Open Access Article
Open Access ArticleThe catalytic conversion of fructose to difructose anhydride
Isabel
Hortal-Sánchez†
a,
Faysal
Ibrahim†‡
 bd,
Edgard A.
Lebrón-Rodríguez
c,
Fabiola Y.
Rodríguez-Rodríguez
a,
Grace
Gooley
bd,
Ruining
Ma‡
b,
Matias
Alvear§
bcd,
Ive
Hermansद
bd,
Edgard A.
Lebrón-Rodríguez
c,
Fabiola Y.
Rodríguez-Rodríguez
a,
Grace
Gooley
bd,
Ruining
Ma‡
b,
Matias
Alvear§
bcd,
Ive
Hermansद
 *bcd and
Nelson
Cardona-Martínez
*bcd and
Nelson
Cardona-Martínez
 *a
*a
aDepartment of Chemical Engineering, University of Puerto Rico, Mayagüez, PR 00681-9000, Puerto Rico. E-mail: isabel.hortal@upr.edu; fabiola.rodriguez9@upr.edu; nelson.cardona@upr.edu
bDepartment of Chemistry, University of Wisconsin, Madison, WI 53706, USA. E-mail: fibrahim@wisc.edu; gooley@wisc.edu; rma68@wisc.edu; alvear@jhu.edu; hermans@jhu.edu
cDepartment of Chemical and Biological Engineering, University of Wisconsin, Madison, WI 53706, USA. E-mail: lebronrodrig@wisc.edu; alvear@jhu.edu; hermans@jhu.edu
dWisconsin Energy Institute, University of Wisconsin–Madison, Madison, Wisconsin 53726, USA. E-mail: fibrahim@wisc.edu; gooley@wisc.edu; alvear@jhu.edu; hermans@jhu.edu
First published on 26th November 2025
Abstract
Difructose anhydride (DFA) is a highly valuable compound, traditionally obtained from inulin or sucrose through enzymatic routes. This work reports a novel eco-efficient process for DFA production from abundantly available fructose in a biomass-derived solvent over a commercial Brønsted acidic beta zeolite. A systematic evaluation of the reaction conditions led to the observation that gamma-valerolactone (GVL) is the most selective solvent giving a DFA yield of 75% under mild reaction conditions. The addition of water as a co-solvent (to improve fructose solubility) suppresses the catalytic activity. Reagent and solvent partitioning was investigated using ssNMR, utilizing the residual dipolar couplings of adsorbed species in the zeolite pores in contrast to the bulk environment. By utilizing a cross-polarization pulse sequence to observe these absorbed species and a direct polarization to observe all species in the sample, we obtained the ratio of the absorbed species to overall species in each sample at varying water content in the reaction mixture. Using this approach, we observed that at the ≥10 volume% water content mark, fructose is no longer able to enter the zeolite pores, coinciding with reaction conditions where DFA is no longer produced. The results of this study illustrate the importance of substrate and solvent partitioning on liquid phase reactions over microporous catalysts.
Green foundation1. High-fructose-corn syrup is a major biomass-based product. This sweetener is however known to have adverse health effects. In this work, we report on an efficient catalytic process to convert fructose into difructose anhydride (DFA) over Brønsted acidic beta zeolite. DFA is also a sweetener itself but has many health benefits. Currently, DFA is only available via expensive enzymatic routes from inulin or sucrose making it too expensive for large scale production. In other words, we provide a much more viable pathway with a lower foodprint from a readily available source.2. We obtained DFA yields of 75% using mild reaction conditions using biomass-derived γ-valerolactone as solvent and a stable green catalyst. The main by-product is water. 3. The reaction demonstrates fundamentally interesting solvent effects in that the fructose uptake is highly dependent on the solvent composition. The method that we demonstrate in this work to investigate the partitioning of compounds (inside/outside the porous catalyst) should help further develop this or other liquid phase reactions over microporous catalysts. |
Introduction
Monosaccharides such as glucose and fructose can be readily derived from the cellulosic fraction of biomass and converted into value-added chemicals. Fructose is a widely available raw material since it is easily obtained from sugar cane,1 corn processing,2 inulin3 and levan depolymerization,4 as well as from glucose isomerization.5–11 Owing to its oxyfunctionalized nature, fructose serves not only as a key feedstock for the production of potential platform chemicals such as 5-hydroxymethylfurfural (HMF), but also as a promising precursor for high-value compounds used in pharmaceuticals, personal care products, and food additives.12 Currently, HMF production is the most studied acid-catalyzed reaction for upgrading fructose that employs Brønsted acid catalysts.13–19 Nonetheless, most research targets platform molecules with the hope that biorefining can become a sustainable pathway to produce commodity chemicals and fuels. With how established petrochemical-based commodity chemical pathways are in the chemical industry, the production of those compounds from biomass is economically challenged. As a result, higher value-added specialty/fine chemicals could be easier entry markets for biomass-derived products. Within this context, the use of heterogeneous catalysts would allow easy separation and regeneration. One of the most utilized classes of heterogeneous catalysts industrially is zeolites, which are active for a variety of sugar chemistries.6,8,11,20–25 Interestingly, some of the porous solid acid-catalyzed fructose to HMF studies report observing difructose anhydrides (DFAs) as an intermediate.26–28 DFAs are higher-value cyclic disaccharides formed from the condensation of two fructose molecules through the formation of two glycosidic linkages. Presently, 15 DFA isomers and their glycosylated derivatives (glycosyl-DFAs) have been identified as having industrial potential in the food, nutraceutical, and pharmaceutical industries. Potential applications range from chemical markers in certain food products, low-calorie sweeteners, to supplements with early studies indicating their potential to prevent the development of cancer, osteoporosis, and anemia. Additionally, their rigidity and hydrophilicity offer possibilities for the development of surfactants, hydrophilic polymers, and complexing agents.29–31Typically, DFAs are currently produced via biosynthetic methods with DFA yields of up to 82%,29 under optimized conditions. However, these processes use expensive tailored DFAs-forming enzymes with costly inulin or levan polysaccharides as substrates.32 The large-scale production of DFAs using those methods is constrained by the isolation and purification of the main products. In this context, chemocatalytic routes, which employ fructose as a feedstock emerge as an innovative and sustainable catalytic strategy for DFA production. The atom economy of these processes (∼90%) is comparable to that of the corresponding biosynthetic processes (∼95%) (Table S1), highlighting their potential as efficient and green alternatives.
However, prior attempts at producing DFAs through catalytic routes showed significant challenges that include low yields at high conversions (due to low selectivity), or technical issues associated with the use of the hazardous, corrosive, and environmentally unfriendly hydrofluoric acid (HF) or resins with high viscosity, which makes homogenization of the reaction mixture challenging.33–36 Moreover, most published research focuses on maximizing HMF yields, and little attention was given to DFA production.27,28,37 The highest DFA yield for a zeolite at a high conversion was 4% for mordenite at 77% conversion.28 A sulfonated carbon catalyst in a 1-butyl-3-methyl-imidazolium chloride and 2-methyltetrahydrofuran biphasic system at 100 °C gave a 28.1% DFA yield at 94% conversion.38 HMF targeted studies usually report relatively high temperatures and comparatively long reaction times, which is likely why DFAs were observed as a short-lived intermediate. Additionally, the catalytic production of furans from carbohydrates in water suffers from low catalytic activity, which increases with the use of polar aprotic solvents, such as gamma-valerolactone (GVL).39,40 GVL is a biomass-derived, biodegradable solvent, recognized as one of the most promising green alternatives for catalytic biomass transformations.41,42 However, because sugar solubility in most polar aprotic solvents is typically low at room temperature, water is commonly used in binary solvent mixtures to ensure adequate solubility.43,44 Solvent selection is moreover important in zeolite-catalyzed biomass conversion processes due to interactions such as competitive adsorption, site-blocking interactions, and various active site restructuring mechanisms. Because of the porous nature of zeolites, molecular sieving of the reaction mixture can take place, partitioning species in or out of the zeolite's pores, which can affect the chemical potential of reactants near the active site.25 This effect is largely influenced by the size and shape selectivity that the specific dimensions of the zeolite pore openings and channels impose on guest molecules. Studies have also illustrated how sugar molecules can take on different conformations in the confinement of zeolite pores.22 The solvent system can also organize differently within the zeolite, affording solvent structures that make for easier access to active sites.45 Nevertheless, the presence of water was particularly noted to be detrimental to some reactions, such as the dehydration of glucose to anhydro-sugars.46 Therefore, herein, we demonstrate a unique approach for selectively dehydrating fructose to difructose anhydride using acid microporous catalysts in a green polar aprotic solvent. Additionally, we investigated how the presence of water alters the reaction pathway when GVL is used as a solvent, inhibiting DFA formation, an aspect not previously explored in literature, which illustrates how important solvent effects are in these systems. To the best of our knowledge, this is the first time reporting a DFA yield of 75% under mild conditions (1.5 wt% fructose in GVL, 50 mL min−1 helium flow, 125 °C and 42 µmol of aluminum sites) with a zeolite catalyst. This result indicates that we can achieve a high catalytic performance while maintaining a high standard, resource-efficient, and environmentally responsible approach as reflected by the high reaction mass efficiency comparable or superior to other technologies. (For more information, refer also to Table S1).
Results and discussion
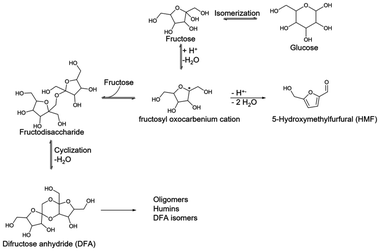
Fructose conversion is a well-studied process that, depending on the reaction conditions, produces various useful molecules (Scheme 1). Many of those studies showed a solvent dependence on rate and product distribution. These effects stem from competitive adsorption, site-blocking, and intermediate stabilization. Fructose itself can be altered by the solvent system due to the various conformations that the molecule can adopt. These conformations can interact differently with the reaction mixture, e.g., the carbonyl of the keto form (ring-opened form) of fructose coordinating to the Brønsted acid proton. Additionally, from a ring-opened form, the dicyclic DFA molecule is not preferentially formed, but isomerization usually dominates.8,9,11 Typically, this ring-opened form is the major conformer in mostly aqueous systems, but it is minimal in organic solvents. But even with the ring-closed forms of fructose, the composition of the reaction mixture can impart complications. The way in which fructose can structure with surrounding solvent molecules can hinder its accessibility to active sites. These structuring complications necessitate considering the nature of the solvent utilized for a given reaction due to interactions with both the zeolite framework and the substrate.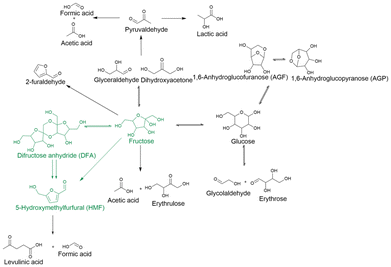 | ||
| Scheme 1 Commonly reported fructose conversion pathways. Note – the double arrow indicates the viewpoint of difructose anhydride being an intermediate to 5-hydroxymethylfurfural. | ||
Effect of the solvent on the reaction outcome
To understand the impact of the solvent environment, we conducted fructose dehydration experiments using H-Alβ25 (additional details are provided in Fig. S6, S7, S8 and Table S3 in the SI) in several pure solvents that potentially promote the closed-ring form of fructose, all of which are mostly polar aprotic. The polar aprotic solvents employed were tetrahydrofuran (THF), dioxane, ethylene carbonate (EC), acetone, and gamma-valerolactone. For comparison, we used water and methanol (MeOH) as polar protic solvents. With the same reaction conditions (140 °C, 1.5 wt% fructose and 2.4 µmol of aluminum sites), we observe that the solvents influence the reaction pathways as fructose conversion and product yields varied significantly (Fig. 1 and Table S3).In water, which is the co-solvent and a byproduct of the dehydration process, we observed no conversion after 15 min of reaction. This agrees with previous studies indicating low reaction rates for carbohydrate dehydration to furans and anhydrosugars in aqueous environments.40,46,48,49 Besides, HMF was preferentially produced over 2-furaldehyde (FAL).48,50 Nonetheless, the formation of humins cannot be discarded, as the solution progressively darkened, and the reaction exhibited poorer carbon balance over time from 100% at 5.8% of conversion after 3 h of reaction to 81% after 24 h and a conversion of 60%.
With THF, the conversion reached 34%, yielding 3.6% DFA, 1.8% HMF, and only 0.3% yield of FAL. In our study, the lack of water initially promoted the production of anhydrous glucose molecules, including 1,6-anhydroglucopyranose (AGP) and 1,6-anhydroglucofuranose (AGF) (8.0% yield), which were generated from the previous isomerization of fructose to glucose (3.2% yield). Notably, the relatively high yield of dihydroxyacetone (4.2%), an intermediate associated with the formation of lactic acid, which typically form under Lewis acidic conditions, suggests that this solvent promoted a different reaction pathway.51–54 Interestingly, we did not detect formic acid that is often reported as a major product when there is water present. This may be attributed to the low conversion achieved under our conditions. Previous studies using pure THF did not report DFA production.48,55
The reaction in methanol produced 47% conversion, with no detection of formic or acetic acid. According to the literature, considerable amounts of formic acid are generated in methanol at high conversion rates, like reactions in water.48 This result contrasts with our findings at lower conversions. Unidentified species might be intermediates for the formation of esters instead of carboxylic acids, as the latter form when using aqueous solutions of aprotic polar solvents and Lewis acids as catalysts.54,56
Furans were the primary products in dioxane with a yield of 19%, while the DFA yield was only 8.4% at a conversion of 66%. As predicted from the literature, the FAL yield was slightly higher than HMF, which might be attributed to the solvent's low basicity and polarity. At these reaction conditions, we did not detect rehydration of HMF to produce formic acid or levulinic acid, which contrasts with observations reported in the literature. This discrepancy may be explained by the different reaction conditions employed in our study.44,48
We observed the highest conversions (higher than 77%) at 15 min for acetone, EC, and GVL. However, despite displaying high conversion (77%) in acetone, the DFA yield was low (3.1%). The isomerization pathway to glucose was important, obtaining 16% yield of AGP and AGF in the absence of water. Yet, the overall mass balance was disappointingly low.
EC, a solid at room temperature, shows high viscosity upon melting and toxicity concerns, representing a challenge in handling, scalability, and sustainability compared to other solvents. The conversion of fructose in EC was 84%, with a DFA yield of 49%. Less than 1% of 1,6-anhydroglucose formed despite a 6.9% glucose yield. Formation of furans was also minimal (yields around 3%).
GVL, being a green solvent, offers additional advantages over other organic solvents, such as lower toxicity, improved safety, and better environmental impact, making it a sustainable choice for biomass conversion processes.57,58 It displayed the highest fructose conversion, 92% after 15 min, with a DFA yield of 47% and a furans yield (HMF and FAL) of 11%.47 As we increased the reaction time to 3 h, the yield of DFA progressively decreased until it was no longer detected. In contrast, the formation of furans increased to 40%, indicating that DFA further converted into furanic compounds over time.27,28,33 The conversion of fructose to HMF and DFA proceeded faster than the reverse reaction from DFAs to fructose, as DFAs reversibly retain fructose via acid catalysis, delaying its degradation and unexpectedly promoting HMF formation.27,33,59,60 The FAL yields were consistently higher than the HMF yields, likely due to the high polarity, low basicity of GVL, and limited reactant accessibility, which is in agreement with previous studies.44,48,49,61 The stability study indicates that the regeneration process retained most of the material's catalytic activity (Fig. 2). This is illustrated by the catalyst crystallinity remaining at 92% via XRD (Fig. S13) and the acidity only decreasing by 4% throughout the reuse and regeneration process (Table S2).
Improving DFA yield and production was possible by employing milder reaction conditions (lower temperature, reduced catalyst loading and increasing fructose concentration), preventing further unwanted reactions (Fig. S6, S7 and S8), such as 1,6-anyhdroglucose formation as a consequence of fructose isomerization or formic or acetic acid as product degradations.46,52,60 This result further illustrates the findings of Dumesic et al. on the utility of GVL for biomass conversion chemistries.62
Evaluation of catalytic performance based on solvent selection shows that GVL and EC are the best solvent choices to target high DFA yields. GVL is the preferred solvent in this case because it is easier to handle compared to EC's solid/waxy nature at lower temperatures.
Effect of water on reactivity
In typical liquid phase reactions, one of the first criteria to evaluate is solvent-reagent miscibility. Thus, many of the reported sugar conversion reactions utilize water-organic mixtures for their solvent systems.22,63–66 Due to the lack of solubility of fructose in pure GVL, we incrementally added water to the system to increase the solubility of the substrate but also move closer to the same regime that other studies operated at.25 By doing so, we observed the negative impact of water with a pronounced deactivation of the system at volume percentages of just over 10% (Fig. 3). Beyond the implications of added water on the system, the trace water that GVL will uptake and the water produced during the reaction could also lead to reactivity complications.To induce a nearly water-free environment within the constraints of our system set up, we bubbled helium gas (50 mL min−1) into the reaction mixture to selectively purge out the water produced during the reaction. This technique aimed to reduce the potential inhibitory effects of ambient and produced water during the reaction on DFA production. In the presence of pure GVL solvent and helium flow, the conversion was 90% after 5 min of reaction and the DFA yield was 75%. This yield remained after an additional 10 min of reaction. In the absence of helium, after 5 min, the conversion was 86%, and the DFA yield was 73%. However, after 15 min, it started to degrade to furans (4.1%) and the DFA yield decreased to 67%. These results may indicate that the water produced during the reaction can lead to the hydrolysis of DFA and push the reaction towards furans. This observation leads us to suggest that the reversibility of DFA production can be impacted by low amounts of water in the reaction mixture, shifting the reaction pathway towards furans (Scheme 2).
Again, leveraging GVL as our preferred solvent, we studied how the incremental addition of water as a cosolvent could impart reaction pathway changes in the system. With only the addition of 2 wt% water to GVL, the conversion decreased from 92% to 86% in the standard 15-minute reaction time. At the 10% water loading condition, the conversion was only 8.7%, with only 1.5% of DFA yield. This decrease in performance illustrates the inhibiting role of water in DFA formation, like the previously reported inhibition with aldehydes and their conversion to anhydrosugars in dioxane.46 At water percentages of 20% and greater, we observed a complete suppression of the catalytic activity, the mechanism of which is still unclear.
To obtain the kinetic reaction order in fructose and the activation energy of the system, we conducted concentration and temperature dependence experiments (Fig. S9 and S10). At our experimental conditions, we obtained an order of 1.7 ± 0.3 in fructose, and we estimated an activation energy of 66 ± 10 kJ mol−1. The obtained kinetic parameters lead us to propose a different reaction pathway rather than DFA being an intermediate to HMF but a competing pathway that involves reversible steps that could lead to HMF (Scheme 2). This proposed pathway requires the activation of fructose at the anomeric carbon to form the fructocarbocation that can undergo dimerization upon contacting another fructose molecule. This dimerized form then undergoes ring-closing dehydration to form DFA, which aligns with the experimentally observed reaction order in fructose.
Effect of water on fructose partitioning
To probe the molecular implications of water addition, we conducted partitioning studies. Given that the reaction rate drops to zero at just over 10% water in the solvent system, understanding the species that can enter the pores of the zeolite at varying water concentrations can provide information about what environment is necessary for catalysis to take place. Utilizing a room temperature mixture of zeolite, GVL, and D2O (in place of water for HPLC detection), we quantified the uptake of species into H-Alβ25 at various increasing water percentages. We observed that the adsorption of fructose into H-Alβ25 only occurred at water concentrations at, or under 10 volume% with decreasing extent of uptake from 2% to 10% water (Fig. 4a). This may be due to water clustering around the Brønsted acid sites within the zeolite,67 not allowing fructose to enter the porous structure of zeolite beta. This fructose uptake follows the observed water-based deactivation of the catalytic system, which shows no catalytic activity at greater than 10 volume% water content in the solvent system. Such observations further corroborate our hypothesis that the fructose is activated at the Brønsted acid sites within the zeolite pore rather than an outer pore catalytic process.To further gain molecular insights into the effect of water on this system, we used a solid-state NMR method to gauge the proportion of adsorbed species versus outer pore species.68 Again, utilizing fructose, GVL and water mixtures with increasing water content, we leveraged the favorable polarization transfer between adsorbed species versus the lack of polarization transfer that outer pore/non-confined species must be able to assess the partitioning of these mixtures. The confinement effects that the micropores of the zeolite impart on adsorbed species cause them to have reduced mobility, which allows for residual dipolar couplings between molecules. This preferentially affords proton magnetization transfer to carbon species confined in the pores rather than in a mobile orientation. The more mobile, rapid tumbling nature of outer pore species does not allow for this effect since they have too short relaxation times (T1) to have residual dipolar couplings. Based on this, we can use a cross-polarization pulse sequence to understand the adsorbed species and a direct polarization pulse sequence to observe all the species in the slurry, confined or not, similarly to the work of Martens and coworkers.68,69 The impact of confinement on the relaxation of species is the major proponent for this magnetization transfer capability.70,71
To observe the extent of adsorption of fructose in the H-Alβ25 zeolite for the various solvent conditions, we used the ratio of the cross-polarization (absorbed) peak area over the direct excitation (total species) Hahn-echo peak area (Fig. S11). To get the largest impact from the cross-polarization effects coming solely from the residual dipolar couplings of the confined fructose, we used the anomeric carbon chemical shift to track fructose since it does not have a directly bound hydrogen that can provide cross-polarization. This experiment yielded a similar exponential decay as the uptake data (Fig. 4b) and aligns well with the deactivation trend from reactivity data (Fig. 2). These data seem to suggest that the uptake of fructose is necessary for the conversion of fructose to DFA, and outer pore catalysis is not the dominant pathway for this system. It is important to note that the 100 vol% water condition shows the most adsorbed fructose, likely due to the cross-polarization enhancement from the surrounding water molecules solvating the fructose molecules in the pore of the zeolite, since there is no water-organic solvent competition for solvating fructose. The higher availability of protons to donate to the anomeric carbon of fructose in a water rich environment within the zeolite pores is the dominant mode for this signal increase rather than any relaxation effects.
Conclusions
In this work, we report on a novel catalytic process for the conversion of fructose to DFA in appreciable yields and provide a molecular understanding of the impact of water as a co-solvent on the uptake of fructose into the porous structure of zeolite beta. We demonstrated that H-Alβ25 is an active catalyst and that biomass-derived GVL is the preferential solvent to drive this reaction pathway towards DFAs. When utilizing incremental concentrations of water as a co-solvent to increase fructose solubility, we observed an inhibition effect with greater than 10 vol% water. Partitioning studies and ssNMR show that at these increasing water concentrations, fructose can no longer enter the pores of zeolite beta, leading to a decrease in fructose conversion. Through the spectroscopic and chromatographic observations obtained in this work, we suggest that intraporous Brønsted acid site accessibility is pivotal to produce DFA through heterogeneously catalyzed routes.Author contributions
I. H-S. and F. R-R. performed reactivity testing and analysis. F. I. performed ssNMR studies and analysis. F. I. and G. G. performed partitioning studies and analysis. E. A. L-R., I. H-S., F. I., R. M., and F. R-R. performed catalyst characterization. I. H-S., F. I., E. A. L-R., I. H., and N. C-M. conceptualized the project. The manuscript was written by I. H-S., and F. I. and revised by E. A. L-R., M. A., I. H., and N. C-M. with input from all authors. N. C-M., and I. H. were responsible for project administration.Conflicts of interest
There are no conflicts to declare.Data availability
The authors declare that all the data supporting the findings of this study are available within the article (and supplementary information (SI) files), or available from the corresponding author on reasonable request.Supplementary information is available. See DOI: https://doi.org/10.1039/d5gc04714e.
Acknowledgements
This work was supported by the NSF-PREM Wisconsin – Puerto Rico Partnership for Research and Education in Materials [grant number DMR-1827894]. A generous gift from Paul J. and Margaret M. Bender enabled the Bruker Avance III 500 spectrometer to be purchased. The solid-state functionality of the Bruker Avance III 500 is thanks to the UW2020 fund from the Wisconsin Alumni Research Fund. The authors would also like to thank Anthony Ayala and Jaymarie Méndez for their help with the execution of the chemical reactions performed in this study and MSc. Ángela Montaño for the XRD pattern acquisition. We thank Dr Juan López for kindly allowing us to use the UV-2600 UV-VIS spectrophotometer. We thank the Structure Commission of the International Zeolite Association (IZA-SC) for providing access to the Database of Zeolite Structures, and Koichi Momma and Fujio Izumi for the development of VESTA, which was used for structure visualization.72,73 The funding sources did not have any involvement in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.References
- M. S. Khatun, M. Hassanpour, S. I. Mussatto, M. D. Harrison, R. E. Speight, I. M. O'Hara and Z. Zhang, Transformation of Sugarcane Molasses into Fructooligosaccharides with Enhanced Prebiotic Activity Using Whole-Cell Biocatalysts from Aureobasidium Pullulans FRR 5284 and an Invertase-Deficient Saccharomyces Cerevisiae 1403-7A, Bioresour. Bioprocess., 2021, 8(1), 85, DOI:10.1186/s40643-021-00438-7.
- A. L. Mokale Kognou, S. Shrestha, Z. Jiang, C. (Charles) Xu, F. Sun and W. Qin, High-Fructose Corn Syrup Production and Its New Applications for 5-Hydroxymethylfurfural and Value-Added Furan Derivatives: Promises and Challenges, J. Bioresour. Bioprod., 2022, 7(3), 148–160, DOI:10.1016/j.jobab.2022.03.004.
- E. Ricca, V. Calabrò, S. Curcio and G. Iorio, The State of the Art in the Production of Fructose from Inulin Enzymatic Hydrolysis, Crit. Rev. Biotechnol., 2007, 27(3), 129–145, DOI:10.1080/07388550701503477.
- E. T. Öner, L. Hernández and J. Combie, Review of Levan Polysaccharide: From a Century of Past Experiences to Future Prospects, Biotechnol. Adv., 2016, 34(5), 827–844, DOI:10.1016/j.biotechadv.2016.05.002.
- J. Dijkmans, D. Gabriëls, M. Dusselier, F. de Clippel, P. Vanelderen, K. Houthoofd, A. Malfliet, Y. Pontikes and B. F. Sels, Productive Sugar Isomerization with Highly Active Sn in Dealuminated β Zeolites, Green Chem., 2013, 15(10), 2777–2785, 10.1039/C3GC41239C.
- J. C. Vega-Vila, J. W. Harris and R. Gounder, Controlled Insertion of Tin Atoms into Zeolite Framework Vacancies and Consequences for Glucose Isomerization Catalysis, J. Catal., 2016, 344, 108–120, DOI:10.1016/j.jcat.2016.09.011.
- W. N. P. van der Graaff, C. H. L. Tempelman, G. Li, B. Mezari, N. Kosinov, E. A. Pidko and E. J. M. Hensen, Competitive Adsorption of Substrate and Solvent in Sn-Beta Zeolite During Sugar Isomerization, ChemSusChem, 2016, 9(22), 3145–3149, DOI:10.1002/cssc.201600800.
- J. W. Harris, M. J. Cordon, J. R. Di Iorio, J. C. Vega-Vila, F. H. Ribeiro and R. Gounder, Titration and Quantification of Open and Closed Lewis Acid Sites in Sn-Beta Zeolites That Catalyze Glucose Isomerization, J. Catal., 2016, 335, 141–154, DOI:10.1016/j.jcat.2015.12.024.
- Y.-P. Li, M. Head-Gordon and A. T. Bell, Analysis of the Reaction Mechanism and Catalytic Activity of Metal-Substituted Beta Zeolite for the Isomerization of Glucose to Fructose, ACS Catal., 2014, 4(5), 1537–1545, DOI:10.1021/cs401054f.
- N. Rai, S. Caratzoulas and D. G. Vlachos, Role of Silanol Group in Sn-Beta Zeolite for Glucose Isomerization and Epimerization Reactions, ACS Catal., 2013, 3(10), 2294–2298, DOI:10.1021/cs400476n.
- M. Moliner, Y. Román-Leshkov and M. E. Davis, Tin-Containing Zeolites Are Highly Active Catalysts for the Isomerization of Glucose in Water, Proc. Natl. Acad. Sci. U. S. A., 2010, 107(14), 6164–6168, DOI:10.1073/pnas.1002358107.
- I. Delidovich and R. Palkovits, Catalytic Isomerization of Biomass-Derived Aldoses: A Review, ChemSusChem, 2016, 9(6), 547–561, DOI:10.1002/cssc.201501577.
- Y. Román-Leshkov and J. A. Dumesic, Solvent Effects on Fructose Dehydration to 5-Hydroxymethylfurfural in Biphasic Systems Saturated with Inorganic Salts, Top. Catal., 2009, 52(3), 297–303, DOI:10.1007/s11244-008-9166-0.
- J. S. Kruger, V. Choudhary, V. Nikolakis and D. G. Vlachos, Elucidating the Roles of Zeolite H-BEA in Aqueous-Phase Fructose Dehydration and HMF Rehydration, ACS Catal., 2013, 3(6), 1279–1291, DOI:10.1021/cs4002157.
- Z. Ma, H. Hu, Z. Sun, W. Fang, J. Zhang, L. Yang, Y. Zhang and L. Wang, Acidic Zeolite L as a Highly Efficient Catalyst for Dehydration of Fructose to 5-Hydroxymethylfurfural in Ionic Liquid, ChemSusChem, 2017, 10(8), 1669–1674, DOI:10.1002/cssc.201700239.
- A. Pande, P. Niphadkar, K. Pandare and V. Bokade, Acid Modified H-USY Zeolite for Efficient Catalytic Transformation of Fructose to 5-Hydroxymethyl Furfural (Biofuel Precursor) in Methyl Isobutyl Ketone–Water Biphasic System, Energy Fuels, 2018, 32(3), 3783–3791, DOI:10.1021/acs.energyfuels.7b03684.
- V. V. Ordomsky, J. van der Schaaf, J. C. Schouten and T. A. Nijhuis, Fructose Dehydration to 5-Hydroxymethylfurfural over Solid Acid Catalysts in a Biphasic System, ChemSusChem, 2012, 5(9), 1812–1819, DOI:10.1002/cssc.201200072.
- Y. Román-Leshkov, J. N. Chheda and J. A. Dumesic, Phase Modifiers Promote Efficient Production of Hydroxymethylfurfural from Fructose, Science, 2006, 312(5782), 1933–1937, DOI:10.1126/science.1126337.
- J. N. Chheda, Y. Román-Leshkov and J. A. Dumesic, Production of 5-Hydroxymethylfurfural and Furfural by Dehydration of Biomass-Derived Mono- and Poly-Saccharides, Green Chem., 2007, 9(4), 342–350, 10.1039/B611568C.
- R. Gounder and M. E. Davis, Titanium-Beta Zeolites Catalyze the Stereospecific Isomerization of d-Glucose to l-Sorbose via Intramolecular C5–C1 Hydride Shift, ACS Catal., 2013, 3(7), 1469–1476, DOI:10.1021/cs400273c.
- E. Nikolla, Y. Román-Leshkov, M. Moliner and M. E. Davis, “One-Pot” Synthesis of 5-(Hydroxymethyl)Furfural from Carbohydrates Using Tin-Beta Zeolite, ACS Catal., 2011, 1(4), 408–410, DOI:10.1021/cs2000544.
- R. Bermejo-Deval, R. S. Assary, E. Nikolla, M. Moliner, Y. Román-Leshkov, S.-J. Hwang, A. Palsdottir, D. Silverman, R. F. Lobo, L. A. Curtiss and M. E. Davis, Metalloenzyme-like Catalyzed Isomerizations of Sugars by Lewis Acid Zeolites, Proc. Natl. Acad. Sci. U. S. A., 2012, 109(25), 9727–9732, DOI:10.1073/pnas.1206708109.
- M. E. Davis, Heterogeneous Catalysis for the Conversion of Sugars into Polymers, Top. Catal., 2015, 58(7), 405–409, DOI:10.1007/s11244-015-0386-9.
- M. J. Cordon, J. W. Harris, J. C. Vega-Vila, J. S. Bates, S. Kaur, M. Gupta, M. E. Witzke, E. C. Wegener, J. T. Miller, D. W. Flaherty, D. D. Hibbitts and R. Gounder, Dominant Role of Entropy in Stabilizing Sugar Isomerization Transition States within Hydrophobic Zeolite Pores, J. Am. Chem. Soc., 2018, 140(43), 14244–14266, DOI:10.1021/jacs.8b08336.
- L. Qi, R. Alamillo, W. A. Elliott, A. Andersen, D. W. Hoyt, E. D. Walter, K. S. Han, N. M. Washton, R. M. Rioux, J. A. Dumesic and S. L. Scott, Operando Solid-State NMR Observation of Solvent-Mediated Adsorption-Reaction of Carbohydrates in Zeolites, ACS Catal., 2017, 7(5), 3489–3500, DOI:10.1021/acscatal.7b01045.
- A. J. Crisci, M. H. Tucker, M.-Y. Lee, S. G. Jang, J. A. Dumesic and S. L. Scott, Acid-Functionalized SBA-15-Type Silica Catalysts for Carbohydrate Dehydration, ACS Catal., 2011, 1(7), 719–728, DOI:10.1021/cs2001237.
- Y. Huang, P.-Y. Chao, T.-Y. Cheng, Y. Ho, C.-T. Lin, H.-Y. Hsu, J.-J. Wong and T.-C. Tsai, Design of Sulfonated Mesoporous Silica Catalyst for Fructose Dehydration Guided by Difructose Anhydride Intermediate Incorporated Reaction Network, Chem. Eng. J., 2016, 283, 778–788, DOI:10.1016/j.cej.2015.08.031.
- T.-Y. Cheng, P.-Y. Chao, Y.-H. Huang, C.-C. Li, H.-Y. Hsu, Y.-S. Chao and T.-C. Tsai, Catalysis of Ordered Nanoporous Materials for Fructose Dehydration through Difructose Anhydride Intermediate, Microporous Mesoporous Mater., 2016, 233, 148–153, DOI:10.1016/j.micromeso.2016.01.015.
- M. Cheng, H. Wu, W. Zhang and W. Mu, Difructose Anhydride III: A 50-Year Perspective on Its Production and Physiological Functions, Crit. Rev. Food Sci. Nutr., 2022, 62(24), 6714–6725, DOI:10.1080/10408398.2021.1904823.
- C. O. Mellet and J. M. G. Fernández, Difructose Dianhydrides (DFAs) and DFA-Enriched Products as Functional Foods, in Carbohydrates in Sustainable Development I, ed. A. P. Rauter, P. Vogel and Y. Queneau, Springer, Berlin, Heidelberg, 2010, pp. 49–77. DOI:10.1007/128_2010_50.
- M. I. García-Moreno, Chemical and Enzymatic Approaches to Carbohydrate-Derived Spiroketals: Di-D-Fructose Dianhydrides (DFAs), Molecules, 2008, 13(8), 1640–1670, DOI:10.3390/molecules13081640.
- M. Cheng, D. Ni, W. Zhang and W. Mu, Tailored Enzymes for Difructose Anhydrides: From Biosynthesis to Degradation, J. Agric. Food Chem., 2024, 72(50), 27654–27667, DOI:10.1021/acs.jafc.4c07830.
- M. H. Tucker, R. Alamillo, A. J. Crisci, G. M. Gonzalez, S. L. Scott and J. A. Dumesic, Sustainable Solvent Systems for Use in Tandem Carbohydrate Dehydration Hydrogenation, ACS Sustainable Chem. Eng., 2013, 1(5), 554–560, DOI:10.1021/sc400044d.
- I. Idri, J.-L. Havet, J. M. Garcia Fernandez, C. Ferroud and C. Porte, Microwave-Assisted Synthesis of Prebiotic Di-D-Fructose Dianhydride-Enriched Caramels, Food Chem., 2012, 134(3), 1527–1532, DOI:10.1016/j.foodchem.2012.03.068.
- M. Audemar, L. Atencio-Genes, C. Ortiz Mellet, F. Jérôme, J. M. Garcia Fernandez and K. De Oliveira Vigier, Carbon Dioxide as a Traceless Caramelization Promotor: Preparation of Prebiotic Difructose Dianhydrides (DFAs)-Enriched Caramels from d -Fructose, J. Agric. Food Chem., 2017, 65(30), 6093–6099, DOI:10.1021/acs.jafc.7b01601.
- J. M. García Fernández, A. Gadelle and J. Defaye, Difructose Dianhydrides from Sucrose and Fructo-Oligosaccharides and Their Use as Building Blocks for the Preparation of Amphiphiles, Liquid Crystals, and Polymers, Carbohydr. Res., 1994, 265(2), 249–269, DOI:10.1016/0008-6215(94)00239-8.
- Y. Han, N. Caillol, F. Allain, A. Mekki-Berrada, G. Pirngruber and K. Larmier, Dehydration of Fructose to 5-HMF Catalyzed by Commercial Faujasite Zeolites: Kinetic Study and Influence of the Properties of the Catalyst, ChemCatChem, 2024, 16(19), e202400257, DOI:10.1002/cctc.202400257.
- A. D. K. Deshan, L. Moghaddam, L. Atanda, H. Wang, J. P. Bartley, W. O. S. Doherty and D. W. Rackemann, High Conversion of Concentrated Sugars to 5-Hydroxymethylfurfural over a Metal-Free Carbon Catalyst: Role of Glucose–Fructose Dimers, ACS Omega, 2023, 8(43), 40442–40455, DOI:10.1021/acsomega.3c05060.
- E. I. Gürbüz, J. M. R. Gallo, D. M. Alonso, S. G. Wettstein, W. Y. Lim and J. A. Dumesic, Conversion of Hemicellulose into Furfural Using Solid Acid Catalysts in γ-Valerolactone, Angew. Chem., Int. Ed., 2013, 52(4), 1270–1274, DOI:10.1002/anie.201207334.
- M. A. Mellmer, C. Sener, J. M. R. Gallo, J. S. Luterbacher, D. M. Alonso and J. A. Dumesic, Solvent Effects in Acid-Catalyzed Biomass Conversion Reactions, Angew. Chem., Int. Ed., 2014, 53(44), 11872–11875, DOI:10.1002/anie.201408359.
- N. I. Haykir and J. Viell, Challenges and Opportunities for Gamma-Valerolactone in Biomass Pretreatment, Ind. Eng. Chem. Res., 2024, 63(28), 12251–12264, DOI:10.1021/acs.iecr.4c01277.
- R. Xu, K. Liu, H. Du, H. Liu, X. Cao, X. Zhao, G. Qu, X. Li, B. Li and C. Si, Falling Leaves Return to Their Roots: A Review on the Preparation of γ-Valerolactone from Lignocellulose and Its Application in the Conversion of Lignocellulose, ChemSusChem, 2020, 13(24), 6461–6476, DOI:10.1002/cssc.202002008.
- T. Wang, M. W. Nolte and B. H. Shanks, Catalytic Dehydration of C6 Carbohydrates for the Production of Hydroxymethylfurfural (HMF) as a Versatile Platform Chemical, Green Chem., 2014, 16(2), 548–572, 10.1039/C3GC41365A.
- Y. Wang, G. Ding, X. Yang, H. Zheng, Y. Zhu and Y. Li, Selectively Convert Fructose to Furfural or Hydroxymethylfurfural on Beta Zeolite: The Manipulation of Solvent Effects, Appl. Catal., B, 2018, 235, 150–157, DOI:10.1016/j.apcatb.2018.04.043.
- J. R. Di Iorio, B. A. Johnson and Y. Román-Leshkov, Ordered Hydrogen-Bonded Alcohol Networks Confined in Lewis Acid Zeolites Accelerate Transfer Hydrogenation Turnover Rates, J. Am. Chem. Soc., 2020, 142(45), 19379–19392, DOI:10.1021/jacs.0c09825.
- Q. Cao, T. Ye, W. Li, J. Chen, Y. Lu, H. Gan, H. Wu, F. Cao, P. Wei and P. Ouyang, Dehydration of Saccharides to Anhydro-Sugars in Dioxane: Effect of Reactants, Acidic Strength and Water Removal in Situ, Cellulose, 2020, 27(17), 9825–9838, DOI:10.1007/s10570-020-03490-2.
- I. Hermans, Catalytic Method For The Production Of Difructose Anhydride, US20240417416A1, 2024.
- R. Li, Q. Lin, Y. Liu, X. Wang, C. Liu, F. Peng and J. Ren, Insights into Solvent Effect on Selective Production of Furfural and 5-Hydroxymethylfurfural from Fructose, J. Catal., 2023, 424, 162–172, DOI:10.1016/j.jcat.2023.05.022.
- M. Asakawa, A. Shrotri, H. Kobayashi and A. Fukuoka, Solvent Basicity Controlled Deformylation for the Formation of Furfural from Glucose and Fructose, Green Chem., 2019, 21(22), 6146–6153, 10.1039/C9GC02600B.
- J. Tan, H. Wang, L. Ma, C. Wang, Q. Liu, Q. Zhang and M. He, Selective Yields of Furfural and Hydroxymethylfurfural from Glucose in Tetrahydrofuran over Hβ Zeolite, RSC Adv., 2018, 8(43), 24534–24540, 10.1039/C8RA04060E.
- M. Dusselier, P. Van Wouwe, F. de Clippel, J. Dijkmans, D. W. Gammon and B. F. Sels, Mechanistic Insight into the Conversion of Tetrose Sugars to Novel α-Hydroxy Acid Platform Molecules, ChemCatChem, 2013, 5(2), 569–575, DOI:10.1002/cctc.201200476.
- T. Flannelly, M. Lopes, L. Kupiainen, S. Dooley and J. J. Leahy, Non-Stoichiometric Formation of Formic and Levulinic Acids from the Hydrolysis of Biomass Derived Hexose Carbohydrates, RSC Adv., 2016, 6(7), 5797–5804, 10.1039/C5RA25172A.
- H.-S. Chen, A. Wang, H. Sorek, J. D. Lewis, Y. Román-Leshkov and A. T. Bell, Production of Hydroxyl–Rich Acids from Xylose and Glucose Using Sn-BEA Zeolite, ChemistrySelect, 2016, 1(14), 4167–4172, DOI:10.1002/slct.201600836.
- J. Dijkmans, M. Dusselier, D. Gabriëls, K. Houthoofd, P. C. M. M. Magusin, S. Huang, Y. Pontikes, M. Trekels, A. Vantomme, L. Giebeler, S. Oswald and B. F. Sels, Cooperative Catalysis for Multistep Biomass Conversion with Sn/Al Beta Zeolite, ACS Catal., 2015, 5(2), 928–940, DOI:10.1021/cs501388e.
- Q. Sun, S. Wang, B. Aguila, X. Meng, S. Ma and F.-S. Xiao, Creating Solvation Environments in Heterogeneous Catalysts for Efficient Biomass Conversion, Nat. Commun., 2018, 9(1), 3236, DOI:10.1038/s41467-018-05534-5.
- M. S. Holm, Y. J. Pagán-Torres, S. Saravanamurugan, A. Riisager, J. A. Dumesic and E. Taarning, Sn-Beta Catalysed Conversion of Hemicellulosic Sugars, Green Chem., 2012, 14(3), 702–706, 10.1039/C2GC16202D.
- D. M. Alonso, S. G. Wettstein and J. A. Dumesic, Gamma-Valerolactone, a Sustainable Platform Molecule Derived from Lignocellulosic Biomass, Green Chem., 2013, 15(3), 584–595, 10.1039/C3GC37065H.
- F. Kerkel, M. Markiewicz, S. Stolte, E. Müller and W. Kunz, The Green Platform Molecule Gamma-Valerolactone – Ecotoxicity, Biodegradability, Solvent Properties, and Potential Applications, Green Chem., 2021, 23(8), 2962–2976, 10.1039/D0GC04353B.
- T.-H. Chan, P.-T. Chen, H.-H. Chang, M.-Y. Lai, M. Hayashi, J.-K. Wang and Y.-L. Wang, Autocatalytic Reaction in Hydrolysis of Difructose Anhydride III, J. Phys. Chem. A, 2011, 115(37), 10309–10314, DOI:10.1021/jp206494r.
- G. S. Svenningsen, R. Kumar, C. E. Wyman and P. Christopher, Unifying Mechanistic Analysis of Factors Controlling Selectivity in Fructose Dehydration to 5-Hydroxymethylfurfural by Homogeneous Acid Catalysts in Aprotic Solvents, ACS Catal., 2018, 8(6), 5591–5600, DOI:10.1021/acscatal.8b01197.
- L. Zhang, G. Xi, Z. Chen, D. Jiang, H. Yu and X. Wang, Highly Selective Conversion of Glucose into Furfural over Modified Zeolites, Chem. Eng. J., 2017, 307, 868–876, DOI:10.1016/j.cej.2016.09.001.
- A. H. Motagamwala, W. Won, C. T. Maravelias and J. Dumesic, An Engineered Solvent System for Sugar Production from Lignocellulosic Biomass Using Biomass Derived Gamma-Valerolactone, Green Chem., 2016, 18(21), 5756–5763, 10.1039/C6GC02297A.
- N. S. Gould and B. Xu, Catalyst Characterization in the Presence of Solvent: Development of Liquid Phase Structure–Activity Relationships, Chem. Sci., 2018, 9(2), 281–287, 10.1039/C7SC03728G.
- R.-J. van Putten, J. N. M. Soetedjo, E. A. Pidko, J. C. van der Waal, E. J. M. Hensen, E. de Jong and H. J. Heeres, Dehydration of Different Ketoses and Aldoses to 5-Hydroxymethylfurfural, ChemSusChem, 2013, 6(9), 1681–1687, DOI:10.1002/cssc.201300345.
- E. Taarning, S. Saravanamurugan, M. Spangsberg Holm, J. Xiong, R. M. West and C. H. Christensen, Zeolite-Catalyzed Isomerization of Triose Sugars, ChemSusChem, 2009, 2(7), 625–627, DOI:10.1002/cssc.200900099.
- Y. Román-Leshkov and M. E. Davis, Activation of Carbonyl-Containing Molecules with Solid Lewis Acids in Aqueous Media, ACS Catal., 2011, 1(11), 1566–1580, DOI:10.1021/cs200411d.
- M. Wang, N. R. Jaegers, M.-S. Lee, C. Wan, J. Z. Hu, H. Shi, D. Mei, S. D. Burton, D. M. Camaioni, O. Y. Gutiérrez, V.-A. Glezakou, R. Rousseau, Y. Wang and J. A. Lercher, Genesis and Stability of Hydronium Ions in Zeolite Channels, J. Am. Chem. Soc., 2019, 141(8), 3444–3455, DOI:10.1021/jacs.8b07969.
- S. Radhakrishnan, P.-J. Goossens, P. C. M. M. Magusin, S. P. Sree, C. Detavernier, E. Breynaert, C. Martineau, F. Taulelle and J. A. Martens, In Situ Solid-State 13C NMR Observation of Pore Mouth Catalysis in Etherification of β-Citronellene with Ethanol on Zeolite Beta, J. Am. Chem. Soc., 2016, 138(8), 2802–2808, DOI:10.1021/jacs.5b13282.
- S. Kerkhofs, F. Saïdi, N. Vandervoort, G. V. den Mooter, C. Martineau, F. Taulelle and J. A. Martens, Silica Capsules Enclosing P123 Triblock Copolymer Micelles for Flurbiprofen Storage and Release, J. Mater. Chem. B, 2015, 3(15), 3054–3061, 10.1039/C5TB00058K.
- D. Weber, J. Mitchell, J. McGregor and L. F. Gladden, Comparing Strengths of Surface Interactions for Reactants and Solvents in Porous Catalysts Using Two-Dimensional NMR Relaxation Correlations, J. Phys. Chem. C, 2009, 113(16), 6610–6615, DOI:10.1021/jp811246j.
- A. Valiya Parambathu, W. G. Chapman, G. J. Hirasaki, D. Asthagiri and P. M. Singer, Effect of Nanoconfinement on NMR Relaxation of Heptane in Kerogen from Molecular Simulations and Measurements, J. Phys. Chem. Lett., 2023, 14(4), 1059–1065, DOI:10.1021/acs.jpclett.2c03699.
- Database of Zeolite Structures, https://www.iza-structure.org/databases/ (accessed 2025-05-27).
- K. Momma and F. Izumi, VESTA 3 for Three-Dimensional Visualization of Crystal, Volumetric and Morphology Data, J. Appl. Crystallogr., 2011, 44(6), 1272–1276, DOI:10.1107/S0021889811038970.
Footnotes |
| † These authors contributed equally to this work. |
| ‡ Current address: Department of Chemistry, Johns Hopkins University, Baltimore, MD 21218, USA. E-mail: hermans@jhu.edu. |
| § Current address: Ralph O'Connor Sustainable Energy Institute, Johns Hopkins University, Baltimore, MD, 21218, USA. E-mail: hermans@jhu.edu. |
| ¶ Current address: Department of Chemical and Biological Engineering, Johns Hopkins University, Baltimore, MD 21218, USA. E-mail: hermans@jhu.edu. |
| This journal is © The Royal Society of Chemistry 2026 |




![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
: