Low indoor light-driven CO2 conversion into visible C4 bioplastic via homogeneous non-metal-based biohybrids under photoexcitation
Wenjing
Wang
 *a,
Mingzhi
Zhang
a,
Meng
Guo
a,
Jiaxin
Wang
a,
Xuelian
Wang
a,
Jianheng
Yin
a,
Liang
Chen
b and
Yaguang
Li
*a,
Mingzhi
Zhang
a,
Meng
Guo
a,
Jiaxin
Wang
a,
Xuelian
Wang
a,
Jianheng
Yin
a,
Liang
Chen
b and
Yaguang
Li
 *c
*c
aCollege of Life Sciences, Engineering Research Center of Ecological Safety and Conservation in Beijing-Tianjin-Hebei (Xiong'an New Area) of MOE, Institute of Life Sciences and Green Development, Hebei University, Baoding 071002, China. E-mail: wangwenjing@hbu.edu.cn
bSchool of Energy, Power and Mechanical Engineering, North China Electric Power University, Baoding 071000, China
cResearch Center for Solar Driven Carbon Neutrality, Engineering Research Center of Zero-carbon Energy Buildings and Measurement Techniques, Ministry of Education, The College of Physics Science and Technology, Hebei University, Baoding, 071002, China. E-mail: liyaguang@hbu.edu.cn
First published on 28th November 2025
Abstract
The conversion of CO2 into C4 bioplastic through biohybrid systems comprising microorganisms and non-metal-based photocatalysts represents a promising strategy for high-value carbon utilization. However, existing non-metal-based biohybrids necessitate the addition of auxiliary agents or heterotrophic carbon sources due to the limited contact and interfacial mass transfer caused by the aggregation of photocatalysts and bacteria. In this work, a homogeneously autotrophic K/O co-doped g-C3N4 (K/O-CN) and Ralstonia eutropha (R. eutropha) biohybrid was developed to efficiently convert CO2 into poly-β-hydroxybutyrate (PHB) without any auxiliary agents. Under low indoor light of 4000 lux (0.75 mW cm−2), the K/O-CN–R. eutropha biohybrid achieved a PHB yield of 49.35 ± 0.85 mg L−1 d−1 surpassing non-metal-based biohybrids with added co-factors and reached a quantum efficiency of 5.88 ± 0.16% exceeding most metal-based biohybrids. The K/O co-doping changed stacked bulk layers to nanorods, which enveloped the bacterial cells with uniform dispersion, establishing a tight interaction, strong coupling, and effective electron transfer between them. Upon photoexcitation, K/O-CN catalyzed H2O splitting to generate H2. Intracellularly, H2 was oxidized to H+, which participated in ATP synthesis and generated additional reducing equivalents (NADPH), thereby enhancing the carbon metabolic cycle and promoting the formation of key intermediates, ultimately driving the conversion of CO2 to PHB. This work offers a streamlined and economically viable pathway for obtaining C4 products from CO2 and providing key insights into interfacial mass transfer and carbon metabolism changes within the biohybrid.
Green foundation1. Our work advances green chemistry by enabling efficient, additive-free CO2-to-C4 bioplastic (PHB) conversion under low indoor light using a novel photocatalyst/bacterium biohybrid. The process allows for simple one-step PHB extraction, supporting low-energy, renewable, and environmentally friendly CO2 utilization.2. This system achieved a PHB yield of 49.35 ± 0.85 mg L−1 d−1, surpassing non-metal-based biohybrids with added co-factors. The system uses a K/O-CN photocatalyst, which is non-toxic to microorganisms, ensuring biosafety and compatibility. Integrated with R. eutropha, a bacterium capable of self-replication, the system maintains high catalytic activity. 3. This system would become even greener when applied to large-scale CO2 utilization and PHB production driven by widespread natural sunlight. |
1. Introduction
Converting carbon dioxide into multi-carbon products enhances economic value and broadens the scope of carbon utilization. Artificial photocatalytic CO2 reduction harnesses solar irradiation to activate photocatalysts for CO2 conversion.1,2 Numerous studies have employed strategies such as heterojunction construction,3–7 composite formation,8,9 and elemental doping10 to enhance the CO2 photoreduction performance of catalysts such as g-C3N4, achieving considerable progress. This approach can effectively convert CO2 into C1 and C2,11 while the efficiency and selectivity for longer-chain products remain markedly low.12,13 Natural carbon fixation of microorganisms offers a highly selective pathway for CO2 conversion into multi-carbon products, yet its energy utilization efficiency is limited to 1%–3%.14,15 The integration of artificial photocatalysis with microbial carbon fixation enables the multi-carbon conversion of CO2 and augments the overall efficiency.16–18 Currently, photocatalyst–microbe biohybrids, including CdS–Methanosarcina barkeri,19 Cd0.8Zn0.2S–Sporomusa ovata (S. ovata),20 CdS–S. ovata,21 Zr-MOF–Moorella thermoacetica (M. thermoacetica),22 and intracellular gold nanocluster–M. thermoacetica,23 can convert CO2 into value-added products such as CH4, acetate, and acetic acid.19–21,23 Photocatalyst–microbe biohybrids enable direct transfer between the photocatalyst and microbe within a single reactor, making the system integrated and reducing operational costs.Poly-β-hydroxybutyrate (PHB, C4H6O2) is a biodegradable plastic that offers a sustainable alternative to conventional petroleum-based plastics. In 2024, the global market size for bioplastics is estimated at approximately 2.09 million tons.24 Converting CO2 into PHB, valued at $4000–$6000 per ton,25 presents a promising strategy for achieving high economic returns in carbon utilization. Ralstonia eutropha (R. eutropha) utilizes CO2 for autotrophic growth, concurrently producing and accumulating hydrophobic PHB within its cytoplasm, which can be easily isolated and extracted. The existing methods to promote PHB production in R. eutropha primarily focus on optimizing culture conditions and genetic engineering. Compared with these methods, photocatalyst–R. eutropha biohybrids require simple operation with the incorporation of a photocatalyst and light to improve PHB production.
Few photocatalyst–R. eutropha biohybrids have been developed for PHB production from CO2 using ZnSe/ZnS,26 metal quantum dots,27 g-C3N4-catalase,28 or organic polymer dots.29 It is still challenging to resolve the biotoxicity of metal photocatalysts and the need for auxiliary additives in non-metal systems to achieve an ideal PHB yield. Non-metal g-C3N4 with stable physicochemical properties and excellent biocompatibility30,31 was used to construct hybrid systems aimed at PHB production. However, existing g-C3N4-based biohybrids primarily employ bulk g-C3N4 to construct a heterotrophic system utilizing fructose rather than CO2 as the carbon source,32 or a g-C3N4-catalase–R. eutropha system with additive H2O2-degrading enzymes.28 The bulk g-C3N4 aggregates with bacterial cells hindering mass transfer at the abiotic–biotic interface. Furthermore, the changes of the overall metabolic profile and the intermediate metabolite in the biohybrids remain unclear.
To address these challenges, this study designs a uniformly dispersed g-C3N4–R. eutropha autotrophic biohybrid to efficiently drive CO2 conversion to PHB without the need for any auxiliary agents under low indoor light irradiation. The system introduces nano-sized, scattered rod-like K/O co-doped g-C3N4 (K/O-CN) as a replacement for bulk g-C3N4, thereby increasing the interface contact and mass transfer at the abiotic–biotic interface. R. eutropha has a unique carbon metabolism pathway to utilize CO2 to produce C4 PHB and is capable of using H2 as an electron donor, which makes it feasible to couple with the K/O-CN in a tandem system. In addition to assessing the final PHB product and coenzyme NADPH, this study conducts a comprehensive analysis of all metabolic intermediates and products within the biohybrid. The green-chemistry advancement of the K/O-CN–R. eutropha biohybrid lies in establishing a novel autotrophic, photo-driven paradigm for PHB production directly from CO2, which operates without the need for any enzymes or electron mediators.
2. Materials and methods
2.1 Synthesis of PCN and K/O-CN
The melamine (99%), lithium chloride (LiCl, 99%), and potassium chloride (KCl, ≥99.5%) used in the synthesis of the photocatalyst were all supplied by Macklin Reagent Co., Ltd. The preparation procedure of K/O-CN is similar to that in the previous report.33 The specific preparation procedure was as follows: 5 g of melamine was placed in a porcelain boat and calcined at 550 °C for 4 h in a tube furnace under air, with a heating rate of 5 °C min−1. 1.90 g yellow pristine carbon nitride was obtained and denoted as PCN. Subsequently, 500 mg of PCN was thoroughly ground with 0.9 g of LiCl and 1.1 g of KCl in an agate mortar. The mixture was then placed in a porcelain boat and calcined at 550 °C for 4 h in a tube furnace under air, with a heating rate of 5 °C min−1. After cooling to room temperature, 0.47 g calcined product was obtained, which was washed 5 times with boiling water and dried in a vacuum oven at 60 °C to obtain K/O-CN. To investigate the effect of doping amount, the co-doped samples were subjected to treatments with varying amounts of LiCl (0.74 g and 1.06 g) and KCl (0.9 g and 1.3 g) and accordingly designated as K/O-CN-1 and K/O-CN-2, respectively. Only K-doped g-C3N4 (K-CN) was prepared by calcining the PCN under an Ar atmosphere rather than in air. Only the O-doped g-C3N4 (O-CN) sample was prepared by calcining the PCN in air without the treatment with LiCl and KCl.2.2 Cultivation of R. eutropha and construction of the photocatalytic biohybrid system
R. eutropha H16 (CGMCC 1.7092) was purchased from the China General Microbiological Culture Collection Center. The strain was sent to Sangon Biotech (Shanghai) Co., Ltd for identification, which confirmed it as R. eutropha. The relevant identification results are provided in Text S1. The composition of the culture media is detailed in Tables S1–S3. For the cultivation of microorganisms, the glycerol stock of R. eutropha stored at −80 °C was thawed and streaked onto a tryptic soy agar (TSA) plate, followed by incubation at 30 °C for 24 h in a constant temperature incubator. A well-grown single colony was then transferred into a tryptic soy broth (TSB) medium and cultured at 30 °C with shaking at 200 rpm under dark conditions for 24 h to enrich the culture. The bacterial culture was subsequently inoculated into a fructose medium at a 5% inoculum volume and incubated at 30 °C with shaking at 200 rpm in the dark for 12 h.For the construction of the biohybrid system, the fructose-pre-cultured bacterial suspension was centrifuged at 4 °C and 5000 rpm for 5 min to collect the bacterial cells. The cells were washed twice with a nitrogen-limited medium and then resuspended in 60 mL of nitrogen-limited medium containing various concentrations of K/O-CN (0.2–1 g L−1), without K/O-CN, or with triethanolamine (TEOA, 5 g L−1), ensuring an OD600 of 0.2. The bacterial suspension was transferred into 100 mL serum bottles (height 5.31 cm, radius 2.45 cm). The bottles were evacuated and injected with a gas mixture (N2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2
H2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO2
CO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O2 = 76
O2 = 76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) until atmospheric pressure was reached. The obtained systems were incubated under autotrophic conditions at 30 °C with shaking at 200 rpm. During the photocatalytic process, the culture was exposed to light at an intensity of 4000 lux. The light was turned off under dark conditions. The light intensity was tuned in the range of 3000–5000 lux. For light–dark cycle experiments, the light and dark conditions were alternated every 12 h to achieve the desired cyclic conditions. For the photocatalytic long-term operation experiment of 6 d, 10 mL of samples were taken every 24 h to measure the PHB content. After sampling, 10 mL of fresh nitrogen-limited medium was added to the reactor. The reactor was then evacuated and purged with a CO2-enriched mixed gas.
4) until atmospheric pressure was reached. The obtained systems were incubated under autotrophic conditions at 30 °C with shaking at 200 rpm. During the photocatalytic process, the culture was exposed to light at an intensity of 4000 lux. The light was turned off under dark conditions. The light intensity was tuned in the range of 3000–5000 lux. For light–dark cycle experiments, the light and dark conditions were alternated every 12 h to achieve the desired cyclic conditions. For the photocatalytic long-term operation experiment of 6 d, 10 mL of samples were taken every 24 h to measure the PHB content. After sampling, 10 mL of fresh nitrogen-limited medium was added to the reactor. The reactor was then evacuated and purged with a CO2-enriched mixed gas.
In the cyclic conditions, the biohybrid system after 48 h of cultivation was centrifuged to recover the photocatalyst, which was then thoroughly washed with deionized water and ethanol, dried, and subsequently reused in a new round of biohybrid reaction with the re-inoculation of R. eutropha. Owing to minor losses of the photocatalyst (approximately 15 wt%) during centrifugation and washing, a small amount of fresh K/O-CN was supplemented to maintain the optimal loading of 0.5 g L−1 in the biohybrid system.
2.3 Detection of PHB
10 mL of cultured bacterial suspension was taken every 12 h and centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min. To maintain the atmosphere and ensure adequate CO2 supply, after each 12 h sampling, the headspace of the reactor was first evacuated, and then a CO2-containing gas mixture (N2
000 rpm for 10 min. To maintain the atmosphere and ensure adequate CO2 supply, after each 12 h sampling, the headspace of the reactor was first evacuated, and then a CO2-containing gas mixture (N2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2
H2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO2
CO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O2 = 76
O2 = 76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) was refilled until atmospheric pressure was reached. The supernatant was discarded, and the cell pellet was washed twice with ultrapure water, followed by centrifugation to collect the cells. The collected cells were dried overnight at 55 °C. The dried cells were then transferred into a pressure-resistant thick-walled tube, and 2 mL of 3% sulfuric acid-acidified methanol and 2 mL of chloroform were added. The mixture was heated at 100 °C for 4 hours, with shaking every hour to ensure thorough cell disruption. After cooling to room temperature, 1 mL of distilled water was added to the tube, and the mixture was vigorously shaken for 1 min and then allowed to settle into layers. The chloroform layer was collected, filtered through a 0.22 μm syringe filter, and transferred into a screw-cap vial for gas chromatography analysis. The analysis was performed using a Fuli GC 9790 Plus II gas chromatograph with an RB-FFAP column and a flame ionization detector (FID). The separation and extraction of PHB are detailed in Text S2.
4) was refilled until atmospheric pressure was reached. The supernatant was discarded, and the cell pellet was washed twice with ultrapure water, followed by centrifugation to collect the cells. The collected cells were dried overnight at 55 °C. The dried cells were then transferred into a pressure-resistant thick-walled tube, and 2 mL of 3% sulfuric acid-acidified methanol and 2 mL of chloroform were added. The mixture was heated at 100 °C for 4 hours, with shaking every hour to ensure thorough cell disruption. After cooling to room temperature, 1 mL of distilled water was added to the tube, and the mixture was vigorously shaken for 1 min and then allowed to settle into layers. The chloroform layer was collected, filtered through a 0.22 μm syringe filter, and transferred into a screw-cap vial for gas chromatography analysis. The analysis was performed using a Fuli GC 9790 Plus II gas chromatograph with an RB-FFAP column and a flame ionization detector (FID). The separation and extraction of PHB are detailed in Text S2.
2.4 Physical, chemical, and biological characterization
The microstructural characterization was conducted using an FEI Nova Nano SEM 450 scanning electron microscope (SEM) and a Talos F200X transmission electron microscope (TEM) with an energy dispersive X-ray spectrometer (EDS) from Thermo Fisher Scientific. N2 adsorption–desorption measurements were conducted on a Micromeritics ASAP 2460 to obtain the Brunauer–Emmett–Teller (BET) surface area and pore size distribution. X-ray diffraction (XRD) analysis was performed using a D-MAX 2500/PC X-ray diffractometer from Rigaku. Fourier-transform infrared (FTIR) spectroscopy was conducted using a Nicolet iS5 spectrometer from Thermo Fisher Scientific. Zeta potential measurements were conducted on a Malvern Zetasizer Nano ZS90 using water as the solvent. X-ray photoelectron spectroscopy (XPS) was carried out using a K-Alpha XPS system from Thermo Fisher Scientific. The potassium content in K/O-CN was determined by inductively coupled plasma optical emission spectroscopy (ICP-OES, PerkinElmer ICP 2100). H2 production testing was performed in a 60 mL quartz reactor under xenon lamp irradiation, with specific conditions detailed in Text S3. The H2 production test in nitrogen-limited culture medium was performed as detailed in Text S4. Ultraviolet–visible diffuse reflectance spectroscopy (UV-vis DRS) was performed using a UV-vis spectrometer (TU-1901) equipped with an integrating sphere from Beijing Persee. Electrochemical tests, including Mott–Schottky analysis, transient photocurrent response (TRP), electrochemical impedance spectroscopy (EIS), and linear sweep voltammetry (LSV), were conducted using a CHI 660E electrochemical workstation from Shanghai Chenhua, with specific conditions provided in Text S5. Photoluminescence (PL) spectra were recorded using an Edinburgh FLS980 spectrometer. For 13CO2 isotope labeling experiment, 13CO2 was used to replace CO2 in the gas mixture and introduced into the reactor of K/O-CN–R. eutropha for PHB production. The culture was cultivated under 4000 lux light conditions for 48 h. PHB was then extracted from both K/O-CN–R. eutropha and K/O-CN–R. eutropha with 13CO2 isotope labeling. The high-resolution solid-state 13C Nuclear Magnetic Resonance (NMR) test was conducted for the extracted PHB using a Bruker AVANCE III 500 MHz by dissolving PHB in the solvent of CDCl3 at 10 mg ml−1.The colony-forming units (CFU) were counted under an upright biological microscope of Nikon Ei. The optical density at a wavelength of 600 nm (OD600) was measured daily using a UV-vis spectrometer (TU-1901) to monitor the growth of bacteria in the system. The NADPH/NADP+ ratio, an indicator of the cellular redox state, was determined by analyzing 5 million cells from various systems grown in a nitrogen-limited medium for 48 h. The concentrations of NADPH and NADP+ were measured using a coenzyme II NADPH content assay kit (MTT colorimetric method) from Solarbio Life Sciences, China, and the ratio was calculated. The CO2 isothermal adsorption tests were performed at 25 °C on a Quantachrome Autosorb iQ from USA. Untargeted metabolomic analysis after 24 h illumination was performed at Paisenno Company using a Thermo Vanquish ultra-high-performance liquid chromatography (UHPLC) system coupled with a Thermo Orbitrap Exploris 120 mass spectrometer (Thermo Fisher Scientific, USA), under specific conditions outlined in Text S6. Multivariate dimensionality reduction analyses, including principal component analysis (PCA) and orthogonal partial least squares-discriminant analysis (OPLS-DA), were conducted using the R software package Ropls.
3. Results and discussion
3.1 Construction of the K/O-CN–R. eutropha biohybrid system
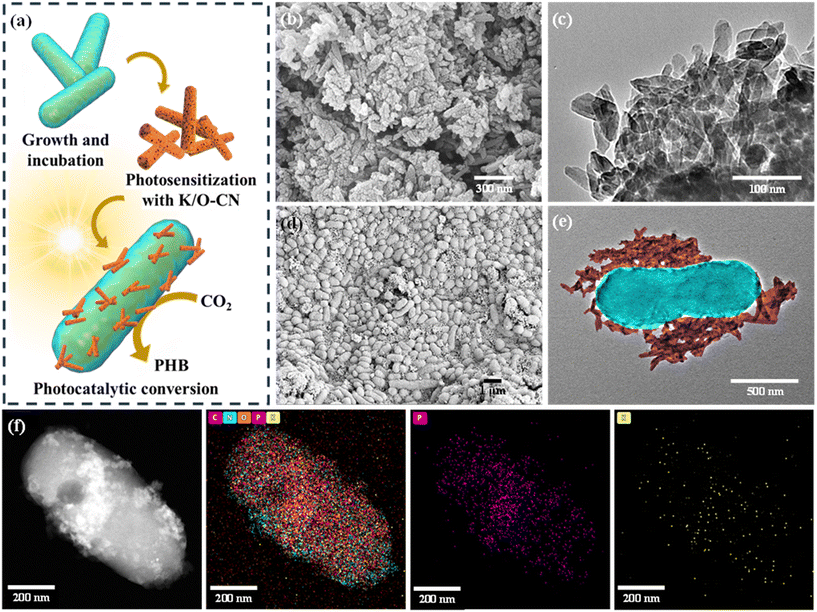
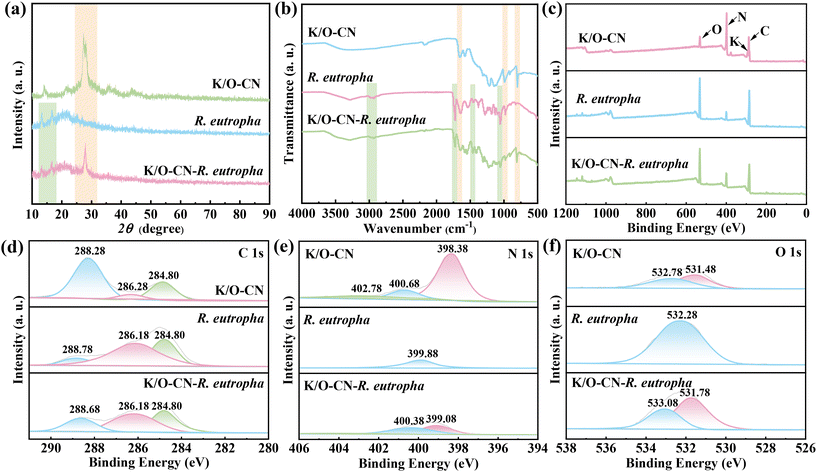
The K/O-CN–R. eutropha biohybrid system was constructed through a mixing-self-assembly approach (Fig. 1a). Initially, the bacteria were inoculated into a fructose medium to promote rapid growth and proliferation, thereby enriching the bacterial biomass. Subsequently, the enriched bacterial cells were introduced into an autotrophic medium containing K/O-CN, facilitating the mixing and self-assembly of the bacteria with K/O-CN. Under illumination, this biohybrid system was driven to catalyze the photoconversion of CO2 into PHB. The morphology and composite state of the K/O-CN–R. eutropha biohybrid system were observed by SEM and TEM. After K/O co-doping treatment, the carbon nitride transformed from stacked blocky layers (Fig. S1 and S2) into nanorods (Fig. 1b and c). Based on N2 adsorption–desorption measurements (Fig. S3a and b), K/O-CN exhibits a significantly larger specific surface area and pore volume than CN, providing more active sites and facilitating CO2 adsorption and mass transport during the reaction. Uniform distributions of K and O were detected in K/O-CN as observed in TEM-EDS mapping (Fig. S4), indicating the successful co-doping of K and O. As shown in Fig. 1d and S5, K/O-CN combined with R. eutropha forms a uniformly dispersed composite system. In the biohybrid system, the R. eutropha cells maintained their original short rod-shaped structure, with the bacterial cells remaining intact. In the pure R. eutropha system, the bacterial surface appeared relatively smooth (Fig. S6 and S7), whereas in the biohybrid system, the nanorod-shaped K/O-CN surrounded the bacterial cells, establishing a close association (Fig. 1e, S8, and S9). TEM-EDS mapping of the biohybrid (Fig. 1f and S10) reveals the presence of common elements C, N, and O shared by both R. eutropha and K/O-CN, along with typical P element in R. eutropha and K element in K/O-CN. The uniform distribution of P and K substantiated the homogeneous integration of R. eutropha and K/O-CN. The homogeneous biohybrid with evenly distributed K/O-CN and R. eutropha enabled effective interaction and mass transfer between them.A comparative analysis was conducted on the crystal structure, functional groups, and chemical states of K/O-CN, R. eutropha, and the K/O-CN–R. eutropha biohybrid. The XRD pattern of PCN (Fig. S11) exhibited a weak diffraction peak at 13.20° and a sharp diffraction peak at 27.40°, corresponding to the in-plane tri-s-triazine motifs ((100) planes) and the conjugated aromatic interlayer stackings ((002) planes), respectively. Similarly, K/O-CN also showed two main diffraction peaks. However, the peak for the (100) planes in K/O-CN shifted to a higher angle, around 14°, and the peak intensity of (002) planes reduced. These changes were attributed to the structural rearrangement and weakened interlayer stacking in K/O-CN due to the rod-like morphology induced by K/O doping.34 Several low-intensity, broad diffraction peaks observed in K/O-CN were attributed to structural distortions or defects induced by the doping of K and O, which led to lattice distortions or the formation of localized amorphous phases within the CN matrix. In the XRD patterns (Fig. 2a), the K/O-CN–R. eutropha biohybrid exhibited three diffraction peaks, with the peak at 27.04° originating from the conjugated aromatic stacking of K/O-CN ((002) planes).35 Additionally, two weak diffraction peaks at 13.32° and 16.78° overlapped with the corresponding structural peaks of R. eutropha, indicating the successful combination of K/O-CN and R. eutropha. For FTIR analysis (Fig. 2b), the K/O-CN–R. eutropha biohybrid showed a characteristic peak at 807 cm−1, corresponding to the bending vibration of triazine units, while the peaks at 980 cm−1 and 1650 cm−1 corresponded to the skeletal vibrations of the CN heterocycles in K/O-CN.36 Moreover, the biohybrid system exhibited characteristic peaks at 1060 cm−1, 1450 cm−1, and 1730 cm−1, corresponding to C–C bending, C–H bending, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations, respectively. The peaks in the range of 3020–2890 cm−1, assigned to the stretching vibrations of C–H groups, originated from R. eutropha. The biohybrid system thus retains the characteristic functional groups of both K/O-CN and R. eutropha without the appearance of new peaks, indicating the absence of new chemical bond formation. Meanwhile, zeta potential measurements in Fig. S12 indicated that the surfaces of K/O-CN and R. eutropha both carried negative charges, suggesting that their interaction was unlikely to be driven by electrostatic attraction. Excluding chemical bonding and electrostatic forces, it could be inferred that K/O-CN and R. eutropha interacted through intermolecular forces, specifically van der Waals interactions, between the surface of the photocatalyst and biomolecules.
O stretching vibrations, respectively. The peaks in the range of 3020–2890 cm−1, assigned to the stretching vibrations of C–H groups, originated from R. eutropha. The biohybrid system thus retains the characteristic functional groups of both K/O-CN and R. eutropha without the appearance of new peaks, indicating the absence of new chemical bond formation. Meanwhile, zeta potential measurements in Fig. S12 indicated that the surfaces of K/O-CN and R. eutropha both carried negative charges, suggesting that their interaction was unlikely to be driven by electrostatic attraction. Excluding chemical bonding and electrostatic forces, it could be inferred that K/O-CN and R. eutropha interacted through intermolecular forces, specifically van der Waals interactions, between the surface of the photocatalyst and biomolecules.
Based on XPS survey analysis in Fig. 2c, K/O-CN, R. eutropha and the biohybrid were primarily composed of the elements C, N, and O, with a small amount of K present in K/O-CN. The atomic percentages of K and O in K/O-CN were found to be 2.25 at% and 7.73 at%, confirming the successful co-doping of K and O. The ICP-OES analysis revealed that the K content in K/O-CN was 1.80 wt%, in good agreement with the value determined by XPS. No Li element was detected in K/O-CN in the XPS survey scan. Meanwhile, no distinct peak was observed in the Li 1s high-resolution spectrum of K/O-CN (Fig. S13). These results indicated that Li element was totally removed from K/O-CN during the process of boiling water cleaning. For the C 1s XPS spectrum (Fig. 2d), the pure bacterial system showed three component peaks ascribed to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (288.78 eV), C–N (286.18 eV), and C
O (288.78 eV), C–N (286.18 eV), and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C (284.80 eV).37,38 After forming the biohybrid with K/O-CN, the C
C (284.80 eV).37,38 After forming the biohybrid with K/O-CN, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O peak in the biohybrid shifted to 288.68 eV,39 moving toward lower binding energy and higher intensity. In the N 1s XPS spectrum (Fig. 2e), unlike the isolated peak of R. eutropha, the biohybrid system showed two fitted peaks with binding energies close to those of K/O-CN. Compared to K/O-CN, the peak corresponding to tertiary N (N–C3) in the biohybrid shifted negatively to 400.38 eV, while the peak corresponding to C–N
O peak in the biohybrid shifted to 288.68 eV,39 moving toward lower binding energy and higher intensity. In the N 1s XPS spectrum (Fig. 2e), unlike the isolated peak of R. eutropha, the biohybrid system showed two fitted peaks with binding energies close to those of K/O-CN. Compared to K/O-CN, the peak corresponding to tertiary N (N–C3) in the biohybrid shifted negatively to 400.38 eV, while the peak corresponding to C–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C shifted positively to 399.08 eV.33 The O 1s XPS spectrum (Fig. 2f) reveals that the C–O and C
C shifted positively to 399.08 eV.33 The O 1s XPS spectrum (Fig. 2f) reveals that the C–O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O peaks in the biohybrid system shifted towards higher binding energies compared to those in K/O-CN, indicating electron loss by K/O-CN during its interaction with R. eutropha. The analysis of these chemical states suggested that there were contact and interactions between K/O-CN and R. eutropha in the biohybrid system. The K 2p high-resolution XPS spectrum of K/O-CN was also recorded, as shown in Fig. S14. The results showed that K/O-CN exhibited two characteristic peaks at 293.00 eV and 295.60 eV, corresponding to the coordination of K+ with nitrogen atoms in the imide groups of carbon nitride and nitrogen atoms in other nitrogen-containing groups, respectively.
O peaks in the biohybrid system shifted towards higher binding energies compared to those in K/O-CN, indicating electron loss by K/O-CN during its interaction with R. eutropha. The analysis of these chemical states suggested that there were contact and interactions between K/O-CN and R. eutropha in the biohybrid system. The K 2p high-resolution XPS spectrum of K/O-CN was also recorded, as shown in Fig. S14. The results showed that K/O-CN exhibited two characteristic peaks at 293.00 eV and 295.60 eV, corresponding to the coordination of K+ with nitrogen atoms in the imide groups of carbon nitride and nitrogen atoms in other nitrogen-containing groups, respectively.
3.2 Photocatalytic performance of CO2 to PHB conversion
H2 production tests indicate that the H2 production performance of K/O-CN was 2.73 times that of PCN (Fig. S15). Compared to the original PCN, K/O-CN had a broader light absorption range (Fig. S16a and b), a narrower bandgap (Fig. S17a and b), and a higher reduction potential (Fig. S18 and S19). Electrochemical and PL tests indicate that K/O-CN also had higher efficiency in charge carrier migration and separation (Fig. S20 and S21) and lower charge transfer resistance (Fig. S22) than PCN. These benefits contributed to its enhancement for H2 production performance. K/O-CN exhibited higher H2 production and better photocatalytic performance than K/O-CN-1 with less K-doping, K/O-CN-2 with higher K-doping, K-CN, and O-CN (Fig. S23). Moreover, as shown in Fig. S24, the pure culture medium did not produce H2, while the system with K/O-CN was able to stably generate H2 under 4000 lux illumination. When the K/O-CN–R. eutropha biohybrid was introduced, the H2 production decreased compared with the system with K/O-CN, indicating that the biohybrid consumed the H2 generated by K/O-CN during photocatalytic water splitting. When PCN was replaced with K/O-CN and combined with R. eutropha to construct a biohybrid system, the CO2 conversion capability of the system was improved (Fig. S25). The PHB yield in K/O-CN–R. eutropha increased by 45% compared to that in PCN–R. eutropha. The extraction and detection processes of the PHB product are described in Text S2 in the SI and section 2.3. These methods effectively extracted PHB and allowed for accurate quantification of its content.To determine the carbon source and the purity of PHB, 13CO2 isotope labeling experiment and high-resolution solid-state 13C NMR test were conducted. The 13C NMR results in Fig. S26 and S27 showed that both PHB from K/O-CN–R. eutropha and K/O-CN–R. eutropha with 13CO2 exhibited characteristic 13C resonances at 168.1, 76.1–66.7, 39.9, and 19.8 ppm, corresponding to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, CH, CH2, and CH3 structural units,40 respectively, which aligned with the typical structure of PHB. No obvious additional impurity peaks were observed, indicating that the extracted PHB was of high purity. Different from the weak resonance signals for most structural units in the PHB from K/O-CN–R. eutropha, the resonance peaks of structural units significantly increased in the PHB from K/O-CN–R. eutropha with 13CO2. This indicated that the incorporation of 13C isotopes from 13CO2 into PHB enhanced its abundance, suggesting that K/O-CN–R. eutropha had produced PHB from CO2.
O, CH, CH2, and CH3 structural units,40 respectively, which aligned with the typical structure of PHB. No obvious additional impurity peaks were observed, indicating that the extracted PHB was of high purity. Different from the weak resonance signals for most structural units in the PHB from K/O-CN–R. eutropha, the resonance peaks of structural units significantly increased in the PHB from K/O-CN–R. eutropha with 13CO2. This indicated that the incorporation of 13C isotopes from 13CO2 into PHB enhanced its abundance, suggesting that K/O-CN–R. eutropha had produced PHB from CO2.
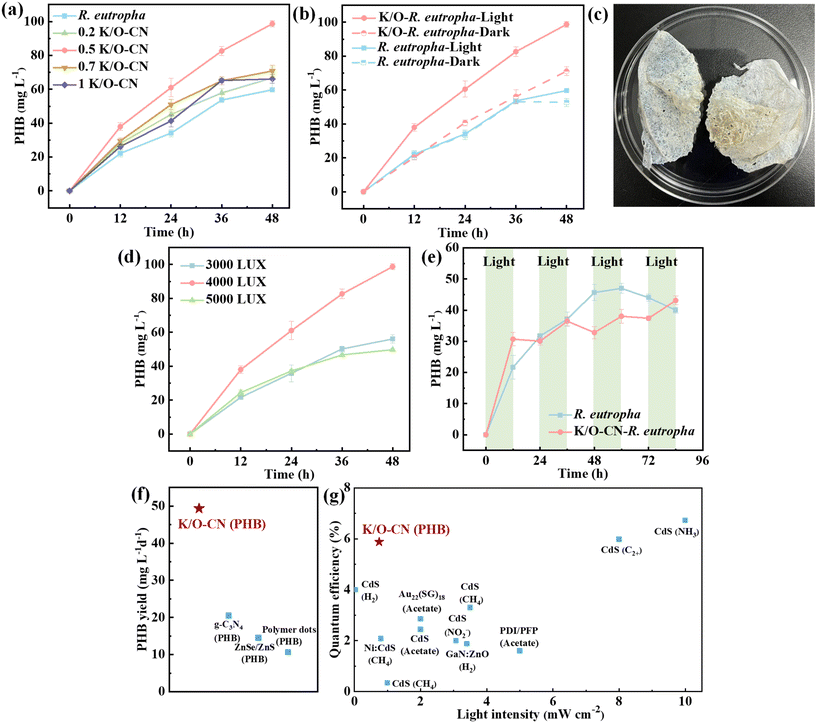
Fig. 3a explores the optimal concentration of K/O-CN for the biohybrid system. Compared to the pure bacterial system, the addition of K/O-CN within the range of 0.2–1 g L−1 enhanced the PHB yield. The maximum accumulation of PHB was achieved with a K/O-CN concentration of 0.5 g L−1. TEM results showed that the biohybrid with a low K/O-CN concentration (0.2 g L−1) did not sufficiently interact with the bacteria (Fig. S28a). At high concentrations of K/O-CN (1 g L−1) in the biohybrid, an excess of photocatalyst aggregated on the bacterial surface (Fig. S28b), which inhibited the bacteria's absorption and utilization of nutrients from the culture medium. To eliminate the influence of carbon from K/O-CN, the PHB production of the biohybrid without supplementing CO2 (N2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2
H2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O2 = 86
O2 = 86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) was tested as shown in Fig. S29. The results indicated that the PHB yield was 0 under these conditions, suggesting that the biohybrid was unable to utilize the carbon from K/O-CN for PHB synthesis. The addition of the sacrificial agent TEOA (5 g L−1) resulted in a moderate increase in PHB yield, but the enhancement was limited (Fig. S30). The limited improvement caused by TEOA addition was due to its potential biotoxicity and the intrinsic metabolic constraints of the bacteria, which prevented further enhancement of PHB synthesis despite the increased electron supply. Considering cost and energy efficiency, the focus remained on research within the sacrificial agent-free system.
4) was tested as shown in Fig. S29. The results indicated that the PHB yield was 0 under these conditions, suggesting that the biohybrid was unable to utilize the carbon from K/O-CN for PHB synthesis. The addition of the sacrificial agent TEOA (5 g L−1) resulted in a moderate increase in PHB yield, but the enhancement was limited (Fig. S30). The limited improvement caused by TEOA addition was due to its potential biotoxicity and the intrinsic metabolic constraints of the bacteria, which prevented further enhancement of PHB synthesis despite the increased electron supply. Considering cost and energy efficiency, the focus remained on research within the sacrificial agent-free system.
Fig. 3b illustrates the impact of light exposure on the PHB production of the K/O-CN–R. eutropha biohybrid. Under low indoor light irradiation (4000 lux, 0.75 mW cm−2), the PHB yield of the K/O-CN–R. eutropha biohybrid reached 49.35 ± 0.85 mg L−1 d−1, representing a 65% increase compared to the pure bacterial system without K/O-CN (29.85 ± 0.25 mg L−1 d−1). This enhancement is huge for the natural carbon metabolism pathway for PHB production. This suggests that the system can greatly improve the efficiency of CO2 utilization, reduce production costs, and accelerate the industrialization of PHB. In the dark, the PHB production of the biohybrid system was similar to that of the pure bacterial system, indicating that the enhancement in CO2 conversion within the biohybrid system was light-dependent. The biohybrid cultured for 48 h was subjected to a single separation and extraction to yield crude PHB, as shown in Fig. 3c. At a light intensity of 4000 lux, the PHB yield of the biohybrid system was significantly higher than that at other light intensities, representing the optimal illumination conditions (Fig. 3d). At illumination intensities below 4000 lux, the light is insufficient to effectively activate the photocatalyst. However, when the light intensity was too high, it generated excessive reactive oxygen species (ROS) from the photocatalyst, which surpassed the bacteria's antioxidant capacity and caused oxidative stress.41
The photosynthetic behavior of the K/O-CN–R. eutropha biohybrid system was studied using a 12 h light–dark cycle (Fig. 3e). Throughout the light–dark cycle, the PHB accumulation in the biohybrid system exhibited an increasing trend and was higher than that in the pure bacterial system. During light exposure, the PHB content in the biohybrid system showed significant accumulation, whereas in the dark, there was a slight decrease in PHB levels due to the bacteria utilizing PHB as an energy source to sustain growth.42 For the pure R. eutropha, the accumulation of PHB was not influenced by light. The PHB production of R. eutropha initially increased and then decreased over time in 96 h, with the decrease being attributed to the depletion of nutrients and the reduction in bacterial growth rate. Due to the continuous alteration of cultivation conditions, which affected bacterial activity and metabolism, the K/O-CN–R. eutropha biohybrid did not exhibit a significant advantage over the R. eutropha system in terms of PHB yield under light–dark conditions. This also indicated that the performance of the biohybrid was light-dependent. The K/O-CN–R. eutropha biohybrid was compared with reported photocatalytic biohybrid systems. For PHB production, the K/O-CN–R. eutropha surpassed non-metal autotrophic biohybrids with added co-factors (Fig. 3f and Table S4). The PHB productivity of the K/O-CN–R. eutropha biohybrid was markedly higher than that of the g-C3N4-based microbial electrosynthesis (MES) system (20.31 mg L−1 d−1),43,44 while it also simplified the reaction configuration and significantly reduced operational costs. The quantum efficiency (QE) of K/O-CN–R. eutropha reached 5.88 ± 0.16%, as calculated using Eqn (1)–(3) (Text S7), which exceeded most metal-based biohybrids (Fig. 3g and Table S5).
We conducted a long-term operation of the K/O-CN–R. eutropha biohybrid system for 6 d in Fig. S31. The results showed that the maximum PHB accumulation occurred between 48 and 72 h, after which the PHB content began to decline. This observation aligns with the typical growth pattern of R. eutropha, which includes the lag phase, exponential phase, stationary phase, and death phase. In the cyclic text (Fig. S32), the PHB yield of the biohybrid system in 48 h after three consecutive cycles of K/O-CN reuse remained above 92.23% of the initial value. Furthermore, XRD and FTIR analyses of K/O-CN before and after the reactions (Fig. S33) confirmed that its crystalline structure and surface functional groups remained unchanged, indicating excellent structural stability and recyclability. Overall, these results demonstrate that K/O-CN in the biohybrid system can be readily separated and effectively reused through a simple recovery process. Therefore, to maximize the PHB yield, rather than long-term operation, the K/O-CN–R. eutropha biohybrid system should be operated for 48 h and then reactivated by re-inoculation and photocatalyst addition.
3.3 Main characteristic analysis of the K/O-CN–R. eutropha biohybrid
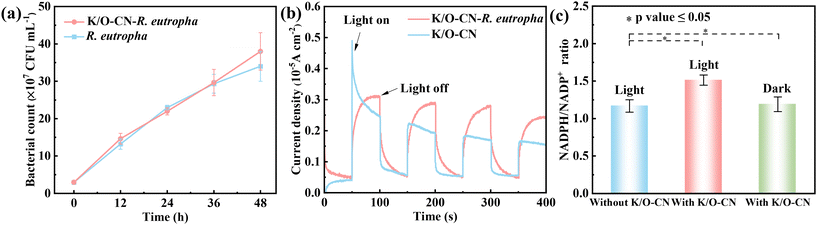
The CFU (Fig. 4a and S34) of K/O-CN–R. eutropha and PCN–R. eutropha presented the same near-linear increase as the pure bacterial system, indicating that the addition of K/O-CN and PCN did not affect the bacterial growth status, and both K/O-CN and PCN were non-toxic and exhibited good biocompatibility. The CFU assays of K/O-CN–R. eutropha with various amounts of K/O-CN (0.2–1 g L−1) presented a similar increasing trend over time, implying that the K/O-CN amount in a suitable range did not affect bacterial activity (Fig. S35). OD measurements were also conducted to monitor bacterial growth. However, the presence of K/O-CN increased light absorption and the optical density, leading to higher OD values for the K/O-CN–R. eutropha system. Therefore, OD testing in Fig. S36 was only used as a supplementary experiment to reflect the overall trend. The OD600 values of both the biohybrid and pure bacterial systems showed an upward trend, indicating continuous bacterial accumulation in both systems. The TPR was used to test the generation and separation characteristics of photo-generated electron–hole pairs in the system (Fig. 4b). Under light, the K/O-CN–R. eutropha biohybrid generated photocurrents, demonstrating that it possessed light-excitation properties. The photocurrent intensity of the biohybrid system was higher than that of pure K/O-CN, demonstrating that the combination with R. eutropha had generated more charge carriers and improved the electron–hole separation efficiency. Notably, the photocurrent of the biohybrid exhibited a curved rather than square-wave response, primarily because the presence of microorganisms altered the charge transfer kinetics of the system. Specifically, the microbial membranes and biomacromolecules slowed down electron transfer and introduced capacitive effects. Compared to pure K/O-CN, the biohybrid system showed a reduced charge transfer resistance (Fig. S37) and increased current density (Fig. S38). The incorporation of the bacteria facilitated charge transfer within the system and accelerated the separation of electron–hole pairs. The CO2 adsorption capacity of the K/O-CN–R. eutropha biohybrid, K/O-CN, and R. eutropha was tested (Fig. S39). The results indicated that the CO2 adsorption capacity of the K/O-CN–R. eutropha biohybrid (4.21 cm3 g−1) was significantly higher than that of pure R. eutropha (0.84 cm3 g−1). This enhancement was attributed to the K/O-CN, which possessed a rod-like porous nanostructure, resulting in a higher CO2 adsorption capacity (9.66 cm3 g−1). The improved CO2 adsorption capacity of the K/O-CN–R. eutropha biohybrid enabled efficient capture and utilization of CO2.During the Calvin–Benson–Bassham (CBB) cycle for PHB production by R. eutropha, NADPH is utilized as a reducing equivalent. Based on this, the NADPH/NADP+ ratio (Fig. 4c) and NADPH quantity (Fig. S40) were measured for both the biohybrid and pure bacterial systems. Under illumination, the NADPH/NADP+ ratio of the K/O-CN–R. eutropha system is 1.51, which was higher than that of the pure bacterial system under light (1.17) and the biohybrid system in the dark (1.19). The combined effects of K/O-CN addition and light exposure enhanced the NADPH/NADP+ ratio in the biohybrid system. The NADPH quantity in the K/O-CN–R. eutropha system under light (0.054 nmol 10−4 cell) was higher than that in both the pure bacterial system under light and the biohybrid system in the dark. These results suggest that the photogenerated electrons from K/O-CN under light were transferred from the extracellular to the intracellular receptors, generating additional NADPH and promoting the energy metabolism of R. eutropha, which in turn increases PHB production.
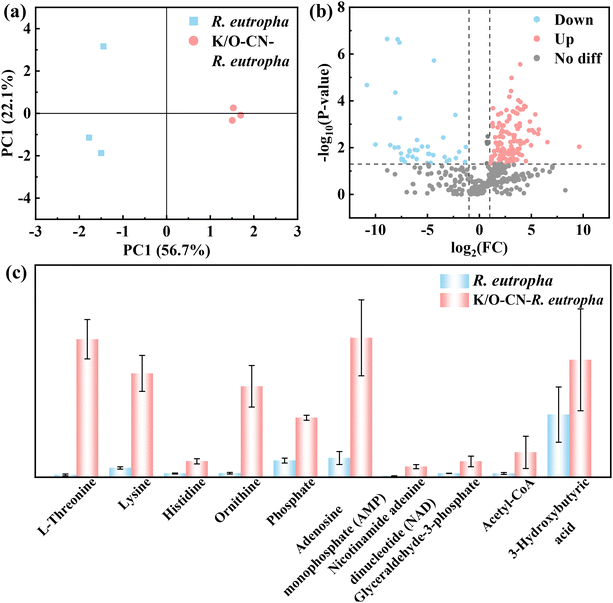
To investigate the CO2 metabolic pathways and changes in metabolic products, untargeted metabolomic analysis was performed on both the biohybrid and pure bacterial systems after illumination. A total of 596 metabolites were identified in both systems, with organic heterocyclic compounds representing the largest proportion (22.3%, Fig. S41). The principal metabolic product, PHB, belongs to this category, which was consistent with the macroscopic experimental results. PCA (Fig. 5a) and OPLS-DA (Fig. S42) score plots indicated that the biohybrid and pure bacterial systems were distributed in different quadrants, with significant metabolic differences, demonstrating that the introduction of K/O-CN under light had a substantial impact on the bacterial metabolic state. Volcano plot analysis (Fig. 5b) showed that many metabolites were upregulated and downregulated in the biohybrid system compared to the pure bacterial system. The types of upregulated metabolites (134) were significantly more than those of downregulated metabolites (40), indicating enhanced metabolism in the biohybrid system. Further analysis of differential metabolites revealed changes related to cell metabolism and PHB production, as shown in Fig. 5c. In the biohybrid system, amino acids such as L-threonine, lysine, and histidine were significantly upregulated, promoting protein synthesis and cell metabolic growth. The increase in ornithine, a participant in one-carbon metabolism, facilitated this process. The rise in phosphate and adenosine monophosphate (AMP) supported the synthesis of cellular energy currency, adenosine triphosphate (ATP). An increase in Nicotinamide Adenine Dinucleotide (NAD), an electron carrier, accelerated intracellular redox reactions. The increase in glyceraldehyde-3-phosphate, a metabolic intermediate, promoted the generation of reducing power (NADPH). The content of acetyl-CoA, a core intermediate in cellular metabolism and PHB synthesis, directly determined the PHB yield.45 The acetyl-CoA content of the biohybrid system was 6.56 times that of pure bacteria. The increase in 3-hydroxybutyric acid, a monomer of PHB, confirmed the increased PHB yield within the biohybrid system.
3.4 Mechanism of photocatalytic CO2 to PHB conversion
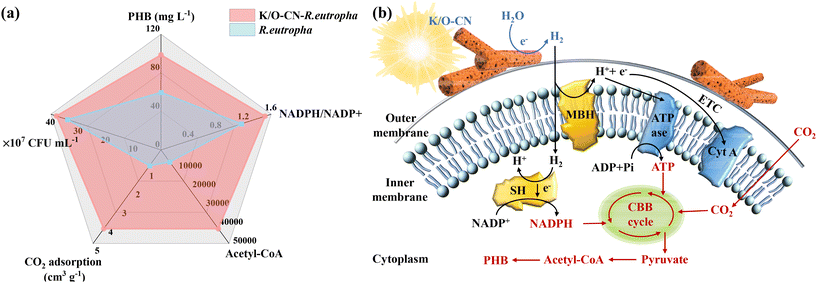
A comparative summary of key parameters for biohybrid and pure bacterial systems (Fig. 6a) reveals that the K/O-CN–R. eutropha biohybrid exhibited superior overall performance. The incorporation of K/O-CN led to several advantages: (1) it was non-toxic and exhibited good biocompatibility; (2) it increased the CO2 adsorption capacity; (3) it generated additional reducing equivalents, enhancing bacterial energy metabolism; (4) it facilitated the production of key metabolic intermediates such as acetyl-CoA. These combined benefits significantly enhanced the K/O-CN–R. eutropha system's ability to utilize CO2 and produce PHB.In summary, the effect of K/O co-doping on PCN involved the following points: (1) after K/O co-doping treatment, the morphology of PCN changed from stacked bulk layers to nanorods; (2) the H2 production performance of K/O-CN was 2.73 times that of PCN; (3) compared to PCN, K/O-CN exhibited a broader light absorption range, a narrower bandgap, and higher reduction potential; (4) K/O-CN showed a smaller charge transfer resistance and higher photogenerated carrier mobility and separation efficiency compared to PCN. Furthermore, the effects of K/O co-doping on the biohybrid for photocatalytic CO2 conversion were reflected in several aspects: (1) the nanorod morphology of K/O-CN prevented the photocatalyst from aggregating into agglomerates with the bacteria, facilitating the effective combination of K/O-CN and R. eutropha; (2) K/O-CN provided more H2 to R. eutropha than PCN, driving R. eutropha to utilize CO2 for PHB production; (3) the CO2 conversion capability of the K/O-CN–R. eutropha biohybrid was improved compared to the PCN–R. eutropha hybrid.
Both photocatalytically generated H2 and injected H2 participated in cellular metabolism and PHB biosynthesis. In the absence of injected H2 (N2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO2
CO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O2 = 86
O2 = 86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4), the cells perished and no PHB was produced (Fig. S43), indicating that injected H2 served as an essential energy source for maintaining cell survival and metabolism. Concurrently, the photocatalytic H2 was rapidly utilized by the bacteria as an in situ source of reducing power and energy for PHB biosynthesis with the upregulation of ATP-related metabolites and NADPH levels.
4), the cells perished and no PHB was produced (Fig. S43), indicating that injected H2 served as an essential energy source for maintaining cell survival and metabolism. Concurrently, the photocatalytic H2 was rapidly utilized by the bacteria as an in situ source of reducing power and energy for PHB biosynthesis with the upregulation of ATP-related metabolites and NADPH levels.
The mechanism underlying the light-driven K/O-CN–R. eutropha biohybrid for CO2 fixation and PHB biosynthesis, along with the mass and charge transfer processes at the abiotic–biotic interface, is elucidated in Fig. 6b. Upon photoexcitation, K/O-CN generates electron–hole pairs, which undergo redox reactions splitting H2O to produce H2. The evolved H2 diffuses into the cell, where a portion is captured by membrane-bound hydrogenase (MBH) located in the inner membrane, catalyzing its oxidation into protons (H+) and electrons (e−). The H+ contributes to ATP synthesis, while the e− enters the electron transport chain (ETC). Another portion of H2 diffuses into the cytoplasm, where soluble hydrogenase (SH) oxidizes it into H+ and e−, driving the reduction of NADP+ to NADPH. ATP and NADPH subsequently function as the energy source and reducing agent, respectively, within the CBB cycle for CO2 fixation. Through a series of metabolic intermediates such as pyruvate and acetyl-CoA, CO2 is ultimately converted into PHB. Without light or a photocatalyst, a small amount of H2 dissolved in the culture medium is utilized by R. eutropha. It is oxidized via MBH and SH into H+ and e−, contributing to ATP and NADPH synthesis and facilitating the conversion of CO2 into PHB.
Based on the inherent metabolic pathways of R. eutropha, the utilization of H2 without light or a photocatalyst is similar to that under light conditions. The key difference lies in the fact that, under light, the H2 generated from water splitting by K/O-CN is utilized, whereas, in the dark, R. eutropha relies on H2 dissolved in the culture medium. Under light conditions, the observed elevation in ATP-related metabolites, the increased NADPH/NADP+ ratio, and the accumulation of key intermediates such as acetyl-CoA and 3-hydroxybutyrate validate that H2 generated from the water splitting by K/O-CN promotes the CO2-to-PHB conversion efficiency in this biohybrid system.
4. Conclusions
This work presents a design of a uniformly autotrophic K/O-CN–R. eutropha biohybrid, capable of driving the efficient conversion of CO2 into visible C4 bioplastic without any auxiliary agents. In this system, rod-shaped K/O-CN surrounded the bacterial cells and bound closely with them, thereby increasing the contact of the abiotic–biotic interface. The strong interactions between K/O-CN and R. eutropha facilitated the mass transfer across the interface. Under low indoor light of 4000 lux (0.75 mW cm−2), the K/O-CN–R. eutropha hybrid achieved a PHB yield of 49.35 ± 0.85 mg L−1 d−1, outperforming non-metal composite systems with the presence of additives. The quantum efficiency of the K/O-CN–R. eutropha system reached 5.88 ± 0.16%, exceeding that of most metal-based biohybrids. The incorporation of K/O-CN in constructing the biohybrid exhibited good biocompatibility, augmented the quantity and separation efficiency of photo-generated charge carriers, produced additional reducing equivalents, and promoted the biosynthesis of key metabolic intermediates such as acetyl-CoA. Upon photoexcitation, K/O-CN decomposed H2O to generate H2, which was subsequently oxidized to participate in ATP synthesis and drive the generation of reducing equivalents like NADPH, thereby accelerating the carbon metabolic cycle and efficiently converting CO2 into high-value PHB.Author contributions
Wenjing Wang: conceptualization, project administration, writing – original draft, and funding acquisition. Mingzhi Zhang: investigation and writing – original draft. Meng Guo: investigation and writing – original draft. Jiaxin Wang: investigation and visualization. Xuelian Wang: data curation and investigation. Jianheng Yin: data curation and investigation. Liang Chen: methodology and funding acquisition. Yaguang Li: supervision, writing – review and editing.Conflicts of interest
There are no conflicts to declare.Data availability
The data that support the findings of this study are available from the corresponding author.Supplementary information (SI) is available. See DOI: https://doi.org/10.1039/d5gc02437d.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (52306135, 52106141), the Science Research Project of Hebei Education Department (BJK2022054), the Natural Science Foundation in Interdisciplinary Research of Hebei University (DXK202109), and the Advanced Talents Incubation Program of Hebei University (521000981377).References
- R. T. Chen, Z. F. Ren, Y. Liang, G. H. Zhang, T. Dittrich, R. Z. Liu, Y. Liu, Y. Zhao, S. Pang, H. Y. An, C. W. Ni, P. W. Zhou, K. L. Han, F. T. Fan and C. Li, Nature, 2022, 610, 296–301 CrossRef CAS.
- K. Sun, Y. Huang, Q. Y. Wang, W. D. Zhao, X. S. Zheng, J. Jiang and H.-L. Jiang, J. Am. Chem. Soc., 2024, 146, 3241–3249 CrossRef CAS.
- G. dos Santos, L. Tian, R. Gonçalves, H. García and L. Rossi, Adv. Funct. Mater., 2025, 35, 2422055 CrossRef CAS.
- J. Cruz, G. Silva, E. Dias, D. Lima, J. Torres, P. Silva and C. Ribeiro, ACS Appl. Mater. Interfaces, 2024, 17, 13029–13036 CrossRef.
- M. F. Lu, Q. Q. Li, C. L. Zhang, X. X. Fan, L. Li, Y. M. Dong, G. Q. Chen and H. F. Shi, Carbon, 2020, 160, 342–352 CrossRef CAS.
- Y. B. Zhong, H. B. Zhu, X. M. Xie, L. Yang, Y. J. Shen, Q. W. Fan, Z. B. Xie and Z. G. Le, Inorg. Chem., 2025, 64, 2706–2715 CrossRef CAS PubMed.
- H. L. Chen, F. Y. Liu, Y. Y. Lin, Z. Zuo, W. T. Wu, Q. Qi, Z. Peng, D. Zou and C. C. Chen, Mater. Today Sustainability, 2023, 23, 100430 CrossRef.
- J. Park, H. Liu, G. Piao, U. Kang, H. W. Jeong, C. Janáky and H. Park, Chem. Eng. J., 2022, 437, 135388 CrossRef CAS.
- P. Zhang, N. Li, L. Li, Y. Yu, R. Tuerhong, X. Su, B. Zhang, L. Han and Y. Han, ChemPhysChem, 2024, 25, e202400075 CrossRef CAS.
- Z. Chen, G. Ding, Z. Wang, Y. Xiao, X. Liu, L. Chen, C. Li, H. Huang and G. Liao, Adv. Funct. Mater., 2025, 35, 2423213 CrossRef CAS.
- L. Wang, Y. Qi, Z. Yang, H. Wu, J. Liu, Y. Tang and F. Wang, Green Energy Resour., 2023, 1, 100036 CrossRef.
- Y. X. Xu, J. L. Qin, F. Wang and H. Z. Wang, Green Chem., 2025, 27, 4489–4503 RSC.
- A. Kumar and R. Ananthakrishnan, Green Chem., 2020, 22, 1650–1661 RSC.
- S. Cestellos-Blanco, H. Zhang, J. M. Kim, Y.-X. Shen and P. D. Yang, Nat. Catal., 2020, 3, 245–255 CrossRef CAS.
- X. Fang, S. Kalathil and E. Reisner, Chem. Soc. Rev., 2020, 49, 4926–4952 RSC.
- K. K. Sakimoto, A. B. Wong and P. D. Yang, Science, 2016, 351, 74–77 CrossRef CAS.
- J. Ye, C. Wang, C. Gao, T. Fu, C. H. Yang, G. P. Ren, J. Lü, S. G. Zhou and Y. J. Xiong, Nat. Commun., 2022, 13, 6612 CrossRef CAS.
- G. Y. Liu, F. Gao, C. Gao and Y. J. Xiong, Chem Catal., 2021, 1, 1367–1377 CAS.
- J. Ye, J. Yu, Y. Y. Zhang, M. Chen, X. Liu, S. G. Zhou and Z. He, Appl. Catal., B, 2019, 257, 117916 CrossRef CAS.
- K. J. Zhang, R. J. Li, J. X. Chen, L. Y. Chai, Z. Lin, L. Zou and Y. Shi, Appl. Catal., B, 2024, 342, 123375 CrossRef CAS.
- Y. He, S. R. Wang, X. Y. Han, J. Y. Shen, Y. W. Lu, J. Z. Zhao, C. P. Shen and L. Qiao, ACS Appl. Mater., 2022, 14, 23364–23374 CrossRef CAS.
- Z. Ji, H. Zhang, H. Liu, O. M. Yaghi and P. D. Yang, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 10582–10587 CrossRef CAS.
- H. Zhang, H. Liu, Z. Q. Tian, D. L. Lu, Y. Yu, S. Cestellos-Blanco, K. K. Sakimoto and P. D. Yang, Nat. Nanotechnol., 2018, 13, 900–905 CrossRef CAS PubMed.
- T. R. Sun, J. J. L. Guzman, J. D. Seward, A. Enders, J. B. Yavitt, J. Lehmann and L. T. Angenent, Nat. Commun., 2021, 12, 4119 CrossRef CAS.
- S. Price, U. Kuzhiumparambil, M. Pernice and P. Ralph, J. Environ. Chem. Eng., 2022, 10, 107502 CrossRef CAS.
- Y. H. Feng, M. Y. Xu, P.-L. Tremblay and T. Zhang, Int. J. Hydrogen Energy, 2021, 46, 21901–21911 CrossRef CAS.
- Y. C. Ding, J. R. Bertram, C. Eckert, R. R. Bommareddy, R. Patel, A. Conradie, S. Bryan and P. Nagpal, J. Am. Chem. Soc., 2019, 141, 10272–10282 CrossRef CAS.
- P.-L. Tremblay, M. Y. Xu, Y. M. Chen and T. Zhang, iScience, 2020, 23, 100784 CrossRef CAS.
- W. Yu, M. V. Pavliuk, A. Liu, Y. Zeng, S. P. Xia, Y. M. Huang, H. T. Bai, F. T. Lv, H. N. Tian and S. Wang, ACS Appl. Mater. Interfaces, 2022, 15, 2183–2191 CrossRef.
- X. C. Wang, K. Maeda, A. Thomas, K. Takanabe, G. Xin, J. M. Carlsson, K. Domen and M. Antonietti, Nat. Mater., 2009, 8, 76–80 CrossRef CAS PubMed.
- C. Y. Feng, J. Luo, C. L. Chen, S. W. Zuo, Y. F. Ren, Z. P. Wu, M. Hu, S. Ould-Chikh, J. Ruiz-Martínez, Y. Han and H. B. Zhang, Energy Environ. Sci., 2024, 17, 1520–1530 RSC.
- M. Y. Xu, P.-L. Tremblay, L. L. Jiang and T. Zhang, Green Chem., 2019, 21, 2392–2400 RSC.
- W. Liu, P. F. Wang, J. Chen, X. Gao, H. Che, B. Liu and Y. H. Ao, Adv. Funct. Mater., 2022, 32, 2205119 CrossRef CAS.
- Y. Pang, P. Li, X. Ma, L. Sun, Y. Liu, D. Qu, L. An and Z. Sun, EES Catal., 2023, 1, 810–831 RSC.
- Y. Xia, Z. H. Tian, T. Heil, A. Y. Meng, B. Cheng, S. W. Cao, J. G. Yu and M. Antonietti, Joule, 2019, 3, 2792–2805 CrossRef CAS.
- L. Chen, J. Y. Yu, Z. Y. Lyu, X. Y. Wen, Y. F. Wang, S. H. Cao and W. Wang, Appl. Surf. Sci., 2024, 642, 158550 CrossRef CAS.
- Z. Wei, M. L. Liu, Z. J. Zhang, W. Q. Yao, H. W. Tan and Y. F. Zhu, Energy Environ. Sci., 2018, 11, 2581–2589 RSC.
- Q. Fan, H. Zhang, D. Liu, C. Yan, H. Zhu, Z. Xie and Z. Le, J. Org. Chem., 2023, 88, 7391–7400 CrossRef CAS PubMed.
- Q. Fan, D. Liu, Z. Xie, Z. Le, H. Zhu and X. Song, J. Org. Chem., 2023, 88, 14559–14570 CrossRef CAS.
- P. Ravindran and N. Vasanthan, Macromolecules, 2015, 48, 3080–3087 CrossRef CAS.
- J.-Y. Zeng, X.-S. Wang, X.-H. Liu, Q.-R. Li, J. Feng and X.-Z. Zhang, Natl. Sci. Rev., 2023, 10, nwad142 CrossRef CAS PubMed.
- M. S. Morlino, R. Serna García, F. Savio, G. Zampieri, T. Morosinotto, L. Treu and S. Campanaro, Biotechnol. Adv., 2023, 69, 108264 CrossRef CAS PubMed.
- K. Zhang, X. Xu, X. L. Li, T. Song, J. Xie and T. Guo, Fuel, 2024, 357, 129740 CrossRef CAS.
- M. R. Galih Pangestu, S. A. Razzak and S. Uddin, Green Energy Resour., 2025, 3, 100131 CrossRef.
- F. Pietrocola, L. Galluzzi, J. M. Bravo-San Pedro, F. Madeo and G. Kroemer, Cell Metab., 2015, 21, 805–821 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2026 |