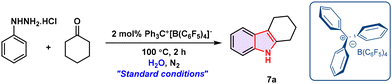An ion-pair as a superacidic precatalyst for the synthesis of indole alkaloids: a novel entry into the Fischer indole synthesis
Pooja
Sivaganesan†
a,
Chibisree
Elanchezhian†
a,
Diksha
Bansal†
a,
Mrinal Kanti
Das
*b and
Saikat
Chaudhuri
 *ac
*ac
aOrganic and Bio-Organic Chemistry Laboratory, CSIR-Central Leather Research Institute (CSIR-CLRI), Chennai–600020, India. E-mail: schaudhuri@clri.res.in
bDepartment of Chemistry, Karimpur Pannadevi College, University of Kalyani, Karimpur, Nadia–741152, WB, India. E-mail: mrinalkdas1991@gmail.com
cAcSIR – Academy of Scientific and Innovative Research, Ghaziabad, Uttar Pradesh–201002, India
First published on 18th November 2025
Abstract
We report the first application of [Ph3C]+[B(C6F5)4]− in ion-pair catalysis to the Fischer indole synthesis, enabling efficient construction of structurally diverse indole frameworks under mild conditions. Using the [Ph3C]+[B(C6F5)4]− ion pair as a highly active and selective catalyst, a broad range of substrates, including sterically hindered and electronically diverse partners, were converted to indoles in good to excellent yields. The method delivers high regioselectivity even in structurally complex settings, as demonstrated in concise total syntheses of paullone, (±)-desbromoarborescidine A, tjipanazole D, and tjipanazole I. Scale-up synthesis was also achieved without loss of efficiency, highlighting the operational simplicity of the protocol. This work establishes ion-pair-catalysed Fischer indole synthesis as a versatile and scalable platform for rapid access to bioactive heterocyclic scaffolds.
Green foundation1. This study introduces a rare example of ion-pair catalysis enabling the rapid construction of carbazole scaffolds under mild, metal-free conditions. The protocol proceeds efficiently in short reaction times and delivers high yields with excellent selectivity, demonstrating operational simplicity and improved environmental compatibility compared to conventional methods.2. Carbazole frameworks are privileged in pharmaceuticals, materials, and natural product synthesis, yet conventional routes often rely on metals, harsh reagents, or prolonged reaction times. This work offers a more practical and sustainable alternative that is scalable and broadly applicable, reinforcing the growing relevance of ion-pair catalysis in heterocyclic synthesis. 3. Looking ahead, further advances in green synthesis will emphasize catalyst economy, energy efficiency, and waste minimization. By integrating ion-pair catalysis with metal-free aqueous conditions, this study contributes a meaningful step toward developing greener carbazole chemistry and motivates continued innovation in sustainable catalytic design. |
Introduction
A defining chapter in the understanding of reactive intermediates was initiated by Gomberg's landmark discovery of the triphenylmethyl radical in 1900.1–3 Subsequent investigations by Norris, Kehrmann, and Wentzel extended this work by demonstrating that the triphenylmethyl system could undergo heterolytic cleavage, generating a stabilized carbocation and revealing its latent ionic nature.4,5 While Gomberg's work firmly established the existence of a persistent organic radical, the pioneering investigations of Norris and Kehrmann provided the first compelling evidence for the generation of carbocations from this system, which was further substantiated by Wentzel's studies on ionic behaviour in solution. Beyond this triad, further mechanistic and spectroscopic explorations by numerous researchers have since deepened the understanding of ion-pair formation, stability, and reactivity.6–9However, it was not until much later that triaryl carbenium ion pairs emerged as effective catalysts in organic synthesis. A notable turning point came with their application in promoting carbon–carbon bond-forming reactions by Mukaiyama and co-workers, such as the aldol reaction, which demonstrated their potential beyond mere reactive intermediates (Fig. 1-I).10,11 This breakthrough catalysed growing interest in exploiting ion-pair systems for broader catalytic purposes. More recently, the field has witnessed rapid expansion, particularly in asymmetric catalysis by Chen and co-workers, where chiral carbocation catalysts have been engineered to impart high levels of enantioselectivity.12 Innovations in this domain by Siegel and Nelson involved the activation of strong C–H and C–F bonds through highly electrophilic ion pairs, significantly expanding the reactivity landscape of traditionally inert bonds.13,14 In parallel, Luo's group demonstrated that the incorporation of chiral phosphate counterions could unlock asymmetric Friedel–Crafts reactions,15 while Oestreich and collaborators extended the scope of this platform to include carbenium-ion-mediated [5 + 1] cycloadditions.16
Of the various ion pair systems explored to date, [Ph3C]+[B(C6F5)4]− stands out as exceptionally versatile and adaptable in mediating diverse organic transformations.17–20 Its weakly coordinating, non-nucleophilic borate counterion enables the generation of highly reactive triaryl carbenium species, unlocking a wide array of catalytic applications.21 Hou and co-workers employed this ion pair in combination with transition metal complexes to facilitate polymerization reactions.22,23 Jutzi demonstrated its utility in stabilizing reactive zirconocene intermediates via a crystalline Brønsted acid form.24 Bergman highlighted its influence on intermolecular hydroamination and hydroarylation processes.25
Building on this, Stokes used the same ion pair to achieve intramolecular hydroarylation of sterically hindered styrenes,26 while Yao extended the method to hydroarylations involving aromatic amines.27 Also, Hashmi harnessed this system for oxidative [2 + 2 + 1] cycloadditions of ynamides,28 and Luo and Loh showcased its ability to activate carbonyl groups through LUMO-lowering interactions, catalysing Diels–Alder reactions with anthracenes.29,30 Building on this momentum, Jia's group has also significantly advanced this field, with several reports demonstrating the catalytic potential of ion pairs across a broad range of C–C and C-heteroatom bond-forming reactions.31,32 Most recently, they developed a rapid and efficient C–S coupling protocol in water using the [Ph3C]+[B(C6F5)4]− ion pair to catalyse dehydrative couplings of thiols and alcohols under metal-free conditions (Fig. 1-II).33
Motivated by these seminal advances, we turned our attention to re-evaluating classical name reactions through the lens of ion-pair catalysis. One such transformation is the Fischer indole synthesis, a well-established method for assembling indole-based frameworks.34–36 Numerous methodologies have been developed for the synthesis of carbazoles,37–41 reflecting the enduring interest in this privileged heterocyclic scaffold. Several ionic-liquid-mediated protocols have also been described,42 highlighting the potential of ionic environments in facilitating such transformations. However, no catalytic protocol employing the discrete ion pair [Ph3C]+[B(C6F5)4]− under aqueous conditions has been reported; herein, we introduce this ion pair as a superacidic precatalyst enabling the efficient synthesis of carbazoles. Given its relevance in constructing diverse heterocycles,43 we envisioned harnessing the reactivity of discrete ion pairs to achieve this transformation under metal-free and mild conditions. To highlight advancements achieved, Fig. 1-III summarizes prior ion-pair-catalysed transformations reported in the literature alongside our novel application of ion-pair catalysis to the Fischer indole synthesis, demonstrating a significant expansion of this catalytic strategy to heterocyclic construction.
In this work, we disclose the first such approach enabled by the [Ph3C]+[B(C6F5)4]− system, which facilitates smooth dehydrative cyclization under aqueous conditions (Scheme 1).
Our efforts are situated within the broader context of heterocyclic synthesis44–46 and thereby stem from the synthetic and pharmacological significance of the resulting carbazole derivatives,47 which are frequently encountered in natural products and medicinally relevant scaffolds due to their diverse biological activities, including anticancer, antimicrobial, and anti-inflammatory effects.48–50 Examples include eburnamonine (1), desbromoarborescidine A (2), carprofene (3), tjipanazole I (4), tjipanazole D (5), and paullone (6) (Fig. 2).
In alignment with this, we previously demonstrated a successful application of the Fischer indole synthesis using pentafluorophenol,51 further inspiring us to probe the potential of ion-pair catalysis for a more sustainable approach in this domain. This study employs water as a green solvent and utilizes the ion pair as a superacidic Brønsted acid precatalyst, providing an efficient and environmentally friendly method to access valuable carbazole scaffolds.
Results and discussion
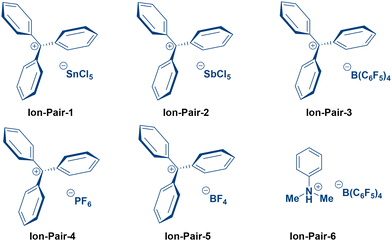
We commenced our investigation into the ion-pair-catalysed Fischer indole synthesis using phenylhydrazine hydrochloride and cyclohexanone as model substrates, with the optimization results summarized in Table 1. Using 10 mol% catalytic loading of ion-pair-1 in HFIP at 80 °C furnished 7a in only 9% yield, and substitution of HFIP with TFE or PhMe similarly gave poor outcomes (entries 1–3, ≤21%). A similar catalytic loading of ion-pair-2 in TFE afforded 7a in just 18% yield (entry 4), whereas switching to ion-pair-3 under the same conditions improved the yield to 51% at the same catalytic loading (entry 5). Replacing TFE with water at 100 °C under otherwise identical conditions dramatically enhanced the efficiency, delivering 7a in 92% yield (entry 6). Shorter reaction times led to reduced yields (entry 7), while extending the reaction time offered no significant improvement from the enhanced yield (entry 8).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a
a
| S. no. | Change from “standard conditions” | 7a yielda (%) |
|---|---|---|
| Reaction conditions: phenylhydrazine hydrochloride (0.25 mmol; 1.0 equiv.) and cyclohexanone (0.26 mmol; 1.05 equiv.) in 2.0 ml of solvent at 100 °C. The reactions were carried out in a 15 ml sealed tube.a Isolated yields from column chromatography. | ||
| 1 | 10 mol% of ion-pair-1 in HFIP at 80 °C | 09 |
| 2 | 10 mol% of ion-pair-1 in TFE at 80 °C | 21 |
| 3 | 10 mol% of ion-pair-1 in PhMe at 80 °C | 10 |
| 4 | 10 mol% of ion-pair-2 in TFE at 80 °C | 18 |
| 5 | 10 mol% catalytic loading in TFE at 80 °C instead of H2O | 51 |
| 6 | 10 mol% catalytic loading | 92 |
| 7 | 10 mol% catalytic loading for 1 h | 72 |
| 8 | 10 mol% catalytic loading for 3 h | 91 |
| 9 | 5 mol% catalytic loading | 92 |
| 10 | Not any | 93 |
| 11 | 1 mol% catalytic loading for 5 h | 61 |
| 12 | Ion-pair-4 instead of ion-pair-3 | 26 |
| 13 | Ion-pair-5 instead of ion-pair-3 | 06 |
| 14 | Ion-pair-6 instead of ion-pair-3 | 40 |
| 15 | Open flask conditions instead of N2 | 18 |
| 16 | O2 atmosphere instead of N2 | 20 |
|
||
Catalyst loading studies revealed that 5 mol% of ion-pair-3 maintained high reactivity (entry 9, 92%), but reducing the loading to 2 mol% unexpectedly furnished the highest yield of 93%, identifying this as the optimal condition (entry 10). Further lowering to 1 mol% resulted in diminished conversion (entry 11, 61%). Screening of alternative ion pairs confirmed the privileged performance of [Ph3C]+[B(C6F5)4]−, which delivered 7a in 93% yield under the optimized aqueous conditions, while SnCl5− (ion-pair-1), SbCl5− (ion-pair-2), PF6− (ion-pair-4), BF4− (ion-pair-5), and ion-pair-6 were markedly less effective (entries 12–14).
Subsequently, we performed the reaction under open-flask and O2 conditions. Although the product formation was detectable, only a modest conversion of about 20% was observed, indicating the critical role of the inert atmosphere in sustaining catalytic efficiency. These findings establish [Ph3C]+[B(C6F5)4]− in water as a uniquely potent and sustainable catalytic platform, combining high efficiency with environmentally benign conditions for the synthesis of heterocycles.
With the optimal reaction parameters in hand, we turned our focus toward evaluating the scope and functional group tolerance of the ion-pair-catalysed Fischer indole synthesis.
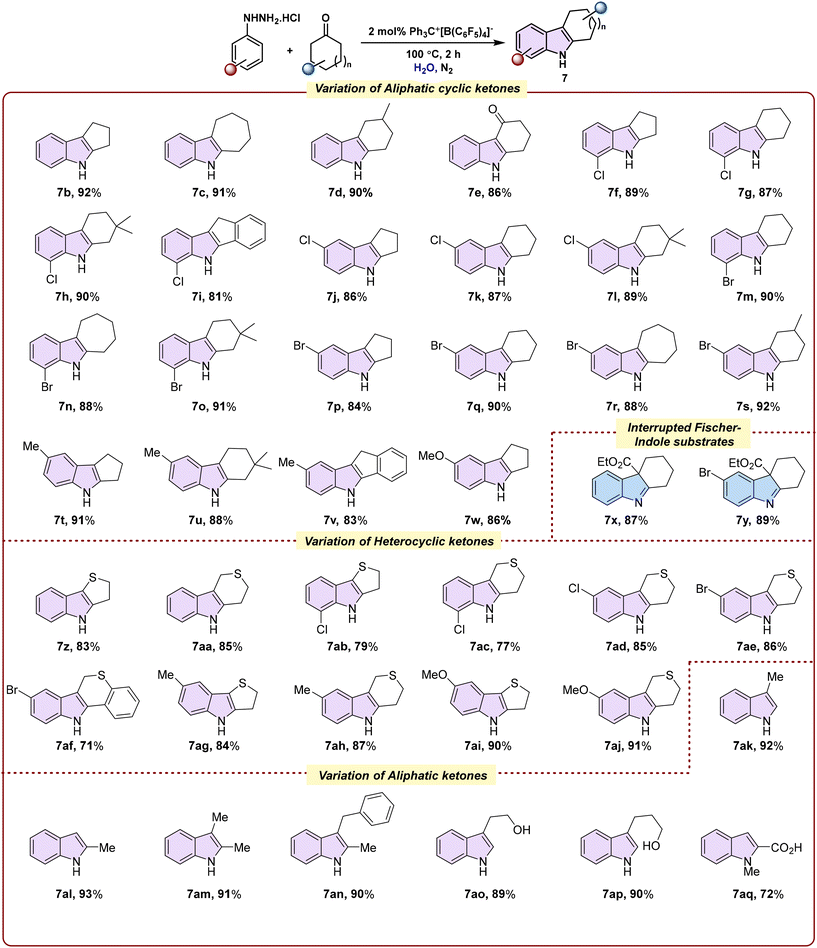
Given the privileged reactivity of [Ph3C]+[B(C6F5)4]− under aqueous conditions, we anticipated that this protocol would accommodate a broad range of hydrazine and ketone substrates, thereby enabling streamlined access to structurally diverse carbazole derivatives. To test this, we examined various substitution patterns on both reaction partners under the optimized conditions (Scheme 2).52
We commenced our investigations by fixing the arylhydrazine hydrochloride component as unsubstituted phenylhydrazine hydrochloride and varying the ketone partner. Cyclopentanone furnished the corresponding carbazole 7b in 92% yield, cycloheptanone provided 7c in 91% yield, and 4-methylcyclohexanone was efficiently transformed into 7d in 90% yield, while the 1,3-cyclohexanone partner yielded 7e in 86% yield. Subsequently, 2-chlorophenylhydrazine hydrochloride was employed with cyclopentanone, cyclohexanone, and 3,3-dimethylcyclohexanone to afford 7f–7h in 87–90% yields. Reaction with 1-indanone yielded 7i in 81% yield, while 4-chlorophenylhydrazine hydrochloride with the same ketones furnished 7j–7l in 86–89% yields, demonstrating the broad applicability of the protocol across diverse arylhydrazine and cyclic ketone substrates.
Further studies with electron-withdrawing arylhydrazine hydrochloride revealed similar trends. 2-Bromophenylhydrazine hydrochloride reacted smoothly with cyclohexanone, cycloheptanone, and 3,3-dimethylcyclohexanone to afford 7m–7o in 88–91% yields. Likewise, 4-bromophenylhydrazine hydrochloride provided 7p–7s in 84–92% yields with cyclopentanone, cyclohexanone, cycloheptanone, and 4-methylcyclohexanone, highlighting the method's tolerance toward sterically and electronically varied hydrazine hydrochloride and ketones.
The scope was further extended to electron-donating arylhydrazines. 4-Methylphenylhydrazine hydrochloride furnished 7t and 7u in 91% and 88% yields with cyclopentanone and 3,3-dimethylcyclohexanone, respectively, while 1-indanone delivered 7v in 83% yield. 4-Methoxyphenylhydrazine hydrochloride reacted with cyclopentanone to provide 7w in 86% yield. In our efforts to access interrupted Fischer indole products, the non-aromatic keto ester ethyl 2-oxocyclohexanecarboxylate was examined. Reaction with phenylhydrazine hydrochloride furnished 7x in 87% yield, and 4-bromophenylhydrazine hydrochloride afforded 7y in 89% yield.
Heterocyclic ketones were also well tolerated. Phenylhydrazine hydrochloride reacted with dihydro-3(2H)-thiophenone and tetrahydro-4H-thiopyran-4-one to give 7z and 7aa in 83% and 85% yields, respectively, while 2-chlorophenylhydrazine hydrochloride afforded 7ab and 7ac in 79% and 77% yields. Tetrahydro-4H-thiopyran-4-one, thiochroman-4-one, and dihydro-3(2H)-thiophenone were further coupled with substituted phenylhydrazines (4-Cl, 4-Br, 4-Me, 4-OMe) to furnish 7ad–7aj in 71–91% yields, highlighting the broad tolerance of heterocyclic ketones under the standard conditions. Finally, various aliphatic ketones were explored with phenylhydrazine hydrochloride. Propionaldehyde afforded 7ak in 92% yield, propanone and 2-butanone gave 7al and 7am in 93% and 91% yields, respectively, and the aromatic ketone 4-phenylbutan-2-one furnished 7an in 90% yield. Aliphatic hydroxyaldehydes, including 4-hydroxybutanal and 5-hydroxypentanal, yielded tryptophan-derived products 7ao and 7ap in 89% and 90% yields. Additionally, N-methylphenylhydrazine hydrochloride reacted with pyruvic acid to deliver the corresponding indole derivative 7aq in 72% yield.
Collectively, these results highlight the broad substrate scope and remarkable functional group tolerance of the ion-pair-catalysed Fischer indole protocol, enabling efficient access to a diverse array of carbazole and indole derivatives from aromatic and aliphatic ketones, heterocyclic ketones, and substituted hydrazine hydrochlorides.
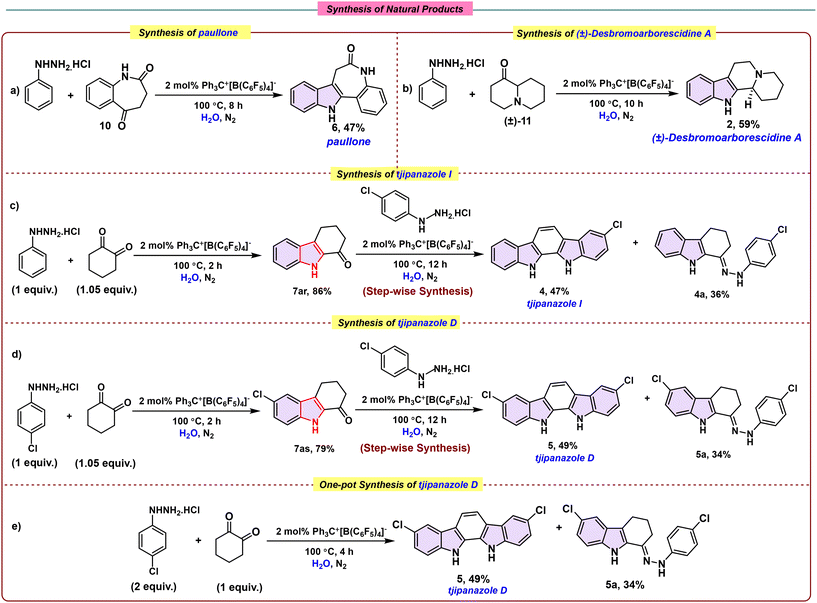
Encouraged by the efficiency and breadth of the ion-pair-catalysed Fischer indole synthesis across diverse substrates, we next sought to demonstrate its utility in the streamlined preparation of complex, bioactive targets. In particular, we targeted the concise synthesis of selected carbazole-based natural products and bioactive analogues, thereby underscoring the method's applicability beyond simple substrates and highlighting its potential as a versatile platform for heterocyclic natural product synthesis. As an initial demonstration, we targeted paullone (6), a bioactive scaffold of notable pharmacological relevance. The required precursor, 3,4-dihydro-1H-benzo[b]azepine-2,5-dione (10), was obtained following a well-established literature protocol.53 Subjecting this intermediate to our optimized ion-pair-catalysed Fischer indole conditions with phenylhydrazine hydrochloride furnished paullone (6) in 47% yield after 8 hours (Scheme 3a). This example underscores the capacity of our strategy to streamline the construction of complex natural products, like heterocycles in a late-stage fashion.52
Building on this success, we applied the optimized ion-pair-catalysed Fischer indole protocol to the synthesis of the natural product (±)-desbromoarborescidine A (2). The required ketone precursor, hexahydro-2H-quinolizin-1(6H)-one ((±)-11), was subjected to phenylhydrazine hydrochloride under the same conditions (Scheme 3b). The reaction proceeded cleanly to afford the desired indolocarbazole framework as a single regioisomer (2), which was isolated in a good yield of 59% and fully characterized.52 The high regioselectivity observed in this transformation underscores the capacity of the [Ph3C]+[B(C6F5)4]− system to direct dehydrative cyclization in structurally complex settings, validating the method's utility for concise access to bioactive carbazole alkaloids.
Following these successes, we further extended the utility of our ion-pair-catalyzed Fischer indole protocol to the synthesis of the natural products tjipanazole I (4) and tjipanazole D (5), underscoring the method's broad applicability to structurally diverse carbazole alkaloids. The respective phenylhydrazine hydrochlorides were initially reacted with 1,2-cyclohexanedione under the optimized conditions, delivering the carbazole intermediates 7ar and 7as in excellent yields of 86% and 79%, respectively. Subsequent treatment of these intermediates with an additional batch of arylhydrazine hydrochloride afforded the natural products tjipanazole I (5) and tjipanazole D (4) in 47% and 49% yields, respectively, accompanied by the corresponding imine side products in 36% and 34% yields, highlighting the efficiency and modularity of the stepwise strategy (Scheme 3c & d). Building on the efficiency of the stepwise protocol, we further investigated a one-pot synthesis of tjipanazole D. In this approach, two equivalents of 4-chlorophenylhydrazine hydrochloride were reacted with one equivalent of 1,2-cyclohexanedione over 12 h, providing tjipanazole D in 49% yield along with the corresponding imine side product in 34%, thereby demonstrating that the transformation can be achieved in a single operational step while maintaining comparable efficiency (Scheme 3e).
We also measured the pH of the solution of the ion pair in water at a molarity of 1.08 × 10−4 M, prior to initiating the reaction. The observed value of 2.92 clearly reflects its superacidic nature, supporting the notion that it generates highly acidic species in situ to drive the catalytic process.
We have proposed a plausible mechanism in which the catalytically active (H2O)nH+[B(C6F5)4]− species is generated in situ under the reaction conditions and is essential for facilitating the Fischer indole synthesis. As depicted in Scheme 4, hydrolysis of the triaryl-carbenium ion pair in water furnishes hydrated (H2O)nH+[B(C6F5)4]− along with triphenylmethanol.
The resulting Brønsted acidic ion pair, containing the weakly coordinating [B(C6F5)4]− counter anion, acts as a strong yet non-nucleophilic proton source that efficiently activates the arylhydrazone intermediate through protonation at nitrogen. This activation promotes tautomerization to the reactive ene-hydrazine species, which undergoes the key [3,3]-sigmatropic rearrangement to generate the cyclohexadienyl iminium intermediate. Stabilization of this highly electrophilic species by the ion pair enables intramolecular electrophilic cyclization to form the indolenine scaffold. Subsequent N–N bond cleavage, accompanied by proton transfers and re-aromatization, affords the indole product while regenerating the (H2O)nH+[B(C6F5)4]− catalyst to complete the cycle.
To further validate the practicality of our ion-pair-catalysed Fischer indole protocol, we performed a scale-up reaction using 4-bromophenyl hydrazine hydrochloride (6.71 mmol, 1.0 equiv.) and 3-methylcyclohexanone (7.05 mmol, 1.05 equiv.) as representative substrates using 2 mol% of [Ph3C]+[B(C6F5)4]− as the catalyst (Scheme 5).52
Under the optimized conditions, the reaction was carried out on a gram scale, affording the corresponding carbazole derivative 6-bromo-2-methyl-2,3,4,9-tetrahydro-1H-carbazole (7at) in an excellent yield of 1.4 g (79%) with no compromise in reaction efficiency or selectivity. This successful scale-up highlights the robustness and operational simplicity of the catalytic system, demonstrating its suitability for larger-scale synthesis and potential applicability in preparative and industrial contexts.
Conclusion
We report the first application of the ion pair [Ph3C]+[B(C6F5)4]− in the Fischer indole synthesis, enabling the efficient and regioselective formation of diverse indole and carbazole frameworks under mild, aqueous, metal-free conditions. The method shows broad functional group tolerance, enables concise total syntheses of bioactive targets, and scales efficiently without loss of performance. Using water as a green solvent and operating via an in situ generated Brønsted acidic ion pair, this protocol offers a practical, sustainable platform for rapid access to valuable heterocycles.Conflicts of interest
There are no conflicts to declare.Data availability
Supplementary information (SI): experimental procedures and characterization data (NMR, IR & HRMS) for all synthesized compounds. Additional spectral images are included to support the findings discussed in the paper. See DOI: https://doi.org/10.1039/d5gc04707b.Acknowledgements
The authors sincerely acknowledge CSIR-CLRI for the infrastructure, facilities, and consistent institutional support that enabled this research. The authors are deeply grateful to the Director, CSIR-CLRI, for his continued encouragement and valuable guidance throughout the course of this work. SC likes to gratefully acknowledge the financial support from ANRF-PMECRG (Anusandhan National Research Foundation), Govt. of India, File No. ANRF/ECRG/2024/002259/CS (GAP2504). MKD gratefully acknowledges the KPDC for providing essential research infrastructure. The CSIR-CLRI communication number allotted for this work is 2187.References
- M. Gomberg, J. Am. Chem. Soc., 1900, 22, 757–771 CrossRef.
- M. Gomberg, J. Am. Chem. Soc., 1901, 23, 496–502 CrossRef.
- M. Gomberg, J. Am. Chem. Soc., 1902, 24, 597–628 CrossRef.
- J. F. Norris, Am. Chem. J., 1901, 25, 117–122 CAS.
- F. Kehrmann and F. Wentzel, Ber. Dtsch. Chem. Ges., 1901, 34, 3815–3819 CrossRef CAS.
- M. R. Feldman and W. C. Flythe, J. Org. Chem., 1978, 43, 2596–2600 CrossRef CAS.
- T. Mukaiyama, S. Kobayashi and S.-I. Shoda, Chem. Lett., 1984, 13, 907–910 CrossRef.
- R. C. Rathore, L. Burns and I. A. Guzei, J. Org. Chem., 2004, 69, 1524–1530 CrossRef CAS PubMed.
- Y. Kitazawa, R. Takita, K. Yoshida, A. Muranaka, S. Matsubara and M. Uchiyama, J. Org. Chem., 2017, 82, 1931–1935 CrossRef CAS PubMed.
- (a) T. Mukaiyama, S. Kobayashi and S.-I. Shoda, Chem. Lett., 1984, 13, 1529–1530 CrossRef; (b) T. Mukaiyama, S. Kobayashi and M. Murakami, Chem. Lett., 1984, 13, 1759–1762 CrossRef; (c) T. Mukaiyama, S. Kobayashi and M. Murakami, Chem. Lett., 1985, 14, 447–450 CrossRef; (d) S. Kobayashi, M. Murakami and T. Mukaiyama, Chem. Lett., 1985, 14, 953–956 CrossRef.
- (a) T. Mukaiyama, H. Nagaoka, M. Murakami and M. Ohshima, Chem. Lett., 1985, 14, 977–980 CrossRef; (b) S. Kobayashi, M. Murakami and T. Mukaiyama, Chem. Lett., 1985, 14, 1535–1538 CrossRef; (c) M. Ohshima, M. Murakami and T. Mukaiyama, Chem. Lett., 1985, 14, 1871–1874 CrossRef; (d) S. Kobayashi, S. Matsui and T. Mukaiyama, Chem. Lett., 1988, 17, 1491–1494 CrossRef; (e) M. Yanagisawa and T. Mukaiyama, Chem. Lett., 2001, 30, 224–225 CrossRef.
- C.-T. Chen, S. D. Chao, K.-C. Yen, C.-H. Chen, I.-C. Chou and S.-W. Hon, J. Am. Chem. Soc., 1997, 119, 11341–11342 CrossRef CAS.
- B. Wigman, S. Popov, A. L. Bagdasarian, B. Shao, T. R. Benton, C. G. Williams, S. P. Fisher, V. Lavallo, K. N. Houk and H. M. Nelson, J. Am. Chem. Soc., 2019, 141, 9140–9144 CrossRef CAS PubMed.
- S. Popov, B. Shao, A. L. Bagdasarian, T. R. Benton, L. Zou, Z. Yang, K. N. Houk and H. M. Nelson, Science, 2018, 361, 381–387 CrossRef CAS.
- (a) J. Lv, Q. Zhang, X. Zhong and S. Luo, J. Am. Chem. Soc., 2015, 137, 15576–15583 CrossRef CAS; (b) Q. Zhang, J. Lv, S. Li and S. Luo, Org. Lett., 2018, 20, 2269–2272 CrossRef CAS.
- T. He, G. Wang, V. Bonetti, H. F. T. Klare and M. Oestreich, Angew. Chem., Int. Ed., 2020, 59, 12186–12191 CrossRef CAS PubMed.
- E. Y. Chen and T. J. Marks, Chem. Rev., 2000, 100, 1391–1434 CrossRef CAS.
- X. Yang, C. L. Stern and T. J. Marks, J. Am. Chem. Soc., 1991, 113, 3623–3625 CrossRef CAS.
- M. Vathauer and W. Kaminsky, Polymer, 2001, 42, 4017–4024 CrossRef CAS.
- V. D. Makhaev, A. N. Galiullin, E. E. Faingol'd, N. M. Bravaya and L. A. Petrova, Russ. Chem. Bull., 2014, 63, 651–656 CrossRef CAS.
- M. Israr, W. H. Kim, D. H. Kim and H. Y. Bae, Chem. Catal., 2024, 4, 100866 CAS.
- (a) X. Shi, M. Nishiura and Z. Hou, Angew. Chem., Int. Ed., 2016, 55, 14812–14817 CrossRef CAS PubMed; (b) G. Song, G. Luo, J. Oyamada, Y. Luo and Z. Hou, Chem. Sci., 2016, 7, 5265–5270 RSC; (c) Y. Luo, H. L. Teng, M. Nishiura and Z. Hou, Angew. Chem., Int. Ed., 2017, 56, 9207–9210 CrossRef CAS PubMed.
- (a) H. Wang, Y. Zhao, M. Nishiura, Y. Yang, G. Luo, Y. Luo and Z. Hou, J. Am. Chem. Soc., 2017, 141, 12624–12633 CrossRef; (b) H. Wang, X. Wu, Y. Yang, M. Nishiura and Z. Hou, Angew. Chem., Int. Ed., 2020, 59, 7173–7177 CrossRef CAS; (c) F. Guo, L. Jiang, K. Diao and Z. Hou, Polym. Chem., 2020, 11, 1314–1320 RSC; (d) Y. Ma, S. J. Lou and Z. Hou, Chem. Soc. Rev., 2021, 50, 1945–1967 RSC.
- P. Jutzi, C. Muller, A. Stammler and H. G. Stammler, Organometallics, 2000, 19, 1442–1444 CrossRef CAS.
- L. L. Anderson, J. Arnold and R. G. Bergman, J. Am. Chem. Soc., 2005, 127, 14542–14543 CrossRef CAS PubMed.
- X. Cai, A. Keshavarz, J. D. Omaque and B. J. Stokes, Org. Lett., 2017, 19, 2626–2629 CrossRef CAS.
- W. Zhu, Q. Sun, Y. Wang, D. Yuan and Y. Yao, Org. Lett., 2018, 20, 3101–3104 CrossRef CAS PubMed.
- H. Jin, H. M. Rudolph, F. Rominger and A. S. K. Hashmi, ACS Catal., 2019, 9, 11663–11668 CrossRef CAS.
- Q. Zhang, J. Lv, S. Li and S. Luo, Org. Lett., 2018, 20, 2269–2272 CrossRef CAS PubMed.
- Z. L. Shen, S. J. Ji and T.-P. Loh, Tetrahedron Lett., 2005, 46, 507–508 CrossRef CAS.
- (a) Z. Zhang, X. Liu, L. Ji, T. Zhang, Z. Jia and T.-P. Loh, ACS Catal., 2022, 12, 2052–2057 CrossRef CAS; (b) Z. Zhang, L. Ji, X. Liu, Z. Li, Y. Lv, Z. Jia and T.-P. Loh, Org. Chem. Front., 2022, 9, 5154–5159 RSC; (c) Z. Zhang, J. Gu, Y. Lv, L. Ji, X. Liu, B. Wu, F. Liu, Z. Jia and T.-P. Loh, Cell Rep. Phys. Sci., 2023, 4, 101246 CrossRef CAS.
- (a) Z. Zhang, J. Gu, L. Ji, X. Liu, T. Zhang, Y. Lv, F. Liu, Z. Jia and T.-P. Loh, ACS Catal., 2022, 12, 14123–14129 CrossRef CAS; (b) Z. Zhang, Y. Lv, L. Ji, P. Chen, S. Han, Y. Zhu, L. Li, Z. Jia and T.-P. Loh, Angew. Chem., Int. Ed., 2024, 63, e202406588 CrossRef CAS PubMed; (c) Z. Zhang, Y. Lv, W. Q. R. Ong, X. Zhao, Z. Jia and T.-P. Loh, Angew. Chem., Int. Ed., 2024, 63, e202408509 CrossRef CAS.
- Y. Zhu, C. Yang, Q. Lin, J. Li, T.-P. Loh, P. Chen and Z. Jia, Org. Lett., 2025, 27, 2110–2115 CrossRef CAS.
- (a) A. Saidykhan, W. H. Martin, R. T. Gallagher, J. Kendrick and R. D. Bowen, J. Mol. Struct., 2023, 1294, 136311 CrossRef CAS; (b) C. Bosch, P. López-Lledó, J. Bonjoch, B. Bradshaw, P. J. Nieuwland, D. Blanco-Ania and F. P. Rutjes, J. Flow Chem., 2016, 6, 240–243 CrossRef CAS; (c) S. Ali Ghumro, R. D. Alharthy, M. Al-Rashida, S. Ahmed, M. I. Malik and A. Hameed, ACS Omega, 2017, 2, 2891–2900 CrossRef CAS PubMed; (d) M. Bashkar, N. Nowrouzi and M. R. Mohammadizadeh, Appl. Organomet. Chem., 2023, 37, e7105 CrossRef CAS; (e) D. Q. Xu, J. Wu, S. P. Luo, J. X. Zhang, J. Y. Wu, X. H. Du and Z. Y. Xu, Green Chem., 2009, 11, 1239–1246 RSC; (f) M. C. da Silva Mendes, B. R. Fazolo, J. M. de Souza, L. G. de Vasconcelos, P. T. de Sousa, E. L. Dall'Oglio and L. C. C. Vieira, Photochem. Photobiol. Sci., 2019, 18, 1350–1358 CrossRef PubMed; (g) D. Bhattacherjee, S. Ram, A. S. Chauhan, Yamini, Sheetal and P. Das, Chem. – Eur. J., 2019, 25, 5934–5939 CrossRef CAS PubMed.
- (a) M. K. Lakshman, D. Sebastian, P. Pradhan, M. C. Neary, A. M. Piette, S. P. Trzebiatowski and P. H. Willoughby, Chem. – Eur. J., 2023, 29, e202302995 CrossRef CAS; (b) H. Y. Bi, C. J. Li, C. Wei, C. Liang and D. L. Mo, Green Chem., 2020, 22, 5815–5821 RSC; (c) S. Ma, D. Long, P. Chen, H. Shi, H. Li, R. Fang and X. She, Org. Chem. Front., 2020, 7, 2689–2695 RSC; (d) O. Dilek, S. Patir, T. Tilki and E. Erturk, J. Org. Chem., 2019, 84, 7901–7916 CrossRef CAS PubMed; (e) A. Dierks, L. Fliegel, M. Schmidtmann and J. Christoffers, Eur. J. Org. Chem., 2020, 7164–7175 CrossRef CAS; (f) A. Khan, M. G. Sarwar and S. Ali, Molecules, 2024, 29, 501 CrossRef CAS PubMed; (g) A. Porcheddu, R. Mocci, M. Brindisi, F. Cuccu, C. Fattuoni, F. Delogu, E. Colacino and M. V. d'Auria, Green Chem., 2022, 24, 4859–4869 RSC.
- (a) S. Kotha, M. Saifuddin and V. R. Aswar, Org. Biomol. Chem., 2016, 14, 9868–9873 RSC; (b) S. Kotha, M. Salman and S. R. Cheekatla, ChemistrySelect, 2024, 9, e202402530 CrossRef CAS; (c) S. Gore, S. Baskaran and B. König, Org. Lett., 2012, 14, 4568–4571 CrossRef CAS PubMed; (d) Y. L. Hu, D. Fang and D. S. Li, Catal. Lett., 2016, 146, 968–976 CrossRef CAS; (e) B. Y. Lim, B. E. Jung and C. G. Cho, Org. Lett., 2014, 16, 4492–4495 CrossRef CAS PubMed; (f) V. H. Thorat, H. Y. Chao, P. H. Yang and J. C. Hsieh, Eur. J. Org. Chem., 2024, e202401194 Search PubMed.
- (a) R. P. Thummel and V. Hegde, J. Org. Chem., 1989, 54, 1720–1725 CrossRef CAS; (b) V. Hegde, P. Madhukar, J. D. Madura and R. P. Thummel, J. Am. Chem. Soc., 1990, 112, 4549–4550 CrossRef CAS; (c) X. Yang, X. Zhang and D. Yin, Org. Process Res. Dev., 2018, 22, 1115–1118 CrossRef CAS; (d) R. A. Duval and J. R. Lever, Green Chem., 2010, 12, 304–309 RSC.
- (a) K. R. Campos, J. C. S. Woo, S. Lee and R. D. Tillyer, Org. Lett., 2004, 6, 79 CrossRef CAS PubMed; (b) I.-K. Park, S.-E. Suh, B.-Y. Lim and C.-G. Cho, Org. Lett., 2009, 11, 5454–5456 CrossRef CAS PubMed; (c) S. Gore, S. Baskaran and B. König, Org. Lett., 2012, 14, 4568–4571 CrossRef CAS PubMed; (d) K. Matsumoto, A. Tanaka, I. Yukio, N. Hayashi, M. Toda and R. A. Bulman, Heterocycl. Commun., 2003, 9, 9–12 CAS; (e) P. Linnepe, A. M. Schmidt and P. Eilbracht, Org. Biomol. Chem., 2006, 4, 302–313 RSC.
- (a) D.-Q. Xu, J. Wu, S.-P. Luo, J.-X. Zhang, J.-Y. Wu, X.-H. Du and Z.-Y. Xu, Green Chem., 2009, 11, 1239–1246 RSC; (b) S. Müller, M. J. Webber and B. List, J. Am. Chem. Soc., 2011, 133, 18534–18537 CrossRef PubMed; (c) K. G. Liu, A. J. Robichaud, J. R. Lo, J. F. Mattes and Y. Cai, Org. Lett., 2006, 8, 5769–5771 CrossRef CAS PubMed.
- (a) S. Govaerts, K. Nakamura, T. Constantin and D. Leonori, Org. Lett., 2022, 24, 7883–7887 CrossRef CAS PubMed; (b) L. Ackermann and R. Born, Tetrahedron Lett., 2004, 45, 9541–9544 CrossRef CAS; (c) B. A. Haag, Z.-G. Zhang, J.-S. Li and P. Knochel, Angew. Chem., Int. Ed., 2010, 49, 9513 CrossRef CAS; (d) S. Wagaw, B. H. Yang and S. L. Buchwald, J. Am. Chem. Soc., 1998, 120, 6621–6622 CrossRef CAS.
- (a) M. Nakazaki and K. Yamamoto, J. Org. Chem., 1976, 41, 1877 CrossRef CAS; (b) T. M. Lipińska and S. J. Czarnocki, Org. Lett., 2006, 8, 367 CrossRef; (c) F. Zhan and G. Liang, Angew. Chem., Int. Ed., 2013, 52, 1266 CrossRef CAS PubMed; (d) G. L. Rebeiro and B. M. Khadilkar, Synthesis, 2001, 370 CrossRef CAS; (e) A. Sudhakaraa, H. Jayadevappab, H. N. H. Kumarc and K. M. Mahadevan, Lett. Org. Chem., 2009, 6, 159 CrossRef; (f) G. Baccolini and P. E. Todesco, J. Chem. Soc., Perkin Trans. 1, 1983, 1, 535–538 RSC.
- (a) D.-Q. Xu, W.-L. Yang, S.-P. Luo, B.-T. Wang, J. Wu and Z.-Y. Xu, Eur. J. Org. Chem., 2007, 1007–1012 CrossRef CAS; (b) R. M. Eduque Jr and E. C. Creencia, Procedia Chem., 2015, 16, 413–419 CrossRef; (c) F. P. Yi, H. Y. Sun, X. H. Pan, Y. Xu and J. Z. Li, Chin. Chem. Lett., 2009, 20, 275–278 CrossRef CAS; (d) P. Maity and A. K. Mitra, J. Ionic Liq., 2025, 100146 CrossRef; (e) T. O. S. Kumar and K. M. Mahadevan, Org. Commun., 2013, 6, 31 Search PubMed; (f) J. Yu, J. Xu, Z. Yu, Y. Jin, J. Li and Y. Lv, J. Flow Chem., 2017, 7, 33–36 CrossRef CAS; (g) W. C. Neuhaus, I. J. Bakanas, J. R. Lizza, C. T. Boon and G. Moura-Letts, Green Chem. Lett. Rev., 2016, 9, 39–43 CrossRef CAS; (h) R. C. Morales, V. Tambyrajah, P. R. Jenkins, D. L. Davies and A. P. Abbott, Chem. Commun., 2004, 158–159 RSC; (i) G. L. Rebeiro and B. M. Khadilkar, Synthesis, 2001, 370–372 CrossRef CAS.
- A. W. Schmidt, K. R. Reddy and H.-J. Knölker, Chem. Rev., 2012, 112, 3193–3328 CrossRef CAS PubMed.
- (a) D. Bansal, G. Nataraj, P. Sivaganesan, M. K. Das and S. Chaudhuri, Chem. – Asian J., 2024, 19, e202401025 CrossRef CAS; (b) P. Sivaganesan, C. Elanchezhian, D. Bansal, J. Sarkar and S. Chaudhuri, New J. Chem., 2024, 48, 20034–20040 RSC; (c) D. Bansal, G. Nataraj, C. Elanchezhian, P. Sivaganesan, M. K. Das and S. Chaudhuri, Chem Asian J., 2025, 20, e202401437 CrossRef CAS PubMed.
- (a) P. Sivaganesan, L. Ganeshan, S. Vl, S. Pal, S. Dam, J. Sarkar and S. Chaudhuri, ChemistrySelect, 2025, 10, e202404705 CrossRef CAS; (b) P. Sivaganesan, S. Vl, A. Sahoo, C. Elanchezhian, G. Nataraj and S. Chaudhuri, ChemistrySelect, 2024, 9, e202403112 CrossRef CAS; (c) D. Bansal, P. Sivaganesan, C. Elanchezhian, G. Nataraj, M. K. Das and S. Chaudhuri, Eur. J. Org. Chem., 2025, e202401488 CrossRef CAS; (d) C. Elanchezhian, D. Bansal, P. Sivaganesan, G. Nataraj, M. K. Das and S. Chaudhuri, Tetrahedron Lett., 2025, 160, 155529 CrossRef CAS.
- (a) P. Sivaganesan, D. Bansal, C. Elanchezhian, G. Nataraj, M. K. Das and S. Chaudhuri, Asian J. Org. Chem., 2025, 14, e202500327 CrossRef CAS; (b) P. Sivaganesan, D. Bansal, N. A. Nazar, M. K. Das and S. Chaudhuri, Chem. – Asian J., 2025, 20, e202500498 CrossRef CAS PubMed; (c) D. Bansal, P. Sivaganesan, G. Nataraj, C. Elanchezhian, M. K. Das and S. Chaudhuri, Tetrahedron Lett., 2025, 165–166, 155660 CrossRef CAS.
- (a) W. Chen and H. Zhang, Sci. China: Chem., 2016, 59, 1065–1078 CrossRef CAS; (b) F. Zhao, N. Li, Y.-F. Zhu and Z.-Y. Han, Org. Lett., 2016, 18, 1506–1509 CrossRef CAS PubMed and references therein.
- (a) S. A. Doggrell and J. C. Hancox, Expert Opin. Drug Saf., 2013, 12, 421–431 CrossRef CAS PubMed; (b) P. Bernardo and C. Chai, Bioorg. Med. Chem. Lett., 2007, 17, 82–85 CrossRef CAS PubMed; (c) S. Peng, L. Wang, J. Huang, S. Sun, H. Guo and J. Wang, Adv. Synth. Catal., 2013, 355, 2550–2557 CrossRef CAS.
- S. Akinga, K. Sugiyama and T. Akiyama, Anti-Cancer Drug Des., 2000, 15, 43–52 Search PubMed.
- D. A. Horton, G. T. Bourne and M. L. Smythe, Chem. Rev., 2003, 103, 893–930 CrossRef CAS PubMed.
- D. Bansal, P. Sivaganesan, C. Elanchezhian, G. Nataraj, M. K. Das and S. Chaudhuri, Chem. – Asian J., 2025, 20, e202500246 CrossRef CAS.
- See the SI for the experimental data of the synthesized compounds.
- J. Huang, Q. Shi, N. Choudhry, H. Li, C. Yang, J. Kalashova, Z. Yan, J. Li, M. C. Reddy, S. G. Gopala and S. Zhang, ACS Med. Chem. Lett., 2022, 13, 1091–1098 CrossRef CAS PubMed.
Footnote |
| † These authors have contributed equally. |
| This journal is © The Royal Society of Chemistry 2026 |