Curcumin encapsulation via protein-stabilized emulsions: comparative formulation and characterization using whey, soy, and pea proteins
Received
26th August 2025
, Accepted 11th October 2025
First published on 13th October 2025
Abstract
Curcumin, a potent polyphenol, has significant limitations in food and pharmaceutical applications due to its poor water solubility, chemical instability, and low oral bioavailability. To address these challenges, this study aimed to develop and systematically characterize stable emulsion-based curcumin delivery systems using a comparative approach. The present study offers novel findings through a direct, side-by-side evaluation of a traditional milk protein (whey protein isolate, WPI) against two plant-based alternatives (soy protein isolate, SPI, and pea protein isolate, PPI) under uniform formulation conditions. The emulsions were formulated with curcumin (0.4%) in butter oil as the lipophilic core, with WPI, SPI, or PPI, and a combination of maltodextrin and gum arabic as wall materials at core-to-wall ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, and 1
2, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. The WPI-based emulsions demonstrated superior physicochemical properties, achieving the lowest particle size (322 ± 5.49 μm) and highest zeta potential (−38.50 mV) at the 1
3. The WPI-based emulsions demonstrated superior physicochemical properties, achieving the lowest particle size (322 ± 5.49 μm) and highest zeta potential (−38.50 mV) at the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio. PPI-based emulsions exhibited the highest antioxidant activity, with 81.38% DPPH inhibition, highlighting their potential as a promising plant-based alternative. Both dairy and plant proteins effectively encapsulated curcumin, with an encapsulation efficiency of 88.16–96.26%. In contrast, the SPI-based emulsions exhibited relatively lower performance across most parameters. FT-IR spectroscopy and fluorescence microscopy confirmed successful curcumin encapsulation and emulsion integrity. These findings highlight WPI as a highly effective encapsulating agent and position PPI as a viable, sustainable, and functional plant-based alternative. This study provides valuable insights for developing stable, cost-effective, and functional curcumin delivery systems to meet the growing market demand for clean-label and plant-based foods and nutraceuticals.
2 ratio. PPI-based emulsions exhibited the highest antioxidant activity, with 81.38% DPPH inhibition, highlighting their potential as a promising plant-based alternative. Both dairy and plant proteins effectively encapsulated curcumin, with an encapsulation efficiency of 88.16–96.26%. In contrast, the SPI-based emulsions exhibited relatively lower performance across most parameters. FT-IR spectroscopy and fluorescence microscopy confirmed successful curcumin encapsulation and emulsion integrity. These findings highlight WPI as a highly effective encapsulating agent and position PPI as a viable, sustainable, and functional plant-based alternative. This study provides valuable insights for developing stable, cost-effective, and functional curcumin delivery systems to meet the growing market demand for clean-label and plant-based foods and nutraceuticals.
Sustainability spotlight
This study supports sustainability by developing effective curcumin encapsulation systems using plant- and dairy-based proteins, reducing dependence on synthetic stabilizers. It shows that soy and pea proteins can match whey protein in stabilizing curcumin, promoting the use of renewable, lower-impact ingredients. The approach enables the creation of functional foods that retain nutrient potency and align with clean-label standards. By leveraging widely available agricultural resources through scalable, cost-efficient methods, the research encourages circular value chains and minimizes waste. These findings contribute to greener food technologies that balance nutritional value, consumer appeal, and environmental responsibility, fostering more resilient and sustainable food systems.
|
1 Introduction
Curcumin, a hydrophobic polyphenol derived from the rhizomes of Curcuma longa, is widely recognized for its multifunctional bioactivities, including powerful antioxidant, anti-inflammatory, antimicrobial, antiviral, and anticancer activities.1,2 However, its practical application in functional foods and pharmaceuticals is constrained by unfavourable physicochemical characteristics. Curcumin exhibits extremely low water solubility at acidic and neutral pH, along with poor chemical stability under alkaline conditions and upon exposure to environmental factors, such as light and heat.3 The poor solubility and cellular permeability classify it as a biopharmaceutical classification system class IV molecule, which makes it difficult to deliver therapeutically relevant concentrations and results in low oral bioavailability.4 To overcome these limitations, encapsulation has emerged as a promising solution to protect curcumin and enhance its stability and bioavailability.5,6 Encapsulation is essential for protecting sensitive bioactive compounds from degradation, thereby enhancing their stability and bioavailability.7 Emulsion-based microencapsulation, in particular, is a technique that has gained significant attention in the food industry because it offers a versatile and scalable method to entrap active ingredients within a protective colloidal structure, making them ideal precursors for developing advanced delivery systems.8–10 It is effective in protecting lipophilic compounds, such as curcumin, from environmental degradation and can improve their sensory properties.11,12 Choudhary et al.13 suggested different encapsulation methods such as inclusion complexes, spray drying, and lipid nanoparticles to enhance the solubility, stability, and bioavailability of α-lipoic acid. Moosavi-Nasab et al.14 extended the shelf life of button mushrooms using aloe vera and gelatin edible coatings containing Shirazi thyme essential oil nanoemulsion and reported that the mushrooms maintained their quality and showed an increased shelf life during 16 days of storage at 5 °C and 90% RH.
Proteins are widely used as emulsifying agents because of their amphiphilic nature, which allows them to form protective films at the oil–water interface. Milk proteins, such as whey protein isolate (WPI), have been extensively studied for their high encapsulation efficiency and emulsion stability.15 However, there is growing interest in sustainable, plant-based alternatives. Plant proteins, such as soy protein isolate (SPI) and pea protein isolate (PPI), are recognized as cost-effective and sustainable, and are functionally capable of stabilizing emulsions through their emulsifying and gelation properties.16,17 Moreover, combining proteins with carbohydrates, such as maltodextrin and gum arabica, has been shown to enhance encapsulation performance and emulsion stability through synergistic interactions.18,19
Despite these advances and innovations, there is a significant research gap, with a noticeable lack of systematic comparative studies evaluating the performance of plant-based proteins, such as SPI and PPI, against conventional milk proteins, such as WPI, under uniform formulation conditions. Most of the existing studies have used a single protein source, such as whey,20,21 soy,22,23 and pea protein,24,25 failing to provide a comprehensive, side-by-side analysis of how different protein types affect the physicochemical, structural, and functional characteristics of curcumin-loaded emulsions. Moreover, most existing studies do not incorporate a comprehensive set of analytical techniques to thoroughly assess the encapsulation performance.
To bridge these critical gaps, this study focused on the comparative formulation and characterization of curcumin-loaded emulsions stabilized by WPI, SPI, and PPI. By providing integrative and comparative experiments, this study advances the understanding of how both dairy- and plant-based proteins function in emulsion-based delivery systems. Ultimately, this study contributes to the development of cost-effective, stable, and functional encapsulation strategies for lipophilic bioactives such as curcumin, supporting the growing demand for plant protein applications in clean-label and functional food innovations.
2 Materials and methods
2.1 Chemicals and ingredients
Pure curcumin powder (≥95% total curcuminoids; residual solvent: 3998 ppm) was obtained from M/s Plant Lipids (Kerala, India). Butteroil was prepared from buffalo milk procured from Dairy Farm, Banaras Hindu University, Varanasi, India, using the creamery butter method with 45% fat cream as per the procedure given by Aneja et al.26 Maltodextrin (20% reducing sugar) and gum arabic were sourced from M/s Hi-Media Laboratories Pvt. Ltd, Mumbai, India. SPI and PPI (containing 90% protein, 1.0% fat, and 0.5% ash) were purchased from ChemKart, Mumbai. WPI (containing 90% protein, 1.0% fat, 1.6% carbohydrates, and 0.5% ash) was obtained from M/s Globex Enterprises, New Delhi. All analytical-grade chemicals and reagents used in this study were procured from certified vendors and freshly prepared following standard laboratory protocols.
2.2 Preparation of curcumin emulsion with high shear homogenization
2.2.1 Preparation of core material.
Butter oil was selected as the lipid phase (core material) due to its high compatibility with curcumin, a lipophilic bioactive that exhibits limited solubility in aqueous systems. The triglyceride-rich matrix of butter oil enhances curcumin solubilization and stability, as confirmed in our previous study, where 59.26 ± 2.03% solubility was achieved at 0.4% (w/v) fortification level.27 In addition, butter oil is a natural dairy fat with GRAS status, confirming its suitability for improved dispersion, stability, and compatibility with dairy-based delivery systems. The mixture was heated to 60 °C and stirred continuously for 10 min using a magnetic stirrer (Narang Scientific, New Delhi) to ensure complete solubilization of curcumin.
2.2.2 Preparation of wall material.
The wall material was formulated using protein isolates (WPI, SPI, or PPI) in combination with carbohydrates (maltodextrin with a dextrose equivalent value of ≤20 and gum arabica) at a fixed ratio of 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. The protein-to-carbohydrate ratio was standardized at 1
10. The protein-to-carbohydrate ratio was standardized at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (w/w) based on preliminary trials conducted with soy and pea proteins, as well as our previous findings employing whey protein concentrate as a wall material for curcumin emulsions,9 to achieve optimal emulsion viscosity. The dry ingredients were accurately weighed and dispersed in milk containing 6% fat and 9% solids-not-fat, followed by mixing with a hand blender (Bajaj HB21, Mumbai, India) to ensure uniform hydration and dispersion of solids prior to high-speed homogenization. The prepared mixture accounted for approximately 80% of the final emulsion volume.
2 (w/w) based on preliminary trials conducted with soy and pea proteins, as well as our previous findings employing whey protein concentrate as a wall material for curcumin emulsions,9 to achieve optimal emulsion viscosity. The dry ingredients were accurately weighed and dispersed in milk containing 6% fat and 9% solids-not-fat, followed by mixing with a hand blender (Bajaj HB21, Mumbai, India) to ensure uniform hydration and dispersion of solids prior to high-speed homogenization. The prepared mixture accounted for approximately 80% of the final emulsion volume.
2.2.3 Formulation of curcumin emulsions.
The curcumin-loaded lipid (core) and aqueous wall phases were mixed at varying core-to-wall ratios (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, and 1
2, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). Microsized emulsions were prepared using a T 25 digital Ultra-Turrax homogenizer (IKA, Germany) operated at 10
3). Microsized emulsions were prepared using a T 25 digital Ultra-Turrax homogenizer (IKA, Germany) operated at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min. The process was carried out at 60 °C to maintain the lipid phase in a molten state, thereby facilitating uniform droplet formation and stable emulsion preparation.9,12 A schematic representation of the formulation process is presented in Fig. 1. The detailed ingredient levels used in each formulation are listed in Table 1.
000 rpm for 10 min. The process was carried out at 60 °C to maintain the lipid phase in a molten state, thereby facilitating uniform droplet formation and stable emulsion preparation.9,12 A schematic representation of the formulation process is presented in Fig. 1. The detailed ingredient levels used in each formulation are listed in Table 1.
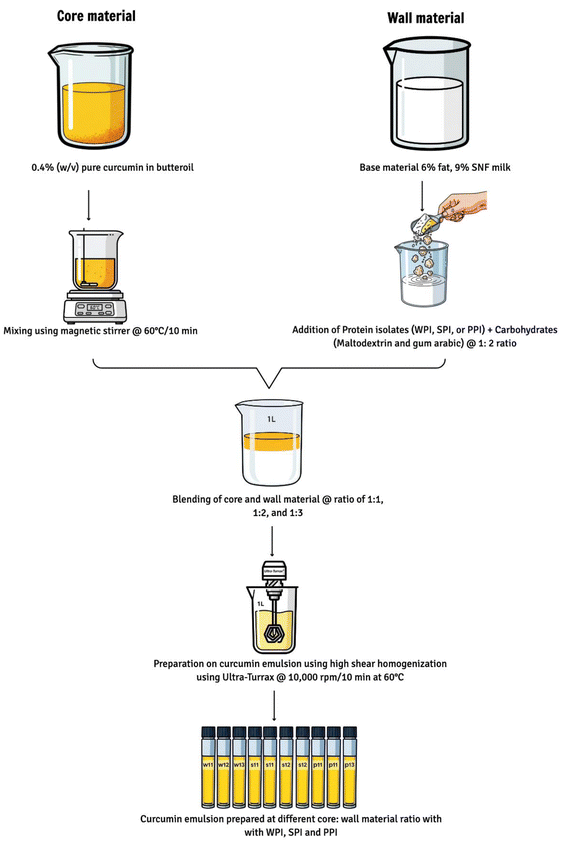 |
| | Fig. 1 Process flow diagram for the formulation of curcumin emulsions (1 litre volume) using whey, soy, and pea protein isolates as wall materials. | |
Table 1 Levels of different ingredients used in the formulation of 1 L curcumin emulsion (35 w/v% total solids) using different protein isolates (whey, soy, and pea protein) in the wall material
Ratios of core![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) wall material wall material |
Ratios of protein : carbohydrates |
Base material (milk with 6% fat and 9% SNF) (in mL) |
Core material (0.4% curcumin supplemented with butteroil) (in g) |
Wall material (protein and carbohydrates in combination, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio) (in g) 2 ratio) (in g) |
| Protein isolates (whey, soy and pea) |
Maltodextrin |
Gum arabica |
Total |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
800.00 |
100.00 |
33.30 |
60.00 |
6.70 |
100.00 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
800.00 |
66.70 |
44.00 |
80.00 |
8.90 |
133.30 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
800.00 |
50.00 |
50.00 |
90.00 |
10.00 |
150.00 |
2.3 Emulsion stability
The stability of the curcumin emulsions was evaluated using a gravity-based method. Ten mL of each emulsion was transferred into a 25 mL graduated cylinder and stored at 25 °C for 24 h without disturbance. The samples were observed for visible phase separation, creaming, or sedimentation. Emulsions without observable separation were considered stable.
2.4 Physicochemical analyses
2.4.1 Total solid content.
The total solids were measured using the oven-drying method, according to the AOAC protocol.28 Approximately 5 g of the emulsion sample was dried at 105 ± 1 °C until a constant weight was reached, and the total solids (%) were calculated by mass difference.
2.4.2 Water activity (aw).
Water activity was measured using a 3TE Aqualab water activity meter (Washington, USA). The emulsion samples were poured into clean measurement cups and placed in the instrument chamber. After temperature equilibration at 25 °C, the aw values were recorded directly from the digital readout. Each measurement was performed in triplicate.
2.4.3 Color analysis.
The color values of the emulsions (L* for lightness, a* for redness/greenness, and b* for yellowness/blueness) were determined using a ColorFlex EZ spectrophotometer (HunterLab, Virginia, USA). The emulsions were placed in a sample cup with a smooth surface to ensure the absence of air bubbles. The instrument was calibrated using a standard white tile before the analysis. Each sample was measured in triplicate.
2.4.4 Particle size and zeta potential.
The particle size distribution and zeta potential were assessed using dynamic light scattering (DLS) with a Zetasizer Nano ZS90 (Malvern Instruments Ltd, UK). For the particle size analysis, 1.5–2 mL of the emulsion was diluted in 400 mL of distilled water. For the zeta potential, 50 mL of emulsion was mixed with 50 mL of Millipore water. Measurements were performed at 25 °C, and the particle size and zeta potential were measured in micrometres (μm) and millivolts (mV), respectively.
2.4.5 Apparent viscosity.
The viscosity was measured at 20 °C using a dynamic rheometer (MCR 52, Anton Paar, Germany) equipped with a stainless-steel cone-plate geometry (CP75-1°, 50 mm diameter). The emulsion samples were equilibrated to the measurement temperature, and the viscosity was recorded at a shear rate of 50 s−1. Each measurement was performed in triplicate.
2.5 2,2-Diphenyl-1-picrylhydrazyl (DPPH) inhibition activity (%)
The antioxidant activity of the curcumin emulsions was evaluated using the DPPH radical scavenging assay.29 The samples were diluted in methanol to a final concentration of 50 mg mL−1. A 2.5 mL aliquot of the sample was mixed with 5 mL of 0.2 mM DPPH solution and incubated in the dark at room temperature for 30 min. The absorbance was measured at 517 nm using a Jasco UV-vis double beam spectrophotometer V-730 (Japan). All measurements were performed in triplicate. The percentage inhibition was calculated using eqn (1).| | 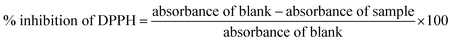 | (1) |
2.6 Encapsulation efficiency
The encapsulation efficiency of the curcumin emulsion was determined as per the method described by Zhan et al.30 with slight modifications. The freshly prepared curcumin emulsion was mixed with 80% (v/v) aqueous ethanol and vortexed for curcumin extraction. The dispersions were centrifuged (3–30K, Sigma, Germany) at 3600 rpm for 20 min to eliminate precipitates. The supernatant was diluted to an appropriate concentration with 80% ethanol, and the absorbance was recorded at 426 nm using a Jasco UV-vis double beam spectrophotometer V-730 (Japan). The encapsulation efficiency was calculated using eqn (2).| | 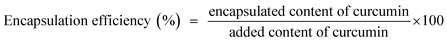 | (2) |
2.7 FT-IR analysis
FT-IR analysis was performed using the potassium bromate (KBr) pellet method for all samples. Freeze-dried emulsion samples (2–3 mg) were mixed with micronized KBr at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, pressed into pellets using a hydraulic press (10 tons pressure), and stored in a desiccator for 2 h. Spectra were recorded on a Spectrum 100-FTIR spectrometer (PerkinElmer, USA) across 4000–400 cm−1 with 25 scans at a resolution of 4 cm−1. The analysis and plotting were conducted using OriginPro 2023 software.
10, pressed into pellets using a hydraulic press (10 tons pressure), and stored in a desiccator for 2 h. Spectra were recorded on a Spectrum 100-FTIR spectrometer (PerkinElmer, USA) across 4000–400 cm−1 with 25 scans at a resolution of 4 cm−1. The analysis and plotting were conducted using OriginPro 2023 software.
2.8 XRD pattern
The crystalline characteristics of the freeze-dried emulsions were analyzed using a Miniflex 600 X-ray diffractometer (Rigaku, Japan) with CuKα radiation (λ = 1.54 Å) operating at 40 kV and 40 mA. The samples were scanned over a 2θ range of 5° to 80° at a rate of 30° min−1. Data processing and graph plotting were performed using OriginPro.
2.9 Microscopy analysis
Fluorescence microscopy was used to visualize curcumin distribution within the emulsions, as described by Yazdi and Corredig.31 Approximately 10 μL of emulsion was placed on a clean glass slide, covered with a cover slip, and observed under a fluorescence microscope (Olympus IX-83, Tokyo, Japan) at 60× magnification.
2.10 Statistical analysis
All data were obtained in triplicate and are reported as mean ± standard deviation. Statistical differences between the groups were analyzed using one-way analysis of variance (ANOVA) at p < 0.05 with the statistical software package SPSS 16.0 software (SPSS INC, Chicago, IL, USA).
3 Results and discussion
3.1 Physicochemical properties of curcumin emulsions
Table 2 shows the total solids (%), water activity, and viscosity (Pa s) of curcumin emulsions prepared with varying core-to-wall ratios and different protein isolates in the wall material.
Table 2 Physicochemical attributes of curcumin emulsion formulations at different core![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) wall material ratios with different protein isolates (WPI, SPI, and PPI) in the wall materiala
wall material ratios with different protein isolates (WPI, SPI, and PPI) in the wall materiala
| Parameters |
Protein isolates |
| Whey |
Soya |
Pea |
Note: values are presented as mean ± SD. Superscript letters (a, b, and c) indicate statistically significant differences (p < 0.05) among different core![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) wall material solid ratios within the same protein wall material solid type. Superscript capital letters (A, B, and C) indicate statistically significant differences (p < 0.05) among different core wall material solid ratios within the same protein wall material solid type. Superscript capital letters (A, B, and C) indicate statistically significant differences (p < 0.05) among different core![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) wall material solid ratios across different protein isolates (WPI, SPI, and PPI) used in the wall material. wall material solid ratios across different protein isolates (WPI, SPI, and PPI) used in the wall material.
|
Core![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) wall wall |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (W11) 1 (W11) |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (W12) 2 (W12) |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (W13) 3 (W13) |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (S11) 1 (S11) |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (S12) 2 (S12) |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (S13) 3 (S13) |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (P11) 1 (P11) |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (P12) 2 (P12) |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (P13) 3 (P13) |
| Total solids (%) |
35.22 ± 0.11aA |
35.02 ± 0.34aA |
34.99 ± 0.24aA |
35.54 ± 0.25aA |
35.72 ± 0.38aA |
35.52 ± 0.17aA |
35.22 ± 0.54aA |
35.57 ± 0.15a |
34.98 ± 0.68aA |
| Water activity (aw) |
0.991 ± 0.001aB |
0.987 ± 0.008aB |
0.994 ± 0.002aB |
0.994 ± 0.003bB |
0.888 ± 0.007aA |
0.885 ± 0.007aA |
0.992 ± 0.003b |
0.890 ± 0.007aA |
0.891 ± 0.007aA |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|
Colour value
|
|
L* |
79.61 ± 0.01cD |
78.80 ± 0.03bC |
77.36 ± 0.06aB |
72.92 ± 1.63aA |
70.70 ± 1.11aA |
70.99 ± 1.34aA |
67.57 ± 3.93aA |
71.81 ± 1.40aA |
70.15 ± 1.63aA |
|
a* |
−4.05 ± 0.13 cC |
−5.43 ± 0.05aA |
−4.90 ± 0.09bB |
1.09 ± 1.98aB |
1.45 ± 1.39aB |
0.89 ± 1.66aB |
4.52 ± 2.86aB |
1.44 ± 2.66aB |
2.46 ± 1.83aB |
|
b* |
69.55 ± 0.10cC |
65.32 ± 0.37bB |
62.77 ± 0.06aA |
74.47 ± 4.45aC |
73.52 ± 3.00aC |
70.62 ± 4.16aC |
69.39 ± 0.36aC |
72.94 ± 2.93aC |
70.58 ± 3.34aC |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|
Particle size
|
|
Z-average (μm) |
350 ± 9.28bB |
322 ± 5.49aA |
420 ± 35.94cB |
584.6 ± 27.79cB |
391.53 ± 26.55aB |
476.2 ± 40.57bB |
512.13 ± 43.36aB |
433.2 ± 37.26aB |
410.13 ± 27.91aB |
| PdI |
0.577 ± 0.068bB |
0.434 ± 0.033aA |
0.531 ± 0.045bB |
0.777 ± 0.10aB |
0.715 ± 0.06aB |
0.819 ± 0.09aB |
0.863 ± 0.144aB |
0.763 ± 0.052aB |
0.663 ± 0.083aB |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|
Zeta potential
|
| Zeta potential (mV) |
−36.8 ± 0.34bB |
−38.5 ± 0.43aA |
−36.23 ± 0.80bB |
−31.96 ± 0.55aC |
−34.66 ± 0.05aC |
−33.5 ± 0.8aC |
−25.63 ± 0.83 cF |
−31.56 ± 0.05aD |
−30.06 ± 0.90bE |
| Electrical conductivity (mS cm−1) |
0.138 ± 0.001bC |
0.112 ± 0.001aA |
0.136 ± 0.001bB |
0.120 ± 0.001bB |
0.116 ± 0.001aB |
0.143 ± 0.001 cC |
0.139 ± 0.001aC |
0.175 ± 0.003bD |
0.141 ± 0.001aC |
| Viscosity (Pa s) |
0.038 ± 0.010 cA |
0.018 ± 0.004bA |
0.012 ± 0.003aA |
0.033 ± 0.007aA |
0.037 ± 0.007aA |
0.026 ± 0.004aA |
0.094 ± 0.035aA |
0.039 ± 0.010aA |
0.022 ± 0.004aA |
| DPPH inhibition (%) |
72.91 ± 3.25bE |
61.37 ± 4.74aD |
58.33 ± 5.48aD |
47.72 ± 0.93 cC |
39.17 ± 2.26bB |
28.55 ± 1.14aA |
81.38 ± 0.51 cG |
77.94 ± 1.82bF |
73.76 ± 2.31aE |
| Encapsulation efficiency (%) |
93.32 ± 0.47aC |
94.04 ± 0.63aC |
92.66 ± 0.22aC |
92.76 ± 0.66bC |
88.16 ± 0.54aA |
91.96 ± 0.35bB |
96.26 ± 0.45bD |
95.78 ± 0.38bD |
94.79 ± 0.31aC |
3.1.1 Total solid content.
The total solid content of all curcumin emulsions ranged from 34.98% to 35.72%, with no significant differences observed among the different protein isolates (WPI, SPI, and PPI) or core-to-wall ratios (p > 0.05). This consistency in dry matter content indicates that the formulation process and compositional adjustments did not significantly alter total solids. These findings align with those of Meena et al.,9 who reported similar results for curcumin emulsions formulated with comparable levels of wall materials. The high solid content (∼35%) is advantageous for potential downstream applications, such as spray drying or incorporation into solid food matrices, where concentrated emulsions are preferred. This characteristic enhances the versatility of emulsions in various food processing techniques.
3.1.2 Water activity (aw).
The water activity (aw) values of the curcumin emulsions ranged from 0.885 to 0.994, with S13 exhibiting the lowest (0.885) and W12 the highest (0.994) values. Soy and pea protein-based emulsions showed significantly lower aw at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 1
2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 core
3 core![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) wall ratios compared to 1
wall ratios compared to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, whereas whey protein emulsions demonstrated minimal changes across ratios. This decline in water activity with increasing wall material indicates an improved water-binding capacity of the protein–carbohydrate matrix, particularly in soy and pea protein systems. The variation in water activity is related to the level and nature of the wall material, especially the protein content.32 Lower aw values (<0.9) are favorable for microbial stability, whereas emulsions with aw ≥0.99 (e.g., W12) may require refrigeration and aseptic handling to prevent microbial spoilage. However, the intrinsic antimicrobial properties of curcumin may contribute to microbial stability and emulsion preservation to a certain extent.33 In this study, water activity in curcumin emulsions was influenced by wall material type and proportion, with higher protein–carbohydrate content improving water-binding, microbial stability, and overall emulsion preservation.
1, whereas whey protein emulsions demonstrated minimal changes across ratios. This decline in water activity with increasing wall material indicates an improved water-binding capacity of the protein–carbohydrate matrix, particularly in soy and pea protein systems. The variation in water activity is related to the level and nature of the wall material, especially the protein content.32 Lower aw values (<0.9) are favorable for microbial stability, whereas emulsions with aw ≥0.99 (e.g., W12) may require refrigeration and aseptic handling to prevent microbial spoilage. However, the intrinsic antimicrobial properties of curcumin may contribute to microbial stability and emulsion preservation to a certain extent.33 In this study, water activity in curcumin emulsions was influenced by wall material type and proportion, with higher protein–carbohydrate content improving water-binding, microbial stability, and overall emulsion preservation.
3.1.3 Viscosity.
The emulsion viscosity ranged between 0.012 and 0.094 Pa s, with the highest value observed in P11 (0.094 Pa s) and the lowest in W13 (0.012 Pa s). Although the viscosities of the SPI and PPI emulsions were not significantly different (p > 0.05), the WPI-based emulsions showed significant variation across the ratios, with W11 exhibiting higher viscosity than W12 and W13. These results suggest that WPI may form more compact and entangled interfacial layers, thereby increasing the bulk viscosity at lower wall-to-material ratios. In contrast, soy and pea proteins may produce looser structures because of their less flexible globular configurations. These trends align with previous studies, indicating that viscosity is influenced by protein solubility, molecular flexibility, and emulsion droplet interactions.34,35 The viscosity of the solution depends on several variables, including the concentration of the emulsifying and stabilizing agents, homogenization pressure, and temperature. These factors also regulate the encapsulation efficiency.34,35 Notably, the viscosity values of the emulsions formulated in this study were lower than those reported by Meena et al.9 and Tonon et al.36 for 30% total solid content.
3.2 Color characteristics
Color is a crucial visual and sensory quality indicator of foods. The analysis of curcumin emulsions with different protein isolates (WPI, SPI, and PPI) as wall materials revealed significant variations in color parameters (L*, a*, and b*) across different core-to-wall material ratios, as shown in Table 2. The L* values (lightness) ranged from 67.57 ± 3.93 (P11) to 79.61 ± 0.01 (W11), with whey-based emulsions appearing significantly lighter. This enhanced lightness may be attributed to the superior color-masking ability of whey proteins, which stems from their excellent film-forming and emulsifying properties.37,38 Soy and pea protein emulsions showed non-significant changes in L* values across different core-to-wall ratios, whereas whey protein emulsions exhibited significant variations. The L* value of the emulsion gel in the present study was lower than that of WPI/PPI-based emulsion gels39 and curcumin-loaded oil body emulsions.40
The a* values (red-green axis) ranged from −5.43 ± 0.05 to +4.52, reflecting the influence of the protein type on the emulsion hue. WPI samples displayed negative a* values, indicating a slight greenish tint, whereas PPI emulsions showed positive values, indicating reddish tones. This difference may be due to variations in the encapsulation efficiency or free curcumin migration.
The b* values (yellowness) were highest for S11 (74.47 ± 4.45) and lowest for W13 (62.77 ± 0.06). The strong yellowness of the soy-based emulsions indicated the presence of unencapsulated curcumin and less effective color masking. Conversely, lower b* values in whey emulsions highlighted WPI's superior encapsulation and pigment masking abilities. These findings align with those of Meena et al.9 and Zou et al.41 for curcumin emulsions, who reported that WPI reduced the visual intensity of encapsulated pigments. Overall, these color parameters play a significant role in the processing, packaging, and acceptability of food products.42 The results of this study provide valuable insights into the color-masking capabilities of different protein isolates in curcumin emulsions, with whey protein demonstrating the most promising results.
3.3 Particle size and zeta potential
3.3.1 Particle size and polydispersity index.
The particle size of the formulation plays an important role in the stability and efficiency of the bioactive compounds present in the emulsion.43Table 2 shows the particle size (μm) and zeta potential (mV) of different combinations of curcumin emulsions with different core-to-wall material ratios and different types of protein isolates in the wall material. The particle size of curcumin emulsion with different protein isolates as wall materials was observed in the range of 322 ± 5.49 to 584.60 ± 27.7 μm. The particle size of the emulsion changed significantly (p < 0.05) among the core![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) wall material ratios, except for PPI (P11, P12, and P13). The smallest particle size (322 ± 5.49 μm) was obtained for the W12 emulsion. The use of different protein isolates (WPI, SPI, and PPI) as wall materials had the least effect on the particle size of the emulsion, and no significant (p > 0.05) changes were observed among the emulsions, except for W12. The particle size of the emulsion was previously reported to be in the range of 356.67–687.80 μm for PPI–pectin complexes for curcumin delivery44 and curcumin emulsions with whey protein concentrate.9 The results of the current study are within the range of those of these studies. Significant changes in particle size were observed among the different core
wall material ratios, except for PPI (P11, P12, and P13). The smallest particle size (322 ± 5.49 μm) was obtained for the W12 emulsion. The use of different protein isolates (WPI, SPI, and PPI) as wall materials had the least effect on the particle size of the emulsion, and no significant (p > 0.05) changes were observed among the emulsions, except for W12. The particle size of the emulsion was previously reported to be in the range of 356.67–687.80 μm for PPI–pectin complexes for curcumin delivery44 and curcumin emulsions with whey protein concentrate.9 The results of the current study are within the range of those of these studies. Significant changes in particle size were observed among the different core![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) wall materials, which were attributed to the formation of larger/smaller oil particles with an increase/decrease in the core material in the emulsion.9,45 The smaller particle size and low polydispersity index indicated that the emulsion with WPI was stable. The droplet size of curcumin emulsions was higher than that reported by Li et al.46 for curcumin nanoparticles and Liang et al.47 for curcumin emulsion prepared with pectin–whey protein complexes.
wall materials, which were attributed to the formation of larger/smaller oil particles with an increase/decrease in the core material in the emulsion.9,45 The smaller particle size and low polydispersity index indicated that the emulsion with WPI was stable. The droplet size of curcumin emulsions was higher than that reported by Li et al.46 for curcumin nanoparticles and Liang et al.47 for curcumin emulsion prepared with pectin–whey protein complexes.
3.3.2 Zeta potential.
Zeta potential is an emulsion property that helps understand the electrostatic forces governing droplet behavior, which ultimately provides stability to the emulsion.48 The zeta potential of emulsions with WPI and PPI changed significantly (p < 0.05) with the varied core![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) wall material ratio, whereas for SPI, it did not change significantly. The highest zeta potential value was observed for the W12 emulsion (−38.50 mV), and a higher zeta potential value indicates stronger repulsion between the particles and better emulsion stability. This may be due to the uniform particle size of the W12 emulsion.49 The zeta potential of the curcumin emulsion varied among the various levels of the protein wall material. A stable emulsion was obtained for WPI based on the higher zeta potential, which could be due to the better emulsifying properties of whey protein.50 Another reason for the higher whey protein zeta potential is its inherent negative charge.51 The zeta potential values obtained in this study were higher than those of curcumin emulsions prepared using a pectin–whey protein complex,47 where the zeta potential was reported as −31.99 mV.
wall material ratio, whereas for SPI, it did not change significantly. The highest zeta potential value was observed for the W12 emulsion (−38.50 mV), and a higher zeta potential value indicates stronger repulsion between the particles and better emulsion stability. This may be due to the uniform particle size of the W12 emulsion.49 The zeta potential of the curcumin emulsion varied among the various levels of the protein wall material. A stable emulsion was obtained for WPI based on the higher zeta potential, which could be due to the better emulsifying properties of whey protein.50 Another reason for the higher whey protein zeta potential is its inherent negative charge.51 The zeta potential values obtained in this study were higher than those of curcumin emulsions prepared using a pectin–whey protein complex,47 where the zeta potential was reported as −31.99 mV.
3.4 Antioxidant activity (DPPH scavenging)
Curcumin can interact with several reactive oxygen species and has been proven to be an effective antioxidant that minimizes the effects of oxidative stress.52Table 2 shows the DPPH inhibition activity (%) for different combinations of curcumin emulsions at different core-to-wall material ratios and different types of protein isolates in the wall material. The highest antioxidant activity was observed for P11 (81.38%), whereas the lowest activity was observed for S13 (28.55%). The antioxidant activity of the emulsion was not significantly different at different core![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) wall material ratios for WP, but significantly varied at different core
wall material ratios for WP, but significantly varied at different core![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) wall material ratios for soy and pea protein. The % DPPH of the emulsion with SPI was less than that of the emulsion reported by Fu et al.53 The DPPH inhibition results of whey and pea proteins were supported by Mohammadian et al.54 and Meena et al.9
wall material ratios for soy and pea protein. The % DPPH of the emulsion with SPI was less than that of the emulsion reported by Fu et al.53 The DPPH inhibition results of whey and pea proteins were supported by Mohammadian et al.54 and Meena et al.9
3.5 Encapsulation efficiency (EE%)
EE is the parameter by which the performance of the encapsulation process is assessed. The high EE value ensures that bioactive compounds have excellent bioaccessibility.55 It is influenced by several factors, such as particle size, wall material properties, and processing parameters (i.e., pressure and intensity of treatment).56 The EE results for different combinations of curcumin emulsions with different core-to-wall material ratios and different types of protein isolates in the wall material are shown in Table 2. The EE of the emulsions exhibited an excellent curcumin encapsulation effect, with an EE value above 88.16%. The core-to-wall material influenced the EE of the emulsions, except for WPI. In addition, the type of protein source also affects EE, but the results are not consistent among the types and their levels in the wall material. The higher EE and high content of soluble curcumin result from the high surface hydrophobicity and curcumin binding capacity of whey protein,56,57 the high content of hydrophobic amino acids and polar and charged residues in soy protein,58 and the hydrophobic interaction of curcumin and tryptophan residues of pea protein.44 The results of the present study are in line with the findings of curcumin emulsions/complexes prepared by Wang et al.,59 Solghi et al.,55 and Vijayan etal.,60 where values of EE (93–98%) are consistent with the present findings.
3.6 FT-IR spectroscopy
The structural properties, nature of bonding and interaction of curcumin with encapsulation materials in curcumin emulsions were evaluated by FT-IR spectroscopy in Fig. 2, and major IR spectral data/peaks are presented in Table 3. The IR spectrum of curcumin emulsion shows a broad range of absorption bands, due to the presence of various functional groups. The IR peaks between 3200 and 2500 cm−1 may be attributed to the stretching vibrations of N–H and the C–H bonds.61 The sharp peak near 2932 cm−1 probably formed due to complexing of the phenolic hydroxyl group of curcumin with amino acid residues of the protein by hydrogen bonding.62 The broad bands observed at 3600–3100 cm−1 are due to interaction between the components; the peaks slightly distorted, probably due to the formation of new hydrogen bonds.61 The distinct IR absorption band at 1700–1600 cm−1 is due to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group stretching vibration of the amide I band of proteins.63 The slight shift (1744 cm−1) of the distinct peaks may be due to changes in protein content during curcumin emulsion preparation. The amide II band at 1580–1545 cm−1 is due to N–H and C–N stretching that represents the vibrational bands in the protein backbone.64 Typical peaks of pure curcumin have been reported at 1627–1462 cm−1 and 1278–1160 cm−1.65 The prominent peak of curcumin disappeared or shifted from this region, which may be due to the formation of complexes during curcumin encapsulation, limiting the stretching vibrations of curcumin.54,65 The IR spectra of emulsions with different functional peaks at 2925–2854, 1543–1535, 1250–1020, and 840–850 cm−1 due to the presence of N–H, N–O, C–N, and C–Cl stretching, respectively, for different functional groups of curcumin and protein isolates. Similar IR spectra and shifting of the curcumin peak upon encapsulation have been reported for curcumin–protein complexes, such as whey protein isolate-curcumin15,61 and curcumin complex with whey, soy and pea protein.60,64,65
O group stretching vibration of the amide I band of proteins.63 The slight shift (1744 cm−1) of the distinct peaks may be due to changes in protein content during curcumin emulsion preparation. The amide II band at 1580–1545 cm−1 is due to N–H and C–N stretching that represents the vibrational bands in the protein backbone.64 Typical peaks of pure curcumin have been reported at 1627–1462 cm−1 and 1278–1160 cm−1.65 The prominent peak of curcumin disappeared or shifted from this region, which may be due to the formation of complexes during curcumin encapsulation, limiting the stretching vibrations of curcumin.54,65 The IR spectra of emulsions with different functional peaks at 2925–2854, 1543–1535, 1250–1020, and 840–850 cm−1 due to the presence of N–H, N–O, C–N, and C–Cl stretching, respectively, for different functional groups of curcumin and protein isolates. Similar IR spectra and shifting of the curcumin peak upon encapsulation have been reported for curcumin–protein complexes, such as whey protein isolate-curcumin15,61 and curcumin complex with whey, soy and pea protein.60,64,65
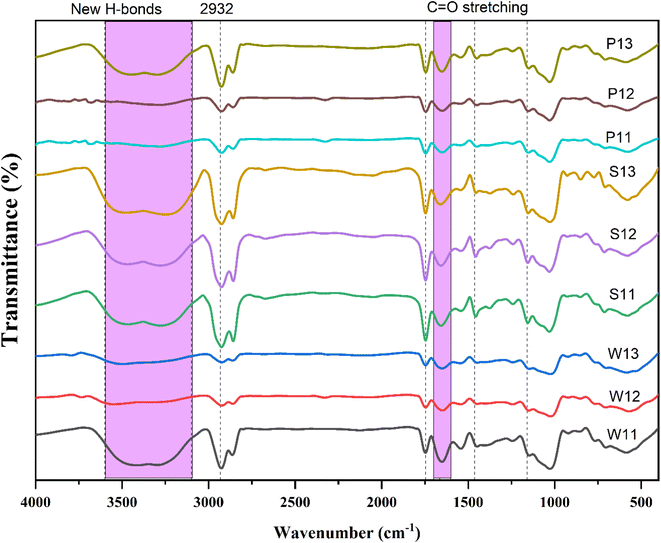 |
| | Fig. 2 FTIR spectra of curcumin emulsions prepared using different concentrations of whey, soy, and pea protein isolates as wall materials. | |
Table 3 Different functional group peaks along with the mode of vibration in the curcumin emulsion formulation at different core![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) wall material ratios with different protein isolates (WPI, SPI, and PPI) in the wall material
wall material ratios with different protein isolates (WPI, SPI, and PPI) in the wall material
| Range (in cm−1) |
W11 |
W12 |
W13 |
S11 |
S12 |
S13 |
P11 |
P12 |
P13 |
Functional group |
| 3800–3700 |
- |
3732 |
3792 |
— |
3740 |
— |
— |
— |
— |
S–S, stretch |
| 3550–3200 |
3424, 3295 |
3537 |
3494 |
3505, 3285 |
— |
3507, 3252 |
3471 |
3285 |
3454, 3285 |
O–H stretching |
| 3000–2800 |
2921, 2858 |
2921, 2858 |
2918, 2855 |
2925, 2856 |
2860 |
2925, 2861 |
2925, 2854 |
2928, 2855 |
2925, 2858 |
N–H and C–H stretching |
| 3300–2500 |
— |
— |
— |
2680 |
— |
2679 |
— |
— |
— |
O–H stretching |
| 2000–1650 |
1651 |
1654 |
1651 |
— |
1667 |
1654 |
1657 |
— |
— |
C–H bending |
| 1750–1735 |
1740 |
1743 |
1743 |
1743 |
1743 |
1740 |
1743 |
1743 |
1743 |
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching O stretching |
| 1662–1626 |
— |
— |
— |
1654 |
— |
— |
1657 |
1644 |
1644 |
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching C stretching |
| 1550–1500 |
1535 |
1540 |
1542 |
— |
— |
— |
1543 |
1542 |
1535 |
N–O stretching |
| 1465–1450 |
1452 |
1450 |
1450 |
1452 |
— |
— |
1452 |
1450 |
1442 |
C–H bending |
| 1420–1330 |
— |
— |
— |
— |
1368 |
1373 |
— |
— |
— |
O–H bending |
| 1250–1020 |
1244 |
1020, 1148, 1240 |
1240, 1144, 1020 |
1151 |
1151 |
1240, 1150, 1022 |
1243, 1149, 1022 |
1246, 1154, 1025 |
1237, 1144, 1025 |
C–N stretching |
| 995–985 |
— |
— |
— |
— |
992 |
— |
— |
— |
— |
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bending C bending |
| 980–960 |
— |
— |
— |
961 |
— |
— |
— |
— |
— |
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bending C bending |
| 850–550 |
850 |
850 |
840 |
— |
850 |
847 |
850 |
845 |
840 |
C–Cl stretching |
| 800–760 |
— |
— |
— |
— |
771 |
775 |
769 |
— |
— |
C–H bending |
| 730–665 |
— |
704 |
— |
718 |
713 |
711 |
710 |
712 |
704 |
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bending C bending |
| 600–500 |
579 |
565 |
579 |
575 |
556 |
575 |
575 |
572 |
575 |
C–I stretching |
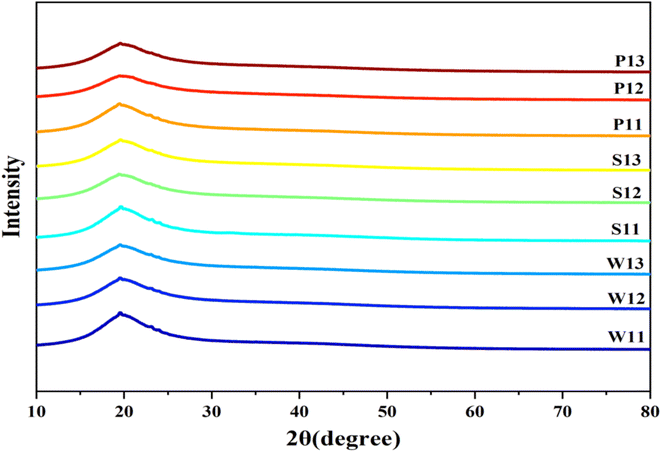
3.7 XRD analysis
The crystallinity of protein isolates i.e. whey, soy and pea, protein–carbohydrates complexes and free or encapsulated curcumin was evaluated by XRD, and the patterns are shown in Fig. 3A. A broad peak at 20° was observed in the XRD patterns of the different emulsions, revealing the presence of β-sheets in the polypeptide chain structure.65 The addition of a broad peak of curcumin emulsions confirmed that curcumin was properly encapsulated in all combinations of proteins, and the complexes formed during encapsulation were amorphous in nature. Guo et al.65 and Fu et al.53 also reported similar findings for curcumin encapsulation using pea protein and soya protein, respectively. The present findings on whey protein are in agreement with those of Mohammadian et al.,54 who reported an amorphous nature for curcumin-loaded whey protein microgels.
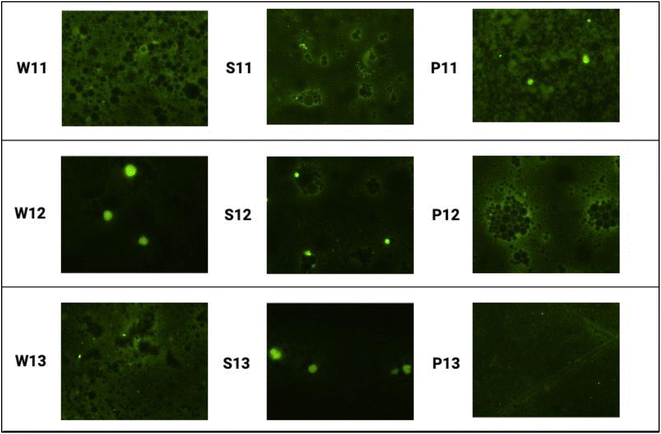
3.8 Fluorescence microscopy
Fig. 3 shows fluorescence images (at 60× magnification) of curcumin emulsions with different protein isolates as wall materials at various core-to-wall ratios. Microscopic analysis of the emulsion revealed the type of emulsion system and droplet size using fluorescence images. The encapsulation efficiency of curcumin can be predicted by fluorescence imaging, as curcumin acts as a natural fluorescent material (emission peak at ∼550 nm wavelength).66 The fluorescence images (Fig. 4) show smaller and more uniform whey protein droplets. The images revealed the presence of free curcumin in both soy and pea proteins, but free curcumin was not observed, whereas the droplet size was uniform for soy and pea protein-based emulsions. In the optical image of the core![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) wall ratio (1
wall ratio (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), the structure is quite compact, which is probably due to protein enrichment. Recently, similar studies were conducted by Singh et al.67 and Wang et al.68 for curcumin-loaded Pickering emulsions, in which the researchers predicted the emulsion stability and interactions between the constituents of the emulsions.
3), the structure is quite compact, which is probably due to protein enrichment. Recently, similar studies were conducted by Singh et al.67 and Wang et al.68 for curcumin-loaded Pickering emulsions, in which the researchers predicted the emulsion stability and interactions between the constituents of the emulsions.
 |
| | Fig. 3 XRD patterns of curcumin emulsions prepared using different concentrations of whey, soy, and pea protein isolates as wall materials. | |
 |
| | Fig. 4 Fluorescence images of curcumin emulsion with different protein isolates as wall materials: WPI (W11: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (core 1 (core![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) wall ratio), W12: 1 wall ratio), W12: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and W13: 1 2 and W13: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3); SPI (S11: 1 3); SPI (S11: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, S12: 1 1, S12: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and S13: 1 2 and S13: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) and PPI (P11: 1 3) and PPI (P11: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, P12: 1 1, P12: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and P13: 1 2 and P13: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) at 60× magnification. 3) at 60× magnification. | |
3.9 Emulsion stability
Emulsion stability is an important practical property of emulsions that is influenced by parameters such as surfactant concentration, particle size distribution, and interactions between the emulsion constituents.69,70 The stability results for the emulsions are presented in Table 4. The emulsion with a core![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) wall material ratio of 1
wall material ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for different protein sources was unstable during the stability test, which could be due to the high amount of core material and low amount of wall material. However, the other emulsion combinations were stable.
1 for different protein sources was unstable during the stability test, which could be due to the high amount of core material and low amount of wall material. However, the other emulsion combinations were stable.
Table 4 Results of emulsion stability for curcumin emulsions prepared using different levels of whey, soy and pea protein isolates as wall materialsa
Core![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) wall wall |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (W11) 1 (W11) |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (W12) 2 (W12) |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (W13) 3 (W13) |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (S11) 1 (S11) |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (S12) 2 (S12) |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (S13) 3 (S13) |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (P11) 1 (P11) |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (P12) 2 (P12) |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (P13) 3 (P13) |
|
✗ – non-stable emulsion and ✓ – stable emulsion.
|
| Emulsion stability |
✗ |
✓ |
✓ |
✗ |
✓ |
✓ |
✗ |
✓ |
✓ |
4 Conclusion
This study successfully developed protein-stabilized emulsions to encapsulate curcumin, demonstrating superior physicochemical properties with whey protein isolate (WPI) and highlighting the high antioxidant activity of pea protein isolate (PPI). These findings provide a robust foundation for creating functional, clean-label delivery systems that address the inherent instability and poor water solubility of curcumin. Future research should build on these results by transitioning from laboratory-scale analyses to biological and industrial validations. This includes testing the formulations in dynamic in vitro gastrointestinal models to assess bioaccessibility and permeability and optimizing process parameters for industrial scale-up. Further investigation into synergistic protein blends of WPI and PPI is warranted to create next-generation emulsions that balance superior physical stability with enhanced nutritional and functional properties. Such work will be crucial for translating these findings into viable and long-term functional foods and nutraceuticals.
Conflicts of interest
The authors declare no conflicts of interest relevant to this article.
Abbreviations
| WPI | Whey protein isolate |
| SPI | Soy protein isolate |
| PPI | Pea protein isolate |
| FT-IR | Fourier-transform infrared spectroscopy |
| XRD | X-ray diffraction |
| DLS | Dynamic light scattering |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| KBr | Potassium bromate |
| EE | Encapsulation efficiency |
Data availability
The data will be provided upon request.
Acknowledgements
The authors gratefully acknowledge financial support from the Institution of Eminence (IoE) Scheme, Banaras Hindu University, Varanasi (UP), for the “IoE Seed grant-II” (Dev. Scheme No. 6031(B) and PFMS Scheme No. 3254), and a “Credit Research Grant for Faculty/Post-Doctoral Fellows” (Scheme No. 6031(A)). The authors also acknowledge the central instrumentation facility BHU (SATHI (BHU)) and BioNEST, BHU.
References
- M. T. El-Saadony, T. Yang, S. A. Korma, M. Sitohy, T. A. Abd El-Mageed and S. Selim,
et al., Impacts of turmeric and its principal bioactive curcumin on human health: pharmaceutical, medicinal, and food applications: a comprehensive review, Front. Nutr., 2023, 9, 1040259 CrossRef PubMed . Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC9881416/.
- B. Jyotirmayee and G. Mahalik, A review on selected pharmacological activities of Curcuma longa L., Int. J. Food Prop., 2022, 25(1), 1377–1398 CrossRef CAS . Available from: https://www.tandfonline.com/doi/pdf/10.1080/10942912.2022.2082464.
- H. Ayub, M. Islam, M. Saeed, H. Ahmad, F. Al-Asmari and M. F. Ramadan,
et al., On the health effects of curcumin and its derivatives, Food Sci. Nutr., 2024, 12(11), 8623–8650 CrossRef CAS PubMed . Available from: https://pubmed.ncbi.nlm.nih.gov/39620006/.
- W. Tiatragoon, F. Laffleur and K. Netsomboon, Development of curcumin-loaded lipid nanocapsules for drug delivery across mucus, J. Drug Delivery Sci. Technol., 2024, 99, 106014 CrossRef CAS . Available from: https://www.sciencedirect.com/science/article/abs/pii/S177322472400683X.
- C. R. Malacrida, S. Ferreira and V. R. Nicoletti, Turmeric oleoresin encapsulated by spray drying in maltodextrin/gelatin and starch/gelatin blends: storage stability and water sorption, Acta Sci., Technol., 2022, 44, 1k Search PubMed . Available from: https://go.gale.com/ps/i.do?p=IFME%26sw=w%26issn=18062563%26v=2.1%26it=r%26id=GALE%7CA690996194%26sid=googleScholar%26linkaccess=fulltext.
- S. S. Patel, H. A. Pushpadass, M. E. E. Franklin, S. N. Battula and P. Vellingiri, Microencapsulation of curcumin by spray drying: Characterization and fortification of milk, J. Food Sci. Technol., 2022, 59(4), 1326–1340 CrossRef CAS PubMed . Available from: https://pubmed.ncbi.nlm.nih.gov/35250058/.
- A. Rezagholizade-shirvan, M. Soltani, S. Shokri, R. Radfar, M. Arab and E. Shamloo, Bioactive compound encapsulation: characteristics, applications in food systems, and implications for human health, Food Chem.:X, 2024, 24, 101953 CAS . Available from: https://www.sciencedirect.com/science/article/pii/S2590157524008411.
- T. Feng, Z. Hu, K. Wang, X. Zhu, D. Chen and H. Zhuang,
et al., Emulsion-based delivery systems for curcumin: encapsulation and interaction mechanism between debranched starch and curcumin, Int. J. Biol. Macromol., 2020, 161, 746–754 CrossRef CAS . Available from: https://www.sciencedirect.com/science/article/abs/pii/S0141813020335303.
- S. Meena, W. Prasad, K. Khamrui, S. Mandal and S. Bhat, Preparation of spray-dried curcumin microcapsules using a blend of whey protein with maltodextrin and gum arabica and its in vitro digestibility evaluation, Food Biosci., 2021, 1, 100990 CrossRef . Available from: https://www.sciencedirect.com/science/article/abs/pii/S2212429221001152.
- C. Bufalini and R. Campardelli, Versatile Emulsion-Based Encapsulation System Production Processes: A Review, Processes, 2025, 13(5), 1409 CrossRef CAS . Available from: https://www.mdpi.com/2227-9717/13/5/1409/htm.
- J. Teixé-Roig, G. Oms-Oliu, M. Artiga-Artigas, I. Odriozola-Serrano and O. Martín-Belloso, Enhanced in vivo absorption and biodistribution of curcumin loaded into emulsions with high medium-chain triglyceride content, Food Res. Int., 2023, 174, 113595 CrossRef . Available from: https://www.sciencedirect.com/science/article/pii/S0963996923011432.
- P. J. Rao, H. Khanum, P. S. Murthy, S. V. Shreelakshmi and M. S. Nazareth, Influence of milk fat on the physicochemical property of nanoencapsulated curcumin and enhancement of its biological properties thereof, J. Food Sci. Technol., 2023, 60(4), 1376–1388 CrossRef CAS PubMed . Available from: https://link.springer.com/article/10.1007/s13197-023-05684-5.
- P. Choudhary, S. Dutta, J. A. Moses and C. Anandharamakrishnan, Recent developments in encapsulation of α-lipoic acid for enhanced bioavailability and stability, Qual. Assur. Saf. Crops Foods, 2023, 15(1), 123–138 CrossRef CAS . Available from: https://www.qascf.com/index.php/qas/article/view/1081/1173.
- M. Moosavi-Nasab, B. Behroozi, H. H. Gahruie and S. Tavakoli, Single-to-combined effects of gelatin and aloe vera incorporated with Shirazi thyme essential oil nanoemulsion on shelf-life quality of button mushroom, Qual. Assur. Saf. Crops Foods, 2023, 15(2), 175–187 CrossRef CAS . Available from: https://qascf.com/index.php/qas/article/view/1241/1239.
- H. Lei, J. Lin, Z. Chen, Z. Shi, D. Niu and X. Zeng,
et al., The behavior of whey protein isolate-curcumin complex at the oil–water interface, Food Hydrocolloids, 2023, 145, 109046 CrossRef CAS . Available from: https://www.sciencedirect.com/science/article/abs/pii/S0268005X23005921.
- X. Zhang, Y. Chen, R. Li, Y. Shi, Y. Zhao and B. Li,
et al., Fabrication of pea protein isolate-stabilized oil-in-water emulsions with high freeze-thaw stability: effect of high intensity ultrasonic on emulsions and interfacial protein structure, Food Hydrocolloids, 2024, 157, 110484 CrossRef CAS . Available from: https://www.sciencedirect.com/science/article/abs/pii/S0268005X24007586.
- E. Galani, I. Ly, E. Laurichesse, V. Schmitt, A. Xenakis and M. D. Chatzidaki, Pea and Soy Protein Stabilized Emulsions: Formulation, Structure, and Stability Studies, Colloids Interfaces, 2023, 7, 30 CrossRef CAS . Available from: https://www.mdpi.com/2504-5377/7/2/30/htm.
- I. Khalifa, M. Li, T. Mamet and C. Li, Maltodextrin or gum Arabic with whey proteins as wall-material blends increased the stability and physiochemical characteristics of mulberry microparticles, Food Biosci., 2019, 31, 100445 CrossRef CAS . Available from: https://www.sciencedirect.com/science/article/abs/pii/S2212429218307594.
- W. Kim, Y. Wang and C. Selomulya, Exploring the relationship between physicochemical stability and interfacial properties in pea/whey protein blend-Stabilised emulsions, Food Hydrocolloids, 2025, 169, 111622 CrossRef CAS . Available from: https://www.sciencedirect.com/science/article/pii/S0268005X2500582X.
- M. Jafari, K. Parastouei and S. Abbaszadeh, Development of curcumin-loaded nanoemulsion stabilized with texturized whey protein concentrate: characterization, stability and in vitro digestibility, Food Sci. Nutr., 2024, 12(3), 1655–1672 CrossRef CAS . Available from: https://www.doi/pdf/10.1002/fsn3.3860.
- Q. Liu, W. Huang, C. Zhou, S. Yang, J. Hong and A. Xie,
et al., Green development of whey protein-based complex system for the intestinal delivery of curcumin: focus on formation mechanism, stability, and in vitro digestive characteristics, Food Chem.:X, 2025, 28, 102563 CAS . Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC12152569/.
- H. Li, X. Zhang, C. Zhao, H. Zhang, Y. Chi and L. Wang,
et al., Entrapment of curcumin in soy protein isolate using the pH-driven method: Nanoencapsulation and formation mechanism, LWT--Food Sci. Technol., 2022, 153, 112480 CrossRef CAS.
- L. Fu, S. Tan, R. Si, Y. Qiang, H. Wei and B. Huang,
et al., Characterization, stability and antioxidant activity of curcumin nanocomplexes with soy protein isolate and pectin, Curr. Res. Food Sci., 2023, 6, 100530 CrossRef CAS . Available from: https://europepmc.org/articles/PMC10290990.
- Y. Zhao, X. Han, H. Yin, Q. Li, J. Zhou and H. Zhang,
et al., Preparation and characterisation of curcumin-loaded pea protein-zein nanocomplexes using pH-driven method, Int. J. Food Sci. Technol., 2022, 57(6), 3589–3603 CrossRef CAS . https://www.doi/pdf/10.1111/ijfs.15683.
- J. Ren, H. Wu, Z. Lu, Q. Qin, X. Jiao and G. Meng,
et al., pH-driven preparation of pea protein isolate-curcumin nanoparticles effectively enhances antitumor activity, Int. J. Biol. Macromol., 2024, 256, 128383 CrossRef CAS , https://www.sciencedirect.com/science/article/abs/pii/S0141813023052820.
-
R. P. Aneja, B. N. Mathur, R. C. Chandan and A. K. Banerjee, Technology Of Indian Milk Products: Handbook On Process Technology Modernization For Professionals, Entrepreneurs And Scientists, 2002, https://api.semanticscholar.org/CorpusID:108035610 Search PubMed.
- S. Meena, A. Raj, J. Meena, B. K. Reddy, D. C. Rai and R. K. Duary,
et al., Study on curcumin fortification to improve the physicochemical properties and anti-oxidative potentialities of butteroil (ghee), Food and Humanity, 2024, 3, 100316 CrossRef
https://www.sciencedirect.com/science/article/pii/S2949824424000910
.
-
AOAC, Official Methods of Analysis, Association of Analytical Chemists, AOAC, Arlington, VA, 17th edn, 2000 Search PubMed.
- W. Brand-Williams, M. E. Cuvelier and C. Berset, Use of a free radical method to evaluate antioxidant activity, LWT–Food Sci. Technol., 1995, 28(1), 25–30 CrossRef CAS
https://www.sciencedirect.com/science/article/abs/pii/S0023643895800085
.
- X. Zhan, L. Dai, L. Zhang and Y. Gao, Entrapment of curcumin in whey protein isolate and zein composite nanoparticles using pH-driven method, Food Hydrocolloids, 2020, 106, 105839 CrossRef CAS , https://www.sciencedirect.com/science/article/pii/S0268005X19321836#bib7.
- Y. S. Rahimi and M. Corredig, Heating of milk alters the binding of curcumin to casein micelles. A fluorescence spectroscopy study, Food Chem., 2012, 132(3), 1143–1149 CrossRef PubMed
https://pubmed.ncbi.nlm.nih.gov/29243593/
.
- L. G. Lima Nascimento, D. Odelli, A. Fernandes de Carvalho, E. Martins, G. Delaplace and P. Peres de sá Peixoto Júnior,
et al., Combination of Milk and Plant Proteins to Develop Novel Food Systems: What Are the Limits?, Foods, 2023, 12, 2385 CrossRef CAS PubMed
https://www.mdpi.com/2304-8158/12/12/2385/htm
.
- C. Dai, J. Lin, H. Li, J. Shen, Z. Shen and Y. Wang,
et al., The Natural Product Curcumin as an Antibacterial Agent: Current Achievements and Problems, Antioxidants, 2022, 11(3), 459 Search PubMed
https://pubmed.ncbi.nlm.nih.gov/35326110/
.
- Y. Liang, H. Patel, L. Matia-Merino, A. Ye and M. Golding, Effect of pre- and post-heat treatments on the physicochemical, microstructural and rheological properties of milk protein concentrate-stabilised oil-in-water emulsions, Int. Dairy J., 2013, 32(2), 184–191 Search PubMed
https://www.sciencedirect.com/science/article/abs/pii/S095869461300143X
.
- L. Tavares, C. P. Zapata Noreña, H. L. Barros, S. Smaoui, P. S. Lima and M. Marques de Oliveira, Rheological and structural trends on encapsulation of bioactive compounds of essential oils: a global systematic review of recent research, Food Hydrocolloids, 2022, 129, 107628 CrossRef CAS
https://www.sciencedirect.com/science/article/abs/pii/S0268005X22001485
.
- R. V. Tonon, C. R. F. Grosso and M. D. Hubinger, Influence of emulsion composition and inlet air temperature on the microencapsulation of flaxseed oil by spray drying, Food Res. Int., 2011, 44(1), 282–289 CrossRef CAS
https://www.sciencedirect.com/science/article/pii/S0963996910003819
.
- J. V. P. Gomes, L. A. de Oliveira, J. d’A. Francisquini, P. C. Anunciação, R. Stephani and L. F. C. de Oliveira,
et al., Morphological characterization of whey protein concentrate admixture of microencapsulated curcumin by spray drying, J. Food Process. Preserv., 2021, 45(2), e15141 CAS.
- S. Solghi, Z. Emam-Djomeh, M. Fathi and F. Farahani, The encapsulation of curcumin by whey protein: assessment of the stability and bioactivity, J. Food Process Eng., 2020, 43(6), e13403 Search PubMed . Available from:https://www.doi/pdf/10.1111/jfpe.13403.
- R. Zhao, M. Zhang, Y. He, W. Wang, D. Li and C. Chang,
et al., Fabrication and characterization of glucono-δ-lactone-induced cold-set emulsion gels stabilized by a whey and pea protein mixture at various mixing ratios for the delivery of curcumin, J. Dairy Sci., 2025, 108(7), 6730–6744 Search PubMed
https://www.sciencedirect.com/science/article/pii/S0022030225003029
.
- J. Zhu, H. Wang, L. Miao, N. Chen, Q. Zhang and Z. Wang,
et al., Curcumin-loaded oil body emulsions prepared by an ultrasonic and pH-driven method: fundamental properties, stability, and digestion characteristics, Ultrason. Sonochem., 2023, 101, 106711 Search PubMed
https://pmc.ncbi.nlm.nih.gov/articles/PMC10749905/
.
- L. Zou, B. Zheng, W. Liu, C. Liu, H. Xiao and D. J. McClements, Enhancing nutraceutical bioavailability using excipient emulsions: influence of lipid droplet size on solubility and bioaccessibility of powdered curcumin, J. Funct. Foods, 2015, 15, 72–83 Search PubMed
https://www.sciencedirect.com/science/article/abs/pii/S1756464615001061
.
- R. Pandiselvam, S. Mitharwal, P. Rani, M. A. Shanker, A. Kumar and R. Aslam,
et al., The influence of non-thermal technologies on color pigments of food materials: an updated review, Curr. Res. Food Sci., 2023, 6, 100529 Search PubMed , https://www.sciencedirect.com/science/article/pii/S2665927123000977.
- G. Zhao, R. Zhang, L. Dong, F. Huang, X. Tang and Z. Wei,
et al., Particle size of insoluble dietary fiber from rice bran affects its phenolic profile, bioaccessibility and functional properties, LWT--Food Sci. Technol., 2018, 87, 450–456 CAS
https://www.sciencedirect.com/science/article/abs/pii/S0023643817306916
.
- Q. Guo, J. Su, X. Shu, F. Yuan, L. Mao and J. Liu,
et al., Production and characterization of pea protein isolate-pectin complexes for delivery of curcumin: effect of esterified degree of pectin, Food Hydrocolloids, 2020, 105, 105777 CAS
https://www.sciencedirect.com/science/article/abs/pii/S0268005X1933022X
.
- X. Huang, Y. Kakuda and W. Cui, Hydrocolloids in emulsions: particle size distribution and interfacial activity, Food Hydrocolloids, 2001, 15(4–6), 533–542 CAS
https://www.sciencedirect.com/science/article/abs/pii/S0268005X01000911
.
- X. Li, T. Xu, C. Wu, G. Fan, T. Li and Y. Wang,
et al., Fabrication and characterization of self-assembled whey protein isolate/short linear glucan core–shell nanoparticles for sustained release of curcumin, Food Chem., 2023, 407, 135124 CAS
https://www.sciencedirect.com/science/article/abs/pii/S0308814622030862
.
- Y. X. Liang, P. H. Li, Y. C. Chiang, H. Y. Song, Y. J. Lai and P. Y. Chiang, Assessment of curcumin self-emulsion containing high methoxyl pectin–whey protein complex: quality stability by thermal, freeze-thaw treatment, and release characteristics, LWT--Food Sci. Technol., 2023, 188, 115398 CAS
https://www.sciencedirect.com/science/article/pii/S0023643823009775
.
- B. Kupikowska-Stobba, J. Domagała and M. M. Kasprzak, Critical Review of Techniques for Food Emulsion Characterization, Appl. Sci., 2024, 14(3), 1069 CAS
https://www.mdpi.com/2076-3417/14/3/1069/htm
.
- E. S. Madivoli, P. G. Kareru, A. N. Gachanja, S. M. Mugo and D. S. Makhanu, Phytofabrication of iron nanoparticles and their catalytic activity, SN Appl. Sci., 2019, 1(8), 1–8 CAS
https://link.springer.com/article/10.1007/s42452-019-0951-0
.
- Y. Pan, L. Liu, J. Li, B. Zhu, X. Li and J. Cheng,
et al., Enhancing the physical stability and bioaccessibility of curcumin emulsions through the interaction of whey protein isolate and soybean lecithin, Food Biosci., 2024, 58, 103676 CAS
https://www.sciencedirect.com/science/article/abs/pii/S2212429224001068
.
- M. Nishanthi, T. Vasiljevic and J. Chandrapala, Properties of whey proteins obtained from different whey streams, Int. Dairy J., 2017, 66, 76–83 CAS
https://www.sciencedirect.com/science/article/abs/pii/S0958694616303466
.
- K. Jakubczyk, A. Drużga, J. Katarzyna and K. Skonieczna-żydecka, Antioxidant potential of curcumin—a meta-analysis of randomized clinical trials, Antioxidants, 2020, 9(11), 1–13 Search PubMed
https://pubmed.ncbi.nlm.nih.gov/33172016/
.
- L. Fu, S. Tan, R. Si, Y. Qiang, H. Wei and B. Huang,
et al., Characterization, stability and antioxidant activity of curcumin nanocomplexes with soy protein isolate and pectin, Curr. Res. Food Sci., 2023, 6, 100530 CAS.
- M. Mohammadian, M. Salami, S. Momen, F. Alavi, Z. Emam-Djomeh and A. A. Moosavi-Movahedi, Enhancing the aqueous solubility of curcumin at acidic condition through the complexation with whey protein nanofibrils, Food Hydrocolloids, 2019, 87, 902–914 CAS
https://www.sciencedirect.com/science/article/abs/pii/S0268005X1831110X
.
- S. Solghi, Z. Emam-Djomeh, M. Fathi and F. Farahani, The encapsulation of curcumin by whey protein: assessment of the stability and bioactivity, J. Food Process Eng., 2020, 43(6), e13403 CAS.
- S. Jafari, S. M. Jafari, M. Ebrahimi, I. Kijpatanasilp and K. Assatarakul, A decade overview and prospect of spray drying encapsulation of bioactives from fruit products: characterization, food application and in vitro gastrointestinal digestion, Food Hydrocolloids, 2023, 134, 108068 CAS
https://www.sciencedirect.com/science/article/abs/pii/S0268005X22005884
.
- I. R. Ariyarathna and D. N. Karunaratne, Microencapsulation stabilizes curcumin for efficient delivery in food applications, Food Packag. Shelf Life, 2016, 10, 79–86 Search PubMed
https://www.sciencedirect.com/science/article/abs/pii/S2214289416301508
.
- F. P. Chen, B. S. Li and C. H. Tang, Nanocomplexation between Curcumin and Soy Protein Isolate: Influence on Curcumin Stability/Bioaccessibility and in Vitro Protein Digestibility, J. Agric. Food Chem., 2015, 63(13), 3559–3569 CAS
https://pubs.acs.org/doi/abs/10.1021/acs.jafc.5b00448
.
- Y. Wang, R. Sun, X. Xu, M. Du, B. Zhu and C. Wu, Structural interplay between curcumin and soy protein to improve the water-solubility and stability of curcumin, Int. J. Biol. Macromol., 2021, 193, 1471–1480 CAS
https://www.sciencedirect.com/science/article/pii/S0141813021023758?via%3Dihub#f0030
.
- U. Kannamangalam Vijayan, N. N. Shah, A. B. Muley and R. S. Singhal, Complexation of curcumin using proteins to enhance aqueous solubility and bioaccessibility: pea protein vis-à-vis whey protein, J. Food Eng., 2021, 292, 110258 CAS
https://www.sciencedirect.com/science/article/abs/pii/S0260877420303496
.
- C. P. Racz, L. Z. Racz, C. G. Floare, G. Tomoaia, O. Horovitz and S. Riga,
et al., Curcumin and whey protein concentrate binding: Thermodynamic and structural approach, Food Hydrocolloids, 2023, 139, 108547 CrossRef CAS.
- H. Lei, J. Lin, Z. Chen, Z. Shi, D. Niu and X. Zeng,
et al., The behavior of whey protein isolate-curcumin complex at the oil-water interface, Food Hydrocolloids, 2023, 145, 109046 CAS
https://www.sciencedirect.com/science/article/pii/S0268005X23005921
.
- H. Yang, S. Yang, J. Kong, A. Dong and S. Yu, Obtaining information about protein secondary structures in aqueous solution using Fourier transform IR spectroscopy, Nat. Protoc., 2015, 10(3), 382–396 CAS
https://www.nature.com/articles/nprot.2015.024
.
- S. Behjati Hosseini, P. Arghavani, J. Hong, H. R. Rahimi, S. Azad-Armaki and R. Yousefi,
et al., Curcumin-loaded pickering emulsions based on soy protein isolate aggregates enhance diabetic wound healing, J. Drug Delivery Sci. Technol., 2024, 101, 106279 Search PubMed
https://www.sciencedirect.com/science/article/pii/S1773224724009481
.
- Q. Guo, I. Bayram, W. Zhang, J. Su, X. Shu and F. Yuan,
et al., Fabrication and characterization of curcumin-loaded pea protein isolate-surfactant complexes at neutral pH, Food Hydrocolloids, 2021, 111, 106214 CrossRef CAS
https://www.sciencedirect.com/science/article/abs/pii/S0268005X20309942
.
- E. Akbari, O. Akhavan, S. Hatamie and R. Rahighi, Curcumin as a green fluorescent label to revive the fluorescence property of functionalized graphene oxide nanosheets, J. Drug Delivery Sci. Technol., 2018, 45, 422–427 CrossRef CAS
https://www.sciencedirect.com/science/article/abs/pii/S1773224717305063
.
- B. G. Singh, N. Bagora, M. Nayak, J. K. Ajish, N. Gupta and A. Kunwar, The Preparation of Curcumin-Loaded Pickering Emulsion Using Gelatin–Chitosan Colloidal Particles as Emulsifier for Possible Application as a Bio-Inspired Cosmetic Formulation, Pharmaceutics, 2024, 16(3), 356 CrossRef CAS PubMed
https://www.mdpi.com/1999-4923/16/3/356/htm
.
- Y. Wang, Y. Jiang and J. Shi, Novel Pickering emulsion stabilized by glycated casein embedding curcumin: Stability, bioaccessibility and antioxidant properties, LWT--Food Sci. Technol., 2024, 194, 115796 CrossRef CAS
https://www.sciencedirect.com/science/article/pii/S0023643824000756
.
- Y. Wei, Y. Xie, Z. Cai, Y. Guo and H. Zhang, Interfacial rheology, emulsifying property and emulsion stability of glyceryl monooleate-modified corn fiber gum, Food Chem., 2021, 343, 128416 CrossRef CAS PubMed
https://www.sciencedirect.com/science/article/abs/pii/S0308814620322780
.
- A. L. R. Costa, A. Gomes, L. B. Cangussu, R. L. Cunha, L. S. de Oliveira and A. S. Franca, Stabilization mechanisms of O/W emulsions by cellulose nanocrystals and sunflower protein, Food Res. Int., 2022, 152, 110930 CrossRef CAS PubMed
https://www.sciencedirect.com/science/article/abs/pii/S0963996921008309
.
|
| This journal is © The Royal Society of Chemistry 2026 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article *a,
Raj Kumar
Duary
a,
Kamalesh Kumar
Meena
*a,
Raj Kumar
Duary
a,
Kamalesh Kumar
Meena
 c and
Shubham
Mishra
a
c and
Shubham
Mishra
a
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, and 1
2, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. The WPI-based emulsions demonstrated superior physicochemical properties, achieving the lowest particle size (322 ± 5.49 μm) and highest zeta potential (−38.50 mV) at the 1
3. The WPI-based emulsions demonstrated superior physicochemical properties, achieving the lowest particle size (322 ± 5.49 μm) and highest zeta potential (−38.50 mV) at the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio. PPI-based emulsions exhibited the highest antioxidant activity, with 81.38% DPPH inhibition, highlighting their potential as a promising plant-based alternative. Both dairy and plant proteins effectively encapsulated curcumin, with an encapsulation efficiency of 88.16–96.26%. In contrast, the SPI-based emulsions exhibited relatively lower performance across most parameters. FT-IR spectroscopy and fluorescence microscopy confirmed successful curcumin encapsulation and emulsion integrity. These findings highlight WPI as a highly effective encapsulating agent and position PPI as a viable, sustainable, and functional plant-based alternative. This study provides valuable insights for developing stable, cost-effective, and functional curcumin delivery systems to meet the growing market demand for clean-label and plant-based foods and nutraceuticals.
2 ratio. PPI-based emulsions exhibited the highest antioxidant activity, with 81.38% DPPH inhibition, highlighting their potential as a promising plant-based alternative. Both dairy and plant proteins effectively encapsulated curcumin, with an encapsulation efficiency of 88.16–96.26%. In contrast, the SPI-based emulsions exhibited relatively lower performance across most parameters. FT-IR spectroscopy and fluorescence microscopy confirmed successful curcumin encapsulation and emulsion integrity. These findings highlight WPI as a highly effective encapsulating agent and position PPI as a viable, sustainable, and functional plant-based alternative. This study provides valuable insights for developing stable, cost-effective, and functional curcumin delivery systems to meet the growing market demand for clean-label and plant-based foods and nutraceuticals.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. The protein-to-carbohydrate ratio was standardized at 1
10. The protein-to-carbohydrate ratio was standardized at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (w/w) based on preliminary trials conducted with soy and pea proteins, as well as our previous findings employing whey protein concentrate as a wall material for curcumin emulsions,9 to achieve optimal emulsion viscosity. The dry ingredients were accurately weighed and dispersed in milk containing 6% fat and 9% solids-not-fat, followed by mixing with a hand blender (Bajaj HB21, Mumbai, India) to ensure uniform hydration and dispersion of solids prior to high-speed homogenization. The prepared mixture accounted for approximately 80% of the final emulsion volume.
2 (w/w) based on preliminary trials conducted with soy and pea proteins, as well as our previous findings employing whey protein concentrate as a wall material for curcumin emulsions,9 to achieve optimal emulsion viscosity. The dry ingredients were accurately weighed and dispersed in milk containing 6% fat and 9% solids-not-fat, followed by mixing with a hand blender (Bajaj HB21, Mumbai, India) to ensure uniform hydration and dispersion of solids prior to high-speed homogenization. The prepared mixture accounted for approximately 80% of the final emulsion volume.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, and 1
2, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). Microsized emulsions were prepared using a T 25 digital Ultra-Turrax homogenizer (IKA, Germany) operated at 10
3). Microsized emulsions were prepared using a T 25 digital Ultra-Turrax homogenizer (IKA, Germany) operated at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min. The process was carried out at 60 °C to maintain the lipid phase in a molten state, thereby facilitating uniform droplet formation and stable emulsion preparation.9,12 A schematic representation of the formulation process is presented in Fig. 1. The detailed ingredient levels used in each formulation are listed in Table 1.
000 rpm for 10 min. The process was carried out at 60 °C to maintain the lipid phase in a molten state, thereby facilitating uniform droplet formation and stable emulsion preparation.9,12 A schematic representation of the formulation process is presented in Fig. 1. The detailed ingredient levels used in each formulation are listed in Table 1.

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) wall material
wall material![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio) (in g)
2 ratio) (in g)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2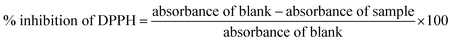
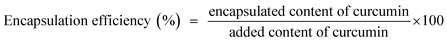
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, pressed into pellets using a hydraulic press (10 tons pressure), and stored in a desiccator for 2 h. Spectra were recorded on a Spectrum 100-FTIR spectrometer (PerkinElmer, USA) across 4000–400 cm−1 with 25 scans at a resolution of 4 cm−1. The analysis and plotting were conducted using OriginPro 2023 software.
10, pressed into pellets using a hydraulic press (10 tons pressure), and stored in a desiccator for 2 h. Spectra were recorded on a Spectrum 100-FTIR spectrometer (PerkinElmer, USA) across 4000–400 cm−1 with 25 scans at a resolution of 4 cm−1. The analysis and plotting were conducted using OriginPro 2023 software.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) wall material ratios with different protein isolates (WPI, SPI, and PPI) in the wall materiala
wall material ratios with different protein isolates (WPI, SPI, and PPI) in the wall materiala
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) wall material solid ratios within the same protein wall material solid type. Superscript capital letters (A, B, and C) indicate statistically significant differences (p < 0.05) among different core
wall material solid ratios within the same protein wall material solid type. Superscript capital letters (A, B, and C) indicate statistically significant differences (p < 0.05) among different core![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) wall material solid ratios across different protein isolates (WPI, SPI, and PPI) used in the wall material.
wall material solid ratios across different protein isolates (WPI, SPI, and PPI) used in the wall material.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) wall
wall![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (W11)
1 (W11)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (W12)
2 (W12)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (W13)
3 (W13)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (S11)
1 (S11)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (S12)
2 (S12)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (S13)
3 (S13)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (P11)
1 (P11)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (P12)
2 (P12)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (P13)
3 (P13)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif)
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif)
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif)
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 1
2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 core
3 core![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) wall ratios compared to 1
wall ratios compared to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, whereas whey protein emulsions demonstrated minimal changes across ratios. This decline in water activity with increasing wall material indicates an improved water-binding capacity of the protein–carbohydrate matrix, particularly in soy and pea protein systems. The variation in water activity is related to the level and nature of the wall material, especially the protein content.32 Lower aw values (<0.9) are favorable for microbial stability, whereas emulsions with aw ≥0.99 (e.g., W12) may require refrigeration and aseptic handling to prevent microbial spoilage. However, the intrinsic antimicrobial properties of curcumin may contribute to microbial stability and emulsion preservation to a certain extent.33 In this study, water activity in curcumin emulsions was influenced by wall material type and proportion, with higher protein–carbohydrate content improving water-binding, microbial stability, and overall emulsion preservation.
1, whereas whey protein emulsions demonstrated minimal changes across ratios. This decline in water activity with increasing wall material indicates an improved water-binding capacity of the protein–carbohydrate matrix, particularly in soy and pea protein systems. The variation in water activity is related to the level and nature of the wall material, especially the protein content.32 Lower aw values (<0.9) are favorable for microbial stability, whereas emulsions with aw ≥0.99 (e.g., W12) may require refrigeration and aseptic handling to prevent microbial spoilage. However, the intrinsic antimicrobial properties of curcumin may contribute to microbial stability and emulsion preservation to a certain extent.33 In this study, water activity in curcumin emulsions was influenced by wall material type and proportion, with higher protein–carbohydrate content improving water-binding, microbial stability, and overall emulsion preservation.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) wall material ratios, except for PPI (P11, P12, and P13). The smallest particle size (322 ± 5.49 μm) was obtained for the W12 emulsion. The use of different protein isolates (WPI, SPI, and PPI) as wall materials had the least effect on the particle size of the emulsion, and no significant (p > 0.05) changes were observed among the emulsions, except for W12. The particle size of the emulsion was previously reported to be in the range of 356.67–687.80 μm for PPI–pectin complexes for curcumin delivery44 and curcumin emulsions with whey protein concentrate.9 The results of the current study are within the range of those of these studies. Significant changes in particle size were observed among the different core
wall material ratios, except for PPI (P11, P12, and P13). The smallest particle size (322 ± 5.49 μm) was obtained for the W12 emulsion. The use of different protein isolates (WPI, SPI, and PPI) as wall materials had the least effect on the particle size of the emulsion, and no significant (p > 0.05) changes were observed among the emulsions, except for W12. The particle size of the emulsion was previously reported to be in the range of 356.67–687.80 μm for PPI–pectin complexes for curcumin delivery44 and curcumin emulsions with whey protein concentrate.9 The results of the current study are within the range of those of these studies. Significant changes in particle size were observed among the different core![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) wall materials, which were attributed to the formation of larger/smaller oil particles with an increase/decrease in the core material in the emulsion.9,45 The smaller particle size and low polydispersity index indicated that the emulsion with WPI was stable. The droplet size of curcumin emulsions was higher than that reported by Li et al.46 for curcumin nanoparticles and Liang et al.47 for curcumin emulsion prepared with pectin–whey protein complexes.
wall materials, which were attributed to the formation of larger/smaller oil particles with an increase/decrease in the core material in the emulsion.9,45 The smaller particle size and low polydispersity index indicated that the emulsion with WPI was stable. The droplet size of curcumin emulsions was higher than that reported by Li et al.46 for curcumin nanoparticles and Liang et al.47 for curcumin emulsion prepared with pectin–whey protein complexes.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) wall material ratio, whereas for SPI, it did not change significantly. The highest zeta potential value was observed for the W12 emulsion (−38.50 mV), and a higher zeta potential value indicates stronger repulsion between the particles and better emulsion stability. This may be due to the uniform particle size of the W12 emulsion.49 The zeta potential of the curcumin emulsion varied among the various levels of the protein wall material. A stable emulsion was obtained for WPI based on the higher zeta potential, which could be due to the better emulsifying properties of whey protein.50 Another reason for the higher whey protein zeta potential is its inherent negative charge.51 The zeta potential values obtained in this study were higher than those of curcumin emulsions prepared using a pectin–whey protein complex,47 where the zeta potential was reported as −31.99 mV.
wall material ratio, whereas for SPI, it did not change significantly. The highest zeta potential value was observed for the W12 emulsion (−38.50 mV), and a higher zeta potential value indicates stronger repulsion between the particles and better emulsion stability. This may be due to the uniform particle size of the W12 emulsion.49 The zeta potential of the curcumin emulsion varied among the various levels of the protein wall material. A stable emulsion was obtained for WPI based on the higher zeta potential, which could be due to the better emulsifying properties of whey protein.50 Another reason for the higher whey protein zeta potential is its inherent negative charge.51 The zeta potential values obtained in this study were higher than those of curcumin emulsions prepared using a pectin–whey protein complex,47 where the zeta potential was reported as −31.99 mV.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) wall material ratios for WP, but significantly varied at different core
wall material ratios for WP, but significantly varied at different core![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) wall material ratios for soy and pea protein. The % DPPH of the emulsion with SPI was less than that of the emulsion reported by Fu et al.53 The DPPH inhibition results of whey and pea proteins were supported by Mohammadian et al.54 and Meena et al.9
wall material ratios for soy and pea protein. The % DPPH of the emulsion with SPI was less than that of the emulsion reported by Fu et al.53 The DPPH inhibition results of whey and pea proteins were supported by Mohammadian et al.54 and Meena et al.9
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group stretching vibration of the amide I band of proteins.63 The slight shift (1744 cm−1) of the distinct peaks may be due to changes in protein content during curcumin emulsion preparation. The amide II band at 1580–1545 cm−1 is due to N–H and C–N stretching that represents the vibrational bands in the protein backbone.64 Typical peaks of pure curcumin have been reported at 1627–1462 cm−1 and 1278–1160 cm−1.65 The prominent peak of curcumin disappeared or shifted from this region, which may be due to the formation of complexes during curcumin encapsulation, limiting the stretching vibrations of curcumin.54,65 The IR spectra of emulsions with different functional peaks at 2925–2854, 1543–1535, 1250–1020, and 840–850 cm−1 due to the presence of N–H, N–O, C–N, and C–Cl stretching, respectively, for different functional groups of curcumin and protein isolates. Similar IR spectra and shifting of the curcumin peak upon encapsulation have been reported for curcumin–protein complexes, such as whey protein isolate-curcumin15,61 and curcumin complex with whey, soy and pea protein.60,64,65
O group stretching vibration of the amide I band of proteins.63 The slight shift (1744 cm−1) of the distinct peaks may be due to changes in protein content during curcumin emulsion preparation. The amide II band at 1580–1545 cm−1 is due to N–H and C–N stretching that represents the vibrational bands in the protein backbone.64 Typical peaks of pure curcumin have been reported at 1627–1462 cm−1 and 1278–1160 cm−1.65 The prominent peak of curcumin disappeared or shifted from this region, which may be due to the formation of complexes during curcumin encapsulation, limiting the stretching vibrations of curcumin.54,65 The IR spectra of emulsions with different functional peaks at 2925–2854, 1543–1535, 1250–1020, and 840–850 cm−1 due to the presence of N–H, N–O, C–N, and C–Cl stretching, respectively, for different functional groups of curcumin and protein isolates. Similar IR spectra and shifting of the curcumin peak upon encapsulation have been reported for curcumin–protein complexes, such as whey protein isolate-curcumin15,61 and curcumin complex with whey, soy and pea protein.60,64,65

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) wall material ratios with different protein isolates (WPI, SPI, and PPI) in the wall material
wall material ratios with different protein isolates (WPI, SPI, and PPI) in the wall material
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching
O stretching![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching
C stretching![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bending
C bending![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bending
C bending![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bending
C bending![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) wall ratio (1
wall ratio (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), the structure is quite compact, which is probably due to protein enrichment. Recently, similar studies were conducted by Singh et al.67 and Wang et al.68 for curcumin-loaded Pickering emulsions, in which the researchers predicted the emulsion stability and interactions between the constituents of the emulsions.
3), the structure is quite compact, which is probably due to protein enrichment. Recently, similar studies were conducted by Singh et al.67 and Wang et al.68 for curcumin-loaded Pickering emulsions, in which the researchers predicted the emulsion stability and interactions between the constituents of the emulsions.

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) wall material ratio of 1
wall material ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for different protein sources was unstable during the stability test, which could be due to the high amount of core material and low amount of wall material. However, the other emulsion combinations were stable.
1 for different protein sources was unstable during the stability test, which could be due to the high amount of core material and low amount of wall material. However, the other emulsion combinations were stable.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) wall
wall![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (W11)
1 (W11)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (W12)
2 (W12)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (W13)
3 (W13)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (S11)
1 (S11)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (S12)
2 (S12)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (S13)
3 (S13)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (P11)
1 (P11)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (P12)
2 (P12)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (P13)
3 (P13)
