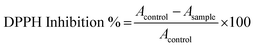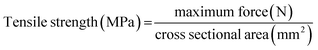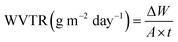Open Access Article
Open Access ArticlePlant-extract-infused edible films as natural antimicrobial and antioxidant packaging for chicken meat
Kanimozhi N. V.,
Sivabharathi M.,
Ragulya K.,
Livetha G. and
Sukumar M. *
*
Centre for Food Technology, A. C. Tech., Anna University, Chennai-600025, India. E-mail: sukumarcbt@gmail.com
First published on 15th December 2025
Abstract
This study reports the development of starch-based edible films enriched with bioactive extracts from Pongamia pinnata and Psidium guajava leaves, valorized as underutilized agro-waste resources. Ultrasound-assisted ethanolic extraction followed by partial purification yielded phenolic- and flavonoid-rich fractions, with Fourier Transform Infrared Spectroscopy (FTIR) confirming quercetin and karanjin as key constituents. The extracts exhibited high bioactivity, with a total phenolic content of 800 µg mL−1 GAE and a flavonoid content of 1295 µg mL−1 QE. Edible films incorporating 5% (v/v) extracts demonstrated improved mechanical and barrier properties; P. guajava films achieved the highest tensile stress (2.57 MPa), while P. pinnata films showed the lowest Water Vapor Transmission Rate (WVTR) (1251.7 g m−2 24 h). Antioxidant activity was confirmed via DPPH assay (IC50 of 49.32 and 54.76 µg mL−1 for P. guajava and P. pinnata, respectively), alongside strong antibacterial activity against Staphylococcus aureus and Bacillus subtilis. Application to chicken meat reduced moisture loss, preserved pH and color, and suppressed microbial growth during short-term storage. Importantly, both plants are traditionally used in food and medicine, and no toxicity concerns were evident at the concentrations tested, supporting their safe use in edible films. Beyond chicken, these films have potential application in other perishable foods such as fish, fruits, and vegetables. Overall, the study demonstrates a sustainable valorization approach for agro-waste leaves and contributes to the development of clean-label, biodegradable packaging aligned with circular economy goals.
Sustainability spotlightThis study presents an eco-friendly alternative to conventional plastic food packaging by developing biodegradable starch films incorporated with polyphenol-rich Psidium guajava and Pongamia pinnata leaf extracts. The films demonstrated strong antioxidant and antimicrobial properties, effectively extending the shelf life of chicken meat. By valorizing underutilized plant by-products and promoting biodegradable packaging, the work directly supports UN Sustainable Development Goals 12 (Responsible Consumption and Production), 13 (Climate Action), and 3 (Good Health and Well-being). The integration of green extraction methods and waste-to-value strategies underscores the sustainable advancement of this research in reducing plastic pollution and enhancing food safety. |
1 Introduction
Meat is highly perishable due to its high moisture content, nutrient composition, and susceptibility to microbial contamination and oxidation.1 Spoilage leads to economic losses, foodborne illnesses, and quality deterioration, necessitating effective packaging solutions. Traditional plastic-based packaging, while effective, contributes to environmental pollution due to its non-biodegradability, creating an urgent need for sustainable alternatives.2Biodegradable edible films are gaining attention as eco-friendly alternatives to synthetic packaging.3 Starch-based films, in particular, are widely studied due to their low cost, abundance, and biodegradability. However, they suffer from poor mechanical strength and high water sensitivity, necessitating modifications. One effective approach is incorporating plant-based bioactive compounds to enhance the antimicrobial and antioxidant properties of edible films, making them more effective in preserving perishable foods like meat.4
Plant-derived extracts are rich in phenolics, flavonoids, and tannins, which offer natural antimicrobial and antioxidant benefits.5 This study utilizes bioactive extracts from Pongamia pinnata and Psidium guajava leaves, two underutilized plant resources rich in natural antimicrobials. P. guajava is particularly known for its high quercetin content, a flavonoid with strong free radical scavenging and antibacterial properties.6 P. pinnata, traditionally used in ethnomedicine, contains karanjin, a furanoflavonoid with documented antimicrobial efficacy against foodborne pathogens.7 While these plants have individually shown preservative potential, their application in starch-based films for direct meat preservation has not been comparatively evaluated in depth.
Although various biodegradable films have been reported, limited studies have examined the incorporation of Pongamia pinnata and Psidium guajava leaf extracts into starch-based films. These underutilized leaves, often treated as agro-waste, are rich in bioactive compounds such as karanjin and quercetin, which can impart antioxidant and antimicrobial properties to packaging materials. The present study aims to valorize these plant resources by developing and characterizing extract-infused starch-based edible films for meat preservation. The objectives include:
• Extracting and characterizing bioactive compounds from Pongamia pinnata and Psidium guajava leaves.
• Developing and optimizing edible films infused with plant extracts.
• Analyzing their antioxidant and antibacterial activities against common meat spoilage bacteria.
• Evaluating their effectiveness in extending meat shelf life.
This research offers a sustainable, cost-effective, and biodegradable packaging alternative with significant benefits: (i) enhancing meat preservation by inhibiting spoilage and oxidation, (ii) reducing plastic waste, contributing to environmental sustainability, and (iii) promoting natural preservatives, reducing reliance on synthetic additives in food packaging.
This study aims to advance sustainable food preservation by developing starch-based edible films enriched with bioactive extracts from Pongamia pinnata and Psidium guajava. Although both plants have been individually studied for their phytochemical and medicinal properties, their application in starch-based food packaging remains limited, and no prior work has compared their functional performance within the same film matrix. The present study introduces two key novelties: (i) the use of partially purified, quercetin and karanjin rich leaf fractions to enhance film functionality and (ii) a direct evaluation of their antimicrobial and physicochemical effects on meat preservation. By valorizing underutilized agro-waste leaves and integrating their bioactive compounds into biodegradable films, this research provides a focused proof-of-concept for clean-label, plant-derived alternatives to synthetic packaging materials.
2 Materials and methods
2.1 Materials
2.2 Ultrasonic extraction
The extraction of bioactive compounds from Pongamia pinnata and Psidium guajava leaves was carried out using ultrasound-assisted extraction (UAE) with a probe-type ultrasonicator.8 Fresh leaves were thoroughly washed with distilled water to remove surface contaminants, shade-dried at room temperature (25 ± 2 °C) for 7–10 days, and ground into a fine powder. For extraction, 10 g of the powdered leaves were mixed with 100 mL of ethanol as the solvent in a 250 mL glass beaker. The mixture was placed in an ice bath to control temperature rise during sonication. A probe-type ultrasonicator (20 kHz, 500 W, 10 mm titanium alloy probe) was used at 40% amplitude in a pulse mode of 10 s ON and 5 s OFF for 30 minutes, maintaining the temperature below 40 °C to prevent the degradation of heat-sensitive compounds. After ultrasonication, the extract was filtered through Whatman no. 1 filter paper and centrifuged at 6000 rpm for 10 minutes to remove residual particulates. The supernatant was then concentrated using a rotary evaporator at 40 °C under reduced pressure to remove the solvent. The final extract was either freeze-dried for long-term storage or stored at 4 °C in an amber-colored bottle until further analysis. The extraction yield was calculated using the formula2.3 Characterization of the extract
where Acontrol is the absorbance of the DPPH solution without the extract, and Asample is the absorbance with the extract. The IC50 value (concentration required to inhibit 50% of the DPPH radicals) was determined by plotting percentage inhibition against extract concentrations using linear regression analysis.11
Each well was loaded with 100 µL of extract solutions at different concentrations: 25 µg mL−1, 50 µg mL−1, and 100 µg mL−1, prepared in DMSO or sterile distilled water. Gentamycin (10 µg mL−1) was used as a positive control, while sterile distilled water served as the negative control. Plates were incubated at 37 ± 2 °C for 24 hours, and the zone of inhibition (mm) was measured using a digital Vernier caliper.12
2.4 Partial purification of bioactive compounds
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio (v/v) and repeated three times (3 × 100 mL) to selectively extract moderately non-polar compounds such as furanoflavonoids. The pooled ethyl acetate fractions were dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure at 40 °C using a rotary evaporator.13 The resulting enriched fraction was dried and stored at 4 °C for further analysis. For compound confirmation, FTIR spectra of the partially purified extract were recorded in the range of 4000–400 cm−1 with a spectral resolution of 4 cm−1 using an ATR-FTIR spectrometer (Sigma-Aldrich, ≥98% purity).
1 ratio (v/v) and repeated three times (3 × 100 mL) to selectively extract moderately non-polar compounds such as furanoflavonoids. The pooled ethyl acetate fractions were dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure at 40 °C using a rotary evaporator.13 The resulting enriched fraction was dried and stored at 4 °C for further analysis. For compound confirmation, FTIR spectra of the partially purified extract were recorded in the range of 4000–400 cm−1 with a spectral resolution of 4 cm−1 using an ATR-FTIR spectrometer (Sigma-Aldrich, ≥98% purity).2.5 Preparation of film incorporated with the leaf extracts
Starch-based edible films were prepared using a solution casting method with modifications. Initially, 3 g of corn starch was gradually dispersed in 100 mL of distilled water and heated to 90 °C with continuous stirring for 30 minutes to allow complete gelatinization. Subsequently, 0.8 mL of glycerol was added as a plasticizer to enhance film flexibility. After thorough mixing, varying concentrations (1%, 3%, 5% and 7% v/v) of Pongamia pinnata or Psidium guajava leaf extracts were incorporated into the starch solution. The mixture was then heated again at 65 °C for 15 minutes to ensure proper integration of the extract. The resulting film-forming solutions were homogenized using a high-speed homogenizer for 2–5 minutes to achieve uniform dispersion. Air bubbles in the homogenized solutions were removed using a vacuum oven. The degassed solutions were poured into sterile Petri dishes and dried under controlled conditions at 25 ± 1 °C and 50 ± 2% relative humidity (RH) for 24 hours to ensure reproducible film formation. Once dried, the films were carefully peeled off and stored at 25 °C in desiccators until further analysis and application.152.6 Characterization of the starch film
where ΔW is the weight gain (g), A is the exposed film area (m2), and t is the time (days).
2.7 Meat wrapping and shelf life analysis
Fresh raw chicken meat samples were cut into uniform pieces weighing 5 grams each. The starch-based edible films incorporated with Pongamia pinnata and Psidium guajava leaf extracts were wrapped tightly around the meat samples, while an unwrapped meat sample served as the control. All samples were kept at room temperature (25 ± 2 °C), and the following parameters were analyzed at 30-minute intervals for a total duration of 3 hours.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CFU per g. For the refrigerated shelf-life study, wrapped and control samples were stored at 4 ± 1 °C and analyzed at predetermined intervals. All analyses were performed in triplicate and results were recorded to assess the preservation efficiency of the extract-incorporated edible films.20
CFU per g. For the refrigerated shelf-life study, wrapped and control samples were stored at 4 ± 1 °C and analyzed at predetermined intervals. All analyses were performed in triplicate and results were recorded to assess the preservation efficiency of the extract-incorporated edible films.202.8 Sensory evaluation
A basic sensory assessment was conducted using eight semi-trained people (n = 8) familiar with sensory methods. Samples (control film-wrapped chicken, P. pinnata film-wrapped, and P. guajava film-wrapped) were coded with three-digit random numbers and presented in randomized order. Evaluations were performed in individual booths under neutral lighting and room temperature (≈25 °C). Panelists rated odor, surface color, texture (mouthfeel/firmness) and overall acceptability using a 9-point hedonic scale (1 = dislike extremely, 9 = like extremely). Water and plain crackers were provided for palate cleansing between samples. Data were collected anonymously; the procedure followed institutional ethical guidelines for voluntary participant testing.3 Results and discussion
3.1 Extraction yield
The efficiency of ultrasound-assisted extraction (UAE) was assessed based on the percentage yield of dried crude extracts obtained from Pongamia pinnata and Psidium guajava leaves. The results showed that UAE yielded 76% for P. pinnata and 78% for P. guajava. These values indicate a high extraction efficiency for both plant materials using UAE.Ultrasound-assisted extraction is known for its ability to enhance mass transfer and disrupt plant cell walls through acoustic cavitation, which facilitates the release of intracellular bioactive compounds. The high yields observed in this study support the effectiveness of UAE in recovering phytochemicals from medicinal leaves. Compared to conventional methods such as Soxhlet or maceration, UAE significantly reduces extraction time and energy consumption while increasing yield.
The slightly higher yield of P. guajava extract compared to P. pinnata may be attributed to its softer leaf structure and higher natural moisture content, which could enhance solvent penetration and compound diffusion. Additionally, the polarity of the ethanol used and its compatibility with polyphenolic compounds might have favored the extraction of bioactive compounds from guava leaves.
These findings are consistent with previous studies,21–24 which reported similar extraction yields ranging from 70% to 80% for UAE of polyphenol-rich plant materials.
3.2 Characterization of the leaf extracts
• Pongamia pinnata: absorbance = 0.69 → concentration = (0.69 − 0.02)/0.001 = 670 µg mL−1 GAE
• Psidium guajava: absorbance = 0.82 → concentration = (0.82 − 0.02)/0.001 = 800 µg mL−1 GAE
The total phenolic content (TPC) was found to be significantly higher in the Psidium guajava leaf extract (800 µg mL−1 GAE) when compared to Pongamia pinnata (670 µg mL−1 GAE). This observation highlights guava leaves as a richer source of polyphenolic compounds, which are well-documented for their antioxidant, anti-inflammatory, and antimicrobial properties. Phenolic compounds act as primary antioxidants by donating hydrogen atoms or electrons and neutralizing free radicals. The elevated TPC in P. guajava aligns with its superior DPPH radical scavenging capacity observed in this study, suggesting a direct correlation between phenolic content and antioxidant efficacy. These findings are consistent with previous reports demonstrating that phenolic-rich plant extracts enhance oxidative stability and can be effectively incorporated into biodegradable films for food preservation.25,26 Thus, the higher phenolic content in the guava extract substantiates its application as a functional additive in the development of antioxidant-enriched active packaging materials.
• Pongamia pinnata: absorbance = 1.8 → concentration = (1.8 − 0.01)/0.002 = 895 µg mL−1 QE
• Psidium guajava: absorbance = 2.6 → concentration = (2.6 − 0.01)/0.002 = 1295 µg mL−1 QE
The total flavonoid content (TFC) analysis revealed a significantly higher flavonoid concentration in the Psidium guajava leaf extract (1295 µg mL−1 QE) compared to Pongamia pinnata (895 µg mL−1 QE). Flavonoids, known for their potent antioxidant and radical scavenging properties, contribute substantially to the prevention of oxidative deterioration in food systems. The elevated TFC in P. guajava supports its enhanced DPPH scavenging ability observed in the current study, indicating a strong correlation between flavonoid concentration and antioxidant activity. These findings align with earlier reports that emphasize the role of flavonoid-rich plant extracts in prolonging shelf-life and improving oxidative stability in food products.27–29 Consequently, the higher flavonoid content in the guava extract underscores its potential application in the formulation of active packaging films intended for meat preservation and other perishable commodities.
 | ||
| Fig. 3 DPPH radical scavenging activity (% inhibition) of Pongamia pinnata and Psidium guajava leaf extracts at different concentrations. | ||
The antioxidant potential of Pongamia pinnata and Psidium guajava leaf extracts was assessed using the DPPH radical scavenging assay. At lower concentrations (25 µg mL−1), P. pinnata exhibited 42.28% inhibition, outperforming P. guajava, which showed 28.12% inhibition, indicating a relatively stronger scavenging potential of P. pinnata at initial dose levels. Interestingly, with increasing concentration, P. guajava demonstrated a marked improvement in activity, surpassing P. pinnata at 75 and 100 µg mL−1, suggesting the presence of dose-dependent bioactive constituents contributing to its radical scavenging efficacy.
The IC50 value, representing the concentration required to inhibit 50% of DPPH radicals, was calculated via linear regression of the concentration vs. % inhibition curve. The IC50 for P. guajava was found to be 49.32 µg mL−1, while for P. pinnata it was slightly higher at 54.76 µg mL−1, indicating that P. guajava possesses marginally superior antioxidant activity. These results may be attributed to its higher total phenolic and flavonoid content, as observed in previous reports,30,31 supporting the hypothesis that antioxidant activity is positively correlated with polyphenolic concentration.
Overall, the findings highlight the potential application of both extracts as natural antioxidants in food preservation and biomedical formulations, offering a sustainable alternative to synthetic additives.
| S. no. | Tested organisms | Zone of inhibition (mm) | |||
|---|---|---|---|---|---|
| 25 µg mL−1 | 50 µg mL−1 | 100 µg mL−1 | Gentamycin | ||
| Pongamia pinnata | |||||
| 1 | Staphylococcus aureus | 12 | 12 | 16 | 31 |
| 2 | Bacillus subtilis | 13 | 15 | 17 | 29 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Psidium guajava | |||||
| 1 | Staphylococcus aureus | 0 | 0 | 0 | 29 |
| 2 | Bacillus subtilis | 10 | 10 | 12 | 25 |
The present study demonstrated that the Pongamia pinnata leaf extract possesses notable antibacterial activity, especially against Bacillus subtilis and Staphylococcus aureus, with the activity increasing at higher concentrations. These results align with previous reports,32–34 which documented significant antimicrobial effects of P. pinnata due to its rich phytochemical profile, including flavonoids and karanjin compounds.
In contrast, the Psidium guajava extract showed selective activity, effectively inhibiting B. subtilis but not S. aureus. This selective activity may be attributed to differences in bacterial cell wall permeability or the concentration of active antimicrobial constituents like quercetin and tannins in the extract. Similar findings were reported,6,35 where the guava extract exhibited higher efficacy against Gram-positive Bacillus species than Staphylococcus.
It is important to note that the P. guajava extract showed no inhibitory activity against Staphylococcus aureus at any tested concentration, which represents a limitation in its broad-spectrum antimicrobial applicability. This lack of efficacy has been similarly observed in previous studies and may be due to the lower permeability of S. aureus cell walls to quercetin-rich extracts. Therefore, P. guajava based films may be more suitable for applications targeting Bacillus species rather than universal pathogen control.
While the activity of both extracts was lower than that of gentamycin, the natural extracts still present promising alternatives, especially for applications in food preservation or packaging, where synthetic antibiotics are not preferred. The results suggest that P. pinnata may serve as a more potent natural antimicrobial agent compared to P. guajava.
3.3 FTIR confirmation of bioactive compounds in plant extracts
To validate the presence of specific bioactive compounds – quercetin in Psidium guajava and karanjin in Pongamia pinnata – the crude extracts were subjected to partial purification using ethyl acetate partitioning. This step enriched the phenolic and furanoflavonoid fractions by removing highly polar and non-phenolic impurities. The resulting fractions were then analyzed using Fourier-transform infrared (FTIR) spectroscopy, a widely accepted method to identify functional groups and confirm the presence of key phytochemicals based on their characteristic vibrational frequencies.The FTIR spectrum of the Psidium guajava ethyl acetate fraction (Fig. 4) showed characteristic absorption peaks corresponding to quercetin and related flavonols. A broad band at ∼3300 cm−1 indicated O–H stretching vibrations of phenolic hydroxyl groups. The strong peak observed at ∼1655 cm−1 corresponded to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching of the flavonol backbone. Prominent aromatic C
O stretching of the flavonol backbone. Prominent aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching bands were noted at ∼1600 cm−1 and ∼1515 cm−1, confirming the presence of conjugated benzene rings. Additional peaks at ∼1460 cm−1 (C–O–H bending), ∼1250 cm−1 (C–O stretching of phenolic ethers), and ∼1050 cm−1 (C–O–C stretching) further supported the presence of flavonoid glycosidic linkages. A distinct band around ∼840 cm−1 represented the C–H out-of-plane bending of substituted aromatic rings. These functional group vibrations collectively confirm that the extraction and partial purification successfully retained quercetin-rich phytochemicals within the P. guajava fraction.36,37
C stretching bands were noted at ∼1600 cm−1 and ∼1515 cm−1, confirming the presence of conjugated benzene rings. Additional peaks at ∼1460 cm−1 (C–O–H bending), ∼1250 cm−1 (C–O stretching of phenolic ethers), and ∼1050 cm−1 (C–O–C stretching) further supported the presence of flavonoid glycosidic linkages. A distinct band around ∼840 cm−1 represented the C–H out-of-plane bending of substituted aromatic rings. These functional group vibrations collectively confirm that the extraction and partial purification successfully retained quercetin-rich phytochemicals within the P. guajava fraction.36,37
The FTIR spectrum of the Pongamia pinnata ethyl acetate fraction (Fig. 5) displayed distinct absorption peaks characteristic of karanjin, a methoxy-furanoflavone abundant in Pongamia leaves. A strong peak at ∼1650 cm−1 corresponded to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching of the γ-pyrone (flavone) ring system. Prominent bands at ∼1600 cm−1 and ∼1510 cm−1 were attributed to aromatic C
O stretching of the γ-pyrone (flavone) ring system. Prominent bands at ∼1600 cm−1 and ∼1510 cm−1 were attributed to aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibrations, confirming the presence of a conjugated benzene framework. The peak at ∼1260–1250 cm−1 represented the C–O stretching of methoxy substituents, a signature functional group of karanjin. Additional peaks at ∼1100–1050 cm−1 indicated the C–O–C stretching of the furan ring, while the band around ∼840 cm−1 corresponded to the C–H out-of-plane bending of substituted aromatic rings. These spectral features closely match the reported FTIR profiles of karanjin, confirming that the partial purification effectively enriched the bioactive methoxy-flavonoid constituents in the extract.38,39
C stretching vibrations, confirming the presence of a conjugated benzene framework. The peak at ∼1260–1250 cm−1 represented the C–O stretching of methoxy substituents, a signature functional group of karanjin. Additional peaks at ∼1100–1050 cm−1 indicated the C–O–C stretching of the furan ring, while the band around ∼840 cm−1 corresponded to the C–H out-of-plane bending of substituted aromatic rings. These spectral features closely match the reported FTIR profiles of karanjin, confirming that the partial purification effectively enriched the bioactive methoxy-flavonoid constituents in the extract.38,39
The FTIR-based confirmation of quercetin and karanjin supports their contribution to the observed antioxidant and antimicrobial activities in this study. The partial purification step further enhanced the interpretability of spectral data by minimizing spectral interference from other matrix components.
3.4 Preparation of bioactive films
Films were developed as shown in Fig. 6: control film without extract, 5% Pongamia pinnata leaf extract film, and 5% Psidium guajava leaf extract film. The 5% extract films exhibited superior transparency and a uniform structure compared to other tested concentrations.The formulation trials for film development revealed that the incorporation of the 5% leaf extract resulted in the most stable and visually uniform bioactive film. Films prepared with lower concentrations (1% and 3%) lacked adequate film-forming integrity, appearing fragile or incomplete, while those with higher concentrations (7%) showed brittleness and non-uniform textures. The 5% formulation maintained transparency, elasticity, and mechanical cohesion, making it ideal for further testing. These findings align with earlier reports where the inclusion of plant extracts at 3–5% concentration enhanced the physical properties and bioactivity of biopolymer-based films.40–42 The optimization trials further indicated that 5% extract loading provided the best balance of mechanical strength, film uniformity, and bioactivity, consistent with previous studies emphasizing that 3–5% incorporation of plant extracts enhances the performance of polysaccharide-based edible films.9,43,44 Thus, the 5% extract concentration was selected for the subsequent analysis of functional properties.
3.5 Characterization of the bioactive film
Preliminary formulation trials were conducted to determine the optimal concentration of P. pinnata and P. guajava leaf extracts for incorporation into starch-based edible films. Films prepared with 1% and 3% extracts appeared structurally weak, exhibiting fragile or partially formed matrices, whereas the 7% extract produced brittle, non-uniform films due to excessive disruption of the starch-glycerol polymer network. In contrast, the 5% (v/v) extract films demonstrated the most desirable characteristics, including uniform surface morphology, good flexibility, and higher mechanical stability. These observations were supported by tensile strength measurements, where 5% extract films recorded the highest stress values for both plants. A detailed comparison of visual attributes, integrity scores, and tensile strength across all concentrations is provided in SI Tables S1 and S2. Based on these results, the 5% extract loading was selected for all subsequent physicochemical, antimicrobial, and meat-wrapping evaluations.| S. no. | Sample | Trial 1 (µm) | Trial 2 (µm) | Trial 3 (µm) | Average thickness (µm) |
|---|---|---|---|---|---|
| 1 | Starch film (control) | 212 | 206 | 210 | 209 |
| 2 | 5% P. pinnata leaf extract film | 164 | 156 | 151 | 157 |
| 3 | 5% P. guajava leaf extract film | 162 | 164 | 161 | 162 |
Similar reductions in thickness upon bioactive incorporation have been reported,43,45,46 suggesting that the integration of plant-based compounds modifies the microstructure of biopolymer films. Additionally, thinner films are often associated with improved transparency and flexibility, which are desirable traits in food packaging. The consistent thickness among all extract-based films indicates good reproducibility in formulation, supporting their suitability for further mechanical and barrier property evaluations.
| S. no. | Sample | Length (mm) | Width (mm) | Max load (N) | Extension at max load (mm) | Tensile stress (MPa) | Tensile strain (mm mm−1) | Modulus (MPa) |
|---|---|---|---|---|---|---|---|---|
| 1 | Starch film (control) | 90 | 20 | 7.44 | 10.25 | 1.74 | 10.87 | 28.14 |
| 2 | 5% P. pinnata leaf extract film | 90 | 20 | 5.51 | 12.10 | 2.47 | 13.44 | 67.19 |
| 3 | 5% P. guajava leaf extract film | 90 | 20 | 8.12 | 21.79 | 2.57 | 24.21 | 62.54 |
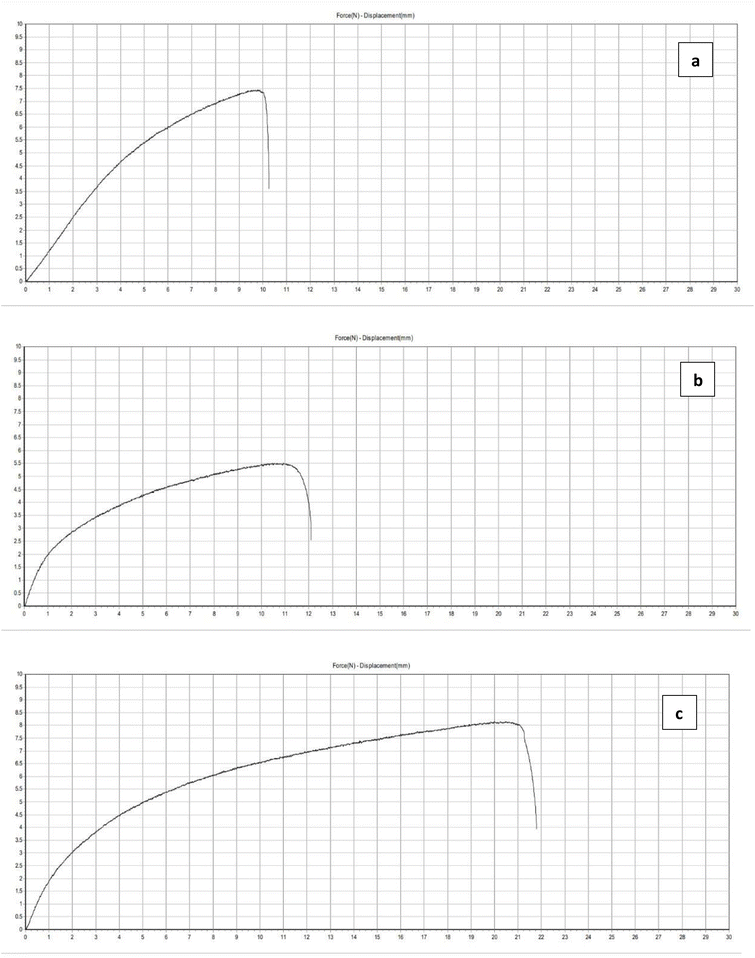 | ||
| Fig. 7 Tensile stress and Young's modulus of control and extract-based films – (a) control film; (b) P. pinnata leaf extract incorporated film; (c) P. guajava leaf extract incorporated film. | ||
The incorporation of plant extracts is known to alter the polymer matrix due to interactions between phenolic compounds and starch chains, which may lead to a more plasticized and cohesive network. The higher strain and tensile values for the P. guajava film may be attributed to the higher flavonoid content, contributing to improved film integrity and resistance to breakage. These results are in agreement with previous studies,47–49 where bioactive compounds enhanced the mechanical flexibility of biopolymer films.
Overall, the findings indicate that extract incorporation, particularly P. guajava, not only improves antioxidant and antimicrobial properties but also enhances the mechanical performance of the films – making them suitable candidates for sustainable food packaging applications.
The tensile properties of the starch film (control) and films incorporated with 5% Pongamia pinnata and Psidium guajava extracts are evaluated. The graph illustrates tensile stress (MPa) and Young's modulus (MPa), indicating enhanced flexibility and mechanical performance with extract incorporation.
| S. no. | Sample | Relative humidity (% RH) | WVTR (g m−2 24 h) |
|---|---|---|---|
| 1 | Starch film (control) | 30 | 1358.08 |
| 2 | 5% P. pinnata leaf extract film | 30 | 1251.70 |
| 3 | 5% P. guajava leaf extract film | 30 | 1350.99 |
 | ||
| Fig. 8 Water vapor transmission rate (WVTR) of control and extract-based starch films – (a) control film; (b) P. pinnata leaf extract incorporated film; (c) P. guajava leaf extract incorporated film. | ||
The reduction in the WVTR observed in the P. pinnata film may be attributed to the presence of phenolic and hydrophobic compounds that could interact with the starch matrix, thereby reducing free hydroxyl groups available for water absorption. This aligns with previous findings,48 where plant extracts altered the moisture barrier by filling voids in the polymer network or creating denser film structures.
Although the P. guajava extract did not significantly reduce the WVTR, its strong antioxidant and antimicrobial effects compensate for this limitation. Thus, the P. pinnata extract may offer added advantage for moisture-sensitive food packaging, while the P. guajava extract provides functional benefits in terms of bioactivity.
Although incorporation of plant extracts reduced the WVTR compared to the control film, the overall values remained high (1251–1358 g m−2 24 h), consistent with the hydrophilic nature of starch-based matrices. These values are considerably higher than those of commercial synthetic packaging materials such as LDPE (5–15 g m−2 24 h) or PVC (10–20 g m−2 24 h), indicating that the developed films offer only limited moisture barrier functionality. This represents a notable limitation for applications involving high-moisture foods such as fresh meat, where rapid water vapor transmission may accelerate surface dehydration. However, since the goal of this study was to explore antimicrobial and antioxidant active packaging rather than replace moisture-proof synthetic packaging, the films can still function as short-term preservative wraps or be combined with secondary moisture-barrier layers in future applications.
3.6 Meat wrapping and analysis
The moisture content of chicken meat after two hours of room-temperature storage is presented in Table 5. The unwrapped control retained the highest moisture level (78.2%), while the samples wrapped with P. pinnata and P. guajava films showed slightly lower values (74% and 75.6%, respectively). This reduction indicates that the starch-based films did not fully prevent moisture migration and allowed some dehydration, which is consistent with their relatively high WVTR values. However, the moisture loss in wrapped samples remained moderate compared to the rapid surface drying typically observed in unwrapped meat, suggesting that the polyphenol-enriched films provided partial, though not complete, moisture regulation. Similar findings were observed,50,51 indicating that plant-based antimicrobial films can slow spoilage even when their moisture barrier properties are limited.| S. no | Samples | Moisture content (%) | L* (lightness) | a* (redness) | b* (yellow) | pH | Water activity |
|---|---|---|---|---|---|---|---|
| 1 | Meat at room temperature after 2 hours | 78.2 | 29.23 | 2.30 | 10.45 | 5 | 0.974 |
| 2 | Meat wrapped in the P. pinnata leaf extract incorporated film | 74 | 47.14 | 8.82 | 20.27 | 6 | 0.932 |
| 3 | Meat wrapped in the P. guajava leaf extract incorporated film | 75.6 | 32.31 | 2.35 | 10.65 | 6 | 0.935 |
Color parameters (L*, a*, b*) are critical quality indicators, as consumers associate freshness with bright appearance and redness. The meat wrapped in the P. pinnata film showed notably improved lightness (L = 47.14) and redness (a = 8.82) compared to the control (L = 29.23, a = 2.30), indicating enhanced oxidative stability and reduced discoloration. This effect is attributed to the antioxidant properties of flavonoids and polyphenols, as previously reported,52 demonstrating color stabilization in meat products with plant-derived phenolics. Conversely, P. guajava-wrapped meat showed a modest increase in L* and a* values (32.31 and 2.35, respectively), indicating slightly less pigment stabilization, though still better than the control.
In terms of pH, both wrapped samples exhibited pH 6.0, compared to 5.0 in the unwrapped control, which suggests lower microbial activity and slower spoilage in the treated samples. Maintenance of near-neutral pH is essential for delaying proteolytic degradation and bacterial proliferation in meat systems, as supported by previous findings,53 which reported similar pH stabilization in meat packaged with active films.
Collectively, these results demonstrate the effectiveness of plant-extract-based biofilms in preserving chicken meat quality and appearance under ambient conditions, supporting their potential use in active food packaging applications.
3.7 Shelf-life evaluation of chicken meat wrapped in bioactive films
Visual microbial analysis of chicken meat stored under ambient conditions revealed significant differences in spoilage progression between the control and bioactive film-wrapped samples. The control (unwrapped) meat (Fig. 9a) showed visible fungal and bacterial growth after 3 hours, indicating rapid microbial deterioration. In contrast, chicken meat samples wrapped with starch films incorporated with Pongamia pinnata (Fig. 9b) and Psidium guajava (Fig. 9c) leaf extracts remained visibly unspoiled, showing no evident microbial colonies within the same period.To address the limitation of short-term ambient testing, a supplementary refrigerated study was performed at 4 ± 1 °C. During cold storage, the control meat exhibited a gradual increase in microbial load, exceeding 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CFU per g by day 3, consistent with typical spoilage kinetics in raw poultry. In contrast, samples wrapped with extract-incorporated films showed significantly slower microbial proliferation. Meat wrapped with P. pinnata films remained below 4
CFU per g by day 3, consistent with typical spoilage kinetics in raw poultry. In contrast, samples wrapped with extract-incorporated films showed significantly slower microbial proliferation. Meat wrapped with P. pinnata films remained below 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CFU per g until day 3 and reached only 5.2
CFU per g until day 3 and reached only 5.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CFU per g by day 5, while P. guajava films maintained counts below 5
CFU per g by day 5, while P. guajava films maintained counts below 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CFU per g through day 4.
CFU per g through day 4.
This marked delay in spoilage in treated samples can be attributed to the antimicrobial efficacy of polyphenolic compounds present in the leaf extracts, which disrupt microbial cell membranes and inhibit enzymatic activity. These results align with the findings, which reported that plant-based antimicrobial packaging films significantly suppressed microbial growth on perishable foods.54 Similarly, reports demonstrated enhanced shelf stability in meat products when wrapped with films containing botanical extracts.55,56
The absence of visual spoilage in the extract-treated films underscores their potential as effective bioactive packaging materials for extending the shelf life of fresh meat under room temperature conditions. Notably, P. pinnata-wrapped meat exhibited slightly better clarity and microbial suppression compared to P. guajava, possibly due to higher antimicrobial activity associated with its specific phytochemical profile. Although the films do not match the long-term barrier properties of commercial packaging, they provide meaningful short-term microbial protection, supporting their application as clean-label, biodegradable meat wraps.
3.8 Sensory analysis
To assess whether the bioactive films imparted any undesirable sensory changes to chicken meat (n = 8), odor, color, texture, and overall acceptability were evaluated using a 9-point hedonic scale. The sensory scores showed no adverse impact from the incorporation of P. pinnata or P. guajava extracts. Sensory evaluation as depicted in Fig. 10 revealed that neither extract-infused film imparted undesirable odor, color, or texture to the chicken meat.The P. guajava film exhibited slightly higher scores for odor (8.8) and overall acceptability (8.2), reflecting its mild aromatic profile and effective preservation of meat freshness. The P. pinnata film also maintained acceptable sensory characteristics, with odor and texture scores of 8.1 and 8.3, respectively. All films preserved the natural color of the meat, with scores between 8.0 and 8.4, demonstrating the films' ability to inhibit surface discoloration during short-term storage.
Texture scores for treated samples were marginally higher than that of the control, indicating reduced moisture loss and firmer meat surface structure consistent with the barrier properties observed in WVTR results. Overall, no off-odors or negative sensory attributes were reported, supporting the suitability of both plant-based films for meat packaging applications. These findings align with previous research showing that phenolic-rich coatings do not impart undesirable flavors when used at low incorporation levels and instead contribute to maintaining product freshness.
In this research, starch-based edible films incorporated with bioactive extracts of Pongamia pinnata and Psidium guajava leaves were successfully developed and evaluated for their physicochemical, antioxidant, and antimicrobial properties. The incorporation of plant extracts enhanced the functional attributes of the films, improving tensile strength, antioxidant activity, and microbial inhibition. Application to chicken meat effectively reduced moisture loss, stabilized pH and color, and lowered microbial load compared to unwrapped controls. Importantly, both plants are traditionally used in food and medicine, and no toxicity concerns have been reported at the tested concentrations, supporting their safe use in edible films. Beyond meat, these films hold potential for other perishable foods such as fish, fruits, and vegetables. Future research may focus on optimizing extract concentrations across different food matrices and exploring blends with other biopolymers or natural agents to enhance performance. These findings reinforce the promise of plant-based edible films as sustainable, biodegradable alternatives for food packaging.
Conflicts of interest
The authors declare no competing interests.Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.All data supporting the findings of this study are included in the manuscript and its supplementary information (SI). Supplementary information: optimization data for starch-based edible films incorporated with Pongamia pinnata and Psidium guajava leaf extracts, including comparative evaluation of different extract concentrations (1%, 3%, 5% and 7% v/v) based on visual appearance, film integrity, and tensile strength measurements, which were used to justify the selection of 5% (v/v) extract concentration for further analyses. See DOI: https://doi.org/10.1039/d5fb00553a.
References
- S. Karanth, S. Feng, D. Patra and A. K. Pradhan, Linking microbial contamination to food spoilage and food waste: the role of smart packaging, spoilage risk assessments, and date labeling, Front. Microbiol., 2023, 14, 1–17, DOI:10.3389/fmicb.2023.1198124.
- G. Atiwesh, A. Mikhael, C. C. Parrish, J. Banoub and T. A. T. Le, Environmental impact of bioplastic use: a review, Heliyon, 2021, 7(9), e07918, DOI:10.1016/j.heliyon.2021.e07918.
- S. S. Nair, J. Trafiałek and W. Kolanowski, Edible Packaging: A Technological Update for the Sustainable Future of the Food Industry, Appl. Sci., 2023, 13(8234), 1–23, DOI:10.3390/app13148234.
- D. Gupta, A. Lall, S. Kumar, T. D. Patil and K. K. Gaikwad, Plant-based edible films and coatings for food-packaging applications: recent advances, applications, and trends, Sustainable Food Technol., 2024, 1428–1455, 10.1039/d4fb00110a.
- M. Takó, E. B. Kerekes, C. Zambrano, A. Kotogan, P. Tamas, J. Krisch and C. Vagvolgyi, Plant phenolics and phenolic-enriched extracts as antimicrobial agents against food-contaminating microorganisms, Antioxidants, 2020, 9(2), 1–21, DOI:10.3390/antiox9020165.
- H. D. Huynh, P. Nargotra, H. M. D. Wang, C. J. Shieh, Y. C. Liu and C. H. Kuo, Bioactive Compounds from Guava Leaves (Psidium guajava L.): Characterization, Biological Activity, Synergistic Effects, and Technological Applications, Molecules, 2025, 30(6), 1–40, DOI:10.3390/molecules30061278.
- G. Bhatt and A. Mukund, Phytochemicals: a Natural Prospect Toward Healthcare—a Tryst with Karanjin, Proc. Natl. Acad. Sci., India, Sect. B, 2025, 95(1), 1–7, DOI:10.1007/s40011-024-01557-9.
- N. V. Kanimozhi and M. Sukumar, Process optimization and kinetic modeling of ultrasound assisted lactic acid production, J. Food Process Eng., 2024, 47(8), 1–12, DOI:10.1111/jfpe.14722.
- M. P. Drisya Raj, N. V. Kanimozhi and M. Sukumar, Preparation and Characterization of Gelatin Based Antimicrobial Edible Films Incorporated with Aloe Vera Gel and Garlic Peel Extract, J. Packag. Technol. Res., 2024, 8(2), 121–128, DOI:10.1007/s41783-024-00166-1.
- A. Hmamou, N. Eloutassi and S. Z. Alshawwa, et al., Total Phenolic Content and Antioxidant and Antimicrobial Activities of Papaver rhoeas L. Organ Extracts Growing in Taounate Region, Morocco, Molecules, 2022, 27(3), 1–12, DOI:10.3390/molecules27030854.
- Z. Asadi, A. Abbasi, A. Ghaemi, E. A. Montazeri and S. Akrami, Investigating the properties and antibacterial, antioxidant, and cytotoxicity activity of postbiotics derived from Lacticaseibacillus casei on various gastrointestinal pathogens in vitro and in food models, GMS Hyg. Infect. Control, 2024, 19, Doc60, DOI:10.3205/dgkh000515.
- N. V. Kanimozhi and N. Balaji, An in vitro study of Anti-Inflammatory, Antioxidant and Antimicrobial Potential of Artemisia pallens, Int. J. Mod. Sci. Technol., 2018, 3(7), 161–165 Search PubMed.
- R. S. Susarla, M. M. Murthy, V. S. K. Bhamidipati Rao, P. P. Chakrabarti, R. B. N. Prasad and S. Kanjilal, A method for isolation of karanjin from the expelled cake of Pongamia glabra, Eur. J. Lipid Sci. Technol., 2012, 114(9), 1097–1101, DOI:10.1002/ejlt.201200016.
- B. Sambandam, D. Thiyagarajan, A. Ayyaswamy and P. Raman, Extraction and isolation of flavonoid quercetin from the leaves of Trigonella foenum-graecum and their anti-oxidant activity, Int. J. Pharm. Pharm. Sci., 2016, 8(6), 120–124 CAS.
- B. Poonkodi, M. Suguna Lakshmi and A. Tamilselvi, et al., Bio-nanocomposite films loaded with lemon leaf extract for bio packaging application, J. King Saud Univ., Sci., 2022, 34(8), 102333, DOI:10.1016/j.jksus.2022.102333.
- J. R. Nastasi, M. A. Fitzgerald and V. Kontogiorgos, Tuning the mechanical properties of pectin films with polyphenol-rich plant extracts, Int. J. Biol. Macromol., 2023, 253(P7), 127536, DOI:10.1016/j.ijbiomac.2023.127536.
- K. A. Nxumalo, O. A. Fawole and A. O. Aremu, Development of Chitosan-Based Active Films with Medicinal Plant Extracts for Potential Food Packaging Applications, Processes, 2024, 12(1), 1–20, DOI:10.3390/pr12010023.
- M. Kurek, N. Benbettaieb, M. Ščetar, E. Chaudy, M. Repajic, D. Klepac, S. Valic, F. Debeaufort and K. Galic, Characterization of food packaging films with blackcurrant fruit waste as a source of antioxidant and color sensing intelligent material, Molecules, 2021, 26(9), 1–15, DOI:10.3390/molecules26092569.
- S. Shi, J. Feng and G. An, et al., Dynamics of heat transfer and moisture in beef jerky during hot air drying, Meat Sci., 2021, 182, 108638, DOI:10.1016/j.meatsci.2021.108638.
- A. Nurmasytha, F. N. Yuliati and P. K. I. Hajrawati, Microbiological analysis of raw chicken meat sold at Maros traditional markets: total plate count and Escherichia coli, IOP Conf. Ser. Earth Environ. Sci., 2021, 788(1), 1–4, DOI:10.1088/1755-1315/788/1/012118.
- S. Ben-Othman, H. Kaldmäe, R. Rätsep, U. Bleive, A. Aluvee and T. Rinken, Optimization of ultrasound-assisted extraction of phloretin and other phenolic compounds from apple tree leaves (Malus domestica borkh.) and comparison of different cultivars from estonia, Antioxidants, 2021, 10(2), 1–13, DOI:10.3390/antiox10020189.
- T. N. Baite, B. Mandal and M. K. Purkait, Ultrasound assisted extraction of gallic acid from Ficus auriculata leaves using green solvent, Food Bioprod. Process., 2021, 128, 1–11, DOI:10.1016/j.fbp.2021.04.008.
- J. Li, Z. Chen, H. Shi, J. Yu, G. Huang and H. Huang, Ultrasound-assisted extraction and properties of polysaccharide from Ginkgo biloba leaves, Ultrason. Sonochem., 2023, 93, 106295, DOI:10.1016/j.ultsonch.2023.106295.
- A. A. Bin Mokaizh, A. H. Nour and K. Kerboua, Ultrasonic-assisted extraction to enhance the recovery of bioactive phenolic compounds from Commiphora gileadensis leaves, Ultrason. Sonochem., 2024, 105, 106852, DOI:10.1016/j.ultsonch.2024.106852.
- A. A. Maria, R. H. Salem, M. A. Salama, A. M. M. Khalil and C. Mark, Antioxidant-Rich Biodegradable Films : Incorporating Date Phenolic Extracts into Polyvinyl Alcohol Biofilms for Strawberry Preservation, J. Food Dairy Sci., 2024, 15, 203–217, DOI:10.21608/jfds.2024.328102.1171.
- N. Oulahal and P. Degraeve, Phenolic-Rich Plant Extracts With Antimicrobial Activity: An Alternative to Food Preservatives and Biocides?, Front. Microbiol., 2022, 12, 1–31, DOI:10.3389/fmicb.2021.753518.
- A. Ghasemzadeh, H. Z. E. Jaafar and A. Rahmat, Antioxidant activities, total phenolics and flavonoids content in two varieties of malaysia young ginger (Zingiber officinale Roscoe), Molecules, 2010, 15(6), 4324–4333, DOI:10.3390/molecules15064324.
- S. Shreshtha, J. Anushi, A. N. Joshi, N. Joshi and H. Anupma, Study of total phenol, flavonoid contents and phytochemical screening of methanolic crude extracts of two weed plants, Ann. Plant Sci., 2017, 6(06), 1645, DOI:10.21746/1651-1654.
- F. S. Al Amri and M. A. Hossain, Comparison of total phenols, flavonoids and antioxidant potential of local and imported ripe bananas, Egypt. J. Basic Appl. Sci., 2018, 5(4), 245–251, DOI:10.1016/j.ejbas.2018.09.002.
- M. Pateiro, J. A. Gómez-Salazar, M. Jaime-Patlán, M. E. Sosa-Morales and J. M. Lorenzo, Plant extracts obtained with green solvents as natural antioxidants in fresh meat products, Antioxidants, 2021, 10(2), 1–21, DOI:10.3390/antiox10020181.
- A. Demidova, T. Nosenko, V. Bahmach, E. Shemanska and S. Molchenko, Study on antioxidants extraction from oak bark and their use for oxidation stability of sunflower oil, Ukr. Food J., 2021, 10(3), 552–563, DOI:10.24263/2304-974X-2021-10-3-9.
- S. Pattanayak and S. Parida, Traditional uses, phyto-chemistry and antimicrobial activity of Pongamia pinnata (L.) Pierre: A review, Indian J. Nat. Sci., 2023, 12(72), 43338–43340 Search PubMed.
- V. K. Bajpai, A. Rahman and S. Shukla, et al., Antibacterial activity of leaf extracts of Pongamia pinnata from India, Pharm. Biol., 2009, 47(12), 1162–1167, DOI:10.3109/13880200903019218.
- V. A. Gargade and D. G. Kadam, In vitro evaluation of antibacterial potential of Pongamia pinnata L. against Xanthomonas axonopodis punicae, phytopathovar of Bacterial blight of Pomegranate (Punica granatum), Int. J. Curr. Microbiol. Appl. Sci., 2015, 4(5), 824–833 CAS . http://www.ijcmas.com.
- L. Purnamasari, M. Victoria Carolino and F. dela Cruz J, The Antibacterial Properties of Psidium guajava Leaf Extract as a Wound Healing Agent of Laboratory Animals: a Review, Biotropika J. Trop. Biol., 2022, 10(2), 154–160, DOI:10.21776/ub.biotropika.2022.010.02.10.
- P. Alugoju, D. Narsimulu, J. U. Bhanu, N. Satyanarayana and L. Periyasamy, Role of quercetin and caloric restriction on the biomolecular composition of aged rat cerebral cortex: an FTIR study, Spectrochim. Acta, Part A, 2019, 220, 117128, DOI:10.1016/j.saa.2019.05.033.
- M. Kokalj Ladan, J. Straus, E. Tavčar Benković and S. Kreft, FT-IR-based method for rutin, quercetin and quercitrin quantification in different buckwheat (Fagopyrum) species, Sci. Rep., 2017, 7(1), 1–8, DOI:10.1038/s41598-017-07665-z.
- M. J. Rekha, B. K. Bettadaiah, S. P. Muthukumar and K. Govindaraju, Synthesis, characterization and anti-inflammatory properties of karanjin (Pongamia pinnata seed) and its derivatives, Bioorg. Chem., 2021, 106, 104471, DOI:10.1016/j.bioorg.2020.104471.
- C. Gnanaraj, M. Govendan and C. Y. Loo, et al., Karanjin: a potential furanoflavonoid for neuroprotection, Phytochem. Rev., 2024, 23(5), 1351–1375, DOI:10.1007/s11101-024-09925-z.
- I. D. L. Silva, L. E. P. T. Moraes Filho, V. F. Caetano, M. F. Andrade, F. Hallwass, A. M. S. S. Brito and G. M. Vinhas, Development of antioxidant active PVA films with plant extract of Caesalpinia ferrea Martius, LWT, 2021, 144, 1–9, DOI:10.1016/j.lwt.2021.111215.
- A. A. Annu and S. Ahmed, Eco-friendly natural extract loaded antioxidative chitosan/polyvinyl alcohol based active films for food packaging, Heliyon, 2021, 7(3), e06550, DOI:10.1016/j.heliyon.2021.e06550.
- N. Nowak, W. Grzebieniarz and A. Cholewa-Wójcik, et al., Effects of Selected Plant Extracts on the Quality and Functional Properties of Gelatin and Furcellaran-Based Double-Layer Films, Food Bioprocess Technol., 2024, 17(5), 1201–1214, DOI:10.1007/s11947-023-03190-2.
- V. Kola and I. S. Carvalho, Plant extracts as additives in biodegradable films and coatings in active food packaging, Food Biosci., 2023, 54, 102860, DOI:10.1016/j.fbio.2023.102860.
- M. Bajić, T. Ročnik, A. Oberlintner, F. Scognamiglio, U. Novak and B. Likozar, Natural plant extracts as active components in chitosan-based films: a comparative study, Food Packag. Shelf Life, 2019, 21, 100365, DOI:10.1016/j.fpsl.2019.100365.
- J. Cheng, F. J. Velez, P. Singh and L. Cui, Fabrication, characterization, and application of pea protein-based edible film enhanced by oregano essential oil (OEO) micro- or nano-emulsion, Curr. Res. Food Sci., 2024, 8, 100705, DOI:10.1016/j.crfs.2024.100705.
- T. Havelt, S. Brettschneider and M. Schmitz, Evaluation of practical applicability and synergistic effects of bio-based food packaging materials combined with plant-based stabilisers, Processes, 2021, 9(10), 1–20, DOI:10.3390/pr9101838.
- P. Rachtanapun, W. Klunklin and P. Jantrawut, et al., Characterization of chitosan film incorporated with curcumin extract, Polymers, 2021, 13(6), 1–15, DOI:10.3390/polym13060963.
- J. Khan, S. Alam, A. Nazir, D. Tian, M. Q. Abbas and Z. Du, Preparation and characterization of chitosan and polyvinyl alcohol active film incorporated with Syzygium guineense plant extract as active packaging materials, Int. J. Biol. Macromol., 2025, 299, 140155, DOI:10.1016/j.ijbiomac.2025.140155.
- M. Tagrida, K. Nilsuwan, S. Gulzar, T. Prodpran and S. Benjakul, Fish gelatin/chitosan blend films incorporated with betel (Piper betle L.) leaf ethanolic extracts: characteristics, antioxidant and antimicrobial properties, Food Hydrocolloids, 2023, 137, 108316, DOI:10.1016/j.foodhyd.2022.108316.
- M. W. Apriliyani, M. A. Purwadi, B. M. Ahmad and L. M. Uula, Physico-chemical and antimicrobial properties of caseinchitosan edible films as food quality and food safety, IOP Conf. Ser. Earth Environ. Sci., 2020, 443(1), 1–9, DOI:10.1088/1755-1315/443/1/012018.
- G. K. Shroti and C. S. Saini, Development of edible films from protein of brewer's spent grain: effect of pH and protein concentration on physical, mechanical and barrier properties of films, Appl. Food Res., 2022, 2(1), 100043, DOI:10.1016/j.afres.2022.100043.
- A. M. Mohite and D. chandel, Formulation of edible films from fenugreek mucilage and taro starch, SN Appl. Sci., 2020, 2(11), 1–9, DOI:10.1007/s42452-020-03710-1.
- J. Yan, S. He, L. Chen, H. Chen, K. Ouyang and W. Wang, Effect of gelatin-chitosan-Cyclocarya paliurus flavonoids edible coating film on the preservation of chilled beef, LWT, 2024, 199, 116138, DOI:10.1016/j.lwt.2024.116138.
- P. Cazón, G. Velazquez, J. A. Ramírez and M. Vázquez, Polysaccharide-based films and coatings for food packaging: a review, Food Hydrocolloids, 2017, 68, 136–148, DOI:10.1016/j.foodhyd.2016.09.009.
- A. Papadochristopoulos, J. P. Kerry, N. Fegan, C. M. Burgess and G. Duffy, Natural anti-microbials for enhanced microbial safety and shelf-life of processed packaged meat, Foods, 2021, 10(7), 1–42, DOI:10.3390/foods10071598.
- P. E. S. Munekata, G. Rocchetti, M. Pateiro, L. Lucini, R. Domínguez and J. M. Lorenzo, Addition of plant extracts to meat and meat products to extend shelf-life and health-promoting attributes: an overview, Curr. Opin. Food Sci., 2020, 31, 81–87, DOI:10.1016/j.cofs.2020.03.003.
| This journal is © The Royal Society of Chemistry 2026 |