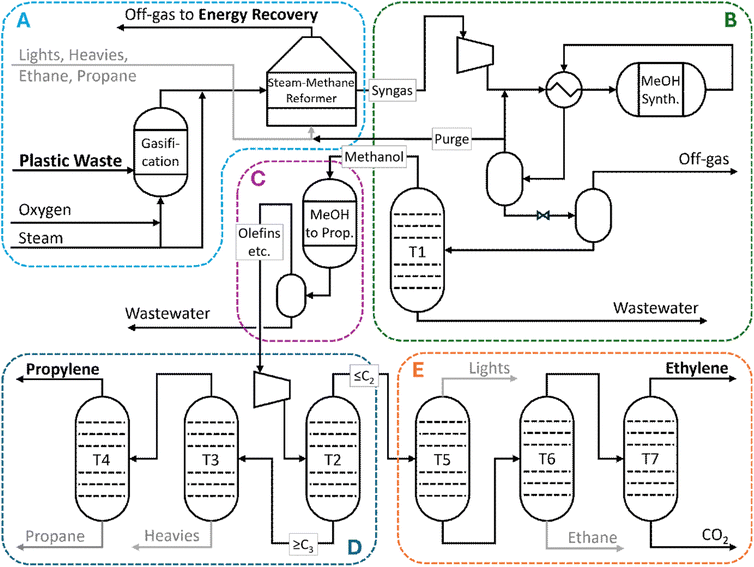
The novel process designed for the base case scenario is illustrated in Fig. 1 (a detailed process flow diagram is included in the SI as Fig. S1). In this process, plastic waste with a composition close to that of municipal plastic waste is fed into a fluidized bed gasification reactor and decomposed into syngas. The syngas must then be upgraded to remove CH4 and increase the H2/CO ratio via SMR, which requires high-temperature heat typically provided by burning natural gas. The upgraded syngas is then converted to methanol, which needs to be separated from reactants and byproducts, and then transformed via a methanol to propylene (MTP) reactor. The main product of the MTP reactions is propylene, but there are significant fractions of propane, ethylene, ethane, and other lighter and heavier components. The separation of these fractions to isolate propylene and ethylene for polyolefin synthesis requires more energy, while disposing of unwanted byproducts via combustion replaces natural gas as the heat source for the SMR. Although not precisely circular, since other plastics included in the feed are not recreated, PP and PE are chosen as the target products due to their simplicity. If gasification proves to be viable for this case, expansion to other polymers should be investigated for future processes.Prior to being fed into the process, plastic waste is collected by a municipal government at a material recovery facility (MRF) and then transported to the location of the recycling plant for a fee. Municipal plastic waste collected in Japan has an average composition of 35% PE, 24% PP, 12% PS, and 29% others by mass.3 The other plastics are modeled as PS and PET with a simplified decomposition mechanism to represent saturated, cyclic, and oxygenated polymer chains as well as possible organic contaminants. In principle, PVC is not collected along with other post-consumer plastic waste in Japan, but chlorinated polymers may still enter the waste recovery system. This process ignores that fraction to simplify the model and give gasification some economic leeway. Chemical and thermal methods are available for treating PVC in the pyrolysis feed.25 While PET has been observed to cause agglomeration during pyrolysis, the use of PET as a representative polymer for this model is considered acceptable because (1) the high temperature of gasification should encourage rapid, complete decomposition and (2) the actual PET content of Japanese municipal plastic waste is actually quite low, as PET has a high collection rate as its own category.26 As the plastic is supplied from the existing waste management system, capital costs for the MRF are not included in calculations. However, the operating costs of this process are reflected in the delivered cost of the plastic waste while material and energy usage are included in the lifecycle analysis.
The plastic waste from the MRF enters section (A), being fed into a fluidized bed gasifier at a rate of 5000 kg h−1 with a mix of steam and pure oxygen as the fluidizing gas. The amount of oxygen is controlled at an equivalence ratio of approximately 2.1 so that incomplete combustion of the contents maintains the temperature of the reactor at 600 °C. Calculations for conversion for this and subsequent reactors are described in the Modeling methodology section, 2.2. The products of gasification are mixed with additional steam, achieving a ratio of approximately 2.6 mol H2O per mol C fed, before entering the steam-methane reforming furnace, which contains a Ni/MgAl2O4 catalyst and is maintained at 900 °C via combustion of the process byproducts.21,22 The stream exiting the SMR has a molar composition of approximately 40% H2, 15% CO, and 10% CO2 with the balance being mostly water. Water is removed via condensation and the resulting stream is compressed in two stages with intercooling to 80 bar and enters section (B). The fresh syngas is mixed with recycled, unreacted syngas and fed into the methanol synthesis reactor. The exothermal MeOH synthesis reactions occur in a Lurgi boiling water reactor, operated at a target temperature of 230 °C over a commercial catalyst.27 The stream exiting the reactor is cooled to 40 °C to condense methanol and water, with 95% of the remaining vapor recycled to the MeOH reactor and 5% purged to the SMR furnace. The pressure of liquid product is reduced to 2 bar and flashed again to remove CO2 that had condensed with the first flash. Methanol at 98.5% molar purity is separated from water in a distillation column (T1) with a recovery rate of 99.5%. This is the feed to the Methanol to Propylene (MTP) process (C).
Methanol from the gasification/MeOH synthesis area is preheated with reactor effluent and reacted in a Lurgi MTP reactor at 400 °C.28 The product of this reaction contains 1% CO2, 52% H2O, 6% C2H4, and 21% C3H6 by mass. After cooling to condense water, this stream is pressurized to 20 bar and sent through a series of distillation columns (sections D and E) for separation. Column T2 separates ≤C2 molecules from ≥C3 molecules. The heavy fraction is then separated into C3 molecules and heavy ends (≥C4) in T3, and the C3 molecules are further separated into propane and propylene in T4. The overall yield of 99.9% pure propylene is approximately 40% of the mass of plastic input. Ethylene at 99.9% purity and approximately 12% overall mass yield can be recovered from the light fraction through a series of three refrigerated columns. T5 (condenser at −126 °C) removes light ends (H2, CH4, CO), T6 (−33 °C) removes ethane, and T7 (−160 °C) separates ethylene from CO2. Light ends, ethane, propane, and heavy ends are recovered in small amounts with low purity and so are not deemed worthwhile to recover in saleable form. They replace natural gas as fuel in the SMR furnace, with excess heat used to generate process steam and electricity.
Propylene and ethylene produced by the process are supplied to a polyolefin plant for processing into plastics. As this process replaces a traditional source of ethylene and propylene, the capital cost of constructing the plastic production facility is not included in calculations. However, the material and energy usage contribute to the operating costs and the corresponding CO2-equivalent emissions are included in the lifecycle analysis.
2.2 Modeling methodology
The base case gasification process described in section 2.1 and variations on it were modeled in Aspen Plus.29 The resulting mass and energy balances are used to estimate the CO2-equivalent emissions and cash flow of the process, while the process conditions are used to size equipment for the calculation of capital expenses. The detailed flow diagram and stream details are included in the SI. As, aside from the first step, the process primarily involves small, nonpolar molecules and water, the Peng–Robinson equation of state with Boston–Mattias correction is used to model phase equilibrium. Due to the rapid nature of the gasification and steam-methane reforming reactions, the gasification reactor and SMR are modeled as equilibrium reactors. The methanol synthesis reactions are modeled using the Vanden Bussche–Froment kinetics in a plug-flow reactor with constant thermal fluid temperature.30 The methanol to propylene reactor is modeled with fixed component yields based on Lurgi MTP processes found in the literature (not counting H2O, 64.6%, the remainder is: 45.4% C3H6, 25.3% C2H6, 18.5% C2H4, 4.1% C4H10 3.3%, C3H8 1.8% C4H8, 1.1% CH4, 0.3%C5H10, 0.2% H2, 0.2% CO on a molar basis).28,31–34 Each distillation column is optimized for recovery and purity of its desired product, with flow rates and temperatures recorded in Table S1. Heat integration is carried out to minimize the consumption of heating and cooling energy, with important points of integration illustrated in Fig. S1. Pumps are given 85% overall efficiency, while compressors and turbines are given 75% overall efficiency.2.3 Evaluation methodology
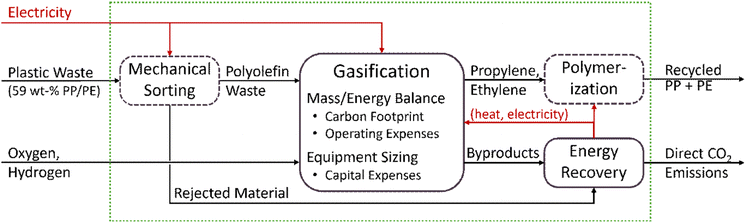
Technoeconomic and Lifecycle analysis are performed based on the boundary illustrated in Fig. 2. It includes mechanical sorting, gasification, and polymerization. Although the strength of gasification is its ability to accept waste with a high degree of mixing and contamination, certain other plastics, particularly polyvinyl chloride (PVC), and contaminants, such as metals, need to be physically removed via mechanical sorting. Mechanical sorting and polymerization are established processes operating at many industrial sites and so are not modeled in detail here. Their contribution to the lifecycle of recycled polyolefins is estimated from literature data. The gasification and energy recovery sections are modeled in detail to determine the mass and energy balance for lifecycle analysis as well as estimate operating and capital expenses.
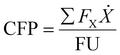 | (1) |
The emission factors for all mass and energy flows crossing the process boundary are included in Table 1. Some, such as electricity and purified oxygen, are obtained from publicly available data while others are calculated based on the energy required for their production. For example, the emission factor of cooling water is derived from the electricity needed to operate the pumps of a circulation loop including cooling towers. Steam production is attributed primarily to the emissions of natural gas combustion in boilers with additional contribution from electricity used for condensate recirculation. The emission factor for plastic waste is calculated based on transportation (50 km by light truck), shredding, and baling. The emission factors for fossil-fuel based PP and PE are calculated based on the lifecycle inventory compiled by Narita et al.35 and are in line with the values reported for other regions.36,37 These factors are applied to the products of the process as avoided emissions, which may be subtracted from the CFP to represent the emissions avoided by replacing current production methods.
| Material (unit) | Emission factor (kg CO2-eq. per unit) | Price ($ per unit) |
|---|---|---|
| a Calculated.b Inventory database for environmental analysis (proprietary).39c Japan electric power information center (2024).40d Low estimate of Honma & Hu (2021).41e Ministry of economy, trade, and industry.42f Turton et al.43 | ||
| Plastic waste (kg) | 0.121a | 0.160d |
| Oxygen (kg) | ∼1b | 0.054e |
| Process water (ton) | ∼0 | 0.177f |
| Waste water (ton) | ∼0 | 0.056f |
| Electricity (kWh) | 0.435c | 0.109c |
| Steam (kWh) | 0.284a | 0.039a |
| Cooling water (kWh) | 0.0046a | 0.0013f |
| Refrigerant (kWh) | 0.261a | 0.065a |
| V.L.T. refrigerant (kWh) | 1.958a | 0.491a |
| Polypropylene (kg) | 2.01a | 1.49e |
| Polyethylene (kg) | 2.02a | 1.53e |
When evaluating the CFP of the gasification process, two functional units can be applied. When comparing gasification with methods of disposing plastic waste that produce different products, a basis of 1 kg waste input is the most appropriate. In this case, products exported from the process generate avoided emissions. When comparing circular processes, however, it is preferable to use the basis of 1 kg product.38 This allows direct comparison of equivalent products, with processing inefficiencies reflected in the CFP increasing or decreasing inversely to yield.
The capital costs and net operating income are then combined into the net present value (NPV), a well-known metric that represents the profitability of a proposed plant over its operating lifetime. NPV is a function of the capital expenses, CAPEX, operating expenses, OPEX, operating income from sale of polyolefins, OPIN, and economic factors (such as internal rate of return, depreciation structure, tax rate, etc.), ECON, up to year n of operation:
| NPV = NPV(CAPEX, OPEX, OPIN, ECON)n | (2) |
| NPV(CAPEX, OPEX, OPIN(MSP), ECON)N = 0 | (3) |
When calculated, it assumes that the price ratio of PP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PE is maintained at the current value of 1
PE is maintained at the current value of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.03. MSP is a useful way to compare the economics of PP and PE produced via recycling with those derived from fossil fuels.
1.03. MSP is a useful way to compare the economics of PP and PE produced via recycling with those derived from fossil fuels.
The NPV and MSP are calculated based on a typical plant lifespan of 15 years. The capital expenses are invested during construction prior to “year zero” and then depreciated linearly over the first 5 years of operation. Additional financial considerations for base case evaluation are a discount rate of 10%, typical for a process that has been well-studied with some commercial implementation, and an annual tax rate of 40%.
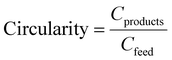 | (4) |
3 Results and discussion
3.1 Base case
The plastic waste feed into the process, based on the average of post-consumer plastic waste in Japan as reported by the PWMI, contains 24% PP and 35% PE, with the mix of other plastics represented by PS (19%) and PET (22%) for modeling purposes. The gasification reactor requires 2662 kg of O2 (approximate equivalence ratio of 0.5) and 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 606 kg of steam to maintain temperature and generate the desired ratio of H2 to CO from the SMR. Single pass conversion of CO into CH3OH in the MeOH reactor is 19% (molar basis), with the recycling of noncondensing gases bringing the overall conversion to 68% on a carbon atom basis (a methanol production rate of 7561 kg h−1). As the MeOH synthesis reactions are exothermal, steam from this reactor is used to heat other operations, such as the reboiler of the methanol distillation column. The fixed conversion of methanol to olefins yields 1600 kg h−1 propylene and 435 kg h−1 ethylene, over 99% of which is recovered via the intensive distillation process. A small amount is lost in the conversion from propylene and ethylene to PP and PE. The overall mass yield of polyolefins (PP + PE) from waste plastic is 40%. Table 2 includes key process metrics for the base case scenario. A process flow diagram with detailed stream information can be found in the SI. The results are consistent (<1% deviation when process conditions are equivalent) with those of previous models based on experimental studies, namely the plastic waste to methanol process of Prifti et al.21,22 and the syn-gas to polypropylene process of Kuusela et al.23
606 kg of steam to maintain temperature and generate the desired ratio of H2 to CO from the SMR. Single pass conversion of CO into CH3OH in the MeOH reactor is 19% (molar basis), with the recycling of noncondensing gases bringing the overall conversion to 68% on a carbon atom basis (a methanol production rate of 7561 kg h−1). As the MeOH synthesis reactions are exothermal, steam from this reactor is used to heat other operations, such as the reboiler of the methanol distillation column. The fixed conversion of methanol to olefins yields 1600 kg h−1 propylene and 435 kg h−1 ethylene, over 99% of which is recovered via the intensive distillation process. A small amount is lost in the conversion from propylene and ethylene to PP and PE. The overall mass yield of polyolefins (PP + PE) from waste plastic is 40%. Table 2 includes key process metrics for the base case scenario. A process flow diagram with detailed stream information can be found in the SI. The results are consistent (<1% deviation when process conditions are equivalent) with those of previous models based on experimental studies, namely the plastic waste to methanol process of Prifti et al.21,22 and the syn-gas to polypropylene process of Kuusela et al.23
| Metric | Flow across boundary | Equivalent emissions [kg CO2-eq. per kg product] | Cost [$ per kg product] | |
|---|---|---|---|---|
| Material inputs | Waste feed | 5000 kg h−1 | 0.30 | 0.40 |
| Oxygen | 2662 kg h−1 | 1.46 | 0.07 | |
| Material outputs | Polypropylene | 1589 kg h−1 | −1.58 | −1.14 |
| Polyethylene | 428 kg h−1 | −0.43 | −0.34 | |
| Direct CO2 emissions | 8404 kg h−1 | 4.17 | ||
| Energy inputs/outputs | Process heating | 9211 kW | 0 | 0 |
| Heat generation | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 754 kW 754 kW |
|||
| Electricity | 5834 kW | 0.49 | 0.12 | |
| Electric. generation | 3571 kW | |||
| Cooling water | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 344 kW 344 kW |
0.03 | 0.01 | |
| Refrigerant | 994 kW | 0.29 | 0.05 |
The largest source of emissions is the combustion of byproducts, which contribute 4.17 kg CO2-eq. per kg product. This is followed by oxygen (1.46 kg kg−1), which is isolated from air via an energy-intensive process, and electricity (0.49 kg kg−1), whose main users are the syngas compressors for MeOH synthesis and air compressors for combustion in the SMR. The balance of heat generation and consumption indicates that no external source of heating is required, but not enough heat is produced to justify exporting it to another process. As such, no heat crosses the process boundary, and the equivalent emissions and cost of process heating are both zero. Electricity generated from residual heat from SMR makes up over half of the electricity requirements of the process, but there are still emissions and costs associated with importing the remaining amount. The total emissions, 6.74 kg CO2-eq. per kg product, are significantly higher than the emissions for fossil-fuel-based production of polyolefins, which are around 2 kg CO2-eq. per kg for both polypropylene and polyethylene. Fig. 3 shows detailed contributions to the carbon footprint, capital expenses, and operating expenses of the process.
The considerable capital expenses are due mainly to the SMR reactor, which accounts for 30% of the overall cost. As a fundamental step in any gasification process, the cost of the SMR reactor is exceedingly difficult to mitigate without changing the feed or intended products. The total capital cost of the plastic-to-methanol portion of the plant, parts (A) and (B) in Fig. 1, is closely aligned to that calculated by Prifti et al.21 for the same process once inflation is accounted for. Other significant capital expenditures are the compressors for syngas before MeOH synthesis and air for the SMR, as well as the distillation columns which present significant contributions to the cost towers and heat exchangers. Operating costs are dominated by the cost of waste collection, contributing $0.40 per kg product out of $0.64 per kg total. This indicates that the process is particularly sensitive to the method of waste acquisition, with government-subsidized or free feed making a large impact on the potential profitability. Other significant contributions come from electricity for the compressors and the purchase of pure O2. With such high capital expenditure, the estimated maintenance costs are correspondingly high, and the complexity of the system requires significant operating labor. These two factors are greater than process operating costs, doubling the total operating expenses and resulting in a net operating income barely above zero.
3.2 Comparison with other plastic disposal methods
The CFP, MSP, and carbon circularity of gasification recycling are compared with alternative end-of-life treatments for plastic waste, summarized in Fig. 4. Using a basis of 1 kg waste allows noncircular methods to be compared, particularly thermal recovery, which produces no product, and mechanical recycling, which produces an inferior product. The process boundary is similar to that shown in Fig. 2, with mechanical sorting and combustion for thermal recovery included. As these processes use older simulations45 in which thermal recovery is not modeled in detail, values for thermal recovery are estimated via direct substitution of the heat of combustion of natural gas with that of the studied plastic waste. For example, 1 kg of unsorted plastic waste replaces 0.70 kg of natural gas (CH4), based on the higher heating values of the constituent plastics.46 Values for mechanical recycling, which includes shredding, washing, drying, milling, wind sifting, and extrusion were obtained from literature47 and correlated with industry observations. Values for solvent recycling are based on models from a previous work, which combined preliminary float-sink separation with a targeted solvent extraction process.45 In the conventional solvent/antisolvent process, PE and PP are targeted by successive extractions with a solvent and precipitated by mixing with an antisolvent. This process requires a large amount of heat energy to separate the solvents for reuse via distillation. In the supercritical (SC) solvent process, supercritical fluids are used in succession to extract PP and PE, with temperature and pressure reduced to induce precipitation. Since most of the fluid is maintained in the supercritical state, energy consumption is much lower than that of the solvent/antisolvent process.In this framework, the processes are credited with avoided emissions, which are emissions that would be produced from an equivalent amount of virgin PP and PE. The product of each recycling process is modified by an equivalence ratio to determine the mass of virgin PP/PE replaced by each process, which is then multiplied by the emissions factor for the production of PP/PE (2.01 kg CO2-eq. per kg). While it can vary drastically based on many factors, the equivalence ratio for mechanical recycling is set at 0.7 kg recycled per kg virgin, which is on the low end of the estimates compiled by Nordahl et al.48 and the high end of those estimated by industrial recyclers. Because of this, the carbon circularity of mechanical recycling is displayed in two parts: the virgin-equivalent portion is given a solid fill while the rest is patterned to represent the difference in quality. The equivalence ratio for solvent-based recycling is 0.99, based on literature and industry reports on the quality of plastic from these processes.49–51 The avoided emissions from product are also affected by the yield, which is 77% of the input PP/PE for mechanical (45% of total waste), 99% of input PP/PE for solvent-based (58% of total waste), and 40% of the total waste input for gasification. For mechanical and solvent-based recycling, rejected waste is sent to a separate facility for thermal recovery; therefore, both emissions from combustion and avoided emissions from energy recovery are included. Other sources of emissions are indirect emissions from materials (e.g. O2 for gasification and solvent makeup for solvent-based methods), electricity consumption including cooling, and heat demand from both direct (natural gas-fired) and indirect (steam) sources.
In terms of CFP, gasification performs worse than any other disposal methods. This is due to the need for pure O2, the energy requirement of SMR, and the low yield of product. It offers a degree of circularity that thermal recovery does not, but due to the low yield it performs worse than any other form of recycling in this comparison. The outlying case is mechanical recycling, which converts a higher portion of the feed into product than gasification but at a lower quality with limited usefulness. The calculated MSP follow the trend of energy usage (and thus CFP), making gasification the most expensive route, and significantly worse than fossil-fuel based PP/PE (approximately $1.5 per kg). Mechanical recycling has a very low MSP due to the simple process with low energy usage, and does not adhere to the trend of MSP following CFP due to lower product quality reducing avoided emissions and relatively larger emissions from burning rejected waste. Clearly, major process changes are needed to make gasification a viable component of a circular plastic ecosystem.
3.3 Options for improving gasification
To improve the economic and environmental performance of the gasification process, several innovative process modifications are investigated and evaluated. These include (1) removing the ethylene recovery section to greatly reduce refrigeration costs and reduce capital expenditure, (2) adding H2 prior to methanol syntheses to increase the H2/CO ratio and improve methanol yield, using H2 produced via conventional steam methane reforming or (3) produced via electrolysis with green electricity, (4) pre-sorting the waste to remove non-polyolefins, thereby decreasing the difference between feed and product and increasing the yield of polyolefins, (5) pre-sorting the waste to remove PP and PE, allowing these fractions to be recycled mechanically while sending the remainder to gasification, and (6) arbitrarily increasing the yield of MTO through hypothetical advances in catalysts or reactor designs. Table 3 summarizes the potential benefit and major drawback of each process variation. The final proposal is speculative, intended to demonstrate the large effect that low polyolefin yield has on the process, but the other five changes are possible with current technology. Fig. 5 illustrates the location of each proposed change on a simplified process diagram, and the flows of major process streams can be found in the SI.| Variant | Brief description | Effects |
|---|---|---|
| (1) | Omit ethylene | + Eliminate costs associated with ethylene separation section |
| – Reduce production of polyolefins | ||
| (2) | Add H2 (SMR) | + Increase conversion from syngas to methanol |
| – Increase CFP with fossil-fuel-based H2 | ||
| (3) | Add H2 (green) | + Increase conversion from syngas to methanol |
| – Increase cost with H2 from electrolysis with green electricity | ||
| (4) | Pre-sorted | + Increase conversion in the gasification process |
| – Decrease overall yield when rejected waste is burned | ||
| (5) | Mechanical + gasification | + Reduce emissions by diverting waste to mechanical process |
| + Increase overall yield by recovering more of original mass | ||
| – Decrease in overall product quality | ||
| (6) | Better MTP | + Increase yield of polyolefins |
| – Decrease amount of energy generated by burning byproducts |
| Material (unit) | Emission factor (kg CO2 per unit) | Price ($ per unit) |
|---|---|---|
| a Parkinson et al. (2019).52b Calculated.c Middle estimate of Honma & Hu (2021).41d Half of value for unsorted waste. | ||
| Hydrogen, SMR (kg) | 13.2a | 1.26a |
| Hydrogen, wind (kg) | 0.88a | 7.86a |
| Waste, sorted (kg) | 1.11b | 0.32c |
| Waste, mech. reject (kg) | 0.06b | 0.08d |
4 Conclusions
The results of this study indicate that gasification on its own has little promise for producing circular polyolefins in an environmentally friendly and economical manner. Various process variants were tested, and some improved the economics, emissions, and/or circularity of gasification, but none were competitive with linear route of conventional polyolefins. The unavoidable factor is the large, high-temperature energy requirement of gasification and SMR (approximately 19 MJ kg−1 waste), which must be provided by partial combustion of reactor contents (autothermal gasification with O2) and combustion of byproducts. As a result, even a process approaching complete circularity, converting all carbon in the plastic waste into polyolefins, will have a high CFP due to the replacement of byproducts with natural gas as means of heating the SMR. Such a process would not even be as circular as expected, since the methane in natural gas represents an additional source of carbon that will be lost in the form of CO2. In order to reduce the CFP below that of the current fossil carbon-based polyolefin lifecycle, a gasification process would likely have to incorporate addition of H2 – preferably green H2, which will not be available at the necessary scale for the foreseeable future – and the CO2 produced from the combustion of byproducts would have to be captured. Both improvements would incur additional costs that would lower the prospects of economical implementation.One option not considered here, the conversion of plastic waste to some other value-added chemical via gasification, warrants further study. While the exclusion of this option is justified when comparing routes for circular polymers, there are many other chemicals that can be produced from syn-gas. Some of these chemicals may be cheaper or have a lower CFP when produced from plastics, relative to their conventional production process, but the loss of potential plastic recycling cannot be ignored. Finding an appropriate way to incorporate the unmitigated emissions of plastic production into the calculation of CFP for other chemicals is a significant consideration for this ongoing work.
While gasification has little prospect as a stand-alone solution for polymer circularity, these results point to specific cases where its implementation should be further explored. As part of a circular polymer ecosystem, gasification could be used to process the rejected waste of mechanical or solvent-based recycling processes. It has been shown that combining mechanical recycling with gasification, while higher in CFP and cost than mechanical recycling alone, enhances circularity at a CFP and MSP lower than that of conventional polyolefins. Similar results would be expected for certain solvent-based routes, with the higher quality of solvent-recycled polymers enhancing the circularity even further. In short, gasification is not a complete solution to the interrelated problems of waste plastic buildup and global warming, but it should continue to be studied as one part of an interconnected circular polymer ecosystem.
Conflicts of interest
There are no conflicts to declare.Data availability
The bulk of the data supporting this article (flow diagrams and stream results) has been included as part of the supplementary information (SI). See DOI: https://doi.org/10.1039/d5gc05081b.Complete data sets are available upon request from the authors.
Acknowledgements
The present work is supported by JST-CREST (JPMJCR24S6 to S. K). Part of the data was obtained as part of the Council for Science, Technology and Innovation (CSTI), the Cross-ministerial Strategic Innovation Promotion Program (SIP), and the third period of the SIP “Circular Economy System” (JPJ012290).References
- P. J. Landrigan, H. Raps, M. Cropper, C. Bald, M. Brunner, E. M. Canonizado, D. Charles, T. C. Chiles, M. J. Donohue, J. Enck, P. Fenichel, L. E. Fleming, C. Ferrier-Pages, R. Fordham, A. Gozt, C. Griffin, M. E. Hahn, B. Haryanto, R. Hixson, H. Ianelli, B. D. James, P. Kumar, A. Laborde, K. L. Law, K. Martin, J. Mu, Y. Mulders, A. Mustapha, J. Niu, S. Pahl, Y. Park, M. L. Pedrotti, J. A. Pitt, M. Ruchirawat, B. J. Seewoo, M. Spring, J. J. Stegeman, W. Suk, C. Symeonides, H. Takada, R. C. Thompson, A. Vicini, Z. Wang, E. Whitman, D. Wirth, M. Wolff, A. K. Yousuf and S. Dunlop, Ann. Glob. Health, 2023, 89, 23 CrossRef PubMed.
- L. Cabernard, S. Pfister, C. Oberschelp and S. Hellweg, Nat. Sustain., 2022, 5, 139–148 CrossRef.
- PWMI, Plastic Products, Plastic Waste and Resource Recovery, Plastic Waste Management Institute (PWMI), 2024 Search PubMed.
- F. Associates, Life cycle impacts for postconsumer recycled resins: PET, HDPE, and PP, The Association of Plastic Recyclers, 2018 Search PubMed.
- M. Baker, M. Stewert, S. Padibjo, C. Stavropoulos, R. Campbell and T. Boseley, An investigation into the technical and market feasibility of recycling post-consumer polypropylene, Polysearch Pty. Ltd, 2005 Search PubMed.
- J. Yu, A. d. C. Munguía-López, V. S. Cecon, K. L. Sánchez-Rivera, K. Nelson, J. Wu, S. Kolapkar, V. M. Zavala, G. W. Curtzwiler, K. L. Vorst, E. Bar-Ziv and G. W. Huber, Green Chem., 2023, 25, 4723–4734 RSC.
- S. H. Zein, A. A. Hussain, O. Y. Yansaneh and A. A. Jalil, Processes, 2022, 10, 2387 CrossRef CAS.
- T. W. Walker, N. Frelka, Z. Shen, A. K. Chew, J. Banick, S. Grey, M. S. Kim, J. A. Dumesic, R. C. Van Lehn and G. W. Huber, Sci. Adv., 2020, 6, eaba7599 CrossRef PubMed.
- P. Zhou, J. Yu, K. L. Sánchez-Rivera, G. W. Huber and R. C. Van Lehn, Green Chem., 2023, 25, 4402–4414 RSC.
- J. N. Hahladakis, C. A. Velis, R. Weber, E. Iacovidou and P. Purnell, J. Hazard. Mater., 2018, 344, 179–199 CrossRef CAS PubMed.
- S. Ugduler, K. M. Van Geem, M. Roosen, E. I. P. Delbeke and S. De Meester, Waste Manage., 2020, 104, 148–182 CrossRef CAS PubMed.
- M. Goto, J. Jpn. Pet. Inst., 2016, 59, 254–258 CrossRef CAS.
- K. Ragaert, L. Delva and K. Van Geem, Waste Manage., 2017, 69, 24–58 CrossRef CAS PubMed.
- M. Hong and E. Y. X. Chen, Green Chem., 2017, 19, 3692–3706 RSC.
- T. Thiounn and R. C. Smith, J. Polym. Sci., 2020, 58, 1347–1364 CrossRef CAS.
- R. R. Bora, R. Wang and F. You, ACS Sustainable Chem. Eng., 2020, 8, 16350–16363 CrossRef CAS.
- S. Kumagai, J. Nakatani, Y. Saito, Y. Fukushima and T. Yoshioka, J. Jpn. Pet. Inst., 2020, 63, 345–364 CrossRef CAS.
- I. Barbarias, G. Lopez, M. Artetxe, A. Arregi, J. Bilbao and M. Olazar, Energy Convers. Manage., 2018, 156, 575–584 CrossRef CAS.
- K. Momono, J. Ishii, S. Hosohara and H. Kijima, ISIJ Int., 2024, 64, 954–963 CrossRef CAS.
- T. Namioka, A. Saito, Y. Inoue, Y. Park, T.-j. Min, S.-a. Roh and K. Yoshikawa, Appl. Energy, 2011, 88, 2019–2026 CrossRef CAS.
- K. Prifti, A. Galeazzi and F. Manenti, Ind. Eng. Chem. Res., 2023, 62, 6167–6175 CrossRef CAS.
- K. Prifti, A. Galeazzi and F. Manenti, Ind. Eng. Chem. Res., 2023, 62, 5083–5096 CrossRef CAS.
- K. Kuusela, V. Uusitalo, J. Ahola and J. Levänen, J. CO2 Util., 2021, 52, 101672 CrossRef CAS.
- W. Hoppe, N. Thonemann and S. Bringezu, J. Ind. Ecol., 2017, 22, 327–340 CrossRef.
- A. López, I. de Marco, B. M. Caballero, M. F. Laresgoiti and A. Adrados, Fuel Process. Technol., 2011, 92, 253–260 CrossRef.
- M. Kawai, J. Nakatani, K. Kurisu and Y. Moriguchi, Resour., Conserv. Recycl., 2022, 180, 10616 CrossRef.
- L. Chen, Q. Jiang, Z. Song and D. Posarac, Chem. Eng. Technol., 2011, 34, 817–822 CrossRef CAS.
- H. Koempel and W. Liebner, Lurgi's Methanol To Propylene (MTP) Report on a successful commercialisation, Lurgi AG, 2007 Search PubMed.
- Aspen Technology, Inc., Aspen Plus, V14 (40.0.0.359), 2022 Search PubMed.
- F. Bisotti, M. Fedeli, K. Prifti, A. Galeazzi, A. Dell'Angelo and F. Manenti, Ind. Eng. Chem. Res., 2022, 61, 2206–2226 CrossRef CAS.
- B. Bozorgi, J. Ahmadpour, A. Mohammad-khah, F. Yaripour and H. Z. Mousavi, Solid State Sci., 2024, 147, 107382 CrossRef CAS.
- M. Vares, A. Sari and F. Yaripour, Fuel, 2025, 383, 133861 CrossRef CAS.
- F. Yaripour, Z. Shariatinia, S. Sahebdelfar and A. Irandoukht, Microporous Mesoporous Mater., 2015, 203, 41–53 CrossRef CAS.
- S. Xu, Y. Zhi, J. Han, W. Zhang, X. Wu, T. Sun, Y. Wei and Z. Liu, Advances in catalysis for methanol-to-olefins conversion, in Advances in Catalysis, Academic Press, 2017, ch. 2, vol. 61, pp. 37–122 Search PubMed.
- N. Narita, M. Sagisaka and A. Inaba, Int. J. Life Cycle Assess., 2002, 7, 277–282 CrossRef CAS.
- S. R. Nicholson, N. A. Rorrer, A. C. Carpenter and G. T. Beckham, Joule, 2021, 5, 673–686 CrossRef CAS.
- PlasticsEurope, Polypropylene (PP) December 2016 Update, 2016 Search PubMed.
- S. L. Nordahl and C. D. Scown, Chem. Sci., 2024, 15, 9397–9407 RSC.
- National Institute of Advanced Industrial Science and Technology, Inventory Database for Environmental Analysis (IDEA), 3.3.0, 2024 Search PubMed.
- T. Otsuki, N. Kishimoto, M. Nabeshima, M. Hirano, E. Sakagami, H. Sasa, H. Yamanaka, T. Aki, R. Fujii, R. Kawamoto and M. Ohashi, The Electric Power Industry in Japan, Japan Electric Power Information Center, Inc., 2024 Search PubMed.
- S. Honma and J.-L. Hu, J. Cleaner Prod., 2021, 284, 125274 CrossRef.
- T. S. D. K. Chosa-tokeibu, Yearbook of Current Production Statistics (Chemical Industry), Research and Statistics Department, Minister's Secretariat, the Ministry of Economy, Trade and Industry (METI): Tokyo, Japan, 2017.
- R. Turton, R. C. Bailie, W. B. Whiting, J. A. Sheaiwitz and D. Bhattacharyya, Analysis, Synthesis, and Design of Chemical Processes, Prentice Hall, Tokyo, 4th edn, 2012 Search PubMed.
- CEPCI, Chemical Engineering, 2024, p. 72 Search PubMed.
- B. Caudle, T. T. H. Nguyen and S. Kataoka, Green Chem., 2025, 27, 1667–1678 RSC.
- R. N. Walters, S. M. Hackett and R. E. Lyon, Fire Mater., 2000, 24, 245–252 CrossRef CAS.
- M. Larrain, S. Van Passel, G. Thomassen, B. Van Gorp, T. T. Nhu, S. Huysveld, K. M. Van Geem, S. De Meester and P. Billen, Resour., Conserv. Recycl., 2021, 170, 105607 CrossRef CAS.
- S. L. Nordahl, N. R. Baral, B. A. Helms and C. D. Scown, Proc. Natl. Acad. Sci. U. S. A., 2023, 120, e2306902120 CrossRef CAS PubMed.
- S. A. Stoian, A. R. Gabor, A.-M. Albu, C. A. Nicolae, V. Raditoiu and D. M. Panaitescu, J. Therm. Anal. Calorim., 2019, 138, 2469–2480 CrossRef CAS.
- J. M. Layman, D. I. Collias, H. Schonemann and K. Williams, United States Pat, 2018/0171094 A1, 2018 Search PubMed.
- J. M. Layman, M. Gunnerson, E. B. Bond, H. Schonemann and K. Williams, United States Pat, 2017/0002119 A1, 2017 Search PubMed.
- B. Parkinson, P. Balcombe, J. F. Speirs, A. D. Hawkes and K. Hellgardt, Energy Environ. Sci., 2019, 12, 19–40 RSC.
| This journal is © The Royal Society of Chemistry 2026 |