Salty taste preference, genetic susceptibility, and risk of metabolic dysfunction-associated steatotic liver disease: insights from three prospective cohorts
Shunming
Zhang†
 a,
Yan
Yan†
b,
Yeqing
Gu
c,
Hongmei
Wu
de,
Qing
Zhang
f,
Li
Liu
f,
Yan
Borné
g,
Lu
Qi
a,
Yan
Yan†
b,
Yeqing
Gu
c,
Hongmei
Wu
de,
Qing
Zhang
f,
Li
Liu
f,
Yan
Borné
g,
Lu
Qi
 *hi,
Tao
Huang
*hi,
Tao
Huang
 *j,
Yu-Ming
Chen
*j,
Yu-Ming
Chen
 *b,
Kaijun
Niu
*b,
Kaijun
Niu
 *de,
Le
Ma
*de,
Le
Ma
 *a and
the China Cohort Consortium
*a and
the China Cohort Consortium
aSchool of Public Health, Xi'an Jiaotong University Health Science Center, Xi'an, China. E-mail: male@mail.xjtu.edu.cn
bGuangdong Provincial Key Laboratory of Food, Nutrition and Health, School of Public Health, Sun Yat-sen University, Guangzhou, China. E-mail: chenyum@mail.sysu.edu.cn
cInstitute of Radiation Medicine, Chinese Academy of Medical Sciences & Peking Union Medical College, Tianjin, China
dSchool of Public Health, Tianjin University of Traditional Chinese Medicine, Tianjin, China. E-mail: nkj0809@gmail.com
eNutritional Epidemiology Institute and School of Public Health, Tianjin Medical University, Tianjin, China
fHealth Management Centre, Tianjin Medical University General Hospital, Tianjin, China
gNutritional Epidemiology, Department of Clinical Sciences Malmö, Lund University, Malmö, Sweden
hDepartment of Epidemiology, Celia Scott Weatherhead School of Public Health and Tropical Medicine, Tulane University, New Orleans, LA, USA. E-mail: lqi1@tulane.edu
iDepartment of Nutrition, Harvard T.H. Chan School of Public Health, Boston, MA, USA
jDepartment of Epidemiology and Biostatistics, School of Public Health, Peking University, Beijing, China. E-mail: huangtao@bjmu.edu.cn
First published on 6th November 2025
Abstract
Background and aims: Animal studies have suggested that high salt intake might increase the risk of metabolic dysfunction-associated steatotic liver disease (MASLD), but results from populations are mixed, in part due to inadequate salt intake measurement. Salty taste preference is the primary factor leading to salt choice and can reflect habitual salt intake. However, no study has investigated the association between salty taste preference and MASLD. This study aimed to determine the association between salty taste preference and risk of MASLD, while considering genetic predisposition to MASLD. Methods: This multicohort study used data from the Tianjin Chronic Low-grade Systemic Inflammation and Health (TCLSIH) cohort (n = 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 869), the Guangzhou Nutrition and Health Study (GNHS) cohort (n = 1225), and the UK Biobank (n = 179
869), the Guangzhou Nutrition and Health Study (GNHS) cohort (n = 1225), and the UK Biobank (n = 179![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 668). Salty taste preference was assessed using self-reported questionnaires. Incident MASLD was ascertained using abdominal ultrasound or electronic health records. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated using Cox proportional hazards models. Results: During follow-up, we documented 3358 MASLD cases in the TCLSIH cohort, 670 cases in the GNHS cohort, and 1780 cases in the UK Biobank. The adjusted HRs (95% CIs) of incident MASLD across the three cohorts were 1.17 (1.05, 1.30), 1.49 (1.18, 1.88), and 1.13 (1.01, 1.28), comparing high with low salty taste preference. Such associations were mediated by 29.8%–49.4% for body mass index and 49.4%–64.5% for waist circumference. Individuals with high salty taste preference and genetic risk had the strongest risk elevation for MASLD, though no significant interaction was observed. Conclusion: Salty taste preference, a proxy for long-term salt intake, was positively associated with risk of MASLD, especially in individuals with high genetic predisposition.
668). Salty taste preference was assessed using self-reported questionnaires. Incident MASLD was ascertained using abdominal ultrasound or electronic health records. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated using Cox proportional hazards models. Results: During follow-up, we documented 3358 MASLD cases in the TCLSIH cohort, 670 cases in the GNHS cohort, and 1780 cases in the UK Biobank. The adjusted HRs (95% CIs) of incident MASLD across the three cohorts were 1.17 (1.05, 1.30), 1.49 (1.18, 1.88), and 1.13 (1.01, 1.28), comparing high with low salty taste preference. Such associations were mediated by 29.8%–49.4% for body mass index and 49.4%–64.5% for waist circumference. Individuals with high salty taste preference and genetic risk had the strongest risk elevation for MASLD, though no significant interaction was observed. Conclusion: Salty taste preference, a proxy for long-term salt intake, was positively associated with risk of MASLD, especially in individuals with high genetic predisposition.
Introduction
Metabolic dysfunction-associated steatotic liver disease (MASLD), formerly known as non-alcoholic fatty liver disease, is the most common chronic liver disease, affecting more than 30% of adults worldwide.1 Of concern, the prevalence of MASLD is projected to increase to 55.7% by 2040.2 MASLD not only confers risks of liver-related adverse outcomes (e.g., cirrhosis and hepatocellular carcinoma) but also increases risks of multiple extrahepatic diseases, including type 2 diabetes, cardiovascular disease (CVD), and chronic kidney disease.3 Therefore, identifying risk factors that influence MASLD onset is essential for developing effective public health strategies to prevent this condition and address the growing disease burden.Dietary salt intake is an important lifestyle behavior. Experimental studies indicate that high salt intake may contribute to MASLD through mechanisms involving activating the aldose reductase pathway in the liver, causing leptin resistance, and stimulating endogenous fructose production and metabolism,4 as well as dysfunctioning the renin–angiotensin–aldosterone system and other pathways.5 However, epidemiological studies conducted in general adults have yielded inconsistent results, showing positive association,6–13 null association,14 or positive association in females but null association in males.15 An important reason for such inconsistent associations lies in varying exposure measurement approaches, as most previous studies used sodium measured by dietary surveys or spot urine samples as a tool to assess long-term salt intake amounts. Noteworthily, sodium (salt) intake varies widely from day to day. Thus, using novel tools that can accurately assess long-term salt intake to study its association with MASLD is important to inform public health initiatives. In this regard, several previous studies have indicated that salty taste preference could be used as a proxy for habitual salt intake.16–20 Salty taste preference does not rely on memory, does not suffer from health-related biases due to social pressure, and has a much higher overall test–retest correlation than the food frequency questionnaire (FFQ).21,22 In addition, salty taste preference is quite stable over time in adults. Therefore, salty taste preference offers a unique instrument for assessing the association between habitual salt intake and MASLD in the general adult population. However, to date, no studies have examined the association between salty taste preference and MASLD. Furthermore, high salt intake causes obesity in mice by activating the aldose reductase–fructokinase pathway in the liver and hypothalamus.4 Population-based studies have also shown that salt intake is positively associated with overweight/obesity,23 which is the main risk factor for MASLD. Meanwhile, previous research has documented that adiposity mediates the association between salt intake and MASLD.9,24 Thus, it is essential to explore the mediating role of adiposity in the association between salty taste preference and MASLD. On the other hand, genetic predisposition also contributes to the development of MASLD.25 However, how genetic factors interact with salty taste preference to affect MASLD remains unclear.
This study aimed to investigate the association between salty taste preference and risk of incident MASLD while exploring the mediation effects of adiposity measures, as well as taking into account genetic predisposition to MASLD in three independent large prospective cohorts. We hypothesized that higher salty taste preference would be associated with a higher risk of MASLD; this association would be amplified by genetic susceptibility; and adiposity would in part mediate the studied association.
Methods
Study population
This multicohort study used data from three independent large prospective cohorts, including the Tianjin Chronic Low-grade Systemic Inflammation and Health (TCLSIH) cohort, the Guangzhou Nutrition and Health Study (GNHS), and the UK Biobank (application number 44430). The TCLSIH cohort is an ongoing, dynamic cohort initiated in May 2013, which consecutively enrolls general adults aged ≥18 years living in Tianjin, located in northern China.26 The GNHS cohort is a population-based sample of adults aged 40–75 years between 2008 and 2013 in Guangzhou, in southern China.27 The UK Biobank is a large population-based cohort of >500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 adults aged 37–73 years recruited in the UK from 2006 to 2010.28 At the baseline, all three cohort participants provided detailed information on their sociodemographic characteristics, lifestyle factors, and health conditions. Participants without data on salty taste preference, with prevalent MASLD or other liver diseases at the baseline, and who were lost to follow-up (only completed baseline visits) were excluded, leaving 16
000 adults aged 37–73 years recruited in the UK from 2006 to 2010.28 At the baseline, all three cohort participants provided detailed information on their sociodemographic characteristics, lifestyle factors, and health conditions. Participants without data on salty taste preference, with prevalent MASLD or other liver diseases at the baseline, and who were lost to follow-up (only completed baseline visits) were excluded, leaving 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 869 TCLSIH cohort participants, 1225 GNHS cohort participants, and 179
869 TCLSIH cohort participants, 1225 GNHS cohort participants, and 179![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 668 UK Biobank participants for the primary analyses. For analyses related to genes, participants without genetic data were excluded. An overview of the study design is presented in Fig. S1. The study was conducted in accordance with the Declaration of Helsinki and was approved by the local ethics committees of each cohort. All participants provided signed informed consent. This manuscript followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines29 and the STROBE-nut guidelines.30
668 UK Biobank participants for the primary analyses. For analyses related to genes, participants without genetic data were excluded. An overview of the study design is presented in Fig. S1. The study was conducted in accordance with the Declaration of Helsinki and was approved by the local ethics committees of each cohort. All participants provided signed informed consent. This manuscript followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines29 and the STROBE-nut guidelines.30
Exposure measurements
In both the TCLSIH and the GNHS cohorts, salty taste preference was assessed at the baseline using a self-reported question “How salty do you usually prefer in your habitual diet?”, which included five response categories: very salty, moderately salty, neutral, moderately light, and very light. Based on the distribution of responses in the study samples, participants were categorized into three groups to enhance statistical power: low (very light and moderately light), medium (neutral), and high (moderately salty and very salty).In the UK Biobank, salty taste preference was measured on a 9-point scale between May 2019 and January 2020. Based on the UK Biobank food preferences questionnaire's introduction (https://biobank.ctsu.ox.ac.uk/showcase/refer.cgi?id=25974), the data can be used with health and behavioral information collected at the baseline because food preferences are stable over time in adults. Participants were asked, “Please rate how much you like salty foods?”, with the response options ranging from 1 point (extremely dislike) to 9 points (extremely like), along with additional categories for “never tried” and “do not wish to answer”. Responses marked as “never tried” or “do not wish to answer” were treated as missing data in the analysis. To maintain the same groups as the TCLSIH cohort and the GNHS cohort and enhance comparability across cohorts, participants were divided into tertiles as follows: low (1–3 points), medium (4–6 points), and high (7–9 points).
To confirm whether salty taste preference can reflect habitual salt intake, we estimated daily salt intake from a validated FFQ in the TCLSIH cohort.31 Furthermore, in the UK Biobank, the concentrations of sodium and potassium in the spot urine samples collected at the baseline were determined using the ion selective electrode method, with measurements on the Beckman Coulter AU5400; the 24 hour sodium excretion (a surrogate for sodium intake) was then estimated from the spot urinary concentrations using the sex-specific INTERSALT equations.32
Assessment of genetic susceptibility
Imputed genetic data were derived from the three cohorts. Detailed information on the genotyping process, imputation, and stringent quality control has been provided in previous studies.33–35 Consistent with recent research,36 we selected five single nucleotide polymorphisms associated with hepatic steatosis to construct a weighted polygenic risk score (PRS) for MASLD37 (Table S1). For the analyses, the PRS was dichotomized at the median.Outcome assessment and follow-up
The primary outcome was incident MASLD, which was defined as ultrasound-diagnosed hepatic steatosis with at least one accompanying cardiometabolic risk factor in both the TCLSIH cohort and the GNHS cohort, or based on the International Classification of Diseases, Tenth Revision, codes K76.0 and K75.8 in the UK Biobank, consistent with our recent work.38 Baseline MASLD was ascertained through liver ultrasound, cardiometabolic risk factors, and alcohol intake in the TCLSIH cohort and the GNHS cohort, whereas the corresponding codes (K76.0 and K75.8) were used for its definition in the UK Biobank. Participants were followed up every 1–2 years in the TCLSIH cohort and every 3 years in the GNHS cohort. The time scale of follow-up was the time from baseline assessment until participants had an incident MASLD diagnosis, death, or at the end of study follow-up, whichever came first. The end of follow-up was defined as December 31, 2019 in the TCLSIH cohort, August 9, 2023 in the GNHS cohort, and December 31, 2022 in the UK Biobank.In an imaging substudy of the UK Biobank between 2014 and 2020, liver assessment using quantitative magnetic resonance imaging included proton density fat fraction (PDFF) for fat content39 and iron-corrected T1 (cT1) time for inflammation and fibrosis.40 We used PDFF-defined MASLD and elevated cT1 as secondary outcomes. The cut-off point for the presence of MASLD was PDFF ≥5%,39 while elevated cT1 was defined as cT1 ≥800 ms.41
Assessment of covariates and potential effect mediators
Covariates included age, sex, ethnicity, household income, employment, educational level, smoking status, alcohol drinking status, physical activity, sedentary time, CVD, cancer, diabetes, hyperlipidemia, hypertension, family history of diseases (including CVD, diabetes, and hypertension), healthy diet score, and total energy intake at baseline visits. Potential effect mediators were baseline body mass index (BMI) and waist circumference. Further details on these variables are provided in SI Methods.Statistical analysis
Baseline characteristics across the three cohorts are presented as means ± standard deviation (SD) for continuous data and as percentages for categorical data.General linear models were applied to analyze the association between salty taste preference and habitual salt intake in the TCLSIH cohort and urinary sodium concentrations in the UK Biobank. Cox proportional hazards models were used to estimate the hazard ratio (HR) and 95% confidence interval (CI) for the association between salty taste preference and risk of incident MASLD across three models. The first model was adjusted for age, sex, ethnicity, household income, employment, and education level. The second model (main model) included additional adjustments for smoking status, alcohol drinking status, physical activity, sedentary time, CVD, cancer, diabetes, hyperlipidemia, hypertension, family history of diseases (including CVD, diabetes, and hypertension), healthy diet score, and total energy intake. The final model was further added to BMI, a potential effect mediator. The proportional hazard assumption was evaluated by incorporating product terms between follow-up time and salty taste preference in the multivariable models, and no violations were observed (P > 0.05). Linear trends were quantified using modeling categories of salty taste preference (low: 1, medium: 2, and high: 3) as an ordinal variable.
Stratified analyses were conducted to evaluate the potential effect modification by age, sex, smoking status, and alcohol drinking status. The multiplicative interaction was evaluated by including multiplication terms of salty taste preference and these stratification variables based on the likelihood ratio test. Furthermore, we conducted a sensitivity analysis by excluding participants with prevalent CVD or cancer at the baseline because these conditions could result in important dietary changes. To assess whether salt intake was responsible for the association between salty taste preference and risk of MASLD, we further adjusted for habitual salt intake in the TCLSIH cohort and estimated 24 hour sodium excretion in the UK Biobank.
Mediation analyses were performed to quantify the contribution of adiposity to the association between salty taste preference and MASLD. The proportion of the association mediated by BMI and waist circumference was calculated using the SAS %MEDIATE macro.
The association between PRS and MASLD was examined using Cox models. PRS-stratified analyses were conducted, and the multiplicative interaction of the salty taste preference with PRS was assessed by including their cross-product interaction term in the aforementioned model 2. For the joint analyses, salty taste preference (low, medium, and high) and PRS (< and ≥ median) were combined into six categories, with combined low genetic risk and salt preference as the reference group. To assess the additive interaction, we calculated relative excess risk due to interaction and attributable proportion due to the interaction between salty taste preference (low or medium vs. high) and genetic risk (low vs. high).42
In the secondary analyses, logistic regression models were used to assess the associations of salty taste preference with PDFF-defined MASLD and elevated cT1 in the UK Biobank, with three models adjusted for the aforementioned covariates.
All analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) and R version 4.5.0. A two-sided P < 0.05 was considered statistically significant.
Results
Baseline characteristics of the sample
Table 1 shows the baseline characteristics of participants across the three cohorts. Overall, the mean age was 40.6 ± 12.4 years in the TCLSIH cohort, 60.3 ± 5.8 years in the GNHS cohort, and 55.8 ± 7.8 years in the UK Biobank. More than half of the participants were female in all three cohorts. Compared with participants without incident MASLD, those with incident MASLD were more likely to have cardiometabolic risk factors and had higher prevalence of cancer and family history of hypertension (Table 1). Furthermore, individuals with higher salty taste preference tended to be younger in the TCLSIH cohort and the UK Biobank, were more likely to be male (in two Chinese cohorts), current smokers, current alcohol drinkers, had lower prevalence of CVD and cancer, and were more likely to adhere to an unhealthy diet in all three cohorts (Table S2).| Characteristics | TCLSIH cohort | GNHS cohort | UK Biobank | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Overall | Incident MASLD | Overall | Incident MASLD | Overall | Incident MASLD | ||||
| No | Yes | No | Yes | No | Yes | ||||
Abbreviations: BMI – body mass index; CVD – cardiovascular disease; DBP – diastolic blood pressure; FBG – fasting blood glucose; GNHS – Guangzhou Nutrition and Health Study; HDL-C – high-density lipoprotein cholesterol; LDL-C – low-density lipoprotein cholesterol; MASLD – metabolic dysfunction-associated steatotic liver disease; MET – metabolic equivalent; PA – physical activity; SBP – systolic blood pressure; TC – total cholesterol; TCLSIH – Tianjin Chronic Low-grade Systemic Inflammation and Health; TG – triglycerides; WC – waist circumference.a Continuous variables were expressed as means ± standard deviations, and categorical variables as %.b ≥10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Yuan in the TCLSIH cohort, ≥3000 Yuan in the GNHS cohort, and ≥52 000 Yuan in the TCLSIH cohort, ≥3000 Yuan in the GNHS cohort, and ≥52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 £ in the UK Biobank.c The TCLSIH cohort and the UK Biobank used MET-hours per week, while the GNHS cohort used MET-hours per day. 000 £ in the UK Biobank.c The TCLSIH cohort and the UK Biobank used MET-hours per week, while the GNHS cohort used MET-hours per day. |
|||||||||
| Number of participants | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 869 869 |
13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 511 511 |
3358 | 1225 | 555 | 670 | 179![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 668 668 |
177![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 888 888 |
1780 |
| Age (years) | 40.6 ± 12.4 | 39.9 ± 12.1 | 43.4 ± 13.0 | 60.3 ± 5.8 | 60.8 ± 6.1 | 60.0 ± 5.5 | 55.8 ± 7.7 | 55.8 ± 7.7 | 56.3 ± 7.6 |
| Sex (male) | 42.9 | 38.5 | 60.5 | 28.1 | 35.0 | 22.4 | 42.8 | 42.8 | 46.2 |
| Ethnicity (White) | — | — | — | — | — | — | 96.8 | 96.8 | 95.8 |
| BMI (kg m−2) | 23.1 ± 3.1 | 22.6 ± 2.9 | 25.1 ± 2.8 | 22.4 ± 2.6 | 21.4 ± 2.4 | 23.2 ± 2.4 | 26.7 ± 4.5 | 26.7 ± 4.5 | 30.9 ± 5.5 |
| WC (cm) | 78.2 ± 9.7 | 76.7 ± 9.3 | 84.3 ± 8.7 | 82.1 ± 7.8 | 79.0 ± 7.2 | 84.7 ± 7.3 | 88.3 ± 13.0 | 88.1 ± 13.0 | 99.4 ± 13.6 |
| TC (mmol L−1) | 4.64 ± 0.86 | 4.60 ± 0.86 | 4.77 ± 0.88 | 5.64 ± 1.05 | 5.63 ± 1.05 | 5.65 ± 1.06 | 5.75 ± 1.11 | 5.75 ± 1.10 | 5.54 ± 1.23 |
| TG (mmol L−1) | 1.08 ± 0.66 | 1.00 ± 0.57 | 1.39 ± 0.84 | 1.32 ± 1.00 | 1.18 ± 0.59 | 1.43 ± 1.23 | 1.66 ± 0.97 | 1.66 ± 0.97 | 2.20 ± 1.20 |
| LDL-C (mmol L−1) | 2.69 ± 0.77 | 2.65 ± 0.77 | 2.86 ± 0.76 | 3.59 ± 0.91 | 3.56 ± 0.86 | 3.62 ± 0.95 | 3.58 ± 0.84 | 3.58 ± 0.84 | 3.49 ± 0.92 |
| HDL-C (mmol L−1) | 1.48 ± 0.38 | 1.52 ± 0.38 | 1.31 ± 0.32 | 1.53 ± 0.42 | 1.60 ± 0.45 | 1.48 ± 0.38 | 1.49 ± 0.38 | 1.49 ± 0.38 | 1.30 ± 0.34 |
| FBG (mmol L−1) | 5.00 ± 0.76 | 4.96 ± 0.73 | 5.16 ± 0.83 | 4.90 ± 1.04 | 4.84 ± 0.99 | 4.96 ± 1.08 | — | — | — |
| 2-Hour postprandial glucose (mmol L−1) | 5.42 ± 0.52 | 5.82 ± 1.54 | 6.32 ± 1.70 | — | — | — | — | — | — |
| Glycosylated hemoglobin A1c (%) | 5.93 ± 1.59 | 5.39 ± 0.50 | 5.52 ± 0.55 | 5.57 ± 0.45 | 5.54 ± 0.47 | 5.59 ± 0.43 | 5.38 ± 0.50 | 5.38 ± 0.50 | 5.63 ± 0.81 |
| SBP (mmHg) | 117.0 ± 14.9 | 115.9 ± 14.6 | 121.4 ± 15.2 | 122.3 ± 17.4 | 121.0 ± 17.7 | 123.0 ± 17.1 | 136.3 ± 18.1 | 136.2 ± 18.1 | 139.7 ± 17.1 |
| DBP (mmHg) | 73.7 ± 10.0 | 73.0 ± 9.9 | 76.6 ± 10.2 | 73.7 ± 10.2 | 73.1 ± 10.7 | 74.2 ± 9.8 | 81.8 ± 10.0 | 81.7 ± 10.0 | 84.3 ± 9.9 |
| High household incomeb | 38.5 | 38.6 | 37.7 | 42.7 | 42.2 | 43.6 | 30.6 | 30.7 | 22.5 |
| Employment | |||||||||
| Managers | 46.8 | 46.6 | 47.5 | — | — | — | — | — | — |
| Professionals | 16.0 | 15.8 | 16.8 | — | — | — | — | — | — |
| Other | 37.2 | 37.6 | 35.7 | — | — | — | — | — | — |
| Education level (≥college) | 73.9 | 74.5 | 71.5 | 26.1 | 27.2 | 25.2 | 44.8 | 44.9 | 34.0 |
| Current smoker | 15.0 | 13.6 | 20.5 | 10.5 | 12.1 | 9.30 | 7.00 | 6.98 | 9.33 |
| Current alcohol drinker | 56.1 | 54.9 | 61.0 | 7.83 | 6.80 | 8.70 | 94.5 | 94.6 | 90.7 |
| PA (weekly or daily MET-hours)c | 19.6 ± 31.5 | 19.1 ± 31.6 | 21.3 ± 31.1 | 35.4 ± 5.97 | 35.6 ± 6.1 | 35.1 ± 5.8 | 41.0 ± 40.3 | 41.0 ± 40.3 | 36.5 ± 40.4 |
| Sedentary time (hours per day) | 6.17 ± 2.93 | 6.24 ± 2.93 | 5.90 ± 2.90 | — | — | — | 4.59 ± 2.27 | 4.58 ± 2.27 | 5.41 ± 2.51 |
| CVD | 3.74 | 3.28 | 5.6 | 20.4 | 21.1 | 19.9 | 5.25 | 5.19 | 11.1 |
| Cancer | 0.95 | 0.84 | 1.37 | 1.22 | 0.90 | 1.50 | 8.02 | 8.00 | 9.16 |
| Diabetes | 3.34 | 2.79 | 5.54 | 7.10 | 6.10 | 7.90 | 3.77 | 3.67 | 13.8 |
| Hyperlipidemia | 34.5 | 31.4 | 47.1 | 41.6 | 38.6 | 44.0 | 14.3 | 14.1 | 29.0 |
| Hypertension | 16.0 | 13.7 | 25.0 | 23.8 | 20.2 | 26.7 | 49.8 | 49.6 | 66.7 |
| Family history of CVD | 30.9 | 29.3 | 37.1 | 9.31 | 9.73 | 9.00 | 56.8 | 56.8 | 60.2 |
| Family history of diabetes | 24.0 | 22.7 | 28.9 | 6.12 | 5.05 | 7.02 | 20.2 | 20.1 | 28.8 |
| Family history of hypertension | 49.7 | 48.3 | 55.3 | 22.7 | 21.3 | 23.9 | 49.5 | 49.5 | 53.4 |
| Healthy diet score | 2.45 ± 1.16 | 2.45 ± 1.16 | 2.45 ± 1.17 | 2.45 ± 1.21 | 2.43 ± 1.20 | 2.47 ± 1.22 | 3.07 ± 1.26 | 3.08 ± 1.26 | 2.85 ± 1.29 |
| Total energy intake (kcal per day) | 2447 ± 1026 | 2439 ± 1022 | 2480 ± 1039 | 1597 ± 497 | 1589 ± 465 | 1604 ± 522 | — | — | — |
Association of salty taste preference with salt intake and urinary sodium concentrations
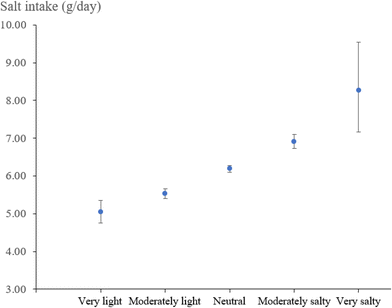
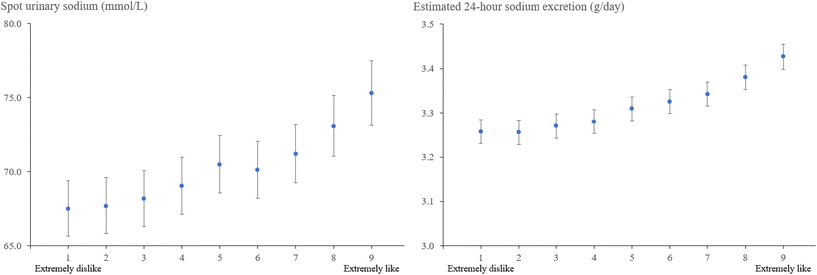
In the TCLSIH cohort, the mostly adjusted geometric means (95% CIs) of habitual salt intake (g per day) across increasing salty taste preference were 5.47 (5.35, 5.58) for low, 6.19 (6.11, 6.28) for medium, and 6.95 (6.77, 7.14) for high, respectively (P for trend <0.0001) (Table S3). Fig. 1 presents the graded association between the original categories of salty taste preference and habitual salt intake. In the UK Biobank, spot urinary sodium concentrations (mmol L−1) were 67.8 (66.0, 69.7), 70.0 (68.2, 72.0), and 72.5 (70.5, 74.5) across low, medium, and high salty taste preference, with P for trend <0.0001; the estimated 24 hour sodium excretion (g per day) across these categories were 3.26 (3.24, 3.29), 3.31 (3.28, 3.34), and 3.37 (3.34, 3.40), respectively (P for trend <0.0001) (Table S4). Fig. 2 displays the graded association between salty taste preference measured on a 9-point scale and urinary sodium concentrations.Association of salty taste preference with risk of incident MASLD
Over the follow-up period of 58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 672 person-years in the TCLSIH cohort, a total of 3358 MASLD cases were recorded; 7038 person-years in the GNHS cohort, 670 cases were documented; and 2
672 person-years in the TCLSIH cohort, a total of 3358 MASLD cases were recorded; 7038 person-years in the GNHS cohort, 670 cases were documented; and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 475
475![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 427 person-years in the UK Biobank, 1780 cases occurred. Table 2 shows the association of salty taste preference with risk of incident MASLD. After adjusting for sociodemographic characteristics, the multivariable HRs (95% CIs) for incident MASLD comparing high with low salty taste preference were 1.16 (1.04, 1.29) in the TCLSIH cohort, 1.55 (1.24, 1.95) in the GNHS cohort, and 1.20 (1.07, 1.36) in the UK Biobank. After further adjusting for lifestyle factors, personal and family history of diseases, and overall diet quality, similar results were observed. In contrast, these associations were attenuated after additional adjustment for BMI; the HRs (95% CIs) comparing high with low salty taste preference were 1.12 (1.00, 1.24) in the TCLSIH cohort, 1.27 (1.00, 1.62) in the GNHS cohort, and 1.07 (0.94, 1.20) in the UK Biobank.
427 person-years in the UK Biobank, 1780 cases occurred. Table 2 shows the association of salty taste preference with risk of incident MASLD. After adjusting for sociodemographic characteristics, the multivariable HRs (95% CIs) for incident MASLD comparing high with low salty taste preference were 1.16 (1.04, 1.29) in the TCLSIH cohort, 1.55 (1.24, 1.95) in the GNHS cohort, and 1.20 (1.07, 1.36) in the UK Biobank. After further adjusting for lifestyle factors, personal and family history of diseases, and overall diet quality, similar results were observed. In contrast, these associations were attenuated after additional adjustment for BMI; the HRs (95% CIs) comparing high with low salty taste preference were 1.12 (1.00, 1.24) in the TCLSIH cohort, 1.27 (1.00, 1.62) in the GNHS cohort, and 1.07 (0.94, 1.20) in the UK Biobank.
| Categories of salty taste preference | P for trendb | |||
|---|---|---|---|---|
| Low | Medium | High | ||
| Abbreviations: GNHS – Guangzhou Nutrition and Health Study; MASLD – metabolic dysfunction-associated steatotic liver disease; TCLSIH – Tianjin Chronic Low-grade Systemic Inflammation and Health. Model 1 was adjusted for age, sex, ethnicity (only in the UK Biobank), household income, employment (only in the TCLSIH cohort), and educational level. Model 2 was further adjusted for smoking status, alcohol drinking status, physical activity, sedentary time (only in the TCLSIH cohort and the UK Biobank), cardiovascular disease, cancer, diabetes, hyperlipidemia, hypertension, family history of diseases (including cardiovascular disease, diabetes, and hypertension), healthy diet score, and total energy intake (only in the TCLSIH cohort and the GNHS cohort). Model 3 was additionally adjusted for body mass index.a Values are hazard ratios (95% confidence intervals) unless otherwise indicated.b P for trend was calculated by using categories of salty taste preference (low: 1, medium: 2, and high: 3) as an ordinal variable. | ||||
TCLSIH cohort (n = 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 869) 869) |
||||
| Number of participants | 4511 | 9579 | 2779 | — |
| Number of cases | 860 | 1869 | 629 | — |
| Person-years | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 936 936 |
33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 505 505 |
9231 | — |
| Incidence per 1000 person-years | 54.0 | 55.8 | 68.1 | — |
| Model 1 | 1.00 (reference) | 1.04 (0.96, 1.13) | 1.16 (1.04, 1.29) | <0.01 |
| Model 2 | 1.00 (reference) | 1.05 (0.97, 1.14) | 1.17 (1.05, 1.30) | <0.01 |
| Model 3 | 1.00 (reference) | 1.03 (0.95, 1.12) | 1.12 (1.00, 1.24) | 0.050 |
| GNHS cohort (n = 1225) | ||||
| Number of participants | 660 | 414 | 151 | — |
| Number of cases | 334 | 239 | 97 | — |
| Person-years | 3943 | 2279 | 816 | — |
| Incidence per 1000 person-years | 84.7 | 104.9 | 118.9 | — |
| Model 1 | 1.00 (reference) | 1.35 (1.14, 1.59) | 1.55 (1.24, 1.95) | <0.01 |
| Model 2 | 1.00 (reference) | 1.35 (1.14, 1.61) | 1.49 (1.18, 1.88) | <0.01 |
| Model 3 | 1.00 (reference) | 1.14 (0.95, 1.36) | 1.27 (1.00, 1.62) | 0.03 |
UK Biobank (n = 179![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 668) 668) |
||||
| Number of participants | 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 310 310 |
73![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 279 279 |
51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 079 079 |
— |
| Number of cases | 538 | 689 | 553 | — |
| Person-years | 761![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 796 796 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 010 010![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 077 077 |
703![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 554 554 |
— |
| Incidence per 1000 person-years | 0.71 | 0.68 | 0.79 | — |
| Model 1 | 1.00 (reference) | 1.00 (0.90, 1.12) | 1.20 (1.07, 1.36) | <0.01 |
| Model 2 | 1.00 (reference) | 0.99 (0.88, 1.11) | 1.13 (1.01, 1.28) | 0.04 |
| Model 3 | 1.00 (reference) | 0.95 (0.85, 1.07) | 1.07 (0.94, 1.20) | 0.31 |
Subgroup analyses and sensitivity analyses
The subgroup analyses by age, sex, smoking status, and alcohol drinking status demonstrated largely consistent results with the primary findings (Table 3). Similar results were also obtained after excluding individuals with prevalent CVD or cancer at the baseline (Table S5). However, after adjusting for habitual salt intake or estimated 24 hour sodium concentration, the elevated risk of MASLD was attenuated in the TCLSIH cohort and was no longer significant in the UK Biobank (Table 4).| Categories of salty taste preference | P for trendb | P for interactionc | |||
|---|---|---|---|---|---|
| Low | Medium | High | |||
| Abbreviations: GNHS – Guangzhou Nutrition and Health Study; MASLD – metabolic dysfunction-associated steatotic liver disease; TCLSIH – Tianjin Chronic Low-grade Systemic Inflammation and Health. Adjusted for age, sex, ethnicity (only in the UK Biobank), household income, employment (only in the TCLSIH cohort and the UK Biobank), educational level, smoking status, alcohol drinking status, physical activity, sedentary time (only in the TCLSIH cohort and the UK Biobank), cardiovascular disease, cancer, diabetes, hyperlipidemia, hypertension, family history of diseases (including cardiovascular disease, diabetes, and hypertension), healthy diet score, and total energy intake (only in the TCLSIH cohort and the GNHS cohort).a Values are hazard ratios (95% confidence intervals) unless otherwise indicated, calculated using Cox proportional hazards models.b P for trend was calculated by using categories of salty taste preference (low: 1, medium: 2, and high: 3) as an ordinal variable.c P for interaction was calculated by adding interaction terms to the Cox models. | |||||
| TCLSIH cohort | |||||
| Age (years) | |||||
| <40 | 1.00 (reference) | 0.99 (0.87, 1.13) | 1.12 (0.96, 1.31) | 0.16 | 0.38 |
| ≥40 | 1.00 (reference) | 1.11 (0.99, 1.23) | 1.21 (1.05, 1.41) | <0.01 | |
| Sex | |||||
| Male | 1.00 (reference) | 1.01 (0.91, 1.13) | 1.10 (0.96, 1.26) | 0.17 | 0.48 |
| Female | 1.00 (reference) | 1.14 (1.01, 1.29) | 1.32 (1.10, 1.58) | <0.01 | |
| Smoking status | |||||
| Current | 1.00 (reference) | 1.11 (0.89, 1.38) | 1.28 (1.00, 1.63) | 0.04 | 0.12 |
| Non-current | 1.00 (reference) | 1.04 (0.95, 1.14) | 1.15 (1.01, 1.29) | 0.04 | |
| Alcohol drinking status | |||||
| Alcohol drinker | 1.00 (reference) | 1.03 (0.94, 1.14) | 1.15 (1.02, 1.30) | 0.85 | 0.41 |
| Non-drinker | 1.00 (reference) | 1.08 (0.93, 1.25) | 1.11 (0.90, 1.37) | 0.03 | |
| GNHS cohort | |||||
| Age (years) | |||||
| <60 | 1.00 (reference) | 1.41 (1.11, 1.78) | 1.69 (1.24, 2.31) | <0.01 | 0.49 |
| ≥60 | 1.00 (reference) | 1.27 (0.98, 1.65) | 1.35 (0.93, 1.96) | 0.04 | |
| Sex | |||||
| Male | 1.00 (reference) | 1.05 (0.71, 1.56) | 1.15 (0.70, 1.88) | 0.60 | 0.19 |
| Female | 1.00 (reference) | 1.42 (1.17, 1.73) | 1.65 (1.25, 2.18) | <0.01 | |
| Smoking status | |||||
| Current | 1.00 (reference) | 1.30 (0.60, 2.80) | 1.42 (0.56, 3.58) | 0.46 | 0.61 |
| Non-current | 1.00 (reference) | 1.38 (1.15, 1.65) | 1.47 (1.14, 1.89) | <0.01 | |
| Alcohol drinking status | |||||
| Alcohol drinker | 1.00 (reference) | 1.59 (0.76, 3.34) | 1.21 (0.50, 2.94) | 0.53 | 0.87 |
| Non-drinker | 1.00 (reference) | 1.34 (1.12, 1.60) | 1.53 (1.19, 1.96) | <0.01 | |
| UK Biobank | |||||
| Age (years) | |||||
| <60 | 1.00 (reference) | 0.95 (0.82, 1.11) | 1.08 (0.93, 1.26) | 0.29 | 0.10 |
| ≥60 | 1.00 (reference) | 1.04 (0.87, 1.23) | 1.21 (1.00, 1.47) | 0.06 | |
| Sex | |||||
| Male | 1.00 (reference) | 1.03 (0.87, 1.23) | 1.27 (1.06, 1.53) | <0.01 | 0.06 |
| Female | 1.00 (reference) | 0.96 (0.83, 1.12) | 1.04 (0.88, 1.22) | 0.69 | |
| Smoking status | |||||
| Current | 1.00 (reference) | 0.95 (0.64, 1.41) | 1.19 (0.80, 1.77) | 0.33 | 0.78 |
| Non-current | 1.00 (reference) | 1.00 (0.89, 1.13) | 1.15 (1.01, 1.31) | 0.03 | |
| Alcohol drinking status | |||||
| Alcohol drinker | 1.00 (reference) | 0.99 (0.88, 1.12) | 1.13 (0.99, 1.28) | 0.06 | 0.98 |
| Non-drinker | 1.00 (reference) | 0.93 (0.64, 1.34) | 1.22 (0.83, 1.79) | 0.39 | |
| Categories of salty taste preference | P for trendb | |||
|---|---|---|---|---|
| Low | Medium | High | ||
| Abbreviations: MASLD – metabolic dysfunction-associated steatotic liver disease; TCLSIH – Tianjin Chronic Low-grade Systemic Inflammation and Health. Model adjusted for age, sex, ethnicity (only in the UK Biobank), household income, employment (only in the TCLSIH cohort), educational level, smoking status, alcohol drinking status, physical activity, sedentary time, cardiovascular disease, cancer, diabetes, hyperlipidemia, hypertension, family history of diseases (including cardiovascular disease, diabetes, and hypertension), healthy diet score, total energy intake (only in the TCLSIH cohort), salt intake (only in the TCLSIH cohort), and estimated 24 hour sodium excretion (only in the UK Biobank).a Values are hazard ratios (95% confidence intervals) unless otherwise indicated.b P for trend was calculated by using categories of salty taste preference (low: 1, medium: 2, and high: 3) as an ordinal variable. | ||||
TCLSIH cohort (n = 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 869) 869) |
1.00 (reference) | 1.04 (0.96, 1.13) | 1.14 (1.03, 1.28) | 0.02 |
UK Biobank (n = 173![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 940) 940) |
1.00 (reference) | 0.96 (0.85, 1.07) | 1.08 (0.95, 1.22) | 0.24 |
Mediation analyses
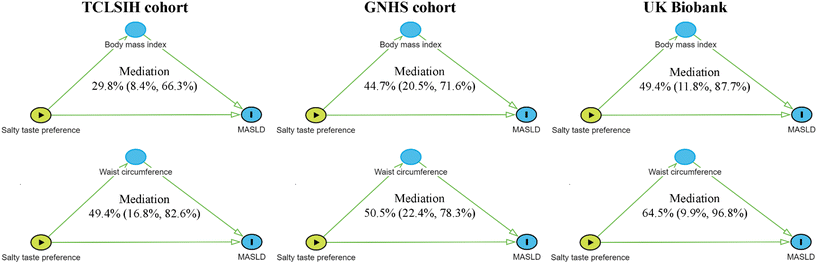
The proportion of association between salty taste preference and MASLD mediated by higher BMI was 29.8% (8.4%, 66.3%) in the TCLSIH cohort, 44.7% (20.5%, 71.6%) in the GNHS cohort, and 49.4% (11.8%, 87.7%) in the UK Biobank (Fig. 3 and Table S7). Waist circumference exhibited a stronger mediating effect than BMI across all cohorts, with proportions of 49.4% (16.8%, 82.6%) in the TCLSIH cohort, 50.5% (22.4%, 78.3%) in the GNHS cohort, and 64.5% (9.9%, 96.8%) in the UK Biobank (Fig. 3 and Table S7).Interactions between salty taste preference and genetic susceptibility
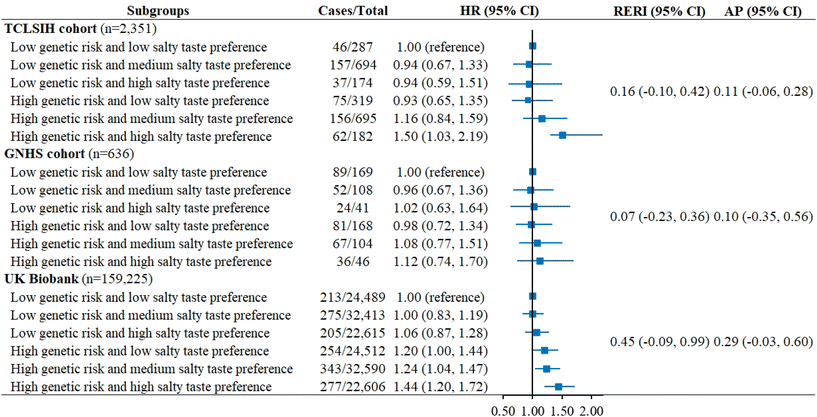
Individuals with high PRS exhibited a higher risk of incident MASLD in all cohorts, although the results were not significant in the GNHS cohort due to the small sample size (Table S8). The significant positive association between salty taste preference and MASLD was observed only in those with high genetic risk, though no significant multiplicative association was observed between salty taste preference and PRS (Table S9; P for interaction ≥0.06). Furthermore, individuals with high genetic risk and high salty taste preference had the highest risk of MASLD when compared to those with low genetic risk and low salty taste preference; however, no statistically significant additive interaction was observed (Fig. 4).Secondary analyses
Table 5 displays the associations of salty taste preference with PDFF-defined MASLD and elevated cT1. In multivariable model 2, compared with participants who had low salty taste preference, those demonstrating high preference had an 18% higher risk of MASLD (odds ratio [OR]: 1.18; 95% CI: 1.11, 1.26; P for trend <0.0001). Additional adjustment for BMI appreciably attenuated such association; the corresponding ORs (95% CIs) across categories of salty taste preference were 1.00 (reference) for low, 1.08 (1.01, 1.15) for medium, and 1.09 (1.02, 1.17) for high (P for trend = 0.02). Similar association patterns were observed for elevated cT1. The adjusted ORs (95% CIs) for elevated cT1 in model 2 were 1.00 (reference) for low, 1.18 (1.03, 1.34) for medium, and 1.18 (1.02, 1.36) for high (P for trend = 0.03).| Categories of salty taste preference | P for trendb | |||
|---|---|---|---|---|
| Low | Medium | High | ||
| Abbreviations: cT1 – corrected T1; MASLD – metabolic dysfunction-associated steatotic liver disease; PDFF – proton density fat fraction. Model 1 was adjusted for age, sex, ethnicity, household income, and educational level. Model 2 was further adjusted for smoking status, alcohol drinking status, physical activity, sedentary time, cardiovascular disease, cancer, diabetes, hyperlipidemia, hypertension, family history of diseases (including cardiovascular disease, diabetes, and hypertension), and healthy diet score. Model 3 was additionally adjusted for body mass index.a Values are odds ratios (95% confidence intervals) unless otherwise indicated.b P for trend was calculated by using categories of salty taste preference (low: 1, medium: 2, and high: 3) as an ordinal variable. | ||||
| PDFF-defined MASLD | ||||
| Number of participants | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 223 223 |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 068 068 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 048 048 |
— |
| Number of cases | 2540 | 3856 | 2812 | — |
| Model 1 | 1.00 (reference) | 1.14 (1.07, 1.21) | 1.23 (1.16, 1.32) | <0.0001 |
| Model 2 | 1.00 (reference) | 1.11 (1.05, 1.18) | 1.18 (1.11, 1.26) | <0.0001 |
| Model 3 | 1.00 (reference) | 1.08 (1.01, 1.15) | 1.09 (1.02, 1.17) | 0.02 |
| Elevated cT1 | ||||
| Number of participants | 8584 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 971 971 |
8592 | — |
| Number of cases | 381 | 630 | 459 | — |
| Model 1 | 1.00 (reference) | 1.19 (1.04, 1.36) | 1.23 (1.07, 1.42) | <0.0001 |
| Model 2 | 1.00 (reference) | 1.18 (1.03, 1.34) | 1.18 (1.02, 1.36) | 0.03 |
| Model 3 | 1.00 (reference) | 1.13 (0.99, 1.30) | 1.10 (0.95, 1.27) | 0.25 |
Discussion
Main findings
In this prospective study of three independent cohorts with varying salty taste preferences (Chinese is among the highest), we observed that greater salty taste preference, reflecting long-term salt intake, was consistently associated with a higher risk of incident MASLD. Such association was in part mediated by adiposity measures, including BMI and waist circumference. Furthermore, this positive association appeared to be stronger in individuals with high genetic risk. Moreover, salty taste preference was positively associated with PDFF-defined MASLD and elevated cT1 levels in a subset of the UK Biobank.Comparison with previous studies
To the best of our knowledge, this is the first study to investigate the association between salty taste preference and risk of MASLD. Our findings are supported by previous cross-sectional studies, in which positive associations of sodium intake measured by dietary survey or urine samples with MASLD were observed.6–11,13 In addition, a prospective cohort study conducted in China showed that perceived high salt intake, as measured by a self-reported question, was associated with a higher risk of MASLD.12 In contrast, a Korean prospective cohort study observed a positive association between sodium intake estimated from spot urine specimens and risk of MASLD among females but not males.15 In another case–control study among Iranian adults, there was no association between dietary sodium intake from FFQ and odds of MASLD.14 The inconsistencies in findings across studies may be due to differences in exposure measurements (estimations of sodium intake vary across studies) and heterogeneity in study designs. Moreover, these studies were limited to drawing causal conclusions due to inaccurate exposure measurements and inadequate covariate adjustment sets (e.g., overall diet quality was not considered in previous studies). A recent Mendelian randomization analysis documented that a high salt diet had a potential causal association with an increased risk of MASLD.43 Of note, Mendelian randomization has unique challenges in studying diet because diet changes over the life course, and food is intercorrelated with many behaviors and the environment.44,45 Salty taste preference serves as a proxy for long-term habits, which helps to overcome this limitation. Salty taste preference is the primary factor leading to an increase in overall salt intake and may be a more comprehensive measure of dietary salt intake than a single day's urine collection or dietary survey. Indeed, we observed that salty taste preference was positively associated with habitual salt intake and urinary sodium concentration in a dose–response manner, supported by several previous studies.16–20 Moreover, our findings indicated that high salty taste preference was associated with a higher risk of MASLD. However, such association was attenuated after adjustment for salt intake as measured using FFQ and became non-significant when adjusted for objectively measured urinary sodium. These findings support the hypothesis that actual sodium intake accounts for much of the observed association between salty taste preference and MASLD.As expected, the association between salty taste preference and the risk of incident MASLD was partly mediated by adiposity. This is supported by a cross-sectional study in Korea, showing that the positive association between dietary sodium intake measured by FFQ and MASLD was partly mediated by BMI and body fat percentage.9 In addition, previous studies have shown a positive association between high salt intake and overweight/obesity.23 The results of our mediation analysis further elucidate that high salty taste preference partly contributes to MASLD through its association with increased adiposity, supported by our recent work.24 Furthermore, waist circumference exhibited a stronger mediating effect than BMI, suggesting that abdominal obesity plays a more important role in the association between salt intake and MASLD.
Potential mechanisms
The potential mechanisms linking salty taste preference to adiposity-mediated MASLD are multifaceted and may involve several biological pathways. First, experimental research in mice indicated that high salt intake activates the aldose reductase–fructokinase pathway in the liver and hypothalamus, leading to endogenous fructose production with the development of leptin resistance and hyperphagia that cause obesity, insulin resistance, and MASLD.4 Second, high salt intake causes accumulation of liver fat due to the dysfunction of the renin–angiotensin–aldosterone system and salt-induced hyperosmolar properties.5 Although these potential mechanisms have been identified, the association between high salty taste preference and MASLD is complex and influenced by various factors. Further research is needed to fully elucidate these mechanisms by integrating multiomics data with behavioral, social, and environmental factors.Public health and clinical implications
Although Chinese have higher salt intake than other populations, such as the UK, largely due to cultural dietary practices,46 the observed association between salty taste preference and MASLD risk is consistent across cohorts from China and the UK. This underscores the need for population-specific salt reduction strategies. In China, where salt intake derives predominantly from cooking practices,46 interventions should prioritize reducing cooking salt use. In contrast, in the UK, where processed foods and discretionary salt use (e.g., table salt addition) contribute more significantly to total intake,46,47 public health efforts could focus on reduction of salt in commercially processed foods and behavior modification, such as gradual reduction in added salt to gradually modify taste preferences. Collectively, our findings support universal recommendations to lower salt intake as a potential strategy for MASLD prevention, tailored to regional dietary patterns.Furthermore, our results suggest that the detrimental effects of salt intake on MASLD are accentuated in individuals with high genetic risk for MASLD. Because self-reported salty taste preference correlates with higher sodium excretion (indicating higher salt intake) and is easy to collect in clinical and public settings, it could be used in public health screenings to identify individuals at risk of MASLD, especially those with high genetic risk. Clinicians should be strongly advised to expand their knowledge about salt reduction strategies and offerings to patients with MASLD.
Strengths and limitations
The strengths of this study include the inclusion of three large cohorts with different ancestries, large sample size, use of a novel tool to assess habitual salt intake compared with previous studies, and adjustment for a wide range of similar covariates. Nonetheless, the study has several limitations. First, data on salty taste preferences were self-reported and collected only once at the baseline, which may lead to misclassification and may not accurately reflect actual sodium intake. However, salty taste preference does not rely on memory, does not suffer from health-related biases due to social pressure, and is relatively stable over time in adults. In addition, the validity of salty taste preference was shown by a dose–response association between this variable and urinary sodium concentrations. Nevertheless, we acknowledge that factors such as social desirability, context (e.g., home cooking vs. processed foods), and cultural interpretations of “salty” across cuisines might have affected our results. Second, the three cohorts operationalized salty taste preference differently, and the GNHS cohort lacked a measure of habitual salt intake. In addition, the categorization of salty taste preference lacks standardization and validated cutoffs across diverse populations. These methodological differences may be a potential source of misclassification bias. Third, salty taste preference might be a marker of an unhealthy lifestyle. However, adjustment for lifestyle factors and subgroup analyses by such factors might help reduce potential confounding. Nevertheless, uncaptured confounding factors are possible. Fourth, hepatic steatosis was diagnosed using liver ultrasound or electronic health records rather than liver biopsy. However, moderate to severe steatosis can be reliably determined with ultrasound, with good sensitivity (84.8%) and specificity (93.6%).48 In addition, the results were not appreciably altered when hepatic steatosis was defined using liver PDFF (accurate and reliable quantification of liver fat content over the entire liver). Nevertheless, we acknowledge that the use of different MASLD diagnostic modalities across cohorts (abdominal ultrasound vs. electronic health records) may introduce bias into risk estimates. Finally, the participants in the cohorts included in this study were from China and the UK, which may limit the generalizability of the findings to other populations.Conclusions
To summarize, this multi-center cohort study indicated that salty taste preference was associated with a higher risk of MASLD, with adiposity as a mediating factor. These findings suggest that interventions aimed at improving people's salt preference may prevent MASLD onset in the general adult population. Moreover, public health efforts should begin with improving salt literacy and awareness.Author contributions
Conceptualization: SZ, YY, LQ, TH, YMC, KN, and LM; data curation: SZ, YY, YG, and HW; formal analysis: SZ and YY; funding acquisition: SZ; investigation: SZ, YY, YG, HW, QZ, and LL; methodology: SZ, YY, and YB; project administration: LQ, TH, YMC, KN, and LM; resources: TH, YMC, KN, and LM; software: SZ, YY, and YB; supervision: LQ, TH, YMC, KN, and LM; validation: YG; visualization: SZ; writing – original draft: SZ and YY; writing – review & editing: YG, HW, QZ, LL, YB, LQ, TH, YMC, KN, and LM.Conflicts of interest
The authors declare that they have no competing interests.Abbreviations
| BMI | Body mass index |
| CI | Confidence interval |
| cT1 | Corrected T1 |
| CVD | Cardiovascular disease |
| FFQ | Food frequency questionnaire |
| GNHS | Guangzhou Nutrition and Health Study |
| HR | Hazard ratio |
| MASLD | Metabolic dysfunction-associated steatotic liver disease |
| OR | Odds ratio |
| PDFF | Proton density fat fraction |
| PRS | Polygenic risk score |
| SD | Standard deviation |
| STROBE | Strengthening the Reporting of Observational Studies in Epidemiology |
| TCLSIH | Tianjin Chronic Low-grade Systemic Inflammation and Health. |
Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5fo04464b.Other relevant data can be obtained from the corresponding authors upon reasonable request. Original data from the UK Biobank used in the present study are available at https://www.ukbiobank.ac.uk/.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (No. 82304128), Young Elite Scientists Sponsorship Program by CAST (No. 2023QNRC001), and Shaanxi Province Postdoctoral Science Foundation (No. 2023BSHEDZZ19). The authors would like to thank all the participants in the Tianjin Chronic Low-grade Systemic Inflammation and Health cohort, the Guangzhou Nutrition and Health Study, and the UK Biobank, and all staff who have worked on collecting data in the study.References
- L. Miao, G. Targher, C. D. Byrne, Y. Y. Cao and M. H. Zheng, Current status and future trends of the global burden of MASLD, Trends Endocrinol. Metab., 2024, 35, 697–707 CrossRef CAS PubMed.
- M. H. Le, Y. H. Yeo, B. Zou, S. Barnet, L. Henry, R. Cheung and M. H. Nguyen, Forecasted 2040 global prevalence of nonalcoholic fatty liver disease using hierarchical bayesian approach, Clin. Mol. Hepatol., 2022, 28, 841–850 CrossRef PubMed.
- N. Stefan, H. Yki-Jarvinen and B. A. Neuschwander-Tetri, Metabolic dysfunction-associated steatotic liver disease: heterogeneous pathomechanisms and effectiveness of metabolism-based treatment, Lancet Diabetes Endocrinol., 2025, 13, 134–148 CrossRef CAS PubMed.
- M. A. Lanaspa, M. Kuwabara, A. Andres-Hernando, N. Li, C. Cicerchi, T. Jensen, D. J. Orlicky, C. A. Roncal-Jimenez, T. Ishimoto, T. Nakagawa, B. Rodriguez-Iturbe, P. S. MacLean and R. J. Johnson, High salt intake causes leptin resistance and obesity in mice by stimulating endogenous fructose production and metabolism, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 3138–3143 CrossRef CAS PubMed.
- J. Xu and F. Mao, Role of high-salt diet in non-alcoholic fatty liver disease: a mini-review of the evidence, Eur. J. Clin. Nutr., 2022, 76, 1053–1059 CrossRef PubMed.
- E. Han, M. K. Kim, S. S. Im, H. S. Kim, T. K. Kwon and B. K. Jang, High Sodium Intake, as Assessed by Urinary Sodium Excretion, Is Associated with Nonalcoholic Fatty Liver Disease or Sarcopenia, Gut Liver, 2023, 17, 456–465 CrossRef CAS PubMed.
- L. Zhou, Y. Yang, Y. Feng, X. Zhao, Y. Fan, J. Rong, L. Zhao and Y. Yu, Association between dietary sodium intake and non-alcoholic fatty liver disease in the US population, Public Health Nutr., 2021, 24, 993–1000 CrossRef PubMed.
- E. H. van den Berg, E. G. Gruppen, H. Blokzijl, S. J. L. Bakker and R. P. F. Dullaart, Higher Sodium Intake Assessed by 24 Hour Urinary Sodium Excretion Is Associated with Non-Alcoholic Fatty Liver Disease: The PREVEND Cohort Study, J. Clin. Med., 2019, 8, 2157 CrossRef CAS PubMed.
- Y. Choi, J. E. Lee, Y. Chang, M. K. Kim, E. Sung, H. Shin and S. Ryu, Dietary sodium and potassium intake in relation to non-alcoholic fatty liver disease, Br. J. Nutr., 2016, 116, 1447–1456 CrossRef CAS PubMed.
- J. H. Huh, K. J. Lee, J. S. Lim, M. Y. Lee, H. J. Park, M. Y. Kim, J. W. Kim, C. H. Chung, J. Y. Shin, H. S. Kim, S. O. Kwon and S. K. Baik, High Dietary Sodium Intake Assessed by Estimated 24 h Urinary Sodium Excretion Is Associated with NAFLD and Hepatic Fibrosis, PLoS One, 2015, 10, e0143222 CrossRef PubMed.
- X. Luo, Y. Li, Y. Zhou, C. Zhang, L. Li, Y. Luo, J. Wang, Y. Duan and J. Xie, Association of Non-alcoholic Fatty Liver Disease With Salt Intake and Dietary Diversity in Chinese Medical Examination Adults Aged 18–59 Years: A Cross-Sectional Study, Front. Nutr., 2022, 9, 930316 CrossRef PubMed.
- X. Shen, C. Jin, Y. Wu, Y. Zhang, X. Wang, W. Huang, J. Li, S. Wu and X. Gao, Prospective study of perceived dietary salt intake and the risk of non-alcoholic fatty liver disease, J. Hum. Nutr. Diet., 2019, 32, 802–809 CrossRef CAS PubMed.
- J. Li, S. Wan, X. Dai, Y. Cui and Z. Lu, The relationship between dietary sodium intake and all-cause mortality in patients with non-alcoholic fatty liver disease: a cohort study from NHANES 2003–2018, Front. Nutr., 2025, 12, 1530025 CrossRef PubMed.
- A. Salehi-Sahlabadi, E. Mirfazli, F. Teymoori, S. Roosta, A. Mokari, M. Azadi and A. Hekmatdoost, The Association Between Dietary Intake of Sodium, Potassium, and Na:K Ratio with the Risk of NAFLD: A Case-Control Study Among Iranian Adults, Int. J. Prev. Med., 2021, 12, 179 CrossRef PubMed.
- J. Lee, J. Y. Lee and Y. J. Yang, Sex-Specific Association between Sodium Intake Estimated by 24-Hour Urinary Sodium Excretion and Nonalcoholic Fatty Liver Disease: The Community-Based Prospective Cohort Study, Nutrients, 2024, 16, 548 CrossRef CAS PubMed.
- Q. He, X. Du, L. Wang, Y. Fang, J. Zhong and R. Hu, Taste Preference for Salt Predicts Salt Intake in a Chinese Population, Nutrients, 2024, 16, 2090 CrossRef CAS PubMed.
- H. Lee, H. J. Cho, E. Bae, Y. C. Kim, S. Kim and H. J. Chin, Not salt taste perception but self-reported salt eating habit predicts actual salt intake, J. Korean Med. Sci., 2014, 29(Suppl 2), S91–S96 CrossRef CAS PubMed.
- R. Takachi, J. Ishihara, M. Iwasaki, Y. Ishii and S. Tsugane, Self-reported taste preference can be a proxy for daily sodium intake in middle-aged Japanese adults, J. Acad. Nutr. Diet., 2014, 114, 781–787 CrossRef PubMed.
- K. Takamura, M. Okayama, T. Takeshima, S. Fujiwara, M. Harada, J. Murakami, M. Eto and E. Kajii, Influence of salty food preference on daily salt intake in primary care, Int. J. Gen. Med., 2014, 7, 205–210 Search PubMed.
- Z. Zhang and X. Zhang, Salt taste preference, sodium intake and gastric cancer in China, Asian Pac. J. Cancer Prev., 2011, 12, 1207–1210 Search PubMed.
- E. Carbonneau, M. Bradette-Laplante, B. Lamarche, V. Provencher, C. Begin, J. Robitaille, S. Desroches, M. C. Vohl, L. Corneau and S. Lemieux, Development and Validation of the Food Liking Questionnaire in a French-Canadian Population, Nutrients, 2017, 9, 1337 CrossRef PubMed.
- C. L. Parr, M. B. Veierod, P. Laake, E. Lund and A. Hjartaker, Test-retest reproducibility of a food frequency questionnaire (FFQ) and estimated effects on disease risk in the Norwegian Women and Cancer Study (NOWAC), Nutr. J., 2006, 5, 4 CrossRef PubMed.
- L. Zhou, J. Stamler, Q. Chan, L. Van Horn, M. L. Daviglus, A. R. Dyer, K. Miura, N. Okuda, Y. Wu, H. Ueshima, P. Elliott, L. Zhao and I. R. Group, Salt intake and prevalence of overweight/obesity in Japan, China, the United Kingdom, and the United States: the INTERMAP Study, Am. J. Clin. Nutr., 2019, 110, 34–40 CrossRef PubMed.
- S. Zhang, Z. Huo, Y. Borne, E. Sonestedt and L. Qi, Adding salt to foods and risk of metabolic dysfunction-associated steatotic liver disease and other chronic liver diseases, Eur. J. Nutr., 2025, 64, 224 CrossRef CAS PubMed.
- E. Trepo and L. Valenti, Update on NAFLD genetics: From new variants to the clinic, J. Hepatol., 2020, 72, 1196–1209 CrossRef CAS PubMed.
- S. Zhang, S. Gan, Q. Zhang, L. Liu, G. Meng, Z. Yao, H. Wu, Y. Gu, Y. Wang, T. Zhang, X. Wang, S. Sun, X. Wang, M. Zhou, Q. Jia, K. Song, L. Qi and K. Niu, Ultra-processed food consumption and the risk of non-alcoholic fatty liver disease in the Tianjin Chronic Low-grade Systemic Inflammation and Health Cohort Study, Int. J. Epidemiol., 2022, 51, 237–249 CrossRef PubMed.
- C. W. Ling, H. Zhong, F. F. Zeng, G. Chen, Y. Fu, C. Wang, Z. Q. Zhang, W. T. Cao, T. Y. Sun, D. Ding, Y. H. Liu, H. L. Dong, L. P. Jing, W. Ling, J. S. Zheng and Y. M. Chen, Cohort Profile: Guangzhou Nutrition and Health Study (GNHS): A Population-based Multi-omics Study, J. Epidemiol., 2024, 34, 301–306 CrossRef PubMed.
- C. Sudlow, J. Gallacher, N. Allen, V. Beral, P. Burton, J. Danesh, P. Downey, P. Elliott, J. Green, M. Landray, B. Liu, P. Matthews, G. Ong, J. Pell, A. Silman, A. Young, T. Sprosen, T. Peakman and R. Collins, UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age, PLoS Med., 2015, 12, e1001779 CrossRef PubMed.
- J. P. Vandenbroucke, E. von Elm, D. G. Altman, P. C. Gotzsche, C. D. Mulrow, S. J. Pocock, C. Poole, J. J. Schlesselman, M. Egger and S. Initiative, Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration, Int. J. Surg., 2014, 12, 1500–1524 CrossRef PubMed.
- C. Lachat, D. Hawwash, M. C. Ocke, C. Berg, E. Forsum, A. Hornell, C. Larsson, E. Sonestedt, E. Wirfalt, A. Akesson, P. Kolsteren, G. Byrnes, W. De Keyzer, J. Van Camp, J. E. Cade, N. Slimani, M. Cevallos, M. Egger and I. Huybrechts, Strengthening the Reporting of Observational Studies in Epidemiology-Nutritional Epidemiology (STROBE-nut): An Extension of the STROBE Statement, PLoS Med., 2016, 13, e1002036 CrossRef PubMed.
- S. Zhang, X. Wu, S. Bian, Q. Zhang, L. Liu, G. Meng, Z. Yao, H. Wu, Y. Gu, Y. Wang, S. Sun, X. Wang, M. Zhou, Q. Jia, K. Song and K. Niu, Association between consumption frequency of honey and non-alcoholic fatty liver disease: results from a cross-sectional analysis based on the Tianjin Chronic Low-grade Systemic Inflammation and Health (TCLSIH) Cohort Study, Br. J. Nutr., 2021, 125, 712–720 CrossRef CAS PubMed.
- P. Elliott, D. C. Muller, D. Schneider-Luftman, R. Pazoki, E. Evangelou, A. Dehghan, B. Neal and I. Tzoulaki, Estimated 24-Hour Urinary Sodium Excretion and Incident Cardiovascular Disease and Mortality Among 398 628 Individuals in UK Biobank, Hypertension, 2020, 76, 683–691 CrossRef CAS PubMed.
- C. Bycroft, C. Freeman, D. Petkova, G. Band, L. T. Elliott, K. Sharp, A. Motyer, D. Vukcevic, O. Delaneau, J. O'Connell, A. Cortes, S. Welsh, A. Young, M. Effingham, G. McVean, S. Leslie, N. Allen, P. Donnelly and J. Marchini, The UK Biobank resource with deep phenotyping and genomic data, Nature, 2018, 562, 203–209 CrossRef CAS PubMed.
- T. Zhang, Y. Gu, G. Meng, Q. Zhang, L. Liu, H. Wu, S. Zhang, X. Wang, J. Zhang, S. Sun, X. Wang, M. Zhou, Q. Jia, K. Song and K. Niu, Genetic Risk, Adherence to a Healthy Lifestyle, and Hyperuricemia: The TCLSIH Cohort Study, Am. J. Med., 2023, 136, 476–483 CrossRef CAS PubMed.
- C. Cheng, F. Xu, X. F. Pan, C. Wang, J. Fan, Y. Yang, Y. Liu, L. Sun, X. Liu, Y. Xu, Y. Zhou, C. Xiao, W. Gou, Z. Miao, J. Yuan, L. Shen, Y. Fu, X. Sun, Y. Zhu, Y. Chen, A. Pan, D. Zhou and J. S. Zheng, Genetic mapping of serum metabolome to chronic diseases among Han Chinese, Cell. Genomics, 2025, 5, 100743 CrossRef CAS PubMed.
- H. Xue, L. Wang, D. Sun, Y. Wu, C. Yu, Y. Huang, S. O. Chan, W. Ling, J. Lv, L. Li, X. Chen, Y. Pang and C. Yu, Associations of Alcohol Consumption and Genetic Predisposition to Hepatic Steatosis With Liver-Related Events: Results From Large Population-Based Cohort Studies, Gastroenterology, 2025, 169, 705–714 CrossRef CAS PubMed.
- C. Bianco, O. Jamialahmadi, S. Pelusi, G. Baselli, P. Dongiovanni, I. Zanoni, L. Santoro, S. Maier, A. Liguori, M. Meroni, V. Borroni, R. D'Ambrosio, R. Spagnuolo, A. Alisi, A. Federico, E. Bugianesi, S. Petta, L. Miele, U. Vespasiani-Gentilucci, Q. M. Anstee, F. Stickel, J. Hampe, J. Fischer, T. Berg, A. L. Fracanzani, G. Soardo, H. Reeves, D. Prati, S. Romeo and L. Valenti, Non-invasive stratification of hepatocellular carcinoma risk in non-alcoholic fatty liver using polygenic risk scores, J. Hepatol., 2021, 74, 775–782 CrossRef CAS PubMed.
- S. Zhang, Y. Yan, X. F. Zeng, Y. Gu, H. Wu, Q. Zhang, L. Liu, Z. Huo, X. Luo, R. Zhang, E. Sonestedt, Y. Borne, L. Qi, T. Huang, M. H. Zheng, Y. M. Chen, K. Niu and L. Ma, Associations of the EAT-Lancet reference diet with metabolic dysfunction-associated steatotic liver disease and its severity: A multicohort study, Hepatology, 2025, 81, 1583–1594 CrossRef PubMed.
- C. Caussy, M. H. Alquiraish, P. Nguyen, C. Hernandez, S. Cepin, L. E. Fortney, V. Ajmera, R. Bettencourt, S. Collier, J. Hooker, E. Sy, E. Rizo, L. Richards, C. B. Sirlin and R. Loomba, Optimal threshold of controlled attenuation parameter with MRI-PDFF as the gold standard for the detection of hepatic steatosis, Hepatology, 2018, 67, 1348–1359 CrossRef CAS PubMed.
- A. Mojtahed, C. J. Kelly, A. H. Herlihy, S. Kin, H. R. Wilman, A. McKay, M. Kelly, M. Milanesi, S. Neubauer, E. L. Thomas, J. D. Bell, R. Banerjee and M. Harisinghani, Reference range of liver corrected T1 values in a population at low risk for fatty liver disease-a UK Biobank sub-study, with an appendix of interesting cases, Abdom. Radiol., 2019, 44, 72–84 CrossRef CAS PubMed.
- E. Jackson, A. Dennis, N. Alkhouri, N. Samala, R. Vuppalanchi, A. J. Sanyal, M. Muthiah, R. Banerjee and A. Banerjee, Cardiac and liver impairment on multiorgan MRI and risk of major adverse cardiovascular and liver events, Nat. Med., 2025, 31, 2289–2296 CrossRef PubMed.
- R. Li and L. Chambless, Test for additive interaction in proportional hazards models, Ann. Epidemiol., 2007, 17, 227–236 CrossRef PubMed.
- Q. Liu, Y. Liu, H. Feng, N. Zhang and Z. Yang, High salt diet causally increases metabolic dysfunction-associated steatotic liver disease risk: A bidirectional mendelian randomization study, Nutr. Res., 2025, 136, 94–104 CrossRef CAS PubMed.
- J. Merino and D. K. Tobias, The unique challenges of studying the genetics of diet and nutrition, Nat. Med., 2022, 28, 221–222 CrossRef CAS PubMed.
- A. Satija, E. Yu, W. C. Willett and F. B. Hu, Understanding nutritional epidemiology and its role in policy, Adv. Nutr., 2015, 6, 5–18 CrossRef PubMed.
- C. A. Anderson, L. J. Appel, N. Okuda, I. J. Brown, Q. Chan, L. Zhao, H. Ueshima, H. Kesteloot, K. Miura, J. D. Curb, K. Yoshita, P. Elliott, M. E. Yamamoto and J. Stamler, Dietary sources of sodium in China, Japan, the United Kingdom, and the United States, women and men aged 40 to 59 years: the INTERMAP study, J. Am. Diet. Assoc., 2010, 110, 736–745 CrossRef CAS PubMed.
- J. Sutherland, P. Edwards, B. Shankar and A. D. Dangour, Fewer adults add salt at the table after initiation of a national salt campaign in the UK: a repeated cross-sectional analysis, Br. J. Nutr., 2013, 110, 552–558 CrossRef CAS PubMed.
- R. Hernaez, M. Lazo, S. Bonekamp, I. Kamel, F. L. Brancati, E. Guallar and J. M. Clark, Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: a meta-analysis, Hepatology, 2011, 54, 1082–1090 CrossRef PubMed.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2026 |