CO2 hydrogenation to CH3OH promoted by strong CuxO–MgO interactions and non-thermal plasma under mild conditions
Qian
Chen
a,
Shengyan
Meng
a,
Xiaohan
Zhai
b,
Li
Wang
b,
Zhaolun
Cui
c,
Dongxing
Li
a,
Chuang
Li
 a,
Chong
Peng
a and
Yanhui
Yi
a,
Chong
Peng
a and
Yanhui
Yi
 *a
*a
aState Key Laboratory of Fine Chemicals, Frontier Science Center for Smart Materials, School of Chemical Engineering, Dalian University of Technology, Dalian 116024, Liaoning, China. E-mail: yiyanhui@dlut.edu.cn
bCollege of Environmental Sciences and Engineering, Dalian Maritime University, Dalian 116026, Liaoning, China
cKey Lab of Materials Modification by Laser, Ion, and Electron Beams, Dalian University of Technology, Dalian 116024, Liaoning, China
First published on 3rd December 2025
Abstract
Herein, we report the strong interaction between CuxO and MgO and its crucial role in promoting plasma-catalytic CO2 hydrogenation to produce CH3OH. Four MgO supports and corresponding CuxO/MgO catalysts were prepared and systematically characterized and tested in plasma-catalytic CO2 hydrogenation. The results show that MgO supports with lower crystallinity and more defects favor stronger interactions with CuxO species, which result in better CO2 conversion and CH3OH selectivity in plasma-catalytic CO2 hydrogenation, achieving 9.1% CO2 conversion and 46.2% CH3OH selectivity under ambient reaction conditions (30 °C and 0.1 MPa). In situ FTIR spectra and catalyst characterization results demonstrate that basic sites of MgO and Oads. species activate CO2 molecules into CO3* species, which are further hydrogenated by H species to form the HCOO* and HCO* species on the MgO support. CuxO sites further promote the subsequent hydrogenation steps to move the reaction forward more easily to form the CH3O* intermediate and the CH3OH product, which could be the main reason why the strong CuxO–MgO interactions promote CH3OH production in plasma-catalytic CO2 hydrogenation.
Green foundation1. This study achieves efficient low-temperature CO2 conversion at 30 °C and ambient pressure via a plasma-driven CuxO/MgO catalytic system. The high-energy electrons from the plasma activate inert CO2 molecules, while the strong CuxO–MgO interaction directs hydrogenation pathways, circumventing traditional high temperature and pressure requirements (200–300 °C and 2–5 MPa).2. A cost-effective Cu-based catalyst exhibits 9.1% CO2 conversion with 46.2% CH3OH selectivity, transforming the greenhouse gas CO2 into methanol—a key organic chemical feedstock. 3. Future work will design bifunctional and tandem catalysts to hydrogenate the byproduct CO into methanol in situ, alongside developing MgO-confined structures to enhance mass transfer efficiency, aiming for higher methanol selectivity. |
1. Introduction
The increase in global population has resulted in an escalating demand for primary energy, more than 70% of which is provided by fossil fuels (coal, natural gas, and oil). This upsurge has led to an increasing atmospheric concentration of carbon dioxide (CO2), which is considered to be the principal driver of adverse climate change and ocean acidification.1Extensive research studies have been directed toward the capture, separation and conversion of CO2 to yield valuable chemicals (e.g., methanol, formic acid, ethanol and hydrocarbons),2–8 among which CO2 hydrogenation to methanol (CH3OH) is an important strategy,9–11 given that CH3OH is a versatile molecule widely used in the chemical and energy industries. However, thermodynamic stability of the CO2 molecule poses challenges in terms of activation under conventional conditions. Thus, the activation of CO2 and the generation of methanol has traditionally been achieved at relatively high temperature and high pressure,9,10,12–15 yet this approach engenders substantial economic burdens.16,17 Additionally, the reactions to produce CO are favorable under high temperature conditions in terms of thermodynamics.18
Non-thermal plasma (NTP), an emerging technology, utilizes energetic electrons that are capable of activating CO2 and H2 to form active species, including radicals and excited (electronic and vibrational) species, which facilitate the CO2 hydrogenation reaction at low temperature and atmospheric pressure.18,19 Additionally, NTP offers high productivity and quick on/off capability, reducing operating costs and enhancing production efficiency. To date, NTP-assisted CO2 hydrogenation has been investigated by many groups,20–25 and excellent performances have been achieved at low temperature and atmospheric pressure using Cu-, Pt- and Fe-based catalysts.18,26–29
MgO, a metal oxide with moderate basicity, is widely used as a catalyst, support, catalytic promoter, flame retardant and adsorbent.1 With suitable basicity, MgO demonstrates moderate adsorption for CO2, making it a popular choice for CO2 capture.30–33 Furthermore, MgO is capable of enhancing the adsorption capacity for CO2 as a promoter, and thus it has been used to improve the performance of CO2 hydrogenation in thermal catalysis.13,34,35
In this study, Cu-based catalysts with MgO as the support were designed aiming to strengthen plasma-catalytic CO2 hydrogenation to CH3OH. Four types of MgO materials were synthesized and used as supports to prepare CuxO/MgO catalysts, which were tested in the plasma-catalytic CO2 hydrogenation reaction. By combining the catalytic performances, the catalyst characterization results, and in situ FTIR spectra, it was found that the strong interactions between CuxO and MgO are favorable for plasma-catalytic CH3OH generation. Experimental details are shown in the SI.
2. Experimental section
2.1 Catalyst preparation
2.2 Synthesis of MgO supports
2.3 Preparation of CuxO/MgO catalysts
The CuxO/MgO catalysts were prepared using an impregnation method with Cu(NO3)2·3H2O as the copper source, as shown in Fig. S5. The amount of CuxO is expressed as the Cu loading, which was 10 wt% for all samples. The impregnated precursor was dried at 110 °C overnight and then calcined in air at 540 °C for 5 h. The prepared catalysts were sieved into target particles of 20–40 mesh for filling in the reaction area.2.4 Catalytic tests
Fig. S6 illustrates the schematic configuration of the plasma-catalytic CO2/H2 conversion system. The reaction occurs within a coaxial dielectric barrier discharge (DBD) reactor designed to generate CO2/H2 plasma, featuring a dual-electrode configuration where a stainless-steel rod of 2 mm diameter serves as the high-voltage central electrode. A water-cooling system circulates deionized water through the inter-cylinder space, simultaneously functioning as the grounding electrode. The discharge gap with discharge length of 60 mm was filled with catalyst granules (0.42–0.85 mm in diameter). Gas feed streams were precisely regulated through calibrated mass flow controllers (MFC, CS200, SevenStar, China) with volumetric verification being conducted using a soap-film flowmeter maintaining flow rates of 18 mL min−1 (CO2) and 54 mL min−1 (H2) under ambient pressure.The plasma-catalytic system employed a DBD reactor powered by an AC high-voltage generator with a frequency range of 7–12 kHz (CTP-2000K, Suman, China). Real-time monitoring of electrical parameters,including applied voltage, discharge current, and external capacitor voltage, was achieved through a two-channel digital oscilloscope (Tektronix, MDO 3024). Plasma power quantification employed the Q-U Lissajous methodology, with operational parameters maintained at an excitation frequency of 9.2 kHz and a net discharge power of 24 W.
A gas chromatograph (Agilent 8860) equipped with a thermal conductivity detector (TCD) connected to a Porapak Q + 5A molecular sieve column (2 m × 2.1 mm) and a flame ionization detector (FID) connected to a HP-PLOT/Q capillary column (30 m × 0.53 mm × 40 μm) was used to analyze the gaseous products. CH4 and CH3OH were analyzed using the FID, while CO2, CO and H2 were analyzed using the TCD. The reaction temperature in the discharge area was close to the circulating water temperature (30 °C).
To evaluate the reaction performance, the CO2 conversion was calculated using eqn (1):
 | (1) |
In the tail gas, CH3OH, CO and CH4 were detected using the gas chromatograph. The selectivity of CH3OH, CO and CH4 was calculated by using eqn (2), (3) and (4), respectively:
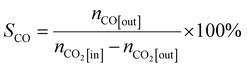 | (2) |
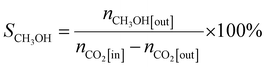 | (3) |
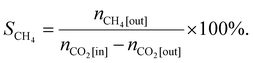 | (4) |
Based on the results of thermogravimetric analysis-mass spectrometry (TGA-MS), as shown in Fig. S10 and Table S11, the coke deposition on the catalyst was found to be negligible.
The carbon balance was calculated using eqn (5):
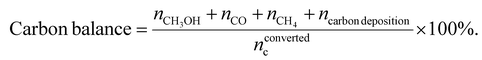 | (5) |
2.5 Catalyst characterization
The crystalline phase of the catalysts was analyzed using a SmartLab 9KW (Rigaku, Japan) powder X-ray diffractometer (PXRD) with Cu Kα radiation (λ = 0.15406 nm) under operational parameters of 240 kV and 50 mA. Diffraction patterns were acquired in the 2θ range of 10°–80° with a scanning rate of 10° min−1 and step size of 0.02°. The specific surface area, N2 adsorption–desorption isotherms, and Barrett–Joyner–Halenda (BJH) pore size distributions were determined using a low-temperature physisorption analyzer (ASAP 3020, Micromeritics, USA), following sample degassing at 350 °C for 5 h under a 10−3 Torr vacuum. The surface morphology and particle size of the catalysts were investigated via field emission scanning electron microscopy (FESEM, SU5000, Hitachi, Japan) at 5 kV accelerating voltage. Atomic-scale microstructure was resolved using a high-resolution transmission electron microscopy system (HRTEM, JEM-F200, JEOL, Japan) operating at 200 kV voltage, demonstrating 0.23 nm point resolution and 0.10 nm lattice resolution. Quasi in situ X-ray photoelectron spectroscopy (quasi in situ XPS) measurements were performed using a spectrometer (PHI 5000 Versaprobe II, ULVAC-PHI, Japan) with a micro-focused monochromatic Al Kα X-ray source and scanning X-ray beam-induced secondary electron image. Binding energy calibration utilized adventitious carbon (C 1s = 284.8 eV) as the internal reference. Immediately after the plasma-catalytic reaction, the entire reactor was transferred into a nitrogen-filled glovebox. Inside the glovebox, the spent catalyst was carefully retrieved and sealed in a sample vial. Prior to XPS scanning, the sealed vial was moved into a dedicated glovebox that was directly connected to the X-ray photoelectron spectrometer. The entire sample transfer process was conducted under an inert atmosphere without exposure to air, which ensured the stability of the Cu valence state and the accuracy of the measurements. Hydrogen temperature-programmed reduction (H2-TPR) and CO2 temperature-programmed desorption (CO2-TPD) experiments were conducted with Quantachrome ChemBET Pulsar TPR apparatus (Anton Paar, Austria). For H2-TPR, 0.15 g samples were pretreated in He (550 °C, 1 h) followed by 10% H2/Ar temperature ramping (50–500 °C, 10 °C min−1) with TCD monitoring. The CO2-TPD involved saturation adsorption (120 mL min−1, 99.99% CO2, 80 min) prior to programmed desorption (50–760 °C, He flow, 10 °C min−1), and the signal of the CO2 desorption was recorded using a TCD detector. Raman spectra of the samples were collected using a micro-confocal Raman spectrometer (InVia Qontor, Renishaw, UK) with a 532 nm excitation laser (5 mW) and an integration time of 10 s. The dispersion, particle size and specific surface area of metallic copper (Cu) were further quantified by N2O chemisorption, conducted on the same apparatus used for the H2-TPR analyses. The detailed experimental procedures and calculation methods are provided in the SI.2.6 Plasma diagnostics
In situ optical emission spectroscopy of the CO2/H2 plasma was performed using a Princeton Instruments SP-2758 intensified ICCD spectrometer equipped with a 300 g mm−1 holographic grating covering the 200–1100 nm spectral range. The spectral resolution was optimized with fixed instrumental parameters: 20 μm entrance slit width and 2 s integration time per acquisition. Dynamic plasma parameters were captured through a digital oscilloscope (DPO 3012, Tektronix, USA) with specialized probes: a P6015A high-voltage passive probe for voltage waveform acquisition and a Pearson Electronics 6585 Rogowski coil current monitor. Power quantification employed the Lissajous graphical method with phase-resolved charge–voltage (Q–U) analysis.2.7 In situ FTIR setup
In situ FTIR measurements were conducted using an FTIR spectrometer (Nicolet iS10, Thermo, USA) equipped with a rapidly recoverable detector containing deuterated triethylene glycol sulfate (DTGS) to observe surface species of catalyst and study the mechanism of CO2 hydrogenation to CH3OH. The catalyst, pressed into a tablet (8 mm), was placed into the custom-designed plasma reaction chamber (Scheme S1). Then, a flow of pure CO2 (18 mL min−1) was introduced for 30 minutes to allow for the saturation of adsorption sites on the catalyst surface, and the spectrum collected at this stage represents the initially adsorbed species. H2 was then introduced to form a CO2/H2 mixture (25 vol% CO2, 75 vol% H2, 18 mL min−1 CO2 and 54 mL min−1 H2), and spectra were recorded for 10 minutes in the absence of plasma. After the signal stabilized, the plasma was ignited while maintaining the CO2/H2 flow, and spectra were then recorded sequentially over time to monitor the dynamic changes of surface species triggered by the plasma. In situ infrared spectra were recorded from 900 to 4000 cm−1 with a resolution of 4 cm−1, and the plasma discharge frequency and input power were set to 9.2 kHz and 24 W, respectively.3. Results and discussion
3.1 Synthesized MgO supports
Four MgO supports were prepared using hydrothermal and sol–gel methods with different preparation procedures (Fig. S1–S4). The synthesized MgO samples were denoted MgO-1, MgO-2, MgO-3 and MgO-4, respectively. Accordingly, employing an impregnation method (Fig. S5), the prepared CuxO/MgO catalysts (ca. 10 wt% loading of Cu) were named CuxO/MgO-1, CuxO/MgO-2, CuxO/MgO-3 and CuxO/MgO-4, respectively.Fig. 1A shows the XRD patterns of the synthesized MgO supports. Diffraction peaks of the (200) and (220) planes of MgO were observed, and the intensity gradually decreased following the sequence MgO-1 > MgO-2 > MgO-3 > MgO-4. That is, the MgO samples show decreasing crystallinity from MgO-1 to MgO-4. The N2-physisorption isotherms are shown in Fig. 1B, and the corresponding texture information (Table S1) indicates an increasing trend for the specific surface area of the synthesized MgO samples, following the sequence MgO-1 < MgO-2 < MgO-3 < MgO-4. In other words, the MgO-1 sample possesses the highest crystallinity but the smallest specific surface area, while the MgO-4 sample has the largest specific surface area. Fig. 1C shows the SEM images of the MgO samples. It can be seen that MgO-1 and MgO-2 feature hexagonal and spherical particles, respectively, both with uniform morphology and high crystallinity. However, the images of MgO-3 and MgO-4 display irregularly shaped particles with relatively low crystallinity.
 | ||
| Fig. 1 Characterization of the synthesized MgO samples. (A) XRD patterns, (B) N2 adsorption isotherms, and (C) SEM images. | ||
3.2 Catalytic performance of CuxO/MgO catalysts
Fig. 2 shows the performance of the plasma-catalytic CO2 hydrogenation reaction employing MgO and CuxO/MgO as catalysts. For the MgO supports, the CO2 conversion is very low (Fig. 2A), and the main product is CO (Fig. 2C). However, the CuxO/MgO catalysts shows much improved performance with both enhanced CO2 conversion (Fig. 2A) and CH3OH selectivity (Fig. 2B), indicating that the CuxO active sites are crucial for the production of CH3OH. Notably, for the CuxO/MgO catalysts employing different MgO supports, the CO2 conversion and CH3OH selectivity gradually increase following the sequence CuxO/MgO-1 < CuxO/MgO-2 < CuxO/MgO-3 < CuxO/MgO-4. However, CO selectivity shows a contrary trend (Fig. 2C). That is, the MgO supports play an important role in plasma-catalytic CO2 hydrogenation to CH3OH, and the CuxO/MgO-4 catalyst shows superior performance, achieving 9.1% CO2 conversion and 46.2% CH3OH selectivity. From Table S3, the (space–time yield) STY of CH3OH achieved in our work ranks at the middle level among the reported plasma-catalysis reactions. The long-term test (Fig. S7) exhibits excellent stability over a 24 hour period for all four CuxO/MgO catalysts, with no significant decline observed in either CO2 conversion or CH3OH selectivity.Fig. 2D shows the performances of “plasma only”, “plasma + MgO-4” and “plasma + CuxO/MgO-4”, which confirms an obvious synergistic effect between plasma and the CuxO/MgO-4 catalysts in driving CO2 hydrogenation to selectively produce CH3OH.
As shown in Table S5, the selectivity of CH4 is 2.6% in the plasma only experiment, and with the introduction of the CuxO/MgO catalyst, the CH4 selectivity was significantly suppressed to 0.5%. The simultaneous increase in CO2 conversion and decrease in CH4 selectivity after introducing the CuxO/MgO catalysts strongly suggests that the catalysts effectively suppress the deep hydrogenation pathway that leads to CH4.
3.3 Catalyst characterization
Fig. 3A shows the XRD patterns of the CuxO/MgO catalysts. Clearly, the samples show nearly identical XRD patterns dominated by the MgO species. Diffraction peaks at 35.55° and 38.73°, associated with the (−111) and (111) planes of CuO, respectively (PDF#05-0661), are observed. However, the intensities are much lower than those of the MgO diffraction peaks, which means Cu species may be highly dispersed on the MgO supports. Fig. 3B shows the H2-TPR profiles of the CuxO/MgO catalysts. Since MgO cannot be reduced at temperatures below 600 °C,13,35,40,41 the observed H2 consumption peaks are attributed to the reduction of Cu species. After peak fitting, the H2-TPR profiles show three peaks denoted α, β and γ, respectively. Generally, the α peak is caused by the reduction of CuO species with strong interactions with the MgO support;42 the β peak corresponds to the reduction of highly dispersed CuOx clusters with smaller sizes; and the γ peak originates from the reduction of Cu2O to metallic Cu0 species.41,43 The γ peak is observed in the CuxO/MgO-3 and CuxO/MgO-4 catalysts but is absent for the CuxO/MgO-1 and CuxO/MgO-2 catalysts, which means that the CuxO/MgO-3 and CuxO/MgO-4 samples may have an increased content of Cu2O. Additionally, the reduction temperature of CuxO shows an increasing trend: CuxO/MgO-1 < CuxO/MgO-2 < CuxO/MgO-3 < CuxO/MgO-4, which suggests that the interaction between CuxO and MgO is gradually enhanced with the decrease of MgO crystallinity. That is, MgO supports with low crystallinity favor strong oxide–support interaction due to the existence of more defects, and the CuxO/MgO-4 catalyst exhibits the strongest oxide–support interaction between CuxO and MgO. Furthermore, this trend correlates perfectly with the significantly improved catalytic performance for CH3OH synthesis.The dispersion of Cu (proportion of surface Cu atoms in relation to the total number of Cu atoms) in the CuxO/MgO-X (X = 1, 2, 3, 4) catalyst was measured by the N2O chemisorption experiment, as shown in Fig. S8, while the corresponding experimental data for specific surface area (SCu) and particle size (DCu) of Cu are shown in Table S6. The dispersion of Cu for the samples CuxO/MgO-1, CuxO/MgO-2, CuxO/MgO-3 and CuxO/MgO-4 was calculated to be 15.7, 18.2, 20.8 and 25.7%, respectively. That is, with the decrease of crystallinity of the MgO supports, the average particle size of Cu in the CuxO/MgO catalysts presents a decreasing trend, and thus the dispersion of Cu shows an increasing trend, as presented in Fig. 3C. For the sample CuxO/MgO-4, the average particle size of Cu was estimated to be around 3.85 nm and the dispersion of the Cu was ca. 25.7%. Overall, the H2-TPR and N2O chemisorption experiments indicate that the oxide–support interactions between CuxO and MgO gradually intensifies following the sequence CuxO/MgO-1 < CuxO/MgO-2 < CuxO/MgO-3 < CuxO/MgO-4, corresponding with the increasing CuxO dispersion.
Fig. 4A and B show the Cu 2p3/2 spectra of the fresh and spent CuxO/MgO samples, respectively (the fitted values and deconvolution details are shown in Tables S7 and S8). For the fresh CuxO/MgO-1 sample (Fig. 4A), the satellite companion peak at 942.6 eV indicates the predominance of divalent Cu species (CuO and Cu2+ in the exchange state). The Cu 2p3/2 peak at 933.9 eV corresponds to the Cu+ species and the Cu 2p3/2 peak at 935.4 eV is attributed to the Cu2+ species.44,45 We can also note that, compared with the CuxO/MgO-1 sample, the binding energies of satellite, Cu2+ and Cu+ are reduced for the samples CuxO/MgO-3 and CuxO/MgO-4, indicating electron shift from MgO to Cu species, which may be caused by interactions between CuxO and MgO. This result is consistent with the conclusion of enhanced interactions from H2-TPR characterization. For the spent CuxO/MgO catalysts, a notable increase in the relative content of Cu+ is observed, suggesting that CuO is partially reduced to Cu2O during the H2/CO2 plasma reaction. Fig. 4C shows the relationship of Cu+ content and reaction performances. The relative content of Cu+ for the spent CuxO/MgO catalysts shows a more pronounced increasing trend (CuxO/MgO-1 < CuxO/MgO-2 < CuxO/MgO-3 < CuxO/MgO-4), which corresponds to CO2 conversion and CH3OH selectivity that are gradually improved, demonstrating the active site role of Cu2O.
Fig. 4D and E show the O 1s spectra of fresh and spent CuxO/MgO samples, respectively (the fitted values and deconvolution details are shown in Tables S9 and S10). The O 1s spectra of the fresh and spent CuxO/MgO samples can be deconvoluted into two peaks, i.e. 529.5 eV and 531.5 eV, corresponding to surface lattice oxygen (Olatt.) and adsorbed oxygen (Oads.) species, respectively.35 Interestingly, the relative content of Oads. gradually increases following the sequence CuxO/MgO-1 < CuxO/MgO-2 < CuxO/MgO-3 < CuxO/MgO-4, and the content of Olatt. shows an inverse trend, irrespective of fresh or spent CuxO/MgO samples. The highest content of Oads. but the lowest content of Olatt. species occurs in the CuxO/MgO-4 sample and originates mainly from the strong CuxO–MgO interactions, which are caused principally by low crystallinity and the presence of more defects on the MgO-4 support. Fig. 4F shows the positive relationship of Oads. species with CO2 conversion and CH3OH selectivity. That is, Cu2O and Oads. species that originated from strong CuxO–MgO interactions play an important role in plasma-catalytic CO2 hydrogenation for CH3OH production.
Fig. 5A shows the Raman spectra of the CuxO/MgO samples. The characteristic band that is peaked at 292 cm−1 corresponds to the Ag modes of CuO.46 The Raman shift bands at 330–760 cm−1 and 830–1380 cm−1 are associated with the MgO characteristic peaks.47–49 The former band is attributed to the longitudinal optical (LO) mode of MgO particles, while the latter is attributed to the MgO symmetric stretching mode of A1 symmetry.49 Notably, the two Raman shift bands of MgO show the characteristic broadening and softening effects from CuxO/MgO-1 to CuxO/MgO-4. That is, the CuxO/MgO-4 sample shows the strongest MgO characteristic peak with the lowest Raman shift. The variations of the position and intensity of MgO characteristic peaks are mainly caused by the gradually enhanced interactions between CuxO and MgO supports, as indicated by the quasi in situ XPS and H2-TPR results.
The CO2 adsorption capacity of the catalysts has been proved to be a critical factor in enhancing the performance of CO2 hydrogenation to CH3OH.13,35Fig. 5B shows the CO2-TPD profiles of the CuxO/MgO samples. The α, β and γ peaks correspond to weak, moderate and strong basic sites in the catalysts, respectively.40 It is evident that the quantity of moderate and strong basic sites in the catalysts continually increase following the sequence CuxO/MgO-1 < CuxO/MgO-2 < CuxO/MgO-3 < CuxO/MgO-4. That is, the CO2 adsorption capacity of the CuxO/MgO catalysts is gradually enhanced with the decrease of MgO crystallinity. From the results of the quasi in situ XPS study, we inferred that the enhanced CO2 adsorption capacity is attributed to the increased content of Oads. in the catalysts with the increase of CuxO–MgO interactions. Fig. 4F indicates that there is a positive correlation between the Oads. content in the catalysts and the performance of plasma-catalytic CO2 hydrogenation to CH3OH. In conjunction with the CO2-TPD, it is inferred that the Oads. enhances the CO2 adsorption capacity of the catalysts, thereby increasing the CO2 conversion and the CH3OH selectivity.
Fig. S9 displays the TEM images of the fresh CuxO/MgO catalysts. The results reveal that the different preparation methods for MgO influences the exposed planes of the CuO crystals. The crystalline lattice distances of 0.232 nm and 0.252 nm correspond to the (111) and (−111) planes of CuO, respectively. Moreover, the size of the CuO particles is observed to be about 5–8 nm, and these results of particle size are in accordance with the results of N2O chemisorption with respect to the particle size (Fig. 3C).
To further elucidate the nature of the active Cu species under reaction conditions, the spent catalysts were examined using TEM, with representative images shown in Fig. 5C–F. Notably, crystalline lattices corresponding to Cu2O were clearly observed in all spent samples. This direct microscopy evidence for the formation of Cu2O during the plasma-catalytic process strongly corroborates our quasi in situ XPS findings (Fig. 4B and C), which indicated a significant increase in the relative content of Cu2O species after the reaction. The convergence of these results provides compelling support for the hypothesis that Cu2O species are the primary active sites for the hydrogenation of CO2 to CH3OH.
3.4 Plasma diagnosis
The plasma discharge behaviors are diagnosed using a two-channel digital oscilloscope. Fig. 6A shows that there is no significant change in the waveform of the voltage for different catalysts. In Fig. 6B, the current waveform for different catalysts is gradually enhanced except for the plasma only, indicating that the catalyst with a larger specific surface area facilitates the surface discharge. As shown in Fig. 6C, the Lissajous figure changes from a parallelogram shape (plasma only) to an oval shape (plasma + CuxO/MgO), indicating a significant shift in the discharge behavior from the crucial filamentary discharges to surface discharges.50 The measured discharge power differs from the input power displayed by the plasma source (24 W), because we measured the actual discharge power across the reactor rather than the input power of the plasma source. The small differences in actual discharge power observed among the different catalysts at the same input power (24 W)arise from changes in the equivalent capacitance of the reactor, which varies with the dielectric properties of the materials packed into the system.The electron energy distribution function (EEDF) of the CO2/H2 plasmas under our experimental conditions was calculated using BOLSIG+.51Fig. 6D shows the mean electron energy as a function of the reduced electric field (E/N). The mean electron energy is 1.7 eV under the condition of plasma only. Clearly, packing of CuxO/MgO catalysts in the CO2/H2 plasma system dramatically enhances the mean electron energy, reaching ca. 4.5–4.7 eV, and a closer inspection reveals a slightly increasing trend from CuxO/MgO-1 to CuxO/MgO-4. This suggests that the catalyst with a stronger oxide–support interaction may slightly enhance the electric field in the discharge gap, leading to a higher mean electron energy. Furthermore, it can be found that the integration of plasma and CuxO/MgO catalysts strongly changes the electron energy distribution in CO2/H2 plasma and the four different CuxO/MgO catalysts show a similar energy distribution as shown in Fig. 6E. We also identified a clear trend of increasing mean electron energy from CuxO/MgO-1 to CuxO/MgO-4. The enhancement of mean electron energy produces more energetic electrons and chemically reactive species for plasma reactions and consequently contributes to the conversion of CO2.
In situ optical emission spectroscopy (OES) was used to identify key species in the CO2/H2 plasma, as presented in Fig. 6F. The CO2/H2 plasma exhibits strong signal intensity, featuring several spectral lines and bands without a catalyst or support, such as the Hα line (656.3 nm, 3d2D → 2p2P0),52 two O atomic spectral lines (777.5 nm, 3s5S0 → 3p5P; 844.7 nm, 3s3S0 → 3p3P),53,54 a band for H2 (580–650 nm, d3Πu → a3Σg+) and a CO band (450 nm → 580 nm, B1Σ → A1Π).55 These findings suggest that H atoms and CO molecules are abundant in the CO2/H2 plasma. After packing the CO2/H2 plasmas with CuxO/MgO catalysts, the spectral intensities were dramatically weakened, which may be caused by both the shielding effect of catalyst granules on light propagation and chemisorption of excited species on catalyst surface. More importantly, the enlarged OES spectra shown in Fig. S11 clearly demonstrate that the hydrogen-related species (Hα at 656.3 nm and H2 band at 580–650 nm) and CO band (450–580 nm) underwent significant intensity decreases. This trend confirms that the CuxO/MgO-4 catalyst, which possesses the strongest CuxO–MgO interaction, is more effective at adsorbing and consuming these plasma-generated active species for the hydrogenation reaction. That is, the gradually decreased OES intensities (from CuxO/MgO-1 to CuxO/MgO-4) were caused by variation of chemisorption of excited species on the catalyst surface. The CuxO/MgO-4 catalyst with strong CuxO–MgO interactions and abundant defects is more conducive to adsorb excited species from CO2/H2 plasma. This should be one of the most important reasons why the CuxO/MgO-4 catalyst exhibits a superior performance compared with the other CuxO/MgO catalysts in plasma-catalytic CO2 hydrogenation to CH3OH.
3.5 Discussion of the reaction mechanism
In situ FTIR studies were conducted to investigate the active intermediates and reaction pathways in plasma-catalytic CO2 hydrogenation to CH3OH on CuxO/MgO catalysts. As shown in Fig. 7A and B, the behavior of the bare MgO support under plasma was first investigated as a control. With increasing CO2 exposure times, the amount of carbonate species (CO3* at 1456, 1540 and 1649 cm−1) on the catalyst surface gradually increased.56,57 Upon plasma ignition, a significant increase in the intensity of gas-phase CO was observed at 2115 and 2170 cm−1,58 concurrently with the emergence of an infrared absorption band at 1338 cm−1, which was assigned to the formyl (HCO*) intermediate.59 Additionally, a weak peak attributed to formate (HCOO*) can be observed at 2841 cm−1.56 This observation suggests that on the MgO support, the reaction proceeds via two parallel pathways: (i) a plasma-induced gas-phase dissociation of CO2 to form CO, which subsequently reacts with H species from gas through a reverse water–gas shift (RWGS) pathway to generate HCO*,18,21 and (ii) the reaction between CO3* on the MgO surface and H species from gas, which follows the formate pathway to produce CH3OH.21,22 Although HCO* and HCOO* are plausible precursors, their subsequent hydrogenation to CH3OH was not detected, presumably due to their extremely low yield on the bare support.In stark contrast, the plasma-catalytic process over the CuxO/MgO catalyst exhibits a distinctly different reaction network. Under identical discharge conditions, the signal for gas-phase CO was significantly suppressed. However, the intensity of the HCO* intermediate was more pronounced than on MgO, and a new peak emerged at 2077 cm−1, corresponding to CO adsorbed on Cu2O active sites (M–CO).60 This suggests that on CuxO/MgO, CO generated from plasma dissociation preferentially adsorbs onto the catalyst surface and reacts with gas-phase H species via an Eley–Rideal (E–R) mechanism to form HCO*, which is then hydrogenated to CH3OH. More importantly, several new, crucial intermediates were observed exclusively on the CuxO/MgO surface. As the plasma discharge proceeded, the intensity of surface CO3* showed a slight increase, which could be attributed to the plasma-enhanced adsorption of CO2.61 Critically, absorption bands corresponding to HCOO* at 1592 cm−1 (ref. 58) and methoxy (CH3O*) at 2820 and 1464 cm−1 (ref. 62 and 63) appeared and intensified over time. Their evolutionary trend mirrored that of the surface CO3*, providing direct evidence for the formate-mediated pathway: CO2 first adsorbs on the surface to form CO3*, which are then hydrogenated to HCOO*, and subsequently to CH3O*, the direct precursor to CH3OH.
By comparing the relative intensities of the key intermediates on CuxO/MgO, the signals for CH3O* and HCOO* were substantially stronger than that for HCO*. This strongly indicates that the formate pathway is the dominant reaction route for CH3OH synthesis on the CuxO/MgO catalyst. These collective observations highlight the multifunctional role of the Cu2O active sites. On the one hand, they adsorb plasma-generated CO, facilitating a minor reaction channel via the Langmuir–Hinshelwood (L–H) mechanism. On the other hand, and more critically, Cu2O active sites are adept at adsorbing H, enabling the hydrogenation of surface carbonates. This process can occur both via an E–R mechanism (adsorbed carbonate reacting with gas-phase H) and an L–H mechanism (adsorbed carbonate reacting with adsorbed H on nearby Cu2O active sites), creating parallel and efficient routes for CH3OH formation (no CH4 production was observed during the entire process from the results of in situ FTIR).
Based on the catalyst characterization results and the in situ FTIR spectra, the possible mechanism of plasma-catalytic CO2 hydrogenation to CH3OH promoted by CuxO–MgO strong interactions is shown in Scheme 1. Base sites of MgO and Oads. species promote activation of the CO2 molecule through chemisorption, leading to formation of CO3* species. Benefiting from the strong hydrogenation capacity of H radicals (generated by plasma), hydrogenation of CO3* species occurs easily to form the HCOO* and HCO* species on the MgO support through the formate and the RWGS pathways, respectively. Furthermore, the subsequent hydrogenation steps proceed more easily in the presence of the Cu2O active sites, leading to formation of the CH3O* intermediate. That is, the synergy of Cu2O active sites and MgO support is crucial for CH3OH generation, which may be explained from two aspects. First, electron transfer from MgO to CuxO (confirmed by quasi in situ XPS) favors the formation of active Cu2O sites. Second, strong interaction between MgO and CuxO means a shorter CuxO–MgO distance and more interfacial sites, which facilitate stepwise hydrogenation on both the MgO support and Cu2O active sites. This may be the main reason why the strong CuxO–MgO interactions promote plasma-catalytic CO2 hydrogenation to produce CH3OH.
 | ||
| Scheme 1 The possible mechanism of plasma-catalytic CO2 hydrogenation to CH3OH (A) MgO support and (B) promoted by the strong CuxO–MgO interaction. | ||
4. Conclusion
Four MgO supports have been synthesized, and corresponding CuxO/MgO catalysts have been prepared to combine with plasma for the CO2 hydrogenation reaction. Catalytic tests and catalyst characterization results show that the MgO support with lower crystallinity and more defects favors stronger interaction with CuxO species, which results in better CO2 conversion and CH3OH selectivity in plasma-catalytic CO2 hydrogenation. The CuxO/MgO-4 catalyst exhibits the best performance, reaching 9.1% CO2 conversion and 46.2% CH3OH selectivity. Furthermore, catalyst characterization and in-situ FTIR spectra demonstrate that strong base sites of MgO and Oads. species activate CO2 molecule into CO3* species, which is further hydrogenated by H species to form the HCOO* and HCO* species on the MgO support, and the presence of the Cu2O active sites promote the subsequent hydrogenation steps that proceed more easily to form the CH3O* intermediate and CH3OH product. This could be the main reason why the strong CuxO–MgO interaction promotes CH3OH production in plasma-catalytic CO2 hydrogenation.Author contributions
Qian Chen: conceptualization, validation, formal analysis, resources, data curation, writing – original draft, and writing – review & editing. Shengyan Meng: conceptualization, validation, formal analysis, and data curation. Xiaohan Zhai: validation, formal analysis, and data curation. Li Wang: resources and data curation. Zhaolun Cui: resources and data curation. Dongxing Li: validation, formal analysis, and data curation. Chuang Li: resources and data curation. Chong Peng: resources and data curation. Yanhui Yi: conceptualization, validation, formal analysis, resources, data curation, writing – original draft, writing – review & editing, supervision, and funding acquisition.Conflicts of interest
There are no conflicts to declare.Data availability
The authors declare that the data supporting the findings of this study are available within the article and its supplementary information (SI). Supplementary information: details of catalyst preparation, the methods of catalytic tests and characterization, plasma diagnostics, in situ FTIR setup, morphological information, standard curve equation, comparison of this work with state-of-the-art plasma-catalysis and thermal-catalysis reported in the literature, the results of stability tests, CH4 selectivity for plasma only and different catalysts, N2O chemisorption, deconvolution details of Cu 2p3/2 and O 1s spectra, TEM for different fresh catalysts, carbon balance analysis and the enlarged in situ OES spectra. See DOI: https://doi.org/10.1039/d5gc02218e.Acknowledgements
We acknowledge the financial support from the National Natural Science Foundation of China [22272015, 22472018, 21503032].References
- O. A. Ojelade and S. F. Zaman, Catal. Surv. Asia, 2019, 24, 11–37 CrossRef.
- Y. Sun, Z. Lin, S. Peng, V. Sage and Z. Sun, J. Nanosci. Nanotechnol., 2019, 19, 3097–3109 CrossRef CAS PubMed.
- L. Fu, Z. Ren, W. Si, Q. Ma, W. Huang, K. Liao, Z. Huang, Y. Wang, J. Li and P. Xu, J. CO2 Util., 2022, 66, 102260 CrossRef CAS.
- S. G. Gizer, O. Polat, M. K. Ram and N. Sahiner, Int. J. Energy Res., 2022, 46, 16241–16263 CrossRef CAS.
- W. Wang, C. Zeng and N. Tsubakia, Green Carbon, 2023, 1, 133–145 CrossRef CAS.
- H. Ju, Y. Zhang, J. Zhang, Z. Yu, Y. Zhang, X. Zhang, X. Guo, J. Liu, Q. Mao, Q. Liu, Y. Zhao, T. Qi, X. Jiang, Z. Guo and S. Chen, Smart Mol., 2025, 3, e20240027 CrossRef CAS PubMed.
- D. Li, M. Ye, C. Ma, N. Li, Z. Gu and Z. Qiao, Smart Mol., 2024, 2, e20240009 CrossRef PubMed.
- T. Xia, Y. Wu, T. Ji, W. Hu, K. Yu, X. He, B. Yin and Y. Liu, Smart Mol., 2025, e20240066 CrossRef CAS PubMed.
- S. Zhang, Z. Wu, X. Liu, K. Hua, Z. Shao, B. Wei, C. Huang, H. Wang and Y. Sun, Top. Catal., 2021, 64, 371–394 CrossRef CAS.
- J. Niu, H. Liu, Y. Jin, B. Fan, W. Qi and J. Ran, Int. J. Hydrogen Energy, 2022, 47, 9183–9200 CrossRef CAS.
- K. Jiang, Y. Xu, F. Cao, B. Li, X. Xu, W. Wang, Y. Tang, L. Wu and Li Tan, Green Carbon, 2025, 3, 139–147 CrossRef.
- S. Dang, B. Qin, Y. Yang, H. Wang, J. Cai, Y. Han, S. Li, P. Gao and Y. Sun, Sci. Adv., 2020, 6, eaaz2060 CrossRef CAS PubMed.
- T. Guo, Q. Guo, S. Li, Y. Hu, S. Yun and Y. Qian, J. Catal., 2022, 407, 312–321 CrossRef CAS.
- C. Wang, Y. Fang, G. Liang, X. Lv, H. Duan, Y. Li, D. Chen and M. Long, J. CO2 Util., 2021, 49, 101542 CrossRef CAS.
- Q. Wu, C. Shen, N. Rui, K. Sun and C. Liu, J. CO2 Util., 2021, 53, 101720 CrossRef CAS.
- X. Jiang, X. Nie, X. Guo, C. Song and J. Chen, Chem. Rev., 2020, 120, 7984–8034 CrossRef CAS PubMed.
- I. Ganesh, Renewable Sustainable Energy Rev., 2014, 31, 221–257 CrossRef CAS.
- L. Wang, Y. Yi, H. Guo and X. Tu, ACS Catal., 2017, 8, 90–100 CrossRef.
- L. Wang, X. Du, Y. Yi, H. Wang, M. Gul, Y. Zhu and X. Tu, Chem. Commun., 2020, 56, 14801–14804 RSC.
- X. Gao, J. Liang, L. Wu, L. Wu and S. Kawi, Catalysts, 2022, 12, 66 CrossRef CAS.
- R. Michiels, Y. Engelmann and A. Bogaerts, J. Phys. Chem., 2020, 124, 25859–25872 CAS.
- M. D. Farahani, Y. Zeng and Y. Zheng, Energy Sci. Eng., 2022, 10, 1572–1583 CrossRef CAS.
- G. Chen, R. Snyders and N. Britun, J. CO2 Util., 2021, 49, 101557 CrossRef CAS.
- S. Xu, H. Chen, C. Hardacre and X. Fan, J. Phys. D:Appl. Phys., 2021, 54, 233007 Search PubMed.
- M. Liu, Y. Yi, L. Wang, H. Guo and A. Bogaerts, Catalysts, 2019, 9, 275 CrossRef.
- Y. Men, Y. Liu, Q. Wang, Z. Luo, S. Shao, Y. Li and Y. Pan, Chem. Eng. Sci., 2019, 200, 167–175 CrossRef CAS.
- S. Meng, L. Wu, M. Liu, Z. Cui, Q. Chen, S. Li, J. Yan, L. Wang, X. Wang, J. Qian, H. Guo, J. Niu, A. Bogaerts and Y. Yi, AICHE J., 2023, e18154 CrossRef CAS.
- Z. Cui, S. Meng, Y. Yi, A. Jafarzadeh, S. Li, E. C. Neyts, Y. Hao, L. Li, X. Zhang, X. Wang and A. Bogaerts, ACS Catal., 2022, 12, 1326–1337 CrossRef CAS.
- Q. Chen, S. Meng, R. Liu, X. Zhai, X. Wang, L. Wang, H. Guo and Y. Yi, Appl. Catal., B, 2024, 342, 123422 CrossRef CAS.
- A. H. Ruhaimi and M. A. A. Aziz, J. Solid State Chem., 2021, 300, 122242 CrossRef CAS.
- A. M. Alkadhem, M. A. A. Elgzoly and S. A. Onaizi, J. Environ. Chem. Eng., 2020, 8, 103968 CrossRef CAS.
- C. Tan, Y. Guo, J. Sun, W. Li, J. Zhang, C. Zhao and P. Lu, Fuel, 2020, 278, 118379 CrossRef CAS.
- A. H. Ruhaimi, M. A. A. Aziz and A. A. Jalil, J. CO2 Util., 2021, 43, 101357 CrossRef CAS.
- S. K. Sharma, T. S. Khan, R. K. Singha, B. Paul, M. K. Poddar, T. Sasaki, A. Bordoloi, C. Samanta, S. Gupta and R. Bal, Appl. Catal., A, 2021, 623, 118239 CrossRef CAS.
- G. Tian, Y. Wu, S. Wu, S. Huang and J. Gao, Chem. Phys. Lett., 2022, 786, 139173 CrossRef CAS.
- W. Zhang, N. Zhou, Y. Zhang, Z. Huang, H. Hu, J. Liang and Y. Qin, J. Appl. Polym. Sci., 2021, 138, e50677 CrossRef.
- C. W. Wong, Y. S. Chan, J. Jeevanandam, K. Pal, M. Bechelany, M. A. Elkodous and G. S. El-Sayyad, J. Cluster Sci., 2019, 31, 367–389 CrossRef.
- P. Kasikamphaiboon and U. Khunjan, Int. J. Chem. Eng., 2018, 8, 1706405 Search PubMed.
- Q. Ao, S. Li, H. Li, H. Guo and X. Liu, Appl. Chem. Ind., 2021, 50, 1855–1858 Search PubMed.
- C. Liu, X. Guo, Q. Guo, D. Mao, J. Yu and G. Lu, J. Mol. Catal. A:Chem., 2016, 425, 86–93 CrossRef CAS.
- S. Goodarznia and K. J. Smith, J. Mol. Catal. A:Chem., 2012, 353, 58–66 CrossRef.
- S. Goodarznia and K. J. Smith, J. Mol. Catal. A:Chem., 2010, 320, 1–13 CrossRef CAS.
- F.-W. Chang, W.-Y. Kuo and K.-C. Lee, Appl. Catal., A, 2003, 246, 253–264 CrossRef CAS.
- C. Mebrahtu, R. Sun, C. H. Gierlich and R. Palkovits, Appl. Catal., B, 2021, 287, 119964 CrossRef CAS.
- F. Bin, X. Wei, B. Li and K. S. Hui, Appl. Catal., B, 2015, 162, 282–288 CrossRef CAS.
- J. Xu, W. Ji, Z. Shen, W. Li, S. Tang, X. Ye, D. Jia and X. Xin, J. Raman Spectrosc., 1999, 30, 413–415 CrossRef CAS.
- S. Demirci, B. K. Yildirim, M. M. Tünçay, N. Kaya and A. N. Güllüoğlu, J. Sol-Gel Sci. Technol., 2021, 99, 576–588 CrossRef CAS.
- R. A. El-Salamony, A. S. Morshedy and A. M. A. E. Naggar, Environ. Technol., 2020, 43, 1860–1869 CrossRef PubMed.
- P. S. Prabhu, P. Kathirvel, D. Maruthamani and S. D. G. Ram, Optics, 2023, 283, 170869 CAS.
- X. Zhang, Y. Liu, M. Zhang, T. Yu, B. Chen, Y. Xu, M. Crocker, X. Zhu, Y. Zhu, R. Wang, D. Xiao, M. Bi, D. Ma and C. Shi, Chem, 2020, 6, 3312–3328 CAS.
- D. Mei, X. Zhu, Y. He, J. D. Yan and X. Tu, Plasma Sources Sci. Technol., 2015, 24, 015011 CrossRef CAS.
- G. Zambrano, H. Riascos, P. Prieto, E. Restrepo, A. Devia and C. Rincón, Surf. Coat. Technol., 2003, 172, 144–149 CrossRef CAS.
- A. V. Kholodkov, K. M. Golant and I. V. Nikolin, Microelectron. Eng., 2003, 69, 365–372 CrossRef CAS.
- T. Czerwiec, J. Gavillet, T. Belmonte, H. Michel and A. Ricard, Surf. Coat. Technol., 1998, 98, 1411–1415 CrossRef CAS.
- A. Granier, M. Vervloet, K. Aumaille and C. Vallée, Plasma Sources Sci. Technol., 2003, 12, 89–96 CrossRef CAS.
- W. Wang, Z. Qu, L. Song and Q. Fu, J. Energy Chem., 2020, 40, 22–30 CrossRef.
- C. Schild, A. Wokaun and A. Baiker, J. Mol. Catal., 1990, 63, 243–254 CrossRef CAS.
- C. Vogt, E. Groeneveld, G. Kamsma, M. Nachtegaal, L. Lu, C. J. Kiely, P. H. Berben, F. Meirer and B. M. Weckhuysen, Nat. Catal., 2018, 1, 127–134 CrossRef CAS.
- Y.-F. Zhao, R. Rousseau, J. Li and D. Mei, J. Phys. Chem., 2012, 116, 15952–15961 CAS.
- Y. Huang, J. Am. Chem. Soc., 1973, 95, 6636–6640 CrossRef CAS.
- S. Xu, S. Chansai, S. Xu, C. E. Stere, Y. Jiao, S. Yang, C. Hardacre and X. Fan, ACS Catal., 2020, 10, 12828–12840 CrossRef CAS.
- J. Wang, G. Li, Z. Li, C. Tang, Z. Feng, H. An, H. Liu, T. Liu and C. Li, Sci. Adv., 2017, 3, e1701290 CrossRef PubMed.
- I. A. Fisher and A. T. Bell, J. Catal., 1997, 172, 222–237 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2026 |


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:



