Open Access Article
Open Access ArticleValorization of areca nut husk and water hyacinth fibers into biodegradable plates for sustainable packaging
Bedanta
Rajbongshi
,
Akuleti
Saikumar
* and
Laxmikant S.
Badwaik
 *
*
Department of Food Engineering and Technology, School of Engineering, Tezpur University, Napaam, Assam, India. E-mail: laxmikantbadwaik@gmail.com; saikumarakuleti019@gmail.com; Fax: +91-03712-267005; Tel: +91-9706368117
First published on 13th October 2025
Abstract
This study investigates the development of biodegradable plates using cellulose fibers derived from areca nut husk and water hyacinth, aiming to create an eco-friendly alternative to conventional plastic plates and mitigate environmental concerns associated with non-degradable packaging. Raw fibers from both sources were treated with an alkali solution to enhance surface properties. Alkali-treated fibers from both sources were blended in various proportions, doped with starch as a binding agent, and processed via thermo-expansion to develop biodegradable plates. Alkali treatment showed significant improvements in the physical properties of both areca nut husk and water hyacinth fibers. Specifically, for areca nut husk, the moisture content dropped from 13.19 ± 1.21% to 9.43 ± 0.97%, bulk density increased from 0.137 ± 0.012 to 0.828 ± 0.030 g cm−3, and the water absorption index decreased from 8.59 ± 0.77 to 6.69 ± 0.32 g g−1. In parallel, water hyacinth fibers showed a reduction in moisture content from 14.76 ± 0.86% to 10.54 ± 0.69%, an increase in bulk density from 0.159 ± 0.008 to 0.413 ± 0.013 g cm−3, and a decline in the water absorption index from 10.34 ± 0.33 to 6.14 ± 0.41 g g−1. Among the tested formulations, plates made from a 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 blend of alkali-treated areca nut husk and water hyacinth fibers exhibited desirable properties like a thickness of 1.93 ± 0.06 mm, grammage of 1315.67 ± 20.55 g m−2, bulk density of 0.18 ± 0.02 g cm−3, moisture content of 10.27 ± 0.26%, water absorptiveness of 481.33 ± 61.04 g m−2, and tensile strength of 3.99 ± 0.31 MPa. Furthermore, coating with beeswax significantly improved the water resistance and overall properties of the plate. Biodegradability tests revealed that plates exposed to soil exhibited nearly 70–75% mass loss within 6 weeks. This innovative approach not only offers a practical solution for sustainable packaging but also addresses the environmental challenges posed by agricultural waste and invasive plant species.
50 blend of alkali-treated areca nut husk and water hyacinth fibers exhibited desirable properties like a thickness of 1.93 ± 0.06 mm, grammage of 1315.67 ± 20.55 g m−2, bulk density of 0.18 ± 0.02 g cm−3, moisture content of 10.27 ± 0.26%, water absorptiveness of 481.33 ± 61.04 g m−2, and tensile strength of 3.99 ± 0.31 MPa. Furthermore, coating with beeswax significantly improved the water resistance and overall properties of the plate. Biodegradability tests revealed that plates exposed to soil exhibited nearly 70–75% mass loss within 6 weeks. This innovative approach not only offers a practical solution for sustainable packaging but also addresses the environmental challenges posed by agricultural waste and invasive plant species.
Sustainability spotlightThe development of biodegradable plates using cellulose fibers of areca nut husk and water hyacinth helps create eco-friendly alternatives to conventional plastic plates and mitigate environmental concerns associated with non-degradable packaging. Alkali treatment enhances the overall properties of fibers. Beeswax coating significantly improved the water resistance of plates and it is suitable for dry and low-moisture food packaging. Utilization of agricultural waste and invasive plants for biodegradable plates is a sustainable approach. This innovative approach not only offers a practical solution for sustainable packaging but also addresses the environmental challenges posed by agricultural waste and invasive plant species. |
1. Introduction
The global packaged food market has achieved remarkable growth in recent years. The ongoing growth of packaged food can be attributed to the convenience, technological progress, and advantages of packaging.1 However, there is growing concern about the environmental impact of packaging, particularly when considering its effects across the entire lifecycle. The current state of the packaging waste management sector falls short of achieving circularity because a significant portion of food packaging is intentionally designed for one-time use and subsequently discarded within a brief time frame. Over the past five decades, plastics have been extensively employed in producing packaging materials owing to their effectiveness and ease of manufacturing. Nevertheless, their resistance to natural decomposition has led to significant detrimental impacts on our planet's delicate environment.2 The food packaging industry is experiencing significant growth within the realm of synthetic plastic packaging.3 In the pursuit of eco-friendly alternatives to replace traditional plastic packaging, there has been a significant focus on developing biodegradable and biomass-based solutions.Biodegradable packaging materials, cutlery, and serving items are gaining attention as sustainable alternatives because they naturally break down in the soil through microbial activity, resulting in reduced environmental pollution. Current research in this field focuses on utilizing agro-based polymers and agricultural by-products to create these biodegradable products.4 While using by-products can help lower raw material expenses, the additional manufacturing processes involved may increase the final price. Despite these challenges, the reuse of agricultural by-products presents a promising avenue, providing both economic and environmental benefits by promoting sustainable packaging production.5 Bio-based materials, especially natural cellulosic fibers, have gained significant interest due to their rapid decomposition, renewability, and ability to address environmental and health concerns. These fibers are widely used as reinforcements in composites to enhance key properties, such as moisture resistance and tensile strength. Increasingly adopted for their lightweight, biodegradable, carbon-neutral, and renewable nature, natural fibers support the creation of stronger, more durable, and more sustainable packaging solutions.6–8
Despite several benefits as reinforcement materials, natural fibers also have drawbacks, such as thermal instability, high moisture absorption, and poor adhesion to hydrophobic polymer matrices, which lead to dimensional instability and reduced mechanical performance.9–11 The hydrophilic nature of fibers originates from hydroxyl (–OH) groups present in cellulose, hemicellulose, and lignin, which readily form hydrogen bonds with water molecules and prevent effective fiber–matrix interaction. This results in weak interfacial bonding, swelling, and reduced composite durability.9,12,13 To overcome these limitations, various effective chemical treatments for surface modification of fibers have been employed. Chemical treatments, such as alkalization, bleaching, and acetylation, reduce the number of hydroxyl groups, remove hemicellulose, lignin, and other impurities, and expose purified cellulose, which enhances fiber reactivity and compatibility with the polymer matrix.14–16 These treatments improve fiber–matrix adhesion, leading to better stress transfer and enhanced composite performance. Studies have shown that alkali treatment with 5–6% NaOH solution significantly improves the mechanical and thermal performance of fiber composites by increasing adhesion and removing hydrophilic components.9,15,16
In the present study, alkali-treated cellulose fibers derived from areca nut husk and water hyacinth were used to develop biodegradable plates. Areca nut (also known as betel nut) is cultivated in over 12 Asian nations, yielding approximately 2.28 million tonnes annually from around 1.31 million hectares of farmland (FAOSTAT, 2023) and supporting the livelihoods of more than 10 million farmers.17,18 India holds a prominent position in the global production of areca nut, occupying the position of the largest producer on a global scale. According to 2017 statistics from the Food and Agriculture Organization (FAO), United Nations, India's output constituted a remarkable 54.07% of the world's total production. However, a substantial issue arises with the byproduct of areca nut processing.19 After kernels are extracted in small-scale facilities, the generated husk (dry or green forms) is often discarded in open areas. In India alone, according to the National Horticulture Board (NHB), the production of areca nut fruit in 2021–22 was recorded at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 371
371![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 940 tonnes, and since the fruit contains around 40% husk, an estimated 548
940 tonnes, and since the fruit contains around 40% husk, an estimated 548![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 776 tonnes of husk are generated annually. The primary constituents of areca nut husk include cellulose (35–64.8%), with notable amounts of hemicellulose, lignin (13–26%), and approximately 7% of pectin.20,21 These husks do not readily decompose due to their lignocellulosic composition, leading to environmental challenges as a large pile of husks accumulates due to inadequate disposal methods.22
776 tonnes of husk are generated annually. The primary constituents of areca nut husk include cellulose (35–64.8%), with notable amounts of hemicellulose, lignin (13–26%), and approximately 7% of pectin.20,21 These husks do not readily decompose due to their lignocellulosic composition, leading to environmental challenges as a large pile of husks accumulates due to inadequate disposal methods.22
Water hyacinth stands out as one of the most pervasive aquatic weeds globally, known for its rapid growth rate of up to 220 kg per ha per day.23–25 It can double its population within 5–15 days, depending on site conditions.26 What makes it particularly troublesome is its exceptional ability to efficiently extract nutrients from water and its high reproductive capacity.27 Agricultural nutrient runoff and excessive fertilization contribute significantly to its massive proliferation, causing the swift degradation of aquatic ecosystems.28 The robust expansion of water hyacinth mats has detrimental effects of reducing dissolved oxygen levels and evapotranspiration, which causes increased water loss.29,30 Moreover, water hyacinth tends to clog irrigation canals and cause rapid silt accumulation, resulting in inefficient flood control measures and lowering water quality for productive water uses.31 Due to these issues, numerous management strategies for tackling this weed have been implemented, involving expensive methods like chemical, physical, or biological controls. However, most of the existing approaches have provided short-term reductions in biomass and are cost-intensive and unsustainable.32 For example, heavy machinery can remove infestations rapidly, but the weeds can regrow within a few weeks in stagnant, nutrient-rich water. Chemical herbicides may show impact in days but can harm non-target aquatic species and pose ecological risks.33,34 Due to these challenges, there is a growing need for more sustainable solutions, such as converting this plant biomass into biodegradable materials through fiber-based valorization. Water hyacinth is a non-wood lignocellulosic material comprising cellulose (65.07%), hemicellulose (15.17%), and lignin (11.38%) as reported by Sumrith et al.35 Its higher cellulose and hemicellulose content, along with lower lignin compared to both woody and non-woody plants, make it ideal for pulp production. This pulp can be utilized in diverse packaging applications due to these unique composition characteristics.36
There has been a remarkable absence of research regarding the prospect of producing biodegradable plates from cellulose fibers derived from areca nut husk and limited exploration of the applicability of water hyacinth in this context. This research work seeks to address two environmental challenges, the management of areca nut husk waste and the proliferation of aquatic weed water hyacinth, by pioneering the development of biodegradable food plates from these sustainable sources. This work investigates the synergistic use of areca nut husk and water hyacinth, a combination that has not been previously explored for biodegradable plate fabrication. Blending different biomass fibers has been reported to improve composite properties by combining the strength of rigid fibers with the flexibility or network-forming ability of softer ones (e.g., paddy straw with pine needles; sugarcane bagasse with bamboo).37,38 Therefore, the proposed research aims to utilize alkali-treated fibers derived from areca nut husk and water hyacinth in conjunction with natural starch-based polymers to develop biodegradable plates with beeswax coating to enhance mechanical strength and water barrier properties. This integrated dual-fiber and surface-modification approach evaluates the structural, functional, and biodegradability characteristics of the plates for sustainable food packaging or serving applications.
2. Materials and methods
2.1. Collection of raw materials
Husks of matured areca nuts were procured from local households in Napaam, Tezpur (26.6528° N, 92.7926° E), Assam, India. Water hyacinth plants with stalks and leaves were collected from the waterbodies near the Tezpur University campus, Tezpur, Assam, India. Commercial food grade tapioca starch was supplied by Kritavin Pvt. Ltd (New Delhi, India), beeswax (solubility: 33.3 mg mL−1 of chloroform, melting range: 61–65 °C, acid value: 5–15, ester value: 75–95, and saponification value: 87–104) and guar gum (presence of starch, acid insoluble matter ≤ 7%, sulphated ash ≤ 1.5%, protein ≤ 10%, and galactomannans ≤ 70%) were supplied by Hi-Media Laboratories Private Ltd (Mumbai, India), glycerol was procured from Merck Life Science Pvt. Ltd (New Delhi, India), and sodium hydroxide was procured from Finar Chemical Limited, Ahmedabad (India).2.2. Preparation of areca nut husk and water hyacinth fibers
After removing the roots, the stalk and leaves of the water hyacinth were separated and cut into small pieces and washed under tap water. The raw areca nut husk and water hyacinth were then dried for 12 h at 70 °C and subsequently soaked separately in 6% NaOH solution at room temperature (25–30 °C) for 24 h and 12 h, respectively. The concentration of alkali solution and soaking duration of fibers were chosen based on the preliminary trials and protocols reported in the literature for treating natural fibers.39–42 After discarding the NaOH solution, fibers were immersed in distilled water for 2 h to remove the residual NaOH and soaked again in distilled water containing a small amount of 2% acetic acid. Finally, the fibers were washed repeatedly 2–3 times to remove the last traces of acid. The pH of each fiber batch was measured and found to be around neutral (7 ± 0.2). If the pH was not neutral, additional washing was carried out to remove any remaining acidic residues, ensuring that the fibers were properly cleaned and safe for further use. Alkali-treated fibers were dried in an oven at 70 °C for 24 h.39 The alkali-treated fibers were pulverized and sieved through a mesh with a size of BSS (410/1969)-52 for uniformity (300 microns). The processed fibers were then carefully stored in distinct PVC airtight containers to maintain their integrity and prevent environmental contamination.432.3. Characterization of fibers
To check the effectiveness of the alkali treatment, the raw and alkali-treated areca nut husk and water hyacinth fibers were analysed for their various properties, including physical properties, functional properties, FTIR, and thermal properties. | (1) |
 | (2) |
2.4. Preparation of biodegradable plates
For the development of the plate, a dough was formulated to provide the structural matrix of the plate. This formulation includes tapioca starch serving as the binding agent, guar gum to prevent starch settling, and glycerol as a plasticizer to provide flexibility to the plate. The addition of guar gum improves matrix uniformity and enhances the homogeneity and moldability of the dough, whereas glycerol reduces intermolecular forces between polymer chains, improves flexibility, and reduces cracking during molding and drying of the plates.50–57 In the first step, a solution was prepared by adding 5 g of tapioca starch, 3 g of guar gum, and 3 mL of glycerol as a plasticizer in 100 mL of distilled water. The resulting solution was homogenized at 1000 rpm for 5 min and subsequently heated to the gelatinization temperature (∼ 65 °C) of the starch.58,59 A dough was prepared by adding 20 g of dried fibers in different ratios into the above solution and used for making plates (Table 1). The total amount of fiber was maintained constant at 20 g across all the formulations.| Plate composition number | Areca nut husk fiber (%) | Water hyacinth fiber (%) |
|---|---|---|
| C1 | 100 | 0 |
| C2 | 80 | 20 |
| C3 | 60 | 40 |
| C4 | 50 | 50 |
| C5 | 40 | 60 |
| C6 | 20 | 80 |
| C7 | 0 | 100 |
The dough was heat-pressed manually, and the process was carried out in a circular-shaped double heat pan press mold (Mazoria, TCB-1, baking machine, India). It is powered by 230 V AC and is equipped with temperature control. Before being subjected to heat pressing, the mold was preheated to 150 °C for 2 min to ensure uniform heating. The prepared dough was placed on the bottom pan and pressed down manually with the upper pan for 7 ± 2 min at 110 °C. The prepared plates were stored for one week in a controlled environmental chamber at 50% RH and 25 °C. This step ensures moisture equilibration and structural stabilization before testing physical and mechanical properties.
2.5. Hydrophobic coating of biodegradable plates
The developed plates were selected for the coating to enhance their water barrier properties and thereby facilitate their application in food packaging or serving purposes. Beeswax was chosen due to its natural abundance, safety for food contact, and demonstrated effectiveness in previous studies and was preferred over other biopolymers such as shellac or chitosan due to its lower cost, easier processing, and higher moisture-barrier properties.43,60,61 Beeswax, after being liquefied, was applied onto the plate surfaces. This was achieved by melting the beeswax in a beaker within a hot water bath set at 70 °C. The waterproof coating was applied using a dipping method, ensuring a one-layer coating to enhance its barrier capability.62 After immersion in the molten beeswax, the plates were held vertically to allow excess wax to drip off before cooling, ensuring a uniform coating.2.6. Properties of the biodegradable plate
| Water absorptiveness (g m−2) = (m2 − m1) × F | (3) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 per test area, with a typical apparatus having a test area of 100 cm2.64
000 per test area, with a typical apparatus having a test area of 100 cm2.64
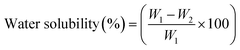 | (4) |
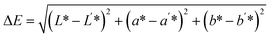 | (5) |
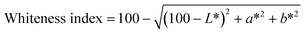 | (6) |
| Tensile strength (MPa) = F/A | (7) |
2.7. Biodegradability test
The test for the biodegradability of the plate samples was carried out by an adapted qualitative test based on the methodology proposed by Engel et al.71 A laboratory-scale soil burial experiment was performed to study the biodegradability of the samples. Vegetable compost (soil) and sand were separately placed in containers, and plate samples (2.5 × 2.5 cm) were buried completely at a depth of 10 cm. Soil was used to simulate natural composting, while sand served as an inert control with lower microbial content. The containers were kept under aerobic conditions at room temperature, with water sprayed daily to maintain soil moisture throughout the experiment. The plate samples were retrieved every 7 days, photographed, and monitored for degradation by measuring the reduction in weight of the samples. The weight loss (% degradation) of the plate samples was calculated using eqn (8).72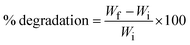 | (8) |
2.8. Statistical analysis
All the experiments were carried out with three replications, and the obtained data were reported as mean ± standard deviation (SD). One-way ANOVA was applied to determine the critical difference of mean and variance among the samples. The Duncan test with equal variances was carried out with a P < 0.05 significance level by using IBM SPSS version 23 software.733. Results and discussion
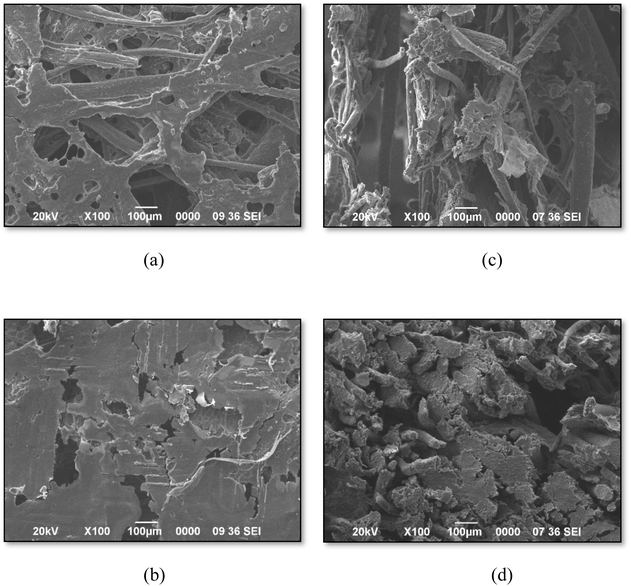
3.1. Physical properties of fibers
The physical properties of raw and alkali-treated areca nut husk and water hyacinth fibers were evaluated to assess their suitability for developing biodegradable plates, and the data are presented in Table 2. The MC, BD, TD, and porosity values were significantly different (p < 0.05) for the raw and alkali-treated fibers from both sources. The alkali treatment removes the hydrophilic non-cellulosic components like hemicellulose, lignin, and pectins from the fiber surface, making it more hydrophobic and reducing the MC.74 The lower MC improves the compatibility of areca nut husk and water hyacinth fibers with polymer matrices in composites. The BD values for raw areca fibers and water hyacinth fibers were 0.137 ± 0.012 and 0.159 ± 0.008 g cm−3, respectively, which increased to 0.828 ± 0.030 and 0.413 ± 0.013 g cm−3 after alkali treatment. This increase in BD suggests a reduction in void spaces between fibers due to the removal of impurities like waxes and hemicelluloses, resulting in a more compact fiber structure.44 True density, on the other hand, showed slight changes for raw areca nut fibers having values of 4.799 ± 0.018 g cm−3, which increased to 6.308 ± 0.333 g cm−3 after alkali treatment, while there were no significant changes for the water hyacinth fibers. In addition, the porosity of the fibers decreased post-treatment, with raw areca fibers and water hyacinth fibers initially showing porosity values of 0.972 ± 0.002 and 0.899 ± 0.004, reducing to 0.867 ± 0.002 and 0.772 ± 0.014, respectively. This decrease in porosity can be attributed to the removal of impurities and the filling of gaps between fibers, resulting in a more densely packed fiber structure.| Parameters | Raw areca nut husk fiber | Alkali-treated areca nut husk fiber | Raw water hyacinth fiber | Alkali-treated water hyacinth fiber |
|---|---|---|---|---|
| a Values are mean ± standard deviation. Different lowercase letters in the same row indicate a statistically significant (p < 0.05) difference (Duncan's multiple range test). | ||||
| Moisture content (%) | 13.191 ± 1.21b | 9.432 ± 0.973a | 14.760 ± 0.861b | 10.540 ± 0.693a |
| Bulk density (g cm−3) | 0.137 ± 0.012a | 0.828 ± 0.030c | 0.159 ± 0.008a | 0.413 ± 0.013b |
| True density (g cm−3) | 4.799 ± 0.018b | 6.308 ± 0.333c | 1.583 ± 0.070a | 1.813 ± 0.060a |
| Porosity | 0.972 ± 0.002d | 0.867 ± 0.002b | 0.899 ± 0.004c | 0.772 ± 0.014a |
| Water absorption index (g g−1) | 8.593 ± 0.769b | 6.690 ± 0.324a | 10.340 ± 0.333c | 6.143 ± 0.409a |
| Oil absorption index (g g−1) | 6.251 ± 0.997d | 3.237 ± 0.997ab | 4.523 ± 0.140b | 1.960 ± 0.022a |
| Water swelling power (g g−1) | 8.540 ± 0.250b | 7.070 ± 0.421a | 11.68 ± 0.345c | 8.097 ± 0.471b |
| Oil swelling power (g g−1) | 8.297 ± 1.034c | 7.471 ± 1.563c | 4.610 ± 0.102b | 2.000 ± 0.095a |
3.2. Functional properties of fibers
The functional properties of areca nut husk and water hyacinth fibers, both in their raw form and after alkali treatment, are presented in Table 2. Alkali treatment enhances the fiber's hydrophobic nature by removing lignin and hemicellulose components, thus increasing cellulose content, crystallinity, and surface roughness.35 The water absorption index (WAI) values for raw areca and water hyacinth fibers were 8.593 ± 0.769 and 10.34 ± 0.333 (g g−1), respectively, whereas alkali treatment reduced the WAI to 6.69 ± 0.324 and 6.143 ± 0.409 (g g−1), respectively. The WAI was significantly influenced by the alkali treatment (p < 0.05), and this decrease indicates a reduction in water uptake, attributed to the removal of hydrophilic components. Furthermore, the reduction in water absorption observed in the alkali-treated fibers is consistent with the results reported in studies on sisal fibers by Sahai et al.75 and Roystonea regia fibers by Goud & Rao,76 indicating a common trend in fiber behaviour post-alkali treatment.Similarly, the oil absorption index (OAI) decreased significantly (p < 0.05) after alkali treatment, with raw areca and water hyacinth fibers having OAI values of 6.251 ± 0.997 and 4.523 ± 0.140 (g g−1), respectively, while alkali-treated fibers showed values of 3.237 ± 0.997 and 1.96 ± 0.022 (g g−1), respectively. Lower OAI values post-treatment signify reduced oil absorption, correlating with increased hydrophobicity.
Furthermore, the water swelling power (WSP) of both fiber types decreased significantly after treatment (p < 0.05), indicating a reduced swelling capacity in water. For raw areca and water hyacinth fibers, the WSP values were 8.540 ± 0.250 and 11.68 ± 0.345 (g g−1), respectively, whereas alkali-treated fibers showed values of 7.07 ± 0.421 and 8.097 ± 0.471 (g g−1), respectively. Similarly, oil swelling power (OSP) decreased significantly (p < 0.05) in alkali-treated water hyacinth fibers, reflecting a reduced oil swelling capacity. However, the OSP of areca nut fibers didn't show a significant change after alkali treatment.
Overall, these results demonstrate that alkali treatment effectively modifies the functional properties of the fibers, making them suitable for the development of lightweight, high-performance polymer plates.
3.3. FTIR analysis of fibers
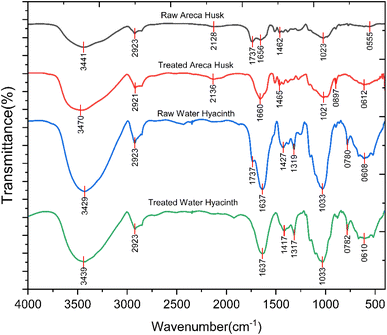
The FTIR spectral analysis reveals significant alterations in peak positions and intensities between raw and alkali-treated areca nut husk and water hyacinth fibers, indicating the efficacy of alkali treatment in modifying the fiber composition. From Fig. 1, in the raw fibers, characteristic peaks at 3441 cm−1 and 2923 cm−1 are due to the stretching vibrations of O–H and C–H bonds, respectively, indicating the presence of cellulose and hemicellulose components. Peaks at 2128 cm−1 and 1737 cm−1 suggest the presence of lignin, while peaks at 1656 cm−1, 1462 cm−1, and 1020 cm−1 may indicate various functional groups within the fiber structure. However, after alkali treatment, a notable shift is observed in some peaks, such as the increase in intensity at 3470 cm−1, indicating an enhanced presence of O–H stretching vibrations due to the removal of hemicellulose. The peak at 2136 cm−1 suggests the partial removal of lignin, corroborated by the decrease in intensity at 1660 cm−1, associated with lignin's aromatic skeletal vibrations. The reduction in peak intensity at 1465 cm−1 further supports lignin degradation, while the consistent intensity at 1021 cm−1 suggests minimal alteration in cellulose content. The appearance of a new peak at 897 cm−1 in alkali-treated fibers may indicate the presence of alkali-insoluble components, potentially residual lignin. Moreover, a peak at 612 cm−1 suggests changes in crystallinity or structural rearrangements induced by alkali treatment.35In the case of raw water hyacinth fibers, characteristic peaks at 3429 cm−1 and 2923 cm−1 correspond to O–H and C–H stretching vibrations, respectively, indicative of cellulose and hemicellulose components. Peaks at 1737 cm−1 and 1637 cm−1 suggest the presence of lignin and additional functional groups within the fiber structure. However, after alkali treatment, slight decreases in peak intensities are observed across several bands while the peak positions remain largely unchanged. Specifically, the decrease in intensity at 1637 cm−1 suggests a reduction in lignin content, indicating lignin removal or modification due to alkali treatment. Similarly, the decrease in intensity at 1417 cm−1 also supports lignin degradation, albeit to a lesser extent. Moreover, the small reductions in peak intensities at 1317 cm−1 and 782 cm−1 further suggest alterations in fiber composition, possibly reflecting the removal or modification of hemicellulose components. Interestingly, the peak intensity at 1033 cm−1 remains relatively constant, indicating minimal changes in cellulose content post-alkali treatment. These findings suggest that alkali treatment induces partial removal or modification of hemicellulose and lignin components in areca nut husk and water hyacinth fibers, leading to subtle alterations in fiber composition and potential improvements in fiber properties.48
3.4. Thermal analysis of fiber
The DSC analysis shows the impact of alkali treatment on the thermal properties of areca nut husk and water hyacinth fibers. For raw areca nut husk fibers, the DSC curve Fig. 2(a) reveals a broad endothermic peak between 50 and 80 °C, which corresponds to the evaporation of moisture within the fibers. This is followed by a smaller exothermic peak at around 340 to 360 °C for raw fibers, indicating the thermal degradation of hemicellulose and lignin. The endothermic peak related to water evaporation becomes less pronounced in alkali-treated fibers, reflecting reduced moisture content. In contrast, the alkali-treated fiber displays a sharper exothermic transition beginning near 250 °C, peaking more distinctly than the raw fiber. This is indicative of a more defined thermal degradation event, primarily associated with cellulose breakdown and partial removal of hemicellulose and lignin, which results in improved fiber crystallinity.77 Similarly, the DSC curve of water hyacinth fibers in Fig. 2(b) shows a prominent endothermic peak between 50 and 100 °C, associated with moisture evaporation. However, the alkali-treated fiber exhibits a sharper exothermic peak at around 250 to 270 °C, suggesting the removal of surface impurities.77 Similar findings were reported by Lemita et al.,78 where the DSC analysis of Strelitzia reginae fibers revealed dehydration peaks below 100 °C and stability between 160 and 260 °C. Raw fibers showed exothermic peaks at around 291.4 °C due to hemicellulose degradation. NaOH treatment improved thermal stability by reducing lignin and hemicellulose content.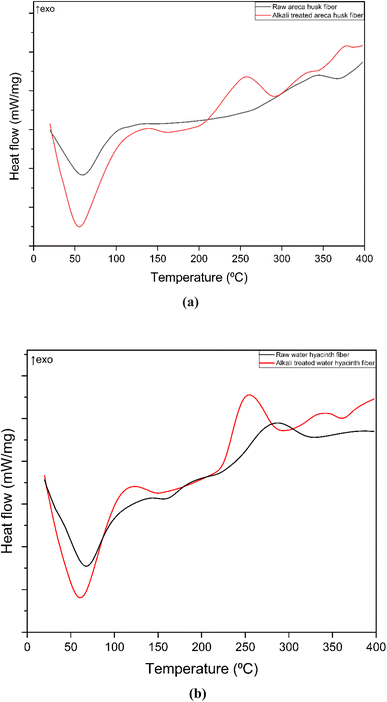 | ||
| Fig. 2 DSC curves of (a) raw and alkali-treated areca nut husk fibers and (b) raw and alkali-treated water hyacinth fibers. | ||
3.5. Physical and functional properties of biodegradable plates
| Plate number | Thickness (mm) | Grammage (g m−2) | Bulk density (g cm−3) | Moisture content (%) | Water absorptiveness (g m−2) | Absorbency (s) |
|---|---|---|---|---|---|---|
| a Values are mean ± standard deviation. Different lowercase letters in the same column indicate a statistically significant (p < 0.05) difference (Duncan's multiple range test). | ||||||
| C1 | 1.66 ± 0.19a | 994.33 ± 38.00a | 0.127 ± 0.019a | 9.27 ± 1.99a | 248.00 ± 50.46a | 5507.00 ± 202.07e |
| C2 | 1.68 ± 0.04a | 1090.00 ± 13.45b | 0.150 ± 0.019b | 10.62 ± 0.25b | 309.67 ± 55.53ab | 4632.00 ± 281.91d |
| C3 | 1.82 ± 0.05ab | 1220.23 ± 23.41c | 0.146 ± 0.006b | 11.18 ± 0.24bc | 388.90 ± 15.50bc | 592.33 ± 90.97c |
| C4 | 1.93 ± 0.06bc | 1315.67 ± 20.55d | 0.184 ± 0.006c | 10.27 ± 0.26b | 481.33 ± 61.04c | 408.00 ± 17.09bc |
| C5 | 1.95 ± 0.12bc | 1276.00 ± 28.16cd | 0.193 ± 0.004c | 13.59 ± 0.11d | 811.67 ± 45.65d | 191.00 ± 52.37ab |
| C6 | 1.98 ± 0.04bc | 1401.67 ± 80.41e | 0.196 ± 0.003c | 12.85 ± 0.24d | 1380.67 ± 117.37e | 181.67 ± 14.05ab |
| C7 | 2.05 ± 0.06d | 1483.33 ± 70.78f | 0.204 ± 0.005c | 12.47 ± 0.35cd | 1934.67 ± 113.57e | 129.33 ± 15.57a |
| Plate number | Color parameters | Tensile strength (MPa) | ||||
|---|---|---|---|---|---|---|
| L* | a* | b* | ΔE | Whiteness index | ||
| C1 | 53.05 ± 0.18e | 7.03 ± 0.07bc | 29.28 ± 0.23e | 0.00 | 44.22 ± 0.06e | 3.14 ± 0.13b |
| C2 | 48.65 ± 0.23d | 7.88 ± 0.10e | 27.89 ± 1.50e | 5.04 ± 0.29a | 41.02 ± 0.89d | 3.25 ± 0.12b |
| C3 | 45.09 ± 1.37c | 7.70 ± 0.28de | 24.16 ± 1.43d | 9.83 ± 0.30b | 39.52 ± 1.85cd | 3.31 ± 0.09b |
| C4 | 43.93 ± 0.82c | 6.83 ± 0.25b | 21.37 ± 1.38c | 12.33 ± 0.72c | 39.60 ± 1.17cd | 3.99 ± 0.31c |
| C5 | 41.59 ± 1.39b | 7.42 ± 0.31cd | 21.11 ± 0.67c | 14.32 ± 0.94d | 37.45 ± 1.51bc | 3.83 ± 0.28c |
| C6 | 39.88 ± 1.39b | 7.14 ± 0.15bc | 17.73 ± 0.78b | 17.73 ± 1.50e | 36.91 ± 1.15b | 3.07 ± 0.33b |
| C7 | 35.37 ± 1.25a | 6.17 ± 0.34a | 12.99 ± 1.19a | 24.30 ± 0.67f | 33.78 ± 1.42a | 2.53 ± 0.14a |
The grammage of the biodegradable plates exhibited variations corresponding to different compositions of areca nut husk and water hyacinth fibers (Table 3). The grammage values ranged from 994.33 ± 38.00 g m−2 for plate C1 (100% areca nut husk fiber) to 1483.33 ± 70.78 g m−2 for plate C7 (100% water hyacinth fiber). These values represent a gradual increase in mass per unit area as the proportion of water hyacinth fiber in the plate increased (p < 0.05). Such variations in grammage are indicative of changes in the density and compactness of the plate materials. As discussed by Iewkittayakorn et al.,64 variations in grammage can influence the thickness of paper products, indicating potential implications for the thickness of these biodegradable plates. Higher grammage increases transportation weight, which leads to higher logistics costs and reduced load capacity, along with more storage, handling, and environmental impacts.3 The observed differences in grammage among the plate formulations in this study may also reflect variations in plate thickness, alongside other physical properties, influenced by the composition of areca nut husk and water hyacinth fibers.
3.6. Hydrophobic coating of the selected plate with optimal composition
The optimal composition was chosen based on some key desirability aspects, such as lower BD (to reduce material handling costs), lower MC (to enhance product stability), and acceptable thickness, water absorptiveness, absorbency, TS, and visual appearance.61 According to the analysis of the desirable parameters for biodegradable plates, the combination C4 emerged as the optimal formulation for developing biodegradable composite plates. This specific blend was selected as the best proportion of fiber for applying a waterproof coating to improve the resistance of the plate against moisture. Consequently, the optimized biodegradable composite plate, coated with beeswax, is illustrated in Fig. 4. The beeswax-coated plates had a uniform, glossy finish and natural tone, which improves their aesthetic and functional appeal for food contact applications.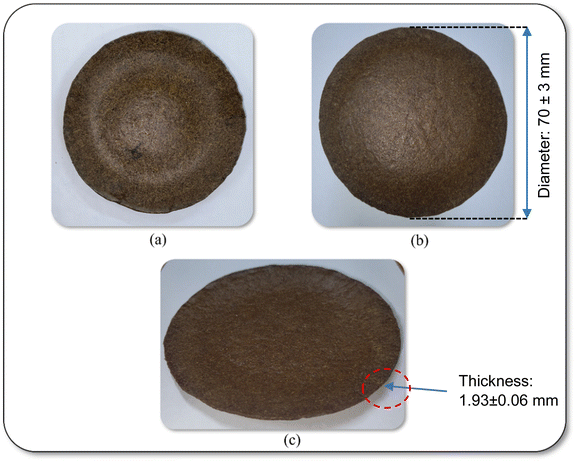 | ||
| Fig. 4 Visual appearance of the plate with final composition (C4) after coating. (a) Top view, (b) bottom view, and (c) side view. | ||
| Sample/parameters | Without coating | With coating | |
|---|---|---|---|
| a Values are mean ± standard deviation. Different lowercase letters in the same row indicate a statistically significant (p < 0.05) difference. | |||
| Thickness (mm) | 1.93 ± 0.06a | 2.01 ± 0.16a | |
| Grammage (g m−2) | 1315.67 ± 20.55a | 1537 ± 35.78b | |
| Bulk density (g cm−3) | 0.184 ± 0.01a | 0.22 ± 0.08a | |
| Water absorptiveness (g m−2) | 481.33 ± 61.04a | 57 ± 9.07b | |
| Absorbency (S) | 408.00 ± 17.09a | >5000b | |
| Water solubility (%) | 25.43 ± 3.11a | 5.32 ± 1.09b | |
| Color parameters | L* | 43.93 ± 0.82a | 38.92 ± 0.81b |
| a* | 6.83 ± 0.25a | 6.76 ± 0.05a | |
| b* | 21.37 ± 1.38a | 16.71 ± 0.29b | |
| ΔE | 12.33 ± 0.72a | 14.14 ± 0.67b | |
| Whiteness index | 39.60 ± 1.17a | 36.31 ± 0.57a | |
| Tensile strength (MPa) | 3.99 ± 0.31a | 5.01 ± 0.42b | |
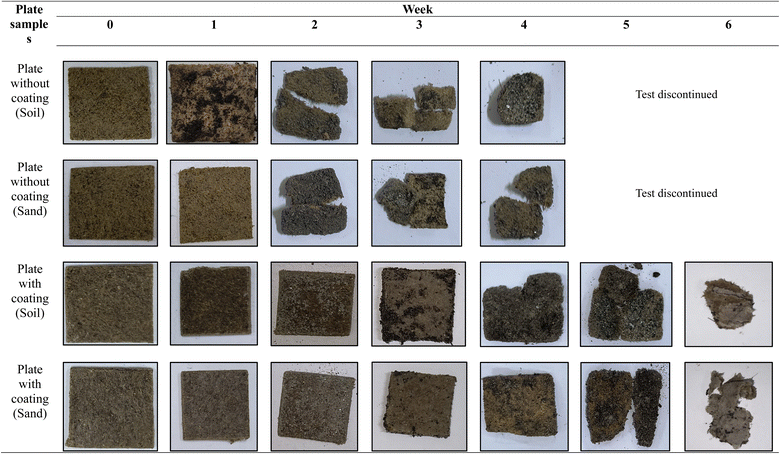
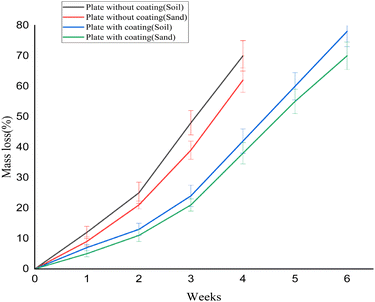
3.7. Biodegradability test
The visual signs and the mass loss of the plates (with and without coating) during the 6 weeks of biodegradation progression are illustrated in Fig. 5 and 6, respectively. From Fig. 6, it was observed that the overall mass loss of plates was higher in soil compared to sand. In addition, non-coated plates degraded more quickly than coated ones, which suggests that coating the plates with beeswax served as a barrier. This barrier restricts the contact of moisture, microbes, and other factors (oxygen and enzymes) with the underlying components of the plate matrix and delays the degradation process.During the biodegradability study, the non-coated samples showed a substantial mass loss of nearly 70% in soil and 60% in sand within just four weeks. By the end of this period, the plates had developed cracks and started disintegrating, making it impractical to continue measurements. As a result, testing of non-coated plates was discontinued beyond the fourth week. In contrast, the coated samples exhibited a relatively slower degradation pattern. Over a six-week period, they showed mass losses of about 75% in soil and 70% in sand, indicating that although coating provided some delay, the material was still significantly biodegradable over time. The primary mechanism for starch biodegradation involves the enzymatic hydrolysis of the polymer chains, resulting in the cleavage of the α-1,4 bonds in the linear chains of amylose and amylopectin.71
Furthermore, the atmospheric heat and activity of microorganisms present in the soil contribute to the degradation process by shortening and weakening the polymer chains of the starch.88 Biodegradation involves diverse microorganisms (such as cellulolytic, hemicellulolytic, pectinolytic, and lignolytic types), which break down biopolymers and convert their carbon into CO2 and restore the carbon cycle.54,89 Additionally, daily spraying of water may have interacted with the OH groups present in the starch, due to which the chains got weakened and accelerated the process of biodegradation. During this process, the molecular interaction among the starch molecules was likely to be disrupted, resulting in the observable degradation of the polymer matrix.90 Consequently, the study shows that the prepared plates can easily be disposed of in gardens and flowerbeds, offering an environmentally friendly solution that reduces pollution from plastic materials and lowers the processing costs from the waste of their disposal, as observed by Sanhawong et al.91
A previous study examined the biodegradation behaviour of corn starch-based foam plates reinforced with banana bunch stalk fibers when buried in both soil and sand for a period of five weeks. The results revealed that samples degraded more rapidly in soil than in sand, as indicated by the higher mass loss in soil. The effect of beeswax coating on the biodegradation process was also reported. Specifically, uncoated plates lost 51.7% of their mass in soil and 43.54% in sand, whereas beeswax-coated plates exhibited lower mass losses of 35.70% in soil and 18.81% in sand.54 In parallel, another study on sugarcane bagasse-based casings reported a similar trend, with approximately 50% mass loss over five weeks. In contrast, commercial expanded polystyrene (EPS) plates showed negligible changes in their mass under the same conditions.92
4. Conclusions
The study investigates the development of biodegradable plates made from cellulose fibers sourced from areca nut husk and water hyacinth, bound with tapioca starch. Alkali treatment improved the properties of the fibers, such as physical, functional, and thermal properties. A composite dough was formulated using gelatinized tapioca starch, guar gum, and glycerol, incorporating fibers in various ratios. The optimal composition, C4 (a 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 ratio of areca nut husk to water hyacinth fiber), exhibited favorable properties including thickness, density, moisture content, water absorptiveness, and tensile strength. Increasing water hyacinth content negatively impacted the plate's hydrophilicity and mechanical strength. To improve the susceptibility against moisture, the plate with optimal composition was coated with beeswax to make it suitable for foods with low and intermediate moisture content. The coated plates demonstrated superior water resistance, positioning them as viable alternatives to expanded polystyrene (EPS) plates and contributing to the reduction of plastic waste. Biodegradability tests revealed that non-coated plates degraded rapidly in both soil and sand environments, with a significant mass loss observed within weeks. Potential challenges in commercial scaling of the plate may include ensuring long-term mold reuse under repeated heating cycles, which could affect consistency and production efficiency. Another concern is the relatively higher cost of beeswax as a coating material when compared to synthetic hydrophobic alternatives. Future work could explore alternative low-cost coatings and the development of automated and high-throughput molding processes to enhance economic feasibility.
50 ratio of areca nut husk to water hyacinth fiber), exhibited favorable properties including thickness, density, moisture content, water absorptiveness, and tensile strength. Increasing water hyacinth content negatively impacted the plate's hydrophilicity and mechanical strength. To improve the susceptibility against moisture, the plate with optimal composition was coated with beeswax to make it suitable for foods with low and intermediate moisture content. The coated plates demonstrated superior water resistance, positioning them as viable alternatives to expanded polystyrene (EPS) plates and contributing to the reduction of plastic waste. Biodegradability tests revealed that non-coated plates degraded rapidly in both soil and sand environments, with a significant mass loss observed within weeks. Potential challenges in commercial scaling of the plate may include ensuring long-term mold reuse under repeated heating cycles, which could affect consistency and production efficiency. Another concern is the relatively higher cost of beeswax as a coating material when compared to synthetic hydrophobic alternatives. Future work could explore alternative low-cost coatings and the development of automated and high-throughput molding processes to enhance economic feasibility.
Ethical review
This study does not involve any human or animal testing.Author contributions
Bedanta Rajbongshi: data curation, formal analysis, investigation; writing – original draft; Akuleti Saikumar: methodology, formal analysis, resources; writing – review & editing. Laxmikant S. Badwaik: conceptualization, project administration, resources, supervision, writing – review & editing.Conflicts of interest
The authors have declared that there is no conflict of interest for this work.Data availability
The data supporting this article have been included within the article.Acknowledgements
The authors are thankful for the support received from DST-FIST and UGC-SAP for carrying out the work.References
- M. Kan and S. A. Miller, Environmental impacts of plastic packaging of food products, Resour. Conserv. Recycl., 2022, 180, 106156, DOI:10.1016/j.resconrec.2022.106156.
- H. Gupta, H. Kumar and A. K. Gehlaut, Preparation and characterization of bio-composite films obtained from coconut coir and groundnut shell for food packaging, J. Mater. Cycles Waste Manag., 2022, 24, 569–581, DOI:10.1007/s10163-021-01343-z.
- L. K. Ncube, A. U. Ude, E. N. Ogunmuyiwa, R. Zulkifli and I. N. Beas, Environmental impact of food packaging materials: A review of contemporary development from conventional plastics to polylactic acid based materials, Materials, 2020, 13, 4994, DOI:10.3390/ma13214994.
- G. Mäder, N. Rüegg, T. Tschichold and S. Yildirim, Utilizing spent coffee grounds as sustainable fillers in biopolymer composites: influence of particle size and content, Sustainable Food Technol., 2025, 3, 1151–1163, 10.1039/D5FB00187K.
- S. Polat, M.-K. Uslu, A. Aygün and M. Certel, The effects of the addition of corn husk fibre, kaolin and beeswax on cross-linked corn starch foam, J. Food Eng., 2013, 116, 267–276, DOI:10.1016/j.jfoodeng.2012.12.017.
- J. Wang, M. Euring, K. Ostendorf and K. Zhang, Biobased materials for food packaging, J. Bioresour. Bioprod., 2022, 7, 1–13, DOI:10.1016/j.jobab.2021.11.004.
- S. Paudel, S. Regmi, S. Bhattarai, A. Fennell and S. Janaswamy, Valorization of grapevine agricultural waste into transparent and high-strength biodegradable films for sustainable packaging, Sustainable Food Technol., 2025, 3, 1218–1231, 10.1039/D5FB00211G.
- H. Pulikkalparambil, S. A. Varghese, V. Chonhenchob, T. Nampitch, L. Jarupan and N. Harnkarnsujarit, Recent advances in natural fibre-based materials for food packaging applications, Polymers, 2023, 15, 1393, DOI:10.3390/polym15061393.
- V. Fiore, G. Di Bella and A. Valenza, The effect of alkaline treatment on mechanical properties of kenaf fibers and their epoxy composites, Composites, Part B, 2015, 68, 14–21, DOI:10.1016/j.compositesb.2014.08.025.
- R. Punyamurthy, D. Sampathkumar, R. P. G. Ranganagowda, B. Bennehalli and C. V. Srinivasa, Mechanical properties of abaca fiber reinforced polypropylene composites: Effect of chemical treatment by benzenediazonium chloride, J. King Saud Univ. Eng. Sci., 2017, 29, 289–294, DOI:10.1016/j.jksues.2015.10.004.
- C. Sawsen, K. Fouzia, B. Mohamed and G. Moussa, Effect of flax fibers treatments on the rheological and the mechanical behavior of a cement composite, Constr. Build. Mater., 2015, 79, 229–235, DOI:10.1016/j.conbuildmat.2014.12.091.
- B. F. Yousif, A. Shalwan, C. W. Chin and K. C. Ming, Flexural properties of treated and untreated kenaf/epoxy composites, Mater. Des., 2012, 40, 378–385, DOI:10.1016/j.matdes.2012.04.017.
- F. Jahan and M. Soni, Effects of chemical treatment on mechanical properties of various natural fiber reinforced composite: a review, Mater. Today Proc., 2021, 46, 6708–6711, DOI:10.1016/j.matpr.2021.04.175.
- R. Sukmawan, K. Kusmono and M. W. Wildan, Study of alkali and acetylation treatments on sisal fibers compatibility with low-amine/epoxy stoichiometric ratio, Results Eng., 2024, 24, 103127, DOI:10.1016/j.rineng.2024.103127.
- M. M. Kabir, H. Wang, K. T. Lau, F. Cardona and T. Aravinthan, Mechanical properties of chemically-treated hemp fibre reinforced sandwich composites, Composites, Part B, 2012, 43, 159–169, DOI:10.1016/j.compositesb.2011.06.003.
- M. P. Harikrishnan, R. Raghunathan, A. S. Warrier, M. Basil, S. K. Sahoo, R. Pandiselvam, T. Venkatesh, S. Pillai, P. Kundu and A. Kothakota, Reinforced water hyacinth based biodegradable cutlery: Green alternative to single-use plastics, Food Packag. Shelf Life, 2023, 40, 101211, DOI:10.1016/j.fpsl.2023.101211.
- P. S. Deshmukh, P. G. Patil, P. U. Shahare, G. B. Bhanage, J. S. Dhekale, K. G. Dhande and V. V. Aware, Effect of mechanical and chemical treatments of arecanut (Areca catechu L.) fruit husk on husk and its fibre, Waste Manage., 2019, 95, 458–465, DOI:10.1016/j.wasman.2019.06.026.
- UN Food and Agriculture Organization, FAOSTAT, Production/Yield quantities of Areca nuts in World, 2023, https://www.fao.org/faostat/en/#data/QCL/visualize, accessed 21 August 2025.
- L. Yusriah, S. M. Sapuan, E. S. Zainudin and M. Mariatti, Characterization of physical, mechanical, thermal and morphological properties of agro-waste betel nut (Areca catechu) husk fibre, J. Clean. Prod., 2014, 72, 174–180, DOI:10.1016/j.jclepro.2014.02.025.
- J. Yuan, H. Zhang, H. Zhao, H. Ren and H. Zhai, Study on dissociation and chemical structural characteristics of areca nut husk, Molecules, 2023, 28, 1513, DOI:10.3390/molecules28031513.
- A. Rajan, J. G. Kurup and T. E. Abraham, Biosoftening of arecanut fiber for value added products, Biochem. Eng. J., 2005, 25, 237–242, DOI:10.1016/j.bej.2005.05.011.
- G. Sunny and T. P. Rajan, Review on areca nut fiber and its implementation in sustainable products development, J. Nat. Fibers, 2021, 19, 4747–4760, DOI:10.1080/15440478.2020.1870623.
- Z. Ismail, S. Z. Othman, K. H. Law, A. H. Sulaiman and R. Hashim, Comparative Performance of Water Hyacinth (Eichhornia crassipes) and Water Lettuce (Pista stratiotes) in Preventing Nutrients Build-up in Municipal Wastewater, Clean: Soil, Air, Water, 2015, 43, 521–531, DOI:10.1002/clen.201200254.
- W. Feng, K. Xiao, W. Zhou, D. Zhu, Y. Zhou, Y. Yuan, N. Xiao, X. Wan, Y. Hua and J. Zhao, Analysis of utilization technologies for Eichhornia crassipes biomass harvested after restoration of wastewater, Bioresour. Technol., 2017, 223, 287–295, DOI:10.1016/j.biortech.2016.10.047.
- A. G. Bayrakci and G. Koçar, Second-generation bioethanol production from water hyacinth and duckweed in Izmir: a case study, Renew. Sustain. Energy Rev., 2014, 30, 306–316, DOI:10.1016/j.rser.2013.10.011.
- G. D. O. Okwadha and D. M. Makomele, Evaluation of water hyacinth extract as an admixture in concrete production, J. Build. Eng., 2018, 16, 129–133, DOI:10.1016/j.jobe.2018.01.002.
- V. B. Barua and A. S. Kalamdhad, Water hyacinth to biogas: a review, Pollut. Res., 2016, 35, 491–501 Search PubMed.
- P. Priya, S. O. Nikhitha, C. Anand, R. S. Dipin Nath and B. Krishnakumar, Biomethanation of water hyacinth biomass, Bioresour. Technol., 2018, 255, 288–292, DOI:10.1016/j.biortech.2018.01.119.
- V. D. Tobias, J. L. Conrad and B. Mahardja, Impacts of water hyacinth treatment on water quality in a tidal estuarine environment, Biol. Invasions, 2019, 21, 3479–3490, DOI:10.1007/s10530-019-02061-2.
- D. Sasaqi, P. Pranoto and P. Setyono, Caraka Tani: J. Sustain. Agric., 2019, 34, 86–100 CrossRef.
- B. A. Adanikin, G. A. Ogunwande and O. O. Adesanwo, Evaluation and kinetics of biogas yield from morning glory (Ipomoea aquatica) co-digested with water hyacinth (Eichhornia crassipes), Ecol. Eng., 2017, 98, 98–104, DOI:10.1016/j.ecoleng.2016.10.067.
- G. M. Dersseh, A. M. Melesse, S. A. Tilahun, M. Abate and D. C. Dagnew, Chapter 19 – Water hyacinth: review of its impacts on hydrology and ecosystem services—Lessons for management of Lake Tana, in Extreme Hydrology and Climate Variability, ed. A. M. Melesse, W. Abtew and G. Senay, Elsevier, 2019, pp. 237–251, DOI:10.1016/B978-0-12-815998-9.00019-1.
- K. H. Thamaga and T. Dube, Remote sensing of invasive water hyacinth (Eichhornia crassipes): A review on applications and challenges, Remote Sens. Appl.: Soc. Environ., 2018, 10, 36–46, DOI:10.1016/j.rsase.2018.02.005.
- A. E. Riner, J. S. Glueckert, C. J. Vuillequez, J. K. Leary, B. P. Sperry and G. E. Macdonald, Evaluation of water hyacinth (Eichhornia crassipes) response to herbicides using unmanned aerial system imagery, Weed Technol., 2025, 39, e59, DOI:10.1017/wet.2025.28.
- N. Sumrith, L. Techawinyutham, M. R. Sanjay, R. Dangtungee and S. Siengchin, Characterization of alkaline and silane treated fibers of ‘Water Hyacinth Plants’ and reinforcement of ‘Water Hyacinth Fibers’ with bioepoxy to develop fully biobased sustainable ecofriendly composites, J. Polym. Environ., 2020, 28, 2749–2760, DOI:10.1007/s10924-020-01810-y.
- A. Karimah, M. R. Ridho, S. S. Munawar, D. S. Adi, I. Ismadi, R. Damayanti, B. Subiyanto, W. Fatriasari and A. Fudholi, A review on natural fibers for development of eco-friendly bio-composite: characteristics, and utilizations, J. Mater. Res. Technol., 2021, 13, 2442–2458, DOI:10.1016/j.jmrt.2021.06.014.
- A. Gupta, G. Singh, P. Ghosh, K. Arora and S. Sharma, Development of biodegradable tableware from novel combination of paddy straw and pine needles: A potential alternative against plastic cutlery, J. Environ. Chem. Eng., 2023, 11, 111310, DOI:10.1016/j.jece.2023.111310.
- C. Liu, P. Luan, Q. Li, Z. Cheng, X. Sun, D. Cao and H. Zhu, Biodegradable, hygienic, and compostable tableware from hybrid sugarcane and bamboo fibers as plastic alternative, Matter, 2020, 3, 2066–2079, DOI:10.1016/j.matt.2020.10.004.
- D. Dhanalakshmi, R. Punyamurthy and B. Basavaraju, A study of the effect of chemical treatments on areca fiber reinforced polypropylene composite properties, Sci. Eng. Compos. Mater., 2017, 24, 501–520, DOI:10.1515/secm-2015-0292.
- S. Chonsakorn, S. Srivorradatpaisan and R. Mongkholrattanasit, Effects of different extraction methods on some properties of water hyacinth fiber, J. Nat. Fibers, 2018, 16, 1015–1025, DOI:10.1080/15440478.2018.1448316.
- N. Muralidhar, K. Vadivuchezhian, V. Arumugam and I. S. Reddy, Flexural modulus of epoxy composite reinforced with arecanut husk fibre (AHF): A mechanics approach, Mater. Today: Proc., 2020, 27, 2265–2268, DOI:10.1016/j.matpr.2019.09.109.
- M. M. Owen, E. O. Achukwu and H. Md Akil, Preparation and mechanical characterizations of water hyacinth fiber based thermoset epoxy composite, J. Nat. Fibers, 2022, 19, 13970–13984, DOI:10.1080/15440478.2022.2113850.
- S. Chaireh, P. Ngasatool and K. Kaewtatip, Novel composite foam made from starch and water hyacinth with beeswax coating for food packaging applications, Int. J. Biol. Macromol., 2020, 165, 1382–1391, DOI:10.1016/j.ijbiomac.2020.10.007.
- P. Senthamaraikannan and M. Kathiresan, Characterization of raw and alkali treated new natural cellulosic fiber from Coccinia grandis L, Carbohydr. Polym., 2018, 186, 332–343, DOI:10.1016/j.carbpol.2018.01.072.
- M. Fazaeli, Z. Emam-Djomeh, A. Kalbasi Ashtari and M. Omid, Effect of spray drying conditions and feed composition on the physical properties of black mulberry juice powder, Food Bioprocess Technol., 2012, 90, 667–675, DOI:10.1016/j.fbp.2012.04.006.
- A. R. P. Kingsly, D. B. Singh, M. R. Manikantan and R. K. Jain, Moisture dependent physical properties of dried pomegranate seeds (Anardana), J. Food Eng., 2006, 75, 492–496, DOI:10.1016/j.jfoodeng.2005.04.033.
- H. Y. Tiong, S. K. Lim, Y. L. Lee, C. F. Ong and M. K. Yew, Environmental impact and quality assessment of using eggshell powder incorporated in lightweight foamed concrete, Constr. Build. Mater., 2020, 244, 118341, DOI:10.1016/j.conbuildmat.2020.118341.
- R. Aidoo, I. N. Oduro, J. K. Agbenorhevi, W. O. Ellis and N. B. Pepra-Ameyaw, Physicochemical and pasting properties of flour and starch from two new cassava accessions, Int. J. Food Prop., 2022, 25, 561–569, DOI:10.1080/10942912.2022.2052087.
- E. Elaveniya and J. Jayamuthunagai, Functional, physicochemical and anti-oxidant properties of dehydrated banana blossom powder and its incorporation in biscuits, Int. J. ChemTech Res., 2014, 6, 4446–4454 CAS.
- B. P. Meshram, P. Jain and K. K. Gaikwad, Innovative Development of Kodo Millet (Paspalum scrobiculatum)-Based Functional Edible Cups Modified with Hibiscus Powder and Guar Gum: An Eco-Efficient Resource Utilization, ACS Food Sci. Technol., 2025, 5, 788–799, DOI:10.1021/acsfoodscitech.4c00985.
- S. K. H. Bukharya, F. K. Choudharya, D. N. Iqbal, Z. Ali, A. Sadiqa, S. Latif, K. M. Al-Ahmary, S. Basheer, I. Ali and M. Ahmed, Development and characterization of a biodegradable film based on guar gum-gelatin@sodium alginate for a sustainable environment, RSC Adv., 2024, 14, 19349–19361, 10.1039/D4RA03985H.
- N. Pal and M. Agarwal, Development and characterization of eco-friendly guar gum-agar-beeswax-based active packaging film for cheese preservation, Int. J. Biol. Macromol., 2024, 277, 134333, DOI:10.1016/j.ijbiomac.2024.134333.
- C. M. Machado, P. Benelli and I. C. Tessaro, Study of interactions between cassava starch and peanut skin on biodegradable foams, Int. J. Biol. Macromol., 2020, 147, 1343–1353, DOI:10.1016/j.ijbiomac.2019.10.098.
- S. Marimuthu, A. Saikumar and L. S. Badwaik, Development and characterization of biodegradable foam plates from corn starch and banana bunch stalks coated with beeswax, Biomass Convers. Biorefin., 2025, 15, 7763–7777, DOI:10.1007/s13399-024-05782-0.
- J. S. Ng, P. L. Kiew and M. K. Lam, et al., Preliminary evaluation of the properties and biodegradability of glycerol- and sorbitol-plasticized potato-based bioplastics, Int. J. Environ. Sci. Technol., 2022, 19, 1545–1554, DOI:10.1007/s13762-021-03213-5.
- J. Tarique, S. M. Sapuan and A. Khalina, Effect of glycerol plasticizer loading on the physical, mechanical, thermal, and barrier properties of arrowroot (Maranta arundinacea) starch biopolymers, Sci. Rep., 2021, 11, 13900, DOI:10.1038/s41598-021-93094-y.
- D. C. M. Ferreira, G. Molina and F. M. Pelissari, Biodegradable trays based on cassava starch blended with agroindustrial residues, Composites, Part B, 2020, 183, 107682, DOI:10.1016/j.compositesb.2019.107682.
- M. Y. Naz, S. A. Sulaiman, B. Ariwahjoedi and K. Z. Shaari, Characterization of modified tapioca starch solutions and their sprays for high temperature coating applications, Sci. World J., 2014, 2014, 375206, DOI:10.1155/2014/375206.
- J. Huang, M. Wei, R. Ren, H. Li, S. Liu and D. Yang, Morphological changes of blocklets during the gelatinization process of tapioca starch, Carbohydr. Polym., 2017, 163, 324–329, DOI:10.1016/j.carbpol.2017.01.083.
- A. Karnwal, G. Kumar, R. Singh, M. Selvaraj, T. Malik and A. R. M. Al Tawaha, Natural biopolymers in edible coatings: Applications in food preservation, Food Chem.: X, 2025, 25, 102171, DOI:10.1016/j.fochx.2025.102171.
- S. Buxoo and P. Jeetah, Feasibility of producing biodegradable disposable paper cup from pineapple peels, orange peels and Mauritian hemp leaves with beeswax coating, SN Appl. Sci., 2020, 2, 1359, DOI:10.1007/s42452-020-3164-7.
- N. J. Changmai and L. S. Badwaik, Effect of Polyvinyl Alcohol, Starch and Modified Bee Wax on Properties of Sweet Lime Pomace Based Biodegradable Containers, J. Packag. Technol. Res., 2021, 5, 107–114, DOI:10.1007/s41783-021-00116-1.
- ISO, 536:2019, Paper and board—Determination of grammage, available online: https://www.iso.org/standard/77583.html, accessed 21 August 2025.
- J. Iewkittayakorn, P. Khunthongkaew, Y. Wongnoipla, K. Kaewtatip, P. Suybangdum and A. Sopajarn, Biodegradable plates made of pineapple leaf pulp with biocoatings to improve water resistance, J. Mater. Res. Technol., 2020, 9, 5056–5066, DOI:10.1016/j.jmrt.2020.03.023.
- N. Kaisangsri, O. Kerdchoechuen and N. Laohakunjit, Biodegradable foam tray from cassava starch blended with natural fiber and chitosan, Ind. Crops Prod., 2012, 37, 542–546, DOI:10.1016/j.indcrop.2011.07.034.
- ASTM, D 644–99 (Reapproved 2002), Standard Test Method for Moisture Content of Paper and Paperboard by Oven Drying, available online: https://file.yzimgs.com/175706/2011090910013559.pdf, accessed 21 August 2025.
- ISO, 535:2023, Paper and board—Determination of water absorptiveness—Cobb method, available online, https://www.iso.org/standard/80320.html, accessed 21 August 2025.
- N. H. P. Rodrigues, J. T. de Souza, R. L. Rodrigues, M. H. G. Canteri, S. M. K. Tramontin and A. C. de Francisco, Starch-based foam packaging developed from a by-product of potato industrialization (Solanum tuberosum L.), Appl. Sci., 2020, 10, 2235, DOI:10.3390/app10072235.
- M. M. Hassan, N. Tucker and M. J. Le Guen, Thermal, mechanical and viscoelastic properties of citric acid-crosslinked starch/cellulose composite foams, Carbohydr. Polym., 2020, 230, 115675, DOI:10.1016/j.carbpol.2019.115675.
- ASTM, D3039/D3039M, Standard Test Method for Tensile Properties of Polymer Matrix Composite Materials, ASTM International, 2014, https://file.yizimg.com/175706/2012061422194947.pdf, accessed 21 August 2025.
- J. B. Engel, A. Ambrosi and I. C. Tessaro, Development of biodegradable starch-based foams incorporated with grape stalks for food packaging, Carbohydr. Polym., 2019, 225, 115234, DOI:10.1016/j.carbpol.2019.115234.
- K. Kapila, S. Kirtania and L. M. Devi, et al., Potential perspectives on the use of poly (vinyl alcohol)/graphene oxide nanocomposite films and its characterization, J. Food Meas. Char., 2024, 18, 1012–1025, DOI:10.1007/s11694-023-02264-1.
- R. Rani and L. S. Badwaik, Functional properties of oilseed cakes and defatted meals of mustard, soybean and flaxseed, Waste Biomass Valorization, 2021, 12, 5639–5647, DOI:10.1007/s12649-021-01407-z.
- N. Muralidhar, V. Kaliveeran, V. Arumugam and I. Srinivasula Reddy, A study on areca nut husk fibre extraction, composite panel preparation and mechanical characteristics of the composites, J. Inst. Eng. (India): Ser. D, 2019, 100, 135–145, DOI:10.1007/s40033-019-00186-1.
- R. S. Sahai, D. Biswas, M. D. Yadav, A. Samui and S. Kamble, Effect of alkali and silane treatment on water absorption and mechanical properties of sisal fiber reinforced polyester composites, Metall. Mater. Eng., 2022, 28, 641–656, DOI:10.56801/MME864.
- G. Goud and R. N. Rao, Effect of fibre content and alkali treatment on mechanical properties of Roystonea regia-reinforced epoxy partially biodegradable composites, Bull. Mater. Sci., 2011, 34, 1575–1581, DOI:10.1007/s12034-011-0361-4.
- G. Manikandan, T. P. Sathishkumar and L. Rajeshkumar, Extraction and characterization of novel biomass-based lignocellulosic fiber Ficus benghalensis bark for potential green material applications, Biomass Convers. Biorefin., 2025, 15, 4835–4847, DOI:10.1007/s13399-024-05829-2.
- L. N. Lemita, S. Deghboudj, M. Rokbi, F. M. Rekbi and R. Halimi, Characterization and analysis of novel natural cellulosic fiber extracted from Strelitzia reginae plant, J. Compos. Mater., 2022, 56, 99–114, DOI:10.1177/00219983211049285.
- C. M. Machado, P. Benelli and I. C. Tessaro, Sesame cake incorporation on cassava starch foams for packaging use, Ind. Crops Prod., 2017, 102, 115–121, DOI:10.1016/j.indcrop.2017.03.007.
- L. R. P. F. Mello and S. Mali, Use of malt bagasse to produce biodegradable baked foams made from cassava starch, Ind. Crops Prod., 2014, 55, 187–193, DOI:10.1016/j.indcrop.2014.02.015.
- D. Behera, S. S. Pattnaik, S. S. Patra, A. K. Barick, J. Pradhan and A. K. Behera, Development and characterization of water hyacinth reinforced thermoplastic starch as sustainable biocomposites, RSC Sustain., 2025, 3, 1807–1818, 10.1039/D4SU00803K.
- C. M. Machado, P. Benelli and I. C. Tessaro, Constrained Mixture Design to Optimize Formulation and Performance of Foams Based on Cassava Starch and Peanut Skin, J. Polym. Environ., 2019, 27, 2224–2238, DOI:10.1007/s10924-019-01518-8.
- J. Yang, Y. Li, X. Li, M. Ji, S. Peng, J. Man, L. Zhou, F. Li and C. Zhang, Starch-fiber foaming biodegradable composites with polylactic acid hydrophobic surface, Int. J. Biol. Macromol., 2024, 267, 131406, DOI:10.1016/j.ijbiomac.2024.131406.
- F. J. Aranda-García, R. González-Núñez, C. F. Jasso-Gastinel and E. Mendizabal, Water absorption and thermomechanical characterization of extruded starch/poly (lactic acid)/agave bagasse fiber bioplastic composites, Int. J. Polym. Sci., 2015, 2015, 343294, DOI:10.1155/2015/343294.
- M. P. Harikrishnan, A. Thampi, A. M. Nandhu Lal, A. S. Warrier, M. Basil and A. Kothakota, Effect of chitosan-based bio coating on mechanical, structural and physical characteristics of microfiber based paper packaging: An alternative to wood pulp/plastic packaging, Int. J. Biol. Macromol., 2023, 253, 126888, DOI:10.1016/j.ijbiomac.2023.126888.
- P. R. Salgado, V. C. Schmidt, S. E. Molina Ortiz, A. N. Mauri and J. B. Laurindo, Biodegradable foams based on cassava starch, sunflower proteins and cellulose fibers obtained by a baking process, J. Food Eng., 2008, 85, 435–443, DOI:10.1016/j.jfoodeng.2007.08.005.
- A. I. Quilez-Molina, J. F. Le Meins, B. Charrier and M. Dumon, Starch-fibers composites, a study of all-polysaccharide foams from microwave foaming to biodegradation, Carbohydr. Polym., 2024, 328, 121743, DOI:10.1016/j.carbpol.2023.121743.
- P. Cerruti, G. Santagata, G. Gomez d'Ayala, V. Ambrogi, C. Carfagna, M. Malinconico and P. Persico, Effect of a natural polyphenolic extract on the properties of a biodegradable starch-based polymer, Polym. Degrad. Stab., 2011, 96, 839–846, DOI:10.1016/j.polymdegradstab.2011.02.003.
- P. Cinelli, E. Chiellini, J. W. Lawton and S. H. Imam, Foamed articles based on potato starch, corn fibers and poly(vinyl alcohol), Polym. Degrad. Stab., 2006, 91, 1147–1155, DOI:10.1016/j.polymdegradstab.2005.07.001.
- C. M. Jaramillo, T. J. Gutiérrez, S. Goyanes, C. Bernal and L. Famá, Biodegradability and plasticizing effect of yerba mate extract on cassava starch edible films, Carbohydr. Polym., 2016, 151, 150–159, DOI:10.1016/j.carbpol.2016.05.025.
- W. Sanhawong, P. Banhalee, S. Boonsang and S. Kaewpirom, Effect of concentrated natural rubber latex on the properties and degradation behavior of cotton-fiber-reinforced cassava starch biofoam, Ind. Crops Prod., 2017, 108, 756–766, DOI:10.1016/j.indcrop.2017.07.046.
- S. Nida, J. A. Moses and C. Anandharamakrishnan, 3D printed food package casings from sugarcane bagasse: a waste valorization study, Biomass Convers. Biorefin., 2025, 15, 1835–1845, DOI:10.1007/s13399-021-01982-0.
| This journal is © The Royal Society of Chemistry 2026 |