Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Nitrous oxide as a green oxidant: a holistic evaluation based on economic, environmental, and safety metrics
Abhinandan Nabera ab,
Gian-Marco Beshara
ab,
Gian-Marco Beshara ab,
Gonzalo Guillén-Gosálbez
ab,
Gonzalo Guillén-Gosálbez *ab and
Javier Pérez-Ramírez
*ab and
Javier Pérez-Ramírez *ab
*ab
aInstitute for Chemical and Bioengineering, Department of Chemistry and Applied Biosciences, ETH Zurich, Vladimir-Prelog-Weg 1, Zurich 8093, Switzerland. E-mail: gonzalo.guillen.gosalbez@chem.ethz.ch; jpr@chem.ethz.ch
bNCCR Catalysis, Zurich 8093, Switzerland
First published on 9th December 2025
Abstract
Sustainable chemical synthesis requires atom-efficient and highly selective oxidation processes. Nitrous oxide, N2O, exhibits unique reactivity in oxidation catalysis due to its ability to deliver selective mono-oxygen species, thereby minimising overoxidation. Industrially, the majority of N2O is produced via the five-step thermal decomposition of ammonium nitrate, a process limited by safety, environmental, and economic concerns. Recent advances in catalyst design offer the one-step direct catalytic oxidation of ammonia (NH3), potentially streamlining production while reducing costs. However, the performance of different N2O production routes across process-based metrics remains poorly understood, making the benefits of the one-step route hypothetical. Furthermore, the majority of existing frameworks for evaluating emerging technologies fail to integrate the three fundamental pillars of sustainability: economic viability, environmental performance, and societal safety. Here, we present an integrated framework encompassing all three pillars of sustainability by combining techno-economic analysis, life cycle assessment, and quantitative safety indicators such as Dow's fire & explosion index and TNT equivalency. Specifically, for N2O, we compare the one-step direct NH3 oxidation process with the conventional five-step route and find that the former, which employs fossil-derived or electrolytic hydrogen-based green NH3, reduces both production costs and carbon footprint by over 20% while significantly lowering safety hazards. In addition, we benchmark N2O against hydrogen peroxide (H2O2), a well-established oxidant, and demonstrate that N2O produced from a fossil/green NH3 blend can match the carbon footprint of H2O2 while offering ca. 40% cost savings and lower safety risks. Given the benefits of one-step N2O, we also demonstrate its potential in a key application: phenol synthesis via direct oxidation of benzene, compared with the conventional cumene route and the H2O2-based direct oxidation. Overall, our findings highlight N2O's potential in oxidation chemistry and underscore the value of our integrated sustainability framework for assessing new technologies.
Green foundation1. By integrating the three pillars of sustainability—economic viability, environmental performance, and safety—our study pioneers a holistic, metrics-based framework for evaluating emerging chemical technologies. Applied to nitrous oxide (N2O), the analysis highlights its potential as a selective oxidant with lower cost, reduced environmental footprint, and improved safety relative to conventional alternatives, while offering a transferable template for the assessment of other sustainable processes2. We show that the one-step ammonia oxidation to N2O cuts costs and carbon footprint by over 20% while improving safety versus the conventional five-step ammonium nitrate route. Benchmarking against hydrogen peroxide, H2O2, a well-established oxidant, N2O from a fossil/green ammonia blend offers equivalent carbon footprint at ∼40% lower cost and hazard potential. We also highlight N2O's potential as a green oxidant in phenol production. 3. The benefits of N2O production rely on promising catalysts demonstrated only at the laboratory scale. Scaling them to industrial levels could realise the potential of the one-step route by lowering costs, emissions, and safety risks, unlocking its promising future for selective oxidations. |
Introduction
Modern chemistry is increasingly driven by the principles of green and sustainable chemistry which prioritise atom-efficient and selective oxidation reactions to minimise waste generation and streamline chemical processes. In this regard, nitrous oxide, N2O, is emerging as a promising and versatile green oxidant in organic synthesis.1,2 Its capacity to transfer a mono-oxygen species to a substrate while releasing only molecular nitrogen (N2)—a benign atmospheric gas—as well as its favourable properties, such as high solubility in non-polar media and thermal stability,3,4 further sets it apart from other oxidants such as dioxygen or peroxides.5 This unique feature minimises overoxidation and by-product formation, making N2O an attractive alternative to conventional, well-established oxidants like hydrogen peroxide (H2O2).5 With moderate reactivity and high thermodynamic driving force for oxygen release, N2O is especially useful in partial oxidation reactions where overoxidation must be avoided. Recent advances in reaction design have significantly expanded the applications of N2O across diverse chemical domains. Typical transformations with N2O include C–H bond hydroxylation, epoxidation and oxidative dehydrogenation (ODH), which enable access to three major product classes: oxygenates (e.g., methanol, dimethyl ether, formaldehyde), hydrocarbons (e.g., olefins), and ring-containing compounds (e.g., phenol and other aromatics).1 Notably, the direct oxidation of benzene to phenol using Fe-ZSM-5 and related zeolites has reached phenol selectivity above 90%.6–12 Parallel research on the N2O-mediated ODH of light alkanes demonstrated high selectivity towards key olefins such as propylene from propane and styrene from ethylbenzene, potentially offering viable alternatives to energy-intensive dehydrogenation with steam or CO2.1,13 There, N2O's moderate reactivity is crucial as it facilitates high alkene selectivity while minimising coking or deep oxidation—longstanding issues in direct dehydrogenation and ODH catalysis, respectively. Moreover, breakthroughs in homogeneous systems have shown that N2O enables phenols formation from aryl halides at ambient conditions using Ni-catalysis, a significant step toward mild, selective oxidations with high functional group tolerance.14 Beyond oxidation, N2O can also serve as a nitrogen donor. Recent work has demonstrated its capability in forming triazenes, azides, and azo dyes under mild conditions, providing atom-efficient alternatives to diazotization or azide-transfer protocols.2,15,16 Together, these developments highlight N2O's growing role in sustainable synthesis—both as a green oxidant and nitrogen source—for greener and more selective production of relevant chemicals across multiple product classes.However, the widespread adoption of N2O has been hindered by the risks associated with its conventional production route, i.e., the thermal decomposition of ammonium nitrate (NH4NO3). This method is not only expensive but also rife with safety and environmental concerns due to the explosive nature of nitrate salts.17 These risks have been tragically highlighted by recent global events such as the 2015 Port of Tianjin explosion, which claimed 173 lives,18 and the 2020 Beirut explosion, which resulted in 218 fatalities and significant property damage.19,20 Moreover, N2O is also well known as a greenhouse gas (GHG) with a global warming potential (GWP) 273 times greater than that of carbon dioxide (CO2),21 further preventing it from being widely recognised as a green oxidant.
The direct catalytic oxidation of ammonia (NH3) to N2O has gained attention as a potentially safer and more economically viable alternative. This innovative approach eliminates reliance on hazardous nitrate intermediates and aligns with advancements in green chemistry principles—specifically atom economy, safer chemistry, and the use of less-hazardous syntheses.22–24 To date, the field of direct NH3-to-N2O conversion is undergoing rapid transformation, driven by the adoption of engineered redox-active supports that mediate oxygen transfer via dynamic vacancy formation and healing. These material innovations underpin the development of stable and selective catalytic systems, achieving high N2O productivity under stoichiometric feed conditions while effectively suppressing undesired by-products, thereby advancing targeted N2O synthesis.25–27 Notably, despite being regarded as a highly polluting gas with a high GWP, when N2O is used in oxidation applications, it ultimately releases benign N2 gas.28 Nonetheless, potential leaks during storage might still occur, but they can be mitigated using gas detection systems, which monitor concentrations in real-time, enabling rapid responses to seal leaks.29
As chemical processes are developed to meet sustainability targets,30 it is essential to evaluate them across multiple criteria to capture all dimensions of performance beyond product selectivity and yield.31 Specifically, the three fundamental pillars of sustainability—economy, environment, and society—should be assessed in an integrated manner. Techno-economic analysis (TEA) can evaluate the competitiveness of a novel route by providing insights into production costs,32,33 while life cycle assessment (LCA) can quantify environmental impacts, such as the carbon footprint, across the value chain.34 Process safety, a key societal consideration, can be assessed using metrics like the Dow Fire and Explosion Index (F&EI)35 or trinitrotoluene (TNT) equivalency,36 which gauge the inherent hazards of production routes.37–39 Routes with significant safety concerns are often eliminated before reaching industrial scale-up, as mitigating these risks typically requires expensive strategies, resulting in higher capital investments and reduced profitability.
Recent studies have widely applied TEA or LCA at the process scale, evaluating novel routes and benchmarking them against business-as-usual practices for chemical production.40–42 Moreover, despite some contributions,43–45 standard feasibility studies in the scientific literature on emerging routes often focus on economic or environmental criteria while omitting safety aspects. The absence of an integrated framework coupling all three dimensions of sustainability, in addition to the lack of systematic metrics for evaluating N2O production technologies benchmarked against well-established oxidants like H2O2, renders the benefits of the one-step route largely speculative. To the best of our knowledge, no previous work has quantitatively combined techno-economic, environmental, and inherent safety assessments into a single holistic framework specifically for N2O production and utilisation. In existing studies, these three pillars are typically treated in isolation or only qualitatively discussed, which masks the overall sustainability profile of chemical production routes and hinders fair benchmarking.
To fill this gap, we present a holistic framework integrating the three pillars of sustainability using techno-economic analysis, life cycle assessment, and quantitative safety metrics, such as the Dow F&EI and TNT equivalency. Specifically focussing on N2O, we compare the conventional five-step and the emerging one-step production routes using these metrics. Across all indicators, the one-step process demonstrates superior performance. Beyond comparing these pathways, we also benchmark the one-step N2O route against H2O2, a well-established oxidant with similar properties, such as being a mono-oxygen donor and forming benign by-products.46–48 This comparison is particularly meaningful because, like H2O2, N2O acts as a selective mono-oxygen donor that aligns closely with green chemistry principles. Our study thus provides the first quantitative comparison of N2O and H2O2 that simultaneously accounts for costs, life cycle impacts, and process safety, thereby moving beyond fragmented evaluations toward a fully integrated sustainability assessment. We find that N2O produced using a blend of fossil/green NH3 (utilising hydrogen, H2, from water electrolysis) could achieve a carbon footprint equivalent to that of H2O2 while offering 40% cost savings and reduced safety risks. We also highlight N2O's potential in key applications, such as phenol production via benzene oxidation, where it can outperform both the conventional cumene process and the H2O2-mediated direct oxidation route.49 Prospective assessments for 2050 further indicate the potential for significant cost and carbon footprint reductions across all evaluated technologies due to a future decarbonised economy.
Overall, these findings position N2O as a promising candidate in oxidation chemistry. Our work also underscores the value of rigorous, data-driven analysis covering all three pillars of sustainability to guide decision-making in green chemistry research more effectively.
Methods
An overview of the aspects covered in this study is provided in Fig. 1. This work integrates process modelling, TEA, LCA, and safety evaluation to systematically compare conventional and emerging routes for N2O, H2O2, and phenol production. For more information on the methodology employed in this work, please refer to the SI. | ||
Fig. 1 Outline of the analysis. First, we compare the five-step and one-step N2O production routes using economic, environmental, and safety metrics (Fig. 2 and Table 1). In the same analysis, we show how emissions can be further reduced using green NH3, i.e., NH3 produced via water electrolysis powered by clean electricity. Next, we extend our analysis to compare N2O with H2O2, another mono-oxygen donor with benign by-products, again in terms of cost, environmental impact, and safety metrics (Fig. 3 and 4, and Table 2). Finally, we explore key applications for N2O, such as the direct production of phenol from benzene oxidation, and compare this route with both the direct oxidation of benzene using H2O2 and the traditional cumene-based process for phenol production (Fig. 5). a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) The reaction of NO2 with water to produce HNO3 is depicted here as a simplified representation of nitrogen oxides absorption; in reality, NO2 forms N2O4, which hydrolyses to HNO3 and NO. The reaction of NO2 with water to produce HNO3 is depicted here as a simplified representation of nitrogen oxides absorption; in reality, NO2 forms N2O4, which hydrolyses to HNO3 and NO. | ||
Process modelling
The process simulations for N2O, H2O2, and phenol production were developed using Aspen Plus v12.1, employing standard process models to simulate each route (refer to Fig. S1–S5 of the SI for the process flow diagrams). For N2O, two routes were analysed: the one-step direct NH3 oxidation and the five-step NH4NO3 decomposition pathway. H2O2 production was modelled via the anthraquinone autoxidation (AO) process, using 2-ethylanthraquinone as the working solution. Phenol production was analysed through the conventional cumene oxidation route and compared with emerging routes involving direct oxidation of benzene using N2O or H2O2. Further details on the process modelling approach and the mass and energy balances are provided in section 1 and Tables S1–S3 of the SI.Techno-economic analysis
The techno-economic assessment (TEA) was based on simulation outputs, including mass and energy flow data and equipment sizing for each production technology. Operational expenditures (OPEX) and capital expenditures (CAPEX) were employed to estimate the production cost associated with each production pathway, using 2022 as the base year and including prospective projections to 2050. Feedstock and utility prices and the full TEA methodology are provided in section 2 and Tables S4, S5 of the SI.Life cycle assessment
The life cycle assessment (LCA) followed ISO 14040/14044 standards50,51 and was performed using an attributional cradle-to-gate approach, assuming a functional unit defined as 1 kg of chemical (N2O, H2O2, or phenol). Foreground data were obtained from process simulations, while background data were extracted from the Ecoinvent v3.10 database.52 Climate change impacts (i.e., GHG emissions) were calculated using the IPCC 2021 GWPs over a 100-year average,21 and the ReCiPe 2016 v1.03 method was used to evaluate impacts on human health, ecosystems, and resource depletion.53 For 2050 projections, a prospective LCA was conducted using the premise v2.1.3 framework.54 Detailed information on the LCA framework, assumptions, and inventories can be found in section 3 and Tables S6–S8 of the SI.Safety assessment
Safety metrics were evaluated using the Dow F&EI and the TNT equivalency method.35,36 The Dow F&EI, applied to N2O, H2O2, and phenol production technologies, quantifies fire and explosion hazards based on material characteristics, process conditions, and equipment configurations. Additionally, the TNT equivalency framework was employed to compare the explosion energy potential of various feedstocks used. Notably, this TNT-based estimate reflects a conservative worst-case, ideal detonation scenario that does not account for preventive and mitigative measures. The preventive and mitigative strategies are incorporated through the Dow F&EI loss control credit factors. Further details on the safety assessment and all numerical results are presented in section 4 and Tables S9, S10 of the SI.Results and discussion
Economic and environmental performance of N2O production from fossil and green NH3
This section evaluates the economic performance and climate change impacts of two N2O production technologies: direct one-step oxidation of NH3 and conventional five-step thermal decomposition of NH4NO3, using both fossil (from natural gas reforming) and green (utilising electrolytic H2) NH3 as inputs. As shown in Fig. 2a, the one-step NH3 oxidation route consistently demonstrates lower production costs compared to the five-step thermal decomposition pathway. The total cost of N2O is 0.6 USD per kg for the one-step fossil scenario and 1.2 USD per kg for the green scenario, compared to 0.9 USD per kg and 1.4 USD per kg for the corresponding five-step cases. The cost advantage of the one-step route is mainly attributed to its lower electricity consumption, since the five-step process requires significant pressure adjustments across its multiple stages. Moreover, the one-step route requires four times lower CAPEX in comparison to the five-step route. Notably, NH3 remains the dominant cost driver across technologies, contributing around 85% to the overall costs on average.Fig. 2b presents the cradle-to-gate climate change impacts associated with each N2O production pathway. Under the fossil-based route, the one-step process has a lower impact of ca. 2.8 kg CO2-eq per kg N2O compared to 3.4 kg CO2-eq per kg for the five-step route. The lower impacts in the one-step route is primarily due to reduced electricity use in the process. This trend remains consistent when green NH3 is used, with impacts 0.7 vs. 1.1 kg CO2-eq per kg N2O for the one- and five-step processes, respectively. Similar to the production costs, NH3 is the largest contributor to the climate change impacts, especially in the fossil scenarios.
Projections for 2050 under a decarbonised electricity mix (purple diamond in Fig. 2) indicate potential reductions in both cost and climate change impacts. The climate change impacts of N2O production drop by 10% and 70% on average in the fossil and green scenarios, respectively. The main reductions in the fossil-based scenarios are due to the decarbonised electricity deployed in the future, while the main reductions in the green scenario are due to both decarbonised electricity and potential efficiency improvements in wind electricity generation, consumed to power the electrolyser unit to produce H2 and subsequently NH3.55 In terms of production costs, future production costs for the green route shows reductions of up to 30%, driven by improved electrolyser efficiency and lower wind generation costs. Further detailed assumptions and limitations of this work are summarised in section 5 of the SI, while the breakdown of production costs and climate change impacts for 2050 is shown in Fig. S6 of the SI.
Regionalised LCA analysis for climate change impacts (Table S11 of the SI) indicate that these trends are robust across major economies, with the one-step route consistently exhibiting lower climate change impacts than the five-step pathway under both fossil and green scenarios. Absolute impacts vary with the region, with the lowest climate change impact of N2O via the one-step process in the fossil scenario occurring in Europe (2.4 kg CO2-eq per kg) and the highest in China (4.5 kg CO2-eq per kg), compared to the global average of 2.8 kg CO2-eq per kg. The high impacts in China are primarily driven by coal-based fossil NH3 production. In the green scenario, which utilises electrolytic H2 for NH3 production, the lowest climate change impact again occurs in Europe (0.5 kg CO2-eq per kg), followed by the United States (0.6 kg CO2-eq per kg) and China (0.8 kg CO2-eq per kg), relative to the global average of 0.7 kg CO2-eq per kg N2O.
Overall, these findings highlight the importance of prioritising innovation in one-step N2O synthesis while also accelerating the deployment of low-carbon NH3 production technologies.
Safety performance of N2O production pathways
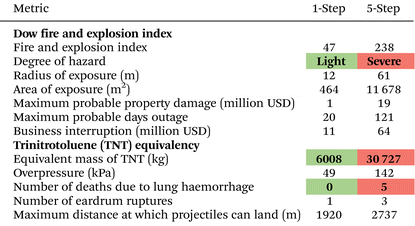
To complement the techno-economic and environmental assessments, we conducted a quantitative risk assessment of both the one- and five-step N2O production technologies using the Dow F&EI and the TNT equivalency method, summarised in Table 1. These frameworks estimate the physical and financial consequences of potential process accidents, providing additional insights into operational safety and business risk.The one-step process is classified as a light hazard (F&EI = 47). In contrast, the five-step route is rated as a severe hazard (F&EI = 238) due to the explosive nature of the nitrate salts. This results into a much larger exposure area and higher projected property damage and business interruption losses. Furthermore, the TNT equivalency highlights the five-step process’ risk, with a TNT equivalent mass over five times higher than that of the one-step route, leading to longer projectile ranges and projected fatalities in the event of an explosion. Thus, beyond its economic and environmental advantages, the one-step route also offers significantly lower safety risks compared to the five-step alternative.
To extend our assessment beyond the production stage, we also evaluated the storage and transportation of N2O using the Dow F&EI and find that both result in light hazard classifications, with F&EI values of 31 and 34, respectively, compared to the production-stage F&EI values of 238 for five-step (severe) and 47 for the one-step (light) N2O production routes. See section 10 and Table S12 of the SI for further details.
Production costs and climate change impacts of N2O and H2O2 as green oxidants
Next, we compare the one-step N2O production pathway with H2O2 due to their shared characteristics as selective mono-oxygen donors with the release of benign by-products, N2 and water, respectively. These mechanistic and environmental commonalities make H2O2 the most directly comparable reference for assessing the green oxidant potential of N2O. This section compares their economic and environmental performance, considering both fossil and green production pathways.According to Fig. 3a, H2O2 produced via the anthraquinone-based AO process is significantly more expensive than N2O across all scenarios. In the fossil case, H2O2 costs reach 1.6 USD per kg, approximately 2.6 times higher than N2O (0.6 USD per kg). Under the green scenario, utilising electrolytic H2 powered by renewable electricity, H2O2 remains 1.7 times more expensive (1.9 vs. 1.2 USD per kg). The higher costs of H2O2 are largely driven by the 2-ethylanthraquinone working solution. In contrast, for N2O production, NH3 feedstock dominates, with minimal contributions from utilities and CAPEX.
Furthermore, Fig. 3b presents the climate change impacts for both oxidants. Under fossil-based production, N2O has a higher climate change impact (2.8 kg CO2-eq per kg) compared to H2O2 (1.3 kg CO2-eq per kg). However, in the green scenario, impacts fall significantly to 0.7 kg CO2-eq per kg for N2O and 0.5 kg CO2-eq per kg for H2O2. In the green scenario, by 2050, N2O's impact further drops to 0.2 kg CO2-eq per kg, slightly outperforming H2O2 (0.4 kg CO2-eq per kg).
To explore cost-effective pathways for reducing the climate change impact of N2O, we evaluated a blended NH3 scenario combining fossil-based and green NH3 feedstocks. As shown in Fig. 4, a mix of 28% fossil and 72% green NH3 enables N2O production to match the climate change impacts of fossil-derived H2O2 (1.3 kg CO2-eq per kg). This blended scenario achieves a 38% lower cost compared to H2O2, highlighting a viable strategy that balances environmental performance while being economically competitive. By 2050, further cost reductions are projected for N2O due to improvements in green NH3 production, reinforcing N2O's competitiveness as a green oxidant. The detailed breakdown of production costs and climate change impacts in 2050 is shown in Fig. S7 of the SI.
 | ||
| Fig. 4 Production costs of N2O and H2O2 are compared under the condition of having the same carbon footprint. To match the climate change impact of fossil-based H2O2, a blend of N2O—comprising 28% fossil-derived and 72% green NH3 (see Fig. 3)—is utilised. By 2050, projections indicate potential reductions in production costs for N2O. | ||
We also compared N2O with molecular oxygen (O2), which is often regarded as the ideal oxidant owing to its abundance and low cost. O2 produced via cryogenic air separation exhibits costs of 0.2–0.4 USD per kg and a climate change impact of around 0.9 kg CO2-eq per kg.52,56 In addition, some applications can directly use air as an oxidant, which further reduces costs. While this makes O2 economically favourable compared to both N2O and H2O2, its diatomic nature limits its reactivity and selectivity. The controlled transfer of a single oxygen atom from O2 is inherently challenging, often resulting in unselective oxidation or complete combustion. Thus, although O2 performs well in terms of cost and moderate environmental impact, its limited selectivity constrains its suitability for selective oxidation processes, reinforcing the relevance of N2O as a green mono-oxygen donor.
Quantitative safety evaluation of N2O and H2O2 production
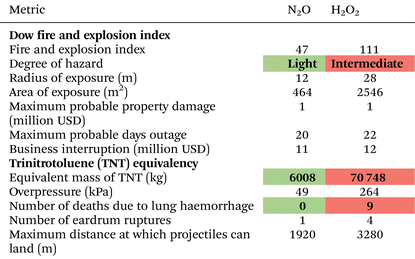
Next, we compare the Dow F&EI and TNT equivalency for the one-step N2O and H2O2 production via the AO process, as summarised in Table 2. The one-step N2O process is classified as a light hazard (F&EI = 47), substantially lower than the intermediate hazard rating of H2O2 (F&EI = 111), resulting in a smaller exposure radius and affected area. Similarly, TNT equivalency reveals a stark contrast in explosion potential, with H2O2 having a TNT equivalent mass of 70 748—more than ten times higher than that of N2O—further resulting in a greater fatality risk. Notably, we do not evaluate separate F&EI values for H2O2 storage or transport, as concentrated H2O2 is typically produced and consumed in situ due to its high reactivity and the impracticality of bulk storage and long-distance transport. Overall, the one-step N2O production pathway poses significantly lower safety risks compared not only to the five-step but also to H2O2 production.Direct oxidation of benzene to phenol using N2O
To support the case of N2O as a viable oxidant, we evaluate its performance in a key application, i.e., phenol production. Today, phenol is a key intermediate in the chemical industry and is traditionally produced via the conventional cumene oxidation process, which involves the alkylation of benzene with propylene to form cumene, followed by oxidation to yield phenol and acetone. Although commercially dominant, this multi-step route is energy-intensive and requires complex separations. In contrast, direct oxidation pathways can convert benzene directly to phenol in a single step using selective oxidants like N2O or H2O2.As shown in Fig. 5a, the direct oxidation of benzene with N2O offers the most cost-effective route to phenol. With a cost of 1.1 USD per kg in the fossil scenario, it reduces costs by ca. 10% compared to the conventional cumene route. In contrast, H2O2-based oxidation is significantly more expensive, at 1.6 USD per kg, due to the high cost of the anthraquinone working solution required to produce H2O2. In the green scenario, where N2O and H2O2 are derived from renewable sources, costs increase to 1.3 USD per kg and 1.8 USD per kg, respectively, maintaining N2O's clear economic superiority.
Fig. 5b compares the cradle-to-gate climate impacts of all routes. The conventional cumene oxidation process shows the highest emissions, at 4.6 kg CO2-eq per kg phenol. Direct oxidation pathways significantly reduce the climate change impacts to 4.3 and 3.3 kg CO2-eq per kg using N2O and to 3.3 and 2.9 kg CO2-eq per kg using H2O2, in the fossil and green scenarios, respectively. By 2050, both alternatives show further reductions in impacts, representing a 10–15% lower carbon footprint on average across scenarios. Additional results showing the breakdown of production costs and climate change impacts are provided in Fig. S8 of the SI. This case study showcases the potential of N2O to serve as a scalable, green oxidant in industrial applications.
For the application stage, we calculated the Dow F&EI for the production of phenol via benzene oxidation using both N2O and H2O2 as oxidants, finding values of around 159 and 169, respectively, in comparison to cumene oxidation, which has an F&EI of 303 due to the high material factor of cumene hydroperoxide, an organic peroxide and chemically unstable oxidant. See section 10 and Table S13 of the SI for more details. Overall, when the production, storage, transportation, and application stages of N2O and H2O2 are considered together, N2O emerges as the more promising oxidising agent based on quantitative safety metrics.
Burden shifting and uncertainty assessment
We extend our analysis to assess burden shifting, i.e., the risk that reducing climate change impacts may inadvertently worsen other environmental impacts. In the one-step vs. five-step N2O production case, the one-step route consistently outperforms the five-step in human health, ecosystem quality, and natural resource impact categories, with the five-step route's higher impacts arising from additional energy use and particulate matter emissions (Fig. S9 of the SI).When comparing the one-step N2O with H2O2 (Fig. S10 of the SI), H2O2 performs better across all impact categories, about 60% lower in the fossil case. This difference becomes less pronounced in the green case using electrolytic H2. The one-step N2O performs worse than H2O2 due to the high contributions of NH3 in all three categories. The higher impacts on human health and ecosystem quality associated with N2O compared to H2O2 are mainly linked to fossil-based NH3 production. Increased CO2, NOx, and SOx emissions from natural gas used as both a feedstock and a heating utility in NH3 production drive these elevated impacts. Using renewable energy for process heating could substantially reduce these burdens. For phenol production, a similar pattern emerges; however, the N2O- and H2O2-based direct oxidation of benzene shows similar results in both cases, due to benzene driving the overall impacts (Fig. S11 of the SI).
Lastly, we performed an uncertainty assessment in these impact categories between the one-step and five-step routes using Monte Carlo sampling with the Ecoinvent pedigree matrix (as defined by default in the background data), which accounts for geographical and temporal correlations in background life cycle inventory (LCI) parameters; results and burden shifting probabilities are shown in Fig. S12–S15 of the SI. The highest probability of burden shifting is observed between the fossil one-step vs. the green five-step N2O production routes, whereas in all other cases the probability of burden shifting in the one-step route is minimal.
Conclusions and outlook
Comprehensive, multi-criteria analysis covering all three sustainability pillars—economic viability, environmental impact, and societal safety—is crucial to ensure that trade-offs do not occur due to improvements in a single dimension. In this study, we integrate techno-economic and environmental assessments with quantitative safety metrics for ex-ante feasibility studies of emerging routes. The same workflow is universally applicable from laboratory to pilot and industrial scales by updating process models and data while retaining a harmonised set of indicators. To the best of our knowledge, this work provides the first holistic and fully quantitative framework that unifies these three pillars specifically for N2O production and utilisation. Our work emphasises that, for N2O production, the one-step NH3 oxidation route consistently outperforms the traditional five-step NH4NO3 decomposition process. Across both fossil-based and green NH3 scenarios, the one-step route delivers over 20% lower production costs and GHG emissions. The one-step route also reveals additional benefits in terms of potential safety hazards. Taken together, lower cost, reduced emissions, and enhanced safety establish the one-step process as a more robust pathway for N2O production.Benchmarking one-step N2O against H2O2, another selective, well-established oxidant, highlights critical trade-offs in the assessed metrics. Under fossil conditions, H2O2 produced via the AO process is ca. 2.6 times more expensive than N2O, largely due to the costly anthraquinone working solution. In terms of climate change impacts, H2O2 outperforms N2O in the fossil scenario (1.3 vs. 2.8 kg CO2-eq per kg), a gap that narrows substantially when green H2 from electrolysis is employed. Notably, by 2050, green N2O achieves a lower climate impact than green H2O2 (0.2 vs. 0.4 kg CO2-eq per kg), underscoring the importance of prospective assessments when evaluating chemical technologies. From a safety perspective, the one-step N2O process demonstrates a lower hazard potential than H2O2. These findings position one-step N2O as a competitive oxidant.
The advantages of N2O extend beyond its production to key industrial applications. In phenol synthesis, the direct oxidation of benzene using N2O emerges as the most economically and environmentally attractive pathway, outperforming both the conventional cumene oxidation process and H2O2-based direct oxidation of benzene. These findings support the broader potential of N2O as a scalable green oxidant for selective mono-oxygenation reactions.
The benefits of the one-step N2O production route rely on the development of robust and efficient catalytic systems. Early pilot-scale studies using Mn–Bi–O/α-Al2O3 catalysts demonstrated the advantages of fluidised-bed reactors, which enabled efficient thermal management and allowed higher NH3 inlet concentrations, resulting in increased N2O productivity.57,58 However, the process was not commercialised. Since 2021, CeO2-based catalysts have shown promising performance, offering up to twofold higher productivity compared to the Mn–Bi–O system and demonstrating remarkable stability even under stoichiometric feed conditions.17,25,26 Nonetheless, future research is needed to improve selectivity and scale these catalysts to industrial levels, which could unlock the full potential of the one-step route, thereby paving the way for their promising application in selective oxidations.
Overall, the methodological framework applied in this work, linking process simulation with TEA, LCA, and quantitative safety metrics, offers a holistic evaluation of emerging chemical technologies across all three sustainability pillars. Applying this integrated framework to N2O and benchmarking it against H2O2 establishes a transferable template for multicriteria evaluation of chemical production routes. Comprehensive application of this framework to other chemical production systems may reveal pathways that provide benefits across multiple metrics. The insights from this study contribute to the development of next-generation oxidants for sustainable chemical synthesis.
Author contributions
A. N.: methodology, visualisation, formal analysis, writing – original draft, writing – review and editing; G. M. B.: methodology, visualisation, writing – original draft, writing – review and editing; G. G.-G.: validation, writing – original draft, writing – review and editing, supervision, and project administration; J. P.-R.: conceptualisation, validation, writing – original draft, writing – review and editing, supervision, and project administration.Conflicts of interest
There are no conflicts to declare.Data availability
The data presented in the figures of this paper are publicly available via Zenodo (https://doi.org/10.5281/zenodo.17518169). The background LCI datasets used in this study are available in the Ecoinvent v3.10 (cut off system model) database under the accessible link (https://ecoinvent.org).Supplementary information (SI) is available. See DOI: https://doi.org/10.1039/d5gc04863j.
Other supporting data are available from the corresponding authors upon request.
Acknowledgements
This publication was created as part of NCCR Catalysis (grant number 225147), a National Centre of Competence in Research funded by the Swiss National Science Foundation.References
- S. Lee, S. Park and J. Lee, ChemSusChem, 2025, e202402728 CrossRef CAS PubMed.
- K. Severin, Chem. Soc. Rev., 2015, 44, 6375–6386 RSC.
- L. C. Yen and J. J. McKetta, J. Chem. Eng. Data, 1962, 7, 288–289 CrossRef CAS.
- M. Galle, D. W. Agar and O. Watzenberger, Chem. Eng. Sci., 2001, 56, 1587–1595 CrossRef CAS.
- I. Gokce, M. O. Ozbek and B. Ipek, J. Catal., 2023, 427, 115113 CrossRef CAS.
- L. Meng, X. Zhu and E. J. M. Hensen, ACS Catal., 2017, 7, 2709–2719 CrossRef CAS.
- A. A. Ivanov, V. S. Chernyavsky, M. J. Gross, A. S. Kharitonov, A. K. Uriarte and G. I. Panov, Appl. Catal., A, 2003, 249, 327–343 CrossRef CAS.
- A. K. Uriarte, M. A. Rodkin, M. J. Gross, A. S. Kharitonov and G. I. Panov, in Studies in Surface Science and Catalysis, ed. R. K. Grasselli, S. T. Oyama, A. M. Gaffney and J. E. Lyons, Elsevier, 1997, vol. 110, pp. 857–864 Search PubMed.
- C. Ouyang, J. Li, Y. Qu, S. Hong and S. He, Green Energy Environ., 2023, 8, 1161–1173 CrossRef CAS.
- G. Mi, J. Li, J. Zhang and B. Chen, Korean J. Chem. Eng., 2010, 27, 1700–1706 CrossRef CAS.
- A. Mancuso, O. Sacco, D. Sannino, V. Venditto and V. Vaiano, Catalysts, 2020, 10, 1424 CrossRef CAS.
- G. I. Panov, G. A. Sheveleva, A. S. Kharitonov, V. N. Romannikov and L. A. Vostrikova, Appl. Catal., A, 1992, 82, 31–36 CrossRef CAS.
- G. M. Beshara, I. Surin, M. Agrachev, H. Eliasson, T. Otroshchenko, F. Krumeich, R. Erni, E. V. Kondratenko and J. Pérez-Ramírez, EES Catal., 2024, 2, 1263–1276 RSC.
- F. Le Vaillant, A. Mateos Calbet, S. González-Pelayo, E. J. Reijerse, S. Ni, J. Busch and J. Cornella, Nature, 2022, 604, 677–683 CrossRef CAS.
- I. R. Landman, F. Fadaei-Tirani and K. Severin, Chem. Commun., 2021, 57, 11537–11540 RSC.
- A. Genoux and K. Severin, Chem. Sci., 2024, 15, 13605–13617 RSC.
- I. Surin, Z. Tang, J. Geiger, S. Damir, H. Eliasson, M. Agrachev, F. Krumeich, S. Mitchell, V. A. Kondratenko, E. V. Kondratenko, G. Jeschke, R. Erni, N. López and J. Pérez-Ramírez, Adv. Mater., 2023, 35, 2211260 CrossRef CAS.
- G. Yu, Y.-S. Duh, X. Yang, Y. Li, Y. Chen, Y. Li, J. Li, R. Chen, L. Gong, B. Yang and J. Huang, Sustainability, 2022, 14, 3429 Search PubMed.
- K. Yammine, J. Daher, J. Otayek, A. Jardaly, J. Mansour, K. Boulos, A. E. Alam, J. Ghanimeh, G. Abou Orm, M. Berberi, E. Daccache, M. Helou, M. Estephan, C. Assi and F. Hayek, Injury, 2023, 54, 448–452 CrossRef PubMed.
- G. Guglielmi, Nature, 2020 DOI:10.1038/d41586-020-02361-x , https://www.nature.com/articles/d41586-020-02361-x.
- Intergovernmental Panel on Climate Change (IPCC), in Climate Change 2022 - Mitigation of Climate Change, Cambridge University Press, 1st edn, 2023, pp. 1727–1790 Search PubMed.
- P. T. Anastas and J. B. Zimmerman, Green Chem., 2019, 21, 6545–6566 RSC.
- A. D. Curzons, D. N. Mortimer, D. J. C. Constable and V. L. Cunningham, Green Chem., 2001, 3, 1–6 RSC.
- H. C. Erythropel, J. B. Zimmerman, T. M. De Winter, L. Petitjean, F. Melnikov, C. H. Lam, A. W. Lounsbury, K. E. Mellor, N. Z. Janković, Q. Tu, L. N. Pincus, M. M. Falinski, W. Shi, P. Coish, D. L. Plata and P. T. Anastas, Green Chem., 2018, 20, 1929–1961 RSC.
- Z. Tang, I. Surin, A. Rasmussen, F. Krumeich, E. V. Kondratenko, V. A. Kondratenko and J. Pérez-Ramírez, Angew. Chem., Int. Ed., 2022, 61, e202200772 CrossRef CAS.
- Q. Yang, I. Surin, J. Geiger, H. Eliasson, M. Agrachev, V. A. Kondratenko, A. Zanina, F. Krumeich, G. Jeschke, R. Erni, E. V. Kondratenko, N. López and J. Pérez-Ramírez, ACS Catal., 2023, 13, 15977–15990 CrossRef CAS PubMed.
- I. Surin, Q. Yang, F. Krumeich, M. Agrachev, T. Otroshchenko, V. A. Kondratenko, E. V. Kondratenko and J. Pérez-Ramírez, Chem. Catal., 2025, 5, 101165 CAS.
- G. I. Panov, K. A. Dubkov and A. S. Kharitonov, in Modern Heterogeneous Oxidation Catalysis, ed. N. Mizuno, Wiley, 1st edn, 2009, pp. 217–252 Search PubMed.
- European Industrial Gases Association, Safe Practices for Storage and Handling of Nitrous Oxide, 2024 Search PubMed.
- D. Faust Akl, D. Poier, S. C. D'Angelo, T. P. Araújo, V. Tulus, O. V. Safonova, S. Mitchell, R. Marti, G. Guillén-Gosálbez and J. Pérez-Ramírez, Green Chem., 2022, 24, 6879–6888 RSC.
- S. Mitchell, A. J. Martín, G. Guillén-Gosálbez and J. Pérez-Ramírez, Angew. Chem., Int. Ed., 2024, e202318676 CAS.
- A. Nabera, I.-R. Istrate, A. J. Martín, J. Pérez-Ramírez and G. Guillén-Gosálbez, Green Chem., 2023, 25, 6603–6611 RSC.
- K. Lee, X. Liu, P. Vyawahare, P. Sun, A. Elgowainy and M. Wang, Green Chem., 2022, 24, 4830–4844 RSC.
- I. Ioannou, S. C. D'Angelo, Á. Galán-Martín, C. Pozo, J. Pérez-Ramírez and G. Guillén-Gosálbez, React. Chem. Eng., 2021, 6, 1179–1194 RSC.
- American Institute of Chemical Engineers, Dow's Fire & Explosion Index Hazard Classification Guide, John Wiley & Sons, Inc, Hoboken, 7th edn, 2010 Search PubMed.
- D. A. Crowl and J. F. Louvar, Chemical process safety: fundamentals with applications, Prentice Hall, Upper Saddle River, NJ, 3rd edn, 2011 Search PubMed.
- G. M. M. James, R. L. Bauer, E. M. Johnson and C. E. Johnson, Sci. Rep., 2025, 15, 15578 CrossRef CAS PubMed.
- D. Chen, C. Wu and J. Li, Process Saf. Environ. Prot., 2023, 180, 752–765 CrossRef CAS.
- T. Homae, Y. Sugiyama, T. Matsumura and K. Wakabayashi, Sci. Technol. Energ. Mater., 2022, 83, 28–31 Search PubMed.
- I. Ioannou, S. C. D'Angelo, A. J. Martín, J. Pérez-Ramírez and G. Guillén-Gosálbez, ChemSusChem, 2020, 13, 6370–6380 CrossRef CAS PubMed.
- S. C. D'Angelo, S. Cobo, V. Tulus, A. Nabera, A. J. Martín, J. Pérez-Ramírez and G. Guillén-Gosálbez, ACS Sustainable Chem. Eng., 2021, 9, 9740–9749 CrossRef.
- N. Von Der Assen and A. Bardow, Green Chem., 2014, 16, 3272–3280 RSC.
- J. Andraos, ACS Sustainable Chem. Eng., 2016, 4, 312–323 CrossRef CAS.
- M. A. Jameel Malik, M. Athar, A. Mohd Shariff and A. Umer, ACS Chem. Health Saf., 2025, 32, 276–287 CrossRef CAS.
- F. Wang and Y. Wang, Procedia Eng., 2012, 45, 139–143 CrossRef CAS.
- K. P. Bryliakov, Chem. Rev., 2017, 117, 11406–11459 CrossRef CAS.
- M. Carraro, M. Gardan, A. Sartorel, C. Maccato and M. Bonchio, Dalton Trans., 2016, 45, 14544–14548 RSC.
- J. Chen, Q. Ma, X. Zheng, Y. Fang, J. Wang and S. Dong, Nat. Commun., 2022, 13, 2808 CrossRef CAS.
- C. Mukarakate, J. D. McBrayer, T. J. Evans, S. Budhi, D. J. Robichaud, K. Iisa, J. ten Dam, M. J. Watson, R. M. Baldwin and M. R. Nimlos, Green Chem., 2015, 17, 4217–4227 RSC.
- International Standards Organization, In ISO 14040:2006 Environmental Management–Life Cycle Assessment–Principles and Framework, 2006.
- International Standards Organization, In ISO 14044:2006 Environmental Management–Life Cycle Assessment–Requirements and Guidelines, 2006.
- G. Wernet, C. Bauer, B. Steubing, J. Reinhard, E. Moreno-Ruiz and B. Weidema, Int. J. Life Cycle Assess., 2016, 21, 1218–1230 CrossRef.
- M. A. J. Huijbregts, Z. J. N. Steinmann, P. M. F. Elshout, G. Stam, F. Verones, M. Vieira, M. Zijp, A. Hollander and R. van Zelm, Int. J. Life Cycle Assess., 2017, 22, 138–147 CrossRef.
- R. Sacchi, T. Terlouw, K. Siala, A. Dirnaichner, C. Bauer, B. Cox, C. Mutel, V. Daioglou and G. Luderer, Renewable Sustainable Energy Rev., 2022, 160, 112311 CrossRef.
- A. Nabera, A. J. Martín, R. Istrate, J. Pérez-Ramírez and G. Guillén-Gosálbez, Green Chem., 2024, 26, 6461–6469 RSC.
- Business Analytiq, Oxygen price index, https://businessanalytiq.com/procurementanalytics/index/oxygen-price-index, (accessed November 10, 2025).
- A. S. Noskov, I. A. Zolotarskii, S. A. Pokrovskaya, V. N. Korotkikh, E. M. Slavinskaya, V. V. Mokrinskii and V. N. Kashkin, Chem. Eng. J., 2003, 91, 235–242 CrossRef CAS.
- A. S. Noskov, I. A. Zolotarskii, S. A. Pokrovskaya, V. N. Kashkin, E. M. Slavinskaya, V. V. Mokrinskii and V. N. Korotkikh, Chem. Eng. J., 2005, 107, 79–87 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2026 |