Open Access Article
Open Access ArticleOptimisation of the microwave-assisted extraction process of bioactive compounds with antioxidant activity from cocoa pod husk (Theobroma cacao L.)
Esteban Jurado-Beizaga a,
Erick Alvarez-Yanamango
a,
Erick Alvarez-Yanamango *bcd,
Oscar Herrera-Calderon
*bcd,
Oscar Herrera-Calderon a and
Alfredo Ibañez
a and
Alfredo Ibañez d
d
aDepartment of Pharmacology, Bromatology and Toxicology, Faculty of Pharmacy and Biochemistry, National University of San Marcos, Lima 15001, Peru
bAgro-industrial Technologies and Processes Research Group (ITEPA PUCP), Pontificia Universidad Católica del Perú, Lima 15001, Peru. E-mail: erick.alvarez@pucp.edu.pe
cEngineering Department, Pontificia Universidad Católica del Perú, Lima 15001, Peru
dInstitute for Omics Sciences and Applied Biotechnology (ICOBA PUCP), Pontificia Universidad Católica del Perú, Lima 15001, Peru
First published on 12th January 2026
Abstract
Cocoa pod husk (CPH), the main by-product of cocoa production, represents up to 80% of the fresh weight of the fruit and is often discarded, despite being a rich source of bioactive compounds such as polyphenols, flavonoids and saponins. The objective of this study was to optimise the microwave-assisted extraction (MAE) of bioactive compounds with the highest antioxidant activity from CPH using the Box–Behnken design (BBD) and Response Surface Methodology (RSM). The effects of microwave power (285, 570, and 855 W), extraction time (2, 3, and 4 min) and liquid/solid ratio (30, 40, and 50 mL g−1) on the Total Phenol Content (TPC), Total Saponin Content (TSC) and antioxidant activity—DPPH Radical Scavenging Capacity (DRSC), ABTS Radical Scavenging Capacity (ARSC) and Ferric Reducing Antioxidant Power (FRAP)—of bioactive compounds were evaluated. The globally optimised conditions (855 W, 3.06 min, and 50 mL g−1) achieved high extraction efficiencies, and yielded a TPC of 2.724 mgGAE g−1, TSC of 0.241 mgEE g−1, and DRSC, ARSC, and FRAP of 81.966, 99.680, and 90.890 µmolTE g−1, respectively, with a desirability index of 0.84. Microwave power emerged as the main factor influencing bioactive recovery, while extraction time and liquid/solid ratio modulated diffusion and prevented thermal degradation. Notably, the protocol was implemented using a modified domestic microwave oven, offering a cost-effective and sustainable alternative to conventional MAE systems. Comprehensive UHPLC-Q-Orbitrap-MS/MS profiling revealed a diverse metabolomic signature, including malic, citric, tartaric, and gluconic acids, and the phenolic compound clovamide, confirming the chemical complexity and functional potential of the extract. The integrated optimisation and profiling framework reinforces the sustainable nature and methodological novelty of MAE in enhancing the value of CPH, supporting its role as a clean-label candidate for functional nutrition, health-promoting products, and circular bioeconomy initiatives.
Sustainability spotlightCocoa pod husk (CPH), an abundant agro-industrial by-product, poses environmental and economic challenges due to its improper disposal, while conventional extraction techniques demand substantial energy and solvent input. This study optimises microwave-assisted extraction (MAE) to sustainably recover antioxidant-rich bioactive compounds—including phenolics and saponins—while significantly reducing resource consumption. The process integrates statistical design methodologies to enhance efficiency and scalability, offering a robust and adaptable valorisation strategy. These findings exemplify circular economy principles by converting agricultural waste into functional ingredients suitable for food, cosmetic, and pharmaceutical applications. The work directly supports SDGs 9 (Industry, Innovation and Infrastructure), 12 (Responsible Consumption and Production), and 13 (Climate Action), reinforcing the contribution of green technologies to agro-industrial sustainability. |
1 Introduction
The cocoa bean is one of the most significant crops worldwide. It is estimated that world production will reach approximately 4.84 million tonnes in the period 2024/25, according to the International Cocoa Organization (ICCO).1 This is evidence of the high global demand and economic impact of cocoa, which is not only limited to the chocolate industry, but also involves its by-products.Cocoa pod husk (CPH) is the main by-product, representing 52–80% of the fresh weight of the fruit.2–8 For every tonne of dry cocoa beans, ten tonnes of wet CPH are generated,9 generating approximately 48.4 million tonnes globally, which could pose environmental problems, but also reflects a critical underutilization of a plant material that, until now, has been considered an unwanted waste by the cocoa and chocolate industries. Therefore, effective management of these wastes is fundamental in attempts to prevent food waste and develop circular economy concepts, within the food–energy–water (FEW) nexus, as their management significantly affects the availability of these three resources.10 CPH is an essential source of bioactive compounds, particularly polyphenols, flavonoids, methylxanthines, and fibres, which have been associated with antioxidant, anti-inflammatory, and cardioprotective properties.9,11–17 Thus, the valorisation of CPH would not only reduce the environmental impact of waste, but also generate integral solutions such as the production of biomass energy, water recovery, development of functional materials, recovery of bioactive compounds with antioxidant activity and enriched composting, with promising applications in the food, cosmetics, and pharmaceutical industries.10,18 Within this framework, agro-industrial by-products, such as CPH, should not be considered as final waste, but as secondary raw materials with economic, environmental and social value. Transforming organic wastes into reusable inputs within the same or other production systems, promotes closed-material cycles.17 Waste management approaches generally include the reuse and valorisation of by-products through innovative processes, their use as functional ingredients rich in bioactive compounds, and the extraction of valuable active ingredients.
Consequently, Microwave-Assisted Extraction (MAE) is positioned as an innovative non-conventional solvent alternative green technology for the recovery of bioactive compounds from CPH presenting significant advantages over conventional solvent extraction.19–23 Its main benefits include a reduction in the volume of solvents used, greater time efficiency, more precise control of operating variables, a decrease in energy consumption and an increase in extraction yield.19 However, its effectiveness does not depend solely on the use of microwaves as an energy source, but is strongly influenced by a series of operating parameters. The most decisive factors are irradiation power, extraction time and solid/liquid ratio.24 Power controls the rate of dielectric heating and, therefore, cell disruption and metabolite release, which must be carefully optimised to avoid thermal degradation of sensitive compounds; and the liquid/solid ratio directly affects the mass transfer efficiency and concentration of the extract.24–26 Therefore, this study aims to optimise the operating parameters of MAE—microwave power, extraction time and liquid/solid ratio—in order to maximise the recovery of bioactive compounds from CPH. In particular, it seeks to improve the extraction efficiency of total phenolic content (TPC) and total saponin content (TSC), as well as antioxidant activity, evaluated using the DPPH radical scavenging capacity (DRSC), ABTS radical scavenging capacity (ARSC) and ferric reducing antioxidant power (FRAP) methods, which was assessed as part of this optimisation process.
As a major novel contribution, unlike previous studies, this work proposes a balanced global optimisation that simultaneously considers all response parameters, using a modified domestic microwave oven as a low-cost, high-efficiency alternative. It also incorporates the identification of the metabolomic profile of the optimised extract, which broadens the understanding of the compounds responsible for bioactivity and establishes a basis for future biotechnological and nutraceutical applications.
2 Materials and methods
2.1 Chemical and analytical instruments
All chemicals used in this study were of analytical grade, including: gallic acid, trolox, acetic acid (glacial) 100%, aluminium chloride hexahydrate, aluminium chloride trihydrate, iron(III) chloride hexahydrate, 2,20-Azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), Folin–Ciocalteu reagent, 2,4,6-tripyridyl-s-triazine (TPTZ), and 2,2-diphenyl-1-picryl-hydrazil (DPPH) were purchased from Merck KGaA (Darmstadt, Germany). Anhydrous sodium carbonate, sodium acetate trihydrate and potassium persulfate were purchased from J.T.Baker (USA). NaOH was purchased from Macron Fine Chemicals (USA). UHPLC-Q-Orbitrap-MS/MS solvents were purchased from Merck (Darmstadt, Germany). Ultrapure water was obtained using a Millipore water purification system (Milli-Q Merck Millipore, Darmstadt, Germany). Absorbance was measured using a Genesys 10S UV-VIS spectrophotometer. UHPLC-Q-Orbitrap-MS/MS was measured using a UHPLC Dionex UltiMate 3000 system coupled to a Q-Exactive orbitrap (Thermo Fisher Scientific, Waltham, MA, USA).2.2 Sample preparation
CPH was obtained from fine aroma cocoa of Amazon origin (Theobroma cacao L.) of the Forastero variety, provided by Kuyay chocolates, located in the Jahuanga zone, Bagua Grande district, Utcubamba province, Amazonas region, Peru (5°46′19.6′ ″S 78°32′57.4′ ″W, 605 m altitude).2.3 Microwave-assisted drying (MAD)
Microwave-assisted drying (MAD) was carried out following the methodology described by Sianoun et al.,27 with slight modifications. Fresh CPH was manually sliced using a stainless-steel knife to produce sheets with a thickness of 5 ± 0.3 mm. The samples were arranged in a single layer on a glass turntable to ensure uniform microwave exposure and prevent overlapping. Drying was performed using a microwave oven (Samsung MG40J5133AT; 95–950 W, 2450 MHz). The drying process was fixed at 95 W (10% power of the microwave oven).The key experimental parameter was the sample-to-power ratio, where the loading density for MAD was maintained at 0.52 g of CPH per watt of microwave power (g W−1). This ratio was achieved by placing 50.10 ± 0.02 g of sample under a microwave power of 95 W. The selected ratio was based on preliminary trials, which indicated that drying under these conditions yielded the highest TPC, increasing from 1.50 mgGAE g−1 in the fresh sample to 2.40 mgGAE g−1 after drying. These values were obtained using ultrasound-assisted extraction (UAE) as the analytical method during preliminary experiments. It should be noted that these TPC values were obtained during preliminary experiments and are not included in the main dataset presented in this article.
All measurements were performed in triplicate. The dried samples were milled using a blade mill (MKM6003, Bosch, Slovenia) and sieved through an ASTM E11 No. 80–60 mesh (Retsch, Germany). Hence, the particle sizes were between 251 and 426 µm. The resulting flour was packaged in trilaminate vacuum bags and stored at −80 °C until further phytochemical and antioxidant activity analysis.
2.4 Microwave-assisted extraction (MAE) optimisation
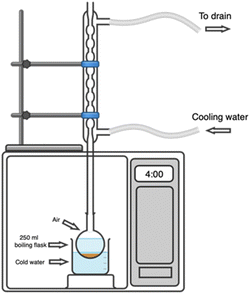
The MAE process for CPH was conducted using a modified microwave oven (Samsung MG40J5133AT), as illustrated in Fig. 1. Bioactive compounds were extracted following the protocol described by Rahayu et al.21 The experimental design included three levels for each variable: microwave power (285, 570, and 855 W), extraction time (2, 3, and 4 min), and liquid/solid ratio (30, 40, and 50 mL g−1). Extractions were performed in a boiling flask using particles whose sizes were in the range between 251 and 426 µm, and an ethanol–water solution (50% v/v) as the solvent. The resulting extracts were centrifuged at 5000 rpm for 10 min, and the residues were washed with the same solvent and centrifuged again at 5000 rpm for 3 min. The final extract volume was adjusted to match the initial extraction volume using the same solvent, and then cooled for subsequent analysis.It is important to note that the microwave system does not have active temperature control. Therefore, the extractions were performed in a boiling flask placed inside a water bath (Fig. 1). In this way, the water bath acted as a thermal buffer, preventing excessive temperature increases. The MAE protocol aimed to maintain the extraction at 80 ± 2 °C. This temperature was defined because preliminary tests showed that extraction temperatures above 90 °C caused condenser saturation, increased margins of error, and were incompatible with the proposed modification model.
2.5 Experimental design
A Box–Behnken design (BBD) with Response Surface Methodology (RSM) was used to optimise and determine the impact of the process variables (A: microwave power, B: extraction time, C: liquid/solid ratio) on the responses: TPC, TSC, ARSC, DPPH and FRAP. These factors and their levels are listed in Table 1. The relationship between the process variables and responses is expressed using an empirical second-order polynomial equation as follows:| Y = β0 + β1X1 + β2X2 + β3X3 + β12X1X2 + β13X1X3 + β23X2X3 + β11X12 + β22X22 + β33X32 + ε |
| Factors | Unit | Levels | ||
|---|---|---|---|---|
| −1 | 0 | +1 | ||
| a Abbreviations: W, watts; min, minutes; mL g−1, millilitres per gram. | ||||
| (A) Microwave power | W | 285 | 570 | 855 |
| (B) Extraction time | min | 2 | 3 | 4 |
| (C) Liquid/solid ratio | mL g−1 | 30 | 40 | 50 |
2.6 Determination of bioactive compounds
Bioactive compounds of CPH extract were evaluated in terms of TPC and TSC. The TPC was determined according to the method of Singleton et al.28 and was expressed as milligrams of gallic acid equivalents per gram (mgGAE g−1) of dried extract sample (DS extract). The TSC was determined according to the method of Nguyen et al.12 and was expressed as milligrams of escin equivalents per gram (mgEE g−1) of DS extract.The identification of secondary metabolites was carried out by UHPLC-Q-Orbitrap-MS/MS following the methodology adapted from Vargas-Arana et al.11 Extracts were prepared by dissolving 10 µL of sample (optimised CPH extract) in 90 µL of acetonitrile (UHPLC grade). Samples were centrifuged at 4200 rpm for 5 min and transferred to a 2 mL glass vial.
An ultra-high-performance liquid chromatograph (UHPLC Dionex UltiMate 3000, Thermo Fisher Scientific, Waltham, MA, USA) equipped with an Acclaim™ C18 reversed-phase HPLC column (5 µm, 4.6 × 150 mm) was used, with a flow rate of 0.7 mL min−1, and the injection volume was 10 µL. The mobile phases were 0.1% formic acid in water (A) and 90% acetonitrile in water (B). The gradient used was (0.00 min, 5% B); (1.00 min, 5% B); (25.00 min, 95% B); (26.00 min, 100% B); (27.00 min, 100% B); (30.00, 5% B); and 2.5 min for column equilibration before each injection.
The Q-Exactive orbitrap (HESI II, Thermo Fisher Scientific, Waltham, MA, USA) was operated in negative ion mode (ESI-), using data-dependent acquisition (DDA). Orbitrap parameters were: AGC target: 5 × 10−6, resolution: 120![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, maximum, IT: 150 ms, range: m/z 100–600, HESI source (1 kV, negative mode), gas heater temp: 140 °C, capillary temperature: 300 °C, carrier gas: N2 (sheath gas flow rate: 10, sweep gas flow rate: 1, S-lens RF level: 80). Parameters for MS2 were: maximum IT: 50 ms, AGC target: 2 × 10−4, resolution: 60
000, maximum, IT: 150 ms, range: m/z 100–600, HESI source (1 kV, negative mode), gas heater temp: 140 °C, capillary temperature: 300 °C, carrier gas: N2 (sheath gas flow rate: 10, sweep gas flow rate: 1, S-lens RF level: 80). Parameters for MS2 were: maximum IT: 50 ms, AGC target: 2 × 10−4, resolution: 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000.
000.
2.7 Determination of antioxidant activity
CPH extract was tested for antioxidant activity utilizing ARSC, DRSC and FRAP. The ARSC assay was performed according to the method described by Re et al.29 The DRSC assay was performed according to the method described by Brand-Williams et al.30 The FRAP assay was performed according to the method described by Benzie et al.31 All antioxidant activity assays were expressed as µmol of Trolox equivalents per gram (µmolTE g−1) of DS extract.2.8 Statistical analysis
Analysis of variance (ANOVA) was carried out to evaluate the statistical significance (p < 0.05) of the factors. Mean comparisons were made using the Tukey HDS test (p < 0.05). All data are presented as mean ± standard deviation (SD). The analyses were repeated thrice unless indicated. The BBD and RSM were performed using Design-Expert version 13 (Stat-Ease Inc., Minneapolis, MN, USA).3 Results and discussion
3.1 Microwave-assisted extraction (MAE) process
The BBD was employed to optimise the independent variables of MAE—microwave power (W), extraction time (min), and liquid/solid ratio (mL g−1)—in relation to the response variables TPC, TSC, DRSC, ARSC, and FRAP. The experiment was conducted using CPH flour with a particle size range of 251–426 µm, and the results are summarised in Table 2.| Run | Microwave power (W) | Extraction time (min) | L/S ratio (mL g−1) | TPC (mgGAE g−1) | TSC (mgEE g−1) | DRSC (µmolTE g−1) | ARSC (µmolTE g−1) | FRAP (µmolTE g−1) |
|---|---|---|---|---|---|---|---|---|
| a The results were expressed as the sample dry extract. Data are presented as mean ± SD from triplicate experiments. Abbreviations: TPC, total phenol content; TSC, total saponin content; DRSC, DPPH radical scavenging capacity; ARSC, ABTS radical scavenging capacity; FRAP, ferric reducing antioxidant power; GAE, gallic acid equivalent; EE, escin equivalent; TE, Trolox equivalent. | ||||||||
| 1 | 570 | 2 | 50 | 2.623 ± 0.013 | 0.239 ± 0.002 | 75.126 ± 1.608 | 94.749 ± 2.165 | 86.333 ± 1.196 |
| 2 | 855 | 2 | 40 | 2.775 ± 0.061 | 0.219 ± 0.002 | 90.536 ± 2.539 | 91.888 ± 3.120 | 87.708 ± 0.778 |
| 3 | 855 | 3 | 50 | 2.712 ± 0.023 | 0.246 ± 0.004 | 81.983 ± 1.439 | 101.433 ± 0.549 | 89.382 ± 1.164 |
| 4 | 570 | 4 | 30 | 2.409 ± 0.036 | 0.218 ± 0.002 | 76.316 ± 1.192 | 92.743 ± 2.220 | 88.621 ± 0.848 |
| 5 | 570 | 3 | 40 | 2.571 ± 0.026 | 0.223 ± 0.006 | 82.257 ± 1.158 | 98.557 ± 1.572 | 89.493 ± 0.905 |
| 6 | 570 | 3 | 40 | 2.551 ± 0.025 | 0.223 ± 0.005 | 82.819 ± 1.083 | 97.212 ± 1.166 | 87.966 ± 1.671 |
| 7 | 570 | 3 | 40 | 2.560 ± 0.020 | 0.226 ± 0.004 | 83.929 ± 2.214 | 98.159 ± 1.584 | 90.024 ± 0.867 |
| 8 | 855 | 4 | 40 | 2.699 ± 0.040 | 0.231 ± 0.003 | 87.841 ± 0.508 | 98.647 ± 2.551 | 94.795 ± 1.011 |
| 9 | 285 | 3 | 30 | 2.193 ± 0.024 | 0.190 ± 0.005 | 78.296 ± 3.422 | 94.716 ± 0.970 | 89.099 ± 1.347 |
| 10 | 855 | 3 | 30 | 2.498 ± 0.032 | 0.197 ± 0.003 | 77.890 ± 1.086 | 97.991 ± 1.515 | 82.067 ± 1.327 |
| 11 | 285 | 3 | 50 | 2.284 ± 0.019 | 0.194 ± 0.004 | 75.199 ± 0.799 | 102.188 ± 1.442 | 85.493 ± 1.046 |
| 12 | 285 | 2 | 40 | 1.985 ± 0.032 | 0.185 ± 0.004 | 76.299 ± 1.243 | 84.762 ± 1.267 | 75.335 ± 0.751 |
| 13 | 285 | 4 | 40 | 2.359 ± 0.028 | 0.201 ± 0.003 | 74.496 ± 1.890 | 89.214 ± 1.205 | 92.578 ± 1.173 |
| 14 | 570 | 2 | 30 | 2.424 ± 0.026 | 0.187 ± 0.002 | 76.128 ± 0.964 | 92.822 ± 0.409 | 71.798 ± 1.676 |
| 15 | 570 | 4 | 50 | 2.616 ± 0.020 | 0.218 ± 0.003 | 85.199 ± 2.767 | 95.843 ± 0.766 | 83.174 ± 1.170 |
| 16 | 570 | 3 | 40 | 2.554 ± 0.023 | 0.225 ± 0.005 | 83.408 ± 1.270 | 99.373 ± 0.628 | 87.600 ± 2.270 |
| 17 | 570 | 3 | 40 | 2.591 ± 0.043 | 0.226 ± 0.003 | 82.080 ± 0.726 | 99.036 ± 1.010 | 87.334 ± 0.678 |
The analysis showed that each response reached its highest individual value under different extraction conditions. The highest TPC (2.775 mgGAE g−1 DS extract) and the greatest DRSC activity (90.536 µmolTE g−1 DS extract) were both obtained at 855 W, 2 min, and 40 mL g−1. In contrast, the maximum TSC (0.246 mgEE g−1 DS extract) occurred at 855 W, 3 min, and 50 mL g−1; the highest ARSC activity (102.188 µmolTE g−1 DS extract) at 285 W, 3 min, and 50 mL g−1; and the maximum FRAP value (94.795 µmolTE g−1 DS extract) at 855 W, 4 min, and 40 mL g−1.
While the individual maxima highlight the distinct sensitivity of each response to specific MAE parameters, these values are not intended to define separate operational targets. Rather, they inform the response surface behaviour that supports the subsequent global optimisation. The focus of this work lies in establishing integrated extraction conditions that ensure overall efficiency across all responses, rather than optimising them independently.
3.2 Optimisation and validation of the extraction of bioactive compounds and antioxidant activity of CPH by MAE
| Responses | R2 | Adj. R2 | p-value (model) | Significance terms | Lack-of-fit p | CV (%) |
|---|---|---|---|---|---|---|
| a The p-value results were expressed as follows: p < 0.05, *. Abbreviations: A, microwave power; B, extraction time; C, liquid/solid ratio; CV, coefficient of variation; TPC, total phenol content; TSC, total saponin content; DRSC, DPPH radical scavenging capacity; ARSC, ABTS radical scavenging capacity; FRAP, ferric reducing antioxidant power. | ||||||
| TPC | 0.932 | 0.917 | <0.0001* | A, C, AB, A2 | <0.0001* | 2.29 |
| TSC | 0.967 | 0.960 | <0.0001* | A, B, C, AC, BC, A2, C2 | 0.0619 | 1.14 |
| DRSC | 0.689 | 0.621 | <0.0001* | A, C2 | <0.0001* | 3.75 |
| ARSC | 0.792 | 0.746 | <0.0001* | A, B2 | <0.0001* | 2.42 |
| FRAP | 0.852 | 0.820 | <0.0001* | B, BC, C2 | <0.0001* | 2.80 |
| Responses | Predicted mean | Std dev | n | SE pred | 95% Pl low | Data mean | 95% PI high | Relative error % |
|---|---|---|---|---|---|---|---|---|
| a The results were expressed as the sample dry extract. Abbreviations: TPC, total phenol content; TSC, total saponin content; DRSC, DPPH radical scavenging capacity; ARSC, ABTS radical scavenging capacity; FRAP, ferric reducing antioxidant power. | ||||||||
| TPC | 2.770 | 0.057 | 5 | 0.038 | 2.692 | 2.724 | 2.847 | 1.67% |
| TSC | 0.246 | 0.004 | 5 | N/A | 0.240 | 0.241 | 0.252 | 1.99% |
| DRSC | 85.683 | 3.021 | 5 | 2.026 | 81.591 | 81.966 | 89.775 | 4.54% |
| ARSC | 102.580 | 2.319 | 5 | 1.555 | 99.439 | 99.680 | 105.720 | 2.91% |
| FRAP | 92.075 | 2.417 | 5 | 1.621 | 88.802 | 90.890 | 95.348 | 1.30% |
Using BBD and RSM methodologies, the response surface graphs revealed that TPC increased with microwave power and liquid/solid ratio, while prolonged extraction time negatively affected yield (Fig. 2). Under specific experimental conditions, the highest individual TPC value (2.775 mgGAE g−1 DS extract) was obtained at 855 W, 40 mL g−1, and 2 min (Table 2). This local maximum reflects the combined curvilinear effects of power and liquid/solid ratio identified in the model, illustrating how each parameter influences TPC recovery.
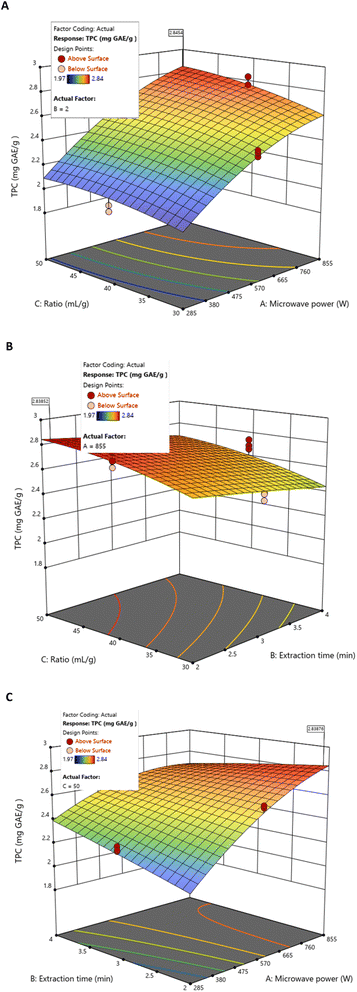 | ||
| Fig. 2 Response surfaces of TPC by MAE as a function of fixed values for (A) extraction time (2 min), (B) microwave power (855 W), and (C) liquid/solid ratio (50 mL g−1). | ||
However, this does not correspond to the overall optimum conditions. When all responses were simultaneously considered, the global optimisation (855 W, 50 mL g−1, 3.06 min) provided a balanced extraction performance, yielding a slightly lower TPC value (2.724 mgGAE g−1 DS extract) (Table 4), consistent with the negative effect of longer extraction times suggested by the AB interaction.
Several studies have supported the influence of extraction variables on TPC during MAE. Rincón et al.20 applied a BBD to optimise MAE parameters for TPC extraction from CPH (100 µm, variety TCS01) using 63% ethanol. Under optimal conditions (400 W, 240 s, and 67 mL g−1), they achieved a TPC of 43.94 mgGAE g−1 DS, identifying microwave power as the most significant factor, followed by the extraction time and liquid/solid ratio. Similarly, Nguyen et al.12 optimised MAE parameters for saponin recovery from CPH (≤1400 µm) using a Central Composite design (CCD). While saponins were the primary target, a TPC of 12.40 mgGAE g−1 DS was reported under optimal conditions (600 W, 6 s min−1 pulsed irradiation, 40 min, 50 mL g−1), identifying methanol concentration and extraction time as key variables influencing phenolic recovery. In a study by Rahayu et al.,21 MAE was conducted on CPH (177 µm, Indonesia) using pure ethanol for varying times (4–10 min) at 800 W and 70 °C. The highest TPC (8.65 mgGAE mL−1 DS) was obtained after 10 min, demonstrating the importance of time, although prolonged exposure may differ in effect depending on the solvent and particle size. Mashuni et al.19 reported a TPC of 853.67 mgGAE L−1 equivalent to 0.853 mgGAE mL−1 DS for CPH (250–600 µm, Forastero variety) extracted with 85% ethanol at 200 W for 20 min, showing low phenolic recovery under relatively low power and long extraction time.
Other studies without formal optimisation designs also reported diverse TPC values depending on variables such as cocoa variety, solvent extraction, particle size, and extraction method. For instance, Nguyen et al.32 reported TPC values between 1.44 and 12.22 mgGAE g−1 DS for CPH (≤1400 µm, Trinitario variety) using water and water bath extraction. Valadez-Carmona et al.14 obtained 1893 mgGAE per 100 g DS equivalent to 18.93 mgGAE g−1 DS for CPH (≤425 µm) extracted by agitation with acetone![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water
water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetic acid (70
acetic acid (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29.5
29.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5), mainly associated with drying treatments prior to extraction. Delgado-Ospina et al.5 found TPC values ranging from 5.4 to 16.6 mgGAE g−1 DS for CPH (500 µm) using sonication with methanol
0.5), mainly associated with drying treatments prior to extraction. Delgado-Ospina et al.5 found TPC values ranging from 5.4 to 16.6 mgGAE g−1 DS for CPH (500 µm) using sonication with methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water and acetone
water and acetone![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water mixtures, following heat pre-treatments. Vargas-Arana et al.11 reported remarkably high TPC (111 mgGAE g−1 extract) from convectively dried CPH (≤425 µm, CCN-51 variety) extracted with methanol + 1% formic acid via sonication. Martínez et al.33 found in CPH (220–640 µm) TPC values of 352.67–365.33 mgGAE per 100 g DS equivalent to 3.52–3.65 mgGAE g−1 DS extracted by agitation with methanol-acetone and 206.67–227 mgGAE per 100 g DS with ethanol equivalent to 2.06–2.27 mgGAE g−1 DS. Comparative analysis with previous studies highlights that TPC extraction from CPH is governed by a complex interaction of factors. This multidimensional dependence highlights the variable but promising nature of MAE in various optimisation strategies.
water mixtures, following heat pre-treatments. Vargas-Arana et al.11 reported remarkably high TPC (111 mgGAE g−1 extract) from convectively dried CPH (≤425 µm, CCN-51 variety) extracted with methanol + 1% formic acid via sonication. Martínez et al.33 found in CPH (220–640 µm) TPC values of 352.67–365.33 mgGAE per 100 g DS equivalent to 3.52–3.65 mgGAE g−1 DS extracted by agitation with methanol-acetone and 206.67–227 mgGAE per 100 g DS with ethanol equivalent to 2.06–2.27 mgGAE g−1 DS. Comparative analysis with previous studies highlights that TPC extraction from CPH is governed by a complex interaction of factors. This multidimensional dependence highlights the variable but promising nature of MAE in various optimisation strategies.
Although our high-power, short-duration MAE approach yielded lower TPC values than those recorded with longer extraction times or higher solvent volumes, it offers an efficient compromise between processing speed and phenolic recovery. It should be noted that the intermediate particle size adopted in this study (251–426 µm) favoured effective mass transfer without negatively affecting operational performance, reaffirming its suitability for the sustainable extraction of phenolic compounds from agro-industrial waste such as CPH.34
On the other hand, comparatively higher TPC values can be attributed to the widespread use of intermediate polarity solvents, such as ethanol and methanol, which not only facilitate deeper penetration into lignocellulosic matrices but also exhibit superior absorption of microwave energy.35 These physicochemical properties favour the solubilisation of bioactive compounds with intermediate polarity, particularly polyphenols.35
BBD and RSM analyses indicated that TSC increased with microwave power and liquid/solid ratio, while extraction time exerted only a minor positive influence (Fig. 3). Under specific experimental conditions, the highest individual TSC value (0.246 mgEE g−1 DS extract) was observed at 855 W, 50 mL g−1, and 3 min (Table 2). This local maximum illustrates the stronger influence of microwave power and liquid/solid ratio on TSC recovery, as well as the relatively limited contribution of extraction time, consistent with the model results.
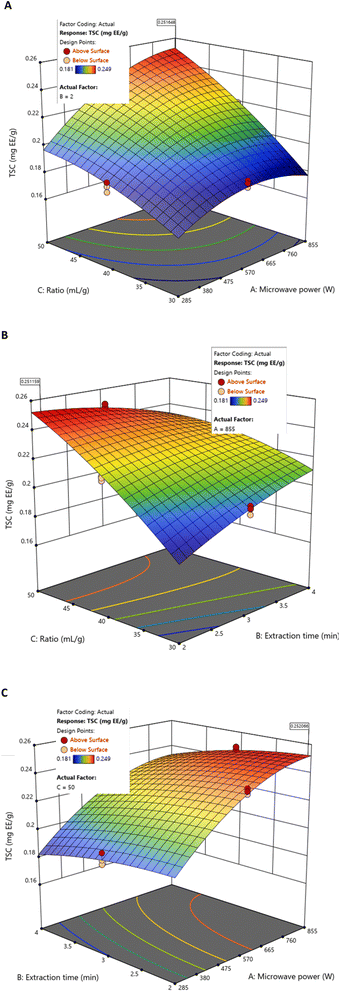 | ||
| Fig. 3 Response surfaces of TSC by MAE as a function of fixed values for (A) extraction time (2 min), (B) microwave power (855 W), and (C) liquid/solid ratio (50 mL g−1). | ||
However, this point does not represent the overall optimal conditions. When all responses were simultaneously considered, the global optimisation (855 W, 50 mL g−1, 3.06 min) yielded a slightly lower TSC value (0.241 mgEE g−1 DS extract) (Table 4), in agreement with the model predictions and the observed curvilinear effects.
The significantly higher yields reported in other studies are strongly influenced by the extraction time, solvent polarity, pre-treatment, and irradiation protocols, which should be considered depending on application priorities-yield vs. efficiency. In this regard, Nguyen et al.12 optimised the MAE parameters for TSC extraction from CPH (≤1400 µm) using a CCD and obtained a significantly higher TSC of 402 mgEE g−1 DS, under conditions of 600 W, 6 s min−1 irradiation, 40 min, and a ratio of 50 mL g−1 (R2 = 0.71, p = 0.038). The substantial difference in yield can be attributed to the use of methanol as a polar solvent, a pre-maceration stage, and prolonged extraction time, all of which improve cell wall rupture and solute diffusion. On the other hand, Nguyen et al.13 reported moderate values (370 mgEE g−1 DS) for CPH (≤1000 µm, Trinitario variety) under MAE conditions of 600 W, 30 min and 5 s min−1 irradiation, with a triple preheating stage (20 s at 600 W), using water as the solvent. These are significantly higher than those obtained in the present study, highlighting how prolonged exposure and thermal cycles can significantly increase extraction efficiency, albeit with greater energy input. On the other hand, from a chemical perspective, water favours the extraction of saponins due to its ability to interact with the hydrophilic sugar chains that characterise these compounds. Saponins are amphiphilic glycosides, with a lipophilic aglycone (sapogenin) and one or more hydrophilic sugar chains.36 This structural duality allows water, under conditions of heating and pressure such as those generated in MAE, to facilitate the solubilisation of the polar fractions and promote the breaking of hydrogen bonds between the saponins and the plant matrix.36
Similarly, conventional extraction methods yielded medium to high saponin yields. In this regard, values of 3.98–118.70 mgEE g−1 DS were reported for CPH using soaking in distilled water for 30 min and bathing in water at 50 °C for 60 min, and UAE (100 W, 60 °C, 50 min) with water as the solvent, after a pre-maceration stage at 31 °C for 30 min.32,37 The high saponin yield could be attributed to the synergistic effect of prolonged sonication and pre-treatment, which alter cell integrity and improve solute diffusion. In addition, ultrasound-induced acoustic cavitation generates microjets and shock waves that increase mass transfer and reduce solvent use.38,39
The model's lack-of-fit was significant (p < 0.0001), indicating room for further refinement (Tables 2 and S3), suggesting that additional variables or nonlinear interactions could further improve its predictive power. However, the acceptable coefficients of determination (R2 = 0.6896; adjusted R2 = 0.6215) and consistent validation results (Table 4) support the reliability of the model for predicting DPPH radical scavenging activity under the MAE conditions studied.
Response surface analyses using BBD and RSM revealed that DRSC increased with microwave power and liquid/solid ratio, while extraction time exerted only a minor positive influence (Fig. 4). Under specific experimental conditions, the highest individual DRSC value (90.536 µmolTE g−1 DS extract) was observed at 855 W, 40 mL g−1, and 2 min (Table 2). This local maximum reflects the strong contribution of microwave power and liquid/solid ratio to DPPH radical scavenging capacity, and is consistent with the significant AC and BC interactions identified in the model.
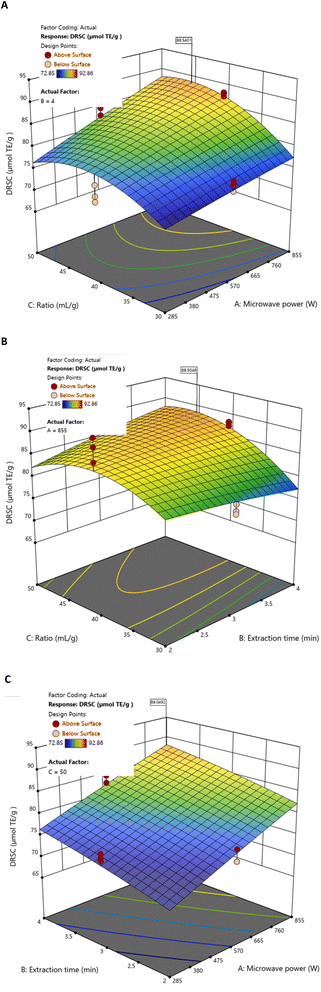 | ||
| Fig. 4 Response surfaces of DRSC by MAE as a function of fixed values for (A) extraction time (4 min), (B) microwave power (855 W), and (C) liquid/solid ratio (50 mL g−1). | ||
However, this individual maximum does not represent the overall optimal conditions. When all responses were jointly considered, the global optimisation (855 W, 50 mL g−1, 3.06 min) resulted in a lower DRSC value (81.966 µmolTE g−1 DS extract) (Table 4), in line with the negative linear trend associated with extraction time and the modest influence of this factor predicted by the model.
In contrast, Rincón et al.20 optimised the MAE parameters in CPH (100 µm, variety TCS01) using BBD and RSM, and reported significantly higher DRSC activity of 430.8 µmolTE g−1 DS under the final optimised conditions with lower microwave power (400 W, 240 s, 67 mL g−1), where microwave power was also the most influential factor. Furthermore, this higher antioxidant activity observed may be due to the finer particle size, solvent composition, and amount of TPC recovered. On the other hand, various non-optimised studies have reported DRSC values ranging from 87.42 to 133 µmolTE g−1 DS,11,40 with substantial variability attributed to differences in extraction method, particle size, solvent type, cacao variety, and pre-treatment strategies. This variability underscores that antioxidant activity is not solely governed by the parameters optimised in this study, but is also shaped by a complex interplay of physical (thermal degradation and Maillard reactions), chemical (enzymatic transformations of polyphenols), and biological (inherent profile of bioactive compounds in different cacao genotypes) factors.41 These multidimensional influences highlight the need for broader integrative approaches when evaluating antioxidant capacity in plant matrices.
BBD and RSM analyses indicated that antioxidant activity increased with microwave power and liquid/solid ratio. Non-linear behaviour was also evident: moderate extraction times enhanced activity, whereas prolonged exposure led to slight reductions, likely due to degradation of thermo-sensitive compounds. Under specific experimental conditions, the individual optimum (855 W, 50 mL g−1, and 3 min) predicted an antioxidant capacity between 102.407 and 102.723 µmolTE g−1 DS extract (Fig. 5). Within the experimental matrix, a similar individual maximum (102.188 µmolTE g−1 DS extract) was obtained at 285 W, 50 mL g−1, and 3 min (Table 2).
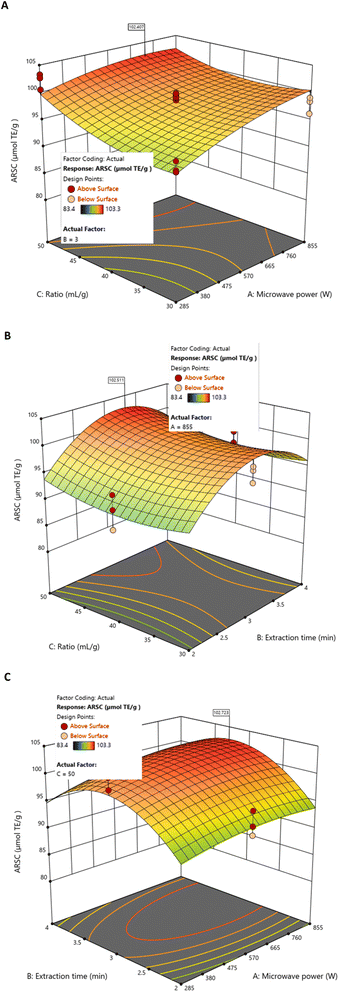 | ||
| Fig. 5 Response surfaces of ARSC by MAE as a function of fixed values for (A) extraction time (3 min), (B) microwave power (855 W), and (C) liquid/solid ratio (50 mL g−1). | ||
This behaviour reflects the dependence of ARSC on the liquid/solid ratio and the existence of an energy saturation zone suggested by the model, where additional increases in microwave power do not proportionally enhance the response.
However, neither of these individual maxima represents the global optimal conditions. When all responses were jointly considered, the overall optimisation (855 W, 50 mL g−1, 3.06 min) yielded an ARSC value of 99.680 µmol TE g−1 DS extract (Table 4), with a relative prediction error of 2.91%, confirming the model's reliability.
Studies using conventional methods and non-optimised protocols show considerable variability in ARSC. For example, Yapo et al.16 reported 51.87 µmolTE g−1 DS in CPH (250 µm) extracted by boiling with 80% ethanol for 20 min. The freeze-dried CPH extract examined by Delgado-Ospina et al.5 yielded a wider range (32.9–140 µmolTE g−1 DS), depending on the polarity and ratio of methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water and acetone
water and acetone![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water mixtures under sonication-stirring. Vargas-Arana et al.11 reported 155.38 µmolTE g−1 DS in convection-dried CPH, citing the role of formic acid (1%) in improving phenolic solubilisation. In units not comparable with our studies, it was found in CPH that MAE is approximately three times higher than UAE in terms of antioxidant activity, which could be attributed to prolonged extraction time, repeated heating cycles, and increased solute diffusion due to the polarity and thermal conductivity of the solvent.13,37 This trend is consistent with other studies comparing UAE and MAE in polyphenol-rich matrices, which reported that MAE produced up to 23% more ARSC compared to UAE, while reducing extraction time sixfold.13,37,42 These findings suggest that MAE typically results in more efficient recovery and better ARSC values due to rapid energy transfer and thermal stimulation of solute diffusion.24
water mixtures under sonication-stirring. Vargas-Arana et al.11 reported 155.38 µmolTE g−1 DS in convection-dried CPH, citing the role of formic acid (1%) in improving phenolic solubilisation. In units not comparable with our studies, it was found in CPH that MAE is approximately three times higher than UAE in terms of antioxidant activity, which could be attributed to prolonged extraction time, repeated heating cycles, and increased solute diffusion due to the polarity and thermal conductivity of the solvent.13,37 This trend is consistent with other studies comparing UAE and MAE in polyphenol-rich matrices, which reported that MAE produced up to 23% more ARSC compared to UAE, while reducing extraction time sixfold.13,37,42 These findings suggest that MAE typically results in more efficient recovery and better ARSC values due to rapid energy transfer and thermal stimulation of solute diffusion.24
What emerges from this comparative analysis is a consistent pattern: ARSC is highly sensitive to extraction kinetics and solvent physicochemistry. More importantly, antioxidant potential appears intricately linked to the phenolic fingerprint including the abundance and accessibility of low-molecular-weight flavonoids and phenolic acids. Factors such as drying intensity, matrix disruption, and solvent polarity not only affect mass transfer but also modulate the liberation of structurally bound polyphenols.43–45
BBD and RSM analyses indicated that microwave power and liquid/solid ratio exerted positive effects on FRAP values. The model also revealed non-linear behaviour, identifying an individual optimum at 285 W, 30 mL g−1, and 4 min, with predicted FRAP values between 95.162 and 95.953 µmolTE g−1 DS extract (Fig. 6).
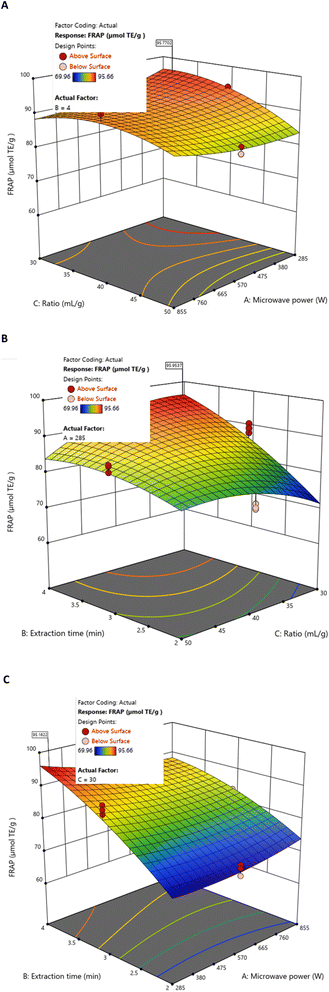 | ||
| Fig. 6 Response surfaces of FRAP by MAE as a function of fixed values for (A) extraction time (4 min), (B) microwave power (285 W), and (C) liquid/solid ratio (30 mL g−1). | ||
Within the experimental domain, the highest individual FRAP value measured (94.795 µmolTE g−1 DS extract) was obtained at 855 W, 40 mL g−1, and 4 min (Table 2). This observation illustrates the strong contribution of extraction time and liquid/solid ratio, as well as the curvilinear effects suggested by the model, particularly the negative quadratic behaviour of the liquid/solid ratio.
Importantly, these individual maxima do not correspond to the global optimal conditions. When all responses were simultaneously considered, the overall optimisation (855 W, 50 mL g−1, 3.06 min) produced a slightly lower FRAP value (90.890 µmolTE g−1 DS extract) (Table 4), consistent with the negative interaction between extraction time and liquid/solid ratio and the curvilinear trend predicted by the model.
In comparison, studies such as that by Rincón et al.20 reported higher values (144.32 µmolTE g−1 DS) under longer conditions (400 W, 240 s, 67 mL g−1), identifying microwave power as the main factor influencing antioxidant recovery, which is consistent with the present findings, and confirming the influence of the energy supplied and the availability of solvent.
Conventional methods, such as water bath or stirring with organic solvents, showed lower values and greater dispersion (53 – 181 µmolTE g−1 DS)5,11,16 demonstrating limited efficiency without energy assistance and the significant influence of drying type, solvent, and plant variety on FRAP activity, likely due to their effect on the preservation and accessibility of phenolic compounds.46 Together, these findings suggest that FRAP activity depends on the interaction between extraction kinetics, the chemical composition of the matrix, and the thermal conditions applied, requiring a multivariable approach for future optimisation, including analysis of the specific phenolic profile and thermal stability of the active compounds.
Overall, the correlation analysis showed significant associations between TPC, TSC, and antioxidant responses (DRSC, ARSC, and FRAP). A strong positive correlation was observed between TPC and DRSC (r = 0.69), indicating that phenolic compounds were primarily responsible for free radical scavenging capacity. Similarly, TPC showed a moderate correlation with ARSC (r = 0.52) and FRAP (r = 0.44), confirming the involvement of phenols in hydrogen transfer (HAT) and single electron transfer (SET) mechanisms.47 In contrast, TSC showed a stronger correlation with FRAP (r = 0.56) and a moderate correlation with ARSC (r = 0.46), suggesting that saponins also contribute to the overall antioxidant potential, especially through electron donation reactions. These results show that the antioxidant activity of CPH extracts obtained by MAE is due to the combined and complementary effect of phenolic compounds and saponins, whose recovery depends largely on microwave power and the liquid/solid ratio used.
3.3 Validation of the models and regression equations
Global multi-response optimisation was carried out considering six responses: bioactive compounds (TPC and TSC) and antioxidant activity (DRSC, ARSC and FRAP). The composite desirability reached 0.840 and an algorithm was applied to identify the combination of factors that maximized the overall response, found to be 855 W, 3.06 min and 50 mL g−1.To verify the predictive accuracy of the MAE model, a confirmation test was conducted in which the predicted values were compared with the newly measured data. Table 4 reports the predicted mean, the experimentally obtained mean, and the 95% prediction intervals for each response. In every case the experimental values lie inside the corresponding interval, demonstrating that the model reliably forecasts future observations within the specified limits. All models exhibited high accuracy, with relative errors below 5% for most responses (TPC: 1.67%; TSC: 1.99%; ARSC: 2.91%; FRAP: 1.30%), except for DRSC (4.54%), which remained within acceptable limits for complex biological matrices (Table 4).
Table 5 summarises the final second-order models, expressed in real (uncoded) units, that describe the influence of microwave power (A), extraction time (B) and liquid/solid ratio (C) on each response CPH extract during MAE.
| Term | TPC | TSC | DRSC | ARSC | FRAP |
|---|---|---|---|---|---|
| β | β | β | β | β | |
| a The p-value results were expressed as follows: p < 0.05, *. Abbreviations: A, microwave power; B, extraction time; C, liquid/solid ratio; TPC, total phenol content; TSC, total saponin content; DRSC, DPPH radical scavenging capacity; ARSC, ABTS radical scavenging capacity; FRAP, ferric reducing antioxidant power. | |||||
| Intercept | 1.314 × 10−1 | −3.204 × 100 | 3.721 × 101 | 4.757 × 101 | −6.083 × 101 |
| A | 3.034 × 10−3* | 4.930 × 10−4* | −5.616 × 10−3* | 3.294 × 10−2* | −2.919 × 10−2 |
| B | 2.936 × 10−1 | 3.876 × 10−1* | −6.475 × 100 | 3.642 ×101 | 4.460 × 101* |
| C | 3.341 × 10−2* | 3.778 × 10−2* | 2.470 × 100 | −1.090 ×100 | 3.946 × 100 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Interactions | |||||
| AB | −4.020 × 10−4* | −2.700 × 10−5 | −7.330 × 10−4 | 1.996 × 10−3 | −9.030 × 10−3 |
| AC | 1.100 × 10−5 | 1.800 × 10−5* | 6.390 × 10−4 | −3.620 × 10−4 | 9.680 × 10−4 |
| BC | 2.500 × 10−4 | −6.218 × 10−3* | 2.470 × 10−1 | 2.833 × 10−2 | −4.996 × 10−1* |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Quadratics | |||||
| A2 | −1.270 × 10−6* | −7.510 × 10−7* | −2.380 × 10−6 | −1.400 × 10−5 | 2.000 × 10−5 |
| B2 | −6.833 × 10−3 | −1.643 × 10−2 | −3.780 × 10−1 | −6.195 × 100* | −2.456 × 100 |
| C2 | −3.930 × 10−4 | −2.870 × 10−4* | −4.330 × 10−2* | 1.763 × 10−2 | −3.548 × 10−2* |
For TPC, microwave power and liquid/solid ratio significantly enhanced recovery, although curvilinear trends suggested potential thermal degradation or saturation effects at elevated levels. Notably, TPC showed a strong negative interaction between power and time, underlining the importance of controlled energy input. In contrast, TSC displayed a complex response profile, with all primary factors, interactions (AC and BC), and quadratic effects contributing significantly. This underscores the intricacy of saponin release mechanisms and suggests a narrower operating window for optimal yield. Antioxidant assays presented divergent behaviours. DRSC and ARSC confirmed microwave power as the primary enhancer of radical scavenging capacity, while ARSC exhibited nonlinear effects of time, possibly due to compound instability. FRAP, conversely, was most responsive to extraction time, with significant interaction and curvature effects, indicating a unique sensitivity among assays to thermal and solvent dynamics.
Overall, the findings indicate that selective optimisation is crucial: while high power favours polyphenols and radical scavenging activities, prolonged exposure may compromise compound integrity. The integration of curvilinear and interaction terms in the models enhances predictive reliability and supports tailored parameter adjustment to maximise functional compound recovery.
3.4 Characterisation of the phytochemical profile in CPH extract obtained by MAE using UHPLC-Q-Orbitrap-MS/MS
Chromatography by UHPLC-Q-Orbitrap-MS/MS in negative ionisation mode allowed the identification of phytochemical compounds from the CPH extract, obtained by MAD and 50% hydroalcoholic extraction based on optimised MAE parameters (855 W, 3.06 min and 50 mL g−1). Several compounds were detected and tentatively identified due to the lack of proper standards. These include organic acids, such as tartaric acid (C4H6O6), [M−H]− m/z 149.0084, citric acid (C6H8O7), [M−H]− m/z 191.0193, gluconic acid (C6H12O7), [M−H]− m/z 195.0506 and malic acid (C4H6O5), [M−H]− m/z 133.0136. Malic and citric acids have previously been reported in CPH, coexisting with other weak acids, which underscores its potential as a source of food-grade pectin.48In agreement with our findings, Ramos-Escudero et al.49 identified malic, tartaric, citric and gluconic acids with molecular ions [M−H]− m/z 133.0163, 149.0108, 191.0214, and 195.053, respectively, using HPLC-ESI-qTOF-MS in hydroalcoholic CPH extracts. Similarly, Abdul Karim et al.50 reported the same acids with molecular ions [M−H]− m/z 133, 149, 191 and 195 by LC-MS/MS in the hydroalcoholic CPH extracts, while Vargas-Arana et al.11 detected tartaric and gluconic acids [M−H]− m/z 149.0086 and 195.0507, respectively, in methanolic CPH extracts analysed by UHPLC-MS.
Additionally, the phenolic compound clovamide (C18H17NO7) with a molecular ion [M−H]− m/z 358.093 was detected and provisionally identified. This compound has also been reported by Gomez et al.51 with [M−H]− m/z 358.2 using HPLC-DAD-ESI-IT-MS/MS in hydroalcoholic CPH extracts.
In summary, UHPLC-Q-Orbitrap-MS/MS analyses confirmed the presence of key organic acids (malic, tartaric, citric, and gluconic) and phenolic compounds such as clovamide in CPH extracts by MAE, in line with previous reports. These results reinforce the potential of CPH as a valuable source of bioactive compounds for applications in the food and nutraceutical industries.
4 Conclusion
Overall, the globally optimised multi-response MAE protocol (855 W, 3.06 min, and 50 mL g−1), with a desirability index of 0.84, demonstrated a rapid, energy-efficient, and scalable approach for recovering a chemically diverse range of bioactive compounds from CPH. The method yielded 2.724 mgGAE g−1 TPC, 0.241 mgEE g−1 TSC, and antioxidant capacities of 81.966, 99.680, and 90.890 µmolTE g−1 for DRSC, ARSC, and FRAP assays, respectively, surpassing conventional extractions in both efficiency and antioxidant recovery.Microwave power was identified as the key driver of extraction efficiency, particularly for phenolic and antioxidant indices, whereas the liquid/solid ratio and extraction time influenced compound recovery by enhancing diffusion before thermal degradation occurred. Importantly, the use of a modified domestic microwave provides an accessible and sustainable alternative to laboratory-scale MAE systems, supporting broader technological adoption in resource-limited contexts.
Beyond its quantitative performance, UHPLC-Q-Orbitrap-MS/MS profiling revealed a complex metabolomic signature, including malic, tartaric, citric, and gluconic acids, as well as clovamide, further substantiating the chemical richness of CPH. Collectively, these findings highlight the methodological novelty and practical feasibility of the optimised MAE protocol, positioning CPH as a valuable clean-label source of bioactives for functional foods and nutraceuticals, while contributing to the circular economy and sustainable revalorisation of agro-industrial by-products.
Author contributions
Esteban Jurado-Beizaga (E. J. B.) and Erick Alvarez-Yanamango (E. A. Y.) conceived and designed the experimentation; E. J. B. performed the experiments and wrote the original manuscript; Oscar Herrera-Calderon (O. H. C.), Alfredo Ibañez (A. I.), and E. A. Y. validated and reviewed the final manuscript.Conflicts of interest
The authors declare no conflicts.Data availability
The data supporting the results of this study are available in the article. Additional datasets generated during this research and the raw output files for the calculations are available from the corresponding author (Erick Alvarez-Yanamango, E-mail: erick.alvarez@pucp.edu.pe) upon request.Supplementary information (SI): ANOVA results for TPC, TSC, DRSC, ARSC and FRAP, regression coefficients and significance of the Box–Behnken models, and interaction curves of MAE parameters. See DOI: https://doi.org/10.1039/d5fb00924c.
Acknowledgements
This research was funded by the National Program for Scientific Research and Advanced Studies (PROCIENCIA) of the National Council of Science, Technology and Technological Innovation (CONCYTEC) through the project with agreement PE501086489-2024-PROCIENCIA, entitled: Development of functional micro and nano additives for the food and cosmetics industry from cocoa (Theobroma cacao L.) waste, applying the circular economy approach. The authors acknowledge the support of the Agro-industrial Technologies and Processes Research Group (ITEPA), the Pontifical Catholic University of Peru and the National University of San Marcos. Erick Alvarez-Yanamango is grateful for the Doctoral Scholarship in Engineering from the Pontifical Catholic University of Peru (PUCP).References
- Statistics – International Cocoa Organization, https://www.icco.org/statistics/, (accessed 29 June 2025).
- L. F. Zambrano-Mite, Y. Villasana, M. L. Bejarano, C. Luciani, D. Niebieskikwiat, W. Álvarez, D. F. Cueva, D. Aguilera-Pesantes and L. M. Orejuela-Escobar, Heliyon, 2023, 9, e17258 CrossRef.
- S. B. Anoraga, R. Shamsudin, M. H. Hamzah, S. Sharif and A. D. Saputro, Heliyon, 2024, 10, e35537 CrossRef.
- L. Porto de Souza Vandenberghe, K. Kley Valladares-Diestra, G. Amaro Bittencourt, A. Fátima Murawski de Mello, Z. Sarmiento Vásquez, P. Zwiercheczewski de Oliveira, G. Vinícius de Melo Pereira and C. Ricardo Soccol, Bioresour. Technol., 2022, 344, 126252 Search PubMed.
- J. Delgado-Ospina, R. Lucas-González, M. Viuda-Martos, J. Fernández-López, J. Á. Pérez-Álvarez, M. Martuscelli and C. Chaves-López, Heliyon, 2021, 7, e06799 CrossRef.
- F. Lu, J. Rodriguez-Garcia, I. Van Damme, N. J. Westwood, L. Shaw, J. S. Robinson, G. Warren, A. Chatzifragkou, S. McQueen Mason, L. Gomez, L. Faas, K. Balcombe, C. Srinivasan, F. Picchioni, P. Hadley and D. Charalampopoulos, Curr. Opin. Green Sustainable Chem., 2018, 14, 80–88 CrossRef.
- N. Muñoz-Almagro, L. Valadez-Carmona, J. A. Mendiola, E. Ibáñez and M. Villamiel, Carbohydr. Polym., 2019, 217, 69–78 CrossRef PubMed.
- L. C. Vriesmann and C. L. de Oliveira Petkowicz, Int. J. Biol. Macromol., 2017, 101, 146–152 CrossRef PubMed.
- G. I. Edo, P. O. Samuel, G. O. Oloni, G. O. Ezekiel, F. O. Onoharigho, O. Oghenegueke, S. C. Nwachukwu, O. A. Rapheal, M. O. Ajokpaoghene, M. C. Okolie, R. S. Ajakaye, W. Ndudi and P. C. Igbodo, Nat. Resour. Hum. Health, 2023, 3, 426–448 CrossRef PubMed.
- K. M. Kibler, D. Reinhart, C. Hawkins, A. M. Motlagh and J. Wright, Waste Manage., 2018, 74, 52–62 CrossRef PubMed.
- G. Vargas-Arana, C. Merino-Zegarra, M. Tang, M. W. Pertino and M. J. Simirgiotis, Antioxidants, 2022, 11, 595 Search PubMed.
- V. T. Nguyen, M. D. Le, T. T. T. Nguyen, T. T. Khong, V. H. Nguyen, H. N. Nguyen, B. N. D. Huynh, H. T. M. Tran and T. S. Trang, J. Food Process. Preserv., 2021, 45, e15134 Search PubMed.
- V. T. Nguyen, A. X. Tran and V. A. T. Le, Powder Technol., 2021, 386, 136–143 CrossRef.
- L. Valadez-Carmona, A. Ortiz-Moreno, G. Ceballos-Reyes, J. A. Mendiola and E. Ibáñez, J. Supercrit. Fluids, 2018, 131, 99–105 CrossRef.
- R. Campos-Vega, K. H. Nieto-Figueroa and B. D. Oomah, Trends Food Sci. Technol., 2018, 81, 172–184 CrossRef.
- B. M. Yapo, V. Besson, B. B. Koubala and K. L. Koffi, Am. J. Food Nutr., 2013, 1, 38–46 Search PubMed.
- J. Korhonen, A. Honkasalo and J. Seppälä, Ecol. Econ., 2018, 143, 37–46 Search PubMed.
- Z. S. Vásquez, D. P. de Carvalho Neto, G. V. M. Pereira, L. P. S. Vandenberghe, P. Z. de Oliveira, P. B. Tiburcio, H. L. G. Rogez, A. Góes Neto and C. R. Soccol, Waste Manage., 2019, 90, 72–83 CrossRef PubMed.
- Mashuni, F. H. Hamid, Muzuni, L. O. Kadidae, M. Jahiding, L. O. Ahmad and D. Saputra, AIP Conf. Proc., 2020, 2243, 030013 CrossRef.
- C. M. Rincón, C. E. Narváez and L.-F. Gutiérrez, Tesis MSc Extracción asistida por microondas de compuestos fenólicos de los subproductos del beneficio del cacao (Theobroma cacao L.), Universidad Nacional de Colombia, 2023 Search PubMed.
- P. P. Rahayu, D. Rosyidi, Purwadi and I. Thohari, Biodiversitas, 2019, 20, 3626–3631 Search PubMed.
- I. Rahmawati, B. A. Fachri, Y. H. Manurung, Nurtsulutsiyah and M. Reza, IOP Conf. Ser.: Earth Environ. Sci., 2021, 743, 012091 CrossRef.
- T. Belwal, C. Cravotto, S. Ramola, M. Thakur, F. Chemat and G. Cravotto, Foods, 2022, 11(6), 798 CrossRef.
- G. V. Barbosa-Cánovas, A. Board, X. D. Chen and R. W. Hartel, Microwave-assisted Extraction for Bioactive Compounds:Theory and Practice, Food Engineering Series Torino, 2013, vol. 4, pp. 24–29 Search PubMed.
- C. H. Chan, R. Yusoff and G. C. Ngoh, Chem. Eng. Technol., 2015, 38, 489–496 CrossRef.
- H. K. Kala, R. Mehta, K. K. Sen, R. Tandey and V. Mandal, Trends Anal. Chem., 2016, 85, 140–152 CrossRef.
- N. Sianoun, P. Pongyeela, N. Chairerk and J. Chungsiriporn, Eng. J., 2023, 27(8), 1–12 Search PubMed.
- V. L. Singleton, R. Orthofer and R. M. Lamuela-Raventós, Methods Enzymol., 1999, 299, 152–178 Search PubMed.
- R. Re, N. Pellegrini, A. Proteggente, A. Pannala, M. Yang and C. Rice-Evans, Free Radical Biol. Med., 1999, 26, 1231–1237 Search PubMed.
- W. Brand-Williams, M. E. Cuvelier and C. Berset, LWT–Food Sci. Technol., 1995, 28, 25–30 CrossRef.
- I. F. F. Benzie and J. J. Strain, Anal. Biochem., 1996, 239, 70–76 CrossRef.
- V. T. Nguyen, T. G. Tran and N. Le Tran, Drying Technol., 2022, 40, 2021–2033 CrossRef.
- R. Martínez, P. Torres, M. A. Meneses, J. G. Figueroa, J. A. Pérez-Álvarez and M. Viuda-Martos, Food Res. Int., 2012, 49, 39–45 CrossRef.
- S. R. Dewi, L. A. Stevens, A. E. Pearson, R. Ferrari, D. J. Irvine and E. R. Binner, Food Bioprod. Process., 2022, 134, 210–222 CrossRef.
- A. Kumar, R. Gehlot, A. Saini and R. Phogat, J. Food Sci., 2024, 89, 9317–9335 CrossRef PubMed.
- R. R. T. Majinda, Methods Mol. Biol., 2012, 864, 415–426 Search PubMed.
- V. T. Nguyen, T. H. N. Tran and C. A. Pham, Waste Biomass Valorization, 2025, 16, 459–470 Search PubMed.
- S. R. Shirsath, S. H. Sonawane and P. R. Gogate, Chem. Eng. Process., 2012, 53, 10–23 CrossRef.
- M. J. Goswami, U. Dutta and D. Kakati, in Ultrasound-Assisted Extraction for Food, Pharmacy, and Biotech Industries, ed. T. Sarkar and S. Pati, Humana, New York, NY, New York, 2024, pp. 103–128 Search PubMed.
- L. Valadez-Carmona, C. P. Plazola-Jacinto, M. Hernández-Ortega, M. D. Hernández-Navarro, F. Villarreal, H. Necoechea-Mondragón, A. Ortiz-Moreno and G. Ceballos-Reyes, Innovative Food Sci. Emerging Technol., 2017, 41, 378–386 CrossRef.
- J. Oracz and E. Nebesny, Int. J. Food Prop., 2016, 19, 1242–1258 CrossRef.
- A. Bouchez, P. Vauchel, S. Périno and K. Dimitrov, Foods, 2023, 12, 1750 CrossRef PubMed.
- Y. Zeng, W. Zhou, J. Yu, L. Zhao, K. Wang, Z. Hu and X. Liu, Antioxidants, 2023, 12, 418 Search PubMed.
- A. Antony and M. Farid, Appl. Sci., 2022, 12, 2107 Search PubMed.
- B. Dulo, T. De Somer, M. Moyo, E. Nakyese, J. Githaiga, K. Raes and S. De Meester, Biomass Convers. Biorefin., 2024, 14, 23565–23579 CrossRef.
- L. Da, S. Borges, G. Pace, P. Lima, F. Artés, M. Euzébio De Souza, L. De Souza Freitas, H. Ibiapina De Jesus, N. De Fátima, A. Santos, M. Roberto Da and S. Melo, Austalian Journal of Crop Science, 2019, 13, 1835–2707 Search PubMed.
- M. Platzer, S. Kiese, T. Tybussek, T. Herfellner, F. Schneider, U. Schweiggert-Weisz and P. Eisner, Front. Nutr., 2022, 9, 882458 CrossRef PubMed.
- J. Paola Jarrín-Chacón, J. Núñez-Pérez, R. del Carmen Espín-Valladares, L. Armando Manosalvas-Quiroz, H. María Rodríguez-Cabrera and J. Manuel Pais-Chanfrau, Foods, 2023, 12, 590 Search PubMed.
- F. Ramos-Escudero, A. Rojas-García, M. de la L. Cádiz-Gurrea and A. Segura-Carretero, Ultrason. Sonochem., 2024, 106, 106887 CrossRef CAS PubMed.
- A. Abdul Karim, A. Azlan, A. Ismail, P. Hashim, S. S. Abd Gani, B. H. Zainudin and N. A. Abdullah, BMC Complementary Altern. Med., 2014, 14, 1–13 CrossRef.
- P. Gomez, C. Reyes and J. G. Figueroa, Molecules, 2025, 30, 3497 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2026 |