Open Access Article
Open Access ArticleExploring the functional food potential of Grand naine banana flour (GBF) as a prospective weaning formulation by developing GBF-based composite flour mixes
Udipta
Hazarika
 a,
Prapti
Saikia
a,
Manisha
Choudhury
a,
Donee
Gohain
b and
Mamoni
Das
a,
Prapti
Saikia
a,
Manisha
Choudhury
a,
Donee
Gohain
b and
Mamoni
Das
 *a
*a
aDepartment of Food Science and Nutrition, College of Community Science, Assam Agricultural University, Jorhat 785013, Assam, India. E-mail: mamoni.das@aau.ac.in
bDepartment of Food Nutrition and Dietetics, Nemcare Group of Institutions, Shantipur, Mirza, Guwahati 781125, Assam, India
First published on 6th October 2025
Abstract
This study was focused on investigating a viable and efficient approach for the utilization and valorization of green banana flour (grand naine cultivar, AAA) (GBF). A series of composite mixes were formulated and tested as a source of affordable, energy-rich weaning food with high market acceptance. The complementary mixes included the following additional ingredients, viz., rice flour, roasted yellow lentil flour, pumpkin seed flour, and sesame seed flour, which afforded four composite mixes (MIX-1 to MIX-4) with saliently increasing concentrations of GBF. Further characterizations revealed that the MIX-4 composite mix had the most favourable characteristics in terms of proximate composition, including protein (15%), moisture (4.48%), and total energy values (401.44 kcal). MIX-4 also showed the highest water-absorption capacity (2.57%) and low solubility (8%), akin to complementary mixes. The high total phenolic content (1.03 mg CE per g) and inhibition capacity (47.94%) of MIX-4 suggest that it has a strong antioxidative capacity as well, which was confirmed by the peroxide and free fatty acid studies done over a period of 60 days. Rheological characteristics further confirmed that the resistant starch in GBF played a significant role in lowering the solubility and enhancing the starch gelatinization of MIX-4. Overall, these results corroborated the functional food potential of GBF-based composite flour mixes for the development of prospective weaning food formulations and for reducing post-harvest economic losses.
Sustainability spotlightGrand naine bananas are one of the major fruit crops cultivated in Assam and the North-East region of India, contributing significantly towards a stable livelihood for farmers. Farmers favor this variety due to its high yield, climatic adaptability, and contribution towards their economic benefits, as banana cultivation is a crucial paradigm and a critical source of income stability and food security. However, owing to the unavailability of proper storage and post-harvest infrastructure, the majority of the harvested bananas undergo rapid degradation and spoilage, resulting in substantial economic losses and income instability among the farmers. As such, this research work is an attempt towards providing a sustainable solution in two key areas: (1) providing a stable livelihood among banana farmers by utilizing raw banana to produce banana flour; (2) providing a banana-flour-based alternative to conventional cereal-based weaning foods with the potential to address nutritional inadequacy, particularly among infants. Processed raw banana flour can provide new avenues for addressing nutritional deficiency among infants in low-income households as a low-cost, healthier weaning formulation (SDG 3). Concurrently, the processing provides economic stability among banana cultivation farmers, thereby reducing crop loss and ensuring responsible consumption (SDG 12). This research serves as a crucial step towards economic empowerment among farmers by accrediting alternative income avenues while enhancing the nutritional profile of food products, thereby fostering a resilient, sustainable agro-food ecosystem in the region. |
1 Introduction
Banana is one of the most cultivated crops globally, and comes in fourth after rice, wheat, and maize in terms of production.1 It plays a vital role in food security while sustaining the global agricultural economy due to its high nutritional value and significant consumption.2 Approximately 1000 distinct banana varieties are grown across 150 nations, with the Cavendish cultivar accounting for 47% of the total production.3,4 Although the fruit is mostly consumed in its ripened/matured form, green (unripe) bananas also form a key component in global dishes and cuisines, as they are exceptionally rich in various nutritional parameters, with starch being the primary component.3,5 Studies by Chang et al. (2022) and Viana et al. (2024) revealed that the nutritional composition of green banana flour is 55.05–65.54% starch, 4.43–5.95% moisture, 3.74–4.49% protein, and 2.30–3.55% ash. This signifies the varied and rich nutritional profile of green bananas, and they have proven to be a crucial food crop resilient to nutritional insecurity in low- and middle-income countries and are a crucial staple food component in various countries.2,5Besides their nutritional prowess, bananas are highly susceptible to spoilage, making their long-term storage quite laborious and demanding. This, coupled with factors such as lack of adequate storage facilities (e.g., cold storage), unpredictable climate patterns, and lack of financial assistance, has led to the monumental degradation of the fruit and economic loss for farmers, producers, and exporters.6 As such, one of the most promising methods that can enhance the shelf life of bananas is processing them into value-added products, such as banana flour, which often involves the utilization of green, unripe banana fruits and can be a suitable alternative towards mitigating food waste, reducing environmental pollution, and increasing profit for farmers.2 A major advantage of using green banana flour is the presence of resistant starch (RS), which is around 36–40% of the total dry weight of starch. Further, it provides functionality and nutritional attributes when utilized as a raw material for the development of novel, composite flours, in the form of weaning/complementary mixes for infants.7 RS found in green banana (resistant starch II) can aid the functional enhancement of product attributes, such as texture, smoothness, and high-temperature storage properties, by acting as a polysaccharide conjugate to stabilize the emulsion conformation within the product system.8 Additionally, RS in weaning products has the potential to act as an infant prebiotic by enhancing the fermentation ability of gut microbiota (incremental abundance of Bifidobacterium and Bacteriodes).9
Complementary foods (CFs) play a significant role in satisfying the nutritional requirements of infants, particularly in the age group of 6–23 months, and they are usually given in liquid or semi-liquid form, which enhances nutrient intake.10 In the North-east region of India, the majority of mothers prefer home-made gruels, which are mostly prepared with rice as the major ingredient. This makes home-made gruels quite deficient in proteins, minerals, amino acids, and vitamins, leading to nutritional deficiency among infants. In India, these deficiencies mostly persist in the form of iron deficiency disorder (55% for children under 5), iron-deficiency anemia (58.60%), and vitamin A deficiency (19%), which gravely impede the cognitive and overall well-being of infants.11,12 Although there are nutrient-rich CFs in the market, a lack of proper weaning food knowledge among parents, as well as low financial capability, has led to the onset of early nutritional disorders, such as diarrhea, among infants.13 As such, the World Health Organization recommends preparing weaning food formulations with a combination of cereals, legumes, fruits, and vegetables for meeting the nutritional needs of infants. Previous studies have successfully reported complementary food formulations using various ingredients, such as banana–finger millet–dates composite flour,14 plantain–cashew–soybean complementary mix,10 and banana–rice–kidney bean-based weaning porridge,15 that can be complemented with breast milk for fulfilling an infant's nutritional needs. Additionally, the use of treatment methods, such as soaking, fermentation, germination, and blanching, can significantly enhance the nutrient composition and decrease the antinutrients present in the food matrix.14,16
To quantify the objectives of this study, two preliminary investigations were conducted. The first one was targeted towards assessing and understanding the reasons for post-harvest loss of the banana crops, for which 50 farmers were surveyed between November 2024 and December 2024 in the following villages around Jorhat, Assam, India: Changmai village, Nagadeva village, Tipomia village, and Sensua village. According to the farmers, the following issues contributed the most towards post-harvest loss of banana harvesting: (i) lack of adequate storage facilities and (ii) lack of proper processing units for value addition. The second preliminary investigation, which focused on quantifying the ingredients required for weaning food development (detailed explanation in Section 2.3), determined that mothers prefer home-made gruels due to low accessibility and availability of premade weaning food products. Focusing on the results from these two preliminary investigations, the low availability of ready-to-eat, affordable weaning food products developed from alternative sources other than cereals was established as the reason that the mothers provide home-made weaning gruels to the infants, often lacking a balanced nutritional profile. Therefore, this study emphasized accomplishing three major objectives, viz., utilization of grand naine banana to prepare minimally processed products and reduce post-harvest loss while increasing profitability among farmers; development of a gluten-free weaning mix utilizing the grand naine banana flour as the principal ingredient to provide a nutritionally enriched, low-cost, easily available alternative weaning product; and a further characterization of the nutritional, functional, morphological, sensorial, and shelf-life properties of the weaning mix, contributing towards its potential market acceptance.
2 Materials and methods
2.1. Preparation of grand naine banana flour (GBF)
The raw grand naine banana (Musa sp.) was procured from the horticulture orchard of Assam Agricultural University, Jorhat, Assam, India at ripening stage 1.17 The processing and preparation of the GBF was done according to the approach provided by Singh & Kaur, (2024) with a few modifications. The raw bananas were initially cleaned to remove any dust and debris. The bananas were then peeled and cut into circular pieces having a thickness of 6.5 mm using a stainless steel knife. The slices were then subjected to blanching (90–100 °C) and simultaneous treatment with 0.05% sodium hypochlorite and 0.1% citric acid to reduce the Maillard reaction (enzymatic browning) for 10 min. Once blanched, the slices were then kept for drying in a convection tray drier (Mevish Pharma Machineries, Maharashtra) at 55 °C ± 2 °C for 7 h until the slices were brittle. The drying temperature was restricted to 55–57 °C as some slices had irreversible colour change at temperatures beyond 60 °C. The convection tray drier works on the principle of atmospheric forced-air drying, which involves the utilization of forced convection heating to remove moisture from the banana slices placed on the perforated trays, with hot circulation enabling moisture absorption from blanched banana slices. The slices were dried until the desired dryness (moisture content below 10%) was achieved. Periodic measurement was done by the difference method until a constant weight of the slices was achieved. For every 1 kg of blanched banana slices that were dried, 200 g of dried slices were obtained, which corresponds to an 80% reduction in the moisture content. Subsequent pulverizing and grinding of the dried slices were done using an electrical grinder, followed by sieving (60-mesh).19 The final, derived GBF was stored in 200 g of HDPE bags at 4 °C until further analysis.2.2. Preparation of additional ingredients
The composite mix was composed of an additional four ingredients: rice flour (Oryza sativa) (RF), yellow lentil flour (Lens culinaris) (PF), sesame seed powder (Sesamum indicum) (SP), and pumpkin seed powder (Cucurbita maxima) (PP). These were procured from the local market in Jorhat, Assam, India. The ingredients were subjected to diligent cleaning to remove any physical contaminants. Additional cleaning using lukewarm water of RF and PF was required to remove debris. Yellow lentils were further subjected to drying and roasting, followed by grinding. Prior to utilization, both SP and PP were respectively subjected to defatting procedure according to the methods given by Fathelrahman et al. (2015) and Dhiman et al. (2018).20,21 All the ingredients were then dried using a convection tray drier (Mevish Pharma Machineries, Maharashtra) at 50 °C ± 2 °C for 60 min. The dried ingredients were then ground, sieved (60-mesh), and stored in 200 g of HDPE bags at 4 °C until further analysis.222.3. Preparation of composite weaning mix
Prior to the formulation of the composite mixes, a preliminary questionnaire-based survey of 60 families residing near the tea gardens and the Assam Agricultural University area was done to determine what type of ingredients would be suited for weaning food development. The survey allowed us to determine that 50% of the families had at least one newborn infant aged between 6 and 24 months. The infants were mostly fed homemade gruel prepared from rice flour and boiled urad dal flour, with a small quantity of milk. Only 10 families used banana flour (once per week) along with the other ingredients to prepare the gruel. The use of these ingredients was based on the families' income and the easy availability of these ingredients. However, none of them reported the use of sesame or pumpkin seed for preparing the gruels. In this study, defatted sesame seed and pumpkin seed flour were utilized as individual ingredients during preparation, as the defatted seeds have the potential to enhance functional properties, such as foaming capacity, emulsification ability, and cogent water-absorption capacities, owing to their higher protein concentration.21,23As this study was focused on the implementation of GBF as an alternative, affordable source, four formulations with varied concentrations of RF, GBF, and PF were prepared, while the concentrations of SP and PP were kept equal for every formulation (Table 1). Numerous trials were conducted to prepare the final selected composite mix formulations. During the evaluation period, concentrations of GBF beyond 30% led to the rapid agglomeration of the mix, which resulted in an undercooked mix. Once the formulations were developed, 25–30 ml of skimmed milk was heated in a low flame until lukewarm (38–40 °C). The formulations (15 g per 1 tbsp) were then added to the milk and cooked with continuous stirring. After 20 minutes, a thick slurry was obtained, which was used for the sensory assessment of the product.
| Formulations → | MIX-1 | MIX-2 | MIX-3 | MIX-4 |
|---|---|---|---|---|
| Ingredients ↓ | ||||
| Rice flour (RF) (g) | 70 | 60 | 50 | 40 |
| Banana flour (GBF) (g) | 10 | 15 | 20 | 25 |
| Yellow lentil flour (PF) (g) | 10 | 15 | 20 | 25 |
| Sesame seeds (SP) (g) | 5 | 5 | 5 | 5 |
| Pumpkin seeds (PP) (g) | 5 | 5 | 5 | 5 |
| Total | 100 g | 100 g | 100 g | 100 g |
2.4. Analysis
For the total phenolic content (TPC) analysis, 0.5 ml of the extract was added to 2.5 ml of DI water, followed by 200 μl of FCR (1 N), after which 2 ml of 7% Na2CO3 was added after 5 min. The solution was kept in incubation for 30 min, and its absorbance was measured at 760 nm using a UV-vis spectrophotometer (AnTech/AN-UV-6500N), with catechol as the standard. The results were expressed as the catechol equivalent (mg CE per 100 g).30 The antioxidant potential was calculated as described by P. S. Kumar et al. (2019), with slight modifications, where the DPPH (2, 2-diphenyl-1-picrylhydrazyl) activity was expressed in terms of the inhibition percentage. In brief, 100 μl (0.1 ml) of the extract was mixed with 3.9 ml of DPPH solution, which was incubated for 30 min, with methanol as the blank. The absorbance was measured at 515 nm and was expressed as % RSA, indicating the difference between the absorbance of the blank and the sample, respectively.19
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio). The pans were hermetically sealed and kept for 1 h for moisture equilibration at 25 °C. The pans were then heated steadily from 20 °C to 130 °C at a heating rate of 0.17 °C s−1 (10 °C min−1), under a nitrogen gas atmosphere. An empty aluminium pan was used as a reference. The resultant DSC thermograms were used to analyse the enthalpy of gelatinization (ΔH), onset temperature (T0), peak temperature (Tp), and conclusion temperature (Tc).31
3 ratio). The pans were hermetically sealed and kept for 1 h for moisture equilibration at 25 °C. The pans were then heated steadily from 20 °C to 130 °C at a heating rate of 0.17 °C s−1 (10 °C min−1), under a nitrogen gas atmosphere. An empty aluminium pan was used as a reference. The resultant DSC thermograms were used to analyse the enthalpy of gelatinization (ΔH), onset temperature (T0), peak temperature (Tp), and conclusion temperature (Tc).31
The sensory assessment (approval no.: AAU/CCSc/FSN/IEC(H)/DR/PR-FSN/24-25/02) was performed at the Department of Food Science and Nutrition, College of Community Science, Assam Agricultural University, Jorhat, Assam, India. Prior to the assessment, 1 tablespoon of each formulation was cooked using 30 ml of milk (Amul) until a thick slurry was obtained (Fig. 2). Thirty semi-trained panellists were randomly selected between the ages of 23–50 years for this purpose. A 5-point hedonic rating scale was used for evaluation, and the formulations were randomly coded to prevent bias and presented in random order to the panellists. The formulations were scored on the basis of their appearance, colour, flavour, taste, and overall acceptability.
3 Results and discussion
3.1. Nutritional composition of the weaning mixes
When the gluten-free composite flours were subjected to nutritional assessment, i.e., proximate analysis, significant differences (p < 0.05) were reported among the samples, with variations in moisture, ash, protein, fiber, fat, carbohydrate, and energy values, as shown in Table 2. The moisture content of the flours ranged from 8.65% to 4.48%. A moisture content higher than 12% is usually detrimental and lowers the shelf life of composite flour products. As such, the low moisture content of the flours will make them less susceptible to microbial growth and enhance the shelf life of the product.37 The highest ash concentration was seen in MIX-4 (2.19%), while MIX-1 reported the lowest ash concentration (1.39%). This increase may be attributed to the fact that the GBF concentration as an ingredient was incremented among the samples in increasing order of MIX-1 → MIX-4, which enhanced the total ash concentration among the flours.38 A similar study by Adegunwa et al. (2017) obtained ash concentrations of plantain–tigernut composite flour between 1.33% and 2.00%, where decreasing the plantain percentage decreased the total ash concentration among the samples. Additionally, MIX-4 reported the highest concentration of protein (15%) and fat (6.64%), which can be attributed to the subsequent increase in the concentration of yellow lentil flour (PF), along with GBF supplementation.37 A study performed by Chitra (2015) on the compositional analysis of banana-based weaning food mixes (type I to type IV) reported higher protein concentrations among type III (15.62%) and type IV (14.75%) samples, where the concentrations of soy flour and banana flour were the highest.39 Thus, it can be inferred that varying the PF concentration could have impacted the final protein concentration in the complementary flour samples. However, the fiber and carbohydrate concentrations were found to be highest in MIX-1, with values of 1.97% and 74.63%, respectively. The overall calculated energy value (EV) was reportedly the highest in MIX-4 (401.44 kcal) and the lowest in MIX-1 (374.30 kcal). Overall, the substitution of RF (70% to 40%) by GBF and PF (from 10% to 25%) was favourable for MIX-4 in terms of the ash, protein, fat, and total EV contents, while MIX-1 had the highest concentration of moisture, fiber, and carbohydrate.| Treatments | MIX-1 | MIX-2 | MIX-3 | MIX-4 |
|---|---|---|---|---|
| a Results are presented as mean value ± standard deviation (n = 3). Values depicted with different alphabets (a–d) along the rows represent significantly different results (p < 0.05) using Duncan's MRT. | ||||
| Proximate profile | ||||
| Moisture (%) | 8.66 ± 0.15d | 8.50 ± 0.11c | 5.91 ± 0.76b | 4.48 ± 0.28a |
| Ash (%) | 1.40 ± 0.33a | 1.64 ± 0.40ab | 1.80 ± 0.36ab | 2.19 ± 0.35b |
| Protein (%) | 8.86 ± 0.08a | 9.28 ± 0.23b | 11.14 ± 0.37c | 15.00 ± 0.18d |
| Fiber (%) | 1.97 ± 0.03d | 1.64 ± 0.05c | 1.44 ± 0.05b | 1.27 ± 0.09a |
| Fat (%) | 4.48 ± 0.04a | 5.64 ± 0.13b | 6.06 ± 0.07c | 6.64 ± 0.13d |
| Carbohydrate (%) | 74.63 ± 0.22d | 73.30 ± 0.15b | 73.64 ± 0.81c | 70.47 ± 0.64a |
| Energy values (kcal) | 374.31 ± 0.89a | 381.09 ± 1.50b | 393.67 ± 3.63c | 401.44 ± 2.78d |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Functional properties | ||||
| WAC (g g−1) | 2.28 ± 0.01a | 2.43 ± 0.03b | 2.47 ± 0.02b | 2.57 ± 0.03c |
| OAC (g g−1) | 1.81 ± 0.04a | 1.97 ± 0.07b | 2.07 ± 0.08bc | 2.16 ± 0.07c |
| BD (g ml−1) | 0.69 ± 0.01a | 0.70 ± 0.01a | 0.72 ± 0.04b | 0.74 ± 0.02c |
| TD (g ml−1) | 0.79 ± 0.01a | 0.80 ± 0.02 ab | 0.83 ± 0.02bc | 0.84 ± 0.01c |
| Carr index (%) | 13.84 ± 2.61a | 14.63 ± 3.80a | 14.20 ± 3.44a | 13.63 ± 2.04a |
| Hausner's ratio | 1.14 ± 0.03a | 1.15 ± 0.04a | 1.14 ± 0.05a | 1.14 ± 0.02a |
| FC (%) | 11.43 ± 0.01d | 9.30 ± 0.01c | 7.23 ± 0.01b | 5.57 ± 0.01a |
| Solubility (%) | 5.33 ± 0.01a | 6.00 ± 0.006a | 6.33 ± 0.02a | 8.00 ± 0.02a |
| Swelling power (g g−1) | 3.42 ± 0.1c | 3.21 ± 0.04b | 3.06 ± 0.05a | 3.04 ± 0.05a |
| Dispersibility (%) | 68.00 ± 0.50a | 70.50 ± 1.32b | 72.38 ± 0.80c | 74.35 ± 1.02d |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Bioactive properties | ||||
| TPC (mg CE per 100 g) | 55.97 ± 0.09a | 81.67 ± 0.04b | 92.08 ± 0.02c | 103.20 ± 0.07d |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Antioxidative properties | ||||
| Inhibition % | 39.66 ± 0.72a | 41.83 ± 1.54ab | 43.66 ± 2.53ab | 47.94 ± 3.47b |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Colour profile | ||||
| L* | 71.03 ± 2.30a | 71.97 ± 1.15a | 78.52 ± 0.54b | 79.03 ± 1.32b |
| a* | −6.70 ± 1.22b | −8.19 ± 0.65a | 1.64 ± 0.71c | 1.88 ± 0.23c |
| b* | 20.93 ± 0.47c | 23.44 ± 1.13d | 10.60 ± 1.03a | 13.06 ± 1.35b |
| Chroma | 21.99 ± 0.81c | 24.84 ± 0.84d | 10.75 ± 0.89a | 13.12 ± 1.30b |
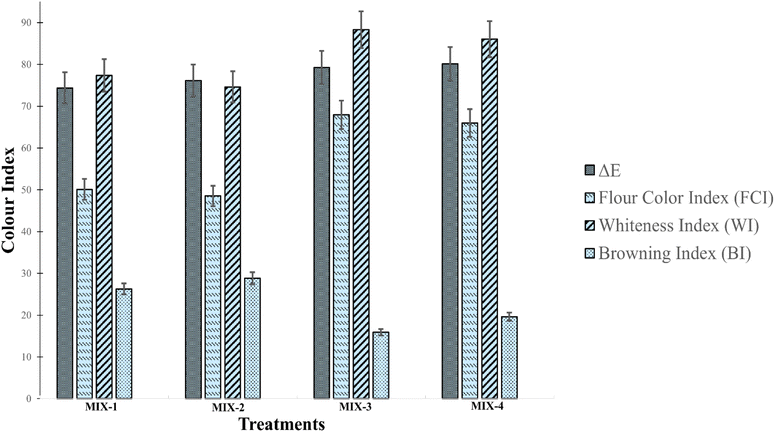
| ΔE | 74.36 ± 2.44a | 76.14 ± 1.00a | 79.26 ± 0.44b | 80.13 ± 1.35b |
| FCI | 50.10 ± 1.84a | 48.53 ± 1.93a | 67.92 ± 1.52b | 65.97 ± 1.72b |
| WI | 77.36 ± 0.74b | 74.60 ± 0.84a | 88.29 ± 0.84d | 86.03 ± 1.22c |
| BI | 26.27 ± 1.79b | 28.82 ± 4.11b | 15.88 ± 1.02a | 19.60 ± 1.70a |
| Starch digestibility (%) | 57.18 ± 1.53a | 57.96 ± 1.77a | 58.54 ± 1.75a | 59.32 ± 1.46a |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Mineral composition | ||||
| Fe (mg per 100g) | 4.65 ± 0.07a | 5.56 ± 0.07ab | 6.20 ± 0.02b | 7.36 ± 0.04c |
The gluten-free flours were characterized for the total iron (Fe) content, as presented in Table 2. Fe is a crucial mineral that is vital for the efficient growth of the central nervous system, including various facets of brain development, such as neurotransmitter functioning, myelination, and glial energy metabolism. Therefore, infants who are exclusively breastfed require iron from complementary foods to fulfil their body's iron needs, as their iron reserve becomes severely depleted within 4–6 months of life.40,41 The Fe content varied between 4.65 and 7.36 mg/100 g, with MIX-4 displaying the highest concentration. This can be attributed to the increasing GBF and PF concentrations, which are good sources of iron.33 The values obtained were in accordance with the results reported by Udomkun et al. (2019), where partial and complete banana flour substitutions yielded Fe concentrations of 5.8 mg/100 g and 4.10 mg/100 g, respectively,42 implying that both banana and legume flour contribute towards enhancing the Fe concentration in the flours.43
3.2. Functional properties of the weaning mixes
Functional properties are essential food characterization properties that largely influence the organoleptic properties, quality, texture, structure, nutritional value, as well as the physical and chemical characteristics, of food. The inherent components, viz., protein, carbohydrate, fiber, fat, minerals, and water, of foods largely determine their functional properties. These properties can be modified under the influence of processing techniques.44,45 The complementary flour mixes in this study were characterized for the following functional properties: water absorption capacity (WAC), oil absorption capacity (OAC), bulk density (BD), tapped density (TD), Carr Index (CI), Hausner's ratio (HR), foaming capacity (FC), solubility, swelling power (SP), and dispersibility, with Table 2 displaying the functional parameter values of the composite flour mixes. Since the formulations were designed as potential weaning-based products, these parameters were studied after preparing the mixes at lukewarm temperatures (38–40 °C).WAC is an essential parameter that determines the texture and smoothness of food. MIX-4 had the highest WAC of 2.57 g g−1, while MIX-1 exhibited the lowest WAC of 2.27 g g−1. The WAC values corresponded with the increase in the GBF and PF percentages of the sample, as higher protein and starch contents enhance the water affinity of the flours.18 Rustagi et al. (2022) observed similar results, where amaranth flour and plantain flour samples exhibited higher WAC than wheat flour, owing to their higher starch content. The OAC ranged from 1.81 g g−1 to 2.16 g g−1, with MIX-4 exhibiting the highest OAC. This may be attributed to the presence of non-polar amino acids, which enhance the oil absorption by inducing hydrophobicity in the sample.46
In terms of BD, MIX-4 exhibited the highest density of 0.74 g ml−1, possibly due to an increasing concentration of GBF in MIX-4, as a higher starch content tends to enhance the BD by influencing the porosity of the particles.46 BD is an essential non-inherent parameter as it assists in determining the packaging and handling requirements of food products. A study by Arzoo et al. (2024), which investigated the influence of processing on BD of finger millet flour, reported that the unprocessed millet flour had the highest BD, implying that processing and addition of other ingredients can contribute towards modifying the particle size. Similarly, MIX-4 exhibited the highest TD (0.84 g ml−1) among all the composite mixes. A higher TD value is usually displayed by small particles, which may be due to the decrease in the stickiness among the particles during dehydration. Additionally, small particles allow for greater availability of the surface area, which enhances the WAC, a position corroborated in this study.29 The Carr index (CI) values allow for understanding the flowability of flour particles, with the CI of the composite mixes ranging between 13.63% and 14.63%. Powder samples with CI < 15 have been characterized to be very good, as low CI values complement properties, such as hydration, bulk density, and tapped density.45 In terms of cohesiveness, which was determined by the Hausner's ratio (HR), all the samples had values lower than 1.2, which shows that the larger flour particles were able to overcome cohesive and frictional forces, easing particle flow.28,45
The measurement of FC allows us to determine the ability of the flour to form foam depending on the existence of soluble protein molecules.47 In this study, MIX-1 exhibited the highest FC with 11.43%, while MIX-4 displayed the lowest FC (5.57%), which may be due to the higher concentration of RF, which increases the starch and fiber contents in the sample. The study by Singh & Kaur (2024) reported similar results, where increasing the unripe banana flour concentration decreased the FC (17% to 15.45%) of the blends.18
While all the samples displayed a similar solubilization property (p > 0.05), MIX-1 exhibited the highest SP (3.42 g g−1), while MIX-4 had the lowest SP (3.04 g g−1). This modification can be ascribed to the decrease in the concentration of RF in MIX-4, as a low SP content corresponds to a low concentration of available starch in the flour. This variation influences the amount of internal starch molecules that are exposed to water. The lower value of SP in MIX-4 indicates strong starch interlinking with the presence of more lipid complexes, which may lead to reduced agglomeration in the sample.48,49 In terms of dispersibility, MIX-4 presented the highest value of 74.35%, while MIX-1 had the lowest value (68%). Dispersibility is the ability of the flour to reconstitute in water. Although the variations were non-significant (p > 0.05), a higher dispersibility percentage indicates that the flour particles were able to mix with water more uniformly (easy reconstitution) with reduced lump formation.28,36
3.3. Phytochemical properties of the weaning mixes
Phytochemicals are essential compounds that impart health benefits in the form of protective action against free oxygen radical species by acting as scavengers and maintaining a balance between oxidants and antioxidants for normal body functioning.50,51 The gluten-free flours presented significant (p < 0.05) bioactive and antioxidative activities in the form of total phenolic content (TPC) and 2,2-diphenyl-1-picrylhydrazyl radical scavenging activity (DPPH), respectively, which are reported in Table 2. The TPC of the flours varied between 55.97 and 103.20 mg CE per 100 g, with MIX-4 displaying the highest TPC among all the treatments. This can be attributed to the presence of more phenolic compounds in the unripe GBF and the enhancement in its concentration from MIX-1 to MIX-4. The inhibition capacity of MIX-4 was the highest as well (47.94%), while MIX-1 displayed the lowest activity (39.66%). Similar results were reported by Singh & Kaur (2024), where the increment of the unripe plantain flour content enhanced the TPC and DPPH activity of the final cake premix blends. Amarasinghe et al. (2021) reported significant TPC and DPPH values in banana-flour-incorporated cookies as well. Two varieties, Pisang awak and Red dacca, were incorporated in the cookies, and the TPC ranged from 1.10 to 3.99 mg GAE per g, while the DPPH varied between 0.04 to 0.15 mg TE per g. Thus, it may be inferred that the concentration of banana flour and its variety play a notable role in delineating the bioactivity of banana-flour-based products.3.4. Colour profile of the weaning mixes
Colour profile plays a crucial role in determining the consumer acceptability of food products. The colour attributes of composite flours are presented in Table 2. The L* values (lightness) of the flours varied significantly (p < 0.05), with MIX-4 displaying the highest L* values, while MIX-1 had the lowest values. Similarly, MIX-4 presented the highest a* values, tending towards a greener color. This can be attributed to the utilization of raw, green GBF, which may have resulted in the higher L* and a* values in MIX-4, and increasing the concentration of GBF in the treatments favoured MIX-4.19 Similar results were achieved by Borbi et al. (2020) in a banana-rich-bean porridge mix, where increasing the rice flour concentration contributed towards L* value enhancements.15 However, MIX-1 and MIX-2 reported the highest values for yellowness (b*), with oven drying contributing towards decreasing the chroma values of the samples. This decrease can be attributed to the Maillard reaction, which resulted in darker colour development. As investigated by Amini Khoozani et al. (2020), although colour variation is imminent, freeze-dried flour displays a lighter and vibrant colour compared to the oven drying variant, due to the mitigation of enzymatic activity and degradation.27 MIX-4 displayed a superior whiteness index (WI) (86.03%) and browning index (BI) (19.60%) (Fig. 2). This may be ascribed to the increasing GBF concentration, which enhances the bioactivity of the sample, resulting in reduced enzymatic browning.52 While colour changes are imminent after banana flour incorporation, processing (blanching, drying) can be a vital factor to determine consumer acceptability, along with improved nutritional characteristics of the processed products.3.5. In vitro starch digestibility
Starch is a major form of carbohydrate that is present predominantly in foods, such as cereals and unripe fruits, and it comprises two major components: amylose and amylopectin. Various factors, such as amylose/amylopectin ratio, resistance to enzymes, lipid percentage, formation of complexes, presence of fiber, and antinutritional factors, play a vital role in determining starch digestibility.53 The total starch digestibility (IVSD%) of the weaning mix samples is presented in Table 2, with values ranging from 57.18% for MIX-1 to 59.32% for MIX-4. Although digestibility was non-significant (p > 0.05) among the mixes, a steady increase in the rate of starch hydrolysis was observed with increasing the content of GBF. This can be attributed to the presence of different forms of starch within the GBF, viz., rapidly digestible starch (RDS), slowly digestible starch (SDS), and resistant starch (RS), which enhance the total starch concentration in the sample. Studies by Kumar et al. (2019) confirmed that grand naine banana flour had a total starch digestibility of 68.97%, with an RS concentration of 32.70%, while Khoza et al. (2021) found that grand naine flour had the highest concentrations of RDS (6.02%) and SDS (13.30%).19,33 Additionally, processing treatments, such as blanching of GBF and cooking of the complementary flour mixes, led to structural rearrangements and formation of complexes with starch (suggested by SEM micrographs), which enhanced the enzyme accessibility to starch and associated complexes, potentially leading to higher rates of starch hydrolysis. Rodriguez et al. (2025) reported similar changes due to processing in the IVSD of infant purees prepared from cereals (wheat, rice, maize) and their autoclaved counterparts. Autoclaved whole grain flours had the highest total hydrolysed starch concentration (29% for wheat, 70% for rice, and 92% for maize), compared to native flours, owing to the structural reconfiguration induced by milling and autoclaving, which enhanced the enzyme susceptibility, thus reducing starch digestibility.54 These results reveal that hydrothermal treatment (blanching) and cooking can modulate starch digestibility while easing RDS availability in infant foods in order to provide a rapid energy source. However, RS, which is readily available in GBF, may lead to the development of gut complications in infants if introduced too early. As such, a comprehensive understanding of starch digestion in infants, the impact of RS on infant microbiome, and a tailored approach towards feeding of weaning food rich in RS is required, which will promote infant intestinal health without compromising gut integrity.9,553.6. Pasting properties
Pasting properties are salient indices that allow us to assess the pasting functionality, i.e., the behaviour and ability of starch granules to resist structural changes under heating and shear conditions. These changes are associated with modifications in the amylose fractions, which are responsible for the pasting characteristics of different samples.56,57 Results of the pasting behaviour of the weaning mixes are given in Table 3. Peak viscosity represents the ability of damaged starch granules to swell freely under heating conditions before their structural breakdown, and the value decreases in the following order: MIX-1 (1694.33 cP) > MIX-2 (1505.50 cP) > MIX-3 (1396.82 cP) > MIX-4 (1200.33 cP). The lower peak viscosity of MIX-4 suggests higher solubility as a result of starch degradation, which can be attributed to the increase in the protein content as the concentration of PF rises, while the higher peak viscosity of MIX-1 implies a greater swelling power of the sample.5,37 Trough viscosity represents the capacity of the weaning mix gels to withstand breakage during cooling. A significant decrease (p < 0.05) was observed among the treatments, which ranged from 1154.07 cP (MIX-4) to 1632.63 cP (MIX-1). This can be due to an increase in the overall PF concentration, which enhanced the protein content. As reported by Wang et al. (2002), increasing the protein content can hinder with peak viscosity of flours.57 The disintegration of starch granules is represented by the breakdown viscosity, which varied between 46.30 cP and 61.70 cP, with MIX-1 having the highest breakdown value. The high concentration of RF and low PF concentration in MIX-1 may account for this high value, suggesting minimal starch breakdown and better resistance to shear stress during cooking.43 However, contrasting final viscosities were observed among the mixes. The final viscosity represents the ability of the flour to form a gel-like structure after cooling and depends upon the starch and amylose-amylopectin ratio. This change can be attributed to an increase in the GBF flour concentration. Although blanching enhances enzymatic hydrolysis, GBF has a relatively high retrogradation rate, which is corroborated by the increasing setback viscosity values among the composite flours.56 As reported by Thakaeng et al. (2021), raw banana flour can only form a paste upon cooling, which complements the values of the final viscosities. All the treatments reported similar peak times, which indicates the cooking ability of the flour.32 The peak temperature indicates the ability of the granules to resist fragmentation and rupture under high temperatures. All the flours had peak temperatures ranging from 73.30 °C to 75.64 °C, suggesting that MIX-1, which had the lowest value, was more prone to breakdown and required less energy.58| Weaning mixes → | MIX-1 | MIX-2 | MIX-3 | MIX-4 |
|---|---|---|---|---|
| Parameters ↓ | ||||
| a Results are presented as the mean of triplicates ± standard deviation (n = 3), with significant values (p < 0.05) depicted with alphabets (a–d) along a row using Duncan's MRT. | ||||
| Peak viscosity (cP) | 1694.33 ± 4.15d | 1505.50 ± 2.50c | 1396.82 ± 2.03b | 1200.33 ± 1.53a |
| Trough viscosity (cP) | 1632.63 ± 3.35d | 1443.83 ± 3.40c | 1341.17 ± 1.61b | 1154.07 ± 1.90a |
| Breakdown viscosity (cP) | 61.70 ± 0.82c | 61.67 ± 3.75c | 55.65 ± 0.56b | 46.27 ± 0.46a |
| Final viscosity (cP) | 1620.67 ± 1.53a | 1923.48 ± 0.50b | 2145.40 ± 1.51c | 2520.17 ± 2.02d |
| Setback viscosity (cP) | 1559.33 ± 1.53a | 1834.50 ± 1.32b | 1995.25 ± 0.95c | 2475.27 ± 0.81d |
| Peak time (min) | 13.07 ± 0.21a | 12.90 ± 0.66a | 13.17 ± 0.42a | 13.03 ± 0.50a |
| Peak temperature (°C) | 73.30 ± 0.79a | 74.00 ± 1.80ab | 74.17 ± 0.76ab | 75.64 ± 0.44b |
3.7. Modulation of thermal properties (DSC)
Thermal analysis techniques allow for the understanding and measurement of the physicochemical parameters of food products as a function of time, temperature, and atmosphere. DSC specifically is used to determine the structural transitions within the sample, which critically influence its physical and chemical attributes.59 As these formulations are a complex flour system, significant changes were observed among the treatments (refer to SI file Fig. 2). Table 4 depicts the onset temperature (To), peak temperature (Tp), conclusion temperature (Tc), gelatinization enthalpy (ΔH), and gelatinization temperature (R) of the formulations. The To of the flours ranged between 45.50 °C and 54.33 °C, while Tp and Tc ranged between 62.72 °C and 70.75 °C and 80.66 °C and 89.50 °C, respectively. The crystallinity of the starch granules critically influences these temperatures. As GBF has RS2 with B-type crystallinity, it has low molecular mobility with a high adsorption rate. However, the hydrothermal treatment (blanching) of GBF disrupted the crystalline structure order, which lowered the gelatinization temperature while improving the digestibility.31,59,60| Parameters → | T o (°C) | T p (°C) | T c (°C) | ΔH (J g−1) | R (°C) |
|---|---|---|---|---|---|
| Weaning mixes ↓ | |||||
| a T o = onset temperature, Tp = peak temperature, Tc = conclusion temperature, ΔH = degree of gelatinization, and R = gelatinization range. The results are presented as the mean of triplicates ± standard deviation (n = 3), with significant values (p < 0.05) depicted with alphabets (a–d) along a column using Duncan's MRT. | |||||
| MIX-1 | 54.33 ± 0.77d | 70.75 ± 1.30b | 89.5 ± 0.38d | 13.99 ± 0.44d | 35.17 ± 1.03ba |
| MIX-2 | 51.17 ± 0.83c | 70.67 ± 0.59b | 86.83 ± 0.33c | 12.79 ± 0.29c | 35.66 ± 1.13b |
| MIX-3 | 47.67 ± 0.41b | 64.25 ± 0.77a | 81.5 ± 0.33b | 9.46 ± 0.45b | 33.83 ± 0.13b |
| MIX-4 | 45.5 ± 0.50a | 62.72 ± 0.36a | 80.67 ± 0.43a | 8.24 ± 0.24a | 35.17 ± 0.17ab |
The gelatinization enthalpy (ΔH) can be influenced by the internal starch fraction array, type, and concentration of flour. The ΔH of the formulations ranged from 8.24 J g−1 to 13.99 J g−1, with MIX-4 displaying the lowest enthalpy. Usually, a decrease in the ΔH values corresponds to an increase in the degree of gelatinization. As the GBF concentration was the highest in MIX-4, blanching may have contributed towards decreasing the crystallinity and molecular order while enhancing starch gelatinization. This was evident from the increasing values of solubility, WAC, and OAC among the treatments.48,60 The increased SP content of MIX-1 may also be due to the arresting of the plasticization process, with increased granular swelling observed as the temperature increased within the sample, resulting in higher ΔH values.61
3.8. Functional-group characterization (FTIR)
The modulations in the functional groups of the weaning mixes were characterized using Fourier Transform Infrared Spectroscopy (FTIR), with the results presented in Fig. 3 for all the treatments. The modulations in the region of 3200–3450 cm−1 correspond to the stretching in the –OH and –NH groups, which validate the presence of phenolic compounds in the samples. The decrease in intensity in the 2800–3000 cm−1 region implies stretching in the –CH group. Moreover, grand naine flour displays characteristic peaks at 1643 and 1002 cm−1, which were observed for the gluten-free flours at 1652 and 1000 cm−1, respectively, corresponding to the –OH, –NH, and –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups.33 However, the peaks were more prominent in MIX-1, which can be ascribed to the increase in the rice flour concentration and the crystallinity in its starch molecules.62,63 The major peaks at 2926, 1652, 1453, and 1160 cm−1 confirm the –CH stretching (alkanes), –C
O groups.33 However, the peaks were more prominent in MIX-1, which can be ascribed to the increase in the rice flour concentration and the crystallinity in its starch molecules.62,63 The major peaks at 2926, 1652, 1453, and 1160 cm−1 confirm the –CH stretching (alkanes), –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching (alkenes), –C
C stretching (alkenes), –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching in carbonates, and –COH linkages of amylose and amylopectin, respectively.52 Additionally, the distinguishable peak at 1022.743 cm−1 corresponds to the amorphous region of starch, which was proportional to the concentration of rice flour. This peak gradually diminished as the concentration reduced from MIX-1 to MIX-4. Similar results by Dankar et al. (2018) confirmed the amorphous conformation of native potato flour samples.63 This result may also be ascribed to the increase in the GBF concentration among the gluten-free flours, which have a high resistant starch content (higher crystallinity).64,65 Another crucial factor that determines the starch conformation is the IR ratio of the bands at 1047/1022 cm−1. A higher ratio represents a more structured order (crystallinity) in the starch molecules. In the case of the weaning mixes, the IR ratio was 0.98 for MIX-1, 1.00 for MIX-2, 1.04 for MIX-3, and 1.05 for MIX-4. As explained by Kumar et al. (2023), alterations in the starch conformation by external modification can lead to dissociation among the crystalline domains. The gradual increase thus indicates increasing crystallinity across the samples, which corresponds to the increasing GBF concentration (rich in resistant starch content) as well.53
O stretching in carbonates, and –COH linkages of amylose and amylopectin, respectively.52 Additionally, the distinguishable peak at 1022.743 cm−1 corresponds to the amorphous region of starch, which was proportional to the concentration of rice flour. This peak gradually diminished as the concentration reduced from MIX-1 to MIX-4. Similar results by Dankar et al. (2018) confirmed the amorphous conformation of native potato flour samples.63 This result may also be ascribed to the increase in the GBF concentration among the gluten-free flours, which have a high resistant starch content (higher crystallinity).64,65 Another crucial factor that determines the starch conformation is the IR ratio of the bands at 1047/1022 cm−1. A higher ratio represents a more structured order (crystallinity) in the starch molecules. In the case of the weaning mixes, the IR ratio was 0.98 for MIX-1, 1.00 for MIX-2, 1.04 for MIX-3, and 1.05 for MIX-4. As explained by Kumar et al. (2023), alterations in the starch conformation by external modification can lead to dissociation among the crystalline domains. The gradual increase thus indicates increasing crystallinity across the samples, which corresponds to the increasing GBF concentration (rich in resistant starch content) as well.53
Protein can be identified in the 1200–1700 cm−1 region of the FTIR spectra, specifically by evaluating the amide I (1600–1700 cm−1), amide II (1500–1600 cm−1), and amide III (1200–1350 cm−1) regions, which correlate with stretching in the –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, –NH, and –CN groups.66 To analyse the hidden peaks within these regions, the second-order derivative of the flours was taken, which revealed the associated amide peaks, confirming the presence of protein among the flours (refer to the supplementary file, Fig. 3A–D). For the amide I region, MIX-1 had peaks at 1632 cm−1 and 1683 cm−1, MIX-2 had peaks in the range of 1631–1691 cm−1, MIX-3 presented peaks between 1637 and 1677 cm−1, and MIX-4 presented peaks at 1631 cm−1 and 1680 cm−1, which confirmed the –C
O, –NH, and –CN groups.66 To analyse the hidden peaks within these regions, the second-order derivative of the flours was taken, which revealed the associated amide peaks, confirming the presence of protein among the flours (refer to the supplementary file, Fig. 3A–D). For the amide I region, MIX-1 had peaks at 1632 cm−1 and 1683 cm−1, MIX-2 had peaks in the range of 1631–1691 cm−1, MIX-3 presented peaks between 1637 and 1677 cm−1, and MIX-4 presented peaks at 1631 cm−1 and 1680 cm−1, which confirmed the –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching. The amide II region, which corresponds to the –NH in-plane bending and –CN stretching, presented significant peaks in the range of 1500–1600 cm−1.67 The external process, extrusion, can physically induce the breakage of the peptide bond in these regions, which can be confirmed by a reduction in the number of peaks.52 The –CN stretching in the amide III region was confirmed by the presence of significant peaks within the 1200–1350 cm−1 range of the spectra.
O stretching. The amide II region, which corresponds to the –NH in-plane bending and –CN stretching, presented significant peaks in the range of 1500–1600 cm−1.67 The external process, extrusion, can physically induce the breakage of the peptide bond in these regions, which can be confirmed by a reduction in the number of peaks.52 The –CN stretching in the amide III region was confirmed by the presence of significant peaks within the 1200–1350 cm−1 range of the spectra.
3.9. Modulations of the microstructural configuration (SEM)
SEM handles the visualization of the changes observed in the weaning mixes by aiding in the microscopic inspection and retrieval of vital information regarding the various macromolecules present, such as starch, along with crucial details, such as size, shape, surface morphology, structural integrity, and complex formation by starch and protein interactions.68 In the current study, significant changes were observed among the formulations, as illustrated in the SEM micrographs. The composite flour granules of all four formulations were irregular, coarse, and scattered. The starch granules in MIX-1 were more elongated and rod-shaped compared to the MIX-4 starch granules. Additionally, the presence of raw GBF enhanced the irregularity on the granule surface, resulting in a rough appearance. This is in agreement with the report by Chang et al. (2022), where the presence of non-starch components enhanced the structural irregularity in the native banana flours.5 It was also observed that as the concentration of GBF increased for each treatment, the granular structure became more intact. This progressive loss in the oval structure can be ascribed to the increase in the GBF concentration, which is rich in different forms of starch, e.g., RDS, SDS, and RS. Raw banana flour is specifically rich in resistant starch type 2 (RS2). RS2 refers to ungelatinized starch granules found in native flours having type B crystallinity, which are distinctly crystalline and compact in nature, making them highly resistant to enzymatic hydrolysis and contributing towards reduced risks of non-communicable diseases.69–71 Moreover, in MIX-4 (Fig. 4G and H), the partial encapsulation of the starch biomass with protein agglomerates was evident by means of the rigid molecular rearrangement, making the matrix tough and rigid, further enhancing the enzymatic hydrolysis resistance.70 Dry grinding of RF contributed significantly towards enhancing the swelling power of MIX-1, as the starch crystallinity was weakened by the increasing content of damaged starch.22,72 Small protein particles were visible on the surface of the starch granules, which had a dense structure with small pores. As the ingredients did not undergo any further processing, such as extrusion, a number of protein aggregates are visible on the surface of the granules, with starch acting as a gelling agent.66 | ||
| Fig. 4 SEM micrographs of the weaning mixes. (A) MIX-1, 10 μm, (B) MIX-1, 30 μm, (C) MIX-2, 10 μm, (D) MIX-2, 30 μm, (E) MIX-3, 10 μm, (F) MIX-3, 30 μm, (G) MIX-4, 10 μm, (H) MIX-4, 30 μm. | ||
3.10. Storage study and sensory assessment
The storage study of the weaning mixes was achieved by means of free fatty acid (FFA) and peroxide value (PV) determinations after every 15 days, over a period of 60 days. Prior to the FFA and PV determinations, the formulations were kept in standard aluminium pouches under ambient conditions (20 °C), while the experiments were conducted at a temperature ranging from 15 °C to 20 °C. FFAs furnish vital information on the storage stability of a product, as they contribute towards off-flavour development (rancidity) as well as act as substrates for enzymatic deterioration in food.73 No FFAs were detected in the formulations initially; however, an increase in the acid values was observed among the flours as the storage period progressed. By the end of the 60th day, the FFA content ranged between 0.15% and 0.253%, with MIX-1 displaying the highest change (Fig. 5A). The presence of a high RF concentration may be due to the FFA increment, as prolonged storage at room temperature (20–25 °C) leads to lipid rancidity via hydrolysis and oxidation, thereby reducing the storage stability of RF.74–76 PV is an indicator of early lipid oxidation, which occurs due to the formation of hydroperoxides. Additionally, it can be used to acknowledge the antioxidant capacity of the product, where a high PV indicates low antioxidant activity.73,77 A significant increment in the PV of the formulations was observed after the 15th day. By the 60th day, the PV ranged between 0.20 and 0.76 meq O2 per kg, with MIX-4 displaying the lowest deterioration (0.20 meq O2 per kg) (Fig. 5B). This may be due to the increase in the GBF concentration, as it contributed towards increasing the TPC and antioxidant capacity of MIX-4.18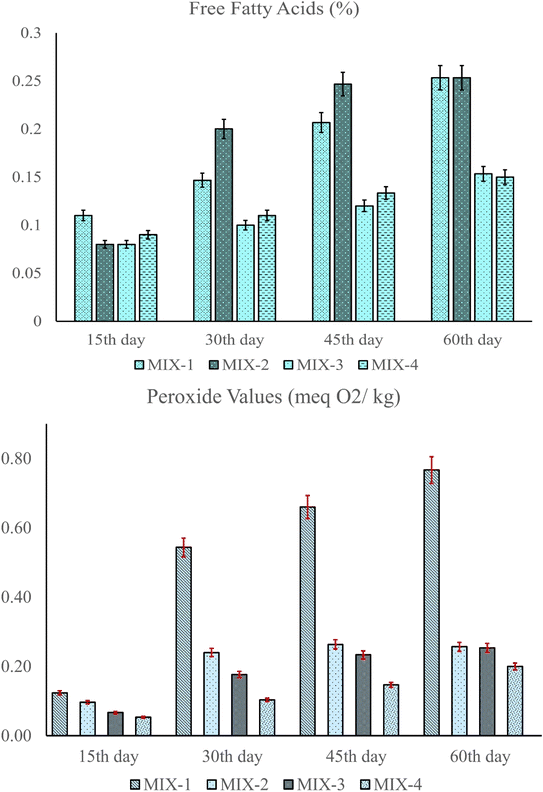 | ||
| Fig. 5 (A) Free fatty acid determination of the formulations after every 15 days. (B) Peroxide value determination of the formulations after every 15 days. | ||
Sensory analysis of the gluten-free flours was conducted in the presence of thirty semi-trained panellists to determine the overall acceptability of the final formulation (Table 5). For all the testing parameters, i.e., appearance, texture, taste, flavour, and overall acceptability, MIX-4 had the highest acceptability, followed by MIX-3. In terms of texture/mouthfeel, MIX-4 had the highest acceptability, which may be attributed to its solubility properties. A low viscosity is a desirable quality for an infant-based weaning/composite mix as it indicates suitability for consumption and easy digestion.10
| Parameters → | Appearance | Taste/flavour | Smell/odour | Texture/mouthfeel | Overall acceptability |
|---|---|---|---|---|---|
| Weaning mixes ↓ | |||||
| a Results are presented as the mean of the triplicates ± standard deviation (n = 30), with significant values (p < 0.05) depicted with alphabets (a–d) along a column using Duncan's MRT. | |||||
| MIX-1 | 3.40 ± 0.85b | 2.77 ± 0.63a | 3.17 ± 0.65a | 2.67 ± 0.55a | 3.37 ± 0.49a |
| MIX-2 | 3.30 ± 0.75a | 3.10 ± 0.75b | 3.17 ± 0.65a | 3.10 ± 0.71b | 3.33 ± 0.55a |
| MIX-3 | 3.70 ± 0.84c | 3.53 ± 0.77c | 3.73 ± 0.83b | 3.50 ± 0.57c | 3.60 ± 0.50b |
| MIX-4 | 3.87 ± 0.63d | 4.23 ± 0.63d | 4.20 ± 0.72c | 4.50 ± 0.63d | 4.07 ± 0.78c |
3.11. Principal component analysis (PCA)
The correlation between the physico-chemical, functional, and rheological properties of the gluten-free composite flours was ascertained using principal component analysis (PCA). In PCA, the similarity and disparity within the properties are represented on the X–Y axes. Principal component 1 (PC 1) comprised 84.10% of the total variations, while principal component 2 (PC 2) constituted 12.50% of the total variations in the composite flour properties, with both PC 1 and PC 2 representing 96.50% of the total variations. The properties whose curves are closer in the plot are positively correlated, while the curves in opposite directions indicate negatively correlated properties. Fig. 6 represents the biplot generated as a result of the PCA, which can be divided into four groups/quadrants. The first quadrant (green solid lines, +XY) consists of the following related properties: pasting temperature, WAC, SV, ash, % RSA, fat, TPC, FV, protein, OAC, dispersibility, solubility, BD, TD, IVSD, and EV. The second quadrant (black-dotted lines, +X −Y) comprises ΔE, L*, a*, FCI, WI, and peak time. The third quadrant (blue-dotted lines, −XY) includes the following related properties: carbohydrate, TV, PV, fiber, BV, FC, To, CI, HR, and SP. The fourth quadrant (red-dotted lines, −X +Y) represents the pasting temperature range, BI, b*, chroma, Tp, Tc, moisture, and ΔH. The PCA biplot reveals that the associated properties in the first quadrant are most likely to belong to MIX-4, as it was characterized by high rheological parameters (pasting temperature, SD, FV, BD). Similar results were reported by Marcel et al. (2021), where banana-maize and plantain-maize blends were associated with favourable rheological properties.58 Additionally, MIX-4 had the highest GBF concentration, implying an increase in the resistant starch content, which enhanced retrogradation. The hydrothermal treatment (blanching) contributed towards an increase in the WAC and OAC attributes as well. Overall, cogent rheological, pasting, and hydration properties can contribute towards the sensory acceptability of a sample, and as a result, MIX-4 was accepted by the panellists.3.12. Nutritional comparison of the selected formulation (MIX-4) with the commercial weaning mix
The characterization of the gluten-free composite flours on the basis of physico-chemical, functional, rheological, and sensorial attributes presented MIX-4 as the most favourable formulation. The concentration and pretreatment of GBF, along with the increase in the PF concentration, contributed significantly towards enhancing the protein content, total fat content, iron content, and energy value of MIX-4. Table 6 discusses the nutritional differences between MIX-4 and Bhimvita (a locally available commercial weaning formulation) based on the Food Safety and Standards Authority of India (FSSAI) guidelines for cereal-based composite foods. MIX-4 has significantly higher total fat, protein, and energy-value concentrations, which are essential nutrients that support a child's growth and development. However, as the concentration of total fat was lower than the guideline value, it is recommended to prepare the formulation in a liquid-milk-based medium to improve the total fat content.78 Thus, locally available GBF can contribute significantly towards improving the nutritional quality of cereal-based composite foods, thereby critically promoting the overall health, development and well-being of children.10| Parameters | MIX-4 (selected) | Commercial formulation (Bhimvita) | FSSAI guidelines78 |
|---|---|---|---|
| Moisture | 4.48% | — | Not more than 5% by weight |
| Ash | 2.19% | — | Not more than 5% by weight |
| Protein | 15.00% | 14.14% | Not less than 15% by weight |
| Total fat | 6.64% | 1.04% | Not less than 7.5% by weight |
| Total fibre | 1.27% | 1.35% | Not more than 1% by weight |
| Carbohydrate | 70.50% | 76.30% | Not less than 55% by weight |
| Iron | 7.36 mg/100 g | 10.70 mg/100 g | Between 3 and 7 mg/100 g |
4 Conclusion
The current study was focused on the development of a gluten-free composite mix (MIX-1 to MIX-4) using grand naine banana flour as the pivotal ingredient, which holds potential as a sustainable weaning food alternative. An exhaustive assessment found that MIX-4 had the most favourable characteristics. MIX-4 was found to be rich in protein (15.00%), fat (6.64%), carbohydrate (70.50%), and iron (7.36 mg/100 g), which was comparable to a commercially available mix (Bhimvita). Pasting and microstructural attributes revealed that MIX-4 contained resistant starch, which can be a favourable prebiotic source for early gut microbiome development among infants, and exhibited a total starch digestibility of 59.32%. These results show that processing methods can enhance the nutritional profile of flours.55 The high solubility and low swelling power of MIX-4 were conducive attributes favoring its use as a weaning formulation, as they make consumption facile and safe with minimal lump formation. Additionally, the favourable TPC (103.20 mg/100 g) and antioxidative (47.94%) characteristics of the mix contributed towards enhancing its shelf-life, as exhibited by the minimal alterations in its FFA and PV over a period of 60 days.Further improvement of the formulation can be achieved by micronutrient fortification and exploration of the influence of processing parameters, such as germination and fermentation, on banana flour. Overall, the objective of developing an affordable, low-cost, energy-rich complementary formulation (MIX-4) using grand naine banana flour with nutritional characteristics within the FSSAI guidelines, which has the potential to fulfil dual motives: address the nutritional inadequacy among infant children in Assam and provide a means of livelihood to banana farmers and producers via the manufacture of a value-added product, was successfully attained in this study.
Ethical statement
The sensory analysis study was approved by the Institutional Ethics Committee for Human Studies-IEC (H), College of Community Science, Assam Agricultural University, Jorhat with approval number: AAU/CCSc/FSN/IEC(H)/DR/PR-FSN/24-25/02. Additionally, informed consent was obtained from all the semi-trained panellists involved in the study prior to sensorial assessments of the weaning mixes in accordance with the above-referred approval given by the Institutional Ethics Committee.Author contributions
Udipta Hazarika: writing – original draft, writing – reviewing and editing, visualization, software, methodology, data curation, and conceptualization. Prapti Saikia: writing – original draft, writing – reviewing and editing, methodology, and data curation. Donee Gohain: writing – reviewing and editing, methodology, and data curation. Manisha Choudhury: validation, supervision, and conceptualization. Mamoni Das: validation, supervision, and conceptualization.Conflicts of interest
The authors declare that they have no conflict of interest related to this research article.Abbreviations
| GBF | Grand naine banana flour |
| CF | Complementary foods |
| RS | Resistant starch |
| RF | Rice flour |
| PF | Pulse/yellow lentil flour |
| SP | Sesame seed flour |
| PP | Pumpkin seed flour |
| EV | Energy value |
| WAC | Water-absorption capacity |
| OAC | Oil-absorption capacity |
| BD | Bulk density |
| TD | Tapped density |
| CI | Carr index |
| HR | Hausner's ratio |
| FC | Foaming capacity |
| SP | Swelling power |
| TPC | Total phenolic capacity |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| RSA | Radical scavenging activity |
| FCI | Flour colour index |
| WI | Whiteness index |
| BI | Browning index |
| IVSD | In vitro starch digestibility |
| RDS | Rapidly digestible starch |
| SDS | Slowly digestible starch |
| DSC | Differential scanning calorimetry |
| ΔH | Degree of gelatinization |
| FTIR | Fourier-transform infrared spectroscopy |
| SEM | Scanning electron microscopy |
| PV | Peroxide value |
| FFA | Free fatty acids |
| PCA | Principal component analysis |
| FSSAI | Food Safety and Standards Authority of India |
Data availability
All the obtained research data have been presented in this article along with the supplementary information file (SI), and the data can be provided upon request. Supplementary information is available. See DOI: https://doi.org/10.1039/d5fb00335k.Acknowledgements
The authors are thankful to Assam Agricultural University, Jorhat, Assam, India for funding and providing the necessary assistance [grant number: No. 1(34.4)/2022/DRA (E)/Part-II (G)/317]. Additionally, the authors would like to thank the National Institute of Pharmaceutical Education and Research, Guwahati (NIPER-G) for assisting with the morphological, rheological, and functional group characterization of the samples.References
- A. Amini Khoozani, J. Birch and A. E. D. A. Bekhit, J. Food Sci. Technol., 2019, 56, 548–559 Search PubMed.
- L. M. Viana, F. S. R. Rodrigues, M. C. B. Santos, A. D. S. Lima, E. H. Nabeshima, M. D. O. Leite, M. A. Martins, C. W. P. de Carvalho, V. G. Maltarollo, L. Azevedo, M. S. L. Ferreira, H. S. D. Martino, M. H. F. Felisberto and F. A. R. de Barros, Food Chem., 2024, 451, 1–11 CrossRef.
- S. Kaashoek, Ž. Malek, N. Bloemendaal and M. C. de Ruiter, Nat. Hazards Earth Syst. Sci., 2025, 25, 1963–1974 CrossRef.
- FAO, Banana Market Review 2024, Rome, 2025 Search PubMed.
- L. Chang, M. Yang, N. Zhao, F. Xie, P. Zheng, J. Simbo, X. Yu and S. kui Du, Food Biosci., 2022, 47, 101624 CrossRef CAS.
- S. Annett, S. Senthil Vinayagam, K. Akhila and P. Rahul, 25th Annual Western Hemispheric Trade Conference, Taylor and Francis, Laredo, 2021, pp. 29–36 Search PubMed.
- A. A. Khoozani, A. E. D. A. Bekhit and J. Birch, Int. J. Biol. Macromol., 2019, 130, 938–946 CrossRef CAS PubMed.
- H. Munir, H. Alam, M. T. Nadeem, R. S. Almalki, M. S. Arshad and H. A. R. Suleria, Food Nutr. Sci., 2024, 12, 3787–3805 CrossRef CAS.
- G. Gopalsamy, E. Mortimer, P. Greenfield, A. R. Bird, G. P. Young and C. T. Christophersen, Nutrients, 2019, 11(6), 1345 CrossRef CAS PubMed.
- W. Ngaha Damndja, E. S. Ngangoum, C. Saidou and S. Mohamadou, Food Chem. Adv., 2023, 3, 100313 CrossRef.
- U. Venkatesh, A. Sharma, V. A. Ananthan, P. Subbiah and R. Durga, J. Nutr. Sci., 2021, 10, e110 CrossRef CAS PubMed.
- E. A. Evans, F. H. Ballen and M. Siddiq, in Handbook of Banana Production, Postharvest Science, Processing Technology, and Nutrition, ed. M. Siddiq, J. Ahmed and M. G. Lobo, Wiley, Croydon, 1st edn, 2020, ch. 1, vol. 1, pp. 1–18 Search PubMed.
- M. Biswas, Indian J. Basic Appl. Med. Res., 2021, 10, 171–179 Search PubMed.
- S. Kabeer, N. Govindarajan, P. Radhakrishnan, H. F. Alharbi, M. M. Essa and M. W. Qoronfleh, Front. Nutr., 2023, 10, 1203955 CrossRef PubMed.
- M. A. Borbi, K. D. Dolan, M. Siddiq, S. Hooper and A. Sami, Legume Sci., 2020, 2(3), e41 CrossRef CAS.
- M. Hazarika, R. Sarma and K. K. Phukon, Agric. Sci. Digest, 2021, 41, 334–337 Search PubMed.
- Y. Mao, S. R. Dewi, S. E. Harding and E. Binner, Food Chem., 2024, 460, 140549 CrossRef CAS PubMed.
- G. Singh and K. Kaur, Food Saf. Health, 2024, 2, 106–116 Search PubMed.
- P. S. Kumar, A. Saravanan, N. Sheeba and S. Uma, LWT, 2019, 116, 108524 Search PubMed.
- N. A. Fathelrahman, N. Elhuda, A. Kheri and I. A. M. Ahmed, Innovative Rom. Food Biotechnol., 2015, 16, 9–20 Search PubMed.
- A. Dhiman K, K. Bavita, S. Attri and P. Ramachandran, Int. J. Chem. Stud., 2018, 6, 167–175 Search PubMed.
- S. R. Kumar, M. B. Sadiq, G. Singh and A. K. Anal, J. Food Sci. Technol., 2022, 59, 2821–2829 CrossRef CAS PubMed.
- S. Abbas, M. K. Sharif, M. Sibt-E-Abbas, T. Fikre Teferra, M. T. Sultan and M. J. Anwar, J. Food Qual., 2022, 2022, 1–9 CrossRef.
- AOAC, J. AOAC Int., 2010, 6, 90022–90025 Search PubMed.
- R. E. Ward and J. F. Legako, in Food Analysis, ed. S. Nielsen, Springer International, Cham, 1st edn, 2017, ch. 21, vol. 1, pp. 371–386 Search PubMed.
- S. Sharma, R. Sharma and B. Singh, Int. J. Food Sci. Technol., 2022, 57, 4744–4753 CrossRef CAS.
- A. Amini Khoozani, J. Birch and A. E. D. A. Bekhit, J. Food Meas. Char., 2020, 14, 1533–1542 CrossRef.
- B. Tiencheu, A. U. Achidi, B. T. Fossi, N. Tenyang, F. T. Ngongang and H. M. Womeni, Am. J. Food Sci. Technol., 2016, 4, 149–159 CAS.
- N. Arzoo, A. K. Shukla, S. Panwar and A. Kumar, Food Humanity, 2024, 2, 100305 Search PubMed.
- E. Elaveniya and J. Jayamuthunagai, Int. J. Chemtech. Res., 2014, 6, 4446–4454 CAS.
- S. R. Kumar, M. B. Sadiq and A. K. Anal, LWT, 2020, 128, 109465 Search PubMed.
- P. Thakaeng, T. Boonloom and S. Rawdkuen, Molecules, 2021, 26(7), 2070 CrossRef CAS PubMed.
- M. Khoza, E. Kayitesi and B. C. Dlamini, Foods, 2021, 10(12), 2894 CrossRef CAS PubMed.
- Y. Bi, Y. Zhang, H. Jiang, Y. Hong, Z. Gu, L. Cheng, Z. Li and C. Li, Food Hydrocoll., 2017, 72, 219–227 CrossRef CAS.
- J. A. John, A. Ravindran, J. A. John and S. Jacob, J. Postharvest Technol., 2021, 2, 58–70 Search PubMed.
- B. Tiencheu, A. Claudia Egbe, A. U. Achidi, E. F. T. Ngongang, N. Tenyang, F. Tonfack Djikeng and B. Tatsinkou Fossi, Food Nutr. Sci., 2021, 9, 4156–4168 CrossRef CAS PubMed.
- M. O. Adegunwa, E. O. Adelekan, A. A. Adebowale, H. A. Bakare and E. O. Alamu, Cogent Chem., 2017, 3(1), 1383707 Search PubMed.
- E. O. Anajekwu, B. Maziya-Dixon, R. Akinoso, W. Awoyale and E. O. Alamu, J. Chem., 2020, 2020(1), 5960346 Search PubMed.
- P. Chitra, Rev. Environ. Health, 2015, 30, 125–130 CAS.
- M. Domellöf, C. Braegger, C. Campoy, V. Colomb, T. Decsi, M. Fewtrell, I. Hojsak, W. Mihatsch, C. Molgaard, R. Shamir, D. Turck and J. Van Goudoever, J. Pediatr. Gastroenterol. Nutr., 2014, 58, 119–129 CrossRef.
- K. Finn, C. Callen, J. Bhatia, K. Reidy, L. J. Bechard and R. Carvalho, Nutrients, 2017, 9, 1–9 CrossRef.
- P. Udomkun, C. Tirawattanawanich, J. Ilukor, P. Sridonpai, E. Njukwe, P. Nimbona and B. Vanlauwe, Food Chem., 2019, 286, 651–658 Search PubMed.
- U. E. Inyang and S. O. Nwabueze, EAS J. Nutr. Food Sci., 2020, 2, 277–288 CrossRef.
- C. Godswill, V. Somtochukwu and C. Kate, Int. J. Adv. Sci. Res., 2019, 5, 2488–9849 Search PubMed.
- N. Savlak, B. Türker and N. Yeşilkanat, Food Chem., 2016, 213, 180–186 CrossRef CAS PubMed.
- S. Rustagi, S. Khan and T. Jain, Food Res., 2022, 6, 411–419 CAS.
- E. R. Ohizua, A. A. Adeola, M. A. Idowu, O. P. Sobukola, T. A. Afolabi, R. O. Ishola, S. O. Ayansina, T. O. Oyekale and A. Falomo, Food Nutr. Sci., 2017, 5, 750–762 CrossRef CAS PubMed.
- F. A. Mabogo, M. E. Mashau and S. E. Ramashia, Int. Food Res. J., 2021, 28, 138–147 CAS.
- S. Sharma, R. Sharma and B. Singh, Int. J. Food Sci. Technol., 2022, 57, 4744–4753 CrossRef CAS.
- R. K. Netshiheni, A. O. Omolola, T. A. Anyasi and A. I. O. Jideani, in Banana Nutrition – Function and Processing Kinetics, ed. A. I. O. Jideani and T. A. Anyasi, IntechOpen, London, 1st edn, 2020, ch. 3, vol. 1, pp. 1–20 Search PubMed.
- N. K. Amarasinghe, I. Wickramasinghe, I. Wijesekara, G. Thilakarathna and S. T. Deyalage, Int. J. Food Sci., 2021, 2020(1), 6681687 Search PubMed.
- S. Pandey, A. Kumar and P. S. Rao, Food Chem., 2021, 339, 127865 CrossRef CAS.
- S. R. Kumar, N. Tangsrianugul, J. Sriprablom, T. Winuprasith, R. Wansuksri and M. Suphantharika, Carbohydr. Polym. Technol. Appl., 2023, 6, 100399 CAS.
- M. D. Rodríguez, N. F. Bongianino, A. E. León and M. C. Bustos, Foods, 2025, 14(8), 1367 CrossRef.
- S. McKeen, W. Young, J. Mullaney, K. Fraser, W. C. McNabb and N. C. Roy, Nutrients, 2019, 11, 1–23 CrossRef.
- H. N. Ayo-Omogie, O. S. Jolayemi and C. E. Chinma, J. Agric. Food Res., 2021, 6, 100214 CAS.
- Z. H. Wang, Y. Wang, H. R. Cui, Y. W. Xia, I. Altosaar and Q. Y. Shu, J. Sci. Food Agric., 2002, 82, 745–752 CrossRef.
- N. R. Marcel, Y. Patrick, E. Seraphine and N. Robert, J. Food Meas. Char., 2021, 15, 59–70 CrossRef.
- L. C. Thomas and S. J. Schmidt, in Food Analysis, ed. S. S. Nielsen, Springer International, Cham, 5th edn, 2017, ch. 30, pp. 529–544 Search PubMed.
- R. Saini, S. Kaur, P. Aggarwal and A. Dhiman, Front. Nutr., 2023, 10, 1178797 CrossRef PubMed.
- R. Grassi de Alcântara, R. Aparecida de Carvalho and F. Maria Vanin, Food Chem., 2020, 326, 126972 CrossRef.
- N. Jan, H. R. Naik, G. Gani, O. Bashir, T. Amin, S. M. Wani and S. A. Sofi, Food Prod., Process. Nutr., 2020, 4(1), 9 CrossRef.
- I. Dankar, A. Haddarah, F. E. L. Omar, M. Pujolà and F. Sepulcre, Food Chem., 2018, 260, 7–12 CrossRef CAS.
- W. Tiboonbun, M. Sungsri-in and A. Moongngarm, World Acad. Sci. Eng. Technol., 2011, 1, 608–611 Search PubMed.
- D. Ishikawa, J. Yang, C. Ichikawa and T. Fujii, Biosci. Biotechnol. Biochem., 2021, 85, 1056–1062 CrossRef.
- M. Jafari, A. Koocheki and E. Milani, J. Cereal. Sci., 2017, 75, 324–331 CrossRef CAS.
- Đ. Tintor, K. Ninković, J. Milošević and N. Polović, Vib. Spectrosc., 2024, 134, 103726 CrossRef.
- V. Sharma and A. Bhardwaj, in Evaluation Technologies for Food Quality, ed. J. Zhong and X. Wong, Elsevier, Duxford, 2019, ch. 30, pp. 743–761 Search PubMed.
- P. Raigond, S. Dutt and B. Singh, in Bioactive Molecules in Food, ed. J. M. Merillon and K. G. Ramawat, Springer Cham, Cham, 1st edn, 2019, ch. 28, pp. 815–846 Search PubMed.
- M. Das, N. Rajan, P. Biswas and R. Banerjee, LWT, 2022, 153, 112391 CrossRef CAS.
- F. A. Hoffmann Sardá, E. B. Giuntini, M. L. P. A. Gomez, M. C. Y. Lui, J. A. E. Negrini, C. C. Tadini, F. M. Lajolo and E. W. Menezes, J. Funct.Foods, 2016, 24, 63–74 CrossRef.
- L. T. Tong, X. Gao, L. Lin, Y. Liu, K. Zhong, L. Liu, X. Zhou, L. Wang and S. Zhou, J. Cereal. Sci., 2015, 62, 45–49 CrossRef CAS.
- J. W. Irwin and N. Hedges, in Understanding and Measuring the Shelf-Life of Food, ed. R. Steele, Elsevier, Cambridge, 2004, ch. 13, pp. 289–316 Search PubMed.
- Q. Zhao, H. Guo, D. Hou, Y. Laraib, Y. Xue and Q. Shen, Cereal Chem., 2021, 98, 935–945 CrossRef CAS.
- L. Zheng, G. Zeng, S. Li, H. Li, X. Wei and H. Lei, Food Res. Int., 2023, 173, 113347 CrossRef CAS PubMed.
- M. Gogoi, M. S. Barooah, P. L. Bordoloi, P. K. Borthakur and B. Neog, Int. J. Chem. Stud., 2020, 8, 2111–2116 CrossRef CAS.
- M. P. Silva, M. J. Martinez, C. Casini and N. R. Grosso, Int. J. Food Sci. Technol., 2010, 45, 1499–1504 CrossRef CAS.
- FSSAI, Food Safety and Standards (Foods for Infant Nutrition) Regulations, 2020, Food Safety and Standards Authority of India, Delhi, 2024 Search PubMed.
| This journal is © The Royal Society of Chemistry 2026 |