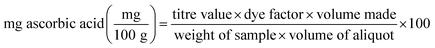Open Access Article
Open Access ArticleDevelopment and quality assessment of an antioxidant-rich Rubus squash: evaluation of physicochemical properties, bioactive compounds, and storage stability
Naseh
Nisar
a,
Sajad Mohd
Wani
 *a,
Mohd Masarat
Dar
b,
Danish
Rizwan
b and
Zahida
Naseem
a
*a,
Mohd Masarat
Dar
b,
Danish
Rizwan
b and
Zahida
Naseem
a
aDivision of Food Science and Technology, Sher-e-Kashmir University of Agricultural Sciences and Technology, Kashmir, Srinagar, Jammu and Kashmir, India 190025. E-mail: wanisajad82@gmail.com; Tel: +91-9858445878
bDepartment of Food Science and Technology, University of Kashmir, Hazratbal, Srinagar, Jammu and Kashmir 190006, India
First published on 28th October 2025
Abstract
Rubus berries, particularly blackberries, are gaining global attention for their rich nutritional and bioactive value. These berries contain essential nutrients and polyphenols, contributing to their health promoting properties. As interest grows in wild edible species, Rubus fruits offer a promising approach to diversify diets. This study focused on developing a Rubus squash as a ready-to-serve beverage. Six squash samples were analyzed for physicochemical parameters, nutritional components, color parameters (L*, a*, and b* values), bioactive compounds, antioxidant activity, and sensory attributes. Specific bioactive compounds (3,4-DHBA, gallic acid, vanillic acid, rutin, quercetin and cyanidin-3-glucoside) were analyzed using HPLC. During storage, TSS, titratable acidity, total sugars and turbidity increased from 40 to 53.09 °Bx, 1.00 to 1.40%, 31.05 to 44.61%, and 947.16 to 1099.15 NTU, respectively, while, vitamin C, phenols, flavonoids, antioxidant activity and anthocyanins decreased, from 23.75 to 14.75 mg/100 mL, 524.03 to 294.53 mg GAE/100 mL, 223.69 to 124.22 mg QE/100 mL and 83.57 to 69.75 mg/100 mL among the treatments, respectively. The bioactive compounds also showed a decline during the storage period, ranging from 26.64 to 16.02 mg L−1, 90.23 to 59.94 mg L−1, 0.65 to 0.46 mg L−1, 4.15 to 3.03 mg L−1, 90.56 to 64.22 mg L−1 and 13.55 to 10.36 mg L−1, respectively, for 3–4 DHBA, gallic acid, vanillic acid, rutin, quercetin and cyanidin-3-glucoside. However, sensory evaluation remained acceptable throughout the storage period. Treatment T4 showed optimal results with maximum vitamin C (20.28 mg/100 mL), DPPH inhibition (77.98%), anthocyanins (75.31 mg/100 mL), phenols (447.95 mg GAE/100 mL), and flavonoids (193.89 mg QE/100 mL). It also scored the highest in sensory attributes and retained maximum bioactive compounds after 6 months of storage. The findings suggest that Rubus squash could serve as a health-promoting, antioxidant-rich beverage, retaining both its sensory appeal and nutritional benefits over time.
Sustainability spotlightIn this study, the utilization of underexploited wild edible berries (Rubus spp.) from the Himalayan region has been promoted. This study addresses the food insecurity and postharvest-losses. By utilizing these nutrient-rich berries for value addition and preservation, the shelf life of these berries is also enhanced. Utilizing these berries for the development of functional foods further aligns with sustainable practices by minimizing food waste. The approach directly aligns with the UN Sustainable Development Goals: SDG 2 (Zero Hunger) by improving access to nutrient-dense food; SDG 12 (Responsible Consumption and Production) by reducing food loss and promoting sustainable resource use; and SDG 15 (Life on Land) by encouraging biodiversity conservation. This research demonstrates how underutilized natural resources can contribute to climate-resilient, sustainable food technologies and enhanced community livelihoods. |
1. Introduction
Global food systems are under stress and increasingly incapable of meeting the nutritional needs of the growing world population particularly marginalized societies.1 Rising costs of fruits and vegetables have intensified the global food crisis, further aggravated by climate change-related factors such as droughts, floods, and temperature extremes. These climate-induced challenges not only reduce crop yields but also degrade their nutritional quality, thereby posing serious threats to food security and the livelihoods of vulnerable populations.2 At the same time, valuable bioresources in the form of wild edible berries often remain underutilized and go to waste. The Himalayan region, known for its rich biodiversity, harbors a wide range of such plants, including Rubus species, which hold immense nutritional and functional potential for sustainable food systems.Rubus belongs to the family Rosaceae, which comprises thousands of species of blackberries and raspberries cultivated worldwide.3 The fruits are consumed freshly and processed into products such as juice, syrup, wine, liqueur, jam, tea, ice cream, desserts, bakery products, etc. With growing awareness about the valuable attributes of fruits, the global consumption of berries and their products has increased greatly. These berries present indispensable components of a healthy diet, providing dietary fiber, minerals (K, Ca, Mg, Fe, Zn, and Mn), vitamins (vitamin C, A, and E), other vital nutrients and phytochemicals, especially polyphenols. Major bioactive compounds reported in Rubus include gallic acid, quercetin, 3,4-dihydroxybenzoic acid, rutin, kampeferol, vanillic acid, and ellaigic acid.4 Since these berries are rich in polyphenols, they are recognized for their potential health benefits, primarily due to their antioxidant and anti-inflammatory properties. Berries exhibit various biological activities, including anti-inflammatory, antioxidant, anticancer, antiviral and antibacterial effects. They have demonstrated benefits in supporting liver and pancreas health, as well as preventing oxidative stress, cancer and cardiovascular diseases such as coronary heart disease and stroke.5Rubus extracts possess bacteriostatic and fungistatic properties, further supporting their health-promoting potential. Traditionally, Rubus has been used as a tonic for blood purification and detoxification, treating conditions like common cold, fever, diabetes and epilepsy. It has also been used to treat ulcers, stomach issues and gastric disorders like gastritis, constipation and dysentery. Additionally, Rubus exhibits wound-healing properties and may act as an anti-fertility, antimicrobial, analgesic and diuretic agents.6
Despite these benefits, Rubus remains underexploited due to the lack of awareness and its highly perishable nature. Developing value-added products could increase consumption and provide health benefits while creating employment and contributing to food security. Value addition would also ensure off-season availability. Among fruit-based beverages, squash is popular. It is a non-alcoholic concentrated beverage made from fruit juice, water and sugar or sugar substitutes, requiring dilution before consumption. Fruit squash is gaining popularity over synthetic beverages due to taste, flavor, nutritive value and storage stability. It can be consumed by children, adults and older individuals to help meet their nutritional needs particularly for micronutrients. It is a fat-free, nutrient dense beverage, rich in vitamins, minerals and naturally occurring phytonutrients that contribute to good health.
There are numerous studies that have been reported for the production of squash from conventional fruits and vegetables or their combinations. There is little or no data available for the development of Rubus squash. The study aims to investigate the potential of Rubus squash as a novel beverage option, exploring its nutritional and phytochemical composition, sensory attributes and potential health benefits.
2. Materials and methods
2.1. Raw materials
Fresh blackberries were harvested from the Zabarwan Range of Himalayas, located in Srinagar, Jammu and Kashmir, India (34.121°N, 74.9040°E). All the chemicals and standards used for experimentation were obtained from Sigma-Aldrich, New Delhi and Hi-Media, Maharashtra, India.2.2. Preparation of squash
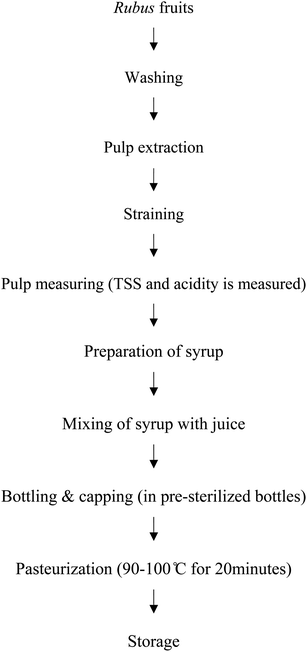
Ripe berries were selected and thoroughly washed with clean water. The berries were then subjected to pulping and the pulp was then mixed with the sugar syrup that was prepared according to the TSS required. Six treatment combinations were developed: T1, T2, and T3: contained 25% pulp with total soluble solids (TSS) 40 °Bx, 45 °Bx and 50 °Bx respectively, while T4, T5, and T6 contained 30% pulp with TSS of 40 °Bx, 45 °B and 50 °Bx respectively. The prepared squash was packaged in glass bottles and was then pasteurized (Chart 1). The samples were stored under ambient conditions for 6 months and analyzed at monthly intervals for physicochemical, nutritional, color, bioactive compounds, antioxidant activity and sensory attributes.2.3. Quality evaluation of squash
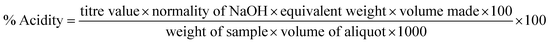 | (1) |
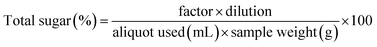 | (2) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15) and diluted to 100 mL in a volumetric flask. The mixture was refrigerated overnight at 4 °C, filtered through Whatman No. 1 filter paper, and absorbance was measured at 535 nm using a UV-vis double beam spectrophotometer (iGENE LABSERVE: IG-28DS). The results were expressed as mg anthocyanin per 100 mL of squash. Anthocyanin content was calculated using eqn (3) and (4).
15) and diluted to 100 mL in a volumetric flask. The mixture was refrigerated overnight at 4 °C, filtered through Whatman No. 1 filter paper, and absorbance was measured at 535 nm using a UV-vis double beam spectrophotometer (iGENE LABSERVE: IG-28DS). The results were expressed as mg anthocyanin per 100 mL of squash. Anthocyanin content was calculated using eqn (3) and (4). | (3) |
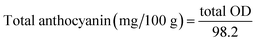 | (4) |
 | (5) |
The results were expressed as mg ascorbic acid per 100 mL of sample and was calculated as:
2.3.6.1. Sample preparation. For all treatments, phenols and flavonoids were extracted using methanol. Squash samples (2 mL) were mixed with methanol (8 mL), and centrifuged at 1680×g for 10 minutes. The supernatant was collected for total phenol and flavonoid analysis.7
2.3.6.2. Total phenolic content (TPC). The TPC of Rubus squash was evaluated using the Folin-Ciocalteu method8 and was quantified as milligrams of gallic acid equivalents per 100 mL (mg GAE/100 mL).
2.3.6.3. Total flavonoid content (TFC). The TFC of the squash extracts was assessed using the Aluminium chloride complex forming assay,9 and expressed as milligrams of quercetin equivalents per 100 mL (mg QE/100 mL).
 | (6) |
2.3.10.1. Extraction and sample preparation. Squash samples were prepared for HPLC analysis following Magiera and Zaręba11 with modifications. The samples (5 mL) were mixed with 80% methanol (20 mL), sonicated for 20 minutes, and centrifuged at 1680×g for 15 minutes. The extraction was repeated twice, and combined filtrates were concentrated using a rotary evaporator at 40 °C. The concentrated samples were stored at −20 °C and reconstituted in 3 mL of mobile phase A and B (1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) before analysis.
1) before analysis.
2.3.10.2. HPLC-DAD analysis. HPLC analysis was performed using an Agilent Technologies 1260 Infinity series system equipped with a quaternary pump, manual injector (20 μL loop), and Diode Array Detector (DAD). Separation was achieved on a Zorbax-SB C18 column (5 μm × 4.6 × 150 mm) using EZ-Chrome software for data acquisition and analysis. The mobile phase consisted of 0.1% trifluoroacetic acid in water (A) and acetonitrile (B) with a gradient elution system of 2% A and 98% B at 1 mL min−1 flow rate. The injection volume was 20 μL, with column temperature maintained at 30 °C and sampler at 4 °C. Mobile phases were filtered and degassed by sonication for 20 minutes before use. Analytes were detected using DAD at wavelengths of 230, 280, 320, and 520 nm.
2.4. Statistical analysis
The means and standard deviations of all triplicate (n = 3) measurements were calculated for each analysis in the current study. Two-way analysis of variance (ANOVA) was conducted to determine significant differences among the mean values. Subsequently, Duncan's LSD test was employed to identify specific differences, utilizing the commercial statistical package SPSS ver.11.5 (SPSS Inc., Chicago, IL, USA), with a significance level set at 5% (p ≤ 0.05). Additionally, Pearson's correlation coefficient (r) was calculated in SPSS to assess correlation between vitamin C, total phenols, total flavonoids, total anthocyanins and antioxidant activity.3. Results and discussion
3.1. Quality evaluation of Rubus squash during storage
| Treatment | S1 | S2 | S3 | S4 | S5 | S6 | S7 |
|---|---|---|---|---|---|---|---|
| a Values are expressed as mean ± standard deviation. b Values with different superscripts (small letters) (within rows) differ significantly (p ≤ 0.05). c Values with different superscripts (capital letters) (within columns) differ significantly (p ≤ 0.05). | |||||||
| TSS°Bx | |||||||
| T1 | 40.00 ± 0aA | 40.16 ± 0.03bA | 40.41 ± 0.04cA | 40.69 ± 0.03dA | 41.04 ± 0.04eA | 41.48 ± 0.03fA | 42.01 ± 0.02gA |
| T2 | 45.00 ± 0aB | 45.21 ± 0.05bB | 45.49 ± 0.03cB | 45.82 ± 0.05dB | 46.21 ± 0.03eB | 46.62 ± 0.06fB | 47.11 ± 0.05gB |
| T3 | 50.00 ± 0aC | 50.25 ± 0.03bC | 50.56 ± 0.04cC | 50.94 ± 0.06dC | 51.37 ± 0.06eC | 51.86 ± 0.04fC | 52.51 ± 0.05gC |
| T4 | 40.00 ± 0aA | 40.25 ± 0.05bA | 40.62 ± 0.05cD | 41.09 ± 0.05dD | 41.67 ± 0.07eD | 42.31 ± 0.04fD | 43.03 ± 0.05gD |
| T5 | 45.00 ± 0aB | 45.25 ± 0.02bB | 45.59 ± 0.04cB | 46.06 ± 0.03dE | 46.64 ± 0.05eE | 47.28 ± 0.03fE | 48.02 ± 0.06gE |
| T6 | 50.00 ± 0aC | 50.25 ± 0.03bC | 50.61 ± 0.04cC | 51.09 ± 0.05dF | 51.68 ± 0.03eF | 52.33 ± 0.05fF | 53.09 ± 0.06gF |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
| Titratable acidity (%) | |||||||
| T1 | 1.00 ± 0Aa | 1.00 ± 0.04Aa | 1.01 ± 0.06Aa | 1.02 ± 0.03Aa | 1.04 ± 0.04Aa | 1.07 ± 0.03Aa | 1.11 ± 0.07Aa |
| T2 | 1.00 ± 0Aa | 1.00 ± 0.08Aa | 1.02 ± 0.07Aa | 1.05 ± 0.08Aab | 1.09 ± 0.06ABab | 1.14 ± 0.04ABab | 1.20 ± 0.03ABb |
| T3 | 1.00 ± 0Aa | 1.00 ± 0.05Aa | 1.03 ± 0.04Aab | 1.07 ± 0.06Aab | 1.12 ± 0.05ABabc | 1.18 ± 0.03ABbc | 1.25 ± 0.11ABCc |
| T4 | 1.00 ± 0Aa | 1.00 ± 0.04Aa | 1.04 ± 0.05Aa | 1.09 ± 0.07Aab | 1.15 ± 0.06ABabc | 1.22 ± 0.08ABbc | 1.30 ± 0.05BCc |
| T5 | 1.00 ± 0Aa | 1.00 ± 0.08Aa | 1.05 ± 0.04Aa | 1.11 ± 0.06Aab | 1.18 ± 0.05ABabc | 1.26 ± 0.08Bbc | 1.35 ± 0.07BCc |
| T6 | 1.00 ± 0Aa | 1.00 ± 0.05Aa | 1.06 ± 0.08Aab | 1.13 ± 0.05Aab | 1.21 ± 0.06Bbc | 1.30 ± 0.07Bcd | 1.40 ± 0.04Cd |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
| Total sugars (%) | |||||||
| T1 | 31.05 ± 0.06aA | 31.28 ± 0.05bA | 31.53 ± 0.04cA | 31.83 ± 0.08dA | 32.18 ± 0.07eA | 32.55 ± 0.05fA | 32.97 ± 0.07gA |
| T2 | 35.23 ± 0.04aB | 35.48 ± 0.05bB | 35.75 ± 0.03cB | 36.06 ± 0.06dB | 36.42 ± 0.07eB | 36.81 ± 0.08fB | 37.26 ± 0.06gB |
| T3 | 39.45 ± 0.03aC | 39.72 ± 0.05bC | 40.05 ± 0.05cC | 40.41 ± 0.04dC | 40.79 ± 0.03eC | 41.21 ± 0.07fC | 41.67 ± 0.07gC |
| T4 | 33.25 ± 0.06aD | 33.51 ± 0.07bD | 33.86 ± 0.04cD | 34.27 ± 0.03dD | 34.75 ± 0.07eD | 35.31 ± 0.05fD | 35.98 ± 0.05gD |
| T5 | 37.32 ± 0.06aE | 38.12 ± 0.04bE | 38.48 ± 0.05cE | 38.82 ± 0.06dE | 39.24 ± 0.04eE | 39.69 ± 0.04fE | 40.17 ± 0.05gE |
| T6 | 42.12 ± 0.07aF | 42.45 ± 0.05bF | 42.81 ± 0.03cF | 43.21 ± 0.05dF | 43.63 ± 0.04eF | 44.10 ± 0.06fF | 44.61 ± 0.04gF |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
| Total anthocyanin content (mg/100 mL) | |||||||
| T1 | 80.72 ± 0.05aA | 79.62 ± 0.03bA | 78.32 ± 0.06cA | 76.97 ± 0.04dA | 75.59 ± 0.05eA | 74.19 ± 0.07fA | 72.74 ± 0.03gA |
| T2 | 79.05 ± 0.03Ab | 77.65 ± 0.04bB | 76.02 ± 0.07cB | 74.64 ± 0.07dB | 73.24 ± 0.05eB | 71.84 ± 0.03fB | 70.51 ± 0.07gB |
| T3 | 78.45 ± 0.04aC | 77.11 ± 0.07bC | 75.77 ± 0.05cC | 74.36 ± 0.03dC | 72.88 ± 0.05eC | 71.35 ± 0.04fC | 69.75 ± 0.06gC |
| T4 | 83.57 ± 0.04aD | 82.35 ± 0.03bD | 81.07 ± 0.05cD | 79.72 ± 0.06Dd | 78.33 ± 0.08eD | 76.86 ± 0.03fD | 75.31 ± 0.05gD |
| T5 | 82.76 ± 0.05aE | 81.51 ± 0.03bE | 80.21 ± 0.06cE | 78.83 ± 0.04dE | 77.38 ± 0.07eE | 75.89 ± 0.05fE | 74.33 ± 0.06gE |
| T6 | 81.45 ± 0.07aF | 80.22 ± 0.05bF | 78.95 ± 0.03cF | 77.62 ± 0.06dF | 76.24 ± 0.05eF | 74.83 ± 0.04fF | 73.35 ± 0.03gF |
| Treatment | S1 | S2 | S3 | S4 | S5 | S6 | S7 |
|---|---|---|---|---|---|---|---|
| a Values are expressed as mean ± standard deviation. b Values with different superscripts (small letters) (within rows) differ significantly (p ≤ 0.05). c Values with different superscripts (capital letters) (within columns) differ significantly (p ≤ 0.05). | |||||||
| Vitamin C (mg/100 mL) | |||||||
| T1 | 20.76 ± 0.03aA | 20.32 ± 0.0Ab | 19.78 ± 0.07Ac | 19.16 ± 0.05Ad | 18.41 ± 0.07Ae | 17.58 ± 0.05Af | 16.68 ± 0.04Ag |
| T2 | 19.85 ± 0.06aB | 19.37 ± 0.06Bb | 18.85 ± 0.04Bc | 18.22 ± 0.07Bd | 17.51 ± 0.08Be | 16.68 ± 0.06Bf | 15.77 ± 0.04Bg |
| T3 | 18.96 ± 0.04aC | 18.45 ± 0.03Cb | 17.91 ± 0.03Cc | 17.27 ± 0.07Cd | 16.52 ± 0.05Ce | 15.67 ± 0.04Cf | 14.75 ± 0.09Cg |
| T4 | 23.75 ± 0.06aD | 23.43 ± 0.04Db | 22.98 ± 0.05Dc | 22.43 ± 0.09Dd | 21.82 ± 0.06De | 21.09 ± 0.07Df | 20.28 ± 0.05Dg |
| T5 | 22.88 ± 0.04aE | 22.47 ± 0.09Eb | 21.93 ± 0.03Ec | 21.29 ± 0.05Ed | 20.58 ± 0.04Ee | 19.76 ± 0.05Ef | 18.82 ± 0.07Eg |
| T6 | 21.84 ± 0.03aF | 21.42 ± 0.06Fb | 20.91 ± 0.05Fc | 20.27 ± 0.04Fd | 19.55 ± 0.03Fe | 18.74 ± 0.07Ff | 17.83 ± 0.07Fg |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
| Total phenolic content (mg GAE/100 mL) | |||||||
| T1 | 433.38 ± 0.03aA | 429.13 ± 0.07bA | 418.51 ± 0.06cA | 404.39 ± 0.05dA | 386.39 ± 0.07eA | 363.27 ± 0.09fA | 335.65 ± 0.12gA |
| T2 | 414.57 ± 0.08aB | 408.62 ± 0.05bB | 400.87 ± 0.13Cb | 386.46 ± 0.07dB | 369.82 ± 0.09eB | 318.72 ± 0.08fB | 318.72 ± 0.11gB |
| T3 | 390.68 ± 0.04aC | 384.83 ± 0.07bC | 376.32 ± 0.09cC | 364.10 ± 0.06dC | 346.88 ± 0.11eC | 324.03 ± 0.05fC | 294.53 ± 0.07gC |
| T4 | 524.03 ± 0.09aD | 521.48 ± 0.06bD | 514.94 ± 0.12cD | 503.49 ± 0.08dD | 487.94 ± 0.05eD | 470.26 ± 0.07fD | 447.95 ± 0.09gD |
| T5 | 504.43 ± 0.08a | 500.78 ± 0.12bE | 495.56 ± 0.06cE | 485.11 ± 0.13dE | 468.69 ± 0.09eE | 449.34 ± 0.05fE | 426.39 ± 0.06gE |
| T6 | 480.28 ± 0.07aF | 476.63 ± 0.09bF | 470.41 ± 0.11cF | 459.36 ± 0.06dF | 442.56 ± 0.08eF | 402.28 ± 0.12fF | 399.83 ± 0.13gF |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
| Total flavonoid content (mg QE/100 mL) | |||||||
| T1 | 191.32 ± 0.06aA | 189.67 ± 0.05bA | 186.78 ± 0.08cA | 181.13 ± 0.11dA | 172.25 ± 0.07eA | 160.43 ± 0.09fA | 143.49 ± 0.11gA |
| T2 | 183.17 ± 0.09aB | 181.39 ± 0.07bB | 178.45 ± 0.05cB | 178.45 ± 0.11dB | 172.58 ± 0.08eB | 163.33 ± 0.06fB | 132.88 ± 0.13gB |
| T3 | 176.51 ± 0.07aC | 174.67 ± 0.09bC | 170.93 ± 0.11cC | 164.35 ± 0.08dC | 154.69 ± 0.12eC | 140.94 ± 0.13fC | 124.22 ± 0.09gC |
| T4 | 223.69 ± 0.11aD | 222.01 ± 0.09aD | 219.68 ± 0.05bD | 216.23 ± 0.12bD | 209.55 ± 0.06cD | 202.21 ± 0.08dD | 193.89 ± 0.12eD |
| T5 | 206.95 ± 0.08aE | 205.23 ± 0.11bE | 202.78 ± 0.09cE | 199.12 ± 0.07dE | 192.34 ± 0.12eE | 184.68 ± 0.06fE | 173.35 ± 0.07gE |
| T6 | 201.15 ± 0.12aF | 199.29 ± 0.06bF | 196.68 ± 0.09cF | 192.68 ± 0.13dF | 185.46 ± 0.06eF | 176.01 ± 0.08fF | 163.39 ± 0.11gF |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
| %DPPH inhibition activity | |||||||
| T1 | 82.98 ± 0.05aA | 81.88 ± 0.04bA | 80.58 ± 0.03cA | 79.28 ± 0.06dA | 77.98 ± 0.07eA | 76.68 ± 0.07fA | 75.38 ± 0.05gA |
| T2 | 81.81 ± 0.04aB | 80.51 ± 0.06bB | 79.21 ± 0.05cB | 77.91 ± 0.07dB | 76.61 ± 0.03eB | 75.31 ± 0.04fB | 74.01 ± 0.05gB |
| T3 | 81.72 ± 0.04aB | 80.42 ± 0.03bB | 79.12 ± 0.05cB | 77.82 ± 0.06dB | 76.52 ± 0.05eB | 75.22 ± 003fB | 73.92 ± 0.07gB |
| T4 | 85.78 ± 0.05aC | 84.48 ± 0.04bC | 83.18 ± 0.06cC | 81.88 ± 0.07dC | 80.58 ± 0.08eC | 79.28 ± 0.06fC | 77.98 ± 0.05gC |
| T5 | 84.87 ± 0.04aD | 83.57 ± 0.11bD | 82.27 ± 0.06cD | 80.97 ± 0.04dD | 79.67 ± 0.04eD | 78.37 ± 0.05fD | 77.07 ± 0.06gD |
| T6 | 84.62 ± 0.05aE | 83.32 ± 0.06bE | 82.02 ± 0.05cE | 80.72 ± 0.07dE | 79.42 ± 0.06eE | 78.12 ± 0.06fE | 76.82 ± 0.04gE |
| Treatment | S1 | S2 | S3 | S4 | S5 | S6 | S7 |
|---|---|---|---|---|---|---|---|
| a Values are expressed as mean ± standard deviation. b Values with different superscripts (small letters) (within rows) differ significantly (p ≤ 0.05). c Values with different superscripts (capital letters) (within columns) differ significantly (p ≤ 0.05). | |||||||
| L* value | |||||||
| T1 | 25.98 ± 0.14aA | 26.59 ± 0.13bA | 27.19 ± 0.12cA | 28.78 ± 0.15dA | 29.35 ± 0.12eA | 30.91 ± 0.14fA | 31.46 ± 0.15gA |
| T2 | 27.84 ± 0.13aB | 28.46 ± 0.13bB | 29.07 ± 0.15cB | 30.67 ± 0.14dB | 31.26 ± 0.13eB | 32.84 ± 0.13fB | 33.41 ± 0.15gB |
| T3 | 29.94 ± 0.12aC | 30.38 ± 0.13bC | 31.21 ± 0.12cC | 32.83 ± 0.15dC | 33.44 ± 0.13eC | 34.04 ± 0.11fC | 35.63 ± 0.11gC |
| T4 | 20.41 ± 0.13aD | 21.79 ± 0.14bD | 22.16 ± 0.17cD | 23.52 ± 0.15dD | 24.87 ± 0.14eD | 25.21 ± 0.12fD | 26.54 ± 0.15gD |
| T5 | 22.63 ± 0.15aE | 23.04 ± 0.11bE | 24.44 ± 0.12cE | 25.83 ± 0.14dE | 26.21 ± 0.13eE | 27.58 ± 0.15fE | 28.65 ± 0.16gE |
| T6 | 24.37 ± 0.14aF | 25.86 ± 0.11bF | 26.34 ± 0.15cF | 27.81 ± 0.13dF | 28.37 ± 0.12eF | 29.72 ± 0.14fF | 30.15 ± 0.13gF |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
| a* value | |||||||
| T1 | 20.99 ± 0.17aA | 20.51 ± 0.14bA | 20.03 ± 0.16cA | 19.57 ± 0.12dA | 19.12 ± 0.16eA | 18.68 ± 0.13fA | 18.25 ± 0.14gA |
| T2 | 19.44 ± 0.13aB | 19.06 ± 0.17abB | 18.69 ± 0.25bcB | 18.33 ± 0.14cdB | 17.98 ± 0.11deB | 17.64 ± 0.13efB | 17.31 ± 0.12fB |
| T3 | 18.97 ± 0.15aC | 18.59 ± 0.12abC | 18.22 ± 0.14bcB | 17.86 ± 0.13cdC | 17.51 ± 0.14deC | 17.17 ± 0.15efC | 16.85 ± 0.17fC |
| T4 | 22.48 ± 0.13aD | 22.16 ± 0.11abD | 21.84 ± 0.17bcC | 21.51 ± 0.15cdD | 21.17 ± 0.16deD | 20.83 ± 0.13efD | 20.45 ± 0.14fD |
| T5 | 22.08 ± 0.11aE | 21.76 ± 0.13abE | 21.43 ± 0.12bcCD | 21.09 ± 0.14cdE | 20.75 ± 0.11deE | 20.40 ± 0.15eE | 20.02 ± 0.16fE |
| T6 | 21.71 ± 0.14aE | 21.39 ± 0.17abE | 21.06 ± 0.16bcD | 20.71 ± 0.12cdF | 20.34 ± 0.15deF | 19.97 ± 0.14eF | 19.57 ± 0.11fF |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
| b* value | |||||||
| T1 | 0.46 ± 0.07ABa | 0.54 ± 0.08ABab | 0.62 ± 0.05Aab | 0.69 ± 0.08ABab | 0.76 ± 0.11ABab | 0.82 ± 0.09ABab | 0.87 ± 0.06ABb |
| T2 | 0.54 ± 0.12ABa | 0.63 ± 0.12ABab | 0.71 ± 0.06Aab | 0.79 ± 0.07Aab | 0.86 ± 0.09Aab | 0.93 ± 0.08Aab | 0.99 ± 0.12Ab |
| T3 | 0.68 ± 0.0.11Ba | 0.75 ± 0.09Ba | 0.82 ± 0.04Aa | 0.88 ± 0.07ADa | 0.93 ± 0.06Aa | 0.98 ± 0.03Aa | 1.02 ± 0.05Aa |
| T4 | 0.27 ± 0.06Aa | 0.31 ± 0.08Aa | 0.37 ± 0.08Aa | 0.42 ± 0.04BCDa | 0.47 ± 0.08Ba | 0.51 ± 0.05Ba | 0.54 ± 0.07Ba |
| T5 | 0.32 ± 0.09ABa | 0.39 ± 0.07ABa | 0.45 ± 0.08Aa | 0.51 ± 0.12Ca | 0.56 ± 0.08ABa | 0.61 ± 0.05ABa | 0.65 ± 0.06ABa |
| T6 | 0.39 ± 0.06ABa | 0.47 ± 0.08ABa | 0.53 ± 0.05Aa | 0.59 ± 0.07Da | 0.64 ± 0.07ABa | 0.68 ± 0.05ABa | 0.72 ± 0.11ABa |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
| Turbidity (NTU) | |||||||
| T1 | 947.16 ± 0.05aA | 947.31 ± 0.08aA | 947.76 ± 0.07bA | 948.39 ± 0.11cA | 949.11 ± 0.09dA | 949.96 ± 0.04eA | 951.01 ± 0.06fA |
| T2 | 961.24 ± 0.08aB | 961.42 ± 0.11aB | 961.93 ± 0.06bB | 962.67 ± 0.08cB | 963.58 ± 0.04dB | 964.61 ± 0.07eB | 965.72 ± 0.12fB |
| T3 | 987.35 ± 0.07aC | 987.56 ± 0.06aC | 988.13 ± 0.09bC | 988.94 ± 0.11cC | 988.87 ± 0.05dC | 990.92 ± 0.12eC | 992.08 ± 0.08fC |
| T4 | 1001.17 ± 0.11aD | 1001.49 ± 0.09bD | 1002.12 ± 0.06cD | 1002.96 ± 0.08dD | 1003.89 ± 0.12eD | 1004.94 ± 0.07fD | 1006.07 ± 0.13gD |
| T5 | 1074.22 ± 0.15aE | 1074.57 ± 0.12bE | 1075.21 ± 0.11cE | 1076.07 ± 0.09dE | 1077.02 ± 0.08eE | 1078.08 ± 0.11fE | 1079.23 ± 0.13gE |
| T6 | 1095.18 ± 0.14aF | 1095.56 ± 0.09bF | 1096.22 ± 0.11cF | 1097.11 ± 0.07dF | 1098.07 ± 0.12eF | 1099.15 ± 0.13fF | 1100.32 ± 0.08gF |
| Treatment | S1 | S2 | S3 | S4 | S5 | S6 | S7 |
|---|---|---|---|---|---|---|---|
| a Values are expressed as mean ± standard deviation. b Values with different superscripts (small letters) (within rows) differ significantly (p ≤ 0.05). c Values with different superscripts (capital letters) (within columns) differ significantly (p ≤ 0.05). | |||||||
| 3–4 DHBA (mg L− 1 ) | |||||||
| T1 | 21.81 ± 0.11aA | 21.59 ± 0.09aA | 21.29 ± 0.04bA | 20.87 ± 0.13cA | 20.33 ± 0.05dA | 19.67 ± 0.06eA | 18.91 ± 0.12fA |
| T2 | 20.47 ± 0.04aB | 20.24 ± 0.07aB | 19.93 ± 0.11bB | 19.50 ± 0.08cB | 18.96 ± 0.12dB | 18.29 ± 0.13eB | 17.51 ± 0.06fB |
| T3 | 19.02 ± 0.08aC | 18.79 ± 0.05bC | 18.47 ± 0.09cC | 18.03 ± 0.07dC | 17.49 ± 0.06eC | 16.81 ± 0.11fC | 16.02 ± 0.09gC |
| T4 | 26.64 ± 0.09aD | 24.46 ± 0.11abD | 24.21 ± 0.13bD | 23.85 ± 0.05cD | 23.36 ± 0.12dD | 22.78 ± 0.07eD | 22.11 ± 0.06fD |
| T5 | 23.95 ± 0.08aE | 23.77 ± 0.11aE | 23.52 ± 0.06bE | 23.16 ± 0.12cE | 22.66 ± 0.09dE | 22.07 ± 0.04eE | 21.39 ± 0.07fE |
| T6 | 23.02 ± 0.08aF | 22.84 ± 0.07aF | 22.59 ± 0.11bF | 22.23 ± 0.09cF | 21.73 ± 0.12dF | 21.13 ± 0.06eF | 20.45 ± 0.04fF |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
| Gallic acid (mg L− 1 ) | |||||||
| T1 | 84.48 ± 0.08aA | 83.29 ± 0.09bA | 81.72 ± 0.12cA | 79.73 ± 0.05dA | 77.20 ± 0.07eA | 74.01 ± 0.06fA | 70.54 ± 0.08gA |
| T2 | 80.46 ± 0.05aB | 79.16 ± 0.08bB | 77.53 ± 0.06cB | 75.45 ± 0.09dB | 72.87 ± 0.11eB | 69.57 ± 0.07fA | 65.99 ± 0.09gB |
| T3 | 74.61 ± 0.06aC | 73.28 ± 0.08bC | 71.63 ± 0.07cC | 69.51 ± 0.06dC | 66.89 ± 0.05eC | 63.56 ± 0.11fC | 59.94 ± 0.09gC |
| T4 | 90.23 ± 0.08aD | 89.38 ± 0.06bD | 88.11 ± 0.05cD | 86.36 ± 0.12dD | 84.09 ± 0.08eD | 81.21 ± 0.05fD | 78.06 ± 0.08gD |
| T5 | 89.02 ± 0.05aE | 88.11 ± 0.07bE | 86.79 ± 0.08cE | 84.98 ± 0.11dE | 82.67 ± 0.06eE | 79.76 ± 0.09fE | 76.54 ± 0.12gE |
| T6 | 87.17 ± 0.05aF | 86.09 ± 0.08bF | 84.64 ± 0.12cF | 82.77 ± 0.07dF | 80.37 ± 0.06eF | 77.29 ± 0.09fE | 73.94 ± 0.11gF |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
| Vanillic acid (mg L− 1 ) | |||||||
| T1 | 0.60 ± 0.06aA | 0.59 ± 0.08aA | 0.58 ± 0.04aA | 0.57 ± 0.07aA | 0.56 ± 0.04aA | 0.54 ± 0.05aA | 0.52 ± 0.06aA |
| T2 | 0.57 ± 0.05aA | 0.56 ± 0.07aA | 0.55 ± 0.08aA | 0.54 ± 0.06aA | 0.53 ± 0.09aA | 0.51 ± 0.12aA | 0.49 ± 0.06aA |
| T3 | 0.54 ± 0.06aA | 0.53 ± 0.08aA | 0.52 ± 0.05aA | 0.51 ± 0.07aA | 0.50 ± 0.12aA | 0.48 ± 0.08aA | 0.46 ± 0.07aA |
| T4 | 0.65 ± 0.11aA | 0.64 ± 0.09aA | 0.63 ± 0.12aA | 0.62 ± 0.08aA | 0.61 ± 0.09aA | 0.60 ± 0.05aA | 0.59 ± 0.12aA |
| T5 | 0.64 ± 0.08aA | 0.63 ± 0.12aA | 0.62 ± 0.06aA | 0.61 ± 0.12aA | 0.60 ± 0.08aA | 0.59 ± 0.06aA | 0.58 ± 0.09aA |
| T6 | 0.63 ± 0.11aA | 0.62 ± 0.09aA | 0.61 ± 0.12aA | 0.60 ± 0.04aA | 0.59 ± 0.07aA | 0.58 ± 0.06aA | 0.57 ± 0.05aA |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
| Rutin (mg L− 1 ) | |||||||
| T1 | 3.81 ± 0.05aAD | 3.73 ± 0.09abAD | 3.65 ± 0.11abAD | 3.57 ± 0.08bcAD | 3.48 ± 0.13cdAD | 3.39 ± 0.08cdAD | 3.30 ± 0.11dAD |
| T2 | 3.68 ± 0.06aAB | 3.60 ± 0.09aAB | 3.52 ± 0.08abAB | 3.44 ± 0.11abAB | 3.35 ± 0.06bcAB | 3.26 ± 0.09bcAB | 3.17 ± 0.12cAB |
| T3 | 3.55 ± 0.08aB | 3.47 ± 0.07abB | 3.39 ± 0.06abB | 3.30 ± 0.05bcB | 3.21 ± 0.11cdB | 3.12 ± 0.09cdB | 3.03 ± 0.13dAB |
| T4 | 4.15 ± 0.08aCD | 4.08 ± 0.11abCD | 4.01 ± 0.07abcCD | 3.94 ± 0.12abcdCD | 3.87 ± 0.09bcdCD | 3.80 ± 0.06cdC | 3.73 ± 0.13dC |
| T5 | 4.06 ± 0.05aCD | 3.99 ± 0.07abCD | 3.92 ± 0.09abcCD | 3.85 ± 0.06abcdCD | 3.78 ± 0.08bcdCD | 3.70 ± 0.12cdCD | 3.62 ± 0.11dBCD |
| T6 | 3.93 ± 0.04aD | 3.86 ± 0.06abD | 3.79 ± 0.08abD | 3.72 ± 0.12abD | 3.64 ± 0.07bcD | 3.56 ± 0.12cD | 3.48 ± 0.09cD |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
| Quercetin (mg L− 1 ) | |||||||
| T1 | 82.45 ± 0.06aA | 81.80 ± 0.05bA | 80.78 ± 0.08cA | 79.40 ± 0.11dA | 77.45 ± 0.05eA | 75.14 ± 0.12fA | 72.34 ± 0.05gA |
| T2 | 78.83 ± 0.04aB | 78.15 ± 0.08bB | 77.09 ± 0.06cB | 75.68 ± 0.09dB | 73.70 ± 0.11eB | 71.34 ± 0.08fB | 68.50 ± 0.09gB |
| T3 | 74.78 ± 0.09aC | 74.07 ± 0.05bC | 72.96 ± 0.08cC | 71.51 ± 0.04dC | 69.49 ± 0.11eC | 67.09 ± 0.06fC | 64.22 ± 0.12gC |
| T4 | 90.56 ± 0.06aD | 90.01 ± 0.08bD | 89.10 ± 0.04cD | 87.85 ± 0.07dD | 86.02 ± 0.05eD | 83.87 ± 0.06fD | 81.19 ± 0.09gD |
| T5 | 88.31 ± 0.11aE | 87.73 ± 0.06bE | 86.78 ± 0.08cE | 85.47 ± 0.05dE | 83.60 ± 0.12eE | 81.39 ± 0.07fE | 78.69 ± 0.08gE |
| T6 | 85.56 ± 0.09aF | 84.95 ± 0.07bF | 83.97 ± 0.12cF | 82.35 ± 0.06dF | 80.45 ± 0.08eF | 78.18 ± 0.09fF | 75.43 ± 0.11gF |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
| Cyanidin-3-glucoside (mg L− 1 ) | |||||||
| T1 | 12.91 ± 0.05aA | 12.81 ± 0.07abA | 12.63 ± 0.08bcA | 12.41 ± 0.11Ca | 12.10 ± 0.09dA | 11.73 ± 0.12eA | 11.30 ± 0.06fA |
| T2 | 12.52 ± 0.11aB | 12.41 ± 0.05abB | 12.23 ± 0.09bcB | 12.00 ± 0.12cB | 11.69 ± 0.04dB | 11.31 ± 0.06eB | 10.88 ± 0.12fB |
| T3 | 12.02 ± 0.08aC | 11.91 ± 0.12abC | 11.72 ± 0.05bcC | 11.49 ± 0.13cC | 11.17 ± 0.06dC | 10.79 ± 0.11eC | 10.36 ± 0.09fC |
| T4 | 13.55 ± 0.11aD | 13.46 ± 0.05aD | 13.33 ± 0.09abD | 13.12 ± 0.12bD | 12.85 ± 0.08cD | 12.52 ± 0.13dD | 12.12 ± 0.06eD |
| T5 | 13.39 ± 0.07aDE | 13.30 ± 0.05aDE | 13.17 ± 0.11abDE | 12.95 ± 0.08bDE | 12.68 ± 0.13cD | 12.34 ± 0.07dDE | 11.93 ± 0.11eDE |
| T6 | 13.18 ± 0.05aE | 13.09 ± 0.13aE | 12.95 ± 0.09abE | 12.73 ± 0.04bE | 12.45 ± 0.06cE | 12.11 ± 0.09dE | 11.70 ± 0.12eE |
This decline can be attributed to several factors. First, the decrease in phenolic compound concentrations may be due to their condensation into brown pigments. These browning reactions can reduce the overall concentration and efficacy of the bioactive phenolic compounds. Additionally, the degradation of antioxidants, such as phenolic compounds, can lead to their oxidation under favorable conditions (e.g., exposure to light and elevated temperatures). This oxidation process can compromise the structural integrity of the bioactive compounds, resulting in a loss of their antioxidant properties. Furthermore, anthocyanins are highly unstable and susceptible to degradation, especially during processing and storage. The degradation of anthocyanins can contribute to the overall decline in phenolic compound concentrations. These conditions can alter the molecular structures of the compounds, resulting in a loss of antioxidant activity and a decline in the overall quality of the stored products. Previous studies have consistently highlighted the impact of storage temperature and conditions on the stability of bioactive compounds, emphasizing the necessity for optimal storage practices to preserve their beneficial effects over time.21Rubus squash contained higher bioactive compounds as compared to blood fruit beverage which contained gallic acid (15.60 mg L−1) and quercetin (4.10 mg L−1).20
| TFC | TPC | Anthocyanins | Vitamin C | %DPPH inhibition | |
|---|---|---|---|---|---|
| TFC | 1 | 0.976 | 0.977 | 0.986 | 0.970 |
| TPC | 1 | 0.983 | 0.995 | 0.986 | |
| Anthocyanins | 1 | 0.994 | 0.976 | ||
| Vitamin C | 1 | 0.979 | |||
| %DPPH | 1 |
4. Conclusion
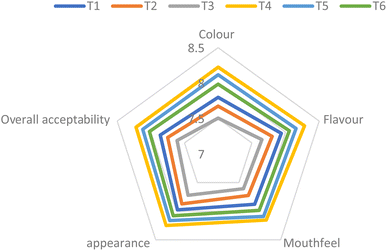
The present study investigated the impact of varying pulp concentration and total soluble solids on the physicochemical, nutritional, and sensory properties of Rubus squash samples during a 6-month storage period. The results demonstrate that both pulp concentration and TSS value significantly (p ≤ 0.05) influenced the quality attributes of the Rubus squash. Treatments with 30% pulp and 45 °Bx TSS (T4) exhibited superior retention of bioactive compounds, antioxidant activity, and sensory properties compared to other formulations. Despite the observed declines in some parameters, all samples remained acceptable for consumption throughout the storage duration.The study provides valuable insights into the stability and shelf-life of squash-based products, emphasizing the importance of optimizing formulation and storage conditions to maximize the preservation of nutritional and sensory qualities. The findings can guide the development of shelf-stable, high-quality squash products with enhanced consumer appeal and health benefits. Therefore, Rubus squash provides a sustainable, antioxidant-rich beverage that not only reduces the wastage of underutilized berries but also meets the increasing consumer demand for nutritious, natural products. Its health-promoting properties and long-term stability align with global efforts to create sustainable, functional food options, positioning it as a valuable addition to the market. For future research, a more detailed evaluation involving flavour and aroma profile should be done. There is scope for the development of blended squash by mixing the berries with other fruits. Also, the fortification of the Rubus squash can be done with vitamin D. Additionally, low sugar or honey can be used to develop Rubus squash as an alternative for health conscious and diabetic people.
Conflicts of interest
The authors declare that they do not have any competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Abbreviations
| 3,4-DHBA | 3,4-Dihydroxybenzoic acid |
| °Bx | Brix |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| GAE | Gallic acid equivalents |
| HPLC | High performance liquid chromatography |
| OD | Optical density |
| POD | Peroxidase |
| PPO | Polyphenol oxidase |
| QE | Quercetin equivalent |
| TFC | Total flavonoid content |
| TPC | Total phenolic content |
| TSS | Total soluble solids |
Data availability
The data used are provided in the manuscript.References
- S. Özokcu, Reimagining Food Security Through Sustainable Agriculture and Reduced Food Waste, 2023 Search PubMed.
- D. J. Gustafson, Indian J. Med. Res., 2013, 138, 398–410 Search PubMed.
- M. Sochor, B. Trávníček and J. C. Manning, S. Afr. J. Bot., 2018, 118, 241–259 CrossRef.
- M. Schulz and J. F. Chim, Food Biosci., 2019, 31, 100438 CrossRef CAS.
- L. Gil-Martínez, N. Mut-Salud, J. A. Ruiz-García, A. Falcón-Piñeiro, M. Maijó-Ferré, A. Baños and A. M. Gómez-Caravaca, Foods, 2023, 12, 1505, DOI:10.3390/foods12071505.
- S. Ranganna, Handbook of Analysis and Quality Control for Fruit and Vegetable Products, Tata McGraw-Hill Education, 1986 Search PubMed.
- S. M. Wani, U. Riyaz, T. A. Wani, M. Ahmad, A. Gani, F. A. Masoodi and S. A. Mir, Cogent Food Agric., 2016, 2, 1176287, DOI:10.1080/23311932.2016.1176287.
- S. M. Wani, F. A. Masoodi, M. Ahmad and S. A. Mir, J. Food Sci. Technol., 2018, 55, 4505–4514 Search PubMed.
- O. U. Shirazi, M. M. A. K. Khattak, N. A. M. Shukri and J. Pharmacogn, Phytochem., 2014, 3, 104–108 Search PubMed.
- W. Brand-Williams, M. E. Cuvelier, C. Berset and L. Wiss, Technol., 1995, 28, 25–30 CAS.
- S. Magiera and M. Zaręba, Food Anal. Methods, 2015, 8, 2665–2674 CrossRef.
- S. Kumar, R. K. Godara, S. Kumar and J. Singh, Int. J. Curr. Microbiol. Appl. Sci., 2018, 7, 827–833 CrossRef.
- N. Ullah, I. M. Qazi, S. Masroor, I. Ali, A. Khan, M. Khan and A. Gillani, J. Food Process. Technol., 2015, 6, 1, DOI:10.4172/2157-7110.1000438.
- R. Sasikumar and A. K. Jaiswal, J. Food Process. Preserv., 2022, 46(12), e17268, DOI:10.1111/jfpp.17268.
- S. S. Purewal, R. Kamboj, K. S. Sandhu, P. Kaur, K. Sharma, M. Kaur and A. K. Siroha, Appl. Food Res., 2022, 2, 100057, DOI:10.1016/j.afres.2022.100057.
- M. Nadeem, S. Tehreem, M. M. A. N. Ranjha, A. Ahmad, A. Din, G. Mueen Ud Din and A. Siddeeg, Int. J. Food Prop., 2022, 25, 661–672 CrossRef CAS.
- S. Mahnoori, J. Singh and N. Gupta, Int. J. Chem. Stud., 2020, 8, 1966–1970 CrossRef CAS.
- D. S. Sharma, R. K. Kaul, M. Sood and N. Gupta, Indian J. Hortic., 2017, 74, 299–302 CrossRef.
- B. M. Muche, R. A. Speers and H. V. Rupasinghe, Front. Nutr., 2018, 5, 100, DOI:10.3389/fnut.2018.00100.
- R. Sasikumar, K. Vivek, S. K. Panda and A. K. Jaiswal, LWT-Food Sci. Technol., 2025, 117929, DOI:10.1016/j.lwt.2025.117929.
- N. S. Mohamad Salin, W. M. Md Saad, H. R. Abdul Razak and F. Salim, Metabolites, 2022, 12, 75 CrossRef CAS.
- N. S. Thakur and A. Thakur, J. Hill Agric., 2017, 8, 87–92 CrossRef.
- R. Sasikumar, R. Nongmaithem, K. Vivek, S. Janghu, K. Govindasam and A. K. Jaiswal, LWT–Food Sci. Technol., 2024, 209, 116796 CrossRef CAS.
- H. Krishna, B. L. Attri, A. Kumar and N. Ahmed, Indian J Trad Know., 2016, 15, 417–423 Search PubMed.
- C. B. Casati, V. Sánchez, R. Baeza, N. Magnani, P. Evelson and M. C. Zamora, Int. J. Food Sci. Technol., 2012, 47, 1728–1736 CrossRef CAS.
- L. Staubmann, A. Mistlberger-Reiner, E. M. Raoui, G. Brunner, L. Sinawehl, M. Winter and M. Pignitter, Food Hydrocolloids, 2023, 138, 108436, DOI:10.1016/j.foodhyd.2022.108436.
- A. Din, S. A. H. Bukhari, A. Salam and B. Ishfaq, Internet J. Food Saf., 2011, 13, 355–360 Search PubMed.
- D. K. Chauhan, V. Puranik and G. K. Rai, Open Access Sci. Rep., 2012, 1, 541–545 Search PubMed.
- S. Akhtar, J. Ali, B. Javed and F. A. Khan, Middle-East J. Sci. Res., 2013, 16, 191–195 Search PubMed.
- N. S. Thakur, G. S. Dhaygude, V. K. Joshi and I. J. Food Ferment, Technol., 2011, 1, 237–246 Search PubMed.
- D. Shobha, H. D. Kumar, T. A. Sreeramasetty, Puttaramanaik, K. P. Gowda and G. B. Shivakumar, J. Food Sci. Technol., 2014, 51, 3154–3162 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2026 |